Abstract
Objective
To evaluate the refractive, keratometric, and biomechanical changes in keratoconus patients following modified accelerated corneal cross-linking (A-CXL).
Methods
This retrospective observational study analyzed clinical data from patients with progressive keratoconus who underwent modified A-CXL with a prolonged riboflavin imbibition period prior to UV irradiation. Preoperative and post-operative data on vision, refraction, tomography using OCULUS Pentacam and biomechanics using Corvis ST were analyzed at various follow-up intervals. Subgroup analysis was conducted based on topographic keratoconus classification. Statistical comparison was performed to assess changes in these parameters over time.
Results
A total of 70 eyes of 55 patients were included. A statistically significant improvement in mean change of logMAR BCVA was noted at 6 and 12 months (p = 0.006 and p = 0.018). Six months following A-CXL, statistically significant improvements were observed in keratometry and pachymetry (p = 0.003 and p < 0.001). Mean changes in biomechanical parameters deformation amplitude ratio (DAR) and Integrated Radius (IR) were found to be significantly decreased at 6 months (p = 0.001 and p = 0.011). Other biomechanical parameters SPA1, SSI and ARTh had no significant changes suggesting stability. Comparative analysis between different stages of keratoconus revealed that patients classified under TKC-4 exhibited greater improvement in keratometry compared to those with TKC-2 and TKC-3 staging.
Conclusion
A-CXL with extended riboflavin exposure was effective in stabilizing both tomographic and biomechanical parameters which resulted in increased corneal stiffness and maintained stability. All KC stages demonstrated corneal stability following A-CXL.
Keywords: Keratoconus, Accelerated corneal collagen cross linking, Refractive changes, Keratometry, Corneal biomechanics
Introduction
Keratoconus (KC) is a progressive, non-inflammatory corneal disease characterized by corneal thinning and conical protrusion [1, 8]. It is usually a bilateral condition, although asymmetry between patient’s eyes is commonly observed. KC occurs approximately in 1 out of 2000 individuals and is associated with irregular astigmatism which typically progresses during puberty and can progress over a period of several years before stabilizing [1, 3, 8]. Sequelae include thinning of the central, inferior paracentral, or inferior mid peripheral cornea. Subsequent change in shape can cause visual distortion, blurriness, and other vision problems such as nearsightedness, light sensitivity, and scarring which can eventually lead to irreversible vision loss [1].
Keratoconus is treated using a variety of strategies. Options for treatment include refractive correction using spectacles, contact lenses or intracorneal ring segment implantation, and, in more advanced cases, deep anterior lamellar keratoplasty (DALK) or penetrating keratoplasty (PKP) [4, 7, 8]. In recent years, corneal collagen crosslinking (CXL) has drawn attention as a treatment for KC. Introduced by Wollensak et al. in 2003, CXL is a treatment to suppress KC progression [1, 8]. It involves applying drops of the photosensitizing agent Vitamin B2 or riboflavin followed by exposure of the treated cornea to ultraviolet light ultraviolet-A (UV-A) interacting photochemically with ambient oxygen in the corneal stroma to produce free oxygen radicals. The corneal stroma's structure is strengthened by this photochemical process, which creates more covalent bonds between the collagen fibrils in the corneal stroma [4]. It brings about changes in collagen causing a stiffening effect by decreasing enzymatic degradation hence stabilizing the stromal layer of the cornea [2]. The procedure has been shown to be effective in slowing or halting the progression of keratoconus, and in some cases, may even improve vision. As CXL slows down keratoconus progression, the need for corneal transplantation procedures has also decreased [3].
The conventional or Dresden protocol is the original CXL protocol. This entails applying the debrided cornea with a 0.1% riboflavin in 20% dextran solution every two minutes for 30 min. Following this pretreatment stage, the cornea is then exposed to UV-A radiation (370 nm) for 30 min at 3 mW/cm2 irradiance, delivering a total energy of 5.4 J/cm2. In light of the lengthy treatment time specified by the Dresden protocol, many researchers have tried to attain equivalent results with shorter treatment durations by modifying either irradiation fluence or altering riboflavin preparations and application. This method is now known as the"Accelerated-CXL"or A-CXL which is based on the Bunsen-Roscoe law of reciprocity which states that increasing the intensity of UV-A irradiation coupled with reducing the exposure time will theoretically deliver a total energy dose equivalent to that given in conventional CXL [1, 6]. One of the earliest clinical studies on A-CXL was done by Kanellopoulos who utilized a UV-A intensity of 7 mW/cm2, with a total exposure time of 15 min, and with a total UV-A dose delivered at 5.4 J/cm2. In more recent studies, high-energy UV-A applications up to 30 mW/cm2 were used, but the overall energy limit was kept constant at 5.4 J/cm2. [5] Different surgeons use their own versions of A-CXL by making their own personal adjustments in time, energy delivery and application of riboflavin solution. We developed our own modified A-CXL technique that uses 0.1% isotonic riboflavin solution instilled every 1 min to soak for a total of 20 min and is then followed by delivering UV-A irradiation which continually emits 30 mW/cm2 of radiation for 4 min for a total energy of 7.2 J/cm2.
The primary purpose of CXL is to strengthen and stabilize the cornea. An efficient way of measuring these effects is through keratometry which analyzes corneal curvature. The highest and lowest corneal powers are taken as keratometry (K) values which are measured in Diopters (D). One parameter of importance is Kmax, the power of the steepest curve which is important in both the assessment and monitoring of KC progression [30]. In addition to keratometry, technology innovations in corneal biomechanics have allowed us to detect and monitor KC and evaluate the results of treatment strategies post CXL. Corneal stiffness is the main pathology in KC making it important to understand the biomechanical properties and the changes occurring in the cornea. Currently, there are two devices that measure corneal biomechanics—the Ocular Response Analyzer (ORA, Reichert, USA) and the Corneal Visualization Scheimpflug Technology (Corvis ST, Oculus, Germany). The ORA is a device that utilizes a jet of air which causes corneal indentation. This air pressure is then decreased returning the cornea to it’s original configuration. The device’s specialized detector system provides information on corneal biomechanics, including corneal hysteresis, corneal resistance factor, deformation amplitude, and speed [2, 5, 9]. The Corvis ST (CST), on the other hand, is a non-contact pneumotonometer that uses an ultra-high-speed Scheimpflug camera to record over 4300 frames per second in order to assess the corneal deformation reaction to a standardized air puff. The CST can be integrated with a Pentacam and this combined system measures the following parameters: Belin/Ambrósio Enhanced Ectasia Total Deviation Display (BAD D), which allows prognosis or assessment of the ectatic disease according to aspects of both the front and back surfaces of the cornea, the Stiffness Parameter at first applanation (SPA1) which is the phase of inward movement of the cornea by the air impulse, and reflects corneal stiffness, the Integrated Radius (IR) which maps the inverse radius in the concave stage of the corneal deformation after air pulse delivery, Ambrósio relational thickness to the horizontal profile (ARTh) reflecting the corneal thickness profile in the temporal-nasal extent thereby describing the relative increase in thickness from the corneal center to the periphery. Lastly, is the Deformation Amplitude Ratio (DAR) which is the ratio of central to peripheral deformation [1, 12].
Analysis of corneal tomography and biomechanical changes after CXL can serve as an effective evaluator of KC therapy. Primarily, this study aims to determine the effects of our modified method of A-CXL on vision, refraction, tomographic and biomechanical parameters by comparing the pre-operative and post-operative measurements. The authors aim to investigate the effects of A-CXL on corneal stiffness and correlate these changes with corneal tomographic and biomechanical parameters following A-CXL as it can provide an insight on how this modified treatment impacts the mechanical strength of the cornea, such as elasticity and stiffness. This study also focuses to report the outcomes of the modified A-CXL technique performed at our refractive center and compare it with the results of other methods presented in the literature. Data obtained can be utilized further to improve outcomes and optimize the treatment procedure.
Methodology
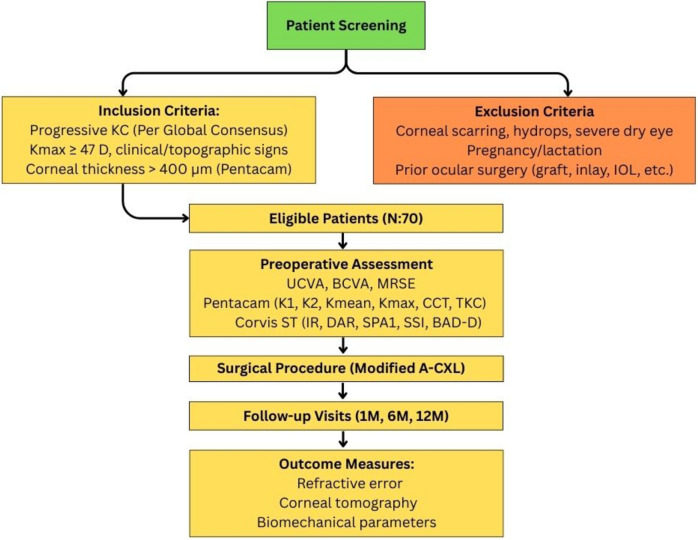
This is a retrospective, non-randomized, single center, observational study of all consecutive cases of progressive KC who underwent A-CXL in a private eye hospital. The study was carried out in accordance with the principles stated in the Helsinki Declaration and approved by the Cardinal Santos Medical Center Research Ethics Review Committee. All patients with progressive KC in one or both eyes, who underwent A-CXL from January 2017 up to December 2022, and with at least a 6-month follow up were included and analyzed in this study (Fig. 1). Due to the retrospective nature of this study, the Ethics Committee agreed to waive the requirement for informed consent.
Fig. 1.
Study Design Flowchart for Modified Accelerated Corneal Collagen Cross-Linking in Keratoconus Patients. This flowchart outlines the patient selection process, inclusion and exclusion criteria, preoperative assessment parameters, surgical procedure, follow-up schedule, and evaluated outcome measures in a retrospective study involving 70 patients undergoing modified A-CXL for progressive keratoconus. Outcome measures include changes in refractive error, corneal tomographic indices, and corneal biomechanical parameters over a 12-month period
In accordance with the Global Consensus on Keratoconus and Ectatic Diseases,[37] KC was diagnosed based on corneal tomographic data and examinations showing abnormal posterior elevation, corneal thickness distribution, clinical non-inflammatory corneal thinning and presence of clinical signs such as Fleischer ring, Vogt striae, Munson sign, Rizzuti sign and Charleoux oil droplet sign. Additionally, KC progression was defined by a consistent change in at least 2 of the following parameters which includes progressive tomographic steepening of the anterior corneal surface, progressive tomographic steepening of the posterior corneal surface, and progressive thinning and/or an increase in the rate of corneal thickness change from the periphery to the thinnest point. The documented changes need to be consistent over time and above the normal or variability such as noise of the measurement system employed. In this study, inclusion criteria are the following: participants must present with both topographic and clinical evidence consistent with a diagnosis of keratoconus. This includes an axial topography pattern indicative of keratoconus, a maximum corneal curvature (Kmax) value of 47.00 diopters or greater, and the presence of central or inferior steepening as demonstrated on the topography map. Finally, corneal pachymetry must show a minimum corneal thickness greater than 400 microns at the thinnest point, as measured by Pentacam. Patients were excluded in the analysis if they had apical corneal scarring, hydrops, severe dry eye, presence of any other corneal or anterior-segment pathology, and history of pregnancy or lactation throughout the study. Furthermore, patients who had undergone ocular surgery that could impact visual, keratometric or pachymetric outcomes such as corneal graft, corneal inlay, intrastromal rings, and refractive surgery including phakic intraocular lens (IOL) were also excluded.
Preoperatively, all wearers of hard and soft contact lenses were advised to discontinue contact lens use 2 weeks, and 1 week respectively before any assessment and surgery. Every patient underwent complete ophthalmic examination including uncorrected visual acuity (UCVA) and best corrected visual acuity (BCVA), sphere, cylinder, manifest refraction spherical equivalent (MRSE), corneal tomography, corneal biomechanical imaging and slit lamp microscopy examination. All visual acuity measurements were converted to the corresponding Logarithm of the Minimum Angle of Resolution (logMAR) values for corresponding statistical analysis. For all patients, demographic data including age, gender, and KC severity classification were recorded. Topographical and tomographical measurements were obtained using Pentacam Scheimpflug Imaging System (Oculus Optikgeräte GmbH, Germany), while the corneal biomechanical parameters were obtained using Corvis ST (CST: Corneal Visualization Scheimpflug Technology, Oculus; Wetzlar, Germany). All patients underwent follow-up examination with repeated clinical and diagnostics assessment which were documented and analyzed during each visit, at one month, six months, and one year following surgery.
Pentacam
Pentacam scans with Quality Specifications (QS) indicated as “OK” were collected from the Topometric/KC-staging printout. The following indices were gathered: flat keratometry (K1), steep keratometry (K2), mean keratometry (Kmean) corresponding to the average of K1 and K2, maximal keratometry (Kmax) corresponding to the steepest point of the anterior corneal surface, central corneal thickness (CCT) pupil center, CCT vertex normal corresponding to the corneal thickness at the apex, CCT thinnest location over anterior corneal surface, and the topographical keratoconus classification (TKC) which corresponds to the categorical severity and consists of TKC-1 (suspect), TKC-2 (mild), TKC-3 (moderate), and TKC-4 (severe).
Corvis ST
The following CST parameters with quality score “OK” were included: (1) The Integrated Radius (IR) which maps the inverse radius in the concave stage of the corneal deformation after the air pulse. A lower value is associated with a more rigid and resilient cornea. (2) The Deformation Amplitude Ratio Max 2 mm (DAR) which indicates the ratio of deformation amplitude at a point 2 mm to the left and right of the cornea's apex. It has been found that the higher the corneal stiffness, the smaller the DAR. (3) Stiffness Parameter A1 (SPA1) is formed during the 1 st applanation and reflects corneal stiffness wherein higher values reflect a stiffer cornea. (4) Stress–Strain Index (SSI) which is a metric developed for evaluating corneal rigidity. The greater the SSI, the greater the corneal stiffness. (5) Belin Ambrosio enhanced ectasia display total deviation value (BAD D) for prognosticating and evaluating corneal ectasia [1, 12]
Corneal cross linking machine and surgical technique
The KXL II system (Glaukos Inc., Burlington, Massachussetts, USA) was used to performed the A-CXL on all patients by an experienced surgeon. Under sterile operating room conditions, a topical anesthetic agent, proparacaine 0.5% (Alcaine; Alcon Laboratories, Inc.), was administered into the eyes prior to the procedure. The epithelial layer was removed with a diluted solution of 20% alcohol. The alcohol solution was dropped into an 8.00-mm optical marker placed on the cornea. After 20 to 30 s, the optical marker was removed, and the 8.0-mm-diameter corneal epithelium was gently peeled with the help of a spatula. A dextran-free isotonic 0.1% riboflavin solution (0.1% solution, VibeX, Avedro Inc., Waltham, Massachusetts, USA) was applied to the cornea every 1 min for 20 min. The machine initially recommended a 10 min application of riboflavin, but the surgeon extended it to 20 min to ensure increased coverage across a larger area. Adequate riboflavin intake was confirmed by a positive Tyndall phenomenon, which shows the presence of a yellow-green flare of the anterior chamber using a handheld slit lamp. If uptake was not adequate, additional riboflavin was administered until the flare was observed. After final confirmation of adequate riboflavin intake, the cornea was aligned under the UV illumination system. Irradiation was conducted at 30 mW/cm2, and the cornea was exposed continuously for 4 min, resulting in a total energy exposure of 7.2 J/cm2 [33, 34]. Throughout the 4-min irradiation, the surgeon made sure that the patient maintained gaze and that the treatment eye was centered. After the procedure, all patients were fitted with bandage contact lenses, and postoperative medications were prescribed which included Vigamox (0.5% Moxifloxacin HCl ophthalmic solution, Alcon Inc., Fort Worth, USA), Pred forte (Predinosolone Acetate ophthalmic solution, Allergan, USA) [31], and Hyabak (Sodium Hyaluronate 0.15%, Sager Pharma, Budapest, Hungary) eye drops four times a day or more for dry eye episodes. Patients were monitored until the corneal epithelium recovered, and the contact lenses were removed 1 week following the procedure.
Statistical analysis
Descriptive statistics were utilized to summarize the general and clinical characteristics of the participants. For categorical variables (nominal/ordinal), frequencies and proportions were calculated. For continuous variables, mean and standard deviation were reported for normally distributed data, while the median and interquartile range (IQR) were used for non-normally distributed data. To evaluate changes over time from baseline to 12 months, the Friedman test was employed to analyze repeated measures within groups. For paired non-parametric data, significant mean changes from baseline to specific follow-up points (baseline vs 1 month, baseline vs 6 months, baseline vs 12 months, 1 month vs 6 months, and so on) were determined using the Wilcoxon signed-rank test. Spearman’s rank correlation coefficient was employed to determine the strength and direction of associations between parameters acknowledging the non-linear and non-parametric nature of the data. All valid data were included in the analysis and a p-value less than 0.05 was considered statistically significant. Missing variables were neither replaced nor estimated and R-4.1.3 was used for data analysis.
Results
A total of 70 eyes from 55 patients were included in the study. Fifteen patients had binocular treatment each with a 6 month treatment interval between each eye while 40 patients had monocular treatment. Subjects’ baseline clinical and demographic profiles are shown in Table 1. The 55 participants had a mean age of 28.98 ± 8.18 years, and most (52.73%) were between the ages of 25 and 34. Subjects aged 19–24 years (21.82%) made up a lower proportion of the sample than those aged 35–44 years (14.55%), with a lesser percentage of subjects ≤ 18 years (7.27%) and 45–55 years (3.64%) in between. Males made up 72.73% of the population while remaining 27.27% were female. The most prevalent TKC stage among the patients who underwent A-CXL was TKC-4 (44.62%), followed by TKC-3 (38.56%), TKC-2 (15.38%), and TKC-1 (1.54%).
Table 1.
Baseline Demographic and Clinical Profile of Subjects
| Mean ± SD; Frequency (%) | |
|---|---|
| Age, years (n = 55) | 28.98 ± 8.18 |
| ≤ 18 | 4 (7.27) |
| 19–24 | 12 (21.82) |
| 25–34 | 29 (52.73) |
| 35–44 | 8 (14.55) |
| 45–55 | 2 (3.64) |
| Sex (n = 55) | |
| Male | 40 (72.73) |
| Female | 15 (27.27) |
| Topographical keratoconus classification (TKC) | |
| Operated Eyes (n = 65) | |
| No Keratoconus (NKC) | 0 |
| Keratoconus Stage 1 (KC-1) | 1 (1.54) |
| Keratoconus Stage 2 (KC-2) | 10 (15.38) |
| Keratoconus Stage 3 (KC-3) | 25 (38.56) |
| Keratoconus Stage 4 (KC-4) | 29 (44.62) |
Visual acuity and refraction
The average visual acuity and refraction of the eyes that underwent A-CXL are displayed in Table 2 with mean change data highlighted in Tables 3 and 4. The logMAR UCVA showed minimal variation over the course of a period of 12 months, ranging from 0.80 ± 0.39 at baseline to 0.83 ± 0.36 at the 12-month mark (p = 0.182). At 12 months, the MRSE demonstrated a myopic shift that was gradually greater than baseline (−6.79 ± 6.12 to −8.45 ± 5.13; p = 0.261). Mean change data shown in Table 3 showed a significant improvement of logMAR BCVA by −0.136 (p = 0.006) at 6 months, suggesting an improvement in visual acuity. This improvement persisted at 12-months, with mean change −0.132 (p = 0.018). The MRSE dropped by −1.718 (p = 0.026) after 6 months, indicating a considerable improvement with a trend toward myopic changes following A-CXL.
Table 2.
Visual Acuity and Refraction, Keratometry and Pachymetry, and Corneal Biomechanical Parameters Before and After Accelerated-Collagen Crosslinking (N:70)
| Baseline | 1 Month | 6 Months | 12 Months | P* | |
|---|---|---|---|---|---|
| Mean ± SD | |||||
| Visual Acuity and Refraction | |||||
| UCVA, logMAR | 0.80 ± 0.39 | 0.82 ± 0.36 | 0.77 ± 0.30 | 0.83 ± 0.36 | 0.182 |
| BCVA, logMAR | 0.51 ± 0.32 | 0.44 ± 0.20 | 0.39 ± 0.19 | 0.41 ± 0.19 | 0.396 |
| Sphere | −5.03 ± 5.45 | −4.47 ± 4.97 | −4.74 ± 5.07 | −5.83 ± 4.83 | 0.219 |
| Cylinder | −4.26 ± 2.80 | −5.50 ± 3.24 | −5.01 ± 3.15 | −5.28 ± 2.70 | 0.647 |
| MRSE | −6.79 ± 6.12 | −6.95 ± 5.42 | −7.55 ± 5.87 | −8.45 ± 5.13 | 0.261 |
| Keratometry and Pachymetry | |||||
| K1 | 51.63 ± 8.62 | 52.82 ± 8.28 | 52.05 ± 9.26 | 51.23 ± 5.79 | 0.054 |
| K2 | 56.40 ± 8.19 | 58.20 ± 8.70 | 57.44 ± 8.35 | 56.22 ± 5.87 | 0.054 |
| Kmean | 53.84 ± 8.41 | 55.33 ± 8.40 | 54.46 ± 8.95 | 53.59 ± 5.75 | 0.012 |
| Kmax | 65.51 ± 10.27 | 67.73 ± 11.38 | 64.92 ± 10.29 | 65.19 ± 7.43 | 0.186 |
| CCT Pupil Center | 467.15 ± 50.75 | 452.92 ± 49.77 | 461.45 ± 55.30 | 459.48 ± 53.99 | 0.015 |
| CCT Vertex Normal | 445.71 ± 57.41 | 425.25 ± 59.22 | 441.81 ± 59.57 | 435.76 ± 59.89 | 0.002 |
| CCT Thinnest Location | 431.35 ± 58.93 | 413.15 ± 61.06 | 415.84 ± 75.60 | 422.52 ± 62.46 | 0.006 |
| Corneal Biomechanics | |||||
| DAR | 6.64 ± 2.30 | 6.61 ± 2.15 | 6.24 ± 1.70 | 6.45 ± 1.19 | 0.098 |
| IR | 13.78 ± 3.93 | 13.70 ± 3.70 | 12.99 ± 4.56 | 13.60 ± 2.82 | 0.145 |
| ARTh | 206.52 ± 111.85 | 193.78 ± 118.11 | 165.83 ± 100.04 | 186.40 ± 106.73 | 0.457 |
| SPA 1 | 53.24 ± 22.03 | 47.05 ± 19.85 | 48.55 ± 19.94 | 51.12 ± 20.68 | 0.373 |
| SSI | 0.74 ± 0.26 | 0.73 ± 0.25 | 0.70 ± 0.25 | 0.66 ± 0.14 | 0.723 |
| BAD D | 13.87 ± 7.28 | 15.27 ± 6.73 | 15.01 ± 6.37 | 14.41 ± 6.05 | 0.659 |
*Statistical analysis used: §–Friedman Test.; UCVA-Uncorrected Visual Acuity; BCVA-Best Corrected Visual Acuity; MRSE-Manifest Refraction Spherical Equivalent;; K1-Flat Keratometry; K2-Steep Keratometry; Kmean-Mean Keratometry; Kmax-Maximal Keratometry; CCT-Central Corneal Thickness; DAR- Deformation Amplitude Ratio; IR- Integrated Radius; ARTh-Ambrósio relational thickness to the horizontal profile; SPA 1 Stiffness Parameter at first applanation; SSI- Stress Strain Index; BAD D-Belin/Ambrósio Enhanced Ectasia Total Deviation Display
Table 3.
Mean Change from Baseline of Visual Acuity, Refraction, Keratometry and Pachymetry, and Corneal Biomechanical Parameters (N:70)
| 1 Month from Baseline | 6 Months from Baseline | 12 Months from Baseline | |||||
|---|---|---|---|---|---|---|---|
| Mean Change | p-value | Mean Change | p-value | Mean Change | p-value | ||
| Vision and Refraction | |||||||
| UCVA, logMAR | −0.005 | 0.987 | −0.102 | 0.124 | −0.038 | 0.425 | |
| BCVA, logMAR | −0.054 | 0.312 | −0.136 | 0.006 | −0.132 | 0.018 | |
| Sphere | 0.625 | 0.494 | −0.729 | 0.141 | −0.892 | 0.221 | |
| Cylinder | −1.202 | 0.009 | −0.757 | 0.214 | −0.950 | 0.260 | |
| MRSE | 0.071 | 0.612 | −1.718 | 0.026 | −1.497 | 0.114 | |
| Keratometry and Pachymetry | |||||||
| K1 | 1.017 | 0.017 | −0.563 | 0.138 | −0.464 | 0.113 | |
| K2 | 1.362 | 0.001 | −0.270 | 0.600 | 0.214 | 0.624 | |
| Kmean | 1.211 | 0.005 | −0.440 | 0.122 | −0.150 | 0.576 | |
| Kmax | 1.56 | 0.043 | −1.493 | 0.090 | −0.614 | 0.142 | |
| CCT Pupil Center | −14.787 | < 0.001 | 0.267 | 0.926 | −9.500 | 0.020 | |
| CCT Vertex Normal | −18.426 | < 0.001 | 1.700 | 0.952 | −9.500 | 0.035 | |
| Thinnest Location | −17.609 | < 0.001 | −6.333 | 0.328 | −9.750 | 0.011 | |
| Corneal Biomechanics | |||||||
| DAR | −0.534 | 0.153 | −1.447 | 0.001 | −0.314 | 0.151 | |
| IR | −0.479 | 0.119 | −1.753 | 0.011 | −0.464 | 0.140 | |
| ARTh | −5.241 | 0.243 | −5.467 | 0.916 | 11.286 | 0.975 | |
| SPA 1 | −4.586 | 0.227 | 6.643 | 0.109 | 2.357 | 0.593 | |
| SSI | 0.024 | 0.349 | 0.013 | 0.443 | −0.043 | 0.917 | |
| BAD D | 0.874 | 0.042 | −0.499 | 0.609 | 0.434 | 0.272 | |
Statistical Analysis Used: Wilcoxon signed-rank test for each pair: [1] Baseline vs 1 Month, [2] Baseline vs 6 Months, [3] Baseline vs 12 Months; UCVA-Uncorrected Visual Acuity; BCVA-Best Corrected Visual Acuity; MRSE-Manifest Refraction Spherical Equivalent; K1-Flat Keratometry; K2-Steep Keratometry; Kmean-Mean Keratometry; Kmax-Maximal Keratometry; CCT-Central Corneal Thickness; DAR- Deformation Amplitude Ratio; IR- Integrated Radius; ARTh-Ambrósio relational thickness to the horizontal profile; SPA 1 Stiffness Parameter at first applanation; SSI- Stress Strain Index; BAD D- Belin/Ambrósio Enhanced Ectasia Total Deviation Display
Table 4.
Mean Change During Postoperative Periods of Visual Acuity and Refraction, Keratometry and Pachymetry, and Corneal Biomechanical Parameters (N:70)
| 6 Months from 1 Month | 12 Months from1 Month | |||
|---|---|---|---|---|
| Mean Change | p-value | Mean Change | p-value | |
| Vision and Refraction | ||||
| UCVA, logMAR | −0.041 | 0.383 | 0.017 | 0.875 |
| BCVA, logMAR | −0.053 | 0.246 | −0.034 | 0.348 |
| Sphere | −0.352 | 0.422 | −0.522 | 0.475 |
| Cylinder | 0.768 | 0.062 | −0.674 | 0.444 |
| MRSE | −0.977 | 0.676 | −0.867 | 0.262 |
| Keratometry and Pachymetry | ||||
| K1 | −1.962 | 0.006 | −1.136 | 0.010 |
| K2 | −1.625 | 0.002 | −1.491 | 0.002 |
| Kmean | −1.934 | 0.001 | −1.268 | 0.002 |
| Kmax | −3.946 | 0.003 | −2.105 | 0.027 |
| CCT Pupil Center | 17.208 | < 0.001 | 4.000 | 0.031 |
| CCT Vertex Normal | 26.458 | < 0.001 | 9.364 | 0.010 |
| CCT Thinnest Location | 17.957 | 0.004 | 8.952 | 0.020 |
| Corneal Biomechanics | ||||
| DAR | −0.300 | 0.529 | 0.129 | 0.876 |
| IR | −1.029 | 0.506 | 0.529 | 0.097 |
| ARTh | 11.143 | 0.683 | −1.235 | 0.887 |
| SPA 1 | 3.538 | 0.421 | 4.000 | 0.246 |
| SSI | −0.057 | 0.376 | −0.100 | 0.018 |
| BAD D | −0.924 | 0.048 | −0.734 | 0.113 |
Statistical Analysis Used: Wilcoxon signed-rank test for each pair: [1] 1 Month vs 6 Months, [2] 1 Month vs 12 Months; UCVA-Uncorrected Visual Acuity; BCVA-Best Corrected Visual Acuity; MRSE-Manifest Refraction Spherical Equivalent; K1-Flat Keratometry; K2-Steep Keratometry; Kmean-Mean Keratometry; Kmax-Maximal Keratometry; CCT-Central Corneal Thickness; DAR- Deformation Amplitude Ratio; IR- Integrated Radius; ARTh-Ambrósio relational thickness to the horizontal profile; SPA 1 Stiffness Parameter at first applanation; SSI- Stress Strain Index; BAD D- Belin/Ambrósio Enhanced Ectasia Total Deviation Display
Keratometry and pachymetry
Mean keratometry measurements are highlighted in Table 2 with corresponding mean change data shown in Tables 3 and 4. One month after A-CXL, Kmax increased from baseline at 65.51 ± 10.27 to 67.73 ± 11.38 indicating increased corneal steepening. This significant decline in keratometry measurements at 1 month is exhibited in Table 3 with the mean change of Kmax increasing by 1.56 (p = 0.043). This was then followed by a significant recovery at 6 and 12 months respectively following the initial decline at 1 month. This recovery in keratometry is depicted in Table 4 (p = 0.003). A similar trend was exhibited by K1, K2 and Kmean wherein there was an initial steepening followed by subsequent recovery detected at 6 and 12 months. Keratometry measurements throughout the 12-month follow-up period demonstrate a trend towards corneal stability rather than improvement as there were no demonstrable signs of steepening among all measured keratometry parameters 1 year following A-CXL.
Pachymetry measurements, including CCT at the apex, thinnest point, and pupil center, are also displayed in Table 2. At baseline, the mean CCT at the thinnest point was 431.35 ± 58.93. A significant preliminary thinning of the cornea was observed one month after A-CXL as highlighted in mean change data presented in Table 3 (p < 0.001). This was subsequently followed by recovery of corneal thickness approaching baseline values, with CCT Thinnest Location values of 415.84 ± 75.60 and 422.52 ± 62.46, at six and twelve months after A-CXL respectively (p = 0.006). Mean change of this recovery is also reflected in Table 4 which demonstrated a statistically significant increase in CCT parameters including CCT Thinnest Location (p = 0.004).
Biomechanical parameters
The CST values for patients are displayed in Table 2 with mean change data shown in Tables 3 and 4 respectively. With a little decline from 6.64 ± 2.30 at baseline to 6.45 ± 1.19 at 12 months, DAR was comparatively steady (p = 0.098). Similarly with IR, baseline readings at 13.78 ± 3.93, decreased slightly to 12.99 ± 4.56 at 6 months, and then increased to 13.60 ± 2.82 at 12 months (p = 0.145). No significant changes were seen in SPA1, which exhibited a modest decline at baseline (53.24 ± 22.03) to 48.55 ± 19.94 at 6 months, and a minor rebound at 12 months (p = 0.373) to 51.12 ± 20.68. Mean change data presented in Table 3 showed that 6 months following A-CXL, DAR and IR significantly decreased by −1.447 (p = 0.001) and −0.479 (p = 0.119) respectively. ARTh had non-significant changes across all time points, with a mean change of −5.241 (p = 0.243) at 1 month and 11.286 (p = 0.975) at 12 months which may suggest a trend in increasing corneal thickness. SPA1 demonstrated an initial reduction from baseline at 1 month −4.586 (p = 0.227) with an increasing change in trend at 12 months following A-CXL with an increase by 2.357 (p = 0.593). SSI showed a slight increase of 0.024 (p = 0.349) at 1 month and a decrease of −0.043 (p = 0.917) at 12 months, both non-significant. BAD D increased significantly by 0.874 (p = 0.042) at 1 month but did not show significant changes at later time points. By 12 months, no statistically significant changes to the biomechanical parameters were noted which may suggest stability at 1 year following A-CXL.
Correlation of corneal biomechanics and visual acuity
The correlation between CST characteristics and visual acuity and refraction of patients 6 and 12 months after A-CXL are displayed in Table 5. At 6 months negative correlations were seen between ARTh and logMAR BCVA (r = −0.526, p = 0.014). Additionally, there was a significant positive correlation between the BAD D and BCVA (r = 0.635, p = 0.002). At 12 months, MRSE had strong negative correlations with DAR and IR respectively (p = 0.043, p = 0.019) while having strong positive correlations with SPA1 and SSI respectively (p = 0.013, p = 0.011).
Table 5.
Correlation between Visual Acuity and Corvis ST Parameters of Patients at 6 months and 12 months post A-CXL (N:70)
| Logmar UCVA | Logmar BCVA | Axis | Sphere | Cylinder | MRSE | |
|---|---|---|---|---|---|---|
| Correlation Coefficient (r), p-value | ||||||
| 6 Months | ||||||
| DAR 2 mm | 0.383, 0.129 | 0.344, 0.127 | 0.253, 0.245 | −0.304, 0.158 | −0.375, 0.078 | −0.435, 0.043 |
| IR | 0.547, 0.023 | 0.263, 0.249 | 0.343, 0.110 | −0.441, 0.035 | −0.245, 0.259 | −0.495, 0.019 |
| ARTh | −0.191, 0.462 | −0.526, 0.014 | −0.233, 0.286 | 0.036, 0.870 | 0.267, 0.217 | 0.214, 0.338 |
| SPA 1 | −0.077, 0.776 | −0.251, 0.286 | −0.149, 0.509 | 0.304, 0.170 | 0.277, 0.212 | 0.437, 0.048 |
| SSI | −0.169, 0.516 | −0.196, 0.394 | −0.279, 0.197 | 0.309, 0.151 | −0.075, 0.735 | 0.233, 0.296 |
| BAD D | 0.105, 0.689 | 0.635, 0.002 | 0.291, 0.178 | 0.043, 0.847 | −0.353, 0.098 | −0.166, 0.460 |
| 12 Months | ||||||
| DAR 2 mm | 0.314, 0.190 | 0.372, 0.106 | 0.115, 0.609 | −0.391, 0.072 | −0.255, 0.252 | −0.471, 0.031 |
| IR | 0.086, 0.727 | 0.174, 0.464 | 0.253, 0.257 | −0.471, 0.027 | −0.201, 0.369 | −0.550, 0.010 |
| ARTh | −0.096, 0.697 | −0.062, 0.796 | −0.152, 0.501 | 0.367, 0.093 | 0.051, 0.821 | 0.404, 0.069 |
| SPA 1 | −0.041, 0.868 | −0.084, 0.726 | 0.168, 0.455 | 0.517, 0.014 | 0.035, 0.877 | 0.533, 0.013 |
| SSI | −0.130, 0.595 | −0.279, 0.233 | −0.355, 0.105 | 0.453, 0.034 | 0.187, 0.406 | 0.543, 0.011 |
| BAD D | 0.109, 0.658 | 0.399, 0.082 | −0.003, 0.990 | −0.400, 0.065 | −0.251, 0.260 | −0.430, 0.051 |
Statistical Analysis Used: Spearman correlation; Correlation coefficient interpretation; 0–0.2, very weak; 0.2–0.4, weak; 0.4–0.6 moderate; 0.6–0.8, strong; 0.8–1.0, very strong; Shaded cells have p < .05. More darkly shaded cells have negative correlation coefficients; DAR- Deformation Amplitude Ratio; IR- Integrated Radius; ARTh-Ambrósio relational thickness to the horizontal profile; SPA 1 Stiffness Parameter at first applanation; SSI- Stress Strain Index; BAD D-Belin/Ambrósio Enhanced Ectasia Total Deviation Display
Correlation of visual acuity and keratometry
Table 6 displays the at 6 and 12 month correlation between the keratometry and visual acuity parameters of the patients after A-CXL. The LogMAR BCVA demonstrated a strong positive correlations with all keratometry measurements 6 and 12 months after A-CXL. It is noteworthy that there was a positive correlation between MRSE and CTT at the Pupil Center and CCT Vertex Normal (p = 0.007, p = 0.007).
Table 6.
Correlation Between Visual Acuity and Keratometry Parameters of Patients at 6 months and 12 months post A-CXL
| Logmar UCVA | Logmar BCVA | Axis | Sphere | Cylinder | MRSE | |
|---|---|---|---|---|---|---|
| Correlation Coefficient (r), p-value | ||||||
| 6 Months | ||||||
| K1 | 0.134, 0.574 | 0.543, 0.002 | 0.348, 0.055 | −0.263, 0.160 | −0.062, 0.740 | −0.340, 0.066 |
| K2 | 0.190, 0.421 | 0.502, 0.006 | 0.418, 0.019 | −0.283, 0.130 | −0.036, 0.847 | −0.334, 0.071 |
| Kmean | 0.084, 0.726 | 0.477, 0.009 | 0.378, 0.036 | −0.278, 0.137 | −0.041, 0.828 | −0.333, 0.072 |
| Kmax | 0.067, 0.778 | 0.515, 0.004 | 0.435, 0.014 | −0.169, 0.372 | −0.100, 0.592 | −0.245, 0.191 |
| CCT Pupil Center | −0.320, 0.169 | −0.079, 0.683 | −0.070, 0.707 | 0.414, 0.023 | 0.125, 0.503 | 0.483, 0.007 |
| CCT Vertex Normal | −0.226, 0.338 | −0.198, 0.302 | −0.156, 0.403 | 0.386, 0.035 | 0.175, 0.347 | 0.484, 0.007 |
| Thinnest Location | −0.148, 0.535 | −0.192, 0.319 | −0.077, 0.682 | 0.261, 0.164 | 0.231, 0.211 | 0.412, 0.024 |
| 12 Months | ||||||
| K1 | 0.334, 0.129 | 0.525, 0.009 | −0.005, 0.979 | −0.504, 0.009 | −0.180, 0.378 | −0.554, 0.004 |
| K2 | 0.297, 0.180 | 0.495, 0.014 | −0.001, 0.996 | −0.384, 0.053 | −0.312, 0.121 | −0.513, 0.009 |
| Kmean | 0.301, 0.174 | 0.521, 0.009 | 0.006, 0.976 | −0.457, 0.019 | −0.239, 0.240 | −0.545, 0.005 |
| Kmax | 0.259, 0.244 | 0.553, 0.005 | 0.048, 0.815 | −0.276, 0.173 | −0.347, 0.082 | −0.382, 0.060 |
| CCT Pupil Center | −0.170, 0.449 | −0.168, 0.434 | −0.186, 0.363 | 0.513, 0.007 | −0.026, 0.901 | 0.525, 0.007 |
| CCT Vertex Normal | −0.171, 0.446 | −0.285, 0.177 | −0.074, 0.721 | 0.570, 0.002 | −0.007, 0.971 | 0.551, 0.004 |
| Thinnest Location | −0.129, 0.568 | −0.255, 0.230 | −0.036, 0.861 | 0.548, 0.004 | −0.088, 0.670 | 0.519, 0.008 |
Statistical Analysis Used: Spearman correlation; Correlation coefficient interpretation; 0–0.2, very weak; 0.2–0.4, weak; 0.4–0.6 moderate; 0.6–0.8, strong; 0.8–1.0, very strong; Shaded cells have p < 0.05. More darkly shaded cells have negative correlation coefficients; UCVA-Uncorrected Visual Acuity; BCVA-Best Corrected Visual Acuity K1-Flat Keratometry; K2-Steep Keratometry; Kmean-Mean Keratometry; Kmax-Maximal Keratometry; CCT-Central Corneal Thickness; DAR- Deformation Amplitude Ratio; IR- Integrated Radius; ARTh-Ambrósio relational thickness to the horizontal profile; SPA 1 Stiffness Parameter at first applanation; SSI- Stress Strain Index; BAD D-Belin/Ambrósio Enhanced Ectasia Total Deviation Display
Correlation of keratometry and corneal biomechanics
The correlation between keratometry and corneal biomechanical parameters in patients who had surgery 6 and 12 months after A-CXL is displayed in Table 7. There were strong relationships between multiple biomechanical and keratometry parameters both 6 and 12 months after CXL surgery. Kmax had strong positive correlations with DAR and IR both at 6 and 12 months (p = 0.001, p = 0.003) as opposed to it having negative correlations with SPA1 and SSI (p = 0.007, p = 0.005). Furthermore, a strong positive associations were detected between all CCT parameters and ARTh (p = 0.001) further supporting that these corneal thickness parameters are reflective of one another.
Table 7.
Correlation Between Keratometry and Biomechanical Parameters of Patients at 6 months and 12 months post A-CXL (N:70)
| DAR | IR | ARTh | SPA 1 | SSI | BAD D | |
|---|---|---|---|---|---|---|
| Correlation Coefficient (r), p-value | ||||||
| 6 Months | ||||||
| K1 | 0.656, 0.001 | 0.683, < 0.001 | −0.459, 0.028 | −0.608, 0.003 | −0.539, 0.008 | 0.575, 0.004 |
| K2 | 0.627, 0.001 | 0.776, < 0.001 | −0.471, 0.023 | −0.624, 0.002 | −0.648, 0.001 | 0.537, 0.008 |
| Kmean | 0.659, 0.001 | 0.727, < 0.001 | −0.473, 0.023 | −0.654, 0.001 | −0.593, 0.003 | 0.560, 0.005 |
| Kmax | 0.519, 0.011 | 0.597, 0.003 | −0.407, 0.054 | −0.515, 0.014 | −0.507, 0.014 | 0.568, 0.005 |
| CCT Pupil Center | −0.409, 0.053 | −0.414, 0.050 | 0.652, 0.001 | 0.458, 0.032 | 0.100, 0.650 | −0.289, 0.181 |
| CCT Vertex Normal | −0.779, < 0.001 | −0.667, 0.001 | 0.648, 0.001 | 0.697, < 0.001 | 0.302, 0.162 | −0.595, 0.003 |
| CCT Thinnest Location | −0.681, < 0.001 | −0.335, 0.118 | 0.832, < 0.001 | 0.721, < 0.001 | 0.090, 0.682 | −0.741, < 0.001 |
| 12 Months | ||||||
| K1 | 0.791, < 0.001 | 0.732, < 0.001 | −0.707, < 0.001 | −0.744, < 0.001 | −0.513, 0.009 | 0.886, < 0.001 |
| K2 | 0.599, 0.002 | 0.600, 0.002 | −0.587, 0.002 | −0.594, 0.002 | −0.438, 0.029 | 0.754, < 0.001 |
| Kmean | 0.721, < 0.001 | 0.692, < 0.001 | −0.647, < 0.001 | −0.697, < 0.001 | −0.506, 0.010 | 0.848, < 0.001 |
| Kmax | 0.630, 0.001 | 0.571, 0.003 | −0.527, 0.007 | −0.604, 0.001 | −0.539, 0.005 | 0.837, < 0.001 |
| CCT Pupil Center | −0.512, 0.009 | −0.633, 0.001 | 0.548, 0.005 | 0.452, 0.023 | 0.464, 0.019 | −0.469, 0.018 |
| CCT Vertex Normal | −0.712, < 0.001 | −0.736, < 0.001 | 0.675, < 0.001 | 0.706, < 0.001 | 0.477, 0.016 | −0.779, < 0.001 |
| CCT Thinnest Location | −0.712, < 0.001 | −0.717, < 0.001 | 0.681, < 0.001 | 0.718, < 0.001 | 0.446, 0.025 | −0.785, < 0.001 |
Statistical Analysis Used: Spearman correlation; Correlation coefficient interpretation; 0–0.2, very weak; 0.2–0.4, weak; 0.4–0.6 moderate; 0.6–0.8, strong; 0.8–1.0, very strong; Shaded cells have p < 0.05. More darkly shaded cells have negative correlation coefficients.; UCVA-Uncorrected Visual Acuity; BCVA-Best Corrected Visual Acuity K1-Flat Keratometry; K2-Steep Keratometry; Kmean-Mean Keratometry; Kmax-Maximal Keratometry; CCT-Central Corneal Thickness; DAR- Deformation Amplitude Ratio; IR- Integrated Radius; ARTh-Ambrósio relational thickness to the horizontal profile; SPA 1 Stiffness Parameter at first applanation; SSI- Stress Strain Index; (BAD D) Belin/Ambrósio Enhanced Ectasia Total Deviation Display
Accelerated-collagen crosslinking and TKC-1
Only one eye out of the 70 operated eyes was preoperatively classified as TKC-1. Mean preoperative refraction was −5.25 ± NA with a demonstrable myopic shift was noted in MRSE at 1 month at −8.00 ± NA followed by a return to baseline measurements at 6 months following A-CXL. Mean Kmax readings at baseline were at 51.10 ± NA which was followed by an initial steepening at 1 month followed by evidence corneal flattening at 6 months closely resembling preoperative values. Similarly, there was an initial decline in corneal thickness at 1 month followed by recovery approaching preoperative pachymetry readings in 6 months. There is limited data on Corvis ST parameters as there were no preoperative parameters available.
TKC staging and keratometry
The subgroup analysis between various TKC stages and their keratometry values one month, six months, and one year after A-CXL are displayed in Table 8. Kmax demonstrated no statistically significant changes across a 12 month post-operative period among the TKC-2 ad TKC-3. However at 12 months following A-CXL, a statistically significant reduction in Kmax was detected among those classified as TKC-4 (p = 0.029). With p-values of 0.013 and 0.001, respectively, CCT values for the pupil center and vertex normal also demonstrated significant variations over time. The cornea's thinnest location also showed a noteworthy decline over time, going from 405.24 ± 54.34 at baseline to 394.18 ± 47.31 at 12 months (p = 0.006).
Table 8.
Keratometry and Pachymetry Values Before and After A-CXL of patients Preoperatively Classified as TKC 2, 3, 4
| Preoperative | 1 month | 6 months | 12 months | p | |
|---|---|---|---|---|---|
| Mean ± SD | |||||
| TKC 2 (n:10) | |||||
| K1 | 46.37 ± 3.06 | 46.55 ± 3.05 | 46.67 ± 3.00 | 46.83 ± 1.80 | 0.896 |
| K2 | 49.58 ± 4.01 | 50.53 ± 3.83 | 49.47 ± 3.84 | 51.97 ± 4.30 | 0.112 |
| Kmean | 47.92 ± 3.41 | 48.43 ± 3.37 | 48.03 ± 3.34 | 49.23 ± 2.66 | 0.852 |
| Kmax | 54.91 ± 5.34 | 58.43 ± 6.38 | 55.47 ± 5.83 | 59.00 ± 5.00 | 0.241 |
| CCT Pupil Center | 505.30 ± 20.98 | 480.17 ± 19.78 | 494.00 ± 9.49 | 491.67 ± 17.01 | 0.615 |
| CCT Vertex Normal | 501.10 ± 20.89 | 475.17 ± 19.08 | 490.33 ± 9.69 | 483.33 ± 22.05 | 0.615 |
| Thinnest Location | 490.00 ± 16.64 | 469.17 ± 17.21 | 478.17 ± 9.28 | 471.67 ± 23.18 | 0.392 |
| TKC 3 (n:25) | |||||
| K1 | 49.20 ± 6.82 | 48.91 ± 6.64 | 50.48 ± 7.36 | 48.62 ± 5.58 | 0.095 |
| K2 | 54.02 ± 6.33 | 54.24 ± 5.86 | 54.93 ± 6.96 | 53.59 ± 5.07 | 0.145 |
| Kmean | 51.47 ± 6.51 | 51.37 ± 6.17 | 52.59 ± 7.10 | 50.95 ± 5.25 | 0.079 |
| Kmax | 61.86 ± 6.06 | 62.13 ± 6.41 | 60.49 ± 9.19 | 61.37 ± 4.90 | 0.473 |
| CCT Pupil Center | 465.36 ± 51.91 | 455.82 ± 46.04 | 443.11 ± 46.00 | 458.21 ± 68.19 | 0.472 |
| CCT Vertex Normal | 451.32 ± 53.40 | 442.24 ± 49.78 | 432.78 ± 58.27 | 444.21 ± 73.17 | 0.472 |
| Thinnest Location | 428.94 ± 56.03 | 428.94 ± 56.03 | 395.00 ± 88.96 | 432.50 ± 72.17 | 0.251 |
| TKC 4 (n:29) | |||||
| K1 | 56.97 ± 6.94 | 58.55 ± 6.83 | 57.49 ± 6.13 | 55.60 ± 4.03 | 0.134 |
| K2 | 61.67 ± 7.35 | 64.12 ± 7.69 | 62.97 ± 7.05 | 60.30 ± 4.87 | 0.116 |
| Kmean | 59.22 ± 7.03 | 61.17 ± 7.13 | 60.09 ± 6.48 | 57.85 ± 4.33 | 0.156 |
| Kmax | 73.56 ± 7.77 | 75.42 ± 10.33 | 72.26 ± 6.92 | 70.99 ± 6.53 | 0.029 |
| CCT Pupil Center | 453.41 ± 52.26 | 441.41 ± 58.10 | 457.21 ± 70.23 | 451.55 ± 40.84 | 0.013 |
| CCT Vertex Normal | 417.79 ± 53.80 | 393.95 ± 60.27 | 422.29 ± 65.49 | 410.64 ± 38.58 | 0.001 |
| CCT Thinnest Location | 405.24 ± 54.34 | 385.95 ± 54.98 | 404.21 ± 75.49 | 394.18 ± 47.31 | 0.006 |
Statistical analysis used: Friedman Test; UCVA-Uncorrected Visual Acuity; BCVA-Best Corrected Visual Acuity K1-Flat Keratometry; K2-Steep Keratometry; Kmean-Mean Keratometry; Kmax-Maximal Keratometry; CCT-Central Corneal Thickness; TKC- Topographical keratoconus classification
TKC staging and corneal biomechanics
CST measurements across a 12 month follow up among the different TKC stages are presented in Table 9. There were no statistically significant changes detected across all recorded CST parameters. Stiffness parameters DAR and IR remained unchanged during the 12 month follow-up period among all TKC stages (p > 0.001). The remaining stiffness parameters SPA1 and SSI also had no demonstrable changes (p > 0.001). Similarly, the CST thickness parameter ARTh had similar results with no significant change among all TKC stages. These findings may suggest that regardless of KC severity, A-CXL promotes corneal stability.
Table 9.
Corvis ST Values Before and After A-CXL of Patients Preoperatively Classified as TKC 2, 3, 4
| Preoperative | 1 month | 6 months | 12 months | p | |
|---|---|---|---|---|---|
| Mean ± SD | |||||
| TKC 2 (n:10) | |||||
| DAR 2 mm | 6.45 ± 1.37 | 5.82 ± 1.73 | 5.26 ± 0.48 | 5.90 ± 1.18 | 0.562 |
| IR | 11.86 ± 1.71 | 12.94 ± 4.96 | 10.62 ± 1.06 | 11.73 ± 2.57 | 0.753 |
| ARTh | 292.00 ± 89.39 | 263.00 ± 80.19 | 269.40 ± 122.79 | 174.67 ± 36.09 | 0.615 |
| SPA 1 | 66.00 ± 12.63 | 62.20 ± 11.71 | 75.40 ± 11.80 | 75.33 ± 21.94 | 0.392 |
| SSI | 0.83 ± 0.15 | 0.96 ± 0.23 | 0.98 ± 0.11 | 0.83 ± 0.21 | 0.682 |
| BAD D | 7.51 ± 2.27 | 8.59 ± 2.68 | 8.04 ± 2.07 | 9.90 ± 0.84 | 0.308 |
| TKC 3 (n:25) | |||||
| DAR 2 mm | 5.26 ± 1.69 | 6.17 ± 1.24 | 5.73 ± 2.29 | 5.98 ± 1.25 | 0.241 |
| IR | 12.35 ± 2.94 | 13.19 ± 2.90 | 11.93 ± 6.16 | 13.02 ± 3.14 | 0.896 |
| ARTh | 223.38 ± 106.19 | 210.31 ± 75.03 | 177.43 ± 88.23 | 220.83 ± 137.23 | > 0.999 |
| SPA 1 | 62.23 ± 22.73 | 51.15 ± 19.07 | 48.33 ± 8.16 | 57.50 ± 17.61 | - |
| SSI | 0.86 ± 0.33 | 0.73 ± 0.15 | 0.63 ± 0.28 | 0.68 ± 0.09 | 0.644 |
| BAD D | 10.55 ± 3.49 | 12.03 ± 2.93 | 13.43 ± 5.55 | 11.83 ± 4.06 | 0.241 |
| TKC 4 (n:29) | |||||
| DAR 2 mm | 7.74 ± 2.46 | 7.49 ± 2.53 | 7.11 ± 1.47 | 7.23 ± 0.78 | 0.112 |
| IR | 15.75 ± 4.27 | 14.74 ± 3.72 | 15.10 ± 3.97 | 14.77 ± 2.15 | 0.145 |
| ARTh | 143.55 ± 52.87 | 139.41 ± 98.46 | 117.11 ± 51.65 | 152.00 ± 64.87 | 0.494 |
| SPA 1 | 40.10 ± 15.99 | 38.35 ± 17.35 | 37.22 ± 16.65 | 36.11 ± 13.31 | 0.896 |
| SSI | 0.61 ± 0.18 | 0.62 ± 0.22 | 0.60 ± 0.19 | 0.59 ± 0.12 | 0.154 |
| BAD D | 19.16 ± 6.60 | 20.25 ± 5.87 | 19.42 ± 5.41 | 18.97 ± 6.68 | 0.896 |
Statistical analysis used: Friedman Test; DAR- Deformation Amplitude Ratio; IR- Integrated Radius; ARTh-Ambrósio relational thickness to the horizontal profile; SPA 1 Stiffness Parameter at first applanation; SSI- Stress Strain Index; (BAD D) Belin/Ambrósio Enhanced Ectasia Total Deviation Display; TKC- Topographical keratoconus classification
TKC and visual acuity
Table 10 exhibits the visual and refractive effects of A-CXL on the different TCK stages following a 12 month follow-up period. Among the different TCK stages, both logMAR UCVA and BCVA had non-significant changes up to 12 months following A-CXL (p > 0.001). A similar pattern was demonstrated by non-significant refractive changes in MRSE seen among the TKC stages (p > 0.001) suggesting stability in refraction despite having minimal changes in vision following A-CXL.
Table 10.
Visual Acuity Values Before and After A-CXL of patients Preoperatively Classified as TKC 2, 3, 4
| Preoperative | 1 month | 6 months | 12 months | p | |
|---|---|---|---|---|---|
| Mean ± SD | |||||
| TKC 2 (n:10) | |||||
| UCVA, logMAR | 0.73 ± 0.34 | 0.73 ± 0.34 | 0.63 ± 0.23 | 1.15 ± 0.21 | - |
| BCVA, logMAR | 0.30 ± 0.24 | 0.39 ± 0.19 | 0.32 ± 0.25 | 0.20 ± NA | - |
| Axis | 82.00 ± 49.34 | 105.00 ± 49.16 | 49.17 ± 21.78 | 55.00 ± 7.07 | 0.562 |
| Sphere | −2.33 ± 2.10 | −2.25 ± 2.57 | −2.71 ± 2.65 | −5.00 ± 4.24 | 0.682 |
| Cylinder | −3.35 ± 1.68 | −3.79 ± 1.47 | −3.04 ± 1.46 | −3.75 ± 3.18 | 0.919 |
| MRSE | −3.48 ± 3.25 | −3.61 ± 3.23 | −3.45 ± 1.83 | −5.00 ± NA | - |
| TKC 3 (n:25) | |||||
| UCVA, logMAR | 0.83 ± 0.42 | 0.91 ± 0.39 | 0.89 ± 0.36 | 0.72 ± 0.31 | 0.229 |
| BCVA, logMAR | 0.49 ± 0.30 | 0.37 ± 0.08 | 0.35 ± 0.18 | 0.32 ± 0.13 | 0.326 |
| Axis | 111.20 ± 56.15 | 106.11 ± 57.41 | 115.00 ± 58.45 | 125.38 ± 58.65 | 0.838 |
| Sphere | −6.19 ± 5.42 | −6.08 ± 5.68 | −6.79 ± 5.23 | −6.50 ± 5.09 | 0.682 |
| Cylinder | −4.75 ± 3.33 | −5.83 ± 3.32 | −5.75 ± 3.72 | −4.50 ± 2.83 | 0.925 |
| MRSE | −8.72 ± 5.54 | −9.00 ± 5.25 | −9.66 ± 5.23 | −8.75 ± 5.17 | 0.858 |
| TKC 4 (n:29) | |||||
| UCVA, logMAR | 0.82 ± 0.32 | 0.83 ± 0.32 | 0.69 ± 0.23 | 0.87 ± 0.41 | 0.877 |
| BCVA, logMAR | 0.63 ± 0.32 | 0.53 ± 0.22 | 0.46 ± 0.15 | 0.50 ± 0.22 | 0.310 |
| Axis | 90.86 ± 55.08 | 112.73 ± 51.68 | 107.00 ± 65.89 | 79.29 ± 63.27 | 0.505 |
| Sphere | −4.45 ± 5.57 | −3.33 ± 4.33 | −4.68 ± 6.33 | −5.41 ± 5.08 | 0.170 |
| Cylinder | −4.12 ± 2.66 | −5.78 ± 3.36 | −5.13 ± 3.15 | −6.16 ± 2.50 | 0.653 |
| MRSE | −5.66 ± 6.44 | −5.78 ± 5.37 | −7.25 ± 6.95 | −8.49 ± 5.56 | 0.071 |
Statistical analysis used: Friedman Test; UCVA-Uncorrected Visual Acuity; BCVA- Best Corrected Visual Acuity; MRSE-Manifest Refraction Spherical Equivalent; TKC-Topographical keratoconus classification
Discussion
Corneal collagen-crosslinking has been proven to halt KC progression and has been well established in literature [23]. The findings presented in this A-CXL study align with the current understanding of the pathophysiology of KC treatment and healing which has been explored in various studies. This has been demonstrated through the overall effect of A-CXL in increasing corneal stiffness secondary to the formation of more chemical bonds within the corneal stroma [24, 25]. As such, irradiation of riboflavin with UV-A produces a photochemical reaction that increases both the inter-fibrillar and intra-fibrillar covalent bonds in the corneal stroma [18]. A review on the epithelial and stromal effects of CXL by Bradford et al. concluded that collagen-crosslinking can lead to mild flattening of the cornea secondary to the strengthening of the corneal stroma which has been strongly associated with halting progression. This has demonstrated that cross-linking can maintain corneal stiffness and postpone the need for more invasive procedures like corneal transplantation [25].
The effectiveness of A-CXL in improving visual acuity has been the subject of numerous studies. Patients with corneal ectasia have often report significant improvements in their visual function after undergoing this procedure. It has been shown that there was either a slight improvement in UCVA and BCVA or a trend towards visual stability following A-CXL in KC while worsening of vision suggests progression [13]. Multiple studies done by Feld et al., Elbaz et al. and Woo et al. respectfully, have demonstrated an observable but non-significant improvement in visual acuity following A-CXL [5, 12, 15]. Studies by Kirgiz et al. and Mita et al. respectfully, have reported significant improvements in post-operative UCVA and BVCA measurements [13, 14].
In the present study, there is an improvement in BCVA by at least 1 line detected as early as 6 months after the crosslinking procedure and this improvement is still evident after 1 year although associated with a slight trend towards a myopic shift. This small but statistically significant improvement in BCVA may be reflective of the induced changes in corneal topography following the crosslinking procedure [12]. Furthermore, it was supported by Latif et al. wherein they proposed that there is an inverse relationship between pre-operative Kmax levels and postoperative BCVA gain. It was postulated that when the pre-operative Kmax increases, the gain in BCVA decreases [29].
Corneal deformation in KC is thought to be a result of unravelling of the corneal lamella leading to lower collagen fibril tension [26]. As a result of this deformation, there is an increase in corneal steepening, a defining factor in progressive KC followed by a change in refractive power. Kmax is defined as the power of the steepest anterior corneal curvature [27]. It is a parameter used for both disease classification and as a predictor of both KC progression and CXL outcomes [11, 12, 27]. A study by Feld et al. exhibited a nonsignificant decrease in Kmax at 6 and 12 months following A-CXL resulting in a reduced anterior surface curvature [12]. A study by Kirgiz et al. which determined the biomechanical and topographic alterations within 1 year following A-CXL demonstrated a significant decrease in the maximum keratometry readings at 12 months [13] while Mita et al. also demonstrated similar outcomes as well as a significant improvement in UCVA 6 months following A-CXL [14]. Elbaz et al. followed up keratoconus patients 1 year after A-CXL stated that A-CXL was effective in stabilizing topographic parameters [15]. Furthermore, Chow et al. compared one year outcomes of conventional and A-CXL in progressive KC and demonstrated stability in keratometry following A-CXL [16].
In our study, there was an initial steepening of all keratometry parameters at 1 month followed by gradual recovery exhibiting flattening of keratometry parameters to below baseline preoperative measurements at 1 year following A-CXL. The trend in these post-operative follow-ups, although not statistically significant, do not point to improvement in corneal steepness but rather a stability of keratometry readings up to 12 months following A-CXL. Similarly, the other keratometry parameters such as K1, K2, and Kmean also demonstrated a similar trend in stability after A-CXL. These subtle findings still reflect a decrease in anterior surface curvature which may have led to the significant improvement in vision [3].
In the subgroup analysis comparing the effects of A-CXL on keratometry readings, it was observed that the mild and moderate TKC stages (TKC-2 & TKC-3) had no significant changes in measured keratometry readings at all given time points. No signs of KC progression were observed. It was however noted that the advanced TKC stage (TKC-4) had a statistically significant reduction in Kmax 1 year after A-CXL treatment. This may suggest that advanced cases of KC demonstrates more corneal flattening compared to the earlier stages. In a study by Chan et al. that looked into the topographic responses between mild to moderate and advanced KC cases after A-CXL stated that this procedure is effective in preventing KC progression in advanced cases. It was suggested that advanced cases were more responsive due to deeper cross-linking penetration because the advanced stages were associated with thinner corneas. Deeper penetration yields a larger area of cross-linking which in turn produces a stronger effect. The same can be said with less progressive cases treated with CXL as there is less penetration leading to a limited area of cornea that is treated [17].
While corneal topography has been known as the gold standard for the evaluation of corneal ectasia, corneal thickness has also been useful in both diagnosis and evaluation as it is a prominent pathologic sequela of KC [22]. As postulated by Holopainen et al., apart from the riboflavin induced thinning due to evaporation and stromal water efflux, UV-A activation of endothelial cell pump gives rise to the increase in pump-site density and activity/turnover which return to normal levels only after a certain amount of time [30].. This explains why corneal thinning was detected immediately after A-CXL and then spontaneously returned to normal or almost normal levels after a given amount of time. CCT at the thinnest point is said to be more sensitive in monitoring progression than apical pachymetry measurements [27].
In a one year study on the effects of CXL on KC and ectatic corneas on pachymetry, Greenstein et al. demonstrated an initial decline in corneal thickness parameters during the first 3 months post-operatively. This was then followed by a gradual recovery which approached baseline values [11]. A study done by Pjano et al. stated that that corneal pachymetry decreases following cross-linking as demonstrated by a statistically significant decrease in corneal thickness in the first 3 months followed by a subsequent increase up to 12 months post-operatively. This finding supports the minimal pachymetry measurement of at least 400 microns before CXL [22]. The current study showed a similar trend of an initial decline in all pachymetry measurements 1 month following A-CXL which was then followed by a statistically significant recovery in 6 and 12 months.
In recent years, the emphasis has turned to monitoring corneal biomechanics following CXL. This may provide more in-depth investigations of the effectiveness of cross-linking within the corneal stroma following surgery. Specifically, the DAR and IR have been highlighted as critical variables for effective recovery following CXL. The IR is the radius of the curvature when the cornea is most concave following contact with compressed air. The DAR on the other hand is a parameter that measures the deformation between the central and peripheral 2 mm from the corneal apex. Both parameters have been found to be independent from intraocular pressure [19]. The altered matrix stiffness in KC plays an important role in maintaining stability [10]. Reduced values of these parameters represent increased corneal rigidity and stiffness [1, 12].
Feld et al. had comparable preoperative and postoperative IR values following A-CXL which indicates stability of corneal stiffness [12]. Vinciguerra et al. demonstrated a significant decrease in both IR and DAR 6 weeks after A-CXL [19]. Jabbarvand et al. demonstrated a significant decrease in both IR and DAR at 6 months following the use of conventional CXL [18]. A four year longitudinal study done by Sedaghat et al. demonstrated a significant decrease in IR which was consistent with stiffening, however no significant changes in DAR were exhibited following conventional CXL [20]. The present study demonstrated a statistically significant decrease in both IR and DAR 6 months following A-CXL.
Biomechanical stiffness parameters also include SPA1. This parameter reflects the inward movement of the cornea after the air impulse and is also reflective of corneal stiffness [1, 12]. The SSI is a biomechanical parameter reflective of corneal stiffness that is independent of IOP and corneal thickness. A demonstrable increase in both of these parameters is associated with a stiffer cornea. Feld et al. noted stability of SPA1 following A-CXL with having post-operative measurements comparable to baseline values [12]. As opposed to these outcomes, Vinciguerra et al. demonstrated a statistically significant increase in SPA1 at 6 months following A-CXL which resulted in a stiffer cornea after A-CXL [19]. Jabbarvand et al. also showed similar increases in SPA1, this time using the conventional CXL [18]. Felter et al. in a 4 year study following KC patients after conventional CXL showed that SPA1 decreased until one year post-operatively and then showed a consistent but not significant increase thereafter. SSI was demonstrated to increase during the first year then decreased to preoperative levels on subsequent follow ups [21].
In the present study, there was no statistically significant change demonstrated in both the SPA1 and SSI parameters which may point to stability of corneal stiffness following A-CXL. Although not statistically significant, SPA1 values were shown to be higher than baseline at 6 and 12 months post-operatively which may demonstrate at trend towards increasing stiffness on long term follow-up periods similar to what was demonstrated by Felter et al. [21]
ARTh is the relative thickness from the center to the periphery. A lower value of this parameter is reflective of a thinner cornea. Feld et al. demonstrated a significant decrease in ARTh at 6 and 12 months following A-CXL in KC patients [12]. Felter et al. observed significantly lower ARTh readings at all time points following conventional CXL in a 4 year follow up study [21]. Jabbarvand et al. demonstrated an increase in ARTh 6 months following the conventional CXL [18]. The present study observed a decreasing trend in ARTh following A-CXL. Feld et al. stated that the decrease in this parameter might be due to the treatment induced thinning and flattening of the corneal apex [12]. Corneal thickness at the thinnest point was lower than baseline at all measured time points in this study and an expected decrease in ARTh values was observed [21].
BAD D is reflective of the prognosis of KC relative to the front and back surfaces of the cornea, pachymetry and reference sphere in comparison to normative data [12]. Flockerzi et al. stated that a BAD D > 0.42 is indicative of KC progression. There was note of an initial increase in BAD D at the first month followed by a gradual return to baseline values on succeeding follow-ups [28]. Feld et al. demonstrated a statistically significant increase in BAD D at 6 months [12]. The current study observed a significant increase in BAD D 1 month followed by a statistically significant decrease following 6 months in relation to the initial 1 month decline. This may indicate that by 6 months, there may already biomechanical evidence of the cornea returning to its preoperative status [28].
The lack of a universally accepted criterion for progression creates challenges in real world practice as it may influence when and how early intervention is considered necessary. Apart from the guidelines set by the Global Consensus on Keratoconus and Ectatic Diseases, Germany’s Federal Joint Committee (G-BA) proposed a criteria for progressive keratoconus consisting of an increase Kmax by ≥ 1D, the increase in the astigmatism determined by ≥ 1D and the decrease in the base curve of the best-fitting contact lens by ≥ 0.1 mm within one year [18, 21]. Alternative methods employ more advanced imaging modalities, such as Scheimpflug tomography while some research focuses on the anterior or posterior surface steepening, as well as corneal thinning. Flockerzi defined KC progression as an increase in Kmax by > 1 D within 12 months, an increase in corneal astigmatism by > 1D or a decrease of the thinnest corneal thickness (TCT) by > 30 µm within 12 months [28]. Jabbarvand et al. defined this as a reduction by 10% in thinnest corneal point or more than 1D increase in Kmax in 6 months follow-up [18]. The mean results of Kmax, CCT, or corneal astigmatism in the present study did not meet the criteria of KC progression described in the literature. Following a 1 year follow up period after A-CXL, there was no evidence of significant KC progression detected thereby suggesting that the treatment goal of halting disease progression seems to have been achieved. There was also no evidence of progression among all TKC stages in all recorded time points and this may also confirm that A-CXL can be effective regardless of the progression criterion used.
One of the study's limitations is it’s retrospective nature and there was no randomization. Second is the limited sample size, which could not adequately reflect a broader KC population. Furthermore, the length of the research study may not have allowed for a thorough evaluation of long-term results, particularly the tomographic and biomechanical characteristics after treatment. The lack of a comparator or control group from the conventional protocol or another modified treatment protocol for A-CXL may make it challenging to contextualize the relative effectiveness of our A-CXL technique in the management of KC. However, the strength of our paper comes from the inclusion of corneal biomechanical analysis after A-CXL adding to previous knowledge on this subject. To the authors knowledge, this is the first study done in the Philippines with detailed inclusion of both corneal tomographic and biomechanical parameters post A-CXL treatment.
For future studies, we recommend a larger sample size and increasing follow-up duration to improve statistical power that will allow thorough knowledge of the long-term corneal stability. Also, comparative studies between different A-CXL protocols could offer a clearer understanding of the optimal treatment parameters, such as various riboflavin solutions and total radiation power delivered in the cornea. With regards to outcome parameters, it is also recommended to include optical correction with RGP lenses, as these may provide more insight on the effects of the cross-linking procedure on the patient’s visual outcomes. Finally, future research could benefit from evaluating patient feedback and satisfaction, ensuring a holistic assessment of the treatment impact on visual satisfaction and the impact of quality of life. With all of these steps, it could greatly contribute significantly to maximizing treatment protocols and personalizing treatment approaches for KC patients.
Conclusions
By analyzing the preoperative and postoperative changes in vision, refraction, keratometry, pachymetry and corneal biomechanics of KC patients, this study further supports that A-CXL is an effective treatment for slowing the course of KC. The outcomes of our modified A-CXL technique seems to have achieved similar outcomes as those in the literature [32]. There was no objective parameter pointing to KC progression among the subgroups of TKC stages treated and there was a statistically significant improvement in spectacle BCVA by 1 line at 6 and 12 after treatment. Despite an initial worsening of keratometry parameters 1 month post-A-CXL, it was followed by a statistically significant recovery that approached baseline readings at 6 and 12 months after A-CXL. There was a statistically significant decline in corneal thickness at 1 month following treatment and a significant recovery in pachymetry measurements at 6 months and 12 months which reflected preoperative data. The measurements of corneal biomechanics showed a trend towards stability among all biomechanical parameters. A statistically significant increase in corneal stiffness parameters IR and DAR was noted at 6 months which reflects an increase in corneal stiffness after the cross-linking procedure. Among the various TKC stages, subgroup analysis revealed that the milder and moderate stages (TKC-2 and TKC-3) exhibited stability, whereas the most advanced stage (TKC-4) showed a statistically significant improvement in Kmax.
Abbreviations
- KC
Keratoconus
- CXL
Corneal Collagen Crosslinking
- D
Diopter
- HOAs
Higher Order Aberrations
- UVA-A
Ultraviolet-A
- A-CXL
Accelerated Corneal collagen crosslinking
- BAD D
Belin/Ambrósio Enhanced Ectasia Total Deviation Display
- SPA1
Stiffness Parameter at first applanation
- SSI
Stress Strain Index
- IR
Integrated Radius
- ARTh
Ambrósio relational thickness to the horizontal profile
- DAR
Deformation Amplitude Ratio
- UCVA
Uncorrected Visual Acuity
- BCVA
Best Corrected Visual Acuity
- MRSE
Manifest Refraction Spherical Equivalent
- logMAR
Minimum Angle of Resolution
- Corvis ST
Corneal Visualization Scheimpflug Technology
- K
Keratometry
- K1
Flat keratometry
- K2
Steep keratometry
- Kmean
Mean keratometry
- Kmax
Maximal keratometry
- CCT
Central corneal thickness
- TCT
Thinnest Corneal Thickness
- TKC
Topographical keratoconus classification
- RGP
Rigid gas-permeable lenses
- ORA
Ocular Response Analyzer
Authors’ contributions
Design and conduct of the study (RTA, EMC); collection (AAG, JCL), management (RTA), analysis (RTA, AAG, EMC, JCL), interpretation of the data (RTA, AAG, EMC, JCL); manuscript preparation (RTA, AAG, EMC), manuscript review (RTA, AAG, EMC, JCL), manuscript approval (RTA, EMC).
Data availability
The datasets generated and/or analyzed during the current study are not publicly available due to compliance with the National Data Privacy Law. However, they are available from the corresponding author upon reasonable request.
Declarations
Ethics approval and consent to participate
In compliance with institutional guidelines for research involving human subjects, the Cardinal Santos Medical Center Research Ethics Review Committee has officially approved the study protocol and waived the need to obtain participants'informed consent. The decision was made on the basis of the study's retrospective design, low participant risk, and use of anonymized data. The decision was made after a careful evaluation of the research protocol and ethical issues, with a strong emphasis on protecting participant rights and welfare. All procedures were carried out in complete conformity to ethical norms and guidelines.
Consent for publication
Not Applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Nishida T, Kojima T, Kataoka T, Isogai N, Yoshida Y, Nakamura T. Evaluation of the Relationship Between the Changes in the Corneal Biomechanical Properties and Changes in the Anterior Segment OCT Parameters Following Customized Corneal Cross-Linking. Clin Ophthalmol. 2022;9(16):1909–23. 10.2147/OPTH.S361836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Salouti R, Khalili MR, Zamani M, Ghoreyshi M, Nowroozzadeh MH. Assessment of the changes in corneal biomechanical properties after collagen cross-linking in patients with keratoconus. J Curr Ophthalmol. 2019;31(3):262–7. 10.1016/j.joco.2019.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.American Academy of Ophthalmology, & Hamill, B. M., MD. (2022). 2021–2022 BCSC (Basic and Clinical Science Course), Book 8: External Disease and Cornea. American Academy of Ophthalmology.
- 4.Dervenis N, Dervenis P, Dragoumis N, Papandroudis A, Zachariadis Z, Balidis M. Accelerated, Pulsed Collagen Cross-Linking versus the Dresden Protocol in Keratoconus: A Case Series. Med Princ Pract. 2020;29(4):332–7. 10.1159/000505598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Woo JH, Iyer JV, Lim L, Hla MH, Mehta JS, Chan CM, Tan DT. Conventional Versus Accelerated Collagen Cross-Linking for Keratoconus: A Comparison of Visual, Refractive, Topographic and Biomechanical Outcomes. Open Ophthalmol J. 2017;29(11):262–72. 10.2174/1874364101711010262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chan TCY, Tsui RWY, Chow VWS, Lam JKM, Wong VWY, Wan KH. Accelerated corneal collagen cross-linking in progressive keratoconus: Five-year results and predictors of visual and topographic outcomes. Indian J Ophthalmol. 2022;70(8):2930–5. 10.4103/ijo.IJO_2778_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kanellopoulos AJ. The impact of keratoconus treatment with the Athens Protocol (partial topography-guided photorefractive keratectomy combined with higher-fluence corneal collagen cross-linking) on quality of life: a long-term study. Clin Ophthalmol. 2019;3(13):795–803. 10.2147/OPTH.S188519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kanellopoulos AJ, Krueger RR, Asimellis G. Cross-linking and corneal imaging advances. Biomed Res Int. 2015;2015: 306439. 10.1155/2015/306439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xanthopoulou K, Seitz B, Belin MW, Flockerzi E. Reliability analysis of successive Corvis ST® measurements in keratoconus 2 years after accelerated corneal crosslinking compared to untreated keratoconus corneas. Graefes Arch Clin Exp Ophthalmol. 2023;261(4):1055–61. 10.1007/s00417-022-05881-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Santodomingo-Rubido J, Carracedo G, Suzaki A, Villa-Collar C, Vincent SJ, Wolffsohn JS. Keratoconus: An updated review. Cont Lens Anterior Eye. 2022;45(3): 101559. 10.1016/j.clae.2021.101559. [DOI] [PubMed] [Google Scholar]
- 11.Greenstein SA, Hersh PS. Corneal Crosslinking for Progressive Keratoconus and Corneal Ectasia: Summary of US Multicenter and Subgroup Clinical Trials. Transl Vis Sci Technol. 2021;10(5):13. 10.1167/tvst.10.5.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Feld S, Flockerzi E, Daas L, Xanthopoulou K, Sideroudi H, Langenbucher A, Seitz B. Die Biomechanik der Hornhaut vor und nach Crosslinking bei Patienten mit Keratokonus [Corneal biomechanics before and after cross-linking in patients with keratoconus]. Ophthalmologie. 2023 Sep;120(9):940–946. German. 10.1007/s00347-023-01839-z. [DOI] [PubMed]
- 13.Kirgiz A, Erdur SK, Cabuk KS, Atalay K, Nacaroglu SA. Alterations in Corneal Biomechanical and Topographic Features After Accelerated Crosslinking: 1-Year Results. Beyoglu Eye J. 2019;4(2):108–14. 10.14744/bej.2019.44154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mita M, Waring GO 4th, Tomita M. High-irradiance accelerated collagen crosslinking for the treatment of keratoconus: six-month results. J Cataract Refract Surg. 2014;40(6):1032–40. 10.1016/j.jcrs.2013.12.014. [DOI] [PubMed] [Google Scholar]
- 15.Elbaz U, Shen C, Lichtinger A, Zauberman NA, Goldich Y, Chan CC, Slomovic AR, Rootman DS. Accelerated (9-mW/cm2) corneal collagen crosslinking for keratoconus-A 1-year follow-up. Cornea. 2014;33(8):769–73. 10.1097/ICO.0000000000000154. [DOI] [PubMed] [Google Scholar]
- 16.Chow VW, Chan TC, Yu M, Wong VW, Jhanji V. One-year outcomes of conventional and accelerated collagen crosslinking in progressive keratoconus. Sci Rep. 2015;25(5):14425. 10.1038/srep14425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chan TC, Chow VW, Jhanji V, Wong VW. Different Topographic Response Between Mild to Moderate and Advanced Keratoconus After Accelerated Collagen Cross-linking. Cornea. 2015;34(8):922–7. 10.1097/ICO.0000000000000483. [DOI] [PubMed] [Google Scholar]
- 18.Jabbarvand M, Moravvej Z, Shahraki K, Hashemian H, Ghasemi H, Berijani S, Amiri Z, Jamali A. Corneal biomechanical outcome of collagen cross-linking in keratoconic patients evaluated by Corvis ST. Eur J Ophthalmol. 2021;31(4):1577–83. 10.1177/1120672120944798. [DOI] [PubMed] [Google Scholar]
- 19.Vinciguerra R, Romano V, Arbabi EM, Brunner M, Willoughby CE, Batterbury M, Kaye SB. In Vivo Early Corneal Biomechanical Changes After Corneal Cross-linking in Patients With Progressive Keratoconus. J Refract Surg. 2017;33(12):840–6. 10.3928/1081597X-20170922-02. Erratum. In: JRefractSurg.2018Jan1;34(1):68. doi:10.3928/1081597X-20171212-01. [DOI] [PubMed]
- 20.Sedaghat MR, Momeni-Moghaddam H, Ambrósio R Jr, Roberts CJ, Yekta AA, Danesh Z, Reisdorf S, Khabazkhoob M, Heidari HR, Sadeghi J. Long-term Evaluation of Corneal Biomechanical Properties After Corneal Cross-linking for Keratoconus: A 4-Year Longitudinal Study. J Refract Surg. 2018;34(12):849–56. 10.3928/1081597X-20181012-02. [DOI] [PubMed] [Google Scholar]
- 21.Felter E, Khoramnia R, Friedrich M, Son HS, Auffarth GU, Augustin VA. Biomechanical changes following corneal crosslinking in keratoconus patients. Graefes Arch Clin Exp Ophthalmol. 2024. 10.1007/s00417-024-06549-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pjano MA, Biscevic A, Grisevic S, Gabric I, Salkica AS, Ziga N. Pachymetry and Elevation Back Map Changes in Keratoconus Patients After Crosslinking Procedure. Med Arch. 2020;74(2):105–8. 10.5455/medarh.2020.74.105-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wollensak G, Spoerl E, Seiler T. Riboflavin/ultraviolet-a-induced collagen crosslinking for the treatment of keratoconus. Am J Ophthalmol. 2003;135(5):620–7. 10.1016/s0002-9394(02)02220-1. [DOI] [PubMed] [Google Scholar]
- 24.Spoerl E, Wollensak G, Seiler T. Increased resistance of crosslinked cornea against enzymatic digestion. Curr Eye Res. 2004;29(1):35–40. 10.1080/02713680490513182. [DOI] [PubMed] [Google Scholar]
- 25.Bradford S, Luo S, Brown D, Juhasz T, Jester J. A review of the epithelial and stromal effects of corneal collagen crosslinking. Ocul Surf. 2023;30:150–9. 10.1016/j.jtos.2023.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Meek KM, Tuft SJ, Huang Y, Gill PS, Hayes S, Newton RH, Bron AJ. Changes in collagen orientation and distribution in keratoconus corneas. Invest Ophthalmol Vis Sci. 2005;46(6):1948–56. 10.1167/iovs.04-1253. [DOI] [PubMed] [Google Scholar]
- 27.Duncan JK, Belin MW, Borgstrom M. Assessing progression of keratoconus: novel tomographic determinants. Eye Vis (Lond). 2016;11(3):6. 10.1186/s40662-016-0038-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Flockerzi E, Xanthopoulou K, Daas L, Feld S, Langenbucher A, Seitz B. Evaluation of Dynamic Corneal Response Parameters and the Biomechanical E-Staging After Accelerated Corneal Cross-Linking in Keratoconus. Asia Pac J Ophthalmol (Phila). 2022;11(6):514–20. 10.1097/APO.0000000000000580. [DOI] [PubMed] [Google Scholar]
- 29.Latif K, Iqbal MS. Visual and Topographical Outcomes Following Accelerated Trans-Epithelial Corneal Crosslinking in Progressive Keratoconus. J Coll Physicians Surg Pak. 2017;27(9):552–5. [PubMed] [Google Scholar]
- 30.Gomes, J. A., Tan, D., Rapuano, C. J., Belin, M. W., Ambrósio, R., Jr, Guell, J. L., Malecaze, F., Nishida, K., Sangwan, V. S., & Group of Panelists for the Global Delphi Panel of Keratoconus and Ectatic Diseases 2015 Global consensus on keratoconus and ectatic diseases Cornea 34 4 359 36910.1097/ICO.0000000000000408 [DOI] [PubMed]
- 31.Agarwal R, Jain P, Arora R. Complications of corneal collagen cross-linking. Indian J Ophthalmol. 2022;70(5):1466–74. 10.4103/ijo.IJO_1595_21.P. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Celik-Buyuktepe T, Ucakhan OO. Comparative Evaluation of Anterior Segment Optical Coherence Tomography Findings Following Accelerated Corneal Crosslinking Protocols Using Different Riboflavin Formulations and Soaking Durations. Curr Eye Res. 2025;50(1):32–40. 10.1080/02713683.2024.2385441. [DOI] [PubMed] [Google Scholar]
- 33.Kang Y, Li S, Liu C, Xu M, Shi S, Liu Y. Accelerated Epithelium-off Corneal Cross-linking With High Ultraviolet Energy Dose (7.2 J/cm2) for Progressive Keratoconus: 2-Year Results in a Chinese Population. J Refract Surg. 2020;36(11):731–739. 10.3928/1081597X-20200820-01. [DOI] [PubMed]
- 34.Fasciani R, Scartozzi L, Bruzio S, Di Stefano G, Mosca L, Guccione L, Ciardiello A, Rizzo S. High-Fluence Epithelium-off Accelerated Pulsed Corneal Cross-linking (15 mW/cm2; 7.2 J/cm2) for Pediatric Keratoconus: A 3-Year Retrospective Analysis. J Refract Surg. 2024;40(3):e148-e155. 10.3928/1081597X-20240208-01. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated and/or analyzed during the current study are not publicly available due to compliance with the National Data Privacy Law. However, they are available from the corresponding author upon reasonable request.