Abstract
Objective
To investigate whether dysfunction of the glymphatic system and altered neurofluidic dynamics contribute to the pathophysiology of classical trigeminal neuralgia (CTN), and to explore the potential interplay between brain-CSF coupling and structural brain changes.
Methods
A total of 131 patients with CTN and 106 age- and sex-matched healthy controls were recruited. All participants underwent multimodal MRI, including high-resolution structural imaging, resting-state functional MRI, and diffusion tensor imaging. Key indices included choroid plexus (CP) volume as a proxy for CSF production, global BOLD-CSF coupling as a measure of functional neurofluidic interaction, and the DTI-based ALPS index reflecting glymphatic clearance. Additional markers included peak width of skeletonized mean diffusivity (PSMD) and global gray/white matter and CSF volume. Partial correlation analyses were performed between imaging metrics and clinical assessments.
Results
CTN patients showed significantly increased CP volume (P = 0.022) and gBOLD-CSF coupling (P < 0.001), along with reduced bilateral ALPS indices (P = 0.002, P = 0.004). PSMD and CSF volume were elevated (P < 0.001, P < 0.001), while gray and white matter volumes were reduced (P = 0.028, P = 0.009). gBOLD-CSF coupling correlated positively with depression, anxiety, and pain-related disability scores (P < 0.001), and negatively with MMSE (P = 0.022).
Conclusion
This study provides multimodal MRI evidence of glymphatic dysfunction and neurofluidic alterations in CTN, supporting a conceptual framework in which disrupted brain-CSF interaction may influence peripheral sensory modulation through a putative brain-CSF-ganglion pathway. These results may inform mechanistic hypotheses and guide future research on the neurofluidic underpinnings of neuropathic pain, potentially providing new insights into the pathogenesis of CTN.
Keywords: Classical trigeminal neuralgia, Glymphatic system, Brain-CSF coupling, Choroid plexus volume, Neurofluidic dynamics
Introduction
Trigeminal neuralgia (TN) is a chronic neuropathic pain condition characterized by recurrent, brief, shock-like pain in the distribution of the trigeminal nerve, often triggered by routine activities such as washing the face or brushing teeth [1, 2]. The annual incidence of TN ranges from 4 to 28 per 100,000 people, with higher prevalence in middle-aged and elderly women [3–5]. According to the third edition of the International Classification of Headache Disorders (ICHD-3), TN is classified into classical trigeminal neuralgia (CTN), secondary trigeminal neuralgia (STN), and idiopathic trigeminal neuralgia (ITN), with CTN being the most common, accounting for approximately 75% of all TN cases [1, 6]. Neurovascular compression (NVC) is widely considered the primary etiological factor in CTN [7]. However, NVC also exists in healthy people [8–12]. Therefore, NVC alone cannot completely explain the occurrence of CTN. An expanding body of functional neuroimaging studies has underscored the contribution of central mechanisms [13, 14]. Given the distinct roles of the glymphatic system within the central nervous system [10, 13–16], emerging evidence suggests its potential involvement in neuropathic pain [17]. However, the exact role of the glymphatic system in CTN remains unclear.
The glymphatic system is a brain-wide perivascular clearance network, anatomically supported by perivascular astrocytic end-feet, a specialized subtype of astrocytes that express the water channel protein aquaporin-4 (AQP4) [18, 19]. It facilitates the dynamic exchange of cerebrospinal fluid (CSF) and interstitial fluid (ISF) within the central nervous system, thereby promoting the efficient removal of metabolic waste and solutes, and contributing to the homeostasis of the neural microenvironment [20, 21]. Astrocytes and AQP4 are integral to glymphatic function, and their dysregulation has been implicated in various neurological conditions, including Alzheimer’s disease, neuromyelitis optica spectrum disorder (NMOSD), and Parkinson’s disease [22–26]. In addition to AQP4, other aquaporins such as AQP1, AQP5, and AQP9 have also been implicated in CSF production, perivascular exchange, and metabolic clearance, further supporting the complexity of glymphatic regulation [27, 28]. During trigeminal nerve injury responses, astrocytes can be rapidly activated, potentially in response to neurovascular compression [29, 30]. Astrocyte activation contributes to neuroinflammation and the persistence of neuropathic pain [17, 22, 29–31], while AQP4-mediated water and solute transport is essential for both waste clearance and nociceptive modulation [17]. Mu et al. detected signs of central nervous system degeneration in the CTN model by measuring reduced levels of docosahexaenoic acid, a key lipid that modulates microglia-mediated neuroinflammation [32]. This inflammatory pathway may in turn impair glymphatic function by disrupting astrocytic AQP4 polarization [33, 34]. Together, these findings suggest that dysfunction of the glymphatic system may be involved in the onset or persistence of CTN. In particular, altered CSF dynamics and disrupted neurofluidic interactions between the brain and trigeminal system may represent an underexplored mechanism in the pathophysiology of CTN.
Beyond metabolic clearance, the glymphatic system operates within a broader neurofluidic context involving CSF production, central coupling, and perivascular transport [35]. A recent study suggested that brain CSF dynamics may functionally interact with peripheral sensory circuits, as CSF-infused solutes activated trigeminal ganglion neurons in animal models [36].These dynamics may influence not only waste removal but also sensory excitability through brain-CSF-nerve interactions. Clinical findings further suggest that peripheral inflammation near the trigeminal nerve may influence central CSF dynamics, as indicated by postoperative reductions of CSF inflammatory markers in trigeminal neuralgia patients [37]. In this context, three neuroimaging markers have been proposed to reflect key components of this interface. Choroid plexus (CP) volume serves as a proxy for CSF production and barrier integrity [38]. The CP forms the blood-CSF barrier via tight junctions between epithelial cells and plays an essential role in CSF secretion, waste clearance, and immune signaling. CP enlargement has been linked to disrupted barrier function, neuroinflammation, and abnormal CSF composition [39–41]. Global BOLD-CSF (gBOLD-CSF) coupling, derived from resting-state fMRI, quantifies the temporal correlation between cortical low-frequency oscillations (< 0.1 Hz) and CSF signal fluctuations at the base of the brain. Initially demonstrated by Kiviniemi et al. [42], this coupling reflects coordinated CSF inflow driven by neural activity, and has since been proposed as a functional index of glymphatic pulsatility and CSF entrainment [43]. The DTI-ALPS index, introduced by Taoka et al., estimates directional water diffusivity along perivascular spaces relative to projection and association fibers [44]. This index has shown strong test-retest reliability, correlates with glymphatic clearance efficiency, and has been applied across various neurological conditions [45, 46]. These markers enable a systems-level assessment of neurofluidic function, encompassing CSF generation, fluid-brain coupling, and solute clearance.
Impaired glymphatic function may lead to white matter injury, likely mediated by reduced interstitial clearance and chronic neuroinflammation. These structural effects may represent a downstream consequence of neurofluidic dysregulation [47]. Together, these processes form a dynamic neurofluidic system whose dysfunction may lead to measurable structural brain changes [48]. To evaluate these potential consequences, we employed the peak width of skeletonized mean diffusivity (PSMD), a diffusion MRI-based biomarker that quantifies microstructural dispersion within the white matter skeleton. PSMD has been extensively validated as a sensitive marker for detecting subtle white matter alterations in various neurological disorders. Owing to its high reproducibility and sensitivity to microstructural disruption, it may also hold promise as a useful biomarker for assessing white matter integrity in pain-related conditions [49].In addition, we assessed gray matter, white matter and CSF tissue volumes, to capture macroscopic structural alterations potentially associated with chronic neurofluidic imbalance. Together, these structural measures complement functional glymphatic indices and help bridge fluid dysregulation and brain integrity within a unified pathophysiological framework.
To systematically investigate glymphatic dysfunction and brain structural alterations in CTN, we conducted a multimodal MRI study involving 131 CTN patients and 106 age- and sex-matched normal controls. Imaging parameters included choroid plexus volume, gBOLD-CSF coupling, DTI-ALPS index, PSMD, and brain tissue volumes. Clinical assessments encompassed pain intensity, cognitive function, and mood status. We hypothesized that CTN is associated with neurofluidic dysregulation and glymphatic dysfunction, which may contribute to white matter microstructural damage and brain atrophy, with these alterations correlating with clinical symptom severity.
Methods
Participants
This study included CTN patients who were prospectively recruited at the Affiliated Hangzhou First People’s Hospital, Westlake University School of Medicine. A subset of the participants (n = 79) had been previously included in our earlier publication in CTN patients [50]. The inclusion and exclusion criteria were identical to those described previously. Additional participants were recruited thereafter to expand the sample size and investigate new research questions related to neurofluidic dynamics.
Clinical and psychological assessments
The assessment procedures were conducted according to the same methodology described in our previous study [50]. Pain characteristics in CTN patients were systematically assessed, including pain intensity, pain episode frequency, and duration of each pain episode. Pain intensity was rated on a 0–10 VAS under clinician supervision.
Neuropsychological assessments included the quality-of-life score of patients with TN (TN QOLS) [51] for evaluating quality of life, and the mini-mental state examination (MMSE) [52], self-rating depression scale (SDS) [53] and self-rating anxiety scale (SAS) [54] were used to assess cognitive function, depression, and anxiety.
MRI protocols
All the CTN patients and HC underwent MRI scanning by a 3.0T MRI scanner (Siemens, MAGNETOM Verio, Germany) and an eight-channel phased-array head coil. All the subjects were asked to close their eyes, stay awake, and breathe quietly until the scan was completed. And all the participants underwent MRI with fMRI(TR = 2000 ms, TE = 30 ms, flip angle = 90°, slice thickness = 3.2 mm, voxel size = 3.438 × 3.438 × 3.2 mm3, field of view = 64 × 64, number of slices = 47)and DTI (TR = 11700 ms, TE = 8630 ms, thickness = 2.0 mm, voxel size = 2.0 × 2.0 × 2.0 mm3, FOV = 256 × 256 mm2, 65 slices, 30 directions); 3D-T1WI (TR = 1900 ms, TE = 2.52 ms, thickness = 1 mm, FOV = 256 × 256 mm2, voxel size = 1 × 1 × 1 mm3, turning angle = 9°, 176 slices); 3D-VIBE (TR = 10 ms, TE = 3.69 ms, flip angle = 12°, FOV = 220 mm × 220 mm2, voxel size = 0.8 × 0.8 × 0.8 mm3, slice thickness = 0.8 mm, 60 slices); and 3D-STIR data acquisition (SPC sequence, TR = 3800 ms, TE = 194 ms, FOV = 230 mm × 230 mm2, voxel size = 0.9 × 0.9 × 0.9 mm3, slice thickness = 0.9 mm, 64 slices).
Data preprocessing
The MRI images were inspected on the picture archiving and communication system (PACS) developed in our hospital and any subjects with structural lesions or suboptimal imaging quality were excluded.
For structural MRI data, image processing was carried out using the Computational Anatomy Toolbox (CAT12, https://neuro-jena.github.io/cat/), an extension of SPM12 that operates within MATLAB (version R2022b). Each participant’s 3D-T1WI scan images were segmented into gray matter, white matter, and CSF using tissue probability maps. These images were then normalized to Montreal Neurological Institute (MNI) space using a linear and nonlinear registration approach, which preserved the unique anatomical features of each brain. This process enabled the calculation of gray matter, white matter, CSF, and total intracranial volume (TIV). Additionally, we examined each volume for potential artifacts that could impact processing, such as issues related to segmentation or normalization.
For rs-fMRI data, preprocessing was conducted using the Data Processing & Analysis for Brain Imaging (DPABI, version 8.0, http://rfmri.org/dpabi/) within the SPM12 framework. The first 10 time points of each time series were discarded to ensure stabilization of the magnetization signal. The remaining 230 time points underwent slice-timing correction to eliminate temporal discrepancies across scans. Spatial smoothing was applied using a 6-mm full-width at half maximum (FWHM) Gaussian kernel, and linear trends were removed from the data. Band-pass filtering was performed to retain signals within the frequency range of 0.01–0.1 Hz. For CSF signals, no spatial smoothing was applied to preserve the accuracy of signals within smaller regions. Neither the whole-brain BOLD signal, CSF signal, nor motion parameters underwent noise regression to prevent the loss of crucial hemodynamic information [43]. Additionally, motion correction was not performed, as accurate motion correction for edge slices in imaging volumes is not feasible when tissue moves in and out of the scanning field [43].
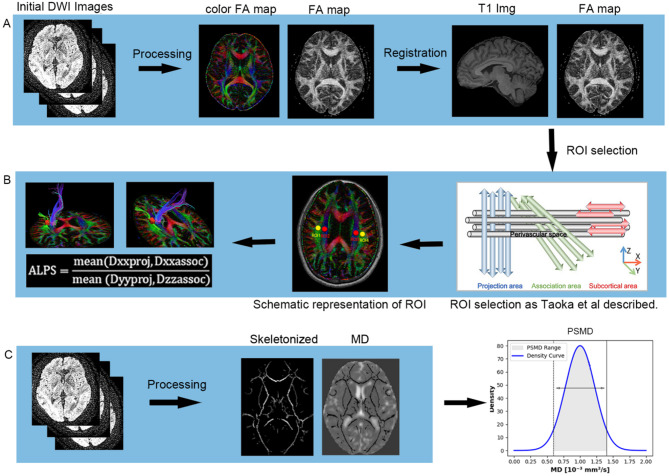
DTI images of all subjects were preprocessed as follows (Fig. 2A): The images were corrected for eddy current distortions and gradient directions using FSL 6.0.7.10. Brain extraction was performed on 3D-T1WI and DTI images using the HD-BET based on deeplearning algorithm [55] and Brain Extraction Tool from FSL to obtain skull-stripped images. Then, the diffusion tensor model was applied at each voxel using the DTIfit program, and values of parameters such as fractional anisotropy (FA) and MD were calculated in FSL. In registration, the linear registration command of FSL was used to register the 3D-T1WI image to the DTI individual space to achieve spatial consistency. Then, the linear and nonlinear registration methods were used to register the DTI image to the MNI template to obtain the spatial transformation matrix file. This registration strategy was adopted to facilitate ALPS index computation in native diffusion space.
Fig. 2.
A.DTI Image Preprocessing and DTI-ALPS Index Calculation: The raw diffusion tensor images underwent brain extraction, eddy current correction, and motion correction to compute the diffusion tensor parameters. These parameters were then registered to standard space using T1-weighted images. B. Extraction of Diffusion Rates Along x, y, and z Axes: Diffusion rates along the x, y, and z axes were extracted from the diffusion tensor images in standard space, projecting fibers and associated tracts. The ALPS index was then calculated as the ratio of these diffusion rates. C. PSMD Calculation Process: The MD map was projected onto the FA skeleton, and a custom-designed mask was applied to obtain the MD skeleton for each subject. PSMD was determined by calculating the difference between the 95th and 5th percentiles of the voxel-based MD skeleton, based on histogram analysis. PSMD: Peak width of the skeletonized mean diffusivity; DTI-ALP: Diffusion tensor imaging along perivascular spaces
Quantification of choroid plexus volume
To achieve accurate choroid plexus segmentation, this study utilized a previously published fully convolutional neural network employing a pre-trained 3D U-Net deep learning model [56]. This model was extensively trained on multiple MRI sequences and exhibited superior performance compared to the classic FreeSurfer automatic segmentation method. The selected T1-weighted images were first non-linearly registered using the Advanced Normalization Tools (ANTs) and then normalized to the MNI 152 T1-weighted template. After registration, the images were cropped within the choroid plexus region based on probability maps provided by the creators of the U-Net model, ensuring consistency in the region of interest (ROI). The trained model was then applied to these cropped images for automatic segmentation, generating choroid plexus masks. We thank the reviewer for this important question. As described in the Methods section, choroid plexus segmentation was performed using a validated deep learning model based on a 3D U-Net architecture. All segmentation results were visually inspected by two experienced radiologist (Rater 1: Jianliang Miao; Rater 2: Lei Pan). Finally, the segmented masks were used to calculate the choroid plexus volume for each subject in standard space.
Coupling of BOLD signal and CSF flow
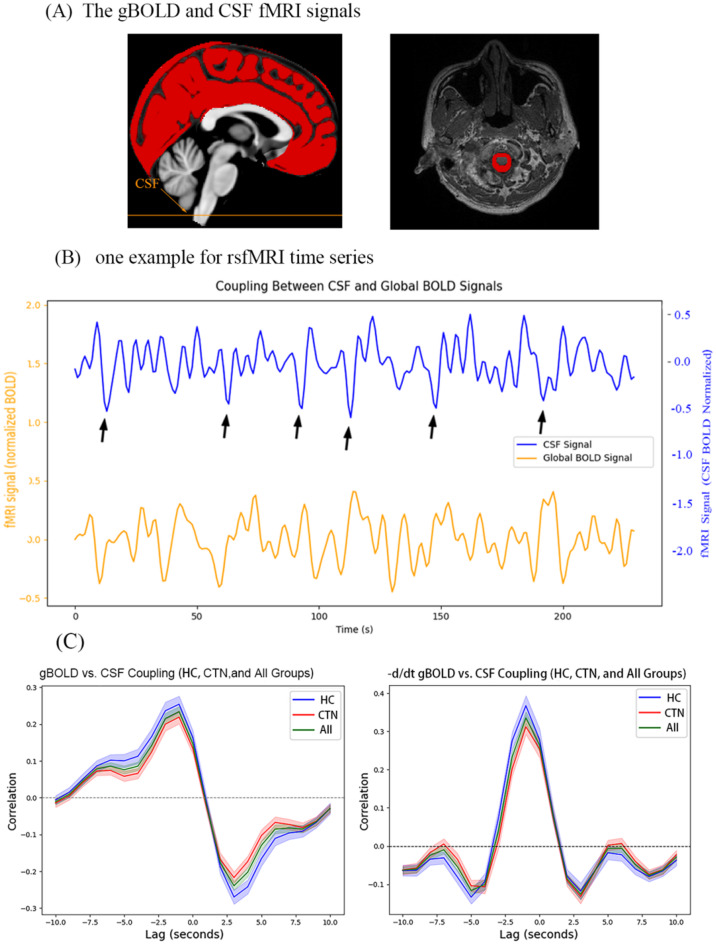
To analyze the coupling between BOLD signal and CSF flow, we first aligned the T1-weighted images with the rs-fMRI data using linear registration. The gBOLD signal was extracted from the cortical gray matter regions defined in the Harvard-Oxford cortical structural atlas (https://neurovault.org/collections/262/) [57]. We excluded subcortical regions due to their increased susceptibility to vascular artifacts in BOLD signals. After completing all preprocessing steps, we extracted and averaged the fMRI signal from each individual’s gray matter mask. Prior to averaging, the fMRI signal was normalized to Z-scores. CSF signal was extracted from the bottom slices of the fMRI data, manually delineated by clinical radiologists. These slices, located at the bottom of the cerebellum, were chosen to maximize sensitivity to the CSF inflow effect. The CSF mask for each participant was manually defined on the bottom slice of the fMRI images, with its position further confirmed using T1-weighted structural images, as shown in Fig. 1A. The Z-standardized mean time series for both gBOLD and CSF signals were then extracted based on each participant’s signal intensity.
Fig. 1.
(A) Extraction of gBOLD signal: The gBOLD signal is extracted from the gray matter regions, while the CSF signal is extracted from the CSF region in the bottom slices of the fMRI acquisition. (B) Representative participant’s gBOLD and CSF signals: The corresponding changes in both signals are shown, demonstrating a marked amplitude correlation. (C) Average gBOLD-CSF cross-correlation functions for the TN, HC, and all participants: The average gBOLD-CSF coupling index shows a positive peak at a lag of -3 s (R = 0.19, P < 0.001, permutation test, n = 10,000), and a significant negative peak at a lag of + 3 s (R = -0.21, P < 0.001, permutation test, n = 10,000). The gray shaded area represents the 95% confidence interval. The negative derivatives of the gBOLD-CSF cross-correlation function show a pattern consistent with systemic coupling between the whole-brain signal and CSF flow. gBOLD, global blood oxygen level-dependent; CSF, cerebrospinal fluid; fMRI, functional magnetic resonance imaging
After extracting the cortical gray matter and CSF signals, to quantify the gBOLD-CSF coupling, we used Pearson’s correlation function to calculate the cross-correlation coefficient between the two signals over a range of time delays (-10 to + 10 s). In our study, we observed that the negative peak at + 3 s and the positive peak at -3 s exhibited similar amplitudes in both the CTN and HC groups. Therefore, we chose the negative peak at + 3 s to quantify the gBOLD-CSF coupling. Additionally, the cross-correlation function between the negative first derivative of the gBOLD signal and the CSF signal was also computed, as described previously [58].
To validate the reliability of the gBOLD-CSF coupling, we employed a permutation strategy in which the gBOLD time series and the CSF time series from different participants were randomly swapped. This process was repeated 10,000 times to establish a null hypothesis distribution based on these correlations. Finally, p-values were calculated from the percentiles of this null distribution. All computations were performed using Python 3.11.10 [59, 60].
DTI-ALPS analysis
ALPS analysis: After data preprocessing, we used FSL to calculate the brain tensor in x (left and right direction), y (anterior and posterior direction) and z (up and down direction) directions for each voxel. Place four 5 mm diameter spherical ROIs in the MNI T1 template (MNI 152_T1_1mm_brain). There were two regions were drawn over the area of projection fibers (ROIproj) with the major fiber running along the z-axis (the center coordinates were (24, − 12, 24) and (− 28, − 12, 24) respectively), and the other two regions over the area of association fibers (ROIassoc) with the major fibers running along the y-axis (the center coordinates were (36, − 12, 24) and (− 40, − 12, 24) respectively) in the bilateral hemispheres 38. The ROI mask was reversely registered to the FA image of each subject by the deformation field generated during the registration process. Manual verification confirmed the accuracy of registration and the location of ROIs by clinical radiology expert in FSL-EYES, which was that the blue voxels were included in the ROIproj and green voxels in the ROIassoc only. Then, the diffusivities along the x-axis (Dxx), y-axis (Dyy), and z-axis (Dzz) were extracted for each ROI. Finally, the ALPS index was calculated as the ratio of diffusivities perpendicular to fiber bundles and parallel to perivascular space (Dxxproj and Dxxassoc) over diffusivities perpendicular to fiber bundles and perpendicular to perivascular space (Dyyproj and Dzzassoc). ALPS index in the left hemisphere (left ALPS index) and right hemisphere (right ALPS index) were calculated respectively using the following formula. ALPS index = mean (Dxxproj, Dxxassoc) / mean (Dyy_proj, Dzz_assoc). The mean ALPS index = (left ALPS index + right ALPS index) /2, as shown in Fig. 2B.
Quantification of PSMD
The calculation of PSMD follows the steps shown in Fig. 2C. PSMD values are automatically computed across the entire brain using a shell script, with no user intervention required (https://www.psmd-marker.com). The script involves several processing steps, including the use of Tract-Based Spatial Statistics (TBSS) [60] for skeletonization of white matter (WM) tracts, while considering the effects of diffusion partial volume on the white matter skeleton. The MD skeleton is then further processed using a standard skeleton with a FA threshold of 0.2 and a custom mask provided by the PSMD toolbox. To exclude the ventricular regions, the resulting MD skeleton is masked with a custom mask. Histogram analysis is then performed on the masked MD skeleton. PSMD is calculated by measuring the difference between the 95th and 5th percentile voxel-based MD values within the white matter skeleton [49].
Statistical analysis
All statistical analyses were performed using SPSS software (version 26.0). The normality of continuous variables, including age, years of education, TIV, ALPS index, gBOLD-CSF coupling index, VAS score, MMSE score, and pain frequency, was assessed using the Shapiro-Wilk test. Normally distributed data are presented as mean ± standard deviation (SD), and intergroup comparisons were conducted using independent samples t-tests. For non-normally distributed data, interquartile range (IQR) was used, and group comparisons were performed using the Mann-Whitney U test. To investigate the potential impact of confounding variables on glymphatic-related indices, regression analysis was used to explore the relationship between age and CP volume, gBOLD-CSF coupling index, and ALPS index. Group comparisons of continuous variables were then performed using a general linear model with ANCOVA, adjusting for age, gender, years of education, and TIV as covariates. Chi-square tests were employed for comparisons of categorical variables. Statistical significance was defined as a two-tailed P-value of < 0.05. To examine the associations between the ALPS index, gBOLD-CSF coupling index, choroid plexus volume, PSMD, GMV/TIV, WMV/TIV, CSF/TIV, and clinical characteristics, partial correlation analysis was conducted, controlling for age, gender, and years of education. A heatmap was generated for visualization.
Results
Demographic and clinical characteristics
This study enrolled 131 CTN patients (89 males, 42 females; age 56 [49-60.25] years) and 106 HC matched for age and gender (75 males, 31 females; age 56 [50–61] years). No significant differences were observed between the two groups in terms of age, gender, education level, or total intracranial volume (TIV) (all P > 0.05). Among the CTN patients, 85 reported right-sided pain, and 52 had pain confined to the V2.3 region. The majority of patients reported severe pain, with a Visual Analog Scale (VAS) score of 9 (7–10). Thirty-seven CTN patients reported pain lasting longer than two minutes, which may be associated with peripheral or central sensitization. Compared to the healthy controls, CTN patients exhibited statistically lower quality of life and higher levels of depression and anxiety, as shown in Table 1. Although a statistically significant difference in MMSE scores was observed, the 1-point group difference is unlikely considered clinically meaningful.
Table 1.
Baseline characteristics of the participants
| HC(n = 106) | CTN(n = 131) | P | |
|---|---|---|---|
| Age | 56(49, 60.25) | 56(50, 61) | 0.513 |
| sex(men) | 75(70.75%) | 89(64.03%) | 0.269 |
| Education | 9(6, 12) | 9(6, 9) | 0.178 |
| TIV | 1419.45 ± 130.86 | 1407.18 ± 118.60 | 0.701 |
| MMSE | 29(28, 30) | 28(26, 29) | < 0.001 |
| TNQOLS | 23.41 ± 0.29 | 49.15 ± 0.80 | < 0.001 |
| SDS | 35(31.25, 41.25) | 45(38.75, 52.5) | < 0.001 |
| SDA | 33.75(31.25, 40) | 40(35, 45) | < 0.001 |
| VAS | N/A | 9(7, 10) | N/A |
| disease_duration | N/A | 3(1.2, 7) | N/A |
TIV, Total Intracranial Volume; MMSE, Mini-Mental State Examination; TNQOLS, Trigeminal Neuralgia Quality of Life Scale; SDS: Self-Rating Depression Scale; SDA: Self-Reported Anxiety Scale; VAS, Visual Analog Scale
Coupling of gBOLD and CSF signal changes
In a representative subject, the peak of the gBOLD signal typically follows the peak of the CSF signal, indicating a potential coupling between the two signals. In some instances, the peak of the CSF signal precedes the peak of the gBOLD signal, as shown in Fig. 1B.
The average cross-correlation function between the gBOLD and CSF signals exhibited a significant positive peak at a lag of -3 s (R = 0.19, P < 0.001, permutation test: n = 10,000) and a significant negative peak at a lag of + 3 s (R = -0.21, P < 0.001, permutation test: n = 10,000). Additionally, the cross-correlation function between the negative derivative of the CSF signal and the gBOLD signal revealed a significant positive peak at a lag of -1.5 s (d/dt R = 0.24, P < 0.001, permutation test: n = 10,000).
We also calculated coupling relationship graphs for the case group, control group, and the entire sample, which were consistent with previous studies. These findings confirm the presence of a significant coupling between the gBOLD and CSF signals in our sample, as illustrated in Fig. 1C.
Intergroup comparison of CP volume, ALPS index, and gBOLD-CSF coupling index
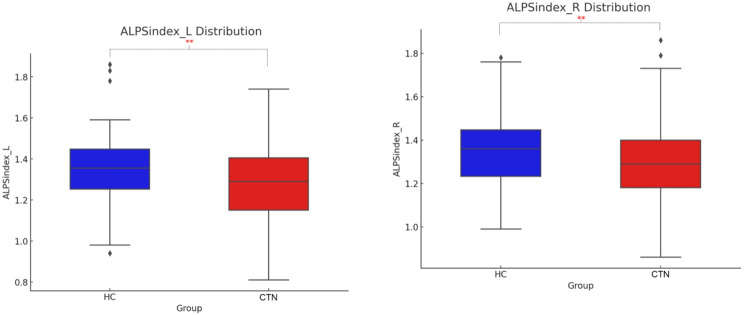
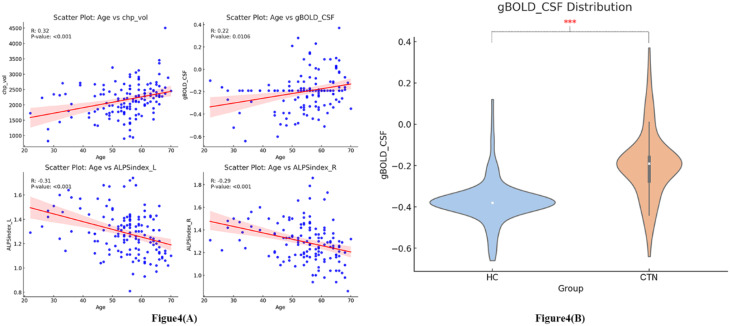
Regression analysis revealed significant predictive relationships between age and CP volume, gBOLD-CSF coupling, as well as the left and right ALPS indices (R = 0.32, P < 0.001; R = 0.22, P = 0.011; R = -0.31, P < 0.001; R = -0.29, P < 0.001), as illustrated in Fig. 4A. Significant differences were found between CTN patients and healthy controls in CP volume, gBOLD-CSF coupling, and the left and right ALPS indices (R = 5.312, P = 0.022; R = 91.812, P < 0.001; R = 10.099, P = 0.002; R = 8.565, P = 0.004), as shown in Table 2; Figs. 3 and 4(B). CTN patients exhibited significantly increased CP volume (F = 5.312, P = 0.022). The gBOLD-CSF coupling index in CTN patients was significantly higher than that of healthy controls (F = 91.812, P < 0.001). In contrast, the left and right ALPS indices in CTN patients were significantly lower compared to healthy controls (F = 10.099, P = 0.002; F = 8.565, P = 0.004).
Table 2.
Structural and glymphatic indices of participants
| HC | CTN | Coefficient value | P | |
|---|---|---|---|---|
| GMV/TIV | 0.441 ± 0.002 | 0.436 ± 0.002 | 4.879 | 0.028 |
| WMV/TIV | 0.365(0.350, 0.373) | 0.357(0.342, 0.370) | 6.898 | 0.009 |
| CSF/TIV | 0.195(0.178, 0.212) | 0.203(0.187, 0.222) | 11.818 | < 0.001 |
| PSMD(10− 3) | 0.296(0.278, 0.313) | 0.307(0.289, 0.331) | 11.891 | < 0.001 |
| Chp_vol | 2035(1676, 2386) | 2203(1787, 2484) | 5.312 | 0.022 |
| ALPSindex_L | 1.356 ± 0.016 | 1.288 ± 0.015 | 10.099 | 0.002 |
| ALPSindex_R | 1.350 ± 0.016 | 1.293 ± 0.015 | 8.565 | 0.004 |
| gBOLD-CSF | -0.380(-0.390, -0.370) | -0.190(-0.160, -0.280) | 91.812 | < 0.001 |
GMV, Gray matter volume; WM, white matter volume; CSF, cerebrospinal fuid; TIV, total intracranial volume; PSMD, peak width of skeletonized mean difusivity; Chp_vo: choroid plexus volume; ALPS, analysis along the perivascular space; gBOLD, global blood oxygen level-dependent
Fig. 3.
Boxplot of ALPS Index for Healthy Controls and CTN Patients. ALPS, analysis along the perivascular space; HC, healthy controls; CTN, Classical Trigeminal Neuralgia
Fig. 4.
(A), Regression analysis results showing the relationship between age and choroid plexus volume, ALPS index, and gBOLD-CSF coupling index. (B), Violin plot of gBOLD-CSF coupling in HC and CTN patients
Brain structural index group comparisons
In CTN patients, both PSMD and CSF volume were significantly increased (F = 11.891, P < 0.001; F = 11.818, P < 0.001). In contrast, brain GMV/TIV and WMV/TIV were significantly reduced (F = 4.879, P = 0.028; F = 6.898, P = 0.009), as shown in Table 2.
Correlation between glymphatic indices and CTN characteristics
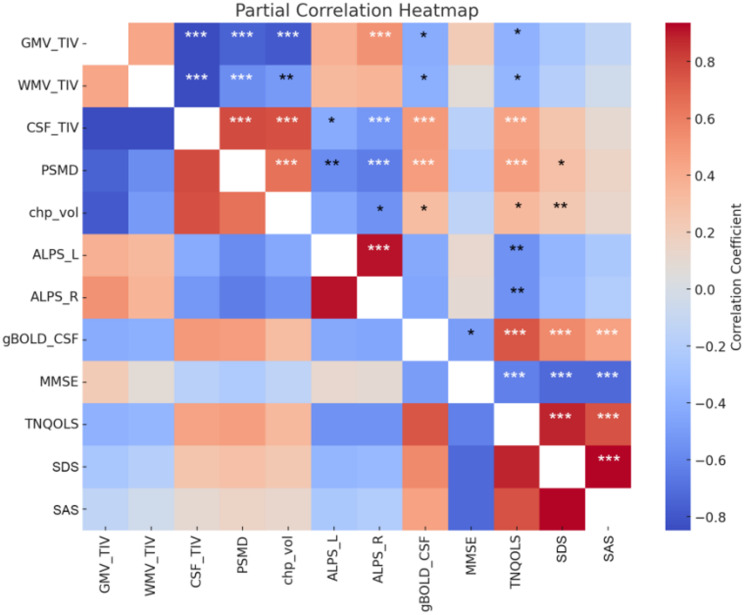
The gBOLD-CSF coupling index was significantly positively correlated with CSF volume, PSMD, CP volume, TNQOLS, SDS, and SAS scores (all P < 0.05), while showing a significant negative correlation with GMV/TIV and WMV/TIV, as well as MMSE scores (all P < 0.05). The left ALPS index was significantly negatively correlated with CSF/TIV, PSMD, and TNQOLS scores (all P < 0.05). The right ALPS index exhibited significant negative correlations with CSF/TIV, PSMD, CP volume, and TNQOLS scores (all P < 0.05), but a significant positive correlation with GMV/TIV (R = 0.223; P < 0.001). CP volume showed significant negative correlations with both GMV/TIV and WMV/TIV (both P < 0.01), and significant positive correlations with CSF/TIV, PSMD, TNQOLS, and SDS scores (all P < 0.05). However, no significant relationships were found between glymphatic indices and VAS scores or disease duration (both P > 0.05). A significant negative correlation was observed between WMV/TIV and VAS scores (R = -0.21, P = 0.014), as shown in Fig. 5. No formal correction for multiple comparisons was applied.
Fig. 5.
Partial Correlation Heatmap Between Glymphatic Function Indices, Brain Structural Indices, and Clinical Scales. ALPS, Analysis Along the Perivascular Space; gBOLD, Global Blood Oxygen Level Dependent; GMV, Gray Matter Volume; WMV, White Matter Volume; CSF, Cerebrospinal Fluid; TIV, Total Intracranial Volume; PSMD, Peak Width of Skeletonized Mean Diffusivity; chp_vol, Choroid Plexus Volume; MMSE, Mini-Mental State Examination; TNQOLS, Trigeminal Neuralgia Quality of Life Scale; SDS, Self-Rating Depression Scale; SDA, Self-Reported Anxiety Scale. No correction for multiple comparisons was applied
Discussion
In this study, we identified converging evidence of glymphatic dysfunction and structural brain alterations in patients with CTN. Using three complementary MRI-based glymphatic markers— CP volume, gBOLD-CSF coupling, and DTI-ALPS index—we observed significant disruptions in CSF production, synchronization, and clearance pathways. These functional abnormalities were accompanied by increased PSMD and reduced gray and white matter volumes, indicating concurrent microstructural disruption and macrostructural atrophy. Importantly, glymphatic metrics were correlated with structural injury and clinical symptoms, supporting a neurofluidic-structural-behavioral model of CTN pathophysiology.
To our knowledge, this is the first study to comprehensively evaluate glymphatic function in CTN. Notably, CP volume was significantly increased in CTN patients compared to healthy controls, suggesting a potential compensatory upregulation of CSF production in response to impaired clearance. The choroid plexus, as a key site of CSF generation and immune regulation, may act to sustain fluid homeostasis when interstitial clearance is compromised. Prior studies have shown that CP enlargement correlates with elevated CSF production and enhanced CSF-ISF exchange, thereby facilitating waste removal [61, 62]. In contrast, CTN patients exhibited reduced gBOLD-CSF coupling and lower DTI-ALPS index values, reflecting impaired fluid pulsatility and perivascular transport. These abnormalities likely reflect disrupted coordination between vascular and fluid oscillations, potentially influenced by astrocytic dysfunction or altered aquaporin-4 polarization, as suggested by prior studies in chronic pain models [63, 64]. The reduced ALPS index further suggests inefficient glymphatic flow along white matter pathways, which may compromise solute clearance and destabilize neuronal network homeostasis [65]. Together, these findings point to a breakdown of the integrated neurofluidic circuit involving CSF production, entrainment, and clearance in CTN.
Structural abnormalities observed in CTN patients likely represent downstream consequences of glymphatic dysfunction and neurofluidic imbalance. Specifically, the PSMD index—a sensitive marker of white matter microstructural integrity—was significantly elevated in CTN and correlated with functional glymphatic metrics. This suggests that impaired CSF-ISF exchange may compromise perivascular clearance, promoting axonal injury, demyelination, or extracellular matrix disruption. In parallel, reductions in gray and white matter volume point to global atrophic processes that may plausibly reflect sustained metabolic stress or inflammation potentially associated with chronic neurofluidic dysregulation. These findings are consistent with studies in other chronic pain conditions. In cancer-related pain, for instance, the ALPS index negatively correlates with pain severity, possibly due to increased AQP4 expression and altered CSF/ISF dynamics driven by tumor-related inflammation [66, 67]. Similarly, patients with chronic migraine—particularly those with medication overuse—show glymphatic impairment correlated with clinical symptom burden [68]. In contrast, no significant glymphatic changes were reported in patients with new daily persistent headache (NDPH) [69], highlighting that neurofluidic responses may be condition-specific. It has been proposed that different pain types influence the glymphatic system through distinct physiological mechanisms, including neuroinflammation, CGRP dysregulation, and cortical spreading inhibition [70]. Taken together, these data position CTN within a broader model in which chronic pain-induced glymphatic disruption contributes to diffuse white matter injury and structural brain deterioration, potentially reinforcing central sensitization.
The mechanisms linking glymphatic dysfunction to central sensitization in CTN likely involve a cascade of neuroinflammatory and glial responses. Chronic pain has been shown to activate astrocytes via the JAK-STAT3 and TNF-α pathways, leading to the loss of AQP4 polarity and reduced CSF-ISF exchange efficiency [71, 72]. In CTN, vascular compression of the trigeminal nerve root may initiate abnormal afferent excitability and glial activation, which in turn amplifies nociceptive input to higher-order brain regions. Key central structures such as the trigeminal spinal nucleus (SpV), locus coeruleus (LC), medial prefrontal cortex (mPFC), and amygdala—known for their roles in pain modulation, mood regulation, and cognitive function—are highly metabolically active and dependent on intact glymphatic clearance [73, 74]. In our cohort, glymphatic impairment was significantly associated with symptoms of depression and anxiety, but not with pain severity or disease duration. This dissociation may reflect a trait–state mismatch, in which neurofluidic metrics capture stable vulnerability traits, whereas pain ratings reflect transient symptom states. Sampling variability or limited range of pain scores may also have constrained the detection of associations. These findings nonetheless support the hypothesis of a brain-CSF-ganglion communication axis in CTN, whereby disrupted neurofluidic dynamics may influence limbic–brainstem integration and contribute to maladaptive affective processing, potentially modulating central pain sensitivity over time.
Taken together, our results support a systems-level model in which glymphatic dysfunction contributes to white matter disintegration and brain atrophy, particularly within circuits implicated in mood and cognition. PSMD emerges as a structural correlate of fluid dysregulation, bridging CSF dynamics and neural network stability. Integration of functional and structural imaging biomarkers may provide novel tools for disease stratification, early detection, and personalized therapeutic targeting in CTN.
Conclusion
This study provides a comprehensive evaluation of glymphatic function and brain structural integrity in CTN patients using multimodal MRI. The observed alterations in CP volume, gBOLD-CSF coupling, ALPS index, and PSMD provide imaging-based evidence supporting a potential link between neurofluidic dysfunction and brain structural degeneration in CTN. These findings offer mechanistic insights and highlight the potential of neurofluidic imaging biomarkers in guiding diagnosis and treatment.
Limitation
First, given the significant impact of sleep on the glymphatic system, sleep disorders may influence its function in the brain. However, sleep quality and insomnia scales were not assessed in this study; future research will include these evaluations. Second, the cross-sectional design of this study precludes the ability to dynamically observe changes in the glymphatic system and brain structure over time. Longitudinal studies are required to establish causal relationships and evaluate the clinical implications.
Acknowledgements
Thanks to all the researchers who participated in this study, all the patients who provided data, and the researchers who provided literature support.
Author contributions
J. Y., H.Y collected the data. F.C., X.G., and Y.Z. analyzed the data. D.W. and L.P. reviewed the data. F.C., Z.Z., and M.J wrote the manuscript. Z.D. and X.G. approved the final draft.
Funding
This study was supported by the Zhejiang Provincial Medical and Health Technology Project (2025KY1058), the Natural Science Foundation of Zhejiang Province (LQN25H090010, Y22H185692), Hangzhou Agriculture and Social Development Scientific Research Guidance Project (20211231Y022), Zhejiang Provincial Traditional Chinese Medicine Science and Technology (2024ZL749), and the Medical and Health Technology Project of Hangzhou (A20231055).
Data availability
The dataset used and analyzed in this study is from Hangzhou First People’s Hospital affiliated with Xihu University in Hangzhou, Zhejiang Province, China, and can be obtained from the corresponding author upon reasonable request.
Declarations
Ethical approval
This study was approved by the local ethics committee of the Affiliated Hangzhou First People’s Hospital, Westlake University School of Medicine (IRB# NO.202107002). All investigations were carried out following the Declaration of Helsinki, and all the participants provided written informed consent.
Consent for publication
This study has obtained informed consents from patients, all human research procedures followed the committee’s ethical standards for human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2008.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Fenyang Chen, Zhiliang Zhang and Jianliang Miao contributed equally to this work.
Contributor Information
Zhongxiang Ding, Email: hangzhoudzx73@126.com.
Xiuhong Ge, Email: gxh478556386@163.com.
References
- 1.Cruccu G, Di Stefano G, Truini A. Trigeminal neuralgia. N Engl J Med. 2020;383(8):754–62. [DOI] [PubMed] [Google Scholar]
- 2.Finnerup NB. Trigeminal neuralgia and the merit of small clinical trials. Lancet Neurol. 2022;21(11):951–3. [DOI] [PubMed] [Google Scholar]
- 3.Jay GW, Barkin RL. Trigeminal neuralgia and persistent idiopathic facial pain (atypical facial pain). Dis Mon. 2022;68(6):101302. [DOI] [PubMed] [Google Scholar]
- 4.Zhao W, Yang L, Deng A, et al. Long-term outcomes and predictors of percutaneous radiofrequency thermocoagulation of Gasserian ganglion for maxillary trigeminal neuralgia: a retrospective analysis of 1070 patients with minimum 2-year follow-up. Ann Med. 2022;54(1):2420–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Andersen ASS, Heinskou TB, Rochat P, et al. Microvascular decompression in trigeminal neuralgia - a prospective study of 115 patients. J Headache Pain. 2022;23(1):145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Headache Classification Committee of the International Headache Society (IHS) The International Classification of Headache Disorders, 3rd edition. Cephalalgia. 2018;38(1):1-211. [DOI] [PubMed]
- 7.Szmyd B, Sołek J, Błaszczyk M, et al. The underlying pathogenesis of neurovascular compression syndromes: A systematic review. Front Mol Neurosci. 2022;15:923089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ge X, Wang L, Pan L, et al. Risk factors for unilateral trigeminal neuralgia based on machine learning. Front Neurol. 2022;13:862973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ge X, Wang L, Wang M, et al. Alteration of brain network centrality in CTN patients after a single triggering pain. Front Neurosci. 2023;17:1109684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ge X, Wang L, Yan J, et al. Altered brain function in classical trigeminal neuralgia patients: ALFF, reho, and DC static- and dynamic-frequency study. Cereb Cortex. 2024;34(1):bhad455. [DOI] [PubMed] [Google Scholar]
- 11.Yan J, Wang L, Pan L, et al. Analyzing the risk factors of unilateral trigeminal neuralgia under neurovascular compression. Front Hum Neurosci. 2024;18:1349186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhao W, Yin C, Ma L, et al. Predictive value of MRI for identifying symptomatic neurovascular compressions in classical trigeminal neuralgia: a PRISMA-compliant meta-analysis. BMC Neurol. 2024;24(1):466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ge X, Wang L, Pan L, et al. Alteration of the cortical morphology in classical trigeminal neuralgia: voxel-, deformation-, and surface-based analysis. J Headache Pain. 2023;24(1):17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ge X, Wang L, Pan L, et al. Amplitude of low-frequency fluctuation after a single-trigger pain in patients with classical trigeminal neuralgia. J Headache Pain. 2022;23(1):117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhu PW, Chen Y, Gong YX, et al. Altered brain network centrality in patients with trigeminal neuralgia: a resting-state fMRI study. Acta Radiol. 2020;61(1):67–75. [DOI] [PubMed] [Google Scholar]
- 16.Liu H, Hou H, Li F et al. Structural and Functional Brain Changes in Patients With Classic Trigeminal Neuralgia: A Combination of Voxel-Based Morphometry and Resting-State Functional MRI Study. Front Neurosci. 202229;16:930765. [DOI] [PMC free article] [PubMed]
- 17.Wang FX, Xu WM, Xu CL, et al. Aquaporins and neuropathic pain. Front Biosci (Landmark Ed). 2023;28(2):35. [DOI] [PubMed] [Google Scholar]
- 18.Peng S, Liu J, Liang C, et al. Aquaporin-4 in glymphatic system, and its implication for central nervous system disorders. Neurobiol Dis. 2023;179:106035. [DOI] [PubMed] [Google Scholar]
- 19.Feng S, Wu C, Zou P, et al. High-intensity interval training ameliorates alzheimer’s disease-like pathology by regulating astrocyte phenotype-associated AQP4 polarization. Theranostics. 2023;13(10):3434–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cai J, Yan Y, Zhang D, et al. Silencing of LncRNA Gm14461 alleviates pain in trigeminal neuralgia through inhibiting astrocyte activation. IUBMB Life. 2020;72(12):2663–71. [DOI] [PubMed] [Google Scholar]
- 21.Murdock MH, Yang CY, Sun N, et al. Multisensory gamma stimulation promotes glymphatic clearance of amyloid. Nature. 2024;627(8002):149–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McGinnis A, Ji RR. The similar and distinct roles of satellite glial cells and spinal astrocytes in neuropathic pain. Cells. 2023;12(6):965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Iliff JJ, Wang M, Liao Y, et al. A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid β. Sci Transl Med. 2012;4(147):147ra111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Feng S, Wu C, Zou P, et al. High-intensity interval training ameliorates alzheimer’s disease-like pathology by regulating astrocyte phenotype-associated AQP4 polarization[J]. Theranostics. 2023;13(10):3434–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pittock SJ, Zekeridou A, Weinshenker BG. Hope for patients with neuromyelitis Optica spectrum disorders—from mechanisms to trials[J]. Nat Reviews Neurol. 2021;17(12):759–73. [DOI] [PubMed] [Google Scholar]
- 26.Si X, Dai S, Fang Y, et al. Matrix metalloproteinase-9 Inhibition prevents aquaporin-4 depolarization-mediated glymphatic dysfunction in parkinson’s disease[J]. J Adv Res. 2024;56:125–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen C, Qin Y, Xu Y, et al. Aquaporins enriched in endothelial vacuole membrane regulate the diameters of microvasculature in hyperglycaemia[J]. Cardiovascular Res. 2024;120(9):1065–80. [DOI] [PubMed] [Google Scholar]
- 28.Xu M, Xiao M, Li S, et al. Aquaporins in nervous System[C]// advances in experimental medicine and biology. Dordrecht: Springer; 2017. pp. 81–103. [DOI] [PubMed] [Google Scholar]
- 29.Asano S, Hayashi Y, Iwata K, et al. Microglia-Astrocyte communication via C1q contributes to orofacial neuropathic pain associated with infraorbital nerve injury. Int J Mol Sci. 2020;21(18):6834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Son JY, Ju JS, Kim YM, et al. TNF-α-Mediated RIPK1 pathway participates in the development of trigeminal neuropathic pain in rats. Int J Mol Sci. 2022;23(1):506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kc E, Islam J, Kim HK, et al. GFAP-NpHR mediated optogenetic Inhibition of trigeminal nucleus caudalis attenuates hypersensitive behaviors and thalamic discharge attributed to infraorbital nerve constriction injury. J Headache Pain. 2023;24(1):137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mu G, Ren C, Zhang Y, et al. Amelioration of central neurodegeneration by docosahexaenoic acid in trigeminal neuralgia rats through the regulation of central neuroinflammation. Int Immunopharmacol. 2023;114:109544. [DOI] [PubMed] [Google Scholar]
- 33.Huang SY, Zhang YR, Guo Y, et al. Glymphatic system dysfunction predicts amyloid deposition, neurodegeneration, and clinical progression in alzheimer’s disease. Alzheimers Dement. 2024;20(5):3251–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Meng JC, Shen MQ, Lu YL, et al. Correlation of glymphatic system abnormalities with parkinson’s disease progression: a clinical study based on non-invasive fMRI. J Neurol. 2024;271(1):457–71. [DOI] [PubMed] [Google Scholar]
- 35.Hablitz LM, Nedergaard M. The glymphatic system: A novel component of fundamental neurobiology. J Neurosci. 2021;41(37):7698–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kaag Rasmussen M, Møllgård K, Bork PAR, et al. Trigeminal ganglion neurons are directly activated by influx of CSF solutes in a migraine model. Science. 2024;385(6704):80–6. [DOI] [PubMed] [Google Scholar]
- 37.Ericson H, Abu Hamdeh S, Freyhult E, et al. Cerebrospinal fluid biomarkers of inflammation in trigeminal neuralgia patients operated with microvascular decompression[J]. Pain. 2019;160(11):2603–11. [DOI] [PubMed] [Google Scholar]
- 38.Damkier HH, Brown PD, Praetorius J. Cerebrospinal fluid secretion by the choroid plexus[J]. Physiol Rev. 2013;93(4):1847–92. [DOI] [PubMed] [Google Scholar]
- 39.Courtney Y, Hochstetler A, Lehtinen MK. Choroid plexus pathophysiology. Annu Rev Pathol. 2025;20(1):193–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Choi JD, Moon Y, Kim HJ, Yim Y, Lee S, Moon WJ. Choroid plexus volume and permeability at brain MRI within the alzheimer disease clinical spectrum. Radiology. 2022;304(3):635–45. [DOI] [PubMed] [Google Scholar]
- 41.Li Y, Zhou Y, Zhong W, et al. Choroid plexus enlargement exacerbates white matter hyperintensity growth through glymphatic impairment. Ann Neurol. 2023;94(1):182–95. [DOI] [PubMed] [Google Scholar]
- 42.Kiviniemi V, Wang X, Korhonen V, et al. Ultra-fast magnetic resonance encephalography of physiological brain activity-Glymphatic pulsation mechanisms? J Cereb Blood Flow Metab. 2016;36:1033–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fultz NE, Bonmassar G, Setsompop K, et al. Coupled electrophysiological, hemodynamic, and cerebrospinal fluid oscillations in human sleep. Science. 2019;366(6465):628–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Taoka T, Masutani Y, Kawai H, et al. Evaluation of glymphatic system activity with the diffusion MR technique: diffusion tensor image analysis along the perivascular space (DTI-ALPS) in alzheimer’s disease cases. Jpn J Radiol. 2017;35(4):172–8. [DOI] [PubMed] [Google Scholar]
- 45.Taoka T, Ito R, Nakamichi R, Nakane T, Kawai H, Naganawa S. Diffusion tensor image analysis along the perivascular space (DTI-ALPS): revisiting the meaning and significance of the method. Magn Reson Med Sci. 2024;23(3):268–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liu X, Barisano G, Shao X, et al. Cross-Vendor Test-Retest validation of diffusion tensor image analysis along the perivascular space (DTI-ALPS) for evaluating glymphatic system function. Aging Dis. 2024;15(4):1885–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sabayan B, Westendorp RGJ. Neurovascular-glymphatic dysfunction and white matter lesions. Geroscience. 2021;43(4):1635–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Venkat P, Chopp M, Zacharek A, et al. White matter damage and glymphatic dysfunction in a model of vascular dementia in rats with no prior vascular pathologies. Neurobiol Aging. 2017;50:96–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Baykara E, Gesierich B, Adam R, et al. A novel imaging marker for small vessel disease based on skeletonization of white matter tracts and diffusion histograms. Ann Neurol. 2016;80(4):581–92. [DOI] [PubMed] [Google Scholar]
- 50.Baykara E, Gesierich B, Adam R, et al. A novel imaging marker for small vessel disease based on skeletonization of white matter tracts and diffusion histograms[J]. Ann Neurol. 2016;80(4):581–92. [DOI] [PubMed] [Google Scholar]
- 51.Luo Y, He M, Li C, Yang H. A research on quality of life score (QOLS) of patients with trigeminal neuralgia (TN). J Infect Public Health. 2019 Sep-Oct;12(5):690–4. [DOI] [PubMed]
- 52.Folstein MF, Folstein SE, McHugh PR. Mini-mental state. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–98. [DOI] [PubMed] [Google Scholar]
- 53.SDSZUNG WW. A SELF-RATING DEPRESSION SCALE. Arch Gen Psychiatry. 1965;12:63–70. [DOI] [PubMed] [Google Scholar]
- 54.Zung WW. A rating instrument for anxiety disorders. Psychosomatics. 1971;12(6):371–9. [DOI] [PubMed] [Google Scholar]
- 55.Isensee F, Schell M, Tursunova I, Brugnara G, Bonekamp D, Neuberger U, Wick A, Schlemmer HP, Heiland S, Wick W, Bendszus M, Maier-Hein KH, Kickingereder P. Automated brain extraction of multi-sequence MRI using artificial neural networks. Hum Brain Mapp. 2019;1:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Eisma JJ, McKnight CD, Hett K, et al. Deep learning segmentation of the choroid plexus from structural magnetic resonance imaging (MRI): validation and normative ranges across the adult lifespan. Fluids Barriers CNS. 2024;21(1):21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Desikan RS, Ségonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, et al. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. NeuroImage. 2006;31:968–80. [DOI] [PubMed] [Google Scholar]
- 58.Jiang D, Liu L, Kong Y, et al. Regional glymphatic abnormality in behavioral variant frontotemporal dementia. Ann Neurol. 2023;94(3):442–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Han F, Brown GL, Zhu Y, et al. Decoupling of global brain activity and cerebrospinal fluid flow in parkinson’s disease cognitive decline. Mov Disord. 2021;36(9):2066–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Smith SM, Jenkinson M, Johansen-Berg H, et al. Tract-based Spatial statistics: Voxelwise analysis of multi-subject diffusion data. NeuroImage. 2006;31(4):1487–505. [DOI] [PubMed] [Google Scholar]
- 61.Mehan WA, Poyiadji N, Paul AB, Buch K. Volumetric changes in the choroid plexus associated with spontaneous intracranial hypotension in patients with spinal CSF leak. AJNR Am J Neuroradiol. 2024;45(8):1162–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Municio C, Carrero L, Antequera D, Carro E. Choroid plexus Aquaporins in CSF homeostasis and the glymphatic system: their relevance for alzheimer’s disease. Int J Mol Sci. 2023;24(1):878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Generoso JS, Thorsdottir S, Collodel A, et al. Dysfunctional glymphatic system with disrupted Aquaporin 4 expression pattern on astrocytes causes bacterial product accumulation in the CSF during Pneumococcal meningitis. mBio. 2022;13(5):e0188622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Manouchehrian O, Ramos M, Bachiller S, Lundgaard I, Deierborg T. Acute systemic LPS-exposure impairs perivascular CSF distribution in mice. J Neuroinflammation. 2021;18(1):34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Song H, Ruan Z, Gao L, et al. Structural network efficiency mediates the association between glymphatic function and cognition in mild VCI: a DTI-ALPS study. Front Aging Neurosci. 2022;14:974114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wang A, Chen L, Tian C, et al. Evaluation of the glymphatic system with diffusion tensor Imaging-Along the perivascular space in Cancer pain. Front Neurosci. 2022;16:823701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhu B, Zhou W, Chen C, et al. AQP4 is an emerging regulator of pathological pain: A narrative review. Cell Mol Neurobiol. 2023;43(8):3997–4005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wu CH, Chang FC, Wang YF, et al. Impaired glymphatic and meningeal lymphatic functions in patients with chronic migraine. Ann Neurol. 2024;95(3):583–95. [DOI] [PubMed] [Google Scholar]
- 69.Zhang X, Wang W, Bai X, et al. Increased glymphatic system activity in migraine chronification by diffusion tensor image analysis along the perivascular space. J Headache Pain. 2023;24(1):147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Vittorini MG, Sahin A, Trojan A, et al. The glymphatic system in migraine and other headaches. J Headache Pain. 2024;25(1):34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tsuda M, Kohro Y, Yano T, et al. JAK-STAT3 pathway regulates spinal astrocyte proliferation and neuropathic pain maintenance in rats. Brain. 2011;134(Pt 4):1127–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Morioka N, Zhang FF, Nakamura Y, Kitamura T, Hisaoka-Nakashima K, Nakata Y. Tumor necrosis factor-mediated downregulation of spinal astrocytic connexin43 leads to increased glutamatergic neurotransmission and neuropathic pain in mice. Brain Behav Immun. 2015;49:293–310. [DOI] [PubMed] [Google Scholar]
- 73.Luo D, Lin R, Luo L, et al. Glial plasticity in the trigeminal root entry zone of a rat trigeminal neuralgia animal model. Neurochem Res. 2019;44(8):1893–902. [DOI] [PubMed] [Google Scholar]
- 74.He B, Wang W, Zhang R, et al. Fluorescence visualization of the neuropathic pain triad in trigeminal neuralgia. J Biophotonics. 2023;16(3):e202200301. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The dataset used and analyzed in this study is from Hangzhou First People’s Hospital affiliated with Xihu University in Hangzhou, Zhejiang Province, China, and can be obtained from the corresponding author upon reasonable request.