Abstract
Background
The effectiveness of whole brain radiation therapy with simultaneous integrated boost (WBRT-SIB) in comparison to whole brain radiation therapy alone (WBRT-alone) is yet unknown, despite the fact that its use in clinic is growing. To investigate the variations in intracranial control and overall survival (OS) between the two approaches, we conducted a matching comparison for patients with brain metastases (BM).
Method
From January 1, 2015, to December 31, 2019, a total of 245 BM patients were eligible for inclusion, including 154 patients who received WBRT-alone (30 Gy/10 fractions) and 91 patients who received WBRT-SIB (40–50 Gy/10 fractions). 1:1 propensity score matching was used to select the patients with balanced baseline characteristics. The intracranial control and OS condition were analyzed using Kaplan-Meier method, Log-rank test, and Cox proportional hazard regression model.
Results
138 patients were matched into the WBRT-SIB group and the WBRT-alone group. Of these, 113 (81.9%) patients had BM originating from the lungs, and 124 (89.9%) patients had more than 3 intracranial lesions. After the initiation of radiotherapy, the WBRT-SIB group and the WBRT-alone group had respective 2-year intracranial progression-free survival (iPFS), local progression-free survival (iLPFS), and distant progression-free survival (iDPFS) of 46.2% and 24.5% (p = 0.017), 49.4% and 29.8% (p = 0.033), and 68.6% and 54.4% (p = 0.040). There was no significant difference in OS (22.2 vs. 19.0 months, p = 0.768). However, in the exploratory subgroup analysis of infratentorial with/without supratentorial metastases (n = 96), the WBRT-SIB group showed a significantly better OS than the WBRT-alone group (24.6 vs.17.2 months, p = 0.040). Furthermore, the Cox proportional hazard model of this subgroup revealed that WBRT-SIB (p = 0.039) and systemic therapy after radiotherapy (p = 0.002) were independent prognostic factors for OS. There was no difference in the incidence of grade 3–4 acute brain radiation reactions between the two groups (24.6% vs. 17.4%, p = 0.290).
Conclusion
WBRT-SIB is a promising strategy for patients with BM. Compared to WBRT alone, WBRT-SIB can significantly prolong the intracranial PFS (including local and distant PFS). Additionally, while WBRT-SIB did not improve OS in the entire cohort, the OS benefit for patients with BM accompanied by infratentorial involvement warrants further exploration.
Keywords: Brain metastases, Whole brain radiation therapy, Simultaneous integrated boost, Prognosis
Introduction
About 20% of cancer patients develop brain metastases (BM), the most prevalent malignancy of the central nervous system. However, the prevalence of BM gradually rises as overall survival (OS) of cancer patients lengthens [1, 2]. The presence of BM indicates a poor prognosis, with a natural course lasting only 1–2 months [3].
Whole brain radiotherapy (WBRT) is a common treatment for BM. However, with a response rate of 45–60%, the median survival following WBRT is just 3–6 months [1–4]. Stereotactic radiosurgery (SRS) is frequently used in patients with brain oligometastasis(less than 4) and can achieve a local control rate of 80–90% and a median OS of 10–12 months [5–7]. The combination of WBRT and SRS may enhance the intracranial control rate [8–10].
In recent years, the advancement of radiation technology and equipment has brought forth new developments in BM radiotherapy. Local treatment for BM is no longer limited to SRS. The technique of WBRT with simultaneous integrated boost (WBRT-SIB) is a new modality that employs Intensity-modulated radiotherapy (IMRT) to concurrently increase the dose of local intracranial metastases during WBRT for patients with brain oligometastasis or multimetastasis [11–15]. WBRT-SIB aims to improve both local lesions and whole brain control at the same time. Additionally, by protecting bilateral hippocampus structures, this technique can reduce the occurrence of cognitive impairment in BM patients caused by WBRT [15–17]. A prospective study reported that 13 patients received IMRT-SIB and were prescribed a WBRT dose of 25/37.5 Gy in 10/15 fractions with a SIB dose of 45/52.5 Gy to the gross lesions. The 1-year local control rate was 92% and the 1-year overall intracranial control rate was 46% [18]. WBRT-SIB can be considered as an alternative treatment of WBRT combined with SRS, especially suitable for managing multiple or large BM.
However, it remains debatable whether WBRT-SIB can significantly increase intracranial control and OS compared to WBRT [19, 20]. Furthermore, given the current difficulty in conducting a prospective randomized controlled trial of WBRT-SIB versus WBRT alone [19], this study attempts to perform a survival analysis from our center’s retrospective data to preliminarily explore the difference in the efficacy of these two techniques and provide more valuable evidence and guidance for clinical application.
Materials and methods
Data collection
A total of 582 BM patients received WBRT-alone or WBRT-SIB at our Center between January 1, 2015, and December 31, 2019. The following clinical data were collected from the hospital system: age, gender, primary tumor site and pathological type, time of BM diagnosis, number and location of BM, maximal diameter of BM, neurological symptoms, resection before brain radiotherapy (RT), extracranial tumor status, Karnofsky performance status (KPS) score, brain radiation technique, dose, and treatment duration. MRIs before and after brain RT all were reviewed. Systemic treatments, including as immunotherapy, chemotherapy, and targeted therapy, were also documented both during and after RT.
Inclusion and exclusion criteria
The inclusion criteria were as follows: (1) primary tumor had a pathological diagnosis, and MRI showed the existence of BM; (2) no prior brain RT; (3) completing WBRT or WBRT-SIB treatment with a prescribed dose of 30 Gy in 10 fractions for WBRT. The exclusion criteria were as follows: (1) MRI revealed leptomeningeal or skull metastasis; (2) lost to follow-up within 3 months and no cerebral imaging review after RT; (3) primary tumor diagnosed as malignant melanoma. Finally, 245 patients in all (91 receiving WBRT-SIB and 154 receiving WBRT-alone) satisfied the above criteria.
Radiotherapy
The WBRT-alone group received brain RT with Three-dimensional conformal radiation therapy(3D-CRT) or IMRT. The WBRT-SIB group received brain RT with IMRT, Volumetric intensity-modulated radiotherapy (VMAT), or Helical tomotherapy (TOMO). The head of patients in the supine position was immobilized using a thermoplastic mask. CT and MR simulations were performed with contrast, respectively. CT and MR in the treatment planning system were fused to outline the radiation target areas, including clinical tumor target of the whole brain (CTV-brain) and intracranial metastatic gross tumor targets (GTV). If a patient had a resected metastasis, the resection cavity was expanded by 3–5 mm to form the respective GTV. The CTV-brain and GTV were expanded by 3 mm to form the respective PTV-brain and PTV-GTV.
WBRT for all patients was prescribed at 30 Gy in 10 fractions over 2 weeks. For the patients with WBRT-SIB, the dose of SIB for metastases or resection cavities (GTV) was delivered at 50 Gy in 10 fractions. If GTVs had large volume (maximal diameter > 3 cm) or localized at sensitive organs and structures (e.g. brainstem, internal capsule, chiasma, and cerebellum), the dose of SIB was reduced at 45–40 Gy in 10 fractions according to the size and location. 24 patients (23 patients in the WBRT-SIB group and 1 patient in the WBRT-alone group) performed hippocampus avoidance during WBRT. The mean dose of hippocampus was limited to 10–18 Gy depending on the RT equipment.
Follow-up
The cut-off date for follow-up was January 30, 2023. Patients underwent brain MR-enhanced review and physical examination routinely 1 month after the treatment and thereafter every 2–3 months. The efficacy of intracranial metastases was evaluated according to Response Assessment in Neuro-Oncology Brain Metastases criteria [20]. Progressive disease was defined as a ≥ 20% increase in the sum of longest diameters of intracranial lesions, the emergence of new intracranial lesions, or significant clinical decline. In addition, for patients undergoing immunotherapy, the imaging follow-up period was extended from 3 to 6 months to rule out pseudoprogression. The Common Terminology Criteria for Adverse Events, Version 5 (CTCAE 5.0) was used to evaluate acute radiation toxicities.
Observation endpoints
The primary endpoints included: (1) intracranial local progression-free survival (iLPFS) was defined as the time between the start of brain radiation therapy and pre-existing intracranial lesions recurrence, death or last follow-up; (2) intracranial distant progression-free survival (iDPFS) was defined as the time between the start of brain radiation therapy and the development of new brain metastasis, death or last follow-up; (3) intracranial progression-free survival (iPFS) was defined as the time between the start of brain radiation therapy and any progression in brain, death or last follow-up; (4) OS was calculated the start of brain radiation therapy until death of any cause or last follow-up. The secondary endpoint was the incidence of acute radiation toxicities during the brain radiotherapy.
Statistics
A 1:1 propensity score matching analysis was administered by logistic regression. The matching covariates included age, KPS, pathological type of primary tumor, number of brain metastases, presence of extracranial metastases, resection of BM, and systemic therapies (including targeted therapy, chemotherapy, and immunotherapy) concurrent or/and following the brain radiotherapy. The caliper value was set at 0.06. The baseline characteristics between the two groups were compared using the chi-square test. Kaplan Meier method was employed to evaluate iLPFS, iDPFS, iPFS, and OS. Log-rank test and Cox proportional hazard regression model were employed to compare and analyze the prognosis differences. Data were administrated using SPSS 26.0 and R programming version 4.1.3.
Results
Baseline characteristics
After 1:1 propensity score matching, there were 69 patients in each treatment group (Fig. 1). The two groups were well-balanced between known prognostic covariates (Table 1). 14(10.1%) patients had 1 ~ 3 cerebral metastases, 101(73.1%) patients had 4 ~ 10 cerebral metastases, and 23(16.7%) patients had more than 10 cerebral metastases. There were 4 patients in each group receiving resection of BM before brain RT. There was no difference between the two groups based on the Diagnosis Specific Graded Prognostic Assessment (DS-GPA) [23] score for BM.
Fig. 1.
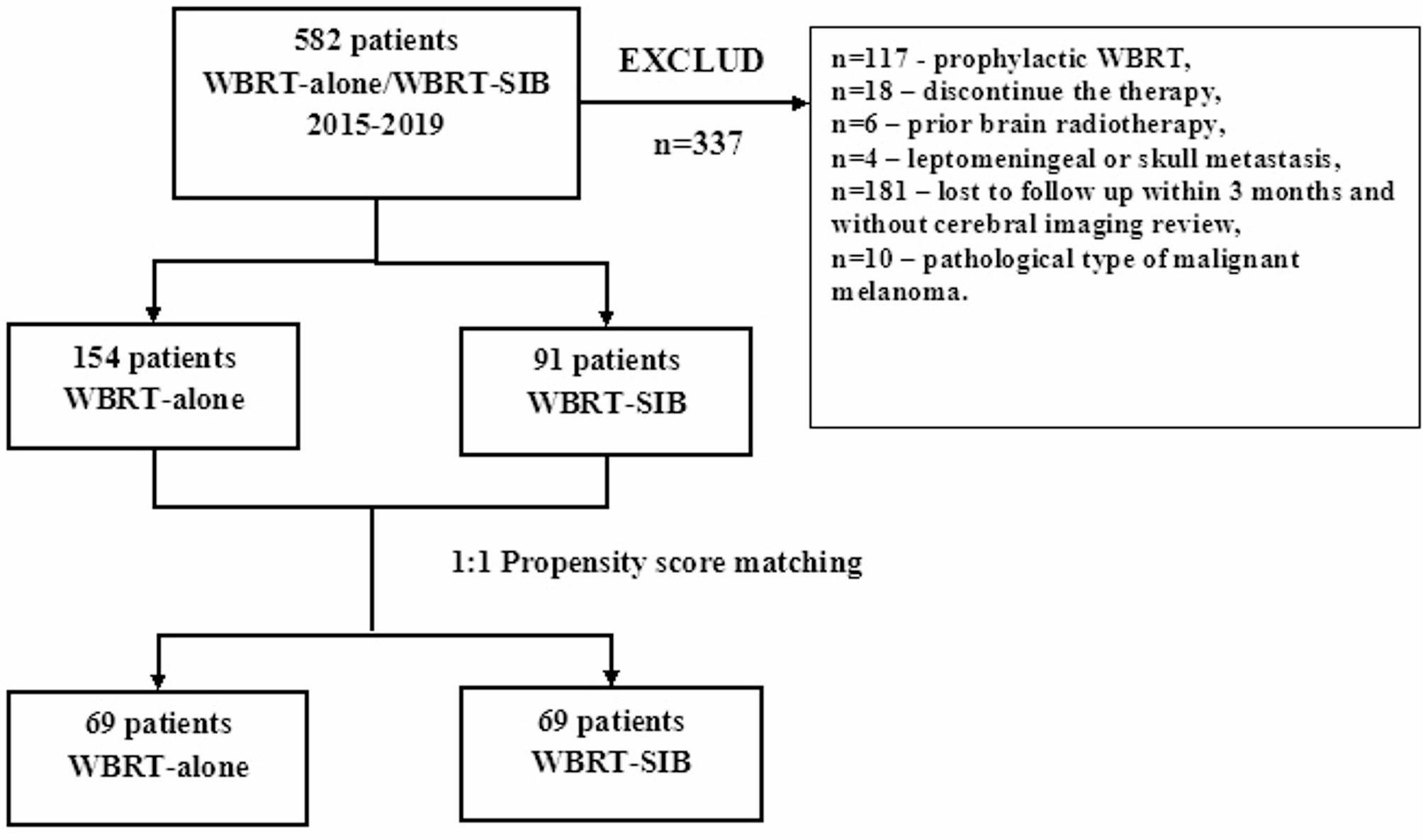
Flow diagram of the study design. WBRT-SIB indicates whole brain radiation therapy with simultaneous integrated boost; WBRT-alone indicates whole-brain radiation therapy
Table 1.
Patient characteristics between WBRT-SIB group and WBRT-alone group
| Factors | Before the 1:1 Propensity Score Match | After the 1:1 Propensity Score Match | ||||
|---|---|---|---|---|---|---|
| WBRT-SIB (%) | WBRT-alone (%) | p value | WBRT-SIB (%) | WBRT-alone (%) | p value | |
| Number of patients | 91(37.1) | 154(62.9) | 69(50.0) | 69(50.0) | ||
| Sex | 0.317 | 0.864 | ||||
| Male | 45(49.5) | 88(57.1) | 36(52.1) | 38(55.1) | ||
| Female | 46(50.5) | 66(43.9) | 33(47.9) | 31(44.9) | ||
| Age | 0.931 | 0.282 | ||||
| ≤ 60 years | 66(72.5) | 114(74.0) | 47(68.1) | 54(78.3) | ||
| > 60 years | 25(27.5) | 40(26.0) | 22(31.9) | 15(21.7) | ||
| Primary tumor | 0.043 | 0.359 | ||||
| Lung cancer | 75(82.4) | 130(84.4) | 58(84.1) | 55(79.7) | ||
| Breast cancer | 7(7.7) | 20(13.0) | 6(8.7) | 11(15.9) | ||
| Othera | 9(9.9) | 4(2.6) | 5(7.2) | 3(4.3) | ||
| Pathological type of primary tumor | 0.005 | 0.218 | ||||
| Adenocarcinoma | 72(79.1) | 107(69.5) | 53(76.8) | 47(68.1) | ||
| Small cell carcinoma | 11(12.1) | 42(27.3) | 10(14.5) | 18(26.1) | ||
| Othera | 8(8.8) | 4(2.6) | 6(8.7) | 4(5.8) | ||
| No. of BM | 1 | 0.778 | ||||
| ≤ 3 | 12(13.2) | 21(13.6) | 8(11.6) | 6(8.7) | ||
| >3 | 79(86.8) | 133(86.4) | 61(88.4) | 63(91.3) | ||
| Location of BM | 0.524 | 0.853 | ||||
| Only supratentorial | 34(37.4) | 50(32.5) | 20(29.0) | 22(31.9) | ||
| Infratentorial b | 57(62.6) | 104(67.6) | 49(71.0) | 47(68.1) | ||
| Longest diameter of BM | 0.18 | 0.468 | ||||
| ≤ 3 cm | 75(82.4) | 138(89.6) | 58(84.1) | 61(88.4) | ||
| > 3 cm | 16(17.6) | 16(10.4) | 11(15.9) | 8(11.6) | ||
| Status of primary tumor before RT | 0.357 | 1 | ||||
| Control | 77(84.6) | 122(79.2) | 62(89.9) | 62(89.9) | ||
| Progression | 14(15.4) | 32(20.8) | 8(10.1) | 7(10.1) | ||
| Existences of extracranial metastases | 0.003 | 1 | ||||
| Yes | 51(56.0) | 102(66.2) | 35(50.7) | 35(50.7) | ||
| No | 40(44.0) | 58(33.8) | 34(49.3) | 34(49.3) | ||
| KPS | 0.372 | 0.849 | ||||
| 90–100 | 63(69.2) | 114(74.0) | 49(71) | 51(73.9) | ||
| ≤ 80 | 28(30.8) | 40(26.0) | 20(29) | 18(26.1) | ||
| Resection of BM before RT | 0.337 | 1 | ||||
| No | 82(90.1) | 145(94.2) | 65(94.2) | 65(94.2) | ||
| Yes | 9(9.9) | 9(5.8) | 4(5.8) | 4(5.8) | ||
| Systemic treatment c | 57(62.6) | 69(44.8) | < 0.001 | 40(58) | 32(46.4) | 0.273 |
| Targeted therapy | 52 | 63 | 35 | 30 | ||
| Bevacizumab | 19 | 39 | 7 | 21 | ||
| Osimertinib | 21 | 12 | 8 | 7 | ||
| Lapatinib | 14 | 4 | 9 | 3 | ||
| Trastuzumab | 6 | 3 | 6 | 1 | ||
| Erlotinib | 9 | 7 | 4 | 3 | ||
| Icotinib | 5 | 3 | 2 | 3 | ||
| Gefitinib | 4 | 6 | 3 | 2 | ||
| Anlotinib | 4 | 5 | 1 | 3 | ||
| Crizotinib | 0 | 3 | 0 | 3 | ||
| Pyrotinib | 3 | 1 | 2 | 0 | ||
| Aletinib | 0 | 2 | 0 | 2 | ||
| Almonertinib | 1 | 4 | 0 | 1 | ||
| Immunotherapy | 5 | 6 | 2 | 2 | ||
| Temozolomide | 12 | 7 | 9 | 3 | ||
| DS-GPA | < 0.001 | 0.324 | ||||
| 3.0–4.0 | 17(18.7) | 39(25.3) | 14(20.3) | 16(23.2) | ||
| 1.5–2.5 | 51(56.0) | 97(63.0) | 38(55.1) | 43(62.3) | ||
| 0–1 | 23(25.3) | 18(11.7) | 17(24.6) | 10(14.5) | ||
Abbreviations: BM, brain metastasis; RT, radiotherapy; KPS, Karnofsky performance status; DS-GPA, Diagnosis-Specific Graded Prognostic Assessment
aWBRT-alone group:1 mediastinal yolk sac tumor, 1 mediastinal small cell carcinoma, 1 esophageal small cell carcinoma, 2 lung squamous cell carcinoma, 1 lung large cell carcinoma. WBRT-SIB group: 1 mediastinal neuroendocrine carcinoma, 1 esophageal squamous carcinoma, 1 renal clear cell carcinoma, 2 cervical squamous cell carcinomas, 2 lung squamous carcinoma, 1 pulmonary mucous epidermoid carcinoma
b 94 patients with both infratentorial and supratentorial metastasis, 1 WBRT-alone patient and 1 WBRT-SIB patient with infratentorial metastasis alone
c Patients received the above systemic therapy from the initiation of radiotherapy to the end of follow-up, 34 of them received 2–4 kinds of systemic therapy drugs
All patients completed the brain RT within 10–34 days. The majority of patients (94.2%) completed RT within 16 days. Mannitol with or without glucocorticoids was administered to 39 patients in the WBRT-SIB group and 36 patients in the WBRT-alone group to treat brain edema during RT.
32 WBRT-SIB patients and 47 WBRT-alone patients developed intracranial progression following brain RT, including both local and distant progression. Subsequently, 6 patients (8.7%) in the WBRT-SIB group and 11 patients (16%) in the WBRT-alone group received local salvage therapies (SRS or surgical resection), respectively. In addition, 43(54.4%) patients with intracranial tumor progression received systemic therapies (targeted therapy /immunotherapy/ temozolomide), including 19 WBRT-SIB patients and 24 WBRT-alone patients.
Intracranial control
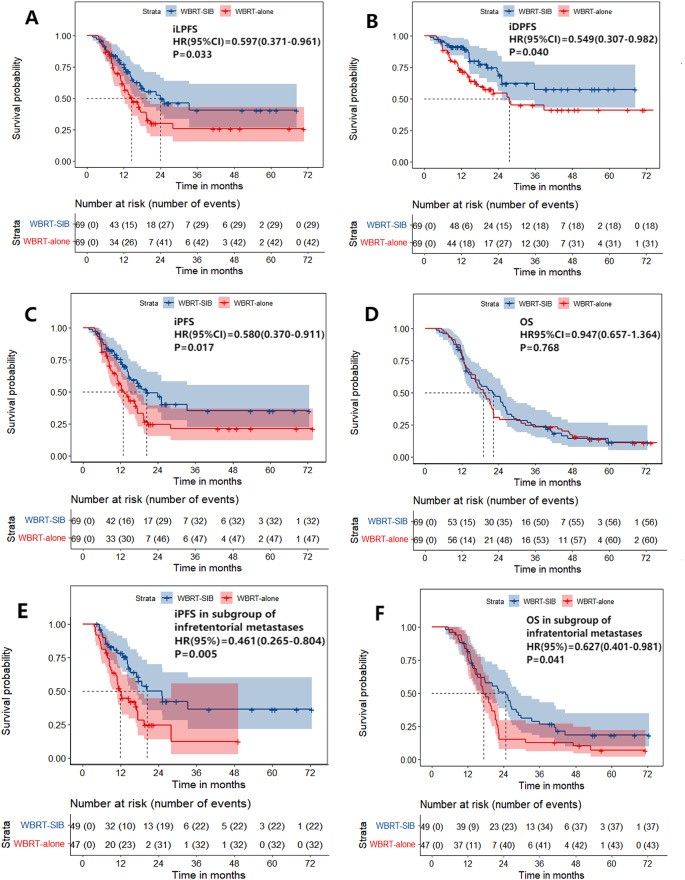
The median follow-up time of 138 patients was 19.5 months (range: 3.5–86.3 months). There were significant differences in iLPFS and iDPFS between the WBRT-SIB group and the WBRT-alone group, with the median time and rates were 24.0 months (95%CI 9.7–38.3) vs. 14.6 months (95%CI 10.3–18.9), 76.9% vs. 60.0% at 1 year and 49.4% vs. 29.8% at 2 years for iLPFS (p = 0.033, Fig. 2A), as well as rates of 91.0% vs. 73.1% at 1 year and 68.6% vs. 54.4% at 2 year for iDPFS (p = 0.040, Fig. 2B), respectively. The median time and rate of iPFS in the WBRT-SIB group were significantly higher than that in the WBRT-alone group, being 20.3 months (95%CI 12.1–28.5) vs. 12.8 months (95%CI 9.6–15.9), and 75.4% vs. 55.0% at 1 year and 46.2% vs. 24.5% at 2year (p = 0.017, Fig. 2C), respectively.
Fig. 2.
(A/B) Intracranial local progression-free survival (iLPFS) and intracranial distant progression-free survival (iDPFS) in WBRT-SIB group were significantly better than WBRT-alone group (p = 0.033, p = 0.040). (C) Intracranial progression-free survival (iPFS) was significantly improved with WBRT-SIB compared with WBRT-alone (12.8 vs. 20.3 months, p = 0.017) (D) There was not significant difference in overall survival (OS) between WBRT-alone group and WBRT-SIB group (p = 0.768), and the median OS were 22.2 months and 19.0 months. (E/F) In the subgroup of infratentorial with or without supratentorial metastasis, iPFS and OS in WBRT-SIB group were significantly better than WBRT-alone group (p = 0.005, p = 0.041)
The subgroup analyses for iPFS showed that iPFS was significantly longer in patients treated with WBRT-SIB in the subgroups of age < 60, adenocarcinoma, multiple (> 3) BM, infratentorial metastases with or without supratentorial metastases (Fig. 2E), primary tumor control, and existence of extracranial metastases, and no systemic treatment (targeted therapy/ immunotherapy/ temozolomide) after the initiation of radiotherapy (Table 2). However, in the subgroup of receiving systemic treatment after the initiation of radiotherapy, there was no significant difference in iPFS between WBRT-SIB and WBRT-alone treatment (p = 0.115). Moreover, among the 23 patients in the WBRT-SIB group who performed hippocampus avoidance during RT, no significant differences were observed in iPFS (22.4 months vs. 33.4 months, p = 0.180), including iLPFS(14.4 months vs. 25.2 months, p = 0.103) and iDPFS(p = 0.515) compared to those without hippocampus avoidance in WBRT-SIB group, and the cumulative incidence of iLPFS and iDPFS at 1 year was 65.2% and 82.9% respectively.
Table 2.
Subgroup analysis for iPFS and OS
| No. of patients | Median iPFS (months) | HR (95%CI) |
P | Median OS (months) | HR (95%CI) |
P | |
|---|---|---|---|---|---|---|---|
| Age ≤ 60 | |||||||
| WBRT-SIB | 47 | 20.3 | 0.488 | 0.006 | 25.5 | 0.771 | 0.247 |
| WBRT-alone | 54 | 12.3 | (0.292–0.817) | 17.9 | (0.497–1.197) | ||
| Adenocarcinoma | |||||||
| WBRT-SIB | 53 | 24 | 0.575 | 0.042 | 24.6 | 1.271 | 0.297 |
| WBRT-alone | 47 | 14.6 | (0.337–0.980) | 21.8 | (0.820–1.971) | ||
| Number of BM > 3 | |||||||
| WBRT-SIB | 61 | 18.7 | 0.575 | 0.023 | 22.1 | 0.934 | 0.726 |
| WBRT-alone | 63 | 12.8 | (0.357–0.925) | 19.5 | (0.635–1.372) | ||
| Infratentorial metastasis | |||||||
| WBRT-SIB | 49 | 20.3 | 0.461 | 0.006 | 24.6 | 0.627 | 0.041 |
| WBRT-alone | 47 | 11.1 | (0.265–0.804) | 17.2 | (0.401–0.981) | ||
| Existences of extracranial metastases | |||||||
| WBRT-SIB | 35 | 25.1 | 0.314 | 0.001 | 17.6 | 0.826 | 0.457 |
| WBRT-alone | 35 | 11.9 | (0.158–0.624) | 16.4 | (0.498–1.370) | ||
| Primary tumor in control | |||||||
| WBRT-SIB | 62 | 24 | 0.511 | 0.005 | 22.2 | 0.975 | 0.897 |
| WBRT-alone | 62 | 12.2 | (0.318–0.822) | 18.9 | (0.663–1.434) | ||
| Systemic treatment after RT | |||||||
| WBRT-SIB | 40 | 22.5 | 0.663 | 0.151 | 24.6 | 1.486 | 0.191 |
| WBRT-alone | 32 | 13.9 | (0.378–1.161) | 24.2 | (0.897–2.486) | ||
| No systemic treatment after RT | |||||||
| WBRT-SIB | 29 | 25.1 | 0.432 | 0.037 | 15.1 | 0.639 | 0.134 |
| WBRT-alone | 37 | 12.8 | (0.188–0.991) | 16.3 | (0.355–1.148) | ||
| Overall | |||||||
| WBRT-SIB | 69 | 20.3 | 0.58 | 0.017 | 22.2 | 0.947 | 0.768 |
| WBRT-alone | 69 | 12.8 | (0.370–0.911) | 18.9 | (0.657–1.364) |
Abbreviations: BM, brain metastases; iPFS, intracranial progression-free survival; OS, overall survival; RT, radiotherapy; HR, hazard ratio; CI, confidence interval
Overall survival
By the date of follow-up, 56 patients in the WBRT-SIB group and 60 patients in the WBRT-alone group had died. The median OS time was 22.2 months (95% CI 16.2–28.1) and 19.0 months (95% CI 15.7–22.7), respectively. The two groups did not show a significant difference in OS rates (78.0% and 79.7% at 1 year, and 47.0% and 30.4% at 2 years, p = 0.768, Fig. 2D).
In the exploratory subgroup analyses for OS, only the subgroup of infratentorial with or without supratentorial metastases showed significantly longer OS in patients received WBRT-SIB (n = 49) compared with patients received WBRT-alone (n = 47), and the median OS time was 24.6 months and 17.2 months (p = 0.041, Fig. 2F; Table 2).
The Cox proportional hazards regression model for the subgroup of infratentorial with or without supratentorial metastasis (n = 96) showed that the patterns of radiotherapy (WBRT-alone vs. WBRT-SIB, hazard ratio = 1.695, p = 0.039) and the systemic therapy after the initiation of radiotherapy (yes vs.no, hazard ratio = 0.471, p = 0.002) were independent prognostic factors for OS (Table 3).
Table 3.
Multivariate analysis for OS in the subgroup of infratentorial with or without supratentorial metastasis (n = 96)
| No of patients | Hazard ratio (95% confidence interval) | P value | |
|---|---|---|---|
| Age | |||
| ≤ 60 years | 69 | 0.735(0.432–1.253) | 0.258 |
| > 60 years | 27 | ||
| Longest diameter of brain metastasis | |||
| > 3 cm | 46 | 1.535(0.941–2.502) | 0.086 |
| ≤ 3 cm | 50 | ||
| Existences of extracranial metastasis | |||
| Yes | 53 | 1.331(0.812–2.182) | 0.256 |
| No | 43 | ||
| Systemic therapy after the initiation of radiotherapy | |||
| Yes | 54 | 0.471(0.290–0.766) | 0.002 |
| No | 42 | ||
| Local salvage SRS or surgical resection | |||
| Yes | 11 | 0.508(0.247–1.045) | 0.065 |
| No | 85 | ||
| Radiotherapy pattern | |||
| WBRT-alone | 47 | 1.695(1.027–2.797) | 0.039 |
| WBRT-SIB | 49 |
Abbreviations: SRS, stereotactic radiosurgery
Toxicity of radiotherapy
According to CTCAE 5.0, 17 patients in the WBRT-SIB group and 11 patients in the WBRT-alone group experienced acute radiation toxicities of grade 3 during RT (Table 4). Only 1 patient in the WBRT-alone group experienced acute toxicity of grade 4 with epilepsy, severe headache, vomiting, and unilateral limb weakness. The incidence of grade 3–4 acute radiation toxicities in WBRT-SIB and WBRT-alone groups were 24.6% and 17.4% (p = 0.290), respectively. Data on delayed radiation brain injury could not be reliably obtained.
Table 4.
Grade 3/4 acute radiation toxicities during radiotherapy
| Symptom | WBRT-alone | WBRT-SIB |
|---|---|---|
| Dizziness | 9 | 13 |
| Headache | 7 | 10 |
| Unilateral limb weakness | 2 | 5 |
| Vomit | 1 | 2 |
| Glossolalia | 2 | 0 |
| Ataxia | 1 | 0 |
| Epilepsy | 1 | 0 |
| Blurring of vision | 0 | 1 |
| Overall | 11 | 17 |
Discussion
In this retrospective study, the baseline characteristics of patients were effectively balanced between the WBRT-SIB group and the WBRT-alone group through propensity score matching. Our results showed that WBRT with simultaneous integrated boost had a significant improvement in iPFS compared with WBRT alone (20.3 vs. 12.8 months, p = 0.017), including iLPFS (76.9% vs. 60% at 1 year, p = 0.033) and iDPFS (91% vs. 73.1% at 1 year, p = 0.040). Lu et al. [21] reported a retrospective study comparing differences between WBRT-alone and WBRT with concurrent or sequential boost for patients with BM from lung cancer. The results revealed that iLPFS was significantly better in the WBRT + boost group (22.3 months vs.17.9 months, p = 0.041), but no significant difference in iDPFS was observed (p = 0.347). They did not perform hippocampal avoidance for any patients. However, their dose of WBRT (40 Gy/20 fractions) exceeded our study. Popp et al. [18] conducted a retrospective study using propensity score matching to compare WBRT-SIB with WBRT-alone for multiple BM. The WBRT-SIB group had better iPFS than the WBRT-alone group (13.5 vs. 6.4 months, p = 0.03). However, the iDPFS rate of the WBRT-SIB group was significantly lower than that of the WBRT-alone group (69% vs. 82% at 1 year, P = 0.016). This could be related to the strict hippocampal dose limitation (D98%≤9 Gy) applied to all WBRT-SIB patients in their study, which resulted in a lower whole-brain biological effective dose in the WBRT-SIB group compared with the WBRT-alone group (33.8 Gy vs. 38.9 Gy, p < 0.001). In our study, however, only 33% of the selected patients in the WBRT-SIB group underwent hippocampus-avoidance. Kenneth et al. [22]published a phase II study of WBRT-SIB with hippocampal dose limitation(WBRT-HSIB) for BM, the cumulative incidence of iPFS and iLPFS were 21.3% and 8.8% at 1 year, respectively, indicating that the main pattern of progression after WBRT-HSIB was intracranial distant progression.
Although some prospective studies [22–24] have shown that hippocampal avoidance in WBRT did not significantly increase new recurrences in the hippocampal areas, the potential risk of progression in these areas warrants close monitoring. Vinai Gondi et al. [25] conducted a retrospective analysis of perihippocampal disease progression in patients receiving HA-WBRT in the Phase II study RTOG 0933. They found that a1-cm3 increase in the aggregate volume of intracranial metastatic disease was significantly associated with the presence of perihippocampal metastasis. A retrospective analysis [26] indicated that NSCLC patients receiving HA-WBRT with extracranial metastases were more likely to develop BM in the hippocampal avoidance region than those without extracranial metastases. whether to limit the hippocampal dose for patients should be determined based on the intracranial and extracranial conditions to minimize the risk of increased brain metastasis progression associated with the hippocampal preservation. In our study, no statistically significant difference was observed in intracranial PFS (including iLPFS and iDPFS) between patients with and without hippocampal preservation in the WBRT-SIB group, suggesting that the selective hippocampal preservation might reduce the influence of hippocampal preservation on the prognosis of the WBRT-SIB group.
Boosting the local dose of BM resulted in a significant improvement in intracranial control rate compared to WBRT alone. RTOG 9508 [27], a phase III clinical trial for patients with 1–3 BM, showed that the treatment of WBRT + SRS could improve significantly iPFS rate at 1 year compared to WBRT-alone (82% vs. 71%, p = 0.0132). However, WBRT-SIB has many advantages over WBRT followed by stereotactic radiosurgery (WBRT + SRS), including its simplicity, shorter treatment time, lower economic cost, being unrestricted by the number and volume of BM (90% of patients in our study were multiple BM), and protection of organs and structures at risk [28]. Moreover, the dose fractionation mode of WBRT-SIB confers radiobiological advantages by reducing tumor cell reproliferation and redistribution [29]. In a retrospective comparison study, Lin et al. [30] found that the WBRT-SIB group(45 Gy/10 fractions) had a significantly longer median iPFS (9.1 vs. 5.9 months, p = 0.001) and a lower iDPFS rate (39.4% vs. 75.0%, p = 0.004) compared to the WBRT + SRS group(WBRT of 30 Gy/10 fractions followed by SRS of 16–24 Gy). Moreover, WBRT-SIB is suitable for medical institutions without specialized equipment for SRS, regardless of the number of brain metastases being multiple or limited.
No significant difference was observed in OS between the WBRT-SIB group and the WBRT-alone group (22.2 months vs. 19.0 months, p = 0.768), which contradicts some previous studies [18, 31]. Currently, brain RT and systemic therapy can extend the median iPFS of BM to over six months. Consequently, extracranial factors contribute to death for these advanced cancer patients rather than intracranial tumor progression. Many previous studies showed no significant difference in OS for BM patients when comparing WBRT and WBRT + SRS, or WBRT-SIB and WBRT + SRS, although these studies indicated that WBRT + SRS and WBRT-SIB were associated with a better iPFS [21, 27, 30, 32, 33]. These studies support the opinion that factors influencing OS are not limited to the control of BM.
The reasons for the inconsistency between our study and previous studies, as well as the lack of OS benefit from the improved iPFS in the WBRT-SIB group, may include: (1) all baseline characteristics in this study showed no statistically significant differences between the two groups after propensity score matching; (2) The WBRT-alone group received more active local treatment measures for BM after intracranial progression comparing with the WBRT-SIB group (SRS/surgery:16% vs. 8.7%). (3) 58%(n = 80) of patients in our study received targeted/immune/temozolomide therapy after RT, which was much higher than in similar studies (approximately 30% in Popp’s study). Additionally, in our study, 34 patients (24.6%) received multiple types of systemic drugs after RT, and 43 patients (31.2%) received systemic drugs after intracranial progression as salvage therapy. On the one hand, some systemic drugs involved in our study have demonstrated the ability to cross the blood-brain barrier and exert an anti-tumor effect, thereby prolonging the OS of patients with BM [34–37]. On the other hand, the disruption of the blood-brain barrier induced by brain RT can facilitate drug delivery to the brain tissue and strengthen the control over BM [38].
It is noteworthy that the subgroup of " infratentorial with or without supratentorial metastasis” was the only subgroup benefiting from SIB in terms of OS, and SIB was independently associated with better OS. This may represent a novel finding. The infratentorial metastasis has a higher risk of obstructive hydrocephalus, compression and injury of the brainstem, and transforamen magna herniation [39], which makes infratentorial with/without supratentorial metastasis more lethal than just supratentorial metastasis. In addition, previous studies showed that infratentorial metastasis is associated with younger age, male gender, lung neuroendocrine and squamous cell carcinomas, high Ki67 expression, and poor prognosis for patients [40]. The infratentorial region is more prone to invasive tumors [41]. In our study, WBRT-SIB can significantly improve the control rate of infratentorial metastasis, thereby translating into better OS for patients. However, the subgroup analysis was exploratory and further prospective studies are needed to determine whether the addition of SIB could improve OS in patients with infratentorial metastasis.
However, the current study has certain limitations. First, there was inevitable selection bias in single-center retrospective study, and multiple endpoints and subgroup analyses may increase the risk of false positives. Second, the baseline characteristics between the two groups were not completely balanced after propensity matching. Moreover, post-radiotherapy treatment was also a prognostic factor, but due to its highly individualized selection, it was difficult to achieve balance between the two groups. Third, the data in our study spanned from 2015 to 2019, with follow-up concentrated in January 2023. Obtaining information on delayed radiation brain injury for most deceased patients was difficult. Despite these limitations, this study can provide a reference for the clinical treatment of BM, but a more scientific and rigorous prospective study is needed to explore and optimize the treatment of BM.
Conclusion
WBRT-SIB is a promising strategy for patients with BM. Compared with WBRT alone, WBRT-SIB can significantly prolong the intracranial PFS (including local and distant PFS), especially for patients with multiple brain metastases. Additionally, while WBRT-SIB did not improve OS in the entire cohort, the OS benefit for patients with BM accompanied by infratentorial involvement warrants further exploration; WBRT-SIB does not increase the acute brain radiation reactions.
Acknowledgements
We thank the specific colleagues and institutions for the contributions of editing and reviewing the manuscript.
Abbreviations
- WBRT-SIB
Whole brain radiation therapy with simultaneous integrated boost
- WBRT
Whole brain radiation therapy
- OS
Overall survival
- BMs
Brain metastase
- iPFS
Intracranial progression-free survival
- iLPFS
Intracrania local progression-free survival
- iDPFS
Intracrania distant progression-free survival
- SRS
Stereotactic radiosurgery
- IMRT
Intensity-modulated radiotherapy
- VMAT
Volume intensity arc therapy
- TOMO
Helical tomotherapy
- RT
Radiotherapy
- KPS
Karnofsky performance status
- 3D-CRT
Three dimensional conformal radiation therapy
- GTV
Gross tumor volume
- CTV
Clinical target volume
- PTV
Planning tumor volume
- CTCAE
The common terminology criteria for adverse events
- DS-GPA
The diagnosis-specific graded prognostic assessment
Author contributions
ZL and XD were responsible for designing, writing the manuscript, and collecting the data. ZL analyzed the data and created the charts. MD and YC provided suggestions for revisions and review of the manuscript. SW designed and revised the manuscript. All authors contributed to the article and approved the submitted version.
Funding
The study was not funded.
Data availability
Data analyzed for this paper cannot be shared on a publicly available repository due to data protection regulations. According to the local ethics committee, only the evaluation of anonymized data is allowed for this study, and it is available from the corresponding author(Shaoxiong Wu, E-mail: wushx@sysucc.org.cn) on reasonable request.
Declarations
Ethics approval and consent to participate
In accordance with ICH-GCP principles and relevant Chinese regulations/guidelines, the research proposal has been reviewed and approved by the Ethics Committee of Sun Yat-sen University Cancer Center for the conduct of this research. The Declaration of Helsinki, Ethical Review of Biomedical Research involving Human Subjects and other relevant international guidelines were strictly followed during the study. Informed consent was obtained from all subjects involved in the study.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Zheqing Liu and Xiaojing Du contributed equally to this work.
References
- 1.Siegel R, Ma J, Zou Z, Jemal A, Cancer statistics. 2014. CA A Cancer J Clinicians. 2014 [cited 2024 Apr 5];64:9–29. Available from: https://acsjournals.onlinelibrary.wiley.com/doi/10.3322/caac.21208
- 2.Larson D, Sahgal A. Adjuvant whole brain radiotherapy: strong emotions decide but rationale studies are needed: in regard to, Brown et al. Int J Radiat Oncol Biol Phys. (2008;70:1305–1309). Int J Radiat Oncol Biol Phys. 2008;72:959. [DOI] [PubMed]
- 3.Verger E, Gil M, Yaya R, Viñolas N, Villà S, Pujol T, et al. Temozolomide and concomitant whole brain radiotherapy in patients with brain metastases: a phase II randomized trial. Int J Radiat Oncol Biol Phys. 2005;61:185–91. [DOI] [PubMed] [Google Scholar]
- 4.Sperduto PW, Wang M, Robins HI, Schell MC, Werner-Wasik M, Komaki R, et al. A phase 3 trial of whole brain radiation therapy and stereotactic radiosurgery alone versus WBRT and SRS with Temozolomide or erlotinib for non-small cell lung cancer and 1 to 3 brain metastases: radiation therapy oncology group 0320. Int J Radiat Oncol Biol Phys. 2013;85:1312–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Roos DE, Smith JG, Stephens SW. Radiosurgery versus surgery, both with adjuvant whole brain radiotherapy, for solitary brain metastases: a randomised controlled trial. Clin Oncol (R Coll Radiol). 2011;23:646–51. [DOI] [PubMed] [Google Scholar]
- 6.Dawe DE, Greenspoon JN, Ellis PM. Brain Metastases in Non–Small-Cell Lung Cancer. Clinical Lung Cancer. 2014 [cited 2024 Apr 5];15:249–57. Available from: https://linkinghub.elsevier.com/retrieve/pii/S1525730414000825 [DOI] [PubMed]
- 7.Jc AMBWAS, Hj T, Fw S. K. Microsurgery plus whole brain irradiation versus Gamma Knife surgery alone for treatment of single metastases to the brain: a randomized controlled multicentre phase III trial. Journal of neuro-oncology. J Neurooncol; 2008 [cited 2024 Apr 5];87. Available from: https://pubmed.ncbi.nlm.nih.gov/18157648/ [DOI] [PubMed]
- 8.Aoyama H, Shirato H, Tago M, Nakagawa K, Toyoda T, Hatano K, et al. Stereotactic radiosurgery plus whole-brain radiation therapy vs stereotactic radiosurgery alone for treatment of brain metastases: a randomized controlled trial. JAMA. 2006;295:2483–91. [DOI] [PubMed] [Google Scholar]
- 9.Rades D, Raabe A, Bajrovic A, Alberti W. Treatment of solitary brain metastasis. Resection followed by whole brain radiation therapy (WBRT) and a radiation boost to the metastatic site. Strahlenther Onkol. 2004;180:144–7. [DOI] [PubMed] [Google Scholar]
- 10.Aoyama H, Tago M, Shirato H. Japanese radiation oncology study group 99 – 1 (JROSG 99 – 1) investigators. Stereotactic radiosurgery with or without Whole-Brain radiotherapy for brain metastases: secondary analysis of the JROSG 99 – 1 randomized clinical trial. JAMA Oncol. 2015;1:457–64. [DOI] [PubMed] [Google Scholar]
- 11.Ni L, Liang X. Feasibility of simultaneous integrated boost with forward intensity-modulated radiation therapy for multiple brain metastases. wo. 2014 [cited 2023 Sep 9];3:187–91. Available from: http://www.termedia.pl/doi/10.5114/wo.2014.43156 [DOI] [PMC free article] [PubMed]
- 12.Jiang A, Sun W, Zhao F, Wu Z, Shang D, Yu Q et al. Dosimetric evaluation of four whole brain radiation therapy approaches with hippocampus and inner ear avoidance and simultaneous integrated boost for limited brain metastases. Radiat Oncol. 2019 [cited 2023 Sep 9];14:46. Available from: https://ro-journal.biomedcentral.com/articles/10.1186/s13014-019-1255-7 [DOI] [PMC free article] [PubMed]
- 13.Ferro M, Chiesa S, Macchia G, Cilla S, Bertini F, Frezza G et al. Intensity Modulated Radiation Therapy With Simultaneous Integrated Boost in Patients With Brain Oligometastases: A Phase 1 Study (ISIDE-BM-1). International Journal of Radiation Oncology*Biology*Physics. 2017 [cited 2023 Sep 9];97:82–90. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0360301616332035 [DOI] [PubMed]
- 14.Liang X, Ni L, Hu W, Chen W, Ying S, Gong Q et al. A planning study of simultaneous integrated boost with forward IMRT for multiple brain metastases. Medical Dosimetry. 2013 [cited 2023 Sep 9];38:115–6. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0958394712001434 [DOI] [PubMed]
- 15.Zhong J, Waldman AD, Kandula S, Eaton BR, Prabhu RS, Huff SB, et al. Outcomes of whole-brain radiation with simultaneous in-field boost (SIB) for the treatment of brain metastases. J Neurooncol. 2020;147:117–23. [DOI] [PubMed] [Google Scholar]
- 16.Lebow ES, Hwang WL, Zieminski S, Wang Y, Niemierko A, Mehan WA et al. Early experience with hippocampal avoidance whole brain radiation therapy and simultaneous integrated boost for brain metastases. J Neurooncol. 2020 [cited 2023 Sep 9];148:81–8. Available from: https://link.springer.com/10.1007/s11060-020-03491-y [DOI] [PubMed]
- 17.Al VPNWFFMSCN. G. Whole brain irradiation with hippocampal sparing and dose escalation on multiple brain metastases: a planning study on treatment concepts. International journal of radiation oncology, biology, physics. Int J Radiat Oncol Biol Phys; 2013 [cited 2023 Sep 9];85. Available from: https://pubmed.ncbi.nlm.nih.gov/22516808/ [DOI] [PubMed]
- 18.Popp I, Rau S, Hintz M, Schneider J, Bilger A, Fennell JT et al. Hippocampus-avoidance whole‐brain radiation therapy with a simultaneous integrated boost for multiple brain metastases. Cancer. 2020 [cited 2022 Oct 7];126:2694–703. Available from: https://onlinelibrary.wiley.com/doi/10.1002/cncr.32787 [DOI] [PubMed]
- 19.Hauswald H, Bernhardt D, Krug D, Katayama S, Habl G, Lorenzo Bermejo J, et al. Whole-brain helical tomotherapy with integrated boost for brain metastases in patients with malignant melanoma - final results of the BRAIN-RT trial. Cancer Manag Res. 2019;11:4669–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lin NU, Lee EQ, Aoyama H, Barani IJ, Barboriak DP, Baumert BG, et al. Response assessment criteria for brain metastases: proposal from the RANO group. Lancet Oncol. 2015;16:e270–278. [DOI] [PubMed] [Google Scholar]
- 21.Lu F, Hou Y, Xia Y, Li L, Wang L, Cao K et al. Survival and intracranial control outcomes of whole-brain radiotherapy (WBRT) alone versus WBRT plus a radiotherapy boost in non-small-cell lung cancer with brain metastases: a single-institution retrospective analysis. Cancer Manag Res. 2019 [cited 2023 Jan 20];11:4255–72. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6512646/ [DOI] [PMC free article] [PubMed]
- 22.Kd W, Jt M et al. T D, K K, A G, S P,. Phase II trial of hippocampal-sparing whole brain irradiation with simultaneous integrated boost for metastatic cancer. Neuro-oncology. Neuro Oncol; 2020 [cited 2023 Apr 6];22. Available from: https://pubmed.ncbi.nlm.nih.gov/32347302/ [DOI] [PMC free article] [PubMed]
- 23.Brown PD, Gondi V, Pugh S, Tome WA, Wefel JS, Armstrong TS, et al. Hippocampal avoidance during Whole-Brain radiotherapy plus memantine for patients with brain metastases: phase III trial NRG oncology CC001. J Clin Oncol. 2020;38:1019–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rodríguez de Dios N, Couñago F, Murcia-Mejía M, Rico-Oses M, Calvo-Crespo P, Samper P, et al. Randomized phase III trial of prophylactic cranial irradiation with or without hippocampal avoidance for Small-Cell lung Cancer (PREMER): A GICOR-GOECP-SEOR study. J Clin Oncol. 2021;39:3118–27. [DOI] [PubMed] [Google Scholar]
- 25.Gondi V, Tome WA, Marsh J, Struck A, Ghia A, Turian JV et al. Estimated risk of perihippocampal disease progression after hippocampal avoidance during whole-brain radiotherapy: Safety profile for RTOG 0933. Radiotherapy and Oncology. 2010 [cited 2023 Sep 9];95:327–31. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0167814010001659 [DOI] [PMC free article] [PubMed]
- 26.Ahn SJ, Kwon H, Kim JW, Park G, Park M, Joo B, et al. Hippocampal metastasis rate based on Non-Small lung Cancer TNM stage and molecular markers. Front Oncol. 2022;12:781818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Andrews DW, Scott CB, Sperduto PW, Flanders AE, Gaspar LE, Schell MC, et al. Whole brain radiation therapy with or without stereotactic radiosurgery boost for patients with one to three brain metastases: phase III results of the RTOG 9508 randomised trial. Lancet. 2004;363:1665–72. [DOI] [PubMed] [Google Scholar]
- 28.Gondi V, Pugh SL, Tome WA, Caine C, Corn B, Kanner A et al. Preservation of Memory With Conformal Avoidance of the Hippocampal Neural Stem-Cell Compartment During Whole-Brain Radiotherapy for Brain Metastases (RTOG 0933): A Phase II Multi-Institutional Trial. J Clin Oncol. 2014 [cited 2023 Mar 13];32:3810–6. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4239303/ [DOI] [PMC free article] [PubMed]
- 29.Borghetti P, Pedretti S, Spiazzi L, Avitabile R, Urpis M, Foscarini F et al. Whole brain radiotherapy with adjuvant or concomitant boost in brain metastasis: dosimetric comparison between helical and volumetric IMRT technique. Radiat Oncol. 2016 [cited 2022 Oct 17];11:59. Available from: http://ro-journal.biomedcentral.com/articles/10.1186/s13014-016-0634-6 [DOI] [PMC free article] [PubMed]
- 30.Lin B, Huang D, Du H, Fan J, Zhang Y, Feng G et al. Whole-Brain Radiation Therapy With Simultaneous Integrated Boost Versus Whole-Brain Radiation Therapy Plus Stereotactic Radiosurgery for the Treatment of Brain Metastasis From Lung Cancer. Front Oncol. 2021 [cited 2022 Oct 7];11:631422. Available from: https://www.frontiersin.org/articles/10.3389/fonc.2021.631422/full [DOI] [PMC free article] [PubMed]
- 31.Du T-Q, Li X, Zhong W-S, Tian J-D, Zhao Y-X, Liu D. Brain metastases of lung cancer: comparison of survival outcomes among whole brain radiotherapy, whole brain radiotherapy with consecutive boost, and simultaneous integrated boost. J Cancer Res Clin Oncol. 2021 [cited 2022 Oct 7];147:569–77. Available from: https://link.springer.com/10.1007/s00432-020-03359-8 [DOI] [PMC free article] [PubMed]
- 32.D R, Mt SJAB, Se KTV. S. A matched-pair analysis comparing whole-brain radiotherapy with and without a stereotactic boost for intracerebral control and overall survival in patients with one to three cerebral metastases. Radiation oncology (London, England). Radiat Oncol; 2017 [cited 2023 Jan 26];12. Available from: https://pubmed.ncbi.nlm.nih.gov/28438175/ [DOI] [PMC free article] [PubMed]
- 33.Rodrigues G, Zindler J, Warner A, Bauman G, Senan S, Lagerwaard F. Propensity-score matched pair comparison of whole brain with simultaneous in-field boost radiotherapy and stereotactic radiosurgery. Radiother Oncol. 2013;106:206–9. [DOI] [PubMed] [Google Scholar]
- 34.Jiang S, Liang H, Liu Z, Zhao S, Liu J, Xie Z, et al. The impact of anlotinib on brain metastases of Non-Small cell lung cancer: post hoc analysis of a phase III randomized control trial (ALTER0303). Oncologist. 2020;25:e870–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lin NU, Borges V, Anders C, Murthy RK, Paplomata E, Hamilton E, et al. Intracranial efficacy and survival with Tucatinib plus trastuzumab and capecitabine for previously treated HER2-Positive breast Cancer with brain metastases in the HER2CLIMB trial. J Clin Oncol. 2020;38:2610–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yang J-J, Zhou C, Huang Y, Feng J, Lu S, Song Y, et al. Icotinib versus whole-brain irradiation in patients with EGFR-mutant non-small-cell lung cancer and multiple brain metastases (BRAIN): a multicentre, phase 3, open-label, parallel, randomised controlled trial. Lancet Respir Med. 2017;5:707–16. [DOI] [PubMed] [Google Scholar]
- 37.Bi N, Ma Y, Xiao J, Zhang H, Xu Y, Tian Y, et al. A phase II trial of concurrent Temozolomide and hypofractionated stereotactic radiotherapy for complex brain metastases. Oncologist. 2019;24:e914–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Allen BD, Limoli CL. Breaking barriers: neurodegenerative repercussions of radiotherapy induced damage on the blood-brain and blood-tumor barrier. Free Radic Biol Med. 2022;178:189–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rogne SG, Helseth E, Brandal P, Scheie D, Meling TR. Are melanomas averse to cerebellum? Cerebellar metastases in a surgical series. Acta Neurol Scand. 2014;130:1–10. [DOI] [PubMed] [Google Scholar]
- 40.Dou Z, Wu J, Wu H, Yu Q, Yan F, Jiang B, et al. The infratentorial localization of brain metastases May correlate with specific clinical characteristics and portend worse outcomes based on Voxel-Wise mapping. Cancers (Basel). 2021;13:324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Emery A, Trifiletti DM, Romano KD, Patel N, Smolkin ME, Sheehan JP. More than just the number of brain metastases: evaluating the impact of brain metastasis location and relative volume on overall survival after stereotactic radiosurgery. World Neurosurg. 2017;99:111–7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data analyzed for this paper cannot be shared on a publicly available repository due to data protection regulations. According to the local ethics committee, only the evaluation of anonymized data is allowed for this study, and it is available from the corresponding author(Shaoxiong Wu, E-mail: wushx@sysucc.org.cn) on reasonable request.