Abstract
Objectives
Diagnosing hypertrophic cardiomyopathy (HCM) can be challenging due to its nonspecific clinical manifestations, variability in electrocardiographic (ECG) patterns, and limited access to echocardiography, the gold standard for diagnosis, often leading to delayed detection. Recent artificial intelligence (AI) advancements have enabled ECG-based algorithms to improve HCM detection. This systematic review and meta-analysis aim to assess the overall diagnostic performance of AI-enhanced ECG in identifying HCM.
Methods
This study followed the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines. Articles were retrieved from PubMed, EBSCO, and Proquest. Inclusion criteria encompassed all studies evaluating AI algorithms for the detection of HCM from 12-lead ECGs. Meta-analysis was performed using R v4.4.1. Bivariate random-effects models were employed to derive pooled estimates of sensitivity, specificity, and the area under the curve (AUC) of the summary receiver operating characteristic (SROC).
Results
A total of five retrospective cohort studies involving 69,343 participants, were included. The pooled sensitivity of AI-enhanced ECG for detecting HCM was 0.84, and the specificity was 0.86. The AI-enhanced ECG demonstrated excellent diagnostic accuracy, with an SROC-AUC of 0.927 in detecting HCM.
Conclusion
AI-enhanced ECG shows promise as a novel screening tool for detecting hypertrophic cardiomyopathy. However, the considerable heterogeneity and the limited number of studies necessitate careful interpretation and highlight the need for additional research in the future.
Keywords: AI-enhanced ECG, Diagnostic, Hypertrophic cardiomyopathy
1. Introduction
Hypertrophic cardiomyopathy (HCM) is a genetic condition affecting cardiac myocytes, resulting in pathological myocardial hypertrophy independent of external factors such as pressure or volume overload [1]. The estimated prevalence of HCM in the general adult population ranges from 0.16 % to 0.29 %, translating to approximately 1 in 625 to 1 in 344 individuals. In the United States, HCM stands as the most common genetic heart disease. The prevalence is estimated at 1 in 500 individuals [2], while in the United Kingdom and Germany, it is reported as 4.15 and 8.61 per 10,000 people, respectively [3]. Maron et al. identified HCM in approximately 100,000 patients, though estimates suggest that the condition may affect around 700,000 people in the United States. These studies suggest that a significant number of HCM patients in the US remain clinically unrecognized, leading to a much lower number of diagnosed cases compared to the estimated prevalence in the general population [4].
Standard 2D echocardiography and magnetic resonance imaging (MRI) are the gold standard diagnostic modalities to diagnose hypertrophic cardiomyopathy (HCM). HCM is defined by a left ventricular wall thickness of at least 15 mm in any myocardial segment, or 13 mm in individuals with a first-degree relative diagnosed with HCM, assuming no abnormal loading conditions or alternative causes of left ventricular hypertrophy (LVH), such as hypertension or valvular heart disease, are present [5,6]. The 12-lead electrocardiogram (ECG) is an important diagnostic tool for assessing patients with cardiovascular symptoms, providing valuable insights into cases of confirmed or suspected hypertrophic cardiomyopathy (HCM) [7]. There are several key ECG findings associated with HCM, such as LVH based on voltage criteria reflecting the thickened ventricular walls, especially in the left ventricle, leading to high-voltage QRS complexes; small, deep Q waves may be present in the inferolateral leads (II, III, aVF, V5–V6), often due to septal hypertrophy; inverted T waves and ST-segment changes are frequently seen, especially in the lateral and anterolateral leads (V4–V6), indicating repolarization abnormalities [7]. However, ECG interpretation requires a high level of expertise, and there are no definitive ECG characteristics unique to HCM. Given its low sensitivity and specificity, AI-enhanced ECG algorithms hold significant promise for identifying occult cardiovascular disease, particularly cardiomyopathies [7].
One of the most clinically challenging scenarios is distinguishing between HCM and physiological cardiac remodeling in athletes, commonly called “Athlete’s heart”. Structural changes seen in well-trained individuals, including increased left ventricular wall thickness, can mimic those observed in HCM [8]. This makes the differentiation between benign adaptation and pathological hypertrophy particularly difficult in pre-participation cardiovascular screening programs [9]. Considering the significant global healthcare burden posed by cardiovascular diseases, particularly hypertrophic cardiomyopathy (HCM), there is great potential for artificial intelligence (AI) applications across various clinical fields within cardiovascular medicine [10]. Recent studies have shown that AI-enhanced ECG is a promising diagnostic modality for the early detection of HCM and may serve as an effective screening tool [11–15]. This finding also has the potential to reduce the number of undiagnosed HCM cases worldwide. This systematic review and meta-analysis aim to evaluate the diagnostic accuracy of AI-enhanced ECG for HCM across all populations.
2. Material and methods
2.1. Study methodology
This study followed the guidance of the Cochrane Handbook for Systematic Reviews of Intervention v6.5. This systematic review was conducted in line with PRISMA statement guidelines [16]. This study was registered on PROSPERO with the registration number CRD42024573038.
2.2. Search strategy
Three databases were chosen for a literature search: PubMed, EBSCO, and ProQuest, which included literature evaluating AI-enhanced ECG for HCM diagnosis. Keywords used within each database were “Hypertrophic Cardiomyopathy”, “ECG”, and “Artificial intelligence” without any limitation on language or publication period. Two independent reviewers (FAT, RS) conducted a literature search independently, searches were done and completed before 22 March 2025. Any disagreement was resolved by consensus. The keywords are provided in Table 1.
Table 1.
PICOTS-SD criteria.
| Patients | Patients diagnosed with Hypertrophic Cardiomyopathy |
|---|---|
| Intervention or Exposure | Artificial-intelligence enhanced electrocardiography |
| Comparator | Echocardiography |
| Outcomes | Diagnostic accuracy (sensitivity, specificity, and area under curve) |
| Time | No publication year restrictions |
| Setting | Subjects visiting medical facilities |
| Study Design | Observational studies (Cohort and Cross-sectional) |
2.3. Study selection
Results collected from the search were imported from the database to EndNote X9, then removing any possible duplicates through all the articles. Three independent reviewers (FAT, LF, DAT) contributed to each phase of the review by thoroughly screening and searching through the titles and abstracts of the relevant articles. Furthermore, the data collection was inserted into a predesigned data extraction form then relevant titles and abstracts were retrieved for full-text review. The following course of action was to solve possible differences between reviewers concerning the study using consensus.
2.4. Eligibility criteria
Certain criteria should be met regarding the eligibility for inclusion: (a) Studies involving the use of artificial intelligence for ECG analysis, with the AI algorithm specifically employing a convolutional neural network (CNN) and (b) involving adults and pediatric patients with hypertrophic cardiomyopathy. The main outcome assessed was diagnostic test accuracy. Exclusion criteria include: (a) Pilot or feasibility studies (b) Studies for which the full text was unable to be retrieved (c) Studies not published in English and (d) Studies that did not provide sufficient data to construct a 2 × 2 contingency table (i.e., true positives, false positives, true negatives, and false negatives). On condition when the related study provided vague details, further follow-up was made by contacting the authors to verify such information. The chosen articles were eventually excluded if the author did not respond.
2.5. Data extraction and risk of bias assessment
Meticulous data extraction was done from each study. These include: (1) First author and year of publication; (2) Study characteristics, composed of study design, location, intervention, and comparison; (3) Subject characteristics; and (4) outcomes. The main outcome was diagnostic test accuracy.
Risk of Bias for the subsequent studies was analyzed with the QUADAS-2, containing four domains. The four domains were patient selection, index test, reference standard, and flow and timing. After that, each study was categorized as low, some concern, or unclear, according to the tool’s recommendation. The risk of bias assessment was managed in pairs (FAT, LF) and disagreements that may stem were revised with a proper review team discussion [17].
2.6. Statistical analysis
Diagnostic test accuracies were pooled as sensitivity, specificity, and the area under the curves (AUCs) of summary receiver operating characteristic (SROC) using a bivariate random effect model. Meta-analysis was done using R v4.4.1. A random-effects model was determined as a result, heterogeneity must be expected. Heterogeneity was assessed between chosen studies using the chi-square test, Cochran Q-test, and I2 value - categorized as might not be important (0–40 %), may represent moderate heterogeneity (30–60 %), may represent substantial heterogeneity (50–90 %) and considerable heterogeneity (75–100 %) [18].
3. Results
3.1. Search strategy
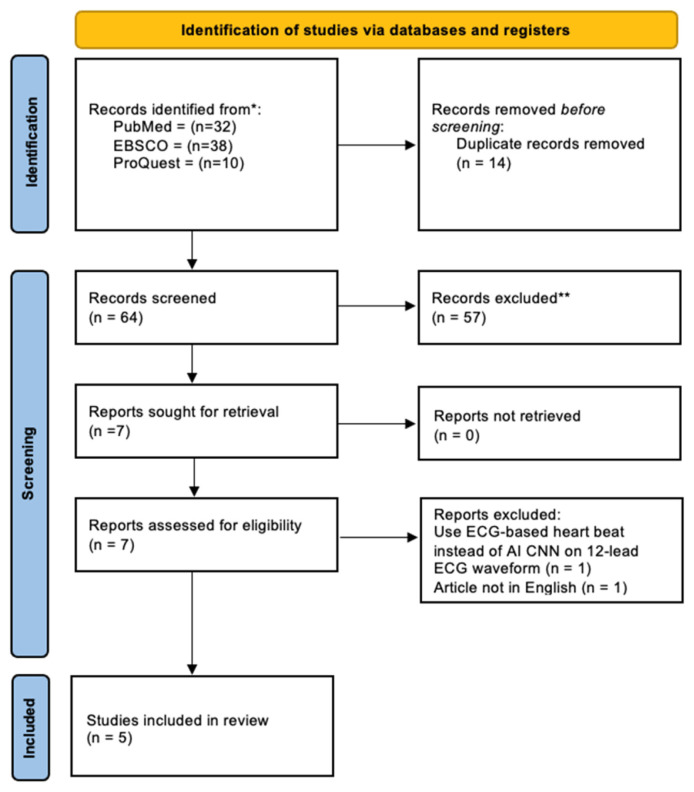
A flow chart illustrating the research selection process and its outcomes is presented in Fig. 1. The initial search strategy resulted in 80 potentially relevant studies. Based on the selection criteria, seven studies were chosen for full-text review. Among these, one study was excluded because it was not published in English, and another study was excluded due to the use of a different diagnostic method (ECG-based heartbeat analysis) which was not relevant to our inclusion criteria. Ultimately, five studies were included in the systematic review, and five of these studies met the criteria for data extraction and were incorporated into the meta-analysis (see Table 2).
Fig. 1.
Prisma 2020 flow diagram of included studies.
Table 2.
Main keyword for search strategy.
| Databases | Keyword |
|---|---|
| PubMed | (((“Cardiomyopathy, Hypertrophic” [Mesh]) OR (“Hypertrophic Cardiomyopathies” OR “Hypertrophic Cardiomyopathy” OR “Hypertrophic Obstructive Cardiomyopathies” OR “Hypertrophic Obstructive Cardiomyopathy”)) AND ((“Electrocardiography” [Mesh]) OR (“ECG” [Title/Abstract] OR “EKG” [Title/Abstract] OR “Cardiography” [Title/Abstract] OR “Cardiographies” [Title/Abstract] OR “Electrocardiogram” [Title/Abstract] OR “Electrocardiograms” [Title/Abstract] OR “Electrocardiograph” [Title/Abstract] OR “Electrocardiographs” [Title/Abstract] OR “12-Lead ECG” [Title/Abstract] OR “12 Lead ECG” [Title/Abstract] OR “12-Lead ECGs” [Title/Abstract] OR “12-Lead EKG” [Title/Abstract] OR “12 Lead EKG” [Title/Abstract] OR “12-Lead EKGs” [Title/Abstract] OR “12-Lead Electrocardiography” [Title/Abstract] OR “12-Lead Electrocardiographies” [Title/Abstract] OR “12 Lead Electrocardiography” [Title/Abstract]))) AND ((“Artificial Intelligence” [Mesh]) OR ((“Artificial Intelligence” [Mesh]) OR (“Intelligence, Artificial” [Title/Abstract] OR “Computer Reasoning” [Title/Abstract] OR “AI (Artificial Intelligence)” [Title/Abstract] OR “Machine Intelligence” [Title/Abstract] OR “Computational Intelligence” [Title/Abstract] OR “Computer Vision Systems” [Title/Abstract] OR “Computer Vision System” [Title/Abstract] OR “Knowledge Acquisition (Computer)” [Title/Abstract] OR “Knowledge Representation (Computer)” [Title/Abstract] OR “Knowledge Representations (Computer)” [Title/Abstract]))) |
| EBSCO | AB (((“Cardiomyopathy, Hypertrophic” OR “Hypertrophic Cardiomyopathies” OR “Hypertrophic Cardiomyopathy” OR “Hypertrophic Obstructive Cardiomyopathies” OR “Hypertrophic Obstructive Cardiomyopathy”) AND (“Electrocardiography” OR “ECG” OR “EKG” OR “Cardiography” OR “Cardiographies” OR “Electrocardiogram” OR “Electrocardiograms” OR “Electrocardiograph” OR “Electrocardiographs” OR “12-Lead ECG” OR “12 Lead ECG” OR “12-Lead ECGs” OR “12-Lead EKG” OR “12 Lead EKG” OR “12-Lead EKGs” OR “12-Lead Electrocardiography” OR “12-Lead Electrocardiographies” OR “12 Lead Electrocardiography”)) AND (“Artificial Intelligence” OR “Intelligence, Artificial” OR “Computer Reasoning” OR “AI (Artificial Intelligence)” OR “Machine Intelligence” OR “Computational Intelligence” OR “Computer Vision Systems” OR “Computer Vision System” OR “Knowledge Acquisition (Computer)” OR “Knowledge Representation (Computer)” OR “Knowledge Representations (Computer)”) |
| Proquest | abstract (“Cardiomyopathy, Hypertrophic” OR “Hypertrophic Cardiomyopathies” OR “Hypertrophic Cardiomyopathy” OR “Hypertrophic Obstructive Cardiomyopathies” OR “Hypertrophic Obstructive Cardiomyopathy”) AND abstract (“Electrocardiography” OR “ECG” OR “EKG” OR “Cardiography” OR “Cardiographies” OR “Electrocardiogram” OR “Electrocardiograms” OR “Electrocardiograph” OR “Electrocardiographs” OR “12-Lead ECG” OR “12 Lead ECG” OR “12-Lead ECGs” OR “12-Lead EKG” OR “12 Lead EKG” OR “12-Lead EKGs” OR “12-Lead Electrocardiography” OR “12-Lead Electrocardiographies” OR “12 Lead Electrocardiography”) AND abstract (“Artificial Intelligence” OR “Intelligence, Artificial” OR “Computer Reasoning” OR “AI (Artificial Intelligence)” OR “Machine Intelligence” OR “Computational Intelligence” OR “Computer Vision Systems” OR “Computer Vision System” OR “Knowledge Acquisition (Computer)” OR “Knowledge Representation (Computer)” OR “Knowledge Representations (Computer)”) |
3.2. Quality assessment
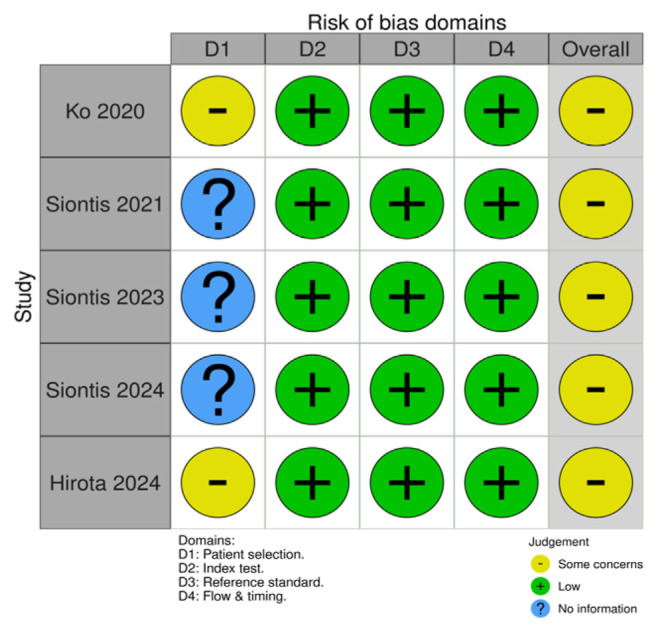
The quality assessment of the studies was conducted using the QUADAS-2 tool for retrospective cohort studies. Two investigators (FAT, LF) independently assessed the risk of bias, and disagreements were resolved through discussion or by consulting a third reviewer (DAT). The inter-rater agreement was measured using Cohen’s kappa statistic, with a value of 0.85, indicating a substantial agreement. The risk of bias (ROB) assessment using the QUADAS-2 tool revealed some concerns across the included studies, particularly in the domain of patient selection (D1). The studies by Ko et al. [13] and Hirota et al. [15] were rated as having “some concerns” due to potential selection bias, as participants were retrospectively chosen from specific cohorts rather than through consecutive or random sampling. This limitation may affect the generalizability of the findings. Similarly, Siontis et al. [11,12,14] had an unclear risk of bias in patient selection due to insufficient details on participants’ recruitment methods (see Figs. 2 and 3).
Fig. 2.
Quadas 2 tool risk of bias.
Fig. 3.
Forest plot of sensitivity and specificity for AI-enhanced ECG in detecting hypertrophic cardiomyopathy. Legends: TP: True positive; FP: False positive; FN: False Negative; TN: True negative.
3.2.1. Characteristics of included studies
All five cohort studies that met the inclusion criteria were included in this review. Together, these studies involved 1925 patients with HCM and 67,418 non-HCM patients (control group). All patients were assessed using an AI-enhanced ECG model based on deep learning convolutional neural networks (CNN), designed to automatically detect HCM features from raw 12-lead ECG waveforms [11–15]. Echocardiography served as the gold standard for diagnostic confirmation. A summary of study characteristics, including study design, sample size, inclusion criteria or HCM definition, type of AI model, and diagnostic accuracy is detailed in Table 3. The participants in Siontis et al. [14] were exclusively pediatric patients aged <18 years, while the other studies primarily assessed adult populations [14]. Across all studies, there was a higher proportion of male participants compared to female participants [11–15].
Table 3.
Characteristics of the included studies
| Study ID | Country and Year of Study | Study Design | Inclusion Criteria | Type of AI model | Participants | Diagnostic Accuracy | |||
|---|---|---|---|---|---|---|---|---|---|
|
|
|
||||||||
| Groups (n) | Gender (Male:Female) | Sensitivity (%) | Specificity (%) | AUC | |||||
| Ko, et al. | USA, 2020 | Retrospective cohort study | Patients aged >18 years old with a diagnosis of HCM based on standard diagnostic criteria who had at least 1 digital, standard, 10-s, 12-lead ECG acquired in the supine position (HCM group); Non-HCM patients who had an ECG and an echocardiogram within the same period of time (Non-HCM/control group) | Deep-learning (Convolutional neural network) | H: 612 N: 12788 |
H: (1703:1357) N: (36.385:27.556) |
87 | 90 | 0.96 |
| Siontis, et al. | USA, 2021 | Retrospective cohort study | Patients aged <18 years old with a diagnosis of HCM based on standard diagnostic criteria (HCM group); Non-HCM patients who had an ECG and an echocardiogram within the same period of time (Non-HCM/control group) | Deep-learning (Convolutional neural network) | H: 300 N: 18439 |
H: (205:95) N: (11844:6595) |
92 | 95 | 0.98 |
| Siontis, et al. | USA, 2023 | Retrospective cohort study | Adult patients with a confirmed diagnosis of Hypertrophic Cardiomyopathy (HCM), according to established diagnostic guidelines, and who have at least one digitally recorded 10-s, 12-lead ECG (HCM group); Non-HCM patients who had an ECG and an echocardiogram within the same period of time (Non-HCM/ control group) | Deep-learning (Convolutional neural network) | H: 100 N: 13294 |
H: (1706:1341) N: (36437:27489) |
80 | 84 | 0.90 |
| Siontis, et al. | USA, 2024 | Retrospective cohort study | Patients with a definite HCM diagnosis by standard European Society of Cardiology (ESC) and Americal College of Cardiology (ACC)/ American Heart Association (AHA) criteria and had at least one 12-lead ECG available in digital format (HCM group); Non-HCM patients who had an ECG and an echocardiogram within the same period of time (Non-HCM/control group) | Deep-learning (Convolutional neural network) | H: 773 N: 3867 |
H: (536:237) N: (36.385:27.556) | 83 | 88 | 0.92 |
| Hirota, et al. | Japan, 2024 | Retrospective cohort study | Adult patients with a confirmed diagnosis of HCM based on one of the following criteria: (1) interventricular septal thickness (IVST) ≥15 mm with no other causes of left ventricular hypertrophy; (2) IVST ≥13 mm with a family history of HCM; or (3) hypertrophy in the apex of the left ventricle (HCM group); Non-HCM patients who had an ECG and an echocardiogram within the same period of time (Non-HCM/control group) | Deep-learning (Convolutional neural network) | H: 140 N: 19030 |
H: (97:43) N: (11388:7642) |
76.4 | 81.4 | 0.854 |
HCM group (H) = Patients with both echocardiographic and clinical diagnosis of HCM; Non-HCM/control group (N) = Non-HCM patients who had an ECG and an echocardiogram within the same period of time. Abbreviations: ECG = Electrocardiogram, HCM = Hypertrophic cardiomyopathy, IVST = Interventricular septal thickness.
All studies in this review utilized the same deep learning-based AI models, specifically CNN designed to detect HCM using standard 12-lead ECGs without incorporating additional demographic or clinical variables. Ko et al. [13] and Siontis et al. [11,12,14] employed the same AI algorithm, originally developed in a single-center cohort study conducted in North America at the Mayo Clinic in 2020 [11–14]. The model relied on deep learning, specifically Convolutional Neural Networks (CNN), for its functionality. In this AI algorithm, 10-s, 12-lead ECGs captured in the supine position using a GE-Marquette machine were digitally stored and processed into a 12×5000 matrix format. The AI model was constructed using the Keras framework, powered by a TensorFlow backend (Google, Mountain View, CA, USA), and programmed in Python (Python Software Foundation, Beaverton, OR, USA). The matrix format organized the data spatially in the first dimension and temporally in the second. Convolutional operations were performed both within individual leads and across multiple leads, enabling the 12-lead ECG data to be analyzed comprehensively [11–14]. Hirota et al. (2024) also utilized a convolutional neural network (CNN)-based algorithm specifically designed to detect hypertrophic cardiomyopathy (HCM) and its subtypes using standard 12-lead ECGs. The study constructed two diagnostic models: a ‘basic diagnosis’ model for general HCM detection and a ‘comprehensive diagnosis’ model, which incorporated subtyping of HCM cases into basal hypertrophy, apex involvement, and dilated-phase HCM (dHCM) [15].
All included studies demonstrated high diagnostic accuracy, with sensitivity (76.4–92 %) and specificity (81.4–95 %). The area under the curve (AUC) values from the included studies ranged from 0.854 to 0.980, further demonstrating the strong diagnostic accuracy of AI-enhanced ECG for HCM diagnosis [11–15].
3.3. Meta-analysis result
The forest plot analysis of sensitivity and specificity for AI-enhanced ECG in detecting HCM demonstrated statistically significant results, with a pooled sensitivity of 0.84 (95 % CI: 0.82–0.86) and a specificity of 0.86 (95 % CI: 0.86–0.87), indicating high diagnostic accuracy. However, substantial heterogeneity (I2 = 93.6 %) was observed across the five included studies. This is likely due to multiple factors, including differences in study populations, ECG acquisition methods, and AI model training processes. Variations in dataset composition, including age distribution and ethnic diversity, may have affected diagnostic performance. Given the limited number of studies, these findings should be considered exploratory rather than definitive, highlighting the need for further standardized validation in diverse populations.
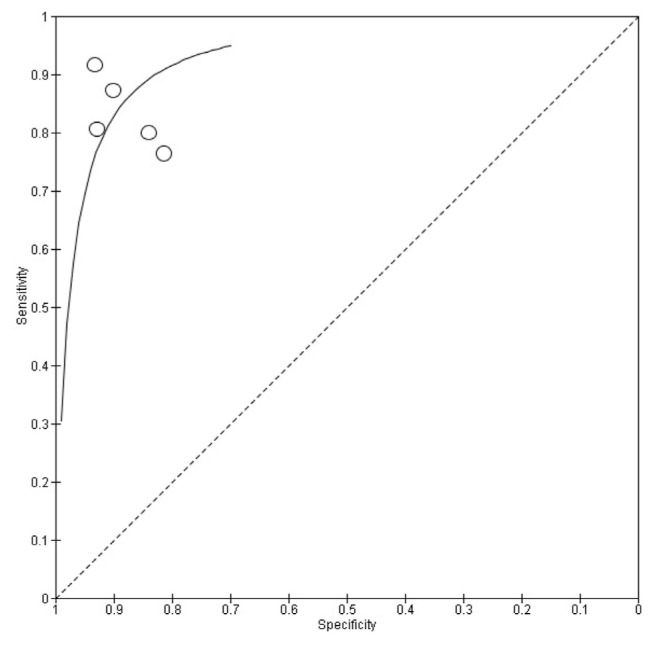
The Summary Receiver Operating Characteristics (SROC) curve (Fig. 4) provides a comprehensive overview of the diagnostic performance of AI-enhanced ECG models for detecting HCM. The area under the curve (AUC) was 0.927, indicating excellent discriminative ability between HCM and non-HCM cases. The curve’s shape highlights the trade-off between sensitivity and specificity across different studies. Most data points are positioned in the upper left quadrant, reflecting high sensitivity and specificity, which align with the pooled estimates of 0.84 (95 % CI: 0.82–0.86) for sensitivity and 0.86 (95 % CI: 0.86–0.87) for specificity. This suggests that AI-driven ECG models reliably detect HCM while minimizing false positives and false negatives. Despite the high overall accuracy, some variability exists across studies, as indicated by the data points. Studies with smaller sample sizes exhibited wider confidence intervals, suggesting potential overfitting or reduced generalizability. In contrast, larger studies, particularly those utilizing deep learning architectures such as convolutional neural networks (CNN), contributed more weight to the pooled estimate, reinforcing the model’s robustness. These results highlight the potential of AI-enhanced ECG analysis as a non-invasive screening tool for HCM. However, to improve its clinical integration and generalizability, further validation across diverse populations and standardized model training protocols are essential.
Fig. 4.
Summary Receiver Operating Characteristic (SROC) plot of Sensitivity and Specificity for AI-enhanced ECG in detecting Hypertrophic Cardiomyopathy.
4. Discussion
This systematic review and meta-analysis aimed to meta-analyze the accuracy of AI-enhanced ECG in diagnosing HCM. Our pooled analysis of five retrospective cohort studies showed that AI-enhanced ECG can identify HCM with high diagnostic accuracy (Sensitivity 84 %; specificity 86 %; AUC 0.927). These findings align with previous research supporting the use of AI-enhanced ECG for detecting cardiovascular diseases, reinforcing its potential as a valuable diagnostic tool in clinical practice [19]. A previous meta-analysis by Lee et al. that evaluated the diagnostic accuracy of AI-enhanced ECG for detecting peripartum cardiomyopathy yielded favorable results, with a sensitivity of 83 % (95 % CI, 0.241–0.263), specificity of 80 % (95 % CI, 0.760–0.859), and an NPV of 97.5 % (95 % CI, 0.953–0.997) [19].
Therefore, the findings from our study are expected to be applicable to a broad population due to several factors: (1) The wide age range of subjects, with both children (<18 years) and adults (>18 years) included in the study, (2) The inclusion of studies covering multiple populations from various countries (multicenter cohorts), which is expected to encompass different races (including East Asian, White, and Black) that may influence ECG results, and (3) The large total sample size, amounting to 69,343 samples [11–15]. In one of the studies we included in our analysis, the results of the subgroup analysis found several factors that could influence the diagnostic accuracy of AI-enhanced ECG [8]. The diagnostic accuracy was found to be superior in the following conditions: female sex (AUC 0.94, compared with males with AUC 0.91, p = 0.001), presence of any ECG abnormalities vs normal ECGs (AUC 0.93 vs AUC 0.84, p < 0.001), presence of LVH criteria vs absence of LVH criteria (AUC 0.93 vs. 0.90, p = 0.012), and absence of tracing artifact vs ECGs with artifact (AUC 0.93 vs. 0.82, p = 0.022) [8].
This research demonstrates the promising potential of using AI in ECG for early detection of hypertrophic cardiomyopathy. AI-enhanced ECG has significant potential in high-priority populations where early detection of HCM is crucial. This includes first-degree relatives of HCM patients, particularly those with pathogenic sarcomere mutations, who may benefit from early screening. Individuals presenting with unexplained left ventricular hypertrophy on routine imaging, unexplained syncope, palpitations, or ventricular arrhythmias could benefit from AI-enhanced ECG for early diagnosis [20]. In sports cardiology, distinguishing between athlete’s heart and HCM remains a diagnostic challenge, and AI-enhanced ECG could provide valuable insights to support clinical decision-making [9].
Beyond improving diagnostic accuracy, AI-enhanced ECG can streamline clinical workflows [21]. It may help triage high-risk patients for echocardiography, ensuring timely imaging. In primary care and community settings with limited access to specialists, it could aid early detection and optimize patient management [22]. By reducing unnecessary imaging in low-risk cases, AI-enhanced ECG may also lower healthcare costs. Furthermore, it enables earlier diagnosis in asymptomatic patients, allowing for more personalized follow-up and intervention [21,22]. These applications highlight its real-world clinical value, extending beyond statistical performance to improve patient outcomes [21].
This meta-analysis has several limitations that should be acknowledged. The relatively small number of included studies (n = 5) may impact the robustness of the pooled estimates. Since all studies were retrospective, potential selection bias remains a concern, particularly as patient cohorts were often derived from specialized centers rather than general populations, limiting the generalizability of AI-enhanced ECG models [11–14]. Furthermore, substantial heterogeneity (I2 = 93.6 %) was observed, likely due to differences in patient selection criteria, ECG acquisition methods, and AI model implementation [11–15]. Although all included studies used echocardiography as the reference standard for HCM diagnosis, variability in echocardiographic interpretation and diagnostic protocols across centers may have influenced patient selection and contributed to heterogeneity. Differences in operator expertise could lead to variations in the identification of LVH and interventricular septal thickness potentially affecting the diagnosis of HCM across studies [23]. While all studies used CNN for ECG interpretation, differences in model training, dataset preprocessing, and validation approaches could affect sensitivity and specificity estimates [24]. Future research should focus on prospective, multicenter validation studies with standardized AI model evaluation protocols to enhance reproducibility and clinical applicability.
5. Conclusion
AI-enhanced ECG could be a potential non-invasive screening tool for HCM. The findings suggest that deep learning-based models, particularly convolutional neural networks (CNN), can effectively analyze standard 12-lead ECGs to identify HCM with high accuracy. While AI-enhanced ECG shows promise for early HCM detection, its integration into clinical practice requires further prospective, multicenter studies with rigorous external validation, optimization and standardization of AI model thresholds, and real-world implementation assessments. Future research should also explore collaborative efforts that integrate AI advancements with existing clinical guidelines to ensure these tools enhance, rather than disrupt, established diagnostic workflows. By integrating AI-enhanced ECG analysis with established clinical frameworks it could improve early identification and patient outcomes.
Acknowledgement
The authors are grateful to all colleagues from Atma Jaya Catholic University of Indonesia for all the support and contributions provided for our study.
List of abbreviations
- AI
Artificial Intelligence
- AUC
Area under curve
- CNN
Convolutional neural network
- CT
Computed tomography
- ECG
Electrocardiography
- HCM
Hypertrophic Cardiomyopathy
- LVH
Left Ventricular Hypertrophy
- MRI
Magnetic Resonance Imaging
- NPV
Negative Predictive Value
- PRISMA
Preferred Reporting Items for Systematic Reviews and Meta-analysis
- SROC
Summary receiver operating characteristic
- UK
United Kingdom
- US
United States
Footnotes
Ethical approval: This systematic review and meta-analysis did not involve the collection of original data from human participants; therefore, ethical approval was not required. The study followed established guidelines for conducting systematic review and meta-analyses based on evidence-based research principles.
Conflict of interest: None declared.
Author contribution: Conception and design of Study: FAT, LFJJ. Literature review: FAT, LFJJ, RS, DAJ. Acquisition of data: FAT, LFJJ, RS. Analysis and interpretation of data: FAT, LFJJ, RS. Research investigation and analysis: FAT, LFJJ. Data collection: FAT, LFJJ, RS, DAJ. Drafting of manuscript: FAT, LFJJ, RS, DAJ. Revising and editing the manuscript critically for important intellectual contents: FAT, LFJJ. Data preparation and presentation: RS, DAJ. Supervision of the research: FAT, LFJJ. Research coordination and management: FAT, LFJJ. Funding for the research: FAT, LFJJ, RS, DAJ.
Funding: This study did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
References
- 1. Marian AJ, Braunwald E. Hypertrophic cardiomyopathy: genetics, pathogenesis, clinical manifestations, diagnosis, and therapy. Circ Res. 2017 Sep 15;121(7):749–70. doi: 10.1161/circresaha.117.311059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Butzner M, Leslie DL, Cuffee Y, Hollenbeak CS, Sciamanna C, Abraham T. Stable rates of obstructive hypertrophic cardiomyopathy in a contemporary era. Front Cardiovasc Med. 2022 Jan 6;8:765876. doi: 10.3389/fcvm.2021.765876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Schultze M, Zema C, Carroll R, Hurst M, Borchert J, Zhong Y, et al. Population estimates of obstructive and non-obstructive hypertrophic cardiomyopathy in the UK and Germany. Eur Heart J. 2022 Oct 1;43(Supplement_2):ehac544–1747. doi: 10.1093/eurheartj/ehac544.1747. [DOI] [Google Scholar]
- 4. Maron MS, Hellawell JL, Lucove JC, Farzaneh-Far R, Olivotto I. Occurrence of clinically diagnosed hypertrophic cardiomyopathy in the United States. AmJ Cardiol. 2016 May 15;117(10):1651–4. doi: 10.1016/j.amjcard.2016.02.044. [DOI] [PubMed] [Google Scholar]
- 5. Task A, Elliott PM, Uk C, Anastasakis A, Germany MA, Germany MB, et al. ESC Guidelines on diagnosis and management of hypertrophic cardiomyopathy. Eur Heart J. 2014;35(39):2733–79. doi: 10.1093/eurheartj/ehu284. [DOI] [PubMed] [Google Scholar]
- 6. Bernard J, Barry J, Joseph A. ACCF/AHA guideline for the diagnosis and treatment of hypertrophic cardiomyopathy. JACC (J Am Coll Cardiol) 2011;58(23):735–1097. doi: 10.1016/j.jacc.2011.06.011. [DOI] [Google Scholar]
- 7. Bernardini A, Crotti L, Olivotto I, Cecchi F. Diagnostic and prognostic electrocardiographic features in patients with hypertrophic cardiomyopathy. Eur Heart J Suppl. 2023 doi: 10.1093/eurheartjsupp/suad074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Palermi S, Cavarretta E, D’Ascenzi F, Castelletti S, Ricci F, Vecchiato M, et al. Athlete’s Heart: a cardiovascular step-by-step multimodality approach. Rev Cardiovasc Med. 2023 May 19;24(5):151. doi: 10.31083/j.rcm2405151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zorzi A, Brunetti G, Corrado D. Differential diagnosis between athlete’s heart and hypertrophic cardiomyopathy: new pieces of the puzzle. Int J Cardiol. 2022 Apr 15;353:77–9. doi: 10.1016/j.ijcard.2022.02.001. [DOI] [PubMed] [Google Scholar]
- 10.Muzammil MA, Javid S, Afridi AK, Siddineni R, Shahabi M, Haseeb M, et al. Artificial intelligence-enhanced electrocardiography for accurate diagnosis and management of cardiovascular diseases. J Electrocardiol. 2024. Jan 28, [DOI] [PubMed]
- 11.Siontis KC, Wieczorek MA, Maanja M, Hodge DO, Kim HK, Lee HJ, et al. Hypertrophic cardiomyopathy detection with artificial intelligence electrocardiography in international cohorts: an external validation study. European Heart Journal-Digital Health. 2024. Apr 15, p. ztae029. [DOI] [PMC free article] [PubMed]
- 12. Siontis KC, Suárez AB, Sehrawat O, Ackerman MJ, Attia ZI, Friedman PA, et al. Saliency maps provide insights into artificial intelligence-based electrocardiography models for detecting hypertrophic cardiomyopathy. J Electrocardiol. 2023 Nov 1;81:286–91. doi: 10.1016/j.jelectrocard.2023.07.002. [DOI] [PubMed] [Google Scholar]
- 13. Ko WY, Siontis KC, Attia ZI, Carter RE, Kapa S, Ommen SR, et al. Detection of hypertrophic cardiomyopathy using a convolutional neural network-enabled electrocardiogram. J Am Coll Cardiol. 2020 Feb 25;75(7):722–33. doi: 10.1016/j.jacc.2019.12.030. [DOI] [PubMed] [Google Scholar]
- 14. Siontis KC, Liu K, Bos JM, Attia ZI, Cohen-Shelly M, Arruda-Olson AM, et al. Detection of hypertrophic cardiomyopathy by an artificial intelligence electrocardiogram in children and adolescents. Int J Cardiol. 2021 Oct 1;340:42–7. doi: 10.1016/j.ijcard.2021.08.026. [DOI] [PubMed] [Google Scholar]
- 15. Hirota N, Suzuki S, Motogi J, Umemoto T, Nakai H, Matsuzawa W, et al. Evaluating convolutional neural network-enhanced electrocardiography for hypertrophic cardiomyopathy detection in a specialized cardiovascular setting. Heart Vessel. 2024 Jun;39(6):524–38. doi: 10.1007/s00380-024-02367-9. [DOI] [PubMed] [Google Scholar]
- 16. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Br Med J. 2021 Mar;29:372. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Whiting PF, Rutjes AW, Westwood ME, Mallett S, Deeks JJ, Reitsma JB, et al. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med. 2011 Oct 18;155(8):529–36. doi: 10.7326/0003-4819-155-8-201110180-00009. [DOI] [PubMed] [Google Scholar]
- 18. Balduzzi S, Rücker G, Schwarzer G. How to perform a metaanalysis with R: a practical tutorial. BMJ Ment Health. 2019 Nov 1;22(4):153–60. doi: 10.1136/ebmental-2019-300117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lee Y, Choi B, Lee MS, Jin U, Yoon S, Jo YY, et al. An artificial intelligence electrocardiogram analysis for detecting cardiomyopathy in the peripartum period. Int J Cardiol. 2022 doi: 10.1016/j.ijcard.2022.01.064. [DOI] [PubMed] [Google Scholar]
- 20. Hong Y, Su WW, Li X. Risk factors of sudden cardiac death in hypertrophic cardiomyopathy. Curr Opin Cardiol. 2022 doi: 10.1097/HCO.0000000000000939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Maleki Varnosfaderani S, Forouzanfar M. The role of AI in hospitals and clinics: transforming healthcare in the 21st century. Bioengineering. 2024 Mar 29;11(4):337. doi: 10.3390/bioengineering11040337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Armoundas AA, Narayan SM, Arnett DK, Spector-Bagdady K, Bennett DA, Celi LA, et al. Use of artificial intelligence in improving outcomes in heart disease: a scientific statement from the American Heart Association. Circulation. 2024 doi: 10.1161/CIR0000000000001201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rigolli M, Anandabaskaran S, Christiansen JP, Whalley GA.Bias associated with left ventricular quantification by multi-modality imaging: a systematic review and meta-analysis Open Heart 2016 10.1136/openhrt-2015-000388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Werner de Vargas V, Schneider Aranda JA, dos Santos Costa R, da Silva Pereira PR, Victória Barbosa JL. Imbalanced data preprocessing techniques for machine learning: a systematic mapping study. Knowl Inf Syst. 2023 doi: 10.1007/s10115-022-01772-8. [DOI] [PMC free article] [PubMed] [Google Scholar]