Abstract
Background
Patients with amyotrophic lateral sclerosis (ALS) often experience spasticity, which can severely affect their ability to perform basic activities like standing and walking, potentially diminishing their already compromised quality of life. Botulinum toxin type A (BTX-A) is a first-line drug for spastic management. However, there are limited reports on its effectiveness in reducing muscle tone among ALS patients, with scarcely any related research conducted in China. We conducted the clinical observation and follow-up study through the relevant ethical post (ChiCTR2200061794). Clinical registration was on July 2, 2022. All participants provided written informed consent.
Case presentation
We report two cases of middle-aged male patients, both diagnosed with ALS, who presented with symptoms such as limb stiffness and walking limitation due to increased muscle tone in the lower limbs. Based on the spasticity of the patient’s lower limbs, the corresponding target muscles were selected for BTX-A treatment under ultrasound guidance, and the patients were evaluated on relevant functional scales before injection (baseline, T0) and at three follow-up visits (T1: 2 weeks, T2: 4 weeks, T3: 8 weeks).
Conclusion
Appropriate BTX-A injected into the target muscles could effectively depress the spasticity of ALS patients without apparent side effects.
Keywords: ALS, BTX-A, Ultrasound, UMN, Lower limb spasticity
Background
Amyotrophic lateral sclerosis (ALS) is a progressive degenerative disease of the central nervous system of unknown pathogenesis that often involves upper motor neurons (UMN) and lower motor neurons (LMN) [1]. When the lesion involves UMN, patients usually show increased muscle tone, hyperreflexia, and positive pathologic signs, which may sometimes be accompanied by clonus. These neurological deficits not only lead to motor dysfunction but also significantly reduce the Barthel Index score, which is characterised by a progressive loss of the ability to perform basic activities of daily living (ADL) such as independent eating, personal hygiene management, and dressing [2]. Conventional treatment regimens for ALS include pharmacologic therapy with glutamatergic nerve conduction modulators (e.g. riluzole) and free radical scavengers (e.g. edaravone), supplemented by multidisciplinary supportive care (including noninvasive ventilatory support, percutaneous gastrostomy nutritional interventions, and rehabilitative training) [3–5]. However, there are significant limitations to these conventional treatments: drug therapy only delays disease progression to a limited extent (riluzole prolongs survival by about 3 months) and is accompanied by adverse drug reactions such as liver function abnormalities and gastrointestinal reactions, supportive therapy, while improving some of the symptoms, does not reverse the neuronal degenerative process [3, 4]. Notably, traditional interventions for ALS-related muscle spasticity (e.g. oral baclofen or tizanidine) often require systemic administration and cause central side effects (e.g. somnolence, cognitive suppression) and have insufficient ability to selectively modulate localised spasticity, making it challenging to balance efficacy and safety [6].
Exploring locally targeted therapeutic strategies has gradually become a research hotspot in this context. Botulinum toxin type A (BTX-A), a neurotoxin, specifically blocks abnormal electrical signalling in spastic muscles by irreversibly inhibiting the release of acetylcholine vesicles from the neuromuscular junction [7]. BTX-A is a first-line medication for treating spasticity, recommended by multiple guidelines for conditions such as muscle tone disorders, adult/child limb spasms, and focal spasms [8–10]. Currently, BTX-A is widely used for various conditions such as dystonia, tic disorders, and other localized symptoms [11]. However, there are limited reports on its effectiveness in reducing muscle tone among patients with ALS, with scarcely any related research conducted in China. In this clinical report, we present two cases of ALS patients who underwent BTX-A injection therapy guided by ultrasound (SONIMAGE HS1 PLUS, Japan). This study received ethical committee approval and was registered with the Chinese Clinical Trial Registry (ChiCTR2200061794). Clinical registration was on July 2, 2022. All participants provided written informed consent.
Case conclusion
Case report 1
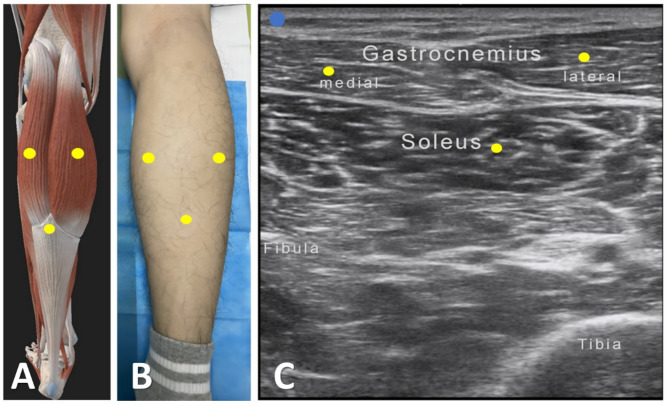
A 45-year-old male patient presented with left upper limb weakness in October 2021, which gradually progressed to weakness in the left lower limb by December of the same year. The patient also exhibited symptoms of stiffness in the limbs but without drooling, difficulty swallowing, or respiratory distress.The patient was diagnosed with ALS according to the revised El Escorial criteria in February 2022, and increased muscle tone was observed in the lower limbs five months later, particularly difficulty in dorsiflexion of the ankle joint. The Modified Ashworth Spasticity Scale (MAS) revealed increased tone in ankle dorsiflexion (Grade II). The patient initially took baclofen but discontinued it due to perceived ineffectiveness. On September 21, 2022, the patient sought medical attention at our hospital. The evaluation showed a severe increase in plantar flexor muscle tone in both lower limbs, with ankle clonus lasting about 30 s. With the patient’s informed consent, we first utilized surface electromyography (sEMG) to assess target muscles for lower limb spasticity, followed by ultrasound-guided localization of the medial gastrocnemius, lateral gastrocnemius, and soleus muscles. Using an in-plane injection technique, we administered botulinum toxin type A (BTX-A, Botox®, Ireland) 100 units per side on September 21, 2022 (Fig. 1; Table 1). The patient did not experience significant discomfort in the first two weeks post-injection. Four weeks post-injection, the patient noted a significant reduction in lower limb muscle tone and no increase in weakness. However, at 4 weeks post-injection, the patient exhibited occasional symptoms of urinary incontinence. Eight weeks post-injection, the patient’s plantar flexor muscle tone returned to normal, ankle clonus occurred approximately once, and the Clinical Spasticity Scale (CSS) score decreased by 10/10 points compared to baseline (Table 2).
Fig. 1.
Intramuscular injection localization techniques. Schematic diagram of the medial and lateral heads of the gastrocnemius and soleus at the point of injection (A). Somatic localisation of the medial and lateral heads of the gastrocnemius and soleus (B). Ultrasonographic localisation of the medial and lateral heads of the gastrocnemius and soleus (C)
Table 1.
General condition, injection site and dose in two patients
| Sex | Age (years) |
Duration of disease | Spasticity | BTX-A dose |
Injection Method | Intramuscular dose per side |
|
|---|---|---|---|---|---|---|---|
| Case1 | M | 45 | 8 months | Plantarflexion & ankle clonus | 200U |
Ultrasound guided |
Medial Gastrocnemius(30u) Lateral Gastrocnemius (30u) soleus (40u) |
| Case2 | M | 63 | 11 months | abduction | 100U |
Ultrasound guided |
Adductor magnus (30u) Adductor longus (20u) |
Table 2.
Changes in outcome measures of the patient (case 1)
| Pretreatment (0wk, T0) |
Posttreatment (2wk, T1) |
Follow-up (4wk, T2) |
Follow-up (8wk, T3) |
|
|---|---|---|---|---|
| CSS(L/R) | 56/56 | 55/55 | 48/48 | 46/46 |
| 10MWT(s) | 105 | 130 | 200 | 256 |
| ALS-FRS | 27 | 27 | 25 | 23 |
| △FS | 3.17 | 3.17 | 3.29 | 3.35 |
| Barthel Index | 40 | 40 | 20 | 15 |
| Adverse reaction | Not | Not | Not | Not |
CSS = Composite Spasticity scale, 10MWT = 10-meter walking test, △FS = rate of disease progression, ALS-FRS = ALS Functional Rating Scale, △FS = (48 - ALS-FRS at ‘‘time of recruitment’’)/duration from onset to injection (months) \0.5;
Case report 2
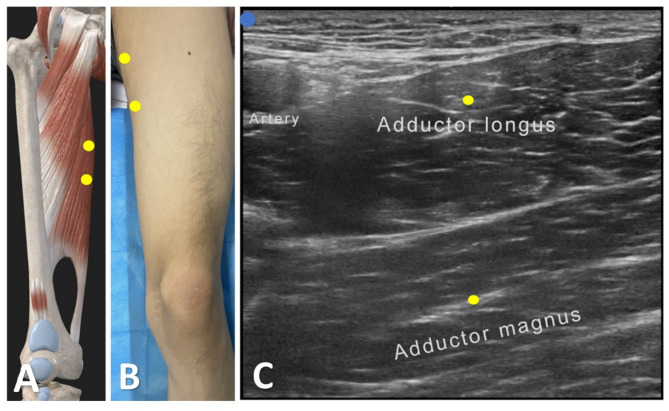
A 63-year-old male patient presented with a progressive weakening of the right lower limb in January 2020, followed by atrophy of the first interosseous muscle in the right hand in August of the same year. By October, there was muscle atrophy in the first interosseous muscle of the left hand. In February 2021, weakness appeared in the left lower limb without accompanying symptoms such as drooling, swallowing difficulties, or respiratory issues. Ultimately, in June 2021, the patient was diagnosed with Amyotrophic Lateral Sclerosis (ALS) according to the revised El Escorial criteria. In January 2022, the patient developed increased muscle tone in the lower limbs, causing difficulty in leg abduction. The MAS showed elevated adductor tone at the hip joint (Grade II). Initially, the patient was prescribed oral tizanidine, but it was discontinued due to poor efficacy. By September 2022, the patient developed walking difficulties and overall fatigue, prompting a visit to our hospital. The evaluation revealed significantly increased tone in the adductor muscles of both lower limbs. After obtaining informed consent, the patient received botulinum toxin type A (BTX-A, Botox®, Ireland) injection therapy on September 19, 2022. We first assessed the target muscles for lower limb spasticity using sEMG, followed by ultrasound-guided localization of the unilateral adductor magnus and adductor longus muscles. The injection was performed using an in-plane technique, with 50 units administered per side. (Fig. 2; Table 1). We conducted follow-up assessments on the patient before treatment (baseline, T0) and after treatment (T1 at 2 weeks, T2 at 4 weeks, and T3 at 8 weeks), utilising various scales for evaluation (Table 3). The patient did not experience any significant discomfort in the first 2 weeks post-injection. At 4 weeks post-injection, the patient noted a significant reduction in lower limb muscle tone. It is noteworthy that the patient experienced mild respiratory difficulty two days after the T3 assessment, possibly related to disease progression. At 8 weeks post-injection, the adductor muscle tone in the patient had essentially returned to normal, and the Clinical Global Impression of Change (CGIC) score had decreased by 8/9 points compared to baseline.
Fig. 2.
Intramuscular injection localization techniques. Schematic diagram of the adductor longus and the adductor magnus at the point of injection (A). Somatic localization of the adductor longus and the adductor magnus (B). Ultrasonographic localization of the adductor longus and the adductor magnus (C)
Table 3.
Changes in outcome measures of the patient (case 2)
| Pretreatment (0wk, T0) |
Posttreatment (2wk, T1) |
Follow-up (4wk, T2) |
Follow-up (8wk, T3) |
|
|---|---|---|---|---|
| CSS(L/R) | 54/55 | 54/55 | 52/53 | 46/46 |
| 10MWT(s) | 300 | 310 | 500 | Can not |
| ALS-FRS | 24 | 21 | 20 | 20 |
| △FS | 1.41 | 1.5 | 1.47 | 1.47 |
| Barthel | 30 | 30 | 25 | 10 |
| Adverse reaction | Not | Not | Not | Not |
CSS = Composite Spasticity scale, 10MWT = 10-meter walking test, △FS = rate of disease progression, ALS-FRS = ALS Functional Rating Scale, △FS = (48 - ALS-FRS at ‘‘time of recruitment’’)/duration from onset to injection (months) \0.5;
Discussion and conclusions
BTX-A exerts its principal therapeutic effect through targeted modulation of neuromuscular junction activity. When administered via localized injection, this neurotoxin demonstrates limited diffusion characteristics while maintaining temporally reversible pharmacological actions. These distinctive pharmacokinetic properties render BTX-A a therapeutically viable intervention strategy for managing diverse clinical syndromes characterized by hyper-functional activity of specific nerve terminal populations, offering both enhanced safety profiles and significant therapeutic efficacy [11]. Currently, BTX-A has a wide range of clinical applications and is recommended by multiple national guidelines for conditions such as dystonia, limb spasticity in adults/children, and focal spasticity. It has not been shown to have significant adverse effects [8–10]. Marvulli et al. treated 15 ALS patients with spasticity of adductor magnus with BTX-A injections and found significant improvement in their muscle spasticity at 90-day follow-up. Furthermore, the combination application of BTX-A with rehabilitation has induced a prolonged therapeutic response, with clinical data demonstrating sustained attenuation of muscular hypertonicity extending up to 3 months post-intervention. This synergistic therapeutic approach appears to potentiate the neuromodulatory effects of BTX-A, thereby optimizing the temporal parameters of spasticity reduction while concurrently enhancing functional recovery outcomes [12, 13]. However, the use of BTX-A in the treatment of ALS remains clinically unclear, with data from very few studies demonstrating its potential efficacy in the treatment of managing sialorrhea and dysphagia due to increased muscle tone in this particular patient population [9, 10]. Currently, the Chinese medical research landscape lacks clinical research investigating the therapeutic application of BTX-A in ALS management. These two rigorously monitored clinical case reports systematically delineate intervention protocols and outcome trajectories. Through this evidence-based framework, we aim to pioneer novel neurorehabilitation strategies while establishing a preliminary mechanistic foundation for optimizing neuromodulatory interventions in motor neuron disease therapeutics.
To enhance the accuracy and safety of the treatment, we employed surface electromyography to identify the target muscles affected by spasticity. Subsequently, we utilised ultrasound guidance for precise injections. In the first case, the patient experienced limb spasticity, and the surface electromyographic evaluation revealed a higher muscle tone in the posterior group of calf muscles compared to the thighs. As a result, we administered injections to the calf gastrocnemius and soleus muscles. In the second case, the patient experienced difficulty walking, and the surface electromyographic evaluation indicated greater tension in the adductor magnus muscle of the thigh than in the calf. Consequently, we administered injections to the adductor magnus and adductor longus muscles of the thigh.
In the first case, the patient primarily presented with a severe increase in plantar flexor tone in both lower limbs and ankle clonus, lasting approximately 30 s. After eight weeks of injection, the patient’s plantar flexor tone returned to normal, the number of ankle clonus decreased to approximately one, and the CSS score decreased by 10/10 points compared to the baseline value. Another patient primarily presented with a severe increase in tension in the adductor muscles of both lower extremities. Eight weeks after the injection, the patient’s adductor tone returned to normal, and the CSS score dropped 8/9 points from the baseline value. In both cases, lower limb spasticity improved one month after the injection. Still, there were no significant changes in the 10MWT, Barthel Index, ALS-FRS, and the rate of disease progression in both patients compared to the baseline value. Vázquez-Costa’s study showed that ankle clonus disappears or significantly decreases in patients who receive BTX-A injections for one month. However, some patients still experience worsened slowing of the step rate in the 10MWT [13]. The lack of significant changes in the remaining indicators reported in the current two cases was initially attributed to disease progression. Both patients developed a temporary inability to control urination and defecation during the follow-up period. However, electromyographic measurements did not detect any weakness in the muscles targeted for injection, indicating these symptoms were unrelated to botulinum toxin administration.
Based on our findings, it could be concluded that BTX-A injections administered at appropriate dosages into target muscles may effectively alleviate myospasm without causing significant side effects.The lack of significant improvement in the 10MWT and ADL may be attributed to the natural disease progression of ALS. However, it has a limited impact on the patient’s ability to walk in the short term. This report has certain limitations, including the small samples, restricted evaluation scales(e.g., quality of life measures) and the short follow-ups. Additionally, these cases only involved BTX-A injections without the inclusion of physiotherapy, as some studies found that physiotherapy shows limited improvement in the ADL of patients with ALS, it may potentially enhance gait function in both short-term and long-term contexts [14, 15].
Overall, there is currently limited research on botulinum toxin for lower limb spasticity in ALS patients. The experiences of these patients may provide some clues and clinical insights for spasticity management in this context, but the findings need to be replicated in larger, controlled studies before clinical implications can be established.
Acknowledgements
We thank the patient for granting permission to publish this information.
Abbreviations
- CSS
Composite spasticity scale
- 10MWT
10-meter walking test
- ALS-FRS
ALS Functional rating scale
- ALS
Amyotrophic lateral sclerosis
- BTX-A
Botulinum toxin type A
- UMN
Upper motor neurons
Author contributions
QD and XQH designed the study. CL and CXW drafted the manuscript. QD, QW and YJH collected the data. BW, LWS and JXQ revised the manuscript. The authors read and approved the final manuscript.
Funding
This work was supported by the Natural Science Foundation of Hubei Province (Joint Fund Program)(No.2024AFD137).
Data availability
Data is provided within the manuscript.
Declarations
Ethics approval and consent to participate
Informed consents were obtained from the patients to publish those cases, and approval for this study was provided by the Research Ethics Committee of The First College of Clinical Medical Science.
Consent for publication
Written informed consents were obtained from the patients to publish those two cases, including any potentially identifiable images or data in this article.
Declaration of large language models
None of Large Language Models was used in manuscript writing.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Qiang Duan, Email: 174499182@qq.com.
Xiaoqun Huang, Email: 624629560@qq.com.
References
- 1.Morfini GA, Bosco DA, Brown H, Gatto R, Kaminska A, Song Y, Molla L, Baker L, Marangoni MN, Berth S, et al. Inhibition of fast axonal transport by pathogenic SOD1 involves activation of p38 MAP kinase. PLoS ONE. 2013;8(6):e65235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abdulla S, Vielhaber S, Kollewe K, Machts J, Heinze HJ, Dengler R, Petri S. The impact of physical impairment on emotional well-being in ALS. Amyotroph Lateral Scler Frontotemporal Degener. 2014;15(5–6):392–7. [DOI] [PubMed] [Google Scholar]
- 3.Jaiswal MK. Riluzole and edaravone: A Tale of two amyotrophic lateral sclerosis drugs. Med Res Rev. 2019;39(2):733–48. [DOI] [PubMed] [Google Scholar]
- 4.Mercadante S, Al-Husinat L. Palliative care in amyotrophic lateral sclerosis. J Pain Symptom Manage. 2023;66(4):e485–99. [DOI] [PubMed] [Google Scholar]
- 5.de Almeida FEO, do Carmo Santana AK, de Carvalho FO. Multidisciplinary care in amyotrophic lateral sclerosis: a systematic review and meta-analysis. Neurol Sci. 2021;42(3):911–23. [DOI] [PubMed]
- 6.Romano M, Bagnato S, Altavista MC, Avanzino L, Belvisi D, Bologna M, Bono F, Carecchio M, Castagna A, Ceravolo R, et al. Diagnostic and therapeutic recommendations in adult dystonia: a joint document by the Italian society of neurology, the Italian academy for the study of parkinson’s disease and movement disorders, and the Italian network on botulinum toxin. Neurol Sci. 2022;43(12):6929–45. [DOI] [PubMed] [Google Scholar]
- 7.Facciorusso S, Spina S, Picelli A, Baricich A, Francisco GE, Molteni F, Wissel J, Santamato A. The role of botulinum toxin Type-A in spasticity: research trends from a bibliometric analysis. Toxins (Basel) 2024, 16(4). [DOI] [PMC free article] [PubMed]
- 8.Restivo DA, Casabona A, Nicotra A, Zappia M, Elia M, Romano MC, Alfonsi E, Marchese-Ragona R. ALS dysphagia pathophysiology: differential botulinum toxin response. Neurology. 2013;80(7):616–20. [DOI] [PubMed] [Google Scholar]
- 9.Simpson DM, Hallett M, Ashman EJ, Comella CL, Green MW, Gronseth GS, Armstrong MJ, Gloss D, Potrebic S, Jankovic J, et al. Practice guideline update summary: botulinum neurotoxin for the treatment of blepharospasm, cervical dystonia, adult spasticity, and headache: report of the guideline development subcommittee of the American academy of neurology. Neurology. 2016;86(19):1818–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Williams G, Singer BJ, Ashford S, Hoare B, Hastings-Ison T, Fheodoroff K, Berwick S, Sutherland E, Hill B. A synthesis and appraisal of clinical practice guidelines, consensus statements and Cochrane systematic reviews for the management of focal spasticity in adults and children. Disabil Rehabil. 2022;44(4):509–19. [DOI] [PubMed] [Google Scholar]
- 11.Pirazzini M, Rossetto O, Eleopra R, Montecucco C. Botulinum neurotoxins: biology, pharmacology, and toxicology. Pharmacol Rev. 2017;69(2):200–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Marvulli R, Megna M, Citraro A, Vacca E, Napolitano M, Gallo G, Fiore P, Ianieri G. Botulinum toxin type A and physiotherapy in spasticity of the lower limbs due to amyotrophic lateral sclerosis. Toxins (Basel) 2019, 11(7). [DOI] [PMC free article] [PubMed]
- 13.Vázquez-Costa JF, Máñez I, Alabajos A, Guevara Salazar M, Roda C, Sevilla T. Safety and efficacy of botulinum toxin A for the treatment of spasticity in amyotrophic lateral sclerosis: results of a pilot study. J Neurol. 2016;263(10):1954–60. [DOI] [PubMed] [Google Scholar]
- 14.Miller RG, Jackson CE, Kasarskis EJ, England JD, Forshew D, Johnston W, Kalra S, Katz JS, Mitsumoto H, Rosenfeld J, et al. Practice parameter update: the care of the patient with amyotrophic lateral sclerosis: drug, nutritional, and respiratory therapies (an evidence-based review): report of the quality standards subcommittee of the American academy of neurology. Neurology. 2009;73(15):1218–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ng L, Khan F, Young CA, Galea M. Symptomatic treatments for amyotrophic lateral sclerosis/motor neuron disease. Cochrane Database Syst Rev. 2017;1(1):Cd011776. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data is provided within the manuscript.