Abstract
Intercalary endoprosthetic devices are a diaphyseal segmental reconstructive option for both primary tumors and skeletal metastases, most used for pathological fractures or failure of internal fixation. Numerous designs have been employed with varying success. These implants require adequate quality and length bone stock, both proximal and distal, to be employed. Alternative reconstructions may include vascularized fibula autograft, allograft reconstruction, acute shortening, and fixation with cement spacer augmentation with planning staged procedures. The reported functional results and complication profile of intercalary endoprostheses are acceptable, but their use is carefully considered, as aseptic loosening remains one of the most common risks for failure. Although successful at short follow-up, these devices are often used for patients with segmental destruction or tumor involvement of the upper extremity, particularly in cases with metastatic bone disease or myeloma. In this review, we discuss the numerous designs, indications and contraindications, alternative options, biomechanics, reported results, and complications.
Intercalary diaphyseal prostheses are used for reconstruction following resection of a diaphyseal segment of a long bone with preservation of the joints at both ends. Indications include reconstruction after resection of primary or metastatic tumors or following failed internal fixation. They are typically used in the humerus, femur, and tibia. When compared with endoprosthetic reconstruction of the distal and proximal femur, proximal humerus, and proximal tibia, their usage and publication of results have been limited. The purpose of this review is to define the elements and variations of intercalary prosthetic designs, discuss indications and contraindications, present alternative reconstructive options, and summarize the literature from the standpoint of results and complications.
Definition and Design of the Intercalary Prosthesis
Their designs vary, but the common underlying theme is a body segment replacing the resected bone connected to two fixation portions (one proximal, one distal). In one early version of humeral intercalary spacers, the body portion was comprised a female: male articulation of two body segments each attached to an intramedullary stem segment (Figure 1). In the humerus, because of a substantial incidence of neurapraxia attributed to the distraction needed to reduce the junction, most humeral spacers have moved to a different design. In the femur, where brief distraction to achieve reduction of the female: male junction is better tolerated, this design is standard for mega prosthesis assembly. Two key advantages of these implants over other intercalary reconstruction options are their ability to provide immediate stability and function, as well as the relatively quick implantation process. The Global Modular Replacement System (GMRS) is still used in the femur (Figure 2). In a subsequent version of the original humeral design, the body portion is comprised two identical lap joint articulating segments secured by set screws and attached to intramedullary stems on each end (Figure 3, A–D). A custom variation on the lap joint technique used female male tapers to join the body segments to separate intramedullary stems.1 In a unique modern design, the body is a separate structure from the intramedullary stems and secured to the stems by screws2 (Figure 4, A–D). The intramedullary fixation is most commonly either cemented or press-fit. Limited reports have been described using either compression osteointegration (Figure 5, A–B) or custom three-dimensional printing with screw fixation3,4 (Figure 6, A–C). Additional separate fixation in one technique includes supplemental spanning plate fixation (Figures 7 and 8). Although the vast majority of these endoprostheses are metallic, an isoelastic prosthesis has been reported.5
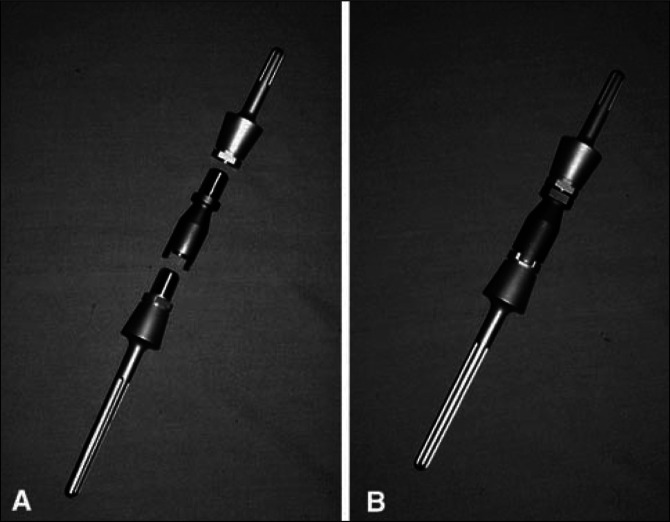
Figure 1.
the original Stryker intercalary humeral spacer design employed a male-female taper that was convenient in terms of achieving the desired rotational alignment of the two segments but required 2.04 cm of distraction to reduce the taper, resulting in 18% incidence of neurapraxia. This original design is shown here preassembled (A) and postassembly (B) (The figure used with permission from Wolters Kluwer Health.6)
Figure 2.

Although the female-male taper design in the Global Modular Replacement System (GMRS, Stryker) system has been abandoned for humeral intercalary spacers, it has been retained for the femoral intercalary spacers, where distraction is not as much of an issue due to the capacity of the thigh soft tissues and sciatic nerve to accommodate it. The intercalary GMRS femoral device shown here (right) has a female body component that can be assembled to any male stem (left) component (with or without body) to serve as an intercalary reconstructive endoprosthesis (Figures used with permission of Stryker)
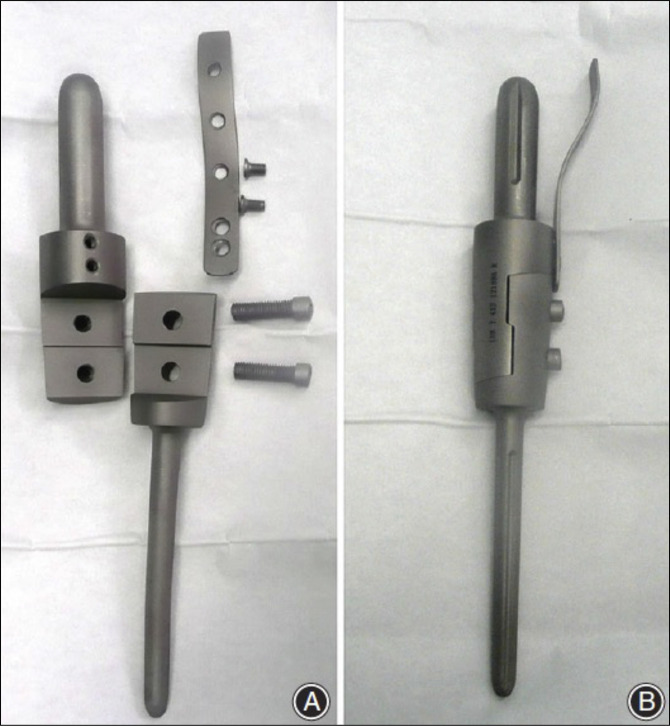
Figure 3.
Image showing lap joint articulating segments A lap joint set screw secured design (A) has replaced the original male-female taper design (Figure 1) for Stryker humeral intercalary endoprosthetic reconstruction to decrease the incidence of neurapraxia attributed to the need for distraction with the earlier design. Intraoperative photographs show the body portions attached to their corresponding stems, which have been cemented in place before the lap joint is closed and secured (B). After the lap joint is closed, two set screws are placed mediolateral and lateral medial (C). Care must be taken to ensure that the desired rotation is achieved before allowing cement to harden and that the final position of the screw holes is accessible. Postoperative anterior-posterior radiograph (D) shows the cemented stems and body in place within the humerus (The figure used with permission from Wolters Kluwer Health).7
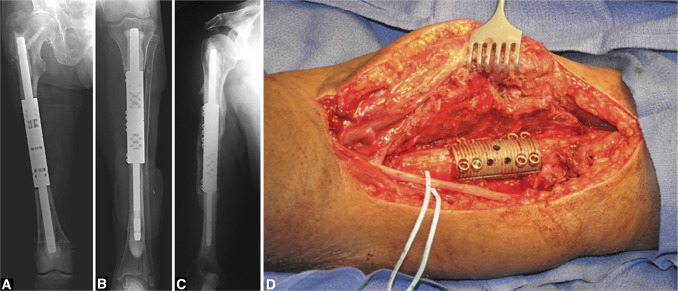
Figure 4.
Radiographs showing the intramedullary diaphyseal segmental defect fixation system (Osteobridge IDSF; Merete) in the (A) femur, (B) tibia, and (C) humerus. In this system, the body segment is independent of the stems and is secured to them with screws. Intraoperative photograph (D) showing the body of the IDSF system in situ (The figure used with permission from Wolters Kluwer Health).2
Figure 5.
Intraoperative photograph (A) showing the compressive osteointegration device (Compress, Biomet, Warsaw, Indiana) in the proximal femur. The use of intercalary osteointegration devices has been reported in the femur. Postoperative anterior-posterior and lateral radiographs (B) showing the implanted compressive osteointegration device in the proximal femur. This device is particularly advantageous when limited intramedullary canal remains after bone resection (Adapted with permission from Wolters Kluwer Health).3
Figure 6.
Images showing custom, three-dimensional, printed, intercalary proximal tibial endoprostheses were used to preserve proximal tibial epiphyseal plate function following intercalary resection of a proximal, tibial, metadiaphyseal osteosarcoma. The (A) design planning, (B) custom device, and (C) postoperative anterior-posterior and lateral radiographs are shown. In (A), both the 3D model (left) and cross-sectional model (right) are shown (This work is licensed under a Creative Commons Attribution CC BY Generic License. It is attributed to Lu M, Li Y, Luo Y, Zhang W, Zhou Y, Tu C. Noncemented three-dimensional-printed prosthetic reconstruction for massive bone defects of the proximal tibia. World J Surg Oncol 2018;16(1):47. doi: 10.1186/s12957-018-1333-6.4 Figures 5–7).
Figure 7.
Image showing a novel lap joint intercalary spacer design (Wego, Beijing, China) supplemented by a plate. The implant components and design plans are shown (Used with permission from John Wiley and Sons, Australia [Zhao LM, Tian DM, Wei Yet al. Biomechanical analysis of a novel intercalary prosthesis for humeral diaphyseal segmental defect reconstruction. Orthop Surg 2018;10(1):23-31. doi: 10.1111/os.12368.1 Figure 2]).
Figure 8.
Lap joint intercalary spacer design supplemented by single plate affixed to body segment by two screws. Disassembled components (A) and assembled device (B) (Used with permission from Chinese Orthopaedic Association and John Wiley and Sons, Australia [Huang HC, Hu YC, Lun DX, Miao J, Wang F, Yang XG, Ma XL. Outcomes of intercalary prosthetic reconstruction for pathological diaphyseal femoral fractures secondary to metastatic tumors. Orthop Surg 2017;9(2):221-228. doi: 10.1111/os.12327]).8
The designs determine the number of implant sizes that must be stocked. Five variables must be considered: (1) length of the body segment, (2) diameter of the body segment, (3) length of the intramedullary stems, (4) diameter of the intramedullary stems, and (5) modularity of the system. To some extent, each of these variables is affected by the anatomic location.9 The size of the defect not only directly determines the length of the body segment but indirectly affects the stem length, especially in the humerus and tibia, where bone length is shorter than in the femur. The diameter of the body segment may be limited to a single choice or separate choices for the upper extremity and lower extremity. Each body diameter requires a correlated matching stem diameter, ultimately requiring numerous available body and stem lengths. One way to decrease the required inventory for these systems is to have one of the two portions of the body segment of a fixed length with variability of the other body segment.2,5 For systems in which the body segment is unique from the stems, the array of body segments is completely independent of the stem lengths and diameters, thereby decreasing the required inventory.9
Methods
A review of the literature in Entrez PubMed as of October 2023, using the search terms “diaphysis endoprosthesis cancer,” identified 22 publications spanning from 1979 to 2021 that reported on intercalary diaphyseal endoprosthetic reconstructions, along with additional case reports. Notably, all reports that included two or more subjects were considered, resulting in a total of 404 reported patients. The clinical outcomes were systematically summarized in tabular format, categorized by implant indications, the distinction between index and revision surgeries, anatomical sites, and functional outcomes as assessed by the Musculoskeletal Tumor Society (MSTS) scoring system. In addition, overall complications were analyzed both collectively and by anatomical site, following the Henderson classification.
Indications and Contraindications
The primary anatomic indication for the usage of intercalary diaphyseal endoprosthetic reconstruction devices is when there has been a segmental resection of a major diaphyseal segment of bone. The proximal and distal joints must be preserved with enough remaining proximal and distal intramedullary canals to allow adequate fixation of the endoprosthesis. The most common site overall for this type of endoprosthetic reconstruction—particularly in the setting of metastases—is the humerus in part because the upper extremity does not undergo the same stresses as a lower extremity weight-bearing bone such as the femur, which is the second most common site for usage. Consequently, there have been fewer reports on femoral and tibial intercalary endoprosthetic reconstruction. In addition, the pattern of disease distribution affects the usefulness of these devices.2 Both humeral and femoral metastases are more common in the proximal metaphysis than the distal diaphysis. Femoral diaphyseal involvement is more commonly dealt with by standard intramedullary nailing. Femoral and tibial intercalary diaphyseal devices are a commonly reported solution for primary bone sarcoma resections, primarily because of the frequent occurrence of tumors in these anatomical locations.9 In addition, the radius has also been noted as a site of interest for such interventions in humans.10
From an oncology standpoint, these devices have been used both following resection of primary bone sarcomas and metastatic disease.10 Among primary bone sarcomas, Ewing sarcoma is the most common diaphyseal tumor, lending itself to intercalary resection and reconstruction. The key for primary bone sarcomas is whether, after achieving an adequate wide resection with a 1 to 2 cm intraosseous margin, there is enough bone left both proximally and distally to allow fixation of the device.10 The amount of bone required to allow adequate fixation varies with the device.
Although equally uncommon, there are also specific situations where intercalary resection is indicated in the setting of metastatic disease or myeloma. Some patients with solitary or oligometastatic renal carcinoma or thyroid carcinoma may benefit from overall survival by surgical removal of the site(s) of bone disease.11–14 In rare instances, similar resections have been performed in the setting of metastatic breast, prostate, and lung carcinoma.14 Also, surgical resection should be considered in solitary plasmacytomas. Of course, treatment in these situations should involve consultation with a multi-disciplinary cancer team.
Regardless of the underlying diaphyseal tumor, when there is failure of initial fixation or local tumor progression, resection and reconstruction should be considered. This occurs particularly after intercalary fixation of impending or actual pathologic fractures. Patients with isolated primary bone tumors who undergo wide resection and reconstruction with allograft fixation are at high risk for long-term complications, including graft failure or failure of internal fixation.15 In such circumstances, intercalary endoprosthetic reconstruction serves as a viable treatment option. Similarly, local progression of radio-resistant tumors may warrant resection and intercalary endoprosthetic reconstruction.
The absolute contraindications to an intercalary endoprosthetic reconstruction include insufficient proximal or distal bone stock, the presence of comorbidities that contraindicate surgery altogether, and the moribund patient. Relative contraindications include insufficient proximal or distal bone to support adequate fixation, expected cure or prolonged survival in a young patient because of the concerns regarding the long-term durability of the device and its fixation, posttraumatic nontumorous conditions, presence of active infection, and rapid progressive primary tumors with extremely poor expected survival.
Alternatives to Endoprosthetic Diaphyseal Reconstruction15
Intercalary Allograft Reconstruction
Intercalary allograft reconstruction involves the use of an allograft diaphyseal segment, typically of the same bone (eg, humerus for humeral defect, femur for femoral defect), although sometimes limited by availability. Fixation may be accomplished with either plate or intramedullary fixation. Often, the intramedullary canal is filled with antibiotic-loaded bone cement for local drug delivery and added strength.16–19 The main advantages of this type of reconstruction are that it is customizable to the exact defect size, and it provides for at least a semibiologic reconstruction, although typically only junctional healing (“spot welding”) is accomplished rather than complete replacement of the bone.16,17 Disadvantages include prolonged time needed for healing of the allograft-host junctions, increased risk of infection, and potential for allograft dissolution/resorption and fracture. Usually, this option is used in the setting of sarcoma, where the prognosis for prolonged survival is good, particularly compared with metastatic disease.
Autogenous Vascularized Autograft
Vascularized autografts, of which the prototypical example is the vascularized fibular graft (VFG), may be used for diaphyseal reconstruction, often with a plate or simple screw fixation. Advantages include a truly biological reconstruction, much quicker healing than an allograft, and the potential for circumferential growth over time. Disadvantages include potential failure to maintain the vascular supply, risk of fracture, and donor site morbidity. Their use as a stand-alone reconstruction is usually limited to the humerus. As for allograft segmental reconstructions, this option is typically reserved for the sarcoma patient with a good prognosis for prolonged survival.18,19
Combined Allograft and Vascularized Fibular Graft Reconstruction
To improve the speed of healing and decrease the likelihood of allograft-host nonunion, VFG may be combined with allografts.17–19 Although this is a more technically demanding procedure, success has been reported. However, as for allograft or VFG reconstructions alone, this technique is primarily indicated for sarcoma patients expected to have prolonged survival.
Shortening and Internal Fixation
Depending on the size of the segmental defect, sometimes the host bone can simply be shortened and fixed with either a plate/screw or intramedullary stabilization technique.15 Although the advantage here is host-to-host healing with a low incidence of nonunion, infection, and fracture, the indications are limited by the defect size, with the main indication being for short-segment defects in the humerus.15
For long-term survivors who have undergone lower extremity shortening procedures, segmental transport may be an option. This technique is primarily indicated in the pediatric population or patients with anticipated prolonged survival, as it restores living, normal bone. However, it requires prolonged treatment and a high level of patient cooperation. Complications can include frequent pin tract infections, delayed healing, and malalignment, and it is generally contraindicated in cases of metastatic disease.15
Cement Augmented Internal Fixation
Cement may be used to replace an intercalary diaphyseal defect similar to an allograft but without the potential for biological healing. In this case, the internal fixation, whether plates or rods, must span the defect and obtain good fixation proximally and distally. Because there is no potential for healing, the biggest risk is that the implant will eventually fail by screw pullout or fatigue fracture. Hence, this technique should be reserved for patients with extremely poor prognosis and limited expected survival.20–22 In addition, the staged Masquelet technique is also considered an appropriate option for managing such defects, as it provides a biological environment that may facilitate eventual healing.
Extracorporeally Devitalized Autografts
Similar to allograft reconstruction, the devitalized bone segment will precisely fit the created defect.15 Unlike allograft, the risk of disease transmission is eliminated. However, this technique should be reserved for either osteoblastic lesions or when at least two-third cortical bone is maintained.15 The possibility of histologic analysis of the tumor is sacrificed. This option is rarely employed in the United States.
Biomechanical Comparisons of Endoprostheses with Other Techniques
The intercalary endoprostheses have been compared favorably with other commonly employed relevant alternative techniques in biomechanical testing in three studies. Compared with plates, intramedullary nails, and external fixators, segmental prosthetic implants were similar in compressive strength to intramedullary nails (markedly better than plates or external fixators) and similar to external fixators in flexion and torsional strength (markedly better than plates and intramedullary nails).23 In light of their findings and the necessity for early mobilization, the authors recommended endoprostheses as the preferred option over other techniques. Another study demonstrated that a male-female, taper-type, cemented intercalary endoprosthesis exhibited superior strength in both nondestructive four-point bending and destructive torsional testing when compared with plate/screw constructs and Rush rod humeral fixation. Notably, the torsional strength of the endoprosthesis approached that of the intact humerus.24
Similarly, in a biomechanical study focusing on a second-generation lap joint spacer, the cemented endoprosthesis displayed statistically significant advantages in peak torque and stiffness compared with intramedullary nails used with either cement or allograft. It is essential to avoid overlengthening to prevent stiffness in adjacent joints.7
Reported Results of Intercalary Endoprostheses
Based on the review of the literature, the most common indication was metastatic disease (64%) among the 15 of 22 reports that included this information (Tables 1 and 2). Most of those placed (92%) were primary rather than revision cases among the 14 of 22 reports that had this information. Eight of 22 reports provided information regarding specific anatomic locations for both primary tumor and metastatic cases. For reports of patients with metastatic disease, the most common location was the humerus (86%) followed by the femur (14%), and no reports provided information for the tibia in the setting of metastatic disease. The most common location for those that were primary tumor surgeries was the femur (71%), followed by the tibia (21%) and humerus (8%).
Table 1.
Reported Results of Diaphyseal Endoprostheses
| Citation | Period | Device | Devices (Patients), N | Indications (N patients) and Mean FU | Index vs Revision Surgery (N patients) | Anatomic Site (N Devices) | Functional Results (MSTS Mean Score) |
| Mahdal et al25CORR2022 | 2012-2021 | Henderson | 25 | NA | NA | Humerus (9)Femur (18) | Overall (82%) |
| Benevenia et al2 CORR 2016 | 2008-2013 | Merete | 44Cemented stems (29)Noncemented (15) | Metastases (33) at 11 monthsPrimary tumors (8) at 19 months | Index (35)Revision (6) | Humerus (18)Femur (21)Tibia (5) | Overall (77%)Cemented (84%)aNoncemented (66%)a |
| Damron et al6,26 CORR 1996, 2008 | 1989-2004First generation: 1989-1998Second generation: 1999-2004 | Stryker | 32First-generation male-female taper (21)Second-generation lap joint (11) | Metastases (29)Primary tumors (3) | Index (23)Revision (9) | Humerus only (32) | Overall preoperation to postoperation:b 9%-71% |
| First generation: 7%-68%Second generation: 14%-73% | |||||||
| Calvert et al3 CORR 2014 | Approximately 2004-2012 | Biomet Compress | 2 intercalary femur devices among 50 overall | Mixed metastases and primary tumors in overall group; no separate description for intercalary | No separate analysis of intercalary devices | Femur (2) | No separate analysis |
| Zhao et al27 J Shoulder Elbow Surg | 2011-2017 | NA | Endoprosthesis alone (4)With plate (5) | Metastatic disease (9) at 14 months | Index surgeries (9) | Humerus (9) | Endoprosthesis alone: 82%With plate: 87% |
| Zheng et al28 BMC Cancer 2019 | Approximately 2010-2017 | Second-generation lap joint type (24% with plates) | 49 | Primary (7)Metastatic (42) at 16 months | Index surgeries (49) | Humerus (13)Femur (30)Tibia (5)Ulna (1) | 67% overall |
| Tedesco et al29 Orthopedics 2017 | NA | Customized anchor plugs for short segment fixation with double compressive osseointegration | 6 | NA | NA | NA | 88% overall |
| Abudu et al30 JBJS-Br 1996 | 1979-1994 | Custom | 18 | Primary only (18) at 65 months | Index surgeries (18) | NA | 77% achieved 80% of preoperative function |
| Guder et al31 Arch Orthop Trauma Surg 2017 | NA | Ultrashort tibial stem | 4 | Primary only (4) at 50 months | Index surgeries (4) | Tibia (4) | 93% |
| Bernthal et al32 Bone Joint J 2019 | 2008-2013 | Custom-cemented cross-pin fixation | 6 | NA | NA | NA | NA |
| Huang et al8 Orthop Surg 2017 | 2011-2015 | Lap joint with single-plate fixation | 16 | Metastatic only (16) at 9 months | Index surgeries (16) | Femur (16) | 85% |
| Szczerba et al33 Ortop Traumatol Rehabil 2019 | 2013-2017 | Modular endoprosthesis | NA (overall larger reports of 82 including other diaphyseal techniques) | Metastatic only (NA) | NA | Humerus (NA)Femur (NA)Tibia (NA) | NA |
| Hanna et al.34 JBJS-Br 210 | 1989-2005 | NA | 23 | Primary only (23) at 97 months | Index surgeries (23) | Femur (23) | 87% for 16/23 with retained prostheses |
| Hamada et al35 J Surg Case Reports 2014 | 2002, 2005 | Custom K-MAX, Kyocera Medical Corp., Kyoto, Japan | 2 | Failure of extracorporeal irradiated autografts (2); duration FU NA | Revision (2) | Femur (2) | 75% (67, 83) |
| Sakellariou et al36 J Long Term Eff Med Implants 2008 | NA | NA | 6 | Primary (2)Metastatic (4) | Index surgeries (6) | Humerus (NA)Femur (NA)Tibia (NA) | 88% |
| Ruggieri et al37 J Surg Oncol 2011 | NA | “Modular intramedullary diaphyseal segmental defect fixation system” | 24 | Primary and metastatic at 29 months | NA | Humerus (NA)Femur (NA)Tibia (NA) | NA |
| Sewell et al38 J Bone Joint Surg Br. 2011 | NA | Custom | 18 | Primary and metastatic at 59 months | NA | Tibia (18) | 77% (TESS 74%) |
| Aldlyami et al9 Int Orthop 2005 | 1979-1999 | Custom | 35 | Primary only at 107 months (35) | Index surgeries (35) | Humerus (3)Femur (29)Tibia (3) | NA |
| Spiegelberg39 JBJS-Br 2009 | NA | Custom (extracortical HA-coated prox plates, distal cemented stems) | 8 | Primary only at 35 months (8) | Index surgeries (8) | Tibia (8) | 79% (Oxford knee score 40) |
| McGrath et al40 Acta Orthop Belg 2011 | 1995-2010 | Custom | 13 | Primary and metastatic at 57 months | Index surgeries (13) | Humerus (13) | 77%TESS (67%) |
| Schürmann et al5 CORR 2000 | 1987-1997 | Isoelastic | 57 | Metastatic only (57) | Index (50)Revision (7) | Humerus (57) | (G or E in 80% using Karnofsky performance) |
| Kuo et al41 CORR 1983 | NA | Titanium fiber metal composite | 17 | Primary and metastatic | NA | NA | (E in 11, S in 5, F in 1) |
CORR, Clinical Orthopaedics and Related Research, FU = follow-up, MSTS = Musculoskeletal Tumor Society, N = number of patients or devices
Cemented versus noncemented P = 0.0017.
Only pain, function, and emotional acceptance categories used; TESS = Toronto Extremity Salvage Score; F, S, G, E = fair, satisfactory, good, excellent.
Table 2.
Summary of Indications for Surgery and Corresponding Anatomic Locations
| Numbers Included in Review | Primary vs Metastases Indication | Primary Versus Revision Surgery | Primary Versus Metastases by Anatomic Location | Primary Versus Revision Cases by Anatomic Location |
| 22 reportsa | 15 of 22 | 14 of 22 | 8 of 22 | 13 of 22 |
| 404 patientsb | 298 of 404 (74%) | 313 of 404 (77%) | 184 of 404 (46%) | 265 of 404 (66%) |
| Ratio of number of patients with parameter to number of patients with information available for that parameterc | Primary tumors: 108 of 298 (36%) | Primary surgery: 289 of 313 (92%) | Primary tumors: 73 of 184 (40%) Femur 52/73 (71%) Tibia 15/73 (21%) Humerus 6/73 (8%) |
Primary surgery: 247 of 265 (93%) Humerus 111/247 (45%) Femur 98/247 (40%) Tibia 38/247 (15%) |
| Metastatic tumors: 190 of 298 (64%) | Revision surgery: 24 of 313 (8%) | Metastatic tumors: 111 of 184 (60%) Humerus 95/111 (86%) Femur 16/111 (14%) |
Revision surgery: 18 of 265 (7%) Humerus 16/18 (89%) Femur 2/18 (11%) |
Each column shows the number of reports with information available for the parameter at the top of the column.
each column shows the number of patients with information available for the parameter at the top of the column.
Each column shows the number of patients with each individual parameter as a fraction of the patients with this information available.
As expected, the mean duration follow-up for isolated metastatic reports (9 to 16 months) was far shorter than that for primary tumor cases (35 to 107 months). Overall functional results using the MSTS/Enneking scoring system range from 66% to 93%. From a purely anatomic perspective, functional results were similar for the humerus (67% to 88%), femur (67% to 87%), and tibia (67% to 93%).
Complications
Reported complications of endoprosthetic reconstructions are presented according to the Henderson et al classification (Table 3). Complications for each report are shown in Table 4 with a summary in Table 5. Overall complication rates in the 22 reports ranged from 6% to 92%. Among the 18 reports that provided information about specific complications, 127 of the total 404 patients (31%) had some type of complication. Primary tumors had a higher average complication rate (48%) in comparison to metastatic disease (12%), which is probably related to the longer duration follow-up in the former. In terms of anatomic location, the femur had the highest average complication rate (37%) followed by the humerus (23%), and no cases reported complications for the tibia. Aseptic loosening (Henderson type II) is the most common complication reported among all of the Henderson failure types (Table 5).
Table 3.
Henderson et al Classification of Complications
| Type of failure | Definition |
| I | Soft-tissue failure |
| II | Aseptic loosening |
| III | Structural failure |
| IV | Infection |
| V | Tumor progression |
Derived from 42.
Table 4.
Diaphyseal Endoprosthetic Complications by Individual Report
| Citation | Device | Overall Implant Complications | Complications By Anatomic Site (%) | Henderson Failure Types (%)a | Notes | Cemented vs Noncemented |
| Mahdal et al25Current Oncology2022 | Henderson (27) | 7/27 (26%)9 humeral18 femoral | Humerus 4/9 (44%) | I (0) | Four out of the five type II cases were in the humerus and one was in the femur | NA |
| II5/27 (18.5%) | ||||||
| Femur3/18 (17%) | III2/27 (7.4%) | |||||
| IV (0) | ||||||
| V (0) | ||||||
| Benevenia et al2 CORR 2016 | MereteCemented stems (29)Noncemented (15) | 12/44 (27%) | Humerus (0)b | I (0) | — | Cemented (21)dNoncemented (33)d |
| Femur12/21 (57%)b | II5/44 (11%); all noncementedc | Between stem and bone | ||||
| Tibia (0%)b | III6/44 (14%); all cementedc | Clamp-rod implant interface | ||||
| IV1/44 (2%) | — | |||||
| V1/44 (2%) | Required amputation | |||||
| Damron et al6,26 CORR 1996, 200 | StrykerCemented stems (32) | 14/32 (44%) | Humerus only (44%) | I 3/32 (9%) | All type I failures due to neurapraxia | All cemented |
| II 5/32 (16%) | ||||||
| III 2/32 (6%) | ||||||
| IV (0) | ||||||
| V 2/32 (6%) | ||||||
| Calvert et al3 CORR 2014 | Biomet Compress stems (n) | NAe | NA | NA | NA | Noncemented only |
| Zhao et al27 J Shoulder Elbow Surg | Grp I: Endoprosthesis alone (4)Grp II: With plate (5) | 2/9 (22%) | Humerus only (22%) | I 1/9 (11%) Grp 1 | No complications in Grp II | Cemented only |
| II 1/9 (11%) Grp I | ||||||
| III (0%) | ||||||
| IV (0%) | ||||||
| V (0%) | ||||||
| Zheng et al28 BMC Cancer 2019 | Cemented (49) with plates (24%) | 11/49 (22%) | Primary4/7 (57%)f | I5/49 (10%) | Type I: 2 radial n. palsies, 3 wound healingType V: only in primary group. | Cemented only |
| II2/49 (4%) | ||||||
| III1/49 (2%) | ||||||
| Metastatic7/42 (17%)f | IV (0%) | |||||
| V3/49 (6%) | ||||||
| Tedesco et al29 Orthopedics 2017 | Customized anchor plugs for short segment fixation with double compressive osseointegration (6) | 3/6 (50%) | NA | I (0%) | All 3 complications required revision | 5/6 cemented (no separate analysis) |
| II (0%) | ||||||
| III3/6 (50%) | ||||||
| IV (0%) | ||||||
| V (0%) | ||||||
| Abudu et al30 JBJS-Br 1996 | Custom (18) | NA | Primary only (NA) | I (NA) | Mechanical loosening, limb shortening secondary DJD “main complications” | NA |
| II (NA) | ||||||
| III (NA) | ||||||
| IV (0%) | ||||||
| V1/18 (5%) | ||||||
| Guder et al31 Arch Orthop Trauma Surg 2017 | Ultrashort tibial stem | 3/4 (75%) | Primary only3/4 (75%) | I2/4 (50%) | Both type I wound complications required revision | NA |
| II (0%) | ||||||
| III (0%) | ||||||
| IV (0%) | ||||||
| V1/4 (25%) | ||||||
| Bernthal et al32 Bone Joint J 2019 | Custom-cemented cross-pin fixation | NA | NA | NA | NA | NA |
| Huang et al8 Orthop Surg 2017 | Lap joint with single-plate fixation | 2/16 (13%) | Metastatic only (13%) | I (0%) | Revision done in type III and type IV (two-stage exchange) | Cemented only |
| II (0%) | ||||||
| III 1/16 (6%) | ||||||
| IV 1/16 (6%) | ||||||
| V 1/16 (6%) | ||||||
| Szczerba et al33 Ortop Traumatol Rehabil 2019 | Modular endoprosthesis included in group of other techniques | NA | Metastatic only (NA for endoprosthetic) | NA | NA | NA |
| Hanna et al34 JBJS-Br 2010 | NA | 9/23 (39%) | Primary only (41%) | I 1/23 (4%) | Implant 5 YSR 85%, 10 YSR 68%. | NA |
| II 1/23 (4%) | ||||||
| III 3/23 (13%) | Revision rate 22%. | |||||
| IV 1/23 (4%) | ||||||
| V 5/23 (22%) | Overall revision surgery 26% | |||||
| Hamada et al35 J Surg Case Reports 2014 | Custom K-MAX, Kyocera Medical corp., Kyoto, Japan | 1/2 (50%) | Metastatic (1)1/2 (50%) | I (0%) | Revision in single type II | NA |
| II 1/2 (50%) | ||||||
| Primary (1)1/2 (50%) | III (0%) | |||||
| IV (0%) | ||||||
| V (0%) | ||||||
| Sakellariou et al36 J Long Term Eff Med Implants 2008 | NA | 1/6 (17%) | NA | I (0%) | Revision in single type II | NA |
| II 1/6 (17%) | ||||||
| III (0%) | ||||||
| IV (0%) | ||||||
| V (0%) | ||||||
| Ruggieri et al37 J Surg Oncol 2011 | “Modular intramedullary diaphyseal segmental defect fixation system” | 8/24 (33%) | Metastatic (NA)Primary (NA) | I (0%) | Type II most common >10-cm bone resection | NA |
| II (NA) | Type III in all femoral reconstructions at prox stem | |||||
| III (NA) | ||||||
| IV (0%) | ||||||
| V (0%) | 1 LLD | |||||
| Sewell et al.38 J Bone Joint Surg Br 2011 | Custom tibial | 13/18 (72%) | NA | I (0%) | Implant 10 YSR 63%4 revisions | NA |
| II4/18 (22%) | ||||||
| III2/18 (11%) | ||||||
| IV1/18 (5%) | ||||||
| V6/18 (33%) | ||||||
| Aldlyami et al.9 Int Orthop 2005 | Custom | 16/35 (46%) | Primary only 16/35 (46%) | I (0%) | Implant 10 YSR 63% | Cemented only |
| II7/35 (20%) | ||||||
| III3/35 (9%) | ||||||
| IV1/35 (3%) | ||||||
| V5/35 (14%) | ||||||
| Spiegelberg39 JBJS-Br 2009 | Custom | 2/8 (25%) | Primary only 2/8 (25%) | I (0%) | Implant survival 75% | Proximal HA-coated plates, distal cemented stems only |
| II (0%) | ||||||
| III1/8 (13%) | ||||||
| IV (0%) | ||||||
| V1/8 (13%) | ||||||
| McGrath et al40 Acta Orthop Belg 2011 | Custom | 12/13 (92%) | Primary only 12/13 (92%) | I (0%) | Implant 10 YSR 47% | NA |
| II4/13 (31%) | ||||||
| III2/13 (15%) | ||||||
| IV (0%) | ||||||
| V6/13 (46%) | ||||||
| Schürmann et al5 CORR 2000 | Isoelastic diaphyseal prosthesis | 10/57 (18%) | Metastatic only10/57 (18%) | I3/57 (5%) | Type III (3/4) prosthesis fracture at locking screw inserted through prosthetic shaft in non-cemented technique | NA |
| II2/57 (4%) | ||||||
| III4/57 (7%) | ||||||
| IV1/57 (2%) | ||||||
| V (0%) | ||||||
| Kuo et al41 CORR 1983 | Titanium fiber metal composite | 1/17 (6%) | NA | NA | NA | NA; Bone graft supplemented prosthesis |
CORR = Clinical Orthopaedics and Related Research, N = number of patients or devices; Henderson failure types (Table 1), prox = proximal.
Type III included periprosthetic fracture and device failure, type V included both local progression and metastases except in primary tumors.
Complications by anatomic site P < 0.001.
Association of complication type with fixation, P = 0.0022.
Cemented versus noncemented, P = 0.39.
Complication rates for overall group of N = 50 implants at multiple sites (no separate analysis of the two femoral intercalary implants in this larger reports.
Complications primary versus metastatic, P = 0.036.
Table 5.
Summary of Overall Reported Complication Ratesa,b,c
| Numbers Included in Review | Overall Complications | Complication by Anatomic Site | Henderson Failure Types |
| 22 reports | 18 of 22 | 14 of 22 | 18 of 22 |
| 404 total patients | 127 total complications 127/404 = 31% Mean complication rate: 37% Median complication rate: 27% Max/min complication rate: 6-92% |
Humerus (n = 64) Overall 20/64 = 31% Mean: 23% Median: 24% Max/min: 0%-44% |
Type I: soft-tissue failure (n = 109) Overall 12/109 = 9% Mean: 2% Median: 0% Max/min: 0-14% |
| Femur (n = 39) Overall 15/39 = 38% Mean: 37% Median: 37% Max/min: 17%-57% |
Type II: Aseptic Loosening (n = 109) Overall 33/109 = 26% Mean: 6% Median: 3% Max/min: 0%-27% |
||
| Tibia (n = 0) Overall = 0% Mean: 0% Median: 0% Max/min: 0% |
Type III: Structural failure (n = 109) Overall 28/109 = 22% Mean: 3% Median: 2% Max/min: 0%-14% |
||
| Primary only (n = 83) Overall 42/83 = 51% Mean: 48% Median: 46% Max/min: 1%-92% |
Type IV: Infection (n = 109) Overall 6/109 = 5% Mean: 0.4% Median: 0% Max/min: 0%-2% |
||
| Metastatic only (n = 73) Overall 12/73 = 16% Mean: 12% Median: 15% Max/min: 1%-18% |
Type V: Tumor progression (n = 109) Overall 30/109 = 24% Mean: 2% Median: 1% Max/min: 0%-10% |
aEach column shows the number of reports with information available for the parameter at the top of the column.
bEach column shows the number of patients with information available for the parameter at the top of the column.
cEach column shows the number of patients with each parameter as a fraction of the patients with this information available.
In the only studies directly comparing cemented versus noncemented constructs, representing a total of 94 prostheses, only two types of prostheses were examined.2,5 Benevenia et al2 reported on 44 endoprostheses using a modular clamp system (OsteoBridge IDSF; Merete) and found that when no cement was used, the most common site of failure was type II failure at the implant-bone interface, whereas for cemented implant failure, it was type III failure at the clamp-rod interface. Schürmann et al5 reported that among 50 isoelastic prostheses, the most common failure mode for noncemented implantation with two locking screws was screw breakage (3 of 19). This occurred rarely with cement (1 of 38), no different than the frequency of implant loosening (1 of 38).
Summary
Numerous options exist for reconstructing diaphyseal segmental bone defects following tumor resection, with metastatic disease being the most common indication reported (64%). Among these cases, the humerus is the predominant site. Intercalary endoprostheses offer a reliable solution that restores fair to excellent function, evidenced by MSTS scores ranging from 66 to 93%. Continued follow-up and detailed documentation of outcomes are essential to clarify the role of these endoprostheses compared with other alternatives, especially as new designs emerge.
Footnotes
Dr. Damron and Ms. Malhotra Editorial Board for JAAOS, CORR, JOR, BMC-MSK, PLOS-one, Medicina; Compensation from JAAOS (Associate Editor); Royalties from Lippincott, Williams, Wilkins, Up-to-Date; Research Support from Stryker; Grant Support from NIH NCI, OREF/MSTS, Carol Baldwin Breast; Cancer Research Fund, Jim and Juli Boeheim; Research Fund, William Smyth Fund.
References
- 1.Zhao LM, Tian DM, Wei Y, et al. : Biomechanical analysis of a novel intercalary prosthesis for humeral diaphyseal segmental defect reconstruction. Orthop Surg 2018;10:23-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Benevenia J, Kirchner R, Patterson F, et al. : Outcomes of a modular intercalary endoprosthesis as treatment for segmental defects of the femur, tibia, and humerus. Clin Orthop Relat Res 2016;474:539-548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Calvert GT, Cummings JE, Bowles AJ, Jones KB, Wurtz LD, Randall RL: A dual-center review of compressive osseointegration for fixation of massive endoprosthetics: 2- to 9-year followup. Clin Orthop Relat Res 2014;472:822-829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lu M, Li Y, Luo Y, Zhang W, Zhou Y, Tu C: Uncemented three-dimensional-printed prosthetic reconstruction for massive bone defects of the proximal tibia. World J Surg Oncol 2018;16:47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schürmann M, Gradl G, Andress HJ, Kauschke T, Hertlein H, Lob G: Metastatic lesions of the humerus treated with the isoelastic diaphysis prosthesis. Clin Orthop Relat Res 2000;380:204-214. [DOI] [PubMed] [Google Scholar]
- 6.Damron TA, Leerapun T, Hugate RR, Shives TC, Sim FH: Does the second-generation intercalary humeral spacer improve on the first? Clin Orthop Relat Res 2008;466:1309-1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Henry JC, Damron TA, Weiner MM, Higgins ME, Werner FW, Sim FH: Biomechanical analysis of humeral diaphyseal segmental defect fixation. Clin Orthop Relat Res 2002;396:231-239. [DOI] [PubMed] [Google Scholar]
- 8.Huang HC, Hu YC, Lun DX, et al. : Outcomes of intercalary prosthetic reconstruction for pathological diaphyseal femoral fractures secondary to metastatic tumors. Orthop Surg 2017;9:221-228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aldlyami E, Abudu A, Grimer RJ, Carter SR, Tillman RM: Endoprosthetic replacement of diaphyseal bone defects. Long-term results. Int Orthop 2005;29:25-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Macmull S, Gokaraju K, Miles J, Blunn GW, Cannon SR, Briggs TW: Use of endoprosthetic diaphyseal replacement: A novel approach to management of extensive metastatic tumor of the midshaft radius. Tech Hand Up Extrem Surg 2010;14:183-186. [DOI] [PubMed] [Google Scholar]
- 11.Stojadinovic A, Shoup M, Ghossein RA, et al. : The role of operations for distantly metastatic well-differentiated thyroid carcinoma. Surgery 2002;131:636-643. [DOI] [PubMed] [Google Scholar]
- 12.Assouad J, Banu E, Brian E, et al. : Strategies and outcomes in pulmonary and extrapulmonary metastases from renal cell cancer. Eur J Cardiothorac Surg 2008;33:794-798. [DOI] [PubMed] [Google Scholar]
- 13.Casadei R, Drago G, Di Pressa F, Donati D: Humeral metastasis of renal cancer: Surgical options and review of literature. Orthop Traumatol Surg Res 2018;104:533-538. [DOI] [PubMed] [Google Scholar]
- 14.Ratasvuori M, Wedin R, Hansen BH, et al. : Prognostic role of en-bloc resection and late onset of bone metastasis in patients with bone-seeking carcinomas of the kidney, breast, lung, and prostate: SSG study on 672 operated skeletal metastases. J Surg Oncol 2014;110:360-365. [DOI] [PubMed] [Google Scholar]
- 15.Zekry KM, Yamamoto N, Hayashi K, et al. : Reconstruction of intercalary bone defect after resection of malignant bone tumor. J Orthop Surg (Hong Kong) 2019;27:2309499019832970. [DOI] [PubMed] [Google Scholar]
- 16.Agarwal M, Puri A, Gulia A, Reddy K: Joint-sparing or physeal-sparing diaphyseal resections: The challenge of holding small fragments. Clin Orthop Relat Res 2010;468:2924-2932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Miller BJ, Virkus WW: Intercalary allograft reconstructions using a compressible intramedullary nail: A preliminary report. Clin Orthop Relat Res 2010;468:2507-2513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Aponte-Tinao L, Farfalli GL, Ritacco LE, Ayerza MA, Muscolo DL: Intercalary femur allografts are an acceptable alternative after tumor resection. Clin Orthop Relat Res 2012;470:728-734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yang J, Li W, Feng R, Li D: Intercalary frozen autografts for reconstruction of bone defects following meta-/diaphyseal tumor resection at the extremities. BMC Musculoskelet Disord 2022;23:890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Prejbeanu R, Vlad Daliborca C, Dumitrascu V, et al. : Application of acrylic spacers for long bone defects after tumoral resections. Eur Rev Med Pharmacol Sci 2013;17:2366-2371. [PubMed] [Google Scholar]
- 21.Siegel HJ, Lopez-Ben R, Mann JP, Ponce BA: Pathological fractures of the proximal humerus treated with a proximal humeral locking plate and bone cement. J Bone Joint Surg Br 2010;92:707-712. [DOI] [PubMed] [Google Scholar]
- 22.Bickels J, Kollender Y, Wittig JC, Meller I, Malawer MM: Function after resection of humeral metastases: Analysis of 59 consecutive patients. Clin Orthop Relat Res 2005;437:201-208. [DOI] [PubMed] [Google Scholar]
- 23.Sakellariou VI, Mavrogenis AF, Babis GC, Soucacos PN, Magnissalis EA, Papagelopoulos PJ: Comparison of four reconstructive methods for diaphyseal defects of the humerus after tumor resection. J Appl Biomech 2012;28:568-578. [DOI] [PubMed] [Google Scholar]
- 24.Chin HC, Frassica FJ, Hein TJ, et al. : Metastatic diaphyseal fractures of the shaft of the humerus. The structural strength evaluation of a new method of treatment with a segmental defect prosthesis. Clin Orthop Relat Res 1989;248:231-239. [PubMed] [Google Scholar]
- 25.Mahdal M, Pazourek L, Apostolopoulos V, Adámková Krákorová D, Staniczková Zambo I, Tomáš T: Outcomes of intercalary endoprostheses as a treatment for metastases in the femoral and humeral diaphysis. Curr Oncol 2022;29:3519-3530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Damron TA, Sim FH, Shives TC, An KN, Rock MG, Pritchard DJ: Intercalary spacers in the treatment of segmentally destructive diaphyseal humeral lesions in disseminated malignancies. Clin Orthop Relat Res 1996;324:233-243. [DOI] [PubMed] [Google Scholar]
- 27.Zhao J, Yu XC, Xu M, et al. : Intercalary prosthetic reconstruction for pathologic diaphyseal humeral fractures due to metastatic tumors: Outcomes and improvements. J Shoulder Elbow Surg 2018;27:2013-2020. [DOI] [PubMed] [Google Scholar]
- 28.Zheng K, Yu XC, Hu YC, et al. : Outcome of segmental prosthesis reconstruction for diaphyseal bone tumors: A multi-center retrospective study. BMC Cancer 2019;19:638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tedesco NS, Van Horn AL, Henshaw RM: Long-term results of intercalary endoprosthetic short segment fixation following extended diaphysectomy. Orthopedics 2017;40:e964-e970. [DOI] [PubMed] [Google Scholar]
- 30.Abudu A, Carter SR, Grimer RJ: The outcome and functional results of diaphyseal endoprostheses after tumour excision. J Bone Joint Surg Br 1996;78:652-657. [PubMed] [Google Scholar]
- 31.Guder WK, Hardes J, Gosheger G, Nottrott M, Streitbürger A: Ultra-short stem anchorage in the proximal tibial epiphysis after intercalary tumor resections: Analysis of reconstruction survival in four patients at a mean follow-up of 56 months. Arch Orthop Trauma Surg 2017;137:481-488. [DOI] [PubMed] [Google Scholar]
- 32.Bernthal NM, Upfill-Brown A, Burke ZDC, et al. : Long-term follow-up of custom cross-pin fixation of 56 tumour endoprosthesis stems: A single-institution experience. Bone Joint J 2019;101-B:724-731. [DOI] [PubMed] [Google Scholar]
- 33.Szczerba P, Guzik G, Bohatyrewicz A, Kotrych D: Bone diaphysis metastases, the ways and results of surgical treatment saving the joints. Ortop Traumatol Rehabil 2019;21:107-115. [DOI] [PubMed] [Google Scholar]
- 34.Hanna SA, Sewell MD, Aston WJ, et al. : Femoral diaphyseal endoprosthetic reconstruction after segmental resection of primary bone tumours. J Bone Joint Surg Br 2010;92:867-874. [DOI] [PubMed] [Google Scholar]
- 35.Hamada K, Naka N, Omori S, et al. : Intercalary endoprosthesis for salvage of failed intraoperative extracorporeal autogeneous irradiated bone grafting (IORBG) reconstruction. J Surg Case Rep 2014;2014:pii: rju014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sakellariou VI, Mavrogenis AF, Papagelopoulos PJ: Limb salvage surgery using the intramedullary diaphyseal segmental defect fixation system. J Long Term Eff Med Implants 2008;18:59-67. [DOI] [PubMed] [Google Scholar]
- 37.Ruggieri P, Mavrogenis AF, Bianchi G, Sakellariou VI, Mercuri M, Papagelopoulos PJ: Outcome of the intramedullary diaphyseal segmental defect fixation system for bone tumors. J Surg Oncol 2011;104:83-90. [DOI] [PubMed] [Google Scholar]
- 38.Sewell MD, Hanna SA, McGrath A, et al. : Intercalary diaphyseal endoprosthetic reconstruction for malignant tibial bone tumours. J Bone Joint Surg Br 2011;93:1111-1117. [DOI] [PubMed] [Google Scholar]
- 39.Spiegelberg BG, Sewell MD, Aston WJ, et al. : The early results of joint-sparing proximal tibial replacement for primary bone tumours, using extracortical plate fixation. J Bone Joint Surg Br 2009;91:1373-1377. [DOI] [PubMed] [Google Scholar]
- 40.McGrath A, Sewell MD, Hanna SA, et al. : Custom endoprosthetic reconstruction for malignant bone disease in the humeral diaphysis. Acta Orthop Belg 2011;77:171-179. [PubMed] [Google Scholar]
- 41.Kuo KN, Gitelis S, Sim FH, et al. : Segmental replacement of long bones using titanium fiber metal composite following tumor resection. Clin Orthop Relat Res 1983;176:108-114. [PubMed] [Google Scholar]
- 42.Henderson ER, Groundland JS, Pala E, et al. : Failure mode classification for tumor endoprostheses: Retrospective review of five institutions and a literature review. J Bone Joint Surg Am 2011;93:418-429. [DOI] [PubMed] [Google Scholar]