Abstract
The vascular theory posits that glaucoma is associated to ocular blood flow alterations. However, there is uncertainty regarding the association of arterial stiffness index (ASI) and pulse pressure (PP) to intraocular pressure (IOP). This study investigates the relationship between ASI and PP, and IOP, using the UK Biobank population. Among 89,930 participants of the UK Biobank population, ASI and PP were assessed, and their correlations with IOP were estimated using multiple linear and logistic regressions adjusted for age, sex, BMI, diabetes, dyslipidemia, mean blood pressure, heart rate, tobacco smoking status, previous CV diseases and antihypertensive therapy. Youden indices were determined for ASI at a value of 9.41 m/s and for PP at 49.18 mmHg to determine IOP > 21mmHg. After adjustment for all covariates, ASI > 9.41 m/s was significantly correlated with IOP levels, B = 0.054 [0.031; 0.076] and with IOP > 21 mmHg, OR = 1.057 [1.004–1.113]. PP > 49.18 mmHg was significantly associated with IOP levels, B = 0.378 [0.345; 0.401] and with IOP > 21 mmHg, OR = 1.558 [1.474–1.648]. Similar results were observed when considering ASI and PP at continuous levels. When considering participants with IOP < 21 mmHg (N = 83,079), ASI > 9.41 m/s and PP > 49.18 mmHg were significantly associated with IOP levels, B = 0.015 [0.008; 0.021], B = 0.286 [0.266; 0.306], respectively. We demonstrated that increased ASI and PP levels are associated with increased IOP. These findings provide new insight into the vascular theory between IOP and ASI.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-025-05639-0.
Keywords: Arterial stiffness, Arterial stiffness index, Atherosclerotic pulse pressure, Intraocular pressure, Glaucoma
Subject terms: Eye diseases, Cardiology, Medical research
Introduction
Glaucoma is a complex disease characterized by progressive loss of retinal ganglion cells and damages to the optic nerve. It is a major public health treat, remaining the leading cause of irreversible blindness worldwide1. Two main theories have been proposed as biological mechanisms of open-angle glaucoma (OAG): the mechanical and vascular theories. The mechanical theory suggests that high intraocular pressure (IOP) puts mechanical stress on retinal ganglion cells, leading to irreversible damage. While elevated intraocular pressure (IOP) and vascular dysregulation are well-established mechanisms, recent studies highlight the role of neuroinflammation and neurotoxicity in POAG progression. Neuroinflammation involves the activation of microglia and astrocytes, releasing pro-inflammatory cytokines such as TNF-α and IL-1β, creating a toxic microenvironment that accelerates RGC loss2,3. Additionally, neurotoxicity, particularly glutamate excitotoxicity, leads to overstimulation of NMDA receptors, resulting in calcium overload, oxidative stress, and neuronal apoptosis4,5.
Among European individuals, the most common form of this disease is primary open-angle glaucoma (POAG)6. High IOP is a major modifiable risk factor for POAG7 with an increased risk of 16% for every mmHg increase in IOP8. Lowering IOP remains the only therapy to slow the progression of vision loss associated with POAG9. However, this theory fails to explain why some patients with lowered IOP do not experience slowed disease progression10. In contrast, the vascular theory posits that glaucoma is associated with alterations in ocular blood flow11 especially vascular dysfunction in normal-tension glaucoma12,13. This theory is supported by the fact that cardiovascular disease is a risk factor for OAG14,15. Vascular dysfunction, such as arteriosclerosis, can alter the microvascular environment of the optic disc and retinal, leading to vascular insufficiency16. Recent studies have shown that elevated pulse wave velocity (PWV) levels, a standard measurement of arterial stiffness (AS) are associated with structural damage progression in OAG10,17. AS, mainly correlated with atherosclerosis, inflammatory processes and hypertension18 is also estimated by arterial stiffness index (ASI)19 and pulse pressure (PP)20and could be considered as a main predictor of target organ damage19. To date, uncertainty remains regarding the association of ASI and PP with IOP. Therefore, this cross-sectional study investigated the relationship between ASI, PP and IOP (1) in the overall study population, (2) according to IOP lower or higher to 21 mmHg, and (3) according to glaucoma disease status, using the UK Biobank population.
Methods
UK biobank population
The UK Biobank cohort initially comprised 9.1 million eligible individuals, but 8.6 million either did not respond or did not provide consent. Consequently, at baseline, the UK Biobank included 502,478 Britons across 22 UK cities, drawn from the UK National Health Service Register between 2006 and 2010. These participants, aged between 38 and 73 years, represented 5.5% of the total UK Biobank cohort. Participants completed questionnaires; a computer-assisted interview; and underwent physical and functional measurements, as well as providing blood, urine, and saliva samples21. Data collected included personalized information from the participants, encompassing socio-economic, behavioral and lifestyle factors; a mental health batter: clinical diagnoses and therapies; genetics; imaging; and physiological biomarkers from blood and urine samples. The details of the cohort protocol are available in the literature22.
Ethical considerations
All participants provided electronic informed consent, and the UK Biobank received ethical approval from the North-West Multi-centre Research Ethics Committee (MREC) covering the whole of the UK. The study was conducted in accordance with the guidelines of the Declaration of Helsinki and approved by the Northwest – Haydock Research Ethics Committee (protocol code: 21/NW/0157, date of approval: 21 June 2021). For details: https://www.ukbiobank.ac.uk/learn-more-about-uk-biobank/about-us/ethics.
Study population
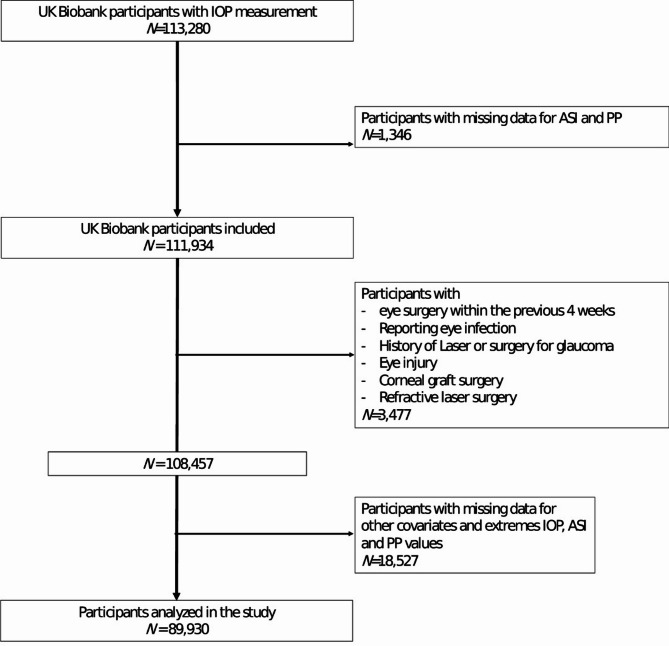
Among the 113,280 volunteers of the UK Biobank with IOP measurement, 1,346 were excluded due to missing data for ASI and PP. Subsequently, 23,350 were excluded due to a history of eye disorders/surgery/laser and missing data for other covariates, and extreme values of IOP, ASI and PP. Therefore, we analyzed data from 89,930 volunteers (Fig. 1).
Fig. 1.
Flowchart.
Intraocular pressure (IOP)
Ophthalmic assessment was not part of the original baseline assessment but was introduced as an enhancement in 2009 at six assessment centers across the UK (Liverpool and Sheffield in North England, Birmingham in the Midlands, Swansea in Wales, and Croydon and Hounslow in Greater London). Self-reported glaucoma status was ascertained by participants who selected “glaucoma” from a list of eye disorders in response to the question, “Has a doctor told you that you have any of the following problems with your eyes?”.
Participant IOP was measured once for each eye using the Ocular Response Analyzer (ORA; Reichert, Corp., Buffalo, NY). Participants who reported eye surgery within the previous four weeks or those with an eye infection were precluded from having IOP measured. The ORA is a non-contact tonometer that measures the force required to flatten the cornea using a jet of air. Unlike conventional non-contact tonometry, the ORA measures two pressures; firstly, when the cornea flattens on inward motion, and secondly when the cornea is flattened on outward motion. The average of these two pressures has been calibrated to derive a Goldmann-correlated IOP (IOPg), and the difference between these two pressures has been shown to be related to the biomechanical properties of the cornea23. A linear combination of these two pressures has been developed to derive a corneal-compensated IOP (IOPcc)24. We used IOPcc in our analyses as it is thought to provide the most accurate assessment of true IOP and is least affected by corneal properties25.
We excluded participants with a history of laser or surgery for glaucoma, eye injury, corneal graft surgery, or refractive laser surgery, as these participants are likely to have IOP altered from physiological levels. To handle extreme values of IOP, we excluded IOP measurements in the top and bottom 0.5 percentiles.
A significant proportion of participants with the highest IOPs in the cohort will have been diagnosed and treated with IOP-lowering medication in the community before entering the current study. Data for pre-treatment IOP were not available, and excluding these participants would have truncated the study’s IOP distribution, thereby reducing statistical power for detecting associations with IOP. We therefore imputed pre-treatment IOP: in study participants reporting current IOP-lowering medication (N = 670), the measured IOP was divided by 0.7 based on the mean IOP reduction achieved by medication. This method has been used in previous studies for IOP26–28. Participant IOP was calculated as the mean of right and left eye values for each participant with data available for both eyes. If data were only available for one eye, that value was considered the participant’s IOP.
Arterial stiffness index (ASI)
Pulse wave arterial stiffness index (ASI) was estimated using a non-invasive method during a volunteer’s visit to a UK Biobank Assessment Centre. Peripheral blood volume was measured by attaching a photoplethysmograph transducer (PulseTrace PCA 2™, CareFusion, USA) to the volunteer’s resting finger, preferably the index finger of the non-dependent hand, although it could be placed on any finger29. Volunteers were asked to breathe in and out slowly five times in a relaxed fashion, and readings were taken over 10–15 s. The carotid-to-femoral pulse transit time was estimated from the dicrotic waveform as the time difference between a forward compound, when the pressure is transmitted from the left ventricle to the finger and a reflected or backward compound as the wave travels from the heart to lower body via the aorta30. ASI was calculated in meters per second (m/s) as: H/PTT, where H is the individual’s height, and PTT is the pulse transit time or the peak-to-peak time between the systolic and diastolic wave peaks in the dicrotic waveform30. This methodology has been validated by independent studies comparing it with carotid femoral PWV, concluding that both measurement methods are highly correlated30–33. ASI is a simple, operator-independent, non-expensive, and rapid method. Extreme outlier ASI values were excluded from the analyses, defined as top and bottom 0.5% extreme values34,35.
Pulse pressure (PP)
Systolic and diastolic blood pressures (SBD, DBP) were measured twice at the assessment center using an automated BP device (Omron 705 IT electronic blood pressure monitor; OMRON Healthcare Europe B.V. Kruisweg 577 2132 NA Hoofddorp), or manually with a sphygmomanometer and an inflatable cuff in association with a stethoscope if the blood pressure device failed to measure BP or if the largest inflatable cuff of the device did not fit around the individual’s arm.
The participant was seated in a chair for all the measurements, which were carried out by nurses trained in performing BP measurements on the left upper arm36. Multiple available measures for each participant were averaged. The Omron 705 IT BP monitor has met the Association for the Advancement of Medical Instrumentation SP10 standard and was validated by the British Hypertension Society protocol, with an overall “A” grade for both SBP and DBP37. However, automated devices tend to measure higher BP compared to manual sphygmomanometers. Therefore, both SBP and DBP measured using the automated device were adjusted using algorithms by Stang et al.38:
For SBP, the following algorithm was performed:
 |
For DBP, the following algorithm was performed:
 |
Pulse pressure (PP) was calculated as: SBP – DBP.
Extreme outlier PP values were excluded from the analyses (defined as top and bottom 0.5% extreme values)39.
Laboratory and clinical parameters
Dyslipidemia was defined as having a fasting plasma total-cholesterol ≥ 6.61 mmol/L, LDL cholesterol ≥ 4.1 mmol/L, triglycerides level > 1.7 mmol/, or taking statins medication. Diabetes status was defined on either receiving anti-diabetic medication, being diabetes diagnosed by a doctor, or having a fasting glucose concentration ≥ 7 mmol/L. Medications were identified through the question: “Do you regularly take any of the following medications?”.
Biological parameters were detailed in the UK Biobank protocol40.
Body mass index was calculated as weight (in kg) divided by heigh2 (meter) and categorized as high (BMI > 30 kg/m2), moderate (BMI between 25 and 30 kg/m2) and low (less than 25 kg/m2).
Current tobacco smokers were defined as participants who responded “yes, on most or all days” or “yes, only occasionally” at the question “do you smoke tobacco now”.
CV diseases were defined by heart attack, angina, and stroke, as diagnosed by a doctor and reported in questionnaires.
Statistical analysis
Characteristics of the study population were described as the means with standard deviation (SD) for continuous variables, and as numbers and proportions for categorical variables. This study explored the correlations between ASI and PP with IOP levels.
Correlations between ASI, PP, and IOP levels were examined using multiple linear regression models, computing regression coefficients (B) and their 95% confidence interval (95% CI), followed by multiple logistic regression models with Odds ratio (OR) and 95% confidence interval for IOP > 21mmHg41. Based on the univariable logistic regression, the maximum Youden index, calculated as:
 |
was chosen to determine the optimal decision thresholds (c) for discrimination for both ASI and PP according to IOP > 21mmHg. Multiple linear and logistic regression models were applied with these Youden indices. Firstly, models were adjusted for age (Model 1). Secondly, models were adjusted for Model 1 + sex, BMI, diabetes, dyslipidemia, mean blood pressure, heart rate, tobacco smoking status, previous CV diseases and antihypertensive therapy (Model 2). Antihypertensive medications were characterized by the question: “Do you regularly take any of the following medications?”. Cardiovascular diseases were defined by myocardial infarction, angina, and stroke as diagnosed by a clinician and reported in questionnaires. Statistics were performed using SAS software (version 9.4; SAS Institute, Carry, NC). A p value < 0.05 was considered statistically significant.
Results
The characteristics of the study population (N = 89,930) are described in Table 1. The study population comprised 1.32% of participants declaring glaucoma disease and 0.75% who were on IOP-lowering medication. In the overall study population, 7.62% had IOP levels higher to 21 mmHg.
Table 1.
Characteristics of the study population.
| Population N = 89,930 |
||
|---|---|---|
| Age (years) | 56.88 | 8.07 |
| IOP (mmHg) | 16.06 | 3.40 |
| IOP > 21 mmHg | 6851 | 7.62% |
| Glaucoma | 1186 | 1.32% |
| IOP lowering medication | 670 | 0.75% |
| ASI (m/s) | 9.40 | 2.90 |
| Pulse pressure (mmHg) | 50.61 | 12.38 |
| Women | 48,136 | 53.18% |
| BMI (kg/m2) | 27.45 | 4.78 |
| SBP (mmHg) | 132.37 | 16.90 |
| DBP (mmHg) | 81.76 | 8.24 |
| Mean BP (mmHg) | 98.63 | 10.32 |
| ASPP-R | 4.61 | 1.84 |
| Heart rate (bpm) | 68.51 | 10.91 |
| Antihypertensive therapy | 19,491 | 21.67% |
| Diabetes | 6812 | 7.57% |
| Dyslipidemia | 51,523 | 57.29% |
| Previous CV disease | 4893 | 5.45% |
| Tobacco smoking | ||
| Current | 8916 | 9.91% |
| Past | 31,230 | 34.73% |
| Never | 49,784 | 55.36% |
ASPP-R: arterial stiffness – pulse pressure – ratio.
When considering the univariable relationship between ASI and IOP > 21mmHg, the Youden index was defined for ASI at the value of 9.41 m/s, p < 0.001 and for PP at the value of 49.18 mmHg, p < 0.001. Participants with IOP > 21mmHg showed higher ASI levels (9.74 (3.04) vs. 9.38 (2.89), p < 0.001) and higher PP levels (55.33 (12.78) vs. 50.23 (12.27), p < 0.001) than others. The same results were observed when considering glaucoma disease status according to IOP levels (Supplementary file 1).
Univariable linear relationship are described in Figs. 2 and 3, and according to the overall study population, the categorization of IOP lower or higher to 21mmHg, and according to the glaucoma disease status.
Fig. 2.
Univariable linear regressions between ASI and PP with IOP in overall study population and according to IOP levels status.
Fig. 3.
Univariable linear regressions between ASI and PP with IOP in population according to glaucoma status and IOP levels status.
In the overall study population, after adjustment for Model 1, ASI was significantly correlated with IOP (B = 0.025 [0.017;0.033]). After adjustment for all covariates (Model 2), ASI remained significantly correlated with IOP (B = 0.018 [0.011;0.026]) and significantly associated with IOP > 21mmHg (OR = 1.018 [1.009–1.027]). The same results were observed when considering ASI > 9.41 m/s with B = 0.051 [0.029;0.074] and OR = 1.057 [1.004–1.113] after adjustment for Model 2 (Table 2).
Table 2.
Linear and logistic regression models for the relationship between ASI and PP with IOP levels and IOP > 21mmHg in the overall study population.
| IOP levels N = 89,930 |
IOP > 21mmHg N = 89,930 |
|||
|---|---|---|---|---|
| Model 1 | Model 2 | Model 1 | Model 2 | |
| ASI levels | 0.025 (0.017;0.033) | 0.018 (0.011;0.026) | 1.021 [1.011–1.031] | 1.018 [1.009–1.027] |
| ASI > 9.41 m/s | 0.051 (0.029; 0.074) | 0.054 (0.031;0.076) | 1.071 [1.018–1.126] | 1.057 [1.004–1.113] |
| PP levels | 0.041 (0.039;0.043) | 0.039 (0.036;0.041) | 1.023 [1.021–1.025] | 1.021 [1.019–1.023] |
| PP > 49.18mmHg | 0.421 (0.398;0.444) | 0.378 (0.354;0.401) | 1.655 [1.567–1.747] | 1.558 [1.474–1.648] |
In the overall study population (N = 89,930), after adjustment for Model 1, PP was significantly correlated with IOP (B = 0.041 [0.039;0.043]). After adjustment for all covariates (Model 2), PP remained significantly correlated with IOP (B = 0.039 [0.036;0.041]) and significantly associated with IOP > 21mmHg (OR = 1.021 [1.019–1.023]). The same results were observed when considering PP > 49.18 mmHg with B = 0.378 [0.354;0.401] and OR = 1.558 [1.474–1.648] after adjustment for Model 2 (Table 2).
When considering participants with IOP < 21mmHg (N = 83,079), ASI was significantly correlated with IOP (for Model 2, B = 0.015 [0.008; 0.021]). The same results were observed for ASI > 9.41 m/s with IOP (for Model 2, B = 0.041 [0.022; 0.060]), with PP with IOP (for Model 2, B = 0.029 [0.027;0.030], and with PP > 49.18 mmHg with IOP (for Model 2, B = 0.275 [0.255; 0.294]) (Table 3). No significant adjusted linear associations were observed between ASI and PP with IOP among participants with IOP > 21mmHg (N = 6,851) (Table 3). However, nonlinear relationships were observed between ASI with IOP (IOP = log(2.956 + 3.156*ASI), p = 0.015) and between PP and IOP (IOP = 21.851 + 0.368*log(PP), p < 0.001) among participants with IOP > 21mmHg.
Table 3.
Linear regression models for the relationship between ASI and PP with IOP levels and IOP > 21mmHg in the population with IOP < 21mmHg and with IOP > 21mmHg.
| IOP < 21mmHg N = 83,079 |
IOP > 21mmHg N = 6,851 |
|||
|---|---|---|---|---|
| Model 1 | Model 2 | Model 1 | Model 2 | |
| ASI levels | 0.017 (0.010;0.023) | 0.015 (0.008;0.021) | 0.006 (−0.012;0.023) | 0.003 (−0.015;0.022) |
| ASI > 9.41 m/s | 0.035 (0.016;0.054) | 0.041 (0.022;0.060) | 0.019 (−0.034;0.737) | 0.009 (−0.046;0.063) |
| PP levels | 0.030 (0.029;0.032) | 0.029 (0.027;0.030) | 0.003 (−0.002;0.007) | 0.001 (−0.004;0.005) |
| PP > 49.18mmHg | 0.309 (0.290;0.329) | 0.286 (0.266;0.306) | 0.007 (−0.051;0.066) | 0.023 (−0.036;0.083) |
When considering glaucoma disease status, only significant correlations were observed in non-glaucoma participants with IOP < 21mmHg (N = 82,516), between ASI and IOP levels (for Model 2, B = 0.015 [0.008; 0.021]), between ASI > 9.41 m/s and IOP levels (for Model 2, B = 0.042 [0.023; 0.061], between PP and IOP levels (for Model 2, B = 0.029 [0.027; 0.031]) and between PP > 49.18 mmHg and IOP levels (for Model 2, B = 0.288 [0.268; 0.308]) (Table 4). No correlation was observed between IOP levels and ASI or PP in participants with glaucoma with IOP > 21mmHg (N = 623), participants with glaucoma with IOP < 21mmHg (N = 563), or in participants with non-glaucoma disease and IOP > 21mmHg (N = 6,228) (Table 4). However, when considering the univariable correlation between PP with IOP, PP showed a significant nonlinear correlation with IOP in non-glaucoma participants with IOP > 21mmHg (log(IOP) = 3.104 + 0.004*Sprt(PP), p < 0.001) and a linear relationship in glaucoma participants with IOP < 21mmHg (p = 0.022) (Fig. 3). When considering the univariable correlation between ASI with IOP, ASI showed a significant nonlinear relationship with IOP in non-glaucoma participants with IOP > 21mmHg (IOP = log(1.74 + 2.53*ASI), p = 0.023) and a trend towards a linear relationship between ASI and IOP in glaucoma participants with IOP < 21mmHg (p = 0.094) (Fig. 3).
Table 4.
Linear regression models for the relationship between ASI and PP with IOP levels and IOP > 21mmHg in the population of glaucoma or not, according to the IOP level.
| Glaucoma with IOP < 21mmHg N = 563 |
Glaucoma with IOP > 21mmHg N = 623 |
|||
|---|---|---|---|---|
| Model 1 | Model 2 | Model 1 | Model 2 | |
| ASI levels | 0.031 (−0.045;0.107) | 0.016 (−0.062;0.094) | 0.033 (−0.043;0.109) | 0.019 (−0.059;0.097) |
| ASI > 9.41 m/s | 0.126(−0.102;0.354) | 0.087 (−0.146;0.321) | 0.138 (−0.086;0.362) | 0.124 (−0.107;0.355) |
| PP levels | 0.009 (−0.010;0.028) | 0.009 (−0.010;0.029) | 0.008 (−0.011;0.026) | 0.005 (−0.013;0.025) |
| PP > 49.18mmHg | 0.185 (−0.051;0.422) | 0.169 (−0.081;0.419) | 0.031 (−0.212;0.274) | 0.062 (−0.1844;0.309) |
| No glaucoma with IOP < 21mmHg N = 82,516 |
No glaucoma with IOP > 21mmHg N = 6,228 |
|||
|---|---|---|---|---|
| Model 1 | Model 2 | Model 1 | Model 2 | |
| ASI levels | 0.017 (0.010;0.023) | 0.015 (0.008;0.021) | 0.002 (−0.014;0.20) | 0.006 (−0.013;0.022) |
| ASI > 9.41 m/s | 0.034 (0.015;0.053) | 0.042 (0.023;0.061) | 0.011 (−0.040;0.062) | 0.040 (−0.013;0.093) |
| PP levels | 0.031 (0.028;0.032) | 0.029 (0.027;0.031) | 0.005 (−0.001;0.009) | 0.003 (−0.001;0.008) |
| PP > 49.18mmHg | 0.311 (0.291;0.330) | 0.288 (0.268;0.308) | 0.041 (−0.015;0.097) | 0.018 (−0.039;0.075) |
Collinearities between continuous variables are described in supplementary file 2.
Supplementary file 3 showed the associations of the confounding factors with IOP levels.
Sensitivity analysis
The correlation analysis reveals a weak positive association between ASI and PP (r = 0.1361), suggesting that these two parameters capture distinct but related aspects of vascular function. Thus, in the overall study population (N = 89,930), after adjustment for all covariates (Model 2), when considering both ASI and PP in the same model, ASI and PP remained significantly correlated with IOP (for ASI, B = 0.016 [0.008;0.023] and for PP, B = 0.013 [0.011;0.016]) and significantly associated with IOP > 21mmHg (for ASI, OR = 1.012 [1.003–1.018] and for PP, OR = 1.011 [1.007–1.014]).
Given this relationship, we further developed the “Arterial Stiffness and Pulse Pressure Ratio” (ASPP-R):
 |
This index aims to provide a more integrated measure of arterial stiffness and vascular load, reflecting both the mechanical properties of the arterial wall and the hemodynamic burden, while accounting for overall blood pressure levels. This approach could enhance the predictive value for cardiovascular outcomes, particularly when both parameters are considered in a combined model.
Thus, in the overall population, after adjustment for all covariates, the ASPP-R remained significantly correlated with IOP (B = 0.077 [0.064;0.091]) and significantly associated with IOP > 21mmHg (OR = 1.051 [1.036–1.065]).
Discussion
This cross-sectional study demonstrated that among a general middle-aged population, ASI and PP were significantly correlated with IOP levels. These findings align with previous studies indicating a positive relationship between PP and IOP25,42,43 and between AS, particularly determined by PWV, and IOP10,44. In line with the vascular theory, we found that the relationship between ASI and PP primarily occurred among normal IOP participants12,13. Furthermore, this study identified that the cutoff of ASI values was 9.41 m/s and that the cutoff of PP values was 49.18 mmHg to determine IOP > 21mmHg. This finding is consistent with some European consortiums which have reported normal references for PWV, as values under the cut-off of 10 m/s45 and with PP indicating normal values under 50 mmHg46.
Arterial stiffness and intraocular pressure
Arterial stiffness is a promising therapeutic target in response to vascular aging47. It occurs when its cells and extracellular matrices undergo several biological modifications, potentially leading to an overall compromise of its flexibility and rigidity. As IOP increases, vessels may be unable to maintain perfusion, leading to optic nerve damages. High pulse pressure, in association with arterial stiffness, could increase the risk of OAG16. This theory suggests that vascular system could be a risk factor of elevated IOP in glaucoma48. Thus, previous investigations have shown that stiffness observed in tissues related to glaucoma was associated with increased IOP49. Furthermore, as observed in this study, some patients might not develop glaucoma despite the presence of elevated IOP50. Numerous clinical studies directly measuring systemic and ocular fluid dynamics of blood flow have confirmed the correlation of vascular dysregulation with many risk factors for glaucoma51.
Pathophysiological relationship between arterial stiffness and intraocular pressure
An increase in IOP can be caused by several resistances to the flow of aqueous humor through the trabecular outflow pathway52. Dysregulation of this tissue through various mechanisms may disrupt the flow of the aqueous humor, potentially initiating an increase in IOP53. A previous study showed that the glaucomatous trabecular meshwork was generally stiffer than that of normal subjects54. Systemic vascular involvement can manifest as trabecular vascular damage leading to an elevation of intraocular pressure, thereby causing reduced permeability of the trabeculum within the eye55.
Moreover, some studies have suggested a possible interaction between arterial stiffness and the peripapillary sclera surrounding the optic nerve head (ONH)53particularly in glaucoma49. The sclera, being the primary load-bearing structure of the eye, responds biomechanically to increased IOP, thereby modulating the extent of force applied to the ONH56. Fundamental investigations have indicated that the structure of the sclera may change in response to elevated blood pressure and increased arterial stiffness56.
We hypothesize that arterial stiffness, indicated by high values of ASI or PP, may lead to the disruption of ocular autoregulation with the loss of axonal fibers due to chronic circulatory failure of the optic nerve head and transient vascular spasms57. Arterial stiffness is a major risk factor for cardiovascular diseases58. Pulse wave velocity (PWV) has traditionally been used as the gold standard for determining arterial stiffness, but it depends critically on the accurate placement of transducers over the arteries and is both time-consuming and complex59. For quantification of arterial stiffness, we assessed ASI and PP as valuable alternatives to PWV, applicable to large-scale epidemiological studies19. The reason why ASI may be significant is a question worthy of further research. Vascular endothelial cells produce and release numerous vasoactive components, which can play a significant role in maintaining vascular functions, including vasodilation, inhibition of platelet adhesion, and the proliferation of vascular smooth muscle cells60. Previous investigations have shown that many risk factors, including age, glycemia, high blood pressure, blood lipid level imbalance, may lead to vascular endothelial damage, which in turn leads to an imbalance of vasoactive factors produced by endothelial cells. These factors may lead in changes in the structure of vascular wall, resulting in decreased vascular compliance61. The reduction in vascular compliance leads to a dysregulation of endothelial dysfunction, which occurs earlier than atherosclerotic plaque formation62. A high ASI level indicates poorer arterial elasticity63and the loss of arterial elasticity could be a risk factor for glaucoma, contributing of the elevation of IOP10,16.
Subgroup inconsistencies
The observed variability in the effect sizes and significance levels of ASI and PP across different subgroups in this study requires further consideration. In particular, the lack of significant associations in participants with glaucoma and IOP > 21 mmHg may reflect distinct pathophysiological mechanisms within this subgroup. Elevated IOP, a hallmark of glaucoma, can significantly alter ocular blood flow and retinal perfusion, potentially masking the effects of systemic arterial stiffness64,65. Additionally, the influence of IOP on optic nerve head biomechanics and axonal transport may further complicate the relationship between systemic vascular measures and intraocular pressure regulation66. The subgroup-specific variability highlights the need for more precise phenotyping in future studies, including the consideration of factors such as baseline IOP, duration of glaucoma, and the presence of vascular comorbidities. It also suggests that systemic vascular markers like ASI and PP may have limited predictive value in certain high-risk populations unless combined with more targeted ocular measurements.
Furthermore, these findings underscore the importance of integrating multimodal assessments, including retinal imaging and ocular perfusion metrics, to capture the full spectrum of vascular dysfunction in glaucoma.
Arterial stiffness and pulse pressure ratio (ASPP-R)
The introduction of the ASPP-R as a novel composite measure offers a promising approach for evaluating vascular health. Traditional measures like ASI and PP, while informative, capture only partial aspects of arterial function. ASI primarily reflects the mechanical stiffness of the arterial wall, which is influenced by structural changes such as collagen deposition and elastin fragmentation19,67 while PP represents the dynamic hemodynamic load exerted on the arterial system68. By combining these two parameters and normalizing by MAP, the ASPP-R provides a more comprehensive index that incorporates both the mechanical properties and the pressure dynamics of the arterial system, potentially enhancing its predictive power for adverse cardiovascular outcomes.
Several studies have demonstrated that increased ASI is independently associated with higher cardiovascular risk, including hypertension, stroke, and heart failure69. Similarly, elevated PP has been linked to greater arterial stiffness and higher all-cause mortality70. However, these markers often capture overlapping but distinct physiological processes, which may explain the relatively modest correlation observed in this study (r = 0.1361). The ASPP-R, by integrating these components, may offer a novel assessment of vascular risk.
Moreover, the normalization by MAP accounts for the overall blood pressure context, reducing the influence of extreme pressure values and potentially improving the specificity of the index for detecting early vascular dysfunction58. This approach aligns with recent evidence suggesting that composite indices combining multiple hemodynamic parameters may provide superior risk stratification in diverse populations71.
Future studies should focus on validating the ASPP-R in larger, prospective cohorts and across different clinical settings to establish its predictive value for long-term cardiovascular outcomes. Additionally, integrating this index with emerging biomarkers of vascular health, such as central aortic pressure and wave reflection indices, could further refine its clinical utility72.
Limitations
The principal strength of this investigation is its very large sample size. Moreover, the use of the Pulse Trace device to measure AS accounts for greater variability in ASI values compared to other available devices73. The UK Biobank study had a low responses rate of 5.5%, and potential volunteer bias may be present. However, given the large sample size and high internal validity of the UK Biobank protocol, these limitations are unlikely to significant impact the observed associations74,75. Additionally, the study cohort consisted of middle-aged English participants, limiting the generalizability of the results to other age groups and ethnicities. The UK Biobank relied on standardized protocols for collecting anthropometric data, ensuring the reproductibility of data collection regardless of when, where, and by whom the measurements were taken. However, our study has several limitations: the ASI values obtained using the UK Biobank methodology were not the gold standard, such as PWV, and do not accurately measure central arterial stiffness. This could bias the observed results. Nonetheless, this ASI measurement has been validated by three independent studies in comparison with PWV, with these studies concluding that both measures are largely correlated31–33. The self-reported nature of glaucoma diagnoses may affect the observed correlations due to potential misclassification errors. Moreover, no specific information was added concerning the severity of glaucoma and the evolution of the disease. Another significant limitation of our study was that only one IOP measurement was performed for each volunteer, making the data more susceptible to measurement errors than if multiple measurements had been taken. However, the standard deviation for IOP was 3.4 mmHg, which is close to that of a previous study that used an average of three measurements, with a standard deviation of 3.7 mmHg76. While the photoplethysmography (PPG) transducer used in this study provides a practical, non-invasive approach for measuring carotid-to-femoral pulse transit time (PTT) and estimating ASI, it also has several inherent limitations. Notably, the use of a single-site PPG sensor may introduce measurement inaccuracies due to factors such as variable arterial wall thickness, sensor positioning, and signal quality77. Additionally, single-site PPG assessments may be less accurate than multi-site measurements, which capture the full propagation of the pulse wave, potentially leading to variability in ASI estimation78.
Conclusion
In conclusion, we observed in this large middle-aged population that arterial stiffness, as measured by ASI and PP, was correlated with IOP, particularly among participants with normal IOP levels. Moreover, we found that the thresholds for ASI and PP indicating high IOP values (exceeding 21 mm Hg) were close to the range of values observed for cardiovascular diseases, such as coronary artery disease. These findings offer insights into the vascular theory through the demonstrated link between IOP and arterial stiffness.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
This research has been conducted using the UK Biobank Resource under Application Number 103608.
Author contributions
Conceptualization: AV, formal analysis: AV, writing – original draft preparation: A.V, editing and writing: AV, AL, MA, CB, JNV. Validation: AV, AL, MA, CB, JNV. Only AV had access to the dataset. The authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data availability
UK Biobank data are available through the UK Biobank Access Management System (UK Biobank Access Management System: http://www.ukbiobank.ac.uk/register-apply/).
Declarations
Competing interests
The authors declare no competing interests.
Ethics of approval statement
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Northwest – Haydock Research Ethics Committee (protocol code: 21/NW/0157, date of approval: 21 June 2021).
Patient consent statement
Written informed consent has been obtained from the patients.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.GBD 2019 Blindness and Vision Impairment Collaborators & Vision Loss Expert Group of the Global Burden of Disease Study. Causes of blindness and vision impairment in 2020 and trends over 30 years, and prevalence of avoidable blindness in relation to VISION 2020: the right to sight: an analysis for the global burden of disease study. Lancet Glob Health. 9, e144–e160 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tezel, G. The immune response in glaucoma: a perspective on the roles of oxidative stress. Exp. Eye Res.93, 178–186 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fernández-Albarral, J. A. et al. Glaucoma: from pathogenic mechanisms to retinal glial cell response to damage. Front. Cell. Neurosci.18, 1354569 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lotery, A. J. Glutamate excitotoxicity in glaucoma: truth or fiction? Eye Lond. Engl.19, 369–370 (2005). [DOI] [PubMed] [Google Scholar]
- 5.Chrysostomou, V., Rezania, F., Trounce, I. A. & Crowston, J. G. Oxidative stress and mitochondrial dysfunction in glaucoma. Curr. Opin. Pharmacol.13, 12–15 (2013). [DOI] [PubMed] [Google Scholar]
- 6.Tham, Y. C. et al. Global prevalence of glaucoma and projections of glaucoma burden through 2040: a systematic review and meta-analysis. Ophthalmology121, 2081–2090 (2014). [DOI] [PubMed] [Google Scholar]
- 7.Leske, M. C. et al. Predictors of long-term progression in the early manifest glaucoma trial. Ophthalmology114, 1965–1972 (2007). [DOI] [PubMed] [Google Scholar]
- 8.de Voogd, S. et al. Incidence of open-angle glaucoma in a general elderly population: the Rotterdam study. Ophthalmology112, 1487–1493 (2005). [DOI] [PubMed] [Google Scholar]
- 9.Garway-Heath, D. F. et al. Latanoprost for open-angle glaucoma (UKGTS): a randomised, multicentre, placebo-controlled trial. Lancet Lond. Engl.385, 1295–1304 (2015). [DOI] [PubMed] [Google Scholar]
- 10.Lee, J. S., Bae, H. W., Park, S., Kim, C. Y. & Lee, S. Y. Systemic arterial stiffness is associated with structural progression in early Open-Angle Glaucoma. Invest. Ophthalmol. Vis. Sci.63, 28 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Flammer, J. et al. The impact of ocular blood flow in glaucoma. Prog Retin Eye Res.21, 359–393 (2002). [DOI] [PubMed] [Google Scholar]
- 12.Drance, S. M., Douglas, G. R., Wijsman, K., Schulzer, M. & Britton, R. J. Response of blood flow to warm and cold in normal and low-tension glaucoma patients. Am. J. Ophthalmol.105, 35–39 (1988). [DOI] [PubMed] [Google Scholar]
- 13.Kashiwagi, K. et al. Systemic circulatory parameters. Comparison between patients with normal tension glaucoma and normal subjects using ambulatory monitoring. Jpn J. Ophthalmol.45, 388–396 (2001). [DOI] [PubMed] [Google Scholar]
- 14.Bae, H. W. et al. Systemic hypertension as a risk factor for open-angle glaucoma: a meta-analysis of population-based studies. PloS One. 9, e108226 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rim, T. H., Lee, S. Y., Kim, S. H., Kim, S. S. & Kim, C. Y. Increased incidence of open-angle glaucoma among hypertensive patients: an 11-year nationwide retrospective cohort study. J. Hypertens.35, 729–736 (2017). [DOI] [PubMed] [Google Scholar]
- 16.Hulsman, C. A. A., Vingerling, J. R., Hofman, A., Witteman, J. C. M. & de Jong, P. T. V. M. Blood pressure, arterial stiffness, and open-angle glaucoma: the Rotterdam study. Arch. Ophthalmol. Chic. Ill. 1960. 125, 805–812 (2007). [DOI] [PubMed] [Google Scholar]
- 17.Lee, T., Bae, H. W., Seong, G. J., Kim, C. Y. & Lee, S. Y. High pulse wave velocity is associated with decreased macular vessel density in Normal-Tension Glaucoma. Invest. Ophthalmol. Vis. Sci.62, 12 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shirwany, N. A. & Zou, M. Arterial stiffness: a brief review. Acta Pharmacol. Sin. 31, 1267–1276 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Said, M. A., Eppinga, R. N., Lipsic, E., Verweij, N. & van der Harst, P. Relationship of arterial stiffness index and pulse pressure with cardiovascular disease and mortality. J. Am. Heart Assoc.7, e007621 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Safar, M. E. Systolic blood pressure, pulse pressure and arterial stiffness as cardiovascular risk factors. Curr. Opin. Nephrol. Hypertens.10, 257–261 (2001). [DOI] [PubMed] [Google Scholar]
- 21.Sudlow, C. et al. UK biobank: an open access resource for identifying the causes of a wide range of complex diseases of middle and old age. PLoS Med.12, e1001779 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bycroft, C. et al. The UK biobank resource with deep phenotyping and genomic data. Nature562, 203–209 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Luce, D. A. Determining in vivo Biomechanical properties of the cornea with an ocular response analyzer. J. Cataract Refract. Surg.31, 156–162 (2005). [DOI] [PubMed] [Google Scholar]
- 24.Luce, D. Methodology for cornea compensated IOP and corneal resistance factor for the Reichert ocular response analyzer. Invest. Ophthalmol. Vis. Sci.47, 2266 (2006). [Google Scholar]
- 25.Chan, M. P. Y. et al. Associations with intraocular pressure in a large cohort: results from the UK biobank. Ophthalmology123, 771–782 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.van der Valk, R. et al. Intraocular pressure-lowering effects of all commonly used glaucoma drugs: a meta-analysis of randomized clinical trials. Ophthalmology112, 1177–1185 (2005). [DOI] [PubMed] [Google Scholar]
- 27.Kovacic, H., Wolfs, R. C. W., Kılıç, E. & Ramdas, W. D. The effect of multiple vitrectomies and its indications on intraocular pressure. BMC Ophthalmol.19, 175 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Khawaja, A. P. et al. Genome-wide analyses identify 68 new loci associated with intraocular pressure and improve risk prediction for primary open-angle glaucoma. Nat. Genet.50, 778–782 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vallée, A. Added value of arterial stiffness index for the 10-year atherosclerotic cardiovascular disease risk determination in a middle-aged population-based study. Clin. Res. Cardiol. Off J. Ger. Card Soc.112, 1679–1689 (2023). [DOI] [PubMed] [Google Scholar]
- 30.Woodman, R. J. et al. Assessment of central and peripheral arterial stiffness: studies indicating the need to use a combination of techniques. Am. J. Hypertens.18, 249–260 (2005). [DOI] [PubMed] [Google Scholar]
- 31.Alty, S. R., Angarita-Jaimes, N., Millasseau, S. C. & Chowienczyk, P. J. Predicting arterial stiffness from the digital volume pulse waveform. IEEE Trans. Biomed. Eng.54, 2268–2275 (2007). [DOI] [PubMed] [Google Scholar]
- 32.Sollinger, D. et al. Arterial stiffness assessed by digital volume pulse correlates with comorbidity in patients with ESRD. Am. J. Kidney Dis. Off J. Natl. Kidney Found.48, 456–463 (2006). [DOI] [PubMed] [Google Scholar]
- 33.Millasseau, S. C., Kelly, R. P., Ritter, J. M. & Chowienczyk, P. J. Determination of age-related increases in large artery stiffness by digital pulse contour analysis. Clin. Sci. Lond. Engl. 1979. 103, 371–377 (2002). [DOI] [PubMed] [Google Scholar]
- 34.Vallée, A. Arterial stiffness determinants for primary cardiovascular prevention among healthy participants. J. Clin. Med.11, 2512 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vallée, A. Arterial stiffness nomogram identification by cluster analysis: A new approach of vascular phenotype modeling. J. Clin. Hypertens. Greenwich Conn. 10.1111/jch.14571 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.UK Biobank. UK Biobank blood pressure. Available at: https://biobank.ctsu.ox.ac.uk/crystal/docs/Bloodpressure.pdf
- 37.Coleman, A., Freeman, P., Steel, S. & Shennan, A. Validation of the Omron 705IT (HEM-759-E) oscillometric blood pressure monitoring device according to the British hypertension society protocol. Blood Press. Monit.11, 27–32 (2006). [DOI] [PubMed] [Google Scholar]
- 38.Stang, A. et al. Algorithms for converting random-zero to automated oscillometric blood pressure values, and vice versa. Am. J. Epidemiol.164, 85–94 (2006). [DOI] [PubMed] [Google Scholar]
- 39.Berger, A. & Kiefer, M. Comparison of different response time outlier exclusion methods: A simulation study. Front. Psychol.12, 675558 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.UK Biobank. Biomarker assay quality procedures: approaches used to minimise systematic and random errors (and the wider epidemiological implications). (2019). https://biobank.ctsu.ox.ac.uk/crystal/crystal/docs/biomarker_issues.pdf
- 41.Dielemans, I. et al. Primary open-angle glaucoma, intraocular pressure, and systemic blood pressure in the general elderly population. Rotterdam Study Ophthalmology. 102, 54–60 (1995). [DOI] [PubMed] [Google Scholar]
- 42.Wu, S. Y. & Leske, M. C. Associations with intraocular pressure in the Barbados eye study. Arch. Ophthalmol. Chic. Ill. 1960. 115, 1572–1576 (1997). [DOI] [PubMed] [Google Scholar]
- 43.Jonas, J. B. et al. Intraocular pressure and associated factors: the central India eye and medical study. J. Glaucoma. 20, 405–409 (2011). [DOI] [PubMed] [Google Scholar]
- 44.Rezaeian, M., Golzan, S. M., Avolio, A. P., Graham, S. & Butlin, M. The association between retinal and central pulse wave velocity in the elderly. Artery Res.26, 148–153 (2020). [Google Scholar]
- 45.Vlachopoulos, C., Aznaouridis, K. & Stefanadis, C. Prediction of cardiovascular events and all-cause mortality with arterial stiffness: a systematic review and meta-analysis. J. Am. Coll. Cardiol.55, 1318–1327 (2010). [DOI] [PubMed] [Google Scholar]
- 46.Asmar, R., Vol, S., Brisac, A. M., Tichet, J. & Topouchian, J. Reference values for clinic pulse pressure in a nonselected population. Am. J. Hypertens.14, 415–418 (2001). [DOI] [PubMed] [Google Scholar]
- 47.Vallée, A. Arterial stiffness and the canonical WNT/β-catenin pathway. Curr. Hypertens. Rep.24, 499–507 (2022). [DOI] [PubMed] [Google Scholar]
- 48.Huck, A. et al. Vascular considerations in glaucoma patients of African and European descent. Acta Ophthalmol. (Copenh). 92, e336–340 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nguyen, C. et al. Studies of scleral Biomechanical behavior related to susceptibility for retinal ganglion cell loss in experimental mouse glaucoma. Invest. Ophthalmol. Vis. Sci.54, 1767–1780 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Heijl, A. et al. Reduction of intraocular pressure and glaucoma progression: results from the early manifest Glaucoma trial. Arch. Ophthalmol. Chic. Ill. 1960. 120, 1268–1279 (2002). [DOI] [PubMed] [Google Scholar]
- 51.Choi, J. & Kook, M. S. Systemic and Ocular Hemodynamic Risk Factors in Glaucoma. BioMed Res. Int. 141905 (2015). (2015). [DOI] [PMC free article] [PubMed]
- 52.Rosenquist, R., Epstein, D., Melamed, S., Johnson, M. & Grant, W. M. Outflow resistance of enucleated human eyes at two different perfusion pressures and different extents of trabeculotomy. Curr. Eye Res.8, 1233–1240 (1989). [DOI] [PubMed] [Google Scholar]
- 53.Borrás, T. A single gene connects stiffness in glaucoma and the vascular system. Exp. Eye Res.158, 13–22 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Last, J. A. et al. Elastic modulus determination of normal and glaucomatous human trabecular meshwork. Invest. Ophthalmol. Vis. Sci.52, 2147–2152 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Van Eijgen, J. et al. The relevance of arterial blood pressure in the management of glaucoma progression: a systematic review. Am. J. Hypertens.hpad11110.1093/ajh/hpad111 (2023). [DOI] [PMC free article] [PubMed]
- 56.Cone-Kimball, E. et al. Scleral structural alterations associated with chronic experimental intraocular pressure elevation in mice. Mol. Vis.19, 2023–2039 (2013). [PMC free article] [PubMed] [Google Scholar]
- 57.Bastelica, P. et al. [Role of the lamina cribrosa in the pathogenesis of glaucoma. A review of the literature]. J. Fr. Ophtalmol. 45, 952–966 (2022). [DOI] [PubMed] [Google Scholar]
- 58.Cecelja, M. & Chowienczyk, P. Role of arterial stiffness in cardiovascular disease. JRSM Cardiovasc. Dis1, (2012). 31;1(4):cvd.2012.012016. [DOI] [PMC free article] [PubMed]
- 59.Tanaka, H. et al. Comparison between carotid-femoral and brachial-ankle pulse wave velocity as measures of arterial stiffness. J. Hypertens.27, 2022–2027 (2009). [DOI] [PubMed] [Google Scholar]
- 60.Gardner, A. W. & Parker, D. E. Predictors of large and small artery elasticity in healthly subjects 9 to 89 years old. Am. J. Hypertens.24, 599–605 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cohn, J. N. Arteries, myocardium, blood pressure and cardiovascular risk: towards a revised definition of hypertension. J. Hypertens.16, 2117–2124 (1998). [PubMed] [Google Scholar]
- 62.Brumback, L. C., Jacobs, D. R., Dermond, N., Chen, H. & Duprez, D. A. Reproducibility of arterial elasticity parameters derived from radial artery diastolic pulse contour analysis: the Multi-Ethnic study of atherosclerosis. Blood Press. Monit.15, 312–315 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wang, H., Wu, X., Gu, Y., Zhou, J. & Wu, J. Relationship of noninvasive assessment of arterial stiffness with 10-Year atherosclerotic cardiovascular disease (ASCVD) risk in a general Middle-Age and elderly population. Int. J. Gen. Med.14, 6379–6387 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Jammal, A. A. et al. Blood pressure and glaucomatous progression in a large clinical population. Ophthalmology129, 161–170 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Asrani, S. G. et al. The relationship between intraocular pressure and glaucoma: an evolving concept. Prog Retin Eye Res.103, 101303 (2024). [DOI] [PubMed] [Google Scholar]
- 66.Wu, X. et al. Role of ocular blood flow in normal tension glaucoma. Adv. Ophthalmol. Pract. Res.2, 100036 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mitchell, G. F. et al. Arterial stiffness and cardiovascular events: the Framingham heart study. Circulation121, 505–511 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Benetos, A. et al. Pulse pressure: a predictor of long-term cardiovascular mortality in a French male population. Hypertens. Dallas Tex. 1979. 30, 1410–1415 (1997). [DOI] [PubMed] [Google Scholar]
- 69.Boutouyrie, P., Chowienczyk, P., Humphrey, J. D. & Mitchell, G. F. Arterial stiffness and cardiovascular risk in hypertension. Circ. Res.128, 864–886 (2021). [DOI] [PubMed] [Google Scholar]
- 70.Domanski, M., Norman, J., Wolz, M., Mitchell, G. & Pfeffer, M. Cardiovascular risk assessment using pulse pressure in the first National health and nutrition examination survey (NHANES I). Hypertens. Dallas Tex. 1979. 38, 793–797 (2001). [DOI] [PubMed] [Google Scholar]
- 71.Mitchell, G. F. Arterial stiffness and wave reflection: biomarkers of cardiovascular risk. Artery Res.3, 56–64 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Townsend, R. R. et al. Clinical Use of Pulse Wave Analysis: Proceedings From a Symposium Sponsored by North American Artery. J. Clin. Hypertens. 17, 503–513 (2015). [DOI] [PMC free article] [PubMed]
- 73.DeLoach, S. S. & Townsend, R. R. Vascular stiffness: its measurement and significance for epidemiologic and outcome studies. Clin. J. Am. Soc. Nephrol. CJASN. 3, 184–192 (2008). [DOI] [PubMed] [Google Scholar]
- 74.Richiardi, L., Pizzi, C., Pearce, N. & Commentary Representativeness is usually not necessary and often should be avoided. Int. J. Epidemiol.42, 1018–1022 (2013). [DOI] [PubMed] [Google Scholar]
- 75.Rothman, K. J., Gallacher, J. E. J. & Hatch, E. E. Why representativeness should be avoided. Int. J. Epidemiol.42, 1012–1014 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Foster, P. J. et al. Intraocular pressure and corneal biomechanics in an adult British population: the EPIC-Norfolk eye study. Invest. Ophthalmol. Vis. Sci.52, 8179–8185 (2011). [DOI] [PubMed] [Google Scholar]
- 77.Elgendi, M. et al. Recommendations for evaluating photoplethysmography-based algorithms for blood pressure assessment. Commun. Med.4, 1–7 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ferizoli, R., Karimpour, P., May, J. M. & Kyriacou, P. A. Arterial stiffness assessment using PPG feature extraction and significance testing in an in vitro cardiovascular system. Sci. Rep.14, 2024 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
UK Biobank data are available through the UK Biobank Access Management System (UK Biobank Access Management System: http://www.ukbiobank.ac.uk/register-apply/).