Abstract
The captivating aroma of basmati rice is highly favoured by consumers across the globe. Unfortunately, the aroma of basmati rice has been gradually diminishing over time due to the excessive use of inorganic fertilizers and the impact of climate change. To understand the microbial community that plays a significant role in aroma enhancement in basmati rice accessions, a systematic study is required. A unique rhizobacteria of basmati rice associated with basmati rice were Actinobacteria, Bacillus subtilis, Burkholderia, Enterobacter, Klebsiella, Lactobacillus, Micrococcus, Pseudomonas, and Sinomonas. The biosynthesis of potential precursors (ornithine, putrescine, proline, and polyamines) of aroma in basmati rice involved various enzymes such as acetylornithine aminotransferase, acetylornithine deacetylase, N-acetylornithine carbomyltransferase, acetylornithine/succinyldiaminopimelate aminotransferase, and ornithine cyclodeaminase. These findings significantly contribute to the existing understanding of the rhizobacteria associated with basmati rice that play a crucial role in enhancing the aroma. The introduction of these cultures into the basmati rice growing areas has the potential to augment the plant growth and enhances the aroma. The present study explored the functional potential of the microbial community associated with aroma improvement in basmati rice. This will also enhance the export potential of the basmati rice in the region on sustainable basis.
Keywords: Metagenomics, Rhizobacteria, Aroma enhancement, Geographical indications (GIs), Basmati rice
Subject terms: Plant ecology, Metagenomics
Introduction
For centuries, basmati rice has been cultivated in the foothills of the North Western Himalayas in the Indian sub-continent, earning it the title of the "queen of fragrance". This aromatic rice holds great significance in the global market as one of the essential commodities. Among the various compounds 2-acetyl-1-pyrroline (2-AP) is considered as one of the key component responsible for its delightful naturally occurring aroma1,2. Basmati rice is highly sought after worldwide due to its unique characteristics, including elongation of grains after cooking, fluffy texture, delicious taste, superior aroma, and distinct flavor3,4. India possesses a rich variety of indigenous aromatic cultivars and landraces5,6. However, the cultivation of basmati rice in India is challenging as it is low yielding and sensitive to photoperiod, limiting its growth to the kharif season (monsoon or wet season) only. The development of aroma in basmati rice grains is influenced by both genetic and environmental factors. While there are over 100 compounds responsible for its aroma, 2-AP stands out as a major biochemical compound contributing to its unique fragrance1,2.
The expression of aroma in basmati rice is also influenced by various factors such as the recessive gene (badh2), micro-climate, soil conditions, and geographical location7. However, the introduction of modern farming practices has led to a slight deterioration in the aroma and other rice grain quality characteristics due to the disruption of the rhizospheric microflora and excessive use of inorganic fertilizers3,7. To enhance the aroma, it is necessary to restore the rhizospheric microflora by introducing beneficial microorganisms8,9. To accomplish this goal, it is essential to replenish the microorganisms present in the rhizosphere with beneficial ones10,11. This action not only enhances the health of the rhizosphere but also boosts plant metabolomics to enhance the aroma of rice12–14. The rhizobacteria of rice are also influenced by factors like pH, electrical conductivity (EC), Mn, Fe, Zn, temperature, moisture, geo-climatic positions of rice fields and crop varieties14.
In recent years, the utilization of high-throughput sequencing has become increasingly prevalent in the examination of the microbiomes of various crops in response to environmental pressures15. Metagenomics, a field that employs culture-independent molecular mechanisms, aids in the analysis of the intricate genomes of microbial communities. By applying genomic studies to entire communities of microbes, metagenomics eliminates the necessity of isolating and culturing individual bacterial community members14. The potential of metagenomics lies in its ability to generate data on microbial interrelationships, which can then be utilized to enhance human health, subsistence agriculture, and energy production. It serves as a tool for exploring the genetically diverse reservoirs of uncultured microbiomes without relying on traditional culturing methods16. The core principle of metagenomics revolves around the direct extraction of DNA from complex samples containing diverse microbiota, thereby revealing the true microbial composition of the environment17. The advent of Next Generation Sequencing (NGS) has further facilitated metagenomic studies through targeted approaches, such as amplifying specific regions of genomic DNA like 16S amplicon and shotgun metagenome sequencing18.
In previous study, the rhizospheric samples were collected from the Basmati 370, screened by using 16S rRNA (27F 5’-AGAGTTTGATCMTGGCTCAG-3’ and 1492R 5’-CGGTTACCTTGTTACGACTT-3’), and identified the potential rhizobacterial species namely, Bacillus aryabhattai, Streptomyces hawaiiensis, Bacillus sp., Bacillus tequilensis and Pseudomonas mosselii that showed a significant increase in shoot length in basmati rice14. In another study, rhizobacteria Enterobacter sp. and Pseudomonas otitidis exhibited highest increase in root length (37 cm/plant) and shoot length (109.75 cm/plant) of Basmati 370 compared to the control16. Chryseobacterium sp. promoted the growth of both the root and shoot in basmati rice in various geographical areas. Previous research has indicated that Acinetobacter sp., Bacillus aryabhattai and Enterobacter ludwugi strains were involved in the production of aroma in Basmati 370, which is a precursor to the aromatic compound proline found in basmati rice14,16. Based on these findings, it can be concluded that PGPR has a significant impact on plant growth and enhancing the aroma in basmati rice14,16. By identifying the core microbiota community in the rhizosphere of rice plants and understanding their response to soil properties, the strategies can be developed to manipulate the microbiota to enhance plant growth traits and aroma in basmati rice. However, the role of the rhizobacterial community in enhancing plant growth and aroma production in Basmati 370 under the GI of basmati has not yet been studied. Although PGPR has been identified in basmati growing areas of Jammu region in previous studies14,16, the unique microbial community responsible for aroma improvement has not been investigated. Therefore, this study aimed to assess the microbial diversity in the rhizosphere of both basmati and non basmati rice varieties in three districts, compare their abundance, and identify the rhizobacteria responsible for plant growth and aroma enhancement. Additionally, the metagenomic study explored the functional potential of the microbial community associated with aroma improvement in basmati rice.
Results
Physicochemical analysis
The microbiota present in the rhizosphere is crucial for the regulation of both the physical and chemical properties of soil, ultimately leading to enhance crop production. The pH of the soil samples collected from Jammu, Samba, and Kathua areas ranged from 8.3 to 8.8, indicating that the soil was slightly alkaline (Table S1). Additionally, the electrical conductivity (EC) values varied from 0.14 ds/m to 0.40. Basmati 370 had higher levels of available nitrogen (N) compared to SJR 5 in the soil samples from Jammu, Samba, and Kathua. Cation exchange was found to be lower in Jammu areas for both basmati and non-basmati rice varieties compared to Samba and Kathua. Micronutrient analysis revealed that basmati rice had higher concentrations of zinc (Zn), iron (Fe), and manganese (Mn) compared to non-basmati rice in all districts (Table S2). The highest concentration of arsenic was observed in Jammu areas for both Basmati 370 and SJR 5, while the lowest concentration was found in Samba areas.
Metagenomics sequencing
In the present metagenomic study, total reads obtained after sequencing were 124,542,696 bp and 154,345,624 bp for Basmati 370 and SJR 5, respectively, in the Jammu area; 129,001,424 bp and 137,656,532 bp for Basmati 370 and SJR 5 in the Samba area; and 158200154 bp and 47,380,704 bp for Basmati 370 and SJR 5 in the Kathua area (Table S3). The mean read length of R1 and R2 for all six samples was 159 bp. After read filtration, the number of reads corresponding to each sample decreased (Table S4). The sequencing quality of all six datasets was found to be greater than 90%, indicating good quality of the sequenced data. The GC content for all six samples was above 65%. Phred score was above 35, indicating more than 99.9% precision of base call. There were no gaps in the sequencing data after filtration, as the Read N content was nil (Supplementary Figure S1 to Figure S4). One possible explanation for this result could be the high complexity of the soil microorganisms.
Assembly of the metagenomes
Total of 15,391 contigs were generated from metagenome assembly of short reads. The N50 value, representing the contig length at which half of the total assembly length is accounted for, was determined to be 1345, while the L50 value, indicating the number of contigs required to reach the N50 length, was found to be 5636 (Table S4). To visualize the growth of contig lengths, a cumulative length plot was constructed, with contigs arranged on the X-axis in descending order of size. The size of the coverage bins in the plot was automatically determined based on the number of contigs and the coverage deviation (Fig. S1). Additionally, the cumulative length plot for aligned contigs illustrated the increase in aligned block lengths (Figure S5). In cases where a contig encountered a misassembly event, QUAST divided it into smaller fragments referred to as aligned blocks. The total length of all contigs amounted to 22,127,513 base pairs (bp), surpassing the minimum threshold of 1000 bp. The largest contig identified in the assembly had a length of 45,769 bp.
Taxonomic comparisons of rhizomicrobiome
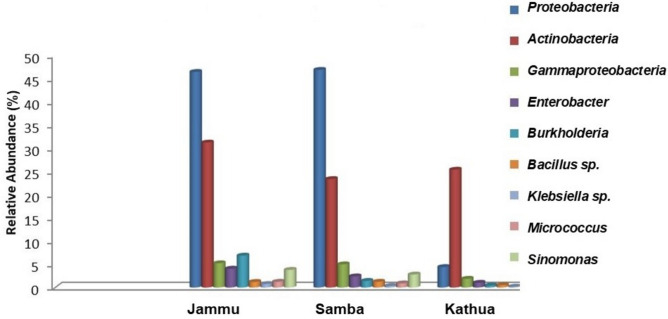
The metagenomic analysis of microbial diversity in the basmati growing regions of Jammu district revealed the highest microbial counts in the R.S Pura area, followed by Samba and Kathua. The predominant microbial groups associated with the rhizosphere of basmati rice areas were Actinobacteria, Burkholderia, Enterobacteria, Proteobacteria, etc. (Fig. 1). Proteobacteria were found to be the most abundant at the phylum level, accounting for 46.43% and 43.05% of reads in the basmati and non-basmati rice fields, respectively. At the class level, Actinobacteria were the most abundant in both the basmati and non-basmati rhizospheric samples, with 34.19% and 35.91% reads in the Jammu area, and 27.19% and 63.98% reads in the Samba area, respectively. In the rhizosphere of Basmati 370 and SJR 5 in the Samba area, Proteobacteria and Actinobacteria were found to be the most abundant at the phylum level, with reads of 46.87% and 59.14%, respectively. At the order level, Actinomycetales were more abundant in both the basmati and non-basmati rice rhizospheres in the Jammu area, while Burkholderiales and Rhizobiales were more abundant in the Samba area. The overall bacterial rhizosphere community composition showed variation between Basmati 370 and SJR 5. The detailed distribution of microbial communities in the rhizospheres of basmati and non-basmati rice fields in the Jammu, Samba, and Kathua districts can be found in Figs. S6–S18.
Fig. 1.
Relative abundance of aroma improving bacteria in rhizosphere of basmati rice in Jammu, Samba and Kathua areas.
Enzymes related to aroma pathways
In the rhizosphere of Basmati 370, several rhizobacteria were found to be responsible for aroma improvement, including Actinobacteria, Bacillus subtilis, Burkholderia, Enterobacter sp., Gamma proteobacteria, Klebsiella, Lactobacillus, Micrococcus, Proteobacteria, Pseudomonas, and Sinomonas (Table 1). The study revealed that Proteobacteria were the most abundant (45%), followed by Actinobacteria (30%) and Burkholderia (7.50%) in the rhizosphere of Jammu area. In contrast, Actinobacteria (30%) exhibited higher abundance compared to Proteobacteria in the Kathua area. A similar trend was observed in the Samba district in which maximum abundance was observed for Proteobacteria (47%). Overall, the rhizobacteria in the Jammu area showed higher abundance compared to Samba and Kathua districts. This difference in abundance may be attributed to the rhizobacteria present in the basmati rice grown in the Ranbir Singh Pura area of Jammu district, which is known to possess a more pronounced aroma compared to other parts of the Jammu region. The microorganisms that have been identified are involved in the biosynthesis of enzymes associated with aroma, which may be essential in the production of precursor molecules that enhance aroma quality (Fig. 2).
Table 1.
Identification of key enzymes related to aroma in different samples.
| Rhizobacteria | Name of the enzyme | Percentage (%) | ||
|---|---|---|---|---|
| Jammu (R S Pura) | Samba | Kathua | ||
| Proteobacteria | Acetylornithine/succinyl diaminopimelate aminotransferase, | 45.00 | 47.00 | 5.00 |
| Actinobacteria | Acetylornithine deacetylase | 30.00 | 22.00 | 25.00 |
| Burkholderia | Tryptophan monooxygenase, Indole-3-acetamide hydrolase, phosphatase | 7.50 | 1.50 | 0.50 |
| Gammaproteobacteria | α-L-arabinofuranosidase | 6.50 | 5.00 | 2.00 |
| Enterobacter |
Gamma-glutamyl putrescine Oxidoreductase |
4.50 | 2.50 | 1.25 |
| Sinomonas sp. | l-methionine demethiolase, l-methionine aminotransferase, and α-keto-γ-methyl-thiobutyric acid demethiolase | 4.00 | 3.00 | 2.50 |
| Bacillus sp. | Fibrinolytic enzyme | 1.25 | 1.50 | 0.50 |
| Micrococcus sp. | Tryptophanase | 1.20 | 0.70 | 1.20 |
| Klebsiella sp. | Spermidine/putrescine import ATP-binding protein PotA | 0.70 | 0.40 | 0.20 |
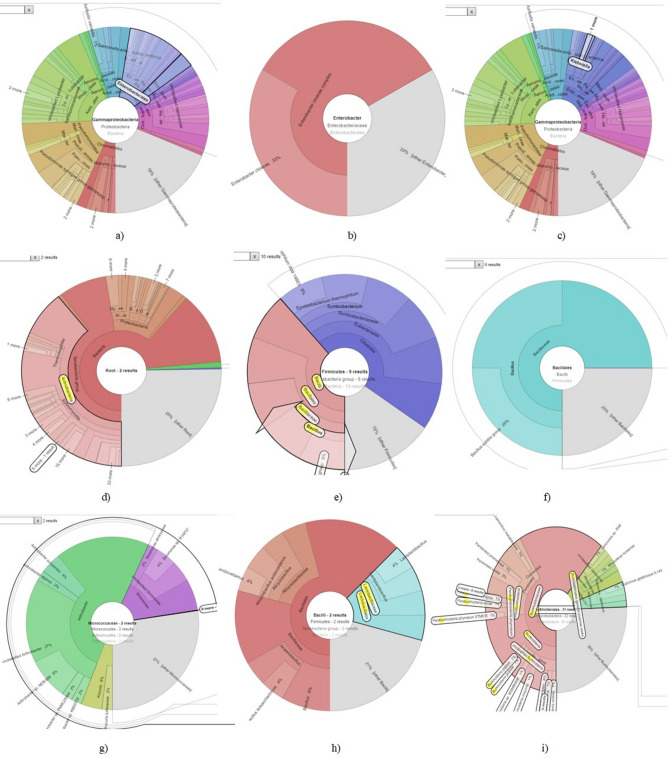
Fig. 2.
Different aroma enhancing bacteria identified in basmati rhizosphere in Kraken Plots (a) and (b) Enterobacter species (c) Pseudomonas species (d) Actinobacteria species (e) and (f) Bacillus species (g) Micrococcus species and (h) Lactobacillus species and (i) Burkholderia species.
Aroma estimation
The research revealed that the bacteria linked to the enhancement of aroma were consistently identified in the rhizospheres of Basmati 370 across all samples obtained from three distinct regions. However, there was a noticeable variation in the intensity of aroma perception in basmati rice among different areas (Table 2). Depending on the sensory evaluation, the samples were rated for aroma using a standard scale (0–3) by a group of five panellists. The sensory test revealed that Basmati 370 had aroma in all three districts (Jammu, Samba, and Kathua), while SJR5 had no aroma presence in any of the samples from these areas. Although Basmati 370 had aroma in all three districts, there was a significant difference in the strength of aroma among the locations. The samples of Basmati 370 had higher aroma content in Jammu compared to Samba and Kathua areas.
Table 2.
Sensory evaluation for aroma in rice samples collected from different areas.
| Sample site/district | Variety | Panellist 1 | Panellist 2 | Panellist 3 | Panellist 4 | Panellist 5 |
|---|---|---|---|---|---|---|
| Jammu | Basmati 370 | 3 | 3 | 3 | 3 | 3 |
| SJR 5 | 0 | 0 | 0 | 0 | 0 | |
| Samba | Basmati 370 | 2 | 2 | 3 | 2 | 2 |
| SJR 5 | 0 | 0 | 0 | 0 | 0 | |
| Kathua | Basmati 370 | 3 | 2 | 3 | 2 | 3 |
| SJR 5 | 0 | 0 | 0 | 0 | 0 |
Where, control signifies non basmati rice variety (SJR 5).
Refinement of bins annotation metric of assembled bins and the distribution from three areas in basmati and non basmati
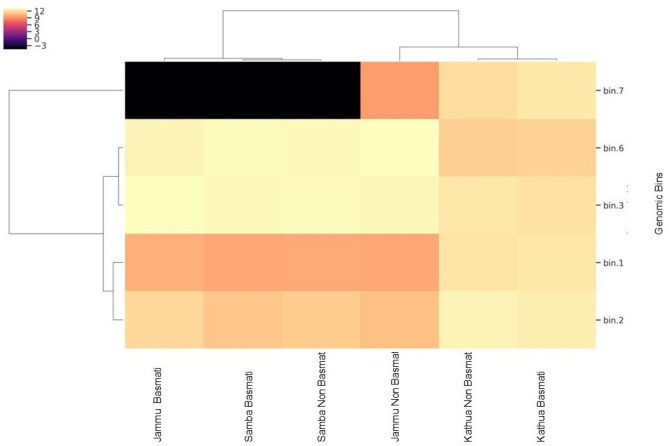
Metagenomic analysis involves several important steps, and one of them is binning, which is as crucial as assembly. The MetaWrap Annotate bins module was used for the functional annotation of the bins. Figure 3 shows the results of binning all contigs obtained from shotgun metagenomics into MAGs with MetaWRAP. Furthermore, CheckM was used for checking the genomic features and calculation of completeness and contamination. Total five bins were constructed, where bins 2 was abundant in basmati (Basmati 370) in Jammu and Samba, and non basmati (SJR 5) in Samba, but almost absent at Kathua areas both in basmati and non basmati (Fig. 3). The results showed that Bin1 contained 619 contigs and 1686 coding sequences (CDS); Bin2 contained 903 contigs and 1641 CDS; Bin3 contained 687 contigs and 1490 CDS; Bin6 contained 271 contigs and 693 CDS; and Bin7 contained 392 contigs and 711 CDS (Table 5S and Fig. 3). However, Bin4 and Bin5 were excluded during the reassembly process due to lower coverage and contamination. These coding sequences were further analyzed for functional annotation, and several hypothetical proteins were identified.
Fig. 3.
Heatmap of identified bins estimated from MAGs with MetaWRAP for basmati and non basmati rhizosphere samples collected from the three locations.
Table 5.
Classification samples for aroma (0–3 scale).
| Scale | Remarks |
|---|---|
| 0 | No aroma |
| 1 | Mild aroma |
| 2 | Aroma |
| 3 | High aroma |
Functional and pathway annotation
By conducting a functional annotation of the shotgun sequences, successfully identified the enzymes that contribute to the pathway of aroma enhancement in bacterial systems. This process of functional annotation allowed for the identification of specific enzymes that play a crucial role in the modulation of aroma in basmati rice. In the study, the rhizosphere of Basmati 370 harbours different aroma enhancing bacteria such as Actinobacteria, Bacillus subtilis, Burkholderia, Enterobacter, Klebsiella, Lactobacillus, Micrococcus, Pseudomonas and Sinomonas as (Table 3). The enzymes participating in the bacterial systems’ aroma enhancement pathway were identified to the corresponding sequences. These enzymes include acetylornithine aminotransferase, acetylornithine deacetylase, acetylornithine/succinyldiaminopimelate aminotransferase, N-acetylornithine carbomyltransferase and ornithine cyclodeaminase (Table 4, Tables 6S,7S and 8S). Importantly, all these enzymes may be involved in biosynthesis of potential precursors (ornithine, putrescene, proline, polyamines) of 2-AP.
Table 3.
Identified microbial groups associated with aroma enhancement in Basmati 370.
| Microorganisms | Functions |
|---|---|
| Actinobacteria | Phosphate solubilization, siderophores production, and nitrogen fixation, Phytopathogen inhibition |
| Proteobacteria | Nitrogen fixation, carbon and sulphur cycling, auxin synthesis, phosphate solubilization |
| Bacillus subtilis | IAA, nitrogen fixation, phosphate solubilization, siderophore production |
| Burkholderia | IAA synthesis, ACC deaminase, phosphate solubilisation |
| Enterobacter | IAA, N2 fixation, phosphate solubilisation |
| Klebsiella pneumoniae | Nitrogen fixation, IAA production |
| Micrococcus sp. | Phosphate solubilization, auxin production, 1-aminocyclopropane-1-carboxylate deaminase activity, and siderophore production |
| Pseudomonas sp. | IAA production, ammonia, phosphate solubilization, siderophore, HCN siderophore, HCN |
| Sinomonas | Phosphate solubilization, salt tolerance |
Table 4.
Enzymes responsible for biosynthesis of precursors of aroma in basmati rice.
| Enzyme | Function |
|---|---|
| Acetylornithine aminotransferase | Arginine metabolism, Lysine and arginine biosynthesis |
| Acetylornithine deacetylase | L-arginine biosynthesis via citrulline, ornithine & polyamine biosynthesis |
| Acetylornithine/succinyldiaminopimelate aminotransferase | Lysine biosynthesis pathway |
| N-acetyl ornithine carbomyltransferase | L-arginine biosynthesis |
| Ornithine cyclodeaminase | Ornithine and putrescine biosynthesis |
Discussion
The rhizosphere is a dynamic and biologically active root zone where various processes take place and serves as a site where organic matter is introduced through rhizodeposition and sloughed-off cells14. Microbial communities within this environment exhibit a high level of diversity and complexity16. These microorganisms play a crucial role in the decomposition of organic matter and the cycling of nutrients. Understanding the specific microorganisms involved in these processes is essential for comprehending biogeochemical cycles and the maintenance of the biosphere19. The present research concentrated on uncovering the rhizobacteria that play a crucial role in the synthesis of precursors associated with aroma, specifically targeting substances like ornithine, putrescine, proline, and polyamines. By elucidating the involvement of these bacteria, the study enhances our understanding of their impact on the biosynthetic processes that generate important aromatic constituents20. The metagenome assembly from short reads is exceptional and the largest contig identified in the assembly had a length of 45,769 bp. At taxonomic levels, the predominant microbial groups associated with the rhizosphere of basmati rice (Basmati 370) areas were Actinobacteria, Burkholderia, Enterobacteria, Proteobacteria, etc. Proteobacteria were found to be the most abundant at the phylum level in the basmati rice (Basmati 370) fields. In the rhizosphere of Basmati 370 and SJR 5 in the Samba area, Proteobacteria and Actinobacteria were found to be the most abundant at the phylum level. Actinobacteria, were more abundant in both the rice varieties rhizospheres in the Jammu area, while Burkholderia were more abundant in the Samba area. The overall bacterial rhizosphere community composition showed variation between Basmati 370 and SJR 5 varieties in different areas. This may be due to the production of variety specific exudates that influence the rhizosphere of specific locations38,39. The rhizobacteria are expected to exhibit a higher degree of adaptation to the rhizosphere of basmati rice (Basmati 370), in contrast to their presence in non basmati rice (SJR 5). This adaptation may be attributed to the unique environmental conditions and biochemical interactions present in the rhizosphere of basmati rice, which could favour the proliferation and activity of specific microbial communities. Previous reports have also indicated variations in bacterial community composition in different rhizospheres of rice crop10,21. Moreover, the dynamics of microbial community and associated functions changes in rhizosphere was also observed over periods of rice growth22,23.
The study revealed that the bacteria responsible for generating aroma were detected in the rhizospheres of Basmati 370 across all the samples gathered from three distinct regions. The sensory test demonstrated that Basmati 370 exhibited a fragrant essence across all three regions, whereas SJR 5 did not possess any aromatic qualities in the samples collected from these specific districts. Basmati 370 exhibited a distinct aroma across the three districts; however, there was a notable variance in the intensity of the aroma between the different locations. In another study, the sensory test showed a distinct difference in the strength of aroma among different aromatic rice varieties24. Due to more rhizobacteria in Jammu area, Basmati 370 had higher aroma content in Jammu area compared to Samba and Kathua areas. Deshmukh et al.25 also reported that the accumulation of the principal aroma compounds in the basmati rice variety is enhanced by the rhizosphere. The enhancement of soil quality is facilitated by the presence of rhizosphere, which has been proven to increase the uptake of nutrients in various studies26. Additionally, rhizosphere plays a significant role in improving the growth of plants and contributes to the aromatic properties of rice grains25.
In the rhizosphere of Basmati 370, a variety of rhizobacteria have been identified that could play a significant role in enhancing the aromatic qualities. These microbial communities interact with the plant roots and contribute to various biochemical processes that may influence the development of aroma compounds (Table 1). The analysis revealed that Proteobacteria were the most abundant (45%), followed by Actinobacteria (30%) and Burkholderia (7.50%) in the rhizosphere of Basmati 370 in Jammu district. In contrast, Actinobacteria (30%) exhibited higher abundance compared to Proteobacteria in the Kathua area. A similar trend was observed in the Samba district. Overall, the rhizobacteria in the Jammu area showed higher abundance compared to Samba and Kathua districts. This difference in abundance may be attributed to the rhizobacteria present in the basmati rice grown in the Ranbir Singh Pura area of Jammu district, which is known to possess a more pronounced aroma compared to other parts of the Jammu region.
A variety of enzymes play a crucial role in the metabolic pathways associated with precursors of aroma such as ornithine, putrescine, proline, and polyamines within bacterial systems. Additionally, the identification of novel sequences, contributed to the understanding of how various precursors can enhance the aroma in rice27. The identified precursors significantly contributed to the enhancement of aroma in basmati during the annotation process, thereby elevating the aromatic qualities of Basmati 370. Previous studies have also highlighted the identification of rhizobacterial isolates and the precursor L-ornithine in rice, contributing to the enhancement of its aromatic qualities7. The ability of these microbes to generate specific enzymes plays a crucial role in the enhancement of various aroma precursor compounds, including ornithine, putrescine, proline, polyamines, etc.20 Numerous studies have clarified the significance of ornithine, putrescine, proline, polyamines precursors in improving the aroma of rice20. In Lactobacillus hilgardii, the immediate precursor of aroma is 1-pyrroline, which is a product of proline catabolism28. Additionally, extensive studies on precursors have shown that in Bacillus cereus where aroma is enhanced through the acetylation of 1-pyrroline29. In rice and Pandanus amaryllifolius Roxb., the biological formation of aroma has been reported from proline or ornithine6,30. In rice, the carboxyl group of proline is substituted with an acetyl group, while the nitrogen present in the pyrroline ring of proline is retained as the nitrogen in the pyrroline structure.31 In addition, comprehensive research on precursors has demonstrated that the increase in aroma associated with proline or ornithine is facilitated by the crucial intermediate 1-pyrroline32. The active degradation product of ornithine, 4-aminobutanal, cyclizes to form 1-pyrroline 4-aminobutanal diethyl acetal is a stable form of 4-aminobutanal that can be hydrolyzed to release the free aldehyde33. Aroma is generated through the thermal transformation of 4-aminobutanal diethyl acetal, which is sourced from ornithine, proline, or putrescine. This process leads to the formation of 1-pyrroline, a compound that is subsequently processed by a bacterial system. Similarly, Lactobacillus sp. was used to enhance the aroma of rice28. Schieberle34 identified a similar metabolic pathway and illustrated how aroma compounds can be enhanced from proline and ornithine in equal proportions through the synthesis of 1-pyrroline. Fothergill and Guest35 also reported an equivalent pathway from ornithine precursor.
The identification of enzymes involved in the pathway of aroma enhancement by bacterial systems was achieved through the functional annotation of the sequences. The enzymes participating in the biosynthetic pathway of aroma production within bacterial systems were identified through a comprehensive functional annotation of the corresponding sequences. This process allowed for a detailed understanding of the enzymatic roles and their contributions to the enhancement of aroma in basmati (Basmati 370). Through the analysis of sequence data, the specific enzymes that facilitate the synthesis of aroma in bacterial organisms were successfully characterized. This functional annotation not only elucidates the enzymatic mechanisms at play but also enhances our overall comprehension of the metabolic processes involved in the aroma biosynthesis. The process of functional annotation revealed the identification of several enzymes linked to the enhancement of aroma in microbial systems (Table 4). These enzymes include acetylornithine aminotransferase, acetylornithine deacetylase, N-acetylornithine carbomyltransferase, acetylornithine/succinyldiaminopimelate aminotransferase, and ornithine cyclodeaminase. In the study, all the enzymes play a crucial role in the production of ornithine, putrescine, proline, and precursor molecules of polyamines, which are essential for enhancing aroma36. Various studies indicated that the enzymes such as acetylornithine aminotransferase, acetylornithine deacetylase, N-acetyl ornithine carbamoyltransferase, and ornithine cyclodeaminase play a significant role in the enhancement of aroma20,36. Different studies have supported the role of amino acids such as proline, putrescine, arginine, lysine, and ornithine as potential precursors aroma in rice37. Furthermore, Bacillus cereus strains were found to induce a significant amount of aroma when grown in a medium supplemented with proline, ornithine, and glutamic acid38. Similarly, Deshmukh et al.39 demonstrated that proline, ornithine, glutamic acid, and putrescine serve as the key precursors for aroma enhancement in aromatic rice. These studies highlight the intricate relationship between microbial metabolism and aroma production, suggesting that targeted manipulation of these precursors could lead to enhancement of aroma. Deshmukh et al.40 showed that the enhancement of aroma in Bacillus cereus is significantly influenced by the acetylation of a specific pyrroline precursor.
Overall, several rhizobacteria were identified from basmati rice like Actinobacteria, Bacillus subtilis, Burkholderia, Enterobacter, Klebsiella, Lactobacillus, Micrococcus, Pseudomonas and Sinomonas. These microorganisms are believed to contribute significantly to the enzymatic processes that facilitate the biosynthesis of key precursors of aroma, such as ornithine, putrescene, proline, and polyamines. Understanding the role of these rhizobacteria in enzyme production and precursor biosynthesis could provide valuable insights aimed at improving the aroma of basmati rice. This knowledge may lead to the development of strategies that harness these beneficial microorganisms to enhance basmati rice quality and yield. Besides the key enzymes that play a crucial role in the enhancement of aroma include acetylornithine aminotransferase, acetylornithine deacetylase, N-acetyl ornithine carbamoyltransferase, and ornithine cyclodeaminase. These enzymes are integral to the biochemical pathways that contribute to aroma in basmati rice. To further explore their potential, these identified rhizobacteria can be subjected to wet lab experiments to assess their suitability as plant growth-promoting rhizobacteria (PGPR) in basmati rice, with the aim of enhancing the region’s export potential.
Conclusion
Several types of bacteria were found in the rhizosphere of basmati rice such as Actinobacteria, Bacillus subtilis, Burkholderia, Enterobacter, Klebsiella, Lactobacillus, Micrococcus, Pseudomonas, and Sinomonas. These microorganisms are believed to play a significant role in enhancing the aroma and promoting plant growth-promoting bacteria activities in basmati rice. The study also found that key enzymes, such as acetylornithine aminotransferase, acetylornithine deacetylase, N-acetyl ornithine carbomyltransferase, acetylornithine aminotransferase, and ornithine cyclodeaminase, are associated with basmati rice. The activity of these enzymes plays a significant role in the biosynthesis of aroma precursors such as ornithine, putrescine, proline, and polyamines. These unique rhizobacteria should also be assessed for PGPR activities to harness their potential for enhancing both the aromatic qualities and the growth performance of basmati rice. The potential PGPRs can be further examined in field trials to determine their effectiveness in promoting the growth of rice crops, particularly basmati rice, which faces challenges due to pesticide residues. Additionally, these isolates can help restore the declining aroma quality of basmati rice. Utilizing beneficial microbes to enhance rice aroma by modifying plant metabolomics and promoting plant growth and health shows promise. However, it is crucial to conduct multi-location field trials and evaluate the interaction of these PGPRs with other native soil microflora.
Methods
Sample collection
The research was carried out at the School of Biotechnology, Sher-e-Kashmir University of Agricultural Sciences and Technology of Jammu, Jammu and Kashmir, India (SKUAST-Jammu), which is located at 32.69° N latitude and 74.65° E longitude. Rice cultivation in this region spans from the plains of Jammu, situated at an elevation of 200 m, to the mid and high hills reaching up to an altitude of 2300 m. The temperature ranges from 6 °C in winter to 45 °C in summer, while the annual precipitation varies between 700 and 1500 mm. The soil composition consists of sandy loam and clay loam with alkaline pH, and the area falls within the sub-tropical zone of the state. The climate is predominantly humid during the crop season, especially prior to the initiation of the panicle stage in the rice crop. The random sampling was done from three areas falling under basmati rice GI viz., Jammu, Kathua and Samba districts of Jammu region, Jammu and Kashmir, India as per Luster et al.41 (Table 9S).
Soil samples from the rhizosphere were obtained from two different rice varieties, namely Basmati 370 (basmati rice variety) and SJR-5 (non basmati rice variety), during the flowering stage of the crop. The rhizosphere samples were collected by a team of agriculture scientists from the farmers’ field in consultation with local farmers. Basmati 370 is a well-known basmati variety known for its attractive appearance and delightful aroma. Both varieties namely, Basmati 370 and SJR 5 have been developed and maintained by Sher-e-Kashmir University of Agricultural Sciences and Technology of Jammu. The cultivation of rice followed recommended agronomical practices. The rhizosphere samples were collected from five randomly selected plants in each replication, carefully uprooting the rice plant and collecting the complete root systems after removing loosely adhering soil (Fig. 19S). These samples were then stored at 4 °C. A total of 27 composite samples, consisting of three composite samples from three different locations were processed in triplicate in the laboratory. Additionally, a total of 6 composite samples, each comprising five soil samples mixed in equal proportions from each replication, were also processed in triplicate. To ensure the purity of the soil, it was sieved through a 2 mm sieve to remove any debris, pebbles, or plastic particles that could potentially interfere with the subsequent experiments. All methods were carried out in accordance with relevant guidelines and regulations.
Physicochemical analysis
The soil sample was subjected to physicochemical analysis using established protocols. Prior to the analysis, the soil samples were air dried and pulverized. To ensure uniformity, the samples were sieved through a 2 mm sieve to remove pebbles, stones, and other unwanted materials that could potentially interfere with the analysis procedures. The resulting homogeneous samples were then sealed in polythene bags for further analysis. The pH values of the samples were determined using a glass electrode pH meter in a soil-to-water ratio of 1:2.5 (w/v). The potassium content was determined using the flame photometric method42,43. The electrical conductivity (EC) was estimated in a 1:2.5 soil-to-water suspension using an EC meter (Spectrum Technologies, Inc.). Organic carbon (OC) was estimated using the wet oxidation method44. For the determination of available nitrogen (N), the alkaline permanganate method, modified from the Kjeldahl method45, was employed. The available phosphorus (P) was determined using the method described by Jackson46. On the other hand, the available potassium (K) and sulfur (S) were determined using the flame photometer (Systronics India Ltd.)47 and turbidimetric method48, respectively. To determine the micronutrients such as copper (Cu), zinc (Zn), iron (Fe), and manganese (Mn), an Atomic Absorption Spectrophotometer (AAS) model Z.2300 (Hitachi, Japan)49 was utilized, following standard methods.
Sensory test for aroma
To conduct sensory testing of aroma, leaf tissues from two different rice varieties, Basmati 370 and SJR 5, were utilized50. A total of six rice leaf samples were collected from various regions including Jammu, Samba, and Kathua. The classification of aroma was carried out through a sensory examination that involved the use of rice leaf tissues immersed in a 1.7% KOH solution. A panel consisting of five individuals who were non-smokers and non-alcoholics was formed. At the flowering stage of the rice varieties, approximately one gram of the first green leaf blade was obtained. Subsequently, the leaves from both varieties were cut into small pieces and placed in petri dishes. To initiate the aroma release, 5 mL of a 1.7% KOH solution was added to each petri dish, which were then kept at room temperature for 30 min. Then the petri dishes were opened and immediately smelled by the panel members. The panellists then rated the aroma samples based on scale (0–3) (Table 5)50.
DNA isolation and preparation of libraries
DNA was extracted from the collected rhizosphere soil samples using HiPura soil DNA purification (HiMedia Pvt. Ltd.). The quality and quantity of the DNA was assessed on the agarose gel and Nanodrop 2000. DNA quality and integrity was checked by 1% agarose gel electrophoresis. The Genomic libraries were prepared using TruSeq™ DNA Nano Illumina library preparation kit (Illumina, Inc. San Diego, CA USA). Final libraries were quantified using Qubit 4.0 Fluorometer (Thermo Fisher Scientific Inc.) using DNA HS assay kit. To identify the insert size of the library, it was queried on Tape station (Agilent Pvt. Ltd.). The concentration was calculated by comparing the area of the sample peak to the known concentration of the top marker. For sequencing, the libraries were pooled and desaturated. Library tubes were filled with libraries for the standard workflow. ExAmp/library mix was loaded onto the flow cell for the Illumina NovaSeq 6000 V1.5 (Illumina, Inc. San Diego, CA USA) and the run performance was monitored using BaseSpace “Sequence Hub”. Different primary metrics such as percent passing filter, aligned percentage, Q30- Q scoring and percent base were taken into consideration for quality results.
Sequence preprocessing and bioinformatic analysis
After the generation of raw sequencing data, the data underwent pre-processing and further analysis utilizing various bioinformatics methodologies. Prior to being loaded onto the Illumina NovaSeq 6000 v 1.5 sequencer flowcell, the libraries were subjected to library preparation and quality assessment procedures. Subsequently, the acquired data underwent quality control (QC) analysis utilizing fastp to ensure accuracy and reliability of the results. To ensure accuracy, the adapters were trimmed, and low-quality bases were eliminated using a sliding window approach. The metagenome assembly was carried out using MEGAHIT and QUAST software tools51. Binning, which involved grouping the assembled contigs based on GC content and coverage, was performed using MaxBin2 and MetaBat2 approaches52. The resulting bins were then consolidated and refined using the MetaWrap binning approach53. Refinement of the bins was conducted if the minimum completion of the bin exceeded 90% and the contamination level was below 30%. The final bin sets were reassembled using two modes, namely permissive and strict, utilizing the SPAdes Genome Assembler54. The reads were initially mapped to the finalized bins using BWA, and the extracted reads were segregated based on the number of single nucleotide polymorphisms (SNPs) in relation to the reference. For the strict mode, reads with less than 2 SNPs were considered, while for the permissive mode, reads with less than 5 SNPs were considered. The reads were individually assembled using SPAdes. The reassembled bins were subsequently reclassified by conducting a Mega-Blast search against the NCBI NT taxonomy database, and the hits were filtered to accurately classify the bins using TAXATOR-TK55.
Pathway annotation
The binning process allows to identify and determine the functional potential of microbial communities with a high level of taxonomic resolution56,57. To annotate the pathways, PROKKA software58 was used. Subsequently, the translated protein sequences were utilized as queries for homology searches via BLAST (https://www.pmc.ncbi.nlm.nih.gov/28451968/) and Hidden Markov Model (HMMER)59. The assembled contigs in the finalized bins were subsequently annotated to determine the gene-models they contained. The identification of open reading frames (ORFs) and RNA regions on the contigs, the conversion of ORFs into protein sequences, the search for protein homologs, and the creation of standard output files were all performed using PROKKA. The Prodigal program within PROKKA was employed to locate and translate genes. The translated protein sequences were then used as queries for homology searches against various public databases (CDD, PFAM, TIGRFAM) as well as PROKKA’s proprietary databases.
To visualize the results, a heatmap was generated using the gplots package60. The samples were then clustered using the hclust function with the ‘Ward’ method, considering different distances/dissimilarities such as Euclidean, Manhattan, and Bray–Curtis. These distances/dissimilarities were calculated using the vegdist function from the vegan package. The metagenome assembly was carried out using MEGAHIT and QUAST51. ANOSIM was employed to test for differences in population structure, utilizing Bray–Curtis dissimilarities within the vegan package61. Binning of the assembled contigs was performed based on GC content and coverage using the MaxBin2 and MetaBat2 approaches52. The resulting bins were further consolidated and refined using the MetaWrap binning approach53. All contig data were binned into metagenome assembled genomes (MAGs) with MetaWrap53 and subjected to the depiction of heatmap. The bins thus obtained were subjected to the calculation of completeness and contamination using CheckM (version 1.1.3)62. The CheckM helped to identify the best contigs among the binned contigs for each lineage, removed contamination and identified wrongly placed contigs to create the final bin set. Bins were considered to be refined if the minimum completion of the bin was > 90% and the contamination less than 30%. Finally, the reassembled bins were classified by conducting a Mega-Blast search against the NCBI NT taxonomy database and filtering the hits for accurate classification using TAXATOR-TK55. However, the data collected on various physicochemical parameters was analyzed using the statistical methodology proposed by Panse and Sukhatme63.
Supplementary Information
Acknowledgements
This study was supported by grants from the School of Biotechnology, Sher-e-Kashmir University of Agricultural Sciences and Technology of Jammu, India.
Author contributions
TKR: Methodology, Data recording, Data analysis, Data curation, Writing- Original draft preparation, Visualization, Investigation RKS: Conceptualization, Supervision, Visualization, Investigation, Software, MG: Validation, Writing- Reviewing and Editing. All authors have read and approved the final manuscript.
Funding
This work was supported by School of Biotechnology, Sher-e-Kashmir University of Agricultural Sciences and Technology of Jammu, India.
Data availability
Sequences of bacteria obtained were deposited in the GenBank nucleotide sequence database under accession no. MT361595 to MT361636. The datasets generated and/or analysed during the current study are available in the NCBI with accession No. PRJNA1016115.
Declarations
Competing interests
The authors declare no competing interests.
Ethical approval
The collection of rice rhizosphere resources and research activities has been conducted in compliance with the Regulations on Resident Instructions and duly approved by the Competent Authority of Sher-e-Kashmir University of Agricultural Sciences and Technology of Jammu, Main Campus, Chatha, Jammu, India.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-025-87889-6.
References
- 1.Okpala, N. E., Mo, Z., Duan, M. & Tang, X. The genetics and biosynthesis of 2-acetyl-1-pyrroline in fragrant rice. Plant Physiol. Biochem.135, 272–276. 10.1016/j.plaphy.2018.12.012 (2019). [DOI] [PubMed] [Google Scholar]
- 2.Yoshihashi, T., Huong, N. T. & Inatomi, H. Precursors of 2-acetyl-1-pyrroline, a potent flavor compound of an aromatic rice variety. J. Agric. Food Chem.50(7), 2001–2004. 10.1021/jf011268s.PMID:11902947 (2002). [DOI] [PubMed] [Google Scholar]
- 3.Salgotra, R.K., Bhat, J.A., Gupta, B.B. & Sharma, S. Determination of genetic relationship among basmati and non-basmati rice (Oryza sativa L.) genotypes from North-West Himalayas using microsatellite markers. Indian J. Biotech. 16, 68–75 (2017).
- 4.Hori, K. & Sun, J. Rice grain size and quality. Rice15, 33. 10.1186/s12284-022-00579-z (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Singh, R. et al. Aromatic Rices. Oxford and IBH; New Delhi, India. Small and Medium Grained Aromatic Rices of India; pp. 155–177 (2000).
- 6.Salgotra, R.K., Gupta, B.B., Bhat, J.A. & Sharma, S. Genetic diversity and population structure of basmati rice (Oryza sativa L.) germplasm collected from North Western Himalayas using trait linked SSR markers. PLoS One10(7): e0131858. 10.1371/journal.pone.0131858 (2015). [DOI] [PMC free article] [PubMed]
- 7.Deshmukh, Y., Khare, P. & Patra, D. Rhizobacteria elevate principal basmati aroma compound accumulation in rice variety. Rhizosphere1, 53–57 (2016). [Google Scholar]
- 8.Sandhu, S. S., Mahal, S. S. & Kaur, A. Physicochemical, cooking quality and productivity of rice as influenced by planting methods, planting density and nitrogen management. Int. J. Food Agric. Vet. Sci.5, 33–40 (2015). [Google Scholar]
- 9.Chinachanta, K., Shutsrirung, A., Herrmann, L., Lesueur, D. & Pathom-aree, W. Enhancement of the aroma compound 2-Acetyl-1-pyrroline in Thai Jasmine Rice (Oryza sativa) by rhizobacteria under salt stress. Biology10(10), 1065. 10.3390/biology10101065 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dhondge, H.V. et al. Exploring the core microbiota in scented rice (Oryza sativa L.) rhizosphere through metagenomics approach, Microb. Res.263, 127157. 10.1016/j.micres.2022.127157 (2022). [DOI] [PubMed]
- 11.Romanczyk, L. J., McClelland, C. A., Post, L. S. & Aitken, W. M. Formation of 2-Acetyl-1-pyrroline by several Bacillus cereus strains isolated from cocoa fermentation boxes. J. Agric. Food Chem.43(2), 469–475 (1995). [Google Scholar]
- 12.Shaikh, M. N. & Nadaf, A. B. Qualitative analysis of 2-Acetyl-1-pyrroline from the rhizosphere fungal species of basmati rice varieties by GC-FID. Int. J. Curr. Res.5(07), 1663–1665 (2013). [Google Scholar]
- 13.Chaparro, J. M. et al. Root exudation of phytochemicals in Arabidopsis follows specific patterns that are developmentally programmed and correlate with soil microbial functions. PLoS One8(2), e55731. 10.1371/journal.pone.0055731 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jasrotia, S., Salgotra, R.K. & Sharma, M. Efficacy of bioinoculants to control of bacterial and fungal diseases of rice (Oryza sativa L.) in northwestern Himalaya. Braz. J. Microb. 52(2), 687–704 (2021). [DOI] [PMC free article] [PubMed]
- 15.Cheng, Z. et al. Metagenomic and machine learning-aided identification of biomarkers driving distinctive Cd accumulation features in the root-associated microbiome of two rice cultivars. ISME Commun.3, 14. 10.1038/s43705-023-00213-z (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jasrotia, S., Salgotra, R.K. & Samnotra, R.K. Identification of basmati rice (Oryza sativa L.) rhizobacteria and their effect on plant growth traits for sustainable development in agriculture. Proc. Indian Natl. Sci. Acad.87, 469–486. 10.1007/s43538-021-00033-6 (2021).
- 17.Ngara, T. R. & Zhang, H. Recent advances in function-based metagenomic screening. Genom. Prot. Bioinf.16(6), 405–415 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ahmad, T. et al. Metagenomic analysis exploring taxonomic and functional diversity of bacterial communities of a Himalayan urban freshwater lake. PLoS One16(3), e0248116. 10.1371/journal.pone.0248116 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 19.Madsen, E. L. Microorganisms and their roles in fundamental biogeochemical cycles. Curr. Opin. Biotech.22, 456–464. 10.1016/j.copbio.2011.01.008 (2011). [DOI] [PubMed] [Google Scholar]
- 20.Zehra, A., Dhondge, H.V., Barvkar, V.T., Singh, S.K. & Nadaf, A.B. Evidence of polyamines mediated 2-acetyl-1-pyrroline biosynthesis in aromatic rice rhizospheric fungal species Aspergillus niger. Braz. J. Microbiol.54(4). 10.1007/s42770-023-01124-w (2023). [DOI] [PMC free article] [PubMed]
- 21.Okabe, A., Toyota, K. & Kimura, M. Seasonal variations of phospholipid fatty acid composition in the floodwater of a japanese paddy field under a long-term fertilizer trial. Soil Sci. Plant Nutr. 46. 10.1080/00380768.2000.10408773 (2000).
- 22.Barreiros, L., Manaia, C.M. & Nunes, O.C. Bacterial diversity and bioaugmentation in floodwater of a paddy field in the presence of the herbicide molinate. Biodegradation22. 10.1007/s10532-010-9417-1 (2011). [DOI] [PubMed]
- 23.Hussain, Q. et al. Microbial community dynamics and function associated with rhizosphere over periods of rice growth. Plant Soil Environ. 58; 10.17221/390/2010- pse (2012).
- 24.Hien, N. L., Yoshihashi, T., Sarhadi, W. A. & Hirata, Y. Sensory test for aroma and quantitative analysis of 2-Acetyl-1-Pyrroline in Asian aromatic rice varieties. Plant Prod. Sci.9(3), 294–297 (2006). [Google Scholar]
- 25.Deshmukh, Y., Khare, P. & Patra, D. D. Rhizosphere elevate principal basmati aroma compound accumulation in rice variety. Rhizosphere1, 53–57 (2016). [Google Scholar]
- 26.Glick, B. R. Plant growth-promoting bacteria: mechanisms and applications. Scientifica963401. 10.6064/2012/963401 (2012). [DOI] [PMC free article] [PubMed]
- 27.Keegan, K. P., Glass, E. M. & Meyer, F. MG-RAST, a Metagenomics service for analysis of microbial community structure and function. Methods Mol. Biol.1399, 207–233. 10.1007/978-1-4939-3369-3_13 (2016). [DOI] [PubMed] [Google Scholar]
- 28.Costello, P. J. & Henschke, P. A. Mousy off-flavor of wine: Precursors and biosynthesis of the causative N-heterocycles 2- ethyltetrahydropyridine, 2-acetyltetrahydropyridine, and 2-acetyl-1- pyrroline by LactoBacillus hilgardii DSM 20176. J. Agrl. Food Chem.50, 7079–7087 (2002). [DOI] [PubMed] [Google Scholar]
- 29.Adams, A. & Kimpe, N. Formation of pyrazines and 2-acetyl-1- pyrroline by Bacillus cereus. Food Chem.101, 1230–1238 (2007). [Google Scholar]
- 30.Thimmaraju, R., Bhagyalakshmi, N., Narayan, M., Venkatachalam, L. & Ravishankar, G. In vitro culture of Pandanus amaryllifolius and enhancement of 2‐acetyl‐1‐pyrroline, the major flavouring compound of aromatic rice, by precursor feeding of L‐proline. J. Sci. Food Agri. 85, 2527–2534 (2005).
- 31.Huang, T.C. et al. Biosynthetic mechanism of 2-acetyl-1-pyrroline and its relationship with Δ1-pyrroline-5-carboxylic acid and methylglyoxal in aromatic rice (Oryza sativa L.) callus. J. Agric. Food Chem. 56, 7399–7404. 10.1021/jf8011739 (2008). [DOI] [PubMed]
- 32.Ghosh, P. & Roychoudhury, A. Differential levels of metabolites and enzymes related to aroma formation in aromatic indica rice varieties: Comparison with non-aromatic varieties. 3 Biotech8, 1–13. 10.1007/s13205-017-1045-6 (2018). [DOI] [PMC free article] [PubMed]
- 33.Muhammad, I. et al. Post-transcriptional regulation of 2-acetyl-1-pyrroline (2-AP) biosynthesis pathway, silicon, and heavy metal transporters in response to Zn in fragrant rice. Front. Plant Sci. 13, 10.3389/fpls.2022.948884 (2022). [DOI] [PMC free article] [PubMed]
- 34.Schieberle, P. The role of free amino acids presents in yeast as precursors of the odorants 2-acetyl-1-pyrroline and 2- acetyltetrahydropyridine in wheat bread crust. Zeitschrift für Lebensmittel Untersuchung und Forschung191, 206–209 (1990). [Google Scholar]
- 35.Fothergill, J. & Guest, J. Catabolism of L-lysine by Pseudomonas aeruginosa. J. Gen. Microb.99, 139–155 (1977). [DOI] [PubMed] [Google Scholar]
- 36.Wei, X., Handoko, D. D., Pather, L., Methven, L. & Elmore, J. S. Evaluation of 2-acetyl-1-pyrroline in foods, with an emphasis on rice flavour. Food Chem.232, 531–544. 10.1016/j.foodchem.2017.04.005 (2017). [DOI] [PubMed] [Google Scholar]
- 37.Lina, G. & Min, Z. Formation and release of cooked rice aroma. J. Cereal Sci.107, 103523. 10.1016/j.jcs.2022.103523 (2022). [Google Scholar]
- 38.Deshmukh, Y., Nadaf, A.B., Khare, P. & Patra, D.D. HS‐SPME‐GC‐FID method for detection and quantification of Bacillus cereus ATCC 10702 mediated 2‐acetyl‐1‐pyrroline. Biotech. Progress30(6), 10.1002/btpr.1989 (2014). [DOI] [PubMed]
- 39.Renuka, N. et al. Co-functioning of 2AP precursor amino acids enhances 2-acetyl-1-pyrroline under salt stress in aromatic rice (Oryza sativa L.) cultivars. Sci. Rep. 12(1), 3911. 10.1038/s41598-022-07844-7 (2022). [DOI] [PMC free article] [PubMed]
- 40.Deshmukh, Y., Khare, P. & Patra, D. D. Mechanism of 2-Acetyl-1-Pyrroline biosynthesis by bacterial system. Int. J. Sci. Res. Publ.5(11), 2250–3153 (2015). [Google Scholar]
- 41.Luster, J., Gottlein, A., Nowack, B. &, Sarret G. Sampling, defining, characterising and modelling the rhizosphere-the soil science tool box. Plant Soil321, 457–482 (2009).
- 42.APHA. Standard Methods for the Examination of Water and Wastewater (23rd edition). Published by America Public Health Association (APHA). American Water Works Association, and Water Environment Federation (2017).
- 43.Arshi, I. & Khan, T. Analysis of soil quality using physicochemical parameters with special emphasis on fluoride from selected sites of Sawai Madhopur Tehsil Rajasthan. Int. J. Environ. Sci. Nat. Res.12(5), 555847 (2018). [Google Scholar]
- 44.Walkley, A. & Black, I. A. An examination of Degtjareff method for determining soil organic matter and a proposed modification of the chromic acid titration method. Soil Sci.37, 29–37 (1934). [Google Scholar]
- 45.Subbiah, B. V. & Asija, G. L. A rapid procedure for the estimation of available nitrogen in soils. Curr. Sci.25, 259–260 (1956). [Google Scholar]
- 46.Jackson, M.L. Soil Chemical Analysis. 134–165 (1967).
- 47.Jackson, M.L. Soil Chemical Analysis. 452–485 (1967).
- 48.Chesnin, L. & Yien, C. H. Turbidimetric determination of available sulphates. Soil Sci. Soc. of Am. J.15, 149–151 (1950). [Google Scholar]
- 49.Lindsay, W. L. & Norvell, W. A. Development of a DTPA soil test for zinc, iron, manganese, and copper. Soil Sci. Soc. of Am. J.42, 421–428 (1978). [Google Scholar]
- 50.Sood, B. C. & Siddiq, E. A. A rapid technique for scent determination in rice. Indian J. Genet. Plant Breed.38, 268–271 (1978). [Google Scholar]
- 51.Dinghua, L., Chi-Man Liu, Ruibang L., Sadakane, K. & Tak-Wah, L. MEGAHIT: an ultra-fast single-node solution for large and complex metagenomics assembly via succinct de Bruijn graph. Bioinformat.31(10), 1674–1676, 10.1093/bioinformatics/btv033 (2015). [DOI] [PubMed]
- 52.Kang, D.D. et al. MetaBAT 2: an adaptive binning algorithm for robust and efficient genome reconstruction from metagenome assemblies. Peer J.26, 7:e7359. 10.7717/peerj.7359 (2019). [DOI] [PMC free article] [PubMed]
- 53.Uritskiy, G. V., DiRuggiero, J. & Taylor, J. MetaWRAP—a flexible pipeline for genome-resolved metagenomic data analysis. Microbiome6, 158. 10.1186/s40168-018-0541-1 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bankevich, A. et al. SPAdes: a new genome assembly algorithm and its applications to single-cell sequencing. J. Comput. Biol.19(5), 455–477. 10.1089/cmb.2012.0021 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Droge, J., Gregor, I. & McHardy, A. C. Taxator-tk: precise taxonomic assignment of metagenomes by fast approximation of evolutionary neighbourhoods. Bioinformat.31(6), 817–824. 10.1093/bioinformatics/btu745 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Quince, C., Walker, A. W., Simpson, J. T., Loman, N. J. & Segata, N. Shotgun metagenomics, from sampling to analysis. Nat. Biotechnol.35, 833–844 (2017). [DOI] [PubMed] [Google Scholar]
- 57.Ejigu, F. G. & Jung, J. Review on the computational genome annotation of sequences obtained by Next-Generation Sequencing. Biology9(9), 295. 10.3390/biology9090295 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Seemann, T. Prokka: rapid prokaryotic genome annotation. Bioinformat.30(14), 2068–2069. 10.1093/bioinformatics/btu153 (2014). [DOI] [PubMed] [Google Scholar]
- 59.Wheeler, T.J. & Eddy, S.R. Nhmmer: DNA homology search with profile HMMs. Bioinformat. 29(19), 2487–2489 (2013). [DOI] [PMC free article] [PubMed]
- 60.Warnes, A.G.R. et al. Package Gplots: Various R Programming Tools for Plotting Data. Available at: http://cran.r-project.org/ (2015).
- 61.Clarke, K. R. Non-parametric multivariate analyses of changes in community structure. Aust. J. Ecol.18, 117–143 (1993). [Google Scholar]
- 62.Parks, D. H., Imelfort, M., Skennerton, C. T., Hugenholtz, P. & Tyson, G. W. CheckM: Assessing the quality of microbial genomes recovered from isolates, single cells, and metagenomes. Genome Res.25, 1043–1055 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Panse, V. G. & Sukhatme, P. V. Statistical Methods for Agricultural Workers (Publication and Information Division of ICAR, 2000). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Sequences of bacteria obtained were deposited in the GenBank nucleotide sequence database under accession no. MT361595 to MT361636. The datasets generated and/or analysed during the current study are available in the NCBI with accession No. PRJNA1016115.