Abstract
Doxorubicin (DOX) is an effective anticancer drug, but its clinical application is limited due to its severe adverse effects on multiple organs and tissues, particularly cardiotoxicity. Studies suggest that metformin and Coenzyme Q10 (CoQ10) may help reduce DOX-induced cardiotoxicity. This study investigated the individual and combined effects of metformin and CoQ10 on DOX-induced cardiotoxicity in rats. 36 male Wistar rats were divided into six groups consisting of N_C, C_Dox (25 mg/kg DOX), C_(Met + Q10) (200 mg/kg metformin + 10 mg/kg CoQ10), T_Met (200 mg/kg metformin + 25 mg/kg DOX), T_Q10 (10 mg/kg CoQ10 + 25 mg/kg DOX), and T_(Met + Q10) (200 mg/kg metformin + 10 mg/kg CoQ10 + 25 mg/kg DOX). DOX administration significantly elevated serum CK-MB, LDH (P < 0.05), and tissue MDA (P < 0.001). It also significantly decreased TAC, CAT, GPx (P < 0.001), and SOD (P < 0.01) in heart tissues. Treatment with metformin and CoQ10 significantly restored the biochemical parameters both in the serum and tissue samples and ameliorated the histopathological damage caused by DOX. In conclusion, the combination of metformin and CoQ10 exerted antioxidant and cardioprotective effects against DOX-induced cardiotoxicity.
Keywords: Metformin, Coenzyme Q10, Doxorubicin, Cardiotoxicity, Cardioprotection
Subject terms: Biochemistry, Clinical pharmacology, Cancer therapy
Introduction
Reports indicated that about 19.3 million newly diagnosed cancer cases and approximately 10 million deaths due to cancer were registered worldwide in 20201. Cancer and heart disease are among the most common causes of mortality and morbidity worldwide2. As living standards improve and life expectancy increases, the incidence of cancer is projected to rise3. It is estimated that by 2070, there will be around 34 million new cases of cancer worldwide4. In recent decades, advances in pharmacology and oncology have led to the development of new drugs for cancer chemotherapy. Although these new agents significantly increase patient survival rates, they are also associated with cardiovascular complications, particularly cardiotoxicity5.
Anthracyclines are the cornerstone of many chemotherapy regimens used to control various cancers6. Anthracycline-induced cardiotoxicity (AIC) is a major complication that significantly increases the risk of mortality and morbidity. AIC limits the maximum effective dose of anthracyclines that can be safely administered7. Although many protective strategies have been evaluated, AIC still remains a significant threat and limitation in patients undergoing anthracycline therapy8–10. Doxorubicin (DOX) is the most commonly used anthracycline in multiple cancer types, such as pediatric cancer, lymphoma, leukemia, breast cancer, and solid tumors. DOX acts by inhibiting topoisomerase II7,11. Despite several investigations, the mechanism of DOX-induced cardiotoxicity remains unclear. However, studies have proposed several mechanisms, including oxidative stress due to elevated reactive oxygen species, mitochondrial dysfunction, release of vasoactive amines, and increased lipid peroxidation12. Therefore, further studies are warranted to identify new effective interventions to prevent DOX-induced cardiotoxicity.
Metformin, a member of the biguanide class, is among the most extensively utilized therapeutic agents in managing diabetes mellitus13. Previous studies have revealed that metformin decreases oxidative stress, inflammation, and apoptosis in cardiac cells and can be used as a cardioprotective agent to avoid DOX-induced cardiotoxicity13–17. Coenzyme Q10 (CoQ10), or ubiquinol, is an endogenous protein that plays a significant role in the mitochondrial respiratory chain. It also prevents lipid peroxidation, regulates membrane permeability, and regenerates vitamin E. Several studies have shown that it can prevent DOX-induced cardiotoxicity18–21. In this study, we investigated the combined cardioprotective effects of CoQ10 and metformin against DOX-induced cardiotoxicity in a rat model, comparing their synergistic action to the individual effects of each compound.
Materials and methods
Thirty-six male Wistar rats weighing 180–250 g and aged 8–10 weeks were obtained from the Pasteur Institute of Iran. Before the experiments began, they were housed for one week to acclimate to their surroundings. The rats were housed under standard laboratory conditions (12-h light/dark cycle, temperature 23 ± 2 °C, humidity 50 ± 10%). Throughout the study, rats were given unrestricted access to water and food.
Drugs and chemicals
Sodium monohydrogen phosphate, sodium acetate, monosodium dihydrogen phosphate, hydrogen peroxide, ferric sulfate, ferric chloride, butanol, bovine serum albumin, and Coomassie blue were purchased from Merck (Germany). Ketamine and TPTZ (2,4,6-Tripyridyl-s-triazine) were purchased from Alphasan (the Netherlands). DOX and CoQ10 were obtained from Acteropharma (Iran). Metformin was obtained from Actoverco (Iran).
Study design
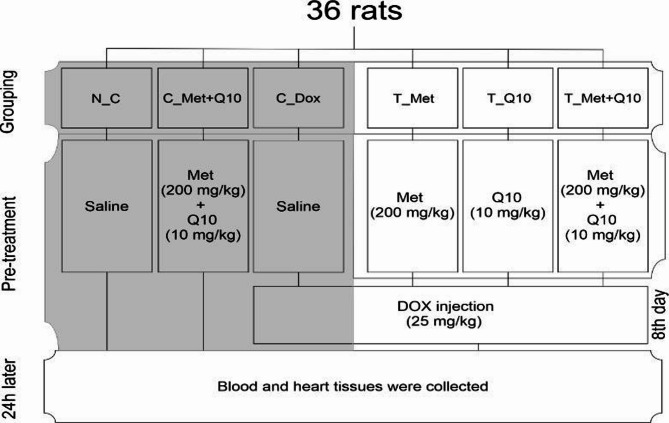
Thirty-six male Wistar rats were randomly divided into six study groups (n = 6): N_C; received normal saline. C_(Met + CoQ10); co-administered of metformin and CoQ10 daily. C_Dox; received normal saline daily and a single dose of DOX. T_Met; received metformin daily and a single dose of DOX. T_Q10; received CoQ10 daily and a single dose of DOX. T_(Met + Q10); co-administered metformin and CoQ10 daily, and a single dose of DOX. Metformin and CoQ10 were administered by gavage in a daily dose of 200 mg/kg and 10 mg/kg, respectively, for 7 days. DOX was administered intraperitoneally at a single dose of 25 mg/kg on the eighth day. Twenty-four hours after the DOX injection, the animals were anesthetized with a combination of ketamine and xylazine (160 µl ketamine 10% + 40 µl xylazine). The blood and hearts of the rats were collected for biochemical and histopathological analyses (Fig. 1). This study was conducted by the ARRIVE guidelines and approved by the Ardabil University of Medical Sciences (Ethics code: IR.ARUMS.AEC.1402.004).
Fig. 1.
A summary of the study design is shown above. N_C; normal control group, C_(Met + Q10); metformin and CoQ10 control group, C_Dox; DOX injury control group, T_Met; metformin pre-treatment group, T_Q10; CoQ10 pre-treatment group, T_(Met + Q10); Pre-treatment group with combination of metformin and CoQ10.
Preparation of tissue lysate and serum
Blood samples were collected directly from the hearts, centrifuged at 3000 rpm for 10 min, and stored in 1.5 ml vials in a −80 °C freezer. These samples were used for subsequent biochemical experiments. Once the blood samples were collected, the hearts were removed and washed with cold normal saline. To prepare tissue lysate, 50 mg of heart tissue was homogenized at 10,000 rpm for 2 min in a cold phosphate buffer solution with pH 7.4. The solution was then centrifuged at 12,000 rpm for 20 min at 4 °C. The supernatant was collected and stored at −80 °C for further biochemical experiments.
Measurement of serum factors
According to the manufacturer’s instructions, CK-MB and LDH levels were measured using standard assay kits by the autoanalyzer (Biochemistry Analyzer BT 1500, Italy).
Measurement of malondialdehyde (MDA)
MDA was measured following the method described by Mihara and Uchiyama, with some modifications. The tissue supernatant (400 µl) was mixed with trichloroacetic acid, and 2400 µl of 1% phosphoric acid was added. Then the test tube was placed in boiling water containing 0.67% thiobarbituric acid for 60 min. After cooling, n-butanol was added, and the samples were centrifuged at 3000 rpm for 10 min. Finally, MDA concentration was determined using the 1,1,3,3-tetraethoxypropane standard curve22.
Measurement of the activity of superoxide dismutase (SOD)
The Zellbio kit was used to measure SOD during the experiment. The SOD reaction depends on free radicals generated by xanthine and xanthine oxidase. These radicals interact with the substrate I.N.T, producing dye molecules. SOD activity was determined by the degree of absorption.
Measurement of the activity of glutathione peroxidase (GPx)
GPx was measured by the Zellbio kit following Valentine and Paglia’s method. Reduced glutathione is oxidized by glutathione peroxidase. Then, oxidized glutathione is converted back to reduced glutathione by glutathione reductase and NADPH, leading to a proportional decrease in light absorption.
Measurement of the activity of tissue catalase (CAT)
The Aebi method was used to measure CAT levels. 50 µl of the centrifuged supernatant liquid was mixed with phosphate buffer in a 500:1 ratio. Next, 2 µl of this solution was added to a cuvette containing 1 ml of 30 mM hydrogen peroxide. The absorption changes at 240 nm over a minute were measured, and the enzyme activity was calculated23.
Total antioxidant capacity assay (TAC)
The ability of the tissues to convert Fe3+ to Fe2+ was evaluated using the FRAP (Ferric Reducing Antioxidant Power Assay) method. This method measures the tissue’s potential to reduce Fe3+ in the presence of TPTZ, forming a blue complex with maximum absorbance at 593 nm. This reaction occurs due to the interaction between Fe2+ and TPTZ24.
Protein assay
The protein concentration was measured using the Bradford assay, which involves the binding of Coomassie Blue dye to proteins25.
Histopathological studies
A small section was cut and fixed to examine the heart tissue in a solution containing 10% formalin. Then, the slices were treated with increasing-grade ethanol solutions to remove any water content, followed by xylene to make them transparent. Next, the slices were embedded in paraffin wax and cut into 5–6 microns thick thin slices. Finally, tissue slices were analyzed for histopathological changes by staining with hematoxylin-eosin and viewed under an Olympus IX71 microscope.
Statistical analysis
The results were presented as “mean ± standard deviation” and analyzed using one-way ANOVA with a significance level of P < 0.05, followed by Tukey’s post hoc test. All statistical analyses were conducted using the GraphPad Prism V9 software.
Results
The effect of metformin, coenzyme Q10, and their combination on the serum LDH and CK-MB levels
The effects of metformin and coenzyme Q10 pre-treatment on serum LDH and CK-MB levels are shown in Table 1. The results showed that Dox Injection significantly increased LDH and CK-MB levels in the serum compared to the normal control group (p < 0.001). However, pre-treatment with metformin, coenzyme Q10, or both significantly decreased LDH and CK-MB levels compared to the damage control group (p < 0.001).
Table 1.
The effect of DOX, metformin, and coenzyme Q10 pre-treatment on serum levels of LDH and CK-MB.
| Serum levels | N_C | C_Met + Q10 | C_Dox | T_Met | T_Q10 | T_Met + Q10 |
|---|---|---|---|---|---|---|
| LDH (U/L) | 594 ± 49.04 | 591 ± 32.04 | * 886 ± 44.67 | # 749.66 ± 50.01 | # 593.5 ± 51.57 | # 426.33 ± 65.85 |
| CK-MB (U/L) | 362 ± 87.53 | 361.25 ± 48.07 | * 587 ± 52.79 | # 390.25 ± 63.18 | # 487.33 ± 41.48 | # 307 ± 44.24 |
Data is ecpressed as the mean ± SD. # indicates a significant difference between the C_Dox and N_C groups (p < 0.005). # indicates a significant difference between the pre-treatment groups and C_Dox (p < 0.005). N_C; normal control group, C_(Met + Q10); metformin and coenzyme Q10 control group, C_Dox; DOX injury control group, T_Met; metformin pre-treatment group, T_Q10; CoQ10 pre-treatment group, T_((Met + Q10); Pre-treatment group with combination of metformin and coenzyme Q10.
Specifically, pre-treatment with coenzyme Q10 alone resulted in a more significant decrease in LDH levels than pre-treatment with metformin alone. Conversely, pre-treatment with metformin alone resulted in a more significant reduction in CK-MB levels than pre-treatment with coenzyme Q10 alone. Furthermore, pre-treatment with the combination of metformin and coenzyme Q10 resulted in a more significant decrease in both LDH and CK-MB levels than their use alone.
The effect of metformin, coenzyme Q10, and their combination on the level of MDA in heart tissue
Figure 2 depicts the impact of DOX and the protective effects of metformin and coenzyme Q10, individually and in combination, on the tissue levels of MDA. The results showed that DOX Injection significantly raised MDA levels in tissue homogenates compared to the normal control group (p < 0.001). However, pre-treatment with metformin, coenzyme Q10, and their combination significantly reduced the MDA levels compared to the DOX group (p < 0.001).
Fig. 2.

The effect of pre-treatment with metformin and coenzyme Q10 on MDA levels of rats treated with DOX. Data is expressed as mean ± standard error. ### represents a significant difference between N_C and C_Dox groups (p < 0. 001). *** represents a significant difference between C_Dox and T_Met groups (p < 0.001). *** represents a significant difference between C_Dox and T_Q10 as well as C_Dox and T_(Met + Q10) groups (p < 0.001). N_C: Normal Control; C_(Met + Q10): metformin and coenzyme Q10 control group; C_Dox: DOX control group; T_Met: metformin test group; T_Q10: coenzyme Q10 test group; T_(Met + Q10): metformin and coenzyme Q10 test group; MDA: malondialdehyde.
The effect of metformin, coenzyme Q10, and their combination on the level of TAC in heart tissue
The effects of DOX on TAC in heart tissue and the protective effects of metformin and coenzyme Q10, separately and in combination, are shown in Fig. 3. The results indicated that DOX injection significantly reduced TAC levels in the tissue homogenate compared to the normal control group (p < 0.001). However, pre-treatment with coenzyme Q10 and the combination of metformin and coenzyme Q10 significantly increased TAC levels compared to the DOX group (p < 0.001), but pre-treatment with metformin alone did not have a significant change.
Fig. 3.
The effect of pre-treatment with metformin and coenzyme Q10 on TAC of rats treated with DOX. Data is expressed as mean ± standard error. ### represents a significant difference between N_C and C_Dox groups (p < 0.001). *** represents a significant difference between C_Dox and T_Q10, as well as C_Dox and T_(Met + Q10) groups (p < 0.0001). N_C: Normal Control; C_(Met + Q10): metformin and coenzyme Q10 control group; C_Dox: DOX control group; T_Met: metformin test group; T_Q10: coenzyme Q10 test group; T_(Met + Q10): metformin and coenzyme Q10 test group; TAC: total antioxidant capacity.
The effect of metformin, coenzyme Q10, and their combination on the level of CAT in heart tissue
Figure 4 demonstrates the impact of DOX and the protective effects of metformin and coenzyme Q10, both alone and in combination, on tissue CAT. The results revealed that DOX injection led to a significant decrease in CAT levels in the tissue homogenate compared to the normal control group (p < 0.001). However, only pretreatment with metformin and coenzyme Q10 combination increased catalase significantly (P < 0.001).
Fig. 4.

The effect of pre-treatment with metformin and coenzyme Q10 on the CAT activity of rats treated with DOX. Data is expressed as mean ± standard error. ### indicates a significant difference between N_C and C_Dox groups (p < 0. 001). *** indicates a significant difference between C_Dox and T_(Met + Q10) groups (p < 0.001). N_C: Normal Control; C_(Met + Q10): metformin and coenzyme Q10 control group; C_Dox: DOX control group; T_Met: metformin test group; T_Q10: coenzyme Q10 test group; T_(Met + Q10): metformin and coenzyme Q10 test group; TAC: total antioxidant capacity.
The effect of metformin, coenzyme Q10, and their combination on the SOD activity in heart tissue
Figure 5 shows the impact of DOX and the protective effects of metformin and coenzyme Q10, as well as their combination, on heart tissue SOD. The SOD activity of heart tissue in the DOX group was significantly lower than that in the normal control group (p < 0.01). However, pre-treatment with metformin and a combination of metformin and coenzyme Q10 significantly increased SOD levels compared to the DOX group (p < 0.05).
Fig. 5.

The effect of pre-treatment with metformin and coenzyme Q10 on tissue SOD activity of rats treated with DOX. Data is expressed as mean ± standard error. ## indicates a significant difference between N_C and C_Dox groups (p < 0.01). * Indicates a significant difference between C_Dox and T_Met groups (p < 0.05). **indicates a significant difference between C_Dox and T_(Met + Q10) groups (p < 0.01). N_C: Normal Control; C_(Met + Q10): metformin and coenzyme Q10 control group; C_Dox: DOX control group; T_Met: metformin test group; T_Q10: coenzyme Q10 test group; T_(Met + Q10): metformin and coenzyme Q10 test group; TAC: total antioxidant capacity.
The effect of metformin, coenzyme Q10, and their combination on the GPx activity in heart tissue
DOX injection significantly (P < 0.0001) decreased GPx enzyme activity in rat heart tissue. Pretreatment with metformin, coenzyme Q10, and the combination of metformin and coenzyme Q10 significantly (P < 0.05) increased the activity of this enzyme in the respective groups (Fig. 6).
Fig. 6.

The effect of pre-treatment with metformin and coenzyme Q10 on tissue GPx activity of rats treated with DOX. Data is expressed as mean ± standard error. ### indicates a significant difference between N_C and C_Dox groups (p < 0.001). * Indicates a significant difference between C_Dox and T_Q10 groups (p < 0.05). *** indicates a significant difference between C_Dox and T_(Met + Q10) as well as C_Dox and T_Met groups (p < 0.01). N_C: Normal Control; C_(Met + Q10): metformin and coenzyme Q10 control group; C_Dox: DOX control group; T_Met: metformin test group; T_Q10: coenzyme Q10 test group; T_(Met + Q10): metformin and coenzyme Q10 test group; GPx: glutathione peroxidase.
The effect of metformin, coenzyme Q10, and their combination on histopathological changes in heart tissue
Histopathological studies showed that the tissue structure of the heart in the N_C group (Fig. 7-A) and the C_(Met + Q10) group (Fig. 7-B) was normal, and a regular pattern of muscle fibers with an oval active nucleus was visible. In the C-Dox group, the destruction of muscle fibers and pyknotic nuclei was observed (Fig. 7-C). In the T_Met (Fig. 7-D), T_Q10 (Fig. 7-E), and T_(Met + Q10) groups (Fig. 7-F), the extent of damage to the tissue structure of the heart was lower than that in the C_Dox group. The comparison between C_Dox and T_Met + Q10 showed the most significant effect in preventing tissue damage.
Fig. 7.
Microscopic heart tissue image in the studied groups (hematoxylin-eosin staining x40). In the N_C group (A) and the C_(Met + Q10) group (B), the normal structure of muscle fibers (1) can be seen. DOX destroyed muscle fibers (2) and pyknosis of nuclei (3) (C). Treatment of rats with metformin (D), Q10 (E), and Q10 + metformin (F) prevented DOX-induced tissue damage.
Discussion
DOX is widely used as a chemotherapeutic agent for the treatment of cancer. Approximately 30% of patients receiving this drug experience cardiotoxicity, which is considered a major adverse effect. The mechanism of cardiotoxicity is not yet fully understood; however, studies suggest that it is multifactorial and involves two major factors: lipid peroxidation and oxidative stress26,27. The present study aimed to investigate the protective effects of metformin and coenzyme CoQ10 individually and in combination against DOX-induced cardiotoxicity in male Wistar rats. The findings provided compelling evidence that metformin and CoQ10, especially in combination, significantly attenuated biochemical, oxidative, and histopathological markers of DOX-induced cardiac injury.
CK-MB and LDH are well-recognized markers for myocardial injury28–30. The significant increase in LDH and CK-MB in the DOX-injured group confirms the induction of cardiotoxicity, consistent with previous studies showing DOX-induced cardiomyocyte necrosis and impaired membrane integrity26,31,32. Pretreatment with metformin or CoQ10 resulted in significant reductions in these markers, which could indicate their protective roles. Also, CoQ10 was more effective in reducing LDH and metformin in lowering CK-MB, suggesting potential differences in their protective mechanisms. These effects are likely related to the role of CoQ10 in mitochondrial stabilization and the impact of metformin on AMP-activated protein kinase (AMPK) activation, which requires additional investigation in future studies33,34. Combination therapy produced the most significant reductions in both markers, suggesting a synergistic cardioprotective effect when both drugs are used concomitantly. These results are also similar to the findings of other studies13,35–38.
MDA is a well-known lipid peroxidation biomarker, indicating oxidative damage caused by ROS (Reactive Oxygen Species) to cell membranes39. In this study, DOX administration significantly increased MDA levels in cardiac tissue, indicating severe oxidative stress and membrane damage, consistent with previous reports on DOX-induced cardiotoxicity. Pretreatment with metformin, CoQ10, or their combination effectively reduced MDA levels, preventing DOX-induced damage. The most reduction was also observed with the combination treatment, likely due to the complementary mechanisms of these drugs, namely the direct inhibition of free radicals by CoQ10 and the indirect antioxidant effects of metformin through AMPK activation and mitochondrial protection. These findings highlight the role of oxidative stress in DOX-induced cardiac injury and the potential of combination treatment with antioxidants to attenuate this damage. Similar studies also had results consistent with the present study. For example, one study evaluated the protective effects of metformin against DOX-induced cardiotoxicity. The findings showed that metformin administration reduced MDA levels after DOX administration17. Another study evaluated the protective effect of CoQ10 against DOX-induced cardiotoxicity. The findings of this study also indicated a decrease in MDA levels after DOX administration19.
TAC represents the capacity of biological systems to reduce oxidative stress by combining enzymatic and nonenzymatic antioxidants40–42. The present study showed that DOX administration significantly reduced TAC levels in rat heart tissue, indicating a decreased antioxidant capacity and a disturbance in redox balance. CoQ10 and CoQ10 + metformin successfully reduced the TAC levels. Metformin alone showed no significant effect. This could maybe suggest a direct effect of CoQ10 on enhancing antioxidant capacity. The improved efficacy of the combination therapy could be due to the ability of CoQ10 to restore endogenous antioxidants and the potential of metformin to improve mitochondrial function and decrease oxidative damage. These findings are consistent with previous studies13,16,18,19,43,44. They also show the need to maintain TAC to prevent DOX-induced cardiotoxicity and support the use of combination antioxidant therapies for improved cardioprotection.
Antioxidant enzymes, including CAT, SOD, and GPx, play an essential role in protecting cells from oxidative damage by neutralizing ROS. These enzymes maintain redox homeostasis and prevent cellular dysfunction45. In this study, DOX administration significantly reduced the activities of CAT, SOD, and GPx enzymes in cardiac tissue, indicating an impairment of antioxidant defense mechanisms. Pre-treatment with metformin or CoQ10 alone showed variable effects—metformin improved SOD and GPx activity, and CoQ10 increased GPx—only their combined pre-treatment significantly restored CAT levels, maybe indicating a synergistic effect. These results were consistent with similar studies13,16,18,19,43,44. In the study by Botelho et al., CoQ10 administration doesn’t reduce CK-MB and LDH levels. It also doesn’t affect CAT and GPx activity. These findings contrast with our results, possibly due to the lower dose of CoQ10. However, they reported that it was effective on SOD level18. The combined treatment also showed the most substantial increase in SOD and GPx expression, likely due to the ability of CoQ10 to stabilize mitochondrial ROS production and the activation of AMPK-mediated antioxidant pathways by metformin. These findings emphasize the importance of preserving enzymatic antioxidant defenses in combating DOX-induced cardiotoxicity and the superior efficacy of metformin and CoQ10 combination pre-treatment in improving cellular redox homeostasis.
Further, histopathological analysis confirmed the biochemical findings. It showed severe myocardial damage (muscle fiber destruction and pyknotic nuclei) in the DOX group, consistent with other studies investigating doxorubicin-induced cardiotoxicity by increasing cardiac biomarkers in rats46. Pretreatment with metformin and CoQ10—especially in combination—preserved tissue architecture. The nearly normal histology observed in the combination group highlights the potential of dual therapy in preventing structural cardiac damage, possibly through combined antioxidant, anti-inflammatory, and metabolic modulatory effects.
These findings have important clinical implications, specifically for cancer patients undergoing anthracycline-based chemotherapy. This combination therapy may deliver higher doses of doxorubicin while minimizing dose-limiting cardiotoxicity. Metformin and CoQ10 safety and cost-effectiveness profiles further support their potential application in clinical practice. However, future human studies are needed to validate optimal dosing regimens and long-term outcomes in oncological settings.
Conclusion
In conclusion, our study demonstrated that metformin and CoQ10, individually and in combination, protect against DOX-induced cardiotoxicity by reducing oxidative stress, enhancing antioxidant defenses, and preserving myocardial structure. The combination therapy exhibited the most robust cardioprotection, highlighting its potential as an adjunctive treatment to prevent chemotherapy-induced cardiac damage. This study’s limitation is the use of male Wistar rats, which may not fully recapitulate human DOX-induced cardiotoxicity and require future studies. Only single doses of metformin and CoQ10 were tested, and long-term effects were not assessed. Assessment of key mechanistic markers (e.g., inflammatory cytokines) was not included, which warrants further investigation.
Acknowledgements
We would like to express our sincere gratitude to Dr. Ramin Salimnejad and Dr. Sonia Sharifi Namin for their invaluable contributions to the pathological studies associated with this research.
Author contributions
F.M., M.O., P.S., and M.B. performed laboratory tests. S.R. did statistical analysis; F.M. prepared the original draft; L.R. and A.F. designed the study, performed the review, editing, and submitting of manuscript. All authors read and confirmed the final version of the manuscript.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Competing interests
The authors declare no competing interests.
Conflict of interest
The authors report no conflicts of interest.
Ethical approval
This study was approved by the Ardabil University of Medical Sciences (Ethics code: IR.ARUMS.AEC.1402.004).
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Aliakbar Fazaeli, Email: aafazaely@gmail.com, Email: a.fazaeli@arums.ac.ir.
Lotfollah Rezagholizadeh, Email: reza34055@gmail.com, Email: l.rezagholizadeh@arums.ac.ir.
References
- 1.Ferlay, J. et al. Cancer statistics for the year 2020: an overview. Int. J. Cancer. 149, 778–789 (2021). [DOI] [PubMed] [Google Scholar]
- 2.Curigliano, G. et al. Cardiotoxicity of anticancer treatments: epidemiology, detection, and management. Cancer J. Clin.66, 309–325 (2016). [DOI] [PubMed] [Google Scholar]
- 3.Fitzmaurice, C. et al. Global, regional, and National cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 29 cancer groups, 1990 to 2016: a systematic analysis for the global burden of disease study. JAMA Oncol.4, 1553–1568 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Soerjomataram, I. & Bray, F. Planning for tomorrow: global cancer incidence and the role of prevention 2020–2070. Nat. Reviews Clin. Oncol.18, 663–672 (2021). [DOI] [PubMed] [Google Scholar]
- 5.Ewer, M. S. & Ewer, S. M. Cardiotoxicity of anticancer treatments. Nat. Reviews Cardiol.12, 547–558 (2015). [DOI] [PubMed] [Google Scholar]
- 6.Sawicki, K. T. et al. Preventing and treating anthracycline cardiotoxicity: new insights. Annu. Rev. Pharmacol. Toxicol.61, 309–332 (2021). [DOI] [PubMed] [Google Scholar]
- 7.Rawat, P. S., Jaiswal, A., Khurana, A., Bhatti, J. S. & Navik, U. Doxorubicin-induced cardiotoxicity: an update on the molecular mechanism and novel therapeutic strategies for effective management. Biomed. Pharmacother.139, 111708 (2021). [DOI] [PubMed] [Google Scholar]
- 8.Carrasco, R., Castillo, R. L., Gormaz, J. G., Carrillo, M. & Thavendiranathan, P. Role of oxidative stress in the mechanisms of anthracycline-induced cardiotoxicity: effects of preventive strategies. Oxidative medicine and cellular longevity (2021). (2021). [DOI] [PMC free article] [PubMed]
- 9.Volkova, M. & Russell, R. Anthracycline cardiotoxicity: prevalence, pathogenesis and treatment. Curr. Cardiol. Rev.7, 214–220 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Swain, S. M., Whaley, F. S. & Ewer, M. S. Congestive heart failure in patients treated with doxorubicin: a retrospective analysis of three trials. Cancer: Interdisciplinary Int. J. Am. Cancer Soc.97, 2869–2879 (2003). [DOI] [PubMed] [Google Scholar]
- 11.Li, D. et al. Role of acetylation in doxorubicin-induced cardiotoxicity. Redox Biol.46, 102089. 10.1016/j.redox.2021.102089 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chatterjee, K., Zhang, J., Honbo, N. & Karliner, J. S. Doxorubicin cardiomyopathy. Cardiology115, 155–162 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Arinno, A. et al. Cardioprotective effects of melatonin and Metformin against doxorubicin-induced cardiotoxicity in rats are through preserving mitochondrial function and dynamics. Biochem. Pharmacol.192, 114743 (2021). [DOI] [PubMed] [Google Scholar]
- 14.Ajzashokouhi, A., Bostan, H., Jomezadeh, V., Hayes, A. & Karimi, G. A review on the cardioprotective mechanisms of Metformin against doxorubicin. Hum. Exp. Toxicol.39, 237–248 (2020). [DOI] [PubMed] [Google Scholar]
- 15.Argun, M. et al. Cardioprotective effect of Metformin against doxorubicin cardiotoxicity in rats. Anatol. J. Cardiol.16, 234 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Asensio-López, M. C., Lax, A., Pascual-Figal, D. A., Valdés, M. & Sánchez-Más, J. Metformin protects against doxorubicin-induced cardiotoxicity: involvement of the adiponectin cardiac system. Free Radic. Biol. Med.51, 1861–1871 (2011). [DOI] [PubMed] [Google Scholar]
- 17.Zilinyi, R. et al. The cardioprotective effect of Metformin in doxorubicin-induced cardiotoxicity: the role of autophagy. Molecules23, 1184 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Botelho, A. F. M. et al. Coenzyme Q10 cardioprotective effects against doxorubicin-induced cardiotoxicity in Wistar rat. Cardiovasc. Toxicol.20, 222–234 (2020). [DOI] [PubMed] [Google Scholar]
- 19.Rahmanifard, M., Vessal, M., Noorafshan, A., Karbalay-Doust, S. & Naseh, M. The protective effects of coenzyme Q10 and Lisinopril against doxorubicin-induced cardiotoxicity in rats: a Stereological and electrocardiogram study. Cardiovasc. Toxicol.21, 936–946 (2021). [DOI] [PubMed] [Google Scholar]
- 20.Shabaan, D. A., Mostafa, N., El-Desoky, M. M. & Arafat, E. A. Coenzyme Q10 protects against doxorubicin-induced cardiomyopathy via antioxidant and anti-apoptotic pathway. Tissue Barriers. 11, 2019504 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sohal, R. S. et al. Effect of coenzyme Q10 intake on endogenous coenzyme Q content, mitochondrial electron transport chain, antioxidative defenses, and life span of mice. Free Radic. Biol. Med.40, 480–487 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Uchiyama, M. & Mihara, M. Determination of malonaldehyde precursor in tissues by thiobarbituric acid test. Anal. Biochem.86, 271–278. 10.1016/0003-2697(78)90342-1 (1978). [DOI] [PubMed] [Google Scholar]
- 23.Aebi, H. In Methods in EnzymologyVol. 105121–126 (Elsevier, 1984).
- 24.Benzie, I. F. & Strain, J. J. The ferric reducing ability of plasma (FRAP) as a measure of antioxidant power: the FRAP assay. Anal. Biochem.239, 70–76 (1996). [DOI] [PubMed] [Google Scholar]
- 25.Bradford, M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem.72, 248–254 (1976). [DOI] [PubMed] [Google Scholar]
- 26.Songbo, M. et al. Oxidative stress injury in doxorubicin-induced cardiotoxicity. Toxicol. Lett.307, 41–48. 10.1016/j.toxlet.2019.02.013 (2019). [DOI] [PubMed] [Google Scholar]
- 27.Wang, C., Sun, S., Jiao, J., Yu, X. & Huang, S. Effects of Nalbuphine on the cardiotoxicity of ropivacaine in rats. Fundam. Clin. Pharmacol.36, 811–817. 10.1111/fcp.12778 (2022). [DOI] [PubMed] [Google Scholar]
- 28.Robinson, D. J. & Christenson, R. H. Creatine kinase and its CK-MB isoenzyme: the conventional marker for the diagnosis of acute myocardial infarction. J. Emerg. Med.17, 95–104 (1999). [DOI] [PubMed] [Google Scholar]
- 29.Farhana, A., Lappin, S. L. & Biochemistry lactate dehydrogenase. (2020). [PubMed]
- 30.Çömez, M. S. et al. Protective effect of Oleuropein on ketamine-induced cardiotoxicity in rats. Naunyn Schmiedebergs Arch. Pharmacol.393, 1691–1699. 10.1007/s00210-020-01870-w (2020). [DOI] [PubMed] [Google Scholar]
- 31.Hekmat, A. S., Navabi, Z., Alipanah, H. & Javanmardi, K. Alamandine significantly reduces doxorubicin-induced cardiotoxicity in rats. Hum. Exp. Toxicol.40, 1781–1795. 10.1177/09603271211010896 (2021). [DOI] [PubMed] [Google Scholar]
- 32.Shekari, M. et al. Cardioprotective effects of sodium thiosulfate against doxorubicin-induced cardiotoxicity in male rats. BMC Pharmacol. Toxicol.2310.1186/s40360-022-00569-3 (2022). [DOI] [PMC free article] [PubMed]
- 33.Agius, L., Ford, B. E. & Chachra, S. S. The Metformin mechanism on gluconeogenesis and AMPK activation: the metabolite perspective. Int. J. Mol. Sci.21, 3240 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Aaseth, J., Alexander, J. & Alehagen, U. Coenzyme Q10 supplementation–In ageing and disease. Mech. Ageing Dev.197, 111521 (2021). [DOI] [PubMed] [Google Scholar]
- 35.Wei, J., Yang, Q., Lin, L. & Zhu, C. Metformin mitigates doxorubicin-induced cardiotoxicity via the AMPK pathway. Nan Fang Yi Ke Da Xue Xue bao = J. South. Med. Univ.43, 1682–1688 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kelleni, M. T., Amin, E. F. & Abdelrahman, A. M. Effect of Metformin and sitagliptin on doxorubicin-induced cardiotoxicity in rats: impact of oxidative stress, inflammation, and apoptosis. J. Toxicol.2015, 424813 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sheta, A., Elsakkar, M., Hamza, M. & Solaiman, A. Effect of Metformin and sitagliptin on doxorubicin-induced cardiotoxicity in adult male albino rats. Hum. Exp. Toxicol.35, 1227–1239 (2016). [DOI] [PubMed] [Google Scholar]
- 38.Mustafa, H. N., Hegazy, G. A., Awdan, E., AbdelBaset, M. & S. A. & Protective role of CoQ10 or L-carnitine on the integrity of the myocardium in doxorubicin induced toxicity. Tissue Cell.49, 410–426 (2017). [DOI] [PubMed] [Google Scholar]
- 39.Gaweł, S., Wardas, M., Niedworok, E. & Wardas, P. Malondialdehyde (MDA) as a lipid peroxidation marker. Wiadomosci Lekarskie (Warsaw Poland: 1960). 57, 453–455 (2004). [PubMed] [Google Scholar]
- 40.Bahrami, M. et al. Examining effects of Metformin and coenzyme Q10 on Doxorubicin-Induced oxidative hepatotoxicity in rats. J. Mazandaran Univ. Med. Sci.34, 1–14 (2024). [Google Scholar]
- 41.Ojarudi, M., Moradi, A., Hajihosseini, R., Mazani, M. & Rezagholizadeh, L. Hepatoprotective and antioxidant activities of combination of Cinnamomum zeylanicum and Zingiber officinale in CCl4-intoxicated rats. J. Kerman Univ. Med. Sci.27, 1–13. 10.22062/jkmu.2020.89591 (2020). [Google Scholar]
- 42.Safarpour, S. et al. Protective effect of Kaempferol and its nanoparticles on 5-Fluorouracil-Induced cardiotoxicity in rats. Biomed. Res. Int.2022 (2273000). 10.1155/2022/2273000 (2022). [DOI] [PMC free article] [PubMed]
- 43.Ikewuchi, J. C. et al. Attenuation of doxorubicin-induced cardiotoxicity in Wistar rats by aqueous leaf-extracts of Chromolaena odorata and Tridax procumbens. J. Ethnopharmacol.274, 114004 (2021). [DOI] [PubMed] [Google Scholar]
- 44.Tarique, M. et al. Formulation development and Pharmacological evaluation of fixed dose combination of Bombyx mori Coccon shell extract, flaxseed oil and coenzyme Q10 against doxorubicin induced cardiomyopathy in rats. Orient. Pharm. Experimental Med.19, 469–483 (2019). [Google Scholar]
- 45.ojarudi, M., Golchin, A., Karamdel, H. R., Valilo, M. & Ranjbarvan, P. Protective effects of Elaeagnus angustifolia L. fruit extract on CCl4-induced oxidative stress and inflammation in rats liver. Avicenna J. Phytomedicine. -10.22038/ajp.2025.25915 (2025).
- 46.Abdelatty, A. et al. Acute and delayed Doxorubicin-Induced myocardiotoxicity associated with elevation of cardiac biomarkers, depletion of cellular antioxidant enzymes, and several histopathological and ultrastructural changes. Life11, 880 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.