Abstract
Purpose
Pancreatic adenosquamous carcinoma (PASC) is a rare and aggressive form of pancreatic cancer whose management often follows its more common pancreatic ductal adenocarcinoma (PDAC) counterpart. While neoadjuvant therapy (NT) is increasingly utilized prior to surgery for PDAC, whether patients with PASC experience similar benefits is unclear.
Methods
Using the National Cancer Database (NCDB), all patients with stage I-III PASC who underwent surgical resection between 2006 and 2020 were included. Patient and tumor characteristics and overall survival (OS) of patients who underwent surgery first (SF) were compared to those who received NT prior to surgery.
Results
Among 1191 patients with PASC who underwent curative intent resection, 208 (17.5%) received NT, whereas 983 (82.5%) underwent SF. Overall, NT was associated with improved OS compared with an SF approach (median 20.7 vs 15.9 months; p = 0.03). On multivariable Cox regression analysis, factors independently associated with improved OS included treatment at an academic/research facility, receipt of NT, and receipt of adjuvant therapy. Factors associated with decreased OS included Black race, positive surgical margins, worse comorbidity score, and higher cancer stage. There was no significant difference in OS between patients who received NT chemotherapy and radiation vs NT chemotherapy alone.
Conclusion
Among patients with localized PASC, the receipt of NT prior to surgical resection was associated with improved OS outcomes. Future research is needed to clarify the optimal neoadjuvant treatment regimen, including the role of preoperative radiation, to enhance response to therapy and improve long-term outcomes.
Keywords: Pancreatic adenosquamous carcinoma, Pancreatic cancer, Neoadjuvant therapy, Surgery first, National Cancer Database
Introduction
Pancreatic adenosquamous carcinoma (PASC) is a rare and aggressive form of pancreatic cancer with both glandular and squamous components [1]. The incidence of PASC is estimated to be 1–4% of all exocrine pancreatic malignancies [2]. PASC shares many similarities with pancreatic ductal adenocarcinoma (PDAC), and as a result, its clinical management often follows that of its more common counterpart. Despite the similarities to PDAC, PASC has a worse prognosis with a median overall survival (OS) of less than 6 months for patients with advanced disease and only 11–20 months for those with localized disease who undergo surgical resection [3]. Unfortunately, many patients with PASC are diagnosed at advanced stages with either metastatic or unresectable disease [3, 4].
Neoadjuvant therapy (NT) is increasingly utilized for patients with PDAC [5, 6]. Potential advantages of NT include possible tumor downstaging that leads to subsequent resection, early treatment of micrometastatic disease, and an in vivo assessment of therapy efficacy [7–9]. Additionally, delivering NT prior to surgery increases the rate of complete multimodality therapy since not all patients will be able to receive adjuvant therapy after surgery due to delayed surgical healing or deconditioning [10–12]. While still controversial for patients with resectable PDAC, NT is a preferred treatment approach for patients with borderline resectable (BR) and locally advanced (LA) disease [7, 8, 13, 14]. On the other hand, the role of NT for other periampullary malignancies, including ampullary cancer, distal cholangiocarcinoma, and duodenal cancer, has not been firmly established [15–18].
Due to the rarity of PASC and the recent trends of using NT, there is limited literature on the role of NT for patients with PASC, although retrospective case series and cohort studies suggest that completing multimodality therapy is associated with improved outcomes [19, 20]. Whether similar benefits of NT observed in patients with PDAC can be expected in patients with localized PASC remains unknown. Therefore, the objective of the current study was to assess the impact of NT prior to surgery on OS for patients with localized PASC. Given that PASC is thought to be particularly radiation-sensitive, the utility of radiation as a component of NT was also explored.
Methods
Study Population
Using the National Cancer Database (NCDB), all patients with stage I-III adenosquamous carcinoma of the pancreas (International Classification of Diseases for Oncology codes C25.0–C25.3, C25.7–C25.9, and histology code 8560) who underwent pancreatectomy (Surgery of the Primary Site Codes 30–80) between 2006 and 2020 were included. The National Cancer Database (NCDB) is a nationwide clinical oncology database that contains data from over 1500 Commission on Cancer accredited facilities [21, 22]. Patients with other histology and individuals who underwent other procedures were excluded.
The following sociodemographic, clinical, and tumor data included in the NCDB were considered for this study: age at diagnosis, sex, race, insurance status, year of diagnosis, household income, treating facility type, distance from the treating facility, time to treatment initiation, tumor grade/differentiation, surgical margins, Charlson–Deyo comorbidity score, NCDB analytic stage group, clinical stage group, clinical T stage, clinical N stage, pathologic stage group, pathologic T stage, pathologic N stage, length of neoadjuvant treatment, receipt of neoadjuvant therapy, type of neoadjuvant chemotherapy, and receipt of adjuvant therapy.
For the purposes of this study, age at diagnosis was reported in years. Sex was reported as male or female. Patient race was classified as White, Black, and other/unknown. Insurance status was reported as private, government (Medicare, Medicaid, and Other Government), and unknown/not insured. Year of diagnosis was reported in terciles (2006–2010, 2011–2015, and 2016–2020). Household income was grouped by 2008–2012 quartiles and reported as less than $38,000, $38,000–47,999, $48,000–62,999, and $63,000 or more. Facility type was categorized as Comprehensive/Community Cancer Program, Academic/Research Program, and Integrated Network Cancer Program based on the primary treating facility when treatment was fragmented [23]. Distance from treating facility was categorized in miles as 0–49, 50–99, > 100, and unknown. Grade/differentiation was categorized as well, moderate, poor, anaplastic, and not determined. Surgical margins were categorized as R0 and R1/R2/unknown. Charlson–Deyo comorbidity score was reported as 0–1 and ≥ 2. Neoadjuvant therapy and adjuvant therapy were classified as neither, chemotherapy only, radiation therapy only, and chemotherapy and radiation therapy. Chemotherapy was classified as single agent, multiagent, and unknown. NT was determined by either reported treatment sequence or treatment initiation timing.
Statistical Analysis
Patients who underwent surgery first (SF) were directly compared with those who received NT prior to surgical resection. The NT group included patients who underwent neoadjuvant chemotherapy, neoadjuvant radiation, and neoadjuvant chemotherapy and radiation. Continuous variables were reported as medians with interquartile ranges and compared using the Mann–Whitney U test. Trends in the use of NT over time were calculated using Kendall’s tau b. Categorical variables were reported as totals and percentages and compared using the χ2 or Fisher’s exact tests, as appropriate. OS was the primary outcome and was defined as the time interval from the date of initial diagnosis until death or last follow-up. OS was censored at the date of last follow-up for living patients. OS was estimated using the Kaplan–Meier method and compared between groups using the log-rank test. Cox proportional hazards regression analysis was used to evaluate associations between patient, tumor, and hospital characteristics and OS. Regression coefficients were reported as hazard ratios (HRs) and corresponding 95% confidence intervals. Variables with p values less than 0.10 on univariable analysis were included in multivariable analysis. A p value of less than 0.05 was considered statistically significant. A subset of patients treated with neoadjuvant chemotherapy versus neoadjuvant chemoradiation was compared as above. All statistical analysis was performed with IBM SPSS Statistics Version 29.0.2.0.
Results
Among 1191 patients with PASC who underwent curative intent resection, 208 (17.5%) received NT, whereas 983 (82.5%) underwent SF. For patients who received NT, 156 (75.0%) received preoperative chemotherapy alone, 49 (23.6%) received chemotherapy and radiation therapy, and 3 (1.4%) received radiation therapy alone. The median length of NT was 119 days (IQR 91–163); 15 (7.3%) patients received single-agent therapy, 183 (89.3%) received multiagent therapy, and 7 (3.4%) were treated with an unrecorded agent. There was an increase in the use of NT over the study period with 11 (5.3%) patients in 2006–2010, 60 (28.8%) of patients in 2011–2015, and 137 (65.9%) of patients in 2016–2020 (τb = 0.222, p < 0.001, Fig. 1). On Kaplan–Meier analysis for the overall cohort, median OS did not significantly change during the study period (2006–2010: 15.9 months, 2011–2015: 16.1 months, and 2016–2020: 17.8 months; p = 0.17).
Fig. 1.
Percentage of patients treated with NT versus SF from 2006 to 2020
Table 1 reports the clinicopathologic characteristics of patients receiving NT compared with individuals undergoing SF. Patients who received NT were younger (median NT 67 [59,73] vs SF 69 [61,76] years, p = 0.007) more likely to be white (p = 0.03), to be diagnosed in a more recent year (p < 0.001), to have higher household income (p = 0.009), to be treated at an academic/research facility (p = 0.002), to experience longer time to treatment initiation (median NT 26 [18, 36] vs SF 18 [4, 24] days, p < 0.001), and to have stage I disease (p < 0.001). NT patients were also more likely to have a worse Charleson-Deyo comorbidity score (p < 0.001), live farther from their treatment facility (p < 0.001), and were less likely to receive any adjuvant therapy (p < 0.001) compared with SF patients.
Table 1.
Patient and tumor characteristics of the total cohort of patients with PASC who received NT vs SF
| Variable | Full cohort (N = 1191) | Surgery first (N = 983) | Neoadjuvant therapy (N = 208) | p value | |||
|---|---|---|---|---|---|---|---|
| Count | % | Count | % | Count | % | ||
| Age at diagnosis (median, IQR) | 68 | (61, 75) | 69 | (61, 76) | 67 | (59, 73) | 0.007 |
| Sex | |||||||
| Male | 640 | 53.7% | 532 | 54.1% | 108 | 51.9% | 0.564 |
| Female | 551 | 46.3% | 451 | 45.9% | 100 | 48.1% | |
| Race | |||||||
| White | 1010 | 84.8% | 821 | 83.5% | 189 | 90.9% | 0.027 |
| Black | 116 | 9.7% | 104 | 10.6% | 12 | 5.8% | |
| Other/unknown | 65 | 5.5% | 58 | 5.9% | 7 | 3.4% | |
| Insurance status | |||||||
| Private | 406 | 34.1% | 335 | 34.1% | 71 | 34.1% | 0.065 |
| Government | 760 | 63.8% | 623 | 63.4% | 137 | 65.9% | |
| Unknown/not insured | 25 | 2.1% | 25 | 2.5% | 0 | 0.0% | |
| Year of diagnosis | |||||||
| 2006–2010 | 249 | 20.9% | 238 | 24.2% | 11 | 5.3% | < 0.001 |
| 2011–2015 | 441 | 37.0% | 381 | 38.8% | 60 | 28.8% | |
| 2016–2020 | 501 | 42.1% | 364 | 37.0% | 137 | 65.9% | |
| Household Income | |||||||
| Less than $38,000 | 145 | 14.1% | 130 | 15.1% | 15 | 9.0% | 0.009 |
| $38,000–$47,999 | 181 | 17.6% | 162 | 18.8% | 19 | 11.4% | |
| $48,000–$62,999 | 267 | 25.9% | 218 | 25.3% | 49 | 29.5% | |
| $63,000 or more | 436 | 42.4% | 353 | 40.9% | 83 | 50.0% | |
| Facility type | |||||||
| Comprehensive/Community Cancer Program | 304 | 25.7% | 271 | 27.7% | 33 | 15.9% | 0.002 |
| Academic/Research | 686 | 57.9% | 547 | 56.0% | 139 | 67.1% | |
| Integrated Cancer Network | 194 | 16.4% | 159 | 16.3% | 35 | 16.9% | |
| Distance from treating facility | |||||||
| 0–49 miles | 813 | 68.5% | 694 | 71.0% | 119 | 57.2% | < 0.001 |
| 50–99 miles | 108 | 9.1% | 89 | 9.1% | 19 | 9.1% | |
| 100 + miles | 104 | 8.8% | 76 | 7.8% | 28 | 13.5% | |
| Unknown | 161 | 13.6% | 119 | 12.2% | 42 | 20.2% | |
| Days to treatment (median, IQR) | 19 | (6, 32) | 18 | (4, 30) | 26 | (18, 36) | < 0.001 |
| Grade/differentiation | |||||||
| Well differentiated | 8 | 0.9% | 8 | 1.0% | 0 | 0.0% | < 0.001a |
| Moderately differentiated | 252 | 28.0% | 226 | 29.0% | 26 | 21.7% | |
| Poorly differentiated | 498 | 55.3% | 450 | 57.7% | 48 | 40.0% | |
| Undifferentiated; anaplastic | 13 | 1.4% | 12 | 1.5% | 1 | 0.8% | |
| Unknown | 129 | 14.3% | 84 | 10.8% | 45 | 37.5% | |
| Surgical margins | |||||||
| R0 | 915 | 76.8% | 752 | 76.5% | 163 | 78.4% | .589 |
| R1/R2/unknown | 276 | 23.2% | 231 | 23.5% | 45 | 21.6% | |
| Charlson–Deyo score | |||||||
| 0–1 | 1058 | 88.8% | 876 | 89.1% | 182 | 87.5% | < 0.001a |
| ≥ 2 | 133 | 11.2% | 107 | 10.9% | 26 | 12.5% | |
| NCDB analytic stage group | |||||||
| Stage I | 184 | 15.4% | 126 | 12.8% | 58 | 27.9% | < 0.001 |
| Stage II | 908 | 76.2% | 770 | 78.3% | 138 | 66.3% | |
| Stage III | 99 | 8.3% | 87 | 8.9% | 12 | 5.8% | |
| Clinical T stage | |||||||
| 0 | 4 | 0.3% | 3 | 0.3% | 1 | 0.5% | < 0.001 |
| 1 | 93 | 7.8% | 83 | 8.4% | 10 | 4.8% | |
| 2 | 422 | 35.4% | 349 | 35.5% | 73 | 35.1% | |
| 3 | 397 | 33.3% | 301 | 30.6% | 96 | 46.2% | |
| 4 | 47 | 3.9% | 23 | 2.3% | 24 | 11.5% | |
| Unknown | 228 | 19.1% | 224 | 22.8% | 4 | 1.9% | |
| Clinical N stage | |||||||
| 0 | 772 | 64.8% | 633 | 64.4% | 139 | 66.8% | < 0.001a |
| 1 | 208 | 17.5% | 149 | 15.2% | 59 | 28.4% | |
| 2 | 5 | 0.4% | 4 | 0.4% | 1 | 0.5% | |
| Unknown | 206 | 17.3% | 197 | 20.0% | 9 | 4.3% | |
| Pathologic T stage | |||||||
| 0 | 4 | 0.3% | 1 | 0.1% | 3 | 1.4% | < 0.001 |
| 1 | 30 | 2.5% | 21 | 2.1% | 9 | 4.3% | |
| 2 | 202 | 17.0% | 185 | 18.8% | 17 | 8.2% | |
| 3 | 790 | 66.3% | 701 | 71.3% | 89 | 42.8% | |
| 4 | 38 | 3.2% | 35 | 3.6% | 3 | 1.4% | |
| Unknown | 127 | 10.7% | 40 | 4.1% | 87 | 41.8% | |
| Pathologic N stage | |||||||
| 0 | 416 | 34.9% | 368 | 37.4% | 48 | 23.1% | < 0.001 |
| 1 | 599 | 50.3% | 527 | 53.6% | 72 | 34.6% | |
| 2 | 47 | 3.9% | 46 | 4.7% | 1 | 0.5% | |
| Unknown | 129 | 10.8% | 42 | 4.3% | 87 | 41.8% | |
| Adjuvant therapy | |||||||
| None | 455 | 38.2% | 329 | 33.5% | 126 | 60.6% | < 0.001 |
| Chemotherapy | 530 | 44.5% | 469 | 47.7% | 61 | 29.3% | |
| Radiation therapy | 15 | 1.3% | 10 | 1.0% | 5 | 2.4% | |
| Chemotherapy and radiation therapy | 191 | 16.0% | 175 | 17.8% | 16 | 7.7% | |
aFisher’s exact test
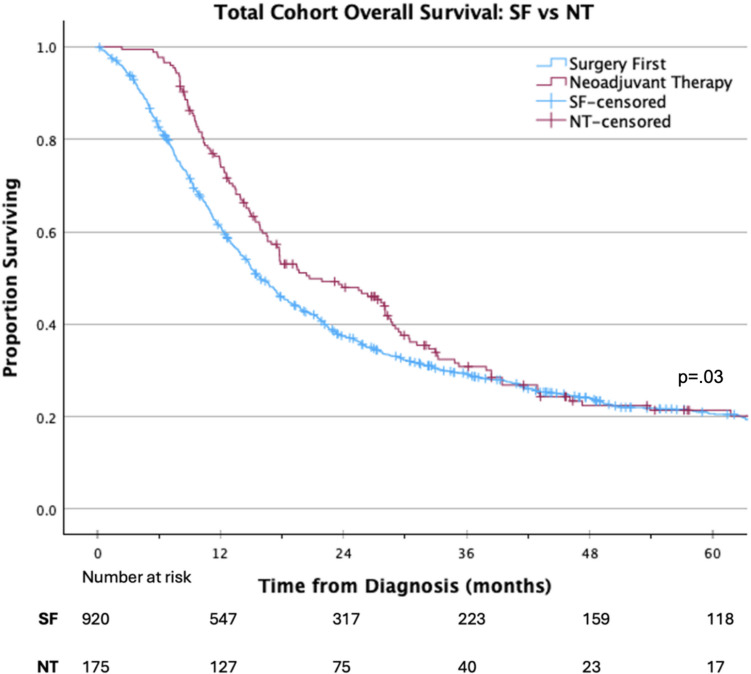
On Kaplan Meier analysis, NT was associated with improved OS compared with a SF approach (median 20.7 vs 15.9 months; p = 0.03; Fig. 2). On multivariable Cox regression analysis, factors independently associated with improved OS included: treatment at an academic/research facility (HR 0.84 95% CI 0.71–0.98, p < 0.05), receipt of NT (HR 0.74 95% CI 0.61–0.91, p < 0.05), and receipt of adjuvant therapy (HR 0.56 95% CI 0.51–0.68, p < 0.001). Factors associated with decreased OS included black race (HR 1.32 95% CI 1.06–1.64, p < 0.05), positive surgical margins (HR 1.54 95% CI 1.31–1.80, p < 0.001), worse Charlson–Deyo comorbidity score (HR 1.33 95% CI 1.07–1.67, p < 0.05), and NCDB analytic stage groups II and III (HR 1.65 95% CI 1.32–2.07, p < 0.001 and HR 2.56 95% CI 1.86–3.52, p < 0.001, respectively) (Table 2).
Fig. 2.
Overall survival of patients with pancreatic adenosquamous carcinoma receiving NT versus SF
Table 2.
Cox proportional hazards analysis for overall survival in the total cohort
| Variable | Univariate analysis, HR (95% CI) | p value | Multivariate analysis, HR (95% CI) | p value |
|---|---|---|---|---|
| Age at diagnosis | 1.00 (1.00–1.01) | 0.168 | ||
| Sex | ||||
| Male | Ref | Ref | ||
| Female | 0.89 (0.78–1.02) | 0.100 | 0.89 (0.77–1.02) | 0.084 |
| Race | ||||
| White | Ref | Ref | ||
| Black | 1.30 (1.04–1.61) | 0.020 | 1.32 (1.06–1.64) | 0.015 |
| Other/unknown | 0.73 (0.53–1.02) | 0.065 | 0.79 (0.56–1.11) | 0.169 |
| Insurance status | ||||
| Private | Ref | Ref | ||
| Government | 1.07 (0.92–1.23) | 0.384 | 0.98 (0.84–1.13) | 0.751 |
| Unknown/not insured | 1.51 (0.97–2.35) | 0.070 | 1.39 (0.89–2.19) | 0.149 |
| Year of diagnosis | ||||
| 2006–2010 | Ref | Ref | ||
| 2011–2015 | 0.94 (0.79–1.11) | 438 | 1.01 (0.85–1.20) | 0.897 |
| 2016–2020 | 0.84 (0.70–1.01) | 0.066 | 0.96 (0.79–1.16) | 0.673 |
| Household income | ||||
| Less than $38,000 | Ref | |||
| $38,000–$47,999 | 1.07 (0.84–1.38) | 0.575 | ||
| $48,000–$62,999 | 1.02 (0.81–1.29) | 0.869 | ||
| $63,000 or more | 0.90 (0.73–1.12) | 0.358 | ||
| Facility type | ||||
| Comprehensive/Community Cancer Program | Ref | Ref | ||
| Academic/Research | 0.85 (0.73–1.00) | 0.048 | 0.84 (0.71–0.98) | 0.032 |
| Integrated Cancer Network | 0.92 (0.75–1.14) | 0.465 | 0.88 (0.71–1.09) | 0.238 |
| Distance from treating facility | ||||
| 0–49 miles | Ref | |||
| 50–99 miles | 0.95 (0.75–1.20) | 0.952 | ||
| 100 + miles | 0.91 (0.71–1.16) | 0.911 | ||
| Unknown | 0.83 (0.67–1.02) | 0.831 | ||
| Grade/differentiation | ||||
| Well differentiated | Ref | |||
| Moderately differentiated | 0.70 (0.33–1.49) | 0.702 | ||
| Poorly differentiated | 0.94 (0.45–1.99) | 0.940 | ||
| Undifferentiated; anaplastic | 0.67 (0.25–1.75) | 0.667 | ||
| Unknown | 0.81 (0.37–1.73) | 0.805 | ||
| Surgical margins | ||||
| R0 | Ref | Ref | ||
| R1/R2/unknown | 1.59 (1.36–1.86) | < 0.001 | 1.54 (1.31–1.80) | < 0.001 |
| Charlson–Deyo score | ||||
| 0–1 | Ref | Ref | ||
| ≥ 2 | 1.37 (1.10–1.70) | 0.005 | 1.33 (1.07–1.67) | 0.012 |
| NCDB analytic stage group | ||||
| Stage I | Ref | Ref | ||
| Stage II | 1.65 (1.33–2.06) | < 0.001 | 1.65 (1.32–2.07) | < 0.001 |
| Stage III | 2.82 (2.06–3.85) | < 0.001 | 2.56 (1.86–3.52) | < 0.001 |
| Neoadjuvant therapy | ||||
| No | Ref | Ref | ||
| Yes | 0.81 (0.67–0.98) | 0.033 | 0.74 (0.61–0.91) | 0.005 |
| Adjuvant therapy | ||||
| No | Ref | Ref | ||
| Yes | 0.66 (0.57–0.75 | < 0.001 | 0.59 (0.51–0.68) | < 0.001 |
P values <.05 on multi-variable Cox analysis are bolded
Table 3 compares patients who received neoadjuvant chemotherapy to those who received neoadjuvant radiation and chemotherapy, both sequential and concurrent. Patients who received neoadjuvant chemotherapy were more likely to be treated in a more recent year and more likely to have a moderately differentiated tumor compared with those who received neoadjuvant chemotherapy and radiation. On Kaplan–Meier analysis, there was no significant difference in OS between patients receiving neoadjuvant chemotherapy versus neoadjuvant chemotherapy and radiation therapy (median 19.7 vs 23.9 months, respectively; p = 0.256; Fig. 3). On multivariable Cox regression analysis of the NT cohort, neoadjuvant radiation was not associated with OS whereas receipt of both adjuvant chemotherapy and radiation therapy (HR 0.34 95% CI 0.17–0.67, p < 0.01) and R1/2 surgical margins were (HR 1.94 95% CI 1.24–3.05, p < 0.01, Table 4).
Table 3.
Patient and tumor characteristics of the NT subset cohort of patients with PASC who received NT chemotherapy vs NT chemotherapy and radiation
| All NT (N = 205) | NT chemotherapy (N = 156) | NT chemotherapy and radiation (N = 49) | p value | ||||
|---|---|---|---|---|---|---|---|
| Variable | Count | % | Count | % | Count | % | |
| Age at diagnosis (median, IQR) | 67 | (59, 73) | 68 | (60, 74) | 62 | (56, 71) | 0.065 |
| Sex | |||||||
| Male | 108 | 52.7% | 84 | 53.8% | 24 | 49.0% | 0.552 |
| Female | 97 | 47.3% | 72 | 46.2% | 25 | 51.0% | |
| Race | |||||||
| White | 186 | 90.7% | 139 | 89.1% | 47 | 95.9% | 0.509a |
| Black | 12 | 5.9% | 11 | 7.1% | 1 | 2.0% | |
| Other/unknown | 7 | 3.4% | 6 | 3.8% | 1 | 2.0% | |
| Insurance status | |||||||
| Private | 70 | 34.1% | 48 | 30.8% | 22 | 44.9% | 0.069 |
| Government | 135 | 65.9% | 108 | 69.2% | 27 | 55.1% | |
| Unknown/not insured | 0 | 0.0% | 0 | 0.0% | 0 | 0.0 | |
| Year of diagnosis | |||||||
| 2006–2010 | 10 | 4.9% | 3 | 1.9% | 7 | 14.3% | < 0.001 |
| 2011–2015 | 60 | 29.3% | 41 | 26.3% | 19 | 38.8% | |
| 2016–2020 | 135 | 65.9% | 112 | 71.8% | 23 | 46.9% | |
| Household income | |||||||
| Less than $38,000 | 15 | 9.1% | 13 | 10.5% | 2 | 4.9% | 0.531a |
| $38,000–$47,999 | 19 | 11.5% | 14 | 11.3% | 5 | 12.2% | |
| $48,000–$62,999 | 48 | 29.1% | 33 | 26.6% | 15 | 36.6^ | |
| $63,000 or more | 83 | 50.3% | 64 | 51.6% | 19 | 46.3% | |
| Facility type | |||||||
| Comprehensive/Community Cancer Program | 32 | 15.7% | 29 | 18.7% | 3 | 6.1% | 0.107 |
| Academic/Research | 138 | 67.6% | 101 | 65.2% | 37 | 75.5% | |
| Integrated Cancer Network | 34 | 16.7% | 25 | 16.1% | 9 | 18.4% | |
| Distance from treating facility | |||||||
| 0–49 miles | 118 | 57.6% | 88 | 56.4% | 30 | 61.2% | 0.680 |
| 50–99 miles | 19 | 9.3% | 16 | 10.3% | 3 | 6.1% | |
| 100 + miles | 28 | 13.7% | 20 | 12.8% | 8 | 16.3% | |
| Unknown | 40 | 19.5% | 32 | 20.5% | 8 | 16.3% | |
| Grade/differentiation | |||||||
| Well differentiated | 0 | 0.0% | 0 | 0.0% | 0 | 0.0% | 0.008a |
| Moderately differentiated | 26 | 22.0% | 24 | 28.2% | 2 | 6.1% | |
| Poorly differentiated | 47 | 39.8% | 29 | 34.1% | 18 | 54.5% | |
| Undifferentiated; anaplastic | 1 | 0.8% | 0 | 0.0% | 1 | 3.0% | |
| Unknown | 44 | 37.3% | 32 | 37.6% | 12 | 36.4% | |
| Surgical margins | |||||||
| R0 | 161 | 78.5% | 118 | 75.6% | 43 | 87.8% | 0.076 |
| R1/R2/unknown | 44 | 21.5% | 38 | 2.4% | 6 | 12.2% | |
| Charlson–Deyo score | |||||||
| 0–1 | 179 | 87.3% | 135 | 86.5% | 44 | 89.8% | 0.559 |
| ≥ 2 | 26 | 12.7% | 21 | 13.5% | 5 | 10.2% | |
| NCDB analytic stage group | |||||||
| Stage I | 57 | 27.8 | 41 | 26.3% | 16 | 32.7% | 0.044 |
| Stage II | 136 | 66.3% | 109 | 69.9% | 27 | 55.1% | |
| Stage III | 12 | 5.9% | 6 | 3.8% | 6 | 12.2% | |
| Clinical T Stage | |||||||
| 0 | 1 | 0.5% | 0 | 0.0% | 1 | 2.0% | .007a |
| 1 | 10 | 4.9% | 7 | 4.5% | 3 | 6.1% | |
| 2 | 71 | 34.6% | 60 | 38.5% | 11 | 22.4% | |
| 3 | 95 | 46.3% | 73 | 46.8% | 22 | 44.9% | |
| 4 | 24 | 11.7% | 12 | 7.7% | 12 | 24.5% | |
| Unknown | 4 | 2.0% | 4 | 2.6% | 0 | 0.0% | |
| Clinical N stage | |||||||
| 0 | 138 | 67.3% | 105 | 67.3% | 33 | 67.3% | 0.373a |
| 1 | 57 | 27.8% | 43 | 27.6% | 14 | 28.6% | |
| 2 | 1 | 0.5% | 0 | 0.0% | 1 | 2.0% | |
| Unknown | 9 | 4.4% | 8 | 5.1% | 1 | 2.0% | |
| Pathologic T stage | |||||||
| 0 | 3 | 1.5% | 0 | 0.0% | 3 | 6.1% | 0.023a |
| 1 | 9 | 4.4% | 7 | 4.5% | 2 | 4.1% | |
| 2 | 17 | 8.3% | 10 | 6.4% | 7 | 14.3% | |
| 3 | 89 | 43.4% | 72 | 46.2% | 17 | 34.7% | |
| 4 | 3 | 1.5% | 2 | 1.3% | 1 | 2.0% | |
| Unknown | 84 | 41.0% | 65 | 41.7% | 19 | 38.8% | |
| Pathologic N stage | |||||||
| 0 | 48 | 23.4% | 27 | 17.3% | 21 | 42.9% | < 0.001a |
| 1 | 72 | 35.1% | 63 | 40.4% | 9 | 18.4% | |
| 2 | 1 | 0.5% | 1 | 0.6% | 0 | 0.0% | |
| Unknown | 84 | 41.0% | 65 | 41.7% | 19 | 38.8% | |
| Adjuvant therapy | |||||||
| None | 125 | 61.0% | 81 | 86.5% | 44 | 89.8% | 0.114a |
| Chemotherapy | 59 | 28.8% | 54 | 34.6% | 5 | 10.2% | |
| Radiation therapy | 5 | 2.4% | 5 | 3.2% | 0 | 0.0% | |
| Both chemotherapy and radiation therapy | 16 | 7.8% | 16 | 10.3% | 0 | 0.0% | |
aFisher’s exact test
Fig. 3.
Overall survival outcomes comparing patients treated with NT chemotherapy versus NT chemotherapy and radiation
Table 4.
Cox proportional hazards analysis for overall survival in the NT subgroup cohort
| Variable | Univariate analysis, HR (95% CI) | p value | Multivariate analysis, HR (95% CI) | p value |
|---|---|---|---|---|
| Age at diagnosis | 1.01 (1.00–1.03) | 0.107 | ||
| Sex | ||||
| Male | Ref | |||
| Female | 0.86 (0.57–1.31) | 0.484 | ||
| Race | ||||
| White | Ref | |||
| Black | 1.27 (0.64–2.50) | 0.493 | ||
| Other/unknown | 0.51 (0.16–1.61) | 0.252 | ||
| Insurance status | ||||
| Private | Ref | |||
| Government | 1.10 (0.76–1.61) | 0.605 | ||
| Year of diagnosis | ||||
| 2006–2010 | Ref | |||
| 2011–2015 | 1.06 (0.50–2.25) | 0.874 | ||
| 2016–2020 | 1.09 (0.52–2.30) | 0.811 | ||
| Household income | ||||
| Less than $38,000 | Ref | |||
| $38,000–$47,999 | 0.68 (0.27–1.66) | 0.393 | ||
| $48,000–$62,999 | 1.07 (0.52–2.20) | 0.845 | ||
| $63,000 or more | 0.95 (0.48–1.88) | 0.887 | ||
| Facility type | ||||
| Comprehensive/Community Cancer Program | Ref | Ref | ||
| Academic/Research | 0.86 (0.53–1.39) | 0.542 | 1.03 (0.63–1.68) | 0.932 |
| Integrated Cancer Network | 0.47 (0.24–0.94) | 0.032 | 0.58 (0.29–1.16) | 0.126 |
| Distance from treating facility | ||||
| 0–49 miles | Ref | |||
| 50–99 miles | 0.78 (0.42–1.44) | 0.424 | ||
| 100 + miles | 0.67 (0.38–1.17) | 0.159 | ||
| Unknown | 0.86 (0.54–1.36) | 0.514 | ||
| Grade/differentiation | ||||
| Moderately differentiated | Ref | |||
| Poorly differentiated | 1.55 (0.89–2.70) | 0.124 | ||
| Undifferentiated; anaplastic | 0.970 | |||
| Unknown | 1.35 (0.76–2.39) | 0.307 | ||
| Surgical margins | ||||
| R0 | Ref | Ref | ||
| R1/R2/unknown | 1.52 (1.00–2.30) | 0.049 | 1.63 (1.06–2.52) | 0.027 |
| Charlson–Deyo score | ||||
| 0–1 | Ref | |||
| ≥ 2 | 1.14 (0.63–2.07) | 0.662 | ||
| NCDB analytic stage group | ||||
| Stage I | Ref | Ref | ||
| Stage II | 1.70 (1.06–2.73) | 0.029 | 1.45 (0.88–2.37) | 0.114 |
| Stage III | 1.61 (0.65–4.00) | 0.305 | 1.19 (0.47–3.00) | 0.719 |
| Neoadjuvant therapy | ||||
| Chemotherapy | Ref | Ref | ||
| Chemotherapy and radiation | 0.79 (0.53–1.19) | 0.258 | 0.71 (0.45–1.12) | 0.141 |
| Adjuvant therapy | ||||
| None | Ref | Ref | ||
| Chemotherapy | 0.75 (0.50–1.14) | 0.179 | 0.70 (0.45–1.09) | 0.119 |
| Chemotherapy and radiation | 0.47 (0.23–0.98) | 0.043 | 0.26 (0.12–0.58) | < 0.001 |
P values <.05 on multi-variable Cox analysis are bolded
Discussion
PASC is an aggressive malignancy with a poor prognosis [1–3]. Multimodal therapy, including chemotherapy, surgical resection, and radiation therapy when applicable, leads to optimal outcomes for patients with localized cancers [5, 16, 25, 26]. While the delivery of chemotherapy and/or radiation therapy prior to surgical resection is increasingly used for patients with PDAC, the role of NT for PASC has been understudied [13, 14, 19, 25]. In this retrospective review of the NCDB, NT prior to surgical resection was associated with improved OS compared with SF even after controlling for confounding factors. Given the limitations associated with a retrospective cancer registry study without intention-to-treat data, as well as the challenges in clinically diagnosing PASC, additional research on optimal treatment sequencing is needed for this aggressive malignancy.
Previous studies have emphasized the importance of multimodality therapy for PASC. Hue et al. utilized the NCDB and reported that receipt of chemotherapy, either in a neoadjuvant or adjuvant manner, was associated with improved OS [19]. Those results build upon other studies that suggest that surgery and chemotherapy are both associated with improved outcomes compared with no treatment and highlight that the best long-term outcomes are observed in patients who are able to receive both treatments as is true for patients with PDAC [3, 4, 27]. Given its relative rarity and in the absence of prospective evidence, current treatment recommendations for PASC are largely based on guidelines for PDAC. Based on several randomized controlled trials, meta-analyses, and expert opinion, NT is now the preferred option for BR and LA PDAC and an acceptable option for potentially resectable PDAC [4, 7, 13, 14, 19, 25, 28].
To the best of our knowledge, the current study is the first to specifically evaluate the role of NT for PASC. Notwithstanding its limitations, given the similarities of our results to prior NCDB studies conducted in patients with PDAC, our findings suggest that the role of NT in PASC might be considered similarly. This is important since NT is often prescribed based on a fine needle aspiration biopsy which may be insufficient to firmly diagnose PASC. In addition to appearing like PDAC on imaging, a threshold of greater than 30% squamous component is used to formally diagnose PASC, but the glandular components are not often equally distributed throughout a tumor [1, 20]. Therefore, many PASC patients may have been treated for presumed PDAC and only diagnosed retrospectively after surgery. When considered with the rarity of PASC and these diagnostic challenges, our finding that the role of NT for PASC may be similar to that of PDAC is reassuring.
A recent study noted that an increased squamous component of PASC is associated with more aggressive behavior and worse outcomes [29]. Radiation therapy is a key treatment component of other squamous histologies, but its importance in treating the squamous component of PASC has not been thoroughly explored and remains controversial [9, 20, 29, 30]. Some studies have suggested that adjuvant chemoradiation may confer a survival benefit for patients with PASC [20]. For that reason, we conducted a subset analysis comparing patients who received neoadjuvant chemotherapy with those who received both neoadjuvant chemotherapy and radiation which revealed no significant difference in OS. Interestingly, among patients receiving NT, the receipt of adjuvant radiation was independently associated with improved OS, suggesting this treatment modality should continue to be investigated in PASC. On the other hand, given the limitations of this retrospective study, this finding could be related to inherent selection biases between patients who were offered adjuvant chemoradiation after NT and surgery and those who were not. Given the mixed literature on the role of adjuvant chemoradiation for both PDAC and PASC, additional research is needed to clarify the role of neoadjuvant and adjuvant radiation for PASC [24, 31].
Several limitations should be acknowledged, particularly considering the retrospective nature of the analysis. Some limitations were inherent to the design of the NCDB, including lack of information on chemotherapy regimen, radiation treatment details, percentage squamous histology, genomic alterations and other squamous differential drivers, as well as anatomic resectability staging. The exact reasons patients were selected for preoperative therapy or SF are multifactorial, unmeasured, and could bias the results. Importantly, this retrospective study is not intention to treat. Only patients who underwent surgical resection could be included. Since not all patients who initiate NT will undergo surgical resection, these findings should be interpreted accordingly [25, 32]. Finally, the overall sample size was limited, particularly for NT subgroup analysis, increasing the likelihood of type II errors.
Notwithstanding these limitations, among patients with localized PASC, the receipt of NT prior to surgical resection was associated with improved OS outcomes. Future research is needed to clarify the optimal neoadjuvant treatment regimen, including the role of preoperative radiation, to enhance response to therapy and optimize short- and long-term outcomes for patients with this aggressive malignancy.
Author Contribution
Study conception and design was by A.W. and J.C. Material preparation, data collection, and analysis were performed by A.W. and D.T. The first draft of the manuscript was written by A.W. and J.C. All authors commented on previous versions of the manuscript and read and approved the final manuscript.
Funding
This work was supported in part by the Ohio State University College of Medicine Roessler research scholarship (A.W.).
Data Availability
The data that support the findings of this study are openly available in the National Cancer Database at https://www.facs.org/quality-programs/cancer/ncdb.
Declarations
Ethics Approval
Due to the deidentified nature of the data, this research is considered exempt from IRB review and informed consent.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Simone CG, Zuluaga Toro T, Chan E, Feely MM, Trevino JG, George TJ. Characteristics and outcomes of adenosquamous carcinoma of the pancreas. Gastrointest Cancer Res GCR. 2013;6:75–9. [PMC free article] [PubMed] [Google Scholar]
- 2.Kwak HV, Hsu DS, Chang A, Kazantsev GB, Peng PD, Spitzer AL, Chang C-K. Pancreatic adenosquamous carcinoma: Experience within an integrated health care system. Perm J. 2022;27:9–12. 10.7812/TPP/22.095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boyd CA, Benarroch-Gampel J, Sheffield KM, Cooksley CD, Riall TS. 415 Patients with adenosquamous carcinoma of the pancreas: A population-based analysis of prognosis and survival. J Surg Res. 2012;174:12–9. 10.1016/j.jss.2011.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Braun R, Klinkhammer-Schalke M, Zeissig SR, Kleihus van Tol K, Bolm L, Honselmann KC, Petrova E, Lapshyn H, Deichmann S, Abdalla TSA, Heckelmann B, Bronsert P, Zemskov S, Hummel R, Keck T, Wellner UF. Clinical outcome and prognostic factors of pancreatic adenosquamous carcinoma compared to ductal adenocarcinoma—Results from the German Cancer Registry Group. Cancers. 2022;14:3946. 10.3390/cancers14163946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aquina CT, Ejaz A, Tsung A, Pawlik TM, Cloyd JM. National trends in the use of neoadjuvant therapy before cancer surgery in the US From 2004 to 2016. JAMA Netw Open. 2021;4: e211031. 10.1001/jamanetworkopen.2021.1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cloyd JM, Shen C, Santry H, Bridges J, Dillhoff M, Ejaz A, Pawlik TM, Tsung A. Disparities in the use of neoadjuvant therapy for resectable pancreatic ductal adenocarcinoma. J Natl Compr Cancer Netw JNCCN. 2020;18:556–63. 10.6004/jnccn.2019.7380. [DOI] [PubMed] [Google Scholar]
- 7.Laurence JM, Tran PD, Morarji K, Eslick GD, Lam VWT, Sandroussi C. A systematic review and meta-analysis of survival and surgical outcomes following neoadjuvant chemoradiotherapy for pancreatic cancer. J Gastrointest Surg Off J Soc Surg Aliment Tract. 2011;15:2059–69. 10.1007/s11605-011-1659-7. [DOI] [PubMed] [Google Scholar]
- 8.Balaban EP, Mangu PB, Khorana AA, Shah MA, Mukherjee S, Crane CH, Javle MM, Eads JR, Allen P, Ko AH, Engebretson A, Herman JM, Strickler JH, Benson AB, Urba S, Yee NS. Locally advanced, unresectable pancreatic cancer: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol Off J Am Soc Clin Oncol. 2016;34:2654–68. 10.1200/JCO.2016.67.5561. [DOI] [PubMed] [Google Scholar]
- 9.Sauer R, Becker H, Hohenberger W, Rödel C, Wittekind C, Fietkau R, Martus P, Tschmelitsch J, Hager E, Hess CF, Karstens J-H, Liersch T, Schmidberger H, Raab R, German Rectal Cancer Study Group. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med. 2004;351:1731–40. 10.1056/NEJMoa040694. [DOI] [PubMed] [Google Scholar]
- 10.Altman AM, Wirth K, Marmor S, Lou E, Chang K, Hui JYC, Tuttle TM, Jensen EH, Denbo JW. Completion of adjuvant chemotherapy after upfront surgical resection for pancreatic cancer is uncommon yet associated with improved survival. Ann Surg Oncol. 2019;26:4108–16. 10.1245/s10434-019-07602-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aloia TE, Lee JE, Vauthey J-N, Abdalla EK, Wolff RA, Varadhachary GR, Abbruzzese JL, Crane CH, Evans DB, Pisters PWT. Delayed recovery after pancreaticoduodenectomy: a major factor impairing the delivery of adjuvant therapy? J Am Coll Surg. 2007;204:347–55. 10.1016/j.jamcollsurg.2006.12.011. [DOI] [PubMed] [Google Scholar]
- 12.DePeralta DK, Ogami T, Zhou J-M, Schell MJ, Powers BD, Hodul PJ, Malafa MP, Fleming JB. Completion of adjuvant therapy in patients with resected pancreatic cancer. HPB. 2020;22:241–8. 10.1016/j.hpb.2019.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cloyd JM, Heh V, Pawlik TM, Ejaz A, Dillhoff M, Tsung A, Williams T, Abushahin L, Bridges JFP, Santry H. Neoadjuvant therapy for resectable and borderline resectable pancreatic cancer: a meta-analysis of randomized controlled trials. J Clin Med. 2020;9:1129. 10.3390/jcm9041129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ghaneh P, Palmer D, et al. Immediate surgery compared with short-course neoadjuvant gemcitabine plus capecitabine, FOLFIRINOX, or chemoradiotherapy in patients with borderline resectable pancreatic cancer (ESPAC5): a four-arm, multicentre, randomised, phase 2 trial. Lancet Gastroenterol Hepatol. 2023;8:157–68. 10.1016/S2468-1253(22)00348-X. [DOI] [PubMed] [Google Scholar]
- 15.Guo M, Beal EW, Miller ED, Williams TM, Tsung A, Dillhoff M, Ejaz A, Pawlik TM, Cloyd JM. Neoadjuvant therapy versus surgery first for ampullary carcinoma: A propensity score-matched analysis of the NCDB. J Surg Oncol. 2021;123:1558–67. 10.1002/jso.26435. [DOI] [PubMed] [Google Scholar]
- 16.Cloyd JM, Wang H, Overman M, Zhao J, Denbo J, Prakash L, Kim MP, Shroff R, Javle M, Varadhachary GR, Fogelman D, Wolff RA, Koay EJ, Das P, Maitra A, Aloia TA, Vauthey J-N, Fleming JB, Lee JE, Katz MHG. Influence of preoperative therapy on short- and long-term outcomes of patients with adenocarcinoma of the ampulla of vater. Ann Surg Oncol. 2017;24:2031–9. 10.1245/s10434-017-5777-7. [DOI] [PubMed] [Google Scholar]
- 17.Cloyd JM, Prakash L, Vauthey J-N, Aloia TA, Chun YS, Tzeng C-W, Kim MP, Lee JE, Katz MHG. The role of preoperative therapy prior to pancreatoduodenectomy for distal cholangiocarcinoma. Am J Surg. 2019;218:145–50. 10.1016/j.amjsurg.2018.08.024. [DOI] [PubMed] [Google Scholar]
- 18.Kaslow SR, Prendergast K, Vitiello GA, Hani L, Berman RS, Lee AY, Correa-Gallego C. Systemic therapy for duodenal adenocarcinoma: An analysis of the National Cancer Database (NCDB). Surgery. 2022;172:358–64. 10.1016/j.surg.2022.03.009. [DOI] [PubMed] [Google Scholar]
- 19.Hue JJ, Katayama E, Sugumar K, Winter JM, Ammori JB, Rothermel LD, Hardacre JM, Ocuin LM. The importance of multimodal therapy in the management of nonmetastatic adenosquamous carcinoma of the pancreas: Analysis of treatment sequence and strategy. Surgery. 2021;169:1102–9. 10.1016/j.surg.2020.11.026. [DOI] [PubMed] [Google Scholar]
- 20.Voong KR, Davison J, Pawlik TM, Uy MO, Hsu CC, Winter J, Hruban RH, Laheru D, Rudra S, Swartz MJ, Nathan H, Edil BH, Schulick R, Cameron JL, Wolfgang CL, Herman JM. Resected pancreatic adenosquamous carcinoma: clinicopathologic review and evaluation of adjuvant chemotherapy and radiation in 38 patients. Hum Pathol. 2010;41:113–22. 10.1016/j.humpath.2009.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bilimoria KY, Stewart AK, Winchester DP, Ko CY. The national cancer data base: a powerful initiative to improve cancer care in the United States. Ann Surg Oncol. 2008;15:683–90. 10.1245/s10434-007-9747-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Merkow RP, Rademaker AW, Bilimoria KY. Practical guide to surgical data sets: National Cancer Database (NCDB). JAMA Surg. 2018;153:850–1. 10.1001/jamasurg.2018.0492. [DOI] [PubMed] [Google Scholar]
- 23.Brown ZJ, Labiner HE, Shen C, Ejaz A, Pawlik TM, Cloyd JM. Impact of care fragmentation on the outcomes of patients receiving neoadjuvant and adjuvant therapy for pancreatic adenocarcinoma. J Surg Oncol. 2022;125:185–93. 10.1002/jso.26706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kersch CN, Grossberg AJ. Perioperative radiation for patients with resectable pancreatic cancer: an updated review after the initial RTOG 0848 results. J Gastrointest Cancer. 2025;56:70. 10.1007/s12029-025-01185-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brown ZJ, Heh V, Labiner HE, Brock GN, Ejaz A, Dillhoff M, Tsung A, Pawlik TM, Cloyd JM. Surgical resection rates after neoadjuvant therapy for localized pancreatic ductal adenocarcinoma: meta-analysis. Br J Surg. 2022;110:34–42. 10.1093/bjs/znac354. [DOI] [PubMed] [Google Scholar]
- 26.Chick RC, Gunderson AJ, Rahman S, Cloyd JM. Neoadjuvant immunotherapy for localized pancreatic cancer: challenges and early results. Cancers. 2023;15:3967. 10.3390/cancers15153967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Im K, Kareddy N, Satyananda V, O’Connor VV. Is pancreatic adenosquamous carcinoma (PASC) a surgical disease? A large healthcare system review. Surg Oncol Insight. 2024;1: 100102. 10.1016/j.soi.2024.100102. [Google Scholar]
- 28.Cloyd JM, Sarna A, Arango MJ, Bates SE, Bhutani MS, et al. Best practices for delivering neoadjuvant therapy in pancreatic ductal adenocarcinoma. JAMA Surg. 2025;160:172–80. 10.1001/jamasurg.2024.5191. [DOI] [PMC free article] [PubMed]
- 29.Tatsuguchi T, Kitahara D, Kozono S, Date K, Shinkawa T, Kuga H, Tamiya S, Nishihara K, Nakano T. Increased proportion of the squamous cell carcinoma components is associated with aggressive behavior and a worse prognosis in resected pancreatic adenosquamous carcinoma. J Gastrointest Cancer. 2024;56:5. 10.1007/s12029-024-01123-6. [DOI] [PubMed] [Google Scholar]
- 30.Li G, Jiang Y, Li G, Qiao Q. Comprehensive analysis of radiosensitivity in head and neck squamous cell carcinoma. Radiother Oncol. 2021;159:126–35. 10.1016/j.radonc.2021.03.017. [DOI] [PubMed] [Google Scholar]
- 31.Cheng C, Luo Z, Xiong W, Shi Z, Tan H. Epidemiology and survival outcomes in adenosquamous carcinoma: a population-based study. Int J Colorectal Dis. 2022;37:1581–92. 10.1007/s00384-022-04198-4. [DOI] [PubMed] [Google Scholar]
- 32.Cloyd JM, Colby S, Guthrie KA, Lowy AM, Chiorean EG, Philip P, Sohal D, Ahmad S. Failure to undergo resection following neoadjuvant therapy for resectable pancreatic cancer: a secondary analysis of SWOG S1505. J Natl Compr Cancer Netw JNCCN. 2024;22: e237099. 10.6004/jnccn.2023.7099. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are openly available in the National Cancer Database at https://www.facs.org/quality-programs/cancer/ncdb.