Abstract
Purpose
Hormone receptor-positive breast cancer is characterized by the expression of estrogen receptor (ER) or progesterone receptor (PR), it is generally associated with less aggressive clinical features and more favorable prognostic outcomes, primarily due to the effectiveness of endocrine therapy. However, the loss of PR expression has been correlated with endocrine resistance and poorer prognosis. To date, there is limited research elucidating the underlying mechanisms distinguishing ER-positive/PR-positive from ER-positive/PR-negative breast cancer. This study aims to investigate the molecular mechanisms associated with these two subtypes and to propose recommendations for precision therapy.
Patients and methods
Fresh tumor tissues from ER + /PR + patients (n = 5) and ER + /PR- patients (n = 5) were subjected to proteomic analysis to identify differentially expressed proteins. Transcriptomic data were obtained from the TCGA database, encompassing 937 breast cancer patients divided into three subgroups: ER + /PR + (n = 627), ER + /PR- (n = 112), and ER-/PR- (n = 198). Clinical characteristics and prognostic data were collected to analyze disease-specific survival (DSS) and overall survival (OS) across the three subtypes. Differential expression data for both transcripts and proteins were extracted, and Cox regression along with Least Absolute Shrinkage and Selection Operator (LASSO) regression were applied to identify key regulatory genes. A risk scoring formula was employed to classify patients into high-risk and low-risk groups. Kaplan–Meier curves, Gene Set Enrichment Analysis (GSEA), immune cell infiltration analysis, and OncoPredict drug sensitivity predictions were conducted to provide insights into the underlying mechanisms and clinical treatment strategies for this patient cohort. The accuracy of this model was further validated using external GEO datasets (GSE21653, GSE20685, and GSE42568). Additionally, we collected data from 97 hormone receptor-positive breast cancer patients who underwent neoadjuvant chemotherapy at our center between January 2021 and December 2023, assessing their response to chemotherapy using the Miller-Payne score.
Results
In the TCGA database, patients with ER + /PR- breast cancer exhibited poorer 5-year DSS and OS compared to those with ER + /PR + status (DSS: P = 0.038; OS: P = 0.052), which was similar to those with ER-/PR- status (DSS: P = 0.47; OS: P = 0.77). 186 differentially expressed proteins (110 up-regulated and 76 down-regulated) were identified based on proteomic analysis. After COX regression and Lasso regression, five key differential genes with prognostic and diagnostic value of ER + /PR + and ER + /PR- patients were finally included, that is HPN, FSCN1, FGD3, LRIG1, and TBC1D7. HPN, FSCN1 and FGD3 can be regarded as a tumor suppressor gene. And LRIG1, and TBC1D7 can be regarded as a risk-associated gene. Patients with high-risk scores had significantly lower survival probabilities compared to those with low-risk scores. Additionally, there were differences in functional pathway enrichment analysis (galactose_metabolism, glycolysis_gluconeogenesis, jak_stat_signaling_pathway, pentose_phosphate_pathway, et al.) and immune cell infiltration (CD8 T cell, Macrophages M1, et al.) between the high-risk and low-risk groups. Drug sensitivity analysis indicated that the low-risk patients may be more sensitive to endocrine drug like fulvestrant, while high-risk patients may be more sensitive to chemotherapy drugs like docetaxel, paclitaxel, and vinorelbine. Of the 97 patients underwent neoadjuvant chemotherapy in our center, the proportion of patients achieving Miller-Payne (MP) score 4 and 5 was higher in ER + /PR- patients (44%) compared to ER + /PR + patients (9.8%).
Conclusion
We confirmed that ER + /PR- breast cancer patients exhibited worse survival compared with ER + /PR + patients. Five key regulatory genes were identified and potential mechanisms and biological pathways were discovered, our prediction of drug sensitivity offers new insights for developing precise pharmacological treatment strategies for ER + /PR- breast cancer.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12885-025-14451-y.
Keywords: Estrogen receptor, Progesterone receptor, Proteomic analysis, Prognosis
Introduction
Breast cancer is a prevalent malignancy among female patients. Recent advances in basic and biomedical sciences have highlighted the heterogeneity of breast cancer, which encompasses multiple molecular subtypes significantly influenced by hormonal receptor status [1, 2]. Breast cancer is classified into several molecular subtypes based on the expression of key biomarkers: ER, PR, Human Epidermal Growth Factor Receptor 2 (HER-2), and Ki67 [3]. Studies involving mouse models of mammary glands, normal human mammary cultures, and clinical research have demonstrated that estrogen and progesterone are the primary proliferative steroid hormones in mammary epithelial cells, playing essential roles in mammary gland development [4]. Estrogen, a lipophilic steroid hormone, exerts its effects through specific binding to ER. Progesterone, a 21-carbon steroid hormone, is involved in the female menstrual cycle, pregnancy, and embryogenesis in humans and other species by binding to progesterone receptors [5].
PR, a member of the steroid nuclear receptor family of ligand-dependent transcription factors, acts by binding to progesterone [6]. PR is encoded by two distinct mRNA transcripts, which are regulated by separate promoters, leading to the production of two functionally distinct proteins: PR-A and PR-B. These two isoforms are typically co-expressed in normal breast tissue in a 1:1 ratio, and the same is true for benign breast lesions. However, in atypical lesions, the expression of either PR-A or PR-B increases significantly as the lesion progresses from a normal to a malignant state. PR-A tends to predominate in ductal carcinoma in situ and invasive breast lesions. Moreover, the PR-A/PR-B ratio can influence the ability of progesterone to modulate gene expression in reproductive tissues and impact the outcome of breast cancer treatment [7–9]. At the molecular level, PR is considered a surrogate marker for functional ER signaling and the expression of ER can regulate the expression of PR, leading to a typical correlation where ER and PR expressions are usually consistent, manifesting as either ER +/PR + or ER-/PR-. However, discordant expression patterns can occur in some breast cancers, such as ER +/PR- or ER-PR +, which may be associated with poorer prognostic outcomes. In such cases, the potential benefit of routine endocrine therapy may be diminished [10, 11]. There remains ongoing debate among experts regarding the existence of ER-PR + breast cancer. Some studies suggest that the appearance of ER-PR + breast cancer could be influenced by factors such as immunohistochemical (IHC) staining techniques and result interpretation, which may lead to false-negative ER results [12].
Nevertheless, the ER +/PR- phenotype represents a distinct subtype of breast cancer, categorized as Luminal B tumors, accounting for approximately 12% of all breast cancer cases [13]. This subtype is characterized by strong and diffuse nuclear expression of ER, accompanied by a lack of PR expression [14]. Luminal B tumors are known for their aggressive behavior, resistance to tamoxifen, greater genomic instability, and higher tumor cell proliferation rates [15]. Hu et al. conducted survival analyses comparing the overall survival of ER +/PR-/HER2- and ER +/PR +/HER2- groups, confirming that the overall survival of the ER +/PR-/HER2- group was significantly poorer than that of the ER +/PR +/HER2- group [16]. Similarly, Li et al. reached a comparable conclusion [17]. In a study previously published by our group [18], we observed that the 5-year disease-free survival (DFS) rate and OS rate for ER +/PR- patients were significantly lower than those for ER +/PR + patients (DFS: 77.0% vs 94.6%; OS: 85.8% vs 97.2%). These results indicate that ER +/PR- breast cancer patients exhibit unique clinical characteristics and prognostic outcomes compared to ER +/PR + breast cancer patients. However, none of these studies have elucidated the underlying mechanisms of this subtype [14].
In this study, to validate our previous findings, we conducted a prognostic analysis of 937 breast cancer patients from the TCGA database. Additionally, to explore the reasons behind the unique prognosis of ER +/PR- breast cancer, we collected fresh tissue samples from both ER +/PR + and ER +/PR- patients and performed proteomic analysis to identify differential proteins and genes. Transcriptomic data were also obtained from the TCGA database, leading to the identification of five key regulatory genes, which were used to construct a risk model. Subsequently, we performed functional enrichment analysis, immune cell infiltration analysis, and drug sensitivity assessments for high-risk and low-risk groups. To further validate the accuracy of the drug sensitivity analysis and the effect of PR status on chemotherapy response, we evaluated the efficacy of neoadjuvant chemotherapy in 97 breast cancer patients treated at our center. In summary, our study aims to investigate the unique characteristics and underlying mechanisms of ER +/PR- breast cancer.
Besides, in this study, a 10% cutoff value for ER and PR expression was selected, following guidelines from classic clinical studies and influential publications [19–21]. Although the most recent ASCO/CAP guidelines recommend a threshold of ≥ 1% for ER/PR positivity [22], these guidelines explicitly differentiate cases with 1% to 10% staining from those with ≥ 10% staining, classifying them as low expression. It has been reported that cases with 1% to 10% staining exhibit gene expression profiles, clinical features, and prognostic outcomes more similar to those with < 10% staining [23–25]. This is particularly relevant, as these patients benefit less from endocrine therapy [26, 27]. Given these factors, including the impact of endocrine therapy, clinical characteristics, and prognosis, as well as the need to expand the sample size, we selected 10% as the cutoff value for hormone receptor expression in our study.
Materials and methods
Patients selection
A total of 937 breast cancer patients from TCGA database (https://cancergenome.nih.gov/) were included in this study. The data, spanning from 1988 to 2013, comprised patients with pathologically confirmed invasive breast cancer. This dataset included information on age, sex, diagnosis date, tumor location, histological type, ER and PR expression status, HER2 status, surgical procedures, TNM staging, and survival data. Based on ER and PR expression status, patients were categorized into three subgroups: ER +/PR + (n = 627), ER +/PR- (n = 112), and ER-/PR- (n = 198). Clinical characteristics and prognostic data for these patients were analyzed, and Kaplan–Meier survival curves were constructed to assess DSS and OS. The statistical significance of the prognostic differences among the three groups was evaluated. To validate the prognostic value of the gene features, we downloaded datasets GSE21653 (n = 236), GSE20685 (n = 327), and GSE42568 (n = 104) from the GEO database (https://www.ncbi.nlm.nih.gov/geo/), excluding patients without complete survival data. The flowchart of our analysis is presented in Fig. 1. Additionally, 97 breast cancer patients who visited the Breast and Thyroid Surgery Department of the Second Affiliated Hospital of Chongqing Medical University between January 2021 and December 2023 were included in the study. All cases were HER2-negative and received standardized neoadjuvant chemotherapy consisting of taxane and anthracycline-based regimens. Using 10% as the cutoff for ER and PR expression, patients were divided into two subgroups: ER +/PR + (n = 72) and ER +/PR- (n = 25). The efficacy of chemotherapy was evaluated post-surgery using the Miller-Payne grading system.
Fig. 1.
Flow chart of data collection, analysis and validation
Fresh tissue collection
Ten patients who did not meet the criteria for neoadjuvant chemotherapy underwent direct modified radical mastectomy following pathological diagnosis. Using 10% as the cutoff for ER and PR expression, these patients were categorized into two subgroups: ER +/PR + (n = 5) and ER +/PR- (n = 5). Tumor and adjacent normal tissues were collected during surgery. Fresh tissue samples were cut into 30 mg portions, quickly placed into specimen tubes, and stored at −80 °C until further processing.
Protein extraction and proteomic analysis
The sample was first ground with liquid nitrogen, then the powder was sonicated for three minutes on ice using a high-intensity ultrasonic processor (Scientz) in lysis buffer. An equal volume of Tris-saturated phenol (pH 8.0) was added, and after centrifugation, proteins were precipitated by adding at least four volumes of ammonium sulfate-saturated methanol, with the supernatant discarded. The protein was then redissolved in 8 M urea, and the protein concentration was determined using a BCA kit according to the manufacturer’s instructions. Pancreatic enzymes were added to digest the protein, and the peptides were subsequently desalted using a Strata X SPE column. The tryptic peptides were then analyzed by mass spectrometry using Liquid Chromatography-Tandem Mass Spectrometry (LC–MS/MS). Data-independent acquisition (DIA) data were processed using the DIA-NN search engine (v.1.8). Raw files obtained from mass spectrometry were used to construct a sample-specific protein database based on the origin of the samples. Database searches were performed, and quality control analysis was conducted at both the peptide and protein levels based on the search results. Quantitative analysis of the proteins was performed, and statistical significance between the two groups was determined using a T-test.
Statistics
First, we screened the genes that overlapped with differentially expressed proteins for survival analysis using univariate Cox regression. Next, we developed a potential risk score through LASSO Cox regression analysis with the “glmnet” R package. Patients were stratified into high-risk and low-risk groups based on the median cutoff value. Kaplan–Meier and ROC curve analyses were then performed to evaluate the prognostic performance of the novel gene signature and the diagnostic value of each individual gene. GSEA (http://www.broadinstitute.org/gsea/index.jsp) was conducted to explore biological functions and pathways using the differentially expressed genes in the two groups. To examine the immune microenvironment between the two groups, we utilized the “CIBERSORT” R package, and the “oncoPredict” R package was used to predict resistance to anti-tumor drugs, as reflected by the half-maximal inhibitory concentration (IC50). All statistical analyses were performed using R (https://www.r-project.org/). All statistical tests were two-sided, and P values < 0.05 were considered statistically significant.
Results
Prognostic analysis of the TCGA cohort
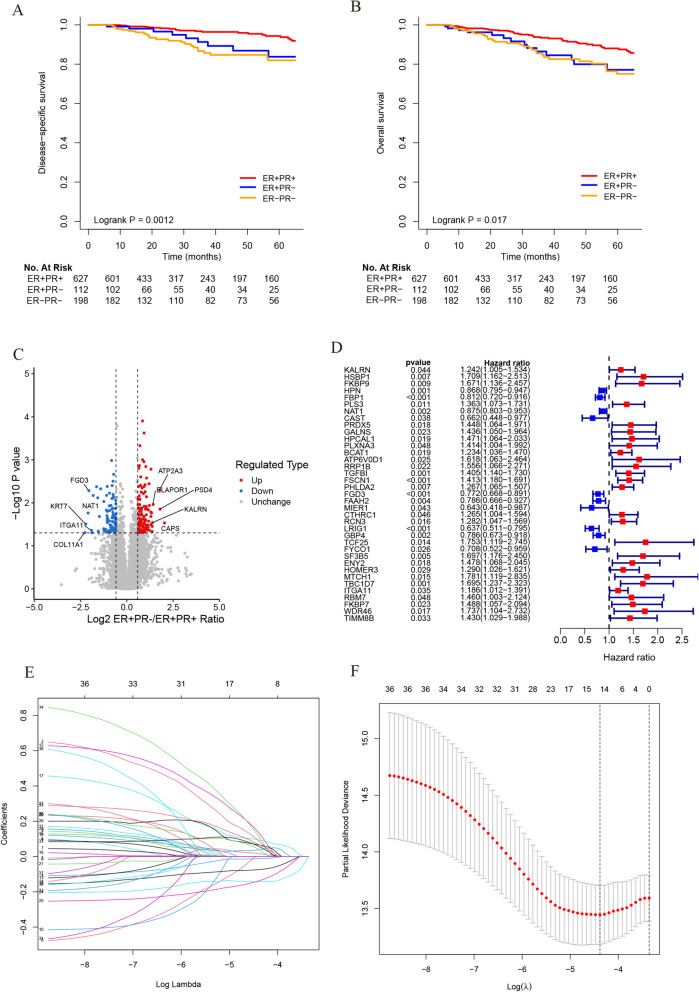
In this study, we performed a prognostic analysis of 937 breast cancer patients from the TCGA database (Fig. 2A-B). The 5-year DSS rate of the ER +/PR- group was significantly lower than that of the ER +/PR + group (83.7% vs 94.3%, P = 0.038), but similar to ER-/PR- group (83.7% vs 81.9%, P = 0.47). Additionally, the ER +/PR + group showed significantly better DSS than the ER-/PR- group (94.3% vs 81.9%, P < 0.001). To better illustrate the prognostic differences, the data from all three groups were plotted together, yielding a P-value of 0.0012. Furthermore, the 5-year OS rate of the ER +/PR- group was lower than that of the ER +/PR + group (77.1% vs 88.0%, P = 0.052), but similar to that of the ER-/PR- group (77.1% vs 75.1%, P = 0.77). The ER +/PR + group exhibited significantly better OS compared to the ER-/PR- group (88.0% vs 75.1%, P = 0.006). When the data from all three groups were uniformly plotted, the result showed a P-value of 0.017. These findings are consistent with our previous retrospective study [18], further validating the unique prognostic characteristics of ER +/PR- breast cancer.
Fig. 2.
Survival analysis of patients in TCGA database and process for screening genes. A Survival analysis of the ER +/PR +, ER +/PR- and ER-/PR- groups. Kaplan–Meier survival curves were plotted and the log-rank test was performed to compare the DSS of these groups in TCGA database. B The OS of these groups. C The volcano map was drawn for 186 differential proteins, the red dots indicate significant upward adjustments, the blue dots indicate significant downward adjustments, and the gray dots indicate no significant differences. D Survival analyses for 37 genes using univariate Cox regression model. E Screening of prognostic model genes using LASSO regression. F Cross-validation of LASSO regression parameter selection
Integrated proteomics and transcriptomics analysis identified 5 key regulatory genes
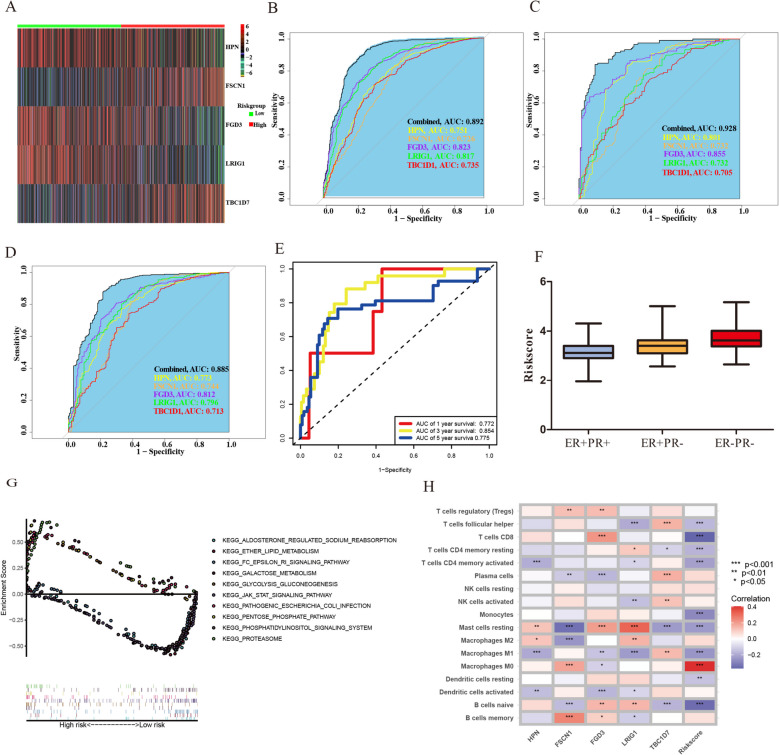
We collected surgical specimens from 10 breast cancer patients treated at our center (5 patients with ER +/PR + and 5 patients with ER +/PR-). LC–MS/MS analysis and subsequent quantitative proteomic analysis revealed a total of 7,263 quantifiable proteins across the 10 samples. A total of 186 differentially expressed proteins (110 up-regulated and 76 down-regulated) were identified, as shown in Fig. 2C. The specific protein names and expression levels are shown in supplementary table. After extracting the related overlapping transcriptomic data from the TCGA database, we performed a univariate Cox proportional hazards analysis, which identified 37 differentially expressed genes with survival significance (Fig. 2D). LASSO Cox regression analysis was then conducted using the expression profile of these 37 genes, resulting in the selection of 14 genes (Fig. 2E-F). To further explore genes associated with PR expression, PR-dependent ROC curves were generated to evaluate the correlation between the targeted genes and PR status. Given the high heterogeneity of cancer, our goal was to identify potentially effective combinations of markers for preliminary validation, rather than for immediate use in high-stakes clinical decisions. Therefore, five genes with AUC greater than 0.7 were selected (Fig. 3B). Ultimately, HPN, FSCN1, FGD3, LRIG1, and TBC1D7 were selected as target genes (Fig. 3A). For external validation, only the GSE21653 dataset had complete records regarding ER and PR status. ROC curves predicting PR status were validated in this cohort, with all AUC values greater than 0.7 (Fig. 3C). Next, we compared the mRNA expression levels of these five genes across the three groups (ER +/PR +, ER +/PR-, and ER-/PR-) in the TCGA database (Figure S1). We can make a preliminary judgment, HPN, FSCN1 and FGD3 can be regarded as a tumor suppressor gene. And LRIG1, and TBC1D7 can be regarded as a risk-associated gene.
Fig. 3.
Identifying target Genes and performing data analysis. A Heatmap showing the gene expression profiles of high-risk group and low-risk group. B PR-dependent ROC curves of risk score in train cohort. C PR-dependent ROC curves of risk score in the external validation, GSE21653. D PR-dependent ROC curves with HER2-negative data of risk score in train cohort. E Time-dependent ROC curves with HER2-negative data of risk score in train cohort on OS. F Risk scores of ER +/PR +, ER +/PR- and ER-/PR- groups. G The biological functions and pathways of dysregulated genes in both groups through KEGG pathway analysis were displayed. F The correlation heatmap between the risk model and the immune microenvironment were displayed
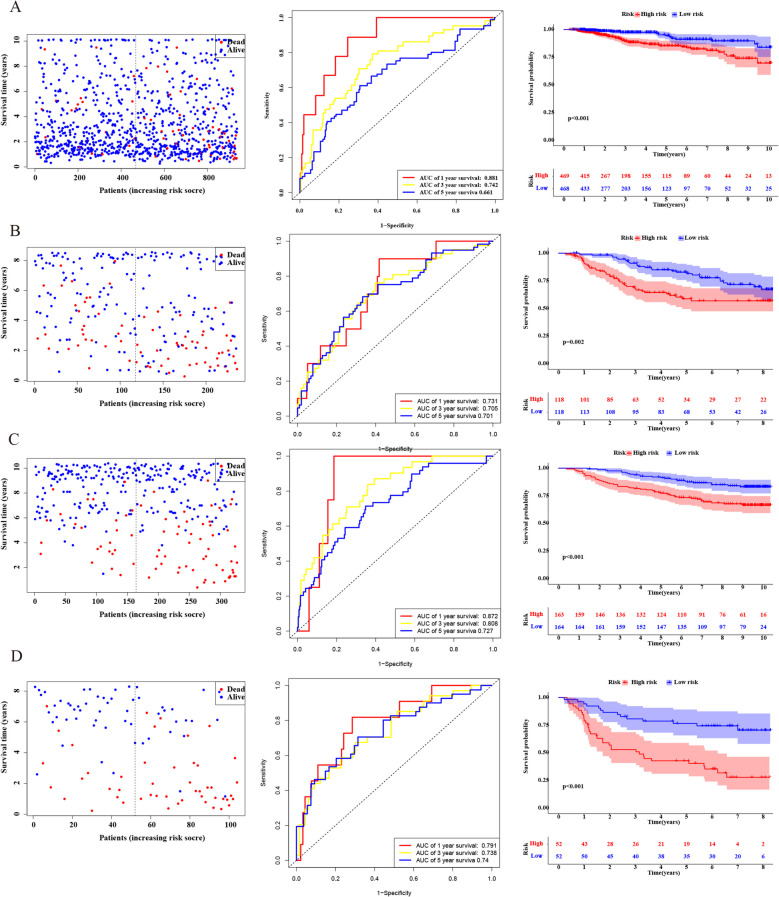
The results indicated that the expression levels of these genes in the ER +/PR- group were intermediate between those in the ER +/PR + and ER-/PR- groups, which aligns with our prognostic analysis. We constructed a risk score-based predictive model using these five genes: risk score = (−0.038 × HPN) + (0.160 × FSCN1) + (−0.137 × FGD3) + (−0.177 × LRIG1) + (0.153 × TBC1D7). Based on the median risk score, patients in the TCGA database were divided into high-risk (n = 469) and low-risk (n = 469) groups. In the TCGA cohort, we observed that high-risk patients had a higher probability of early cumulative mortality compared to low-risk patients (Fig. 4A, left panel). Time-dependent ROC analysis was conducted at 1, 3, and 5 years to assess the model’s accuracy. The AUC for 1-year survival was 0.881, 0.742 at 3 years, and 0.661 at 5 years (Fig. 4A, middle panel). Kaplan–Meier survival curves demonstrated that the survival probability of high-risk patients was significantly lower than that of low-risk patients (P < 0.001) (Fig. 4A, right panel). Using the same risk score formula, we validated the model’s accuracy in the GSE 21653, GSE 20685, and GSE 42568 cohorts (Fig. 4B-D).
Fig. 4.
Prognosis analysis of patients in different cohorts. A The probability of early cumulative mortality between high-risk patients and low-risk patients (left panel), Time-dependent ROC analysis at 1, 3, and 5 years (middle panel), Kaplan–Meier survival curves between high-risk patients and low-risk patients (right panel) were displayed in the TCGA cohort. B in the GSE 21653 cohort, C in the GSE 20685 cohort, D in the GSE 42568 cohort
When we initially constructed the prediction model, we did not consider the potential effect of HER2 on the outcome. After reviewing the relevant literature, we concluded that HER2 may influence the clinical characteristics and prognosis of patients [28–30], and it may also lead to negative expression of PR [31–33]. Additionally, HER2-positive patients receive targeted therapy and it will affect our assessment of prognosis [28, 34]. To enhance the accuracy of the model, we excluded data from HER2-positive cases, included only HER2-negative patients, and constructed PR-dependent ROC curves. The AUC for the five genes remained above 0.7 (Fig. 3D), and the AUC for 1-year survival was 0.772, 0.854 at 3 years, and 0.775 at 5 years (Fig. 3E), indicating that the effects of these five genes on prognosis were independent of HER2 status. Our calculations revealed that the ER +/PR + group had the lowest risk score, the ER-/PR- group had the highest, and the ER +/PR- group had an intermediate risk score (Fig. 3F).
Gene set enrichment analysis and immune infiltration analysis
We explored the biological functions and pathways of dysregulated genes in both groups through KEGG pathway analysis. The GSEA results indicated that genes enriched in the high-risk group were associated with pathways related to glycolysis/gluconeogenesis, galactose metabolism, pathogenic Escherichia coli infection, the pentose phosphate pathway, and the proteasome. In contrast, genes in the low-risk group were enriched in pathways related to aldosterone-regulated sodium reabsorption, ether lipid metabolism, Fc epsilon RI signaling, JAK-STAT signaling, and the phosphatidylinositol signaling system (Fig. 3G). The correlation heatmap revealed the relationship between the risk model and the immune microenvironment. Notably, there were negative correlations between the risk score and several immune cell types, including follicular helper T cells, CD8 + T cells, resting and activated memory CD4 + T cells, monocytes, resting mast cells, M1 macrophages, resting dendritic cells, and naive B cells. In contrast, the risk score was positively correlated with M0 macrophages (Fig. 3H).
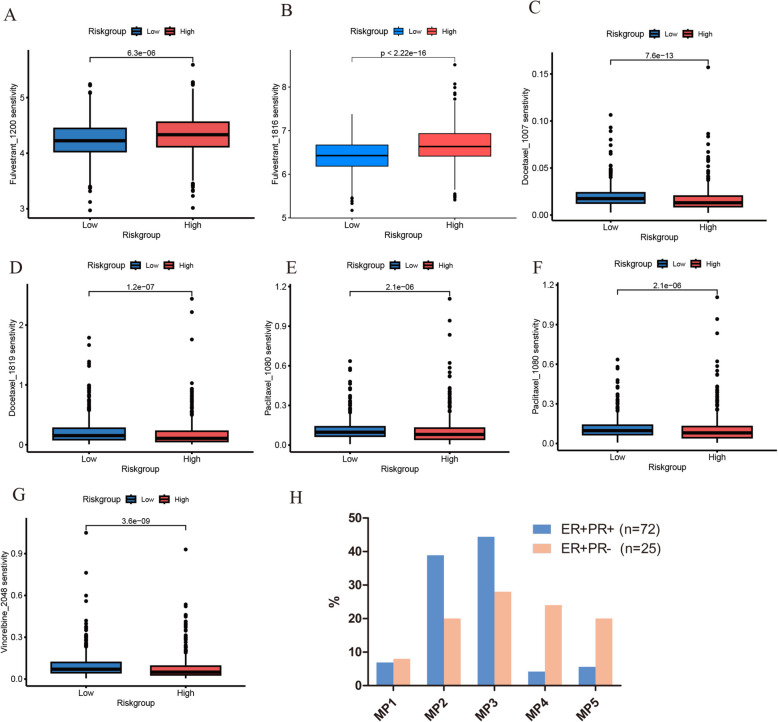
Drug sensitivity analysis
Given the heterogeneity of breast cancer, drug sensitivity can vary significantly among patients. Therefore, understanding the differences in drug sensitivity among breast cancer patients with varying risk levels can provide valuable insights for personalized clinical treatment. The drug sensitivity analysis revealed that the IC50 of Fulvestrant was higher in the high-risk group compared to the low-risk group, while the IC50 of Docetaxel, Paclitaxel, and Vinorelbine was higher in the low-risk group compared to the high-risk group. This suggests that high-risk patients may have better efficacy with chemotherapeutic drugs and relatively lower sensitivity to endocrine therapy (Fig. 5A-G). To further validate the effect of PR status on chemotherapy response, we assessed the efficacy of neoadjuvant chemotherapy in 97 breast cancer patients treated at our center. The proportion of patients in the ER +/PR- group achieving MP grades 4 (24%) and 5 (20%) was higher compared to the ER +/PR + group (grade 4, 4.2%; grade 5, 5.6%). This indicates that ER +/PR- breast cancer patients responded more effectively to chemotherapy than those in the ER +/PR + group, as shown in Fig. 5H.
Fig. 5.
The estimated IC50s of clinical chemotherapeutic drugs and endocrine therapy drugs in high-risk and low-risk groups. A, B The estimated IC50s of Fulvestrant. C, D The estimated IC50s of Docetaxel. E, F The estimated IC50s of Paclitaxel. G The estimated IC50s of Vinorelbine. H The efficacy of neoadjuvant chemotherapy of the ER +/PR + and ER +/PR- groups was displayed.
Discussion
Breast cancer is one of the most common cancers among women globally and remains the leading cause of cancer-related deaths in women. It exhibits significant biological heterogeneity and can be classified into distinct subtypes based on molecular profiling. These subtypes vary in clinical-pathological characteristics, prognosis, treatment strategies, and disease outcomes [35]. Precision treatment based on molecular subtypes of breast cancer is increasingly becoming a central focus of research. While hormone receptor-positive breast cancer responds favorably to endocrine therapy, a subset of ER-positive patients still experiences relapse. Identifying these patient populations is of critical clinical importance. The role of PR in endocrine responsiveness, however, remains controversial [36]. Compared to ER +/PR + breast cancer, the ER +/PR- subtype exhibits greater genomic instability and higher tumor cell proliferation rates. Its clinical features and prognosis are generally intermediate between the ER +/PR + and ER-/PR- subtypes [33]. Since PR is primarily regulated by ER at the transcriptional level, the underlying biology and mechanisms driving PR loss in ER +/PR- breast cancer are of particular interest. Historically, the predictive value of PR has been linked to its ER-dependent activity. As such, the absence of PR has been considered a marker of non-functional ER, contributing to resistance to endocrine therapy [37]. PR negativity may arise from mechanisms such as primary loss of the PR gene copy number, PR promoter methylation, and miRNA-mediated downregulation of PR. While these mechanisms have been explored, they have not been fully elucidated [31, 38]. Therefore, gaining a deeper understanding of the biological mechanisms driving ER +/PR- breast cancer and developing improved treatment strategies is essential. Our previous research demonstrated that ER +/PR- breast cancer has distinct clinical characteristics and prognosis, falling between the ER +/PR + and ER-/PR- subtypes.
In our study, we developed a novel risk model that incorporates five key regulatory genes, which aids in identifying high-risk breast cancer patients and guiding personalized treatment strategies, offering significant clinical value. The robustness of this model was validated using three external GEO cohorts, and ROC analysis confirmed the predictive efficacy of these five genes for PR status. The identified genes were HPN, FGD3, LRIG1, FSCN1, and TBC1D7. HPN plays a crucial role in regulating TGF-β signaling in breast cancer by releasing latent TGF-β from its extracellular matrix (ECM) storage [39]. Studies have shown that HPN is essential for the oncogenic Ras-induced loss of epithelial integrity and tumor progression in breast cancer models [40]. Its role in promoting the TGF-β pathway in both normal mammary glands and WAP-Myc-driven mammary tumors highlights its potential as a therapeutic target for pharmacological intervention in the TGF-β pathway [41]. Therefore, HPN can be regarded as a tumor suppressor gene. FGD3 contains a Fascin domain, and is involved in cytoskeletal reorganization and the regulation of cell motility. FGD3 has an inhibitory effect on cell migration in both tumor and normal cells. Reduced expression of FGD3 correlates with increased cell migration risk, while higher expression is associated with reduced cell migration [42]. The role of FGD3 in breast cancer was first elucidated by Cheng et al., who identified it as a major protective gene in this context [43]. Therefore, FGD3 can be regarded as a tumor suppressor gene. LRIG1 belongs to the LRIG family of single-pass transmembrane proteins [44]. In breast cancer, LRIG1 expression is reduced compared to normal tissues. Recent studies have shown that LRIG1 copy number loss is prevalent in triple-negative and HER2-positive breast cancers [45]. Moreover, LRIG1 expression negatively correlates with the invasive behavior of breast cancer cells [46]. Therefore, LRIG1 can be regarded as a tumor suppressor gene. FSCN1 is involved in cytoskeletal reorganization by binding and cross-linking actin filaments, a crucial function for cell migration and invasion. It may interact with multiple signaling pathways that influence tumor biological characteristics. Overexpression of FSCN1 has been observed in breast cancer cells, suggesting that inhibiting FSCN1 expression could be a potential therapeutic strategy [47]. Therefore, FSCN1 can be regarded as a risk-associated gene. TBC1D7 is involved in intracellular membrane trafficking and signal transduction. It contains a TBC1 domain, which regulates small GTPases’ activity. TBC1D7 plays a key role in the regulation of the endosomal and transport vesicle systems, which is crucial for material transport and cellular signal transduction. Through modulation of pathways like mTOR, which are involved in cell proliferation and survival, TBC1D7 may influence breast cancer cell behavior and contribute to tumor growth and metastasis [48]. Therefore, TBC1D7 can be regarded as a risk-associated gene.
Subsequently, we conducted functional enrichment analysis, immune cell infiltration profiling, and drug sensitivity assessments for both high-risk and low-risk groups. Significant differences in biological pathways and immune cell infiltration characteristics were observed between the two groups. To provide new insights into the clinical treatment of these patients, we found that low-risk patients exhibited greater sensitivity to fulvestrant compared to high-risk patients. In contrast, high-risk patients may benefit more from chemotherapy drugs, such as docetaxel, paclitaxel, and vinorelbine, suggesting that these agents could lead to better clinical outcomes in this group.
Our study has several potential limitations. First, although we identified five key genes, the exact relationship between these genes and PR status remains unclear. Additionally, the number of clinical specimens collected was relatively small, which could impact the accuracy of the differential protein analysis. Future research is required to further explore the underlying mechanisms driving the development of ER +/PR- breast cancer and to establish more precise treatment strategies.
Conclusions
In summary, our study confirms that ER +/PR- breast cancer presents a unique prognosis, positioned between the ER +/PR + and ER-/PR- subtypes. We have identified five key differentially expressed genes, which provide valuable insights into the underlying mechanisms and biological pathways. Additionally, our drug sensitivity predictions offer new perspectives for developing precise pharmacological treatment strategies tailored to ER +/PR- breast cancer.
Supplementary Information
Supplementary Material 1: Figure S1. The mRNA expression levels of targeted genes among three groups (ER +/PR +, ER +/PR-, and ER-/PR-) in the TCGA database.
Acknowledgements
We thank the TCGA and GEO database for providing their platforms and contributors for their valuable data sets.
Abbreviations
- ER
Estrogen receptor
- PR
Progesterone receptor
- DSS
Disease-specific survival
- OS
Overall survival
- LASSO
Least Absolute Shrinkage and Selection Operator
- GSEA
Gene Set Enrichment Analysis
- MP score
Miller-Payne score
- HER-2
Human epidermal growth factor receptor 2
- IHC
Immunohistochemistry
- DFS
Disease-free survival
- LC–MS/MS
Liquid Chromatography-Tandem Mass Spectrometry
- DIA
Data-independent acquisition
- IC50
Half-maximal inhibitory concentration
- ECM
Extracellular matrix
Authors’ contributions
J.M. and Y. F. planned the study. Z.J.L. collected the fresh tissue. Y.F. and Z.J.L. analyzed the data and wrote the manuscript. J.Y.reworked figures and revised typos and grammatical issues in revised manuscript. All authors have read and agreed to the published version of the manuscript. Y.F. and J.M. contributed equally.
Funding
This research was supported by the Natural Science Foundation of Chongqing (CSTB2024NSCQ-MSX0260). The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Data availability
Data are available upon reasonable request. Access to datasets from The Second Affiliated Hospital of Chongqing Medical University should be requested directly from the institution via their data access request forms. All experiments and implementation details are described thoroughly in the Materials and methods section.
Declarations
Ethics approval consent to participate
All experiments were performed in accordance with relevant guidelines and regulations. All experiments were performed in accordance with relevant guidelines and regulations. The studies involving human participants were reviewed and approved by Ethics Committee of the Second Affiliated Hospital of Chongqing Medical University. The patients/participants provided their written informed consent to participate in this study.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Yang Feng and Jia Ming contributed equally to this work.
Contributor Information
Yang Feng, Email: 304859@hospital.cqmu.edu.cn.
Jia Ming, Email: mingjia@cqmu.edu.cn.
References
- 1.Jin X, Zhou YF, Jiang YZ, Shao ZM. Molecular classification of hormone receptor-positive HER2-negative breast cancer. Nat Genet. 2023;55(10):1696–708. [DOI] [PubMed] [Google Scholar]
- 2.Waks AG, Winer EP. Breast cancer treatment: a review. JAMA. 2019;321(3):288–300. [DOI] [PubMed] [Google Scholar]
- 3.Merino Bonilla JA, Torres Tabanera M, Ros Mendoza LH. Breast cancer in the 21st century: from early detection to new therapies. Radiologia. 2017;59(5):368–79. [DOI] [PubMed] [Google Scholar]
- 4.Trabert B, Sherman ME, Kannan N, Stanczyk FZ. Progesterone and Breast Cancer. Endocr Rev. 2020;41(2):320–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Taraborrelli S. Physiology, production and action of progesterone. Acta Obstet Gynecol Scand. 2015;94(Suppl 161):8–16. [DOI] [PubMed] [Google Scholar]
- 6.Gellersen B, Fernandes MS, Brosens JJ. Non-genomic progesterone actions in female reproduction. Hum Reprod Update. 2009;15(1):119–38. [DOI] [PubMed] [Google Scholar]
- 7.Zheng ZY, Bay BH, Aw SE, Lin VC. A novel antiestrogenic mechanism in progesterone receptor-transfected breast cancer cells. J Biol Chem. 2005;280(17):17480–7. [DOI] [PubMed] [Google Scholar]
- 8.Kastner P, Krust A, Turcotte B, Stropp U, Tora L, Gronemeyer H, Chambon P. Two distinct estrogen-regulated promoters generate transcripts encoding the two functionally different human progesterone receptor forms A and B. EMBO J. 1990;9(5):1603–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mote PA, Bartow S, Tran N, Clarke CL. Loss of co-ordinate expression of progesterone receptors A and B is an early event in breast carcinogenesis. Breast Cancer Res Treat. 2002;72(2):163–72. [DOI] [PubMed] [Google Scholar]
- 10.Liu XY, Ma D, Yu KD, Jiang YZ, Shao ZM. Genomic landscape and endocrine-resistant subgroup in estrogen receptor-positive, progesterone receptor-negative, and HER2-negative breast cancer. Theranostics. 2018;8(22):6386–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zattarin E, Leporati R, Ligorio F, Lobefaro R, Vingiani A, Pruneri G, Vernieri C. Hormone receptor loss in breast cancer: molecular mechanisms, clinical settings, and therapeutic implications. Cells. 2020;9(12):2644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kunc M, Biernat W, Senkus E. Lost but not least-novel insights into progesterone receptor loss in estrogen receptor-positive breast cancer. Cancers (Basel). 2021;13(19):4755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yildiz-Aktas IZ, Dabbs DJ, Cooper KL, Chivukula M, McManus K, Bhargava R. The effect of 96-hour formalin fixation on the immunohistochemical evaluation of estrogen receptor, progesterone receptor, and HER2 expression in invasive breast carcinoma. Am J Clin Pathol. 2012;137(5):691–8. [DOI] [PubMed] [Google Scholar]
- 14.Li Z, Tu Y, Wu Q, Wang Z, Li J, Zhang Y, Sun S. Clinical characteristics and outcomes of single versus double hormone receptor-positive breast cancer in 2 large databases. Clin Breast Cancer. 2020;20(2):e151–63. [DOI] [PubMed] [Google Scholar]
- 15.Thakkar JP, Mehta DG. A review of an unfavorable subset of breast cancer: estrogen receptor positive progesterone receptor negative. Oncologist. 2011;16(3):276–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hu T, Chen Y, Liu Y, Zhang D, Pan J, Long M. Classification of PR-positive and PR-negative subtypes in ER-positive and HER2-negative breast cancers based on pathway scores. BMC Med Res Methodol. 2021;21(1):108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li Y, Yang D, Yin X, Li H, Ren G. Clinicopathological characteristics and breast cancer-specific survival of patients with single hormone receptor-positive breast cancer. JAMA Netw Open. 2020;3(1):e1918160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu J, Gan M, Lin Z, Deng Q, Deng J, Zeng B, Shi Y, Ming J. Clinical features and prognosis analysis of hormone receptor-positive, HER2-negative breast cancer with differential expression levels of estrogen and progesterone receptors: a 10-year retrospective study. Breast J. 2022;29(2022):5469163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Viale G, Regan MM, Maiorano E, Goldhirsch A, Gusterson BA, Coates AS. Prognostic and predictive value of centrally reviewed expression of estrogen and progesterone receptors in a randomized trial comparing letrozole and tamoxifen adjuvant therapy for postmenopausal early breast cancer: BIG 1–98. J Clin Oncol. 2007;25(25):3846–52. [DOI] [PubMed] [Google Scholar]
- 20.Sikov WM, Berry DA, Perou CM, Hahn OM, Carey LA, Hudis CA, Winer EP. Impact of the addition of carboplatin and/or bevacizumab to neoadjuvant once-per-week paclitaxel followed by dose-dense doxorubicin and cyclophosphamide on pathologic complete response rates in stage II to III triple-negative breast cancer: CALGB 40603 (Alliance). J Clin Oncol. 2015;33(1):13–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dong W, Fujii T, Ning J, Iwase T, Qin J, Ueno NT, Shen Y. Reassessing estrogen receptor expression thresholds for breast cancer prognosis in HER2-negative patients using shape restricted modeling. Sci Rep. 2025;15(1):5590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Allison KH, Hammond MEH, Dowsett M, Viale G, Weisberg TF, McShane LM, Wolff AC. Estrogen and progesterone receptor testing in breast cancer: ASCO/CAP guideline update. J Clin Oncol. 2020;38(12):1346–66. [DOI] [PubMed] [Google Scholar]
- 23.Raghav KP, Hernandez-Aya LF, Lei X, Do KA, Hortobagyi GN, Gonzalez-Angulo AM. Impact of low estrogen/progesterone receptor expression on survival outcomes in breast cancers previously classified as triple negative breast cancers. Cancer. 2012;118(6):1498–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen T, Zhang N, Moran MS, Su P, Haffty BG, Yang Q. Borderline ER-positive primary breast cancer gains no significant survival benefit from endocrine therapy: a systematic review and meta-analysis. Clin Breast Cancer. 2018;18(1):1–8. [DOI] [PubMed] [Google Scholar]
- 25.Balduzzi A, Bagnardi V, Rotmensz N, Mastropasqua G, Goldhirsch A, Colleoni M. Survival outcomes in breast cancer patients with low estrogen/progesterone receptor expression. Clin Breast Cancer. 2014;14(4):258–64. [DOI] [PubMed] [Google Scholar]
- 26.Early Breast Cancer Trialists’ Collaborative Group (EBCTCG), Davies C, Godwin J, Gray R, Wang YC, Dowsett M, Ingle J, Peto R. Relevance of breast cancer hormone receptors and other factors to the efficacy of adjuvant tamoxifen: patient-level meta-analysis of randomised trials. Lancet. 2011;378(9793):771–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Honma N, Horii R, Iwase T, Saji S, Younes M, Ito Y, Akiyama F. Proportion of estrogen or progesterone receptor expressing cells in breast cancers and response to endocrine therapy. Breast. 2014;23(6):754–62. [DOI] [PubMed] [Google Scholar]
- 28.Harbeck N, Gnant M. Breast cancer. Lancet. 2017;389(10074):1134–50. [DOI] [PubMed] [Google Scholar]
- 29.Cortazar P, Zhang L, Untch M, Rastogi P, Eiermann W, von Minckwitz G. Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. Lancet. 2014;384(9938):164–72. [DOI] [PubMed] [Google Scholar]
- 30.Krug K, Jaehnig EJ, Satpathy S, Carr SA, Ellis MJ, Gillette MA, Clinical Proteomic Tumor Analysis Consortium. Proteogenomic landscape of breast cancer tumorigenesis and targeted therapy. Cell. 2020;183(5):1436-1456.e31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cui X, Schiff R, Arpino G, Osborne CK, Lee AV. Biology of progesterone receptor loss in breast cancer and its implications for endocrine therapy. J Clin Oncol. 2005;23(30):7721–35. [DOI] [PubMed] [Google Scholar]
- 32.Agrawal A, Robertson JF, Cheung KL, Gutteridge E, Ellis IO, Nicholson RI, Gee JM. Biological effects of fulvestrant on estrogen receptor positive human breast cancer: short, medium and long-term effects based on sequential biopsies. Int J Cancer. 2016;138(1):146–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fei F, Siegal GP, Wei S. Characterizing clinicopathologic features of estrogen receptor-positive/progesterone receptor-negative breast cancers. Clin Breast Cancer. 2022;22(7):e788–97. [DOI] [PubMed] [Google Scholar]
- 34.de Azambuja E, Holmes AP, Piccart-Gebhart M, Gelber RD, Eidtmann H, Baselga J. Lapatinib with trastuzumab for HER2-positive early breast cancer (NeoALTTO): survival outcomes of a randomised, open-label, multicentre, phase 3 trial and their association with pathological complete response. Lancet Oncol. 2014;15(10):1137–46. [DOI] [PubMed] [Google Scholar]
- 35.McAnena PF, McGuire A, Ramli A, et al. Breast cancer subtype discordance: impact on post-recurrence survival and potential treatment options[J]. BMC Cancer. 2018;18(1):282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rakha EA, El-Sayed ME, Green AR, Paish EC, Powe DG, Gee J, et al. Biologic and clinical characteristics of breast cancer with single hormone receptor positive phenotype. J Clin Oncol. 2007;25:4772–8. [DOI] [PubMed] [Google Scholar]
- 37.Liu XY, Ma D, Xu XE, Jin X, Yu KD, Jiang YZ, Shao ZM. Genomic landscape and endocrine-resistant subgroup in estrogen receptor-positive, progesterone receptor-negative, and HER2-negative breast cancer. Theranostics. 2018;8(22):6386–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Godbole M, Chandrani P, Gardi N, Dhamne H, Patel K, Yadav N, Gupta S, Badwe R, Dutt A. Mir-129-2 mediates down-regulation of progesterone receptor in response to progesterone in breast cancer cells. Cancer Biol Ther. 2017;18(10):801–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Belitškin D, Pant SM, Munne P, Klefström J. Hepsin regulates TGFβ signaling via fibronectin proteolysis. EMBO Rep. 2021;22(11):e52532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tervonen TA, Pant SM, Belitškin D, Klefström J. Oncogenic Ras disrupts epithelial integrity by activating the transmembrane serine protease Hepsin. Cancer Res. 2021;81(6):1513–27. [DOI] [PubMed] [Google Scholar]
- 41.Belitškin D, Munne P, Pant SM, Klefström J. Hepsin promotes breast tumor growth signaling via the TGFβ-EGFR axis. Mol Oncol. 2024;18(3):547–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hayakawa M, Matsushima M, Hagiwara H, Kitagawa M. Novel insights into FGD3, a putative GEF for Cdc42, that undergoes SCF(FWD1/beta-TrCP)-mediated proteasomal degradation analogous to that of its homologue FGD1 but regulates cell morphology and motility differently from FGD1. Genes Cells. 2008;13(4):329–42. [DOI] [PubMed] [Google Scholar]
- 43.Cheng WY, Ou Yang TH, Anastassiou D. Development of a prognostic model for breast cancer survival in an open challenge environment. Sci Transl Med. 2013;5(181):181ra50. [DOI] [PubMed] [Google Scholar]
- 44.Hedman H, Henriksson R. LRIG inhibitors of growth factor signalling - double-edged swords in human cancer? Eur J Cancer. 2007;43(4):676–82. [DOI] [PubMed] [Google Scholar]
- 45.Thompson PA, Ljuslinder I, Tsavachidis S, Brewster A, Sahin A, Hedman H, et al. Loss of LRIG1 locus increases risk of early and late relapse of stage I/II breast cancer. Cancer Res. 2014;74(11):2928–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yokdang N, Hatakeyama J, Wald JH, Sweeney C. LRIG1 opposes epithelial-to-mesenchymal transition and inhibits invasion of basal-like breast cancer cells. Oncogene. 2016;35(22):2932–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang CQ, Tang CH, Wang Y, Su CM. FSCN1 gene polymorphisms: biomarkers for the development and progression of breast cancer. Sci Rep. 2017;7(1):15887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dibble CC, Cantley LC. Regulation of mTORC1 by PI3K signaling. Trends Cell Biol. 2015;25(9):545–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material 1: Figure S1. The mRNA expression levels of targeted genes among three groups (ER +/PR +, ER +/PR-, and ER-/PR-) in the TCGA database.
Data Availability Statement
Data are available upon reasonable request. Access to datasets from The Second Affiliated Hospital of Chongqing Medical University should be requested directly from the institution via their data access request forms. All experiments and implementation details are described thoroughly in the Materials and methods section.