Abstract
Breast cancer is the most common neoplasm among women, and sleep disturbances are prevalent symptoms that affect their quality of life. Despite evidence suggesting that sleep problems are common in this population, there is a lack of research that comprehensively analyses these aspects. This study focuses on assessing sleep quality, insomnia severity and its impact on several variables, including health-related quality of life, in women diagnosed with breast cancer. To assess sleep quality, insomnia severity and its impact on several variables, including health-related quality of life, in women diagnosed with breast cancer. An observational study was conducted with 245 women diagnosed with breast cancer. Participants with conditions that could interfere with sleep were excluded. Standardised questionnaires, such as the Pittsburgh Sleep Quality Index and the Insomnia Severity Index, were used. In addition, the European Organization for the Research and Treatment of Cancer Quality of Life and Breast Cancer Questionnaires were administered and sociodemographic, clinical and treatment-related variables were recorded. Almost 66% of the participants reported poor sleep quality. Sleep disturbances were associated with a significant deterioration in participants’ quality of life, affecting both their daily functioning and intensity of the symptoms experienced. Women in chemotherapy treatment showed greater severity of insomnia (8.30 ± 6.36; p < 0.001) and significantly worse sleep quality (8.80 ± 4.91; p < 0.001). A greater number of chemotherapy cycles was also associated with an increase in insomnia symptoms (p = 0.015). Women with breast cancer experience a high prevalence of sleep disturbances, which negatively impacts their quality of life. The need to systematically identify and address sleep problems throughout treatment and recovery is emphasised, as these disturbances can affect patients’ overall well-being and functional ability.
Keywords: Breast cancer, Sleep disturbances, Quality of life, Functional impact, Comprehensive oncological care
Subject terms: Breast cancer, Quality of life, Sleep disorders
Introduction
Breast cancer (BC) is the most frequently diagnosed cancer in women, accounting for an estimated 2.3 million new cases, representing 11.7% of all cancer cases. Among the various symptoms experienced by women with BC, sleep problems are prevalent1. In Spain, the estimated incidence rate of breast cancer in 2024 is 147.3 cases per 100,000 women (IC 95%: 130.1–165.9)2.
While numerous studies have explored the relationship between symptom clusters in BC and quality of life (HRQoL)3–5studies focusing primarily on sleep, i.e. investigating factors potentially contributing to poor sleep health, such as physical and psychological distress symptoms, and lifestyle stressors, are lacking. The measurement of specific dimensions of sleep health is crucial for a comprehensive understanding of the types of sleep issues that women with BC may encounter6.
Sleep disturbance in women with BC is an often-overlooked and potentially modifiable risk factor that may contribute to poor survival, reduced HRQoL, and medication non-adherence7. Despite its high prevalence, sleep disturbances remain underassessed in clinical practice, limiting timely interventions that could improve patient outcomes. The overall prevalence of poor sleep quality in women with BC is estimated to be 62%8, presenting both before and during treatment, and sometimes persisting for years afterward9,10. Sleep disturbances in BC patients have been associated with various factors such as timing of diagnosis11 and surgery12. BC itself is a well-established risk factor for sleep disturbances, as both the disease and its treatments disrupt normal sleep patterns and exacerbate pre-existing sleep problems. While other cancer-related symptoms resolve, once established insomnia tends to persist and impairs daytime functioning and quality of life. It is also a risk factor for comorbid conditions, to which cancer survivors are already more prone. For those patients who experienced insomnia even before their diagnosis, the majority support the notion that having cancer has exacerbated their sleep difficulties, specifically insomnia13. Prevalence rates of sleep disorders are also found to be up to three times higher in patients undergoing chemotherapy compared to the general population12,14.
Several types of sleep and wakefulness disorders exist, with insomnia being the most common, followed by hypersomnolence. Insomnia is defined as the inability to initiate sleep, difficulty maintaining sleep, or waking prematurely at least three days a week for a minimum of three months15. Hypersomnolence includes a group of disorders in which the primary complaint is excessive daytime sleepiness not caused by a circadian rhythm sleep-wake disorder or disturbed sleep due to another untreated sleep disorder such as sleep apnea16. While hypersomnia refers to specific disorders such as idiopathic hypersomnia, the term hypersomnolence is used to describe symptoms that include excessive sleepiness and increased sleep duration. Daytime sleepiness is understood as the inability to stay awake and alert during most daytime situations16. The consequences of insomnia are numerous and can negatively impact both psychological and physical functioning. Notably, persistent sleep disturbances may contribute to long-term declines in HRQoL and overall health status, even after treatment has concluded. Insomnia may contribute to the onset of fatigue, anxiety, depression, cognitive disturbances, immunosuppression, it can affect HRQoL, and it can even influence the course of the disease17–19. However, it has also been demonstrated that the presence of symptoms such as anxiety, pain, dyspnea, or fatigue can trigger or worsen sleep disorders12,14.
Two groups of symptoms have been identified in women undergoing treatment for BC: an upper gastrointestinal group and a psychoneurological group20. The latter is further divided into several subgroups, the first subgroup corresponding to psychoemotional symptoms (state anxiety and trait anxiety), the second group corresponding to symptoms of pain, fatigue, and cognitive impairment, and the third subgroup to symptoms related to sleep disturbance21. It is common for these symptoms not to appear in isolation, which means that the presence of one of them can trigger the appearance or exacerbation of other symptoms in its group22. Additionally, we know that the presence of this cluster of symptoms during the active oncologic treatment process has a significant impact on various dimensions of quality of life, and on emotional, physical, cognitive, and social functioning21.
Focusing on a potential explanation of the mechanisms underlying the relationship between sleep problems and cancer, there is substantial scientific evidence on the pathways linking cancer and the brain and how this leads to altered sleep patterns. Tumors disrupt normal homeostatic processes, resulting in aberrant changes in physiology and behavior that are detrimental to health23. However, disentangling cause and effect in cancer-related sleep disruption is challenging, complicating the identification of direct links between cancer progression and sleep disturbances. Various mechanisms have been studied to explain the relationship between cancer and sleep problems. For instance, the effects triggered by cytokines and growth factors secreted by tumors at the cerebral level, alterations in metabolism and subsequent energy balance mediated by hormones such as ghrelin or leptin, and changes in blood glucose and amino acid concentrations along with pH levels23.
The interaction between sleep and BC treatments is of fundamental importance, as the response to chemotherapy and immunotherapy requires proper immune system functioning significantly influenced by sleep24. Addressing sleep disturbances in clinical practice is crucial for optimizing treatment efficacy and improving patient well-being. Among possible interventions for sleep problems in women with BC, the effectiveness of cognitive-behavioral therapy has been demonstrated25. Lifestyle changes, such as physical exercise26 or dietary changes27have been shown to have positive effects.
Numerous studies have attempted to identify the association between HRQoL, various symptoms, and different treatment trajectories. BC survivors exhibit more severe symptoms and worse HRQoL during treatment, although these improve after treatment. However, these patients maintain an overall lower health status than the general population, even long after the completion of treatment28.
The objectives of this study are to evaluate sleep quality and its relationship with clinical and sociodemographic variables, analyze the prevalence of sleep disturbances (insomnia and excessive daytime sleepiness), and examine their relationship with sociodemographic, clinical, and HRQoL variables in BC patients.
Methods
Design
We conducted an observational study utilizing a cross-sectional design with a non-probability sampling approach at Badajoz University Hospital in Spain, spanning the period from 2021 to 2023. The primary focus of the investigation was on women diagnosed with BC actively undergoing oncological treatment. This study was reported according to the STROBE checklist.
Study setting, sampling and selection criteria
The sample consisted of 245 patients. Participants were selected through intentional or convenience non-probabilistic sampling. All patients met the selection criteria and all participants voluntarily provided informed consent prior to their involvement in the study, thereby upholding ethical standards and respecting the individuals who contributed to the research.
In terms of inclusion criteria, participants were required to meet specific conditions, while a comprehensive set of exclusion criteria was meticulously applied: being a minor, surpassing the age of 85 years, lacking registration as a patient at Badajoz University Hospital, failure to provide informed consent, presenting a neurological or cognitive impairment that could impede the proper execution of the assessment, having a history of prior treatment for a different type of primary cancer, possessing a diagnostic record of comorbidity associated with depression, anxiety and/or cognitive impairment, encountering linguistic or communicative barriers, having a pre-existing diagnosis of a psychiatric disorder, and currently undergoing psychopharmacological and/or psychotherapeutic treatment.
Instruments and measures
Sociodemographic questionnaire
An ad hoc questionnaire was designed for comprehensive data. It covered aspects such as gender, age, marital status, number of children, educational level, responsibility in the care of dependents, and employment status.
Medical history
A tailored questionnaire, informed by individual medical records and guided by the Oncology Medical Unit, was devised for a comprehensive data collection initiative. This instrument encompassed various patient-related parameters, including current status, time elapsed since diagnosis, body fat mass (BMI) and its classification according to the criteria of the Spanish Society for the Study of Obesity (SEEDO)29comorbidities, menopausal details, breast involvement, TNM classification, histological grade, molecular markers (RE, RP, Her2, Ki67), family cancer history, adjuvant-neoadjuvant therapy, surgery type, oncological treatment, sleep quality, insomnia, daytime sleepiness and HRQoL. The questionnaire aimed to yield a nuanced understanding of each patient’s circumstances and experiences within the context of BC.
Pittsburgh sleep quality index (PSQI)
The PSQI is a comprehensive self-report questionnaire utilized for the evaluation of sleep quality in adults. This assessment encompasses various dimensions of sleep experienced over the preceding month, encompassing aspects such as sleep duration, disturbances, onset latency, efficiency, medication utilization, and daytime dysfunction. Each of these components is rated on a scale from 0 to 3, with elevated scores indicative of more pronounced sleep-related difficulties. The sum of these component scores yields a comprehensive PSQI score, with a potential range from 0 to 21. According to Buysse et al.30 a total score of 5 is the cut-off point; thus a score equal to or less than 5 indicates good sleepers.
Insomnia severity index (ISI)
The ISI is a reliable self-report tool for measuring insomnia perception. With seven items rated on a 0 to 4 scale, it assesses sleep onset, maintenance, early awakening, satisfaction with sleep, daily functioning interference, impairment noticeability, and distress level. Total scores range from 0 to 28, indicating absence (0–7), sub-threshold (8–14), moderate (15–21), or severe (22–28) insomnia31.
Epworth sleepiness scale (ESS)
The ESS is a commonly used questionnaire designed for the evaluation of daytime sleepiness in individuals32. It comprises eight self-report items, each depicting various typical situations. Respondents are tasked with rating their propensity to fall asleep on a scale ranging from 0 to 3, where 0 denotes no likelihood of falling asleep and 3 signifies a substantial likelihood. The individual scores for each item are subsequently summated to yield a comprehensive ESS score, which falls within the range of 0 to 24. Elevated scores on this scale indicate a heightened level of daytime sleepiness33. A score ≥ 10 points would indicate daytime sleepiness32. The score is classified as follows: 0–9 normal, 10–12 mild sleepiness, 13–16 moderate sleepiness and > 16 severe sleepiness.
European organization for the research and treatment of cancer quality of life questionnaire (EORTC QLQ-C30)
The EORTC QLQ-C30 is a 30-item questionnaire designed to assess the impact of cancer on individuals’ quality of life. It comprises three scales: global health status (GHS) and HRQoL, functional scales covering physical (PF), role (RF), emotional (EF), cognitive (CF), and social functioning (SF); and symptom scales addressing fatigue (FA), nausea and vomiting (NV), pain (PA), dyspnea (DY), insomnia (SL), appetite loss (AP), constipation (CO), diarrhea (DI), and financial difficulties (FI). Higher scores on functional scales indicate healthier functioning, and an elevated GHS score signifies a better quality of life. Conversely, higher scores on symptom scales or items denote more pronounced symptomatology or problems34.
Quality of life questionnaire of breast cancer (QLQ-BR23)
The QLQ-BR23 serves as a supplementary questionnaire specifically tailored for individuals diagnosed with BC. It encompasses 23 questions, which are further classified into two scales. The functional scales: body image (BRBI), sexual functioning (BRSEF), sexual enjoyment (BRSEE), future perspective (BRFU). Otherwise, the symptom scales are: systemic therapy side effects (BRST), breast symptoms (BRBS), arm symptoms (BRAS), and upset by hair loss (BRHL). The scoring methodology applied to QLQ-BR23 mirrors that used for the functional and symptom scales or single items within the QLQ-C3035.
Data collection
A proficient researcher administered detailed clinical interviews, distributing study questionnaires to each participant. All documentation transpired in-person within the Medical Oncological Service, closely supervised by the researcher.
Data analysis
The variables were studied both from a descriptive and an inference point of view. The socio-demographic and clinical characteristics of the total number of enrolled women were analyzed with descriptive statistics in terms of mean ± standard deviation and percentages. Some results are expressed in terms of median and interquartile range. Specifically, the time since diagnosis was treated as an independent variable, and its influence on other variables was scrutinized via linear regression. A multiple correspondence analysis (MCA) was performed to detect the principal associations in the consumption of medication. The inference was carried out by Student’s t-test, one way ANOVA test, and chi-square test as required. The correlation between the quantitative variables was calculated by means of Pearson’s correlation coefficient.
All statistical analyses were conducted utilizing IBM Corp.‘s IBM SPSS Statistics for Windows, Version 22.0, released in 2013, based in Armonk, NY. The significance level (α) for all analyses was set at p ≤ 0.05.
Results
Socio-demographic and clinical characteristics
A total of 245 women with an average age of 53.31 ± 10.83 years (range 29–83 years), diagnosed with BC under treatment, took part in our study. The sociodemographic and clinical variables of the sample are detailed in Table 1. The average time since diagnosis of BC was 23.02 ± 42.87 months (median = 5, IQR = 17). However, for 73.4% of the sample, no more than 12 months had elapsed since diagnosis.
Table 1.
Social, demographic and clinical characteristics of the patients included in the study (mean age ± standard deviation).
| Variable | Categories | N (%)/M ± SD |
|---|---|---|
| Age | 53.31 ± 10.83 | |
| Marital status | Married | 178 (72.7) |
| Single | 25 (10.2) | |
| Divorced | 19 (7.7) | |
| Widowed | 23 (9.4) | |
| Education level | No studies | 20 (8.2) |
| Elementary school | 66 (26.9) | |
| Middle school | 34 (13.9) | |
| High school | 58 (23.7) | |
| Higher education | 67 (27.3) | |
| Employment situation | Currently in employment | 26 (10.6) |
| Temporary sick leave | 108 (44.1) | |
| Permanent sick leave | 19 (7.8) | |
| Unemployed | 50 (20.4) | |
| Retired | 42 (17.1) | |
| BMI | 25.87 ± 5.15 | |
| Underweight (< 18.5) | 9 (3.7) | |
| Normal weight (18.5–24.9) | 109 44.5) | |
| Overweight grade I (25.0-26.9) | 31 (12.7) | |
| Overweight grade II (pre-obese) (27.0-29.9) | 44 (18) | |
| Obesity type I (30.0-34.9) | 37 (15.1) | |
| Obesity type II (35,0-39.9) | 8 (3.3) | |
| Obesity type III (morbid) (40.0-49.9) | 4 (1.6) | |
| Obesity type IV (extreme) (≥ 50) | 3 (1.2) | |
| Comorbidity | Yes | 154 (62.9) |
| No | 91 (37.1) | |
| Family history of BC | Yes | 93 (38) |
| No | 152 (62) | |
| Tumor staging | 0 | 10 (4.1) |
| I | 70 (28.6) | |
| II | 90 (36.7) | |
| III | 45 (18.4) | |
| IV | 30 (12.2) | |
| Grade | 1 | 34 (17) |
| 2 | 57 (28.5) | |
| 3 | 109 (54.5) | |
| Estrogen receptors | Yes | 195 (79.6) |
| No | 50 (20.4) | |
| Progesterone receptors | Yes | 145 (59.2) |
| No | 100 (40.8) | |
| Molecular subtype | Luminal A/ Luminal B HER2 negative-like | 126 (51.4) |
| Luminal B HER2 positive-like/HER2-type | 93 (38) | |
| Triple negative | 26 (10.6) | |
| Breast | Left | 133 (54.3) |
| Right | 93 (38) | |
| Both | 19 (7.8) | |
| Current situation | Initial treatment | 179 (73.1) |
| Relapse | 43 (17.6) | |
| Under review | 23 (9.4) | |
| Therapy | Neoadjuvant | 46 (18.8) |
| Adjuvant | 199 (81.2) | |
| Menopause | Natural | 103 (43.3) |
| Drug-induced menopause | 69 (28.4) | |
| Intervention-induced menopause | 14 (5.7) | |
| Reproductive stage | 56 (22.9) | |
| Treatment | Surgery | 184 (75.1) |
| Chemotherapy | 209 (85.3) | |
| Radiotherapy | 105 (42.9) | |
| Hormonotherapy | 84 (34.3) | |
| Immunotherapy | 53 (21.6) | |
| Treatment combinations | Chemotherapy | 33 (13.4) |
| Surgery | 8 (3.3%) | |
| Surgery and chemotherapy | 44 (18) | |
| Other combinations | 160 (65.3) | |
| Surgical treatment | Conservative surgery | 121 (49.4) |
| Uni- or bilateral mastectomy | 63 (25.7) | |
| Without surgical treatment | 61 (24.9) | |
| Chemotherapy cycles | Chemotherapy cycles < 4 | 146 (59.6) |
| Chemotherapy cycles ≥ 4 | 99 (40.4) |
In the chemotherapy group, the average number of cycles in our sample was 5.90 ± 7.85. We established a cut-off point at ≥ 4 cycles, which gave the following result: 59.6% (n = 146) of patients had received < 4 cycles, and 40.4% (n = 99) had received > 4 cycles.
With regard to CT treatment and antineoplastic agents, we highlight the combinations with the highest results. For the joint analysis, medication consumed by less than 5% of the patients was not taken into account. MCA was performed to detect the principal associations in the consumption of medication; these associations were subsequently confirmed by the χ2 test. We highlight those which were highly significant (p < 0.001) (Table 2). The patients in the chemotherapy group were, therefore, treated with regimens of standard dose polychemotherapy, and the majority (59.2%; n = 145) received a combination of two or three cytotoxic agents such as doxorubicin (anthracycline agent), cyclophosphamide (alkylating agent), and docetaxel (taxane). The next largest group was pertuzumab and/or trastuzumab treatment (18.8%; n = 46).
Table 2.
Results of the most common treatment combinations.
| Group | Drugs | n | % |
|---|---|---|---|
| 1 | Denosumab | 3 | 1.2% |
| 2 | Doxorubicin + Cyclophosphamide + Docetaxel | 145 | 59.2% |
| 3 | Pertuzumab + Trastuzumab | 46 | 18.8% |
| 4 | Paclitaxel | 27 | 11% |
| 5 | Carboplatin | 18 | 7.3% |
| 6 | Other combinations of antineoplastic agents | 42 | 21% |
Prevalence of sleep disturbances
The mean sleep quality score (mean value of the PSQI) of the breast cancer patients was 8.44 ± 4.82 (Table 3). According to a threshold value of five on the PSQI, almost two thirds of the sample (65.3%; n = 160) of breast cancer women were considered as poor sleepers compared to 34.7% (n = 85) of the sample who were considered good sleepers.
Table 3.
PSQI scores and sleep disturbance scores of the sample.
| Full score | Min | Max | Score ≥ 2 (%) | M ± SD | |
|---|---|---|---|---|---|
| Global score | 21 | 0 | 20 | 8.44 ± 4.82 | |
| Sleep quality dimensions | |||||
| D1: Subjective sleep quality | 3 | 0 | 3 | 38.9 | 1.33 ± 0.83 |
| D2: Sleep latency | 3 | 0 | 3 | 47.4 | 1.51 ± 1.09 |
| D3: Sleep duration | 3 | 0 | 3 | 37.9 | 1.13 ± 1.12 |
| D4: Habitual sleep efficiency | 3 | 0 | 3 | 40.5 | 1.27 ± 1.23 |
| D5: Sleep disturbance | 3 | 0 | 3 | 40 | 1.47 ± 0.66 |
| D6: Hypnotic drugs | 3 | 0 | 3 | 34.8 | 1.06 ± 1.37 |
| D7: Daytime dysfunction | 3 | 0 | 3 | 13.7 | 0.75 ± 0.72 |
| Sleep quality | N (%) | ||||
|---|---|---|---|---|---|
| Poor sleepers | 160 (65.3) | ||||
| Good sleepers | 85 (34.7) | ||||
| Insomnia | N (%)/M ± SD | ||||
|---|---|---|---|---|---|
| 7.89 ± 6.22 | |||||
| No insomnia | 136 (55.5) | ||||
| Insomnia | 109 (44.5) | ||||
| Subclinical insomnia | 71 (29) | ||||
| Moderate clinical insomnia | 31 (12.7) | ||||
| Severe clinical insomnia | 7 (2.8) | ||||
| Daytime sleepiness | N (%)/M ± SD | ||||
|---|---|---|---|---|---|
| 5.80 ± 4.16 | |||||
| No daytime sleepiness | 202 (82.4) | ||||
| Daytime sleepiness | 43 (17.6) | ||||
| Mild daytime sleepiness | 23 (9.4) | ||||
| Moderate daytime sleepiness | 17 (6.9) | ||||
| Severe daytime sleepiness | 3 (1.2) | ||||
Evaluation of the PSQI component scores revealed that 38.9% of patients rated their subjective sleep quality as poor or very poor (D1). Additionally, 22.1% of participants required 31 to 60 min to fall asleep, while 25.3% needed more than 60 min (D2) (Table 3). The average sleep duration was 6.48 h per night; 16.3% of participants slept fewer than 5 h per day, and 21.6% slept between 5 and 6 h per day (D3). Furthermore, 40.5% exhibited a habitual sleep efficiency of less than 75% (D4), and 34.8% reported taking sleep medication in the past month (D6).
In this study, 40% of participants experienced sleep disturbances at least once per week (D5), with the most common reasons being the need to use the bathroom (57.2%) and waking up in the middle of the night or early morning (49%). Additionally, 46.3% of the target population reported poor daytime well-being, with 13.7% rating their well-being as quite poor, accompanied by diurnal dysfunctions such as fatigue and lack of energy (D7). The mean scores for each PSQI dimension are presented in Table 3.
Regarding sleep disturbances related to insomnia, the study determined a mean ISI score of 7.89 ± 6.22 for the sample, indicating a prevalence of insomnia in 44.5% of patients (n = 109). When categorizing ISI scores, we found that 29% of participants (n = 71) exhibited subclinical insomnia, 12.7% (n = 31) had moderate clinical insomnia, and 2.9% (n = 7) showed severe clinical insomnia (Table 3).
When assessing symptoms of daytime sleepiness, the ESS revealed that 17.6% of patients (n = 43) experienced excessive daytime sleepiness. Specifically, 9.4% (n = 23) of patients reported mild daytime sleepiness, 6.9% (n = 17) reported moderate daytime sleepiness, and 1.2% (n = 3) reported severe daytime sleepiness (Table 3).
Health-related quality of life
The results for the various functional and symptomatic HRQoL scales, as measured by the QLQ-C30 questionnaire, are presented in Table 4.
Table 4.
Results of the EORTC-QLQ-C30.
| EORTC QLQ-C30 | M ± SD |
|---|---|
| Global health status (GHS) | 63.55 ± 24.57 |
| Functional scales | |
| Physical functioning (PF) | 78.96 ± 22.20 |
| Role functioning (RF) | 76.46 ± 29.17 |
| Emotional functioning (EF) | 73.09 ± 23.37 |
| Cognitive functioning (CF) | 78.44 ± 29.38 |
| Social functioning (SF) | 75.91 ± 26.58 |
| Symptom scales/items | |
| Fatigue (FA) | 31.28 ± 27.21 |
| Nausea and vomiting (NV) | 7.50 ± 16.63 |
| Pain (PA) | 28.96 ± 29.74 |
| Dyspnea (DY) | 10.45 ± 24.20 |
| Insomnia (SL) | 3535 ± 35.72 |
| Appetite loss (AP) | 12.78 ± 24.52 |
| Constipation (CO) | 21.48 ± 30.64 |
| Diarrhea (DI) | 10.19 ± 21.75 |
| Financial difficulties (FI) | 17.02 ± 27.95 |
The GHS gave a mean of 63.55 ± 24.57. Higher scores on the functional scale items reflected better quality of life, whereas the symptom items reflected that the higher the score, the worse the symptom intensity. In this sense, scores were high on most quality of life dimensions (> 75 on the functional scales), with the exception of GHS and EF.
Regarding the items evaluated on the symptom scales we observed that the most disturbing quality of life symptoms were SL (35.35 ± 35.72), FA (31.28 ± 27.21), PA (28.96 ± 29.74) and CO (21.48 ± 30.64).
The QLQ-BR23 module allowed assessment of symptoms specifically related to breast cancer, treatment-associated side effects, and quality of life domains affected by both disease and treatment. Table 5 shows the results.
Table 5.
Results of the QLQ-BR23.
| EORTC QLQ-BR23 | M ± SD |
|---|---|
| Functional scales | |
| Body image (BRBI) | 78.36 ± 25.54 |
| Sexual functioning (BRSEF) | 70.67 ± 33.29 |
| Sexual enjoyment (BRSEE) | 69.13 ± 38.81 |
| Future perspective (BRFU) | 54.81 ± 34.31 |
| Symptom scales/items | |
| Systemic therapy side effects (BRST) | 26.83 ± 2038 |
| Breast symptoms (BRBS) | 18.11 ± 20.61 |
| Arm symptoms (BRAS) | 17.74 ± 22.50 |
| Upset by hair loss (BRHL) | 20.17 ± 34.96 |
From a functional perspective, the BRFU scale was the most affected, with a mean score of 54.81 ± 34.31. In terms of symptomatology, BRST and BRHL were the symptoms that had the most negative impact on patients’ HRQoL.
Factors related with sleep disturbances
Sociodemographic, clinical characteristics and sleep disturbances
We found no relationship between sleep quality, the presence of insomnia, or excessive daytime sleepiness and any of the sociodemographic variables studied, except age.
When analyzing sleep quality scores, we found a significant negative correlation with age (r=-0.190; p = 0.003), indicating poorer sleep quality among younger patients. Specifically, younger patients (mean age 49.97 ± 10.23 years) reported worse perceived sleep quality in D1 (p = 0.002) and a greater frequency of sleep disturbances in D5 over the past month (mean age 48.5 ± 9.56 years). A similar pattern was observed for insomnia (r=-0.175; p = 0.006) (Fig. 1), with younger patients exhibiting a higher symptomatic perception of insomnia for those meeting the insomnia threshold (cut-off score of 8).
Fig. 1.
Correlation/relationship between age and ISI scores.
The mean age for the group without insomnia was 54.60 ± 11.72 years, compared to 51.74 ± 9.44 years for the group with insomnia (p = 0.035) (Fig. 1).
With regard to excessive daytime sleepiness, the ESS scores showed no relationship with any of the sociodemographic variables, except age, with which it correlated inversely (r=-0.258; p < 0.001) (Fig. 2). Similar to findings in the insomnia study, hypersomnolence perception was higher among younger patients, with a cut-off point of 10 for this variable. The mean age for the group without excessive daytime sleepiness was 54.51 ± 10.74, while for the group with excessive daytime sleepiness it was 47.70 ± 9.48 (p < 0.001) (Fig. 2).
Fig. 2.
Relationship between age and ESS score.
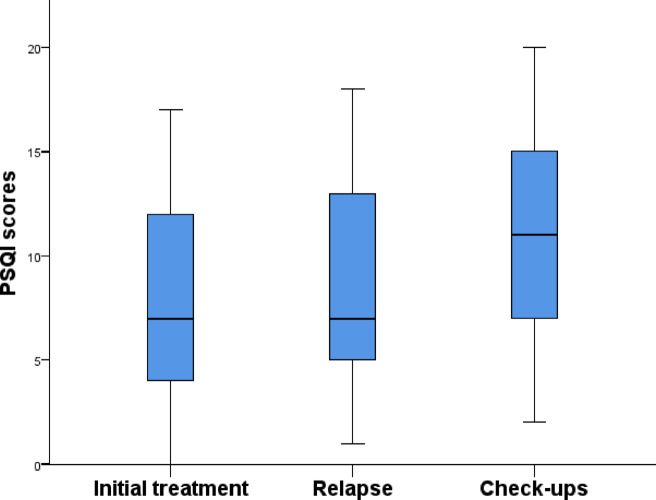
Pertaining to the current situation, a statistically significant relationship was found with perceived sleep quality as measured by the PSQI (p = 0.006). Patients under review scored higher on the PSQI (mean = 11.26 ± 4.99), indicating that this group had worse sleep quality compared to patients in initial treatment (mean = 7.99 ± 4.62) (Fig. 3). In fact, the χ2 test revealed a significant association between patients under review and poorer sleep quality (p = 0.023), with 91.3% of patients under review group being classified as poor sleepers, compared to 62.6% in the initial treatment group and 62.8% in the relapse group. A similar pattern was observed for insomnia, where the χ2 test showed a significant association between patients under review and clinical insomnia (p = 0.035). Specifically, 65.5% of patients under review reported suffering from insomnia, compared to 40.2% in initial treatment and 53.5% in relapse.
Fig. 3.
Relationship between current situation and PSQI.
We found no association with the rest of the clinical variables in the study.
Therapeutic management and sleep disturbances
The association between therapeutic management and sleep disturbances was only significant in our sample for the group of patients treated with different chemotherapy regimens versus the other types of therapy described in Table 1. Chemotherapy treatment was found to be associated with higher ISI scores (mean 8.30 ± 6.36 in the chemotherapy group vs. 5.47 ± 4.74 in the non-chemotherapy group; p < 0.001) (Fig. 4) and with clinically significant insomnia (χ2=5.000; p = 0.019) with 47.8% of chemotherapy patients experiencing insomnia compared to 27.8% in the non-treatment group.
Fig. 4.
Relationship between chemotherapy treatment and ISI scores.
Patients who received chemotherapy reported poorer sleep quality (mean 8.80 ± 4.91) compared to those who did not (mean 6.36 ± 3.37) (p < 0.001) (Fig. 5). The results also indicated that chemotherapy had a more negative impact than other treatments, particularly on the following dimensions: D1 perceived sleep quality (χ2 = 6.781, p = 0.009), D3 duration (χ2 = 7.915, p = 0.015), D4 efficiency (χ2 = 7.388, p = 0.025), and D5 sleep disturbances (χ2 = 5.918, p = 0.019). In this group, patients exhibited less efficient sleep (< 85%), lower sleep quality, more disturbances in the past month, and sleep duration of less than 7 h. A similar trend was observed regarding the number of chemotherapy cycles received. A significant correlation was found between sleep disturbances and the number of chemotherapy cycles, with higher PSQI and ISI scores associated with a greater number of cycles (r = 0.794, p < 0.001; r = 0.239, p < 0.001, respectively). Specifically, a higher number of chemotherapy cycles was linked to poorer sleep quality and increased clinical insomnia (p = 0.015, p = 0.047, respectively). In the analysis of sleep quality by dimension, a higher number of cycles was associated with worse scores in D1 perceived sleep quality (p = 0.026), D3 duration (p = 0.035), D4 efficiency (p = 0.017), and D5 sleep disturbances (p = 0.011).
Fig. 5.
Relationship between chemotherapy treatment and PSQI scores.
Receiving 4 or more cycles of chemotherapy was associated with insomnia (χ2 = 4.087; p = 0.029). Specifically, 53.7% of patients who had received 4 or more cycles of chemotherapy exhibited symptoms consistent with insomnia.
Likewise, in the pharmacological group of chemotherapy, patients treated with Group 3 (pertuzumab + trastuzumab) experienced higher levels of excessive daytime sleepiness compared to those who did not receive this treatment (p = 0.037) (Fig. 6).
Fig. 6.
Relationship between treatment with chemotherapy: Group 3 Pertuzumab_Trastuzumab and ESS scores.
Impact of sleep disturbances on health-related quality of life
The results corresponding to the study of the impact of sleep disturbances on HRQoL are shown in Table 6. Total scores from the three scales, cut-off points, and categories are reflected.
Table 6.
Results of the impact of sleep disturbances on HRQoL.
| PSQI | ISI | ESS | PSQI > 5 | ISI > 8 | ESS > 10 | ISI Categories | ESS Categories | |||
|---|---|---|---|---|---|---|---|---|---|---|
| GHS | r | -0.293 | -0.232 | -0.382 | – | – | – | – | – | |
| p | < 0.001* | < 0.001* | < 0.001* | < 0.001* | < 0.001* | < 0.001* | < 0.001* | < 0.001* | ||
|
Functional scales QLQ-C30 |
PF | r | -0.329 | -0.364 | -0.141 | – | – | – | – | – |
| p | < 0.001* | < 0.001* | 0.027** | < 0.001* | < 0.001* | 0.024** | < 0.001* | 0.011** | ||
| RF | r | -0.251 | -0.239 | -0.381 | – | – | – | – | – | |
| p | < 0.001* | < 0.001* | < 0.001* | < 0.001* | < 0.001* | < 0.001* | < 0.001* | < 0.001* | ||
| EF | r | -0.417 | -0.450 | -0.091 | – | – | – | – | – | |
| p | < 0.001* | < 0.001* | 0.157 | < 0.001* | < 0.001* | 0.018** | < 0.001* | 0.023** | ||
| CF | r | -0.233 | -0.295 | -0.375 | – | – | – | – | – | |
| p | < 0.001* | < 0.001* | < 0.001* | < 0.001* | < 0.001* | < 0.001* | < 0.001* | < 0.001* | ||
| SF | r | -0.258 | -0.204 | -0.361 | – | – | – | – | – | |
| p | < 0.001* | < 0.001* | < 0.001* | < 0.001* | < 0.001* | 0.016** | < 0.001* | 0.014** | ||
|
Functional scales QLQ-BR23 |
BRBI | r | -0.307 | -0.394 | -0.193 | – | – | – | – | – |
| p | < 0.001* | < 0.001* | 0.002** | < 0.001* | < 0.001* | 0.043** | < 0.001* | 0.008** | ||
| BRSEF | r | 0.011 | -0.120 | -0.104 | – | – | – | – | – | |
| p | 0.897 | 0.061 | 0.003** | 0.609 | 0.260 | 0.105 | 0.210 | 0.320 | ||
| BRSEE | r | 0.074 | -0.010 | -0.028 | – | – | – | – | – | |
| p | 0.245 | 0.086 | 0.661 | 0.548 | 0.937 | 0.144 | 0.230 | 0.280 | ||
| BRFU | r | -0.260 | -0.201 | 0.080 | – | – | – | – | – | |
| p | < 0.001* | 0.002** | 0.211 | < 0.001* | < 0.001* | 0.907 | < 0.001* | 0.425 | ||
|
Symptom scales QLQ-C30 |
FA | r | 0.354 | 0.487 | 0.258 | – | – | – | – | – |
| p | < 0.001* | < 0.001* | < 0.001* | < 0.001* | < 0.001* | < 0.001* | < 0.001* | < 0.001* | ||
| NV | r | 0.002 | 0.045 | 0.222 | – | – | – | – | – | |
| p | 0.974 | 0.449 | < 0.001* | 0.317 | 0.482 | < 0.001* | 0.106 | 0.306 | ||
| PA | r | 0.341 | 0.383 | 0.135 | – | – | – | – | – | |
| p | < 0.001* | < 0.001* | 0.035** | < 0.001* | < 0.001* | 0.007** | < 0.001* | < 0.001* | ||
| DY | r | 0.265 | 0.294 | 0.208 | – | – | – | – | – | |
| p | < 0.001* | < 0.001* | < 0.001* | 0.017** | < 0.001* | 0.008** | < 0.001* | 0.422 | ||
| SL | r | 0.668 | 0.751 | 0.052 | – | – | – | – | – | |
| p | < 0.001* | < 0.001* | 0.418 | < 0.001* | < 0.001* | < 0.001* | < 0.001* | < 0.001* | ||
| AP | r | 0.177 | 0.248 | 0.161 | – | – | – | – | – | |
| p | 0.005** | < 0.001* | 0.012** | 0.021** | < 0.001* | 0.016** | < 0.001* | 0.530 | ||
| CO | r | 0.291 | 0.313 | 0.099 | – | – | – | – | – | |
| p | < 0.001* | < 0.001* | 0.122 | < 0.001* | < 0.001* | 0.039** | < 0.001* | 0.456 | ||
| DI | r | 0.159 | 0.276 | 0.113 | – | – | – | – | – | |
| p | 0.013** | < 0.001* | 0.077 | 0.010** | < 0.001* | 0.005** | < 0.001* | 0.125 | ||
| FI | r | 0.326 | 0.348 | 0.111 | – | – | – | – | – | |
| p | < 0.001* | < 0.001* | 0.082 | < 0.001* | < 0.001* | 0.016** | < 0.001* | 0.009** | ||
|
Symptom scales QLQ-BR23 |
BRST | r | 0.430 | 0.523 | 0.179 | – | – | – | – | – |
| p | < 0.001* | < 0.001* | 0.005** | < 0.001* | < 0.001* | 0.032** | < 0.001* | 0.025** | ||
| BRBS | r | 0.300 | 0.280 | 0.174 | – | – | – | – | – | |
| p | < 0.001* | < 0.001* | 0.006** | < 0.001* | < 0.001* | < 0.001* | < 0.001* | < 0.001* | ||
| BRAS | r | 0.260 | 0.344 | 0.246 | – | – | – | – | – | |
| p | < 0.001* | < 0.001* | < 0.001* | < 0.001* | < 0.001* | < 0.001* | < 0.001* | < 0.001* | ||
| BRHL | r | 0.171 | 0.237 | 0.135 | – | – | – | – | – | |
| p | 0.007** | < 0.001* | 0.035** | 0.039** | 0.002** | 0.006** | < 0.001* | 0.012** |
* p-value ≤ 0.001. ** p-value ≤ 0.05.
HRQoL, insomnia, and excessive daytime sleepiness scores were inversely correlated with all the functional scales of the QLQ-C30, supporting the impact of these symptoms on the quality of life of our patients. A similar pattern was observed with the BR23 module, with a few exceptions: no correlation was found between the BRSEF and BRSEE functional scales and sleep quality or excessive daytime sleepiness, although BRSEF did correlate with the ESS excessive daytime sleepiness scores. Additionally, no correlation was found between BRFU scores and sleepiness.
Both PSQI and ISI scores showed correlations with all symptomatic scales, except for NV, which only correlated with ESS scores. These findings highlight the strong relationship between sleep disturbances, poor sleep, and symptomatic perceptions that influence HRQoL. However, excessive daytime sleepiness scores did not correlate with several symptoms on the quality of life scales: SL, CO, DI, and FI.
When examining the distribution of the sample with respect to the categorical variables of sleep quality, insomnia, and excessive daytime sleepiness at their respective cut-off points, the t-test confirmed that poorer sleep quality, insomnia, and excessive daytime sleepiness were associated with lower scores on the functional scales of both the QLQ-C30 and BR23, with the exception of the BRSEF and BRSEE scales. A similar association was observed for the symptom scales, except for NV.
Further analysis using ANOVA of the different ISI and ESS categories revealed the following:
Worse GHS is associated with increased levels of subclinical and moderate insomnia, as well as moderate and severe daytime sleepiness.
Higher ISI and ESS scores were linked to greater functional impairments in physical, emotional, cognitive, and social domains.
For the breast cancer-specific module, a relationship was found between insomnia categories and self-image and future concerns. Daytime sleepiness severity (moderate and severe) was only related to the BRBI scale.
All categories of insomnia exhibit a worse perception of all symptoms except NV. Severe and moderate daytime sleepiness were associated with FA, PA, SL, FI, BRBI, BRST, BRAS, and BRHL.
Discussion
Sociodemographic and clinical variables
The sample distribution in terms of sociodemographic characteristics is virtually consistent with previous studies analyzing age, marital status, education level, and employment status. The average age of the sample is 53.31 years, similar to recent studies3,36,37.
Regarding tumor characteristics, there is a higher incidence in the left breast (54.3%), consistent with previous findings38. Stage II (37.7%) is the most frequent, followed by stage I (28.6%), mirroring literature data39–41. Histological grade III (51.4%) is most common, consistent with immunohistochemical studies41. Hormone receptor positivity is between 60 and 80%, and 36.4% are HER2 positive, in line with previous samples42.
Concerning therapeutic management, nearly half underwent breast-conserving surgery, while around a quarter had radical surgery, as in previous studies43. Adjuvant treatment was received by 81.2%, consistent with BC samples41,44,45. The majority are undergoing initial oncological treatment, including chemotherapy (85.3%), radiotherapy (42.9%), hormone therapy (34.3%), and immunotherapy (21.6%), which are typical in BC therapeutic approaches46. Notably, the combination of doxorubicin-cyclophosphamide and docetaxel is the most frequent polychemotherapy treatment (59.2%)37,47.
Furthermore, 78% are menopausal, with drug-induced menopause in 30%. This group experiences symptoms affecting both physical and emotional aspects, which have a negative effect on their quality of life48.
Sleep disturbances
The prevalence of insomnia is around 45%, higher than recent studies using the same instruments on BC populations49although this percentage is similar to that found in a prospective observational study of 170 women diagnosed with breast cancer, which was 46%50. Objective sleep/wake rhythms, self-reported sleep quality, and insomnia symptoms indicate common sleep disturbances in cancer patients51. Poor sleep quality is clinically concerning, associated with reduced quality of life, diminished functioning, increased pain, lower energy, and mental health issues52.
A noteworthy 34.7% of the sample exhibited good sleep quality, categorized as proficient sleepers. In contrast, almost two-thirds of the participants, specifically 65.3%, displayed poor sleep quality, classified as inadequate sleepers measured by the PSQI. These findings align with previous studies that highlight the poor sleep quality exhibited by women suffering from this pathology53. In fact, a recent study by Reynolds-Cowie and Fleming54 found that the impact of insomnia was significant for all study participants and that many reported that the consequences of sleep deprivation were often more overwhelming than the impact of cancer treatment.
This finding highlights the need for sleep management in breast cancer chemotherapy patients. Sleep disturbances can lead to various medical and mental illnesses, resulting in cognitive decline, decreased appetite, and weakened immunity55.
In addition, the PSQI components revealed that 47.4% of participants reported that sleep latency was greater than 30 min, 40.5% had usual sleep efficiency less than 75%, 38.9% of the respondents described the quality of their sleep as poor or very poor, and 37.9% reported that sleep duration was less than 6 h. However, despite the high prevalence of sleep disturbances in the population, the majority (65.3%) did not use sleep medications. Our results are in line with previous studies, such as the one carried out in a sample of 337 breast cancer patients, using the PSQI56. It was found that 55.8% of sample had a significant sleep deficit, 76.2% reported sleep latency greater than 30 min, 47.8% of patients reported sleeping less than 6 h, 43.38% of patients had usual sleep efficiency scores of less than 75%, and 56.4% described their sleep quality as fairly or very poor.
With regard to dimension 3 sleep duration, the recommendations provided by the National Sleep Foundation57 advise that adults between 26 and 65 years old should sleep between 7 and 9 h per night, so in this study, almost 40% of the sample did not meet these recommendations.
Relationship between sleep disturbances, sociodemographic and clinical variables
A significant, negative correlation was found between insomnia and age, indicating a higher perception of symptomatic insomnia in younger patients. Previous studies have identified age as a significant predictor of symptom intensity in the psychoneurological group, particularly sleep disturbances. This study shows a negative association between age and insomnia, in line with other studies6. Consistent with recent literature, in a study on sleep disturbance and cancer-related fatigue symptom cluster in breast cancer patients undergoing chemotherapy58younger participants were more likely to be in the group with sleep complaints and fatigue compared to the group with minimal symptoms prior to chemotherapy. The effect of age on the intensity of insomnia could be attributed to several age-related characteristics. Firstly, younger patients generally tend to have higher levels of education and may be more proactive in self-assessing their symptoms. Secondly, it is possible that younger women may have faced greater physical and psychological burdens from domestic, familial, and professional responsibilities, as well as from their jobs, and therefore may not obtain sufficient rest. On the other hand, other studies suggest that breast cancer at younger ages is often characterized by being more aggressive and having worse outcomes. These women experience long-term and late effects of cancer treatments, such as fertility problems and treatment-associated amenorrhea, in addition to depression and fear of recurrence, more frequently than older women59.
Additionally, a statistically significant relationship was observed between perceived sleep quality (PSQI) and the current situation. Patients in the review situation report worse sleep quality than those in initial treatment. We found little evidence related to this finding, which leads us to suppose that it is possibly linked to time since diagnosis, chemotherapy duration, and uncertainty about disease recurrence. In this regard, Desai et al.60 found a higher prevalence of insomnia depending on the time elapsed. Women who had been diagnosed with breast cancer between two and five years previously were significantly more likely to report insomnia than those diagnosed within two years of their study entry. In this regard, Duzova et al.61 have recently associated sleep quality with time since diagnosis in breast cancer survivors. In any case, lack of sleep has been described as a persistently distressing complaint for patients long after completion of active cancer treatment54. Therefore, this aspect is often underestimated, underscoring the need for increased awareness among health professionals to effectively address long-term sleep disturbances throughout the cancer patient trajectory. This problem is highlighted by scientific evidence54 supporting that a lack of clinician understanding of the importance of sleep health and the limited availability of evidence-based insomnia interventions, such as cognitive behavioural therapy for insomnia (CBT-I), stand out as important gaps in cancer care.
Relationship between sleep disturbances and therapeutic management
The association between treatment modality and sleep disturbances was significant in women undergoing chemotherapy, as they experienced more intense symptoms related to insomnia and sleep quality. Chemotherapy treatment was found to be correlated with higher scores of insomnia and poor sleep quality, leading us to hypothesize that breast cancer patients encounter increased sleep problems, both in terms of quality and insomnia, during the active treatment phase and also subsequently. These findings concur with reports from other authors indicating that insomnia is more common in patients post-chemotherapy compared to those not undergoing chemotherapy62,63and that patients not receiving chemotherapy are much less likely to develop insomnia compared to those undergoing chemotherapy. Indeed, in the observational study by Fleming et al., chemotherapy was identified as the main risk factor for persistent insomnia50.
Furthermore, clinically significant insomnia and poor sleep quality were found to be associated with a higher number of chemotherapy cycles, specifically insomnia with a cut-off point of 4 or more cycles. There is evidence to suggest that sleep disturbances worsen after the initiation of chemotherapy and accumulate over the course of treatment. In line with this, our results are consistent with those found by Putri & Makiyah64who reported that chemotherapy treatment was a significant predictor for the psychoneurological group experiencing sleep disturbances at different stages of treatment, and that patients receiving chemotherapy had more intense symptoms in this regard.
In a study by Ancoli-Israel et al.65 on sleep, fatigue, depression, and circadian activity rhythms in women with breast cancer, disruptions in circadian activity rhythms were often observed in cancer patients. Compared to healthy controls, breast cancer patients undergoing chemotherapy experienced greater circadian disruption (increased daytime napping), poorer sleep quality, more fatigue, more depressive symptoms, more disrupted circadian activity rhythms, and poorer quality of life at baseline. Additionally, from the fourth cycle onward, all the symptoms worsened: patients exhibited poorer sleep, increased fatigue, more depressive symptoms, and more disrupted circadian activity rhythms compared to their own baseline levels and healthy controls. Our results corroborate these findings, as we found that a higher number of chemotherapy cycles corresponded to poorer sleep quality and clinical insomnia. Moreover, starting from the fourth chemotherapy cycle, there was a clear deterioration in symptoms consistent with clinical insomnia. These results confirm the significant temporal effect of chemotherapy treatment, as pointed out by recent studies58.
In an assessment of sleep pattern disturbances in breast cancer patients undergoing adjuvant treatment with chemotherapy and/or radiotherapy through polysomnography conducted by Eldin et al.66 the severity of insomnia for patients receiving chemotherapy, compared to those without treatment and the control group, statistically correlated. Higher scores of insomnia symptoms were associated with chemotherapy treatment. The comparison between patients who received treatment, those who did not, and the control groups showed a significant reduction in total sleep time, decreased sleep efficiency, and prolonged sleep latency for the chemotherapy group. Excessive daytime sleepiness was also significant for patients receiving chemotherapy, both before treatment and in the respective control groups.
The abovementioned study provides evidence that the severity of insomnia increases with the number of chemotherapy cycles and differs among chemotherapy drugs66. Specifically, patients receiving pertuzumab and/or trastuzumab exhibit higher levels of excessive daytime sleepiness, consistent with drug profiles describing this symptom as very common (10%)67.
Relationship between sleep disturbances and quality of life
Sleep problems were found to correlate with diminished quality of life. Scores for sleep quality, insomnia, and excessive daytime sleepiness exhibited an inverse correlation with all functional scales of the QLQ-C30, confirming the impact of these symptoms on the quality of life of our patients. A similar pattern emerged in the BR23 module, with some exceptions: there was no correlation for the BRSEF and BRSEE functional scales with sleep quality. These results show that all the sleep indicators statistically significantly predict physical and mental HRQoL as compared to other studies that found that all sleep outcomes, except insufficient sleep duration, were associated with physical HRQoL, but not with mental HRQoL68. Previous studies have also observed a relationship between sleep disturbance and general HRQoL in breast cancer patients at varying stages of the clinical care trajectory, including with objectively measured sleep69.
Both PSQI and ISI scores correlated with all the symptomatic scales. These data highlight the close relationship existing between sleep disturbances, poor sleep, and symptomatic perceptions that impact quality of life. These results are supported by previous findings which indicate that poorer sleep quality correlates with a lower quality of life, physical and functional well-being sub-scales being the most strongly associated, and overall, that individuals with sleep problems fared worse than those with normal sleep patterns21,65,68.
Specifically, our results obtained from the symptom scales of the QLQ-C30-BR23 module are supported by recent studies analyzing quality of life using this module50which align with our findings. Patients with high scores for systemic therapy side effects were much more likely to develop insomnia, as were patients with high arm symptom scores.
Our findings contribute to this research by demonstrating the independent association between self-reported sleep problems and physical HRQoL in women treated for cancer using standardised measures of these constructs. As such, unassessed and untreated sleep problems may undermine the physical domains of HRQoL throughout the cancer care trajectory, as shown by current studies68. Insomnia has been shown to have a detrimental and pervasive impact on the quality of life of cancer survivors, persisting even long into survivorship54.
The data presented here are useful for identifying patients most likely to experience sleep disturbances, while minimising confounding factors related to mixed cancer sites and various treatment regimens. Athough by focusing specifically on the evolution of insomnia in breast cancer clearer comparisons within groups are obtained, there is still variation in terms of disease characteristics, treatment regimens, and follow-up and post-graduate support. This diversity may explain why symptom management strategies within oncology services are often fragmented and piecemeal. It may also explain the well-documented absence of effective sleep management programmes for cancer patients, despite considerable evidence supporting cognitive behavioral therapy as a first-line treatment for cancer-associated insomnia50. This absence may be explained by a lack of appreciation of the extent to which insomnia episodes will persist or remit over time. Therefore, the importance of assessing the evolution of insomnia in cancer patients is evident and special attention should be paid to the post-treatment survival phase and may also be an optimal time to offer therapeutic intervention to those suffering from persistent sleep disorders after cancer54.
Strengths of this study include the recruitment of a clinical sample of breast cancer patients directly from an Oncology Medical Unit, with very few exclusion criteria. This emphasizes the generalizability of study outcomes. However, the study has limitations that may affect the generalizability and reliability of its findings. The cross-sectional design used cannot identify causal paths between variables. Another situation is that reliance on self-report measures of sleep quality may lead to bias in reporting. Additionally, there were no measures of body fat, sedentary lifestyle, pain or sleep disordered breathing, which may play an important role in sleep disturbance. In addition, the exclusion of patients with mood disorders may limit the generalisability of our results. However, this decision was made to minimise confounding, as the association between these disorders and sleep disturbances had already been demonstrated in a previous study by our group. These limitations need to be addressed in future research to gain a more robust understanding of the impact of treatment characteristics and sleep quality to the recovery process and quality of life.
Conclusions
Women with breast cancer experience a high prevalence of sleep disturbances, which negatively impacts their quality of life. Nearly three-thirds of the sample exhibited poor sleep quality, and almost half displayed symptoms consistent with insomnia. Younger patients and those who received chemotherapy as a treatment modality, particularly those undergoing 4 cycles or more and on a pertuzumab and trastuzumab therapeutic regimen, exhibited a significant deterioration in sleep patterns as manifested by poorer sleep quality and a higher prevalence of insomnia symptoms. The classification of groups with varying symptom management needs and the distinction of groups based on baseline sociodemographic/medical variables can help to recognise patients at risk of an increased symptom load, while simultaneously minimising confounding factors related to different treatment regimens.
These findings clarify which patients may benefit from additional assessment and early intervention to prevent or reduce sleep disturbances and associated daytime impairment minimising symptom burden during chemotherapy such as cognitive behavioural therapy for insomnia (CBT-I) recommended as first-line treatment for insomnia but rarely available for cancer survivors according to the evidence. All the aforementioned data can inform targeted interventions to improve patients’ overall cancer experiences. Furthermore, the existence of sleep problems was associated with a decrease in both physical and mental HRQoL, so improving sleep through specific interventions should improve their HRQoL.
Acknowledgements
To all patients and professionals involved in the study, without whose contribution this study would not have been possible.
Author contributions
Conceptualization: ND-G, CFL-J, JM-F, MAM-P, MN-D, MCC. Methodology: ND-G, CFL-J, JM-F, MAM-P, MN-D, MCC. Validation: ND-G, CFL-J, JM-F, MAM-P, MN-D, MCC. Formal análisis: JM-F, MAM-P. Investigation: ND-G, CFL-J, JM-F, MAM-P, MN-D, MCC. Data curation: ND-G and MCC. Writing—original draft preparation: ND-G, CFL-J, JM-F, MAM-P, MN-D, MCC. Writing—review and editing: ND-G, CFL-J, JM-F, MAM-P, MN-D, MCC. Visualization: ND-G, MAM-P, MCC. Supervision: ND-G, MAM-P, MCC.
Funding
This work was funded by a grant from the Regional Government of Extremadura, co-financed by the European Regional Development Fund (GR24011).
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Declarations
Competing interests
The authors declare no competing interests.
Ethical approval
The study was conducted in accordance with the Declaration of Helsinki and current legislation (Organic Law 3/2018 of 5 December on the Protection of Personal Data and Guarantee of Digital Rights and the Regulation (EU) 2016/679 of the European Parliament and Council of 27 April 2016, on data protection (GDPR), and the conditions established by Law 14/2007 on biomedical research.). The protocol was approved by the Ethics in Clinical Investigation Committee of Badajoz, Health Area of Badajoz (05/07/2018). Informed consent was obtained from all subjects involved in the study.
Consent to participate
Informed consent was obtained from all individual participants included in the study.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Sung, H. et al. Global Cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. Cancer J. Clin.71 (3), 209–249. 10.3322/CAAC.21660 (2021). [DOI] [PubMed] [Google Scholar]
- 2.Red Española de Registros de Cáncer (REDECAN). Estimaciones de la Incidencia del Cáncer en España, 2024 (1st ed., Vol. 1). (2024).
- 3.Berger, A. M., Kumar, G., LeVan, T. D. & Meza, J. L. Symptom clusters and quality of life over 1 year in breast Cancer patients receiving adjuvant chemotherapy. Asia-Pacific J. Oncol. Nurs.7 (2), 134–140. 10.4103/apjon.apjon_57_19 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee, L., Ross, A., Griffith, K., Jensen, R. E. & Wallen, G. R. Symptom clusters in breast Cancer survivors: A latent class profile analysis. Oncol. Nurs. Forum. 47 (1), 89–100. 10.1188/20.ONF.89-100 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.So, W. K. W. et al. The symptom cluster of fatigue, pain, anxiety, and depression and the effect on the quality of life of women receiving treatment for breast cancer: a multicenter study. Oncol. Nurs. Forum. 36 (4), E205–E214. 10.1188/09.ONF.E205-E214 (2009). [DOI] [PubMed] [Google Scholar]
- 6.Hwang, Y. & Knobf, M. T. Sleep health in young women with breast cancer: a narrative review. Support. Care Cancer. 30 (8), 6419–6428. 10.1007/S00520-022-06953-3 (2022). [DOI] [PubMed] [Google Scholar]
- 7.Gonzalez, B. D. et al. Prevalence, risk factors, and trajectories of sleep disturbance in a cohort of African-American breast cancer survivors. Support. Care Cancer. 29 (5), 2761–2770. 10.1007/S00520-020-05786-2/TABLES/3 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cheng, W. H., Teo, R. H., Cheng, L. J., Lau, Y. & Lau, S. T. Global prevalence of sleep disturbances among breast cancer survivors: A systematic review with meta-analysis. Sleep. Health. 9 (5), 704–716. 10.1016/J.SLEH.2023.04.004 (2023). [DOI] [PubMed] [Google Scholar]
- 9.Grayson, S. et al. Factors associated with sleep disturbances in women undergoing treatment for early-stage breast cancer. Support. Care Cancer. 30 (1), 157–166. 10.1007/s00520-021-06373-9 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Strollo, S. E., Fallon, E. A., Gapstur, S. M. & Smith, T. G. Cancer-related problems, sleep quality, and sleep disturbance among long-term cancer survivors at 9-years post diagnosis. Sleep Med.65, 177–185. 10.1016/J.SLEEP.2019.10.008 (2020). [DOI] [PubMed] [Google Scholar]
- 11.Fontes, F., Severo, M., Gonçalves, M., Pereira, S. & Lunet, N. Trajectories of sleep quality during the Fi Rst three years after breast cancer diagnosis. Sleep Med.34, 193–199. 10.1016/j.sleep.2017.03.022 (2017). [DOI] [PubMed] [Google Scholar]
- 12.Cai, T., Huang, Y., Huang, Q., Xia, H. & Yuan, C. Symptom trajectories in patients with breast cancer: an integrative review. Int. J. Nurs. Sci.9 (1), 120–128. 10.1016/j.ijnss.2021.12.011 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Clara, M. I., van Straten, A., Savard, J., Canavarro, M. C. & Allen Gomes, A. Web-based cognitive-behavioral therapy for insomnia in cancer survivors: the OncoSleep randomized trial. Sleep. Med.129, 67–74. 10.1016/j.sleep.2025.02.021 (2025). [DOI] [PubMed] [Google Scholar]
- 14.Chang, W. P. & Chang, Y. P. Meta-Analysis of changes in sleep quality of women with breast cancer before and after therapy. Breast Care. 15 (3), 227–235. 10.1159/000502943 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders DSM-5-TR (5th edn.,text rev.) (2022).
- 16.American Academy of Sleep Medicine. International Classification of Sleep Disorders. (2014).
- 17.Caplette-Gingras, A., Savard, J., Savard, M. H. & Ivers, H. Is insomnia associated with cognitive impairments in breast cancer patients? Behav. Sleep. Med.11 (4), 239–257. 10.1080/15402002.2012.672940 (2013). [DOI] [PubMed] [Google Scholar]
- 18.Hayley, A. C. et al. Prevalence of excessive daytime sleepiness in a sample of the Australian adult population. Sleep Med.15 (3), 348–354. 10.1016/j.sleep.2013.11.783 (2014). [DOI] [PubMed] [Google Scholar]
- 19.Mercier, J., Savard, J. & Bernard, P. Exercise interventions to improve sleep in cancer patients: a systematic review and meta-analysis. Sleep Med. Rev.36, 43–56. 10.1016/j.smrv.2016.11.001 (2016). [DOI] [PubMed] [Google Scholar]
- 20.Kim, H. J., Barsevick, A. M., Tulman, L. & McDermott, P. A. Treatment-Related symptom clusters in breast cancer: A secondary analysis. J. Pain Symptom Manag.36 (5), 468–479. 10.1016/J.JPAINSYMMAN.2007.11.011 (2008). [DOI] [PubMed] [Google Scholar]
- 21.Durán-Gómez, N. et al. Prevalence of Psychoneurological symptoms and symptom clusters in women with breast Cancer undergoing treatment: influence on quality of life. Semin. Oncol. Nurs.39 (4), 151451. 10.1016/j.soncn.2023.151451 (2023). [DOI] [PubMed] [Google Scholar]
- 22.So, W. K. W. et al. Symptom clusters experienced by breast cancer patients at various treatment stages: A systematic review. Cancer Med.10 (8), 2531–2565. 10.1002/cam4.3794 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Walker, W. H. & Borniger, J. C. Molecular mechanisms of cancer-induced sleep disruption. Int. J. Mol. Sci.20 (11), 2780. 10.3390/ijms20112780 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mogavero, M. P., DelRosso, L. M., Fanfulla, F., Bruni, O. & Ferri, R. Sleep disorders and cancer: state of the Art and future perspectives. Sleep Med. Rev.56, 101409. 10.1016/j.smrv.2020.101409 (2021). [DOI] [PubMed] [Google Scholar]
- 25.Edinger, J. D. et al. Behavioral and psychological treatments for chronic insomnia disorder in adults: an American academy of sleep medicine clinical practice guideline. J. Clin. Sleep Med.17 (2), 255–262. 10.5664/JCSM.8986 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yang, H., Yang, Z., Pan, H. & Zhou, Q. Effects of physical activity on sleep problems in breast cancer survivors: a meta-analysis. Support. Care Cancer. 29, 4023–4032. 10.1007/s00520-020-05914-y (2021). [DOI] [PubMed] [Google Scholar]
- 27.George, M. A., Lustberg, M. B. & Orchard, T. S. Psychoneurological symptom cluster in breast cancer: the role of inflammation and diet. Breast Cancer Res. Treat.184, 1–9. 10.1007/s10549-020-05808-x (2020). [DOI] [PubMed] [Google Scholar]
- 28.Tran, T. X. M. et al. Long-term trajectory of postoperative health-related quality of life in young breast cancer patients: a 15-year follow-up study. J. Cancer Surviv.17 (5), 1416–1426. 10.1007/s11764-022-01165-4 (2022). [DOI] [PubMed] [Google Scholar]
- 29.Rubio Hererra, M. A. et al. Consenso SEEDO 2007 Para La evaluación Del sobrepeso y La obesidad y El establecimiento de criterios de intervención terapéutica. Revista Esp. De Obesidad. 5, 135–175. 10.1016/S0025-7753(07)72531-9 (2007). [Google Scholar]
- 30.Buysse, D. J. et al. The Pittsburgh sleep quality index: a new instrument for psychiatric practice and research. Psychiatry Res., 28(2), 193–213. 10.1016/0165-1781(89)90047-4. (1989). [DOI] [PubMed] [Google Scholar]
- 31.Morin, C. M., Belleville, G., Bélanger, L. & Ivers, H. The insomnia severity index: psychometric indicators to detect insomnia cases and evaluate treatment response. Sleep34 (5), 601–608. 10.1093/sleep/34.5.601 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Johns, M. W. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep14, 540–545. 10.1093/sleep/14.6.540 (1991). [DOI] [PubMed] [Google Scholar]
- 33.Ferrer, M. et al. Measurement of the perceived impact of sleep problems: the Spanish version of the functional outcomes sleep questionnaire and the Epworth sleepiness scale. Med. Clin.113 (7), 250–255 (1999). [PubMed] [Google Scholar]
- 34.Arraras, J. I. et al. The EORTC QLQ-C30 (version 3.0) quality of life questionnaire: validation study for Spain with head and neck cancer patients. Psycho-oncology11 (3), 249–256. 10.1002/PON.555 (2002). [DOI] [PubMed] [Google Scholar]
- 35.Arraras, J. I. et al. The EORTC breast Cancer quality questionnaire (QLQ-BR23): A psychometric study with Spanish patients. Behav. Psychol.9 (1), 81–98 (2001). [Google Scholar]
- 36.Kim, K. & Park, H. Factors affecting anxiety and depression in young breast cancer survivors undergoing radiotherapy. Eur. J. Oncol. Nurs.50, 101898. 10.1016/j.ejon.2021.101898 (2021). [DOI] [PubMed] [Google Scholar]
- 37.Whisenant, M., Wong, B., Mitchell, S. A., Beck, S. L. & Mooney, K. Trajectories of depressed mood and anxiety during chemotherapy for breast Cancer. Cancer Nurs.43 (1), 22–31. 10.1097/NCC.0000000000000670 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Petruseviciene, D., Surmaitiene, D., Baltaduoniene, D. & Lendraitiene, E. Effect of Community-Based occupational therapy on Health-Related quality of life and engagement in meaningful activities of women with breast Cancer. Occup. Therapy Int.2018 (1), 6798697. 10.1155/2018/6798697 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Alonso-Molero, J. et al. Quality of life in a cohort of 1078 women diagnosed with breast cancer in spain: 7-year follow-up results in the mcc-spain study. Int. J. Environ. Res. Public Health. 17 (22), 1–16. 10.3390/ijerph17228411 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nakamura, Z. M. et al. Serial assessment of depression and anxiety by patients and providers in women receiving chemotherapy for early breast Cancer. Oncologist26 (2), 147–156. 10.1002/onco.13528 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Villar, R. R. et al. Quality of life and anxiety in women with breast cancer before and after treatment. Rev. Latinoam. Enferm.25, e2958. 10.1590/1518-8345.2258.2958 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pegram, M., Jackisch, C. & Johnston, S. R. D. Estrogen/HER2 receptor crosstalk in breast cancer: Combination therapies to improve outcomes for patients with hormone receptor-positive/HER2-positive breast cancer. NPJ Breast Cancer9 (1), 45. 10.1038/s41523-023-00533-2 (2023). [DOI] [PMC free article] [PubMed]
- 43.Arraras, J. I. et al. An evaluation study of the determinants of future perspective and global Quality of Life in Spanish long-term premenopausal early-stage breast cancer survivors. Contemp. Oncol.20 (2), 165–170. 10.5114/wo.2016.60073 (2016). [DOI] [PMC free article] [PubMed]
- 44.Criscitiello, C. et al. Health-related quality of life among patients with HR+/HER2– early breast cancer. Clin. Ther.43 (7), 1228–1244.e4. 10.1016/j.clinthera.2021.04.020 (2021). [DOI] [PubMed]
- 45.Ward Sullivan, C. et al. Differences in symptom clusters identified using symptom occurrence rates versus severity ratings in patients with breast cancer undergoing chemotherapy. Eur. J. Oncol. Nurs.28, 122–132. 10.1016/j.ejon.2017.04.001(2017). [DOI] [PMC free article] [PubMed]
- 46.Wang, J. & Wu, S. G. Breast cancer: an overview of current therapeutic strategies, challenge, and perspectives. Breast Cancer: Targets Therapy. 15, 721–730. 10.2147/BCTT.S432526 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jung, M. S. et al. Cognitive dysfunction and symptom burden in women treated for breast cancer: a prospective behavioral and fMRI analysis. Brain Imaging Behav.11 (1), 86–97. 10.1007/s11682-016-9507-8 (2017). [DOI] [PubMed]
- 48.Passildas, J. et al. Impact of chemotherapy-induced menopause in women of childbearing age with non-metastatic breast cancer – Preliminary results from the MENOCOR study. Clin. Breast Cancer19 (1), e74–e84. 10.1016/j.clbc.2018.10.003 (2019). [DOI] [PubMed]
- 49.Haque, R. et al. Insomnia and susceptibility to depressive symptoms and fatigue in diverse breast Cancer survivors. J. Women’s Health. 30 (11), 1604–1615. 10.1089/jwh.2019.8135 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fleming, L. et al. Insomnia in breast cancer: a prospective observational study. Sleep42 (3), zsy245. 10.1093/sleep/zsy245 (2019). [DOI] [PubMed] [Google Scholar]
- 51.Jakobsen, G., Gjeilo, K. H., Hjermstad, M. J. & Klepstad, P. An update on prevalence, assessment, and risk factors for sleep disturbances in patients with advanced cancer—Implications for health care providers and clinical research. Cancers14 (16), 3933. 10.3390/cancers14163933 (2022). [DOI] [PMC free article] [PubMed]
- 52.Grassi, L. et al. Insomnia in adult patients with cancer: ESMO clinical practice guideline. ESMO Open8 (6), 102047. 10.1016/j.esmoop.2023.102047 (2023). [DOI] [PMC free article] [PubMed]
- 53.Emre, N. & Yılmaz, S. Sleep quality, mental health, and quality of life in women with breast cancer. Indian J. Cancer. 61 (2), 299–304. 10.4103/ijc.IJC_859_20 (2024). [DOI] [PubMed] [Google Scholar]
- 54.Reynolds-Cowie, P. & Fleming, L. Living with persistent insomnia after cancer: A qualitative analysis of impact and management. Br. J. Health Psychol.26, 33–49. 10.1111/bjhp.12446 (2021). [DOI] [PubMed] [Google Scholar]
- 55.Kugbey, N., Asante, O., Meyer-Weitz, A. & K., & Depression, anxiety and quality of life among women living with breast cancer in ghana: mediating roles of social support and religiosity. Support. Care Cancer. 28 (6), 2581–2588. 10.1007/s00520-019-05027-1 (2020). [DOI] [PubMed] [Google Scholar]
- 56.Kherchi, O. et al. Relationship between sleep quality and anxiety-depressive disorders in Moroccan women with breast cancer: A cross-sectional study. Iran. J. Public Health52 (7), 1457–1465. 10.18502/ijph.v52i7.13247 (2023). [DOI] [PMC free article] [PubMed]
- 57.Hirshkowitz, M. et al. National sleep foundation’s sleep time duration recommendations: Methodology and results summary. Sleep Health1 (1), 40–43. 10.1016/j.sleh.2014.12.010 (2015). [DOI] [PubMed]
- 58.Fox, R. S. et al. Sleep disturbance and cancer-related fatigue symptom cluster in breast cancer patients undergoing chemotherapy. Support. Care Cancer28 (2), 845–855. 10.1007/s00520-019-04834-w (2020). [DOI] [PMC free article] [PubMed]
- 59.Beverly Hery, C. M. et al. Factors associated with insomnia symptoms over three years among premenopausal women with breast cancer. Breast Cancer Res. Treat.202 (1), 155–165. 10.1007/s10549-023-07058-z (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Desai, K. et al. Prevalence and risk factors for insomnia among breast cancer patients on aromatase inhibitors. Support. Care Cancer. 21 (1), 43–51. 10.1007/s00520-012-1490-z (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Duzova, U. S., Duzova, M. & Altinel, B. The effect of sleep quality on attitudes toward death in breast cancer survivors. Support. Care Cancer. 32 (10), 666. 10.1007/s00520-024-08865-w (2024). [DOI] [PubMed] [Google Scholar]
- 62.Costa, A. R. et al. Impact of breast cancer treatments on sleep disturbances - A systematic review. Breast23 (6), 697–709. 10.1016/j.breast.2014.09.003 (2014). [DOI] [PubMed] [Google Scholar]
- 63.Mansano-Schlosser, T. C. & Ceolim, M. F. Association between poor clinical prognosis and sleep duration among breast cancer patients. Rev. Latinoam. Enferm.25 (e2899). 10.1590/1518-8345.1826.2899 (2017). [DOI] [PMC free article] [PubMed]
- 64.Putri, D. S. R. & Makiyah, S. N. N. Factors affecting sleep quality of breast cancer patients with chemotherapy. Open. Access. Macedonian J. Med. Sci.9 (T4), 130–136. 10.3889/oamjms.2021.5816 (2021). [Google Scholar]
- 65.Ancoli-Israel, S. et al. Sleep, fatigue, depression, and circadian activity rhythms in women with breast cancer before and after treatment: A 1-year longitudinal study. Support. Care Cancer22 (9), 2535–2545. 10.1007/s00520-014-2204-5 (2014). [DOI] [PMC free article] [PubMed]
- 66.Eldin, E. S. T. et al. Evaluation of sleep pattern disorders in breast cancer patients receiving adjuvant treatment (chemotherapy and/ or radiotherapy) using polysomnography. J. B U ON. 24 (2), 529–534 (2019). [PubMed] [Google Scholar]
- 67.Agencia Española de Medicamentos y Productos Sanitarios. CIMA. 2024. https://cima.aemps.es/cima/publico/home.html. Accessed 25 April 2024.
- 68.Edmed, S. L. et al. Sleep and health-related quality of life in women following a cancer diagnosis: results from the women’s wellness after Cancer program in Australia. Support. Care Cancer. 30 (12), 10243–10253. 10.1007/s00520-022-07429-0 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Liu, L. et al. Decreased health-related quality of life in women with breast cancer is associated with poor sleep. behavioral sleep medicine. 11 (3), 189–206. 10.1080/15402002.2012.660589 (2013). [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.