Abstract
Small interfering RNA (siRNA) therapeutics are a new class of drugs that is rapidly expanding to tackle various diseases. Extrahepatic delivery of siRNAs, especially to the parenchyma of solid tumors, is challenging with multiple strategies being explored such as lipid nanoparticle based delivery and ligand conjugation strategies. Here, we report that an albumin-binding dendritic siRNA (D-siRNA) boosts blood circulation time following systemic administration, leading to improved delivery and silencing activity in a melanoma tumor model, in comparison to non-albumin binding lipophilic siRNAs. D-siRNAs increased the tumor-to-liver delivery ratio, including both immune and non-immune cell types within the tumor parenchyma. Using D-siRNAs to target JAK1 expression as an adjuvant to immune checkpoint inhibitors, we found that D-siRNAs was able to enhance PD1 antibody treatment and slow tumor progression of melanoma. Thus, this work demonstrates the utility of D-siRNAs as a systemically administered tumor delivery strategy, enabling the use of siRNAs as chemotherapeutic agents. Further mechanistic studies into the role of JAK1 in melanoma pathology and progression may expand this into additional targets as potential treatments.
Keywords: MT: Delivery Strategies, siRNA therapeutics, delivery, cancer, tumor delivery, immunotherapy, albumin binding, lipid conjugation, melanoma
Graphical abstract

D-siRNAs, dendritic siRNAs with albumin-binding properties, show promise for systemic siRNA delivery in cancer therapy. Fakih et al. demonstrated enhanced circulation, tumor delivery, and activity of D-siRNAs in a melanoma model, suggesting their potential as a therapeutic and delivery strategy for solid tumors.
Introduction
Small interfering RNAs (siRNAs) are an emerging drug class that can silence the expression of disease-related genes with high specificity and durability.1,2,3 Since the initial approval in 2018 for the first liver siRNA drug, many liver-targeted siRNA therapies have been approved by the Food and Drug Administration (FDA) and European Medicines Agency (EMA), with additional therapies currently under clinical evaluation for extrahepatic tissues such as muscle, skin, and the CNS.2,3 siRNA applications in the oncology space have not been as successful, with multiple setbacks in clinical trials.4,5 The challenges that siRNA faces in oncology pertain to optimizing molecule stability, dosing, and most importantly robust delivery to tumors.4 Strategies to overcome the delivery barrier include the use of nanoparticles or conjugation strategies that alter siRNA pharmacokinetics and biodistribution.2,3
Leveraging albumin binding is a promising strategy to enhance siRNA delivery, particularly to solid tumors.6,7 This is due to many of albumin’s inherent characteristics: (1) prolonged circulation that boosts biodistribution,6,8 (2) albumin’s natural accumulation within tumors as an energy source,9 and (3) superior tissue penetration compared to other carriers.6,10 The clinical success of Abraxane, an FDA-approved albumin-bound chemotherapeutic, highlights the potential of this strategy.7 Some studies have also reported that albumin binding enhanced productive tumor delivery of siRNA to multiple breast cancer models.11 We recently reported the development of an albumin-binding dendritic siRNA (D-siRNA) platform that enables robust and selective albumin binding in vivo, in comparison to traditional lipid-conjugated siRNAs (e.g., docosanoic acid [DCA-siRNA]).12,13 Our previous studies demonstrated that systemically administered D-siRNA achieves broad tissue distribution without compromising siRNA gene-silencing activity.12 Moreover, local administration in a glioblastoma xenograft model resulted in effective siRNA delivery.14 Here, we demonstrate that the albumin-binding properties of D-siRNA extend plasma circulation after systemic administration (i.e., intravenous [i.v.] or subcutaneous [s.c.]), leading to enhanced siRNA durability and productive delivery to diverse cell types within the tumor parenchyma of a melanoma model. By targeting JAK1 with D-siRNAs as an adjuvant therapy to immune checkpoint inhibitor PD-1 antibody,15,16 D-siRNAJak1 supported slowing the melanoma tumor growth rate compared to PD-1 antibody treatment alone. This proof-of-concept study validates the potential of albumin-binding D-siRNAs as a promising tumor delivery vehicle for future oncology therapeutics.
Results
Dendritic conjugate improves plasma circulation time of siRNAs
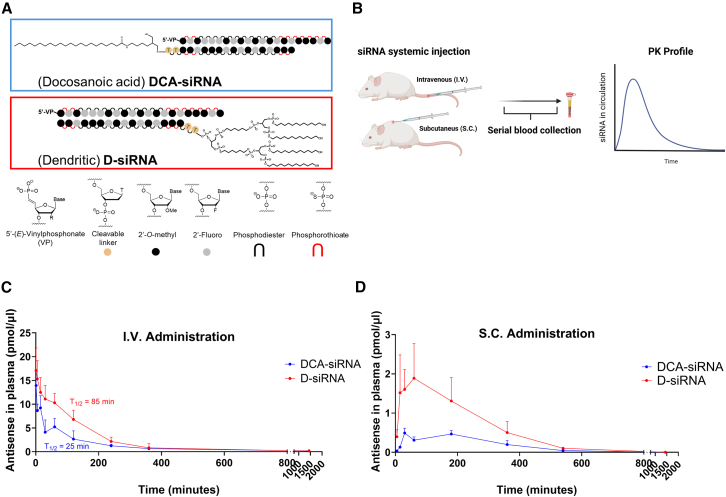
Our previous work demonstrated that dendritic conjugated D-siRNA exhibits exclusive binding to albumin upon i.v. or s.c. administration, contrasting with linear lipid-conjugated DCA-siRNA’s broader lipoprotein affinity.12,17 Given albumin’s extended circulation half-life, we hypothesized D-siRNA would exhibit additional prolonged plasma circulation.10,18 To test this, we administered D-siRNA and DCA-siRNA via both i.v. and s.c. routes, quantifying plasma siRNA concentrations over time (Figures 1A and 1B). As predicted, D-siRNA displayed a significantly increased circulation time compared to DCA-siRNA in both administration methods (Figures 1C and 1D). Specifically, i.v. administration revealed a D-siRNA plasma half-life of approximately 85 min, a ∼3.4-fold increase over DCA-siRNA’s 25 min. Subcutaneous administration further enhanced D-siRNA’s area-under-curve and circulation time, yielding a ∼3.3-fold increase. These results confirm that albumin-bound D-siRNA achieves extended circulation regardless of administration route, potentially improving albumin-mediated extrahepatic tumor distribution.
Figure 1.
Assessing plasma circulation of D-siRNA and DCA-siRNA
(A) Structures of D-siRNA and DCA-siRNA (B) study schematic to quantify plasma circulation, where siRNAs are administered systemically followed by serial blood collection to quantify siRNAs at various time points. Mice (n = 4) were injected either intravenously (i.v.) at a dose of 20 nmol or subcutaneously (s.c.) at a dose of 15 nmol of either D-siRNA or DCA-siRNA. siRNAs in circulation were quantified via PNA assay and plotted for (C) i.v. and (D) s.c. administration. One-phase decay fitting via GraphPad Prism was conducted for i.v. data, where half-life is calculated showing D-siRNA (85.35 min) with a ∼3.4-fold increase compared to DCA-siRNA (25.63 min). Area under curve for s.c. data was calculated in GraphPad Prism, with a ∼3.3-fold increase with D-siRNA (n = 4, mean ± SD). Subset of data in (D), particularly DCA-siRNA plasma circulation, has been previously reported by Yamada et al.19
D-siRNAs have improved distribution and activity to solid tumors
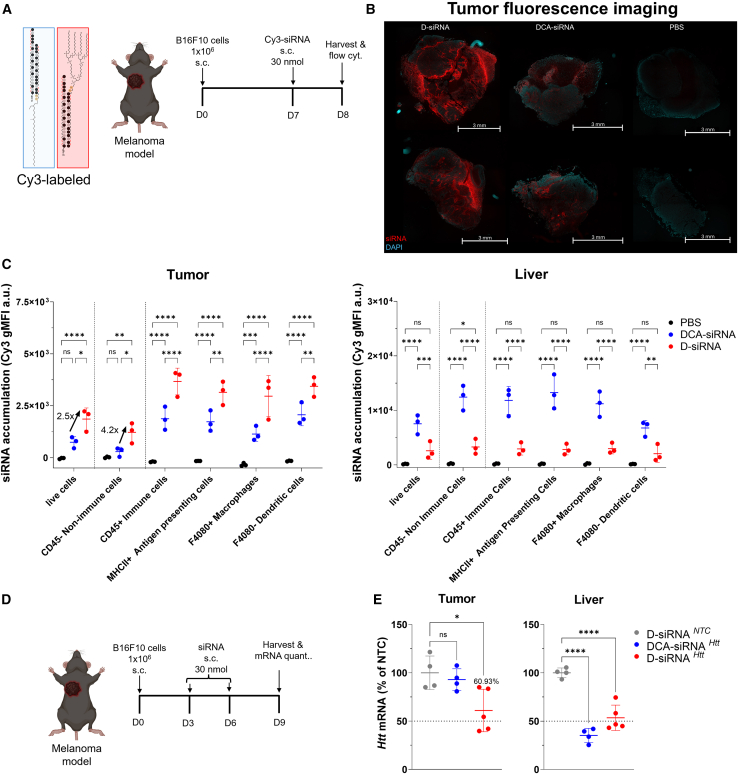
To evaluate tumor delivery following improved plasma circulation, we utilized a B16F10 melanoma tumor model.20 Once palpable tumors (∼5 × 5 mm) formed, Cy3-labeled D-siRNAs and DCA-siRNAs (30 nmol) were s.c. injected (Figure 2A). Twenty-four hours post-injection, tumors were harvested for fluorescence microscopy and flow cytometry analysis (Figures 2B and 2C). Fluorescence imaging revealed enhanced D-siRNA distribution within tumors, evidenced by increased Cy3 signal intensity (in red) (Figure 2B). Flow cytometry of single-cell suspensions was conducted to quantify cellular uptake, demonstrating a ∼2.5-fold overall increase (∗p = 0.01) in D-siRNA delivery compared to DCA-siRNA (Figure 2C, gating strategy provided in Figure S1). Notably, D-siRNA delivery to CD45-negative tumor parenchyma cells increased ∼4.2-fold (∗p = 0.03), whereas delivery to antigen-presenting cells, macrophages, and dendritic cells increased overall by ∼2-fold. This demonstrates that D-siRNA is able to improve delivery to major cell types in the tumor tissue, including parenchyma cell types and resident immune cells. Conversely, DCA-siRNA exhibited higher liver uptake. These findings demonstrate that D-siRNA significantly improves delivery to both tumor parenchyma and resident immune cells, resulting in an enhanced tumor-to-liver ratio compared to DCA-siRNA.
Figure 2.
In vivo delivery and efficacy of D-siRNA and DCA-siRNA in a melanoma model
(A) Schematic of study assessing the delivery of Cy3-labeled D-siRNA and DCA-siRNA to melanoma tumors (n = 3/group). (B) Fluorescent microscopy imaging of sample tumors. (C) Flow cytometry quantification of siRNA delivery to tumor and liver tissues to various cell types. Data represented as geometric mean fluorescent intensity (gMFI) (n = 3, mean ± SD; two-way ANOVA with Tukey’s multiple comparison tests; n.s.: not significant; ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, ∗∗∗∗p < 0.0001). (D) Schematic of study to measure mRNA silencing within tumor and liver tissues. Htt targeting siRNAs were administered at two doses (30 nmol/dose; s.c.), followed by tissue harvest 3 days after last dose and (E) mRNA quantification of Htt mRNA in tumor and liver. Htt mRNA levels were quantified by QuantiGene 2.0 assay (n = 4–5, mean ± SD; one-way ANOVA with Dunnet’s multiple comparison tests; n.s.: not significant; ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001).
To determine whether improved tumor delivery translated to functional gene silencing, we evaluated Htt knockdown in tumor-bearing mice. Htt gene was used as a control because it is ubiquitously expressed in tumor tissues, allowing the knockdown to be better measured without being significantly impacted by tumor-infiltrating immune cell populations. Mice received two doses (30 nmol/dose, ∼15 mg/kg) of Htt-targeting siRNAs (D-siRNAHtt, DCA-siRNAHtt, or non-targeting D-siRNANTC) on days 3 and 6 after tumor inoculation. Tumors and livers were harvested on day 9, 3 days after the final dose (Figure 2D). mRNA quantification revealed robust Htt silencing (∼40%, ∗p = 0.01) in tumors treated with D-siRNA, whereas DCA-siRNA showed minimal effect. Both D-siRNA and DCA-siRNA effectively silenced Htt in the liver, with DCA-siRNA demonstrating slightly higher efficacy. These results align with the biodistribution data, suggesting that enhanced D-siRNA tumor accumulation might be attributed to the effective gene silencing.
JAK1-targeting D-siRNAs support immunotherapy in melanoma
Recent work has reported benefits of interferon gamma pathway inhibition in multiple cancers to complement immunotherapy, including lung cancer and melanoma.15,16,21 Combining JAK1/2- or JAK1-specific inhibitors with anti-PD1 immune checkpoint inhibitors (ICIs) has demonstrated improved tumor response and reduced T cell exhaustion.15 We recently developed a potent JAK1-selective siRNA capable of robust systemic JAK1 silencing in both mouse and human tissues.22 To investigate whether JAK1-targeting siRNAs could augment PD1-based ICI, we treated melanoma-bearing mice with anti-PD1 (250 μg, i.p., every other day) in combination with either D-siRNAJak1, DCA-siRNAJak1, or non-targeting D-siRNANTC (60 nmol, ∼30 mg/kg, s.c. every other day) (Figure 3A). Mice receiving the D-siRNAJak1 significantly improved the response to PD-1 treatment, resulting in slower tumor growth compared to D-siRNANTC (∗p = 0.04), whereas DCA-siRNAJak1 did not result in a significant effect (Figure 3B). Notably, this enhanced efficacy was further highlighted when tumor volume was normalized to the initial volume, demonstrating robust growth inhibition (Figure 3C). A replicate study was also conducted, showing similar and consistent results of D-siRNAJak1 supplementing PD-1 ICI treatment (Figure S2). These findings suggest that D-siRNAs effectively deliver JAK1-targeting siRNA payloads to melanoma tumors, thereby enhancing ICI efficacy.
Figure 3.
Targeting JAK1 with siRNAs as an adjuvant to PD1 immunotherapy
(A) Schematic of study assessing the impact of JAK1 inhibition using D-siRNA or DCA-siRNA in combination with PD-1 immunotherapy as a treatment for melanoma (n = 4–7/group). (B) Plot of tumor volumes and (C) relative tumor growth rate (tumor volume ratio to first day of treatment). Each line represents a mouse; arrows indicate treatment (two-way ANOVA comparison to D-siRNANTC; n.s.: not significant; ∗p < 0.05).
Discussion
The recent clinical success of siRNA therapeutics has fueled significant interest in expanding their application beyond the liver, particularly for targeted delivery to solid tumors.2,3 The potential for siRNA in cancer treatment is well recognized, offering selective target inhibition, low toxicity, and potent efficacy compared to other modalities like small molecule or peptide inhibitors.23 However, clinical translation of siRNA in oncology has been hindered by challenges in achieving sufficient and effective delivery to the tumor parenchyma.3,5,6 Utilizing albumin as a carrier is a promising strategy to overcome these limitations, as it prolongs drug circulation and enhances accumulation in the tumor microenvironment due to the reliance of tumor cells on albumin for proliferation.6 Lipid conjugation to siRNAs has also been explored to promote interactions with plasma proteins and facilitate extrahepatic delivery, including some instances of tumor microenvironment targeting.17,24 In this study, we demonstrate that a selective albumin-binding dendritic conjugate (D-siRNA) significantly enhances plasma circulation and enables productive delivery to a diverse range of cell types within the solid tumor microenvironment, including both malignant and resident immune cells. Notably, unlike previous studies where lipid conjugation primarily targeted cells within the microenvironment, excluding malignant cells,24 our albumin-binding D-siRNAs achieved effective delivery and gene silencing in both immune and non-immune tumor cells. In contrast, the non-albumin-selective DCA-siRNA did not support significant delivery or silencing. This highlights the critical role of strong and selective albumin binding in achieving comprehensive and functional siRNA delivery throughout solid tumors. A previous research study, conducted in immunocompromised mice, demonstrated enhanced tumor delivery using engineered di-lipidic conjugates with improved albumin-binding properties,11 which also supports this strategy. By utilizing immunocompetent wild-type mice, our work provides a more clinically relevant assessment of D-siRNA efficacy.
JAK inhibition has recently been reported to play a crucial role supporting immunotherapy treatment in multiple cancers, such as Hodgkin lymphoma and non-small cell lung cancer.15,16 In these malignancies, chronic inflammation drives elevated expression of interferon-stimulated genes within both malignant and resident immune cells, resulting in high levels of programmed death ligand 1 (PD-L1).21 Prior investigations employed small molecule inhibitors targeting JAK1/JAK2 to augment PD-1 immunotherapy.15,16 In contrast, our study presents the first demonstration of JAK1-selective inhibition using siRNAs, leading to significant slowing of tumor growth and improved PD-1 ICI response. The ability of albumin-binding D-siRNAs to effectively internalize into both immune and non-immune cells within the tumor parenchyma is pivotal. This is because PD-L1 overexpression occurs across these diverse cell populations, benefiting from JAK1 downregulation in both cell types to enhance therapeutic outcomes.21 A key technical challenge in this study is to accurately quantify JAK1 silencing within the tumor tissues at the study endpoint. This difficulty could be attributed to significant immune cell infiltration, as these cells also express considerable JAK1, introducing expression variability that is hard to deconvolute. However, the substantial tumor growth inhibition observed suggests that JAK1 silencing in the resident tumor cells remains effective, likely mirroring the levels achieved in our short-term HTT-siRNA study. Overall, this pilot work offers a phenotypic observation of JAK1 modulation in tumor parenchyma to enhance anti-tumor immunotherapy, but future studies are required to further examine T cell infiltration and exhaustion to confirm the hypothesized mechanisms by which JAK1 inhibition enhances ICI efficacy in melanoma.15,16
In the comparison of albumin vs. low-density lipoprotein (LDL) binding conjugates (D-siRNA vs. DCA-siRNA, respectively), we observe a more favorable tumor-to-liver distribution ratio by the former. This resulted in an increased tumor accumulation that functionally translated into improved gene silencing and slowing of tumor growth. However, it is crucial to acknowledge that tumor accumulation of the injected dose remained lower than liver accumulation. Additional chemical engineering efforts are still needed to further enhance functional tumor delivery and minimize hepatic exposure. Nevertheless, our work demonstrates that modulation of serum binding properties is a promising strategy for optimizing tumor-targeted siRNA delivery.
This work provides proof of concept that albumin-binding D-siRNAs enable improved and functional delivery to tumor sites, supporting their development as systemically administered adjuvants to cancer immunotherapy. Although demonstrated in a melanoma model, testing in other cancer models will further validate D-siRNAs as a robust platform for functional delivery to diverse solid tumors. Consequently, this will unlock opportunities to silence a wider array of targets critical for tumor progression across multiple cancer types.
Materials and methods
Oligonucleotide synthesis, deprotection, and purification
Oligonucleotides were synthesized and purified as previously described.12,25 Briefly, oligonucleotides at a 10-μmol using a MerMade 12 (BioAutomation) synthesizer. Standard RNA 2′-O-methyl and 2′-fluoro modifications were applied for improving siRNA stability (ChemGenes and Hongene). The sense strand with docosanoic acid was made using docosanoic-acid-functionalized controlled pore glass (CPG) (Hongene) as previously described.25 The sense strand with the dendritic conjugate was synthesized on CPG functionalized with UnyLinker (ChemGenes), and commercially available amidites (Cy3, C6, C12, and symmetrical branching from ChemGenes) were used to build the dendritic moiety on the 5′ end as previously described.12,13 All sense strands had a 2dT spacer in between the strand and the conjugate. Antisense strands were synthesized on CPG functionalized with an Unylinker (ChemGenes), and custom 5’-(E)-vinyl phosphonate phosphoramidite (ChemGenes) was used to introduce at the 5′ end. All strands were cleaved and deprotected using 28% aqueous ammonium hydroxide solution with 3% Diethylamine for 20 h at 55°C, followed by drying under vacuum at 60°C, and resuspended in Millipore H2O. Compounds were purified using an Agilent Prostar System (Agilent Technologies, Santa Clara, CA) on a C18 column for lipid-conjugated sense strands and on an ion-exchange column for antisense strands. Pure compounds were desalted by amicon filtration with 3 kDa cutoff and characterized by liquid chromatography-mass spectrometry (LC/MS) analysis on an Agilent 6530 accurate-mass quadrupole time-of-flight (Q-TOF) LC/MS (Agilent Technologies). All synthesized siRNA sequences are provided in Table S1.
In vivo mouse studies
Wild-type strain FVB/NJ female mice age 8–10 weeks (The Jackson Laboratory) were used for the pharmacokinetics and plasma circulation of lipid conjugated siRNAs. Wild-type C57BL/6 male mice of age 8–12 weeks (inbred inhouse) were used for the fluorescent siRNA tumor and cell-type distribution, mRNA silencing, and melanoma tumor model. Animal studies were performed in accordance with the guidelines of University of Massachusetts Chan Medical School Institutional Animal Care and Use Committee (IACUC). All procedures were approved under protocols #202000010 (Khvorova Laboratory) and #201900330 (Harris Laboratory), in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. The colonies were maintained and housed at pathogen-free animal facilities at UMass Chan Medical School with 12 h light/12 h dark cycle at controlled temperature (23 ± 1°C) and humidity (50% ± 20%) with free access to food and water.
Plasma clearance and pharmacokinetics of siRNAs
Plasma circulation of siRNA was conducted via serial blood microsampling as previously described.26 Briefly, FVB/NJ female mice age 8–10 weeks (n = 4) were injected either i.v. (20 nmol siRNA) or s.c. (15 nmol siRNA) with either DCA or D-siRNA, followed by blood microsampling (10–20 μL). The saphenous vein was punctured using a 30G1/2 sterile lancet, and blood droplets were collected using Microvette CB300 K2R tubes (Sarstedt, 16.444.100). For i.v. injection, blood was sampled at 1 min, 5 min, 15 min, 30 min, 1 h, 2 h, 4 h, 6 h, and 24 h; for s.c. injection blood was collected at 5 min, 15 min, 30 min, 1 h, 3 h, 6 h, 9 h, and 24 h. Blood was then centrifuged for 10 min at 10,000 RPM at 4°C to isolate plasma. Plasma was then lysed (2 μL plasma in 200 μL homogenizing buffer [Affymetrix] containing 0.2 mg mL−1 proteinase K [Invitrogen]) at 65°C for 30 min. Quantification of oligonucleotides in plasma was conducted via PNA-hybridization assay as previously described.19,26 The probe used to hybridize to the antisense strand was purchased from PNAbio, with a sequence: Alexa 488-OO-AAATATCAGTAAAGAGATTAA. DCA-siRNA plasma circulation data in the s.c. study have been previously reported by Yamada et al.19
Melanoma tumor model
Melanoma tumor model was generated as previously described.20 The murine melanoma cell line B16F10 was obtained from American Type Culture Collection (ATCC) and used for tumor generation. Cells were cultured in DMEM (Corning) supplemented with 10% fetal bovine serum (FBS) and 100 U/mL penicillin/streptomycin (PS) and maintained at 37°C in a humidified atmosphere of 5% CO2. Cells used were between passages 4 and 9 to abrogate the heterogeneity introduced by long-term culture. For inoculation, cells (0.5–1 × 106) were resuspended in 100 μL PBS before s.c. injection into wild-type C57BL/6 male mice of age 8–12 weeks in the upper right flank. Tumor sizes were monitored by measuring in two dimensions using a caliper, and tumor volume is calculated based on the formula: V (mm3) = (LxW2)/2.
Fluorescence imaging and flow cytometry for tumor delivery
Wild-type C57BL/6 male mice of age 8–12 weeks (n = 3) were inoculated in the upper right flank with 1 × 106 B16F10 melanoma cells, as previously described.20 When tumors were palpable (∼5 mm × 5 mm), Cyanine-3-labeled siRNAs were injected s.c., followed by organ collection (tumor and liver) for imaging and flow cytometry. For imaging, tissues were incubated overnight in formalin, followed by washing with PBS, and provided to histology core for slicing and mounting using ProLong Gold Antifade Mountant with DAPI staining for nuclei.
For flow cytometry, tissues were minced and pushed through 70 μm strainer, followed by incubation with DMEM media (Corning) supplemented with 1 mg/mL Collagenase D (Sigma Aldrich) and 0.5 mg/mL DNase1 (Sigma Aldrich) at 37°C for 30 min. Digestion was stopped by transferring to ice, followed by centrifugation at 800 g for 10 min, and suspending in 2mL RBC lysis buffer hybrid max (Sigma Aldrich) to eliminate red blood cells. After 5-min incubation, lysis buffer was neutralized with DMEM media, and cells were centrifuged at 800 g for 10 min and resuspended in FACS staining buffer (Biolegend). The following dyes and anti-mouse antibodies were used for staining (Biolegend), with gating strategy provided in Figure S1: Live/dead Zombie NIR (#423105), APC-Cy7 anti-CD45 (#127525), Brilliant Violet 711 anti- I-A/I-E (MHCII) (#107643), Brilliant Violet 421 anti-F40/80 (#123131), and Pacific Blue anti-CD11c (#117321). InVivoMAb anti-mouse CD16/CD32 clone 2.4G2 (BioXcell, #BE0307) was used as an Fc Blocker during staining. Post staining, cells were washed and fixed with 4% paraformaldehyde, followed by resuspension in FACS buffer before readout.
mRNA silencing in tumor model
Wild-type C57BL/6 male mice of age 8–12 weeks (n = 5) were inoculated in the upper right flank with 1 × 106 B16F10 melanoma cells. When tumors were palpable (∼5 mm × 5 mm) at day 3, Htt-targeting siRNAs were administered s.c. on day 3 and day 6 at a dose of 30 nmol (150 μL of 200 μM). Mice were sacrificed at day 9, and tumor and liver tissues collected and stored in RNAlater (Sigma-Aldrich) at 4°C overnight. mRNA levels were then quantified using the QuantiGene (QG) 2.0 assay (Affymetrix), after 1 × 3 mm punch biopsies were taken and homogenized as previously described.12 Briefly, biopsies were placed in QIAGEN collection microtubes holding 3 mm tungsten beads and lysed in 400 μL homogenizing buffer (Affymetrix) containing 0.2 mg/mL proteinase K (Invitrogen) using a QIAGEN TissueLyser II. Samples were then incubated for 1 h at 55°C–60°C. Lysates and diluted probe sets (mouse Htt or mouse Hprt) were added to the branched DNA (bDNA) capture plate, to quantify mRNA levels via luminescence as previously described.12,19
Tumor growth suppression with PD1 antibody and Jak1-siRNA in melanoma model
Wild-type C57BL/6 male mice of age 8–12 weeks mice were inoculated in the upper right flank with 0.5 × 106 B16F10 melanoma cells. When tumors were palpable (∼5 mm × 5 mm) at day 7, mice were randomized (n = 7 for D-siRNAJak1 and D-siRNANTC and n = 4 for DCA-siRNAJak1) and dosed with siRNAs targeting Jak1 (60 nmol) s.c. every other day, in addition to intraperitoneal injection of PD-1 antibody (250 μg) every other day. Tumor volumes were calculated by measuring length and width using calipers every other day. Mice were humanely euthanized when endpoint of study is reached, typically when tumor sizes exceeded a volume of 1,200 mm3 and mice health was compromised with low body score. Ulcerated tumor-bearing mice were excluded, and two independent experiments were conducted.
Statistical analysis
Data were analyzed using GraphPad Prism 10 software (GraphPad Software, Inc.). Sample size and statistical methods used for each experiment are described in the corresponding figure legends. Differences in all comparisons were considered significant at p < 0.05.
Data availability
All data from this work are included. Request for additional data or materials should be directed to the corresponding authors Dr. John E. Harris or Dr. Anastasia Khvorova.
Acknowledgments
Figures were created with BioRender.com. This project was supported by National Institutes of Health (grant no. R35 GM131839 and S10 OD020012 to A.K. and K99 AR082987 to Q.T.) and UMass System’s Office of Technology Commercialization & Ventures (OTCV) Technology Development Fund (awarded to H.H.F., Q.T., J.E.H., and A.K.). H.H.F. was partially supported for parts of the project, by the Natural Sciences and Engineering Research Council of Canada of Canada (Discovery and NSERC CREATE Training grant) and the Fonds de Recherche du Quebec AUDACE program.
Author contributions
H.H.F., H.F.S., J.E.H., and A.K. conceived the project. H.H.F. synthesized all siRNA compounds. H.H.F., Q.T., A.S., K.O, K.Y.G., and N.M. conducted the in vivo studies. M.O.R. assisted with in vivo studies and prepared all in vitro reagents. H.H.F. prepared the manuscript draft; Q.T., H.F.S., J.E.H., and A.K. reviewed and edited the manuscript. All authors have proofread and approved the manuscript for publication.
Declaration of interests
Q.T., H.H.F., K.O., H.F.S., J.E.H., and A.K. are listed as inventors of RNAi technology patents or patent applications related to this work.
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.omtn.2025.102579.
Contributor Information
John E. Harris, Email: john.harris@umassmed.edu.
Anastasia Khvorova, Email: anastasia.khvorova@umassmed.edu.
Supplemental information
References
- 1.Khvorova A. siRNAs-A New Class of Medicines. JAMA. 2023;329:2185–2186. doi: 10.1001/jama.2023.4570. [DOI] [PubMed] [Google Scholar]
- 2.Belgrad J., Fakih H.H., Khvorova A. Nucleic Acid Therapeutics: Successes, Milestones, and Upcoming Innovation. Nucleic Acid Therapeut. 2024;34:52–72. doi: 10.1089/nat.2023.0068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tang Q., Khvorova A. RNAi-based drug design: considerations and future directions. Nat. Rev. Drug Discov. 2024;23:341–364. doi: 10.1038/s41573-024-00912-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Das M., Musetti S., Huang L. RNA Interference-Based Cancer Drugs: The Roadblocks, and the "Delivery" of the Promise. Nucleic Acid Therapeut. 2019;29:61–66. doi: 10.1089/nat.2018.0762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tian Z., Liang G., Cui K., Liang Y., Wang Q., Lv S., Cheng X., Zhang L. Insight Into the Prospects for RNAi Therapy of Cancer. Front. Pharmacol. 2021;12 doi: 10.3389/fphar.2021.644718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hoogenboezem E.N., Duvall C.L. Harnessing albumin as a carrier for cancer therapies. Adv. Drug Deliv. Rev. 2018;130:73–89. doi: 10.1016/j.addr.2018.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cho H., Jeon S.I., Ahn C.H., Shim M.K., Kim K. Emerging Albumin-Binding Anticancer Drugs for Tumor-Targeted Drug Delivery: Current Understandings and Clinical Translation. Pharmaceutics. 2022;14 doi: 10.3390/pharmaceutics14040728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schilling U., Friedrich E.A., Sinn H., Schrenk H.H., Clorius J.H., Maier-Borst W. Design of compounds having enhanced tumour uptake, using serum albumin as a carrier—part II. In vivo studies. Int. J. Rad. Appl. Instrum. B. 1992;19:685–695. doi: 10.1016/0883-2897(92)90103-6. [DOI] [PubMed] [Google Scholar]
- 9.Stehle G., Sinn H., Wunder A., Schrenk H.H., Stewart J.C., Hartung G., Maier-Borst W., Heene D.L. Plasma protein (albumin) catabolism by the tumor itself—implications for tumor metabolism and the genesis of cachexia. Crit. Rev. Oncol. Hematol. 1997;26:77–100. doi: 10.1016/s1040-8428(97)00015-2. [DOI] [PubMed] [Google Scholar]
- 10.Kratz F. Albumin as a drug carrier: design of prodrugs, drug conjugates and nanoparticles. J. Contr. Release. 2008;132:171–183. doi: 10.1016/j.jconrel.2008.05.010. [DOI] [PubMed] [Google Scholar]
- 11.Hoogenboezem E.N., Patel S.S., Lo J.H., Cavnar A.B., Babb L.M., Francini N., Gbur E.F., Patil P., Colazo J.M., Michell D.L., et al. Structural optimization of siRNA conjugates for albumin binding achieves effective MCL1-directed cancer therapy. Nat. Commun. 2024;15:1581. doi: 10.1038/s41467-024-45609-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fakih H.H., Tang Q., Summers A., Shin M., Buchwald J.E., Gagnon R., Hariharan V.N., Echeverria D., Cooper D.A., Watts J.K., et al. Dendritic amphiphilic siRNA: Selective albumin binding, in vivo efficacy, and low toxicity. Mol. Ther. Nucleic Acids. 2023;34 doi: 10.1016/j.omtn.2023.102080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lacroix A., Fakih H.H., Sleiman H.F. Detailed cellular assessment of albumin-bound oligonucleotides: Increased stability and lower non-specific cell uptake. J. Contr. Release. 2020;324:34–46. doi: 10.1016/j.jconrel.2020.04.020. [DOI] [PubMed] [Google Scholar]
- 14.Sarli S.L., Fakih H.H., Kelly K., Devi G., Rembetsy-Brown J.M., McEachern H.R., Ferguson C.M., Echeverria D., Lee J., Sousa J., et al. Quantifying the activity profile of ASO and siRNA conjugates in glioblastoma xenograft tumors in vivo. Nucleic Acids Res. 2024;52:4799–4817. doi: 10.1093/nar/gkae260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mathew D., Marmarelis M.E., Foley C., Bauml J.M., Ye D., Ghinnagow R., Ngiow S.F., Klapholz M., Jun S., Zhang Z., et al. Combined JAK inhibition and PD-1 immunotherapy for non-small cell lung cancer patients. Science. 2024;384 doi: 10.1126/science.adf1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zak J., Pratumchai I., Marro B.S., Marquardt K.L., Zavareh R.B., Lairson L.L., Oldstone M.B.A., Varner J.A., Hegerova L., Cao Q., et al. JAK inhibition enhances checkpoint blockade immunotherapy in patients with Hodgkin lymphoma. Science. 2024;384 doi: 10.1126/science.ade8520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Osborn M.F., Coles A.H., Biscans A., Haraszti R.A., Roux L., Davis S., Ly S., Echeverria D., Hassler M.R., Godinho B.M.D.C., et al. Hydrophobicity drives the systemic distribution of lipid-conjugated siRNAs via lipid transport pathways. Nucleic Acids Res. 2019;47:1070–1081. doi: 10.1093/nar/gky1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Godinho B.M.D.C., Knox E.G., Hildebrand S., Gilbert J.W., Echeverria D., Kennedy Z., Haraszti R.A., Ferguson C.M., Coles A.H., Biscans A., et al. PK-modifying anchors significantly alter clearance kinetics, tissue distribution, and efficacy of therapeutics siRNAs. Mol. Ther. Nucleic Acids. 2022;29:116–132. doi: 10.1016/j.omtn.2022.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yamada K., Hariharan V.N., Caiazzi J., Miller R., Ferguson C.M., Sapp E., Fakih H.H., Tang Q., Yamada N., Furgal R.C., et al. Enhancing siRNA efficacy in vivo with extended nucleic acid backbones. Nat. Biotechnol. 2024 doi: 10.1038/s41587-024-02336-7. [DOI] [PubMed] [Google Scholar]
- 20.Fukuda K., Okamura K., Riding R.L., Fan X., Afshari K., Haddadi N.S., McCauley S.M., Guney M.H., Luban J., Funakoshi T., et al. AIM2 regulates anti-tumor immunity and is a viable therapeutic target for melanoma. J. Exp. Med. 2021;218 doi: 10.1084/jem.20200962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Benci J.L., Xu B., Qiu Y., Wu T.J., Dada H., Twyman-Saint Victor C., Cucolo L., Lee D.S.M., Pauken K.E., Huang A.C., et al. Tumor Interferon Signaling Regulates a Multigenic Resistance Program to Immune Checkpoint Blockade. Cell. 2016;167:1540–1554.e12. doi: 10.1016/j.cell.2016.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tang Q., Fakih H.H., Zain Ui Abideen M., Hildebrand S.R., Afshari K., Gross K.Y., Sousa J., Maebius A.S., Bartholdy C., Søgaard P.P., et al. Rational design of a JAK1-selective siRNA inhibitor for the modulation of autoimmunity in the skin. Nat. Commun. 2023;14:7099. doi: 10.1038/s41467-023-42714-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yue P., Turkson J. Targeting STAT3 in cancer: how successful are we? Expet Opin. Invest. Drugs. 2009;18:45–56. doi: 10.1517/13543780802565791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ganesh S., Kim M.J., Lee J., Feng X., Ule K., Mahan A., Krishnan H.S., Wang Z., Anzahaee M.Y., Singhal G., et al. RNAi mediated silencing of STAT3/PD-L1 in tumor-associated immune cells induces robust anti-tumor effects in immunotherapy resistant tumors. Mol. Ther. 2024;32:1895–1916. doi: 10.1016/j.ymthe.2024.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Biscans A., Coles A., Haraszti R., Echeverria D., Hassler M., Osborn M., Khvorova A. Diverse lipid conjugates for functional extra-hepatic siRNA delivery in vivo. Nucleic Acids Res. 2019;47:1082–1096. doi: 10.1093/nar/gky1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Godinho B.M.D.C., Gilbert J.W., Haraszti R.A., Coles A.H., Biscans A., Roux L., Nikan M., Echeverria D., Hassler M., Khvorova A. Pharmacokinetic Profiling of Conjugated Therapeutic Oligonucleotides: A High-Throughput Method Based Upon Serial Blood Microsampling Coupled to Peptide Nucleic Acid Hybridization Assay. Nucleic Acid Therapeut. 2017;27:323–334. doi: 10.1089/nat.2017.0690. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data from this work are included. Request for additional data or materials should be directed to the corresponding authors Dr. John E. Harris or Dr. Anastasia Khvorova.