Abstract
Netherton syndrome (NS) is a rare autosomal recessive genodermatosis caused by pathogenic mutations in the serine protease inhibitor Kazal-type 5 (SPINK5) gene, leading to impaired skin barrier function and immune dysregulation. It is characterized by congenital ichthyosis, trichorrhexis invaginata, and severe atopy. Herein, we present the case of a young boy with NS, confirmed by genetic analysis revealing a homozygous splice site mutation in SPINK5 (c.1302+5G>C). The patient was treated with intravenous immunoglobulin due to frequent infections that required multiple hospital admissions. When he was 6 years old, dupilumab was added to reduce skin inflammation and improve skin barrier function before food reintroduction. He demonstrated substantial clinical benefits, with marked relief from pruritus resulting in better quality of life. Additionally, he exhibited notable hair growth. Serologically, total his serum IgE levels decreased from 1078 IU/mL to 55.8 IU/mL following dupilumab therapy. This case highlights the potential benefits of an integrated therapeutic approach in management of this challenging condition.
Key words: Netherton syndrome, trichorrhexis invaginata, bamboo hair, SPINK5, IVIG, dupilumab, food allergy
Netherton syndrome (Online Mendelian Inheritance in Man [OMIM] no. 256500) is a rare autosomal recessive genodermatosis characterized by congenital ichthyosis, trichorrhexis invaginata (hair shaft defects), and severe atopy with a high IgE level.1 NS is caused by pathogenic mutations in the serine protease inhibitor Kazal-type 5 (SPINK5) gene, which encodes for the serine protease inhibitor lympho-epithelial Kazal-type inhibitor (LEKTI).1 LEKTI plays a critical role in maintaining skin barrier function and immune regulation by modulating proteolytic activity and inflammatory pathways.1, 2, 3
The estimated incidence of NS is approximately 1 in 200,000 newborns.1 Patients typically present at birth or during early infancy with generalized, scaly erythroderma that can resemble erythrodermic psoriasis and complicate diagnosis.4 Patients with NS are at risk of severe complications such as sepsis, failure to thrive, and hypernatremic dehydration.3,4 A hallmark of NS is trichorrhexis invaginata (also known as "bamboo hair"), in which the hair shaft invaginates on itself, creating a characteristic appearance.4 Additionally, patients often exhibit significant atopic manifestations, including eczema and markedly elevated serum IgE levels. Although most patients present with the classic triad of symptoms, in rare cases, ichthyosis linearis circumflexa may be the only clinical manifestation of NS.5 To date, there is no specific or curative treatment for NS, and management remains focused on symptomatic relief and prevention of complications.1,3 Herein, we present the case of a young boy with NS who demonstrated significant clinical improvement through a comprehensive therapeutic regimen, including immunoglobulin replacement therapy, dupilumab, and targeted food elimination.
Case presentation
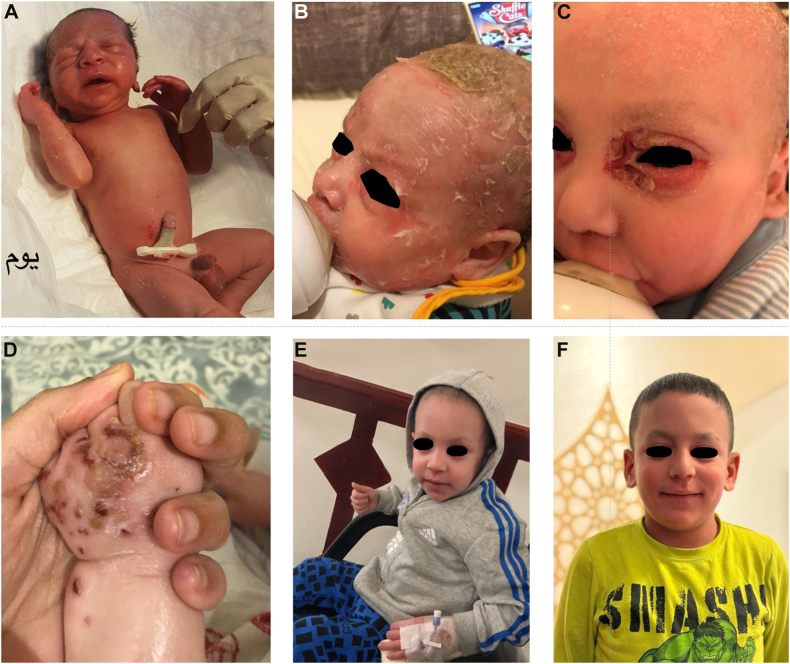
A 7-month-old Saudi boy, born to first-degree consanguineous parents, presented to the clinic with generalized erythema, scaling, pruritus, and sparse hair. He was delivered at 38 weeks via spontaneous vaginal delivery. At birth, he was noted to have severe generalized erythema that prompted neonatal intensive care unit admission for observation; he was discharged on day 5 (Fig 1, A). In the subsequent weeks, the patient’s erythema persisted, and he developed scales over his scalp and extremities, along with perioral fissuring (Fig 1, B). His parents also noted sparse hair on his scalp, eyebrows, and eyelashes, characterized by short, brittle hair prone to breakage. Dermatologic examination, including trichoscopy, revealed trichorrhexis invaginata, a hallmark feature of NS. Whole exome sequencing identified a likely pathogenic homozygous splice site variant in SPINK5, c.1302+5G>C in intron 14, confirming a diagnosis of NS.
Fig 1.
A, Generalized erythroderma at birth. B, Thick scaly plaques over the scalp with persistent erythema and sparse hair. C and D, Recurrent skin infections at age of 6 months. E, Resolution of erythema and scale with hair regrowth after 1 year of IVIG and food elimination. F, Significant resolution of skin lesions and reduction of itch after 5 years of IVIG and food elimination, along with 2 years of dupilumab therapy.
At the age of 18 months, the patient began undergoing intravenous immunoglobulin replacement therapy (IVIG), 500 mg/kg every 4 weeks, because of frequent cutaneous infections that required multiple antibiotics and hospital admissions (Fig 1, C and D). The patient's mother reported notable clinical improvements following the start of IVIG, including improved skin barrier integrity, decreased fissuring, reduced scaling, and fewer superficial bacterial skin infections. An IgE food allergy panel was done; the result was positive for egg and garlic. Targeted food elimination helped maintain dermatologic improvement and prevented flare-ups (Fig 1, E).
When the patient was 6 years old, dupilumab, 300 mg every 4 weeks, was added as an adjunct therapy to reduce skin inflammation and pruritic, as well as to improve skin barrier function before food reintroduction. This decision was based on the potential beneficial effect in modulating immune responses associated with allergic inflammation. The patient demonstrated substantial clinical benefits, with marked relief from pruritus and chronic itching. Relieving the itch helped improve his sleep quality, playtime, focus, and overall quality of life. Additionally, the patient exhibited notable hair growth, with longer strands that resisted immediate breakage. His eyebrow growth improved, particularly in the medial two-thirds, alongside the emergence of sparse eyelashes. Fig 1, F shows significant improvement in the condition of the patient’s skin 2 years after initiation of monthly dupilumab and 6 years of monthly IVIG. Serologically, the patient’s total serum IgE levels decreased from 1078 IU/mL to 55.8 IU/mL (reference range 25-449.7 IU/mL) following dupilumab therapy. Additionally, his levels of food-specific IgE antibodies, such as those for egg and garlic, showed improvement.
Discussion
NS is a rare autosomal recessive disorder that is caused by mutations in the SPINK5 gene and characterized by a triad of congenital ichthyosis, trichorrhexis invaginata (bamboo hair), and severe atopy, as seen in our patient.1 Deficiency of SPINK5 results in unopposed kallikrein activity, leading to the production of proinflammatory mediators such as IL-17, IL-36, and IL-20, along with neutrophilic infiltration.6 NS should be considered in the differential diagnosis of newborns presenting with erythroderma, particularly in regions with high consanguinity rates, such as Saudi Arabia. Early diagnosis is critical, as it can result in life-threatening complications, including hypernatremic dehydration, failure to thrive, and sepsis.3
Treatment for NS is not standardized and has traditionally relied on supportive therapies such as antihistamines, topical corticosteroids, retinoids, emollients, antibiotics, and phototherapy.7,8 More recently, IVIG and biologics such as dupilumab, secukinumab, infliximab, and ixekizumab have shown variable success in small studies.8 Emerging targeted therapies, including kallikrein inhibitors, are currently in the development phase.7
In our patient, a combination of IVIG, dupilumab, and targeted food elimination led to significant clinical improvement. Interestingly, the patient’s pruritus improved markedly after initiation of dupilumab, resulting in an overall enhancement in quality of life. This observation aligns with recent case reports demonstrating the efficacy of dupilumab in managing NS.7 Biologics targeting IL-17A, such as secukinumab and ixekizumab, have also shown promise in improving outcomes in case reports.7
NS is classified as an inborn error of immunity within the spectrum of hyper-IgE syndromes. Immunologically, NS is associated with reduced numbers of memory B cells, decreased numbers of naive CD4+ T cells, elevated IgE levels, and impaired response to polysaccharide antigens.9 Although IgG levels are typically normal, IVIG has been shown to correct some immunologic abnormalities, reduce skin infections, and enhance skin barrier integrity.9 Our patient demonstrated improved skin integrity (reduced fissuring and scaling) and decreased frequency of bacterial infections with maintenance IVIG at a dose of 500 mg/kg every 4 weeks.
In conclusion, this report has highlighted the successful management of NS in a pediatric patient by using a comprehensive treatment approach, including IVIG, dupilumab, and dietary modifications. Continued research is needed to develop more targeted therapies for this rare condition.
Disclosure statement
Disclosure of potential conflict of interest: The authors declare that they have no relevant conflicts of interest.
Footnotes
Written informed consent for participation and for publication of this case report was obtained from the parent.
References
- 1.Barbati F., Giovannini M., Oranges T., Lodi L., Barni S., Novembre E., et al. Netherton syndrome in children: management and future perspectives. Front Pediatr. 2021;9 doi: 10.3389/fped.2021.645259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chavanas S., Bodemer C., Rochat A., Hamel-Teillac D., Ali M., Irvine A.D., et al. Mutations in SPINK5, encoding a serine protease inhibitor, cause Netherton syndrome. Nat Genet. 2000;25 doi: 10.1038/75977. [DOI] [PubMed] [Google Scholar]
- 3.Chiticariu E., Hohl D. Netherton syndrome: insights into pathogenesis and clinical implications. J Invest Dermatol. 2020;140:1129–1130. doi: 10.1016/j.jid.2019.11.007. [DOI] [PubMed] [Google Scholar]
- 4.Boskabadi H., Maamouri G., Mafinejad S. Netherton syndrome, a case report and review of literature. Iran J Pediatr. 2013;23:611–612. [PMC free article] [PubMed] [Google Scholar]
- 5.Guerra L., Fortugno P., Pedicelli C., Mazzanti C., Proto V., Zambruno G., Castiglia D. Ichthyosis linearis circumflexa as the only clinical manifestation of Netherton syndrome. Acta Derm Venereol. 2015;95:720–724. doi: 10.2340/00015555-2075. [DOI] [PubMed] [Google Scholar]
- 6.Petrova E., López-Gay J.M., Fahrner M., Leturcq F., de Villartay J.P., Barbieux C., et al. Comparative analyses of Netherton syndrome patients and Spink5 conditional knock-out mice uncover disease-relevant pathways. Commun Biol. 2024;7:152. doi: 10.1038/s42003-024-05780-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Petrova E., Hovnanian A. Advances in understanding of Netherton syndrome and therapeutic implications. Expert Opinion on Orphan Drugs. 2020;8:455–487. [Google Scholar]
- 8.Nouwen A.E.M., Schappin R., Nguyen N.T., Ragamin A., Bygum A., Bodemer C., et al. Outcomes of systemic treatment in children and adults with Netherton syndrome: a systematic review. Front Immunol. 2022;13 doi: 10.3389/fimmu.2022.864449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eränkö E., Ilander M., Tuomiranta M., Mäkitie A., Lassila T., Kreutzman A., et al. Immune cell phenotype and functional defects in Netherton syndrome. Orphanet J Rare Dis. 2018;13:213. doi: 10.1186/s13023-018-0956-6. [DOI] [PMC free article] [PubMed] [Google Scholar]