Abstract
Social determinants of health, including neighborhood and built environment factors, play a crucial but underexplored role in epilepsy incidences. Among these, air pollution emerges as a potentially-preventable driver of epilepsy and adverse health outcomes. Evidence is accumulating on the effects of air pollution on the brain, especially in stroke and neurodegenerative disorders; however, the specific impact on epilepsy remains underresearched, potentially due to the complexities of studying this condition. The present narrative review addresses a critical knowledge gap by exploring: 1) the role of air pollution in epilepsy epidemiology; 2) the biological mechanisms of air pollution in the brain in the context of epilepsy; and 3) how air pollution affects the management of people living with epilepsy. We outline vital questions and actionable interventions regarding the role of air pollution in epilepsy.
Keywords: Epilepsy, Air Pollution, Particulate Matter, Social Determinants of Health, Epidemiology
INTRODUCTION
Air pollution is a potentially-modifiable environmental risk factor. It is a potentially-preventable driver of epilepsy and a mediator of unequal health outcomes. Despite growing evidence linking air pollution to adverse brain outcomes, its specific roles in epilepsy remain poorly understood. This knowledge gap persists due to several roadblocks: the inherent complexity of epilepsy as a heterogeneous condition for diagnosis, the episodic nature of seizures, and limited interdisciplinary collaboration between environmental health and neurological research. Compounding this issue, the broader landscape of epilepsy is evolving. For instance, the incidence of conditions such as hippocampal sclerosis, historically associated with refractory focal epilepsy, has declined over recent decades, 1 2 potentially reflecting changes in environmental exposures or healthcare practices. At the same time, rising socioeconomic inequality 3 and climate change threaten to exacerbate epilepsy outcomes 4 and the neurological impacts of air pollution.
Compared to other brain disorders, such as stroke or neurodegenerative diseases, the role of air pollutants in epilepsy has received much less attention. Nevertheless, emerging evidence suggests that air pollution significantly influences known risk factors for epilepsy, including inflammation, oxidative stress, and neurodevelopmental disruption, as well as outcomes in people living with epilepsy. Unlike other conditions, a predisposition to abnormal brain excitability is a core characteristic of epilepsy; yet, the specific mechanisms through which pollutants may exacerbate this vulnerability remain poorly studied. For instance, pollutants carried by particulate matter (PM), including heavy metals, persistent organic pollutants, and airborne microplastics (MPs), may interact with neurochemical pathways to heighten seizure susceptibility or interfere with antiseizure medications. These aspects, though critical, are underexamined ( Table 1 ).
Table 1. What is air pollution?
| Air pollution is a heterogeneous mixture of gaseous components and particulate matter (PM). A distinction should be made between ambient and indoor air pollution because the sources and constituents significantly differ. The primary gaseous air pollutants are nitrogen oxides (NOX), sulfur dioxide (SO2), carbon monoxide (CO), ozone (O3), carbonyl compounds, and organic solvents. Particulate matter is made up of solid and liquid particles and categorized by size: coarse particles (PM10, diameter < 10 μm), fine particles (PM2.5, diameter < 2.5 μm), and ultrafine particles (PM0.1, diameter < 0.1 μm). Each PM category includes components from smaller-sized PM categories. The composition and sources of PM are diverse. Anthropogenic sources include biomass solid-fuel burning, fossil fuel combustion, vehicle emissions, industrial processes and agricultural activities. Natural sources include wildfires, dust storms, volcanoes, ocean splashes and biological aerosols. 80% of ambient PM comprises organic carbon (OC), elemental carbon (EC), salt, mineral dust, nitrite (NO3-), sulphate (SO42-), and ammonium (NH4 + ). Where people live and how they spend their time governs their exposure to different compositions and concentrations of air pollutants. Additionally, airborne microplastics are recognized as significant pollutants. Microplastics (MPs) are plastic particles smaller than 5 mm, derived from the degradation of larger plastics, primarily through solar ultraviolet (UV) radiation. Microplastics include polyethylene (PE), polymethyl methacrylate (PMMA), and polyethylene terephthalate (PET). Particulate matter and microplastics also act as vectors for other chemical compounds, particularly heavy metals and persistent organic pollutants (POPs). Common POPs include polychlorinated biphenyls (PCBs), Di(2-ethylhexyl)phthalate (DEHP), polybrominated diphenyl ethers (PBDEs), perfluoroalkyl substances (PFAS), bisphenol A (BPA), polychlorinated dibenzo-p-dioxins (PCDDs), and p-benzoquinone (PBQ). |
Epilepsy is often a symptom of underlying determinants of health, adding to the complexity. The roles of social determinants of health (SDHs) in the epidemiology, biology and management of epilepsy are poorly understood relative to genetic or molecular underpinnings. Thus, understanding how air pollution influences the epidemiology, biology, and management of epilepsy is critical for developing effective interventions and addressing growing disparities. In the present study, we attempt to synthesize the seemingly-disparate streams of evidence into a coherent framework. We outline actionable questions to address the role of air pollution in epilepsy.
EPIDEMIOLOGY
Air pollution increases the risk of significant structural insults that predispose to epilepsy ( Figure 1 ). Short- and long-term exposure to ambient air pollutants (nitrogen dioxide [NO2], sulfur dioxide [SO2], ozone [O3], carbon oxide [CO], PM2.5, and PM10) increases the incidence of stroke. 5 The risk of brain tumors increases, dose-dependently, with exposure to specific pollutants. 6 Common sources of brain metastases—lung, breast, colon, thyroid and skin cancers—are increasingly linked to air pollutant exposure. This also applies to dementia, as one recent meta-analysis 7 shows the dose-related risk of dementia with PM2.5. The association with head trauma is not well assessed, with only one study 8 from Taiwan reporting that short-term exposure to PM2.5 and nitrogen oxides (NOx) is associated with an increased risk of traumatic intracranial hemorrhage. The mechanisms here are likely complex, with contribution of transient neuropsychological impairment that can increase the risk of head trauma.
Figure 1.
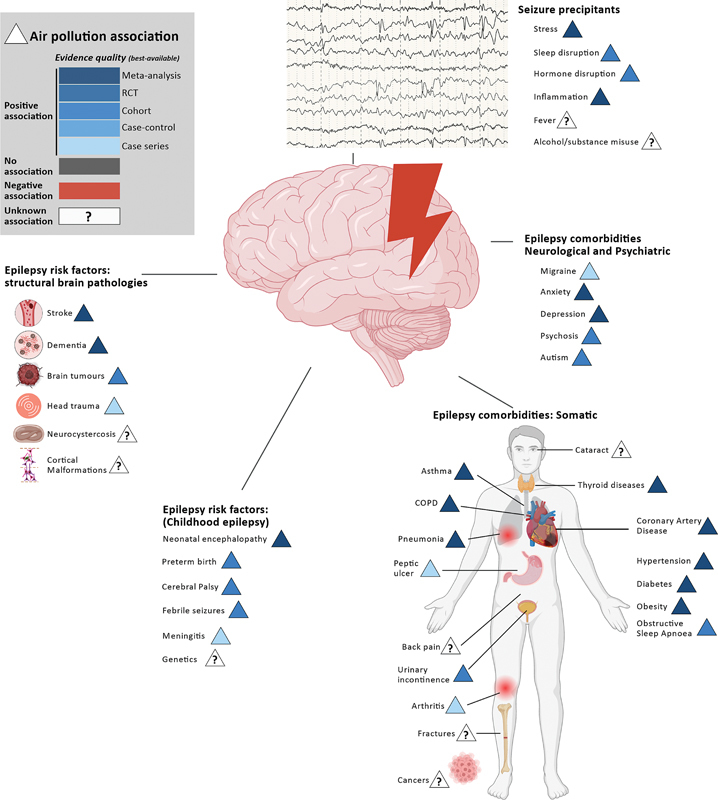
Notes: The circle indicates poverty; the triangle indicates air pollution; and different shades of blue indicate the quality of the best available evidence for positive associations. Epilepsy risk factors, comorbidities, and seizure precipitants associated with exposure to air pollutants.
The risk factors for childhood epilepsy are increasingly associated with exposure to air pollutants ( Figure 1 ). Long-term exposure to PM2.5 increases the risk of neonatal encephalopathy. 9 Exposure to PM2.5 and PM10 in the 1 st trimester, and to PM2.5, PM10, and O3 in the 3 rd trimester, increases the risk of preterm birth. 10 11 In a Canadian cohort study, 12 the average prenatal PM2.5 exposure increased the risk of cerebral palsy among full-term births A Danish cohort study 13 showed exposure to NO2 increases the risk of febrile seizures. Exposure to diesel exhaust particles increases susceptibility to invasive pneumococcal disease, a common cause of meningitis. 14 No study has explored the potential associations between air pollutants and cortical malformation, despite accumulating evidence of harmful effects on normal cortical development.
Hardly any investigations have explored air pollution exposure directly with epilepsy incidences and relative risks. One study 15 examined the association between the incidence and prevalence of epilepsy using Global Burden of Disease data with broad environmental indices of the surveyed countries. This study 15 suggested that the Air Quality Index was negatively associated with epilepsy prevalence but not incidence after adjusting for socioeconomic confounders. Interestingly, the association with the Pollution Emissions Index, which is typically measured at the source, was normal in this study. 15 This highlights the importance of investigating other environmental factors (such as weather conditions) that can concentrate pollution in certain areas. A single-center, case-control study 16 conducted in the Netherlands reported no association between new epilepsy diagnosis and the annual average O2, O3, PM2.5, and PMN10 exposure for the year of presentation to the clinic. Notably, the median levels of pollutants for cases and controls were low (such as PM2.5 of 14.2 μg/m 3 ).
Poverty could confound the relationship between air pollution and epilepsy by being independently associated with both variables. Studies 17 globally show that poorer neighborhoods are more likely to be exposed to higher pollutant levels. In low- and middle-income countries (LMICs), increasing air pollution parallels rising urbanization. Indoor air pollution is a significant concern, 18 particularly in low-income households that rely on biomass fuels such as wood, charcoal, and dry cow dung for cooking and heating. Living in poverty is also associated with exposure to harmful chemicals from various sources: pesticides used in agriculture, industrial pollutants, and chemicals from poorly-regulated waste disposal. Ever ubiquitous microplastics are ever more present in poorer regions. Prevalent and unique to LMICs are slums with extremely high levels of average PM2.5 that can go up to 110ug/m 3 (such as in Korogocho, Nairobi, Kenya). 19 Ventilation is often inadequate, and burning biomass solid fuel is nearly universal. Unable to move up the fuel ladder, households resort to unorthodox fuels, such as plastic, or employ ineffective interim solutions, such as forced-draft stoves.
PATHOPHYSIOLOGY
Animal models of air pollutant-related seizures
In a kainic acid (KA)-induced mice epilepsy model, 20 PM2.5 exposure (6 mg/kg, once every 3 days for 31 days) increased seizure frequency, duration and severity. Exposure to carbon black and zinc oxide nanoparticles increased seizure susceptibility and severity, as well as spontaneous recurrent seizures, in a KA- and pentylenetetrazole (PTZ)-induced mice model of epilepsy. 21 Acute (1 hour) O3 exposure reduced the latency to the first seizure and increased the spread of seizure in a rat amygdaloid kindling model. 22 However, 15-day (once-a-day) O3 exposure increased the latency to the first seizure in a PTZ-induced mouse model. 23 In a febrile seizure rat model, acute exposure to low concentrations of SO2 alleviated neuronal damage, while high concentration aggravated neuronal damage. 24 These seemingly contradictory results require further investigation. One possibility is that the brain can adapt to chronic or low dose exposure but is sensitive to acute fluctuations in or high levels of pollution.
Additionally, some persistent organic pollutants (POPs) and heavy metals can be proconvulsive. Bisphenol A (BPA) at doses above 250 ug/kg showed proconvulsant activity in penicillin-induced seizure models in male rats. 25 Prenatal exposure to polychlorinated biphenyl (PCB) increases the susceptibility to audiogenic seizures in adult rats. 25 Perfluoroalkyl substances (PFASs) provoke a seizurogenic effect in developing zebrafish larvae. 26 Prolonged cadmium exposure exacerbated seizure severity in a KA-induced seizure mouse model. 27 Exposure to PM2.5 increased hippocampal lead, aluminum, and manganese content in rats. 28 Prolonged exposure to lead resulted in dose-dependent increases in PTZ-induced seizure thresholds in rats and mice. 29 Arsenic decreased the latency to seizure onset and the time of death in a PTZ-induced seizure mouse model. 30 Methylmercury (MeHg) poisoning during development increased seizure susceptibility in various animal models. 31 Intracranial exposure to nickel and cobalt induced focal seizures in rodents, 32 and in, monkeys, cadmium, copper, nickel, antimony, mercury, and cobalt. 33
Mechanisms that predispose to hyperexcitability
Changes in neuronal excitability and excitatory-inhibitory neurotransmission balance contribute to seizure generation ( Figure 2 ). Air pollutants, especially CO, in people with epilepsy increased subclinical seizures on electroencephalograms (EEGs). 34 Human volunteers exposed to diesel exhaust for 30 minutes showed increased median power frequency in the frontal cortex. 35 Limited evidence 36 shows the potential of specific pollutants (such as SO2, some PCBs, polybrominated diphenyl ether [PBDE], and di(2-ethylhexyl)phthalate [DEHP]) to increase neuronal excitability ( Table 2 ). The effects are pollutant-specific, taking the different impacts of PCB19, PCB126, and PCB77 on human voltage-gated potassium channel 1.3 (Kv1.3). They are also concentration-specific (for example, MEHP inhibits calcium channels at lower doses and sodium and potassium channels at higher doses 37 ).
Figure 2.

Air pollutants may predispose to seizures and epilepsy by disrupting normal neuronal function, network excitability, and cortical development. Air pollutants can affect the brain at all developmental stages. (Top Right) Acting directly in the brain, they can cause direct and indirect damage through oxidative stress, inflammation, mitochondrial dysfunction, altered myelination and alterations in normal cell signaling. Air pollutants indirectly affect the brain through systemic inflammation driven by chronic lung inflammation and damage to vital organs essential for healthy brain function. (Middle Right) Network excitability may be increased through multiple complex pathways that result in net excitatory-inhibitory imbalance, such as through modulation of glutamatergic, GABAergic transmission, ion channel, and neuronal excitability. Pollutants also indirectly modulate seizure threshold through autonomic disturbance, inflammation, sleep disturbance, and endocrine disruption. (Bottom) In the developing brain, disruption of normal neuronal migration pathways, increases in somatic mutation rates, maternal immune activation, placental impairment, and maternal thyroid dyshomeostasis are potential mechanisms through which air pollutants can cause permanent structural changes (that is, cortical malformations, MCDs).
Table 2. Specific pollutant effects on neuronal excitability.
| Pollutant | Model system | Effect | Reference (see Supplementary Material*) |
|---|---|---|---|
| Sulfur dioxide (SO2) | Rat, hippocampal neuron |
Shifts stead stage inactivation curve of transient outward potassium currents. Increases neuronal excitability. |
1 |
| Rat, dorsal root ganglia | Inhibits voltage-gated sodium channel. Increases neuronal excitability. |
2 | |
|
Polycyclic aromatic hydrocarbon (PAH):
benzo[a]pyrene |
Mouse, hippocampal neuron | Represses synaptic vesicle exocytosis. Inhibits P/Q-type calcium channel. |
3 |
| Polychlorinated biphenyl (PCB) | Xenopus laevis oocyte | PCB19 inhibited human voltage-gated potassium channel 1.3 (Kv1.3) currents. PCB126 right-shifted steady-state activation curve of human Kv1.3. PCB77 enhances human Kv1.3 currents. |
4–6 |
| Polybrominated diphenyl ether | Rat, hippocampal neuron | PBDE 209 dose-dependent inhibition of voltage gated sodium channel. Decreases neuronal excitability. |
7 |
| Bisphenol A (BPA) | Mouse, dorsal root ganglia | BPA inhibits voltage-gated sodium channel. | 8 |
| Di(2-ethylhexyl) phthalate (DEHP) | Rat, hippocampal neuron | DEHP at 0.1 M and 0.3 M inhibit voltage-gated potassium channel of CA1 neurons. Mono-2-ethylhexyl phthalate (MEHP), the main active metabolite of DEHP, inhibits calcium channel at low dose, and sodium and potassium channels at higher dose of CA3 neurons. |
9,10 |
Note: Supplementary Material containing further references available at: https://www.arquivosdeneuropsiquiatria.org/wp-content/uploads/2025/03/ANP-2024.0360-Supplementary-Material.docx .
The effects on excitatory glutamatergic and inhibitory gamma-aminobutyric acid (GABA)-ergic neurotransmitters are complex ( Table 3 ). The outcomes depend on the specific pollutant, exposure duration and protocol, model system tested, region of the brain, and sex of the animal. The source of pollutant matters: 24-hour exposure to PM2.5 collected from Los Angeles, 38 United States, increased the levels of N-methyl-D-aspartate receptors (NMDARs), whereas, from Shanxi, China, decreased NMDAR levels. 39 The duration of SO2 exposure above Environmental Protection Agency (EPA) standard levels also increases glutamatergic transmission after 1 week 40 but decreases it after 90 days. 41 Sex differences are essential: 6 days of exposure to PM2.5 increased hippocampal glutamate in males but decreased hippocampal GABA in females, for example. 42 Differential effects on different brain areas are not well assessed but exist:.10 days of exposure to polycyclic aromatic hydrocarbon (PAH) benzo(a)pyrene increased NMDA-R by 17 times in the hippocampus whilst decreasing by 4 to 35 times in frontal cortices. 42
Table 3. Specific pollutant effect on excitatory and inhibitory neurotransmission.
| Pollutant | Model system | Effect | Reference (see Supplementary Material *) |
|---|---|---|---|
| Particulate matter (PM)2.5 | Mouse, hippocampal neurons | PM2.5 exposure (Shanxi, China) increases glutamatergic transmission via ROS-NF-kB. | 11 |
| Macrophage and microglia cell cultures (human-derived) | PM2.5 exposure (Beijing, China) increases glutamatergic transmission via microglia-derived glutamate production. | 12 | |
| Rat, hippocampal neurons |
24–48 hours of exposure to PM2.5 (Los Angeles, United States) increased N-methyl-D-aspartate receptor (NMDA-R) levels | 13 | |
| Rat, tracheal perfusion | 12-week (1/week) exposure to PM2.5 (Shanxi, China) increases mGluR1 expression in male rats. | 14 | |
| Rat, Hippocampal slice |
2 hours of exposure of PM2.5 (Los Angeles, United States) reduced synaptic function via nitrosylation of NMDA-R in CA1 but not dentate gyrus. | 15 | |
| Mouse, primary cortical neuron culture | 24-hour exposure to PM2.5 (from Shanxi, China) decreased NMDA-R NR2B, PSD95 expression. PM2.5 collected in winter showed the strongest effect. | 16 | |
| Mouse, hippocampus | 6-day (4 hours/day) exposure to PM2.5 (Boston, United States) to early postnatal (PND4-7,10-13) increased glutamate in the hippocampus of male subjects and caused loss of GABAergic neurons in the hippocampus of female subjects. | 17 | |
| Diesel exhaust particle | Mouse, inhalation | 8-week (5 hours/day, 5 days/week) exposure increases VGLUT1 levels in the prefrontal and temporal cortex. 2-month exposure (5 hours/day, 5 days/week) increases VGAT after 8 weeks in the prefrontal cortex and olfactory bulb, but not in the temporal cortex. |
18 |
| Mouse, prenatal exposure | Prenatal 5-day exposure reduces NMDA-R expression in the hippocampus of male offspring. | 19 | |
| Cerebral organoid | Decreased relative GABA expression levels. | 20 | |
| Mouse, inhalation | 2-month exposure (every 2 days) reduced GABA in the frontal cortex and hippocampus in a dose-dependent manner. | 21 | |
| Gasoline, vaporous | Mouse | Acute exposure decreases GABA. Chronic exposure does not increase GABA. |
22 |
| Sulfur dioxide (SO2) | Rat, inhalation | 1-week exposure to SO2 above Environmental Protection Agency (EPA) standards increases glutamate release, NDMA-R and PSD95 expression levels. | 23 |
| Rat, inhalation | 90-day exposure to SO2 above EPA standards decreases GluR1, GluR2, NR1, NR2A and NR2B expression in a dose-dependent manner. | 24 | |
|
Polycyclic aromatic hydrocarbon (PAH):
benzo[a]pyrene |
Rat | 7-week exposure (daily) decreases glutamate levels, reduces GluR1 and GluR2 | 25 |
| Mice, intraperitoneal | 10-day (1/day) exposure – NMDA-R1 expression increased by 17-fold in the hippocampus and decreased by 4-35-fold in the frontal cortex. | 26 | |
| Mice | 3-day exposure increases NMDA-R. | 27 | |
| Mice, prenatal exposure | Prenatal exposure reduces NMDA-R NR2B subunit expression. Prenatal 3-day exposure downregulates AMPA-R expression. |
28 29 |
|
| Mice, inhalation | Prenatal 3-day (4 hours/day, E14-E17) exposure increases prefrontal glutamate concentration. | 30 | |
| Rat, hippocampus | Increases gamma-aminobutyric acid (GABA) receptor expression. | 31 | |
|
PAH
1-nitropyrene |
Mouse, prenatal exposure |
Reduced inhibitory synaptic transmission in the medial prefrontal cortex. | 32 |
| Perfluoroalkyl substances (PFAS) | Mouse, hippocampus | 3-month (1/day) exposure Increased glutamate levels. | 33 |
| Chicken embryo cerebellar granule neuron | PFOS exposure increases glutamate transmission. | 34 | |
| hiPSC | PFOS and PFOA inhibit human GABA-A receptor. | 35 | |
| Polybrominated diphenyl ether (PBDE) | Mice | PBDE209 activates NMDA-R. PBDE47 prenatal exposure reduces AMPA-R. |
36 |
| Xenopus laevis oocyte | 6-OH-BDE-47 potentiates human GABA-A receptor. | 37 | |
| Di(2-ethylhexyl) phthalate (DEHP) | Mouse, neuroblastoma cell line | Decreases synapsin1 and PSD95. | 38 |
| Inhibits GABA-A currents. | 39 | ||
| Polychlorinated biphenyl (PCB) | Rat, X. Laevis oocyte | PCB138 and PCB180 prenatal exposure reduces glutamatergic transmission via NMDA-R. PCB52 increased GABA transmission. |
40,41 |
| X. Laevis oocyte | PCB19, PCB28, PCB47, PCB51, PCB52, PCB95, and PCB100 potentiate human GABA-A receptors. PCB95 increases excitatory transmission. |
37 | |
| 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) | Rat | TCDD prenatal exposure reduces glutamatergic transmission via NMDA-R. | 42 |
| Toluene | Rat, hippocampal neurons | Acute toluene exposure increases the amplitude of NMDA-R currents and decreases GABA-R currents. | 43 |
| Heavy metals | Various | Manganese (Mn), lead (Pb), and mercury (Hg) can inhibit astrocyte glutamate transporter, increasing glutamate in the synaptic cleft. | 44 |
Note: Supplementary Material containing further references available at: https://www.arquivosdeneuropsiquiatria.org/wp-content/uploads/2025/03/ANP-2024.0360-Supplementary-Material.docx .
The predisposition to seizures arises through mechanisms which increase the positive excitatory feedback loop. This can occur through the loss of inhibitory interneurons. Prenatal exposure to diesel, carbon black nanoparticles, MeHg, and 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) disrupts the normal development of fast-spiking parvalbumin (PV) interneurons. 43 Chronic inhalation of diesel particles increased the expression of perineuronal nets (found around PV interneurons) in adult mice's temporal cortex and transiently decreased PV interneuron numbers. 44 Another important mechanism is axon sprouting, which creates de novo recurrent excitatory circuits. Dose-related SO2 exposure resulted in mossy fiber sprouting in a rat febrile seizure model. 24 Other non-specific mechanisms associated with epileptogenesis and linked to air pollution include neuroinflammation, metal dyshomeostasis, disruption of normal blood-brain barrier, reactive astrocyte activation, and alterations in the gut microbiome. 45
Impact on cortical development
Air pollutants disrupt normal cortical development, potentially causing cortical malformations such as heterotopias and focal cortical dysplasia ( Figure 2 ). Ultrafine particles, MPs associated with heavy metals, and POPs can cross the placental and immature blood-brain barriers. 45 Prenatal exposure to diesel exhaust disrupts normal reelin and heat shock pathways, which are critical for neuronal migration. 46 Volatile organic compounds, PAHs, PMs, and airborne MPs destabilized microtubule networks. 47 Heavy metals (such as cobalt, mercury, and cadmium) and POPs directly impair neuronal migration. 48 Lower thyroxine levels in first-trimester pregnant women are associated with air pollution exposure. 49 Transient modest maternal thyroid deficiency causes heterotopias. 50 Impaired placentation, the most common cause of chronic fetal hypoxia, can lead to polymicrogyria and heterotopia 51 Numerous meta-analyses 52 have linked air pollution with disorders of impaired placentation: preeclampsia, gestational hypertension, and intrauterine growth restriction. Air pollutants elevate maternal and fetal inflammation. A Mexican study, 53 particularly relevant to epilepsy, found higher titers of anti-glutamic acid decarboxylase 65 (GAD65) antibodies in children exposed to high PM2.5 levels in metropolitan areas.
MANAGEMENT OF EPILEPSY
Diagnosis and treatment
How air pollution affects time until diagnosis, misdiagnosis rates, and access to timely neurodiagnostic tests and specialist assessments are unknown. Air pollutants may affect the interpretation of neuroimaging findings. For example, quantitative hippocampal volume measures are commonly used in the assessment of temporal lobe epilepsy during the preoperative evaluation, but current assessment protocols do not currently consider the finding that PM2.5 levels are inversely associated with hippocampal volume, which may need to be taken into account when interpreting the significance of reduced volume. 54
Air pollutants are associated with common precipitants of seizures ( Figure 1 ): stress, sleep disturbance, inflammation, and endocrine disruption. Short-term PM exposure increases systemic inflammatory markers; 55 PM2.5, NO2, SO2 and O3 is associated with poor sleep quality; 56 and PM2.5, PAH and BPA decrease progesterone levels. 57 However, epidemiological evidence of increased seizure risk from pollutants is scarce and inconclusive. A small meta-analysis showed that short-term exposure to O3, NO2, and CO increased seizure risk, though the effect size was minimal for O3 and NO2. 58 A Danish cohort study 13 showed that exposure to NO2 was associated with a 5% higher risk of childhood febrile seizures.
What is more apparent is increased healthcare service use: PM10 is associated with increased emergency ambulance calls for epilepsy; 58 short-term exposure to NO2, SO2, CO, PM2.5, and PM10 increase hospitalization; 58 O3 is associated with reduced risk of hospitalization; 58 and NO2 and SO2 also increased visits to the outpatient epilepsy clinic in a single-center study. 59 The two studies 60 61 that explored seasonality showed the strongest associations in the winter months. Only two studies 62 63 have explored long-term exposure to pollutants and hospitalization. No studies have investigated the role of indoor household pollution.
Outcomes
Air pollution may play a role in the effectiveness and toxicity of antiseizure medications (ASMs). Relevant to many common renally-excreted ASMs, (such as levetiracetam), short- and long-term air pollution exposures impair renal function. 64 Long-term exposure is associated with higher levels of liver enzymes and increased odds of metabolic dysfunction-associated fatty liver disease. 65 Particulate matter has also been shown to inhibit some cytochrome P450s 66 and induce others. 67 At least 3 PAHs induce cytochrome P450 family 1 ( CYP1 ) expression, which can be further augmented by valproate. 68 This is concerning because the most widely available first-line ASMs in LMICs (such as phenobarbital and carbamazepine) are not enzyme-neutral. Pollutants also interact directly with proteins associated with drug resistance, such as P-glycoprotein (P-gp) and multidrug resistant 1 (MDR1). Six hours of exposure to diesel exhaust particles increases P-gp levels, 53 while 24 hours of exposure decreases these levels. 69 Two weeks of exposure to ultrafine particles increased ATP binding cassette subfamily B member 1 ( ABCB1 [MDR1]) transporter levels in a mouse cortex. 70 Some pollutants, such as TCDD and PAH, can activate aryl hydrocarbon-receptor pathway, which increases MDR1 expression, 71 while others, such as microplastics and BPA, inhibit this pathway. 72
Seizure control alone does not guarantee a good outcome: air pollution is associated with an increased risk of developing multimorbidity 73 ( Figure 1 ). It increases the risk and severity of many neuropsychiatric comorbidities that impact quality of life and seizure control. Long-term exposure to PM2.5, PM10, NO2, O3 and CO increases the risk of migraine. 74 Short-term exposure to NO2 and SO2 is a potential precipitant of acute migraine attacks. 75 Several studies 76 have associated long-term air pollution with the risk of depression and anxiety. Short-term exposure to SO2 and NO2 increases the risk of hospitalization for depression and anxiety. 75 People with epilepsy have a higher suicide risk, and short-term exposure to NO2, SO2, and PM10 is associated with increased suicide risk, with the effect particularly pronounced among women and individuals with lower levels of schooling. 77 Neurodevelopmental outcomes, critical in children with epilepsy, are linked to prenatal exposure to air pollutants. These include intellectual ability, attention, and fine psychomotor development. Autistic spectrum disorder and attention deficit hyperactivity disorder (ADHD)-like behavior are also implicated. 78
Pollution-associated increases in respiratory and cardiovascular diseases are consequential. Untreated asthma increases the risk of epileptic seizures. 79 Chronic obstructive pulmonary disease (COPD) is an independent risk factor for stroke-related seizures. 80 Drugs commonly used to treat more severe respiratory diseases, such as montelukast, theophylline, and systemic corticosteroids, have potential drug interactions with common first-line ASMs. Air pollutant exposure increases arrhythmias and sudden cardiac death, and it has been hypothesized 81 that air pollution is an aggravating event in sudden unexpected death in epilepsy (SUDEP). Importantly, pneumonia and ischemic heart disease are the most common causes of premature death in people with epilepsy, even when seizure-free for decades. 82
ADDRESSING THE ROLE OF AIR POLLUTION IN EPILEPSY
Emerging evidence suggests that air pollution may play a role as a risk factor in epilepsy. Within the symptom-component cause model of epilepsy, various factors act alone or in combination to contribute to the condition. We presented a body of evidence showing that air pollution is associated with increased rates and severity of many of these component causes, including the main risk factors for childhood and adult epilepsy. Preclinical studies demonstrate the potential of specific pollutants to contribute to generating a hyperexcitable brain that is predisposed to seizures and associated neuropsychiatric comorbidities. However, we need a far greater and more nuanced understanding of the role of air pollution in epilepsy.
Our current understanding is lacking in scope, specificity and scale:
Scope: Key aspects and populations are missing from current epidemiological studies. Most studies do not report or control for indices of socioeconomic status (SES), a significant confounder. Temporal exposure characteristics are often unclear and masked by averages; we need to know peak and cumulative exposure at least. 83 Knowing when the risk of seizure is highest, such as hours or days after exposure, is crucial if we were to prevent seizures. The concentration-response associations are also unclear, and we do not know if the relationship is non-linear or if a threshold effect exists. Critical areas are missing from the field. First, how do pollutants influence epileptogenesis after the initial structural insult (such as acute stroke)? A potential window of opportunity to influence the outcome. Second, indoor pollution levels can be much higher, more toxic and more associated with SES. 18 What are the effects of indoor pollution? Third, what is the relationship between pollutants and drug resistance in epilepsy? Preclinical studies show potential interaction with many ASM molecular targets, pharmacokinetics, and drug-resistance proteins.
Specificity: Air pollutant mixtures vary widely worldwide in their composition and concentrations due to different emission sources. A single pollutant and a mixture of pollutants are likely to affect outcomes differentially. This may also explain differences in biological responses when PM is taken from different locations and seasons in preclinical studies. Equally, epilepsy is heterogeneous. Focal epilepsy and generalized epilepsy have different biology, natural history, and responsiveness to ASMs. Preclinical studies hint at possible differences in pollutant effects on other brain areas. Pollutant effects on frontal lobe epilepsy versus temporal lobe epilepsy, or absence of seizures versus myoclonic seizures, could be very different. Precision medicine can benefit from more specific epidemiological studies.
Scale: Scale is essential for understanding the full impact of air pollution on epilepsy risk and outcomes across diverse populations and regions. There are not many epidemiological studies on air pollution and epilepsies. The current studies are mostly small, single-centre, retrospective, and concentrated on a few countries, often near academic centers. The exposure timeframe is frequently too short, especially relative to the time course of the development of epilepsy (months to years). The range of pollution concentration is not broad enough. Two-thirds of people with epilepsy live in LMICs, where average PM2.5 concentrations are multitudes higher than in high-income countries (HICs). In contrast, the mean PM2.5 level of 49 ug/m 3 was calculated in a study 58 that included a recent meta-analysis reporting the 90 to 160 ug/m 3 range and conducted in 2023 in Dehli. 84 Premature and incorrect conclusions can be drawn if studies only focus on areas of relatively low pollution.
In conclusion, the role of air pollution in epilepsy represents an urgent and underexplored area of research, with a narrow window of opportunity to act. The broader trend of rising socioeconomic inequality 3 and climate change 4 is likely to adversely impact epilepsy outcomes and worsen the effects of air pollution. A more precise understanding at the epidemiological and mechanistic levels may enable us to mitigate and prevent the harmful effects. As seizures are a threshold phenomenon, even small interventions could lead to significant improvements.
Conflict of Interest JWS reports personal fees from Eisai, UCB, and Angelini Pharma, and grants from UCB and Angelini Pharma, outside the submitted work. All other authors have no conflicts to report.
Authors' Contributions
Conceptualization: PJ, JSJ, XZ, DD, JWS; Writing – original draft: PJ; Writing – review & editing: PJ, JSJ, XZ,DD, JWS.
Editor-in-Chief: Hélio A. G. Teive. https://orcid.org/0000-0003-2305-1073
Associate Editor: Marina Koutsodontis Machado Alvim. https://orcid.org/0000-0002-6780-0761
Members of the SdHIELD Research Group: Prem Jareonsettasin (University College London), John S. Ji (Tsinghua University), Ding Ding (Fudan University), Annalena Lange (University of Bonn), Theodor Rüber (University of Bonn), Rhys H Thomas (Newcastle University), Kathryn Bush (Newcastle University), Owen Pickrell (Swansea University), Jimmy Li (Université de Montréal), Mark Keezer (Université de Montréal), Gagandeep Singh (Dayanand Medical College & Hospital/University College London), and Ley Sander (University College London).
Data Availability Statement
This is a review article, and no new data were generated or analyzed during this study. All data supporting the findings are included in the manuscript, which is based on publicly available literature and sources cited within the review.
References
- 1.Vassallo P, Gursal V, Xiong W, Zhou D, De Tisi J, Thijs R D et al. Temporal Trends in Hippocampal Sclerosis Surgery: An Observational Study From a Tertiary Epilepsy Centre. Eur J Neurol. 2025;32(01):e70041. doi: 10.1111/ene.70041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jehi L, Friedman D, Carlson C, Cascino G, Dewar S, Elger C et al. The evolution of epilepsy surgery between 1991 and 2011 in nine major epilepsy centers across the United States, Germany, and Australia. Epilepsia. 2015;56(10):1526–1533. doi: 10.1111/epi.13116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bush K J, Cullen E, Mills S, Chin R FM, Thomas R H, Kingston A et al. Assessing the extent and determinants of socioeconomic inequalities in epilepsy in the UK: a systematic review and meta-analysis of evidence. Lancet Public Health. 2024;9(08):e614–e628. doi: 10.1016/S2468-2667(24)00132-4. [DOI] [PubMed] [Google Scholar]
- 4.Sisodiya S M, Gulcebi M I, Fortunato F, Mills J D, Haynes E, Bramon E et al. Climate change and disorders of the nervous system. Lancet Neurol. 2024;23(06):636–648. doi: 10.1016/S1474-4422(24)00087-5. [DOI] [PubMed] [Google Scholar]
- 5.Toubasi A, Al-Sayegh T N. Short-term exposure to air pollution and ischemic stroke: a systematic review and meta-analysis. Neurology. 2023;101(19):e1922–e1932. doi: 10.1212/WNL.0000000000207856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hassanipour S, Nikbakht H A, Amrane A, Arab-Zozani M, Shojaie L, Rostami S, Badeenezhad A. The relationship between air pollution and brain cancer: a systematic review and meta-analysis. Ann Glob Health. 2023;89(01):45. doi: 10.5334/aogh.3889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Abolhasani E, Hachinski V, Ghazaleh N, Azarpazhooh M R, Mokhber N, Martin J. Air pollution and incidence of dementia: a systematic review and meta-analysis. Neurology. 2023;100(02):e242–e254. doi: 10.1212/WNL.0000000000201419. [DOI] [PubMed] [Google Scholar]
- 8.Liao K-H, Chan T-C, Wu C-C, Huang W-C, Hsu C-W, Chuang H-C et al. Association between short-term air pollution exposure and traumatic intracranial hemorrhage: pilot evidence from Taiwan. Front Neurol. 2023;14:1.087767E6. doi: 10.3389/fneur.2023.1087767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tang Z, Jia J. PM 2.5 -related neonatal encephalopathy due to birth asphyxia and trauma: a global burden study from 1990 to 2019 . Environ Sci Pollut Res Int. 2023;30(12):33002–33017. doi: 10.1007/s11356-022-24410-w. [DOI] [PubMed] [Google Scholar]
- 10.Liu Y, Xu J, Chen D, Sun P, Ma X. The association between air pollution and preterm birth and low birth weight in Guangdong, China. BMC Public Health. 2019;19(01):3. doi: 10.1186/s12889-018-6307-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Smith R B, Beevers S D, Gulliver J, Dajnak D, Fecht D, Blangiardo M et al. Impacts of air pollution and noise on risk of preterm birth and stillbirth in London. Environ Int. 2020;134:105290. doi: 10.1016/j.envint.2019.105290. [DOI] [PubMed] [Google Scholar]
- 12.Zhang Y, Hu Y, Talarico R, Qiu X, Schwartz J, Fell D B et al. Prenatal Exposure to Ambient Air Pollution and Cerebral Palsy. JAMA Netw Open. 2024;7(07):e2420717. doi: 10.1001/jamanetworkopen.2024.20717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hjortebjerg D, Andersen A MN, Ketzel M, Raaschou-Nielsen O, Sørensen M. Exposure to traffic noise and air pollution and risk for febrile seizure: a cohort study. Scand J Work Environ Health. 2018;44(05):539–546. doi: 10.5271/sjweh.3724. [DOI] [PubMed] [Google Scholar]
- 14.Shears R K, Jacques L C, Naylor G, Miyashita L, Khandaker S, Lebre F et al. Exposure to diesel exhaust particles increases susceptibility to invasive pneumococcal disease. J Allergy Clin Immunol. 2020;145(04):1272–1.284E9. doi: 10.1016/j.jaci.2019.11.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sarmadi M, Rahimi S, Rezaiemanesh M R, Yektay S. Association between water and sanitation, air and emission pollution and climate change and neurological disease distribution: A study based on GBD data. Chemosphere. 2021;285:131522. doi: 10.1016/j.chemosphere.2021.131522. [DOI] [PubMed] [Google Scholar]
- 16.Slinger G, Verbeek S T, Van Diessen E, Noorlag L, Braun K PJ, Shen Y et al. Air pollution and childhood epilepsy diagnosis at a first seizure clinic in The Netherlands: A case-control study. Environ Adv. 2024;16:100541. doi: 10.1016/j.envadv.2024.100541. [DOI] [Google Scholar]
- 17.Singer JdM, André CDSd, André PAd, Rocha F MM, Waked D, Vaz A M et al. Assessing socioeconomic bias of exposure to urban air pollution: an autopsy-based study in Sao Paulo, Brazil. The Lancet Regional Health Americas. 2023;22:100500. doi: 10.1016/j.lana.2023.100500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hu Y, Ji J S, Zhao B. Deaths Attributable to Indoor PM 2.5 in Urban China When Outdoor Air Meets 2021 WHO Air Quality Guidelines . Environ Sci Technol. 2022;56(22):15882–15891. doi: 10.1021/acs.est.2c03715. [DOI] [PubMed] [Google Scholar]
- 19.Muindi K, Kimani-Murage E, Egondi T, Rocklov J, Ng N. Household air pollution: sources and exposure levels to fine particulate matter in Nairobi slums. Toxics. 2016;4(03):12. doi: 10.3390/toxics4030012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mei H, Wu D, Yong Z, Cao Y, Chang Y, Liang J et al. PM 2.5 exposure exacerbates seizure symptoms and cognitive dysfunction by disrupting iron metabolism and the Nrf2-mediated ferroptosis pathway . Sci Total Environ. 2024;910:168578. doi: 10.1016/j.scitotenv.2023.168578. [DOI] [PubMed] [Google Scholar]
- 21.Ke P, Liu J, Chen C, Luo S, Gu H, Gu J et al. Zinc Oxide Nanoparticles Exacerbate Epileptic Seizures by Modulating the TLR4-Autophagy Axis. Int J Nanomedicine. 2024;19:2025–2038. doi: 10.2147/IJN.S442623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Escalante-Membrillo C, Paz C.Development of an experimental model of epilepsy in rats exposed to ozone Toxicol Lett 199793(2-3):103–107. 10.1016/s0378-4274(97)00077-5 [DOI] [PubMed] [Google Scholar]
- 23.Mallok A, Vaillant J D, Soto M TD, Viebahn-Hänsler R, Pérez A F et al. Ozone protective effects against PTZ-induced generalized seizures are mediated by reestablishment of cellular redox balance and A1 adenosine receptors. Neurol Res. 2015;37(03):204–210. doi: 10.1179/1743132814Y.0000000445. [DOI] [PubMed] [Google Scholar]
- 24.Han Y, Yi W, Qin J, Zhao Y, Zhang J, Chang X. Dose-dependent effect of sulfur dioxide on brain damage induced by recurrent febrile seizures in rats. Neurosci Lett. 2014;563:149–154. doi: 10.1016/j.neulet.2013.12.042. [DOI] [PubMed] [Google Scholar]
- 25.Poon E, Bandara S B, Allen J B, Sadowski R N, Schantz S L. Developmental PCB exposure increases susceptibility to audiogenic seizures in adulthood. Neurotoxicology. 2015;46:117–124. doi: 10.1016/j.neuro.2014.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee H, Tran C M, Jeong S, Kim S S, Bae M A, Kim K T. Seizurogenic effect of perfluorooctane sulfonate in zebrafish larvae. Neurotoxicology. 2022;93:257–264. doi: 10.1016/j.neuro.2022.10.007. [DOI] [PubMed] [Google Scholar]
- 27.Chang Y, Jiang X, Dou J, Xie R, Zhao W, Cao Y et al. Investigating the potential risk of cadmium exposure on seizure severity and anxiety-like behaviors through the ferroptosis pathway in epileptic mice: An integrated multi-omics approach. J Hazard Mater. 2024;480:135814. doi: 10.1016/j.jhazmat.2024.135814. [DOI] [PubMed] [Google Scholar]
- 28.Li Q, Zheng J, Xu S, Zhang J, Cao Y, Qin Z et al. The neurotoxicity induced by PM 2.5 might be strongly related to changes of the hippocampal tissue structure and neurotransmitter levels . Toxicol Res (Camb) 2018;7(06):1144–1152. doi: 10.1039/c8tx00093j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mesdaghinia A, Yazdanpanah H, Seddighi M, Banafshe H R, Heydari A. Effect of short-term lead exposure on PTZ-induced seizure threshold in mice. Toxicol Lett. 2010;199(01):6–9. doi: 10.1016/j.toxlet.2010.07.012. [DOI] [PubMed] [Google Scholar]
- 30.Khodayar M J, Kalantari H, Samimi A, Alboghobeish S, Moghadam P T, Zeinvand-Lorestani M. Arsenic and oxidative stress in pentylenetetrazole-induced seizures in mice. Journal of Basic and Clinical Pathophysiology. 2019;7:1–6. doi: 10.22070/jbcp.2019.4045.1108. [DOI] [Google Scholar]
- 31.Yuan Y. Methylmercury: a potential environmental risk factor contributing to epileptogenesis. Neurotoxicology. 2012;33(01):119–126. doi: 10.1016/j.neuro.2011.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cooper R M, Legare C E, Teskey G C.Changes in (14)C-labeled 2-deoxyglucose brain uptake from nickel-induced epileptic activity Brain Res 2001923(1-2):71–81. 10.1016/s0006-8993(01)03034-7 [DOI] [PubMed] [Google Scholar]
- 33.Chusid J G, Kopeloff L M. Epileptogenic effects of pure metals implanted in motor cortex of monkeys. J Appl Physiol. 1962;17:697–700. doi: 10.1152/jappl.1962.17.4.697. [DOI] [PubMed] [Google Scholar]
- 34.Chen Z, Yu W, Xu R, Karoly P J, Maturana M I, Payne D E et al. Ambient air pollution and epileptic seizures: A panel study in Australia. Epilepsia. 2022;63(07):1682–1692. doi: 10.1111/epi.17253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Crüts B, Van Etten L, Törnqvist H, Blomberg A, Sandström T, Mills N L, Borm P J. Exposure to diesel exhaust induces changes in EEG in human volunteers. Part Fibre Toxicol. 2008;5:4. doi: 10.1186/1743-8977-5-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kim J-H, Hwang S, Park S I, Lee H J, Jung Y J, Jo S H. 3,3′,4,4′-tetrachlorobiphenyl (PCB77) enhances human Kv1.3 channel currents and alters cytokine production. Korean J Physiol Pharmacol. 2024;28(04):323–333. doi: 10.4196/kjpp.2024.28.4.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lu Y, Wang H, Yang J, Jiang W, Xin H, Luo Y et al. Distinct Role of Mono-2-ethylhexyl Phthalate in Neuronal Transmission in Rat CA3 Hippocampal Neurons: Involvement of Ion Channels. Molecules. 2022;27(10):3082. doi: 10.3390/molecules27103082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu F, Huang Y, Zhang F, Chen Q, Wu B, Rui W et al. Macrophages treated with particulate matter PM2.5 induce selective neurotoxicity through glutaminase-mediated glutamate generation. J Neurochem. 2015;134(02):315–326. doi: 10.1111/jnc.13135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen M, Li B, Sang N. Particulate matter (PM 2.5 ) exposure season-dependently induces neuronal apoptosis and synaptic injuries . J Environ Sci (China) 2017;54:336–345. doi: 10.1016/j.jes.2016.10.013. [DOI] [PubMed] [Google Scholar]
- 40.Yao G, Yun Y, Sang N. Differential effects between one week and four weeks exposure to same mass of SO2 on synaptic plasticity in rat hippocampus. Environ Toxicol. 2016;31(07):820–829. doi: 10.1002/tox.22093. [DOI] [PubMed] [Google Scholar]
- 41.Yao G, Yue H, Yun Y, Sang N. Chronic SO2 inhalation above environmental standard impairs neuronal behavior and represses glutamate receptor gene expression and memory-related kinase activation via neuroinflammation in rats. Environ Res. 2015;137:85–93. doi: 10.1016/j.envres.2014.11.012. [DOI] [PubMed] [Google Scholar]
- 42.Allen J L, Liu X, Weston D, Prince L, Oberdörster G, Finkelstein J N et al. Developmental exposure to concentrated ambient ultrafine particulate matter air pollution in mice results in persistent and sex-dependent behavioral neurotoxicity and glial activation. Toxicol Sci. 2014;140(01):160–178. doi: 10.1093/toxsci/kfu059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Umezawa M, Onoda A, Korshunova I, Jensen A CØ, Koponen I K, Jensen K A et al. Maternal inhalation of carbon black nanoparticles induces neurodevelopmental changes in mouse offspring. Part Fibre Toxicol. 2018;15(01):36. doi: 10.1186/s12989-018-0272-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kim S Y, Kim J K, Park S H, Kim B-G, Jang A-S, Oh S H et al. Effects of inhaled particulate matter on the central nervous system in mice. Neurotoxicology. 2018;67:169–177. doi: 10.1016/j.neuro.2018.06.001. [DOI] [PubMed] [Google Scholar]
- 45.Cory-Slechta D A, Sobolewski M. Neurotoxic effects of air pollution: an urgent public health concern. Nat Rev Neurosci. 2023;24(03):129–130. doi: 10.1038/s41583-022-00672-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chang Y-C, Daza R, Hevner R, Costa L G, Cole T B. Prenatal and early life diesel exhaust exposure disrupts cortical lamina organization: Evidence for a reelin-related pathogenic pathway induced by interleukin-6. Brain Behav Immun. 2019;78:105–115. doi: 10.1016/j.bbi.2019.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Leem J, Kim S, Kim J-S, Oh J S. ROS-independent cytotoxicity of 9,10-phenanthrenequinone inhibits cell cycle progression and spindle assembly during meiotic maturation in mouse oocytes. J Hazard Mater. 2022;436:129248. doi: 10.1016/j.jhazmat.2022.129248. [DOI] [PubMed] [Google Scholar]
- 48.Huang Y, Guo X, Lu S, Chen Q, Wang Z, Lai L et al. Long-term exposure to cadmium disrupts neurodevelopment in mature cerebral organoids. Sci Total Environ. 2024;912:168923. doi: 10.1016/j.scitotenv.2023.168923. [DOI] [PubMed] [Google Scholar]
- 49.Li J, Liao J, Hu C, Bao S, Mahai G, Cao Z et al. Preconceptional and the first trimester exposure to PM 2.5 and offspring neurodevelopment at 24 months of age: Examining mediation by maternal thyroid hormones in a birth cohort study . Environ Pollut. 2021;284:117133. doi: 10.1016/j.envpol.2021.117133. [DOI] [PubMed] [Google Scholar]
- 50.O'Shaughnessy K L, Thomas S E, Spring S R, Ford J L, Ford R L, Gilbert M E. A transient window of hypothyroidism alters neural progenitor cells and results in abnormal brain development. Sci Rep. 2019;9(01):4662. doi: 10.1038/s41598-019-40249-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Larroche J C, Girard N, Narcy F, Fallet C. Abnormal cortical plate (polymicrogyria), heterotopias and brain damage in monozygous twins. Biol Neonate. 1994;65(06):343–352. doi: 10.1159/000243900. [DOI] [PubMed] [Google Scholar]
- 52.Decrue F, Townsend R, Miller M R, Newby D E, Reynolds R M. Ambient air pollution and maternal cardiovascular health in pregnancy. Heart. 2023;109(21):1586–1593. doi: 10.1136/heartjnl-2022-322259. [DOI] [PubMed] [Google Scholar]
- 53.Hartz A MS, Bauer B, Block M L, Hong J-S, Miller D S. Diesel exhaust particles induce oxidative stress, proinflammatory signaling, and P-glycoprotein up-regulation at the blood-brain barrier. FASEB J. 2008;22(08):2723–2733. doi: 10.1096/fj.08-106997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Balboni E, Filippini T, Crous-Bou M, Guxens M, Erickson L D, Vinceti M.The association between air pollutants and hippocampal volume from magnetic resonance imaging: A systematic review and meta-analysis Environ Res 2022204(Pt A):111976. 10.1016/j.envres.2021.111976 [DOI] [PubMed] [Google Scholar]
- 55.Tang H, Cheng Z, Li N, Mao S, Ma R, He H et al. The short- and long-term associations of particulate matter with inflammation and blood coagulation markers: A meta-analysis. Environ Pollut. 2020;267:115630. doi: 10.1016/j.envpol.2020.115630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cao B, Chen Y, McIntyre R S. Comprehensive review of the current literature on impact of ambient air pollution and sleep quality. Sleep Med. 2021;79:211–219. doi: 10.1016/j.sleep.2020.04.009. [DOI] [PubMed] [Google Scholar]
- 57.Wei D, Li S, Liu X, Zhang L, Liu P, Fan K et al. Long-term exposure to particulate matter and residential greenness in relation to androgen and progesterone levels among rural Chinese adults. Environ Int. 2021;153:106483. doi: 10.1016/j.envint.2021.106483. [DOI] [PubMed] [Google Scholar]
- 58.Antaya T C, Espino-Alvarado P H, Oiamo T, Wilk P, Speechley K N, Burneo J G. Association of outdoor air and noise pollution with unprovoked seizures and new onset epilepsy: A systematic review and meta-analysis. Epilepsia. 2024;65(07):1847–1867. doi: 10.1111/epi.18010. [DOI] [PubMed] [Google Scholar]
- 59.Xu C, Fan Y-N, Kan H-D, Chen R-J, Liu J-H, Li Y-F et al. The novel relationship between urban air pollution and epilepsy: a time series study. PLoS One. 2016;11(08):e0161992. doi: 10.1371/journal.pone.0161992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yalçın G, Sayınbatur B, Toktaş İ, Gürbay A. The relationship between environmental air pollution, meteorological factors, and emergency service admissions for epileptic attacks in children. Epilepsy Res. 2022;187:107026. doi: 10.1016/j.eplepsyres.2022.107026. [DOI] [PubMed] [Google Scholar]
- 61.Chiang K-L, Lee J-Y, Chang Y-M, Kuo F-C, Huang C-Y. The effect of weather, air pollution and seasonality on the number of patient visits for epileptic seizures: A population-based time-series study. Epilepsy Behav. 2021;115:107487. doi: 10.1016/j.yebeh.2020.107487. [DOI] [PubMed] [Google Scholar]
- 62.Fluegge K, Fluegge K. Air pollution and risk of hospitalization for epilepsy: the role of farm use of nitrogen fertilizers and emissions of the agricultural air pollutant, nitrous oxide. Arq Neuropsiquiatr. 2017;75(09):614–619. doi: 10.1590/0004-282X20170107. [DOI] [PubMed] [Google Scholar]
- 63.Min J, Lee W, Bell M L, Kim Y, Heo S, Kim G E et al. Hospital admission risks and excess costs for neurological symptoms attributable to long-term exposure to fine particulate matter in New York State, USA. Environ Res. 2023;229:115954. doi: 10.1016/j.envres.2023.115954. [DOI] [PubMed] [Google Scholar]
- 64.Kuźma Ł, Małyszko J, Bachórzewska-Gajewska H, Kralisz P, Dobrzycki S. Exposure to air pollution and renal function. Sci Rep. 2021;11(01):11419. doi: 10.1038/s41598-021-91000-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Shen Q, Liu Y J, Qiu T T, Loon K S, Zhou D. Microplastic-induced NAFLD: Hepatoprotective effects of nanosized selenium. Ecotoxicol Environ Saf. 2024;272:115850. doi: 10.1016/j.ecoenv.2023.115850. [DOI] [PubMed] [Google Scholar]
- 66.Reed J R, Dela Cruz A LN, Lomnicki S M, Backes W L. Inhibition of cytochrome P450 2B4 by environmentally persistent free radical-containing particulate matter. Biochem Pharmacol. 2015;95(02):126–132. doi: 10.1016/j.bcp.2015.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhang S, Zhang J, Guo D, Peng C, Tian M, Pei D et al. Biotoxic effects and gene expression regulation of urban PM 2.5 in southwestern China . Sci Total Environ. 2021;753:141774. doi: 10.1016/j.scitotenv.2020.141774. [DOI] [PubMed] [Google Scholar]
- 68.Ozawa S, Yamaori S, Aikawa K, Kamijo S, Ohmori S. Expression profile of cytochrome P450s and effects of polycyclic aromatic hydrocarbons and antiepileptic drugs on CYP1 expression in MOG-G-CCM cells. Life Sci. 2020;258:118140. doi: 10.1016/j.lfs.2020.118140. [DOI] [PubMed] [Google Scholar]
- 69.Aquino G V, Dabi A, Odom G J, Lavado R, Nunn K, Thomas K et al. Evaluating the effect of acute diesel exhaust particle exposure on P-glycoprotein efflux transporter in the blood-brain barrier co-cultured with microglia. Curr Res Toxicol. 2023;4:100107. doi: 10.1016/j.crtox.2023.100107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Puris E, Saveleva L, Górová V, Vartiainen P, Kortelainen M, Lamberg H et al. Air pollution exposure increases ABCB1 and ASCT1 transporter levels in mouse cortex. Environ Toxicol Pharmacol. 2022;96:104003. doi: 10.1016/j.etap.2022.104003. [DOI] [PubMed] [Google Scholar]
- 71.Vogel C FA, Van Winkle L S, Esser C, Haarmann-Stemmann T. The aryl hydrocarbon receptor as a target of environmental stressors - Implications for pollution mediated stress and inflammatory responses. Redox Biol. 2020;34:101530. doi: 10.1016/j.redox.2020.101530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Morgan S E, DeLouise L A. Effects of microplastic interaction with persistent organic pollutants on the activity of the aryl hydrocarbon and estrogen receptors. Chemosphere. 2024;365:143338. doi: 10.1016/j.chemosphere.2024.143338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Keezer M R, Sisodiya S M, Sander J W. Comorbidities of epilepsy: current concepts and future perspectives. Lancet Neurol. 2016;15(01):106–115. doi: 10.1016/S1474-4422(15)00225-2. [DOI] [PubMed] [Google Scholar]
- 74.Portt A E, Orchard C, Chen H, Ge E, Lay C, Smith P M. Migraine and air pollution: A systematic review. Headache. 2023;63(09):1203–1219. doi: 10.1111/head.14632. [DOI] [PubMed] [Google Scholar]
- 75.Szyszkowicz M, Colman I, Wickham M, Rowe B H. Ambient sulphur dioxide and emergency department visits for migraine and depression. Int Neuropsychiatr Dis J. 2013;1:77–88. doi: 10.9734/INDJ/2013/5245. [DOI] [Google Scholar]
- 76.Yang T, Wang J, Huang J, Kelly F J, Li G. Long-term exposure to multiple ambient air pollutants and association with incident depression and anxiety. JAMA Psychiatry. 2023;80(04):305–313. doi: 10.1001/jamapsychiatry.2022.4812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lin G-Z, Li L, Song Y F, Zhou Y X, Shen S Q, Ou C Q. The impact of ambient air pollution on suicide mortality: a case-crossover study in Guangzhou, China. Environ Health. 2016;15(01):90. doi: 10.1186/s12940-016-0177-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Tartaglione A M, Camoni L, Calamandrei G, Chiarotti F, Venerosi A. The contribution of environmental pollutants to the risk of autism and other neurodevelopmental disorders: A systematic review of case-control studies. Neurosci Biobehav Rev. 2024;164:105815. doi: 10.1016/j.neubiorev.2024.105815. [DOI] [PubMed] [Google Scholar]
- 79.Uysalol M, Yıldız R, Özünal Z G. Is Seizure an Adverse Effect of Salbutamol in the Pediatric Population? Balkan Med J. 2022;39(05):340–344. doi: 10.4274/balkanmedj.galenos.2022.2022-3-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.De Reuck J, Proot P, Van Maele G. Chronic obstructive pulmonary disease as a risk factor for stroke-related seizures. Eur J Neurol. 2007;14(09):989–992. doi: 10.1111/j.1468-1331.2007.01829.x. [DOI] [PubMed] [Google Scholar]
- 81.Scorza C A, Calderazzo L, Arida R M, Cavalheiro E A, Scorza F A. Environmental air pollution is an aggravating event for sudden unexpected death in epilepsy. Arq Neuropsiquiatr. 2013;71(10):807–810. doi: 10.1590/0004-282X20130092. [DOI] [PubMed] [Google Scholar]
- 82.Neligan A, Bell G S, Johnson A L, Goodridge D M, Shorvon S D, Sander J W.The long-term risk of premature mortality in people with epilepsy Brain 2011134(Pt 2):388–395. 10.1093/brain/awq378 [DOI] [PubMed] [Google Scholar]
- 83.Ji J S. Air pollution and cardiovascular disease onset: hours, days, or years? Lancet Public Health. 2022;7(11):e890–e891. doi: 10.1016/S2468-2667(22)00257-2. [DOI] [PubMed] [Google Scholar]
- 84.Tiwari S, Kumar A, Mantri S, Dey S. Modelling ambient PM2.5 exposure at an ultra-high resolution and associated health burden in megacity Delhi: exposure reduction target for 2030. Environ Res Lett. 2023;18(04):44010. doi: 10.1088/1748-9326/acc261. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This is a review article, and no new data were generated or analyzed during this study. All data supporting the findings are included in the manuscript, which is based on publicly available literature and sources cited within the review.


