Abstract
Inflammatory bowel disease (IBD) presents a significant clinical challenge, yet the way bioactive gases are implicated remains elusive. We detect elevated colonic Nos2 levels in both IBD patients and mice undergoing diverse colitis. Additionally, Nos2 deficiency significantly aggravates anti-CD40-induced colitis, along with an increase in GM-CSF production by ILC3s. We identified a previously unappreciated role of the crucial ILC3 regulator, AhR, in promoting Cyp4f13 expression to allow ILC3s to bind with externally derived nitric oxide (NO). This further restrains Cyp4f13-catalyzed ROS generation and thereby diminishes NF-κB activation strictly necessary for GM-CSF production. Accordingly, the exacerbated anti-CD40-induced colitis due to defective NO generation in Nos2 deficient mice is efficiently recovered by a Cyp4f13 inhibitor, HET0016. Importantly, IBD patients with elevated NO binding to colonic ILC3s show decreased disease activity. Thus, our findings uncover a crucial regulatory mechanism for restraining colitogenic GM-CSF production in ILC3s and underscores its implication in IBD therapy.
Subject terms: Innate lymphoid cells, Crohn's disease, Extracellular signalling molecules, Cytokines
Metabolic processes lead to the production of bioactive gases, such as nitric oxide, which may play roles in the gut immune homeostasis. Here authors show that genomic deletion of Nos2 in mice aggravates colitis in experimental models via an Aryl-Hydrocarbon-Receptor-mediated pathway in type 3 innate lymphoid cells.
Introduction
Immune regulation is crucial for gut homeostasis1. The gut immune system needs to reach a delicate equilibrium between defending against pathogenic invasion and tolerating commensal and dietary antigens. Any disruption to this balance will give rise to inflammatory disorders in the gut2, such as inflammatory bowel disease (IBD). IBD, which consists of ulcerative colitis (UC) or Crohn’s disease (CD), is a chronic and recurrent intestinal inflammation that is prevalently found in industrialized countries. The onset of IBD is triggered by a complex interplay of multiple factors, including genetic mutations, environmental influences, and dysregulated immunity. Especially, uncovering the mechanisms beneath the dysregulated immunity in IBD patients is essential for developing effective therapeutic strategies. Nevertheless, the current immune-related therapeutic approaches, like the blocking of tumor necrosis factor (TNF), interleukin (IL)−12, and IL-233,4, have not achieved satisfactory outcomes, underscoring the need for further exploration of unrecognized immune regulations in the gut.
Recently, the metabolic regulation of immune cells has attracted significant attention. Metabolites derived by both hosts and microbiota from dietary components are abundantly presented in the intestinal environment. These metabolites have substantial effects on the activation and function of immune cells within the gut. Among them, bioactive gases generated from various metabolic processes, such as nitric oxide (NO), hydrogen sulfide (H2S), and carbon monoxide (CO), serve as distinctive elements5,6. These bioactive gases are known to show crucial impacts on a variety of physiological processes, including immune responses. Due to their gaseous property, the approaches for studying them mainly restricted to evaluating the enzymes related to their generation and examining their cellular levels through detection probes5. It is reported that NO is associated with the pathogenesis of IBD7. NO is synthesized by nitric oxide synthase (NOS), which includes three types, neuronal NOS (nNOS) expressed in nerve cells, inducible NOS (iNOS) present in immune cells, and endothelial NOS (eNOS) existing in endothelial cells. Among them, iNOS is recognized as a potential biomarker for IBD8. However, our knowledge about their regulatory roles during the development of IBD remains rather unclear.
Innate lymphoid cells (ILCs) are a newly discovered immune cell population, exerting crucial roles in regulating gut homeostasis9. Functionally, ILCs resemble the CD4+ T helper (Th) cells and are categorized into three subsets, namely type 1 ILCs (ILC1s), ILC2s, and ILC3s9,10. ILC1s mainly express interferon-gamma (IFN-γ) and TNF, ILC2s primarily secrete IL-5, IL-13, IL-4, while ILC3s predominantly generate IL-22, along with small amounts of IL-17 and granulocyte-macrophage colony-stimulating factor (GM-CSF)9. Among these, the majority of ILC3s are enriched in the gut. They are further subdivided into diverse subgroups, such as NKp46+ ILC3, T-bet+NKp46- ILC3s, and T-bet- ILC3s that are derived from the common PLZF+ and GATA3-dependent ILC progenitors, and lymphoid tissue inducer (LTi) cells which develop independently from the LTi progenitors11,12. Despite this disparity, these ILC3 subgroups exhibit notable functional redundancy13. The activation of ILC3s is mainly driven by inflammatory cytokines, like IL-1β and IL-23 generated by macrophages and dendritic cells (DCs)14. Additionally, this process is also regulated by a series of essential transcriptional regulators, such as retinoic acid-related orphan receptor gamma t (RORγt), aryl hydrocarbon receptor (AhR), and signal transducer and activator of transcription 3 (STAT3)15–17. Activated ILC3s serve as the predominant source of IL-22 in the gut, which plays vital roles in maintaining gut homeostasis by acting on the epithelial barrier18,19. However, IL-22 levels are usually found to be elevated in IBD patients20. In murine studies, IL-22 shows both beneficial effects in alleviating dextran sodium sulfate (DSS)-induced colitis and Citrobacter rodentium (C. rodentium) infection, as well as detrimental influences in worsening chronic colitis18,21,22. Moreover, GM-CSF, another effector cytokine of ILC3s, is considered the major contributor to anti-CD40-induced colitis, a commonly used mouse model for IBD study23–25. Thus, the impacts of ILC3 activation on IBD progression remains ambiguous, and uncovering the regulatory mechanisms that drive the beneficial or detrimental effects of activated ILC3s will further enrich our understanding of the delicate immune balance in the gut.
Here, we note that both IBD patients and colitis mice show a consistent increase in the levels of colonic nitric oxide synthase 2 (Nos2) that is responsible for catalyzing NO generation. Additionally, Nos2 deficiency leads to an aggravated production of GM-CSF in colonic ILC3s and an aggravated anti-CD40-induced colitis in mice, indicating a crucial role of Nos2-derived NO in mitigating the pro-inflammatory response of ILC3s. Mechanistically, colonic ILC3s are found to bind with externally generated NO in the gut primarily via an AhR-driven cytochrome P450 member Cyp4f13. This binding represses the catalytic activity of Cyp4f13, resulting in a decreased generation of ROS and a subsequent reduction in NF-κB activation. By using an inhibitor of Cyp4f13 N-hydroxy-N′-(4-butyl-2 methylphenyl) formamidine (HET0016), we efficiently attenuate the ‘AhR-Cyp4f13-NF-κB’ axis, restrain the excessive GM-CSF production by colonic ILC3s, and significantly ameliorate the anti-CD40-induced colitis in Nos2 deficient mice. Notably, the binding of NO to colonic ILC3s and its association with the severity of IBD are also observed in humans, highlighting the clinical significance of our findings.
Results
ColonicNos2displays a consistent increase in various colitis circumstances
To appraise the correlation between bioactive gas generation in the gut and the occurrence of IBD, the publicly available RNA-sequencing (RNA-seq) data (GSE165512) of gut tissues from healthy control (HC) individuals and UC patients were revisited. Among the enzymes responsible for catalyzing the production of bioactive gases such as NO, H2S, and CO, it was noted that NOS2 and NOS3, which are involved in NO generation, were significantly upregulated in both UC patients (Fig. 1A, B). Additionally, among these enzymes, only NOS2 manifested an overtly highest expression in the gut across various tissues (Fig. S1A). Subsequently, the expression changes of these bioactive gas-related enzymes were evaluated during a variety of mouse colitis situations, including anti-CD40-induced, DSS-induced, T cell transfer (TCT)-induced, and 2,4-dinitrobenzene sulfonic acid (DNBS)-induced colitis, as well as the spontaneous colitis in Il10−/− mice and the ulcerative colitis in Tbx21−/−Rag2−/− mice (TRUC), employing the publicly available RNA-seq data (GSE208395)20 (Fig. 1C). Notably, the expression of colonic Nos2, rather than Nos3, showed a consistent increase across these diverse colitis conditions. We further verified that colon specimens from UC patients displayed elevated levels of NOS2 compared to those of the healthy cohort (Fig. 1D and Table S1). Further, the expression of these bioactive gas-related enzymes in mouse colons during anti-CD40-induced and DSS-induced colitis was examined using quantitative PCR (qPCR) (Figs. 1E, F and S1B, C). Consistently, only the colonic Nos2 levels exhibited a significantly increase under both colitis conditions. Taken together, these findings imply that the upregulation of colonic Nos2 is associated with the incidence of colitis in both humans and mice.
Fig. 1. Colonic NOS2 levels are elevated during colitis conditions.
A Diagram illustrating enzymatic catalysis of the generation of bioactive gases NO, H2S, and CO (left), and box plots showing the expression levels of these related enzymes in colon tissues of HC individuals, and UC and CD patients (right). B Bar chart visualizes the fold changes of gene expression shown in (A). C Expression alterations of the bioactive gas-related enzymes in colon tissues of mice experiencing various colitis conditions compared to that in steady-state mice. D Real-time qPCR analysis for the relative expression of colonic NOS2 to HPRT1 in HC individuals and UC patients. E Schematics for anti-CD40-induced and DSS-induced colitis. F Expression of NO-related enzymes in colons of mice experiencing anti-CD40-induced and DSS-induced colitis, and the corresponding steady-state mice. Data are representative of more than three (D) or two (F) independent experiments. Data are presented as box plots showing median, quartile, minimum and maximum values (A), bar graph showing mean ± s.d. (C, F), or violin plots showing median and quartile values (D). Statistical significances are determined by two-sided unpaired Student’s t-test (A, B), two-sided Mann-Whitney U test (D, F), or calculated via limma with multiple correction using the Benjamini-Hochberg procedure (C). ns not significant; *p < 0.05; **p < 0.01; ***p < 0.001.
Nos2deficiency exacerbates anti-CD40-induced colitis
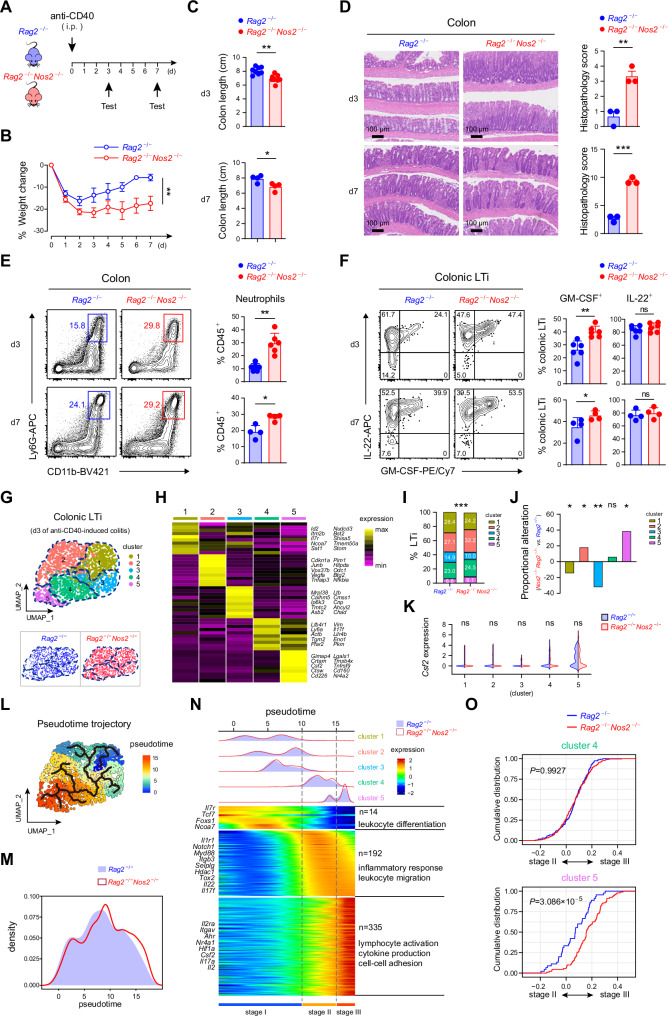
In order to illuminate the role of Nos2 in regulating gut homeostasis, the influence of Nos2 deficiency on anti-CD40-induced and DSS-induced colitis in mice were evaluated (Figs. 2A and S1A). We observed an obviously aggravated body weight loss and colon length shortening on both day 3 and day 7 during anti-CD40-induced colitis in the context of Nos2 deficiency (Nos2−/−Rag2−/−) (Fig. 2B, C). In contrast, during DSS-induced colitis, Nos2−/− mice showed negligible alterations compared to wild-type mice (Fig. S2B, C). The anti-CD40-induced colitis is a unique mouse model for investigating the role of innate immunity during colon inflammation23. Thus, the impacts of Nos2 deficiency on the innate immune response during anti-CD40-induced colitis were further examined. The inflamed colons in Nos2−/−Rag2−/− mice exhibited excessive immune cell infiltration and epithelial damage (Fig. 2D). Consistently, an increased accumulation of neutrophils was detected (Fig. 2E). In previous studies, the primary cause of anti-CD40-induced colitis was attributed to the overexpressed GM-CSF by ILC3s24,25. In line with this, the colonic ILC3s in Nos2−/−Rag2−/− mice demonstrated a remarkably enhanced generation of GM-CSF on both day 3 and day 7 of anti-CD40-induced colitis, while their IL-22 production remained unaltered (Fig. S2D). Particularly, LTi cells, being the predominant ILC3 subgroup in the colon and the major source of GM-CSF within ILC3s (Fig. S2E), showed a more enhanced GM-CSF production (Fig. 2F).
Fig. 2. Nos2 deficiency leads to aggravated anti-CD40-induced colitis.
A Schematics illustrating anti-CD40-induced colitis in Rag2-/- and Rag2-/-Nos2-/- mice. B Body weight alteration during anti-CD40-induced colitis (n = 4 per group). Examination of Rag2-/- and Rag2-/-Nos2-/- mice with anti-CD40-induced colitis on day 3 (d3) and d7, including colon length (d3, n = 8 per group; d7, n = 4 per group) (C), H&E staining showing the pathological changes of colons (n = 3 per group) (D), flow cytometric analysis of neutrophil infiltrations in colons (d3, n = 6 per group; d7, n = 4 per group) (E), and activation of colonic LTi cells as indicated by their production of IL-22 and GM-CSF (d3, n = 6 per group; d7, n = 4 per group) (F). G UMAP visualizing the classification of colonic LTi cells from Rag2-/- and Rag2-/-Nos2-/- mice experiencing anti-CD40-induced colitis on d3. H Heatmap profiling of the top 10 specifically expressed genes among the five LTi clusters. Specifically expressed genes were based on their significantly higher expression in one cluster compared to the other four clusters. I Percentages of each LTi cluster in Rag2-/- and Rag2-/-Nos2-/- mice. J Proportional alterations of the five LTi clusters in Rag2-/-Nos2-/- mice compared to Rag2-/- mice. K Split violin plots comparing Csf2 expression in the five LTi clusters from Rag2-/- and Rag2-/-Nos2-/- mice. L Pseudotime trajectory of LTi cells. M Cell density of colonic LTi cells along the pseudotime trajectory. N Heatmap showing the dynamic changes in gene expression along the pseudotime (lower panel). The distribution of LTi clusters during the activation (divided into three stages), along with the pseudotime trajectory (upper panel). O Empirical cumulative distribution function (ECDF) plots showing relative expression of genes annotated to cluster 4 (top) and cluster 5 (bottom) in LTi cells from Rag2-/- (black) and Rag2-/-Nos2-/- (red) mice, in comparison with the gene expression difference between stage II and stage III. Data are representative of two (G–O) or at least three (A–F) independent experiments. Data are presented as mean ± s.d., and statistical significances are determined by two-sided paired Student’s t-tests (B), two-sided Mann-Whitney U test (C, E, F, K, O), two-sided unpaired Student’s t-tests (D), two-sided chi-square test (I) or Fisher’s exact test (J). ns not significant; *p < 0.05; **p < 0.01; ***p < 0.001.
On day 3 of anti-CD40-induced colitis, the colonic LTi cells from Rag2−/− and Nos2−/−Rag2−/− mice were sort-purified and subjected to single-cell RNA-seq (scRNA-seq) analysis to further clarify the influence of Nos2 deficiency on their GM-CSF production (Fig. 2G). These LTi cells were unbiasedly categorized into five clusters according to transcriptional profiles, with cluster 5 showing the highest Csf2 (encoding GM-CSF) expression (Fig. 2G, H). In line with the previous observation (Fig. 2F), we noticed a significant proportional increase of cluster 5 in the LTi cells from Nos2−/−Rag2−/− mice (Fig. 2I, J). Nevertheless, Csf2 expression was not affected by Nos2 deficiency across the five LTi clusters (Fig. 2K). Thus, the results indicated that the increased GM-CSF expression was primarily due to the altered proportions of LTi clusters in Nos2−/−Rag2−/− mice. To further elucidate this alteration, a pseudotime trajectory analysis was performed (Fig. 2L). Consistently, we observed an enhanced progression of LTi cells in Nos2−/−Rag2−/− mice towards the terminus that was predominantly composed of Csf2 high expressing cluster 5 (Fig. 2M). Additionally, based on gene expression dynamics along the trajectory, we roughly allocated the LTi cells to three stages (Fig. 2N). While stage I, mainly consisting of clusters 1, 2, and 3, exhibited an inactive trait, stage II and III represented the active states of LTi cells as indicated by the increased expression of Il22, Il17f, Il17a, and Csf2. Stage II, primarily constituted by cluster 4, already presented an enhanced Il22 expression and exhibited ‘inflammatory response’ and ‘leukocyte migration’ features. Whereas, stage III ultimately showed an augmented expression of Csf2, as well as several ILC3 activation-related transcription factors Ahr, Nr4a1, and Hif1a, and showed ‘lymphocyte activation’, ‘cytokine production’, and ‘cell-cell adhesion’ features. The gene expression dynamics, together with the previous flow cytometric observation, suggested that, compared to Il22 expression, Csf2 expression relied strictly on further intensified LTi activation (Fig. 2F, N). Using an empirical cumulative distribution function, we noted that while Nos2 deficiency did not affect the progression of cluster 4, it significantly proceeded cluster 5 towards stage III, corroborating the increased Csf2 expression in colonic LTi cells from Nos2−/−Rag2−/− mice (Fig. 2O).
Overall, Nos2 deficiency leads to the exacerbation of anti-CD40-induced colitis, accompanied by the excessive GM-CSF production in colonic LTi cells.
External nitric oxide effectively restrains GM-CSF expression in LTi cells
In line with the elevated levels of colonic Nos2 during anti-CD40-induced colitis, we detected increased NO levels in LTi cells under the colitis condition by employing a widely recognized fluorescent probe for cellular NO, DAF-FM DA (Figs. 3A, B and S3A, B), while they were reduced in Nos2 deficient mice (Fig. S3C). However, based on a transcriptome comparison among various ILC subsets13,26–32, as well as neurons, macrophages, and endothelial cells33–35 that exhibited characteristic expressions of Nos1, Nos2, and Nos3, Nos2 was almost undetectable in both LTi cells and NKp46+ ILC3s (Fig. S3D). Correspondingly, neither the expression of iNOS (encoded by Nos2) nor cellular NO was detected in unstimulated or IL-23 and IL-1β-stimulated ILC3s cultured in vitro (Fig. S3E). Additionally, NO levels in LTi cells were not affected by the iNOS substrate L-arginine (L-Arg) or inhibitor N(ω)-nitro-L-arginine methyl ester (L-NAME) (Fig. S3F, G). Therefore, these results indicated that although NO was present in LTi cells, it was not endogenously generated by the cells.
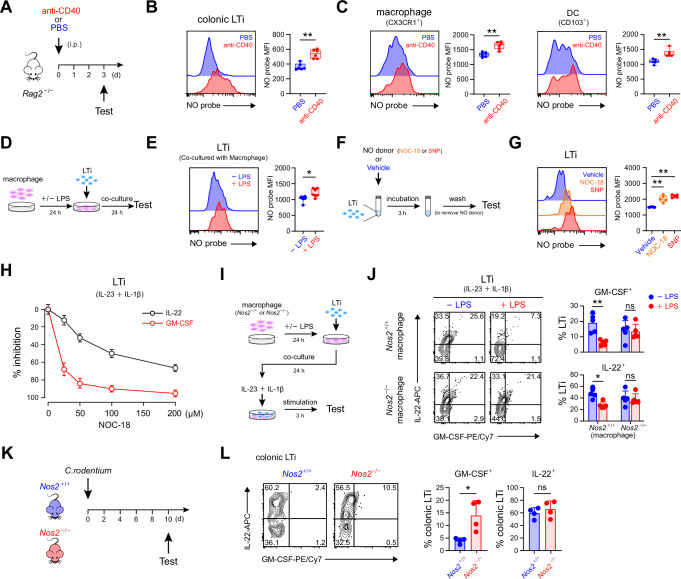
Fig. 3. Binding of external NO to LTi cells efficiently inhibits their GM-CSF production.
A Schematics of anti-CD40-induced colitis or control operation. B Flow cytometric detection of NO in colonic LTi cells of mice with or without anti-CD40-induced colitis on day 3 (left) and statistical calculation for the mean fluorescent intensity (MFI) of NO (n = 3 per group) (right). C NO detected in colonic CX3CR1+ macrophage (left) and CD103+ dendritic cell (right) in mice with or without anti-CD40-induced colitis on day 3 (n = 6 per group). D Schematics of NO detection in LTi cells co-cultured with macrophages. E NO in LTi cells cocultured with macrophages in the presence or absence of LPS stimulation (n = 7 per group). F Schematics of NO detection after administering LTi cells with the NO donor. G NO detected in LTi cells after administered by the NO donors or vehicle (n = 5 per group). H Titration of the influence of the NO donor NOC-18 on the production of GM-CSF and IL-22 in LTi cells (n = 3 per group). I Schematics for assessing the influence of NO derived from LPS-stimulated macrophages on the activation of LTi cells by IL-23 and IL-1β stimulation. J Examination of GM-CSF and IL-22 production by LTi cells after co-cultured with Nos2+/+ or Nos2-/- macrophages with or without LPS stimulation (n = 5 per group). K Schematics for C. rodentium infection of Nos2+/+ or Nos2-/- mice. L Examination of GM-CSF and IL-22 production by colonic LTi cells of Nos2+/+ or Nos2-/- mice on day 10 post C. rodentium infection (n = 4 per group). Data are representative of two (B, C, H, J, L) or at least three (E, G) independent experiments. Data are presented as box plots showing median, quartile, minimum and maximum values (B, C, E, G) or bar graph showing mean ± s.d. (H, J, L), and statistical significances are determined by two-sided Mann-Whitney U test (B, C, E, G, J, L). ns, not significant; *p < 0.05; **p < 0.01.
Subsequently, the possibility of LTi cells binding with externally generated NO was explored. The elevated levels of colonic Nos2 during anti-CD40-induced colitis indicated an increase in NO generation within the inflamed colonic environment (Fig. 1F). Especially, we noticed increased NO levels in both CX3CR1+ macrophages and CD103+ DCs that were known to interact with and activate ILC3s in the colonic environment14 (Figs. 3C and S3H). In another circumstance, when mice were supplied with L-Arg in their drinking water, it also augmented intestinal NO generation, as evidenced by significantly elevated NO levels in intestinal macrophages (Fig. S3I). Correspondingly, this also led to increased NO levels in both NKp46+ ILC3s and LTi cells, but not in ILC2s (Fig. S3J). To directly validate whether the NO generated from macrophages could be bound by LTi cells, the two types of cells were sort-purified and cultured together (Fig. 3D). In comparison to unstimulated macrophages, when macrophages were stimulated by LPS to increase their iNOS expression and NO generation, we also observed significantly elevated NO levels in the co-cultured LTi cells (Fig. 3E). Further, using NO donors such as 3,3-bis-(aminoethyl)-1-hydroxy-2-oxo-1-triazene (NOC-18) or sodium nitroprusside (SNP), we verified the direct binding of externally generated NO with LTi cells (Fig. 3F, G). Thus, to distinguish this type of NO detected in LTi cells, hereinafter it was referred to as NOb (bound nitric oxide).
Next, we examined the influence of external NO on GM-CSF expression in LTi cells (Fig. S3K). It was noted that NO donors markedly inhibited LTi activation, as indicated by the reduced production of GM-CSF and IL-22 (Fig. S3L, M). Further, through dose titration, we observed that the GM-CSF expression in LTi cells was more susceptible to the inhibitory effect of external NO (Fig. 3H). In line with this, upon LTi activation by IL-23 and IL-1β, the presence of LPS-stimulated macrophages to generate NO significantly suppressed the production of GM-CSF, but not IL-22, in LTi cells, while presence of unstimulated macrophages or Nos2−/− macrophages did not bring about any changes (Fig. 3I, J). Thus, these findings, along with the increased GM-CSF production by the colonic LTi cells from Nos2−/−Rag2−/− mice undergoing anti-CD40-induced colitis (Fig. 2F), highlighted the specific role of external NO in effectively restraining GM-CSF expression by LTi cells within the colonic environment. Additionally, during Citrobacter rodentium (C. rodentium) infection that typically activates colonic ILC3s, we detected a remarkable increase in colonic Nos2 expression on day 10 (Fig. S3N, O). Correspondingly, at this timepoint, colonic LTi cells in Nos2−/− mice exhibited enhanced GM-CSF production compared to wild-type mice, while their IL-22 production remained almost unaltered (Fig. 3K, L).
Collectively, these data suggest that increase of colonic Nos2 levels leads to the binding of externally generated NO to LTi cells, which effectively restrains their GM-CSF production.
GM-CSF of intestinal NKp46+ILC3s is also restricted by external nitric oxide
In mice received L-Arg, we also noticed a notable increase in NO levels within intestinal NKp46+ ILC3s (Fig. S3J), raising the possibility that they were also capable of binding with external NO. Indeed, we confirmed that externally generated NO from both NO donors and LPS-stimulated macrophages led to elevated NOb levels in NKp46+ ILC3s (Fig. S3P, Q). Moreover, the majority of intestinal non-LTi ILC3 subgroups, particularly NKp46+ ILC3s, presented significant NOb levels even in steady state, while LTi cells only exhibited minimal NOb levels (Fig. S3R, S). Nevertheless, the NOb levels in NKp46+ ILC3s were unlike derived from iNOS, as they remained unaltered in Nos2−/− mice (Fig. S3T). Thus, NO generation catalyzed by other NOSes might be accountable for the notably NOb levels in NKp46+ ILC3s in steady-state intestine. Among intestinal ILCs in steady state, the NOb+ cells were predominantly composed of T-bet+ non-LTi ILC3s including NKp46+ ILC3s, while the NOb- cells were mainly constituted by other ILC subsets such as ILC2s (Fig. S3U, V). Similar to LTi cells, NKp46+ ILC3s showed reduced activation, especially in terms of GM-CSF production, in the presence of NO donors (Fig. S3W). Dose titration of the NO donor demonstrated that GM-CSF expression in NKp46+ ILC3s was more prone to the inhibitory effect (Fig. S3X). Further, when comparing the responsiveness of sort-purified LTi cells, and NOblow and NObhigh NKp46+ ILC3s from steady-state intestine, it was observed that NObhigh NKp46+ ILC3s displayed a significantly decreased capacity in GM-CSF production compared to the other two ILC3 subgroups, while there was no significant variance in their IL-22 production (Fig. S3Y, Z). Therefore, intestinal NKp46+ ILC3s are also capable of binding with external NO, which results in a restriction in their GM-CSF production.
AhR-driven expression of Cyp4f13 accounts for external nitric oxide binding to ILC3s
We further investigated the molecular mechanism beneath the binding of external NO to ILC3s and its influence on GM-CSF production. Based on the previous scRNA-seq analysis (Fig. 2N), we noticed that the expression of Csf2 along the pseudotime trajectory displayed a delayed upsurge compared to Il22 (Fig. 4A), which was consistent with the flow cytometric observation that GM-CSF only emerged in IL-22high LTi cells (Fig. 2F). When assessing the activities of transcription factors that were specifically expressed in the terminal stage III or were known to be involved in regulating ILC3 activation, we also found that the regulatory activity of AhR demonstrated a consistent change with the expression dynamics of Csf2, implying a crucial association of AhR during the process of GM-CSF production (Fig. 4B). Indeed, we verified that GM-CSF production was dramatically reduced in both NKp46+ ILC3s and LTi cells in the context of Ahr deficiency (Ahrfl/flRorcCre) (Fig. 4C). Moreover, the NOb levels of both ILC3 subgroups, but not ILC2s, were significantly reduced in Ahrfl/flRorcCre mice (Figs. 4D and S4A). On the other hand, an AhR agonist, indigo, notably enhanced the ability of in vitro cultured ILC3s to bind with external NO derived from the NO donor (Figs. S4B and 4C). Thus, these findings indicated that the molecule related to the binding of external NO to ILC3s was driven by AhR.
Fig. 4. AhR-driven Cyp4f13 is accountable for external nitric oxide binding to ILC3s.
A Dynamic expression of Csf2 and Il22 along the pseudotime trajectory (2l). B Regulatory activities of the indicated transcription factors along the pseudotime trajectory. C Flow cytometric examination of GM-CSF production by IL-23 and IL-1β activated NKp46+ ILC3s and LTi cells from Ahrfl/fl or Ahrfl/flRorcCre mice (left), and statistical calculation of the percentage of GM-CSF+ cells (n = 5 per group) (right). D Detection of NO in intestinal NKp46+ ILC3 and LTi cells from Ahrfl/fl or Ahrfl/flRorcCre mice (left), and statistical calculation of the MFI of NO (n = 4 per group) (right). E Differentially expressed genes (TPM > 10, fold change >2, P value < 0.05) between NKp46+ ILC3s of Ahrfl/fl or Ahrfl/flRorcCre mice (X axis), as well as between ILC3s and ILC2s (Y axis). F Heatmap profiling of the AhR-driven NKp46+ ILC3-specific genes. G Predicted AhR binding motif within the chromatin accessibility site at the Cyp4f13 locus in LTi cells (left), and validation of this putative AhR binding via CUT&RUN-qPCR assay (right). H Detection of NO in intestinal NKp46+ ILC3s (left) and LTi cells (right) from wild-type (Cyp4f13+/+) and Cyp4f13-/- mice and statistical calculation of the MFI of NO (n = 4 per group). I Schematics for detection of NO binding ability of NKp46+ ILC3s and LTi cells from wild-type (Cyp4f13+/+) and Cyp4f13-/- mice. Detection of NO in NKp46+ ILC3s (J) or LTi cells (K) from wild-type (Cyp4f13+/+) and Cyp4f13-/- mice after incubated with the NO donors, and statistical calculation of the MFI of NO (n = 5 per group). L Schematics for detection of NO binding ability of HET0016 or the corresponding vehicle treated NKp46+ ILC3s and LTi cells from wild-type (Cyp4f13+/+) and Cyp4f13-/- mice. Detection of NO in HET0016 or the corresponding vehicle treated NKp46+ ILC3s (M) or LTi cells (N) from wild-type (Cyp4f13+/+) and Cyp4f13-/- mice, and statistical calculation of the MFI of NO (n = 5 per group). Data are representative of two (A, B, E, F) or at least three (C, D, G, H, J, K, M, N) independent experiments. Data are presented as box plots showing median, quartile, minimum and maximum values (D, H, J, K, M, N) or bar graph showing mean ± s.d. (C, G), and statistical significances are determined by two-sided Mann-Whitney U test (C, D, H, J, K, M, N) or two-sided unpaired Student’s t-tests (G). ns not significant; *p < 0.05; **p < 0.01; ***p < 0.001.
To uncover the molecule accountable for the binding of external NO to ILC3s, we conducted an RNA-seq comparison between Ahr sufficient (Ahrfl/fl) and deficient (Ahrfl/flRorcCre) NKp46+ ILC3s (Fig. 4E). Additionally, given that external NO binding was particularly manifested in ILC3s but not in ILC2s (Fig. S3P), we also employed the publicly available RNA-seq data to determine the specifically expressed genes in NKp46+ ILC3s compared to ILC2s13,28–32 (Fig. 4E). These AhR-driven NKp46+ ILC3-specific genes (235) included cytoplasmic proteins (62), secretory factors (19), membrane proteins (72), and nuclear proteins (82) (Fig. 4F). Within them, a candidate gene, Cyp4f13, caught our attention due to its potential to interact with NO. The known NO-interacting molecules, such as NOSes, nitric oxide reductases (NORs), and soluble guanylate cyclase (sGC), all possessed a common ferrous heme structure for interacting with NO and forming heme-nitrosyl. Cyp4f13 belonged to the cytochrome P450 family which typically contained the heme structure. Based on the interaction of NOR with NO (PDB# 1CL6), we predicted that Cyp4f13 also had the potential to bind with NO through the iron ion (Fe2+) in its heme structure (Fig. S4D). Firstly, we confirmed the reduced Cyp4f13 expression in Ahr-deficient NKp46+ ILC3s through quantitative PCR (qPCR) (Fig. S4E). Additionally, using a cleavage under targets and release using nuclease (CUT&RUN)-qPCR assay, we observed that AhR could directly bind to a chromatin opening region around the promoter of Cyp4f13 (Fig. 4G). Further, we demonstrated that interfering with Cyp4f13 expression in an ILC3 cell line, MNK3, led to a reduction in their ability to bind external NO (Fig. S4F). Therefore, to assess the involvement of Cyp4f13 in the external NO binding to ILC3s, a Cyp4f13 deficient mouse strain (Cyp4f13−/−) was constructed (Fig. S4G). Consistently, both intestinal NKp46+ ILC3s and LTi cells from Cyp4f13−/− mice exhibited reduced NOb levels (Fig. 4H). In addition, in vitro cultured Cyp4f13−/− ILC3s also demonstrated a significantly diminished ability to bind with external NO derived from the NO donor (Fig. 4I–K).
Cyp4f13 could also interact with its inhibitor, HET0016, which we found to have the potential to block the external NO binding (Fig. S4H). Indeed, administration of HET0016 decreased the capacity of both NKp46+ ILC3s and LTi cells to bind with NO derived from the NO donor (Fig. 4L–N). Moreover, we noticed a further reduction in NOb levels in Cyp4f13−/− ILC3s after the HET0016 administration (Fig. 4L–N). This finding suggested that other cytochrome P450 members targeted by HET0016 might also contribute to the external NO binding in ILC3s, yet further validation was still required. In line with this, among the cytochrome P450 members that showed significant expression (TPM > 1) in NKp46+ ILC3s, several other members displayed reduced expression in the Ahr deficient context, although their expression levels were not as high as Cyp4f13 (Fig. S4I).
Altogether, our findings suggest that the AhR-driven expression of Cyp4f13, as well as other cytochrome P450 members, is accountable for the binding of external NO to ILC3s.
HET0016 efficiently alleviates anti-CD40-induced colitis
Given the binding of external NO to Cyp4f13 and its impact on GM-CSF expression in ILC3s, we further explicated the role of Cyp4f13 in GM-CSF production. Upon IL-23 and IL-1β stimulation, both in vitro cultured NKp46+ ILC3s and LTi cells demonstrated decreased production of GM-CSF in the Cyp4f13−/− context, while their IL-22 expression remained unchanged, indicating that Cyp4f13 was particularly required for GM-CSF production by ILC3s (Figs. 5A and S5A). Consistently, inhibiting the biological function of Cyp4f13 through HET0016 administration also reduced GM-CSF production by both in vitro cultured NKp46+ ILC3s and LTi cells (Figs. 5B, C and S5B).
Fig. 5. HET0016 treatment alleviates anti-CD40-induced colitis.
A Flow cytometric examination of GM-CSF and IL-22 production by IL-23 and IL-1β stimulated LTi cells form wild-type (Cyp4f13+/+) and Cyp4f13-/- mice (left), and statistical calculation of the percentages of GM-CSF+ and IL-22+ cells (n = 4 per group). B Schematics for assessing the influence of HET0016 on the activation of LTi cells. C Flow cytometry examination of the influence of HET0016 (100 μM) or the corresponding vehicle on the production of GM-CSF and IL-22 by IL-23 and IL-1β stimulated LTi cells (left), and statistical calculation of the percentages of GM-CSF+ and IL-22+ cells (n = 5 per group). D–I Rag2-/- or Rag2-/-Nos2-/- mice with anti-CD40-induced colitis were treated with HET0016 (10 mg/kg per day) or the corresponding vehicle, followed by examination on day 3. D Schematics for the HET0016 or vehicle administration. E Statistical calculation of body weight loss of the mice on day 3 (n = 4 per group). F Statistical calculation of colon length of the mice (n = 4 per group). G H&E staining of colon tissues of the mice (left), and statistical calculation of the histopathology scores (n = 3 per group). H Flow cytometry examination of colonic neutrophil infiltration (left), and statistical calculation of the percentages of neutrophils (n = 4 per group). I Flow cytometry examination of GM-CSF and IL-22 production by colonic LTi cells (left), and statistical calculation of the percentages of GM-CSF+ and IL-22+ cells (n = 4 per group). Data are representative of at least three independent experiments. Data are presented as bar graph showing mean ± s.d. (A, C, E–I), and statistical significances are determined by two-sided Mann-Whitney U test (A, C, E, F, H, I) or two-sided unpaired Student’s t-tests (G). ns not significant; *p < 0.05; **p < 0.01.
Considering the role of HET0016 in inhibiting both Cyp4f13 and other cytochrome P450 members with potential for external NO binding, and the consequent influence on GM-CSF expression by ILC3s, we wondered whether it could restore the aggravated anti-CD40-induced colitis in Nos2−/−Rag2−/− mice. Indeed, administration of HET0016 to the mice led to significant improvement in body weight loss and colon length shortening on day 3 of the colitis (Fig. 5D–F). Additionally, histological staining revealed that HET0016 efficiently reversed immune cell infiltration and epithelial injury in the inflamed colon (Fig. 5G). Concurrently, we observed a decreased infiltration of neutrophils and a reduction in GM-CSF production by colonic LTi cells (Fig. 5H, I). Therefore, our results underscore the therapeutic potential of the cytochrome P450 inhibitor HET0016 in treating the development of IBD associated with GM-CSF production (Fig. S5C).
Cyp4f13 promotes GM-CSF expression in ILC3s through enhancing NF-κB activity
Cyp4f13 is accountable for the ω-hydroxylation of its substrates. For instance, it catalyzes the conversion of arachidonic acid (AA) to 20-hydroxyeicosatetraenoic acid (20-HETE) (Fig. S6A). In line with the role of Cyp4f13 in facilitating GM-CSF production by LTi (Fig. 5A), supplementation of AA to strengthen the ω-hydroxylation reaction further augmented the GM-CSF production (Fig. 6A, B). In contrast, in the contexts of Cyp4f13 deficiency or HET0016 administration, the effect of AA on promoting GM-CSF production in LTi cells was absent, confirming that the ω-hydroxylase functionality of Cyp4f13 was associated with this process (Fig. 6A–D).
Fig. 6. Cyp4f13 promotes the production of GM-CSF by ILC3s through activating NF-κB.
A Schematics for assessing the influence of AA on the activation of LTi cells from wild-type (Cyp4f13+/+) or Cyp4f13-/- LTi cells. B Flow cytometry examination of the impact of AA (AA-BSA conjugate) or the corresponding vehicle BSA on the production of GM-CSF and IL-22 by IL-23 and IL-1β stimulated wild-type (Cyp4f13+/+) or Cyp4f13-/- LTi cells (left), and statistical calculation of the percentages of GM-CSF+ and IL-22+ cells (n = 5 per group) (right). C Schematics for assessing the influence of AA on the activation of LTi cells pre-treated with HET0016 or the corresponding vehicle. D Flow cytometry examination of the impact of AA or BSA on the production of GM-CSF and IL-22 expression by IL-23 and IL-1β stimulated LTi cells pre-treated with HET0016 or the corresponding vehicle (left), and statistical calculation of the percentages of GM-CSF+ and IL-22+ cells (n = 6 per group) (right). E Schematics for identification of the AA-Cyp4f13 regulated genes based on RNA-seq analysis on AA or BSA treated wild-type (Cyp4f13+/+) or Cyp4f13-/- LTi cells. F Heatmap profiling of the expression of AA-Cyp4f13 regulated genes (TPM > 5, fold change > 2, P adj <0.05). G Sankey diagram visualization of predicted transcription factors accountable for the regulation of both AA-Cyp4f13 regulated genes (left) and Csf2 or Il22 (right). H CUT&RUN-sequencing analysis of NF-κB binding site at the Csf2 locus. I Schematics for assessing the role of NF-κB during the activation of ILC3s. J Flow cytometry examination of GM-CSF and IL-22 production by IL-23 and IL-1β stimulated ILC3s after treating with low and high doses of NF-κB inhibitors PDTC or TPCR or the corresponding vehicle (left), and statistical calculation of the percentages of GM-CSF+ and IL-22+ cells (n = 5 per group) (right). K Schematics for assessing the influence of AA on ROS generation in ILC3 and the effect of HET0016 on this process. L Examination of the influence of AA on ROS generation in ILC3s and the effect of HET0016 on the process (left), and statistical calculation of the MFI of ROS staining (n = 4 per group) (right). M Schematics for assessing the influence of ROS scavenger NAC on the NF-κB activity of IL-23 and IL-1β stimulated ILC3s from NF-κBluc mice. N Statistical calculation of NF-κB activities in IL-23 and IL-1β stimulated ILC3s after treated by NAC (50 μM) or the corresponding vehicle (n = 4 per group). O Schematics for titrating the influence of NAC on IL-23 and IL-1β stimulated ILC3s pre-treated with AA. P Titration of the influence of NAC on the production of GM-CSF and IL-22 by LTi cells (n = 3 per group). Q Schematics for assessing the influence of NO and HET0016 on the NF-κB activity of IL-23 and IL-1β stimulated ILC3s pre-treated with AA. R Statistical calculation of NF-κB activities in SNP, HET0016, or the corresponding vehicle treated ILC3s pre-incubated with AA or BSA (n = 4 per group). S Statistical calculation of NF-κB activities in NOC-18, SNP, or the corresponding vehicle treated ILC3s (n = 4 per group). T Graphical illustration of the role of Cyp4f13 catalyzed AA ω-hydroxylation in activating NF-κB through enhancing ROS generation, followed by elevated GM-CSF expression, and the inhibitory effect of external NO and HET0016 on this process. Data are representative of two (H, L, N, R, S) or at least three (B, D, J, P) independent experiments. Data are presented as box plots showing median, quartile, minimum and maximum values (L) or bar graph showing mean ± s.d. (B, D, J, N, P, R, S), and statistical significances are determined by two-sided Mann-Whitney U test (B, D, J, L, N, R, S). ns not significant; *p < 0.05; **p < 0.01.
To uncover the underlying mechanism, the transcriptome changes in both Cyp4f13 sufficient and deficient LTi cells after AA treatment were examined via RNA-seq (Figs. 6E and S6B). We identified 224 genes that were upregulated by Cyp4f13-mediated AA ω-hydroxylation, as they were upregulated by AA-treatment in the presence of Cyp4f13 while remaining unaltered in the absence of Cyp4f13 (Fig. 6E, F and Supplementary Data 1). Further, based on the existence of binding motifs in their gene loci, we deduced eight transcription factors that were potentially accountable for the expression of these genes (Fig. 6G). Additionally, four of these transcription factors, NF-κB, ETS1, RUNX3, and BATF, were also potentially involved in regulating Csf2 expression (Fig. 6G). Among them, NF-κB particularly caught our attention, as ω-hydroxylation was usually accompanied by the generation of ROS that was well-known to enhance the transcription factor activity of NF-κB36. We confirmed the binding of NF-κB to the Csf2 locus in ILC3s through a cleavage under targets and release using nuclease (CUT&RUN) assay (Fig. 6H). Then, the correlation between NF-κB activation and GM-CSF production in ILC3s was evaluated by using NF-κB inhibitors, such as pyrrolidinedithiocarbamate ammonium (PDTC) and N-tosyl-l-phenylalanine chloromethyl ketone (TPCK). Upon IL-1β and IL-23 stimulation, NF-κB activity was effectively suppressed by these inhibitors (Fig. S6G). Consequently, while IL-22 was scarcely affected in the presence of the inhibitors at a low dosage, GM-CSF production already showed a notably decrease, indicating that the expression of GM-CSF in ILC3s particularly depended on a sufficiently high NF-κB activity (Fig. 6I, J).
Next, the generation of ROS through Cyp4f13-catalyzed AA ω-hydroxylation and its impact on NF-κB activity and GM-CSF production in ILC3s were evaluated. We noted that supplementation of AA to both NKp46+ ILC3s and LTi cells increased their ROS levels, yet the change was not witnessed in the absence of Cyp4f13, thereby confirming the role of Cyp4f13-mediated ω-hydroxylation in raising the ROS levels in ILC3s (Fig. S6C, D). Additionally, administration of HET0016 to inhibit the ω-hydroxylase activity of Cyp4f13 in ILC3s reversed the AA-induced ROS to a level even lower than that in bovine serum albumin (BSA)-treated ILC3s, indicating that the Cyp4f13-mediated ω-hydroxylation might typically be present in ILC3s (Fig. 6K, L). Subsequently, to assess the influence of ROS on NF-κB activation, a reporter mouse tool that converts NF-κB activity into luciferase expression (NF-κBluc) was employed. We detected that the ROS scavenger, N-acetylcysteine (NAC), significantly decreased the NF-κB activity in ILC3s (Fig. 6M, N). Furthermore, when ILC3s were subjected to different dosages of NAC, we observed that the production of GM-CSF, as compared to IL-22, was more prone to ROS clearance, phenocopying the influence of external NO (Fig. 6O, P). On the other hand, the metabolite product of the Cyp4f13-catalyzed AA ω-hydroxylation, 20-HETE, had no impact on GM-CSF production in ILC3s (Fig. S6E, F). Finally, since both external NO and HET0016 could interact with the ferrous heme catalytic center of Cyp4f13 and thereby inhibit its ω-hydroxylase activity, their effects on the NF-κB activation in ILC3s were also assessed. In line with the increased ROS levels, supplementation of AA to ILC3s significantly enhanced their NF-κB activity, while both the NO donor and HET0016 could reverse this increase (Fig. 6Q, R). Moreover, even without AA supplementation, the NF-κB activity was also reduced by the NO donors, suggesting that the role of Cyp4f13-mediated ω-hydroxylation in enhancing NF-κB activity was ordinarily present in ILC3s (Fig. 6S).
Overall, our findings uncover a crucial role of Cyp4f13 in enhancing NF-κB activity and subsequential GM-CSF production in ILC3s via generating ROS, and illuminate the effect of external NO and HET0016 in antagonizing this process (Fig. 6T).
External NO binding to colonic ILC3s associates with reduced IBD activity
The elevation of colonic NOS2 was conserved in UC patients and mice with colitis, raising the possibility that a similar impact of NOS2-derived NO on colonic ILC3s might also exist in IBD patients. In line with this speculation, we observed that human ILC3s, rather than ILC2s or T and B cells, were also capable of binding with external NO derived from the NO donor (Fig. 7A). Additionally, HET0016 could efficiently attenuate the external NO binding, suggesting that cytochrome P450 members were also responsible for this process (Fig. 7B, C). Indeed, several of the cytochrome P450 members showed significant expression in human ILC3s, and some of them, such as CYP4F3, exhibited both sequential and structural similarities to Cyp4f13 (Fig. S7A, B). Further, we observed that supplementation of AA significantly raised the ROS levels in human ILC3s, while HET0016 efficiently inhibited this increase, evidencing that the cytochrome P450 members in human ILC3s exerted the ω-hydroxylase activity (Fig. 7D). Moreover, we detected that HET0016 also significantly suppressed the production of GM-CSF by human ILC3s (Fig. 7E). These findings demonstrate the evolutionary conservation of Cyp450-mediated regulatory mechanisms between murine and human ILC3s.
Fig. 7. External NO binding to colonic ILC3s ameliorates the activity of IBD.
A Detection of the ability of ILC3s, ILC2s, and T and B (T/B) cells in PBMC to bind with externally generated NO from SNP. B Schematics for detection of external NO binding ability of HET0016 or the corresponding vehicle treated human ILC3s. C Detection of NO binding ability of human ILC3 pretreated with HET0016 or the corresponding vehicle (left) and statistical calculation of the MFI of NO (n = 5 per group). D Detection of ROS in BSA or AA incubated human ILC3s pre-treated with HET0016 or the corresponding vehicle (left) and the statistical calculation of the MFI of ROS staining (n = 5 per group) (right). E Flow cytometry examination of GM-CSF expression by IL-23 and IL-1β stimulated human ILC3 pre-treated with HET0016 or the corresponding vehicle (left) and statistical calculation of the percentages and GM-CSF MFI of GM-CSF+ cells (n = 6 per group) (right). F Flow cytometry showing examination of NO levels in ILC3s from human colon and utilization of NO levels in T/B cells for background normalization. G Statistical calculation of rNOb levels of HC individuals and UC patients (HC, n = 16; UC, n = 22). H Correlation between Mayo score and rNOb levels of UC patients (n = 22). I H&E staining showing colon tissues of UC patients with low (rNOb <1.24) and high (rNOb > 1.24) rNOb levels. J Assessment of neutrophil infiltration in UC patients with low (rNOb <1.24) and high (rNOb > 1.24) rNOb levels using Geboes Score (rNOb <1.24, n = 14; rNOb > 1.24, n = 8). K Correlation between the concentration of fecal calprotectin (top) or C-reactive protein (bottom) and rNOb levels of UC patients (n = 22). Data are representative of at least three independent experiments. Data are presented as box plots showing median, quartile, minimum and maximum values (C, C, G) or bar graph showing mean ± s.d. (J), and statistical significances are determined by two-sided Mann-Whitney U tests (C, D, G, J), two-sided Wilcoxon U test (E) or Pearson correlation tests (H, K). *p < 0.05; **p < 0.01.
Given the increased colonic NOS2 levels in IBD patients (Fig. 1A, D), we further assessed whether the NOb levels of their colonic ILC3s were also correspondingly increased. To eliminate potential variations in NOb detection across different experimental samples, the background NO detected in T and B cells of the same sample was used as a reference. Thus, a relative NOb value (rNOb), calculated as the NOb levels in ILC3s divided by the NO levels detected in T and B cells (rNOb = NOb in ILC3s / NO in T and B cells), was employed for the examination (Fig. 7F). As a result, colonic ILC3s from UC patients indeed displayed significantly increased rNOb levels compared to those from HC individuals (Fig. 7G). Particularly, we noticed that a portion of these patients presented distinctly higher colonic ILC3 rNOb levels (rNOb >1.24, referred to as rNObhigh) than the remaining patients and HC individuals (rNOb ≤1.24, referred to as rNOblow), and they presented reduced disease activities as evaluated by the mayo score (Fig. 7H). Additionally, these patients also demonstrated reduced neutrophil infiltration in the colon based on histological staining and quantitative assessment using the Geboes Scoring (Figs. 7I, J and S7C). Furthermore, these rNObhigh UC patients also exhibited decreased levels of fecal calprotectin and C-reactive protein, corroborating their reduced disease activities (Fig. 7K).
Collectively, these observations underscore the clinical significance of our findings, and indicate its potential implications for diagnosing IBD disease activity.
Discussion
Our study has uncovered an ‘AhR-Cyp4f13-NF-κB’ axis that exerts a particular regulatory role on the production of GM-CSF in ILC3s. In a previous study, it was shown that ILC3s are the predominant source of GM-CSF in the gut37,38. Our scRNA-seq data suggest that Csf2 and Il22 display markedly distinct expression dynamics. The upsurge in Csf2 expression is significantly delayed compared to that of Il22 during ILC3 activation. In line with this, flow cytometric detection also reveals that GM-CSF expression is primarily present when ILC3s exhibit the highest IL-22 expression. Therefore, the expression regulations of the two cytokines should be different. However, the regulation of GM-CSF expression remains largely elusive compared to that of IL-22. Based on the similarity between the dynamic changes in GM-CSF expression and AhR transcription factor activity during ILC3 activation, an Ahrfl/flRorcCre mouse strain is utilized to elucidate the role of AhR in GM-CSF production. To avoid any confounding effects in Ahrfl/flRorcCre mice, such as the influence of Ahr deficiency on Th17 or Treg cells, we mainly use in vitro experiments with ILC3s isolated from these mice. AhR upregulates the expression of Cyp4f13 in ILC3s, which in turn raises the ROS levels via its ω-hydroxylase activity. Subsequently, the increased ROS enhances the activation of NF-κB, which can directly bind to the Csf2 locus to promote its expression. Eventually, we demonstrate that the production of GM-CSF in ILC3s is strictly dependent on adequate levels of ROS and NF-κB activity. However, future studies on Cyp4f13 deficient mice are still required to assess the impact of Cyp4f13 on GM-CSF production in vivo and its translational potential. Altogether, these findings uncover a new ‘AhR-Cyp4f13-NF-κB’ axis in ILC3s, which guarantees sufficient ROS and NF-κB activity levels for them and thereby promotes the production of GM-CSF.
GM-CSF is typically regarded as a vital pro-inflammatory cytokine that is related to the progression of numerous inflammatory diseases39, through multiple mechanisms, including the recruitment and activation of myeloid cells and the modulation of Th1/Th2 balance. Significantly, ILC3s can exacerbate inflammation by producing GM-CSF, which may further influence T cell responses and alter Th1/Th2 balance, thereby playing a potential role in inflammatory bowel disease (IBD) progression. Our discovery of the ‘AhR-Cyp4f13-NF-κB’ axis has revealed a new approach for treating GM-CSF-related inflammations. Notably, Cyp4f13, or other AhR-responsive cytochrome P450 members with similar roles in the axis, has the potential to serve as a therapeutic target. By using HET0016 to inhibit the catalytic activity of Cyp4f13, we have shown the restoration of anti-CD40-induced colitis in Nos2 deficiency mice. More significantly, this treatment only specifically inhibits the production of GM-CSF in colonic ILC3s, while their IL-22 expression remains unaltered. We have also demonstrated the effect of HET0016 in counteracting the production of GM-CSF in human ILC3s, indicating the potential application of this inhibitor or its modified substances in treating GM-CSF-related inflammations, including IBD. Further preclinical validation using more physiologically relevant models such as organoid is required to establish its therapeutic applicability.
In this study, we observed the augmented levels of colonic NOS2 in UC patients, as well as a consistent elevation in colonic Nos2 expression in mice experiencing various colitis. Despite the well-defined increments of iNOS and the NO derived therefrom in inflamed tissues, which are regarded as significant components of the host’s response to noxious stimuli and virulent pathogens, their contribution to the development of colitis remains largely uncertain40,41. To elucidate the functional role of Nos2 in intestinal inflammation, we employed two well-established murine colitis models: anti-CD40- and DSS-induced colitis. While the involvement of Nos2 across other colitis models require further investigation, our results demonstrate that Nos2 ameliorates anti-CD40- but not DSS-induced colitis. Based on the further elucidated mechanisms, we find that the influence of colonic NO on GM-CSF production by ILC3s is consistent in mice and humans, and thereby we find that the rNOb levels exhibit high accuracy in predicting the disease activity of IBD patients. Overall, our findings prominently emphasize the significance of Nos2 upregulation during the progression of colitis in preventing a more aggravated inflammation situation, and have defined a new criterion, rNOb, that shows potential for clinical application in the future.
We have also revealed a new interaction pattern between NO and immune cells. The NO probe DAF-FM DA is typically utilized to detect the cellularly generated NO through Nos enzymes. Unexpectedly, although no Nos enzyme expression is detected, ILC3s still demonstrate remarkable NO levels. Our study further explicates that the NO in ILC3s is externally derived and binds to the cells via AhR-responsive cytochrome P450 members, mainly Cyp4f13 in mice, as manifested by the significantly reduced NO binding in Ahr deficiency ILC3s. This interaction adopts a similar manner as that between NO and hemoglobin, relying on a ferrous heme structure, but was previously unrecognized in immune cells6. Additionally, other characteristics of these cytochrome P450 members, such as their cellular locations or structural features, are presumably also required for their interaction with NO. Thus, further exploration of the properties of these cytochrome P450 members accountable for NO binding is still needed in the future. The regulation also bears a resemblance to the well-defined AhR-Cyp1a1 axis presented in the gut epithelium42. Although AhR has long been considered a fundamental regulator for ILC3s, the existence of an AhR-cytochrome P450 axis within its regulatory network has been largely overlooked in previous studies17.
The binding of external NO to ILC3s is also influenced by the distribution of these cells within the tissue environment. As previously reported, NKp46+ ILC3s and LTi cells show distinct tissue distribution patterns in the gut, with the former mainly spreading in the lamina propria, while the latter is predominantly concentrated in lymphoid follicles43. Correspondingly, our findings also suggest that their NOb levels in the steady-state intestine are different. NKp46+ ILC3s exhibit significantly high NOb levels, while LTi cells only show minimal external NO binding. Additionally, in line with the negligible Nos2 expression in the steady-state intestine, the NOb in NKp46+ ILC3s does not originate from Nos2 since its levels remain unaltered in Nos2−/− mice. During anti-CD40-induced colitis, it was reported that ILC3s, presumably LTi cells, egressed from lymphoid follicles and redistributed within anatomically distinct villus inflammatory foci, in close proximity to the inflammatory myeloid cells24,25. Consistent with this, here we detect considerably elevated NOb levels in LTi cells during anti-CD40-induced colitis. Given that both ILC3 subgroups have the capacity to bind with external NO, these observations imply that the tissue distribution is crucial in determining the NOb levels in ILC3s. In other words, to bind externally generated NO, ILC3s need to locate in close proximity to the NO-generating cells, which are typically iNOS-expressing macrophages or DCs during tissue inflammation. In addition to iNOS, other NOSes such as nNOS and eNOS can also produce NO in the gut. It may be interesting to explore and compare the influence of different NOSes on ILC3s and gut homeostasis.
Methods
Human samples collection
A total of 16 HC and 22 UC colon tissue samples were obtained and cryopreserved from adult volunteers and patients. Informed consent was obtained from all the participants, and these tissues were obtained following the protocol approved by the Medicine Ethics Committee of Peking University Third Hospital (project #: 2023-734-01). The collected tissues were processed for qPCR and flow cytometry to analysis NO binding and GM-CSF production of ILC3. The baseline characteristics of the participants for these samples are listed in Table S1. Blood samples were collected from healthy volunteers.
Mouse strains
All mouse studies were done following the protocols approved by the Institutional Animal Care and Use Committee of Peking University Health Science Center. All mice were bred and maintained in a specific-pathogen-free facility under a 12-h light/12-h dark cycle, with an ambient temperature of 20–24 °C and a humidity of 30–70 %. Mice aged 6–8 weeks, with matching age and sex, were utilized for experiments. There was no gender bias among the mice used in all experiments. C57BL/6J mice were purchased from the Department of Laboratory Animal Science, Peking University Health Science Center. Rag2−/− (stock # T011534) and Cyp4f13−/− (stock # T046466) mice were purchased from the Model Animal Research Center of Nanjing University. Nos2−/− (stock # 002596), Rorcfl/fl (stock # 008771), Rorc-Cre (stock # 022791), and NF-κBluc (stock # 027529) mice were purchased from the Jackson Laboratories. Ahrfl/fl mice (stock # 006203) were maintained in our laboratory and can also be purchased from the Jackson Laboratories. To generate Nos2−/−Rag2−/− mice, Nos2−/− mice were crossed with Rag2−/− mice. To generate Ahrfl/flRorc-Cre mice, Ahrfl/fl mice were crossed with Rorc-Cre mice. Strict adherence to animal welfare and ethical considerations was ensured throughout the study. Experimental and control mice were co-housed before euthanasia. Mice were euthanized by gradual displacement of chamber air with compressed CO2 at a flow rate of 30–70% chamber volume per minute until respiratory arrest, followed by ≥1 minute of exposure to ensure death. Mouse genotypes and data analyses were performed blindly. No mice were excluded from the analyses.
Tissue digestion
For mice tissues, small intestines and colons were collected after euthanasia, and the contents were emptied. For human tissues, colon biopsy samples were obtained from HC individuals and UC patients following the protocol approved by the Medicine Ethics Committee of Peking University Third Hospital. The tissues were sliced into small pieces and processed by first incubating in 5 mM ethylenediaminetetraacetic acid (EDTA), 1 mM dithiothreitol (DTT), and 2% fetal bovine serum (FBS) for 20 min at 37 °C with shaking to remove intestinal epithelial cells. Next, the samples were vortexed and the epithelial fraction was discarded. Subsequently, enzymatic digestion of the remaining tissues was performed in RPMI containing 0.05 mg/ml collagenase IV, 0.5 mg/ml dispase, and 0.1 mg/ml deoxyribonuclease (DNase) I for 45 min at 37 °C with shaking. The digested tissues were subjected to mechanical dissociation and minced through a 40 μm cell strainer. The filtered cells were collected by centrifugation at 540 ×g for 6 min and then resuspended in ice-cold flow buffer (PBS containing 2% FBS).
Flow cytometry
The single-cell suspensions after tissue digestion were stained in flow buffer with corresponding antibodies for surface molecules for 30 min at 4 °C in the dark. Viability staining was performed using eFluorTM 506 dye (eBioscience). For intranuclear transcription factor staining, the Foxp3/Transcription Factor Staining Buffer Set (eBioscience) was used. For intracellular cytokine staining, the cells of digested tissues were resuspended in RPMI containing 10% FBS and 10 ng/ml IL-7, and stimulated with 1 ng/ml IL-23 and 1 ng/ml IL-1β or Cell Stimulation Cocktail (eBioscience) at 37 °C for 3 h. And, 2 mM monensin was added after the first 30 min. Thereafter, the cells were subjected to surface molecule staining, fixation in 2% formaldehyde for 20 min at room temperature, and permeabilization with 0.1% Triton X-100 for 10 min at room temperature. Intranuclear and intracellular antibody staining was performed for 1 h at 4 °C in the dark. For flow cytometry, samples were acquired on a BD LSRFortessa (BD Biosciences) flow cytometer. Flow cytometry data were analyzed with FlowJo software (FlowJo) and GraphPad Prism software (GraphPad).
The gating strategies for NKp46+ ILC3s, LTi cells, and ILC2 in mice were respectively live lineage-CD127+KLRG-1-c-kit+NKp46+, live lineage-CD127+KLRG-1-c-kit+CCR6+, and live lineage-CD127+KLRG-1+. The lineage markers for mouse ILC staining included CD3ε, CD5, CD19, B220, Gr-1 and NK1.1, unless specifically stated. The gating strategy for ILC3s in humans was live CD45+lineage-CD127+CRTH2-c-kit+. The lineage markers for human ILC staining included CD3 and CD19.
Cellular NO and ROS detection
Resuspended cells after tissue digestion, or in vitro cultured ILCs, were stained with corresponding antibodies for surface molecules, along with the viability dye, for 30 min at 4 °C in the dark. Then, the cell sample was washed by centrifugation and incubated with 5 μM DAF-FM DA (Beyotime, S0019S) for NO detection or 10 μM DCFH-DA (Beyotime, S0033S) for ROS detection at 37 °C for 20 min. Afterwards, the cell sample was washed, resuspended in flow buffer, and subjected to flow cytometry analysis. The gating strategies for viable NKp46+ ILC3s, LTi cells, and ILC2 in mice were respectively live lineage-CD127+KLRG-1-c-kit+NKp46+, live lineage-CD127+KLRG-1-c-kit+CCR6+, and live lineage-CD127+KLRG-1+. The lineage markers for the staining of mouse ILCs included CD3ε, CD5, CD19, B220, Gr-1 and NK1.1, unless specifically stated. The gating strategy for viable ILC3s in humans was live CD45+lineage-CD127+CRTH2-c-kit+. The lineage markers for the staining of human ILCs included CD3 and CD19.
Flow cytometry staining reagents
Antibodies specific to mouse Gr-1 (RB6-8C5, 1:400), IL-7R (A7R34, 1:400), Ly6G (1A8-Ly6G, 1:600), CD11b (M1/70, 1:400), IL-22 (IL22JOP, 1:400), T-bet (4B10, 1:400) and iNOS (CXNFT, 1:400) were purchased from eBioscience. Antibodies specific to mouse CD3ɛ (145-2C11, 1:400), CD5 (53-7.3, 1:400), CD19 (6D5, 1:400), B220 (RA3-6B2, 1:400), NK1.1 (PK136, 1:400), CD45.2 (104, 1:400), CD11c (N418, 1:400), MHC-II (M5/114.15.2, 1:2000), F4/80 (BM8, 1:400), CX3CR1 (QA16A03, 1:400), CD103 (2E7, 1:400), c-kit (ACK2, 1:400), KLRG-1 (2F1, 1:400), CCR6 (29-2L17, 1:500), NKp46 (29A1.4, 1:400), GM-CSF (MP1-22E9, 1:400), antibodies specific to human CD45 (2D1, 1:400), IL-7R (A019D5, 1:400), c-Kit (104D2, 1:400), CRTH2 (BM16, 1:400), GM-CSF (BVD2-21C11, 1:400), and BV785-Streptavidin (1:400) were purchased from BioLegend. Antibodies specific to mouse GATA3 (L50-823, 1:200), RORγt (Q31-378, 1:400), antibodies specific to human CD3 (SK7, 1:400), CD19 (HIB19, 1:400) and BUV737-Streptavidin (1:400) were purchased from BD Biosciences.
Anti-CD40 induced colitis
Anti-CD40 induced colitis in Rag2−/− and Nos2−/−Rag2−/− mice was conducted by intraperitoneally injecting 50 μg monoclonal anti-CD40 antibody (clone FGK4.5, cat #BE0016-2). For the administration of HET0016 or the corresponding vehicle (30% 2-Hydroxypropyl-β-cyclodextrin (HPβCD)), mice were given intraperitoneal injections of 10 mg/kg HET0016 or the equivalent volume of vehicle daily for three days. Body weight of each mouse was weighed every day. On day 3 or 7, mice were sacrificed and colon length of each mouse was measured. Then, colon samples were digested and subjected to antibody staining, following with flow cytometry analysis or cell sorting.
DSS-induced colitis
DSS-induced colitis in wild-type and Nos2−/− mice on a B6 background was conducted by administering mice with 2% (w/v) DSS (M. W. 40,000-50,000) in drinking water for 5 days, followed by a switch to regular drinking water. Body weight of each mouse was monitored daily. Mice were sacrificed and colon length was measured on day 7.
C. rodentium infection
Wild-type and mice were infected with 1 × 1010 colony forming units (CFU) of C. rodentium (strain DBS100) via oral gavage. Body weight of each mouse was monitored daily. On day 3 and day 10 post infection, mice were sacrificed, and colons were collected and digested for flow cytometry analysis.
L-arginine administration
B6 mice were given 1% (w/v) L-arginine in drinking water for 2 weeks. Then, mice were sacrificed, and small intestines were collected and digested for flow cytometry analysis.
Histology and pathology scoring
Colons from mice experiencing anti-CD40-induced colitis were longitudinally sliced, and the luminal content was removed by washing in cold PBS. The opened colon was rolled up to form a Swiss-roll, followed by fixation in 4% paraformaldehyde (PFA) for 12 h. Then, the fixed tissue was dehydrated in 30% sucrose and embedded in paraffin. Tissue sections were stained with hematoxylin and eosin (H&E). Scoring of histopathology was performed blinded using the method described by Izcue et al.44. Briefly, each sample was graded semiquantitatively from 0 to 3 for the four following criteria: degree of epithelial hyperplasia, goblet cell depletion, lamina propria infiltrate and epithelial cell damage. Scores for each criteria were added to get histopathology score.
Cell sorting
Small intestine or colon tissues were digested to acquire single-cell suspensions. The samples were stained with corresponding antibodies for surface molecules for 30 min at 4 °C in the dark. If NO detection was needed, the samples were subsequently incubated with DAF-FM DA for 20 min at 37 °C in the dark. Afterwards, the samples were washed and subjected to high-purity cell sorting on a FACSAria III Cell Sorter (BD Biosciences).
Cell culture
Sort-purified ILC3s were cultured in RPMI supplemented with 10% FBS, 10 ng/ml IL-7 (PeproTech), 10 ng/ml IL-2 (PeproTech), and 10 ng/ml SCF (PeproTech). Cells were cultured at 5% CO2 and 37 °C.
L-arginine and L-NAME treatment
LTi cells were treated with 200 mg/L L-arginine or 10 mM L-NAME at 37°C for 3 h. Then, cells were harvested for NO detection.
NO donor treatment
NKp46+ ILC3s and LTi cells were treated with 1 mM SNP or 100 μM NOC-18 for 3 h. Subsequently, the NO donor was removed by washing the cells. Then, the cell samples were stained with antibodies corresponding to surface molecules, followed by NO detection. To measure the influence of the NO donor on the activation of NKp46+ ILC3s or LTi cells, the cells were stimulated with 1 ng/ml IL-23 and 1 ng/ml IL-1β for 3 h in the presence of the NO donors. Afterwards, intracellular cytokine staining was performed.
Indigo treatment
To measure NO binding ability of ILC3s, cells were treated with 100 μM indigo for 12 h at 5% CO2 and 37 °C, and 1 mM SNP was added during the last 3 h. Subsequently, the NO donor was removed through washing the cells. Then, the cells were stained by antibodies corresponding to surface molecules, followed by NO detection.
HET0016 administration
For assessing the influence of HET0016 on antagonizing the NO binding ability of mouse or human ILC3s, the cell samples were treated with 100 μM HET0016 for 12 h at 5% CO2 and 37 °C, and 1 mM SNP was added during the last 3 h. Subsequently, the NO donor was removed by washing the cells. Then, the cells were stained by antibodies corresponding to surface molecules, followed by NO detection.
For evaluating the impact of HET0016 on the activation of ILC3s, the cell samples were treated with 100 μM HET0016 for 6 h. Subsequently, the cells were stimulated with 1 ng/ml IL-23 and 1 ng/ml IL-1β for 3 h and 37 °C, followed with intracellular cytokine staining.
Co-culture of ILC3s and macrophages
Peritoneal macrophages of wild type or Nos2−/− mice were obtained, washed with cold PBS, and resuspended in DMEM with 10% FBS. The macrophages were cultured in the presence or absence of 200 mg/ml LPS at 37 °C for 24 h. Subsequently, the cell culture medium was replaced with RPMI containing 10% FBS, 10 ng/ml IL-7, 10 ng/ml IL-2, and 10 ng/ml SCF, and sort-purified NKp46+ ILC3s or LTi cells were added. ILC3s and macrophages were co-cultured for 24 h, followed by NO detection or intracellular cytokine staining.
AA treatment
AA-BSA conjugate (referred to as AA in this study) was prepared as described previously45. In brief, AA and BSA were mixed in 150 mM NaCl at a 6:1 molar ratio, and the solution was incubated at 37 °C for 1 h and adjusted pH to 7.4. Then the solution was aliquoted and frozen at −20 °C.
To assess the impacts of AA on ILC3s, the cells were treated with 50 μM AA or the equivalent BSA vehicle, along with other reagents if mentioned, for 30 min at 37 °C followed by ROS detection, or for 6 h at 37 °C followed by intracellular cytokine staining. For intracellular cytokine staining, the cells were stimulated by 1 ng/ml IL-23 and 1 ng/ml IL-1β for 3 h, with monensin added for the last 2.5 h. Thereafter, the cytokine production in ILC3s were detected through intracellular cytokine staining.
NAC treatment
To assess the impacts of NAC on ILC3s, the cells were stimulated with 1 ng/ml IL-23 and 1 ng/ml IL-1β for 3 h in the presence of 1 μM, 10 μM, 100 μM or 500 μM NAC. Subsequently, intracellular cytokine staining was performed.
Luciferase assay
1 × 105 sort-purified ILC3s from NF-κBluc mice were stimulated with 10 ng/ml IL-23 and 10 ng/ml IL-1β for 30 min. Thereafter, NF-κB activity in the activated ILC3s was measured by luciferase assays, employing a luciferase reporter gene assay kit (Yeason) according to the manufacturer’s instructions. The NF-κB activity in unstimulated ILC3s was used for normalization. To assess the effects of NAC, NO donors, AA, or HET0016 on the NF-κB activity of ILC3s, the sort-purified cells were pre-treated with 50 μM NAC, 100 μM NOC-18, 1 mM SNP, 50 μM AA, 100 μM HET0016, or the corresponding control vehicles for 3 h, followed by stimulation and luciferase activity assay.
NF-κB inhibition
To measure the influence of NF-κB inhibition, sort-purified ILC3s were pre-treated with 10 μM PDTC or 10 μM TPCK for 1 h, followed by stimulation with 1 ng/ml IL-23 and 1 ng/ml IL-1β for 3 h. Then, the cells were subjected to intracellular cytokine staining.
Real-time qPCR
Total RNAs were extracted from human colon tissues or the distal colons of mice. The GoScript Reverse Transcriptase System (Promega) was used for reverse transcription of messenger RNA according to the manufacturer’s instructions. The resultant complementary DNAs (cDNAs) were then subjected to real-time qPCR analysis using a GoTaq qPCR and RT-qPCR Systems kit (Promega). Hprt1 was used as an internal control.
Public data acquisition
Gene expression profiles (RNA-Seq) of 84 biopsies from patients with Crohn’s disease, 40 biopsies from patient with Ulcerative colitis, and 46 control biopsies were retrieved from GEO database under accession number GSE165512. Gene expression profiles (RNA-Seq) of mouse colon tissue from multiple colitis models were retrieved from GEO database under accession number GSE208395. Gene expression profiles (RNA-Seq) of multiple cell types in small intestine for examining NOS expression were retrieved from GEO database under accession number GSE116092, GSE205894, GSE104708, GSE47851, GSE119461, GSE71198, GSE72909, GSE109125, GSE107776, GSE140290, GSE184748. Putative TF target genes of AhR and RORγt were retrieved from GEO database under accession number GSE222621 and GSE40918. Chromatin accessibility profiles (ATAC-Seq) of mouse intestinal LTi were retrieved from GEO database under accession number GSE116091. Gene expression profiles (RNA-Seq) of human intestinal ILC3 were retrieved from GEO database under accession number GSE126107.
Bulk RNA-seq
To analysis the transcriptome profile of intestinal NKp46+ cells in Ahrfl/fl or Ahrfl/fl Rorc-Cre mice, small intestines were digested and sort purified, followed by RNA-seq. To assess the impact of AA and Cyp4f13 on LTi cells, sort-purified LTi cells from Cyp4f13+/+ or Cyp4f13−/− mice were treated with 50 μM AA or 8.5 μM BSA as controls for 6 h with IL-23 and IL-1β stimulation conducted for the last 3 h, followed by RNA-seq. Cells were directly sorted into the lysis buffer of Single Cell Full Length mRNA-Amplification Kit (N712, Vazyme). According to the manufacturer’s protocols, the samples were reverse transcribed and amplified. The RNA-seq libraries were constructed using TruePrep DNA Library Prep Kit V2 for Illumina (TD502, Vazyme). The libraries were performed on the Illumina NovaSeq platform with paired-end 150-bp reads.
Bulk RNA-Seq data analysis
RNA-Seq reads were aligned to the mouse reference genome (mm10) using HISAT2 (v.2.2.1), and then quantified by featureCounts (v.1.6.3). Transcripts-per-million (TPM) values for gene-level counts were calculated using scuttle (v.1.4.0) package. Differential gene expression analysis was performed by DESeq2 (v.1.34.0) package. Gene Ontology (GO) analysis was performed by over-representation test with clusterProfiler (v.4.2.2) package. For RNA-Seq data from public database, batch effect removal was performed by the limma (v.3.50.3) package.
Single-cell RNA-seq with DNBelab C4 system
To profile gene expression of colonic LTi cells from Rag2−/− and Rag2−/−Nos2−/− mice, the mice were first injected with 50 μg anti-CD40 intraperitoneally. On day 3, the mice were euthanized and the colons were digested for high-purity LTi cell sorting. Cells were converted to barcoded scRNA-seq libraries through steps including droplet encapsulation, emulsion breakage, mRNA captured bead collection, reverse transcription, cDNA amplification and purification. Indexed sequencing libraries were constructed according to the manufacturer’s protocol. The sequencing libraries were quantified by Qubit ssDNA Assay Kit (Thermo Fisher Scientific) and Agilent Bioanalyzer 2100. The sequencing libraries were further sequenced by the DNBSEQ-T7 sequencing platform at Annoroad Gene Technology Co., Ltd. using pair-end sequencing. The sequencing reads contained 30-bp read 1 (including the 10-bp cell barcode 1, 10-bp cell barcode 2 and 10-bp unique molecular identifiers (UMI)), 100-bp read 2 for gene sequences and 10-bp barcodes read for sample index.
scRNA-Seq data processing
Raw reads were aligned against mm10 genome and GENCODE vM23 gene annotation using DNBC4tools (v2.1.2) run to generate gene-cell count matrices.
Seurat package (v.4.3.0) was utilized for the analysis of Single-cell RNA-Seq data. Quality of scRNA-seq data was assessed based on three metrics: (1) the UMI counts per cell should be more than 1000 less than 15000; (2) features per cell should be more than 500; (3) proportion of mitochondrial genes should be less than 10%. First, the gene–cell count matrix was normalized using the NormalizeData function. Next, High Variable Features (HVFs) were selected using the FindVariableFeatures function, the principal components (PCs) were calculated using the RunPCA function, top 10 PCs were used for UMAP dimension and nearest neighborhood graphs building, and the Louvain algorithm was applied for clustering using the FindClusters function (resolution = 0.8). Cluster-specific marker genes were identified by performing differential expression testing using the FindMarkers function. The activity of gene sets in single cells was calculated using the AddModuleScore function.
Monocle3 package (v.1.3.4) was adopted to infer the activation trajectory of LTi cells. New CellDataSet objects were built from the cluster-annotated Seurat object using the new_cell_data_set function. Learn_graph was used for learning principal graph from UMAP dimension and building cell trajectory, and order_cells was used for calculating pseudotime. DifferentialGeneTest was used to test gene expression according pseudotime, and gene with q value < 0.001 were divided into three stages. Mann-Whitney U test was used to analyze the influence of Nos2 deficiency on the progression of LTi cells from stage II to stage III.
CUT&RUN
CUT&RUN libraries were established using CUT&RUN Assay Kit (HD102, Vazyme). Briefly, 10,0000–200,000 ILC3 were wash and incubated with ConA beads, followed by anti-NFkB-p65 (8242S, Cell Signaling Technology) and anti-AhR (BML-SA210-0025, Enzo Biochem) antibody incubation at a dilution of 1:25 overnight at 4 °C. Then, samples were washed and incubated with pG-MNase for 1 h at 4 °C. Ca2+ was added and reaction was performed at 0 °C for 90 min. The reaction was then stopped and DNA was released at 37 °C for 30 min. DNA in supernatant was collected by column-based method following DNA library generation using Vazyme kits (HD102 and N322) or PCR. The library was quantified by Qubit, pooled, and sequenced on an Illumina NovaSeq. CUT&RUN DNA fragments and ILC3 total DNA as input were quantified by PCR. Primers for Cyp4f13, forward 5’-ACCAATCCCTCCAACCCTCAG-3’, reverse 5’-CCCACCTAAGCCTATGACTCTC-3’. The relative quantification of each sample was calculated by comparing the intensity of the gel bands obtained from CUT&RUN DNA fragments and input samples.
ATAC-Seq and CUT&RUN data processing
ATAC-Seq and CUT&RUN reads were aligned to the mouse reference genome (mm10) using Bowtie2 (v.2.4.4). The aligned reads were then sorted by SAMtools (v.1.6) and deduplicated with PICARD (v.2.18.23). CUT&RUN peaks were called by MACS2 (v.2.2.6) and annotated to their neighboring genes with HOMER (v.4.9.1). Library tracks were visualized using IGV (v.2.16.2).
Motif analysis was conducted with FIMO software in MEME Suite (v5.0.5), using a P value threshold of 0.0001. Transcription factor binding motifs are annotated by HOCOMOCO (v11) database.
Protein BLAST
To identify human proteins similar to murine Cyp4f13, we performed a protein BLAST search using the NCBI BLAST tool (https://blast.ncbi.nlm.nih.gov/blast/Blast.cgi). The amino acid sequence of murine Cyp4f13 was used as the query, and the search was limited to the Homo sapiens (human, taxid:9606) database. The protein scores were shown using heatmap.
Structure analysis
The PDB structure files of NOR (PDB ID: 1CL6) and CYP4B1 (PDB ID: 6C94) were downloaded from Protein Data Bank (https://www.rcsb.org/), while structures of Cyp4f13 and CYP4F3 were predicted using AlphaFold246. Figures were prepared in Chimera47 and PyMOL (https://pymol.org/2/).
Statistics
All samples were compared using Prism v7 software (GraphPad) by either a two-sided unpaired Student’s t-test, a two-sided paired Student’s t-test a two-sided Mann-Whitney U test or a two-sided Wilcoxon U test. Results represent either the mean and error bars depict the s.d., violin plots showing median and quartile values and boxplots showing median, quartile, minimum and maximum values Descriptive statistics are provided in the figure legends. P value of <0.05 was considered to be significant. No statistical method was used to predetermine sample size, but the sample size used was based on the results from our previous studies. No data were excluded from the analyses. The experiments were randomized and blinded to allocation during experiments and outcome assessment.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Supplementary information
Description of Additional Supplementary Files
Source data
Acknowledgements
We thank J. Zhu from National Institute of Health, Z. Fan from Institute of Biophysics of Chinese Academy of Sciences, B. Su from Shanghai Jiao Tong University, J. Qiu from Shanghai Institute of Nutrition and Health for their helpful discussion. This work was supported by the National Key Research & Development Program of China (2022YFA1103602, C.Z.), the National Natural Science Foundation of China (U23A20167, 32170896, 31770957, and 91842102, C.Z.), the Shenzhen Innovation Committee of Science and Technology (JCYJ20220818100401003, C.Z.), the Natural Science Foundation of Beijing (Z240019, C.Z.) and International Institute of Population Health, Peking University Health Science Center (JKCJ202305, J.L.).
Author contributions
C.Z. conceived and conducted the project. X.Z. perform the majority of experiments. J.L. performed endoscopies to obtain colon biopsies. Y.Z. performed bulk RNA-seq and scRNA-seq analysis. L.H. helped with bioinformatics analysis. D.W. helped with bulk RNA-seq and CUT&RUN libraries. R.L. performed structure analysis. J.W. provided critical reagent. P.L. and G.A. helped with some experiments. R.C. performed histopathological analysis of UC biopsies. T.S. helped with colon biopsies obtain. P.Z. provided critical reagents. L.B. and C.J. discussed and analyzed the results. C.Z. and X.Z. designed the experiments and wrote the manuscript. C.Z. supervised the project.
Peer review
Peer review information
Nature Communications thanks Nobuhito Taniki and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Data availability
All data reported in this paper will be shared by the lead contact upon request. Bulk RNA-seq, scRNA-seq and CUT&RUN experiments are deposited at GSA with accession number GSA: CRA015773. This study did not generate any unique codes. All other data can be made available from the authors on reasonable request. Source data are provided with this paper.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Xingyu Zhao, Jun Li, Yime Zhang.
Supplementary information
The online version contains supplementary material available at 10.1038/s41467-025-60969-x.
References
- 1.Mowat, A. M. & Agace, W. W. Regional specialization within the intestinal immune system. Nat. Rev. Immunol.14, 667–685 (2014). [DOI] [PubMed] [Google Scholar]
- 2.Blander, J. M., Longman, R. S., Iliev, I. D., Sonnenberg, G. F. & Artis, D. Regulation of inflammation by microbiota interactions with the host. Nat. Immunol.18, 851–860 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kobayashi, T. & Hibi, T. Improving IBD outcomes in the era of many treatment options. Nat. Rev. Gastroenterol. Hepatol.20, 79–80 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Neurath, M. F. Cytokines in inflammatory bowel disease. Nat. Rev. Immunol.14, 329–342 (2014). [DOI] [PubMed] [Google Scholar]
- 5.Kalantar-Zadeh, K., Berean, K. J., Burgell, R. E., Muir, J. G. & Gibson, P. R. Intestinal gases: influence on gut disorders and the role of dietary manipulations. Nat. Rev. Gastroenterol. Hepatol.16, 733–747 (2019). [DOI] [PubMed] [Google Scholar]
- 6.Lundberg, J. O. & Weitzberg, E. Nitric oxide signaling in health and disease. Cell185, 2853–2878 (2022). [DOI] [PubMed] [Google Scholar]
- 7.Liu, J., Chen, X., Wang, A. & Su, D. A mitochondria-targeted nitric oxide probe with large Stokes shift for real-time imaging and evaluation of inflammatory bowel disease in situ. Anal. Chim. Acta1332, 343372 (2024). [DOI] [PubMed] [Google Scholar]
- 8.Xing, C. et al. NOS2 as a prognostic biomarker for early-onset colorectal cancer based on public data and clinical validation analysis. Sci. Rep.15, 4300 (2025). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vivier, E. et al. Innate lymphoid cells: 10 years on. Cell174, 1054–1066 (2018). [DOI] [PubMed] [Google Scholar]
- 10.Spits, H. et al. Innate lymphoid cells—a proposal for uniform nomenclature. Nat. Rev. Immunol.13, 145–149 (2013). [DOI] [PubMed] [Google Scholar]
- 11.Klose, C. S. N. et al. A T-bet gradient controls the fate and function of CCR6−RORγt+ innate lymphoid cells. Nature494, 261–265 (2013). [DOI] [PubMed] [Google Scholar]
- 12.Constantinides, M. G., McDonald, B. D., Verhoef, P. A. & Bendelac, A. A committed precursor to innate lymphoid cells. Nature508, 397–401 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rankin, L. C. et al. Complementarity and redundancy of IL-22-producing innate lymphoid cells. Nat. Immunol.17, 179–186 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Longman, R. S. et al. CX3CR1+ mononuclear phagocytes support colitis-associated innate lymphoid cell production of IL-22. J. Exp. Med.211, 1571–1583 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Withers, D. R. et al. Transient inhibition of ROR-γt therapeutically limits intestinal inflammation by reducing TH17 cells and preserving group 3 innate lymphoid cells. Nat. Med.22, 319–323 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guo, X. et al. Induction of innate lymphoid cell-derived interleukin-22 by the transcription factor STAT3 mediates protection against intestinal infection. Immunity40, 25–39 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Qiu, J. et al. The aryl hydrocarbon receptor regulates gut immunity through modulation of innate lymphoid cells. Immunity36, 92–104 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sonnenberg, G. F., Monticelli, L. A., Elloso, M. M., Fouser, L. A. & Artis, D. CD4+ lymphoid tissue-inducer cells promote innate immunity in the gut. Immunity34, 122–134 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wu, D. et al. PD-1 signaling facilitates activation of lymphoid tissue inducer cells by restraining fatty acid oxidation. Nat. Metab.4, 867–882 (2022). [DOI] [PubMed] [Google Scholar]
- 20.Pavlidis, P. et al. Interleukin-22 regulates neutrophil recruitment in ulcerative colitis and is associated with resistance to ustekinumab therapy. Nat. Commun.13, 5820 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wu, D. et al. Proline uptake promotes activation of lymphoid tissue inducer cells to maintain gut homeostasis. Nat. Metab.5, 1953–1968 (2023). [DOI] [PubMed] [Google Scholar]
- 22.Gunasekera, D. C. et al. The development of colitis in Il10−/− mice is dependent on IL-22. Mucosal Immunol.13, 493–506 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Uhlig, H. H. et al. Differential activity of IL-12 and IL-23 in mucosal and systemic innate immune pathology. Immunity25, 309–318 (2006). [DOI] [PubMed] [Google Scholar]
- 24.Pearson, C. et al. ILC3 GM-CSF production and mobilisation orchestrate acute intestinal inflammation. Elife5, e10066 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Song, C. et al. Unique and redundant functions of NKp46+ ILC3s in models of intestinal inflammation. J. Exp. Med.212, 1869–1882 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pokrovskii, M. et al. Characterization of transcriptional regulatory networks that promote and restrict identities and functions of intestinal innate lymphoid cells. Immunity51, 185–197.e186 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Asahi, T. et al. Liver type 1 innate lymphoid cells lacking IL-7 receptor are a native killer cell subset fostered by parenchymal niches. Elife12, e84209 (2023). [DOI] [PMC free article] [PubMed]
- 28.Huang, Y. et al. S1P-dependent interorgan trafficking of group 2 innate lymphoid cells supports host defense. Science359, 114–119 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yagi, R. et al. The transcription factor GATA3 is critical for the development of all IL-7Rα-expressing innate lymphoid cells. Immunity40, 378–388 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li, S. et al. Aryl hydrocarbon receptor signaling cell intrinsically inhibits intestinal group 2 innate lymphoid cell function. Immunity49, 915–928.e915 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhong, C. et al. Group 3 innate lymphoid cells continuously require the transcription factor GATA-3 after commitment. Nat. Immunol.17, 169–178 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yoshida, H. et al. The cis-regulatory atlas of the mouse immune system. Cell176, 897–912.e820 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schmid, C. D. et al. ALK1 controls hepatic vessel formation, angiodiversity, and angiocrine functions in hereditary hemorrhagic telangiectasia of the liver. Hepatology77, 1211–1227 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rocha-Resende, C. et al. Immunomodulatory role of non-neuronal cholinergic signaling in myocardial injury. JCI Insight5, e128961 (2019). [DOI] [PMC free article] [PubMed]
- 35.Obata, Y. et al. Neuronal programming by microbiota regulates intestinal physiology. Nature578, 284–289 (2020). [DOI] [PubMed] [Google Scholar]
- 36.Morgan, M. J. & Liu, Z. -g Crosstalk of reactive oxygen species and NF-κB signaling. Cell Res.21, 103–115 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mortha, A. et al. Microbiota-dependent crosstalk between macrophages and ILC3 promotes intestinal homeostasis. Science343, 1249288 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li, J. et al. Activation of DR3 signaling causes loss of ILC3s and exacerbates intestinal inflammation. Nat. Commun.10, 3371 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wicks, I. P. & Roberts, A. W. Targeting GM-CSF in inflammatory diseases. Nat. Rev. Rheumatol.12, 37–48 (2016). [DOI] [PubMed] [Google Scholar]
- 40.Kolios, G., Rooney, N., Murphy, C. T., Robertson, D. A. & Westwick, J. Expression of inducible nitric oxide synthase activity in human colon epithelial cells: modulation by T lymphocyte derived cytokines. Gut43, 56–63 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shi, C. et al. A nanoparticulate dual scavenger for targeted therapy of inflammatory bowel disease. Sci. Adv.8, eabj2372 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schiering, C. et al. Feedback control of AHR signalling regulates intestinal immunity. Nature542, 242–245 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jarade, A. et al. Inflammation triggers ILC3 patrolling of the intestinal barrier. Nat. Immunol.23, 1317–1323 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Izcue, A. et al. Interleukin-23 restrains regulatory T cell activity to drive T cell-dependent colitis. Immunity28, 559–570 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gotoh, K. et al. Metabolic analysis of mouse bone-marrow-derived dendritic cells using an extracellular flux analyzer. STAR Protoc.2, 100401 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Varadi, M. et al. AlphaFold Protein Structure Database: massively expanding the structural coverage of protein-sequence space with high-accuracy models. Nucleic Acids Res.50, D439–d444 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pettersen, E. F. et al. UCSF ChimeraX: Structure visualization for researchers, educators, and developers. Protein Sci.30, 70–82 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Description of Additional Supplementary Files
Data Availability Statement
All data reported in this paper will be shared by the lead contact upon request. Bulk RNA-seq, scRNA-seq and CUT&RUN experiments are deposited at GSA with accession number GSA: CRA015773. This study did not generate any unique codes. All other data can be made available from the authors on reasonable request. Source data are provided with this paper.