Abstract
Objectives
To verify whether propofol-assisted deep extubation is associated with fewer complications in pediatric patients undergoing dental treatment.
Materials and methods
This prospective study enrolled 60 pediatric candidates undergoing elective dental interventions, with randomized allocation to either deep extubation (DE; n = 30) or awake extubation (AE; n = 30) protocols. The primary endpoint was the incidence of at least 1 respiratory adverse event, whereas time to extubation (TOE), time to wake-up (TOW), hemodynamic fluctuations during extubation, incidence of emergence agitation (EA), nasal obstruction, epistaxis, sore throat, and hoarse voice after extubation were the secondary endpoints.
Results
The DE group reported significantly lower incidence of at least 1 respiratory adverse event (0.0% VS 23.3%, P = 0.016). The TOE was significantly shorter in the DE group, averaging 2.78 ± 0.87 min, compared to 5.50 ± 1.01 min in the AE group (P < .001). The TOW was longer in the DE group, with an average of 15.03 ± 3.44 min compared to 10.63 ± 1.52 min in the AE group (P < .001). The average value of mean arterial pressure (AVMAP) during extubation was lower in the DE group at 74.70 ± 13.35 mmHg, compared to 87.43 ± 15.31 mmHg in the AE group (P < .001). The average value of heart rate (AVHR) in the DE group was 108.37 ± 13.41 bpm, while in the AE group, it was 127.93 ± 20.74 bpm (P < .001). Additionally, the rates of sore throat and hoarse voice were significantly lower in the DE group (6.7% and 3.3%) than in the AE group (27% and 30%).
Conclusions
For pediatric patients undergoing dental treatment, propofol-assisted deep extubation is superior, allowing for less extubation time without increasing airway complications. This technique provides a smoother extubation with fewer hemodynamic fluctuations and lower incidences of voice hoarseness and persistent coughing, provided that certain rules for deep extubation are followed.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12871-025-03170-3.
Keywords: Deep extubation, Mouth rehabilitation, General anesthesia
Introduction
Tracheal extubation remains one of the most challenging and high-risk phases in perioperative anesthetic management [1]. Deep extubation (DE), defined by the deliberate maintenance of profound hypnotic and analgesic states during airway removal, has been increasingly adopted as a strategic approach to mitigate periextubation complications such as coughing, laryngospasm, and hemodynamic instability [2–4]. This technique demonstrates particular advantages in pediatric populations and uncooperative patients [5, 6]. Current evidence suggests that DE effectively reduces airway irritation and associated complications during extubation while improving patient comfort and procedural efficiency [2, 3, 7–13].
However, in many of those articles, patients receiving DE require inhalation anesthetics. During DE with desflurane, 12 (48.0%) of the 25 participants experienced coughing and breathholding [8], while tracheal extubation in deeply anesthetized pediatric patients using only high-concentration sevoflurane [9] was accompanied by more respiratory complications and a higher incidence of emergence agitation (EA). Based on a systematic review [3], DE reduces the overall airway complication risk but increases airway obstruction relative to awake extubation (AE) among pediatric patients under general anesthesia (GA).
Our previous retrospective study [6] indicated that propofol-assisted DE was appropriate for uncooperative pediatric patients [14] in the outpatient department. Airway obstruction (glossoptosis) would not occur following our DE procedure. These results provided the rationale for implementing this trial to evaluate the clinical efficacy of propofol-assisted DE in pediatric dental surgery. We hypothesize that propofol- assisted DE optimizes extubation conditions while reducing respiratory complication rates.
Materials and methods
Ethical approval was obtained from the Institutional Review Board of Baoding Second Hospital (Approval No. KY2023044) and prospectively registered on January 17, 2024 (www.chictr.org.cn; No. ChiCTR2400079951). Written informed consents were provided by legal guardians of all recruited children prior to study initiation. The trial complied with the Declaration of Helsinki and was conducted according to the relevant CONSORT guidelines (Supplementary file 1).
Subjects
Per the Guideline on Behavior Guidance for the Pediatric Dental Patient [14], uncooperative pediatric patients are defined as those with behavioral challenges (psychological/emotional immaturity, physical/mental disabilities affecting cooperation, and severe anxiety, fear, or communication barriers) and medical necessity (complex surgical interventions and GA required to reduce psychological trauma and medical risks).
Subjects eligible for the study were evaluated following the eligibility criteria one day prior to surgery at Baoding Second Hospital. Inclusion criteria: age 3 to 8 years old, eligible for nasotracheal intubation, American Society of Anesthesiologists (ASA) status I–II, and written informed consents. Exclusion criteria: ASA > II; contraindications for nasotracheal intubation; anticipated difficult airways; preoperative upper respiratory tract infection and a diagnosis of adenoid or tonsillar hypertrophy.
Randomization and blinding
A random number table was produced by SAS 8.0 for random allocation (simple randomization), which was then sealed in sequentially numbered, opaque envelopes by an unblinded statistician to ensure allocation concealment. Thereafter, subjects were assigned to the DE or AE group (1:1) according to the envelope provided at the end of the surgery before extubation. Only the attending anesthesiologist knew the randomization profile. The recruited children, investigators, and outcome analysts remained blinded to group assignments throughout the trial duration.
Anesthesia procedure
Patients without preoperative pharmacotherapy were escorted to the ambulatory surgical unit for standardized physiological monitoring, including ECG, pulse oximetry (SpO₂), capnography (ETCO₂), and noninvasive blood pressure. Anesthesia and dental interventions followed protocolized approaches aligned with institutional guidelines validated in prior study [6]. Opioid and muscle relaxant-free GA was applied to all the participants.
Sevoflurane (up to 8% end-tidal sevoflurane (ETsevo)) was inhaled via a facemask to induce anesthesia. Once patients lost consciousness and involuntary movements, dexamethasone (0.10 mg/kg) and propofol (2–3 mg/kg) were administered via titration method after intravenous catheter insertion. After spontaneous breathing ceased, nasotracheal intubation was performed through the wider nasal cavity prepared with an adrenaline swab. Patients were positioned supine with a doughnut pillow to maintain a neutral head position. Intubation was achieved using UEscope (Zhejiang UE Medical Corp., Zhejiang, China) and a Shiley RAE Nasal tube (Covidien, Ireland), with size determined by the Khine formula [15]. Experienced attending anesthesiologist (> 10 years of experience) performed intubation, confirmed by capnography waveforms and bilateral breath sounds.
Anesthesia maintenance was achieved through protocol-guided titration of sevoflurane (1–2% ETsevo) co-administered with propofol (2–4 mg/kg/h), targeting systolic blood pressure maintained within ± 20% of preoperative baseline and spontaneous ventilation preserved with respiratory rate (RR) 14–18 breaths/min, tidal volume (Vt) 6–8 mL/kg, and ETCO₂ 45–55 mmHg. Dental procedures (extraction, root canal, cavity filling) were performed. Rubber dam isolated most exogenous substances. Local anesthesia was given before stimulation. High-flow oxygen (8-10L/min) replaced sevoflurane 5 min before treatment completion. Post-procedure, instrument counts were confirmed, and oral secretions were evacuated using a saliva ejector. To minimize laryngospasm risk, tracheal suction catheter advancement was restricted to clinically necessary cases.
Awake extubation
Propofol and sevoflurane were discontinued 5 min before treatment ended. Extubation occurred when ETsevo < 0.1% with signs like facial grimace, adequate respiration, eye-opening, coughing with open mouth, or purposeful movements. In recovery, patients were placed laterally with oxygen supply and SpO2 monitoring until fully awake.
Deep extubation
The anesthetic protocol was executed in three sequential phases: (1) Sevoflurane administration was terminated 5 min before treatment ended. Propofol infusion (8–10 mg/kg/h) was maintained under dual endpoint monitoring before extubation: ETsevo < 0.1% and sustained lateral positioning without gross movement/coughing. (2) Physiological stability was confirmed through continuous assessment of respiratory parameters (RR 14–18 breaths/min, Vt 8–10 mL/kg, and ETCO₂ 45–55 mmHg). If gross movement/coughing occurred, 1 mg/kg propofol was added. (3) After extubation, the patient’s head was tilted back, oxygen was administered via facemask, and the patient was moved to postanesthesia care unit (PACU) laterally with continuous SpO2 monitoring until fully awake.
Complication management:
① In case of laryngospasm, 100% O₂ was administered via facemask under positive pressure, followed by 1 mg/kg propofol intravenously.
② Persistent post-extubation cough triggered the implementation of a standardized airway management protocol: immediate oropharyngeal secretion clearance, lateral decubitus positioning with thoracic percussion to enhance secretion drainage, and supplemental oxygen administration via facemask until respiratory stability was achieved.
③ When breath holding or glossoptosis occurred, oropharyngeal secretions were cleared, the modified jaw-thrust maneuver (MJTM) [16] was performed, the patient was positioned laterally, and oxygen was given via facemask under positive pressure.
④ Mild epistaxis was not threatening. Severe epistaxis should be managed with adrenaline drop while placing the patients in the lateral position on the same side as epistaxis.
Patients stayed in PACU till the Aldrete score was 9 or 10 [17]. Every patient was followed up by a blinded nurse till 24 h post-discharge.
Data of parameters below were obtained:
The primary endpoint was incidence of at least one respiratory adverse event, equal to the count of positive participants, who experienced at least one of coughing, laryngospasm, breath holding, glossoptosis, oxygen desaturation, and hypoxemia [18].
The secondary endpoints were comprised of time to extubation (TOE), time to wake-up (TOW), heart rate (HR), SpO2 and mean arterial pressure (MAP) at specific intervals, incidence of EA, nasal obstruction, epistaxis, sore throat, and hoarse voice after extubation.
Definitions of outcomes
Laryngospasm was defined as a complete airway obstruction characterized by sustained tonic adductor muscle activity and increased tension in the submental muscle group [1]. Glossoptosis was identified by posterior displacement of the tongue base accompanied by snoring sounds and visible respiratory effort. Coughing was described as a consecutive episode of vigorous and sustained coughs (> 10 s). Breathholding refers to the act of stopping or delaying the normal process of breathing lasting more than 10 s, not owning to laryngospasm or glossoptosis.
TOE refers to the duration between the completion of the operation and extubation. TOE involved in the procedure consisted of cleaning the oral cavity, putting the subject in his/her lateral decubitus position, and fine-tuning the anesthesia depth. TOW refers to the period between extubation and open eyes. Oxygen desaturation referred to 90–95% SpO2, while hypoxemia was 90% SpO2 for 10 s or longer.
Sugiyama et al. provided the criteria for recording epistaxis (Table 1) [19]. The identification and quantification of EA were completed by the PAED scale (Table 2) [20, 21].
Table 1.
Epistaxis severity
| Severity | Definition |
|---|---|
| None | No blood on tube or posterior pharyngeal wall surface |
| Mild | Obvious blood seen on tube or posterior pharyngeal wall surface |
| Moderate | Blood pooling on posterior pharyngeal wall |
| Severe | Excessive blood during pharynx impeding nasotracheal intubation that requires immediate orotracheal intubation |
Table 2.
The PAED scale
| 1. The child makes eye contact with the caregiver |
| 2. The child’s actions are purposeful |
| 3. The child is aware of his/her surroundings |
| 4. The child is restless |
| 5. The child is inconsolable |
The periextubation data about hemodynamic surveillance were collected from the anesthesia information management system, and stored at 1-min intervals from the end of surgery to being transferred to PACU.
Items 1, 2, and 3 are rated below: 4 not at all, 3 just a little,2 quite a bit, 1 very much, and 0 extremely. Whereas Items 4 and 5 are rated below: 0 not at all, 1 just a little, 2 quite a bit, 3 very much, and 4 extremely. All item scores were added up to acquire the total PAED scale score of 0–20. The emergence delirium degree showed a direct increase with the total score. EA was the PAED score ≥ 10.
Hypothesis statement and calculation of sample size
The assumptions below were applied to calculate sample size: the DE and AE groups would exhibit significantly different rates of overall respiratory adverse events. For our preliminary pilot study(unpublished), the incidence of airway-related complications was 2.6% for the DE group and 35% for the AE group. When α and β errors were set at 5% and 10%, power analysis using PASS 11 (NCSS, Kaysville, UT) suggested that a total sample size of 52 was necessary, comprising 26 participants in each group. After factoring in a 10% dropout rate, the total number of subjects required was determined to be 30 for each group.
Statistical analysis
Quantitative variables following a normal distribution were expressed as mean ± standard deviation (SD), while those not were described using median and interquartile range (Q1, Q3). Categorical variables were presented as percentages. For comparisons between groups, the t-test was utilized for normally distributed continuous data, and the Mann–Whitney U test for non-normally distributed continuous data. Categorical data were compared using either the Chi-square test or Fisher’s exact test, as appropriate. Ranked data were analyzed using the rank-sum test. A two-sided P-value of < 0.050 was considered statistically significant. All statistical analyses were performed using SPSS software version 23.0 (IBM Corp., Armonk, NY, USA).
Results
This trial was carried out between May 2024 and September 2024 and enrolled 60 participants, including 30 in the DE whereas 30 in the AE groups. Figure 1 displays the trial flowchart.
Fig. 1.
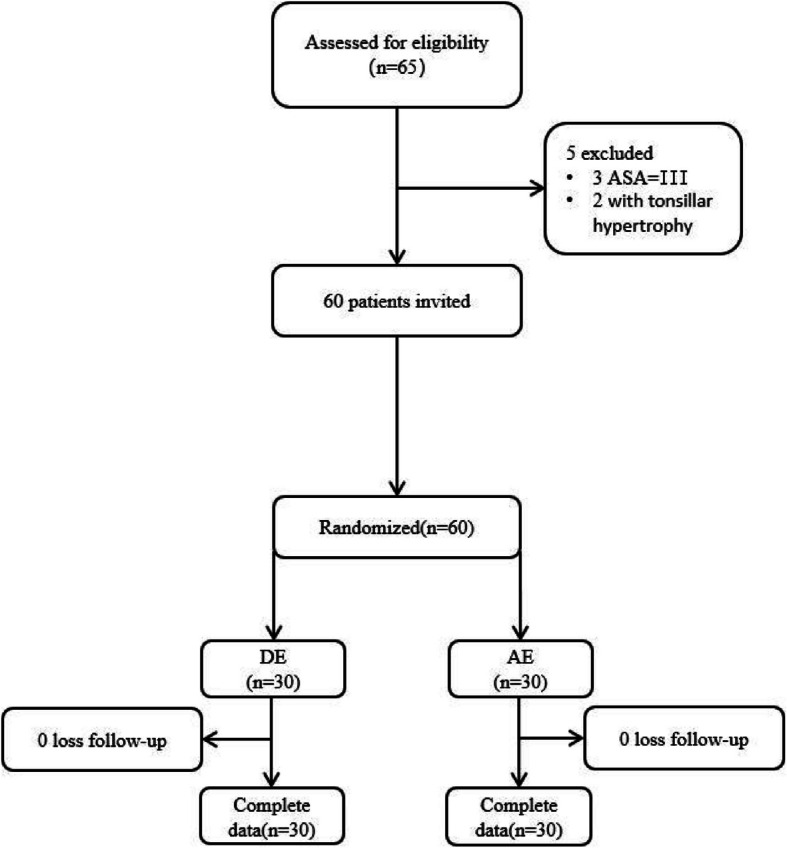
A trial flowchart
Patient characteristics are shown in Table 3. Demographic profiles, preoperative variables, and treatment categories were comparable between the two groups.
Table 3.
Basic features and preoperative data
| DE (n = 30) | AE (n = 30) | P | |
|---|---|---|---|
| Age (yr) | 4.8 ± 1.4 | 5.5 ± 1.5 | 0.736 |
| Female | 10 (33%) | 17 (57%) | 0.069 |
| BMI | 17.35 ± 4.12 | 18.63 ± 3.95 | 0.354 |
| ASA status | 0.671 | ||
| Grade I | 26 | 28 | |
| Grade II | 4 | 2 | |
| Duration of the procedure | 2.12 ± 0.49 | 2.55 ± 0.66 | 0.071 |
| Dental extraction | 15 (50%) | 22 (73%) | 0.063 |
| Root canal therapy | 29 (97%) | 27 (90%) | 0.612 |
BMI body mass index, ASA American Society of Anesthesiologists
The incidence of at least 1 respiratory adverse event for each group was 0.0% VS 23.3% (7 positive in AE) (P = 0.016), while statistical analysis revealed no significant difference in the rate of separate respiratory adverse event (Table 4).
Table 4.
Trial outcomes
| DE (n = 30) | AE (n = 30) | P | |
|---|---|---|---|
| Incidence of at least 1 respiratory adverse event | 0 | 7 (23.3%) | 0.016 |
| Coughing | 0 | 3 | 0.236 |
| Laryngeal spasm | 0 | 1 | > 0.999 |
| Breath holding | 0 | 3 | 0.236 |
| Glossoptosis | 0 | 0 | > 0.999 |
| Oxygen desaturation | 0 | 5 | 0.062 |
| Hypoxemia | 0 | 1 | > 0.999 |
| TOE (m) | 2.78 ± 0.87 | 5.50 ± 1.01 | < 0.001 |
| TOW (m) | 15.03 ± 3.44 | 10.63 ± 1.52 | < 0.001 |
| AVMAP (mmHg) | 74.70 ± 13.35 | 87.43 ± 15.31 | < 0.001 |
| AVHR (bpm) | 108.37 ± 13.41 | 127.93 ± 20.74 | < 0.001 |
| Nasal obstruction | 3 (10%) | 5 (16.7%) | 0.706 |
| Sore throat | 2 (6.7%) | 8 (27%) | 0.038 |
| Hoarse Voice | 1 (3.3%) | 9 (30%) | 0.006 |
| Emergence agitation | 1 | 2 | > 0.999 |
| Epistaxis after extubation | > 0.999 | ||
| None | 28 | 27 | |
| Mild | 1 | 3 | |
| Moderate | 1 | 0 | |
| Severe | 0 | 0 |
AVMAP Average Value of MAP, AVHR Average Value of HR, bpm beat per minute
The TOE was significantly shorter in the DE group, averaging 2.78 ± 0.87 min, compared to 5.50 ± 1.01 min in the AE group (P < 0.001). The TOW was longer in the DE group, with an average of 15.03 ± 3.44 min compared to 10.63 ± 1.52 min in the AE group (P < 0.001). The DE group demonstrated significantly lower average value of mean arterial pressure (AVMAP) (74.70 ± 13.35 vs 87.43 ± 15.31 mmHg) and average value of heart rate (AVHR) (108.37 ± 13.41 vs 127.93 ± 20.74 bpm) compared with the AE group (both P < 0.001) during extubation. Additionally, the DE group showed significantly reduced postoperative sore throat (6.7% vs 27%) and hoarse voice (3.3% vs 30%) incidence, whereas both groups exhibited comparable incidences of EA, nasal obstruction, and epistaxis (Table 4). Every child was smoothly discharged approximately 2 h postoperatively.
Discussion
Our results suggest that DE was superior, allowing for less extubation time without increasing airway complications for pediatric dental patients. This technique provides a smoother extubation with fewer hemodynamic fluctuations and lower incidences of voice hoarseness and persistent coughing, provided that certain rules for deep extubation are followed. Based on the strict selection criteria and adequate rescue measures, patients would experience a more comfortable treatment process.
A randomized controlled trial (RCT) assessing respiratory complications during pediatric adenotonsillectomy [4] reported that, the AE group exhibited 3.1-fold higher complication rates versus DE (62% vs 20%; P < 0.001), which was aligned with our comparison (23.3% vs 0.0%, P = 0.016). In a systematic review comparing the airway complication rate in pediatric patients of the DE and AE groups following GA [3], DE lowered the overall risk of airway complications but increased the airway obstruction risk relative to AE. Instead, none of the respiratory adverse events (coughing, laryngospasm, breath holding, glossoptosis, oxygen desaturation, and hypoxemia) happened in the DE group of our research. The reasons are as follows:① Different types of elective surgeries;② Different extubation protocols for DE.
We strongly recommend propofol-assisted deep extubation in lateral decubitus position to achieve smooth extubation [22]. It is essential to recognize that the success of deep extubation depends on enhancing overall anesthesia and dental treatment processes [6]. Patients with adenotonsillar hypertrophy ≥ 1, class III obesity, micrognathia, obstructive sleep apnea syndrome (OSAS), or other indicators of a difficult airway are not suitable candidates for this technique. The use of a rubber dam can be vital for isolating treatment regions, as it prevents the accumulation of exogenous fluids and debris within the oropharynx, while also allowing for accurate tracking of all medical instruments to minimize the pulmonary aspiration incidence. The lateral decubitus position of patients helps to avoid glossoptosis, and reduces the risk of pulmonary aspiration. Moreover, it is vital to choose an appropriate timing for extubation. Cuff pressure can be extensively used for assessing the anesthesia depth, so long as the stable respiratory pattern is kept in the endotracheal tube deflation and reinflation processes [8, 22, 23]. The lateral decubitus position of patients prior to extubation has become the standardized operating procedure, serving as an approach to determining whether the patient is under the deep anesthesia state. Furthermore, this position contributes to preventing blood or secretions from entering the pharynx, therefore decreasing the aspiration and perioperative hypoxia risk.
A new method of using propofol for DE, which is rarely reported, was applied in this situation [2–4, 7–13, 24–26]. While sevoflurane offers improved intraoperative control over anesthesia depth and helps prevent inadvertent awareness during surgery, it carries a risk of EA (with the incidence rates reaching up to 80%) [27]. For participants receiving tonsillectomy, DE was guaranteed through single intravenous premedication of 1 or 2 μg/kg dexmedetomidine with inhaled low-concentration sevoflurane, and this might decrease the EA rate (0%), which was much lower than that of high-concentration sevoflurane alone (25%) [9]. In our RCT, the incidences of EA were 3.3% in the DE group, and 6.7% in the AE group, consistent with our previous retrospective research findings (2.6%) [6], both of which were attributed to propofol [28]. In a meta-analysis that involved pediatric patients, propofol could prevent EA when it was administered via a continuous infusion [29]. This is one of the advantages of using sevoflurane in combination with propofol, which may mainly be explained by propofol’s rapid pharmacokinetics. Mild airway hyperreactivity could be observed following propofol anesthesia [13]. Propofol exhibits antiemetic properties at the level of general anesthesia. It is not only inexpensive but can be easily administered and titrated with steady-state infusion devices [5], and it allows for quicker recovery without lingering residual anesthetic effects. In addition, propofol has been increasingly suggested to be effective in terminating episodes of laryngospasm [6, 30–33]. This mechanism may partially account for the decreased incidence of laryngospasm in the DE group relative to the recent European findings reported in the APRICOT study (3.1%, 95% CI: 2.9–3.3) [34].
Pediatric propofol administration raises safety concerns due to its association with propofol infusion syndrome (PRIS). The US Food and Drug Administration specifically cautions against extended propofol-mediated sedation to pediatric patients in intensive care settings due to the risk of PRIS. Japanese retrospective research identified heightened pediatric susceptibility to PRIS, attributable to elevated anesthetic propofol levels, slower metabolic clearance, and amplified lipid metabolism dependence compared with adults [35, 36]. The guidelines impose strict dosing thresholds (≤ 4 mg/kg/h) and 48-h administration limits for ventilator-dependent pediatric cases. The accumulative doses in our study meet that requirement. Importantly, epidemiologic evidence confirms the exceptional rarity of PRIS occurrences [35]. PRIS surveillance requires vigilance for triad manifestations: intractable metabolic acidosis, pathognomonic ECG abnormalities, and myoglobinuria; with mandatory monitoring of acid–base balance and muscle injury biomarkers during prolonged high-dose infusions, prioritizing multimodal sedation strategies. Per our dental anesthesia protocol [6] and international sedation and analgesia guideline [37], propofol should be titrated to desired endpoints in small, incremental doses.
Pediatric patients have a higher demand for oxygen, a lower functional residual capacity, and elevated CO2 ratios than adults. These factors increase their vulnerability to perioperative hypoxia [38]. DE, focusing on reducing stress responses in recovery, can facilitate smoother extubation with fewer hemodynamic fluctuations and lower incidences of voice hoarseness and sore throat (P < 0.05). This approach may lead to a more comfortable experience for pediatric patients and potentially enhance the operating room efficiency with a faster TOE. At the same time, a longer TOW is beneficial to uncooperative pediatric patients.
We did not utilize opioids in our anesthesia procedure. The impact of opioids on pain management is significantly affected by their association with various side effects and complications. The common adverse reactions related to opioid use are central nervous system depression, nausea, vomiting, drug tolerance, and withdrawal syndromes. Fewer frequent adverse reactions may encompass hyperalgesia, delayed gastric emptying, hormonal and immunologic issues, myoclonus, and muscle rigidity [39]. Findings of a meta-analysis of randomized trials related to opioid versus opioid-free analgesia (OFA) [40], indicate that postoperative opioid prescription fails to demonstrate superior analgesia efficacy while does lead to opioids-related complications. For elective minor and moderate surgeries, the evidence suggests that clinicians may opt for opioid-free analgesia in these situations. A study examining whether OFA could reduce postoperative nausea and vomiting (PONV) indicated that OFA decreased the incidence by 50% following thoracoscopic lung resection [41]. During DE, it is critical to avoid nausea, vomiting, and respiratory depression. Caution should be exercised when prescribing opioids, especially for patients with a higher risk of apnea and airway obstruction [42].
The successful clinical techniques inherently require teamwork rather than individual efforts. There have been reports of metal or transparent crowns being misplaced in the pharynx [43–45]. Therefore, meticulous and careful oral procedures, along with adequate hemostasis, are vital before performing DE. If a crown accidentally enters the pharynx, it becomes imperative to retrieve it using a video laryngoscope. Employing high-speed suction, four-handed dentistry, and ligation using a suitably fitted rubber dam are efficient strategies for avoiding aspiration and deglutition of materials or loose instruments during dental practices. Alexander and Delholm also recommended utilizing dental floss for securing the rubber dam clamp [46]. If a prosthesis is not expelled from the airway, an ENT consultation for bronchoscopy should be arranged.
This study has several limitations: (1) Patients with mental health disorders were excluded, requiring further validation of DE in this population; (2) Focused on low-trauma pediatric dental surgeries under GA, DE's efficacy in procedures requiring intensive opioid analgesia remains unverified; (3) Continuous intraoperative monitoring of anesthetic agents'hemodynamic and respiratory effects was omitted; (4) The potential advantages of DE combined with adjuvant agents (e.g., dexmedetomidine) for outpatient/infant populations warrant systematic investigation.
Conclusions
For pediatric patients undergoing dental treatment, propofol-assisted deep extubation proves advantageous, facilitating less extubation time without increasing airway complications. This technique enables a smoother extubation with fewer hemodynamic fluctuations and reduced incidences of voice hoarseness and persistent coughing, as long as specific guidelines for DE are adhered to. With stringent selection criteria and effective rescue measures in place, patients are likely to have a more comfortable treatment experience.
Supplementary Information
Authors’ contributions
Xiang Zhang conceived the ideas. Wei Cui and Yun Liu participated in the study design. Ying Shang collected and annotated all the data. Xiang Zhang and Yun Liu participated in formal analysis and drafted and critically revised the manuscript. All authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Funding
This research was supported by the Young Clinical Research Fund of the Chinese Stomatological Association (CSA-A2021-02).
Data availability
The data used to support the findings of this study are available from the corresponding author upon request.
Declarations
Ethics approval and consent to participate
Ethical approval for the present RCT was provided by the Ethics Committee of Baoding Second Hospital (no. KY2023044). The study was registered at www.chictr.org.cn (no. ChiCTR2400079951), and informed written consent was obtained from the legal guardians of all included participants before the study. All methods were performed in accordance with the relevant guidelines and regulations of the Helsinki Declaration.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Wei Cui and Xiang Zhang have contributed equally to this work as co-first authors.
References
- 1.Sasaki CT, Suzuki M. Laryngeal spasm: a neurophysiologic redefinition. Ann Otol Rhinol Laryngol. 1977;86(2 pt. 1):150–7. [DOI] [PubMed] [Google Scholar]
- 2.Ramgolam A, Hall GL, Zhang G, Hegarty M, von Ungern-Sternberg BS. Deep or awake removal of laryngeal mask airway in children at risk of respiratory adverse events undergoing tonsillectomy-a randomised controlled trial. Br J Anaesth. 2018;120(3):571–80. [DOI] [PubMed] [Google Scholar]
- 3.Koo CH, Lee SY, Chung SH, Ryu JH. Deep vs. Awake extubation and LMA removal in terms of airway complications in pediatric patients undergoing anesthesia: a systemic review and meta-analysis. J Clin Med. 2018;7(10):353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.von Ungern-Sternberg BS, Davies K, Hegarty M, Erb TO, Habre W. The effect of deep vs. awake extubation on respiratory complications in high-risk children undergoing adenotonsillectomy: a randomised controlled trial. Eur J Anaesthesiol. 2013;30(9):529–36. [DOI] [PubMed] [Google Scholar]
- 5.Wu X, Liu Y, Li B, Zhou D, Cheng T, Ma T, et al. Safety of deep intravenous propofol sedation in the dental treatment of children in the outpatient department. J Dent Sci. 2023;18(3):1073–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang X, Wang XD, Cui W, Gao SC, Yang XD, Xia B. Safety of propofol-assisted deep extubation in the dental treatment of children-a retrospective, observational study. BMC Anesthesiol. 2024;24(1):213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Iyilikci L, Tezcan G, Gokel E. Comparison of complications and hemodynamic changes during and after removal of laryngeal mask airway in deep anesthesia versus awake patients. Turk Anesteziyoloji ve Reanimasyon. 1999;27(8):423–6. [Google Scholar]
- 8.Kim MK, Baek CW, Kang H, Choi GJ, Park YH, Yang SY, et al. Comparison of emergence after deep extubation using desflurane or desflurane with remifentanil in patients undergoing general anesthesia: a randomized trial. J Clin Anesth. 2016;28:19–25. [DOI] [PubMed] [Google Scholar]
- 9.Di M, Han Y, Yang Z, Liu H, Ye X, Lai H, et al. Tracheal extubation in deeply anesthetized pediatric patients after tonsillectomy: a comparison of high-concentration sevoflurane alone and low-concentration sevoflurane in combination with dexmedetomidine pre-medication. BMC Anesthesiol. 2017;17(1):28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Valley RD, Freid EB, Bailey AG, Kopp VJ, Georges LS, Fletcher J, et al. Tracheal extubation of deeply anesthetized pediatric patients: a comparison of desflurane and sevoflurane. Anesth Analg. 2003;96(5):1320–4. [DOI] [PubMed] [Google Scholar]
- 11.Valley RD, Ramza JT, Calhoun P, Freid EB, Bailey AG, Kopp VJ, et al. Tracheal extubation of deeply anesthetized pediatric patients: a comparison of isoflurane and sevoflurane. Anesth Analg. 1999;88(4):742–5. [DOI] [PubMed] [Google Scholar]
- 12.Qiu J, Xie M, Chen J, Chen B, Chen Y, Zhu X, et al. Tracheal extubation under deep anesthesia using transnasal humidified rapid insufflation ventilatory exchange vs. Awake extubation: an open-labeled randomized controlled trial. Front Med (Lausanne). 2022;9:810366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huang RC, Hung NK, Lu CH, Wu ZF. Removal of laryngeal mask airway in adults under target-controlled, propofol-fentanyl infusion anesthesia: awake or deep anesthesia? Medicine (Baltimore). 2016;95(17):e3441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guideline on Behavior Guidance for the Pediatric Dental Patient. Pediatr Dent. 2016;38(6):185–98. https://pubmed.ncbi.nlm.nih.gov/27931459/. [PubMed]
- 15.Khine HH, Corddry DH, Kettrick RG, Martin TM, McCloskey JJ, Rose JB, et al. Comparison of cuffed and uncuffed endotracheal tubes in young children during general anesthesia. Anesthesiology. 1997;86(3):627–31; discussion 27A. [DOI] [PubMed] [Google Scholar]
- 16.Park D, Kim JS, Heo SJ. The effect of the modified jaw-thrust maneuver on the depth of sedation during drug-induced sleep endoscopy. J Clin Sleep Med. 2019;15(10):1503–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Aldrete JA. The post-anesthesia recovery score revisited. J Clin Anesth. 1995;7(1):89–91. [DOI] [PubMed] [Google Scholar]
- 18.Karam C, Zeeni C, Yazbeck-Karam V, Shebbo FM, Khalili A, Abi Raad SG, et al. Respiratory adverse events after LMA® mask removal in children: a randomized trial comparing propofol to sevoflurane. Anesth Analg. 2023;136(1):25–33. [DOI] [PubMed] [Google Scholar]
- 19.Sugiyama K, Manabe Y, Kohjitani A. A styletted tracheal tube with a posterior-facing bevel reduces epistaxis during nasal intubation: a randomized trial. Can J Anaesth. 2014;61(5):417–22. [DOI] [PubMed] [Google Scholar]
- 20.Sikich N, Lerman J. Development and psychometric evaluation of the pediatric anesthesia emergence delirium scale. Anesthesiology. 2004;100(5):1138–45. [DOI] [PubMed] [Google Scholar]
- 21.Zou Y, Liu SH, Xue FS. Emergence agitation or delirium in children. J Anesth. 2022;36(1):156. [DOI] [PubMed] [Google Scholar]
- 22.Hu C, Yu H, Ye M, Shen X. Sevoflurane in combination with remifentanil for tracheal extubation after otologic surgery. Am J Health Syst Pharm. 2014;71(13):1108–2011. [DOI] [PubMed] [Google Scholar]
- 23.Juang J, Cordoba M, Ciaramella A, Xiao M, Goldfarb J, Bayter JE, et al. Incidence of airway complications associated with deep extubation in adults. BMC Anesthesiol. 2020;20(1):274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jung H, Kim HJ, Lee YC, Kim HJ. Comparison of lateral and supine positions for tracheal extubation in children: a randomized clinical trial. Anaesthesist. 2019;68(5):303–8. [DOI] [PubMed] [Google Scholar]
- 25.Heidari SM, Abbasi S, Rahimi M. Removal of laryngeal mask airway: awake or deep anesthesia? J Res Med Sci. 2005;10(2):59–62. [Google Scholar]
- 26.Splinter WM, Reid CW. Removal of the laryngeal mask airway in children: deep anesthesia versus awake. J Clin Anesth. 1997;9(1):4–7. [DOI] [PubMed] [Google Scholar]
- 27.Kuratani N, Oi Y. Greater incidence of emergence agitation in children after sevoflurane anesthesia as compared with halothane: a meta-analysis of randomized controlled trials. Anesthesiology. 2008;109(2):225–32. [DOI] [PubMed] [Google Scholar]
- 28.Chidambaran V, Costandi A, D’Mello A. Propofol: a review of its role in pediatric anesthesia and sedation. CNS Drugs. 2015;29(7):543–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dahmani S, Stany I, Brasher C, Lejeune C, Bruneau B, Wood C, et al. Pharmacological prevention of sevoflurane- and desflurane-related emergence agitation in children: a meta-analysis of published studies. Br J Anaesth. 2010;104(2):216–23. [DOI] [PubMed] [Google Scholar]
- 30.Wu X, Liu Y, Li B, Zhou D, Cheng T, Ma T, et al. Safety of deep intravenous propofol sedation in the dental treatment of children in the outpatient department. J Dent Sci. 2023;18(3):1073–8. [DOI] [PMC free article] [PubMed]
- 31.Afshan G, Chohan U, Qamar-Ul-Hoda M, Kamal RS. Is there a role of a small dose of propofol in the treatment of laryngeal spasm? Paediatr Anaesth. 2002;12(7):625–8. [DOI] [PubMed] [Google Scholar]
- 32.Soares RR, Heyden EG. Treatment of laryngeal spasm in pediatric anesthesia by retroauricular digital pressure. Case report. Rev Bras Anestesiol. 2008;58(6):631–6. [DOI] [PubMed] [Google Scholar]
- 33.Batra YK, Ivanova M, Ali SS, Shamsah M, Al Qattan AR, Belani KG. The efficacy of a subhypnotic dose of propofol in preventing laryngospasm following tonsillectomy and adenoidectomy in children. Paediatr Anaesth. 2005;15(12):1094–7. [DOI] [PubMed] [Google Scholar]
- 34.Habre W, Disma N, Virag K, Becke K, Hansen TG, Jöhr M, et al. Incidence of severe critical events in paediatric anaesthesia (APRICOT): a prospective multicentre observational study in 261 hospitals in Europe. Lancet Respir Med. 2017;5(5):412–25. [DOI] [PubMed] [Google Scholar]
- 35.Takeshita R, Yasunami K, Watanabe F, Tokutsu K, Muramatsu K, Horishita T, et al. Propofol infusion syndrome (PRIS) incidence and related symptoms in children: a retrospective analysis of a nationwide hospital-based database in Japan. Br J Anaesth. 2024;133(3):684–6. [DOI] [PubMed] [Google Scholar]
- 36.Hara T, Ozawa A, Shibutani K, Tsujino K, Miyauchi Y, Kawano T, et al. Practical guide for safe sedation. J Anesth. 2023;37(3):340–56. [DOI] [PubMed] [Google Scholar]
- 37.Practice Guidelines for Moderate Procedural Sedation and Analgesia 2018: A Report by the American Society of Anesthesiologists Task Force on Moderate Procedural Sedation and Analgesia, the American Association of Oral and Maxillofacial Surgeons, American College of Radiology, American Dental Association, American Society of Dentist Anesthesiologists, and Society of Interventional Radiology. Anesthesiology. 2018;128(3):437–79. 10.1097/ALN.0000000000002043. [DOI] [PubMed]
- 38.Monaco F, D’Andria Ursoleo J, Lerose CC, Barucco G, Licheri M, Della Bella PE, et al. Anaesthetic management of paediatric patients undergoing electrophysiology study and ablation for supraventricular tachycardia: a focused narrative review. J Clin Anesth. 2024;93:111361. [DOI] [PubMed] [Google Scholar]
- 39.Benyamin R, Trescot AM, Datta S, Buenaventura R, Adlaka R, Sehgal N, et al. Opioid complications and side effects. Pain Physician. 2008;11(2 Suppl):S105–20. [PubMed] [Google Scholar]
- 40.Fiore JF Jr, El-Kefraoui C, Chay MA, Nguyen-Powanda P, Do U, Olleik G, et al. Opioid versus opioid-free analgesia after surgical discharge: a systematic review and meta-analysis of randomised trials. Lancet. 2022;399(10343):2280–93. [DOI] [PubMed] [Google Scholar]
- 41.Feng CD, Xu Y, Chen S, Song N, Meng XW, Liu H, et al. Opioid-free anaesthesia reduces postoperative nausea and vomiting after thoracoscopic lung resection: a randomised controlled trial. Br J Anaesth. 2024;132(2):267–76. [DOI] [PubMed] [Google Scholar]
- 42.Vitale L, Rodriguez B, Baetzel A, Christensen R, Haydar B. Complications associated with removal of airway devices under deep anesthesia in children: an analysis of the Wake Up Safe database. BMC Anesthesiol. 2022;22(1):223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jin SB, Ju MH. Case report of bronchial aspiration of metal casting during dental treatment. Taehan Chikkwa Uisa Hyophoe Chi. 1989;27(10):965–8. [PubMed] [Google Scholar]
- 44.Seals ML, Andry JM, Kellar PN. Pulmonary aspiration of a metal casting: report of case. J Am Dent Assoc. 1988;117(5):587–8. [DOI] [PubMed] [Google Scholar]
- 45.Adewumi A, Kays DW. Stainless steel crown aspiration during sedation in pediatric dentistry. Pediatr Dent. 2008;30(1):59–62. [PubMed] [Google Scholar]
- 46.Alexander RE, Delhom JJ Jr. Rubber dam clamp ingestion, an operative risk: report of case. J Am Dent Assoc. 1971;82(6):1387–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data used to support the findings of this study are available from the corresponding author upon request.


