Abstract
Managing lupus nephritis (LN) has been challenging despite therapeutic advancements, as 30% of patients face end-stage kidney disease. Current laboratory tests for diagnosing LN activity are insufficient. Recognizing the potential for severe complications in patients with LN, there is crucial need to identify innovative biomarkers for early diagnosis of this disease. Hence, the objective of this study was to evaluate diagnostic efficacy of miRNAs for LN. The protocol has been registered on PROSPERO (CRD42023460747). This systematic review and meta-analysis was performed following the PRISMA guidelines. A comprehensive search for relevant literature was carried out across PubMed, Scopus, Embase, and Hinari. The methodological quality of the articles was assessed using the QUADAS-2 tool. Stata 14.0 software was used to calculate pooled sensitivity, specificity, and other diagnostic parameters related to miRNAs for the diagnosis of LN utilizing a random effects model. The assessment of heterogeneity among studies involved the application of the Cochran-Q test and I2 statistic tests. Subsequently, subgroup analyses and meta-regression analysis were conducted to investigate the primary sources of heterogeneity. To evaluate publication bias, Deeks’ funnel plot was used. Additionally, Fagan’s nomogram and likelihood ratio scattergram were used to assess the clinical utility of miRNAs for LN. Additionally, a sensitivity analysis was conducted to evaluate the robustness and reliability of the results. In this meta-analysis, 23 studies from 11 publications were included, encompassing a total of 526 LN patients and 490 controls. The selected studies pertained low risk of bias. The overall pooled values for sensitivity, specificity, PLR, NLR, DOR, and AUC were 0.85 (95% CI 0.78–0.89), 0.83 (95% CI 0.75–0.89), 4.93 (95% CI 3.47–7.07), 0.19 (95% CI 0.13–0.26), 26.35 (95% CI 16.39–42.36), and 0.91 (95% CI 0.88–0.93), respectively. Furthermore, miR-181a, miR-223 and miR-146a demonstrated superior diagnostic efficacy with promising clinical utility in excluding and confirming LN. Additionally, findings from the subgroup analysis indicated that miRNA panels, upregulated miRNAs, and miRNAs used in the diagnosis of African patients exhibited superior diagnostic performance. Circulating microRNAs (miRNAs) show promise as noninvasive biomarkers for the early diagnosis of LN. Furthermore, miRNA panels and upregulated miRNAs exhibited improved diagnostic efficacy for LN diagnosis. However, substantiating these findings will necessitate a substantial number of prospective studies and multicenter studies in the near future.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-025-07860-3.
Keywords: MiRNAs, Noncoding RNAs, Diagnostic biomarkers, Lupus nephritis, LN, Systemic lupus erythematous, Meta-analysis
Subject terms: Immunology, Molecular biology, Nephrology, Pathogenesis
Introduction
Systemic lupus erythematosus (SLE) is a multifaceted systemic autoimmune disease characterized by a variety of clinical manifestations affecting numerous organs and systems, mainly through inflammation and autoantibody production1,2. Among its serious complications, lupus nephritis (LN), which encompasses inflammation of the kidneys, is the foremost cause of SLE-associated morbidity and mortality worldwide3. Although LN management has improved recently, identifying a major challenge is that LN management is a strong predictor of end-stage renal disease (ESRD) in approximately 10 to 30% of patients4,5. Owing to its diverse clinical presentation, timely and accurate diagnosis of LN is still a substantial challenge. Diagnostic tests, including serial creatinine analysis, routine urine analysis and albumin–creatinine ratio tests, commonly aid in subsequent SLE patients for the development of LN. Currently, renal biopsy is considered the “gold standard” for LN diagnosis and subtype characterization6. Renal biopsy is indeed an invasive procedure resulting in potential complications, and in some instances, a repeated renal biopsy may be required for monitoring established LN, all of which collectively make it an unsuitable diagnostic modality7,8. Thus, an improved noninvasive diagnostic approach capable of monitoring and detecting LN at an early stage is needed, and the exploration of advanced and innovative techniques is urgently needed for accurate, specific and sensitive diagnostic biomarkers9,10.
Recently, various LN biomarkers, including urinary or serum cytokines/chemokines (IL-10, IL-17, IP-10, and monocyte chemoattractant protein-1 (MCP-1)), growth factors and cell adhesion molecules (CAMs), have been reported to aid in LN diagnosis11,12. However, these biomarkers do not explicitly reflect LN type, severity or prognosis and simply reflect generalized renal inflammation rather than LN specificity.
Circulating microRNAs (miRNAs) are a class of small noncoding RNAs (approximately 19 to 24 nucleotides) that play critical roles in the posttranscriptional regulation of gene expression13. Dysregulation of miRNAs, including miR-146a, miR-126, miR-21, miR-223, and miR-181, is reportedly associated with the onset and progression of various forms of autoimmune diseases, including SLE, indicating their potential clinical significance as promising diagnostic biomarkers14–16. Moreover, miRNAs are stable in body fluids, have noninvasive potential, easily estimate expression levels, and exhibit up- and downregulations that reflect LN disease-specific alterations in gene expression patterns, all of which make them preferable diagnostic markers for assessing the onset and pathogenesis of LN, unlike tissue-based biomarkers17. However, comprehensive and up-to-date evidence-based data are still lacking.
This systematic review and meta-analysis aimed to comprehensively assess the existing studies on circulating miRNAs through identifying trends, evaluating the quality of studies, determining the clinical applicability of miRNA markers, and exploring potential causes of heterogeneity to provide wide-ranging scientific evidence on the use of miRNAs as potential diagnostic biomarkers for LN in patients with SLE, with the aim of enhancing early detection of LN and facilitating proper and timely management of SLE patients.
Methods
Registration
This study was reported according to the Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA) statement guidelines18 (Supplementary material 1). The protocol was registered on the Prospective Register of Systematic Reviews (PROSPERO) with registration number CRD42023460747.
Search strategy
A thorough search strategy was implemented across multiple electronic databases, such as PubMed, Embase, Hinari, and Scopus, with the aim of identifying all pertinent studies. The following keywords were utilized: “miRNAs” OR “microRNAs” OR “miRNA” OR “microRNA” OR “miR*” AND “biomarker” OR “diagnostic biomarkers” OR “potential biomarkers” OR “promising biomarkers” OR “candidate biomarkers” AND “lupus nephritis” OR “renal disease” OR “renal damage” OR “kidney disease” OR “kidney damage” AND “systemic lupus erythematosus” OR “SLE”. The most recent search was carried out on September 5, 2023. Furthermore, the reference lists of relevant studies were meticulously scanned independently to prevent the oversight of any potential studies. Supplementary material 2 contains the specific search details.
Inclusion and exclusion criteria
All the studies to be considered in our analysis had to meet specific requirements. The inclusion criteria were as follows: observational studies, including case‒control, cross-sectional, or cohort studies, that focused on evaluating the diagnostic accuracy of circulating miRNAs in patients with SLE who have LN compared to healthy individuals or SLE patients without LN. Additionally, studies should use either plasma or serum samples to analyze miRNA profiles and provide comprehensive data, including key parameters such as specificity, sensitivity, and sample size, which are necessary for calculating true positives (TPs), false positives (FPs), true negatives (TNs), and false negatives (FNs). Conversely, studies that did not meet the inclusion criteria were excluded if they were review articles, meta-analyses, letters, commentaries, or abstracts; did not provide adequate information to construct a two-by-two table; or were conducted on subjects other than humans.
Study selection, data extraction and quality assessment
The studies retrieved from the search results were imported into EndNote 20 software to eliminate duplicates. Subsequently, two independent reviewers (EA and MAB) thoroughly screened the title, abstract, and full-text quality of each selected paper, adhering to the eligibility criteria. In the event of any discrepancies between the two reviewers, a third reviewer (AG) was involved in the discussion to determine the final selection of articles for the review.
The extraction of the following data was carried out by two independent reviewers (HD and AW) using Microsoft Office Excel software: (1) first author, year of publication, country, miRNA profile, regulatory mode of miRNAs, specimen, internal reference control, sample size (both LN and healthy individuals or SLE), diagnostic methods and cutoff values; and (2) diagnostic parameters such as sensitivity, specificity and area under curve to calculate the TP, FP, TN, and FN. The Microsoft Excel spreadsheet was organized with subsections that were created through consensus among all the reviewers. Both reviewers conducted a thorough verification of their findings, and in instances where discrepancies emerged between them, they engaged in discussions to arrive at a mutual agreement or sought the guidance of the third reviewer (EE).
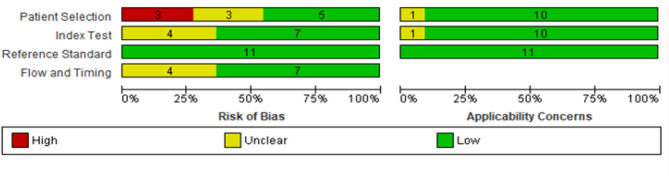
The methodological rigour of the chosen eligible articles was evaluated using the Quality Assessment of Diagnostic Accuracy Studies 2 (QUADAS-2) scoring system19. The QUADAS-2 tool encompasses patient selection criteria, assessment of the index test, reference standard, and flow and timing of the studies to assess both the risk of bias and applicability. Two authors (ZM and MT) independently assessed the initial QUADAS-2 items, and any disparities were resolved with the involvement of a third author (HE).
Data analysis
The data were analyzed using Stata 14.0 software (Stata Corp., USA). Parameters such as TP, FP, FN, and TN were used to calculate the pooled sensitivity, specificity, positive likelihood ratio (PLR), negative likelihood ratio (NLR), diagnostic ratio (DOR), and corresponding 95% confidence interval (CI) using the random effects model20. Forest plots of summary statistics were constructed using the data from the enrolled studies. Summary receiver operating characteristic (SROC) curves were subsequently generated to compute the area under the curve (AUC), evaluating the collective diagnostic efficacy of the miRNAs. To examine the presence of a threshold effect, both the Spearman correlation coefficient and the SROC curve were employed. A characteristic “shoulder-arm” shape in the SROC curve or a significantly positive correlation coefficient suggested the presence of a threshold effect within the heterogeneity. The Cochran-Q test and the I2 statistic were further used to analyze the heterogeneity of nonthreshold effects among the studies, with p values less than 0.05 for the Cochran-Q test and I2 > 50%, indicating significant heterogeneity between studies21. When statistical heterogeneity existed between studies, subgroup analysis and meta-regression analysis were adopted to determine the possible sources of heterogeneity. Sensitivity analysis was performed to determine the robustness of the meta-analysis results. Deeks’ funnel plot was used to assess the presence of publication bias. A p value of < 0.05 was considered to indicate statistical significance. Moreover, the clinical effectiveness of the miRNAs in distinguishing LN patients from controls was assessed using a Fagan plot and likelihood ratio scattergram.
Results
Study selection process
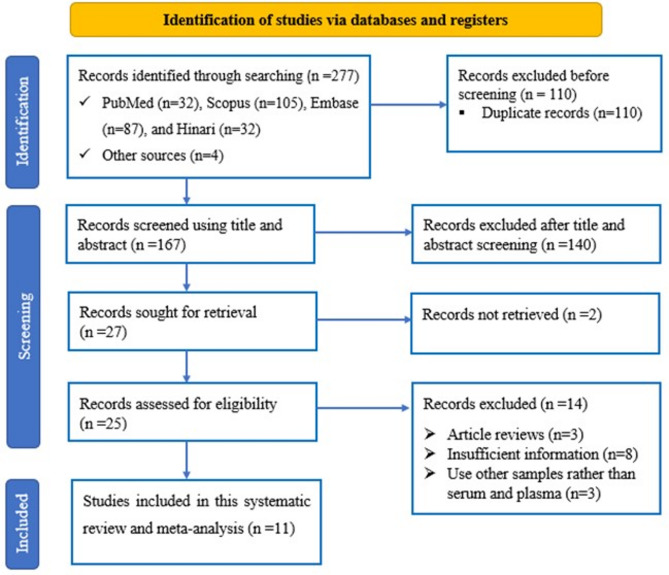
The PRISMA flow diagram, which outlines the study identification, inclusion, and exclusion criteria, is depicted in Fig. 1. In total, 273 studies were initially identified through database searches, and an additional 4 studies were found via manual searching within the references of relevant studies. After eliminating 110 duplicated articles, the titles and abstracts of the remaining records were screened for relevance. Subsequently, 106 articles were removed based on their title review, and an additional 34 articles were excluded after the abstract review. The full texts of 25 studies were subsequently evaluated for eligibility based on the study selection criteria. Fourteen of these articles were excluded because they did not meet the inclusion criteria. Ultimately, 11 studies met the criteria and were included in the meta-analysis.
Fig. 1.
Flow diagram of the study selection process.
Characteristics of the included studies and quality assessment
In this study, 11 articles included a total of 526 LN patients and 490 control individuals, encompassing both SLE patients and healthy individuals. Geographically, 3 studies were from China22–24, 4 from Egypt9,25–27, 3 from Iran28–30, and one from Colombia31. Regarding the types of samples, six studies opted for plasma samples, while five utilized serum samples for quantifying miRNA levels. Seven studies employed individuals with SLE as the control group, three studies utilized healthy participants as the control group, and one study used both groups as control groups. Concerning the internal reference control, U6 was utilized in 3 studies, while miR-191-5p was employed in another 3 studies. Additionally, 13 miRNAs were upregulated, while 6 were downregulated across these studies. All the investigations relied on qRT‒PCR for miRNA identification. The sensitivity and specificity of miRNAs for detecting LN varied within ranges of 47.7–100% and 34–100%, respectively. Table 1 comprehensively summarizes the characteristics and details of these studies. Additionally, the quality of the studies was evaluated for bias risk and applicability concerns utilizing the QUADAS-2 tool. All the included articles produced good results, confirming the reliability of the synthesized metabolites in the meta-analysis. Figure 2 depicts the quality assessment of the included literature.
Table 1.
Characteristics of the included studies.
| Authors | Year | County | miRNAs | Expression | Specimen | Method | Reference | Participants | Cutoff | Sen (%) | Spe (%) | AUC | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Case | No | Control | No | ||||||||||||
| Guo et al.22 | 2016 | China | miR-21 | Up | Plasma | qRT‒PCR | U6 | LN | 44 | HC | 24 | 0.009 | 63.6 | 87.5 | 0.807 |
| Guo et al.22 | 2016 | China | miR-126 | Up | Plasma | qRT‒PCR | U6 | LN | 44 | HC | 24 | 0.044 | 59.1 | 95.8 | 0.788 |
| Guo et al.22 | 2016 | China | miR-148a | UP | Plasma | qRT‒PCR | U6 | LN | 44 | HC | 24 | 0.028 | 47.7 | 100 | 0.776 |
| Zhang et al.23 | 2018 | China | miR-203 | Down | Serum | qRT‒PCR | U6 | LN | 35 | HC | 74 | NA | 76.82 | 81.56 | 0.897 |
| Wu et al.24 | 2021 | China | miR-485-5p | Up | Serum | qRT‒PCR | U6 | LN | 116 | SLE | 65 | 1.846 | 68.1 | 90.8 | 0.841 |
| Tawfik et al.25 | 2019 | Egypt | miR-146a | UP | Serum | qRT‒PCR | SNORD 68 | LN | 28 | HC | 28 | 2.1 | 92.8 | 78.5 | 0.837 |
| Tawfik et al.25 | 2019 | Egypt | miR-155 | UP | Serum | qRT‒PCR | SNORD 68 | LN | 28 | HC | 28 | 0.98 | 100 | 64.2 | 0.642 |
| Abdul-Maksoud et al.26 | 2021 | Egypt | miR-181a | UP | Serum | qRT‒PCR | NA | LN | 49 | SLE | 67 | NA | 92.3 | 80.6 | 0.876 |
| Abdul-Maksoud et al.26 | 2021 | Egypt | miR-223 | Down | Serum | qRT‒PCR | NA | LN | 49 | SLE | 67 | NA | 79.3 | 82.7 | 0.758 |
| Abdul-Maksoud et al.26 | 2021 | Egypt | miR-181a, 223 | NA | Serum | qRT‒PCR | NA | LN | 49 | SLE | 67 | NA | 94.3 | 92.3 | 0.93 |
| Higazi et al.9 | 2023 | Egypt | miR-146a | Down | Plasma | qRT‒PCR | miRNA-16 | LN | 46 | SLE | 44 | 1.2 | 91.6 | 100 | 0.950 |
| Kahwa et al.27 | 2023 | Egypt | miR-145 | Down | Serum | qRT‒PCR | U6 snRNA | LN | 30 | SLE | 30 | 0.22 | 73.3 | 76.7 | 0.71 |
| Vahed et al.28 | 2018 | Iran | miR-125a | Up | Plasma | qRT‒PCR | miR-191-5p | LN | 26 | HC | 26 | NA | 92 | 34 | 0.67 |
| Vahed et al.28 | 2018 | Iran | miR-142-3p | Down | Plasma | qRT‒PCR | miR-191-5p | LN | 26 | HC | 26 | NA | 80 | 55 | 0.62 |
| Vahed et al.28 | 2018 | Iran | miR-146 | Up | Plasma | qRT‒PCR | miR-191-5p | LN | 26 | HC | 26 | NA | 56 | 96 | 0.75 |
| Vahed et al.28 | 2018 | Iran | miR-155 | Up | Plasma | qRT‒PCR | miR-191-5p | LN | 26 | HC | 26 | NA | 88 | 67 | 0.82 |
| Vahed et al.28 | 2018 | Iran | miR-125, miR-142-3p, miR-146, miR-155 | NA | Plasma | qRT‒PCR | miR-191-5p | LN | 26 | HC | 26 | NA | 88 | 78 | 0.89 |
| Nakhjavani et al.29 | 2019 | Iran | miR-21 | Up | Plasma | qRT‒PCR | miR-191-5p | LN | 26 | HC | 26 | NA | 86 | 63 | 0.912 |
| Nakhjavani et al.29 | 2019 | Iran | miR-150 | Down | Plasma | qRT‒PCR | miR-191-5p | LN | 26 | HC | 26 | NA | 69 | 72 | 0.640 |
| Nakhjavani et al.29 | 2019 | Iran | miR-423 | Up | Plasma | qRT‒PCR | miR-191-5p | LN | 26 | HC | 26 | NA | 96 | 66 | 0.811 |
| Nakhjavani et al.29 | 2019 | Iran | miR-21, 150,423 | NA | Plasma | qRT‒PCR | miR-191-5p | LN | 26 | HC | 26 | NA | 79 | 83 | 0.929 |
| Etemadi et al.30 | 2019 | Iran | miR-192 | Up | Plasma | qRT‒PCR | miR-191-5p | LN | 26 | HC | 26 | NA | 88 | 99 | 0.950 |
| Navarro-Quiroz et al.31 | 2016 | Colombia | miR-221-5p, 380-3p, 556-5p, 758-3p, 3074-3p | NA | Plasma | qRT‒PCR | hsa-miR-26b-5p | LN | 100 | SLE & HC | 80 | NA | 97 | 70.3 | NA |
HC: healthy control; LN: lupus nephritis; NA: not available; SLE: systemic lupus erythematosus; qRT‒PCR: quantitative real-time polymerase chain reaction.
Fig. 2.
Risk of bias assessment of studies using QUADAS-2.
Diagnostic values of MiRNAs for LN
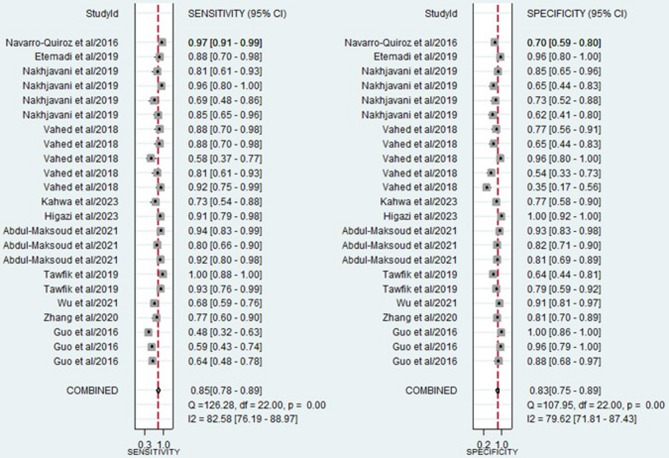
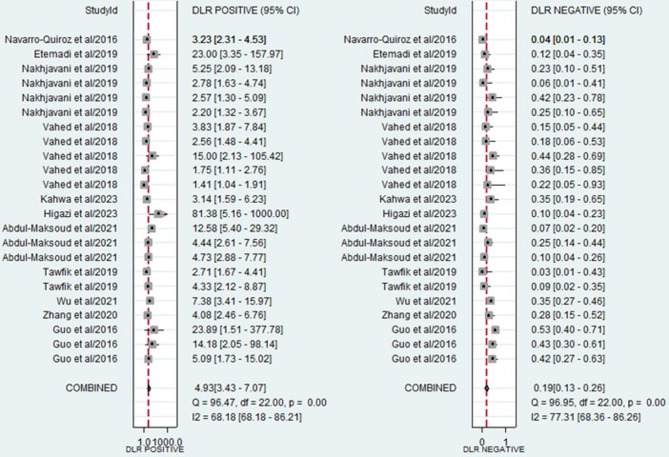
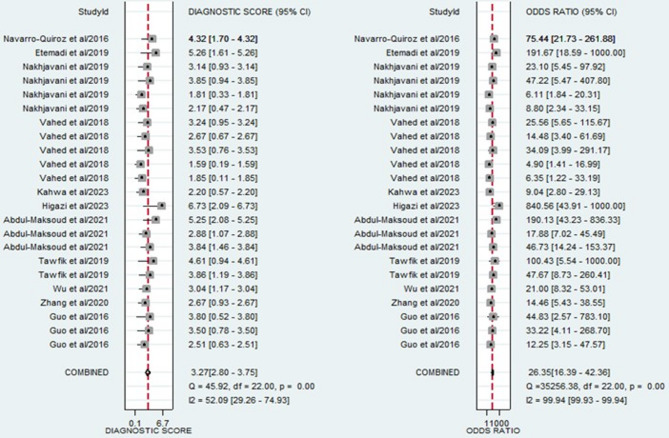
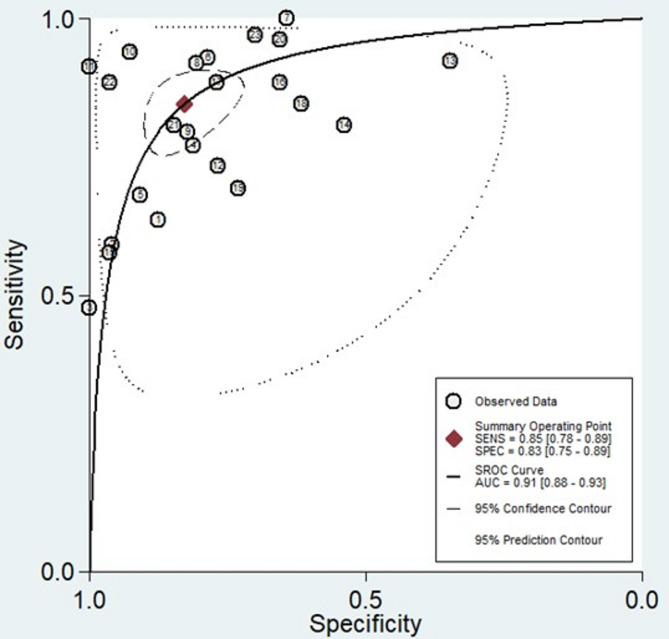
The forest plots, which display the pooled diagnostic values for miRNAs in the diagnosis of LN, are provided below. Additionally, the pooled results are detailed as follows: sensitivity, 0.85 (95% CI: 0.78–0.89); specificity, 0.83 (95% CI: 0.75–0.89) (Fig. 3); PLR, 4.93 (95% CI: 3.47–7.07); NLR, 0.19 (95% CI: 0.13–0.26) (Fig. 4); and DOR, 26.35 (95% CI: 16.39–42.36) (Fig. 5). In light of the evident heterogeneity among the studies concerning sensitivity (I2 = 82.58%) and specificity (I2 = 79.62%), a bivariate random effects model was used to estimate the diagnostic performance of miRNAs for LN. Subsequently, the SROC curve was constructed, resulting in an AUC of 0.91 (95% CI = 0.88–0.93). This AUC value underscores the outstanding diagnostic efficacy of miRNAs, thereby demonstrating their ability to differentiate LN patients from individuals with SLE and those from the healthy population (Fig. 6).
Fig. 3.
Forest plot of the pooled sensitivity and specificity of miRNAs for diagnosing LN.
Fig. 4.
Forest plot of the pooled PLR and NLR of miRNAs for diagnosing LN.
Fig. 5.
Forest plot of the pooled DOR of miRNAs for diagnosing LN.
Fig. 6.
SROC, 95% confidence contour and 95% prediction contour.
Investigating the origins of heterogeneity
Our attention was drawn to the presence of heterogeneity, prompting an investigation into its sources. Initially, we assessed whether the threshold effect played a role in the observed heterogeneity. However, our findings revealed a Spearman correlation coefficient of − 0.46 (P = 0.25) and the absence of the characteristic “shoulder-arm” shape, which is typically representative of the threshold effect in the SROC curve (Fig. 6). This finding indicated the exclusion of the threshold effect in our current study. Furthermore, we identified heterogeneity stemming from factors other than the threshold effect, which we termed the nonthreshold effect. Notably, the DORs from each study and the summary DOR did not conform to a linear distribution. This nonlinear pattern was evident in the I2 statistic, which was 99.94% (P = < 0.001) (Fig. 5), suggesting that the nonthreshold effect may have contributed to the observed heterogeneity. To assess the sources of heterogeneity, subgroup analysis and meta-regression were also conducted.
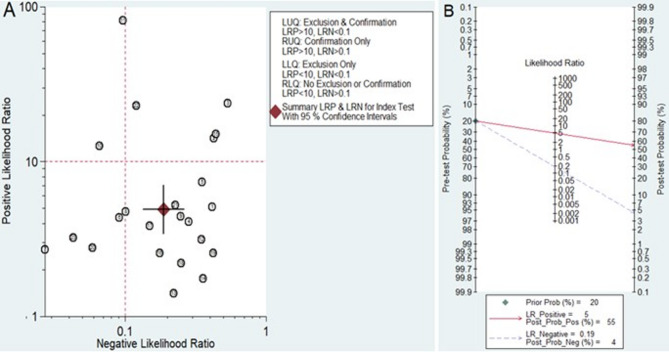
Clinical utility of MiRNAs for diagnosing LN
Playing a significant role in the clinical decision-making process during diagnosis is considered an important aspect of biomarkers. To evaluate the clinical utility of miRNAs in diagnosing LN, a likelihood ratio scattergram was constructed using the combination of the PLR and NLR. A high diagnostic accuracy was indicated by a PLR > 10 and an NLR < 0.1. The results indicated that two studies conducted by Abdul-Maksoud et al. (including miR-181a and miR-223)26 and Higazi et al. (including miR-146a)9 demonstrated the superior diagnostic efficacy of these miRNAs (Fig. 7A). Subsequently, a Fagan plot was generated to evaluate the clinical value of the miRNAs in the diagnosis of LN. Initially, the pretest probability was set at 20%. The subsequent presentation of a positive test resulted in a posttest probability of 55%, with a PLR of 4.93. Conversely, the NLR was determined to be 0.19, and a negative test reduced the posttest probability to 4% (Fig. 7B).
Fig. 7.
Assessment of the clinical applicability of miRNAs for the diagnosis of LN. (A) Likelihood ratio scattergram. (B) Fagan’s nomogram.
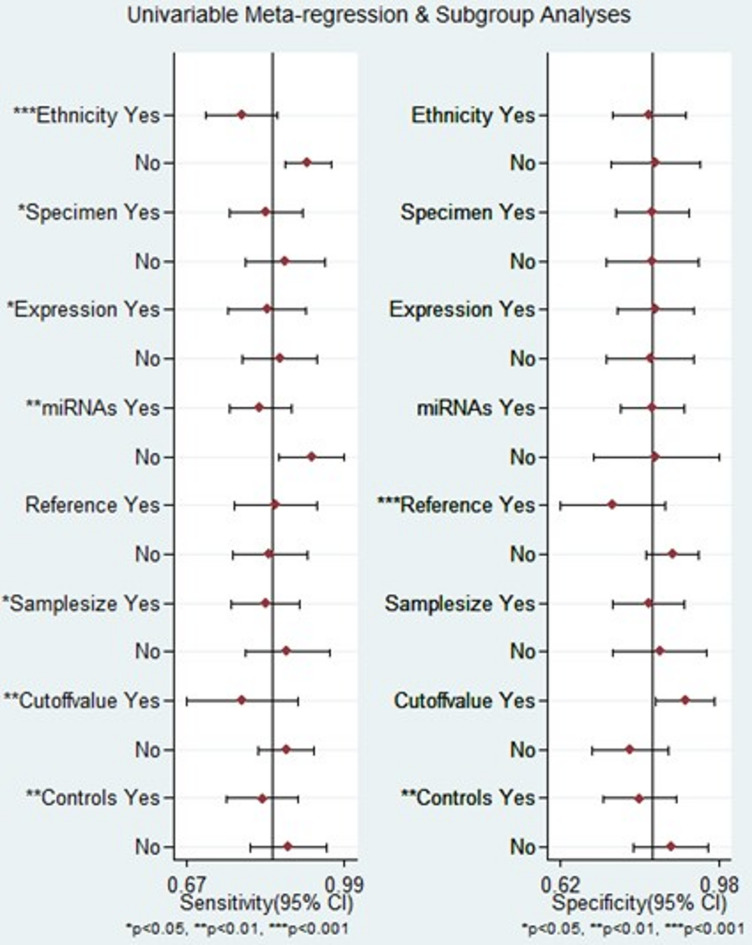
Subgroup analysis and meta-regression
To identify sources of heterogeneity among studies, subgroup analysis and meta-regression analysis were conducted considering factors such as ethnicity, specimen type, miRNA expression, miRNA profiling, internal reference control, sample size, cutoff value setting, and comparison groups. According to our subgroup analysis, circulating miRNAs exhibited superior diagnostic accuracy for diagnosing LN among African patients. The sensitivity, specificity, PLR, NLR, DOR, and AUC were 0.90 (95% CI = 0.83–0.95), 0.85 (95% CI = 0.74–0.92), 6.1 (95% CI = 3.3–11.0), 0.11 (95% CI = 0.06–0.21), 53 (95% CI = 20–141), and 0.94 (95% CI = 0.92–0.96), respectively. In contrast, the performance of miRNAs for diagnosing LN in Asian patients was found to be less favorable than that in African patients. The combined results indicated sensitivity (0.77, 95% CI = 0.69–0.84), specificity (0.82, 95% CI = 0.71–0.90), PLR (4.4, 95% CI = 2.8–6.9), NLR (0.27, 95% CI = 0.21–0.35), DOR (16, 95% CI = 10–24), and AUC (0.86, 95% CI = 0.82–0.89).
In addition, it is important to note that the diagnostic effectiveness of the miRNA panel significantly surpassed that of a single miRNA. The sensitivity, specificity, PLR, NLR, DOR, and AUC were 0.92 (95% CI = 0.85–0.96), 0.83 (95% CI = 0.71–0.91), 5.4 (95% CI = 3.2–9.4), 0.09 (95% CI = 0.05–0.18), and 60 (95% CI = 26–136), respectively. The AUC for the miRNA panel was 0.95 (95% CI = 0.92–0.96). Interestingly, miRNA expression levels were found to be associated with diagnostic value. The results indicated that upregulated miRNAs displayed significant diagnostic efficacy with an AUC of 0.90 (95% CI = 0.87–0.93), in contrast to downregulated miRNAs, which had an AUC of 0.85 (95% CI = 0.82–0.88).
Notably, compared with healthy individuals, miRNAs exhibited superior diagnostic value in distinguishing LN patients from SLE patients. This was evident in terms of sensitivity (0.93, 95% CI = 0.91–0.95), specificity (0.89, 95% CI = 0.80–0.94), PLR (7.6, 95% CI = 3.9–14.8), NLR (0.17, 95% CI = 0.10–0.31), DOR (44, 95% CI = 15–133), and AUC (0.93, 95% CI = 0.91–0.95). Moreover, in studies that did not employ optimal truncation values, the combined results included a sensitivity, specificity, PLR, NLR, DOR, and AUC of 0.87 (95% CI = 0.81–0.91), 0.77 (95% CI = 0.68–0.85), 3.9 (95% CI = 2.7–5.5), 0.17 (95% CI = 0.12–0.24), 23 (95% CI = 13–40), and 0.90 (95% CI = 0.87–0.92), respectively. In contrast, studies that reported cutoff values demonstrated a more favorable diagnostic performance, with a sensitivity of 0.79 (95% CI: 0.61–0.90), specificity of 0.91 (95% CI = 0.79–0.96), PLR of 8.6 (95% CI = 3.9–18.9), NLR of 0.23 (95% CI = 0.12–0.44), DOR of 38 (95% CI = 15–98), and AUC of 0.93 (95% CI = 0.90–0.95), as outlined in Table 2.
Table 2.
Summary estimates of subgroup analysis for diagnostic test accuracy.
| Subgroup | No of studies | Sensitivity (95% CI) | Specificity (95% CI) | PLR (95% CI) | NLR (95% CI) | DOR (95% CI) | AUC (95% CI) |
|---|---|---|---|---|---|---|---|
| Ethnicity | |||||||
| Asian | 15 | 0.77 (0.69, 0.84) | 0.82 (0.71, 0.90) | 4.4 (2.8, 6.9) | 0.27 (0.21, 0.35) | 16 (10, 24) | 0.86 (0.82, 0.89) |
| African | 7 | 0.90 (0.83, 0.95) | 0.85 (0.74, 0.92) | 6.1 (3.3, 11.0) | 0.11 (0.06, 0.21) | 53 (20, 141) | 0.94 (0.92, 0.96) |
| Specimen | |||||||
| Plasma | 15 | 0.83 (0.74, 0.79) | 0.84 (0.71, 0.92) | 5.3 (2.8, 9.7) | 0.20 (0.13, 0.31) | 26 (13, 53) | 0.90 (0.87, 0.92) |
| Serum | 8 | 0.87 (0.77, 0.93) | 0.83 (0.77, 0.87) | 5.0 (3.8, 6.6) | 0.16 (0.09, 0.29) | 32 (16, 63) | 0.90 (0.87, 0.92) |
| Expression | |||||||
| Upregulated | 13 | 0.83 (0.72, 0.91) | 0.84 (0.72, 0.91) | 5.1 (3.0, 8.5) | 0.20 (0.13, 0.31) | 26 (16, 41) | 0.90 (0.87, 0.93) |
| Downregulated | 6 | 0.79 (0.71, 0.86) | 0.82 (0.64, 0.93) | 4.5 (1.9, 10.7) | 0.25 (0.16, 0.40) | 18 (5, 65) | 0.85 (0.82, 0.88) |
| miRNAs | |||||||
| Single | 19 | 0.82 (0.74, 0.88) | 0.83 (0.73, 0.90) | 4.8 (3.1, 7.4) | 0.22 (0.16, 0.30) | 22 (13, 37) | 0.89 (0.86, 0.92) |
| Panel | 4 | 0.92 (0.85, 0.96) | 0.83 (0.71, 0.91) | 5.4 (3.2, 9.4) | 0.09 (0.05, 0.18) | 60 (26, 136) | 0.95 (0.92, 0.96) |
| Reference | |||||||
| miR-191-5p | 10 | 0.84 (0.76, 0.90) | 0.74 (0.60, 0.85) | 3.2 (2.1, 5.1) | 0.22 (0.15, 0.31) | 15 (8, 27) | 0.87 (0.84, 0.90) |
| U6 | 5 | 0.64 (0.54, 0.72) | 0.92 (0.82, 0.97) | 8.2 (3.7, 18.2) | 0.39 (0.31, 0.49) | 21 (9, 46) | 0.82 (0.79, 0.85) |
| Sample size | |||||||
| < 100 | 17 | 0.83 (0.73, 0.89) | 0.83 (0.71, 0.90) | 4.8 (2.9, 8.1) | 0.21 (0.14, 0.30) | 23 (13, 43) | 0.90 (0.87, 0.92) |
| ≥ 100 | 6 | 0.88 (0.76, 0.94) | 0.83 (0.77, 0.89) | 5.3 (3.8, 7.3) | 0.15 (0.08, 0.29) | 35 (18, 69) | 0.91 (0.88, 0.93) |
| Cutoff value | |||||||
| Given | 8 | 0.79 (0.61, 0.90) | 0.91 (0.79, 0.96) | 8.6 (3.9, 18.9) | 0.23 (0.12, 0.44) | 38 (15, 98) | 0.93 (0.90, 0.95) |
| NA | 15 | 0.87 (0.81, 0.91) | 0.77 (0.68, 0.85) | 3.9 (2.7, 5.5) | 0.17 (0.12, 0.24) | 23 (13, 40) | 0.90 (0.87, 0.92) |
| Control groups | |||||||
| HC | 16 | 0.82 (0.73, 0.88) | 0.80 (0.69, 0.88) | 4.1 (2.7, 6.2) | 0.23 (0.16, 0.32) | 18 (12, 28) | 0.88 (0.85, 0.90) |
| SLE | 6 | 0.85 (0.74, 0.91) | 0.89 (0.80, 0.94) | 7.6 (3.9, 14.8) | 0.17 (0.10, 0.31) | 44 (15, 133) | 0.93 (0.91, 0.95) |
HC: healthy control; SLE: systemic lupus erythematous.
The outcomes of the meta-regression analysis are depicted in Fig. 8. Factors such as ethnicity, specimen type, miRNA expression, miRNA profiling, sample size, cutoff value setting, and comparison groups were identified as contributors to heterogeneity in terms of sensitivity (p < 0.05). Additionally, the use of an internal reference control and comparison groups were found to impact specificity (p < 0.05).
Fig. 8.
Meta-regression analysis for sensitivity and specificity.
Sensitivity analysis
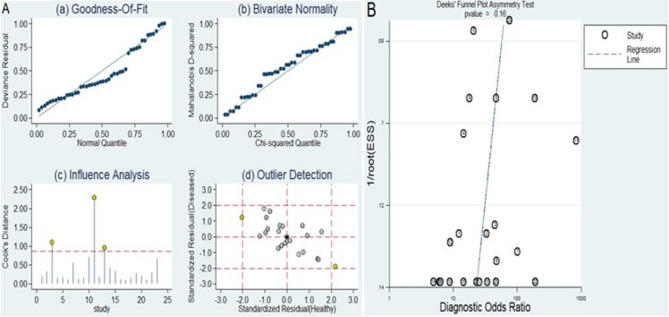
The outcomes of the sensitivity analysis are displayed in Fig. 9A. Examination of goodness of fit (Fig. 9A (a)) and assessment of bivariate normality (Fig. 9A (b)) both indicated the robustness of our chosen model. Influence analysis revealed that the studies were conducted by Guo et al.22, Higazi et al.9, and Vahed et al.28 carried the most substantial weight (Fig. 9A (c)). The outlier detection suggested that the heterogeneity may be attributed to the data corresponding to the studies by Guo et al. (miR-148a)22 and Vahed et al. (miR-125a)28 (Fig. 9A (d)). The exclusion of two outlier studies resulted in a high sensitivity (0.85, 95% CI = 0.79–0.90), specificity (0.83, 95% CI = 0.76–0.87), PLR (4.9, 95% CI = 3.6–6.6), NLR (0.18, 95% CI = 0.13–0.26), DOR (27, 95% CI = 17–44), and AUC (0.91, 95% CI = 0.88–0.93). Importantly, these alterations did not lead to any significant changes in the combined diagnostic efficacy.
Fig. 9.
Sensitivity analysis and assessment of publication bias. (A) Sensitivity analysis. (B) Deeks’ funnel plot for publication bias analysis.
Publication bias
Deeks’ funnel plot was generated to evaluate the potential presence of publication bias within this study. The studies exhibited a relatively symmetric distribution on both sides of the regression line, with a p value of 0.16. Importantly, these differences were not statistically significant, suggesting that there was no evidence of publication bias in the included dataset (Fig. 9B).
Discussion
Systemic lupus erythematosus can potentially impact any organ, but it most commonly affects the kidneys. The presence of autoantibodies and immune complexes in renal compartments is a contributing factor to the development of LN32. Renal complications are observed in up to 70% of SLE patients and serve as a crucial predictor of morbidity and mortality in SLE patients33. Despite advancements in therapeutic approaches, managing lupus nephritis remains a challenge, with approximately 30% of patients progressing to end-stage kidney disease4. Conversely, existing laboratory tests lack the ability to accurately diagnose lupus nephritis activity and assess therapeutic response, presenting a challenge in evaluating potential new treatments34. Given the potential severe complications stemming from renal involvement and the limited treatment options available, there is a pressing need to identify novel biomarkers capable of monitoring and predicting the progression of renal complications35.
Recently, there have been numerous reports on a diverse range of new LN biomarkers and their connection to the development or activity of this disease. These include serum and urinary cytokines, adhesion molecules, growth factors, long noncoding RNAs, and miRNAs10,36. MiRNAs, crucial genetic regulators, actively participate in the pathophysiology of various diseases37. Disrupted miRNA expression can potentially disturb vital cellular processes. Several miRNAs, both in blood serum, urine, and renal tissue, have been found to be abnormally expressed in LN patients38. Extensive research and reviews in the last decade have delved into the role of miRNAs in LN, with several widely recognized miRNAs implicated in the development and progression of LN39–42. Consequently, utilizing circulating miRNA expression signatures in monitoring SLE could prove advantageous, as these signatures could serve as cost-effective and noninvasive biomarkers for screening for the development of disease complications, including LN43. Therefore, this meta-analysis aimed to assess the pooled diagnostic accuracy of circulating miRNAs for LN.
In the current meta-analysis, a total of 11 diagnostic accuracy studies were included, encompassing 526 patients with LN and 490 individuals in the control group. The results showed a pooled sensitivity of 0.76 (95% CI = 0.70–0.81), specificity of 0.80 (95% CI = 0.74–0.85), PLR of 3.75 (95% CI = 2.82–5.01), NLR of 0.30 (95% CI = 0.24–0.39), DOR of 12.32 (95% CI = 7.65–19.83) and AUC of 0.85 (95% CI = 0.81–0.88) for the diagnosis of LN. The combined sensitivity and specificity demonstrated a moderate level of accuracy for the diagnostic test, suggesting that miRNAs possess sufficient statistical capability to identify or exclude suspected cases, thereby enhancing clinical diagnosis. A PLR of 3.75 indicated a 3.75-fold improvement in the ability to accurately diagnose LNs, while an NLR of 0.30 signified a 70% reduction in the likelihood of successful LN diagnosis when miRNA expression was reversed. Moreover, the DOR of 12.32 (> 1.0) in our analysis suggested that dysregulated miRNAs achieved a relatively high overall accuracy in diagnosing LN. Additionally, a higher AUC reflects superior performance in terms of balancing sensitivity and specificity, with a value of 0.91 in our study, indicating an overall high diagnostic accuracy of miRNAs in detecting LN.
To identify sources of heterogeneity between studies, subgroup analysis and meta-regression analysis were conducted considering factors such as ethnicity, specimen type, miRNA expression, miRNA profiling, internal reference control, sample size, cutoff value setting, and comparison groups. According to our subgroup analysis, circulating miRNAs exhibited superior diagnostic accuracy for diagnosing LN among African patients (AUC = 0.94) compared with Asian patients (AUC = 0.86). The variation in the incidence of LN among different races and regions may be attributed to differences in epigenetic factors and genetic predispositions. In addition, the diagnostic effectiveness of the miRNA panel (AUC = 0.95) significantly surpassed that of a single miRNA (AUC = 0.89). These findings are in line with that of other recent meta-analysis44. This might be attributed to the fact that every biological factor is a component of a complex matrix of interconnected biological occurrences. Consequently, the relationships of each factor play a role in influencing their predictive capabilities. When multiple miRNAs are associated with disease progression through different mechanisms, incorporating them into the same predictive model can increase the accuracy as their contributions become additive44.
Interestingly, upregulated miRNAs showed a greater diagnostic efficacy than downregulated miRNAs, with an AUC of 0.90 and an AUC of 0.85, respectively; these results are consistent with those of studies by WU et al. and He et al.45,46. This could be ascribed to the greater specificity of upregulated miRNAs to the disease, along with their higher expression levels in patients with LN. Additionally, the increased detectability of upregulated miRNAs compared to that of downregulated miRNAs contributed to this phenomenon. In addition, the miRNAs exhibited superior diagnostic value in distinguishing LN patients from SLE patients (AUC = 93) compared to healthy individuals (AUC = 88). Nonetheless, only six of the included studies included SLE patients as a control group. However, further research may be needed to determine the underlying mechanisms behind the above observations. Additionally, miRNAs utilizing U6 as the internal reference control for relative quantitative analysis exhibited a lower diagnostic accuracy (AU: 0.82) than miRNAs that used miR-191-5p as the internal reference control (AU: 0.87). Support for this observation comes from a study by Xiang et al.47, which concluded that U6 is unsuitable as an internal reference control for standardizing miRNA quantitative detection due to its instability. Therefore, to enhance the practical utility of miRNAs as diagnostic biomarkers in clinical settings, it is exceedingly important to choose a unified, suitable, and stable internal reference in the future.
Regarding clinical applications, the Fagan diagram shows promising results for circulating miRNAs as a diagnostic marker for LN. The results indicate that the posttest probability of LN increases from 20 to 55% when the test is positive, suggesting that the test has significant diagnostic value for LN. Additionally, we utilized a likelihood ratio scattergram, which combines the PLR and NLR, to assess the clinical applicability of the nomograms. The results are situated in the first quadrant, where a PLR > 10 and an NLR < 0.1 indicate high applicability. Notably, a miRNA panel (for miR-181a and miR-223) from a study by Abdul-Maksoud et al.26 and a miRNA-14a from a study by Higazi et al.9 demonstrated superior diagnostic efficacy. Consequently, both single-miRNA (miR-146a) and single-miRNA panels, such as miR-181a and miR-223, may hold more promise and merit further investigation in future research.
This meta-analysis offers several key strengths. First, this is the first comprehensive synthesis assessing the diagnostic utility of miRNAs in LN. Second, meta-regression and subgroup analysis were used to investigate sources of heterogeneity. Third, the absence of publication bias, as evidenced by the Deeks’ funnel plot asymmetry test, enhances the reliability of the findings. Despite these insights, the study has several limitations. Firstly, substantial heterogeneity is observed across the included studies, even after performing subgroup and meta-regression analyses. This heterogeneity arises from differences in patient populations, miRNA types, measurement techniques, sample types, sample sizes, cutoff values, internal reference controls, and study designs. Such variability may influence the pooled sensitivity and specificity estimates, potentially leading to an overestimation of the diagnostic performance of miRNAs and limiting the generalizability of the findings across different clinical settings and populations. Secondly, many of the included studies have small sample sizes, which may further contribute to overestimated diagnostic accuracy. Thirdly, the inclusion of a limited number of studies constrains the overall scope of the study. Fourthly, due to the insufficiency of similar miRNAs for result pooling, identifying specific single miRNAs or miRNA panels as optimal diagnostic biomarkers for LN remains difficult. Fifthly, although standardized protocols were employed within individual studies, notable variability across studies persists, leading to inconsistencies in miRNA quantification and interpretation. Additionally, the predominantly cross-sectional design of the included studies limits the ability to assess the temporal relationship between miRNA levels and disease progression or treatment response. Furthermore, although Deeks’ funnel plot did not show significant asymmetry, the potential impact of unpublished negative or inconclusive studies cannot be excluded. Given the novelty and growing interest in miRNAs as diagnostic biomarkers, positive findings are more likely to be published, which could inflate pooled estimates and obscure true variability in diagnostic performance, leading to overly optimistic conclusions. Lastly, the limited representation of the present study population from certain countries may compromise the global applicability of the diagnostic performance of miRNAs for LN. Addressing these concerns calls for expanded international prospective multicenter research in this domain.
In conclusion, circulating miRNAs are promising noninvasive biomarkers for diagnosing LN. In particular, miRNA panels, upregulated miRNAs, miRNAs that distinguishing LN patients from SLE patients, and miRNAs for diagnosing LN among African patients demonstrated enhanced diagnostic efficacy for LN. To validate the diagnostic efficacy of specific miRNAs identified in our meta-analysis, we emphasize the need for larger, well-powered, multicenter prospective studies with well-defined cohorts of LN patients and appropriate control groups. Longitudinal studies are necessary to assess temporal changes in miRNA levels, determine their correlation with disease progression, and establish their utility as biomarkers for monitoring disease activity over time. Additionally, larger sample sizes are crucial to enhance statistical power and ensure generalizability across diverse populations. Standardizing miRNA measurement techniques through the adoption of uniform protocols in future research will minimize variability, enhance reproducibility, and ensure the comparability and reliability of findings. Encouraging multicenter collaborations is essential to pool resources and data, produce more robust studies, validate miRNA biomarkers across different demographics and clinical settings, and ultimately facilitate their translation into clinical practice. For clinical practice, we suggest integrating miRNA testing as a complementary diagnostic tool alongside existing routine laboratory tests for LN, developing clinical guidelines that include miRNA testing for early detection and monitoring of LN, and educating clinicians on the potential benefits and limitations of miRNA-based diagnostics to facilitate informed decision-making in routine protocols.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Author contributions
E.A and M.A.B conceptualized and planned the study, conducted literature searches, screened and extracted the data, and assessed the quality of the included articles. They also performed the statistical analyses and contributed to manuscript writing. A.G, H.D, A.W, Z.M, E.E, M.T, and H.E participated in the article searches; data screening and extraction; and quality assessment of the included data and provided support for the analysis, review, and editing of the manuscript. The final draft of the manuscript was reviewed and approved by all authors before submission for publication.
Funding
The authors received no financial support for the study.
Data availability
“All data generated or analyzed during this study are included in this published article and its supplementary information files”.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Ermiyas Alemayehu and Melaku Ashagrie Belete have contributed equally to this work and share first authorship.
References
- 1.Fava, A. & Petri, M. Systemic lupus erythematosus: Diagnosis and clinical management. J. Autoimmun.96, 1–13 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barber, M. R. et al. Global epidemiology of systemic lupus erythematosus. Nat. Rev. Rheumatol.17 (9), 515–532 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Trotter, K., Clark, M. R. & Liarski, V. M. Overview of pathophysiology and treatment of human lupus nephritis. Curr. Opin. Rheumatol.28 (5), 460 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Anders, H-J. et al. Lupus nephritis (Primer). Nat. Rev. Disease Primers6(1). (2020). [DOI] [PubMed]
- 5.Besouw, M. T. et al. Pediatric lupus nephritis presenting with terminal renal failure. Acta Clin. Belg.71 (6), 455–457 (2016). [DOI] [PubMed] [Google Scholar]
- 6.Fanouriakis, A. et al. 2019 update of the joint European league against rheumatism and European renal Association–European dialysis and transplant association (EULAR/ERA–EDTA) recommendations for the management of lupus nephritis. Ann. Rheum. Dis.79 (6), 713–723 (2020). [DOI] [PubMed] [Google Scholar]
- 7.ParikhSV, Alvarado, A., Malvar, A. & Rovin, B. H. editors (eds) itors. The kidney biopsy in lupus nephritis: Past, present, and future. Semin. Nephrol.35 5 466–477 (2015). [DOI] [PubMed] [Google Scholar]
- 8.Khosroshahi, H. T., Abedi, B., Daneshvar, S., Sarbaz, Y. & Shakeri Bavil, A. Future of the renal biopsy: Time to change the conventional modality using nanotechnology. Int. J. Biomed. Imaging. (2017). [DOI] [PMC free article] [PubMed]
- 9.Higazi, A. M. et al. Potential role of Circulating miRNA-146a and serum Kallikrein 1 as biomarkers of renal disease in biopsy-proven lupus nephritis patients. Egypt. Rheumatologist. 45 (1), 73–80 (2023). [Google Scholar]
- 10.Mihaylova, G., Vasilev, V., Kosturkova, M. B., Stoyanov, G. S. & Radanova, M. Long non-coding RNAs as new biomarkers in lupus nephritis: A connection between present and future. Cureus12(7). (2020). [DOI] [PMC free article] [PubMed]
- 11.Palazzo, L., Lindblom, J., Mohan, C. & Parodis, I. Current insights on biomarkers in lupus nephritis: A systematic review of the literature. J. Clin. Med.11 (19), 5759 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Aragón, C. C. et al. Urinary biomarkers in lupus nephritis. J. Transl. Autoimmun.3, 100042 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shang, R., Lee, S., Senavirathne, G. & Lai, E. C. MicroRNAs in action: Biogenesis, function and regulation. Nat. Rev. Genet. 1–18. (2023). [DOI] [PMC free article] [PubMed]
- 14.Honarpisheh, M., Köhler, P., von Rauchhaupt, E. & Lech, M. The involvement of microRNAs in modulation of innate and adaptive immunity in systemic lupus erythematosus and lupus nephritis. J. Immunol. Res. (2018). [DOI] [PMC free article] [PubMed]
- 15.Li, W. et al. Circulating Exosomal MicroRNAs as biomarkers of systemic lupus erythematosus. Clinics75. (2020). [DOI] [PMC free article] [PubMed]
- 16.Wang, H., Peng, W., Ouyang, X., Li, W. & Dai, Y. Circulating MicroRNAs as candidate biomarkers in patients with systemic lupus erythematosus. Transl. Res.160 (3), 198–206 (2012). [DOI] [PubMed] [Google Scholar]
- 17.Song, J., Zhao, L. & Li, Y. Comprehensive bioinformatics analysis of mRNA expression profiles and identification of a miRNA–mRNA network associated with lupus nephritis. Lupus29 (8), 854–861 (2020). [DOI] [PubMed] [Google Scholar]
- 18.Page, M. J. et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Int. J. Surg.88, 105906 (2021). [DOI] [PubMed] [Google Scholar]
- 19.Freeman, K. et al. Quality Assessment of Diagnostic Accuracy Studies-2 Quality Appraisal Tool and Guidance Notes. Multiplex Tests To Identify Gastrointestinal Bacteria, Viruses and Parasites in People with Suspected Infectious Gastroenteritis: A Systematic Review and Economic Analysis (NIHR Journals Library, 2017). [DOI] [PMC free article] [PubMed]
- 20.Jackson, D., White, I. R. & Thompson, S. G. Extending dersimonian and laird’s methodology to perform multivariate random effects meta-analyses. Stat. Med.29 (12), 1282–1297 (2010). [DOI] [PubMed] [Google Scholar]
- 21.Higgins, J. P., Thompson, S. G., Deeks, J. J. & Altman, D. G. Measuring inconsistency in meta-analyses. Bmj327 (7414), 557–560 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guo, S. et al. Clinical correlation of plasma miR-21, miR-126 and miR-148a in patients with lupus nephritis. Int. J. Clin. Exp. Med.9 (2), 2905–2912 (2016). [Google Scholar]
- 23.Zhang, H. et al. B cell-related Circulating MicroRNAs with the potential value of biomarkers in the differential diagnosis, and distinguishment between the disease activity and lupus nephritis for systemic lupus erythematosus. Front. Immunol.9, 1473 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wu, Q., Qin, Y., Shi, M. & Yan, L. Diagnostic significance of Circulating miR-485-5p in patients with lupus nephritis and its predictive value evaluation for the clinical outcomes. J. Chin. Med. Assoc. 84 (5), 491–497 (2021). [DOI] [PubMed] [Google Scholar]
- 25.Tawfik, N. et al. Serum miRNA-146a and miRNA-155 as novel biomarkers in lupus nephritis activity with systemic lupus erythematosus. Am. J. Biochem.9, 21–34 (2019). [Google Scholar]
- 26.Abdul-Maksoud, R. S. et al. Circulating miR‐181a and miR‐223 expression with the potential value of biomarkers for the diagnosis of systemic lupus erythematosus and predicting lupus nephritis. J. Gene. Med.23 (5), e3326 (2021). [DOI] [PubMed] [Google Scholar]
- 27.Kahwa, S. M., Shaker, O. G., Eissa, B. M. & Wahb, A. M. Tumor necrosis factor-α, its related immunoregulatory long non-coding RNA (THRIL) and micro-RNA 145 as potential biomarkers in lupus nephritis patients. Egypt. Rheumatologist. 45 (4), 299–302 (2023). [Google Scholar]
- 28.Vahed, S. Z. et al. Altered levels of immune-regulatory MicroRNAs in plasma samples of patients with lupus nephritis. BioImpacts BI. 8 (3), 177 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nakhjavani, M. et al. Plasma levels of miR-21, miR-150, miR-423 in patients with lupus nephritis. Iran. J. Kidney Dis.13 (3), 198 (2019). [PubMed] [Google Scholar]
- 30.Etemadi, J., Motavalli, R., Mahmoodpoor, F. & Abediazar, S. Elevated levels of plasma microRNA-192 in patients with lupus nephritis. Immunopathol. Persa. 5 (1), e02–e (2019). [Google Scholar]
- 31.Navarro-Quiroz, E. et al. High-throughput sequencing reveals Circulating MiRNAs as potential biomarkers of kidney damage in patients with systemic lupus erythematosus. PLoS ONE. 11 (11), e0166202 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Almaani, S., Meara, A. & Rovin, B. H. Update on lupus nephritis. Clin. J. Am. Soc. Nephrol. CJASN. 12 (5), 825 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Furie, R. et al. Two-year, randomized, controlled trial of Belimumab in lupus nephritis. N. Engl. J. Med.383 (12), 1117–1128 (2020). [DOI] [PubMed] [Google Scholar]
- 34.Malvar, A. et al. Histologic versus clinical remission in proliferative lupus nephritis. Nephrol. Dialysis Transpl. 32 (8), 1338–1344 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dias, R., Hasparyk, U. G., Lopes, M. P. & de Barros, J. L. V. M. Simões e Silva AC. Novel biomarkers for lupus nephritis in the OMICS era. Curr. Med. Chem.28 (29), 6011–6044 (2021). [DOI] [PubMed] [Google Scholar]
- 36.Ghorbaninezhad, F. et al. Tumor necrosis factor–α in systemic lupus erythematosus: Structure, function and therapeutic implications. Int. J. Mol. Med.49 (4), 1–13 (2022). [DOI] [PubMed] [Google Scholar]
- 37.Zhang, M., Xiong, F., Zhang, S., Guo, W. & He, Y. Crucial roles of miR-625 in human cancer. Front. Med.9, 845094 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Khoshmirsafa, M. et al. Elevated expression of miR-21 and miR‐155 in peripheral blood mononuclear cells as potential biomarkers for lupus nephritis. Int. J. Rheum. Dis.22 (3), 458–467 (2019). [DOI] [PubMed] [Google Scholar]
- 39.Zhang, L-H. et al. MiR-146b-5p targets IFI35 to inhibit inflammatory response and apoptosis via JAK1/STAT1 signaling in lipopolysaccharide-induced glomerular cells. Autoimmunity54 (7), 430–438 (2021). [DOI] [PubMed] [Google Scholar]
- 40.So, B. Y., Yap, D. Y. & Chan, T. M. MicroRNAs in lupus Nephritis–Role in disease pathogenesis and clinical applications. Int. J. Mol. Sci.22 (19), 10737 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li, H. et al. Epigenetic regulation of RCAN1 expression in kidney disease and its role in podocyte injury. Kidney Int.94 (6), 1160–1176 (2018). [DOI] [PubMed] [Google Scholar]
- 42.Ye, H. et al. microRNA-199a May be involved in the pathogenesis of lupus nephritis via modulating the activation of NF-κB by targeting Klotho. Mol. Immunol.103, 235–242 (2018). [DOI] [PubMed] [Google Scholar]
- 43.Nagy, D. et al. MicroRNA-126 and 146a as potential biomarkers in systemic lupus erythematosus patients with secondary antiphospholipid syndrome. Egypt. Rheumatologist. 42 (3), 201–206 (2020). [Google Scholar]
- 44.Guévremont, D., Roy, J., Cutfield, N. J. & Williams, J. M. MicroRNAs in parkinson’s disease: A systematic review and diagnostic accuracy meta-analysis. Sci. Rep.13 (1), 16272 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wu, N. et al. Circulating MicroRNAs as diagnostic biomarkers for melanoma: A systematic review and meta-analysis. BMC Cancer. 23 (1), 1–14 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.He, J., Jiang, Y., Liu, L., Zuo, Z. & Zeng, C. Circulating Micrornas as promising diagnostic biomarkers for patients with glioma: A meta-analysis. Front. Neurol.11, 610163 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Xiang, M. et al. U6 is not a suitable endogenous control for the quantification of Circulating MicroRNAs. Biochem. Biophys. Res. Commun.454 (1), 210–214 (2014). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
“All data generated or analyzed during this study are included in this published article and its supplementary information files”.