Abstract
Background
Endometrial carcinoma (EC) is a common gynecological malignancy with a complex pathogenesis. PDS5B is revealed to be dysregulated and play critical roles in multiple cancers, while its role in EC remains largely unknown. The aim of this study was to explore the expression profile and biological function of the PDS5B in EC.
Methods
In this study, differential gene expression analysis in EC was performed using public databases and relevant analytical tools. The expression of PDS5B was verified by western blot and qRT-PCR in cell and tissue samples. The effects of PDS5B overexpression on EC cell proliferation, invasion and apoptosis, as well as the interaction of PDS5B with YTHDC1 and the regulation of PTEN stability were further explored by in vitro experiments. The effects of PDS5B on tumorigenesis and metastasis were explored using in vivo models.
Results
PDS5B was down-regulated in EC, and overexpression of PDS5B significantly inhibited cell growth, and promoted apoptosis. In vitro results revealed that PDS5B interacted with YTHDC1 in EC cells and YTHDC1 regulated PTEN expression and stability via m6A modification, affecting the biological behavior of EC cells. In in vivo nude mouse models, overexpression of PDS5B significantly inhibited EC tumor development and metastasis in nude mice.
Conclusions
PDS5B interacts with YTHDC1 and further regulates PTEN in endometrial cancer cells, thereby inhibiting the malignant development of the tumor. These findings indicate the potential part of PDS5B in the development of EC and provide new molecular targets for the treatment of EC.
Supplementary Information
The online version contains supplementary material available at 10.1007/s12672-025-03021-0.
Keywords: Endometrial carcinoma, PDS5B, PTEN, YTHDC1, M6A
Introduction
Endometrial carcinoma (EC) is a common malignant tumor of the female reproductive tract and the fifth most common malignant disease among women worldwide, severely affecting patients’ quality of life [1–3]. With the improvement of screening and testing techniques, the incidence of EC has been increasing annually and is trending towards a younger age [4]. In addition, increasing age, racial differences, high body mass index (BMI), metabolic syndrome, and family history all contribute to an increased risk of EC [5]. Some data suggest that the 5-year survival rate for patients with early-stage EC is upper than 90%, and only 20% for patients with advanced disease [6]. Due to its high aggressiveness and recurrence rate, EC seriously affects patient prognosis. Generally, EC can be classified into type I (estrogen-dependent) and type II (non-estrogen-dependent), of which type I accounts for about 80-85%, and is often accompanied by mutations in β-catenin, PTEN and KRAS [7]. As different pathological criteria can lead to divergent pathologies in 10-20% of endometrial cancers [5, 8], the more precise molecular typing of TCGA is often used as an important complement to histological typing [9, 10]. Currently, the principle of treatment for EC is based on surgical treatment, and hormonal therapy, chemotherapy and radiotherapy are mainly applicable to early-stage patients, with poorer efficacy in advanced-stage patients [11]. The combination of TCGA molecular typing is expected to provide prospective ideas in the selection of surgical options [12]. Therefore, elucidating the molecular mechanisms of EC development and searching for new potential biomarkers are important for the clinical diagnosis, treatment and prognosis of EC.
With the progress of molecular biology techniques, scholars have gradually recognized that abnormalities in molecular regulation play a crucial part in the development of endometrioid carcinoma, which is also the basis for molecular typing and targeted drug development [13]. Precocious Dissociation of Sister Chromatids 5B (PDS5B) belongs to the PDS5 family, and is related to sister chromatid adhesion protein [14]. It plays an important role in cell division, especially in maintaining chromosome stability and promoting proper chromosome segregation [15]. It has been found that cells with PDS5B knockdown exhibit defective chromosome morphology [16]. PDS5B plays a part in a range of cellular processes, including DNA damage repair, gene transcription and DNA replication [17]. In addition, aberrant function of PDS5B has been associated with the development of certain types of cancer. Loss and low expression of PDS5B has been discovered in a range of malignant tissues and tumor cell lines, including esophageal cancer [18], gastric cancer [19], prostate cancer [20]. However, few studies have reported the expression pattern and role of PDS5B in EC.
In this study, we screened and validated the gene expression profiles of our gene of interest, PDS5B, in EC tissues and cells. We also explored the effects of PDS5B overexpression on EC cells in vitro and tumor growth as well as metastasis in vivo. In addition, we further explored the underlying mechanism of PDS5B in EC, and found PDS5B affect the malignant progression of EC by regulating the YTHDC1/PTEN pathway.
Materials and methods
Clinical tissue samples
Endometrial tissue samples from EC patients (n = 3) and normal population (n = 3) were obtained from Changzhou Geriatric Hospital Affiliated to Soochow University, Changzhou No. 7 People’s Hospital. The study was approved by the Joint Ethics Committee of the Ministry of Health.
Cell culture
The human EC cell lines (Ishikawa, ECC-1) and the human endometrial stromal cell line SHT290 were cultured in MEM medium. v). The EC cell line HEC-1-A was cultured in McCoy’s 5 A medium. vi). The medium was supplemented with 10% fetal bovine serum and 100 U/mL penicillin-streptomycin, and the culture conditions were 37 °C, 5% CO2. Relevant information is in Supplementary Table 3.
Animal study
Five-week-old C57BL/C nude mice were purchased from the Animal Experiment Centre of Changzhou Geriatric Hospital Affiliated to Soochow University, Changzhou No. 7 People’s Hospital, placed in an SPF environment, and kept for one week for acclimation. Control mice (n = 5) were injected subcutaneously with Ishikawa cells transfected with empty plasmid (5 × 106 cells/200 µL, and the experimental group was injected with the same dose of PDS5B plasmid cells. Tumor volume was assessed every 7 days and when the tumor volume reached 300 mm3, the mice were executed to remove the tumor for subsequent assays.
An orthotopic xenograft model was constructed for monitoring endometrial tumor metastasis in vivo using five-week-old C57BL/C nude mice injected in situ with Ishikawa cells. The control group (n = 5) was injected in situ with Ishikawa cells transfected with empty plasmid (5 × 106 cells/200 µL), and the experimental group (n = 5) was injected with the same dose of PDS5B plasmid cells. Injections were performed by anaesthetizing the mice and fixing them in a supine position, and the cells were inoculated in parallel along the mid-abdominal opening to locate the uterine cavity. Tumor metastasis in mice implanted with Ishikawa cells was assessed using bioluminescence imaging after four weeks. The number of metastatic lung nodules was detected using H&E staining after the mice were executed. The animal experimental protocol was confirmed by the Commission’s institutional laboratory and complied with the rules of Declaration of Helsinki principles.
RNA interference and overexpression
For PDS5B, specific amplification primers were designed, the amplification product and GV538 vector were digested and ligated, and the constructed overexpression vector of PDS5B was subcloned into lentiviral vector. pcDNA3.1 was used as a negative control.
For YTHDC1, PTEN designed specific hairpin primers and after annealing the hairpin primers were inserted into the pLentiGuide-puro vector and the interference vector was placed in 293T cells in culture, and after the cells were cultured to a density of 70-80%, PsPaX2 (2.2 µg), pMD2.G (3 µg) and Lipofectamine 3000 were added to infect the EC cell line. After 48 h, supernatants containing packaged lentivirus were harvested. For the construction of the IshikawaPTEN cells, PTEN cDNA sequence was cloned into pcDNA 3.1 vector plasmid, and Ishikawa cells were transfected with PTEN overexpression vector (pcDNA3.1/PTEN) or control empty pcDNA3.1 vector for 48 h using Lipofectamine 3000. Relevant information is in Supplementary Tables 1 and 3.
PCR amplification
Total RNA was extracted from EC and normal tissues and cells using TRIzol® reagent. Reverse transcription reagent was added immediately to transcribe the RNA into cDNA, and amplification reagent was added to amplify the target genes. RT-PCR amplification conditions were set up on the StepOnePlus PCR system. We detected gene expression using the 2−ΔΔCT method with GADPH as the internal control. The related information is shown in Supplementary Tables 1 and 3.
Western blot
Tissues and transfected cell lines were lysed separately with lysis buffer and denatured proteins were separated by polyacrylamide microgels. Proteins were transferred to nitrocellulose membranes by wet transfer and blocked with TBST buffer containing 5% skimmed milk for one hour. The membranes were incubated with the appropriate antibodies and color development solutions were added to correctly visualize the markers by the Luminata Crescendo detection system. The information used is shown in Supplementary Tables.
Actinomycin D treatment
Actinomycin D powder was dissolved in DMSO to a final concentration of 5 mg/mL, and mixed. Then 5 µg/mL actinomycin D was incubated with EC cells, and the cells were collected after incubation for 0, 2, 4 and 8 h. Total RNA was extracted and the expression of PTEN mRNA was determined.
Assays for cell proliferation
The EC cell lines transfected with different plasmids were inoculated into 6-well plates (2 × 103/well) and cultured for 7 days to form colonies, then crystal violet solution was added. The colony spheres were washed and dried, and the colony groups were captured and observed by Epson scanner.
The EC cell lines transfected with different plasmids were inoculated into 6-well plates (1 × 104/well), and after growing to a certain concentration (70-80%), EdU medium was added to the adherent cells and incubated for 2 h. Then the cells were fixed, permeabilized and stained according to the instructions of the fluorescence assay, and then observed under a fluorescence microscope. Reagent sources are detailed in Supplementary Table 3.
Assays for cell apoptosis
The cells in each group were fixed with 4% formaldehyde and osmotically stabilized for 10 min. TUNEL working solution was added and incubated away from light. The cells were stained with DAPI and mounted in glycerol, observed by confocal microscope and photographed for further analysis.
Cells in the logarithmic growth phase were inoculated into 6-well plates, and the cell suspension was collected by digesting the adherent cells, centrifuged to obtain a cell pellet, and mixed with FITC and PI dyes according to the instructions in the kit, and then tested for apoptosis by flow cytometry. Reagent sources are listed in Supplementary Table 3.
CCK-8 assay
5 × 103 EC cells transfected with different vector plasmids were inoculated into 24-well medium and cultured for 24, 48 and 72 h, respectively, and the OD values were determined by a microplate reader after adding CCK-8. The reagent sources are listed in Supplementary Table 3.
Wound-healing treatment
After 24 h of incubation in serum-containing medium, the cells (5 × 105/well) are replaced with serum-free medium and incubated for 30 min. The wells were scratched with a sterilized pipette tip perpendicular to the horizontal line at the back of the wells, washed gently with D-Hanks medium, and then incubated with the medium for 24 h before being immediately imaged.
Transwell assays
For migration assay, cells were passaged to prepare a suspension with a final concentration of 1 × 106 cells/ml, 200 µL of the suspension was added to the upper layer of the chamber placed in a 24-well plate, and the lower layer was added with 800 mL of 30% FBS, incubated in the incubator for 24 h. Then fixed with 4% paraformaldehyde and stained with 0.5% crystal violet, and counted by an inverted contrast microscope.
For cell invasion test, 100 µL of matrix gel (1 mg/mL) was evenly spread in the chambers before the cell suspension was added to the chambers, and the rest of the steps were the same as the cell migration assay mentioned above, while the incubation time of the chambers in the incubator was prolonged to 48 h. Reagent sources are detailed in Supplementary Table 3.
RIP assay
EC cells (1 × 107) were lysed using IP lysate, and the supernatant of cell lysate was collected. The magnetic bead protein A/G was incubated with 5 µg of YTHDC1 antibody at 25 °C for 1 h. IgG was used as a negative control for RIP. Spare lysate supernatant was added to the above buffer and incubated overnight at 4 °C. RIP buffer was washed three times and 10% of the magnetic beads were collected to test the efficiency of immunoprecipitation. The remaining precipitate was resuspended with proteinase K. RNA purification was performed after 30 min of oscillatory incubation at 55 °C. The enrichment was detected by amplification using PTEN primers. The related information is shown in Supplementary Tables 1 and 2.
MeRIP assay
Total RNA (300 µg) was extracted using the RNA kit and processed for enzymatic digestion using recombinant dnazyme I. The m6A antibody was incubated with magnetic bead protein A/G magnetic beads (25 °C, 30 min). The remaining RNA was added to the above buffer and incubated overnight (4 °C). The RNA was purified by elution twice and detected by amplification using the PTEN primer.
Immunohistochemistry
Tumor tissues from xenograft mice were treated with xylene and ethanol to confer H2O2 blocking enzyme activity. Antigen retrieval was conducted with sodium citrate buffer. Samples were incubated overnight with PCNA, Ki67 antibody and then with the corresponding secondary antibody. Captured tissue sections were scored using ImageJ. Supplementary Table 2 lists the relevant information.
Hematoxylin-Eosin staining
Sections of lung nodules were decarbonized with xylene, then dehydrated with ethanol and embedded with paraffin. Stained sections were dehydrated with ethanol, processed with xylene and dried naturally, and the images obtained were analyzed by microscopic examination. The number of metastatic nodules in the lungs was measured using ImageJ.
Statistical analysis
Statistical analyses were performed using SPSS 21.0 or GraphPad Prism 8. Analyses were performed using Student’s t-test and one-way ANOVA. Pearson correlation analysis was used to explore the correlation between the expression of two genes. Data are presented as mean ± standard deviation (SD) of at least three independent experiments. Two-sided tests were performed and a P value of less than 0.05 was considered statistically significant.
Results
PDS5B is down-regulated in EC
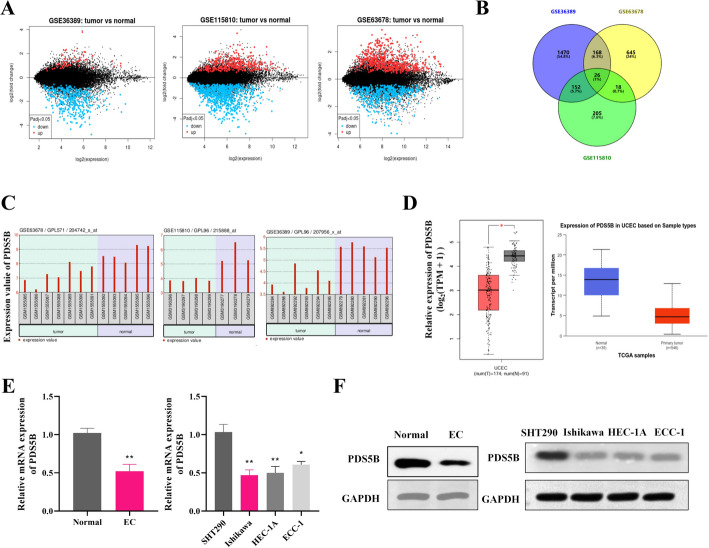
EC-related gene expression profiles GSE36389, GSE115810 and GSE63678 were analyzed using the GEO2R online analysis tool, and a number of up-regulated and down-regulated genes were screened out. There were 26 shared differentially expressed genes in the 3-group database (Fig. 1A-B). Among them, PDS5B was significantly down-regulated in EC in the three datasets (Fig. 1C). PDS5B was also shown to be down-regulated in UCEC in the GEPIA and TCGA database (Fig. 1D). Furthermore, we also demonstrated the low expression of PDS5B in EC tissues and cell lines (Ishikawa, HEC-1A, ECC-1) using quantitative assays (Fig. 1E-F).
Fig. 1.
PDS5B expression is down-regulated in EC. A MA plots of differentially expressed genes in EC patients and normal patients in the GSE36389, GSE115810 and GSE63678 datasets in the GEO database. B Common differentially expressed genes in the three datasets. C Expression of PDS5B gene in the three datasets. D Expression of PDS5B gene in GEPIA and TCGA databases. E qRT-PCR and F western blot detection of PDS5B expression in clinical patient tissues (n = 3) and EC cell lines (n = 3). *p < 0.05, **p < 0.01
Low expression of PDS5B promotes proliferation and migration and inhibits apoptosis in EC
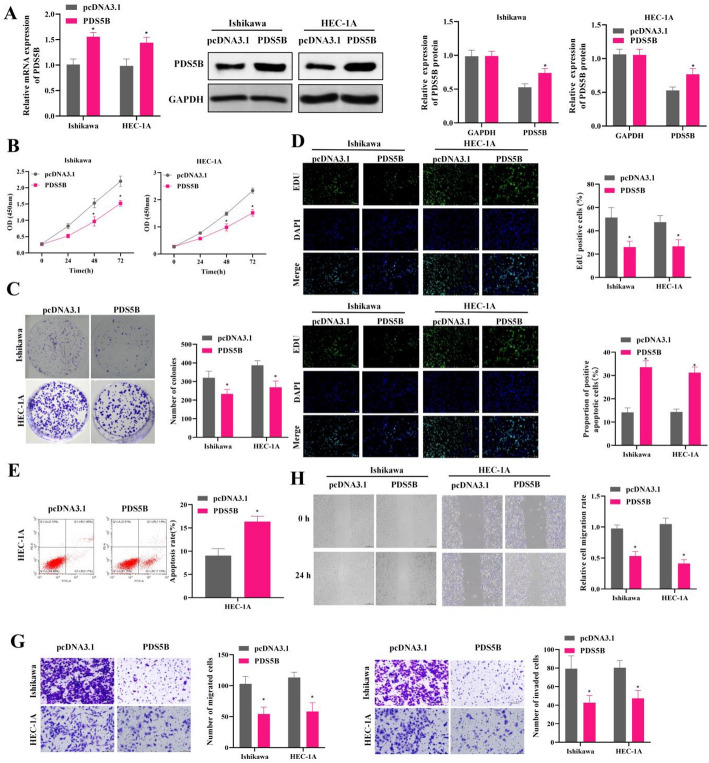
To further explore the effects of PDS5B overexpression on EC cell malignancy, EC cells were transfected with PDS5B overexpression vectors. Quantitative analysis exhibited that PDS5B expression was significantly up-regulated in EC cells, indicating the successful construction of PDS5B overexpression cell lines (Fig. 2A). CCK-8, colony formation and EdU assays demonstrated that overexpression of PDS5B significantly inhibited the proliferation and viability of EC cells (Fig. 2B-D). Flow cytometry analysis and TUNEL assays exhibited that overexpression of PDS5B increased the apoptosis level of EC cells (Fig. 2E-F). Transwell and wound healing assay revealed that overexpression of PDS5B decreased the migratory and invasive ability of EC cells (Fig. 2G-H).
Fig. 2.
Low expression of PDS5B promotes proliferation and migration and inhibits apoptosis in EC. A qRT-PCR and western blot detection of PDS5B expression in Ishikawa and HEC-1 A cell line. B CCK-8 assay for cell viability. C Colony formation assay for cell proliferation. D EdU assay for cell proliferation. Bar scale = 100 μm. E TUNEL assay for cell apoptosis. Bar scale = 100 μm. F Flow cytometry for apoptosis. G Transwell assay for migration and invasion. H Wound healing assay for migration. *P < 0.05
PDS5B interacts with YTHDC1
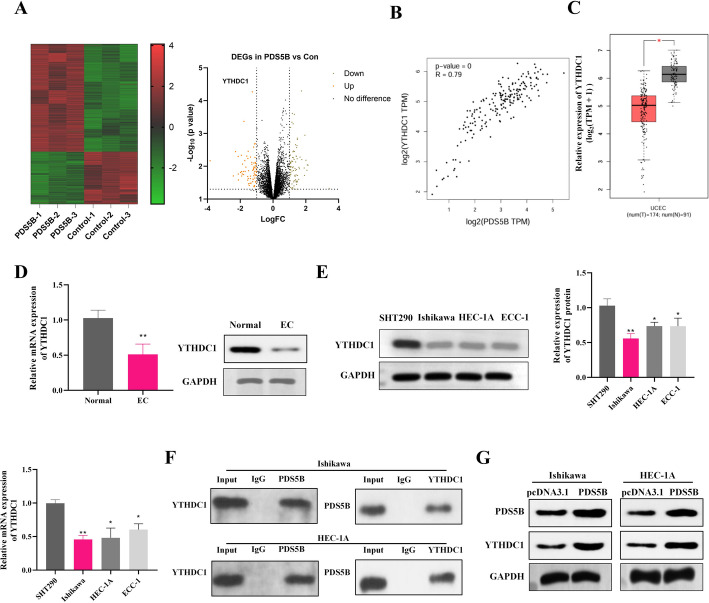
RNA-seq was performed on lshikawa cells overexpressing PDS5B or not overexpressing PDS5B, and the DEGs between the two groups are shown in the volcano plot (Fig. 3A). Among these up-regulated DEGs, YTHDC1 showed most significant differential expression. Studies have shown that YTHDC1 is a potential marker for the diagnosis and prognosis of endometrial cancer [21], thus we chose the YTHDC1 gene for subsequent studies. The GEPIA database showed a positive correlation between PDS5B and YTHDC1 in UCEC, and the YTHDC1 gene was significantly under-expressed in UCEC (Fig. 3B-C). Western blot and qRT-PCR results further confirmed that YTHDC1 expression was significantly down-regulated in EC tissues and EC cell lines (Fig. 3D-E). Co-IP assay indicated that PDS5B interacted with YTHDC1 in EC cells (Fig. 3F). Moreover, YTHDC1 expression was also significantly increased after overexpression of PDS5B, which indicated the positive regulation of PDS5B on YTHDC1 expression in EC cells (Fig. 3G).
Fig. 3.
PDS5B interacts with YTHDC1. A Schematic representation of RNA-seq of lshikawa cells overexpressing PDS5B or control. B GEPIA database showing PDS5B correlation with YTHDC1 in endometrial cancer. C GEPIA data showing the expression of YTHDC1 in endometrial cancers. qRT-PCR and western blot detection of YTHDC1 expression in EC D tissues and E cell lines. F Co-IP to detect the interaction of PDS5B with YTHDC1. G Western blot detection of YTHDC1 expression after overexpression of PDS5B. *p < 0.05, **p < 0.01
Targets of m6A modification of PTEN by YTHDC1 in EC
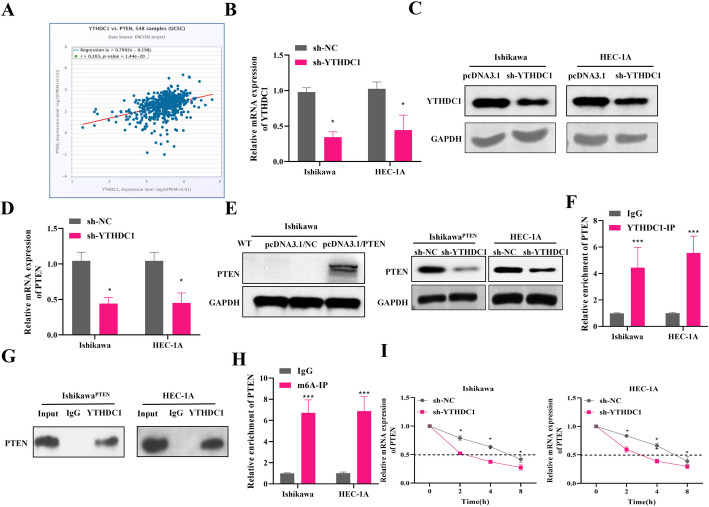
A previous study has indicated that YTHDC1 positively regulates the expression of tumor suppressor PTEN in prostate cancer [22]. We also explored the expression correlation between YTHDC1 and PTEN in Uterine Corpus Endometrial Carcinoma (UCEC) using the starBase database. The results showed that expression of YTHDC1 and PTEN were positive correlated in UCEC patients (r = 0.383, p-value = 1.44e-20) (Fig. 4A). To further investigate the relationship between PTEN and YTHDC1 in EC, we knocked down YTHDC1 in EC cells. Quantitative results revealed that the expression of YTHDC1 in EC cells was significantly down-regulated after knockdown of YTHDC1, indicating the successful construction of sh-YTHDC1 cell line (Fig. 4B-C). Further observation of PTEN expression revealed that knockdown of YTHDC1 down-regulated PTEN mRNA expression (Fig. 4D). Meanwhile, we constructed the Ishikawa cells with PTEN re-expression (IshikawaPTEN), and found that the protein levels of PTEN were decreased in IshikawaPTEN and HEC-1 A cells transfected with sh-YTHDC1, suggesting that YTHDC1 regulated the endogenous and exogenous expression of PTEN (Fig. 4E). Furthermore, the RIP results confirmed that YTHDC1 interacted with PTEN in EC cells (Fig. 4F-G). Further, total cellular RNA was immunoprecipitated with m6A antibody to determine whether PTEN mRNA contained m6A modification. MeRIP-qPCR analysis showed that PTEN mRNA was precipitated by m6A antibody, indicating that YTHDC1 modulates PTEN expression by m6A modification of PTEN (Fig. 4H). To test whether knockdown of YTHDC1 affected the stability of PTEN mRNA, we used actinomycin D to inhibit transcription in EC cell lines. We found that sh-YTHDC1 further accelerated the degradation of PTEN mRNA (Fig. 4I). The above findings indicate that YTHDC1 maintains the stability of PTEN mRNA modified by m6A.
Fig. 4.
Targets of m6A modification of PTEN by YTHDC1 in EC. A The expression correlation between YTHDC1 and PTEN in UCEC in starBase database. B qRT-PCR and C western blot detection of YTHDC1 expression after YTHDC1 knockdown. D qRT-PCR detected PTEN mRNA expression after YTHDC1 knockdown. E Western blot detected PTEN protein expression in wild-type Ishikawa cells, Ishikawa cells transfected with pcDNA3.1/NC, or Ishikawa cells transfected with pcDNA3.1/PTEN for PTEN overexpression (IshikawaPTEN cells). Western blot also measured PTEN expression in IshikawaPTEN and HEC-1 A cells after YTHDC1 knockdown. F-G RIP to detect the interaction of YTHDC1 with PTEN. H MeRIP to detect m6A level of PTEN in cells. I Actinomycin D assay to detect the stability of PTEN after YTHDC1 knockdown. NC, negative control; *P < 0.05, ***P < 0.001
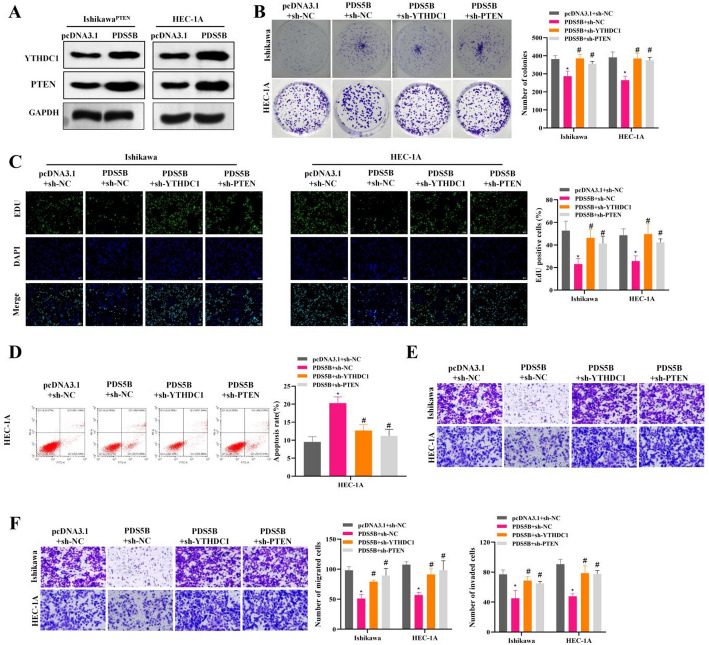
PDS5B inhibits malignant behaviors of EC cells by regulating YTHDC1/PTEN
To further investigate the regulatory mechanism of PDS5B in EC, we further explored the effects of PDS5B overexpression with or without knockdown of YTHDC1 and PTEN on EC cells. The results that overexpression of PDS5B significantly up-regulated the expression levels of YTHDC1 and PTEN (Fig. 5A). Colony formation and EdU assays exhibited that overexpression of PDS5B inhibited the proliferation and viability of EC cells, while sh-YTHDC1 or sh-PTEN reversed the inhibitory effect of PDS5B on cell proliferation and viability (Fig. 5B-C). In addition, overexpression of PDS5B promoted apoptosis, whereas sh-YTHDC1 or sh-PTEN significantly rescued the elevated rate of apoptosis (Fig. 5D). Results of Transwell assays showed that sh-YTHDC1 or sh-PTEN reversed the inhibition of PDS5B on the migratory and invasive abilities of EC cells (Fig. 5E-F). The above results suggest that PDS5B regulates EC cell malignancy via the YTHDC1/PTEN pathway.
Fig. 5.
PDS5B inhibits malignant progression of EC through YTHDC1/PTEN. A Western blot detection of YTHDC1/PTEN expression after PDS5B overexpression. B Colony formation assay for cell proliferation. C Cell proliferation was detected by EdU. Bar scale = 100 μm. D Apoptosis was detected by flow cytometry. E-F Transwell assay for migration and invasion. *P < 0.05, *compared with pcDNA 3.1 + sh-NC, #P < 0.05, #compared with PDS5B + sh-NC
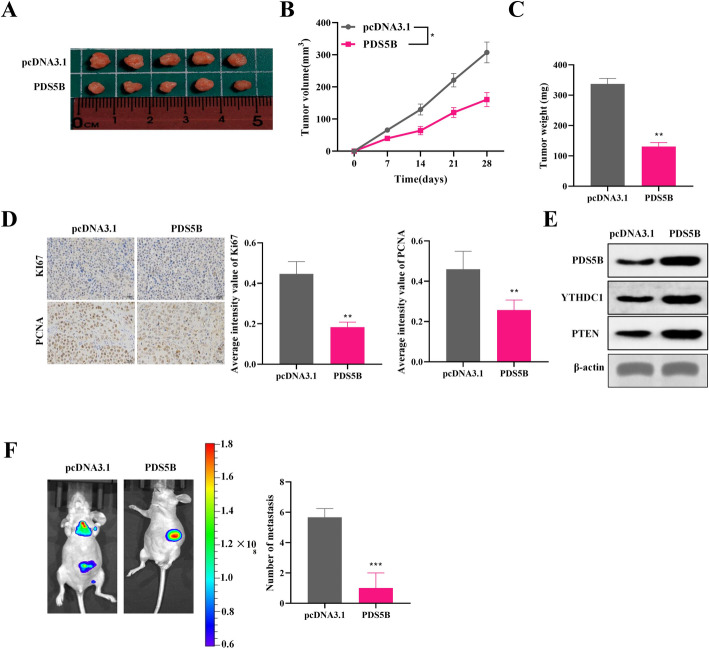
PDS5B inhibits EC cell growth and metastasis in vivo
PDS5B-overexpressed EC cells were injected subcutaneously into BALB/c nude mice and formed tumors. The results revealed that overexpression of PDS5B significantly suppressed the growth in tumor volume and weight, (Fig. 6A-C).IHC analysis also exhibited that overexpression of PDS5B suppressed the expression of Ki67 and PCNA, suggesting that PDS5B overexpression inhibited PDS5B (Fig. 6D). Western blot results showed that PDS5B, YTHDC1 and PTEN were highly expressed in the tumor tissues of nude mice in the PDS5B overexpression group (Fig. 6E). The results of the spontaneous metastasis model revealed that overexpression of PDS5B inhibited the intraperitoneal metastatic ability of Ishikawa cells (Fig. 6F). The above results suggest that overexpression of PDS5B suppresses EC progression by up-regulating YTHDC1/PTEN expression in vivo.
Fig. 6.
PDS5B inhibits EC cell growth and metastasis in vivo. A Schematic of mouse xenograft tumors (n = 5). B Tumor volume and C tumor mass. D IHC staining to analyze the protein expression levels of Ki67 and PCNA. Bar scale = 100 μm. E Western blot detection of PDS5B, YTHDC1 and PTEN expression in tumor tissue. F Monitoring in situ tumor metastasis by in vivo imaging system and H&E staining. **p < 0.01, ***p < 0.001
Discussion
In this study, we found that PDS5B was the common DEG in several EC-related datasets and its expression was significantly down-regulated in EC. The low expression of PDS5B was further confirmed in both EC tissues and EC cells. Therefore, we hypothesized that PDS5B plays a critical part in EC. Several studies have shown that PDS5B is closely related to tumorigenesis and progression. A study has revealed the relation between PDS5B expression and survival of ovarian cancer patients [23]. Xu et al. have shown that up-regulating the expression of PDS5B could inhibit the viability, migration and invasion of NSCLC cells. Furthermore, low expression of PDS5B may be associated with lymph node metastasis in lung cancer patients [24]. Ma et al. have found that PDS5B shows anti-tumor activity in pancreatic cancer cells, and overexpression of PDS5B inhibits cell viability and induces apoptosis [25]. These results are consistent with the findings in our study that overexpression of PDS5B expression inhibited EC cell viability, proliferation and migration and promoted apoptosis.
To further explore the mechanism of PDS5B in EC, we investigated the set of genes positively correlated with PDS5B using the UALCAN database, and found that PDS5B and YTHDC1 are a pair of related factors in EC. YTHDC1 is an m6A RNA methylation regulator, which is closely associated with the clinical features of cancer and endocervical adenocarcinoma (CESC), and can be identified as a potential marker for the diagnosis and prognosis of EC [26]. Ma et al. have predicted the interaction between potential therapeutic compounds and YTHDC1 interactions to deepen the study of its molecular mechanism [21]. Therefore, we speculate that PDS5B is involved in the regulation of EC together with YTHDC1. Analysis of the GEPIA database, validation results of the sample cell lines have demonstrated the mutual interaction. m6A RNA methylation regulators can play a critical part in EC, suggesting that PDS5B influences EC progression by regulating the methylation modification level of downstream molecules through YTHDC1 [27].
YTHDC1 has been found to regulate PTEN expression through methylation modification of PTEN, which has a determinant role in the activation of the downstream PI3K/AKT pathway [22, 28]. Whether such a regulatory mechanism also exist in EC is unclear. PTEN is a multifunctional lipid phosphatase with tumor-suppressing ability [29]. Mone et al. have first found PTEN to be absent in endometrial intraepithelial neoplasia (EIN) [30]. However, PTEN expression is also absent in some normal endometrial glands, which may be an early driver [31]. The above study initially revealed the low expression of PTEN in EC. Further, we investigated whether YTHDC1 affects EC progression by regulating PTEN. We found that sh-YTHDC1 inhibited PTEN expression and significantly reduced the half-life of PTEN. In addition, sh-YTHDC1 significantly increased the m6A level of PTEN. Here, we confirmed that our previous conjecture that YTHDC1 regulated PTEN expression by modulating PTEN m6A modification. Both METTL3 and YTHDC1 are m6A RNA methylation regulators, and β-elemene inhibits the m6A modification of PTEN through METTL3, which promotes the expression of PTEN to delay lung cancer progression [32]. PTEN, as an oncogene, is involved in regulating apoptosis in a range of cells and plays a critical part in cell cycle, growth, migration and apoptosis through PI3K/AKT signaling [31]. In this study, either sh-YTHDC1 or sh-PTEN reversed the inhibitory effects of PDS5B on EC cells, and we therefore concluded that PDS5B inhibited the malignant development of EC cells through the YTHDC1/PTEN axis.
To further explore the tumor-suppressive effects of PDS5B, we found that overexpression of PDS5B enhanced the expression of YTHDC1 and PTEN. Tumor growth and the rate of tumor metastasis were inhibited in vivo. These results confirmed that PDS5B inhibited the malignant progression of EC and exerted anti-cancer effects through the YTHDC1/PTEN axis.
We should acknowledge that this study also possesses some limitations. First, the sample size is relatively small in this study, which should be increased in future research to further confirm the applicability and value of our findings. Second, though we have demonstrated the changed expression of YTHDC1/PTEN in mouse tumor tissues, the effects of YTHDC1/PTEN knockdown on EC tumor growth and metastasis in vivo were not explored, and should be validated in the future research.
Conclusions
In summary, the present study revealed the important role of PDS5B in endometrial cancer, with its overexpression connected to the enhanced inhibition of tumor proliferation, migration and apoptosis. Mechanically, PDS5B may inhibit the malignant progression of endometrial cancer by interacting with YTHDC1, which in turn regulates the m6A modification of PTEN. These findings not only enhance our understanding of the molecular mechanisms of endometrial cancer, but also provide valuable information for the progress of new therapeutic strategies.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
Not applicable.
Author contributions
QG contributed to the study conception and design. Experimental operation, data collection and analysis were performed by HW, YQ and JL. The first draft of the manuscript was written by HW. All authors read and approved the final manuscript.
Funding
This work was supported by the Science and Technology Project of Changzhou Geriatric Hospital Affiliated to Soochow University, Changzhou No. 7 People’s Hospital.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Declarations
Ethics approval and consent to participate
This study was performed according to the principles of the Declaration of Helsinki, and was approved by the Joint Ethics Committee of the Ministry of Health. Informed consent was obtained from all individual participants included in the study. This study was approved by the Laboratory Animal Ethics Committee of Changzhou Geriatric Hospital Affiliated to Soochow University, Changzhou No. 7 People’s Hospital, and complied with the rules of Declaration of Helsinki principles. The maximal tumor size permitted by the ethics committee was 1000 mm3 and the maximal tumor burden was not exceeded in this study.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Sakna NA, Elgendi M, Salama MH, et al. Diagnostic accuracy of endometrial sampling tests for detecting endometrial cancer: a systematic review and meta-analysis. BMJ Open. 2023;13(6):e072124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jónsdóttir B, Wikman A, Sundström Poromaa I, et al. Advanced gynecological cancer: quality of life one year after diagnosis. PLoS ONE. 2023;18(6):e0287562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Siegel RL, Giaquinto AN, Jemal A. Cancer statistics, 2024. CA Cancer J Clin. 2024;74(1):12–49. [DOI] [PubMed] [Google Scholar]
- 4.Xia C, Dong X, Li H, et al. Cancer statistics in China and united states, 2022: profiles, trends, and determinants. Chin Med J (Engl). 2022;135(5):584–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Colombo N, Creutzberg C, Amant F, et al. ESMO-ESGO-ESTRO consensus conference on endometrial cancer: diagnosis, treatment and Follow-up. Int J Gynecol Cancer. 2016;26(1):2–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017;67(1):7–30. [DOI] [PubMed] [Google Scholar]
- 7.Rutgers JK. Update on pathology, staging and molecular pathology of endometrial (uterine corpus) adenocarcinoma. Future Oncol. 2015;11(23):3207–18. [DOI] [PubMed] [Google Scholar]
- 8.Lou Y, Liao J, Shan W et al. Menopausal status combined with serum CA125 level significantly predicted concurrent endometrial Cancer in women diagnosed with atypical endometrial hyperplasia before surgery (†). Diagnostics (Basel). 2021;12(1):6. [DOI] [PMC free article] [PubMed]
- 9.Kandoth C, Schultz N, Cherniack AD, et al. Integrated genomic characterization of endometrial carcinoma. Nature. 2013;497(7447):67–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Corr B, Cosgrove C, Spinosa D, et al. Endometrial cancer: molecular classification and future treatments. BMJ Med. 2022;1(1):e000152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Abu-Rustum N, Yashar C, Arend R, et al. Uterine neoplasms, version 1.2023, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2023;21(2):181–209. [DOI] [PubMed] [Google Scholar]
- 12.Kommoss S, McConechy MK, Kommoss F, et al. Final validation of the promise molecular classifier for endometrial carcinoma in a large population-based case series. Ann Oncol. 2018;29(5):1180–8. [DOI] [PubMed] [Google Scholar]
- 13.Yen TT, Wang TL, Fader AN, et al. Molecular classification and emerging targeted therapy in endometrial Cancer. Int J Gynecol Pathol. 2020;39(1):26–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maffini M, Denes V, Sonnenschein C, et al. APRIN is a unique Pds5 paralog with features of a chromatin regulator in hormonal differentiation. J Steroid Biochem Mol Biol. 2008;108(1–2):32–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Arruda NL, Bryan AF, Dowen JM. PDS5A and PDS5B differentially affect gene expression without altering cohesin localization across the genome. Epigenetics Chromatin. 2022;15(1):30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Losada A, Yokochi T, Hirano T. Functional contribution of Pds5 to cohesin-mediated cohesion in human cells and Xenopus egg extracts. J Cell Sci. 2005;118(Pt 10):2133–41. [DOI] [PubMed] [Google Scholar]
- 17.Zhang N, Coutinho LE, Pati D. PDS5A and PDS5B in cohesin function and human disease. Int J Mol Sci. 2021;22(11):5868. [DOI] [PMC free article] [PubMed]
- 18.França JA, Diniz MG, Bernardes VF, et al. Cohesin subunits, STAG1 and STAG2, and cohesin regulatory factor, PDS5b, in oral squamous cells carcinomas. J Oral Pathol Med. 2017;46(3):188–93. [DOI] [PubMed] [Google Scholar]
- 19.Kim MS, An CH, Yoo NJ, et al. Frameshift mutations of chromosome cohesion-related genes SGOL1 and PDS5B in gastric and colorectal cancers with high microsatellite instability. Hum Pathol. 2013;44(10):2234–40. [DOI] [PubMed] [Google Scholar]
- 20.Mo W, Zhang J, Li X, et al. Identification of novel AR-targeted MicroRNAs mediating androgen signalling through critical pathways to regulate cell viability in prostate cancer. PLoS ONE. 2013;8(2):e56592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ma J, Yang D, Ma XX. Immune infiltration-related N6-methyladenosine RNA methylation regulators influence the malignancy and prognosis of endometrial cancer. Aging. 2021;13(12):16287–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Su Y, Wang B, Huang J, et al. YTHDC1 positively regulates PTEN expression and plays a critical role in cisplatin resistance of bladder cancer. Cell Prolif. 2023;56(7):e13404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Couturier AM, Fleury H, Patenaude AM, et al. Roles for APRIN (PDS5B) in homologous recombination and in ovarian cancer prediction. Nucleic Acids Res. 2016;44(22):10879–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xu H, Zhou W, Zhang F, et al. PDS5B inhibits cell proliferation, migration, and invasion via upregulation of LATS1 in lung cancer cells. Cell Death Discov. 2021;7(1):168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ma J, Cao T, Cui Y, et al. miR-223 regulates cell proliferation and invasion via targeting PDS5B in pancreatic Cancer cells. Mol Ther Nucleic Acids. 2019;14:583–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wu H, Dong H, Fu Y, et al. Expressions of m6A RNA methylation regulators and their clinical predictive value in cervical squamous cell carcinoma and endometrial adenocarcinoma. Clin Exp Pharmacol Physiol. 2021;48(2):270–8. [DOI] [PubMed] [Google Scholar]
- 27.Zhang Y, Yang Y. Effects of m6A RNA methylation regulators on endometrial cancer. J Clin Lab Anal. 2021;35(9):e23942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang X, Chen Q, Bing Z, et al. Low expression of m6A reader YTHDC1 promotes progression of ovarian cancer via PIK3R1/STAT3/GANAB axis. Int J Biol Sci. 2023;19(14):4672–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen CY, Chen J, He L, et al. PTEN: tumor suppressor and metabolic regulator. Front Endocrinol (Lausanne). 2018;9:338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Monte NM, Webster KA, Neuberg D, et al. Joint loss of PAX2 and PTEN expression in endometrial precancers and cancer. Cancer Res. 2010;70(15):6225–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lac V, Nazeran TM, Tessier-Cloutier B, et al. Oncogenic mutations in histologically normal endometrium: the new normal? J Pathol. 2019;249(2):173–81. [DOI] [PubMed] [Google Scholar]
- 32.Feng Y, Li C, Liu S, et al. β-Elemene restrains PTEN mRNA degradation to restrain the growth of lung Cancer cells via METTL3-Mediated N(6) Methyladenosine modification. J Oncol. 2022;2022:3472745. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.