Abstract
Background
In acute kidney injury (AKI), inflammatory crosstalk between tubular epithelial cells (TECs) and immune cells drives disease progression. Although the Axl-SOCS3 axis in myeloid cells typically suppresses inflammation, TEC-specific SOCS3 deletion paradoxically protects against AKI, suggesting a cell type-specific pro-inflammatory role.
Methods
We induced AKI via bilateral ischemia/reperfusion (IRI) in mice. The Axl-specific pharmacological inhibitor R428 was administered via subcutaneous injection immediately post-IRI, with plasma and kidney samples collected 24 h later. To assess the effects of SOCS3 in TECs, small interfering RNA was used to silence SOCS3 in cisplatin injured HK2 cells. Axl/SOCS3 expression levels were assessed in human AKI biopsies.
Results
In AKI patients and IRI mice, Axl was upregulated in interstitial immune cells, while SOCS3 increased in TECs. Axl inhibition by R428 attenuated renal injury, reducing inflammatory infiltration, NF-κB p65 phosphorylation, and TEC SOCS3 expression. Notably, SOCS3 knockdown in TECs suppressed NF-κB activation and IL-1β/IL-6 production, implicating Axl-SOCS3 as a pro-inflammatory amplifier in AKI.
Conclusion
The Axl-SOCS3 axis exacerbates AKI by reinforcing NF-κB-driven inflammation in TECs, creating a vicious cycle between immune cells and TECs. Targeting this cross-cellular pro-inflammatory pathway offers a promising therapeutic strategy for AKI.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12882-025-04222-z.
Keywords: Acute kidney injury, Inflammation, Tubular epithelial cells, Axl, Suppressor of cytokine signaling 3
Background
Acute kidney injury (AKI) remains a life-threatening disease affecting 10–15% of hospitalized patients, with no targeted therapies clinically available [1]. The disease progression is driven by a self-amplifying inflammatory circuit between injured tubular epithelial cells (TECs) and immune cells: Not only do damaged TECs release DAMPs and cytokines to recruit immune cells, but infiltrating immune cells further activate TECs through pro-inflammatory mediators, creating a vicious cycle [2]. Central to this pathological crosstalk is the NF-κB pathway – a master regulator of innate/adaptive immunity that drives transcription of IL-1β, IL-6, et al., and promotes M1 macrophage polarization. Notably, NF-κB is robustly activated in both TECs and interstitial immune cells during IRI-AKI, and TEC-specific inhibition of NF-κB p65 significantly attenuates tubular apoptosis and macrophage infiltration [3], underscoring TECs as active contributors to renal inflammation.
The Axl receptor tyrosine kinase, a member of the Tyro3, Axl, and Mer (TAM) family [4], exhibits dual regulatory roles in inflammation that are cell-context dependent [5–9]. While primarily expressed in myeloid cells, such as dendritic cells (DCs) and macrophages [10, 11]. Its expression is strongly upregulated in response to toll-like receptor (TLR) activation, driving the intracellular expression of suppressor of cytokine signaling (SOCS) proteins 1 and 3, which function to terminate inflammatory responses to pathogens [10, 12]. However, our transcriptomic data revealed a paradoxical upregulation of SOCS3, a canonical NF-κB target gene, as a top differentially expressed gene in AKI kidneys [13]. Intriguingly, TEC-specific SOCS3 deletion not only reduced renal inflammation but also increased M2 macrophages [14], suggesting SOCS3 may sustain NF-κB-driven pro-inflammatory responses in TECs during AKI.
The context-dependent duality of Axl signaling manifests strikingly across organ systems: in hepatic and cerebral ischemia-reperfusion injury (IRI), Axl exerts cryoprotection by suppressing oxidative stress and inflammation [7, 8], whereas in the kidney microenvironment, it paradoxically promotes pathogenesis [5, 6, 9]. Notably, though virtually undetectable in healthy kidneys [6], Axl becomes functionally activated in renal diseases through divergent NF-κB-mediated mechanisms, i.e. inducing mesangial proliferation in IgA nephropathy [5] and sustaining IL-6-driven inflammation in lupus nephritis [9]. This kidney-specific signal reprogramming (from cytoprotective to pro-inflammatory) coincides with the established role of NF-κB in mediating immune cell-TEC crosstalk during AKI [3], raising a pivotal mechanistic question: Could Axl function as the master regulator of this pathogenic dialogue, specifically through SOCS3-dependent control of NF-κB activation within TECs? This unexplored axis may hold the key to understanding the self-perpetuating inflammatory cycle of AKI.
Methods
Experimental animals
Male C57BL/6J mice, 8 weeks old, were purchased from SpePharm Biotechnology (Beijing, China). All mice were maintained in animal facilities under specific pathogen-free conditions.
Induction of AKI in C57BL/6J mice
After adaptive feeding for one week, mice were randomly allocated to four groups: sham + vehicle, sham + R428, IRI + vehicle, and IRI + R428. Each group consisted of at least five mice. IRI was induced bilateral in kidneys, as previously described [15]. Briefly, mice were anesthetized with inhaled oxygenated isoflurane and placed on a 37 °C heating pad. Bilateral dorsal incisions were made to expose the kidneys, and renal pedicles were clamped for 28 min using non-traumatic microvascular clips. Complete ischemia was verified by a color change in the kidneys from red to dark purple. Upon removal of the clips, the kidney color reverted from purple to red. Sham-operated mice underwent an identical procedure, excluding renal pedicle clamping. Immediately post-reperfusion, mice received a single subcutaneous injection of the highly selective Axl inhibitor R428 (25 mg/kg in 200 µL of 0.5% hydroxypropylmethylcellulose/0.1% Tween 80 vehicle; Cat# A8329; Apexbio), a dosing regimen selected based on its well-characterized pharmacokinetics (Cmax = 2.6 µmol/L, t1/2 = 4 h at this dose) and established efficacy in myocardial ischemia-reperfusion models [16, 17]. As rigorously validated by Holland et al., R428 exhibits: (1) sub-nanomolar Axl specificity (EC50 = 14 nmol/L) with > 50-fold selectivity over Tyro3/Mer kinases, (2) negligible off-target activity (< 10% inhibition across 133-kinase panel), and (3) excellent in vivo safety profiles [17]. Mice were sacrificed 24 h after IRI by cervical dislocation, with plasma and kidney samples collected for further analysis. Plasma creatinine (Cr) and blood urea nitrogen (BUN) levels were measured using a biochemical analyzer (Cat# BS-240VET; Mindray).
Renal histology
Kidney tissues were fixed in 10% buffered formalin, embedded in paraffin, and stained with periodic acid-Schiff (PAS). Tubulointerstitial damage was semi-quantitatively scored by two renal pathologists who were blinded to experimental groups. The scores were based on a scale from 0 to 4+, reflecting the percentage of affected cortical and outer medullary regions, including brush border loss, tubular necrosis, and renal tubular casts (0 = no lesion, 1+ = <25%, 2 + = 25–50%, 3 + = 50–75%, 4 + = 75–100%).
Cell lines and culture conditions
HK2 cells, an immortalized human proximal tubular epithelial cell line (Cat# CL-0109; Procell), were cultured in DMEM supplemented with 10% fetal bovine serum and 1% penicillin-streptomycin in a humidified atmosphere (5% CO₂, 95% air) at 37 °C.
SiRNA knockdown
HK-2 cells were plated in culture plates (6-well for Western blot, 12-well for immunofluorescence, and 96-well for CCK-8 assays) 24 h prior to transfection. Cells were transfected with 100 nmol/L OnTargetPlus SmartPool siRNA against human SOCS3 (Cat# L-004299-00; Dharmacon) or non-targeting control siRNA (Cat# D-001810-10; Dharmacon) using Lipofectamine 2000 (Cat# 11668030; Invitrogen) according to the manufacturer’s protocol. After 24 h transfection, cells were washed with PBS and treated with 20 µmol/L cisplatin for 48 h. SOCS3 knockdown efficiency was confirmed by qRT-PCR before cisplatin treatment.
Cell counting kit-8 (CCK-8) assay
To assess cell viability, 5 × 103 cells per well were seeded into 96-well plates, with six wells used for each assayed group. After 48 h treatment of cisplatin, cell numbers were evaluated using the cell counting kit-8 (CCK-8, Cat# CK04; Dojindo). Ten microliters of CCK-8 reagent were added to each well, followed by a 2-hour incubation at 37 °C. Absorbance at 450 nm was then measured using a spectrophotometer (Bio-rad).
Immunofluorescence staining
Immunofluorescence staining of kidney sections and cultured HK2 cells was performed on paraffin-embedded tissue sections and cell slides, respectively. Following fixation and antigen retrieval, nonspecific binding was blocked with 3% bovine serum albumin. Tissue sections were incubated overnight at 4°C with the following primary antibodies: anti-Axl (1:400, Cat# 5180; Bioss), anti-SOCS3 (1:300, Cat# 14025; Proteintech), and anti-F4/80 (1:100, Cat# 34028; Bioss). Co-staining was conducted with anti-Ecadherin (1:300, Cat# 14472; CST) to highlight Axl or SOCS3 localization. Cell slides were incubated with anti-Ki67 (1:300, Cat# 9129; CST) overnight at 4°C. After three PBS washes, sections and slides were incubated with secondary antibodies, washed, and mounted with a fluorescent medium containing 4’,6-diamidino-2-phenylindole (Cat# ZLI-9557; ZSGB-BIO). Staining was visualized using a fluorescence microscope (Leica). For quantitative analysis, six to ten high-power fields from each cell slide or kidney section (outer medulla and cortex) were imaged to count Ki67 or Axl-positive cells and SOCS3-positive tubules.
Immunohistochemistry staining
Immunohistochemistry of kidney sections was performed on paraffin-embedded tissues using primary antibodies against Axl (1:400, Cat# 5180; Bioss), SOCS3 (1:300, Cat# 14025; Proteintech), and Ly6G (1:200, Cat# 238132; Abcam). After incubation with diaminobenzidine-labeled secondary antibodies, six to ten randomly selected high field fields per section were visualized under a microscope (Leica).
Western blot
Total protein was extracted from kidney tissue and HK2 cells on ice using radioimmunoprecipitation assay (RIPA) buffer (Cat# P0013B; Beyotime) with protease and phosphatase inhibitors (Cat# P1046; Beyotime). Protein concentrations were determined with a Pierce BCA Protein Assay Kit (Cat# 23227; Thermo). Proteins were denatured, separated by SDS-PAGE, and transferred to polyvinylidene difluoride membranes. Membranes were blocked with 5% BSA in TBS with 0.1% Tween 20 (TBST) for 1 h at room temperature, then incubated overnight at 4 °C with primary antibodies: anti-Axl (1:1000, Cat# 5180; Bioss), anti-SOCS3 (1:1000, Cat# 14025; Proteintech), anti-p-p65 (1:1000, Cat# 3033; CST), anti-p65 (1:1000, Cat# 8242; CST), anti-Arginase-1 (Arg-1, 1:1000, Cat# 93668; CST), anti-β-actin (1:1000, Cat# 4970; CST), and anti-GAPDH (1:10000, Cat# 181602; Abcam). After washing three times with TBST, the membranes were incubated with horseradish peroxidase (HRP)-conjugated secondary antibodies for 1 h at room temperature. After three washes with TBST, the membranes were incubated in Immobilon® ECL Ultra Western HRP Substrate (Cat# WBULS0500; Millipore), and images were captured by an ImageQuant LAS 4000 Mini system (GE, Healthcare). The relative intensity of the protein bands was quantified by digital densitometry using ImageJ software (Media Cybernetics). The level of GAPDH or β-actin was used as an internal standard.
Quantitative real-time polymerase chain reaction (qRT-PCR)
Total RNA from HK2 cells was extracted using TRIzol® reagent following the manufacturer’s instructions (Cat# 15596026; Thermo). RNA concentrations were determined by photometric measurements. cDNA was synthesized from 2 µg total RNA using Fast King RT Enzyme (Cat# KR118; TIANGEN). The mRNA expression of SOCS3, IL-1β, IL-6, and β-actin was quantified using SYBR Green PCR Master Mix (Cat# FP209; TIANGEN) on an ABI Vii7 system. Relative expression was calculated by the 2-ΔΔCt method. Sequences of the primer pairs are listed in Supplementary Table S1.
Human specimens
Kidney samples were obtained from three AKI patients who underwent kidney biopsies at Tianjin Medical University General Hospital and pathologically diagnosed with acute tubular injury, with normal adjacent kidney tissue from renal cell carcinoma patients serving as controls. The AKI diagnosis followed Kidney Disease: Improving Global Outcomes (KDIGO) guidelines.
Statistical analysis
Data were analyzed using GraphPad Prism (version 10.0) and are presented as mean ± SEM for normally distributed variables or median ± interquartile range (IQR) for non-normally distributed data. Normality was assessed using the Shapiro-Wilk test, with variance homogeneity evaluated by F-test. Parametric data were analyzed by unpaired Student’s t-test (equal variance) or Welch’s t-test (unequal variance) for two-group comparisons, and one-way ANOVA with Tukey’s post hoc test (homogeneous variance) or Games-Howell test (heterogeneous variance) for multi-group comparisons. Non-parametric data were analyzed using the Mann-Whitney U test (two groups) or Kruskal-Wallis test with Dunn’s post hoc correction (multiple groups). All P-values were two-tailed, and P < 0.05 was considered significant.
Results
Axl and SOCS3 are involved in IRI-induced AKI
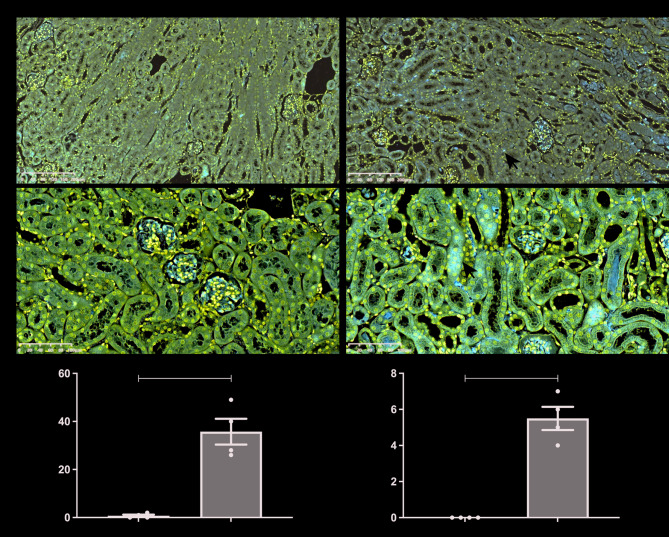
We first evaluated the expression of Axl and SOCS3 in kidneys of mice with AKI induced by IRI. Immunohistochemical staining revealed that both Axl and SOCS3 were minimally detected in the kidneys of sham-operated mice (Fig. 1A and B). In contrast, in IRI-AKI mice, Axl was predominantly localized to cells in the renal interstitium (Fig. 1A), while SOCS3 was mainly observed in the cytoplasm of TECs (Fig. 1B). Quantitative analysis showed a significant increase in the number of Axl-positive cells and SOCS3-positive tubules in the kidneys of IRI mice compared to sham controls (Fig. 1C and D). These results suggest that both Axl and SOCS3 are involved in the pathogenesis of AKI.
Fig. 1.
Expression patterns of Axl and SOCS3 in kidneys following IRI-induced AKI. (A-B) Representative immunohistochemical staining of Axl (A) and SOCS3 (B) in renal tissues. Arrows indicate positively stained cells/tubules. Scale bars: 200 μm (A), 100 μm (B). (C) Quantitative analysis of Axl-positive cells/100× (n = 4 mice/group). (D) Quantitative analysis of SOCS3-positive tubules/200× (n = 4). Data are shown as mean ± SEM. ** P < 0.01
Inhibition of Axl ameliorates IRI-induced AKI
To investigate the role of Axl in AKI, we assessed renal tissue damage and renal function in IRI mice following treatment with R428, a specific pharmacological inhibitor of Axl [17]. PAS staining revealed severe tubular injury, characterized by dilation, necrosis, cast formation, and loss of brush border in the renal cortical region of IRI mice, which was significantly attenuated by R428 treatment (Fig. 2A). Renal histology improved following R428 administration, as indicated by a decrease in renal pathological scores compared to IRI mice treated with the vehicle (Fig. 2C). Plasma Cr and BUN levels, which were significantly elevated in IRI mice, were substantially reduced in R428-treated mice (Fig. 2D and E). We next examined the expression of Axl in kidney of mice by immunofluorescence, co-staining with Ecadherin, a marker for epithelial cell tight junctions [13], to outline the renal tubules for highlighting the location of Axl expression. Figure 2B and F showed Axl was predominantly localized in interstitial cells and the Axl positive cells were markedly reduced following R428 treatment. Furthermore, Western blot showed the similar trend of Axl expression despite the difference is not significant (Fig. 2G and H). These data indicate that Axl inhibition alleviates renal dysfunction and pathological damage, suggesting a detrimental role of Axl in AKI.
Fig. 2.
Effects of Axl inhibitor on mice with IRI-induced AKI. (A) PAS-stained kidney sections showing tubular injury. Scale bar, 200 μm. (B) Immunofluorescence co-staining of Axl (green) and E-cadherin (red). The arrows indicate the positive staining of Axl. Scale bar, 100 μm. (C) Semiquantitative renal pathological scores (n = 5). (D-E) Plasma Cr (D) and BUN (E) levels (n = 4). (F) Quantification of Axl-positive cells/200× (n = 4). (G) Representative Western blot of Axl expression. (H) Densitometric analysis of Axl/β-actin ratio (n = 6). Data are shown as mean ± SEM or median ± interquartile range (IQR). *P < 0.05, **p < 0.01, and P****Р<0.0001
Inhibition of Axl alleviates renal inflammation during IRI-induced AKI
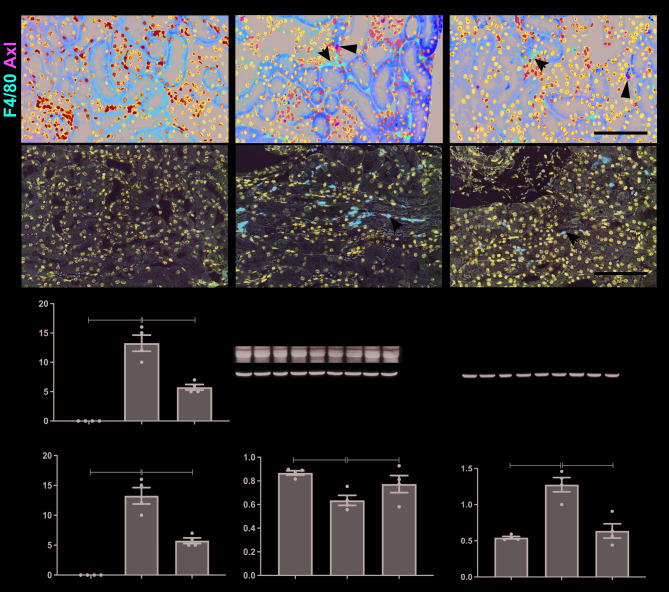
The recruitment of macrophages and neutrophils, along with the activation of the NF-κB pathway, are hallmark features of inflammation in AKI. Immunofluorescence staining showed a significant increase in the infiltration of F4/80-positive macrophages in the kidneys of IRI mice compared to sham controls, and co-staining with Axl reflected that F4/80 positive macrophages is not the source of Axl in renal interstitium (Fig. 3A). Treatment with R428 significantly reduced the number of F4/80-positive macrophages (Fig. 3C). Similarly, the infiltration of Ly6G-positive neutrophils was markedly reduced in IRI kidneys following R428 administration (Fig. 3B and D). Western blot analysis revealed that the protein expression of Arg-1, a marker of anti-inflammatory M2 macrophages, was reduced in IRI kidneys, while R428 treatment significantly increased Arg-1 expression (Fig. 3E and F). Additionally, IRI induced the expression of phosphorylated p65 (p-p65), a key indicator of NF-κB pathway activation, which was significantly reversed by Axl inhibition with R428 (Fig. 3G and H). These results suggest that Axl inhibition reduces inflammation in AKI.
Fig. 3.
Effects of Axl inhibitor on renal inflammation in mice with IRI-induced AKI. (A) Immunofluorescence co-staining of F4/80 (red) and Axl (green). Arrows indicate F4/80-positive macrophages. (B) Ly6G immunohistochemistry showing neutrophil infiltration. (C-D) Quantification of F4/80- (C) and Ly6G- (D) positive cells/400× (n = 4). (E-F) Western blot analysis (E) and quantification (F) of Arg-1 expression. (G-H) Western blot analysis (G) and quantification (G) of p-p65 expression (n = 4). Scale bar, 100 μm. Data are shown as mean ± SEM. **P < 0.01
Axl Inhibition decreases IRI-induced SOCS3 expression in TECs
To explore the relationship between Axl and SOCS3 in IRI-induced AKI, we assessed SOCS3 expression in kidney tissues by immunofluorescence and Western blot. Co-staining of SOCS3 with E-cadherin, a marker of epithelial cell tight junctions, revealed that SOCS3 expression in TECs was elevated in the kidneys of IRI mice, but was significantly reduced after R428 treatment (Fig. 4A). The number of SOCS3-positive tubules in the kidneys of IRI mice treated with R428 was significantly lower compared to untreated IRI mice (Fig. 4B). This was further confirmed by Western blot analysis, which showed a marked reduction in SOCS3 protein levels following Axl inhibition (Fig. 4C and D). These findings suggest that Axl mediates SOCS3 expression in TECs during AKI.
Fig. 4.
Reduction of SOCS3 expression in TECs of IRI-induced AKI mice by Axl inhibition. (A) Immunofluorescence co-staining of SOCS3 (green) and Ecadherin (red). The arrows indicate the positive staining of SOCS3. Scale bar, 100 μm. (B) Quantification of SOCS3-positive cells/400× (n = 4). (C-D) Western blot analysis (C) and quantification (D) of SOCS3 expression (n = 4). Data are shown as mean ± SEM or median ± interquartile range (IQR). *P < 0.05
Knockdown of SOCS3 in tecs prevents cisplatin-induced inflammation
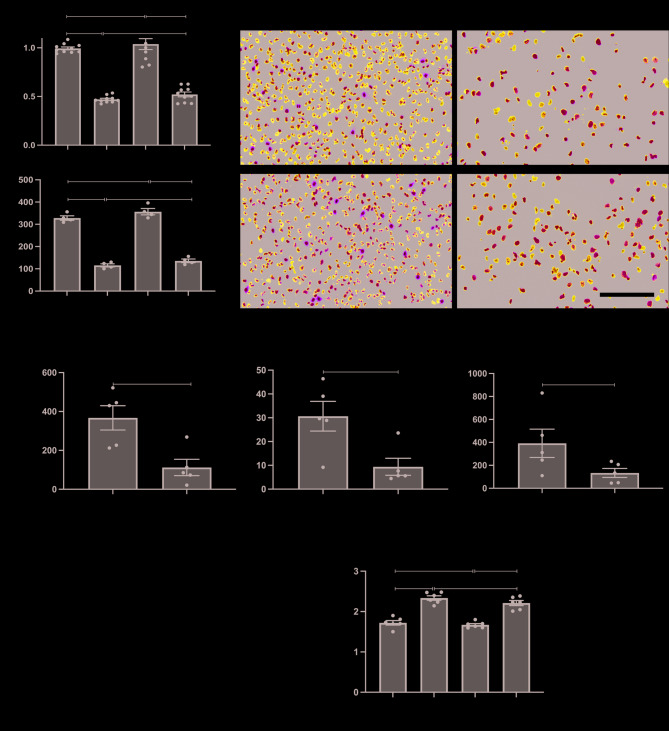
To evaluate the role of SOCS3 in TECs during AKI, we knocked down SOCS3 using small interfering RNA (siSOCS3) in HK2 cells treated with cisplatin to simulate AKI in vitro. Knockdown of SOCS3 in cisplatin-injured HK2 cells did not significantly affect cell survival, as measured by CCK-8 assays (Fig. 5A), nor did it enhance proliferation, as indicated by Ki67 immunofluorescence staining (Fig. 5B and C). However, RT-PCR analysis revealed that cisplatin treatment induced a significant increase in SOCS3 expression and inflammatory factors, including IL-1β and IL-6, which were substantially reduced following SOCS3 knockdown (Fig. 5D). Furthermore, a slightly decrease trend in NF-κB p65 phosphorylation levels was observed in cisplatin-injured HK2 cells following SOCS3 knockdown (Fig. 5E and F). These results suggest that although SOCS3 knockdown does not promote TEC proliferation, it can suppress inflammation induced by TEC injury.
Fig. 5.
Impact of SOCS3 knockdown in cisplatin-treated HK2 cells. (A) Cell viability assessed by CCK-8 assay. (B) Representative Ki67 immunofluorescence (green). (C) Quantification of Ki67-positive cells/400× (n = 4). Scale bar, 100 μm. (D) The relative mRNA expression of SOCS3, IL-1β, and IL-6 determined by qRT-PCR (n = 5). (E-F) Western blot analysis (E) and quantification (F) of p-p65 levels (n = 6). Data are shown as mean ± SEM. *P < 0.05, **P < 0.01
Expression of Axl and SOCS3 in kidneys of patients with AKI
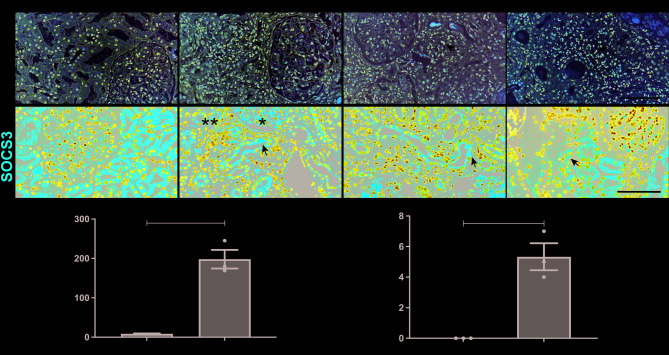
To further validate the role of Axl and SOCS3 in human AKI, we performed immunofluorescence and immunohistochemical analyses on kidney biopsy samples from three patients with biopsy-proven acute tubular injury. Our study included three representative AKI cases encompassing the spectrum of disease etiologies: ischemic AKI (Patient 1: NSAID-induced renal hypoperfusion), mixed ischemic/nephrotoxic AKI (Patient 2: volume depletion with gentamicin/acetaminophen toxicity), and pure nephrotoxic AKI (Patient 3: herbal medicine-induced tubular injury). This clinical stratification directly parallels our experimental approach, with the IRI mouse model recapitulating ischemic injury and cisplatin injured HK2 cells modeling nephrotoxic damage. The clinical characteristics of these AKI patients at the time of biopsy are summarized in Supplementary Table S2. Normal kidney samples, obtained from patients undergoing nephrectomy for renal cell carcinoma, exhibited minimal to no Axl staining. In contrast, kidney samples from AKI patients displayed enhanced Axl staining, primarily localized to the renal interstitium (Fig. 6A). A similar pattern was observed for SOCS3, which was predominantly localized in TECs (Fig. 6B). These findings further support the upregulation of Axl and SOCS3 in the pathogenesis of AKI in humans.
Fig. 6.
Expressions of Axl and SOCS3 in kidneys of patients with AKI. (A) Representative Axl immunohistochemistry (400×). (B) SOCS3 immunofluorescence (400×). The arrows indicate the positive staining of Axl and SOCS3. Scar bar, 100 μm. (C-D) Quantification of Axl-positive cells (C) and SOCS3-positive tubules/400× (n = 1–3 patients). Data are shown as mean ± SEM. *P < 0.05
Discussion
Inflammation plays a pivotal role in the pathogenesis of AKI, where injured tubular epithelial cells (TECs) initiate a self-perpetuating cycle of immune cell recruitment and proinflammatory cytokine release that exacerbates renal injury [2, 18]. While Axl expression is negligible in healthy kidneys [6], our study reveals its significant upregulation during AKI with distinct cellular localization - predominantly in interstitial cells for Axl and TECs for SOCS3 (Figs. 1 and 6). We demonstrate that pharmacological inhibition of Axl with R428 not only attenuated renal structural damage and functional impairment, but also reduced SOCS3 expression in TECs (Figs. 2, 3 and 4). Complementary in vitro studies showed that SOCS3 knockdown in cisplatin-injured TECs suppressed NF-κB activation and decreased production of IL-1β and IL-6, without significantly affecting cell viability (Fig. 5). These findings collectively identify a novel proinflammatory cascade in AKI wherein Axl activation drives SOCS3 upregulation in TECs, leading to NF-κB-mediated cytokine production and subsequent immune cell infiltration. The compartmentalized nature of this pathway (interstitial Axl to tubular SOCS3) and its functional impact on renal inflammation position the Axl/SOCS3 axis as a promising therapeutic target for AKI intervention.
While TAM receptors typically suppress inflammatory responses, accumulating evidence reveals Axl’s paradoxical proinflammatory functions in specific pathological contexts [16, 19–23]. TAM receptors are widely expressed across tissues, with Axl being the most abundant, particularly in dendritic cells (DCs), macrophages, NK cells, fibroblasts, and endothelial cells [24, 25]. The expression of Axl is highly regulated by the inflammatory environment [26]. In AKI, we observed Axl upregulation primarily in renal interstitial cells (Figs. 1 and 6), mirroring findings in cardiac ischemia where DAMP-mediated TLR activation induces myeloid Axl expression [16]. Our demonstration that R428-mediated Axl inhibition attenuated renal injury while suppressing inflammatory cell infiltration, M1 polarization, and NF-κB activation (Figs. 2 and 3) aligns with reports in hypertension-induced renal injury and myocardial IRI [16, 20]. The proinflammatory mechanism involves: (1) Axl’s enhancement of M1 macrophage polarization (LPS/IFN-γ-induced) while suppressing M2 markers [26], and (2) NF-κB activation, as evidenced by studies in glomerulonephritis [9] and TGF-β-stimulated podocytes [27]. This establishes Axl as a key amplifier of the NF-κB-mediated inflammatory cascade that drives AKI progression [3], challenging its conventional anti-inflammatory reputation and highlighting its context-dependent functionality.
While Gas6, the primary ligand for Axl, has demonstrated protective effects in sepsis-induced [28] and IRI-induced AKI models [29], this apparent contradiction with our findings can be explained by several key considerations. First, Axl activation can occur through both Gas6-dependent and -independent mechanisms, including receptor dimerization [30]. Second, Gas6 exhibits pan-TAM receptor activity, with particularly strong binding to MerTK [11], which exerts dominant anti-inflammatory effects that may mask Axl’s proinflammatory functions. This functional dichotomy is evident in glomerulonephritis models, where MerTK knockout exacerbates disease severity while Axl knockout is protective [31], a pattern replicated in myocardial IRI [16] and chronic liver disease [32]. Importantly, our use of R428 selectively targets Axl without affecting MerTK signaling, thereby unmasking Axl’s intrinsic proinflammatory role while preserving MerTK-mediated protection. This specificity may explain the therapeutic benefits we observed, including potential anti-fibrotic effects suggested by R428’s efficacy in unilateral ureteral obstruction models [33]. The net effect of Gas6 administration likely reflects a balance between its activation of protective MerTK signaling and potentially detrimental Axl activation, with the outcome depending on the relative expression and activation states of these receptors in specific disease contexts.
Our study elucidates a novel intercellular signaling axis wherein Axl activation in interstitial cells drives SOCS3 upregulation in TECs, creating a proinflammatory feedback loop. Three lines of evidence support this mechanism: (1) Axl inhibition significantly reduced SOCS3 expression specifically in TECs (Figs. 4 and 6), demonstrating Axl-dependence; (2) SOCS3 knockdown in injured TECs attenuated NF-κB activation and proinflammatory cytokine production without affecting cell survival (Fig. 5); and (3) this TEC-intrinsic SOCS3-NF-κB axis promotes macrophage M1 polarization, as evidenced by both our data and independent studies showing SOCS3-overexpressing TECs induce M1 polarization in coculture [14]. The resulting cytokine milieu (IL-1β, IL-6) then recruits additional inflammatory cells, further activating interstitial Axl expression - thereby completing a self-sustaining inflammatory circuit. This model aligns with established findings that NF-κB knockout in TECs suppresses proinflammatory gene networks [3], and explains how compartmentalized Axl (interstitial) and SOCS3 (tubular) expression can coordinately amplify renal inflammation through paracrine signaling.
While our study provides evidence for the role of Axl/SOCS3 signaling in AKI, it has some limitations. First, we did not investigate the effect of Gas6, the ligand of Axl, on IRI-AKI and Axl/SOCS3 signaling. Although Giangola et al. [29] reported that Gas6 protects against IRI-induced renal damage, Gas6-dependent activation is not the sole mechanism of Axl activation, as Axl overexpression can also lead to its activation [4]. Furthermore, Gas6 can bind and activate MerTK, which exerts dominant anti-inflammatory effects. Compensatory overexpression of Axl has been observed in Gas6 knockout models both in vivo [34] and in vitro [19]. Thus, the role of Gas6 on Axl-SOCS3 signaling during AKI requires further investigation. Second, we used R428 (also known as BGB324 or Bemcentinib), a pharmacological inhibitor of Axl, to achieve specific inhibition of Axl at nanomolar concentrations [17]. However, further studies are needed to investigate whether genetic approaches to activate Axl exacerbate AKI.
While this study establishes the pathogenic role of the Axl-SOCS3 axis in AKI, certain limitations should be acknowledged. First, although we employed the highly selective Axl inhibitor R428 [17] and demonstrated its therapeutic efficacy, complementary genetic approaches (e.g., conditional knockout) could further strengthen these findings. Second, while we focused on Axl’s ligand-independent activation pathways [4], the potential modulation by Gas6, which also activates anti-inflammatory MerTK [11], warrants future investigation, particularly given the compensatory overexpression of Axl has been observed in Gas6 knockout models both in vivo [34] and in vitro [19]. We also recognize that the specific identity of Axl + interstitial cells (F4/80-) remains to be fully characterized; ongoing single-cell sequencing studies in our lab aim to resolve this question. Importantly, the conserved Axl-SOCS3-NF-κB signaling across distinct injury models (ischemic IRI and nephrotoxic cisplatin) strengthens the generalizability of our conclusions, while the human tissue correlations underscore clinical relevance. These limitations conversely highlight critical future directions: (1) cell-specific Axl manipulation, (2) Gas6-MerTK-Axl signaling dynamics, and (3) therapeutic optimization of R428’s timing/dosing in AKI progression.
Conclusion
Our study reveals the Axl-SOCS3 signaling axis as a clinically relevant mediator of renal inflammation in AKI, where pharmacological Axl inhibition attenuates renal injury through TEC-specific reduction of SOCS3 expression and subsequent suppression of NF-κB activation and proinflammatory cytokine production. The conservation of this pathway across species, from murine IRI models to human AKI specimens, coupled with the anti-inflammatory effects observed upon SOCS3 knockdown in injured TECs, establishes Axl-SOCS3 as both a pathophysiological mechanism and a potential therapeutic target. These findings provide a foundation for developing cell-type-specific interventions to modulate this signaling cascade in AKI management.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
Not applicable.
Author contributions
Xin Kang performed the experiments and drafted the manuscript. Qiuhua Gu and Xi Cheng helped collect and assembly the data. Qiuhua Gu conducted the tissue embedding, sectioning and PAS staining. Junya Jia conceived and supervised the study, made the semi-quantitation of renal injury, helped assembly the data and revised the manuscript. Tiekun Yan made the semi-quantitation of renal injury and revised the manuscript. All the authors approved the final manuscript.
Funding
This study was funded by the Tianjin Education Commission Scientific Research Project (No. 2021KJ214), and Tianjin Key Medical Discipline (Specialty) Construction Project (TJYXZDXK-071C).
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
All animal experiments were approved by the Institutional Animal Care and Use Committee of Tianjin Medical University General Hospital (Approval Number: IRB2022-ZWFL-070). All patients provided written informed consent. The clinical protocols were performed in accordance with the principles of the Declaration of Helsinki and approved by the Ethics Committee of Tianjin Medical University General Hospital (Approval Number: IRB2020-YX-114-01).
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Xin Kang, Email: kangxin6452@163.com.
Junya Jia, Email: jiajunya@126.com.
References
- 1.Ronco C, Bellomo R, Kellum JA. Acute kidney injury. Lancet. 2019;394(10212):1949–64. [DOI] [PubMed] [Google Scholar]
- 2.Bonventre JV, Yang L. Cellular pathophysiology of ischemic acute kidney injury. J Clin Invest. 2011;121(11):4210–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Markó L, Vigolo E, Hinze C, Park JK, Roël G, Balogh A, Choi M, Wübken A, Cording J, Blasig IE, et al. Tubular epithelial NF-κB activity regulates ischemic AKI. J Am Soc Nephrol. 2016;27(9):2658–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhu C, Wei Y, Wei X. AXL receptor tyrosine kinase as a promising anti-cancer approach: functions, molecular mechanisms and clinical applications. Mol Cancer. 2019;18(1):153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bian Q, Anderson JC, Zhang XW, Huang ZQ, Ebefors K, Nyström J, Hall S, Novak L, Julian BA, Willey CD, et al. Mesangioproliferative kidney diseases and Platelet-Derived growth Factor-Mediated AXL phosphorylation. Kidney Med. 2021;3(6):1003–e10131001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fiebeler A, Park JK, Muller DN, Lindschau C, Mengel M, Merkel S, Banas B, Luft FC, Haller H. Growth arrest specific protein 6/axl signaling in human inflammatory renal diseases. Am J Kidney Dis. 2004;43(2):286–95. [DOI] [PubMed] [Google Scholar]
- 7.Niu X, Cheng Y, Zhang M, Du L, Wu X, Lu C, Li X, Liu S, Zhao A, Zhang S, et al. Neuroprotective effects of Omentin-1 against cerebral hypoxia/reoxygenation injury via activating GAS6/Axl signaling pathway in neuroblastoma cells. Front Cell Dev Biol. 2021;9:784035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang Z, Liu D, Yan Q, Liu F, Zhan M, Qi S, Fang Q, Yao L, Wang W, Zhang R, et al. Activated AXL protects against hepatic Ischemia-reperfusion injury by upregulating SOCS-1 expression. Transplantation. 2022;106(7):1351–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhen Y, Ren Y, Medvedovic M, Adams DE, Wang D, Shao WH. Axl regulated survival/proliferation network and its therapeutic intervention in mouse models of glomerulonephritis. Arthritis Res Ther. 2022;24(1):284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lemke G. Biology of the TAM receptors. Cold Spring Harb Perspect Biol. 2013;5(11):a009076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rothlin CV, Carrera-Silva EA, Bosurgi L, Ghosh S. TAM receptor signaling in immune homeostasis. Annu Rev Immunol. 2015;33:355–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rothlin CV, Ghosh S, Zuniga EI, Oldstone MB, Lemke G. TAM receptors are pleiotropic inhibitors of the innate immune response. Cell. 2007;131(6):1124–36. [DOI] [PubMed] [Google Scholar]
- 13.Kang X, Chen Y, Xin X, Liu M, Ma Y, Ren Y, Ji J, Yu Q, Qu L, Wang S, et al. Human amniotic epithelial cells and their derived exosomes protect against Cisplatin-Induced acute kidney injury without compromising its antitumor activity in mice. Front Cell Dev Biol. 2021;9:752053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Susnik N, Sörensen-Zender I, Rong S, von Vietinghoff S, Lu X, Rubera I, Tauc M, Falk CS, Alexander WS, Melk A, et al. Ablation of proximal tubular suppressor of cytokine signaling 3 enhances tubular cell cycling and modifies macrophage phenotype during acute kidney injury. Kidney Int. 2014;85(6):1357–68. [DOI] [PubMed] [Google Scholar]
- 15.Ren Y, Chen Y, Zheng X, Wang H, Kang X, Tang J, Qu L, Shao X, Wang S, Li S, et al. Human amniotic epithelial cells ameliorate kidney damage in ischemia-reperfusion mouse model of acute kidney injury. Stem Cell Res Ther. 2020;11(1):410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.DeBerge M, Glinton K, Subramanian M, Wilsbacher LD, Rothlin CV, Tabas I, Thorp EB. Macrophage AXL receptor tyrosine kinase inflames the heart after reperfused myocardial infarction. J Clin Invest 2021, 131(6). [DOI] [PMC free article] [PubMed]
- 17.Holland SJ, Pan A, Franci C, Hu Y, Chang B, Li W, Duan M, Torneros A, Yu J, Heckrodt TJ, et al. R428, a selective small molecule inhibitor of Axl kinase, blocks tumor spread and prolongs survival in models of metastatic breast cancer. Cancer Res. 2010;70(4):1544–54. [DOI] [PubMed] [Google Scholar]
- 18.Rabb H, Griffin MD, McKay DB, Swaminathan S, Pickkers P, Rosner MH, Kellum JA, Ronco C. Inflammation in AKI: current understanding, key questions, and knowledge gaps. J Am Soc Nephrol. 2016;27(2):371–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bárcena C, Stefanovic M, Tutusaus A, Joannas L, Menéndez A, García-Ruiz C, Sancho-Bru P, Marí M, Caballeria J, Rothlin CV, et al. Gas6/Axl pathway is activated in chronic liver disease and its targeting reduces fibrosis via hepatic stellate cell inactivation. J Hepatol. 2015;63(3):670–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Van Beusecum JP, Barbaro NR, Smart CD, Patrick DM, Loperena R, Zhao S, de la Visitacion N, Ao M, Xiao L, Shibao CA, et al. Growth arrest Specific-6 and Axl coordinate inflammation and hypertension. Circ Res. 2021;129(11):975–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Palazzo L, Lindblom J, Mohan C, Parodis I. Current Insights on Biomarkers in Lupus Nephritis: A Systematic Review of the Literature. J Clin Med 2022, 11(19). [DOI] [PMC free article] [PubMed]
- 22.Adams DE, Zhen Y, Qi X, Shao WH. Axl Expression in Renal Mesangial Cells Is Regulated by Sp1, Ap1, MZF1, and Ep300, and the IL-6/miR-34a Pathway. Cells 2022, 11(12). [DOI] [PMC free article] [PubMed]
- 23.Shibata T, Habiel DM, Coelho AL, Kunkel SL, Lukacs NW, Hogaboam CM. Axl receptor Blockade ameliorates pulmonary pathology resulting from primary viral infection and viral exacerbation of asthma. J Immunol. 2014;192(8):3569–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miao YR, Rankin EB, Giaccia AJ. Therapeutic targeting of the functionally elusive TAM receptor family. Nat Rev Drug Discov. 2024;23(3):201–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang WN, Li XP, Wang PF, Zhu L, Xiao XH, Dai YJ. Comprehensive analysis of the novel Omicron receptor AXL in cancers. Comput Struct Biotechnol J. 2022;20:3304–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zagórska A, Través PG, Lew ED, Dransfield I, Lemke G. Diversification of TAM receptor tyrosine kinase function. Nat Immunol. 2014;15(10):920–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Möller-Hackbarth K, Dabaghie D, Charrin E, Zambrano S, Genové G, Li X, Wernerson A, Lal M, Patrakka J. Retinoic acid receptor responder1 promotes development of glomerular diseases via the nuclear Factor-κB signaling pathway. Kidney Int. 2021;100(4):809–23. [DOI] [PubMed] [Google Scholar]
- 28.Chen LW, Chen W, Hu ZQ, Bian JL, Ying L, Hong GL, Qiu QM, Zhao GJ, Lu ZQ. Protective effects of growth Arrest-Specific protein 6 (Gas6) on Sepsis-Induced acute kidney injury. Inflammation. 2016;39(2):575–82. [DOI] [PubMed] [Google Scholar]
- 29.Giangola MD, Yang WL, Rajayer SR, Kuncewitch M, Molmenti E, Nicastro J, Coppa GF, Wang P. Growth arrest-specific protein 6 protects against renal ischemia-reperfusion injury. J Surg Res. 2015;199(2):572–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pagani S, Bellan M, Mauro D, Castello LM, Avanzi GC, Lewis MJ, Sainaghi PP, Pitzalis C, Nerviani A. New Insights into the Role of Tyro3, Axl, and Mer Receptors in Rheumatoid Arthritis. Dis Markers 2020, 2020:1614627. [DOI] [PMC free article] [PubMed]
- 31.Zhen Y, Priest SO, Shao WH. Opposing roles of tyrosine kinase receptors Mer and Axl determine clinical outcomes in experimental Immune-Mediated nephritis. J Immunol. 2016;197(6):2187–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zagórska A, Través PG, Jiménez-García L, Strickland JD, Oh J, Tapia FJ, Mayoral R, Burrola P, Copple BL, Lemke G. Differential regulation of hepatic physiology and injury by the TAM receptors Axl and Mer. Life Sci Alliance 2020, 3(8). [DOI] [PMC free article] [PubMed]
- 33.Hoel A, Osman T, Hoel F, Elsaid H, Chen T, Landolt L, Babickova J, Tronstad KJ, Lorens JB, Gausdal G, et al. Axl-inhibitor Bemcentinib alleviates mitochondrial dysfunction in the unilateral ureter obstruction murine model. J Cell Mol Med. 2021;25(15):7407–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fourcot A, Couchie D, Chobert MN, Zafrani ES, Mavier P, Laperche Y, Brouillet A. Gas6 deficiency prevents liver inflammation, steatohepatitis, and fibrosis in mice. Am J Physiol Gastrointest Liver Physiol. 2011;300(6):G1043–1053. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.