Abstract
Obtaining estimates of demographic parameters are fundamental for managing species. However, survey timing and duration influences the precision and accuracy of estimates. We used motion-activated camera images to investigate the effect of survey duration, timing, camera density and on- or off-trail placement on detection rates, sex and age ratios, and relative abundance estimates of moose (Alces alces) in Isle Royale National Park (IRNP), Michigan, USA. Variations in detection rates reflected moose life history patterns and suggested the optimal times to estimate demographic ratios and population relative abundance. We recommend camera surveys of 25-days during mid-June–mid-July and early December–early January to produce consistent and precise calf: cow and bull: cow ratios. On-trail cameras returned greater detection rates and relative abundance estimates, but decreased precision for summer bull: cow and calf: cow ratios than off-trail cameras. Subsampling camera densities to 3 cameras/km2 decreased precision and consistency for density and ratio estimates. We recommend estimating moose relative abundance during early December–early January, using > 3 cameras/km2 placed on and off-trail. Pairing life history events with high detection rates can be used to identify optimal survey periods and could be applied to other species.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-025-05603-y.
Keywords: Moose, Alces alces, Age ratios, Detection rate, Relative abundance, Ungulate
Subject terms: Ecology, Ecology, Behavioural ecology, Conservation biology, Ecological modelling, Population dynamics
Reliable estimates of sex and age ratios, and relative abundance are fundamental to monitoring wildlife populations and making management decisions1,2. Sex and age ratios such as juvenile: adult female or adult male: adult female are commonly used to infer demographic trends3 and population growth for various ungulate species (e.g., elk3 Cervus canadensis, caribou4 Rangifer tarandus). Specifically, summer- and winter-derived juvenile: adult female ratios can index productivity and recruitment, respectively5. Precise relative abundance estimates can be more critical as they are used to establish hunting quotas6, monitor long-term population trends1, or influence the decision to introduce new individuals, predators, or competitors7.
Estimating demographic parameters can be difficult if the ability to detect or differentiate age or sex classes varies temporally due to animal movements8 or life history9. For example, adult male moose, along with other male cervids, are generally detected more frequently during the breeding season than females or calves due to greater movements by males10,11. These increased movements can result in increased detections leading to overestimation of males during the breeding season12, inflating relative abundance estimates and skewing population-level sex or age ratios. However, the timing of surveys to estimate ungulate population trends often coincides with factors such as hunting season or preferred weather conditions to reduce survey costs, rather than timing based on life history.
We suggest that considering timing of life history events among sex and age classes could improve detection probability and estimate population characteristics more precisely. Changes in life history events can result in differences in species’ sex/age class patterns of mobility, resulting in potential differences and increased variability in detection probabilities8. For example, moose Alces alces adult females (hereafter cows) generally give birth from May to June and restrict their home ranges and mobility to protect their low-mobility young (hereafter calves)13. Cow and calf mobility increases with calf age, with greatest mobility during late June–October. Adult males (hereafter bulls) undergo rutting and breeding from mid September to late October14 and increase mobility compared to cows. Cow and bull activity and mobility are similar after the breeding season, emphasizing foraging before winter10,15. During late winter, when ambient temperatures are lowest, mobility decreases to conserve energy16. Selecting standardized periods where mobility is similar across sex or age classes should improve precision within and across years for population ratios calculations typically used to estimate fecundity (late fall and winter).
In addition to mobility, body characteristics change seasonally that can affect the ability to correctly identify sex or age classes, which could lead to decreased precision and consistency when calculating population characteristics. Distinguishing juveniles from adult ungulates becomes more difficult as juveniles mature. While some ungulate species (e.g., white-tailed deer Odocoileus virginianus, elk, mule deer O. hemionus) offspring have temporary spots, not all juvenile ungulates (e.g., moose, caribou, pronghorn Antilocapra americana) have this trait and identification relies on rapidly changing body sizes to differentiate between juveniles and adults which can cause potential misidentification or categorizing as unknown. For example, moose calves weigh 12–20 kg at birth17 and can increase body mass 1.3–1.6% per day17. By January–March, calves can weigh 160–225 kg18 compared to adults weighing 360–600 kg17. Another common identifiable trait is using antlers to identify adult male cervids; however, shed or undeveloped antlers could lead to misidentification between males and females. For moose, bulls do not grow antlers until mid-April–early May, followed by rapid growth resulting in complete antler development by August or September17, and antler loss during late December–late January5. Misidentification between calves and bulls and a potential increase for unknown identifications can occur in the early winter as calves (< 1 year) can produce variable antler characteristics while having closer body mass to adults5.
To facilitate more precise and consistent population characteristics, survey designs would ideally occur during life history events that increase probability of identifiable body characteristics among target sex and age classes. However, the most common survey method for collecting information on moose populations is aerial surveys during mid to late winter, which coincides when moose are least mobile19,20. Behavior differences in summer between sex and age classes can generate biased estimates, limiting the collection of population estimates to occur in winter21. Additionally, aerial surveys rely on specific weather and flight conditions, which can further constrain timing and measurement across consecutive days22,23. Hunter observations also have been used to collect moose occurrence data20,24; however, this method usually occurs when moose are breeding and there are behavioral differences between sex and age classes25 that can reduce accuracy of detectability, relative abundance, and ratio estimates. In contrast, remote cameras could be deployed during more appropriate survey periods to obtain summer and winter population characteristics while being cost-effective and potentially more reliable26. While the influence of survey length on detection rate, species richness, and occupancy has been investigated27, understanding survey timing, duration, and influence of survey design are still needed to optimize precision of detection rates, sex and age ratios, and relative abundance.
In the United States, federal, state, and tribal agencies operate under diverse laws, policies, and regulations that influences method selection and execution. For example, the Wilderness Act (1964) prohibits the use of motorized and mechanized equipment and installations in designated wilderness areas28, which limits the use of certain survey methodologies, such as aerial or long-term remote camera surveys. However, the Wilderness Act does allow the use of prohibited equipment when their use meets the stated purpose of the Act (i.e., to preserve wilderness character)28. Many agencies are required to complete a minimum requirements analysis when prohibited methodologies are proposed28. Balancing the requirements of laws, policies, and regulations with research objectives often requires evaluation of numerous methodologies to assess which is most appropriate. While remote cameras do impede the goals of the Wilderness Act, they are non-invasive and adaptable to diverse survey designs and could reduce impacts compared with alternatives like aerial surveys.
We investigated timing, sampling duration, and camera density to estimate moose detection rates, relative abundance, and sex and age ratios to identify periods of increased precision using moose detections from remote cameras in a designated wilderness, Isle Royale National Park (IRNP), Michigan, USA. We predicted that seasonal variation in detection rates would reflect moose life history events, as movement influences detection probability8. Thus, we predicted that variability in relative abundance would be reflected in detection rates as relative abundance estimates are influenced by detection rates. We predicted early December–early January would be optimal to estimate relative abundance and calf: cow (i.e., recruitment) and bull: cow ratios as bull, calf, and cow movements are similar17 and can be differentiated using body size and antlers or pedicels post-shedding of antlers. We expected that bull: cow and calf: cow ratios could also be estimated in late June–late July when calves and cows become more mobile post-calving and bull antlers are more developed. We predicted that survey durations of 25- to 30-days would produce the most consistent and lowest variation for bull: cow and calf: cow ratios as this interval can increase the probability of detecting27 moose while limiting potential seasonal changes in life history. Lastly, we investigated the effects of camera density and placement on- and off-trail on survey precision and duration.
Results
Detection rate
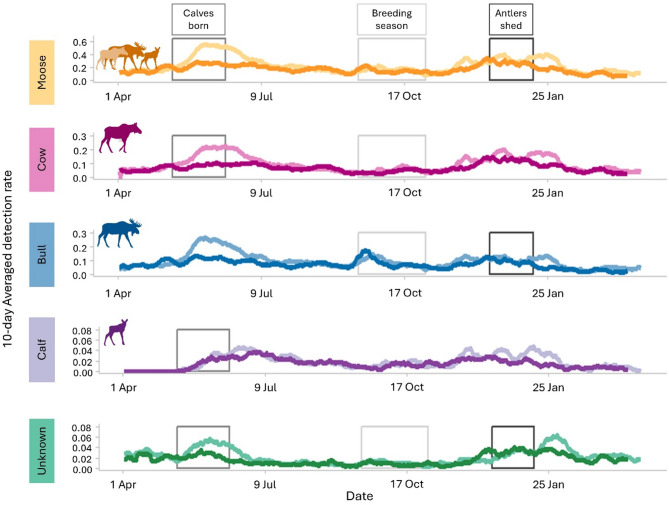
Overall, bull, cow, and calf detection rates increased from early-June to mid-July, and mid-November to mid-January (Fig. 1). Additionally, bulls had increased detections from late September to mid-October. Unknown sex and age detections were greatest from mid-June to early July and late December to late January. All moose sex and age classes had lower detection rates in 2021 than 2020 during early June–mid July and mid-November–early February (Fig. 1).
Fig. 1.
Moose mean daily detection rates across 10-days using on- and off-trail cameras (n = 156), Isle Royale National Park, Michigan, USA, 2020 (lighter colors) and 2021 (darker colors). Moose detections included unknown age and sex. Moose neonates are born, breeding season, and shedding of antlers occur approximately mid-May-early June, mid-September–late October, and mid-December–early January14, respectively.
On-trail cameras detected more moose than off-trail cameras (Supplementary Fig. S1). On and off-trail cameras had higher bull, cow, calf, and unknown detections during early June–mid-July and for bulls mid-September–mid-October. Moose detections on-trail were greater in 2020 than in 2021. Only on-trail detection rates increased mid-November–early February. Overall detection rates decreased as camera density per km2 decreased; however, all camera density subsamples produced similar variation in detection rates across seasons (Supplementary Fig. 2).
Index of moose relative abundance
We found the index of relative abundance estimates were most consistent (i.e., similar estimates and confidence intervals) across cameras on- and off-trail, camera density subsamples, and years when 60-day survey windows started in December (Figs. 2 and 3). With a start period of 1 December, we estimated 2.2 moose/km2 in 2020 (95% CI = 1.8–2.7), and 1.8 moose/km2 (95% CI = 1.5–2.2) in 2021. For all models estimating relative abundance, 95% confidence intervals overlapped from 1 November to 15 December then diverged after 1 January. On-trail camera derived relative abundance estimates were greater and did not overlap with off-trail cameras (Fig. 2). For camera density subsamples, we found 5 (100%) and 4 (80%) cameras/km2 had similar estimates and confidence intervals (Fig. 3) which became increasingly variable at lower camera densities.
Fig. 2.
Moose relative abundance/km2 estimations (95% confidence intervals) across all pooled (blue; n = 156) and on- (pink; n = 98) or off-trail (orange; n = 58) cameras, Isle Royale National Park (544 km2), Michigan, USA, 2020 and 2021. Estimations calculated from moose detections within 60-day periods.
Fig. 3.
Moose relative abundance/km2 estimates (95% confidence intervals) using camera density subsets of 5 (100%), 4 (80%), 3 (60%), and 1 (20%)/km2, Isle Royale National Park (544 km2), Michigan, USA, 2020–2021. Estimations calculated from moose detections within 60-day periods. Camera numbers have equal proportions of on- and off-trail cameras and are scaled to 1 km2.
Age and sex ratios
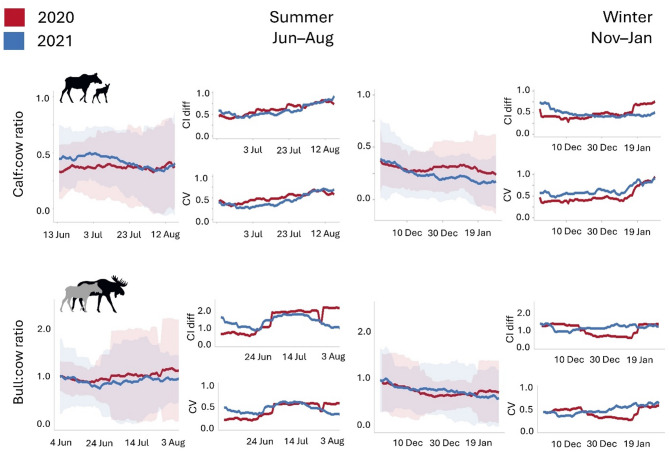
Calf: cow and bull: cow ratios calculated using 25-day survey periods during mid June–mid July and early December–early January were least variable (Fig. 4). However, winter derived ratios were more precise than summer ratios. Bull: cow ratios were more variable than calf: cow ratios, especially in summer. Ratios calculated outside mid June–mid July and early December–early January produced large confidence interval differences, and coefficients of variation. For calf: cow and bull: cow ratios, surveys < 20 days had increased variability in mean ratio estimates, and > 30 days had gradual increases in confidence interval differences and coefficient of variation (Supplementary Fig. S3, S4).
Fig. 4.
Daily mean calf: cow and bull: cow ratios across 25-day moving windows (95% confidence intervals) in summer (June–August) and early winter (November–January), Isle Royale National Park, Michigan, USA, 2020 (red) and 2021 (blue). For each set of ratios, differences in 95% confidence intervals (CI diff – [upper CI – lower CI]) and coefficient of variation (CV) are plotted for 2020–2021. Periods with low CI diff values and CV indicate higher precision.
For summer calf: cow ratios, our most precise estimates were on 21 June 2020 (0.39 95% CI: 0.18–0.60) and 1 July 2021 (0.50 95% CI: 0.28–0.72) and for winter, 11 December 2020 (0.26 95% CI: 0.11–0.42) and 9 January 2021 (0.23 95% CI: 0.03–0.43) (Supplementary Tab. S5). The most precise bull: cow ratios occurred on 19 June 2020 (0.87 95% CI: 0.61–1.13) and 1 July 2021 (0.81 95% CI: 0.39–1.23) and for winter, 11 January 2021 (0.64 95% CI: 0.37–0.90) and 11 December 2021 (0.76 95% CI: 0.34–1.18) (Supplementary Tab. S6). Except for summer bull: cow ratios, calf: cow and bull: cow ratios calculated using on- and off-trail and cameras densities of 4 and 5/km2 produced similar ratios with 95% overlapping confidence intervals differences (Supplementary Fig. S7,S8). Summer off-trail bull: cow ratios had greater confidence interval differences and coefficient of variation than on-trail (Supplementary Fig. S7).
Discussion
We estimated the effects of survey timing, sampling durations, design, and density on precision and consistency when calculating moose detection rates, relative abundance, and sex and age ratios from remote cameras. We found support for our prediction that variation in detection rates reflected moose life history events. Detection rates increased following calving when calves became mobile in late June–July and also increased post-rut when moose increased their mobility for foraging during December–January. Further, we found optimal times to estimate relative abundance and sex and age ratios occurred during periods (e.g., mid-June to mid-July and early December to early January) with high but consistent detection rates across age and sex categories. We found support for our prediction that 25-day periods would produce a consistent and low variation for ratios. Windows < 25 days had increased daily variability and windows > 30 days had increased differences in confidence intervals and coefficient of variations indicating decreased precision. Our prediction that on-trail placement and low densities of cameras increase variability was supported while also finding camera densities of 4 and 5/km2 produced similar relative abundance, detection rates, and sex and age ratios.
Detection rate
Moose detection rates for sex and age classes varied temporally and our prediction that variation in detections would reflect moose life history events, as movement influences detection probability8, was supported. Aside from the breeding season, all sex and age classes exhibited similar patterns across seasons and between years. Detection rates for all classes increased in early June, midway through calving season from late May to mid-June. We predicted this delayed increase as calves and cows have limited mobility after birth, and mobility increases within a few weeks13. Additionally, increase in higher quality-forage and increased foraging could increase mobility in late May29 and explain why bull and cow detection rates increase during this period compared to late winter. During the breeding season in September–October, only bulls had increased detection rates. Bulls increase mobility and allocate energy to mate with multiple cows rather than other activities such as foraging10,30. In contrast, cows in estrus maintain a breeding area and exhibit lower mobility rates than bulls4. During late November–late January, all classes had increased detection rates with similar variation. During this post-rut period, moose increase foraging to increase body mass before forage quality and quantity further declines10. Mobility and home range size of bulls, cows, and calves are most similar at this time10,15 and likely explain similar detection patterns we observed. After January, all moose detection rates declined, which was expected as moose mobility decreases to conserve energy expenditures in late winter16. While overall seasonal variations between years were similar, 2020 had greater overall detections than 2021, particularly during summer. This difference could be related to decreased park visitation in 2020 due to COVID-19 pandemic restrictions; mean detection rates of moose overall and on-trail decreased in summer as visitation increased then peaked31 or from wolf predation as IRNP’s wolf population doubled from 2020 to 2021 (~ 12 to 24 minimum individuals).
Index of moose relative abundance
We found support for our prediction that variability in relative abundance estimates reflected variability in detection rates.Additionally, we found support that early December–early January would be an optimal survey period based on similar estimates and confidence intervals and more similarities among moose demographic life history patterns. Our greatest relative abundance estimates across all pooled sites, camera density subsamples, on- and off-trail, and years occurred during a 60-day survey period beginning in early December. This period also corresponded to the greatest and most consistent measurements of detection rates across demographic groupings and when these groupings are most behaviorally similar due to their emphasis on foraging14. Estimates initiated before 15 November or after 1 January generated lower moose relative abundance estimates and 95% confidence intervals did not overlap with estimates from surveys starting in December. During mid-November, moose transition from breeding season to focus on foraging in preparation for winter10,14,15. The lower estimates in mid-November could be due to capturing the transitional period between these periods. From late January to March, moose movements decrease to conserve energy, particularly in deep snow conditions16. This change could have resulted in lower observeddetection rates and influenced our relative abundance estimates. While we are unable to test model accuracy differences and across the Isle Royale, our results suggest that cameras can be useful for year-to-year comparisons in relative abundance. Cameras are easy to maintain and survey designs can be repeatable. Although we did not estimate moose relative abundance during summer, behavioral differences among bulls, cows, and calves during summer and reduced observability can influence estimates from aerial surveys21. Further assessments testing the use of instantaneous sampling estimators using a representative sampling across a whole survey area or a larger representative sample would further inform potential limitations towards using an instantaneous sampling estimator.
Age and sex ratios
Our predictions that calf: cow and bull: cow ratios could be optimally estimated in early December–early January and late June–late July were supported. Estimates outside these periods exhibited greater detection variability and greater differences in confidence intervals and coefficients of variance. Differences in seasonal behavior across sex and age classes can influence the reliability of sex and age ratios3,12. Additionally, we found winter ratios to be more consistent and precise than summer ratios, especially for bull: cow ratios. While all classes forage during summer, cows spend considerable energy protecting and feeding calves13,14. Consequently, cows and calves have more restricted home ranges than bulls17, which undoubtedly influences detection probability. We found an increase in unknown age- and sex-categorized moose detections starting mid-January and between mid-May–late June, which could have caused increased variability in ratio estimates. During these periods, most unknown classifications occurred even with the moose fully visible in sequences. The increase of unknown classifications coincided with male antler loss after early January and before well-developed antlers in August17.
Survey design
We found support that camera survey durations of 25 to 30 days would produce consistent and low variation in bull: cow and calf: cow ratios. Moose life history events such as breeding season and calving, are often relatively brief (i.e., 3–4 weeks)16. Pairing the 25-day survey window with life history events and increases in detection rates can reduce variability observed in shorter survey periods (i.e., < 20 days) and improve ability for across-year comparisons.
In addition to survey length, we found camera placement (i.e., on- and 50 m off-trail) and camera density influenced precision of moose detection rates, sex and age ratios, and relative abundance estimates. On and off-trail camera placement can result in differences in detectability for some species, with on-trail placements having often greater detections32,33. Generally, we found that on-trail camera placements had higher detection rates for all moose sex and age classes but only during calving, breeding, and winter foraging seasons. While off-trail camera detections slightly increased detections in calving, breeding and winter foraging seasons, overall detections were consistent each year. However, on- and off-trail camera placements demonstrated temporal variability in detection rates, sex and age ratios, and relative abundance. We did not compare off-trail cameras > 50 m from trails with on-trail cameras or the effects of cameras on a spatially limited trail network. Our survey design limited our ability to sample within 50 m of trails though variability in detections could differ at distances further from trails. In another study, detection rates were similar for 9 of 12 species (e.g., white-tailed deer and black bear [Ursus americanus]) at 0, 25, and 200 m off trail, excluding coyote (Canis latrans), bobcat (Lynx rufus), and chipmunk (Tamias striatus) that were detected more frequently on trails with increased human activity34. When using instantaneous sampling models, using images from cameras placed on-trails can bias abundance estimates35. Camera sites selected for ISE models should be representative of the sampling area to reduce bias35. Further assessments of these camera distributions would further inform potential effects on moose population estimates.
Summer calf: cow ratios and winter calf: cow and bull: cow ratios were similar in estimates and precision. Summer bull: cow ratios were highly variable but greater for off-trail, likely due to mobility differences between bulls and cows. In summer, cows are with calves and have more restricted movements and home ranges than bulls13. Additionally, unlike bulls, cows allocate considerable energy toward lactation and protecting their young instead of foraging13,14. Movement patterns and behavior differences between bulls and cows in summer can lead to overestimating bull: cow ratios due to more detections of bulls than cows16. The main difference between on and off-trail estimates occurred when calculating relative abundance, as on-trail estimates were greater than off-trail estimates. If management goals are to increase detection probability and obtain age and sex ratios, on-trail-only placements can be effective; however, if using cameras to estimate relative abundance, using on and off-trail camera placements or using an area based on sampling (e.g., power analysis) in models could generate more representative estimates.
We found that changes in relative abundance and detection rate reflected variation in moose life history patterns. Differences in relative abundance and detection rates could also be influenced by potential data biases from our camera array, moose movement rates, and moose moving between on- and off-trail camera locations. Models including the instantaneous sampling estimator, require that movement rates not influence species detectability35. However, depending on time of year, species movement rates change, such as moose males having greater movements than females during September–November10. Changes movements can markedly impact the accuracy of estimators, especially when detection probabilities are low8. To reduce the influence of movement rates, the sampling area and spacing of cameras should reflect the movement characteristics of the focal species8. Observed changes in relative abundance, detection rate, and age/sex ratios were likely influenced by changes in movement rates due to life history. As our study shows detection rates vary with life history, studies should prioritize when behavior is most stable across the focal species demographics (e.g., sex or age classes of interest), which we demonstrated produce the greatest and most consistent detection rates throughout the year (i.e., December to January for moose). Periods of greater detection probability, stabilized movement rates, and similar behavior patterns in survey windows paired with sampling designs accounting for movement patterns will reduce bias for relative abundance or density estimators8. Lastly, variation in detection and movement rates throughout the year across demographic groupings highlights the importance of surveying during the same period across years to facilitate long-term monitoring.
We found that 5 and 4 cameras/km2 produced consistent and similar values of sex and age ratios and relative abundance. If obtaining estimates of abundance is a management goal, consideration should be made regarding how abundance is calculated, as some methodology requires denser camera placement to meet assumptions. One benefit to using cameras, as demonstrated with our design, is that cameras can be deployed at the same sampling locations generating a replicable design. However, many methods typically used to survey moose can be difficult to replicate to the same location, time of year, or in relation to moose life history.
Based on our results, we suggest that the timing of typical moose survey methods (e.g., aerial surveys, hunter observations, pellet surveys) has not been optimal in relation to moose life history20. For example, aerial, snow-track and pellet surveys often occur in winter and rely on adequate snow cover9,20,22. Because of the moose behavior, weather requirements, and winter holidays, these surveys generally occur from late January-early March21,22, which are when moose are least mobile. Hunter observations are less consistent measures and often occur during the moose breeding season, which could result in bias from differences in detections by moose age and sex19,25,36. Additionally, many of these survey methods do not account for imperfect detection (i.e., sightability)21. Using remote cameras can allow for better-timed surveys that correspond with moose life history rather than winter or opportunistic samples. Camera placement can be consistent across years, improving comparability for annual trends. Many camera-related analytical approaches to calculate population metrics such as density or occupancy have calibration for imperfect detection.
Placement of cameras within the field is also important to minimize obstruction and imperfection detection. Placement and orientation of cameras should ensure the greatest probability of detecting focal species. We placed our cameras 1.5 m off ground angled 45 degrees towards the ground, north facing, which allowed us to obtain sex and age class information on moose easily while minimizing false triggers that could fill SD cards and deplete battery life. The camera height minimized temporary obstruction from snow and fast-growing vegetation typical in our system. Additionally, maximizing camera detection distances and viewsheds can also minimize imperfect detection and help standardize differences between on and off trail cameras37. Small changes in a camera’s viewshed area can generate error in density estimates that are extrapolated across areas much larger than the collective viewshed areas38. Detailed information where moose are in relation to distance from cameras or each camera’s area of detection can allow for non-individual derived relative abundance or density estimators, like the instantaneous sampling used in this study and other space to event (TTE and STE) models35. Lastly, cameras can be deployed with minimal maintenance while collecting sex and age moose information and other bycatch species detections.
Depending on research or management goals, remote cameras may provide the best “minimum tool” with respect to the minimum requirements obligation of the Wilderness Act28. While cameras are a prohibited installation, they can be less noticeable and invasive to wildlife and recreationists compared to aircraft. Survey method selection requires careful evaluation of tradeoffs when considering protected area goals and objectives in the context of law, policy, and regulation. In the case of IRNP, surveying moose using remote cameras could offer an alternative methodology that better preserves wilderness character compared to traditional aerial surveys. Our results suggest cameras can be deployed and retrieved during late fall and early spring, avoiding overlap with human visitation. Evaluating methodologies is important to provide options that best meet al.l management objectives.
Considering that IRNP goals include maximizing human wilderness experiences while maximizing precision of moose demographic estimates, we recommend: (1) deploying remote cameras during the winter, particularly from November–January and ensuring cameras are not placed during peak visitation, (2) using a 25-day survey period to calculate calf: cow and bull: cow ratios and using a 60-day survey period to calculate abundance during early December–early January, (3) the camera array should have an approximately equal ratio of on-and off-trails and ≥ 4 cameras/km2 or have representative sampling. Combining life history and detection rate patterns can be used to optimize periods for estimating demographic parameters. Our approach to estimating moose relative abundance and sex and age ratios can serve as a framework for monitoring medium- to high-density moose populations and potentially other ungulate species considering life history events.
Study area
Isle Royale National Park (IRNP) is an archipelago comprising of 558 km2 with the main island, Isle Royale, comprising 535 km2 in northwestern Lake Superior, 24 km from the Canadian mainland in the transitional zone of temperate northern hardwoods and boreal forest biomes39 (Fig. 5). Approximately 99% of the IRNP is designated wilderness and is open to park visitors from 15 April– 31 October yearly.
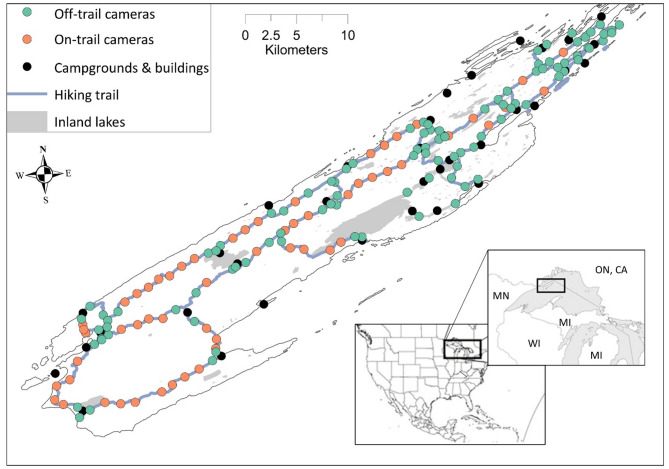
Fig. 5.
Camera locations (n = 156), Isle Royale National Park, Michigan, USA, 1 April–31 March 2020–2022.
In the early 1900s, moose colonized IRNP and persisted without major predators until gray wolves colonized in the 1940s40. The decline of IRNP’s wolf population from 50 to 14 individuals41, then to two related individuals by 201840,42, assisted in the increase moose abundance, resulting in over-browsed understory conditions, particularly for moose’s preferred forage, balsam fir43. As no hunting occurs in IRNP, wolves were introduced during 2018–2020 to restore ecosystem processes, including wolf predation of moose40. Moose abundance in IRNP during 2020 and 2022 were 1876 and 1039 (95% CI = 800–1349), respectively40,44.
Methods
Data organization and collection
We used images collected from 15 January 2020 to 13 January 2022 from 156 infrared remote cameras (Stealth Cam DS4K; Irving, Texas, USA) positioned along or within 50 m of trails throughout Isle Royale, the main island within IRNP (Fig. 5). Along each human hiking trail segment, we spaced cameras 350–1600 m apart where cameras nearest trail intersections (100–300 m from an intersection) were placed on-trail (n = 98) and those further, 50 m perpendicular from trails (n = 58). On-trail cameras can have increased detections of certain wildlife species33,34, however, IRNP has a high density of hiking trails that are well distributed across the park (Fig. 5). We included on- and off-trail cameras to provide a more accurate representation around the study area’s trail network. Additionally, camera locations were selected to represent proportionally the area of the 14 ecological groupings (e.g., northern shrublands, rock barrens, boreal hardwood forests) on IRNP45. Within each ecological group, camera locations remained consistent throughout the study. At each camera location, we positioned cameras to maximize detection area and minimize visual obstruction to reduce obstruction differences between on and off-trail cameras. To reduce obstruction that could impair detection46, we removed vegetation 10 m in front of each camera during each check. We positioned cameras 1.5 m above ground to avoid covering by snow and vegetation (e.g., Rubus parviflorus) and oriented each to detect animals 4–15 m distant. Additionally, cameras were placed 1.5 m above ground to increase chances of photographing a moose’s head at close range (< 5 m), allowing for sex identification. We programmed cameras to take five images each detection with a trigger speed of ≤ 0.5 s and no delay between detections. Species detected were initially identified using program RECONN.AI (Michigan Aerospace, Ann Arbor, Michigan), which uses regional convolutional neural network models to identify species from images. We manually checked the AI-processed moose identification records to ensure accuracy and grouped images taken within a 10-minute interval generating group-size counts, using direction of travel and species demographic characteristics to identify individuals in each time intervals. Most images were of moose moving in a consistent direction, however, when uncertain of the number of individuals, we used minimum counts. We categorized moose as bull, cow, calf (< 10.5-months old), and combined (all detections including unknown sex or age). We assigned year ranges as 1 April–31 March to include all calving and late winter seasons and labeled the year to match the start year. To test effects of lower camera densities, we randomly subset the 2020 and 2021 datasets retaining 100% (5 cameras/ km2), 80% (4), 60% (3), and 20% (1) of cameras to investigate the influence of camera densities on population estimates. Additionally, we ran our estimations across all pooled sites (n = 156), on-trail (n = 98), and off-trail (n = 58) only camera placements. For each moose demographic ratios, we calculated a moving 10-day averaged daily detection rate (number of detections/day) across all sites pooled, on-trail cameras only, and off-trail cameras only each year (Fig. 1). For daily detection rates, we calculated all moose combined, adult female, adult male, juveniles, and unknown moose based on the number of individuals observed at a camera site each day averaged across all sites combined, on-trail cameras only, and off-trail cameras only within a 10-day period.
Gender/Age ratiosand recruitment estimates
From 1 April to 31 March 2020–2021, we calculated grand means of daily estimates of calf: cow ratios (daily average of the number of calf detections / number of cow detections per site) and daily estimates of bull: cow ratios (daily mean of the number of bull detections/number of cow detections at each site). We excluded 2 days when bulls or calves were detected but females were not (i.e., inf) and 2 days when no moose were detected across all sites. To identify periods to estimate early season calf: cow ratios5, late season calf: cow ratios, and early- and late-season bull: cow ratios, we initially plotted daily calf: cow and bull: cow ratio estimates across the entire year to identify a period in summer (14 June–17 August) and winter (25 November–29 January) with greater consistency and lower confidence interval differences (Fig. 4). We generated daily detection histories for 10- to 50-day intervals. The start day of each interval was the first day of each period. For example, the 10-day and 50-day 1 April detection histories contained dates 1 April to 10 April and 1 April to 21 May, respectively. We then repeated this step for 2 April, 3 April, etc., until the last day of each period, subset, camera-placement type, and year. We calculated mean demographic ratios across days, standard error, and 95% confidence intervals from each detection history.
To compare mean demographic ratio values within survey intervals (i.e., actual dates) and interval lengths (i.e., 10-day, 15-day, 20-day, and 25-day), we calculated coefficients of variation (CV) and the compared the differences between the upper and lower 95% confidence intervals. We assumed lower differences in confidence intervals, and CVs indicated increased consistency and precision.
Instantaneous sampling Estimation modeling
We estimated cow and bull moose relative abundance using on-trail, off-trail, and all cameras using an instantaneous sampling estimation (ISE) model in R package spaceNtime35. The ISE model is a relative abundance or density estimator that incorporates species’ count information and the amount of space (viewable area) sampled before a species of interest is detected on cameras. The model uses multiple spatial and temporal replicates to estimate relative abundance using the mean count nij at location i = 1, 2, …, total number of cameras (M) and sampling occasion (j) j = 1, 2, … when divided by a cameras’ viewable area35. During each camera check, we estimated the minimum and maximum distance (m) the camera could detect a person. We placed temporary markers 5 and 10 m from cameras and verified in the field that each viewable area included both markers. Camera detection distances at sites varied minimally during the study as cameras were permanently attached to trees and were only moved when the tree or camera was damaged. We then estimated each camera’s viewable area, including camera angle of view (Stealth camera DS4K: θ = 43.54 degrees) into the circular sector area equation ((θ/360°) * π(detection distance)2) where we subtracted the minimum detection area from the maximum to get the total area. We assumed detections on and off-trail represented the area around Isle Royale’s trail network and extrapolated relative abundance to a buffered area around all trails (28 km2 or 60 m out from a trail in each direction multiplied by the total length of trails).
For ISE models, we used intervals of 2s window every 30 s following Ausband et al.47 to generate a count (group size per sequence) histories using the build_occ() and ise_build_eh() functions35,47. Although Ausband et al.47 used a Space to event (STE) model, this model uses a similar structure and recommendations for use as does the ISE model when generating encounter histories35,47. When using motion-activated cameras, it is recommended that ISE and STE models use extremely short windows and period lengths35,47. Additionally, we ran multiple models testing different sized windows and intervals and found that intervals of 2s window every 30s produced the most consistent outputs and confidence intervals across all cameras and our subsets. As we used 10-minute intervals when classifying moose sequences, we used the initial timestamp of the first detected animal per sequence when running models to reduce overcounting. We estimated relative abundance and 95% confidence intervals using 60-day survey windows that started the 1st or 15th of each month during 1 November–31 March each year. We divided the outputs by a buffered surveyed area around the trail network (28 km2) to estimate an index of relative abundance (number of moose/km2). We used 60-days to ensure we had adequate data to estimate relative abundance while also ensuring life history events were fully within the survey window as timing of life history events can vary regionally and across years and study sites48.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
This project was supported by the US National Park Service, Boone and Crockett Program at Michigan State University, the National Park Foundation, and Edna Bailey Sussman Foundation. We thank Isle Royale National Park Staff for assistance in project logistics and the wolf project’s postdocs and field and data technicians for their assistance with data collection and processing throughout this project.
Author contributions
All authors worked on review, and editing of the manuscript. HMB lead the investigation, data curation, methodology, formal analysis and main writing. MR, JB, LP, and HMB obtained resources for the study. MR, JB, and HMB acquired funding. HMB, JB, KK, and MR worked on the conceptualization of the manuscript.
Data availability
The data that support the findings of this study are available from the corresponding author, HMB, upon reasonable request.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Yoccoz, N. G., Nichols, J. D. & Boulinier, T. Monitoring of biological diversity in space and time. Trends Ecol. Evol.16, 446–453 (2001). [Google Scholar]
- 2.Lindenmayer, D. B. et al. Value of long-term ecological studies. Austral Ecol.37, 745–757 (2012). [Google Scholar]
- 3.Harris, N. C., Kauffman, M. J. & Mills, L. S. Inferences about ungulate population dynamics derived from age ratios. J. Wildl. Manage.72, 1143–1151 (2008). [Google Scholar]
- 4.DeCesare, N. J. et al. Estimating ungulate recruitment and growth rates using age ratios. J. Wildl. Manage.76, 144–153 (2012). [Google Scholar]
- 5.Van Ballenberghe, V. Productivity estimates of moose populations: a review and re- evaluation. Alces: J. Devoted Biology Manage. Moose. 15, 1–18 (1979). [Google Scholar]
- 6.Garel, M., Bonenfant, C., Hamann, J. L., Klein, F. & Gaillard, J. M. Are abundance indices derived from spotlight counts reliable to monitor red deer Cervus elaphus populations? Wildl. Biol.16, 77–84 (2010). [Google Scholar]
- 7.Van Kleunen, L. B. et al. Decision-making under uncertainty for species introductions into ecological networks. Ecol. Lett.26, 983–1004 (2023). [DOI] [PubMed] [Google Scholar]
- 8.Keiter, D. A. et al. Effects of scale of movement, detection probability, and true population density on common methods of estimating population density. Sci. Rep.7, 9446 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Samuel, M. D., Garton, E. O., Schlegel, M. W. & Carson, R. G. Visibility bias during aerial surveys of elk in northcentral Idaho. J. Wildl. Manage.51, 622–630 (1987). [Google Scholar]
- 10.Miquelle, D. G. Why don’t bull moose eat during the rut? Behav. Ecol. Sociobiol.27, 145–151 (1990). [Google Scholar]
- 11.de Vos, A., Brokz, P. & Geist, V. A review of social behavior of the North American cervids during reproductive period. Am. Midl. Nat.77, 390–417 (1967). [Google Scholar]
- 12.Solberg, E. J., Rolandsen, C. M., Heim, Linnell, J. D. C., Herfindal, I. & Sæther, B. E. Age and sex-specific variation in detectability of moose (Alces alces) during the hunting season: implications for population monitoring. Eur. J. Wildl. Res.56, 871–881 (2010). [Google Scholar]
- 13.Ballard, W. B., Spraker, T. H. & Taylor, K. P. Causes of neonatal moose calf mortality in South central Alaska. J. Wildl. Manage.45, 335–342 (1981). [Google Scholar]
- 14.Bowyer, R., Ballenberghe, V. & Kie, J. Moose (Alces alces). in 931–964 (2003).
- 15.Borowik, T., Kowalczyk, R., Maślanko, W., Duda, N. & Ratkiewicz, M. Annual movement strategy predicts within-season space use by moose. Behav. Ecol. Sociobiol.75, (2021).
- 16.Ditmer, M. A. et al. Moose at their bioclimatic edge alter their behavior based on weather, landscape, and predators. Curr. Zool.64, 419–432 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ecology and Management of the North American Moose. The Journal of Wildlife Management (University Press of Colorado, 2007).
- 18.Solberg, E. J., Heim, M., Grøtan, V., Sæther, B. E. & Garel, M. Annual variation in maternal age and calving date generate cohort effects in moose (Alces alces) body mass. Oecologia154, 259–271 (2007). [DOI] [PubMed] [Google Scholar]
- 19.Moll, R. J. et al. A review of methods to estimate and monitor moose density and abundance. Alces58, (2022).
- 20.Rönnegård, L., Sand, H., Andrén, H., Månsson, J. & Pehrson, Å. Evaluation of four methods used to estimate population density of moose Alces alces. Wildl. Biol.14, 358–371 (2008). [Google Scholar]
- 21.Gasaway, W. C., Dubois, S. D. & Harbo, S. J. Biases in aerial transect surveys for moose during May and June. J. Wildl. Manage.49, 777–784 (1985). [Google Scholar]
- 22.Gasaway, W. C., DuBois, S. D., Reed, D. J. & Harbo, S. J. Estimating Moose Population Parameters from Aerial Surveys. (1986). http://hdl.handle.net/11122/1498
- 23.Brinkman, T. J., Kellie, K. A., Reinking, A. K. & Liston, G. E. Boelman, N. T. Changing snow conditions are challenging moose (Alces alces) surveys in Alaska. Wildl. Soc. Bull.48, e1555 (2024). [Google Scholar]
- 24.Crum, N. J., Fuller, A. K., Sutherland, C. S., Cooch, E. G. & Hurst, J. Estimating occupancy probability of moose using Hunter survey data. J. Wildl. Manage.81, 521–534 (2017). [Google Scholar]
- 25.Rolandsen, C. M., Solberg, E. J., Tufto, J., Sæther, B. E. & Heim, M. Factors affecting detectability of moose Alces alces during the hunting season in Northern Norway. Alces39, 79–88 (2003). [Google Scholar]
- 26.Burton, A. C. et al. Wildlife camera trapping: a review and recommendations for linking surveys to ecological processes. J. Appl. Ecol.52, 675–685 (2015). [Google Scholar]
- 27.Kays, R. et al. An empirical evaluation of camera trap study design: how many, how long and when? Methods Ecol. Evol.11, 700–713 (2020). [Google Scholar]
- 28.House of Representatives, Congress. 16 U.S.C. 1131 – National Wilderness Preservation System & U.S. Government Publishing Office. (2011). https://www.govinfo.gov/app/details/USCODE-2011-title16/USCODE-2011-title16- chap23-Sect. 1131.
- 29.Risenhoover, K. L. Winter activity patterns of moose in interior Alaska. J. Wildl. Manage.50, 727–734 (1986). [Google Scholar]
- 30.Solberg, E. J. et al. Age and sex-specific variation in detectability of moose (Alces alces) during the hunting season: implications for population monitoring. Eur. J. Wildl. Res.56, 871–881 (2010). [Google Scholar]
- 31.Boone, H. M. et al. Dynamic Recreational Trail Use Alters Mammal Diel and Space Use during and after Covid- 19 in a U.S. National Park. Biological Conservation (in review).
- 32.Cusack, J. J. et al. Random versus game Trail-Based camera trap placement strategy for monitoring terrestrial mammal communities. PLoS One. 10, e0126373 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kolowski, J. M. & Forrester, T. D. Camera trap placement and the potential for bias due to trails and other features. PLoS One. 12, e0186679 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kays, R. et al. Does hunting or hiking affect wildlife communities in protected areas? J. Appl. Ecol.54, 242–252 (2016). [Google Scholar]
- 35.Moeller, A. K. & Lukacs, P. M. SpaceNtime: an R package for estimating abundance of unmarked animals using camera-trap photographs. Mammalian Biology. 102, 581–590 (2022). [Google Scholar]
- 36.Ericsson, G. & Wallin, K. Hunter observations as an index of moose Alces alces population parameters. Wildl. Biol.5, 177–185 (1999). [Google Scholar]
- 37.Moeller, A. K., Waller, S. J., DeCesare, N. J., Chitwood, M. C. & Lukacs, P. M. Best practices to account for capture probability and viewable area in camera-based abundance Estimation. Remote Sens. Ecol. Conserv.9, 152–164 .
- 38.Cusack, J. J. et al. Applying a random encounter model to estimate Lion density from camera traps in Serengeti National park, Tanzania. J. Wildl. Manage.79, 1014–1021 . [DOI] [PMC free article] [PubMed]
- 39.Romanski, M. C. et al. Wolves and the Isle Royale environment: restoring an Island ecosystem 2018–2020. (2020). https://digitalcommons.mtu.edu/michigantech-p/15560
- 40.Hoy, S. R., Perterson, R. O. & Vucetich, J. A. Ecological studies of wolves on Isle Royale annual report 2021–2022. (2022).
- 41.Peterson, R. O., Thomas, N. J., Thurber, J. M., Vucetich, J. A. & Waite, T. A. Population limitation and the wolves of Isle Royale. J. Mammal. 79, 828–841 (1998). [Google Scholar]
- 42.Hedrick, P. W., Kardos, M., Peterson, R. O. & Vucetich, J. A. Genomic variation of inbreeding and ancestry in the remaining two Isle Royale wolves. J. Hered.108, 120–126 (2017). [DOI] [PubMed] [Google Scholar]
- 43.National Park Service. Geospatial data for vegetation mapping inventory project of Isle Royale National Park. (2020).
- 44.Sovie, A. R. et al. Using distance sampling to estimate moose abundance in Isle Royale National Park. Alces (2024).
- 45.Sanders, S. & Kirschbaum, J. Woody species response to altered herbivore pressure at Isle Royale National park. Ecosphere14, e4623 (2023). [Google Scholar]
- 46.Moll, R. J. et al. The effect of camera-trap viewshed obstruction on wildlife detection: implications for inference. Wildl. Res.47, 158–165 (2020). [Google Scholar]
- 47.Ausband, D. E. et al. Estimating Wolf abundance from cameras. Ecosphere13, e3933 (2022). [Google Scholar]
- 48.Saether, B. E., Andersen, R., Hjeljord, O. & Heim, M. Ecological correlates of regional variation in life history of the moose Alces Alces. Ecology77, 1493–1500 (1996). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author, HMB, upon reasonable request.