Abstract
Background
Bell’s palsy (BP) is the most prevalent acute mono-neuropathy associated with facial nerve failure, accounting for 60–75% of all occurrences of facial paralysis. The primary pathophysiological mechanism leading to BP is recognized as the confinement of the facial nerve within the facial canal, caused by edema. The link between vitamin D and neurological disorders has drawn more scientific attention in recent years, and vitamin D plays a role as a neuro-immuno-modulator. While some studies suggest an association between vitamin D and the incidence of BP, others report no significant impact. This systematic review aims to critically assess the relationship between vitamin D levels and the severity of BP.
Method
A comprehensive search was conducted across Scopus, PubMed, and Web of Science databases for articles published from inception until September 29, 2024. The inclusion criteria for this meta-analysis encompassed articles that reported serum vitamin D levels and vitamin D deficiency in patients with BP, compared with the control group, specifically those presenting original data from cross-sectional, case–control, and cohort studies. Vitamin D levels in BP patients were reported as mean ± SD. Meta-analyses were performed using Stata version 17 software (Stata Corp, College Station, TX), with effect sizes expressed as standardized mean differences (Hedges g) and 95% confidence intervals (CIs).
Result
Three studies reported the mean vitamin D levels among BP subjects, with the pooled mean vitamin D level of 15.98 ng/ml (CI = 13.83–18.06). No significant difference was observed for grade 2 vs controls (Hedges'g = 0.05, 95% CI = [-0.38 to 0.49], P value = 0.79, I2 = 0%), and grade 3 vs. controls (Hedges'g = -0.18, 95% CI = [-0.55 to 0.18], P value = 0.32, I2 = 0%). In contrast, the meta-analysis showed a significantly decreased value for vitamin D across grade 4 of the disease (Hedges'g = -0.72, 95% CI = [-1.18 to -0.27], P value = 0.001, I2 = 0%).
Conclusion
In this systematic review and meta-analysis, we evaluate the relationship between serum vitamin D levels in patients with BP. Our meta-analysis showed a significant decrease in Vitamin D levels in patients with high-grade BP. Our meta-analysis results show that a high grade of BP is associated with lower serum vitamin D levels. Given that inflammation is involved in the pathophysiological background of BP, the anti-inflammatory effect of vitamin D can be a possible mechanism for the benefit of this vitamin in BP.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12883-025-04268-4.
Keywords: Bell’s palsy (BP); Vitamin D; 1,25-dihydroxy vitamin D; Facial paralysis
Introduction
Bell's palsy (BP), which bears the name of Scottish anatomist Sir Charles Bell, is a peripheral facial nerve disorder that develops suddenly [1]. BP patients often first notice facial weakness, sometimes accompanied by drooling. Concurrently, patients may experience ear pain and cheek paresthesia on the same side. Taste abnormalities and, less commonly, hyperacusis are additional symptoms [2]. It is the most prevalent acute mononeuropathy and the most common diagnosis associated with facial nerve failure, accounting for 60–75% of all occurrences of facial paralysis [3]. Its annual incidence rate varies from 11.5 to 53.3 per 100,000 people in different communities. This condition affects people of all ages and genders and is similarly prevalent in both sexes [4]. The severity of facial nerve palsy is typically assessed using the six-point House-Brackmann (HB) scale, ranging from grade I (normal function) to grade VI (complete paralysis) [2]. History taking and clinical neurological examination may not necessarily indicate the causes but are enough for typical peripheral facial nerve palsy cases. In Atypical cases, more diagnostic evaluations, including Magnetic Resonance Imaging (MRI) or Lumbar puncture (LP), are needed, like in areas of Lyme serology [5]. The primary pathophysiological mechanism leading to BP is recognized as the confinement of the facial nerve within the facial canal, caused by edema. Nevertheless, the specific trigger for this inflammation remains undiscovered [6]. Several potential risk factors have been proposed, including Herpes Simplex Virus (HSV) infection, impaired blood flow in the vasa nervorum, ischemic nerve damage, autoimmune responses, and genetic susceptibility. Among these factors, HSV infection is considered the most prominent [6]. Meanwhile, recent studies show that there is an association between vitamin D and peripheral neuropathy [7, 8] and the relationship between vitamin D and the health of peripheral nerves has been recently noticed [8].
Vitamin D is a fat-soluble steroid hormone that UV radiation produces on the skin. It stimulates the production of central nervous system genes and maintains calcium homeostasis. Research shows that 1,25-(OH)D targets various nervous system tissues and is a neuro-immuno-modulator. Vitamin D is linked to neurological disorders like multiple sclerosis [9], Parkinson's disease [10], and Alzheimer's disease [11], with patients in these conditions typically having lower serum vitamin D levels than healthy controls. Vitamin D influences various immune-related cells in the nervous system, including microglia and astrocytes. These cells facilitate communication between the immune system and the CNS, playing a crucial role in modulating the nervous system's immune microenvironment. [12, 13]. Additionally, vitamin D stimulates microglia and astrocytes to release specific factors that suppress neuroinflammation [14, 15].Vitamin D, a neurotrophic hormone, protects the brain by enhancing VDRs expression and reducing L-type calcium channel expression. It improves axonogenesis, sensory neural response, and electrophysiological recovery. Ergocalciferol stimulates axonal regeneration and enlarges axonal diameter, possibly due to its regulatory impact on neurotrophic factors. However, its role in myelination remains unexplored, as VDR is found in oligodendrocytes and Schwann cells [16].
Increasing evidence has shown that individuals with BP have lower levels of 25-hydroxyvitamin (25[OH]D) relative to healthy controls, and vitamin D deficiency has been proposed to be linked to disease grading [17–19]. At the same time, some studies suggest no association between vitamin D level and BP severity [20]. Thus, considering all the applications of vitamin D, it is essential to see if it has a role in predicting the future of BP and grading [21–24]. This systematic review and meta-analysis aim to critically assess the relationship between vitamin D levels and the incidence and severity of facial paralysis in BP patients.
Method
The present systematic review and meta-analysis adhered to the PRISMA [25]guidelines and was prospectively registered with the Open Science Framework (OSF), under the assigned registration DOI: https://doi.org/10.17605/OSF.IO/3B4VY.
Search strategy
A comprehensive search was conducted across Scopus, PubMed, and Web of Science databases, to identify clinical trials examining the association between vitamin D and Bell’s palsy in articles published from inception until 29 September 2024. Each article was evaluated separately. We reviewed the titles and abstracts of potential studies according to our inclusion and exclusion criteria to eliminate irrelevant publications. The search strategy employed the terms “vitamin D” and “25-Hydroxyvitamin D2” “Hydroxycholecalciferols” and “Cholecalciferol” and “Dihydrotachysterol” and “Ergocalciferolsl” and “Facial paralysis” and “Facial neuropathy” and “Facial nerve injury” and “Hemifacial spasm” and “Facial palsy” and “Bell’s palsy” (All items are detailed in Supplementary Table 1). EndNote software was utilized to manage and remove duplicate articles. Additionally, reference lists of the selected articles were manually reviewed to ensure thoroughness.
Study selection
Two reviewers (KST and MM) independently screened the articles by titles and abstracts. Articles that did not meet the eligibility criteria, as well as duplicates, were excluded. The full texts of the remaining articles were then reviewed to determine their inclusion. Any discrepancies were resolved through discussion between the reviewers to reach a consensus.
Inclusion and exclusion criteria
The inclusion criteria for this meta-analysis encompassed human and original studies that investigated the effect of serum Vitamin D on the severity of facial paralysis in patients with BP compared to patients without BP, specifically those presenting primary studies from cross-sectional, case–control, and cohort studies. Conversely, review articles, meta-analyses, conference abstracts, or unpublished and gray literature were excluded. One of the included articles was in Indonesian, which was translated to English by Chat GPT 4.
Data extraction
The following data were extracted from the eligible studies:
Information about study specifics, including author name, publication year, country, and study design.
Details regarding case studies, such as total sample size, age of BP patients and control groups, and sex of patients in both groups.
Information on vitamin D serum levels and measurement methods, including serum vitamin D levels in patients with BP and control groups and the percentage of vitamin D deficiency in both groups.
Key findings of each study concerning vitamin D levels and their role in BP grading and severity.
Quality assessment
The methodological quality of the included studies was assessed using the Joanna Briggs Institute (JBI) Critical Appraisal Checklists for cross-sectional, case–control, and cohort studies [26]. These tools provide a structured framework to evaluate studies'reliability, validity, and applicability based on their design and objectives.
The answers to the checklist were used to determine each study's overall quality. Studies were categorized as high quality if their JBI they received a “yes” answer for more than 70% of questions, medium quality if they received a “yes” answer for between 50 and 70% of questions, and low quality if received a “yes” for less than 50% of questions. The included studies were all of high quality. The assessment findings are summarized in Table 1.
Table 1.
Quality assessment
| Author, Year | Q1 | Q2 | Q3 | Q4 | Q5 | Q6 | Q7 | Q8 | - | - | - |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Uysal (2021) [19] | ✓ | ✓ | ! | ✓ | ✓ | ✓ | ! | ✓ | - | - | - |
| Ocak (2020) [20] | ✓ | ✓ | ✓ | ✓ | ! | × | ✓ | ✓ | - | - | - |
| JBI Checklist Questions for the case–control studies. Questions of the checklist are indexed in the table as Q1-Q8, and are written here: Q1) Were the criteria for inclusion in the sample clearly defined? Q2) Were the study subjects and the setting described in detail? Q3) Was the exposure measured validly and reliably? Q4). Were objective, standard criteria used for measurement of the condition? Q5) Were confounding factors identified? Q6) Were strategies to deal with confounding factors stated? Q7) Were the outcomes measured validly and reliably? Q8) Was appropriate statistical analysis used? Yes =, No = ×, not applicable =! | |||||||||||
| Author, Year | Q1 | Q2 | Q3 | Q4 | Q5 | Q6 | Q7 | Q8 | Q9 | Q10 | Q11 |
| Göker (2020) [17] | ✓ | ✓ | ! | ✓ | ✓ | × | ! | ✓ | ✓ | ✓ | ✓ |
| JBI Checklist Questions for the cohort studies. Questions of the checklist are indexed in the table as Q1-Q11, and are written here: Q1) Were the two groups similar and recruited from the same population? Q2) Were the exposures measured similarly to assign people to both exposed and unexposed groups? Q3) Was the exposure measured in a valid and reliable way? Q4) Were confounding factors identified? Q5) Were strategies to deal with confounding factors stated? Q6) Were the groups/participants free of the outcome at the start of the study (or at the moment of exposure)? Q7) Were the outcomes measured validly and reliably? Q8) Was the follow-up time reported and sufficient to be long enough for outcomes to occur? Q9) Was follow-up complete, and if not, were the reasons to loss to follow-up described and explored? Q10) Were strategies to address incomplete follow-up utilized? Q11) Was appropriate statistical analysis used? Yes =, No = ×, not applicable =! | |||||||||||
| Author, Year | Q1 | Q2 | Q3 | Q4 | Q5 | Q6 | Q7 | Q8 | Q9 | Q10 | - |
| Sinta (2023) [18] | ✓ | ✓ | ! | ✓ | ✓ | × | ! | ✓ | ✓ | ✓ | - |
| JBI Checklist Questions for the cross-sectional studies. Questions of the checklist are indexed in the table as Q1-Q10, and are written here: Q1) Were the groups comparable other than the presence of disease in cases or the absence of disease in controls? Q2) Were cases and controls matched appropriately? Q3) Were the same criteria used for identification of cases and controls? Q4) Was exposure measured in a standard, valid and reliable way? Q5) Was exposure measured in the same way for cases and controls? Q6) Were confounding factors identified? Q7) Were strategies to deal with confounding factors stated? Q8) Were outcomes assessed in a standard, valid and reliable way for cases and controls? Q9) Was the exposure period of interest long enough to be meaningful? Q10) Was appropriate statistical analysis used? Yes =, No = ×, not applicable =! | |||||||||||
Statistical analysis
Vitamin D levels in BP patients were reported as mean ± SD. Meta-analyses were performed using Stata version 17 software (StataCorp, College Station, TX), with effect sizes expressed as standardized mean differences (Hedges g) and 95% confidence intervals (CIs). Heterogeneity among the studies was assessed using Higgins'I2 test [27], where a heterogeneity level below 40% is deemed insignificant, as per the Cochrane Handbook. In such cases, a fixed-effects approach was used for the analysis, while a fixed-effects approach was used if I2 exceeded 40%.
Due to the limited pooled studies, meta-regression and subgroup analyses were not feasible. P-values below 0.05 were considered statistically significant.
Since a maximum of two studies were included in each pair of meta-analyses, the publication bias tests could not be statistically conducted.
Results
Search results
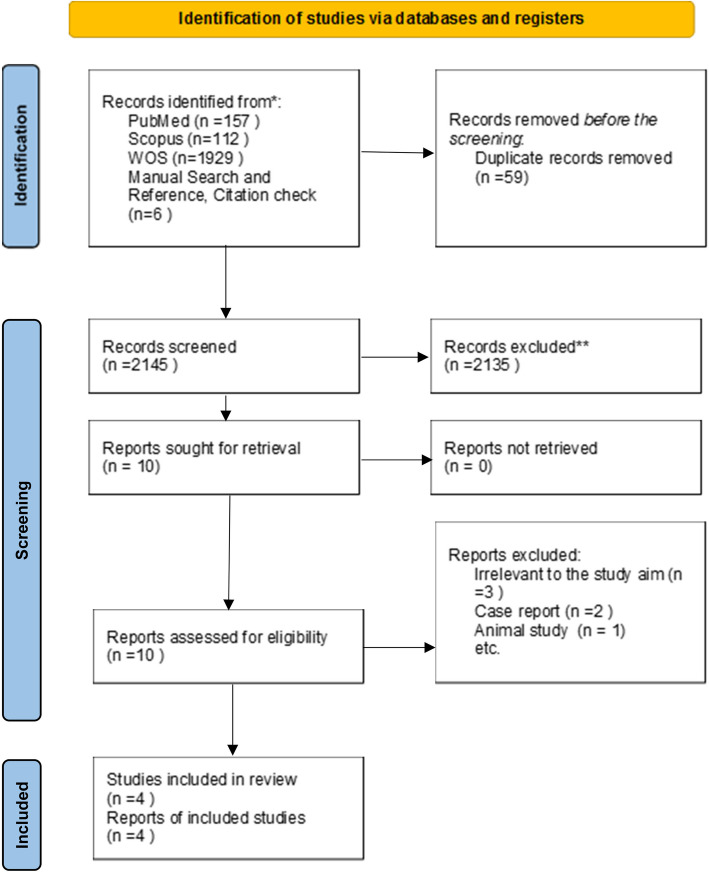
Following the systematic searches in four databases, 2204 studies were recognized. Fifty-nine duplicate studies were omitted. After the screening of the title and abstract independently by two reviewers, 2135 studies were excluded. The remaining articles were screened based on the eligibility criteria. Finally, four studies were included in a systematic review, and four studies provided data for the meta-analysis. Full text of one study translated to English by chat gpt4 [18].
PRISMA flow diagram showing the studies at each stage of the screening process. (Fig. 1).
Fig. 1.
PRISMA diagram flow chart
Study characteristics
Table 2 presents the key characteristics of the included studies. The severity of BP in patients was assessed using the HB scale. All the studies measured the level of 25-hydroxyvitamin D [17–20]. The sample sizes of the studies included in the analysis varied from 20 to 91 participants. In total, 193 individuals were examined for the case versus control comparison. The mean age of participants in this analysis ranged from 38.77 to 42.25 years. Regarding gender composition among participants, most groups had a predominance of females. The exception was the patient group in one study, which included 19 females and 30 males [17], and the control group in the Ocak et al. study with 20 females and 28 males [20].
Table 2.
Characteristic findings of the included studies
| Author | Year | Country | Study design | Number of patients with Bell's Palsy | Number of the control group | Age in Bell's Palsy (Mean Age: years) | Age in control (Mean Age: years) | Vitamin D method | Male with Bell's Palsy | Male in control group | Female with Bell's Palsy | Female in control group | Serum vitamin D in Bell's Palsy (ng/mL) | Serum vitamin D in control group (ng/mL) | Vitamin D deficiency% in Bell's Palsy | Main findings |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Göker et al. [17] | 2020 | Turkey | Prospective Cohort Study | 49 | 42.2 ± 13.6 | chemiluminescence method (using the Beckman Coulter UniCel DxI 800 (Beckman Coulter Inc., CA, USA) device) | 30 | 19 |
Grade 2 (n = 11): 24.0 ± 9.4 Grade 3 (n = 21): 18.2 ± 6.0 Grade ≥ 4 (n = 17): 10.8 ± 4.4 |
35%: Grade ≥ 4 group | In individuals with Bell's palsy, vitamin D may promote healing, prevent peripheral facial paralysis, and hasten the return of face neuronal function. Particularly in regions with severe vitamin D insufficiency, this supports public health initiatives to raise vitamin D status, including dietary supplements | |||||
| Uysal et al. [19] | 2021 | Turkey | Retrospective case–control study | 52 | 50 | 42.25 ± 16.09 | 40.76 ± 14.52 | 25 | 25 | 27 | 25 | 14.37 ± 7.60 | 18.84 ± 9.38 | 42.3%: Grade ≥ 4 group | Vitamin D levels may be linked to BP development, and low levels can increase disease severity. BP patients should be checked for vitamin D deficiency and treated if detected. Increased awareness can accelerate low-grade patient admission to health institutions | |
| Ocak et al. [20] | 2020 | Turkey | Prospective case–control study | 43 | 48 | 41.88 | 38.77 | 20 | 28 | 23 | 20 | 15.96 ± 9.00 | 18.61 ± 19.01 | 23.3%: Grade ≥ 4 group | Vitamin D concentrations in the BP and control groups were comparable. Vitamin D levels were decreased in patients with advanced HB grades in the Bells Palsy group. The findings suggested a potential impact of vitamin D levels on Bells Palsy prognosis. Recuperation may be negatively impacted by low vitamin D levels, especially in patients with advanced grades | |
| Sinta et al. [18] | 2023 | Indonesia | Cross-sectional study | 20 | 48.8 ± 12.97 | Chemiluminescent Microparticle Immunoassay (CMIA) and Electro-Chemiluminescence Immunoassay (ECLIA) methods | 10 | 10 | 18.06 ± 6.53 | 65% | The clinical severity of Bell's palsy is significantly correlated with blood vitamin D levels. The patient's clinical severity increases with decreasing blood levels of vitamin D |
Two studies administered standard therapy for participants with vitamin D deficiency (loading dose: 50,000 IU cholecalciferol per week for 8 weeks, maintenance dose: 1500 IU) [17, 20]. Two types of analyses were conducted between the studies. One analysis compared cases and controls across the two studies [19, 20]. Other analyses were performed separately between patients with BP grades 2 to 4 and the controls, as well as comparisons between different grading levels [17–20].
Comparing vitamin D levels between patients and control
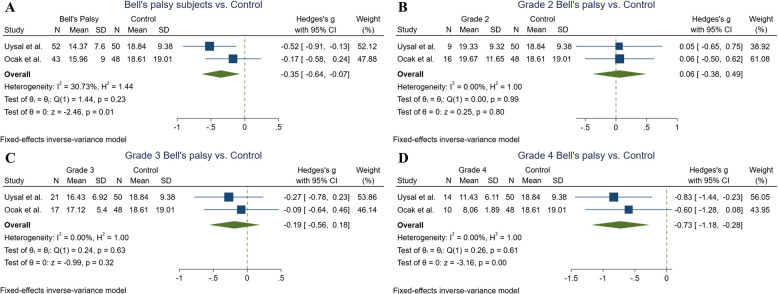
A total of three studies reported the mean vitamin D levels among BP subjects [18–20]. with the pooled mean vitamin D level of 15.98 ng/ml (CI = 13.83–18.06). Two of them compared the results to the healthy individuals [19, 20]. Uysal et al. conducted a study on 52 patients and 50 controls and revealed significantly lower vitamin D levels among patients (18.84 ± 9.38) compared to the controls (14.37 ± 7.60) (P values = 0.007) [19]. However, Ocak et al. did not reach a significant difference regarding the comparison of the 43 patients and 48 controls [20]. Our meta-analysis result showed significantly lower vitamin D levels in BP patients compared to the control group (Hedges'g = −0.35, 95% CI = [−0.64 to −0.07], P value = 0.01, I2 = 30.73%) (Fig. 2A).
Fig. 2.
Forest plots indicating the Bell’s palsy and its subgroups base on the grading system compared to the healthy controls
In terms of disease grading based on the HB scale grading system, all of the included studies reported the vitamin D level across these subgroups of patients [17–20]. At the same time, two of them also had the results of the healthy [19, 20].
Therefore, meta-analyses were conducted to evaluate the difference between BP subgroups regarding the grading system and the healthy controls. No significant difference was observed for grade 2 vs. controls (Hedges'g = 0.05, 95% CI = [−0.38 to 0.49], P value = 0.79, I2 = 0%), and grade 3 vs. controls (Hedges'g = −0.18, 95% CI = [−0.55 to 0.18], P value = 0.32, I2 = 0%) according to Fig. 2B and C. In contrast, the meta-analysis showed a significantly decreased value for vitamin D across grade 4 of the disease compared to the control group (Hedges'g = −0.72, 95% CI = [−1.18 to −0.27], P value = 0.001, I2 = 0%) (Fig. 2D).
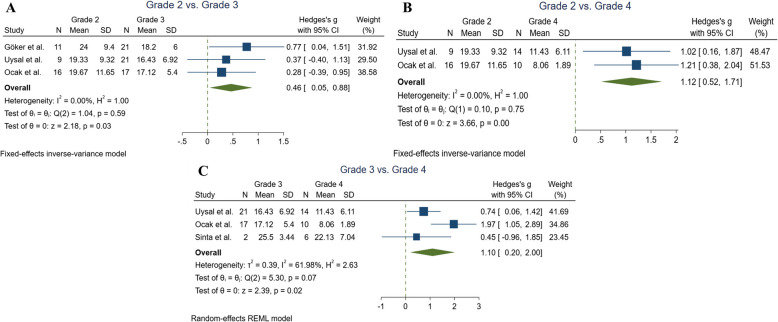
Comparing vitamin D levels between Bell’s palsy subgroups based on the grading system
In the total of three studies, inter-group comparisons were performed based on the grading system [17–19]. Uysal et al. reported that the mean vitamin D level in 9 subjects with grade 2 (19.33 ± 9.32) was statistically significantly higher than in 14 individuals with grade 4 (11.43 ± 6.11), with a P-value of 0.002 [19].
Similarly, Goker et al. observed that 11 patients with grade 2 (24.0 ± 9.4) and 21 subjects with grade 3 (18.2 ± 6.0) had significantly greater vitamin D levels compared to 17 patients with grade 4 (10.8 ± 4.4), with a P-value of 0.01 > [17].
Sinta et al. also found that higher clinical severity was associated with lower vitamin D levels, as the mean blood vitamin D levels for 12 subjects with grade 5, 6 subjects with grade 4, and 2 subjects with grade 3 were 14.77 ± 3.98, 22.13 ± 7.04, and 25.5 ± 3.45 ng/mL, respectively [18].
Therefore, we conducted meta-analyses to compare these subgroups. As shown in Fig. 3, vitamin D levels decrease with increasing disease severity. Patients with grade 2 had significantly higher vitamin D levels compared to those with grade 3 (Hedges'g = 0.46, 95% CI = [0.04 to 0.87], P = 0.02, I2 = 0%), and grade 4 (Hedges'g = 1.11, 95% CI = [0.51 to 1.71], P < 0.001, I2 = 0%) (Fig. 3A and B). Furthermore, patients with grade 3 BP exhibited greater vitamin D levels than those with grade 4 (Hedges'g = 1.09, 95% CI = [0.19 to 2.00], P = 0.01, I2 = 61.98%) (Fig. 3C).
Fig. 3.
Forest plots indicating the between group comparisons of Bell’s palsy subgroups based on the grading system
Discussion
The present study indicates that vitamin D levels were significantly lower in BP patients compared to healthy controls. While no significant differences were observed for grades 2 and 3, patients with grade 4 BP had markedly reduced vitamin D levels. Additionally, vitamin D levels progressively declined with increasing disease severity. The results of the study suggest a potential association between vitamin D deficiency and BP progression, but not causation, and the relationship between vitamin D levels and BP severity is still under investigation. This study contributes to the growing body of research by systematically examining the relationship between serum vitamin D levels and BP severity, with a focus on disease grading. More than fifty percent of peripheral facial paralysis cases are attributable to BP, characterized by the sudden onset of unilateral paresis or paralysis affecting all muscles of facial expression [28, 29]. The results of the study by Goker et al. showed that vitamin D levels were significantly lower in patients who had an HB Scale Grade ≥ 4 compared to other patients. Also, the initial vitamin D levels in the patients who did not show complete recovery during the follow-up period were significantly lower than the patients in the group who had complete recovery [17].
This condition typically presents without accompanying symptoms of CNS involvement, and its onset can range from a few hours to several days. While BP generally follows a benign clinical course, it may raise concerns about potential sequelae in affected patients [19]. Previous research has examined various factors that may influence the prognosis of this condition. Previous studies have reported mixed results, with some suggesting a significant association between vitamin D levels and BP severity, while others found no such link. Although the precise pathophysiology remains unclear, potential etiologies include vascular causes or viral infections, such as HSV, Varicella zoster virus, Cytomegalovirus, or Epstein-Barr virus [19]. The study of Ulusoy et al. also showed that the ratio of neutrophils to lymphocytes, as well as the ratio of platelets to lymphocytes, was higher in patients with BP than in others, which indicates the inflammatory role of these markers [30].
In addition to these, various factors have been identified as prognostic markers, including procalcitonin levels, serum vitamin D levels, blink reflex, vestibular evoked myogenic potentials, and high-frequency ultrasonography of the facial nerve [22, 23, 31, 32]. While BP is generally self-limiting, instances where complete recovery is not attained can significantly impair a patient's quality of life. In rare cases, the clinical manifestations may recur, affecting either the ipsilateral or contralateral facial region [24]. Thus, timely and appropriate intervention is critical upon diagnosis [20]. Vitamin D deficiency, influenced by factors such as age, obesity, sunlight exposure, and skin tone, may contribute to variations in susceptibility and severity of BP. Factors contributing to vitamin D insufficiency include living in northern or southern latitudes and avoiding sunlight. In both the north and south hemispheres, the prevalence of BP rises as latitude increases. However, high-latitude populations consuming vitamin D-rich diets (e.g., oily fish) may counteract the deficiency risk, complicating the interpretation of geographical trends in BP [21].
Recent research has demonstrated that low levels of vitamin D and sun exposure, either past or present, are independent risk factors for the onset of BP. There is growing evidence that immunosuppression brought on by UV light is distinct from that brought on by the vitamin D system [33].
In one study, the prevalence of vitamin D hypovitaminosis was anticipated, as all participants resided between 36° and 42° northern latitudes and 26° and 45° eastern longitudes during the autumn-spring period [17, 34].
Several biological mechanisms, such as the role of vitamin D in nerve regeneration and immune modulation, may explain the observed link between vitamin D levels and BP severity. The highly active vitamin D metabolite is produced by the VDR, an enzyme that is widely expressed in human brains. VDR is also responsible for the production of vitamin D's active form, 1, 25-hydroxyvitamin D. Studies on the effects of VDR gene transcription in neuronal cells have shown that vitamin D and VDRs are required for brain development, anxiety prevention, glial-derived neurotrophic factor induction, and nerve growth factor synthesis induction [35, 36]. Vitamin D also regulates genes involved in the myelination process, with Prx being essential for peripheral nerve myelin maintenance and Tspan2 necessary for oligodendrocyte differentiation into myelin-forming glia [37, 38]. Vitamin D is undoubtedly a key molecule in nerve regeneration. An animal study by Montava et al. demonstrated the positive effects of vitamin D3 on myelination and recovery following traumatic facial nerve injury [39]. Research suggests that vitamin D interacts with immune pathways, potentially influencing the inflammatory processes implicated in BP [40, 41]. Both in vivo and in vitro research support a link between lower vitamin D levels and several neurological diseases [42].
Also, review studies have suggested that adequate levels of vitamin D can prevent facial paralysis and reduce the severity of the disease [34].
Chabas et al.'s animal study demonstrated that vitamin D3 (cholecalciferol) administered after a left peroneal nerve incision led to locomotor and electrophysiological recovery in the nerve, increasing axon count, diameter, and neuritis myelination [43]. While literature suggests a potential role for vitamin D in nerve function, further research is needed to clarify its impact on preventing BP or mitigating its severity.
We propose that inadequate vitamin D levels could negatively impact nerve regeneration in the context of BP severity and progression [5, 44–46]. One study indicated that vitamin D levels did not influence the onset of BP, as the average levels were comparable between the study and control groups. However, within the BP cohort, patients classified as HB scale grade 4 exhibited lower vitamin D levels than the other grades, and their recovery rates were also reduced. Additionally, patients with higher vitamin D levels generally demonstrated better recovery outcomes than those with lower levels. These results imply that vitamin D may play a significant role in the prognosis of BP, with lower levels potentially hindering recovery, especially in patients with more advanced disease stages [20].
Some limitations of this systematic review should be considered. Because we lacked information in the majority of cases, we were unable to rule out the influence of medication and vitamin D supplementation. Second, the level of circulating 25(OH)D was determined utilizing various assay procedures. Third, we were unable to combine the results based on ethnicity or solar exposure because most studies do not include this information. Blood vitamin D levels, which form the basis of the meta-analyses, should have already expressed the effects of sunlight exposure and ethnicity, which are upstream factors influencing vitamin D status and serve as partial surrogates for sunlight exposure and typical intake or supplementation levels in a population.
More limitations
The study includes data from only four studies, which limits the generalizability of the findings and increases the risk of random error. Also, all included studies are from Turkey and Indonesia, reducing the global applicability of the findings. Populations from different regions, especially those with differing dietary habits, sunlight exposure, and genetic predispositions, are not represented. The included studies do not account for the long-term outcomes of BP patients, limiting the understanding of vitamin D's role in recovery or recurrence. Variability in participant characteristics (e.g., age, gender, baseline health conditions) and disease grading systems could confound the results, and these factors were not adequately controlled. Although some biological pathways are mentioned, the study does not profoundly investigate the mechanistic role of vitamin D in nerve regeneration or neuroinflammation.
One of the other limitations is that the cross-sectional nature of the included studies does not establish a causal relationship between low vitamin D levels and the severity of BP. Also, vitamin D levels are influenced by seasonal changes in sunlight exposure, but this was not accounted for in the analysis, and the threshold for defining vitamin D deficiency or insufficiency may vary across studies, leading to potential misclassification bias. This limitation affects the current study due to the lack of standardized reporting of vitamin D deficiency across the included studies, preventing a meta-analysis of its role as a potential risk factor for Bell’s palsy.
Future research should focus on addressing the limitations of existing studies by conducting longitudinal and interventional trials to establish a causal relationship between vitamin D levels and BP severity, recovery, and recurrence. Randomized controlled trials are particularly needed to evaluate the efficacy of vitamin D supplementation as a therapeutic intervention. Expanding the geographic and demographic scope of studies is crucial to improve the generalizability of findings, particularly by including diverse populations with varying dietary habits, sunlight exposure, and genetic backgrounds. Standardizing the measurement methods for serum 25(OH) D levels and employing uniform definitions of vitamin D deficiency will enhance comparability across studies. Additionally, exploring the mechanistic pathways through which vitamin D influences nerve regeneration and neuroinflammation, including its interaction with neurotrophic factors and vitamin D receptors, could provide valuable insights into its role in neurological conditions. Future research should also account for seasonal and environmental factors influencing vitamin D levels and consider stratified analyses based on age, sex, and comorbidities to identify populations most likely to benefit from targeted interventions. These advancements will help refine the understanding of vitamin D's role in BP and inform more effective clinical and public health strategies.
Conclusion
In this systematic review and meta-analysis, we evaluate the relationship between serum vitamin D levels in patients with BP. Vitamin D may influence the severity of BP. Our meta-analysis results show that a high grade of BP is associated with lower serum vitamin D levels. Given that inflammation is involved in the pathophysiological background of BP, the anti-inflammatory effect of vitamin D can be a possible mechanism for the benefit of this vitamin in BP.
Supplementary Information
Abbreviations
- BP
Bell’s palsy
- HSV
Herpes Simplex Virus
- UV
Ultraviolet
- NMOS
Neuromyelitis Optica Spectrum Disorder
- CNS
Central Nervous System
- VDR
Vitamin D Receptors
- OSF
Open Science Framework
- CMIA
Chemiluminescent Microparticle Immunoassay
- Prx
Periaxin Gene
- PAMP
Pathogen Associated Molecular Patterns
- TLR
Toll-Like Receptor
- TREM
Triggering Receptor Expressed on Myeloid cells
- HB scale
House-Brackmann scale
Authors’ contributions
E.F. conceived the design, revised the manuscript, drafted, and acquired the data. M.M drafted, analyzed the results, and revised. SH.H revised, drafted, wrote the protocol. M.MB searched, drafted, and designed. K.ST drafted and screened. A.HB and F.A drafted and designed. E.SH drafted, revised and extracted the data. M.KhA revised and controlled the aspects.
Funding
None.
Data availability
The detailed data of the manuscript is available upon request from the corresponding authors.
Declarations
Ethics approval and consent to participate
IRB Information: not applicable.
Research involving human participants and, or animals: Not applicable.
Consent for publication
Not applicable. This manuscript does not contain any individual person’s data in any form (including images or personal/clinical details) that would require consent for publication.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Elaheh Foroughi and Mehrdad Mozafar contributed equally to this work and share the first authorship.
Contributor Information
Mani Khorsand Askari, Email: Mani.Askari@utoledo.edu.
Erfan Shahabinejad, Email: erfanshn14@gmail.com.
References
- 1.Baugh RF, Basura GJ, Ishii LE, Schwartz SR, Drumheller CM, Burkholder R, et al. Clinical practice guideline: Bell’s Palsy executive summary. Otolaryngol Head Neck Surg. 2013;149(5):656–63. [DOI] [PubMed] [Google Scholar]
- 2.Adour KK. Current concepts in neurology: diagnosis and management of facial paralysis. N Engl J Med. 1982;307(6):348–51. [DOI] [PubMed] [Google Scholar]
- 3.Bosco D, Plastino M, Bosco F, Consoli A, Labate A, Pirritano D, et al. Bell’s palsy: a manifestation of prediabetes? Acta Neurol Scand. 2011;123(1):68–72. [DOI] [PubMed] [Google Scholar]
- 4.Monini S, Lazzarino AI, Iacolucci C, Buffoni A, Barbara M. Epidemiology of Bell’s palsy in an Italian Health District: incidence and case-control study. Acta Otorhinolaryngol Ital. 2010;30(4):198. [PMC free article] [PubMed] [Google Scholar]
- 5.Selesnick SH, Patwardhan A. Acute facial paralysis: evaluation and early management. Am J Otolaryngol. 1994;15(6):387–408. [DOI] [PubMed] [Google Scholar]
- 6.Celik O, Ulkumen B, Eskiizmir G, Can F, Pabuscu Y, Kamiloglu U, et al. The ratio of facial nerve to facial canal as an indicator of entrapment in Bell’s palsy: a study by CT and MRI. Clin Neurol Neurosurg. 2020;198: 106109. [DOI] [PubMed] [Google Scholar]
- 7.Bajaj S, Singh RP, Dwivedi NC, Singh K, Gupta A, Mathur M. Vitamin D levels and microvascular complications in type 2 diabetes. Indian J Endocrinol Metab. 2014;18(4):537–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Celikbilek A, Gocmen AY, Tanik N, Borekci E, Adam M, Celikbilek M, et al. Decreased serum vitamin D levels are associated with diabetic peripheral neuropathy in a rural area of Turkey. Acta Neurol Belg. 2015;115(1):47–52. [DOI] [PubMed] [Google Scholar]
- 9.Duan S, Lv Z, Fan X, Wang L, Han F, Wang H, et al. Vitamin D status and the risk of multiple sclerosis: a systematic review and meta-analysis. 2014;570:108–13. [DOI] [PubMed] [Google Scholar]
- 10.Luo X, Ou R, Dutta R, Tian Y, Xiong H. Shang HJFiN. Association between serum vitamin D levels and Parkinson’s disease: a systematic review and meta-analysis. 2018;9:909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Annweiler C, Llewellyn DJ. Beauchet OJJoAsD. Low serum vitamin D concentrations in Alzheimer’s disease: a systematic review and meta-analysis. 2013;33(3):659–74. [DOI] [PubMed] [Google Scholar]
- 12.Koduah P, Paul F, Dörr JM. Vitamin D in the prevention, prediction and treatment of neurodegenerative and neuroinflammatory diseases. Epma j. 2017;8(4):313–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang W, Li Y, Meng X. Vitamin D and neurodegenerative diseases Heliyon. 2023;9(1): e12877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.de Siqueira EA, Magalhães EP, de Menezes R, Sampaio TL, Lima DB, da Silva MC, et al. Vitamin D3 actions on astrocyte cells: a target for therapeutic strategy in Parkinson’s disease? Neurosci Lett. 2023;793: 136997. [DOI] [PubMed] [Google Scholar]
- 15.Shi Y, Shi Y, Jie R, He J, Luo Z, Li J. Vitamin D: The crucial neuroprotective factor for nerve cells. Neuroscience. 2024;560:272–85. [DOI] [PubMed] [Google Scholar]
- 16.Cui X, Gooch H, Petty A, McGrath J. Eyles D. Vitamin D and the brain: Genomic and non-genomic actions. Mol Cell Endocrinol; 2017. p. 453. [DOI] [PubMed] [Google Scholar]
- 17.Göker AE, Karaketir S, Alagöz MH, Başkadem Yılmazer A, Sarı H, Bircan HS, et al. The effect of vitamin D levels on prognosis of patients with facial paralysis. Turkish J Ear Nose Throat. 2020;30(4):118–22. [Google Scholar]
- 18.Sinta M, Sulistyani S, Ratnasari Y, Milyarona FP. Peran kadar vitamin d darah terhadap derajat keparahan klinis pasien Bell’s palsy. Jurnal Ilmu Medis Indonesia. 2023;3(1):1–8. [Google Scholar]
- 19.Uysal A, Güntel M. Determining the relationship between disease stage, duration of admission time, and vitamin D levels in patients with idiopathic facial paralysis. J Contemp Med. 2021;11(3):392–5. [Google Scholar]
- 20.Ocak E, Uyar MS, Kocaoz D, Mirici E, Acar A. Role of vitamin d deficiency on the onset and prognosis of Bell’s palsy. ENT Updates. 2020;10(3):439–43. [Google Scholar]
- 21.Kampman MT, Brustad M. Vitamin D: a candidate for the environmental effect in multiple sclerosis - observations from Norway. Neuroepidemiology. 2008;30(3):140–6. [DOI] [PubMed] [Google Scholar]
- 22.Li S, Guo RJ, Liang XN, Wu Y, Cao W, Zhang ZP, et al. High-frequency ultrasound as an adjunct to neural electrophysiology: evaluation and prognosis of Bell’s palsy. Exp Ther Med. 2016;11(1):77–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mancini P, De Seta D, Prosperini L, Nicastri M, Gabriele M, Ceccanti M, et al. Prognostic factors of Bell’s palsy: multivariate analysis of electrophysiological findings. Laryngoscope. 2014;124(11):2598–605. [DOI] [PubMed] [Google Scholar]
- 24.PERDOSSI. Panduan Praktik Klinis. Jakarta: Perhimpunan Dokter Spesialis Saraf. 2016.
- 25.Page MJ, Mckenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 Statement: an updated guideline for reporting systematic review. bmj. 2021;3(29):372. [DOI] [PMC free article] [PubMed]
- 26.Barker TH, Stone JC, Sears K, Klugar M, Leonardi-Bee J, Tufanaru C, et al. Revising the JBI quantitative critical appraisal tools to improve their applicability: an overview of methods and the development process. JBI Evidence Synthesis. 2023;21(3):478–93. [DOI] [PubMed] [Google Scholar]
- 27.Thorlund K, Imberger G, Johnston BC, Walsh M, Awad T, Thabane L, et al. Evolution of heterogeneity (I2) estimates and their 95% confidence intervals in large meta-analyses. PLoS ONE. 2012;7(7): e39471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fisch U. Management of intratemporal facial nerve injuries. J Laryngol Otol. 1980;94(1):129–34. [DOI] [PubMed] [Google Scholar]
- 29.Murthy JM, Saxena AB. Bell’s palsy: Treatment guidelines. Ann Indian Acad Neurol. 2011;14(Suppl 1):S70-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ulusoy B, Bozdemir K, Kale H, Korkmaz MH. The role of inflammation markers in predicting the prognosis of Bell’s palsy. Euro Res J. 2019;5(4):629–35. [Google Scholar]
- 31.Chung JH, Lee SK, Kim SH, Yeo SG, Park MS, Byun JY. Neurotological parameters and prognosis of Bell’s palsy patients. Audiol Neurootol. 2015;20(2):117–21. [DOI] [PubMed] [Google Scholar]
- 32.Kilicaslan S, Uluyol S, Gur MH, Arslan IB, Yagiz O. Diagnostic and prognostic value of procalcitonin levels in patients with Bell’s palsy. Eur Arch Otorhinolaryngol. 2016;273(6):1615–8. [DOI] [PubMed] [Google Scholar]
- 33.Lucas RM, Ponsonby AL, Dear K, Valery PC, Pender MP, Taylor BV, et al. Sun exposure and vitamin D are independent risk factors for CNS demyelination. Neurology. 2011;76(6):540–8. [DOI] [PubMed] [Google Scholar]
- 34.Şenkal E, Ünüvar E, Seren L, Göl C, Durankuş F. D Vitamini Bakılmasının Gerekliliği ve Düzeylerinin Yorumu. Çocuk Dergisi. 2018;18(3):97–102. [Google Scholar]
- 35.Simon KC, Munger KL, Xing Y, Ascherio A. Polymorphisms in vitamin D metabolism related genes and risk of multiple sclerosis. Mult Scler. 2010;16(2):133–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Smolders J, Damoiseaux J, Menheere P, Tervaert JW, Hupperts R. Association study on two vitamin D receptor gene polymorphisms and vitamin D metabolites in multiple sclerosis. Ann N Y Acad Sci. 2009;1173:515–20. [DOI] [PubMed] [Google Scholar]
- 37.Marchesi C, Milani M, Morbin M, Cesani M, Lauria G, Scaioli V, et al. Four novel cases of periaxin-related neuropathy and review of the literature. Neurology. 2010;75(20):1830–8. [DOI] [PubMed] [Google Scholar]
- 38.Terada N, Baracskay K, Kinter M, Melrose S, Brophy PJ, Boucheix C, et al. The tetraspanin protein, CD9, is expressed by progenitor cells committed to oligodendrogenesis and is linked to beta1 integrin, CD81, and Tspan-2. Glia. 2002;40(3):350–9. [DOI] [PubMed] [Google Scholar]
- 39.Montava M, Garcia S, Mancini J, Jammes Y, Courageot J, Lavieille JP, et al. Vitamin D3 potentiates myelination and recovery after facial nerve injury. Eur Arch Otorhinolaryngol. 2015;272(10):2815–23. [DOI] [PubMed] [Google Scholar]
- 40.Kim TH, Lee B, Kwon E, Choi SJ, Lee YH, Song GG, et al. Regulation of TREM-1 expression by 1,25-dihydroxyvitamin D3 in human monocytes/macrophages. Immunol Lett. 2013;154(1–2):80–5. [DOI] [PubMed] [Google Scholar]
- 41.Sadeghi K, Wessner B, Laggner U, Ploder M, Tamandl D, Friedl J, et al. Vitamin D3 down-regulates monocyte TLR expression and triggers hyporesponsiveness to pathogen-associated molecular patterns. Eur J Immunol. 2006;36(2):361–70. [DOI] [PubMed] [Google Scholar]
- 42.Abou-Raya S, Helmii M, Abou-Raya A. Bone and mineral metabolism in older adults with Parkinson’s disease. Age Ageing. 2009;38(6):675–80. [DOI] [PubMed] [Google Scholar]
- 43.Chabas JF, Stephan D, Marqueste T, Garcia S, Lavaut MN, Nguyen C, et al. Cholecalciferol (vitamin D₃) improves myelination and recovery after nerve injury. PLoS ONE. 2013;8(5): e65034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Barlow PG, Svoboda P, Mackellar A, Nash AA, York IA, Pohl J, et al. Antiviral activity and increased host defense against influenza infection elicited by the human cathelicidin LL-37. PLoS ONE. 2011;6(10): e25333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Peitersen E. Bell’s palsy: the spontaneous course of 2,500 peripheral facial nerve palsies of different etiologies. Acta Otolaryngol Suppl. 2002;549:4–30. [PubMed] [Google Scholar]
- 46.Zhao Y, Ran Z, Jiang Q, Hu N, Yu B, Zhu L, et al. Vitamin D alleviates rotavirus infection through a microrna-155-5p mediated regulation of the TBK1/IRF3 signaling pathway in vivo and in vitro. Int J Mol Sci. 2019;20(14). 10.3390/ijms20143562. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The detailed data of the manuscript is available upon request from the corresponding authors.