Abstract
Background
Our previous study revealed that circ0043898 is downregulated in esophageal cancer (EC), and its overexpression attenuates the progression of EC. The objective of this article is to explore whether circ0043898 inhibits tumor progression by inhibiting cancer stem cells (CSCs) in EC.
Methods
PCDH-circ0043898 plasmid was transfected into EC cells, and the effect of overexpression was verified by qRT-PCR. Immunofluorescence, flow cytometry, and stem cell spheroidization were used to detect CSCs phenotype changes. RNA sequencing was adopted to identify downstream regulatory genes and p-PI3K, PI3K, and KRAS expressions were validated by western blot. To investigate whether KRAS functions downstream of circ0043898 in regulating cancer stemness, we co-transfected EC cells with both KRAS and circ0043898 plasmids and examined CSCs phenotypes.
Results
circ0043898 was overexpressed in the EC cells, and reduced stem cell markers (CD44 and CD133) and the number of stem cell spheroidization. In addition, overexpression of circ0043898 changed many genes expression, including reduced p-PI3K, PI3K, and KRAS expressions. Moreover, overexpression of KRAS attenuated the effect of overexpressed circ0043898 on CSCs phenotype.
Conclusions
Overexpression of circ0043898 reduced CSCs markers and the number of stem cell spheroidization. However, the overexpression of KRAS attenuated the inhibition effect of overexpressed circ0043898 on CSCs marker and the number of stem cell spheroidization. These findings identify a potential therapeutic target for the EC.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12885-025-14358-8.
Keywords: circ0043898, KRAS, Esophageal cancer, Stemness, CircRNAs
Introduction
In 2020, 604,100 patients worldwide were diagnosed with esophageal cancer (EC), ranking seventh out of 36 common cancers, in which, China accounts for over 50% of global cases; additionally, there is 544,076 EC deaths, ranking sixth out of 36 common cancers [1]. The current treatment methods for EC include surgical resection, radiotherapy, chemotherapy, and immunotherapy [2]. Although these methods improve the survival rate of EC patients, due to the lack of early diagnosis, they are often found in the middle to late stages and often accompanied by metastasis [3]. EC prognosis is still poor, so more research is needed to find new treatment methods [4].
Cancer stem cells (CSCs) are a group of cells derived from tumor cells with stem cell like characteristics (self-renewal and differentiation potential) [5]. CSCs can trigger tumor formation again, and CSCs have been proven to exist in EC [6]. The biomarker of CSCs including CD44, CD133, epithelial cell adhesion molecule, SOX2, Oct-3/4, and Nanog [7]. However, the biomarkers of different cancer stem cells may vary [7]. For EC, the biomarker of CSCs including CD44, CD133, CD271, CD14, CD90, CXCR4, ALDH, ICAM1, and SCAR Homolog [8, 9, 10]. CSCs are involved in various processes, such as tumor angiogenesis, drug resistance, and tumor recurrence [11]. Recent studies have also explored G-quadruplex (G4)-based approaches to target oncogenic drivers associated with CSC-related resistance and tumor relapse [12, 13]. In other words, treatment strategies targeting CSCs can help inhibit the progression of EC, reduce EC recurrence, and increase cancer cell sensitivity to drugs.
Circular RNAs (circRNAs) have covalently closed-loop structures [14], which can resist acid exonuclease mediated degradation and therefore have higher stability than linear RNAs [15]. CircRNAs are involved in regulating gene expression related to CSCs and activating downstream pathways, thus potentially becoming therapeutic targets or clinical biomarkers [16, 17]. For example, circRNA IPO11 recruits TOP1 to the GLI1 promoter to promote TOP1 transcription and activate the Hedgehog pathway, driving self-renewal of liver CSCs and promoting liver cell metastasis [18]. The combination of antisense oligonucleotides targeting circRNA IPO11 and the TOP1 inhibitor exerts a synergistic anti-tumor effect [18]. Inhibiting circRNA CDR1 can reduce stem genes (SOX2, OCT4, and Nanog) in non-small cell lung cancer cells, and increase sensitivity to cisplatin [19]. Furthermore, circRNAs are increasingly recognized as both diagnostic biomarkers and potential therapeutic tools in various solid tumors [20].
In our previous study [21], we demonstrated that circ0043898 was downregulated in EC tissues. Circ0043898 overexpression could prevent EC cell progression. In vivo experiments further confirmed that circ0043898 overexpression suppressed tumor formation. These findings indicate that circ0043898 may act as a tumor suppressor in EC. However, its downstream regulatory mechanisms remain largely unclear. Given that cellular proliferation, migration, and invasion are functional hallmarks often linked to cancer stemness [22, 23], we speculated that the tumor-suppressive effects of circ0043898 observed in our previous work might involve modulation of CSC-related properties. In EC, CSCs have been strongly associated with enhanced metastatic potential, therapeutic resistance, and tumor recurrence, making them a critical driver of disease progression [24, 25]. These insights prompted us to investigate whether circ0043898 suppresses EC progression by targeting CSC phenotypes and to further explore the underlying regulatory mechanisms involved.
KRAS, a well-characterized oncogene, is frequently mutated or amplified in esophageal carcinoma and plays a pivotal role in regulating tumor proliferation, metastasis, and therapeutic resistance through multiple downstream pathways, including PI3K/AKT and MAPK [26, 27]. These pathways have also been implicated in maintaining CSC phenotypes and enhancing cellular plasticity in EC. Recent studies also have highlighted that PI3K/AKT sustains CSC phenotypes in EC by enhancing stemness-associated markers such as CD44 and CD133, promoting EMT, and conferring resistance to conventional therapies [24, 28, 29, 30]. Aberrant activation of this pathway has been linked to increased self-renewal capacity, invasiveness, and recurrence of EC. Therefore, targeting PI3K/AKT signaling is considered a promising strategy for eliminating CSCs and improving therapeutic outcomes. In parallel, pan-KRAS inhibitors have shown potential in blocking oncogenic KRAS signaling and suppressing tumor growth in preclinical models [31].
Although the oncogenic function of KRAS in EC is well established, its potential regulation by circRNAs has rarely been reported. Emerging evidence suggests that circRNAs may regulate target genes by sponging miRNAs or interfering with protein-RNA interactions [32, 33, 34]. Therefore, we hypothesize that circ0043898 may exert its suppressive effects by downregulating KRAS expression and inhibiting downstream PI3K/AKT signaling, thus attenuating the stem-like characteristics of EC cells. Accordingly, this study aimed to explore whether circ0043898 regulates EC stemness through modulation of KRAS and the PI3K/AKT pathway, providing new insight into its tumor-inhibitory mechanism.
Materials and methods
Cell culture
ECA109 (MeisenCTCC, cat. CTCC-007-0162) and Kyse-520 (MeisenCTCC, cat. CTCC-007-0181) cells were maintained in DMEM supplemented with 10% FBS at 37 °C with 5% CO₂.
Overexpression of circ0043898 and KRAS
The overexpression plasmids were synthesized by General Bio (Anhui, China). The plasmid PCDH-CMV-EF1A-EGFP-T2A-PURO was obtained from Jiangsu Sesofei Biotechnology Co., LTD. Transfections were performed using Lipofectamine 3000 (Invitrogen, USA) for either 24–48 h depending on the subsequent experimental requirements. For assays evaluating cancer stemness characteristics, including Western blot, flow cytometry, and immunofluorescence (IF), cells were harvested at 48 h post-transfection. A previous study has demonstrated that changes in stemness-associated markers can be reliably detected within 48 h following gene modulation [35], supporting the use of this time frame for assessing stemness-related changes.
qRT-PCR
Total RNA was extracted using TriQuick Reagent (Solarbio, Beijing, China). cDNA was synthesized with 5× RT SuperMix for qPCR (APEXBio, cat. K1074), and quantitative amplification was carried out using 2× SYBR Green qPCR Master Mix (APEXBio, cat. K1070) and primers (Table 1).
Table 1.
Primer sequences
| Primer name | Sequences (5’-3’) | Length (bp) |
|---|---|---|
| H-GAPDH-F | GAGTCAACGGATTTGGTCGT | 185 |
| H-GAPDH-R | GACAAGCTTCCCGTTCTCAG | |
| H-circ0043898-F1 | GGCAAATATGGGTGCAAAGGC | 163 |
| H-circ0043898-R1 | AGAACAGGTGTCCAGCTTTTTGA | |
| H-PI3KCB-F | GACTTTGCGACAAGACTGCC | 220 |
| H-PI3KCB-R | ATCACTCATCTGTCGCAGGC | |
| H-KRAS-F | AGAGTGCCTTGACGATACAGC | 134 |
| H-KRAS-R | ACCTGCTGTGTCGAGAATATCC |
Western blot
ECA109 and Kyse-520 cells were transfected with either PCDH-NC or PCDH-circ0043898 plasmids. After 48 h, cells were harvested for total protein extraction using RIPA (Beyotime, Shanghai, China). After quantification, the protein (35 µg) were separated and transferred onto PVDF membranes. Membranes were blocked with 5% BSA for 1 h, followed by overnight incubation at 4 °C with primary antibodies specific to KRAS (Proteintech, cat 12063-1-AP), PI3K (ABclonal, cat A18355), phospho-PI3K (ABclonal, cat AP0854), CD44 (ABclonal, cat A19020), and CD133 (ABclonal, cat A0219). GAPDH (Proteintech, cat 60004-1-Ig) was used as an internal control. After washing, membranes were incubated with secondary antibodies (Biosharp, Cat. No. BL001A or Cat. No. BL003A) for 1 h. After washing, protein bands were visualized using enhanced chemiluminescence (ECL, Beyotime). Band intensity was quantified using ImageJ software to assess protein level.
IF detection
Cells were fixed with 4% paraformaldehyde (Solarbio, Beijing, China) for 2 h and then washed three times with PBS (Solarbio). Cells were incubated overnight at 4 °C with primary antibodies against CD44 (ABclonal, cat. no. A19020) and CD133 (ABclonal, cat. no. A0219). After incubation, cells were washed and incubated with secondary antibodies for 1 h. Finally, after washing, images were captured using a fluorescence microscope (Mshot, Guangzhou, China).
Flow cytometry
ECA109 and Kyse-520 cells were transfected with the corresponding plasmids. After 48 h, cells were digested with 0.5% trypsin and harvested by centrifugation at 8000 rpm for 10 min. The cell pellet was washed and 1 × 10⁶ cells were resuspended in 100 µL PBS. Antibodies against hCD133-APC (R&D Systems, cat. no. FAB11331A-100) and human/mouse CD44-FITC (Elabscience, cat. no. E-AB-F1100C) were added. After incubation, unbound antibodies were removed by washing twice with PBS. Finally, cells were analyzed using a flow cytometer. To ensure experimental consistency, all cells used for flow cytometry and immunofluorescence were derived from parallel cultures transfected simultaneously under identical conditions.
Stem cell spheroid formation
After transfecting the corresponding plasmids into ECA109 and Kyse-520 cells using Lipofectamine 3000 for 24 h, cells were gently washed once with PBS to remove residual culture medium. Cells were then digested with 0.5% trypsin and harvested by centrifugation at 8000 rpm for 10 min. The cell pellet was resuspended in serum-free medium consisting of advanced DMEM/F12 (Gibco, cat. no. 12634-010), 2% B27 supplement (Gibco, cat. no. 17504044), 20 ng/mL EGF (Peprotech, cat. no. AF-100-15), and 20 ng/mL FGF (Peprotech, cat. no. 100-18B-50UG). Cells were plated at a density of 10,000 cells per well in low-adhesion 6-well plates and incubated continuously for 7 days. The formation of spheroids was monitored and documented using a phase-contrast microscope.
RNA sequencing and functional analysis
EC cells (ECA109, Kyse-520) were transfected with empty PCDH plasmid (PCDH-NC group) or PCDH-circ0043898 plasmid (PCDH-circ0043898 group), respectively. Samples were collected 24 h after transfection and subjected to RNA sequencing. Differentially expressed genes (DEGs) were identified using a fold change threshold of ≥ 2 and an adjusted p-value (padj) < 0.05. A total of 309 overlapping DEGs between the two cell lines were identified and subsequently used for GO and KEGG enrichment analyses. Among the top 15 enriched pathways, the Ras pathway and PI3K-AKT pathway were prominently represented. Based on the enrichment results and previous literature [36], KRAS and PI3K were selected as key candidate genes for downstream validation by qRT-PCR and Western blot.
Statistical analysis
In addition to RNA sequencing data, all experiments were independently repeated at least three times (n = 3). Data are presented as the mean ± SD. Statistical analysis was performed using GraphPad Prism 8.0 with Student’s t-test or ANOVA. Tukey’s post hoc test was applied following ANOVA to assess differences between specific groups. P < 0.05 was considered statistically significant.
Results
Overexpression of circ0043898 reduces stem cell markers and weakens the ability of stem cells to form spheres in EC cells
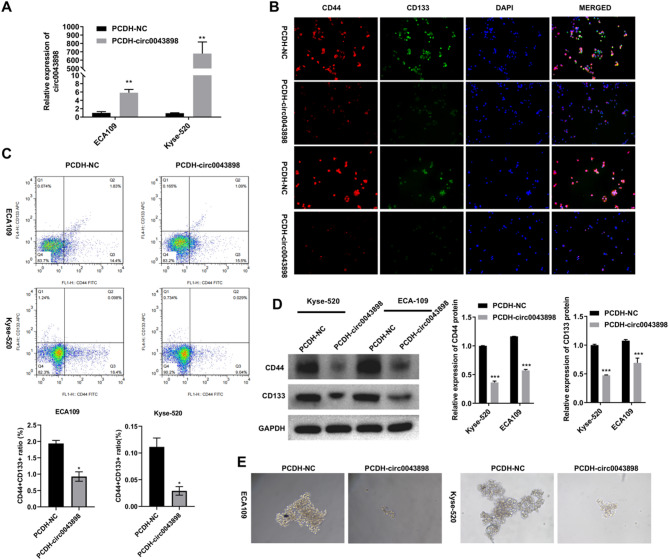
Two EC cell lines, ECA109 and Kyse-520, were transfected with either the empty PCDH plasmid (PCDH-NC group) or the PCDH-circ0043898 overexpression plasmid (PCDH-circ0043898 group). At 24 h post-transfection, total RNA was collected for qRT-PCR to assess circ0043898 expression levels and to observe the ability of cells to form spheroids. At 48 h post-transfection, cells were harvested for IF staining and flow cytometry analysis to examine stemness markers CD44 and CD133. In qRT-PCR, circ0043898 expression was elevated in both cell lines following transfection with PCDH-circ0043898, indicating successful overexpression (Fig. 1A). IF data revealed that CD44 and CD133 expression was markedly decreased in the overexpression group, suggesting reduced stem-like properties (Fig. 1B). These findings were further supported by flow cytometry, which showed a decreased proportion of CD44⁺CD133⁺ double-positive cells after circ0043898 overexpression (Fig. 1C). To validate these results at the protein level, we performed Western blot analysis and observed a consistent reduction in CD44 and CD133 protein expression in the PCDH-circ0043898 group (Fig. 1D). Moreover, the ability of EC cells to form stem cell-like spheroids was notably impaired upon circ0043898 overexpression, as shown by the reduced number and size of spheres in both cell lines (Fig. 1E).
Fig. 1.
Overexpression of circ0043898 reduced stem cell markers and weakened the ability of stem cells to form spheres in EC cells. EC cells (ECA109, Kyse-520) were transfected with PCDH empty plasmid (named PCDH-NC group) and PCDH-circ0043898 plasmid (named PCDH-circ0043898 group), respectively. After 24 h, samples were collected for qRT-PCR to detect circ0043898 and the observation of stem cell sphere formation ability. After 48 h, samples were collected for IF, flow cytometry, and Western blot to detect stemness marker genes CD44 and CD133. (A) qRT-PCR detection of circ0043898 expression. (B) IF detection of CD44 and CD133 expressions. (C) Flow cytometry detection of CD44⁺CD133⁺ double-positive cell population. (D) Western blot analysis of CD44 and CD133 protein expression following circ0043898 overexpression. Blots were cut prior to antibody incubation. (E) Representative images of stem cell spheres in EC cells. *P < 0.05, **P < 0.01, ***P < 0.001
RNA sequencing to monitor the gene expression changes and related functional analysis after overexpression of circ0043898
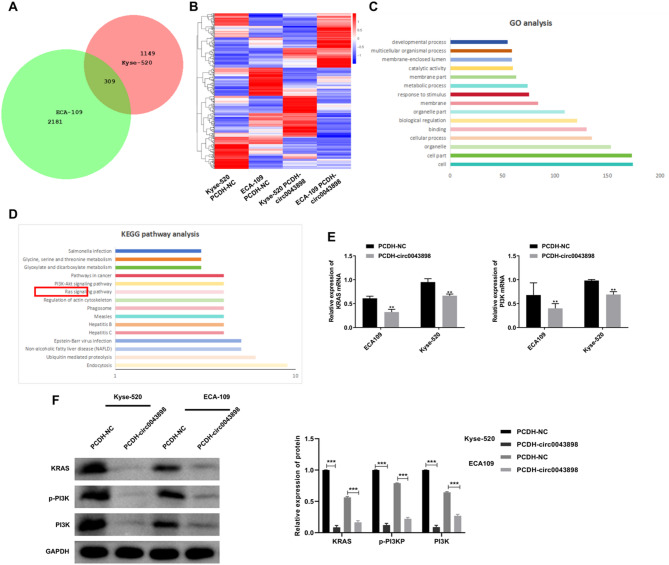
RNA sequencing analysis revealed that a total of 2,490 genes in ECA109 cells and 1,458 genes in Kyse-520 cells were altered upon circ0043898 overexpression. Among these, 309 genes were commonly differentially expressed, comprising 159 downregulated and 150 upregulated genes, as determined by the criteria of Fold Change ≥ 2 and padj < 0.05 (Fig. 2A). The expression profiles of these 309 genes across the four experimental conditions (ECA109/PCDH-NC, ECA109/PCDH-circ0043898, Kyse-520/PCDH-NC, and Kyse-520/PCDH-circ0043898) were visualized in a heatmap, demonstrating distinct clustering patterns (Fig. 2B). GO enrichment analysis of the 309 shared genes indicated significant involvement in biological categories such as cellular components, particularly “cell,” “cell part,” and “organelle” (Fig. 2C). KEGG pathway enrichment further revealed that the DEGs were enriched in multiple cancer-associated signaling cascades, including the PI3K-AKT and Ras pathways (Fig. 2D). Subsequent qRT-PCR validation demonstrated that the mRNA levels of KRAS and PI3K, representative genes of the Ras pathway, were suppressed following circ0043898 overexpression (Fig. 2E). Consistently, Western blot results confirmed a marked decrease in protein levels of KRAS, PI3K, and phosphorylated PI3K in both EC cell lines upon circ0043898 upregulation (Fig. 2F).
Fig. 2.
RNA sequencing reveals gene expression changes and functional enrichment following circ0043898 overexpression in EC cells. ECA109 and Kyse-520 cells were transfected with empty PCDH plasmid (named the PCDH-NC group) and PCDH-circ0043898 plasmid (named the PCDH-circ0043898 group), respectively. Samples were collected for RNA sequencing 24 h post-transfection. (A) Venn diagram showing DEGs in Kyse-520 and ECA109 cells following circ0043898 overexpression. (B) Heatmap of DEGs in Kyse-520 and ECA109 cells. (C) Top 15 GO functional enrichment analysis of 309 commonly DEGs in Kyse-520 and ECA109 cells. (D) Top 15 KEGG pathway enrichment analysis of 309 commonly DEGs. (E) qRT-PCR detection of KRAS and PI3K mRNA levels in EC cells following circ0043898 overexpression. (F) Western blot analysis of KRAS, PI3K, and p-PI3K protein expression in EC cells following circ0043898 overexpression. Blots were cut prior to antibody incubation. ***P < 0.001
Overexpression of KRAS enhanced the stemness of EC cells inhibited by overexpression of circ0043898
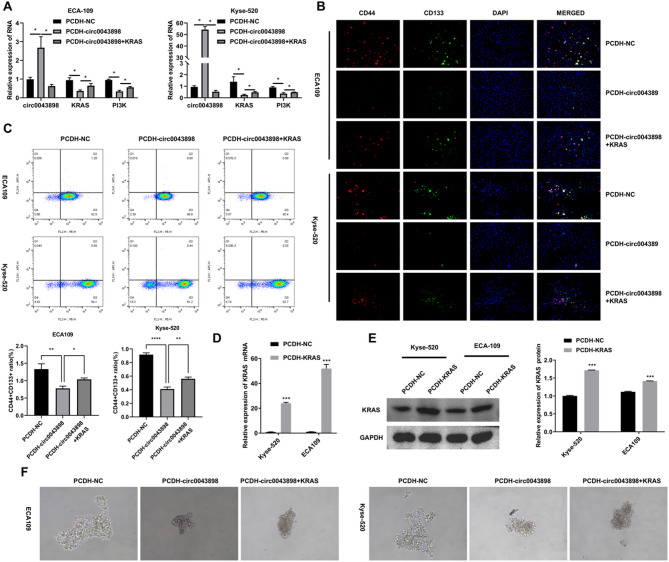
ECA109 and Kyse-520 cells were transfected with PCDH-NC (named PCDH-NC group), PCDH-circ0043898 plasmid (named PCDH-circ0043898 group), PCDH-circ0043898 and PCDH-KRAS plasmid (named PCDH-circ0043898 + KRAS group), respectively. After 24 h of transfection, qRT-PCR and stem cell spheroid formation were performed. After 48 h of transfection, IF and flow cytometry were used to detect stemness marker genes CD44 and CD133. Figure 3A suggested that overexpression of KRAS inhibited the upregulation of circ0043898 expression induced by PCDH-circ0043898, but increased KRAS and PI3K, which are inhibited by overexpression of circ0043898. The results of Fig. 3B and C indicated that overexpression of KRAS can increase stemness gene markers CD3 and CD144, which were inhibited by overexpression of circ0043898. Figure 3D suggested that overexpression of KRAS increased the number of stem cells in EC cells inhibited by overexpression of circ0043898.
Fig. 3.
Overexpression of KRAS reversed the stemness inhibition induced by circ0043898 in EC cells. ECA109 and Kyse-520 cells were transfected with PCDH-NC (PCDH-NC group), PCDH-circ0043898 (PCDH-circ0043898 group), or co-transfected with PCDH-circ0043898 and PCDH-KRAS (PCDH-circ0043898 + KRAS group). After 24 h of transfection, samples were collected for qRT-PCR analysis and stem cell spheroid formation assays. After 48 h of transfection, IF and flow cytometry were performed to assess stemness markers CD44 and CD133. (A) qRT-PCR analysis of circ0043898, KRAS, and PI3K mRNA expression. (B) IF detection of CD44 and CD133 protein expression. (C) Flow cytometry analysis of CD44⁺CD133⁺ double-positive cell ratios. (D) qRT-PCR analysis confirming KRAS overexpression. (E) Western blot detection of KRAS expression in each group. Blots were cut prior to antibody incubation. (F) Representative images of stem cell spheroid formation in each group. *P < 0.05, **P < 0.01, ***P < 0.001
Overexpression of KRAS reverses the inhibitory effects of circ0043898 on the stemness of EC cells
ECA109 and Kyse-520 cells were transfected with PCDH-NC (PCDH-NC group), PCDH-circ0043898 (PCDH-circ0043898 group), or co-transfected with PCDH-circ0043898 and PCDH-KRAS (PCDH-circ0043898 + KRAS group). At 24 h post-transfection, total RNA was extracted for qRT-PCR, and stem cell spheroid formation assays were initiated. At 48 h, cells were harvested for IF and flow cytometry to assess stemness markers CD44 and CD133. qRT-PCR results demonstrated that KRAS overexpression reversed the suppressive effect of circ0043898 on KRAS and PI3K mRNA levels, while the upregulation of circ0043898 remained unaffected, suggesting that KRAS may act downstream of circ0043898 in regulating this axis (Fig. 3A). IF analysis further showed that KRAS overexpression restored CD44 and CD133 expression, which had been diminished by circ0043898 alone (Fig. 3B). Flow cytometry results confirmed these observations, with increased CD44⁺CD133⁺ cell populations in the co-transfection group compared to the circ0043898-only group (Fig. 3C). To validate the efficiency of KRAS overexpression, additional qRT-PCR and Western blot experiments were performed. Both mRNA and protein levels of KRAS were elevated in the PCDH-KRAS transfection group, confirming the success of the overexpression (Fig. 3D and E). Finally, spheroid formation assays revealed that the ability of EC cells to form stem cell-like spheres suppressed by circ0043898 was markedly rescued by co-overexpression of KRAS, suggesting functional restoration of stemness (Fig. 3F).
Discussion
Base on the above results, this study found that overexpression of circ0043898 inhibited CSCs markers (CD44 and CD133) in EC cells and stem cell spheroidization. In addition, overexpression of circ0043898 alters many downstream genes and participates in the regulation of multiple pathways, including the classic cancer cell pathways PI3K-AKT and Ras. Overexpression of KRAS can reverse the effect circ0043898 overexpression on CSCs markers and stem cell spheroidization.
CSCs have similar characteristics to stem cells, which may have already entered the bloodstream or other locations in the early stages of tumors, then self-renewal and multi-directional differentiation according to changes in the body environment, leading to cancer recurrence and metastasis [37]. CircRNAs participate in the regulation of CSCs. For example, circRNA Gprc5a can be encoded as a short peptide and promote the self-renewal of CSCs (stem cell spheroidization increased) in bladder cancer [38]. CircRNA CDR1as increases proliferation and stemness of hepatoblastoma (stem cell spherulation and CD144 expression increased) by regulating the miR-7-5p/KLF4 axis [39]. The highly malignant EBV associated cancer cell line SNU-4th exhibits the characteristics of CSCs (CD44 + CD24-), increases circRNA LMP2A to maintain a stem cells phenotype through the miR-3908/TRIM59/p53 axis (increased stem cell sphericity and stem cell markers Sox2, Klf4, Bmi1, and Oct4) [40]. Moreover, CSCs enhance immune escape, and clinical research and development of immunotherapies targeting for CSCs have begun [41, 42]. In this study, overexpressed circ0043898 inhibited stem cell marker, CD44 and CD133, as well as reduced the number of stem cell spheroids. These results indicated that circ0043898 regulated the stemness of CSCs in EC. However, the potential mechanism still unclear.
Then, RNA sequencing was performed to find the downstream gene regulated by circ0043898. Enrichment of DEGs in pathways of PI3K-AKT and Ras. The PI3K/AKT pathway is an intracellular pathway involved in proliferation, metabolism, migration, drug resistance, and angiogenesis [43]. Moreover, the PI3K/AKT pathway typically exhibits excessive activation in various types of tumors, including EC [43, 44]. At present, some inhibitors targeting the PI3K/AKT pathway have been used in clinical cancer treatment, although there are still many issues that need to be addressed, including which drugs should be used to treat specific types of cancer [45]. In this study, overexpressed circ0043898 inhibited PI3K, and p-PI3K. This indicated that overexpressed circ0043898 maybe decrease the stemness of EC through PI3K/AKT pathway.
The RAS gene family consists of three members, including KRAS, HRAS, and NRAS, with 90% identity in its G domain, possessing conserved structures and biochemical properties [46]. In which, KRAS is involved in the proliferation, differentiation, and survival of cancer cells through direct interactions with the effector, such as RAF family member CRAF [47]. Pan KRAS inhibitor, which targets KRAS, may exert therapeutic effects on KRAS-driven cancer, such as EC, lung cancer, colorectal cancer, and pancreatic cancer [31, 48, 49]. In this study, overexpressed circ0043898 inhibited KRAS, indicating that overexpressed circ0043898 reduced the stemness in EC through inhibiting KRAS. Although our study did not directly investigate the molecular mechanism by which circ0043898 regulates KRAS, previous literature suggests that circRNAs can modulate gene expression through miRNA sponging or RNA-protein interactions [32, 34]. Based on this, we speculate that circ0043898 may suppress KRAS expression via acting as a miRNA sponge, a hypothesis supported by the typical regulatory pattern of many tumor-suppressive circRNAs. Future studies involving RNA immunoprecipitation (RIP), dual-luciferase reporter assays, or miRNA profiling will be necessary to elucidate whether circ0043898 interacts with specific miRNAs that target KRAS or binds directly to relevant regulatory proteins. This may help clarify the upstream mechanisms responsible for the KRAS/PI3K/AKT suppression observed in our study.
However, this study was only confirmed the overexpressed KRAS reversed the role of circ0043898 overexpression on the stemness in vitro, so further in vivo studies are needed for further confirmation. These findings also raise the question of whether KRAS, in turn, could influence circ0043898, potentially forming a regulatory feedback loop. Although our current work does not explore this possibility, it remains an interesting direction for future investigation. Further research is warranted to determine whether KRAS activation could modulate the transcription or biogenesis of circ0043898, which may provide new insights into the dynamic interplay between oncogenic signaling and circRNA regulation in EC. This study thus provides a basis for such follow-up investigations.
In addition to mechanistic insights, our findings may have translational potential. Given that circ0043898 negatively regulates KRAS expression and suppresses cancer stemness, future studies could assess the clinical significance of circ0043898 expression levels in EC specimens. Tumors with low circ0043898 expression may represent a subtype characterized by elevated KRAS activity and enhanced stemness properties, which could be more responsive to KRAS-targeted therapies. Stratifying patients based on circ0043898 status may inform individualized treatment strategies and improve therapeutic outcomes in EC. Building on these insights, further research could explore therapeutic strategies that modulate circ0043898 expression in EC. One potential approach is to develop synthetic circ0043898 mimics or delivery systems aimed at restoring its function in tumors with low endogenous expression. Alternatively, combined use of circ0043898-based therapies and KRAS inhibitors may enhance the suppression of CSC traits. However, several challenges need to be addressed before clinical application can be realized, including the efficient and selective delivery of circRNAs, maintaining their stability in vivo, and accounting for inter-patient variability. Future studies using in vivo models and clinical specimens will be essential to evaluate the feasibility, safety, and effectiveness of these strategies.
Conclusions
Overexpression of circ0043898 inhibited CSC markers (CD44 and CD133) and reduced stem cell spheroid formation in EC cells. This inhibitory effect was reversed by KRAS overexpression, suggesting that circ0043898 may suppress CSC properties through downregulation of KRAS. These findings reveal a novel circ0043898–KRAS regulatory axis that contributes to the control of CSC characteristics and may represent a potential therapeutic target for EC. Together, this study provides mechanistic insight and supports the potential of circRNA-based strategies in future EC therapy.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Author contributions
Conceptualization, Methodology, Formal analysis, Investigation, Writing - Review & Editing: Wei Wang, Yuankai Song, Shiqiang Liu, Xinming Xiong, and Xin YangWriting - Original Draft: Wei Wang.
Funding
This study was supported by the grants from the GuangDong Basic and Applied Basic Research Foundation of China (2023A1515220144).
Data availability
The data generated in the present study are included in the figures and tables of this article.
Declarations
Ethics approval and consent to participate
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Zhu H, et al. Esophageal cancer in China: practice and research in the new era. Int J Cancer. 2023;152:1741–51. 10.1002/ijc.34301. [DOI] [PubMed] [Google Scholar]
- 2.Waters JK, Reznik SI. Update on management of squamous cell esophageal Cancer. Curr Oncol Rep. 2022;24:375–85. 10.1007/s11912-021-01153-4. [DOI] [PubMed] [Google Scholar]
- 3.Wang Y, Yang W, Wang Q, Zhou Y. Mechanisms of esophageal cancer metastasis and treatment progress. Front Immunol. 2023;14:1206504. 10.3389/fimmu.2023.1206504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lewis S, Lukovic J. Neoadjuvant therapy in esophageal Cancer. Torac Surg Clin. 2022;32:447–56. 10.1016/j.thorsurg.2022.06.003. [DOI] [PubMed] [Google Scholar]
- 5.Fu Z, et al. CELF6 as an oncogene in colorectal cancer: targeting Stem-Cell-Like properties through modulation of HOXA5 mRNA stability. Front Biosci (Landmark Ed). 2024;29:395. 10.31083/j.fbl2911395. [DOI] [PubMed] [Google Scholar]
- 6.Liu X, et al. EMT and Cancer cell stemness associated with chemotherapeutic resistance in esophageal Cancer. Front Oncol. 2021;11:672222. 10.3389/fonc.2021.672222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Walcher L, et al. Cancer stem Cells-Origins and biomarkers: perspectives for targeted personalized therapies. Front Immunol. 2020;11:1280. 10.3389/fimmu.2020.01280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu K, et al. Etiology, cancer stem cells and potential diagnostic biomarkers for esophageal cancer. Cancer Lett. 2019;458:21–8. 10.1016/j.canlet.2019.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lin Y, et al. CD14, a novel surface marker of esophageal cancer stem cells. Oncol Rep. 2023;49. 10.3892/or.2022.8450. [DOI] [PubMed]
- 10.Wu Q, et al. Cancer stem cells in esophageal squamous cell cancer. Oncol Lett. 2019;18:5022–32. 10.3892/ol.2019.10900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Trevellin E, Pirozzolo G, Fassan M, Vettor R. Prognostic value of stem cell markers in esophageal and esophagogastric junction cancer: a meta-analysis. J Cancer. 2020;11:4240–9. 10.7150/jca.33699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chaudhuri R, Prasanth T, Biswas D, Mandal S, Dash J. Combating multidrug-resistance in S. pneumoniae: a G-quadruplex binding inhibitor of efflux pump and its bio-orthogonal assembly. NAR Mol Med. 2024;1:ugae005. 10.1093/narmme/ugae005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fatma K, Thumpati P, Panda D, Velayutham R, Dash J. Selective recognition of c-KIT 1 G-Quadruplex by structural tuning of heteroaromatic scaffolds and side chains. ACS Med Chem Lett. 2024;15:388–95. 10.1021/acsmedchemlett.3c00537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wu K, Cheng K. Hsa_circFOXP1 contributes to breast Cancer progression through regulating miR-338-3p. Clin Exp Obstet Gynecol. 2024;51. 10.31083/j.ceog5105112.
- 15.Amaya L, et al. Circular RNA vaccine induces potent T cell responses. Proc Natl Acad Sci USA. 2023;120:e2302191120. 10.1073/pnas.2302191120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lagunas-Rangel FA. Circular RNAs and their participation in stemness of cancer. Medical oncology (Northwood, London, England) 37;42 (2020). 10.1007/s12032-020-01373-x [DOI] [PubMed]
- 17.Zhou P, Chen X, Shi K, Qu H, Xia J. The characteristics, tumorigenicities and therapeutics of cancer stem cells based on circrnas. Pathol Res Pract. 2022;233:153822. 10.1016/j.prp.2022.153822. [DOI] [PubMed] [Google Scholar]
- 18.Gu Y, et al. Circular RNA circIPO11 drives self-renewal of liver cancer initiating cells via Hedgehog signaling. Mol Cancer. 2021;20:132. 10.1186/s12943-021-01435-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhao Y, Zheng R, Chen J, Ning D. CircRNA CDR1as/miR-641/HOXA9 pathway regulated stemness contributes to cisplatin resistance in non-small cell lung cancer (NSCLC). Cancer Cell Int. 2020;20:289. 10.1186/s12935-020-01390-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.He M, Pan Y, You C, Gao H. CircRNAs in cancer therapy tolerance. Clin Chim Acta. 2024;558:119684. 10.1016/j.cca.2024.119684. [DOI] [PubMed] [Google Scholar]
- 21.Wang W, et al. Circ0043898 acts as a tumor inhibitor and performs regulatory effect on the Inhibition of esophageal carcinoma. Cancer Biol Ther. 2018;19:1117–27. 10.1080/15384047.2018.1480889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hassn Mesrati M, Syafruddin SE, Mohtar MA, Syahir A. CD44: A multifunctional mediator of Cancer progression. Biomolecules. 2021;11. 10.3390/biom11121850. [DOI] [PMC free article] [PubMed]
- 23.Yan F, et al. Hypoxia promotes non-small cell lung cancer cell stemness, migration, and invasion via promoting Glycolysis by lactylation of SOX9. Cancer Biol Ther. 2024;25:2304161. 10.1080/15384047.2024.2304161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Das PK, Islam F, Smith RA, Lam AK. Therapeutic strategies against Cancer stem cells in esophageal carcinomas. Front Oncol. 2020;10:598957. 10.3389/fonc.2020.598957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gupta P, et al. Expression of CD44 and CD133 stem cell markers in squamous cell carcinoma of esophagus. Indian J Pathol Microbiol. 2021;64:472–8. 10.4103/ijpm.Ijpm_682_20. [DOI] [PubMed] [Google Scholar]
- 26.Huang L, Guo Z, Wang F, Fu L. KRAS mutation: from undruggable to druggable in cancer. Signal Transduct Target Ther. 2021;6:386. 10.1038/s41392-021-00780-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bortoletto AS, Parchem RJ. KRAS hijacks the MiRNA regulatory pathway in Cancer. Cancer Res. 2023;83:1563–72. 10.1158/0008-5472.Can-23-0296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kai JD, et al. MYH9 is a novel cancer stem cell marker and prognostic indicator in esophageal cancer that promotes oncogenesis through the PI3K/AKT/mTOR axis. Cell Biol Int. 2022;46:2085–94. 10.1002/cbin.11894. [DOI] [PubMed] [Google Scholar]
- 29.Ramalingam PS, Premkumar T, Sundararajan V, Hussain MS, Arumugam S. Design and development of dual targeting CAR protein for the development of CAR T-cell therapy against KRAS mutated pancreatic ductal adenocarcinoma using computational approaches. Discov Oncol. 2024;15:592. 10.1007/s12672-024-01455-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hussain MS, et al. Circular RNAs in the KRAS pathway: emerging players in cancer progression. Pathol Res Pract. 2024;256:155259. 10.1016/j.prp.2024.155259. [DOI] [PubMed] [Google Scholar]
- 31.Kim D, et al. Pan-KRAS inhibitor disables oncogenic signalling and tumour growth. Nature. 2023;619:160–6. 10.1038/s41586-023-06123-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Misir S, Wu N, Yang BB. Specific expression and functions of circular RNAs. Cell Death Differ. 2022;29:481–91. 10.1038/s41418-022-00948-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yang L, Wilusz JE, Chen LL. Biogenesis and regulatory roles of circular RNAs. Annu Rev Cell Dev Biol. 2022;38:263–89. 10.1146/annurev-cellbio-120420-125117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhao X, Zhong Y, Wang X, Shen J, An W. Advances in circular RNA and its applications. Int J Med Sci. 2022;19:975–85. 10.7150/ijms.71840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhou T, et al. SOX9-activated FARSA-AS1 predetermines cell growth, stemness, and metastasis in colorectal cancer through upregulating FARSA and SOX9. Cell Death Dis. 2020;11:1071. 10.1038/s41419-020-03273-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Petty RD, et al. Gefitinib and EGFR gene copy number aberrations in esophageal Cancer. J Clin Oncol. 2017;35:2279–87. 10.1200/jco.2016.70.3934. [DOI] [PubMed] [Google Scholar]
- 37.Han T, et al. Significant circrnas in liver cancer stem cell exosomes: mediator of malignant propagation in liver cancer? Mol Cancer. 2023;22:197. 10.1186/s12943-023-01891-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gu C, et al. circGprc5a promoted bladder oncogenesis and metastasis through Gprc5a-Targeting peptide. Mol Therapy Nucleic Acids. 2018;13:633–41. 10.1016/j.omtn.2018.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen L, et al. CircRNA CDR1as promotes hepatoblastoma proliferation and stemness by acting as a miR-7-5p sponge to upregulate KLF4 expression. Aging. 2020;12:19233–53. 10.18632/aging.103748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gong LP, et al. Epstein-Barr virus-derived circular RNA LMP2A induces stemness in EBV-associated gastric cancer. EMBO Rep. 2020;21:e49689. 10.15252/embr.201949689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Noh KH, et al. Cancer vaccination drives Nanog-dependent evolution of tumor cells toward an immune-resistant and stem-like phenotype. Cancer Res. 2012;72:1717–27. 10.1158/0008-5472.can-11-3758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rojas LA, et al. Personalized RNA neoantigen vaccines stimulate T cells in pancreatic cancer. Nature. 2023;618:144–50. 10.1038/s41586-023-06063-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang M, et al. PLCD3 promotes malignant cell behaviors in esophageal squamous cell carcinoma via the PI3K/AKT/P21 signaling. BMC Cancer. 2023;23:921. 10.1186/s12885-023-11409-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.He Y, et al. Targeting PI3K/Akt signal transduction for cancer therapy. Signal Transduct Target Therapy. 2021;6:425. 10.1038/s41392-021-00828-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Engelman JA. Targeting PI3K signalling in cancer: opportunities, challenges and limitations. Nat Rev Cancer. 2009;9:550–62. 10.1038/nrc2664. [DOI] [PubMed] [Google Scholar]
- 46.Parker JA, Mattos C, The K-Ras N-R, Isoforms H-R. Unique conformational preferences and implications for targeting oncogenic mutants. Cold Spring Harbor Perspect Med. 2018;8. 10.1101/cshperspect.a031427. [DOI] [PMC free article] [PubMed]
- 47.Wang P, Laster K, Jia X, Dong Z, Liu K. Targeting CRAF kinase in anti-cancer therapy: progress and opportunities. Mol Cancer. 2023;22:208. 10.1186/s12943-023-01903-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Skoulidis F, et al. Sotorasib for lung cancers with KRAS p.G12C mutation. N Engl J Med. 2021;384:2371–81. 10.1056/NEJMoa2103695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kang M, et al. MicroRNA-193b acts as a tumor suppressor gene in human esophageal squamous cell carcinoma via target regulation of KRAS. Oncol Lett. 2019;17:3965–73. 10.3892/ol.2019.10039. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data generated in the present study are included in the figures and tables of this article.