Abstract
Introduction
The prevalence of knee osteoarthritis (OA) is rising worldwide, leading to disability and a reduced quality of life, particularly in elderly patients. While there are several treatment options, there is little consensus in the scientific community over which methods are most effective. Viscosupplementation with hyaluronic acid (HA) has been found to reduce pain in patients with knee OA over a period of up to 6 months, with little to no side effects. The aim of this prospective open-label, uncontrolled, observational, single-site study was to assess the efficacy and safety of a single hybrid HA injection over a period of 6 months in subpopulations of patients with low to severe symptomatic knee OA in everyday clinical practice.
Methods
Fifty patients who met the inclusion criteria participated in the study. A single intra-articular ultrasound-guided injection of hybrid HA (Sinovial®) was administered. Patients submitted Visual Analog Scale (VAS) and Western Ontario and McMaster Universities Arthritis Index (WOMAC) questionnaires at 28, 42, 84, and 168 days post-treatment.
Results
VAS scores measured at rest and when walking indicate an improvement during follow-up, particularly at 28 and 42 days, compared to baseline. Similarly, the most notable improvement of the WOMAC score was observed within the first 42 days after injection. While decrease in pain and joint function improvement were not as pronounced at the end of follow-up, they were still statistically better than at baseline. Overall patient satisfaction was high.
Conclusion
Treatment with a single injection of hybrid HA was demonstrated to be safe and effective in patients with varying degrees of knee OA. Patients with medial knee OA responded better to treatment than patients with patellofemoral OA, which provides information on which types of patients are best suited to this intervention.
Trial Registration
ClinicalTrials.gov identifier, NCT06652893. Retrospectively registered October 10, 2024.
Keywords: Hybrid HA, Osteoarthritis, VAS, Viscosupplementation, WOMAC
Key Summary Points
| Knee osteoarthritis (OA) is a growing health concern, particularly in ageing populations. |
| Viscosupplementation with hyaluronic acid (HA) has been shown to reduce pain and improve joint function. |
| The aim of this study was to observe whether a single injection of hybrid HA reduced pain in patients with low to severe knee OA over a period of 6 months. |
| According to results for the VAS and WOMAC scores, pain was reduced in most patients, particularly within the first few weeks after treatment, and no adverse events were reported. |
Introduction
Osteoarthritis (OA) is a degenerative joint disease characterized by the breakdown of cartilage and changes in the subchondral bone, which lead to the loss of volume and altered physical characteristics of articular cartilage. The primary symptoms of OA include pain, which worsens with activity and improves with rest, stiffness lasting for a short period (less than 30 min) upon resuming activity, swelling, tenderness around the joint, decreased range of motion with joint crepitus, muscle weakness, and even deformation of the joint. Symptoms can vary in intensity and may affect any joint; however, knee joints are the most commonly affected areas. OA is present mainly in elderly patients and may substantially affect overall health and quality of life (QoL) [1, 2]. The prevalence of OA has dramatically increased globally, from 247.5 million cases in 1990 to 527.8 million cases in 2019 [3].
Although OA is typically easy to diagnose through a combination of clinical evaluation, patient history, and imaging tests such as X-rays or MRI, the treatment of this disease is challenging. Treatment focuses on managing symptoms and improving joint function through a combination of lifestyle changes, physical therapy, medication, and eventually surgery [2, 4]. OA is a chronic disease that requires intervention with both non-pharmacological and pharmacological treatment modalities, and inevitably, disease progression may necessitate successive treatments [4]. Among the options for the pharmacological treatment of OA, paracetamol, non-steroidal anti-inflammatory drugs (NSAIDs), and cyclooxygenase 2 inhibitors (COX-2) are commonly used. Intra-articular viscosupplementation therapy with hyaluronic acid (HA) is a very good alternative for patients who are intolerant to oral and topical analgesics [5–7].
Intra-articular injections of HA have been proven to reduce pain and improve joint function, especially in OA, which is characterized by pain and functional impairment [7]. Viscosupplementation with both low molecular weight HA (L-HA) and high molecular weight HA (H-HA) in the same product is more similar to synovial fluid and could provide better results than linear HA, as natural synovial fluid encompasses hyaluronians of different molecular weights, which suggests their cooperation in maintaining articular homeostasis [8]. The combined use of H-HA (1100–1400 kDa) bound to L-HA (80–100 kDa) by weak hydrogen bonds in a dynamic hybrid complex has been shown in in vitro studies to stimulate chondrocyte proliferation and to decrease their catabolism more efficiently than linear HA. Moreover, hybrid HA has displayed higher potential as an anti-inflammatory agent than linear HA [9]. Hybrid cooperative complexes have also proved to be more stable to bovine testicular hyaluronidase (BTH) digestion than linear HA [10]. In clinical studies, hybrid HA has been shown to reduce pain both in an active and inactive state 6 months post-treatment in major joint OA, independently when compared with baseline or when compared to linear HA [8].
The aim of the present study is to assess the efficacy and safety of a single hybrid HA injection over a period of 6 months in subpopulations of patients with low to severe symptomatic knee OA in everyday clinical practice. Our hypothesis is that the total reduction of pain obtained 6 months after treatment with viscosupplementation with hybrid HA is significantly different as compared to baseline.
Methods
Study Design
This was a prospective open-label, uncontrolled, a case series, single-site study performed between December 2022 and July 2023 on patients treated for symptomatic OA of the knee with HA infiltration, with a single hybrid HA injection in an outpatient setting.
The study was performed in accordance with the ethical guidelines of the Declaration of Helsinki and was approved by the Local Bioethics Committee in Lodz, Poland. Collected information and data were managed according to the guidelines of Good Clinical Practice (GCP). All participants signed an informed consent form at the time of enrollment to collect clinical data. All patients also gave their consent to the use of patient reported outcomes (PROs). The study was registered on ClinicalTrials.gov with NCT06652893.
Setting
Consecutive adult patients with knee OA and with a medical indication for viscosupplementation with hybrid HA who fulfilled the inclusion criteria were recruited for the study. The inclusion criteria were a medical indication for viscosupplementation therapy with hybrid HA; patients aged between 45 and 80 years; diagnosis of unicompartmental femorotibial gonarthrosis or patellofemoral arthrosis, gonarthrosis symptoms for at least 6 months; patients who failed to respond to analgesics and/or regular NSAIDs, or were proved to be intolerant to the regular use of analgesics, NSAIDS, or weak opioids; grade 2 to 3 OA in the Kellgren–Lawrence grading scale [11]; mechanical axis deviation on involved leg less than 10° as measured on standing radiograms; pain when walking measured by the Visual Analog Scale (VAS; 0–100 mm) of at least 40 mm on the targeted knee; and contralateral knee pain when walking with VAS of less than 10 mm [12]. The exclusion criteria were inability to understand the aim of the study, inability to provide acceptable consent, pregnancy, serious psychiatric disorders, systemic or neurological disorders, allergy or intolerance to hybrid HA, and secondary gonarthrosis due to systematic and inflammatory disorders.
Intervention
A single intra-articular injection of hybrid HA complex Sinovial® HL (IBSA Pharmaceuticals, Italy, patent number WO/2012/032151) in the knee was performed from the lateral approach to the suprapatellar recess under ultrasound control. For the ultrasound-guided infiltration method, a sterile linear probe with a 20-G guide was used to visualize the joint suprapatellar recess and avoid intrasynovial infiltration. The patient was positioned supine with the knee in full extension. From the lateral approach to the suprapatellar recess, HA was then injected into the knee joint, verifying intra-articular positioning in real time via ultrasound imaging (direct visualization of viscous fluid or air bubbles).
Patients did not receive anti-inflammatory or pain-relieving drugs in the week before the first injection. Patients were evaluated at five time points: at baseline and at 28, 42, 84, and 168 days after the intervention. At each evaluation, clinical data relating to the status of the disease and PROs were collected. A time window of 7 days was allowed before or after planned study visits.
Outcomes
The primary outcome was to evaluate the efficacy of hybrid HA over a period of 6 months, assessed as a reduction in pain measured by VAS (0–100 mm VAS). The secondary outcomes included evaluation of the efficacy of hybrid HA, assessed by changes of the total Western Ontario and McMaster Universities Arthritis Index (WOMAC), a scale widely used in the evaluation of hip and knee OA, and changes in pain at rest and at walking (0–100 mm VAS) The average improvement of the WOMAC scores was independently correlated with the type of gonarthrosis and Kellgren–Lawrence grade, gender, and the presence of osteophytes.
To evaluate the effect of HA viscosupplementation in patients with knee OA, the following PROs were used: knee function (physical function, stiffness, pain) measured using the WOMAC score [13], pain at rest and pain when walking measured using VAS (0–100 mm) [12], and patient satisfaction regarding pain relief and therapeutic efficacy evaluated by the independent researcher (second author), measured using a five-point Likert scale: 1, not at all satisfied; 2, slightly satisfied; 3, somewhat satisfied; 4, very satisfied; 5, extremely satisfied [14]. The diagnosis of knee OA and classification was based on the American College of Radiology’s (ACR) Kellgren–Lawrence Grading Scale [11]. As this was an observational study, other medical interventions could not be excluded, in line with everyday clinical practice. All additional treatments and interventions were recorded during follow up. The patients were instructed to engage in normal, everyday activities.
The safety was monitored by collecting all possible side effects like persistent pain after injection (longer than 12 h), swelling, redness, limitation of range of motion, impairment of knee function.
Study Size
The number of participants to be included was based on the following assumptions and requirements: the minimum clinically important difference (MCID) was calculated to ascertain the smallest change in an outcome that patients perceived as beneficial. MCID for pain assessment in non-surgical therapies for osteoarthritis was presented on the VAS (0–100 mm), with values presented in the literature ranging from 10 to 19.9 points. MCID ranges of 8.4–19.9, 10–30, and 15–20 points were also reported for the VAS (0–100 mm) [15]. There are no direct reports of MCID after HA therapy in the literature [16]. Study assumptions were as follows: the expected standard deviation (SD) of the total VAS score was 20 mm [17] and the desired margin of error was 6 mm, α = 0.05, power (1 − β) = 0.8. On the basis of these values, a minimum of 35 subjects were to be included in the study. Accounting for dropout rate as high as at least 20%, we planned to include 50 subjects in the study.
Data Analysis
Data from PROs were stored at the study site in a data management system, using S-scales Software (Justine S Design, Poland). VAS and WOMAC scores, at baseline and 168 days following injections, were compared by paired t tests and one-way analysis of variance (ANOVA). Missing data (e.g., lost to follow-up) were omitted in the analysis. All tests were two-sided with α = 0.05 and power (1 − β) = 0.8.
Results
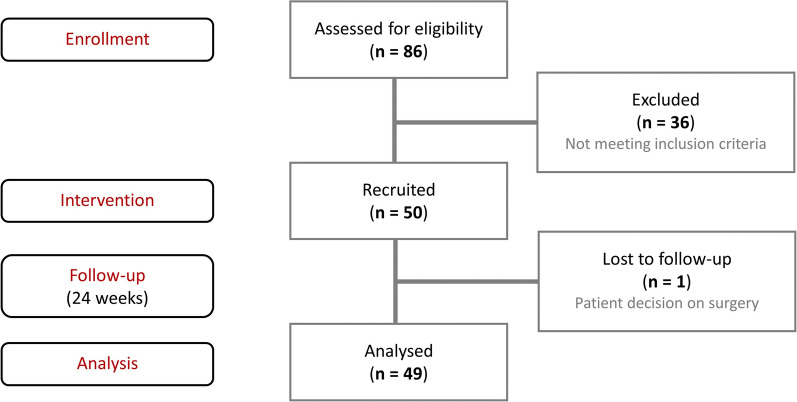
Out of 86 consecutive patients examined in the orthopedic outpatient clinic, 50 patients fulfilled the inclusion criteria and were selected for the study. One patient resigned after 28 days of follow-up and decided to have surgery on her knee. The remaining 49 patients finished the study protocol (Fig. 1). The baseline characteristics of the study population are presented in Table 1. All the patients were white.
Fig. 1.
Study design and patient disposition. n number of patients
Table 1.
Baseline characteristics of the study population
| Variable | Study group (n = 50) |
|---|---|
| Age, years | 49.6 (14.1) 20–84 |
| BMI | 38.4 (12.5) 19–62 |
| Gender | |
| Women | 39 (78%) |
| Men | 11 (22%) |
| Sport activity | |
| Yes | 5 (10%) |
| No | 45 (90%) |
| Work status | |
| Working | 29 (58%) |
| Retired | 21 (42%) |
n number of patients, BMI body mass index
Primary Outcome
The results for VAS pain at rest are presented in Table 2 and Fig. 2. The improvement was observed within the first 3 months post-treatment, but was statistically significant only within the first 6 weeks. After 3 months, a decrease of the VAS score was observed. The results for VAS pain when walking are presented in Table 3 and Fig. 3. The improvement was the highest within the first 4 weeks after treatment, with some statistical improvement after 6 weeks. This effect persisted until the end of follow-up.
Table 2.
VAS results for pain at rest
| VAS | n | Mean | Median | Minimum | Maximum | SD | p value* |
|---|---|---|---|---|---|---|---|
| Baseline | 50 | 57 | 55 | 16 | 89 | 13.7 | |
| 28 days | 50 | 47 | 50 | 0 | 68 | 14.4 | 0.0003 |
| 42 days | 49 | 43 | 42 | 2 | 82 | 16.8 | 0.03 |
| 84 days | 49 | 41 | 39 | 0 | 82 | 21.5 | 0.2 |
| 168 days | 49 | 44 | 45 | 0 | 84 | 22.6 | 0.03 |
n number of patients, SD standard deviation, VAS Visual Analog Scale (0–100 mm)
*p values < 0.05 were considered statistically significant
Fig. 2.
VAS results for pain at rest. VAS Visual Analog Scale, SE standard error, SD standard deviation
Table 3.
VAS results for pain when walking
| VAS | n | Mean | Median | Minimum | Maximum | SD | p value* |
|---|---|---|---|---|---|---|---|
| Baseline | 50 | 59 | 63 | 15 | 91 | 19.3 | |
| 28 days | 50 | 45 | 48 | 13 | 78 | 17.9 | 0.0001 |
| 42 days | 49 | 40 | 41 | 0 | 77 | 17.6 | 0.04 |
| 84 days | 49 | 40 | 38 | 0 | 91 | 21.8 | 0.8 |
| 168 days | 49 | 39 | 35 | 1 | 78 | 22.8 | 0.4 |
Data are presented as mean (SD) range
n number of patients, SD standard deviation, VAS Visual Analog Scale (0–100 mm)
*p values < 0.05 were considered statistically significant
Fig. 3.
VAS results for pain at walking. VAS Visual Analog Scale, SE standard error, SD standard deviation
Secondary Outcomes
The overall patient satisfaction rate, assessed using a Likert scale was as follows: 35% very satisfied (17 patients), 35% satisfied (17 patients), 22% moderately satisfied (11 patients), and 8% dissatisfied (4 patients).
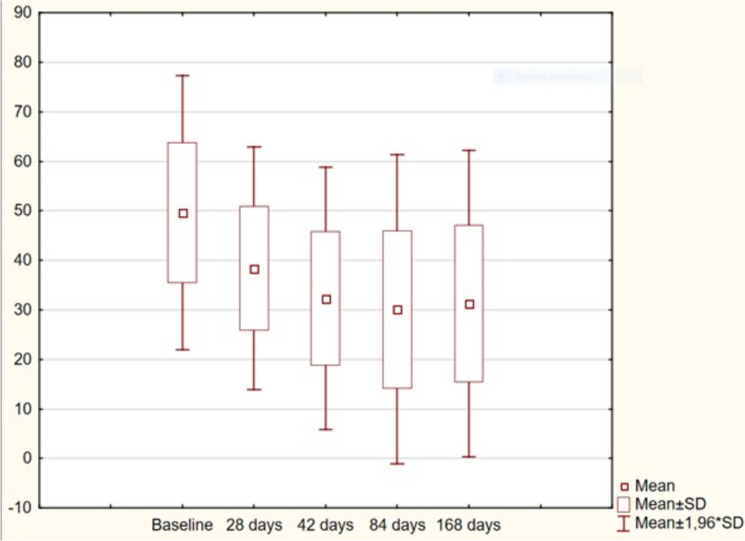
The results for WOMAC scores are presented in Table 4 and Fig. 4. The most notable improvement of the WOMAC score was observed within the first 6 weeks after injection. There was further statistically significant improvement up until 84 days (3 months) of follow-up; and up until the end of follow-up 168 days (6 months), no deterioration of the WOMAC score was noted. The results from 42, 84, and 168 days post-treatment were still better than at baseline and on day 28. The distribution of WOMAC subscales is presented in Fig. 5.
Table 4.
Results of the WOMAC score
| WOMAC | n | Mean | Median | Minimum | Maximum | SD | p value* |
|---|---|---|---|---|---|---|---|
| Baseline | 50 | 49.6 | 51 | 20 | 84 | 14.1 | |
| 28 days | 50 | 38.4 | 38 | 19 | 62 | 12.5 | 0.0001 |
| 42 days | 49 | 32.3 | 29 | 10 | 64 | 13.5 | 0.0003 |
| 84 days | 49 | 30.0 | 30 | 6 | 64 | 15.9 | 0.002 |
| 168 days | 49 | 31.2 | 29 | 7 | 65 | 15.8 | 0.27 |
n number of patients, SD standard deviation, WOMAC the Western Ontario and McMaster Universities Arthritis Index
*p values < 0.05 were considered statistically significant
Fig. 4.
Results of the WOMAC score. WOMAC Western Ontario and McMaster Universities Arthritis Index, SD standard deviation
Fig. 5.
Results of the WOMAC subscales. WOMAC Western Ontario and McMaster Universities Arthritis Index. Overall—total WOMAC, Difficulty-Physical Function subscale
In further analysis, the average improvement of WOMAC scores was compared with the type and grade of gonarthrosis (Table 5). Patients with medial gonarthrosis had the best response to hybrid HA treatment. The lowest WOMAC improvement was observed in patients with patellofemoral gonarthosis. No statistically significant difference in the WOMAC score from baseline was observed between radiological Kellgren–Lawrence grades II and III of OA (Table 5).
Table 5.
Comparison of the type and grade of gonarthrosis and average improvement of WOMAC score from baseline
| Gonarthrosis | Average improvement of WOMAC score from baseline | |||
|---|---|---|---|---|
| Day 28 | Day 42 | Day 84 | Day 168 | |
| Type | ||||
| Medial | 22* | 21 | 22 | 22.5* |
| Lateral | 20.5* | 18 | 19 | 17* |
| Patellofemoral | 10* | 14.5 | 12 | 12* |
| Grade (Kellgren–Lawrence Scale) | ||||
| II | 12 | 19 | 20 | 19 |
| III | 12 | 13 | 20 | 19 |
WOMAC Western Ontario and McMaster Universities Arthritis Index
*Results significant at p < 0.05
The female patients generally responded better to hybrid HA treatment, but a statistically significant difference was found only at 84 days of follow-up (Table 6). The presence of osteophytes was a strong negative predictive factor of a lack of WOMAC improvement at all time points of follow-up (Table 7). There was a positive correlation between increased body mass index (BMI) and average improvement from the baseline in WOMAC score within the first 28 days (Spearman’s correlation 0.3, p < 0.05). The age of the patients and duration of symptoms were not correlated with all scores measured nor with the improvement in WOMAC score.
Table 6.
Comparison of gender and average improvement of WOMAC score from baseline
| Gender | Average improvement of WOMAC score from baseline | |||
|---|---|---|---|---|
| Day 28 | Day 42 | Day 84* | Day 168 | |
| Women | 12 | 17 | 21 | 19 |
| Men | 12 | 13 | 17 | 16 |
WOMAC Western Ontario and McMaster Universities Arthritis Index
*Results significant at p < 0.05
Table 7.
Comparison of the presence of osteophytes and an average improvement of the WOMAC score from baseline
| Osteophytes | Average improvement of the WOMAC score from baseline | |||
|---|---|---|---|---|
| Day 28* | Day 42 | Day 84** | Day 168** | |
| No | 15 | 18 | 24 | 22 |
| Yes | 7 | 13 | 12 | 12 |
WOMAC Western Ontario and McMaster Universities Arthritis Index
*Results significant at p < 0.05
**Results significant at p < 0.001
Additional Treatments
During 168 days follow-up patients refrain from physiotherapy and other medical interventions.
Safety
No complications or adverse events were reported after hybrid HA injection or during the study.
Discussion
The last decade has shown a growing interest in the use of HA formulations as viscosupplementation for OA. HA occurs naturally in the synovial fluid of joints, ensuring their adequate lubrication. However, the different types of HA used in studies make it difficult to compare findings and indicate which formulation provides the most clinical benefit [7, 17, 18]. Hybrid HA has been demonstrated to be safe and effective in the treatment of knee OA owing to its increased viscoelasticity, anti-inflammatory properties, and longer half-life [19]. Low molecular weight HA has anti-inflammatory properties, while high molecular weight HA has viscosupplementary effects. The short and long chains are linked by hydrogen bonds, and the hybrid HA has been shown to be more resistant to enzymes, despite their lack of cross-linking agents [20, 21].
The results of this study are in alignment with other studies in the field [21]. A large randomized clinical trial of 692 patients demonstrated that a single injection of hybrid HA resulted in a marked decrease in the VAS pain score, which continued throughout a follow-up period of 24 weeks [22]. A review of viscosupplementation in knee OA found that the most robust response to hybrid HA injections takes place within 5–13 weeks after treatment, yet patients report a health-related QoL for up to 6 months [7].
The efficacy of HA injections in treating knee OA has been documented, primarily focusing on pain relief and functional improvement. As detailed by Lu et al. [23], HA injections not only enhance the lubricating properties of the synovial fluid but also promote the restoration of the damaged HA layer on articular cartilage surfaces, potentially alleviating arthritic symptoms. Gupta et al. highlighted significant improvements in pain and joint function among patients who received HA injections, particularly in cases where conservative treatments, such as acetaminophen, were ineffective [24]. Furthermore, a meta-analysis indicates that intra-articular HA injections lead to considerable reductions in pain scores and improvements in functional outcomes for patients with knee OA [25].
Hybrid HA formulations, which may include cross-linking agents or combined viscosupplementation techniques, have also exhibited promising results. Scaturro et al. demonstrated that a single injection of hybrid HA in overweight patients yielded significant improvements in knee pain and function, which were maintained for up to 25 weeks post-injection [26]. These findings align with those reported by Zhang et al., who noted the long-term benefits of HA injections despite variations in individual responses [27]. These results collectively support the notion that HA injections can serve as a pivotal intervention for managing knee OA when conventional therapies fail.
Safety remains a major consideration in the application of intra-articular injections. An important factor in the use of hybrid HA is its tolerability and favorable safety profile. Within the reviewed literature, HA injections have generally been reported to have a favorable safety profile. A consensus statement reiterated that the incidence of adverse effects is low, as HA is biocompatible [28]. As was the case in this study, there were no adverse events reported, with side effects limited to temporary pain and swelling at the injection site [7, 29]. HA may also be safer for patients with comorbidities, such as obesity and diabetes, as it does not have the same side effects as other medications used in viscosupplementation, such as corticosteroids [16]. However, some studies have reported rare complications, such as joint infections resulting from improper injection techniques [30]. Most adverse reactions were transient and minor, consisting of localized pain or swelling, underscoring the importance of utilizing ultrasound-guided injections for improved accuracy and reduced complications [31]. Studies suggest that using ultrasound guidance may enhance the therapeutic benefits while minimizing procedural risks [32].
The ultrasound-guided administration of hybrid HA may be viewed as a best practice technique, particularly in overweight patients [16, 24].
In our study only the lateral access to the knee joint was utilized with good outcome. The recent study by Farì et al. [33] compared medial and lateral HA injection approaches and no statistically significant difference was found between medial and lateral injections in terms of efficacy in pain relief and functional improvement. As safety profiles were similar for both techniques they conclude that the choice between medial or lateral injection may depend on clinician preference or patient anatomy, as neither demonstrated superiority.
Furthermore, the evaluation of different hybrid HA formulations is crucial. Hylan G-F 20, a cross-linked HA, has been shown to effectively manage OA symptoms in patients who had not responded adequately to other therapies [34]. Such preparations can enhance the duration of relief compared to standard HA. While hybrid formulations have exhibited prolonged effects, there remains some debate on their efficacy relative to other treatments [25].
Moreover, hybrid HA injections have been compared against alternative treatments. Studies show that, while HA is beneficial, emerging therapies like platelet-rich plasma (PRP) also demonstrate substantial efficacy in knee OA management, prompting a comparison of joint-specific injections [35]. Notably, Raeissadat et al. conducted a study that emphasized the cost-utility of various intra-articular options, confirming that HA remains a valuable option amidst evolving treatment paradigms [36].
Although this was a small study, our findings indicate that some patient subpopulations experienced increased clinical benefit compared to others: patients with medial and lateral types of OA had greater improvement than those with patellofemoral OA, assessed using WOMAC scores. Patients with osteophytes (bone spurs) also showed less improvement compared to patients without osteophytes. In a recent review, Chevalier and Sheehan concluded that viscosupplementation with intra-articular HA injections may benefit certain subpopulations more than others, e.g., younger patients with less severe disease and normal BMI [37]. This real-world study demonstrated the overall improvement of OA symptoms for all types of patients, yet incorporating various subpopulations in the design of future studies may provide additional information on the efficacy of hybrid HA viscosupplementation.
Recapitulating these findings, we observed a narrative supporting the efficacy and relative safety of hybrid HA injections in knee OA treatment. Multiple clinical trials consistently underscore the ability of HA to improve joint function and reduce pain, thereby enhancing life quality for individuals suffering from this degenerative condition. HA shows promising senomorphic potential in preclinical studies, but further clinical research is needed to confirm its mechanisms in humans [38].
If proven, HA could become a dual-purpose therapy—not just for symptom relief but also for modifying OA disease progression. As the medical community continues to explore and refine treatment approaches, hybrid HA will likely remain a cornerstone treatment modality for knee OA, particularly as our understanding of its bioactivity and structural properties evolves.
Limitations of the Study
This study was observational, open-label, and uncontrolled, and therefore had inherent limitations in terms of susceptibility to bias, confounding and restricting the ability to define causality. However, this study was designed to evaluate the daily clinical practice regarding heterogeneous patient populations and the medical interventions they received. Data generated in a real-life setting is essential to assess and improve clinical practice worldwide and to complement controlled trials.
Conclusions
Intra-articular hybrid HA injections for symptomatic knee OA present an effective, generally safe option for management—especially in patients who have failed conservative treatments. The ultrasound-guided viscosupplementation technique was found to be safe and well tolerated by patients. The strongest effect of treatment was found in patients with medial gonarthrosis with no osteophytes present. Future research should continue to explore hybrid formulations and injection techniques to enhance the safety and efficacy of this intervention.
Acknowledgements
The authors wish to thank patient participants for their involvement in the study.
Medical Writing/Editorial Assistance
Editorial assistance was provided by Proper Medical Writing, Warsaw, Poland. Medical writing assistance was founded by IBSA International SA. No AI assistance was used to prepare the manuscript.
Author Contributions
Marcin E Domzalski: study conception and design, study preparation, data analysis, manuscript preparation. Klaudia Marchewa: study preparation, data collection, manuscript preparation.
Funding
This research was sponsored equally by SPORTO Clinic and IBSA International SA. The journal’s Rapid Service Fee was funded by the authors.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Conflict of Interest
Marcin E. Domzalski and Klaudia Marchewa declare that they have no competing interests.
Ethical Approval
The study was performed in accordance with the ethical guidelines of the Declaration of Helsinki and was approved by the Local Bioethics Committee in Lodz, Poland. Collected information and data were managed according to the guidelines of Good Clinical Practice (GCP). All participants signed an informed consent form at the time of enrollment to collect clinical data. All patients also gave their consent to the use of patient reported outcomes (PROs). The study was registered on ClinicalTrials.gov with NCT06652893.
References
- 1.Geng R, Li J, Yu C, et al. Knee osteoarthritis: current status and research progress in treatment (Review). Exp Ther Med. 2023;26:481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Katz JN, Arant KR, Loeser RF. Diagnosis and treatment of hip and knee osteoarthritis: a review. JAMA. 2021;325:568–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Long H, Liu Q, Yin H, et al. Prevalence trends of site-specific osteoarthritis from 1990 to 2019: findings from the global burden of disease study 2019. A&R. 2022;74(7):1172–83. 10.1002/art.42089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kucharz EJ, Szántó S, Ivanova Goycheva M, et al. Endorsement by Central European experts of the revised ESCEO algorithm for the management of knee osteoarthritis. Rheumatol Int. 2019;39:1117–23. [DOI] [PubMed] [Google Scholar]
- 5.Balazs EA, Denlinger JL. Viscosupplementation: a new concept in the treatment of osteoarthritis. J Rheumatol Suppl. 1993;39:3–9. [PubMed] [Google Scholar]
- 6.Peck J, Slovek A, Miro P, et al. A comprehensive review of viscosupplementation in OA of the knee. Orthop Rev (Pavia). 2021;13(2):25549. 10.52965/001c.25549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Abate M, Pelotti P, De Amicis D, Di Iorio A, Galletti S, Salini V. Viscosupplementation with hyaluronic acid in hip osteoarthritis (a review). Ups J Med Sci. 2008;113:261–77. [DOI] [PubMed] [Google Scholar]
- 8.Abate M, Salini V. Efficacy and safety study on a new compound associating low and high molecular weight hyaluronic acid in the treatment of hip osteoarthritis. Int J Immunopathol Pharmacol. 2017;30:89–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stellavato A, De Novellis F, Reale S, De Rosa M, Schiraldi C. Hybrid complexes of high and low molecular weight: evaluation using an in vitro model of osteoarthritis. J Biol Regul Homeost Agents. 2016;30:7–16. [PubMed] [Google Scholar]
- 10.Stellavato A, Corsuto L, D’Agostino A, et al. Hyaluronan hybrid cooperative complexes as a novel frontier for cellular bioprocesses re-activation. PLoS ONE. 2016;11:e0163510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kohn MD, Sassoon AA, Fernando ND. Classifications in brief: Kellgren–Lawrence classification of osteoarthritis. Clin Orthop Relat Res. 2016;474:1886–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Åström M, Thet Lwin ZM, Teni FS, Burström K, Berg J. Use of the visual analogue scale for health state valuation: a scoping review. Qual Life Res. 2023;32:2719–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gandek B. Measurement properties of the Western Ontario and McMaster Universities Osteoarthritis Index: a systematic review. Arthritis Care Res (Hoboken). 2015;67:216–29. [DOI] [PubMed] [Google Scholar]
- 14.Joshi A, Kale S, Chandel S, Pal DK. Likert scale: explored and explained. Br J Appl Sci Technol. 2015;7:396–403. [Google Scholar]
- 15.Concoff A, Rosen J, Fu F, et al. A comparison of treatment effects for nonsurgical therapies and the minimum clinically important difference in knee osteoarthritis: a systematic review. JBJS Rev. 2019;7:e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bert J, Kenney J, Sgaglione NA, et al. Viscosupplementation for osteoarthritis of the knee: a key opinion leader panel discussion. J Manag Care Spec Pharm. 2018;24:S2–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huang TL, Tsai CH. Safety and efficacy of single CHAP Hyaluronan injection versus three injections of linear Hyaluronan in pain relief for knee osteoarthritis: a prospective, 52-week follow-up, randomized, evaluator-blinded study. BMC Musculoskelet Disord. 2021;22:572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.D’Agostino A, Stellavato A, Busico T, et al. In vitro analysis of the effects on wound healing of high- and low-molecular weight chains of hyaluronan and their hybrid H-HA/L-HA complexes. BMC Cell Biol. 2015;16:19. 10.1186/s12860-015-0064-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Szczęsny G, Tomaszewski W, Domżalski M. Evolution of the hyaluronic acid in viscosupplementation—from linear particles to hybrid complexes. Ortop Traumatol Rehabil. 2021;23(3):229–38. 10.5604/01.3001.0014.9625. [DOI] [PubMed] [Google Scholar]
- 20.Papalia R, Russo F, Torre G, et al. Hybrid hyaluronic acid versus high molecular weight hyaluronic acid for the treatment of osteoarthritis in obese patients. J Biol Regul Homeost Agents. 2017;31(4 Suppl 2):103–9. [PubMed] [Google Scholar]
- 21.Domżalski M, Migliore A. A review of the clinical effectiveness and safety of hybrid cooperative complexes in intra-articular viscosupplementation. Rheumatol Ther. 2022;9:957–74. 10.1007/s40744-022-00450-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Migliore A, Blicharski T, Plebanski R, et al. Knee osteoarthritis pain management with an innovative high and low molecular weight hyaluronic acid formulation (HA-HL): a randomized clinical trial. Rheumatol Ther. 2021;8(4):1617–36. 10.1007/s40744-021-00363-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lu L, Dai C, Zhang Z, et al. Treatment of knee osteoarthritis with intra-articular injection of autologous adipose-derived mesenchymal progenitor cells: a prospective, randomized, double-blind, active-controlled, phase IIb clinical trial. Stem Cell Res Ther. 2019. 10.1186/s13287-019-1248-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gupta A, Maffulli N, Rodriguez H, et al. Safety and efficacy of umbilical cord-derived Wharton’s jelly compared to hyaluronic acid and saline for knee osteoarthritis: study protocol for a randomized, controlled, single-blind, multi-center trial. J Orthop Surg Res. 2021. 10.1186/s13018-021-02475-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Montañez-Heredia E, Irízar S, Huertas P, et al. Intra-articular injections of platelet-rich plasma versus hyaluronic acid in the treatment of osteoarthritic knee pain: a randomized clinical trial in the context of the spanish national health care system. Int J Mol Sci. 2016;17(7):1064. 10.3390/ijms17071064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Scaturro D, Vitagliani F, Terrana P, et al. Intra-articular hybrid hyaluronic acid injectiontreatment in overwight patients with knee hip osteoarthritis. Appl Sci. 2021;11(18):8711. 10.3390/app11188711. [Google Scholar]
- 27.Zhang H, Wang C, Li H, Huang Y, Li Z. Intra-articular platelet-rich plasma versus hyaluronic acid in the treatment of knee osteoarthritis: a meta-analysis. Drug Des Dev Ther. 2018;12:445–53. 10.2147/dddt.s156724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Campos G, Sousa E, Hamdan P, et al. Brazilian consensus statement on viscosupplementation of the knee (COBRAVI). Acta Ortopédica Brasil. 2019;27(4):230–6. 10.1590/1413-785220192704218616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Abate M, Salini V. Safety and tolerability of intra-articular hyaluronic acid (Sinovial®/GELSYN-3tm) injections in the treatment of knee osteoarthritis. J Biol Homeost Agents. 2017;31(4):1139–45. [PubMed] [Google Scholar]
- 30.Kunugiza Y, Tani M, Tomita T, Yoshikawa H. Staphylococcal scalded skin syndrome after intra-articular injection of hyaluronic acid. Mod Rheumatol. 2010;21(3):316–9. 10.1007/s10165-010-0391-6. [DOI] [PubMed] [Google Scholar]
- 31.Park Y, Lee S, Nam H, Lee J, Nam S. Comparison of sonographically guided intra-articular injections at 3 different sites of the knee. J Ultrasound Med. 2011;30(12):1669–76. 10.7863/jum.2011.30.12.1669. [DOI] [PubMed] [Google Scholar]
- 32.Cohernchujit B, Tharakulphan S, Apivatgaroon A, Prasetia R. Accuracy comparisons of intra-articular knee injection between the new modified anterolateral approach and superolateral approach in patients with symptomatic knee osteoarthritis without effusion. Asia-Pac J Sports Med Arthrosc Rehabil Technol. 2019;17:1–4. 10.1016/j.asmart.2019.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Farì G, Mancini R, Dell’Anna L, et al. Medial or lateral, that is the question: a retrospective study to compare two injection techniques in the treatment of knee osteoarthritis pain with hyaluronic acid. J Clin Med. 2024;13(4):1141. 10.3390/jcm13041141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pal S, Thuppal S, Reddy K, et al. Long-term (1-year) safety and efficacy of a single 6-ml injection of hylan G-F 20 in indian patients with symptomatic knee osteoarthritis. Open Rheumatol J. 2014;8(1):54–68. 10.2174/1874312901408010054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vílchez-Cavazos F, Millán-Alanís J, Blázquez-Saldaña J, et al. Comparison of the clinical effectiveness of single versus multiple injections of platelet-rich plasma in the treatment of knee osteoarthritis: a systematic review and meta-analysis. Orthop J Sports Med. 2019. 10.1177/2325967119887116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Raeissadat S, Rahimi M, Rayegani S, Moradi N. Cost-utility analysis and net monetary benefit of platelet rich plasma (PRP), intra-articular injections in compared to plasma rich in growth factors (PRGF), hyaluronic acid (HA) and ozone in knee osteoarthritis in Iran. BMC Musculoskelet Disord. 2023. 10.1186/s12891-022-06114-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chevalier X, Sheehan B. Predictors of clinical benefit with intra-articular hyaluronic acid in patients with knee osteoarthritis—a narrative review. Curr Rheumatol Rev. 2024;20(4):379–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bernetti A, Agostini F, Paoloni M, et al. Could hyaluronic acid be considered as a senomorphic agent in knee osteoarthritis? A systematic review. Biomedicines. 2023;11(10):2858. 10.3390/biomedicines11102858. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.