Abstract
Background
The etiology of intrahepatic cholestasis of pregnancy (ICP) is not fully understood; however, genetic, hormonal, environmental factors, and inflammation are believed to contribute to its development. This study aimed to investigate the relationship between systemic immune-inflammation index (SII), systemic inflammatory response index (SIRI), and neutrophil-to-monocyte ratio (NMR) levels with disease severity in ICP and to evaluate their potential in predicting adverse perinatal outcomes.
Materials and methods
A total of 240 pregnant women who delivered at Bursa City Hospital between 2019 and 2024 were retrospectively analyzed. Based on fasting serum bile acid (SBA) levels, patients were divided into three groups: control (SBA < 10 µmol/L), mild (SBA 10–40 µmol/L), and severe (SBA ≥ 40 µmol/L) cholestasis. Inflammatory indices were calculated using complete blood count. Blood samples had been collected at the time of diagnosis, between 28 and 36 weeks of gestation during the third trimester, and prior to the initiation of any treatment. Intergroup comparisons and correlation analyses were conducted. The predictive value of these indices was assessed using receiver operating characteristic (ROC) analysis.
Results
No significant differences were found between groups in WBC and other complete blood count parameters. Only NMR was significantly higher in the severe cholestasis group compared to the control group (p = 0.0057). SII and SIRI levels did not differ significantly among the groups. Gestational age at delivery, birth weight, Apgar scores, and neonatal intensive care unit (NICU) admission rates were significantly worse in the cholestasis groups than in controls (p < 0.001). SII showed a negative correlation with gestational age and 5th-minute Apgar score. In ROC analysis, SII predicted preterm birth with an AUC of 0.600 (cut-off: 897.35; sensitivity: 57%; specificity: 67%) and predicted low 5th-minute Apgar scores with an AUC of 0.669 (cut-off: 1248.38; sensitivity: 75%; specificity: 83%).
Conclusion
The SII and the SIRI may not be reliable markers for the diagnosis or severity assessment of ICP. However, the SII may serve as a potential predictor for preterm birth and low Apgar scores. NMR was significantly elevated in severe ICP patients, suggesting its potential diagnostic value, which should be supported by further studies.
Keywords: Intrahepatic cholestasis of pregnancy (ICP), Systemic immune-inflammation index (SII), Systemic inflammatory response index (SIRI), Neutrophil-to-Monocyte ratio (NMR)
Background
Intrahepatic cholestasis of pregnancy (ICP) is the most common liver disorder specific to pregnancy, typically occurring in the second and/or third trimester and characterized by pruritus and elevated serum bile acid concentrations that rapidly resolve after delivery [1]. Its incidence varies widely, ranging from less than 1–28% [2]. Although the exact etiology remains unclear, genetic, hormonal, and environmental factors, along with inflammation, are believed to contribute to its pathogenesis. The accumulation of bile acids may trigger an immune response, thereby activating inflammatory processes and resulting in hepatocellular injury. Inflammatory indices derived from complete blood count parameters provide simple, cost-effective, and readily accessible tools for evaluating systemic inflammation. Among these, the Systemic Immune-Inflammation Index (SII) and the Systemic Inflammatory Response Index (SIRI) have emerged as novel markers. These indices have shown prognostic value in various oncological, cardiovascular, and obstetric conditions [3–5]. The neutrophil-to-monocyte ratio (NMR), another marker based on leukocyte subtypes, has demonstrated high sensitivity and specificity in predicting disease activity in systemic inflammatory disorders such as rheumatoid arthritis. It has been shown to have a comparable discriminative capacity to traditional inflammatory markers such as C-reactive protein and erythrocyte sedimentation rate [6].
The aim of this study is to investigate the relationship between SII, NMR, and SIRI levels and disease severity in patients with intrahepatic cholestasis of pregnancy. Additionally, this study seeks to evaluate the potential of these parameters to predict adverse perinatal outcomes, including gestational age at delivery, birth weight, low Apgar scores, and neonatal intensive care unit (NICU)admission.
Materials and methods
This retrospective, cross-sectional, case-control study was conducted on pregnant women who received antenatal care and delivered at the Department of Obstetrics and Gynecology, Division of Perinatology, Bursa City Hospital, Turkey, between 2019 and 2024.
The study was approved by the Ethics Committee of Bursa City Hospital (Approval date: March 19, 2025; Decision number: 2025-6/8).
The study included pregnant women who were diagnosed with intrahepatic cholestasis and had complete blood count (CBC) and biochemical test results available at the time of diagnosis, as well as healthy pregnant women with similar gestational ages and available test results. A total of 240 pregnant women were included and categorized into three groups according to fasting serum bile acid (SBA) levels: Control Group (SBA < 10 µmol/L, n = 123), Mild Cholestasis Group (SBA 10–40 µmol/L, n = 75), and Severe Cholestasis Group (SBA ≥ 40 µmol/L, n = 42) [7]. Patients with multiple gestations, major fetal anomalies, a history of systemic infections or inflammatory diseases, preeclampsia, HELLP syndrome, acute or chronic liver disease, or missing data were excluded from the study.
The mild and severe patient groups had received their diagnosis of intrahepatic cholestasis between the 28th and 36th weeks of gestation, during the third trimester. These patients had been started on ursodeoxycholic acid treatment following diagnosis. Inflammatory indices were calculated from blood samples that were obtained at the time of diagnosis, before the initiation of treatment.
Data were retrospectively retrieved from the hospital’s electronic medical record system. The following variables were collected:
Demographic data: Maternal age, gestational age, body mass index (BMI), gravida, parity.
Biochemical parameters: ALT, AST, total bilirubin, direct bilirubin, SBA.
Complete blood count parameters: leukocytes, neutrophils, lymphocytes, monocytes, platelets.
Neonatal outcomes: gestational age at delivery, birth weight, Apgar scores (1st and 5th minute), NICU admission.
The following inflammatory indices were calculated:
SII = (Platelet × Neutrophil) / Lymphocyte.
NMR = Neutrophil / Monocyte.
SIRI = (Neutrophil × Monocyte) / Lymphocyte.
Statistical analyses were performed using IBM SPSS Statistics version 26.0. Continuous variables were presented as mean ± standard deviation. Group comparisons were performed using one-way ANOVA for normally distributed data, and the Kruskal–Wallis test for non-normally distributed data.
The normality of continuous variables was assessed using the Shapiro-Wilk test. Although some variables exhibited mild deviations from normality, one-way ANOVA was preferred based on its robustness and the comparability of group sizes, allowing consistent reporting of data as mean ± standard deviation.
Categorical variables were analyzed using the Chi-square test or Fisher’s exact test. A p-value of < 0.05 was considered statistically significant. Additionally, receiver operating characteristic (ROC) curves and area under the curve (AUC) values were calculated to assess the predictive ability of the inflammatory indices for preterm birth and low Apgar scores. The sample size was calculated using G*Power 3.1 software. Based on prior studies, a medium effect size (f = 0.30), alpha level of 0.05, and statistical power (1–β) of 0.80 were assumed. The analysis determined that a minimum of 111 participants would be required to detect a statistically significant difference among the three groups.
Results
There were no statistically significant differences among the groups in terms of maternal age, gravida, parity, body mass index (BMI), or gestational age (p > 0.05). Liver enzyme levels (AST, ALT) and direct bilirubin levels were significantly higher in the severe cholestasis group (p < 0.001). As expected, fasting serum bile acid levels showed significant differences across the groups (p < 0.001). No significant differences were found between the ICP and control groups in terms of WBC, neutrophil, monocyte, or lymphocyte counts. Among the inflammatory indices derived from complete blood count parameters, only the neutrophil-to-monocyte ratio (NMR) demonstrated a statistically significant difference between the groups (p = 0.0057). Post-hoc analysis revealed that the NMR was significantly higher in the severe cholestasis group compared to the healthy control group, while no significant difference was observed between the mild and severe cholestasis groups. SII and SIRI values did not differ significantly among the groups (p > 0.05) (Table 1).
Table 1.
Demographic, biochemical and inflammatory markers by group
| Variable | Control (n:123) |
Mild (n:75) |
Severe (n:42) |
p-value1 |
|---|---|---|---|---|
| Age | 29.47 ± 5.70 | 29.25 ± 5.14 | 29.51 ± 5.31 | 0.985 |
| Gravida | 2.21 ± 1.25 | 2.09 ± 1.26 | 2.27 ± 1.52 | 0.7484 |
| Parity | 1.02 ± 1.06 | 0.81 ± 0.97 | 1.02 ± 1.25 | 0.3637 |
| BMI | 28.29 ± 4.04 | 29.59 ± 5.44 | 28.54 ± 5.77 | 0.1698 |
| Gestational Age** | 33.57 ± 3.55 | 32.70 ± 4.05 | 32.20 ± 4.32 | 0.1807 |
| AST (U/L) | 19.77 ± 14.70a | 61.51 ± 58.53b | 88.63 ± 143.22c | < 0.001 * |
| ALT (U/L) | 18.29 ± 19.01a | 94.24 ± 110.19b | 140.85 ± 313.74c | < 0.001 * |
| Direct Bilirubin (mg/dL) | 0.13 ± 0.08a | 0.26 ± 0.25b | 0.61 ± 0.69c | < 0.001 * |
| WBC (10^3/µL) | 9.03 ± 2.28 | 9.08 ± 2.68 | 9.08 ± 2.30 | 0.9258 |
| Platelet (10^3/µL) | 245.55 ± 64.87 | 247.41 ± 79.13 | 252.88 ± 62.64 | 0.7183 |
| Neutrophil (10^3/µL) | 6.27 ± 1.95 | 6.74 ± 2.74 | 6.60 ± 2.41 | 0.2896 |
| Monocyte (10^3/µL) | 0.71 ± 0.23 | 0.69 ± 0.29 | 0.62 ± 0.20 | 0.2644 |
| Lymphocyte (10^3/µL) | 1.95 ± 0.60 | 1.88 ± 0.74 | 1.85 ± 0.68 | 0.6705 |
| SBA (µmol/L) | 3.70 ± 2.29a | 18.53 ± 7.40b | 76.39 ± 40.05c | < 0.001 * |
| SII | 865.17 ± 499.53 | 985.43 ± 693.97 | 980.93 ± 451.46 | 0.0871 |
| NMR | 9.32 ± 3.17a | 10.48 ± 5.18 | 11.11 ± 3.67b | 0.0057 * |
| SIRI | 2.54 ± 1.61 | 2.99 ± 2.98 | 2.56 ± 1.42 | 0.5999 |
Values are presented as mean ± standard deviation (SD)
One-way analysis of variance (ANOVA) test
*p < 0.05 was considered statistically significant
Dunnett’s T3 post-hoc test: c > b > a
**Gestational week refers to the week of diagnosis for ICP patients and the corresponding matched gestational age for control subjects
Abbreviations: BMI: Body Mass Index; AST: Aspartate aminotransferase; ALT: Alanine aminotransferase; SBA: Serum bile acid; SII: Systemic immune-inflammation index; SIRI: Systemic inflammatory response index; NMR: Neutrophil-to-Monocyte Ratio; WBC: White blood cell count
Gestational age at delivery and birth weight were significantly lower in the cholestasis groups compared to the control group (p < 0.001). Similarly, the 1st and 5th minute Apgar scores were significantly lower in the cholestasis groups than in controls (p < 0.001). The highest rate of NICU admission was observed in the severe cholestasis group (control: 0%, mild: 1.5%, severe: 5.0%; p < 0.001) (Table 2).
Table 2.
Neonatal outcomes by group
| Variable | Control (n:123) | Mild (n:75) |
Severe (n:42) |
p-value |
|---|---|---|---|---|
| Gestational Age at Birth | 38.44 ± 1.74b | 36.26 ± 2.30a | 36.23 ± 1.70a | < 0.001 * 1 |
| Birth Weight (g) | 3305 ± 495b | 2783 ± 548a | 2834 ± 378a | < 0.001 * 1 |
| 1st Minute Apgar | 8.88 ± 0.62b | 8.26 ± 1.86a | 8.18 ± 1.81a | < 0.001 * 1 |
| 5th Minute Apgar | 9.82 ± 0.52b | 9.22 ± 1.81a | 9.20 ± 1.71a | < 0.001 * 1 |
| NICU Admission | 1 (%0.8)a | 2 (%2.7) | 5 (%11.9)b | 0.0024 * 2 |
Values are presented as mean ± standard deviation (SD)
One-way analysis of variance (ANOVA) test. 2Chi-square test
*p < 0.05 was considered statistically significant
Dunnett’s T3 post-hoc test: b > a
Abbreviations: NICU: Neonatal intensive care unit; Apgar: Appearance, Pulse, Grimace, Activity, and Respiration score
SII showed a weak but statistically significant negative correlation with gestational age at delivery (ρ = -0.16, p = 0.0181) and 5th minute Apgar score (ρ = -0.14, p = 0.0352). No statistically significant correlations were found between neonatal outcomes and NMR or SIRI (Table 3).
Table 3.
Correlation between inflammatory markers and neonatal outcomes in all groups
| Inflammatory Markers | Gestational Age at Birth r (p) |
Birth Weight r (p) |
1st Minute Apgar r (p) |
5th Minute Apgar r (p) |
|---|---|---|---|---|
| SII | -0.16(0.0181*) | -0.12 (0.0647) | -0.08 (0.2539) | -0.14(0.0352*) |
| NMR | -0.08 (0.2328) | 0.02 (0.7844) | -0.05 (0.4330) | -0.01 (0.8350) |
| SIRI | -0.13 (0.0615) | -0.11 (0.1143) | -0.05 (0.4643) | -0.08 (0.2325) |
Correlations were evaluated using Spearman’s rho coefficient
*p < 0.05 was considered statistically significant
Abbreviations: SII: Systemic immune-inflammation index; SIRI: Systemic inflammatory response index; NMR: Neutrophil-to-Monocyte Ratio; Apgar: Appearance, Pulse, Grimace, Activity, and Respiration score
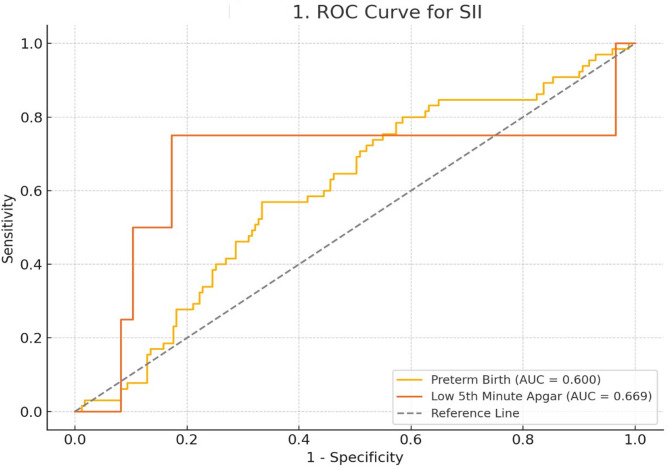
ROC analysis was used to assess the ability of SII to predict preterm birth and low 5-minute Apgar scores, with the results illustrated in Fig. 1. For preterm birth, the area under the curve (AUC) was 0.600, with a cut-off value of 897.35, sensitivity of 57%, and specificity of 67%. For low 5-minute Apgar scores, the AUC was 0.669, with a cut-off value of 1248.38, sensitivity of 75%, and specificity of 83%.
Fig. 1.
ROC curve of SII for predicting preterm birth and low 5th minute apgar score
AUC for preterm birth = 0.600 (cut-off: 897.35; sensitivity: 57%; specificity: 67%). AUC for low 5th-minute Apgar score = 0.669 (cut-off: 1248.38; sensitivity: 75%; specificity: 83%)
Abbreviations: SII– Systemic immune-inflammation index; ROC– Receiver operating characteristics; AUC– Area under the curve
Discussion
Intrahepatic cholestasis of pregnancy (ICP) is the most common pregnancy-specific liver disorder and is associated with significant maternal and fetal complications, particularly when diagnosis or management is delayed [8]. The pathophysiology of ICP is multifactorial, with hormonal, genetic, and environmental contributors, but an emerging body of evidence highlights the role of inflammation in its development and progression. Elevated levels of circulating endotoxins in ICP have been shown to stimulate the release of proinflammatory cytokines—such as interleukin-6 (IL-6) and tumor necrosis factor-alpha (TNF-α)—mainly from peripheral blood monocytes, suggesting the presence of a systemic inflammatory response [9, 10]. However, direct measurement of these cytokines requires specialized laboratory techniques that may not be routinely available in many clinical settings.
Therefore, our study aimed to evaluate the clinical utility of simple, readily accessible, and cost-effective inflammatory indices—specifically, the systemic immune-inflammation index (SII), systemic inflammation response index (SIRI), and neutrophil-to-monocyte ratio (NMR)—alongside standard hematologic and biochemical parameters, in the diagnosis and severity assessment of ICP.
The elevation of bile acids in ICP directly affects hepatocytes and stimulates the release of proinflammatory mediators, resulting in increased, extravasated, and activated neutrophils and other inflammatory cells [11]. This inflammatory cascade can lead to leukocyte extravasation and activation, theoretically resulting in altered systemic hematologic profiles [12]. In our study, we found no statistically significant differences in WBC, neutrophil, monocyte, or lymphocyte counts between the ICP and control groups. This finding contrasts with studies by Abide et al., which reported increased WBC levels in ICP, and by Silva et al., which observed decreased WBC and neutrophil levels [13, 14]. These conflicting observations suggest that while ICP may involve a localized hepatic inflammatory process, it may not consistently elicit systemic leukocytosis detectable through standard complete blood count parameters.
Interestingly, our analysis revealed a significantly elevated NMR in the severe cholestasis group compared to the control group, even though no significant differences were observed between the mild and severe groups. This finding is noteworthy because it may reflect a relative increase in neutrophils or decrease in monocytes in response to more severe hepatic inflammation, suggesting a shift in leukocyte subpopulations rather than absolute cell count elevations. Prior studies have supported the potential of NMR as an inflammation-based biomarker in various disease states, including pregnancy-related disorders [6, 15]. Thus, our results add to the growing body of evidence that NMR may be a sensitive index for identifying heightened inflammatory activity in severe ICP.
Previous studies have proposed that SII and SIRI are reflective of both local immune activity and systemic inflammatory response [16, 17]. In our study, although the mean values of SII and SIRI were higher in the ICP groups compared to controls, the differences were not statistically significant. This is consistent with findings by Callıoğlu et al. [18]. These findings may suggest that while SII and SIRI are theoretically robust markers of systemic inflammation, their diagnostic performance in ICP may be limited due to the organ-specific and possibly subclinical nature of inflammation in this disorder.
As predicted in earlier studies, perinatal outcomes—including gestational age at delivery, birth weight, Apgar scores, and NICU admission—differed significantly among the groups. Perinatal outcomes worsened with increasing disease severity [19, 20]. In our study, SII levels showed a significant negative correlation with gestational age and 5th minute Apgar scores. This suggests that SII may serve as a useful inflammatory marker for predicting adverse perinatal outcomes in ICP, corroborating previous findings that associate higher SII levels with poorer perinatal prognosis [21]. In contrast, SIRI and NMR levels did not show significant correlations with perinatal outcomes in our cohort, which may reflect the need for larger sample sizes or stratification based on disease severity to uncover subtle associations.
This study has several strengths and limitations. One of its main strengths is the specific stratification of ICP patients based on serum bile acid levels, allowing for a more nuanced analysis of disease severity. Additionally, the simultaneous evaluation of hematological, biochemical, and systemic inflammatory indices provides a comprehensive perspective on the inflammatory profile of ICP. The correlation of these indices with perinatal outcomes further enhances the clinical relevance of the findings.
However, the retrospective nature of the study introduces inherent limitations, including potential selection bias and incomplete data. Furthermore, potential confounding factors that could influence inflammatory indices—such as physiological changes related to gestational age—could not be entirely excluded. Another limitation is the absence of direct comparisons with established inflammatory cytokines such as IL-6 and TNF-α, which are considered gold standards for assessing systemic inflammation. Despite these limitations, this study contributes valuable insights into the potential role of easily accessible hematologic markers in the clinical evaluation of ICP and offers a foundation for future large-scale prospective research.
Conclusion
According to the results of our study, SII and SIRI levels do not appear to be reliable markers for diagnosing ICP or distinguishing its severity at this stage. However, the significant negative correlation observed between SII levels and both gestational age at delivery and 5th minute Apgar scores suggests that SII may hold potential as a predictive marker for preterm birth and low Apgar scores in ICP patients. These findings underscore the value of leveraging simple, cost-effective hematologic indices in the clinical management of ICP, especially in resource-limited settings. Additionally, since we found that the NMR ratio was particularly elevated in the severe disease group, its utility as a diagnostic marker may also warrant further investigation in larger-scale studies.
Acknowledgements
None.
Abbreviations
- ICP
Intrahepatic cholestasis of pregnancy
- SII
Systemic immune-inflammation index
- SIRI
Systemic inflammatory response index
- NMR
Neutrophil-to-Monocyte ratio
- SBA
Serum bile acid
- ROC
Receiver operating characteristics
- NICU
Neonatal intensive care unit
- AUC
Area under the curve
- Apgar
Appearance, Pulse, Grimace, Activity, and Respiration score
Author contributions
E.S: Conceptualization, Methodology, Writing– Original Draft Preparation, Project Administration, Formal Analysis. M.R.Ö: Resources, Writing– Review & Editing, E.B.S.Y: Resources, Writing– Review & Editing, Supervision. M.B: Conceptualization, Methodology, Writing– Review & Editing, Project Administration, Supervision, Formal Analysis. All authors reviewed and approved the submitted version.
Funding
The authors received no financial support for this article’s research, authorship, and publication.
Data availability
The datasets used and analysed during the current study are available from the corresponding author upon reasonable request.
Declarations
Ethics approval and consent to participate
This study was approved by the Ethics Committee of Bursa City Hospital (Approval date: March 19, 2025; Decision number: 2025-6/8). Due to the retrospective design of the study and the use of anonymized patient data, the requirement for informed consent was waived by the Ethics Committee. The study was conducted in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Clinical trial number
Not applicable.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Clinical Updates in Women’s Health Care Summary. Liver disease: reproductive considerations. Obstet Gynecol. 2017;129(1):236. [DOI] [PubMed] [Google Scholar]
- 2.Geenes V, Williamson C. Intrahepatic cholestasis of pregnancy. World J Gastroenterol. 2009;15(17):2049–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dziedzic EA, Gąsior JS, Tuzimek A, Paleczny J, Junka A, Dąbrowski M, et al. Investigation of the associations of novel inflammatory Biomarkers-Systemic inflammatory index (SII) and systemic inflammatory response index (SIRI)-With the severity of coronary artery disease and acute coronary syndrome occurrence. Int J Mol Sci. 2022;23(17):9553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Qi Q, Zhuang L, Shen Y, Geng Y, Yu S, Chen H, et al. A novel systemic inflammation response index (SIRI) for predicting the survival of patients with pancreatic cancer after chemotherapy. Cancer. 2016;122(14):2158–67. [DOI] [PubMed] [Google Scholar]
- 5.Shen C, Hu W, Wu T, Wang G, Qiao L, Gao T. Analysis of changes in platelet parameters and inflammatory markers in intrahepatic cholestasis of pregnancy before disease development. Am J Transl Res. 2024;15(12):7448–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Obaid JMAS, Almjydy MMA, Garban MAQ, Al-Hebari FSQ, Al-Washah NAH. Neutrophil-to-monocyte ratio is the better new inflammatory marker associated with rheumatoid arthritis activity. Health Sci Rep. 2023 2;6(8):e1478. [DOI] [PMC free article] [PubMed]
- 7.Mays JK. The active management of intrahepatic cholestasis of pregnancy. Curr Opin Obstet Gynecol. 2010;22:100–3. [DOI] [PubMed] [Google Scholar]
- 8.Allen K, Jaeschke H, Copple BL. Bile acids induce inflammatory genes in hepatocytes: a novel mechanism of inflammation during obstructive cholestasis. Am J Pathol. 2011;178(1):175–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zeng W, Hou Y, Gu W, Chen Z. Proteomic biomarkers of intrahepatic cholestasis of pregnancy. Reprod Sci. 2024;31:1573–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu W, Chen L, Miao K, You Y, Li J, Lu J, et al. Identification and validation of diagnostic biomarkers for intrahepatic cholestasis of pregnancy based on untargeted and targeted metabolomics analyses of urine metabolite profiles. BMC Pregnancy Childbirth. 2023;23:828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kondrackiene J, Kupcinskas L. Intrahepatic cholestasis of pregnancy-current achievements and unsolved problems. World J Gastroenterol. 2008;14:5781–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Omeroglu I, Golbasi H, Bayraktar B, Golbasi C, Yildirim Karaca S, Demircan T, et al. Modified myocardial performance index for evaluation of fetal heart function and perinatal outcomes in intrahepatic pregnancy cholestasis. Int J Cardiovasc Imaging. 2023;39(5):907–14. [DOI] [PubMed] [Google Scholar]
- 13.Yayla Abide Ç, Vural F, Kılıççı Ç, Bostancı Ergen E, Yenidede İ, Eser A, et al. Can we predict severity of intrahepatic cholestasis of pregnancy using inflammatory markers? Turk J Obstet Gynecol. 2017;14(3):160–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Silva J, Magenta M, Sisti G, Serventi L, Gaither K. Association between complete blood count components and intrahepatic cholestasis of pregnancy. Cureus. 2020;12:e12381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tang F, Dai P, Wei Q, Gan K, Wang Z, Chen H, et al. The Neutrophil-to-Monocyte ratio and Platelet-to-White blood cell ratio represent novel prognostic markers in patients with pancreatic Cancer. Gastroenterol Res Pract. 2021;2021:6693028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang J, Zhou D, Dai Z, Li X. Association between systemic immune inflammation index and diabetic depression. Clin Interv Aging. 2021;16:97–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tian T, Lu J, Zhao W, Wang Z, Xu H, Ding Y, et al. Associations of systemic inflammation markers with identification of pulmonary nodule and incident lung cancer in Chinese population. Cancer Med. 2022;11:2482–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Çallıoğlu N, Tuna G, Tandoğan Ö, Ersan F, Atalay S, Bilirer KK. Systemic immune-inflammatory index and platelet-to-lymphocyte ratio in intrahepatic cholestasis of pregnancy. Saudi Med J. 2024;45(11):1217–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ovadia C, Seed PT, Sklavounos A, Geenes V, Di Ilio C, Chambers J, et al. Association of adverse perinatal outcomes of intrahepatic cholestasis of pregnancy with biochemical markers: results of aggregate and individual patient data meta-analyses. Lancet. 2019;2(10174):899–909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shao Y, Chen S, Li H, Tang Q, Xu D. Gebeliğin intrahepatik Kolestazının maternal Safra Asidi profili ve alt tip analizi. Orphanet J Rare Dis 2021 7; 16(1):259. [DOI] [PMC free article] [PubMed]
- 21.Sahin R, Tanacan A, Serbetci H, Agaoglu Z, Karagoz B, Haksever M, et al. The role of first-trimester NLR (neutrophil to lymphocyte ratio), systemic immune-inflammation index (SII), and, systemic immune-response index (SIRI) in the prediction of composite adverse outcomes in pregnant women with systemic lupus erythematosus. J Reprod Immunol. 2023;158:103978. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and analysed during the current study are available from the corresponding author upon reasonable request.