Abstract
Background
Numerous clinical studies have suggested that cinnamon supplementation may be effective for cardiovascular disease risk factors, but the findings are controversial. This comprehensive systematic review and meta-analysis aimed to assess the impact of cinnamon supplementation on cardiovascular disease risk factors.
Methods
Relevant studies were identified through electronic searches of databases, including Web of Science, PubMed, Central, Scopus, and Embase, up to July 2024.
Results
Forty-nine studies were included. Cinnamon supplementation significantly reduced WC (SMD = − 0.40; 95% (CI): − 0.73, − 0.06), DBP (SMD = − 1.04; 95% CI: − 1.54, − 0.55), SBP (SMD = − 0.85; 95% CI: − 1.54, − 0.16), fasting glucose (SMD = − 1.28; 95% CI: − 1.65, − 0.90), fasting insulin (SMD = − 0.26; 95% CI: − 0.50, − 0.02), HbA1c (SMD = − 0.71; 95% CI: − 1.02, − 0.40), HOMA-IR (SMD = − 0.54; 95% CI: − 0.82, − 0.26), postprandial blood glucose (SMD = − 2.28; 95% CI: − 3.48, − 1.08), CRP (SMD = − 0.78; 95% CI: − 1.28, − 0.27), LDL-C (SMD = − 0.71; 95% CI: − 1.02, − 0.40), total cholesterol (TC) (SMD = − 1.15; 95% CI: − 1.55, − 0.75), triglycerides (TG) (SMD = − 0.91; 95% CI: − 1.25, − 0.56), and MDA (SMD = − 0.76; 95% CI: − 1.07, − 0.45). Additionally, cinnamon supplementation significantly elevated HDL-C levels (SMD = 0.56; 95% CI: 0.23, 0.89).
Conclusion
Cinnamon supplementation demonstrated significant benefits in improving cardiovascular risk factors. These findings suggest its potential as an adjunct therapy for improving cardiovascular disease risk factors.
Supplementary Information
The online version contains supplementary material available at 10.1186/s41043-025-00967-3.
Keywords: Nutritional supplement, Anthropometric indices, Lipid profile, Glycemic indices, Blood pressure
Introduction
Cardiovascular diseases (CVDs) remain a leading cause of global mortality, with risk factors such as obesity, dyslipidemia, diabetes, and hypertension contributing significantly to their burden [1]. In 2023, the World Heart Federation reported that CVDs accounted for approximately 20.5 million deaths annually, underscoring the need for effective preventive strategies [2]. Lifestyle interventions, including dietary modifications, have gained attention for their potential to mitigate these risk factors [3, 4]. Among dietary components, spices like cinnamon (Cinnamomum verum and related species) have been investigated for their therapeutic effects on cardiovascular health due to their bioactive compounds [5–7].
Cinnamon, derived from the bark of trees in the Cinnamomum genus of the Lauraceae family, is widely used in culinary and medicinal applications across Asia, Australia, and South America [8]. Its key bioactive constituents, including cinnamaldehyde, cinnamic acid, and cinnamate, exhibit antioxidant, anti-inflammatory, and antidiabetic properties that may benefit cardiovascular health [9, 10]. Recent meta-analyses on cinnamon supplementation have shown mixed results. Fateh et al. (2024) found that while cinnamon had no significant effect on low-density lipoprotein cholesterol (LDL-C) levels and total cholesterol (TC), it significantly reduced triglycerides (TG) at doses below 500 mg/day [11]. Another review, covering 28 studies on patients with type 2 diabetes mellitus (T2DM), reported significant improvements in fasting blood glucose (FBG), hemoglobin A1c (HbA1c), and homeostatic model assessment for insulin resistance (HOMA-IR) with doses of 2 g/day or more, alongside benefits for lipid profiles and body mass index (BMI) [12]. However, a review of 23 studies found no significant changes in LDL-C or high-density lipoprotein cholesterol (HDL-C), suggesting limited impact on these specific cardiovascular markers [13]. Additionally, an umbrella review of seven meta-analyses highlighted substantial reductions in body weight and BMI at doses of 3 g/day or higher, although effects on waist circumference (WC) were less notable [14]. A more recent analysis confirmed that cinnamon supplementation could lower TG and LDL-C and increase HDL-C, but had no effect on FBG [15]. These inconsistencies highlight the need for a comprehensive, high-quality GRADE-assessed meta-analysis that integrates both subgroup and dose–response analyses to clarify cinnamon's true impact on cardiovascular risk factors.
Despite growing interest in cinnamon as a complementary therapy, consensus is lacking regarding its optimal dosing, long-term safety, and mechanisms of action across diverse populations. Moreover, most previous reviews have focused on single outcomes (e.g., glycemic control or lipid profile) rather than evaluating a broad spectrum of cardiovascular risk markers collectively. To address this literature gap, we conducted a systematic review and meta-analysis assessing the effects of cinnamon supplementation on multiple cardiovascular risk factors—including lipid profile, glycemic indices, blood pressure, and anthropometric measures—with an emphasis on dose–response relationships. By analyzing diverse subgroups and integrating dose–response data, we aim to clarify optimal dosages and therapeutic contexts for cinnamon use in CVD prevention. Our rigorous application of the GRADE framework ensures high-quality evidence, offering valuable guidance for healthcare professionals and policymakers in developing evidence-based strategies for cardiovascular health management.
Methods
Protocol and registration
This systematic review and meta-analysis was conducted according to a pre-specified protocol and adhered to the 2015 Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [16]. The study is registered with the International Prospective Register of Systematic Reviews (PROSPERO), ensuring transparency and rigorous research standards. The registration number is CRD42024607218, available for public verification.
Literature search and study selection
A comprehensive literature search was conducted through July 2024 across several databases: Cochrane Central Register of Controlled Trials (Central), Web of Science, Medline, Scopus, and Embase. The search strategy targeted studies investigating cinnamon’s effects on health markers and used relevant MeSH and EMTREE terms such as “cinnamon” and “clinical trial” (Supplementary Table 1). We also examined references in key articles, systematic reviews, and meta-analyses for additional studies.
The primary outcomes assessed were body fat percentage (BF), BMI, WC, body weight, waist-to-hip ratio (WHR), diastolic (DBP) and systolic blood pressure (SBP), fasting glucose, fasting insulin, postprandial glucose, HbA1c, HOMA-IR, quantitative insulin sensitivity check index (QUICKI), C-reactive protein (CRP), interleukin-6 (IL-6), HDL-C, TC, TG, alanine transaminase (ALT), aspartate transaminase (AST), alkaline phosphatase (ALP), blood urea nitrogen (BUN), malondialdehyde (MDA), and total antioxidant capacity (TAC).
Inclusion/exclusion criteria and data extraction
Randomized controlled trials (RCTs) involving human participants were included if they used a placebo-controlled, parallel, or crossover design and were published in English with at least one week of intervention. Exclusion criteria included non-RCTs, animal and in vitro studies, observational studies (e.g., cohort, case–control, cross-sectional), reviews, case reports, conference abstracts, and editorials. Studies combining cinnamon with other interventions were excluded unless the effects of cinnamon could be independently analyzed. EndNote X7 software was used to consolidate search results and manage duplicates.
Two reviewers (H.M. and A.A.) independently screened titles and abstracts, followed by full-text reviews to assess eligibility according to inclusion criteria. Discrepancies were resolved with input from a third reviewer (A.J.). Data extraction was performed independently by two authors (A.J. and V.M.) using a standardized form to capture study characteristics, population demographics, intervention specifics, and outcome measures.
Quality assessment and evidence grading
The methodological quality of included studies was assessed using the Cochrane Risk of Bias tool, covering sequence generation, allocation concealment, blinding, incomplete outcome data, selective reporting, and other biases [17]. Two reviewers (H.M. and M.A.) conducted this assessment, with disagreements resolved through discussion or third-party consultation (A.J.).
The certainty of the evidence was evaluated using the GRADE approach, taking into account risk of bias, consistency, precision, directness, and additional factors (e.g., dose–response relationship, effect size) [18]. Evidence quality was rated as high, moderate, low, or very low for each outcome, with two reviewers (A.J. and H.M.) conducting assessments and a third reviewer (V.M.) consulted for unresolved discrepancies.
Statistical analysis
All statistical analyses were conducted using Stata version 17.0 (Stata Corp, College Station, Texas). Pre- and post-intervention means, standard deviations (SDs), and sample sizes from both intervention and control groups were used to estimate the effects of cinnamon supplementation. Standardized mean differences (SMDs) were calculated using a random-effects model to account for heterogeneity across studies, with the DerSimonian-Laird method applied for weighting [19].
When only baseline and endpoint values were reported without change SDs, the Follmann method was used to estimate SDs, assuming a correlation coefficient (R) of 0.5 [20]. For studies reporting standard errors, SDs were calculated as SD = SE × sqrt(n). Heterogeneity was assessed using the I2 statistic, and prediction intervals were calculated to evaluate clinical relevance [21].
Subgroup analyses were conducted based on population characteristics, intervention duration, and cinnamon dosage to identify sources of heterogeneity. Meta-regression was used to examine the relationship between cinnamon dosage (g/day), duration, and cardiovascular outcomes [22]. A nonlinear model was applied to evaluate the dose–response association between cinnamon supplementation and health outcomes [23], offering insights into the effects of varying dosages and intervention durations on outcomes and helping to determine the dosage that yields the greatest benefit.
Leave-one-out sensitivity analysis was performed to test the robustness of findings by sequentially omitting each study and recalculating the pooled estimate. Egger's test was employed to evaluate publication bias.
Results
Study selection
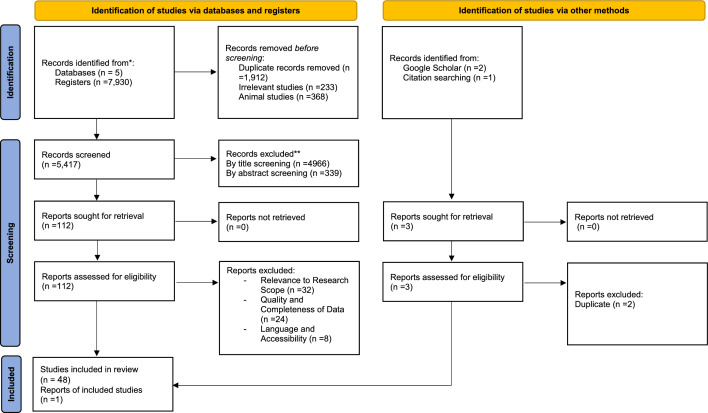
The study selection process is illustrated in Fig. 1. Initially, 7,930 studies were identified through database searches. After removing 1,912 duplicates, 233 irrelevant studies, and 368 animal studies, 5,417 studies remained for title and abstract screening. Of these, 5,305 studies were excluded as irrelevant, resulting in 112 full-text articles for further assessment. After evaluating these, 64 studies were excluded due to non-relevant outcomes (Supplementary Table 2 shows detailed exclusion reasons). Ultimately, 49 studies with a total of 3,038 participants were included in the systematic review and meta-analysis [24–72].
Fig. 1.
Flowchart of study selection for inclusion trials in the systematic review
Study characteristics
The characteristics of the included studies are summarized in Table 1. Supplementary Figs. 1, 2, 3, 4,5,6 and 7 present the SMDs and 95% CIs for outcomes including BF, BMI, WC, weight, WHR, DBP, SBP, fasting glucose, fasting insulin, glucose, HbA1c, HOMA-IR, postprandial blood glucose, QUICKI, CRP, IL-6, HDL-C, LDL-C, TC, TG, ALP, ALT, AST, BUN, MDA, and TAC, along with their changes.
Table 1.
Characteristic of included studies in the meta-analysis
| Studies. Year (Ref.) | Country | Study Design | Health Condition | Sample Size (Sex) | Sample Size (INT/CON) |
Trial Duration (Week) |
Means Age & BMI (INT/CON) | Dose of Supplement (mg/d) | Type of Supplement (INT/CON) | Outcomes |
|---|---|---|---|---|---|---|---|---|---|---|
| Khan et al. 2003[24] | Pakistan | R, PC | T2DM |
60 (B) |
10/10 | 6 |
Age 52.0 ± 5.85/ 52.0 ± 6.87 BMI: NR |
1000 |
Capsule: (Cinnamomum cassia)/ Placebo (Wheat flour) |
Fasting serum glucose, Fasting serum TG, Fasting serum cholesterol & Fasting serum LDL |
| 10/10 |
Age 52.0 ± 5.85/ 52.0 ± 6.87 BMI: NR |
3000 | ||||||||
| 10/10 |
Age 52.0 ± 5.85/ 52.0 ± 6.87 BMI: NR |
6000 | ||||||||
| Mang et al. 2006[25] | Germany | R, PC, DB | T2DM |
65 (B) |
33/32 | 16 |
Age: 62.8 ± 8.37/ 63.7 ± 7.17 BMI: 29.6 ± 4.64/30.1 ± 5.22 |
3000 |
Capsule: Aqueous cinnamon extract (Cinnamomum cassia)/ Placebo (Microcrystalline cellulose) |
FPG, HbA1c, TC, LDL, HDL & TG |
| Vanschoonbeek et al. 2006[26] | Netherlands | R, PC, DB | T2DM |
25 (F) |
12/13 | 6 |
Age: 62 ± 6.92/ 64 ± 7.21 BMI: 30.7 ± 3.81/ 30.1 ± 5.04 |
1500 |
Capsule: (Cinnamomum cassia) + Sulfonylurea derivatives or thiazolidinediones with or without metformin or metformin derivatives only or diet only/ Placebo (Wheat flour) + Sulfonylurea derivatives or thiazolidinediones with or without metformin or metformin derivatives only or diet only |
Plasma Glucose, Plasma Insulin, HbA1c, TC, LDL, HDL, TG & HOMA-IR |
| Ziegenfuss et al. 2006[27] | USA | R, PC, DB | Pre-diabetic |
22 (B) |
12/10 | 12 |
Age: 46.3 ± 8.8 / 45.6 ± 11.1 BMI: 32.3 ± 5.7/ 34.4 ± 12.6 |
500 |
Capsule: Cinnulin PF® (Water-soluble cinnamon extract)/Placebo |
BUN, ALP, AST, ALT Cholesterol, TG, HDL, VLDL, LDL, FBG, SBP & % fat |
| Blevins et al. 2007[28] | USA | R, PC | T2DM |
57 (B) |
29/28 | 12 |
Age: 63.6/ 58 BMI: 32.5 ± 1.7/ 32 ± 1.5 |
1000 |
Capsule: Cinnamon (C. cassia)/ Placebo (Wheat flour) |
BMI, Glucose, HbA1c, TC, HDL, LDL, TG & Insulin |
| Crawford et al. 2009[29] | USA | R, SB | T2DM |
109 (B) |
55/54 | 12 |
Age: 60.5 ± 10.7/ 59.9 ± 9.2 BMI: 31.9 ± 6.4/ 32.9 ± 6.4 |
1000 |
Capsule: Cinnamomum cassia + Usual care with management changes / Usual care with management changes |
HbA1c |
| Roussel et al. 2009[30] | USA | R, PC, DB | Impaired fasting glucose |
21 (B) |
11/10 | 12 |
Age: 45.8 ± 3.6/ 45.6 ± 2.7 BMI: 25 to 45/ 25 to 45 |
500 |
Capsule: Dried aqueous extract of Cinnamomon cassia (Cinnulin PF)/Placebo |
Plasma MDA, Fasting Glucose & Fasting Insulin |
| Soni et al. 2009[31] | India | R | T2DM |
30 (M) |
15/15 | 6 |
Age: 40–60/ 40–60 BMI: NR |
2000 |
Capsule: Cinnamon (Cinnamomum Cassia)/No supplement |
FBG & PBG |
| Akilen et al. 2010[32] | UK | R, PC, DB | T2DM |
58 (B) |
30/28 | 12 |
Age: 54.90 ± 10.14/ 54.43 ± 12.53 BMI: 33.36 ± 4.20/ 32.13 ± 8.31 |
2000 |
Capsule: Cinnamon powder (Cinnamomum cassia)/ Placebo (Starch with 80% amylose and 20% amylopectin) |
HbA1c, FPG, HDL, LDL, Serum TG, TC, Weight, WC, BMI, SBP & DBP |
| Khan et al. 2010[33] | Pakistan | R, PC | T2DM |
14 (B) |
7/7 | 4 |
Age: 40 ≤/ 40 ≤ BMI: NR |
1500 |
Capsule: Cinnamon/ placebo (Maize flour) |
Fasting serum glucose, Fasting serum TG, Fasting serum cholesterol, Fasting serum HDL & Fasting serum LDL |
| Haghighian et al. 2011 [34] | Iran | R, PC, DB | T2DM |
60 (B) |
30/30 | 8 |
Age: 59.1 ± 12.1/ 54.6 ± 13.1 BMI: 29 ± 3/ 29 ± 7 |
1500 |
Capsule: Whole cinnamon + Usual diabetic medicine / Placebo + Usual diabetic medicine |
Weight, BMI, FBG, TC, TG, LDL & HDL |
| Wainstein et al. 2011 [35] | Israel | R, PC | T2DM |
59 (B) |
29/30 | 12 |
Age: 61.7 ± 6.3/ 64.4 ± 15.4 BMI: 29.8 ± 4.3/ 30.9 ± 6.9 |
1200 |
Capsule: Cinnamon (C. cassia) + Metformin and/or sulfonylurea and lifestyle interventions/ Placebo (Microcrystalline cellulose) + Metformin and/or sulfonylurea and lifestyle interventions |
Weight, BMI, WC, FPG, FPI, HbA1c, Adiponectin, SBP, DBP, Pulse, TC, HDL, LDL, TG |
| Khan et al. 2012[36] | Pakistan | R, PC | T2DM |
14 (B) |
7/7 | 4 |
Age: 40 ≤/ 40 ≤ BMI: NR |
1500 |
Capsule: Cinnamon (Cinnamomum Cassia)/ Placebo (Maize flour) |
ALT, ALP, Bilirubin & Creatinine |
| Lu et al. 2012 [37] | China | R, PC, DB | T2DM |
66 (B) |
23/10 |
Age: 62.4 ± 7.9/ 60 ± 5.9 BMI: NR |
120 |
Tablet: Cinnamon extract (Cinnamomum aromaticum) + Gliclazide (diamicron, 30 mg per tablet) / Placebo + Gliclazide (diamicron, 30 mg per tablet) |
HbA1c, Fasting glucose, TG, TC, HDL, LDL, AST & ALT | |
| 23/10 |
Age: 58.9 ± 6.4/ 60 ± 5.9 BMI: NR |
360 | ||||||||
| Sharma et al. 2012[38] | India | R, PC, DB | T2DM |
150 (B) |
50/25 | 12 |
Age: 30 ≤/30 ≤ BMI: 25.86 ± 3.93/ 27.34 ± 38.96 |
3000 |
Cinnamon/ Placebo |
FBS, HbA1c, TC, TG, LDL, HDL |
| 50/25 |
Age: 30 ≤/30 ≤ BMI: 26.53 ± 4.53/27.34 ± 38.96 |
6000 | ||||||||
| Vafa et al. 2012 [39] | Iran | R, PC, DB | T2DM |
37 (B) |
19/18 | 8 |
Age: 54.11 ± 10.37/ 55.67 ± 7.98 BMI: 29.23 ± 3.98/ 28.59 ± 3.54 |
3000 |
Capsule: Cinnamon (Cinnamomum Zeylanicum) + Metformin: 1–1/5 gr/d, Gliclazide: 160–240 mg/d/ Placebo (Wheat flour) + Metformin: 1–1/5 gr/d, Gliclazide: 160–240 mg/d |
FPG, Insulin, HbA1c, TG, TC, LDL, HDL, Apo A-1, Apo B, Weight, BMI, Fat body mass, DBP & SBP |
| Aldallal et al. 2013[40] | Iraq | R, PC | T2DM |
48 (M) |
8/9 | 12 |
Age: 43.6 ± 2.6/ 43.6 ± 2.6 BMI: 27.3 ± 1.7/ 27.3 ± 1.7 |
1000 |
Capsule: Cinnamon/ Placebo (wheat flour) |
FBG, PBG, HbA1c, BUN, AST, ALT & ALP |
| 8/8 |
Age: 43.6 ± 2.6/ 43.6 ± 2.6 BMI: 27.3 ± 1.7/ 27.3 ± 1.7 |
2000 | ||||||||
| 7/8 |
Age: 43.6 ± 2.6/ 43.6 ± 2.6 BMI: 27.3 ± 1.7/ 27.3 ± 1.7 |
4000 | ||||||||
| Hasanzade et al. 2013 [41] | Iran | R, PC, DB | T2DM |
71 (B) |
35/36 | 8 |
Age: 53.7 ± 9.7/ 54.7 ± 8.1 BMI: 27.1 ± 3.2/ 28.7 ± 4 |
1000 |
Capsule: Cinnamon (Cinnamomum cassia)/Placebo |
FBS & HbA1c |
| Zahmatkesh et al. 2013 [42] | Iran | R, PC, DB | T2DM |
56 (B) |
28/28 | 6 |
Age: 56.1 ± 9.8/ 53.1 ± 8.4 BMI: 29.6.3 ± 4/ 30.7 ± 3.3 |
2000 |
Capsule: Cinnamon + diet or edible antidiabetes drugs / Placebo (Pea flour) + diet or edible antidiabetes drugs |
HbA1c, FBS, Cholesterol, TG, HDL & LDL |
| Askari et al. 2014 [43] | Iran | R, PC, DB | NAFLD |
45 (B) |
23/22 | 12 |
Age: 20–65/ 20–65 BMI: 29.9 ± 3.9/ 30.3 ± 4.1 |
1500 |
Capsule: Cinnamon/ Placebo (wheat flour) |
BMI, WC, FBS, QUICK-I, HOMA, Cholesterol, LDL, HDL, TG, ALT, AST, GGT & hs-CRP |
| Azimi et al. 2014 [44] | Iran | R, PC, SB | T2DM |
79 (B) |
40/39 | 8 |
Age: 54.15 ± 6.32/ 53.64 ± 8.11 BMI: 28.78 ± 1.26/ 28.40 ± 1.24 |
3000 |
Sticks: Cinnamon (Cinnamomum verum) + 3 glasses of black tea/Placebo (3 glasses of black tea) |
Weight, BMI, WC, F2-Isoprostan, hs-CRP, FBS, Insulin, HbA1c, Cholesterol, TG, LDL & HDL |
| Hosseini et al. 2014[45] | Iran | R, PC, DB | T2DM |
47 (B) |
24/23 | 8 |
Age: 52 ± 6.87/ 52 ± 5.8 BMI: NR |
3000 |
Capsule: Cinnamon / Placebo (wheat flour) |
FBS, TG, TC, LDL, VLDL, HDL |
| Kort et al. 2014 [46] | USA | R, PC, DB | PCOS |
17 (F) |
11/6 | 24 |
Age: 26.95 ± 4/ 27.86 ± 5 BMI: 33.0 ± 5.6/ 31.4 ± 6.0 |
1500 |
Capsule: Cinnamon/ Placebo |
HOMA-IR, QUICK-I |
| Wickenberg et al. 2014 [47] | Sweden | R, PC, DB | IGT |
17 (B) |
9/8 | 12 |
Age: 73 ± 2/ 72 ± 2 BMI: 25.7 ± 1.3/ 28.6 ± 1.9 |
12,000 |
Capsule: Cinnamon (C. cassia)/ Placebo (cellulose) |
Fasting glucose, Fasting insulin, HbA1c, Cholesterol, LDL, HDL, TG, AST, ALT & ALP |
| Tangvarasittichai et al. 2015 [48] | Thailand | R, PC, DB | T2DM |
106 (B) |
49/57 | 8 |
Age: 57.5 ± 1.1/ 56.9 ± 1.2 BMI: 24.73 ± 4.04/ 24.87 ± 3.72 |
1500 |
Capsule: Cinnamon (Cinnamomum cassia) + Metformin, sulphonyleuraes or both / Placebo + Metformin, sulphonyleuraes or both |
Glucose, Insulin, HOMA-IR, QUICK-I, MDA, TAC, hs-CRP |
| Anderson et al. 2016 [49] | China | R, PC, DB | Prediabetic |
137 (B) |
64/73 | 8 |
Age: 61.3 ± 0.8/ 61.3 ± 0.8 BMI: 24.8 ± 0.4/ 25.8 ± 0.3 |
500 |
Capsule: Dried water extract of cinnamon (Cinnamomum cassia)/ Placebo (Dark broen (baked) wheat flour) |
BMI, Fasting glucose, 2-h Glucose, Fasting Insulin, 2-h Insulin, HOMA-IR, SBP, DBP, TC, TG, LDL & HDL |
| Azimi et al. 2016 [50] | Iran | R, PC, SB | T2DM |
79 (B) |
40/39 | 8 |
Age: 54.15 ± 6.32/ 53.64 ± 8.11 BMI: 28.78 ± 1.26/ 28.40 ± 1.24 |
3000 |
Powder: Cinnamon (Cinnamomum verum) + 3 glasses of Black tea/ Placebo (3 glasses of Black tea) |
Weight, BMI, Waist, sICAM-1, SBP & DBP |
|
Mirfeizi et al 2016 [51] |
Iran | R, PC, TB | T2DM |
72 (B) |
27/45 | 12 |
Age: 52 ± 13/ 54 ± 12 BMI: 28.36 ± 3.27/ 28.94 ± 4.45 |
1000 |
Capsule: Cinnamon/ Placebo (Starch) |
BMI, FBG, 2-h PPG, HbA1c, Serum Insulin, HOMA-IR, TG, Cholesterol, LDL & HDL |
| Sengsuk et al. 2016[52] | Thailand | R, PC, DB | T2DM |
99 (B) |
49/50 | 8 |
Age: 57.2 ± 1.1/ 56.9 ± 1.2 BMI: 24.73 ± 4.04/ 24.86 ± 3.74 |
1500 |
Capsule: (Cinnamomum cassia)/ Placebo |
SBP, DBP, Glucose, BUN, Creatinine, TC, TG, HDL, LDL & HbA1c |
| Gupta Jain et al. 2017 [53] | India | R, PC, DB | MetS |
116 (B) |
58/58 | 16 |
Age: 44.3 ± 7.2/ 45.1 ± 8.4 BMI: 33.6 ± 5.4/ 31.2 ± 4.4 |
3000 |
Capsule: Cinnamon/ Placebo (Wheat flour) |
Weight, BMI, WC, WHR, %BF, SBP, DBP, FBG, HbA1c, PPG, TC, Serum TG, HDL, LDL & hs-CRP |
| Talaei et al. 2017 [54] | Iran | R, PC, DB | T2DM |
39 (B) |
20/19 | 8 |
Age: 58.90 ± 7.93/ 56.26 ± 9.46 BMI: 26.41 ± 3.06/ 29.02 ± 5.53 |
3000 |
Capsule: Cinnamon (Cinnamomum zeylanicum Blume) + Metformin/ Placebo (Microcrystalline cellulose) + Metformin |
FBG, FI, HOMA-IR, HbA1c, TAC & MDA |
| Borzoei et al. 2018 [55] | Iran | R, PC, DB | PCOS |
84 (F) |
42/42 | 8 |
Age: 29.3 ± 6.14/ 30.2 ± 6.69 BMI: 30.7 ± 5.04/ 31.61 ± 4.84 |
1500 |
Capsule: Cinnamon/ Placebo (wheat flour) |
Weight, BMI, Glucose, Insulin, HOMA-IR, Adiponectin, TC, TG, LDL & HDL |
| Borzoei et al. 2018 [56] | Iran | R, PC, DB | PCOS |
84 (F) |
42/42 | 8 |
Age: 29.26 ± 6.14 / 30.17 ± 6.69 BMI: 30.75 ± 5.04/ 31.61 ± 4.84 |
1500 |
Capsule: Cinnamon/ Placebo (Wheat flour) |
BMI, TAC & MDA |
| Hajimonfarednejad et al. 2018 [57] | Iran | R, PC, DB | PCOS |
59 (F) |
29/30 | 12 |
Age: 28.62 ± 5.74/ 26.53 ± 6.35 BMI: 27.63 ± 4.30/ 26.09 ± 4.56 |
1500 |
Capsule: Cinnamon/ Placebo (450 mg of starch & 50 mg of cinnamon powder) |
Weight, BMI, WC, FBS, Insulin, 2 h PPG, HOMA‐IR, HbA1C, TG, TC, LDL & HDL |
| Pishdad et al. 2018 [58] | Iran | R, PC, DB | Dyslipidemia |
60 (F) |
30/30 | 8 |
Age: 30–59/ 30–59 BMI: 31.25 ± 2/ 30.2 ± 4 |
3000 |
Capsule: Cinnamomum Verum/ Placebo (Microcrystalline cellulose) |
Weight, BMI, WC, HDL, TC & TG |
| Shishehbor et al. 2018 [59] | Iran | R, PC, DB | RA |
36 (F) |
18/18 | 8 |
Age: 44.66 ± 11.22/ 49.11 ± 7.45 BMI: 28.59 ± 5.56/ 29.59 ± 5.43 |
2000 |
Capsule: Cinnamon/ Placebo (Starch) |
Weight, BMI, CRP, TNF- α, ESR, SBP, DBP, FBS, TG, TC, HDL, LDL, ALT, AST |
| Mirmiran et al. 2019 [60] | Iran | R, PC, DB | T2DM |
39 (B) |
20/19 | 8 |
Age: 58.90 ± 7.93/ 56.26 ± 9.46 BMI: 26.41 ± 3.06/ 29.02 ± 5.53 |
3000 |
Capsule: Cinnamon extract/ Placebo (Microcrystalline cellulose) |
FBG, HbA1c, TAC, FI, HOMA-IR, MDA, ICAM-1& VCAM-1 |
| Zare et al. 2019 [61] | Iran | R, PC, TB | T2DM |
138 (B) |
69/69 | 12 |
Age: 52.1 ± 9.7/ 53.2 ± 8.5 BMI: 29.9 ± 12.3/ 29.3 ± 17.1 |
1000 |
Capsule: Cinnamon (Cinnamomum verum)/ Placebo (Starch) |
BMI, Weight, %BF, Body muscle, Visceral fat, FPG, 2 h PPG, HbA1c, Insulin, HOMA-IR, TG, TC, LDL & HDL |
| Davari et al. 2020 [62] | Iran | R, PC, DB | T2DM |
39 (B) |
20/19 | 8 |
Age: 58.9 ± 7.93/ 56.26 ± 9.46 BMI: 26.41 ± 3.06/ 29.02 ± 5.53 |
3000 |
Capsule: Cinnamon extract/ Placebo (Microcrystalline cellulose) |
FBS, FI, HbA1c, HOMA-IR, hs-CRP, TNF-α, IL-6 |
| Romeo et al. 2020 [63] | Korea | R, PC, DB | Prediabetic |
54 (B) |
27/27 | 12 |
Age: 50.4 ± 11.5/ 54.1 ± 8 BMI: 28.2 ± 5.0/ 25.5 ± 3.3 |
1500 |
Capsule: Cinnamon extract/ Placebo (cellulose (91.5%), caramel food coloring (8.4%), and cinnamon incense (0.1%) did not contain any active substances) |
FPG, Glycated albumin, HbA1c, Insulin, HOMA-IR & HOMA-B |
| Zare et al. 2020 [64] | Iran | R, PC, TB | T2DM |
138 (B) |
69/69 | 12 |
Age: 52.17 ± 9.7/53.2 ± 8.5 BMI: 29.92 ± 102.17/ 29.33 ± 142.12 |
1000 |
Capsule: Cinnamon/ Placebo (Starch) |
FBG, HbA1c, Insulin & HOMA-IR |
| Zareie et al. 2020 [65] | Iran | R, PC, DB | Migraine |
43 (B) |
21/22 | 8 |
Age: 37.13 ± 7.8/ 39.36 ± 6.87 BMI: NR |
1800 |
Capsule: Cinnamon/ Placebo (100 mg of corn starch) |
IL-6 |
| Delaviz et al. 2021 [66] | Iran | RCT | Progressive-relapsing MS |
41 (B) |
21/20 | 8 |
Age: 44.22 ± 11.8/ 42.94 ± 9.55 BMI: 26.03 ± 4.16/ 24.54 ± 4.55 |
2000 |
Capsule: Cinnamon, Placebo (Wheat flour) |
BMI, WC, Weight, IL-6 & hs-CRP |
| Neto et al. 2021 [67] | Brazil | R, PC, TB | T2DM |
140 (B) |
71/69 | 12 |
Age: 61.7 ± 11.7/ 60.8 ± 10.8 BMI: NR |
3000 |
Capsule: Cinnamon (Cinnamomum verum)/ Placebo (750 mg of microcrystalline cellulose) |
HbA1c, Fasting venous glycemia, HOMA-IR & Insulin |
| Shirzad et al. 2021 [68] | Iran | R, PC, DB | Stage 1 HTN |
37 (B) |
19/18 | 12 |
Age: 54.4 ± 10.2/ 49.8 ± 9.07 BMI: 28.4 ± 3.52/ 26.4 ± 3.02 |
1500 |
Capsule: Cinnamon (Cinnamomum zeylanicum)/ Placebo (containing 500 mg lactose powder) |
SBP, DBP, BMI, LDL, HDL, TG, TC & FBS |
| Dastgheib et al. 2022 [69] | Iran | R, PC, DB | PCOS |
42 (F) |
20/22 | 8 |
Age: 30.2 ± 5.72/ 30 ± 6.37 BMI: 28.61 ± 4.65/ 27.52 ± 5.09 |
1500 |
Capsule: Cinnamon (Cinnamomum zeylanicum extract)/ Placebo (rice flour capsule) |
Weight, BMI, WC, WHR, FBS, Insulin, HOMA-IR, TG, TC, LDL & HDL |
| Al Dhaheri et al. 2024[70] | UAE | R, PC, DB | MetS |
47 (B) |
25/22 | 12 |
Age: 27.84 ± 12.04/ 28.82 ± 11.7 BMI: 33.53 ± 9.96/ 33.94 ± 5.84 |
3000 |
Powder: Cinnamon (Cinnamomum)/ Placebo |
SBP, DBP, TC, TG, HDL, LDL, FBG, HbA1c, Weight, BMI, WC, WHR, Fat mass, %BF & Fat-free mass |
| Peivandi et al. 2024[71] | Iran | R, PC, DB | PCOS |
39 (F) |
19/20 | 24 |
Age: 18–38/ 18–38 BMI: 28.34 ± 3.77/ 30.31 ± 5.56 |
1500 |
Capsule: Cinnamon/ Placebo (Starch) |
BMI, WC, HC, TG, HDL, HOMA-IR & QUICK-I |
| Zareie, 2024 [72] | Iran | R, PC, DB | Migraine |
43 (B) |
21/22 | 8 |
Age: 37.13 ± 7.6/ 39.36 ± 6.85 BMI: 26.12 ± 15.35/ 26.87 ± 22.37 |
600 |
Capsule: Cinnamon powder/ Placebo (100mg of corn starch) |
Weight, BMI, WC, HC & WHR |
Footprint: B, Both Sex; BMI, Body Mass Index; CON, Control Group; DB, Double-Blinded; DBP, Diastolic Blood Pressure; F, Female; FBS, Fasting Blood Sugar; HbA1c, Hemoglobin A1C; HDL, High-Density Lipoprotein; HC, Hip Circumference; HOMA-IR, Homeostatic Model Assessment of Insulin Resistance; hs-CRP, High-Sensitivity C-Reactive Protein; INT, Intervention Group; LDL, Low-Density Lipoprotein; MDA, Malondialdehyde; MetS, Metabolic Syndrome; NAFLD, Non-Alcoholic Fatty Liver Disease; PCOS, Polycystic Ovary Syndrome; QUICKI, Quantitative Insulin Sensitivity Check Index; RCT, Randomized Controlled Trial; SBP, Systolic Blood Pressure; T2DM, Type 2 Diabetes Mellitus; TC, Total Cholesterol; TG, Triglycerides; TB, Triple-Blinded; WC, Waist Circumference; WHR, Waist-to-Hip Ratio; BUN, Blood Urea Nitrogen; ALP, Alkaline Phosphatase; AST, Aspartate Aminotransferase; ALT, Alanine Aminotransferase; VLDL, Very-Low-Density Lipoprotein; FBG, Fasting Blood Glucose; FPG, Fasting Plasma Glucose; PBG, Postprandial Blood Glucose; FPI, Fasting Plasma Insulin; Apo A-1, Apolipoprotein A-I; Apo B, Apolipoprotein B; IGT, Impaired Glucose Tolerance; GGT, Gamma-Glutamyl Transferase; TAC, Total Antioxidant Capacity; 2 h PPG, 2 h Postprandial Glucose; sICAM-1, Soluble intercellular adhesion molecule-1; FI; Fasting Insulin; TNF- α, Tumor Necrosis Factor alpha; ESR, Erythrocyte Sedimentation Rate; %BF, Body Fat Percentage; VCAM-1, Vascular cell adhesion protein 1; RA, Rheumatoid Arthritis; MS, Multiple Sclerosis; HTN, Hypertension
The studies were published between 2003 [24] and 2024 [70–72] and were conducted in Pakistan [24, 33, 36], Germany [25], the Netherlands [26], the USA [27–30, 46], India [31, 38, 53], the UK [32], Israel [35], China [37, 49], Iran [34, 39, 41–45, 50, 51, 54–62, 64–66, 68, 69, 71, 72], Iraq [40], Sweden [47], Thailand [48, 52], Korea [63], Brazil [67], and the UAE [70]. Participants in the intervention groups had a mean age ranging from 18 to 73 years. Cinnamon doses varied from 120 mg/day to 12,000 mg/day, with intervention durations ranging from 4 to 24 weeks. Intervention group sample sizes ranged from 7 to 100 participants. Nine studies included only female participants [26, 46, 55–59, 69, 71], two included only males [31, 40], and the remainder included both genders. The populations studied included individuals with T2DM [24–26, 28, 29, 31–42, 44, 45, 48, 50–52, 54, 60–62, 64, 67], prediabetes [27, 49, 63], impaired fasting glucose [30], non-alcoholic fatty liver disease (NAFLD) [43], polycystic ovary syndrome (PCOS) [46, 55–57, 69, 71], impaired glucose tolerance (IGT) [47], metabolic syndrome (MetS) [53, 70], dyslipidemia [58], rheumatoid arthritis (RA) [59], migraine [65, 72], progressive relapsing multiple sclerosis (MS) [66], and stage 1 HTN [68].
Qualitative data assessment
Using the Cochrane Risk of Bias Assessment tool, 43 studies were rated as high risk of bias [24–47, 49–51, 54, 55, 58–66, 68–72], five as moderate risk [32, 52, 53, 57, 67], and one study as low risk [48] (Table 2).
Table 2.
Quality of included studies in the meta-analysis
| Study, Year (Ref.) | Random sequence generation | Allocation concealment | Blinding of participants & personnel | Blinding of outcome assessment | Incomplete outcome data | Selective outcome reporting | Other sources of bias | Overall quality |
|---|---|---|---|---|---|---|---|---|
| Khan, 2003[24] | L | H | H | H | U | L | L | Poor |
| Mang, 2006[25] | U | H | L | H | H | L | L | Poor |
| Vanschoonbeek, 2006[26] | H | H | L | H | L | L | L | Poor |
| Ziegenfuss, 2006[27] | U | H | L | H | L | L | L | Poor |
| Blevins, 2007[28] | U | H | H | H | H | L | L | Poor |
| Crawford, 2009[29] | L | L | H | H | H | L | L | Poor |
| Roussel, 2009[30] | U | H | U | H | L | L | L | Poor |
| Soni, 2009[31] | H | H | H | H | L | L | L | Poor |
| Akilen, 2010[32] | L | L | L | H | L | L | L | Fair |
| Khan, 2010[33] | U | H | H | H | L | L | L | Poor |
| Haghighian, 2011 [34] | U | H | U | H | U | L | L | Poor |
| Wainstein, 2011 [35] | L | H | L | H | L | L | L | Poor |
| Khan, 2012[36] | L | H | H | H | L | L | L | Poor |
| Lu, 2012 [37] | U | H | L | H | L | L | L | Poor |
| Sharma, 2012[38] | U | H | L | H | L | L | L | Poor |
| Vafa, 2012 [39] | U | H | L | H | H | L | L | Poor |
| Aldallal, 2013[40] | L | H | L | H | H | L | L | Poor |
| Hasanzade, 2013 [41] | L | H | L | H | L | L | L | Poor |
| Zahmatkesh, 2013 [42] | L | H | U | H | H | L | L | Poor |
| Askari, 2014 [43] | L | H | L | H | L | L | L | Poor |
| Azimi, 2014 [44] | L | H | H | H | L | L | L | Poor |
| Hosseini, 2014[45] | U | H | L | H | L | L | L | Poor |
| Kort, 2014 [46] | L | H | L | H | L | L | L | Poor |
| Wickenberg, 2014 [47] | L | L | U | H | H | L | L | Poor |
| Tangvarasittichai, 2015 [48] | L | L | L | L | L | L | L | Good |
| Anderson, 2016 [49] | L | H | L | H | H | L | L | Poor |
| Azimi, 2016 [50] | L | H | L | H | L | L | L | Poor |
| Mirfeizi, 2016 [51] | U | H | L | U | L | L | L | Poor |
| Sengsuk, 2016[52] | L | H | L | L | L | L | L | Fair |
| Gupta Jain, 2017 [53] | L | L | L | H | L | L | L | Fair |
| Talaei, 2017 [54] | U | H | L | H | H | L | L | Poor |
| Borzoei, 2018 [55] | L | H | U | H | L | L | L | Poor |
| Borzoei, 2018 [56] | L | H | U | H | L | L | L | Poor |
| Hajimonfarednejad, 2018 [57] | L | H | L | L | L | L | L | Fair |
| Pishdad, 2018 [58] | L | H | U | H | L | L | L | Poor |
| Shishehbor, 2018 [59] | L | H | L | H | L | L | L | Poor |
| Mirmiran, 2019 [60] | L | H | L | H | H | L | L | Poor |
| Zare, 2019 [61] | L | H | U | U | L | L | L | Poor |
| Davari, 2020 [62] | U | H | L | H | H | L | L | Poor |
| Romeo, 2020 [63] | L | H | L | H | L | L | L | Poor |
| Zare, 2020 [64] | L | H | U | U | L | L | L | Poor |
| Zareie, 2020 [65] | L | H | L | H | H | L | L | Poor |
| Delaviz, 2021 [66] | L | H | L | H | H | L | L | Poor |
| Neto, 2021 143] | L | H | L | L | L | L | L | Fair |
| Shirzad, 2021 [68] | L | H | L | H | L | L | L | Poor |
| Dastgheib, 2022 [69] | L | H | L | H | H | L | L | Poor |
| Al Dhaheri, 2024[70] | L | H | H | H | H | L | L | Poor |
| Peivandi, 2024[71] | L | H | L | L | H | L | L | Poor |
| Zareie, 2024 [148) | L | H | L | H | H | L | L | Poor |
Footprint: H, high risk of bias; L, low risk of bias; U, unclear risk of bias
Effects of cinnamon supplementation on anthropometric indices
Cinnamon supplementation resulted in a statistically significant reduction in WC (SMD = − 0.40; 95% CI: − 0.73, − 0.06; P = 0.020; I2 = 80.5%, P < 0.001). However, no significant effect was observed for BF (SMD = − 0.19; 95% CI: − 0.88, 0.50; P = 0.590; I2 = 88.9%, P < 0.001), BMI (SMD = − 0.13; 95% CI: − 0.40, 0.13; P = 0.320; I2 = 83.8%, P < 0.001), weight (SMD = − 0.08; 95% CI: − 0.41, 0.25; P = 0.629; I2 = 85.3%, P < 0.001), and WHR (SMD = − 0.28; 95% CI: − 0.61, 0.06; P = 0.102; I2 = 37.8%, P = 0.185) (Supplementary Fig. 1).
Sensitivity analyses for BMI, WC, and WHR indicated stable results upon exclusion of individual studies. However, removing Al Daheri et al. [70] significantly affected BF (SMD = − 0.56; 95% CI: − 0.95, − 0.17), and excluding Pishdad et al. [58] impacted the findings for weight (SMD = − 0.25; 95% CI: − 0.46, − 0.03). Publication bias was not detected for BF (Egger’s P = 0.460), BMI (Egger’s P = 0.345), WC (Egger’s P = 0.233), and weight (Egger’s P = 0.065), though significant asymmetry was found for WHR (Egger’s P = 0.045).
Effects of cinnamon supplementation on blood pressure
Studies indicated that cinnamon supplementation led to a significant decrease in diastolic blood pressure (DBP) (SMD = − 1.04; 95% CI: − 1.54, − 0.55; P < 0.001; I2 = 88.6%, P < 0.001) and systolic blood pressure (SBP) (SMD = − 0.85; 95% CI: − 1.54, − 0.16; P = 0.016; I2 = 94.3%, P < 0.001) (Supplementary Fig. 2).
Sensitivity analyses for DBP confirmed stable outcomes, while excluding Al Daheri et al. [70] significantly altered the SBP results (SMD: − 0.61; 95% CI: − 1.27, 0.04). No publication bias was detected for DBP (Egger's P = 0.803) or SBP (Egger's P = 0.357).
Effects of cinnamon supplementation on glycemic profile
Cinnamon supplementation demonstrated a significant effect on fasting glucose (SMD = − 1.28; 95% CI: − 1.65, − 0.90; P < 0.001; I2 = 93.5%, P < 0.001), fasting insulin (SMD = − 0.26; 95% CI: − 0.50, − 0.02; P = 0.035; I2 = 78.0%, P < 0.001), HbA1c (SMD = − 0.71; 95% CI: − 1.02, − 0.40; P < 0.001; I2 = 89.3%, P < 0.001), HOMA-IR (SMD = − 0.54; 95% CI: − 0.82, − 0.26; P < 0.001; I2 = 80.4%, P < 0.001) and postprandial blood glucose (SMD = − 2.28; 95% CI: − 3.48, − 1.08; P < 0.001; I2 = 94.4%, P < 0.001). Nevertheless, trials showed no significant impact on the QUICKI (SMD = − 0.33; 95% CI: − 0.72, 0.07; P = 0.103; I2 = 24.0%, P = 0.268) (Supplementary Fig. 3).
Sensitivity analyses across fasting glucose, fasting insulin, HbA1c, HOMA-IR, and postprandial glucose indicated stable results. However, removing Peivandi et al. [71] notably changed the QUICKI results (SMD = − 0.50; 95% CI: − 0.86, − 0.14). Publication bias was absent for fasting insulin (Egger's P = 0.131), HbA1c (Egger's P = 0.096), HOMA-IR (Egger's P = 0.135) and QUICKI (Egger's P = 0.392), though significant asymmetry was noted for fasting glucose (Egger's P < 0.001) and postprandial glucose (Egger's P < 0.001).
Effects of cinnamon supplementation on inflammatory biomarkers
Studies assessed CRP levels, demonstrating a significant reduction (SMD = − 0.78; 95% CI: − 1.28, − 0.27; P = 0.003; I2 = 84.3%, P < 0.001). For IL-6, three studies reported no significant reduction (SMD = − 0.37; 95% CI: − 0.90, 0.16; P = 0.168; I2 = 53.1%, P = 0.119) (Supplementary Fig. 4).
Sensitivity analyses for both CRP and IL-6 confirmed the stability of these findings across different study exclusions. No publication bias was detected for CRP (Egger’s P = 0.275) or IL-6 (Egger’s P = 0.438).
Effects of cinnamon supplementation on lipid profile
Cinnamon supplementation had a significant effect on lipid profile indices including, HDL-C (SMD = 0.56; 95% CI: 0.23, 0.89; P = 0.001; I2 = 90.6%, P < 0.001), LDL-C (SMD = − 0.71; 95% CI: − 1.02, − 0.40; P < 0.001; I2 = 88.9%, P < 0.001), TC (SMD = − 1.15; 95% CI: − 1.55, − 0.75; P < 0.001; I2 = 93.1%, P < 0.001) and TG (SMD = − 0.91; 95% CI: − 1.25, − 0.56; P < 0.001; I2 = 91.3%, P < 0.001) (Supplementary Fig. 5).
Sensitivity analyses for these lipid parameters confirmed the robustness of these results, with no detected publication bias for HDL-C (Egger’s P = 0.056). However, significant asymmetry was observed for LDL (Egger’s P = 0.022), TC (Egger’s P < 0.001) and TG (Egger’s P = 0.001).
Effects of cinnamon supplementation on liver function tests
Our finding revealed that cinnamon had no considerable impact on ALP (SMD = − 0.07; 95% CI: − 0.47, 0.32; P = 0.720; I2 = 0.0%, P = 0.488), ALT (SMD = − 0.59; 95% CI: − 1.45, 0.28; P = 0.182; I2 = 88.9%, P < 0.001), AST (SMD = − 0.29; 95% CI: − 0.96, 0.39; P = 0.406; I2 = 82.4%, P < 0.001), and BUN (SMD = − 0.50; 95% CI: − 1.74, 0.74; P = 0.428; I2 = 90.0%, P < 0.001) (Supplementary Fig. 6).
Sensitivity analyses for ALP, ALT, and BUN showed that excluding any studies did not alter overall results, although excluding Shishehbor et al. [59] significantly impacted ALT (SMD = − 0.84; 95% CI: − 1.59, − 0.08). Publication bias was not detected for ALT (Egger’s P = 0.314), AST (Egger’s P = 0.989), or BUN (Egger’s P = 0.371); however, asymmetry was noted for ALP (Egger’s P = 0.028).
Effects of cinnamon supplementation on oxidative stress parameters
Studies on MDA indicated a significant reduction (SMD = − 0.76; 95% CI: − 1.07, − 0.45; P < 0.001; I2 = 34.3%%, P = 0.193) (Supplementary Fig. 7). In contrast, no significant effect was reported for TAC (SMD = 0.52; 95% CI: − 0.05, 1.08; P = 0.073; I2 = 79.1%%, P = 0.002) (Supplementary Fig. 7).
Sensitivity analysis for MDA confirmed the stability of these results. However, excluding studies by Talaei et al. (SMD = 0.71, 95% CI: 0.15, 1.27) [54] and Mirmiran et al. (SMD = 0.68, 95% CI: 0.07, 1.28) [60] significantly changed the effect on TAC. No publication bias was detected for MDA (Egger’s P = 0.904), though significant asymmetry was observed for TAC (Egger’s P = 0.008).
Subgroup analysis
Subgroup analysis based on health conditions (T2DM or prediabetes vs. other conditions: PCOS, NAFLD, MetS, dyslipidemia, HTN, MS, RA, migraine, IGT, and impaired fasting blood glucose), cinnamon dosage (> 2000 mg/day vs. ≤ 2000 mg/day), intervention duration (≥ 8 weeks vs. < 8 weeks), participant age (≥ 50 years vs. < 50 years), baseline BMI (Healthy/Overweight [< 30 kg/m2] vs. Obese [≥ 30 kg/m2]), cinnamon type (C. cassia vs. C. verum vs. C. zeylanicum), and continent (Asia vs. Europe or America) is presented in Table 3.
Table 3.
Description of the analysis and subgroup results of Cinnamon supplementation on cardiovascular disease risk factors
| StudiesN | ParticipantN | SMD (95%CI) | P-value | Heterogeneity | |||
|---|---|---|---|---|---|---|---|
| P heterogeneity | I2 | P between sub-groups | |||||
| Analysis and subgroup results of Cinnamon supplementation on BF | |||||||
| Overall effect | 5 | 360 | − 0.19 (− 0.88, 0.50) | 0.590 | < 0.001 | 88.9% | |
| Analysis and subgroup results of Cinnamon supplementation on BMI | |||||||
| Overall effect | 23 | 1505 | − 0.13 (− 0.40, 0.13) | 0.320 | < 0.001 | 83.8% | |
| Health Condition | |||||||
| Prediabetes/Diabetes | 10 | 772 | − 0.28 (− 0.81, 0.25) | 0.305 | < 0.001 | 92.0% | 0.394 |
| Other conditions | 13 | 733 | − 0.03 (− 0.21, 0.15) | 0.707 | 0.130 | 31.6% | |
| Cinnamon dosage (mg/day) | |||||||
| 2000 > | 14 | 952 | − 0.30 (− 0.65, 0.06) | 0.099 | < 0.001 | 85.5% | 0.102 |
| 2000 ≤ | 9 | 553 | 0.12 (− 0.23, 0.48) | 0.502 | < 0.001 | 76.1% | |
| Intervention duration (weeks) | |||||||
| 8 ≥ | 12 | 778 | − 0.07 (− 0.21, 0.07) | 0.347 | 0.980 | 0.0% | 0.760 |
| 8 < | 11 | 727 | − 0.16 (− 0.71, 0.40) | 0.580 | < 0.001 | 92.2% | |
| Intervention Age (year) | |||||||
| 50 ≥ | 12 | 696 | − 0.04 (− 0.23, 0.15) | 0.676 | 0.099 | 36.5% | 0.449 |
| 50 < | 11 | 809 | − 0.25 (− 0.74, 0.25) | 0.330 | < 0.001 | 91.3% | |
| Baseline BMI | |||||||
| Healthy/Overweight (< 30 kg/m2) | 16 | 999 | − 0.23 (− 0.52, 0.08) | 0.142 | < 0.001 | 81.4% | 0.337 |
| Obese (≥ 30 kg/m2) | 7 | 506 | 0.07 (− 0.46, 0.60) | 0.787 | < 0.001 | 88.1% | |
| Not given | – | – | – | – | – | – | |
| Cinnamon type | |||||||
| C.cassia | 3 | 174 | − 0.03 (− 1.34, 1.29) | 0.968 | < 0.001 | 94.2% | 0.657 |
| C.verum | 3 | 218 | 0.06 (− 0.21, 0.32) | 0.674 | 0.995 | 0.0% | |
| C.zeylanicum | 3 | 116 | − 0.17 (− 0.54, 0.20) | 0.362 | 0.506 | 0.0% | |
| Not given | 14 | 997 | − 0.19 (− 0.54, 0.16) | 0.291 | < 0.001 | 86.2% | |
| Continent | |||||||
| Asia | 21 | 1390 | − 0.14 (− 0.39, 0.11) | 0.262 | < 0.001 | 80.2% | 0.956 |
| Europe/America | 2 | 115 | − 0.08 (− 2.42, 2.27) | 0.950 | < 0.001 | 97.1% | |
| Analysis and subgroup results of Cinnamon supplementation on WC | |||||||
| Overall effect | 13 | 767 | − 0.40 (− 0.73, − 0.06) | 0.020 | < 0.001 | 80.5% | |
| Health Condition | |||||||
| Prediabetes/Diabetes | 4 | 275 | − 0.09 (− 0.33, 0.15) | 0.466 | 0.908 | 0.0% | 0.090 |
| Other conditions | 9 | 492 | − 0.56 (− 1.06, − 0.07) | 0.026 | < 0.001 | 85.3% | |
| Cinnamon dosage (mg/day) | |||||||
| 2000 > | 6 | 287 | − 0.09 (− 0.32, 0.15) | 0.475 | 0.880 | 0.0% | 0.047 |
| 2000 ≤ | 7 | 480 | − 0.71 (− 1.28, − 0.14) | 0.015 | < 0.001 | 88.6% | |
| Intervention duration (weeks) | |||||||
| 8 ≥ | 6 | 344 | − 0.19 (− 0.40, 0.02) | 0.080 | 0.533 | 0.0% | 0.225 |
| 8 < | 7 | 423 | − 0.59 (− 1.21, 0.02) | 0.059 | < 0.001 | 88.9% | |
| Intervention Age (year) | |||||||
| 50 ≥ | 9 | 492 | − 0.56 (− 1.06, − 0.07) | 0.026 | < 0.001 | 85.3% | 0.090 |
| 50 < | 4 | 275 | − 0.09 (− 0.33, 0.15) | 0.466 | 0.908 | 0.0% | |
| Baseline BMI | |||||||
| Healthy/Overweight (< 30 kg/m2) | 9 | 486 | − − 0.10 (− 0.28, 0.08) | 0.273 | 0.855 | 0.0% | 0.032 |
| Obese (≥ 30 kg/m2) | 4 | 281 | − 1.16 (− 2.11, − 0.21) | 0.017 | < 0.001 | 92.1% | |
| Not given | – | – | – | – | – | – | |
| Cinnamon type | |||||||
| C.cassia | 2 | 117 | − 0.19 (− 0.55, 0.18) | 0.315 | 0.806 | 0.0% | 0.470 |
| C.verum | 3 | 218 | − 0.15 (− 0.44, 0.14) | 0.322 | 0.307 | 15.4% | |
| C.zeylanicum | 1 | 42 | − 0.02 (− 0.62, 0.59) | 0.958 | – | – | |
| Not given | 7 | 390 | − 0.67 (− 1.30, − 0.03) | 0.041 | < 0.001 | 88.4% | |
| Continent | |||||||
| Asia | 12 | 709 | − 0.42 (− 0.78, − 0.05) | 0.026 | < 0.001 | 82.1% | 0.568 |
| Europe/America | 1 | 58 | − 0.23 (− 0.75, 0.28) | 0.378 | – | – | |
| Analysis and subgroup results of Cinnamon supplementation on Weight | |||||||
| Overall effect | 16 | 1038 | − 0.08 (− 0.41, 0.25) | 0.629 | < 0.001 | 85.3% | |
| Health Condition | |||||||
| Prediabetes/Diabetes | 7 | 510 | − 0.22 (− 0.65, 0.20) | 0.297 | < 0.001 | 81.7% | 0.442 |
| Other conditions | 9 | 528 | 0.04 (− 0.48, 0.56) | 0.887 | < 0.001 | 87.9% | |
| Cinnamon dosage (mg/day) | |||||||
| 2000 > | 7 | 485 | − 0.22 (− 0.64, 0.20) | 0.305 | < 0.001 | 80.6% | 0.456 |
| 2000 ≤ | 9 | 553 | 0.03 (− 0.48, 0.54) | 0.905 | < 0.001 | 88.1% | |
| Intervention duration (weeks) | |||||||
| 8 ≥ | 10 | 561 | 0.15 (− 0.28, 0.58) | 0.490 | < 0.001 | 83.9% | 0.047 |
| 8 < | 6 | 477 | − 0.46 (− 0.87, − 0.04) | 0.031 | < 0.001 | 79.0% | |
| Intervention Age (year) | |||||||
| 50 ≥ | 9 | 528 | 0.04 (− 0.48, 0.56) | 0.887 | < 0.001 | 87.9% | 0.442 |
| 50 < | 7 | 510 | − 0.22 (− 0.65, 0.20) | 0.297 | < 0.001 | 81.7% | |
| Baseline BMI | |||||||
| Healthy/Overweight (< 30 kg/m2) | 11 | 673 | − 0.20 (− 0.49, 0.09) | 0.168 | < 0.001 | 70.2% | 0.410 |
| Obese (≥ 30 kg/m2) | 5 | 365 | 0.20 (− 0.70, 1.09) | 0.671 | < 0.001 | 93.9% | |
| Not given | – | – | – | – | – | – | |
| Cinnamon type | |||||||
| C.cassia | 2 | 117 | − 0.06 (− 0.43, 0.30) | 0.729 | 0.447 | 0.0% | 0.340 |
| C.verum | 3 | 218 | 0.83 (− 0.57, 2.22) | 0.245 | < 0.001 | 95.5% | |
| C.zeylanicum | 2 | 79 | − 0.27 (− 0.72, 0.17) | 0.232 | 0.619 | 0.0% | |
| Not given | 9 | 624 | − 0.33 (− 0.66, 0.01) | 0.046 | < 0.001 | 74.5% | |
| Continent | |||||||
| Asia | 15 | 980 | − 0.07 (− 0.43, 0.28) | 0.690 | < 0.001 | 86.3% | 0.673 |
| Europe/America | 1 | 58 | − 0.21 (− 0.72, 0.31) | 0.433 | − | − | |
| Analysis and subgroup results of Cinnamon supplementation on WHR | |||||||
| Overall effect | 4 | 248 | − 0.28 (− 0.61, 0.06) | 0.102 | 0.185 | 37.8% | |
| Analysis and subgroup results of Cinnamon supplementation on DBP | |||||||
| Overall effect | 10 | 703 | − 1.04 (− 1.54, − 0.55) | < 0.001 | < 0.001 | 88.6% | |
| Health Condition | |||||||
| Prediabetes/Diabetes | 6 | 467 | − 0.90 (− 1.54, − 0.25) | 0.01 | < 0.001 | 90.3% | 0.461 |
| Other conditions | 4 | 236 | − 1.27 (− 2.04, − 0.50) | < 0.001 | < 0.001 | 84.4% | |
| Cinnamon dosage (mg/day) | |||||||
| 2000 > | 4 | 330 | − 0.64 (− 1.43, 0.14) | 0.109 | < 0.001 | 90.9% | 0.169 |
| 2000 ≤ | 6 | 373 | − 1.32 (− 1.88, − 0.76) | < 0.001 | < 0.001 | 82.3% | |
| Intervention duration (weeks) | |||||||
| 8 ≥ | 5 | 386 | − 1.24 (− 1.99, − 0.49) | 0.001 | < 0.001 | 90.4% | 0.474 |
| 8 < | 5 | 317 | − 0.85 (− 1.59, − 0.12) | 0.023 | < 0.001 | 89.1% | |
| Intervention Age (year) | |||||||
| 50 ≥ | 3 | 199 | − 1.71 (− 2.03, − 1.38) | < 0.001 | 0.785 | 0.0% | 0.008 |
| 50 < | 7 | 504 | − 0.79 (− 1.38, − 0.20) | 0.008 | < 0.001 | 89.3% | |
| Baseline BMI | |||||||
| Healthy/Overweight (< 30 kg/m2) | 7 | 482 | − 0.92 (− 1.55, − 0.29) | 0.004 | < 0.001 | 89.9% | 0.392 |
| Obese (≥ 30 kg/m2) | 3 | 221 | − 1.34 (− 2.08, − 0.60) | < 0.001 | 0.003 | 82.7% | |
| Not given | – | – | – | – | – | – | |
| Cinnamon type | |||||||
| C.cassia | 2 | 117 | − 0.37 (− 0.89, 0.15) | 0.163 | 0.157 | 50.1% | < 0.001 |
| C.verum | 1 | 79 | − 2.02 (− 2.57, − 1.48) | < 0.001 | – | – | |
| C.zeylanicum | 2 | 74 | − 0.19 (− 0.65, 0.27) | 0.409 | 0.718 | 0.0% | |
| Not given | 5 | 433 | − 1.44 (− 2.09, − 0.79) | < 0.001 | < 0.001 | 88.3% | |
| Continent | |||||||
| Asia | 9 | 645 | − 1.09 (− 1.63, − 0.55) | < 0.001 | < 0.001 | 89.6% | 0.246 |
| Europe/America | 1 | 58 | − 0.64 (− 1.17, − 0.11) | 0.018 | – | – | |
| Analysis and subgroup results of Cinnamon supplementation on SBP | |||||||
| Overall effect | 11 | 725 | − 0.85 (− 1.54, − 0.16) | 0.016 | < 0.001 | 94.3% | |
| Health Condition | |||||||
| Prediabetes/Diabetes | 7 | 489 | − 0.47 (− 1.30, 0.37) | 0.270 | < 0.001 | 94.4% | 0.137 |
| Other conditions | 4 | 236 | − 1.53 (− 2.65, − 0.41) | 0.007 | < 0.001 | 91.8% | |
| Cinnamon dosage (mg/day) | |||||||
| 2000 > | 5 | 352 | − 0.81 (− 1.57, − 0.05) | 0.037 | < 0.001 | 90.2% | 0.920 |
| 2000 ≤ | 6 | 373 | − 0.88 (− 2.12, 0.36) | 0.163 | < 0.001 | 96.3% | |
| Intervention duration (weeks) | |||||||
| 8 ≥ | 5 | 386 | − 0.29 (− 1.42, 0.84) | 0.613 | < 0.001 | 96.0% | 0.142 |
| 8 < | 6 | 339 | − 1.32 (− 2.10, − 0.54) | 0.001 | < 0.001 | 89.6% | |
| Intervention Age (year) | |||||||
| 50 ≥ | 4 | 221 | − 2.04 (− 2.89, − 1.19) | < 0.001 | 0.001 | 81.3% | 0.002 |
| 50 < | 7 | 504 | − 0.20 (− 0.96, 0.55) | 0.598 | < 0.001 | 93.8% | |
| Baseline BMI | |||||||
| Healthy/Overweight (< 30 kg/m2) | 7 | 482 | − 0.28 (− 1.08, 0.53) | 0.500 | < 0.001 | 94.1% | 0.011 |
| Obese (≥ 30 kg/m2) | 4 | 243 | − 1.88 (− 2.81, − 0.95) | < 0.001 | < 0.001 | 87.4% | |
| Not given | – | – | – | – | – | – | |
| Cinnamon type | |||||||
| C.cassia | 2 | 117 | − 0.66 (− 1.03, − 0.29) | 0.001 | 0.353 | 0.0% | < 0.001 |
| C.verum | 1 | 79 | 1.64 (1.13, 2.16) | < 0.001 | – | – | |
| C.zeylanicum | 2 | 74 | 0.01 (− 0.45, 0.47) | 0.970 | 0.970 | 0.0% | |
| Not given | 6 | 455 | − 1.63 (− 2.48, − 0.78) | < 0.001 | < 0.001 | 93.1% | |
| Continent | |||||||
| Asia | 9 | 645 | − 0.73 (− 1.53, 0.07) | 0.072 | < 0.001 | 95.2% | 0.377 |
| Europe/America | 2 | 80 | − 1.40 (− 2.64, − 0.16) | 0.027 | 0.036 | 77.3% | |
| Analysis and subgroup results of Cinnamon supplementation on Fasting glucose | |||||||
| Overall effect | 39 | 1998 | − 1.45 (− 1.88, − 1.02) | < 0.001 | < 0.001 | 93.8% | |
| Health Condition | |||||||
| Prediabetes/Diabetes | 30 | 1578 | − 1.90 (− 2.43, − 1.37) | < 0.001 | < 0.001 | 94.5% | < 0.001 |
| Other conditions | 9 | 420 | − 0.34 (− 1.02, 0.35) | 0.333 | < 0.001 | 90.1% | |
| Cinnamon dosage (mg/day) | |||||||
| 2000 > | 19 | 1072 | − 0.86 (− 1.25, − 0.47) | < 0.001 | < 0.001 | 87.5% | 0.003 |
| 2000 ≤ | 20 | 926 | − 2.28 (− 3.13, − 1.44) | < 0.001 | < 0.001 | 95.9% | |
| Intervention duration (weeks) | |||||||
| 8 ≥ | 17 | 786 | − 1.25 (− 1.81, − 0.69) | < 0.001 | < 0.001 | 91.7% | 0.356 |
| 8 < | 22 | 1212 | − 1.65 (− 2.29, − 1.01) | < 0.001 | < 0.001 | 94.8% | |
| Intervention Age (year) | |||||||
| 50 ≥ | 15 | 630 | − 4.01 (− 5.35, − 2.67) | < 0.001 | < 0.001 | 96.6% | < 0.001 |
| 50 < | 24 | 1368 | − 0.68 (− 1.00, − 0.36) | < 0.001 | < 0.001 | 86.6% | |
| Baseline BMI | |||||||
| Healthy/Overweight (< 30 kg/m2) | 26 | 1517 | − 1.16 (− 1.69, − 0.63) | < 0.001 | < 0.001 | 94.6% | 0.094 |
| Obese (≥ 30 kg/m2) | 5 | 264 | − 1.32 (− 2.07, − 0.57) | 0.001 | < 0.001 | 84.5% | |
| Not given | 8 | 217 | − 2.68 (− 3.96, − 1.41) | < 0.001 | < 0.001 | 91.4% | |
| Cinnamon type | |||||||
| C.cassia | 9 | 316 | − 1.68 (− 2.83, − 0.54) | 0.004 | < 0.001 | 93.9% | < 0.001 |
| C.verum | 1 | 79 | 0.02 (− 0.42, 0.46) | 0.935 | – | – | |
| C.zeylanicum | 3 | 116 | − 0.13 (− 0.56, 0.29) | 0.541 | 0.262 | 25.3% | |
| Not given | 26 | 1487 | − 1.68 (− 2.22, − 1.13) | < 0.001 | < 0.001 | 94.4% | |
| Continent | |||||||
| Asia | 34 | 1815 | − 1.67 (− 2.15, − 1.19) | < 0.001 | < 0.001 | 94.3% | 0.007 |
| Europe/America | 5 | 183 | − 0.27 (− 1.16, 0.63) | 0.559 | < 0.001 | 85.7% | |
| Analysis and subgroup results of Cinnamon supplementation on Fasting insulin | |||||||
| Overall effect | 7 | 349 | 0.09 (− 0.22, 0.39) | 0.582 | 0.095 | 44.3% | |
| Analysis and subgroup results of Cinnamon supplementation on Glucose | |||||||
| Overall effect | 5 | 371 | − 0.39 (− 1.17, 0.40) | 0.339 | < 0.001 | 92.1% | |
| Analysis and subgroup results of Cinnamon supplementation on HbA1c | |||||||
| Overall effect | 30 | 1876 | − 0.71 (− 1.02, − 0.40) | < 0.001 | < 0.001 | 89.3% | |
| Health Condition | |||||||
| Prediabetes/Diabetes | 26 | 1637 | − 0.63 (− 0.94, − 0.32) | < 0.001 | < 0.001 | 87.6% | 0.513 |
| Other conditions | 4 | 239 | − 1.15 (− 2.65, 0.36) | 0.135 | < 0.001 | 95.5% | |
| Cinnamon dosage (mg/day) | |||||||
| 2000 > | 14 | 964 | − 0.49 (− 0.88, − 0.10) | 0.013 | < 0.001 | 87.0% | 0.162 |
| 2000 ≤ | 16 | 912 | − 0.95 (− 1.45, − 0.44) | < 0.001 | < 0.001 | 91.2% | |
| Intervention duration (weeks) | |||||||
| 8 ≥ | 9 | 483 | − 0.14 (− 0.50, 0.22) | 0.434 | < 0.001 | 73.4% | 0.002 |
| 8 < | 21 | 1393 | − 1.02 (− 1.42, − 0.61) | < 0.001 | < 0.001 | 91.0% | |
| Intervention Age (year) | |||||||
| 50 ≥ | 8 | 420 | − 2.89 (− 4.00, − 1.77) | < 0.001 | < 0.001 | 94.0% | < 0.001 |
| 50 < | 22 | 1456 | − 0.28 (− 0.51, − 0.05) | 0.019 | < 0.001 | 77.5% | |
| Baseline BMI | |||||||
| Healthy/Overweight (< 30 kg/m2) | 21 | 1258 | − 0.70 (− 1.09, − 0.31) | < 0.001 | < 0.001 | 89.6% | 0.662 |
| Obese (≥ 30 kg/m2) | 6 | 412 | − 0.87 (− 1.73, − 0.01) | 0.047 | < 0.001 | 93.6% | |
| Not given | 3 | 206 | − 0.53 (− 0.82, − 0.25) | < 0.001 | 0.585 | 0.0% | |
| Cinnamon type | |||||||
| C.cassia | 7 | 396 | 0.05 (− 0.30, 0.40) | 0.781 | 0.009 | < 0.001 | |
| C.verum | 1 | 79 | − 0.08 (− 0.52, 0.36) | 0.731 | – | – | |
| C.zeylanicum | 1 | 37 | − 0.50 (− 1.15, 0.16) | 0.137 | – | – | |
| Not given | 21 | 1364 | − 1.07 (− 1.47, − 0.67) | < 0.001 | < 0.001 | 90.5% | |
| Continent | |||||||
| Asia | 23 | 1405 | − 0.96 (− 1.36, − 0.57) | < 0.001 | < 0.001 | 90.7% | 0.001 |
| Europe/America | 7 | 471 | − 0.11 (− 0.44, 0.23) | 0.530 | 0.008 | 65.2% | |
| Analysis and subgroup results of Cinnamon supplementation on HOMA-IR | |||||||
| Overall effect | 17 | 1211 | − 0.54 (− 0.82, − 0.26) | < 0.001 | < 0.001 | 80.4% | |
| Health Condition | |||||||
| Prediabetes/Diabetes | 11 | 925 | − 0.44 (− 0.81, − 0.07) | 0.019 | < 0.001 | 85.8% | 0.189 |
| Other conditions | 6 | 286 | − 0.77 (− 1.10, − 0.44) | < 0.001 | 0.128 | 41.6% | |
| Cinnamon dosage (mg/day) | |||||||
| 2000 > | 13 | 954 | − 0.62 (− 0.96, − 0.28) | < 0.001 | < 0.001 | 83.4% | 0.172 |
| 2000 ≤ | 4 | 257 | − 0.33 (− 0.58, − 0.08) | 0.009 | 0.610 | 0.0% | |
| Intervention duration (weeks) | |||||||
| 8 ≥ | 8 | 509 | − 0.45 (− 0.70, − 0.20) | < 0.001 | 0.086 | 43.9% | 0.486 |
| 8 < | 9 | 702 | − 0.63 (− 1.09, − 0.17) | 0.007 | < 0.001 | 87.4% | |
| Intervention Age (year) | |||||||
| 50 ≥ | 6 | 286 | − 0.77 (− 1.10, − 0.44) | < 0.001 | 0.128 | 41.6% | 0.189 |
| 50 < | 11 | 925 | − 0.44 (− 0.81, − 0.07) | 0.019 | < 0.001 | 85.8% | |
| Baseline BMI | |||||||
| Healthy/Overweight (< 30 kg/m2) | 13 | 945 | − 0.58 (− 0.91, − 0.24) | 0.001 | < 0.001 | 83.1% | 0.817 |
| Obese (≥ 30 kg/m2) | 3 | 126 | − 0.30 (− 1.23, 0.63) | 0.527 | 0.008 | 79.4% | |
| Not given | 1 | 140 | − 0.47 (− 0.81, − 0.14) | 0.006 | – | – | |
| Cinnamon type | |||||||
| C.cassia | 2 | 131 | − 0.41 (− 1.00, 0.19) | 0.183 | 0.154 | 50.8% | 0.836 |
| C.verum | – | – | – | – | – | – | |
| C.zeylanicum | 1 | 42 | − 0.67 (− 1.29, − 0.05) | 0.036 | – | – | |
| Not given | 14 | 1038 | − 0.55 (− 0.88, − 0.22) | 0.001 | < 0.001 | 83.6% | |
| Continent | |||||||
| Asia | 14 | 1029 | − 0.62 (− 0.93, − 0.30) | < 0.001 | < 0.001 | 82.3% | 0.157 |
| Europe/America | 3 | 182 | − 0.20 (− 0.68, 0.29) | 0.420 | 0.192 | 39.4% | |
| Analysis and subgroup results of Cinnamon supplementation on Insulin | |||||||
| Overall effect | 13 | 1031 | − 0.45 (− 0.74, − 0.15) | 0.003 | < 0.001 | 81.0% | |
| Health Condition | |||||||
| Prediabetes/Diabetes | 10 | 846 | − 0.36 (− 0.72, 0.01) | 0.058 | < 0.001 | 84.7% | 0.113 |
| Other conditions | 3 | 185 | − 0.74 (− 1.04, − 0.44) | < 0.001 | 0.941 | 0.0% | |
| Cinnamon dosage (mg/day) | |||||||
| 2000 > | 10 | 775 | − 0.59 (− 0.92, − 0.26) | < 0.001 | < 0.001 | 79.1% | 0.004 |
| 2000 ≤ | 3 | 256 | 0.02 (− 0.22, 0.27) | 0.852 | 0.974 | 0.0% | |
| Intervention duration (weeks) | |||||||
| 8 ≥ | 6 | 373 | − 0.43 (− 0.81, − 0.06) | 0.025 | 0.010 | 66.8% | 0.908 |
| 8 < | 7 | 658 | − 0.47 (− 0.93, − 0.01) | 0.047 | < 0.001 | 87.5% | |
| Intervention Age (year) | |||||||
| 50 ≥ | 3 | 185 | − 0.74 (− 1.04, − 0.44) | < 0.001 | 0.941 | 0.0% | 0.113 |
| 50 < | 10 | 846 | − 0.36 (− 0.72, 0.01) | 0.058 | < 0.001 | 84.7% | |
| Baseline BMI | |||||||
| Healthy/Overweight (< 30 kg/m2) | 9 | 725 | − 0.39 (− 0.71, − 0.07) | 0.016 | < 0.001 | 76.8% | 0.072 |
| Obese (≥ 30 kg/m2) | 3 | 166 | − 0.82 (− 1.72, 0.08) | 0.075 | 0.001 | 85.1% | |
| Not given | 1 | 140 | 0.04 (− 0.29, 0.38) | 0.797 | – | – | |
| Cinnamon type | |||||||
| C.cassia | 3 | 188 | − 0.88 (− 1.74, − 0.03) | 0.044 | 0.002 | 84.4% | 0.329 |
| C.verum | 1 | 79 | − 0.02 (− 0.46, 0.42) | 0.930 | – | – | |
| C.zeylanicum | 2 | 79 | − 0.40 (− 1.25, 0.45) | 0.359 | 0.059 | 71.9% | |
| Not given | 7 | 685 | − 0.35 (− 0.71, 0.01) | 0.060 | < 0.001 | 81.0% | |
| Continent | |||||||
| Asia | 10 | 809 | − 0.43 (− 0.72, − 0.14) | 0.004 | < 0.001 | 74.8% | 0.864 |
| Europe/America | 3 | 222 | − 0.53 (− 1.70, 0.63) | 0.367 | < 0.001 | 92.3% | |
| Analysis and subgroup results of Cinnamon supplementation on Postprandial blood glucose | |||||||
| Overall effect | 8 | 463 | − 2.28 (− 3.48, − 1.08) | < 0.001 | < 0.001 | 94.4% | |
| Analysis and subgroup results of Cinnamon supplementation on QUICKI | |||||||
| Overall effect | 3 | 162 | − 0.33 (− 0.72, 0.07) | 0.103 | 0.268 | 24.0% | |
| Analysis and subgroup results of Cinnamon supplementation on CRP | |||||||
| Overall effect | 7 | 462 | − 0.78 (− 1.28, − 0.27) | 0.003 | < 0.001 | 84.3% | |
| Analysis and subgroup results of Cinnamon supplementation on IL-6 | |||||||
| Overall effect | 3 | 123 | − 0.37 (− 0.90, 0.16) | 0.168 | 0.119 | 53.1% | |
| Analysis and subgroup results of Cinnamon supplementation on Fasting cholesterol | |||||||
| Overall effect | 4 | 74 | − 3.93 (− 6.23, − 1.64) | 0.001 | < 0.001 | 88.1% | |
| Analysis and subgroup results of Cinnamon supplementation on Fasting LDL | |||||||
| Overall effect | 4 | 74 | − 2.52 (− 4.37, − 0.68) | 0.007 | < 0.001 | 88.0% | |
| Analysis and subgroup results of Cinnamon supplementation on Fasting TG | |||||||
| Overall effect | 4 | 74 | − 2.67 (− 4.18, − 1.16) | 0.001 | 0.001 | 81.0% | |
| Analysis and subgroup results of Cinnamon supplementation on HDL | |||||||
| Overall effect | 30 | 1780 | 0.56 (0.23, 0.89) | 0.001 | < 0.001 | 90.6% | |
| Health Condition | |||||||
| Prediabetes/Diabetes | 19 | 1198 | 0.65 (0.20, 1.09) | 0.005 | < 0.001 | 92.3% | 0.507 |
| Other conditions | 11 | 582 | 0.42 (− 0.06, 0.91) | 0.086 | < 0.001 | 86.9% | |
| Cinnamon dosage (mg/day) | |||||||
| 2000 > | 17 | 1014 | 0.29 (0.04, 0.54) | 0.021 | < 0.001 | 72.1% | 0.075 |
| 2000 ≤ | 13 | 766 | 1.00 (0.26, 1.74) | 0.008 | < 0.001 | 95.2% | |
| Intervention duration (weeks) | |||||||
| 8 ≥ | 12 | 733 | 0.16 (− 0.16, 0.49) | 0.319 | < 0.001 | 78.0% | 0.025 |
| 8 < | 18 | 1047 | 0.87 (0.35, 1.39) | 0.001 | < 0.001 | 93.2% | |
| Intervention Age (year) | |||||||
| 50 ≥ | 12 | 700 | 1.12 (0.31, 1.93) | 0.007 | < 0.001 | 95.5% | 0.042 |
| 50 < | 18 | 1080 | 0.25 (0.02, 0.47) | 0.031 | < 0.001 | 68.4% | |
| Baseline BMI | |||||||
| Healthy/Overweight (< 30 kg/m2) | 19 | 1198 | 0.54 (0.10, 0.97) | 0.016 | < 0.001 | 92.0% | 0.485 |
| Obese (≥ 30 kg/m2) | 8 | 469 | 0.75 (0.08, 1.42) | 0.028 | < 0.001 | 91.0% | |
| Not given | 3 | 113 | 0.31 (− 0.09, 0.70) | 0.125 | 0.661 | 0.0% | |
| Cinnamon type | |||||||
| C.cassia | 5 | 216 | 0.34 (− 0.32, 1.01) | 0.313 | < 0.001 | 81.5% | 0.004 |
| C.verum | 2 | 139 | − 0.35 (− 0.82, 0.12) | 0.140 | 0.166 | 47.8% | |
| C.zeylanicum | 3 | 116 | 0.02 (− 0.53, 0.58) | 0.934 | 0.107 | 55.3% | |
| Not given | 20 | 1309 | 0.81 (0.37, 1.25) | < 0.001 | < 0.001 | 92.6% | |
| Continent | |||||||
| Asia | 24 | 1536 | 0.62 (0.23, 1.01) | 0.002 | < 0.001 | 92.1% | 0.420 |
| Europe/America | 6 | 244 | 0.35 (− 0.17, 0.88) | 0.184 | 0.002 | 73.3% | |
| Analysis and subgroup results of Cinnamon supplementation on LDL | |||||||
| Overall effect | 28 | 1674 | − 0.56 (− 0.86, − 0.25) | < 0.001 | < 0.001 | 88.6% | |
| Health Condition | |||||||
| Prediabetes/Diabetes | 19 | 1191 | − 0.50 (− 0.92, − 0.08) | 0.019 | < 0.001 | 91.3% | 0.405 |
| Other conditions | 9 | 483 | − 0.71 (−0.98, − 0.45) | < 0.001 | 0.056 | 47.3% | |
| Cinnamon dosage (mg/day) | |||||||
| 2000 > | 16 | 975 | −0.45 (− 0.76, − 0.13) | 0.006 | < 0.001 | 81.8% | 0.460 |
| 2000 ≤ | 12 | 699 | − 0.71 (− 1.32, − 0.10) | 0.023 | < 0.001 | 92.7% | |
| Intervention duration (weeks) | |||||||
| 8 ≥ | 11 | 666 | − 0.29 (− 0.73, 0.15) | 0.197 | < 0.001 | 86.6% | 0.152 |
| 8 < | 17 | 1008 | − 0.73 (− 1.14, − 0.32) | < 0.001 | < 0.001 | 88.9% | |
| Intervention Age (year) | |||||||
| 50 ≥ | 10 | 601 | − 1.04 (− 1.65, − 0.42) | 0.001 | < 0.001 | 91.1% | 0.026 |
| 50 < | 18 | 1073 | − 0.27 (− 0.55, 0.01) | 0.054 | < 0.001 | 79.0% | |
| Baseline BMI | |||||||
| Healthy/Overweight (< 30 kg/m2) | 18 | 1152 | − 0.71 (− 1.14, − 0.28) | 0.001 | < 0.001 | 91.6% | 0.181 |
| Obese (≥ 30 kg/m2) | 7 | 409 | − 0.35 (− 0.84, 0.13) | 0.156 | < 0.001 | 81.5% | |
| Not given | 3 | 113 | − 0.16 (− 0.55, 0.23) | 0.409 | 0.746 | 0.0% | |
| Cinnamon type | |||||||
| C.cassia | 5 | 216 | − 0.19 (− 0.60, 0.21) | 0.348 | 0.085 | 51.2% | 0.053 |
| C.verum | 1 | 79 | 0.10 (− 0.34, 0.54) | 0.658 | – | – | |
| C.zeylanicum | 3 | 116 | − 0.22 (− 0.78, 0.34) | 0.438 | 0.103 | 55.9% | |
| Not given | 19 | 1263 | − 0.73 (− 1.15, − 0.32) | 0.001 | < 0.001 | 91.3% | |
| Continent | |||||||
| Asia | 22 | 1430 | − 0.70 (− 1.06, − 0.34) | < 0.001 | < 0.001 | 90.2% | 0.001 |
| Europe/America | 6 | 244 | 0.05 (− 0.20, 0.31) | 0.683 | 0.513 | 0.0% | |
| Analysis and subgroup results of Cinnamon supplementation on TC | |||||||
| Overall effect | 29 | 1742 | − 0.91 (− 1.31, − 0.52) | < 0.001 | < 0.001 | 93.0% | |
| Health Condition | |||||||
| Prediabetes/Diabetes | 19 | 1199 | − 1.08 (− 1.66, − 0.50) | < 0.001 | < 0.001 | 95.0% | 0.272 |
| Other conditions | 10 | 543 | − 0.71 (− 1.02, − 0.40) | < 0.001 | 0.002 | 65.6% | |
| Cinnamon dosage (mg/day) | |||||||
| 2000 > | 16 | 975 | − 0.62 (− 1.02, − 0.21) | 0.003 | < 0.001 | 88.3% | 0.136 |
| 2000 ≤ | 13 | 767 | − 1.28 (− 2.06, − 0.50) | 0.001 | < 0.001 | 95.6% | |
| Intervention duration (weeks) | |||||||
| 8 ≥ | 12 | 734 | − 0.54 (− 1.06, − 0.03) | 0.038 | < 0.001 | 90.7% | 0.116 |
| 8 < | 17 | 1008 | − 1.17 (− 1.74, − 0.59) | < 0.001 | < 0.001 | 94.0% | |
| Intervention Age (year) | |||||||
| 50 ≥ | 11 | 661 | − 1.66 (− 2.51, − 0.80) | < 0.001 | < 0.001 | 95.4% | 0.011 |
| 50 < | 18 | 1081 | − 0.45 (− 0.81, − 0.08) | 0.018 | < 0.001 | 87.7% | |
| Baseline BMI | |||||||
| Healthy/Overweight (< 30 kg/m2) | 18 | 1160 | − 1.24 (− 1.84, − 0.64) | < 0.001 | < 0.001 | 95.2% | 0.121 |
| Obese (≥ 30 kg/m2) | 8 | 469 | − 0.44 (− 0.94, 0.06) | 0.083 | < 0.001 | 84.6% | |
| Not given | 3 | 113 | − 0.64 (− 1.04, − 0.25) | 0.001 | 0.953 | 0.0% | |
| Cinnamon type | |||||||
| C.cassia | 5 | 216 | − 0.20 (− 0.79, 0.39) | 0.506 | 0.002 | 76.4% | 0.005 |
| C.verum | 2 | 139 | − 0.11 (− 1.23, 1.00) | 0.845 | 0.001 | 90.5% | |
| C.zeylanicum | 3 | 116 | − 0.17 (− 0.54, 0.19) | 0.350 | 0.860 | 0.0% | |
| Not given | 19 | 1271 | − 1.34 (− 1.90, − 0.79) | < 0.001 | < 0.001 | 94.9% | |
| Continent | |||||||
| Asia | 23 | 1498 | − 1.15 (− 1.62, − 0.68) | < 0.001 | < 0.001 | 94.1% | < 0.001 |
| Europe/America | 6 | 244 | 0.02 (− 0.37, 0.40) | 0.937 | 0.060 | 52.8% | |
| Analysis and subgroup results of Cinnamon supplementation on TG | |||||||
| Overall effect | 30 | 1780 | − 0.73 (− 1.08, − 0.39) | < 0.001 | < 0.001 | 91.2% | |
| Health Condition | |||||||
| Prediabetes/Diabetes | 19 | 1198 | − 0.97 (− 1.46, − 0.47) | < 0.001 | < 0.001 | 93.5% | 0.075 |
| Other conditions | 11 | 582 | − 0.39 (− 0.78, 0.02) | 0.061 | < 0.001 | 81.6% | |
| Cinnamon dosage (mg/day) | |||||||
| 2000 > | 17 | 1014 | − 0.42 (− 0.75, − 0.10) | 0.011 | < 0.001 | 83.5% | 0.060 |
| 2000 ≤ | 13 | 766 | − 1.15 (− 1.84, − 0.46) | 0.001 | < 0.001 | 94.5% | |
| Intervention duration (weeks) | |||||||
| 8 ≥ | 12 | 733 | − 0.48 (− 0.95, − 0.00) | 0.050 | < 0.001 | 89.3% | 0.210 |
| 8 < | 18 | 1047 | − 0.91 (− 1.40, − 0.42) | < 0.001 | < 0.001 | 92.2% | |
| Intervention Age (year) | |||||||
| 50 ≥ | 12 | 700 | − 1.18 (− 1.95, − 0.42) | 0.002 | < 0.001 | 94.9% | 0.076 |
| 50 < | 18 | 1080 | − 0.44 (− 0.74, − 0.14) | 0.004 | < 0.001 | 82.1% | |
| Baseline BMI | |||||||
| Healthy/Overweight (< 30 kg/m2) | 19 | 1198 | − 0.82 (− 1.33, − 0.31) | 0.002 | < 0.001 | 93.9% | 0.826 |
| Obese (≥ 30 kg/m2) | 8 | 469 | − 0.64 (− 1.03, − 0.25) | 0.001 | < 0.001 | 74.5% | |
| Not given | 3 | 113 | − 0.62 (− 1.14, − 0.11) | 0.018 | 0.199 | 38.0% | |
| Cinnamon type | |||||||
| C.cassia | 5 | 216 | − 0.23 (− 0.50, 0.04) | 0.088 | 0.828 | 0.0% | 0.007 |
| C.verum | 2 | 139 | − 0.07 (− 0.40, 0.27) | 0.694 | 0.389 | 0.0% | |
| C.zeylanicum | 3 | 116 | − 0.03 (− 0.94, 0.89) | 0.953 | 0.003 | 83.2% | |
| Not given | 20 | 1309 | − 1.06 (− 1.54, − 0.58) | < 0.001 | < 0.001 | 93.6% | |
| Continent | |||||||
| Asia | 24 | 1536 | − 0.86 (− 1.27, − 0.44) | < 0.001 | < 0.001 | 93.0% | 0.029 |
| Europe/America | 6 | 244 | − 0.31 (− 0.56, − 0.06) | 0.016 | 0.987 | 0.0% | |
| Analysis and subgroup results of Cinnamon supplementation on ALP | |||||||
| Overall effect | 6 | 101 | − 0.07 (− 0.47, 0.32) | 0.720 | 0.488 | 0.0% | |
| Analysis and subgroup results of Cinnamon supplementation on ALT | |||||||
| Overall effect | 10 | 248 | − 0.59 (− − 1.45, 0.28) | 0.182 | < 0.001 | 88.9% | |
| Health Condition | |||||||
| Prediabetes/Diabetes | 7 | 150 | − 0.55 (− 0.98, − 0.12) | 0.012 | 0.164 | 34.6% | 0.922 |
| Other conditions | 3 | 98 | − 0.71 (− 3.77, 2.37) | 0.653 | < 0.001 | 97.2% | |
| Cinnamon dosage (mg/day) | |||||||
| 2000 > | 6 | 164 | − 0.98 (− 2.00, 0.04) | 0.060 | < 0.001 | 87.5% | 0.241 |
| 2000 ≤ | 4 | 84 | 0.02 (− 1.30, 1.34) | 0.977 | < 0.001 | 86.9% | |
| Intervention duration (weeks) | |||||||
| 8 ≥ | 2 | 50 | 0.81 (− 0.78, 2.40) | 0.318 | 0.014 | 83.6% | 0.057 |
| 8 < | 8 | 198 | − 0.93 (− 1.76, − 0.10) | 0.027 | < 0.001 | 84.7% | |
| Intervention Age (year) | |||||||
| 50 ≥ | 7 | 165 | − 0.58 (− 1.86, 0.71) | 0.377 | < 0.001 | 92.2% | 0.896 |
| 50 < | 3 | 83 | − 0.67 (− 1.24, − 0.11) | 0.020 | 0.249 | 28.0% | |
| Baseline BMI | |||||||
| Healthy/Overweight (< 30 kg/m2) | 6 | 146 | − 0.62 (− 2.15, 0.91) | 0.425 | < 0.001 | 93.5% | 0.682 |
| Obese (≥ 30 kg/m2) | 1 | 22 | − 0.29 (− 1.13, 0.56) | 0.508 | – | – | |
| Not given | 3 | 80 | − 0.73 (− 1.24, − 0.21) | 0.006 | 0.334 | 8.8% | |
| Cinnamon type | |||||||
| C.cassia | 2 | 31 | − 0.02 (− 0.73, 0.69) | 0.956 | 0.951 | 0.0% | 0.275 |
| C.verum | – | – | – | – | – | – | |
| C.zeylanicum | – | – | – | – | – | – | |
| Not given | 8 | 217 | − 0.73 (− 1.78, 0.33) | 0.177 | < 0.001 | 91.2% | |
| Continent | |||||||
| Asia | 8 | 209 | − 0.70 (− 1.79, 0.39) | 0.206 | < 0.001 | 91.2% | 0.398 |
| Europe/America | 2 | 39 | − 0.16 (− 0.79, 0.47) | 0.621 | 0.661 | 0.0% | |
| Analysis and subgroup results of Cinnamon supplementation on AST | |||||||
| Overall effect | 9 | 234 | − 0.29 (− 0.96, 0.39) | 0.406 | < 0.001 | 82.4% | |
| Analysis and subgroup results of Cinnamon supplementation on BUN | |||||||
| Overall effect | 5 | 169 | − 0.50 (− 1.74, 0.74) | 0.428 | < 0.001 | 90.0% | |
| Analysis and subgroup results of Cinnamon supplementation on MDA | |||||||
| Overall effect | 5 | 289 | − 0.76 (− 1.07, − 0.45) | < 0.001 | 0.193 | 34.3% | |
| Analysis and subgroup results of Cinnamon supplementation on TAC | |||||||
| Overall effect | 4 | 268 | 0.52 (− 0.05, 1.08) | 0.073 | 0.002 | 79.1% | |
Footprint: ALP, Alkaline Phosphatase; ALT, Alanine Aminotransferase; AST, Aspartate Aminotransferase; BF, Body fat; BMI, Body Mass Index; BUN, Blood Urea Nitrogen; CI, Confidence Interval; CRP, C-Reactive Protein; DBP, Diastolic Blood Pressure; FBS, Fasting Blood Sugar; HbA1c, Hemoglobin A1C; HDL, High-Density Lipoprotein; HOMA-IR, Homeostatic Model Assessment of Insulin Resistance; IL-6, Interleukin-6; INT, Intervention Group; LDL, Low-Density Lipoprotein; MDA, Malondialdehyde; QUICKI, Quantitative Insulin Sensitivity Check Index; SBP, Systolic Blood Pressure; SMD, Standardised Mean Difference; TAC, Total Antioxidant Capacity; TC, Total Cholesterol; TG, Triglycerides; WC, Waist Circumference; WHR, Waist-to-Hip Ratio
Bold formatting has been used to denote statistically significant findings (p < 0.05)
Cinnamon supplementation showed a greater impact on fasting glucose, HbA1c, HDL-C, TG, and ALT in T2DM or prediabetic participants, while WC and SBP were more significantly affected in other health conditions. DBP, HOMA-IR, LDL-C, and TC improved significantly across both health conditions. No substantial effects were observed on BMI, weight, and fasting insulin in these subgroups.
For dosages > 2000 mg/day, significant effects were observed in SBP and fasting insulin. In contrast, doses ≤ 2000 mg/day had a more notable effect on WC, DBP, and HbA1c. Both dosage groups showed significant effects on fasting glucose, HOMA-IR, HDL-C, LDL-C, TC, and TG, while BMI, weight, and ALT were unaffected.
Regarding intervention duration, studies lasting < 8 weeks showed significant effects on weight, SBP, HbA1c, HDL-C, TG, and ALT. Both duration groups saw improvements in DBP, fasting glucose, HOMA-IR, LDL, TC, and TG, while BMI, WC and fasting insulin remained unaffected in both categories.
Participants aged ≤ 50 showed significant improvements in WC, and SBP, while those over 50 exhibited significant reductions in ALT. Both age groups demonstrated significant effects on DBP, fasting glucose, HbA1c, HOMA-IR, HDL-C, LDL-C, TC, and TG. No significant changes in BMI, weight, and fasting insulin were observed in either age group.
For baseline BMI, cinnamon supplementation significantly impacted HOMA-IR, LDL-C, and TC in healthy or overweight individuals, while significant improvements in WC and SBP were observed in obese participants. Both BMI groups showed effects on DBP, fasting glucose, HbA1c, HDL-C, and TG. No significant effects were seen for BMI, weight, fasting insulin, and ALT in either category.
Regarding cinnamon types, C. cassia significantly reduced fasting glucose, LDL-C, TC, and TG. C. verum lowered DBP, and both C. cassia and C. verum affected SBP, while C. zeylanicum impacted HOMA-IR. No significant effects were found for BMI, WC, weight, fasting insulin, HbA1c, HDL-C, and ALT across these cinnamon types.
Regionally, cinnamon supplementation yielded significant effects on WC, fasting glucose, fasting insulin, HbA1c, HOMA-IR, HDL-C, LDL-C, and TC in studies conducted in Asia. SBP was more significantly affected in studies from Europe or America, with DBP and TG showing significant improvements across both regions. BMI, weight, and ALT levels remained unaffected across regions.
Meta-regression and non-linear dose–response analysis
Meta-regression analysis showed a linear relationship between dosage and significant change in fasting insulin level (P = 0.02). Additionally, a non-linear dose–response regression model was employed to explore the relationship between cinnamon supplementation and cardiovascular outcomes. A significant dose-dependent reduction was observed for BMI (P = 0.026), fasting insulin (P < 0.001), LDL (P = 0.005) TC (P = 0.011), and TG (P = 0.016), with approximate optimal doses of 600, 1000, 6000, 6000, and 6000 mg/day, respectively. Also, a significant association was noted between cinnamon dose and HDL-C increase (P < 0.001), with an optimal dose for HDL-C enhancement at around 6000 mg/day. (Supplementary Figs. 8,9,10 and 11).
GRADE assessment
The GRADE profile for cinnamon supplementation outcomes is summarized in Table 4, with the quality of evidence rated as very low across all outcomes.
Table 4.
GRADE profile of cinnamon supplementation on cardiovascular risk factors
| Outcomes | Risk of bias | Inconsistency | Indirectness | Imprecision | Publication Bias | Number (INT/CON) |
SMD (95%CI) | Quality of evidence |
|---|---|---|---|---|---|---|---|---|
| BF | Seriousa | Very seriousb | Not serious | Very seriousc, d | None | 183/177 | − 0.19 (− 0.88, 0.50) |
⨁◯◯◯ Very low |
| BMI | Seriousa | Very seriousb | Not serious | Seriousc | None | 743/762 | − 0.13 (− 0.40, 0.13) |
⨁◯◯◯ Very low |
| WC | Seriousa | Very seriousb | Not serious | Not serious | None | 385/382 | − 0.40 (− 0.73, − 0.06) |
⨁◯◯◯ Very low |
| Weight | Seriousa | Very seriousb | Not serious | Seriousc | None | 521/517 | − 0.08 (− 0.41, 0.25) |
⨁◯◯◯ Very low |
| WHR | Seriousa | Not serious | Not serious | Very seriousc, d |
Publication bias strongly Suspectede |
124/124 | − 0.28 (− 0.61, 0.06) |
⨁◯◯◯ Very low |
| DBP | Seriousa | Very seriousb | Not serious | Not serious | None | 350/353 | − 1.04 (− 1.54, − 0.55) |
⨁◯◯◯ Very low |
| SBP | Seriousa | Very seriousb | Not serious | Not serious | None | 362/363 | − 0.85 (− 1.54, − 0.16) |
⨁◯◯◯ Very low |
| Fasting glucose | Seriousa | Very seriousb | Not serious | Not serious |
Publication bias strongly Suspectede |
1029/969 | − 1.45 (− 1.88, − 1.02) |
⨁◯◯◯ Very low |
| Fasting insulin | Very seriousa | Not serious | Not serious | Very seriousc, d |
Publication bias strongly Suspectede |
172/177 | 0.09 (− 0.22, 0.39) |
⨁◯◯◯ Very low |
| Glucose | Seriousa | Very seriousb | Not serious | Very seriousc, d | None | 181/190 | − 0.39 (− 1.17, 0.40) |
⨁◯◯◯ Very low |
| HbA1c | Seriousa | Very seriousb | Not serious | Not serious | None | 972/904 | − 0.71 (− 1.02, − 0.40) |
⨁◯◯◯ Very low |
| HOMA-R | Seriousa | Very seriousb | Not serious | Not serious | None | 591/620 | − 0.54 (− 0.82, − 0.26) |
⨁◯◯◯ Very low |
| Insulin | Seriousa | Very seriousb | Not serious | Not serious | None | 503/528 | − 0.45 (− 0.74, − 0.15) |
⨁◯◯◯ Very low |
| Postprandial blood glucose | Seriousa | Very seriousb | Seriousf | Not serious |
Publication bias strongly Suspectede |
221/242 | − 2.28 (− 3.48, − 1.08) |
⨁◯◯◯ Very low |
| QUICKI | Seriousa | Not serious | Seriousf | Very seriousc, d | None | 79/83 | − 0.33 (− 0.72, 0.07) |
⨁◯◯◯ Very low |
| CRP | Seriousa | Very seriousb | Seriousf | Not serious | None | 229/233 | − 0.78 (− 1.28, − 0.27) |
⨁◯◯◯ Very low |
| IL-6 | Very seriousa | Seriousb | Very seriousf | Very seriousc, d | None | 62/61 | − 0.37 (− 0.90, 0.16) |
⨁◯◯◯ Very low |
| Fasting cholesterol | Very seriousa | Very seriousb | Very seriousf | Seriousd |
Publication bias strongly Suspectede |
37/37 | − 3.93 (− 6.23, − 1.64) |
⨁◯◯◯ Very low |
| Fasting LDL | Very seriousa | Very seriousb | Very seriousf | Seriousd |
Publication bias strongly Suspectede |
37/37 | − 2.52 (− 4.37, − 0.68) |
⨁◯◯◯ Very low |
| Fasting TG | Very seriousa | Very seriousb | Very seriousf | Seriousd | None | 37/37 | − 2.67 (− 4.18, − 1.16) |
⨁◯◯◯ Very low |
| HDL | Seriousa | Very seriousb | Not serious | Not serious | None | 921/859 | 0.56 (0.23, 0.89) |
⨁◯◯◯ Very low |
| LDL | Seriousa | Very seriousb | Not serious | Not serious | None | 866/808 | − 0.56 (− 0.86, − 0.25) |
⨁◯◯◯ Very low |
| TC | Seriousa | Very seriousb | Not serious | Not serious | None | 903/839 | − 0.91 (− 1.31, − 0.52) |
⨁◯◯◯ Very low |
| TG | Seriousa | Very seriousb | Not serious | Not serious |
Publication bias strongly Suspectede |
922/858 | − 0.73 (− 1.08, − 0.39) |
⨁◯◯◯ Very low |
| ALP | Very seriousa | Not serious | Not serious | Very seriousc, d |
Publication bias strongly Suspectede |
51/50 | − 0.07 (− 0.47, 0.32) |
⨁◯◯◯ Very low |
| ALT | Very seriousa | Very seriousb | Not serious | Very seriousc, d | None | 138/110 | − 0.59 (− 1.45, 0.28) |
⨁◯◯◯ Very low |
| AST | Very seriousa | Very seriousb | Not serious | Very seriousc, d | None | 131/103 | − 0.29 (− 0.96, 0.39) |
⨁◯◯◯ Very low |
| BUN | Very seriousa | Very seriousb | Seriousf | Very seriousc, d | None | 84/85 | − 0.50 (− 1.74, 0.74) |
⨁◯◯◯ Very low |
| MDA | Seriousa | Not serious | Seriousf | Seriousd | None | 142/147 | − 0.76 (− 1.07, − 0.45) |
⨁◯◯◯ Very low |
| TAC | Seriousa | Very seriousb | Seriousf | Very seriousc, d |
Publication bias strongly Suspectede |
131/137 | 0.52 (− 0.05, 1.08) |
⨁◯◯◯ Very low |
Footprint: ALP, Alkaline Phosphatase; ALT, Alanine Aminotransferase; AST, Aspartate Aminotransferase; BF, Body fat; BMI, Body Mass Index; BUN, Blood Urea Nitrogen; CI, Confidence Interval; CRP, C-Reactive Protein; DBP, Diastolic Blood Pressure; FBS, Fasting Blood Sugar; HbA1c, Hemoglobin A1C; HDL, High-Density Lipoprotein; HOMA-IR, Homeostatic Model Assessment of Insulin Resistance; IL-6, Interleukin-6; INT, Intervention Group; LDL, Low-Density Lipoprotein; MDA, Malondialdehyde; QUICKI, Quantitative Insulin Sensitivity Check Index; SBP, Systolic Blood Pressure; SMD, Standardised Mean Difference; TAC, Total Antioxidant Capacity; TC, Total Cholesterol; TG, Triglycerides; WC, Waist Circumference; WHR, Waist-to-Hip Ratio
Explanations
a. Downgraded since more than 50% of the participants were from high-risk bias studies
b. The I2 value was > 50% (or Heterogeneity among the studies was high)
c. Downgraded since the 95% CI crosses the threshold of interest
d. Downgraded since the participants included were less than 400 people
e. Publication Bias was detected through Egger and Begg's test. (p-value < 0.05)
f. Downgraded for indirectness in the country
Discussion
To our knowledge, this is the first GRADE-assessed systematic review and dose–response meta-analysis to evaluate the effects of cinnamon supplementation on metabolic and cardiovascular factors. Our review included 49 RCTs with a total of 3,038 participants, revealing that cinnamon improved WC, blood pressure, glycemic indices, CRP, lipid profile, and MDA in adults. Notably, cinnamon supplementation selectively reduced WC without significantly affecting overall body weight, BMI, or body fat, potentially due to its capacity to enhance insulin sensitivity, reduce visceral fat, and exert anti-inflammatory effects that may specifically target abdominal fat [24, 73]. Further studies are needed to confirm these findings and elucidate the underlying mechanisms. The reduction in CRP without corresponding changes in IL-6 may reflect cinnamon's influence on systemic inflammation without impacting IL-6 pathways. Furthermore, our findings on liver enzymes indicate that cinnamon should not be regarded as a liver function enhancer. The observed decrease in MDA without an increase in TAC suggests a reduction in lipid peroxidation rather than an overall boost in antioxidant capacity. Our results must be interpreted with precaution due to the high heterogeneity in most of the studied factors.
Subgroup analysis revealed that certain factors, including higher doses (≥ 2000 mg/day) and longer durations (> 8 weeks), were associated with larger effect sizes. Non-linear regression indicated a significant dose–response relationship for fasting insulin, LDL-C, TC, TG, and HDL-C, with the optimal dose for most beneficial effects, aside from insulin, identified as 6000 mg/day. The acceptable daily intake of coumarin in Europe is set at 0.1 mg/kg/day to ensure safe consumption [74]. Differences in cinnamon's effects can be attributed to compositional variations; Cassia contains high coumarin levels (1 mg/g), a compound with potential toxicity, while Ceylon (C. verum/zeylanicum) has minimal coumarin (< 0.01 mg/g), making it safer for regular or high-dose use [75]. Additionally, Cassia has a higher essential oil content, especially cinnamaldehyde, whereas Ceylon offers a broader range of antioxidant polyphenols [75–77].
Younger adults (≤ 50 years) experienced greater benefits from cinnamon supplementation than older adults (> 50 years). This aligns with a large observational study finding lifestyle interventions had a more substantial impact on cardiometabolic markers in younger adults [78]. Younger individuals typically have greater physical and cognitive abilities to benefit from lifestyle modifications, while older adults may face more health complications and have a reduced capacity for adaptation due to age-related decline in bodily functions and cognitive processing.
Beneficial effects of cinnamon on metabolic and cardiovascular health can be related to the multiple molecular mechanisms attributed to its bioactive compounds, primarily cinnamaldehyde, cinnamic acid, and polyphenols. One key mechanism is enhancement of insulin sensitivity and regulation of glucose metabolism by cinnamon. Cinnamaldehyde can activate insulin receptors and promote glucose transporter 4 (GLUT4) translocation in muscle and fat tissues, facilitating greater glucose uptake [79]. Moreover, cinnamon activates the phosphorylation process in insulin signaling cascade, leading to the activation of intracellular cascade events [80]. This activity lowers blood glucose levels and improves insulin resistance, potentially reducing visceral fat, which is associated with insulin resistance and abdominal obesity. Cinnamic acid also supports glucose regulation by improving glucose uptake [81].
Regarding lipid metabolism, bioactive compounds of cinnamon inhibit hepatic Hydroxymethylglutaryl-CoA (HMG-CoA) reductase, a key enzyme in cholesterol biosynthesis, resulting in decreased LDL-C and increased HDL-C levels [82]. Cinnamaldehyde and cinnamic acid have also been shown to enhance lipid oxidation and reduce triglyceride levels [83]. Moreover, cinnamon reduces cholesterol and fatty acid absorption in gut cells by inhibiting Niemann-Pick C1-like 1 and Cd36 mRNA receptors [80]. Furthermore, cinnamon upregulates the expression of genes that suppress triglyceride accumulation, cholesterol levels, and apolipoprotein-B48 synthesis at the mRNA level [84].
The anti-inflammatory properties of cinnamon contribute further to its metabolic benefits. Cinnamaldehyde and cinnamic acid can inhibit the nuclear factor-kappa B (NF-Κb) pathway, a master regulator of inflammation, leading to reduced production of pro-inflammatory cytokines [85, 86]. Our study showed a significant reduction in CRP, which supports the ability of cinnamon in reducing systemic inflammation. The fact that IL-6 was unaffected by cinnamon may indicate selective anti-inflammatory actions, possibly targeting specific pathways influenced by active components of cinnamon rather than broad cytokine suppression. Therefore, additional studies focusing on other inflammatory pathways are needed to determine the molecular mechanisms of anti-inflammatory actions of cinnamon. Antioxidant effects are another important aspect of cinnamon, particularly due to its high polyphenol content, including proanthocyanidins [87]. These antioxidants directly scavenge reactive oxygen species (ROS), reducing oxidative stress markers like MDA [88].
Several molecular mechanisms have been identified to explain the hypotensive effects of cinnamon. HTN is often associated with increased oxidative stress and elevated inflammatory markers, and cinnamon, as a potent antioxidant, helps to reduce ROS, thus mitigating oxidative stress [89]. Furthermore, the antioxidant properties of cinnamon contribute to a decrease in the production of nitric oxide (NO), which serves both as a powerful vasodilator and a marker of oxidative stress [90]. However, cinnamon may exert its anti-hypertensive effects through other pathways. Transient receptor potential ankyrin 1 (TRPA1) channels in arteries, when activated by cinnamon, stimulate the release of calcitonin gene-related peptide, resulting in vasodilation. Therefore, cinnamon has been shown to exert a hypotensive effect by inhibiting calcium release from intracellular stores, leading to vascular smooth muscle relaxation and reduced blood pressure [91, 92].
The RoB 2 assessment for the 49 RCTs included in the meta-analysis reveals significant methodological limitations, with 43 studies rated as poor quality, primarily due to high risk of bias in allocation concealment (44 studies) and blinding of outcome assessment (38 studies). These weaknesses, compounded by variable issues in random sequence generation (11 unclear, 1 high risk) and blinding of participants/personnel (18 highs, 5 unclear), suggest potential selection, performance, and detection biases that undermine the reliability of the findings. Despite these concerns, all studies demonstrated low risk in selective outcome reporting and other sources of bias, indicating transparent reporting and absence of additional methodological flaws. Only one study was rated as good quality, with five rated as fair, highlighting the rarity of robust methodology.
Given the very low certainty of evidence and the high risk of bias in the included studies, the potential beneficial effects of cinnamon on the assessed outcomes should be interpreted with caution and considered as possible rather than definitive.
The predominance of poor-quality studies, reflected in the very low GRADE ratings, necessitates cautious interpretation of cinnamon’s effects on cardiovascular risk factors and underscores the urgent need for high-quality RCTs with improved randomization, concealment, and blinding to provide more definitive evidence.
Clinical implications of findings
The results of this meta-analysis offer meaningful clinical insights for healthcare professionals managing patients at risk of cardiovascular and metabolic diseases. The significant reductions observed in fasting plasma glucose, HbA1c, HOMA-IR, fasting insulin, and postprandial blood glucose levels suggest that cinnamon supplementation can serve as an effective adjunctive therapy for improving glycemic control in adults with T2DM, prediabetes, metabolic syndrome, and related disorders. These glycemic benefits were consistently observed across a range of doses and intervention durations, highlighting cinnamon’s potential as a complementary strategy alongside conventional glucose-lowering therapies.
Furthermore, the significant improvements in both SBP and DBP indicate that cinnamon supplementation may offer additional cardiovascular protection, particularly for individuals with coexisting hypertension or elevated blood pressure—a common comorbidity in patients with metabolic dysfunction. A small reduction in blood pressure may seem clinically insignificant to an individual patient, but it can have significant effects on population health. Large meta-analyses show that the risk of cardiovascular disease is consistently reduced with decreasing blood pressure. Etihad et al. report that a 10 mmHg reduction in SBP reduces major cardiovascular events by approximately 20% [93, 94]. While no significant effects were observed on body weight, BMI, or body fat, the modest but significant reduction in WC underscores cinnamon’s possible role in targeting central adiposity, which is strongly linked to cardiometabolic risk. The modest decreases in WC, lipid profile, and glycemic indices associated with cinnamon supplementation indicate that it may serve as a supportive option for cardiovascular risk factor management [95, 96]. While modest, it may carry clinical implications depending on the population studied and the context of the intervention. Nonetheless, the clinical relevance of these findings should be viewed with caution. Although the reductions in outcomes are statistically significant, they may fall short of the minimal clinically important difference (MCID) for certain populations.
Clinicians should consider cinnamon supplementation, particularly in populations with impaired glucose metabolism and elevated blood pressure, as part of an integrative care approach. Although the supplementation appears generally safe and well-tolerated across diverse adult populations, practitioners should be mindful of the variability in individual responses, as well as the high heterogeneity in the included trials. Personalizing the dose and duration of cinnamon intake based on patient characteristics, therapeutic goals, and tolerability is advisable to maximize potential benefits.
Despite the observed clinical benefits, it is essential to acknowledge that data on the potential adverse effects and long-term safety of cinnamon supplementation remain limited. While most trials included in this meta-analysis reported no serious adverse events and described cinnamon as generally well-tolerated, systematic reporting of safety outcomes was inconsistent and often lacking in detail. Concerns about coumarin content—particularly in Cassia cinnamon—warrant consideration, as high doses over extended periods may pose risks of hepatotoxicity and other toxicological effects [97]. Moreover, potential interactions with antidiabetic and antihypertensive medications, as well as variability in cinnamon species, formulations, and purity, should be carefully evaluated in clinical practice. Future trials with rigorous safety assessments and longer intervention durations are needed to more definitively establish the long-term safety profile of cinnamon supplementation. Until such data are available, clinicians should exercise caution, prioritize standardized extracts with low coumarin content, and monitor patients regularly when recommending cinnamon as an adjunctive therapy.
Strengths and limitations
This meta-analysis represents a robust and comprehensive evaluation of cinnamon supplementation’s effects on cardiovascular risk factors, underpinned by a meticulous methodological framework. By adhering to the PRISMA guidelines and registering the protocol with PROSPERO, we ensured transparency, reproducibility, and alignment with the highest standards of systematic review conduct. The extensive literature search, spanning multiple databases (Cochrane, Web of Science, Medline, Scopus, Embase) and supplemented by manual reference screening, minimized the risk of overlooking relevant studies, capturing 49 RCTs with 3,038 participants. The application of the GRADE framework to assess evidence quality provided a rigorous evaluation of the certainty of findings, enhancing their reliability for clinical and research applications. Advanced statistical approaches, including meta-regression, non-linear dose–response analyses, and subgroup analyses, allowed us to elucidate dose-dependent effects and identify optimal dosages, offering nuanced insights into cinnamon’s therapeutic potential across diverse populations, health conditions, and cinnamon types (C. cassia, C. verum, C. zeylanicum). This comprehensive scope, coupled with the inclusion of a wide range of outcomes (anthropometric, glycemic, lipid, inflammatory, and oxidative stress markers), positions our study as a significant advancement over prior meta-analyses that focused on narrower endpoints, such as glycemic control or lipid profiles alone.
Our study offers distinct advantages over previous meta-analyses in this field. Unlike earlier reviews that often concentrated on specific outcomes, such as glycemic control in T2DM or lipid modulation, our analysis encompasses a broad spectrum of cardiovascular risk factors, including blood pressure, waist circumference, and inflammatory biomarkers like CRP. This holistic approach provides a more complete understanding of cinnamon’s multifaceted benefits, particularly for populations with T2DM, prediabetes, PCOS, and metabolic syndrome. The inclusion of 49 RCTs, compared to smaller datasets in prior studies, enhances the statistical power and generalizability of our findings. Additionally, our subgroup analyses based on age, baseline BMI, cinnamon type, and geographic region reveal critical patterns in treatment response, offering practical guidance for personalized interventions. The use of non-linear dose–response modeling further distinguishes our work by identifying optimal dosing strategies, a feature absent in many prior analyses. By addressing these gaps, our study contributes essential evidence to support the integration of cinnamon supplementation into evidence-based clinical practice and public health strategies.
Despite these strengths, several limitations must be acknowledged to contextualize our findings. The high heterogeneity observed across outcomes (I2 > 80% for most parameters) reflects variability in study designs, participant characteristics, cinnamon formulations, and intervention durations, which may influence the precision of our effect estimates. Although we employed a random-effects model and conducted subgroup and sensitivity analyses to mitigate this, residual confounding cannot be entirely ruled out. The exclusion of non-English studies introduces potential language bias, possibly limiting the applicability of our findings in diverse cultural and geographic contexts. Publication bias, detected for some outcomes, suggests that negative or null results may be underrepresented, potentially overestimating cinnamon’s effects. The reliance on SMDs rather than weighted mean differences (WMDs) for outcomes with heterogeneous units (e.g., insulin, inflammatory markers) was necessary but may reduce clinical interpretability. Furthermore, the lack of standardization in cinnamon preparations (e.g., differences in coumarin content between C. cassia and C. verum) and the absence of detailed reporting on cinnamon extraction methods or plant parts used in many studies introduce uncertainty into dose–response interpretations. The short duration of most included trials (4–24 weeks) limits insights into the long-term safety and efficacy of cinnamon supplementation, particularly at higher doses. Finally, the very low GRADE ratings for our outcomes, underscore the need for future high-quality, large-scale RCTs to confirm our findings and address these methodological shortcomings.
Future research directions
Future research should focus on addressing the identified gaps in the current evidence base for cinnamon supplementation to enhance its clinical applicability and safety profile. Given the significant heterogeneity observed across outcomes (I2 > 80% for most parameters), large-scale, high-quality RCTs with standardized cinnamon formulations and clearly defined coumarin content are essential to reduce variability and improve the precision of effect estimates. Long-term studies extending beyond 24 weeks are needed to evaluate the sustained efficacy and safety of cinnamon, particularly at higher doses (e.g., 6000 mg/day, optimal for lipid profiles), and to assess potential risks such as hepatotoxicity associated with coumarin in C. cassia. Investigating optimal dosing strategies tailored to specific populations and considering factors like age (≤ 50 vs. > 50 years), baseline BMI, and geographic region could refine therapeutic recommendations. Additionally, studies should explore potential drug interactions between cinnamon and common medications for diabetes, hypertension, and dyslipidemia to ensure safe integration into clinical practice.
The molecular mechanisms underlying cinnamon’s beneficial effects on glycemic control, lipid profiles, blood pressure, and inflammation warrant further exploration. Advanced omics approaches (e.g., transcriptomics, metabolomics) could elucidate the roles of bioactive compounds like cinnamaldehyde, cinnamic acid, and polyphenols in modulating insulin signaling, lipid metabolism, and inflammatory pathways, such as NF-κB. The selective reduction in CRP without affecting IL-6 suggests specific anti-inflammatory pathways that require further investigation to understand cinnamon’s targeted effects. Research into cinnamon’s impact on gut microbiota and its potential role in mediating metabolic benefits could provide novel insights into its mechanism of action, particularly for visceral fat reduction (e.g., waist circumference). Studies comparing different cinnamon forms (e.g., whole powder, extracts, or isolated compounds) and delivery methods (e.g., capsules, functional foods) could optimize bioavailability and therapeutic outcomes, addressing the current lack of standardization in intervention protocols.
Further investigation is needed to explore cinnamon’s effects in understudied subpopulations, such as individuals with gestational diabetes, rheumatoid arthritis, or NAFLD, where preliminary evidence suggests potential benefits. The observed regional differences (e.g., stronger effects on glycemic and lipid outcomes in Asian studies) highlight the need for global, multi-center trials to assess the influence of genetic polymorphisms, dietary patterns, and environmental factors on treatment response. Research into synergistic effects of cinnamon with other natural compounds (e.g., turmeric, ginger) or conventional therapies could enhance its cardiometabolic benefits, potentially leading to combination therapies. Additionally, studies examining cinnamon’s broader health impacts, such as its antioxidant properties (evidenced by reduced malondialdehyde) or potential neuroprotective and anticancer effects, could expand its therapeutic applications. Cost-effectiveness analyses comparing cinnamon supplementation with standard treatments would provide critical data for policymakers and healthcare providers, supporting its integration into public health strategies. Finally, developing cinnamon-based nutraceuticals or functional foods through interdisciplinary research could facilitate practical dietary interventions, ensuring accessibility and scalability for diverse populations.
Conclusions
This systematic review and meta-analysis provides robust evidence supporting the effectiveness of cinnamon supplementation in ameliorating various risk factors for cardiovascular diseases. The significant reductions observed in WC, blood pressure, fasting glucose, and lipid profiles, along with improvements in inflammatory markers and oxidative stress parameters, underscore cinnamon's potential as a complementary approach to conventional therapies. Given its favorable safety profile and accessibility, healthcare practitioners may consider incorporating cinnamon supplementation into dietary recommendations for individuals at risk of cardiovascular diseases. Future research should focus on elucidating the mechanisms underlying these effects and evaluating long-term outcomes to establish optimal dosages and formulations for clinical practice.
Supplementary Information
Acknowledgements
None.
Abbreviations
- ALP
Alkaline phosphatase
- ALT
Alanine aminotransferase
- AMP
Adenosine monophosphate
- AST
Aspartate aminotransferase
- BF
Body fat
- BMI
Body mass index
- BUN
Blood urea nitrogen
- CENTRAL
Central register of controlled trials
- CI
Confidence interval
- CIN
Cinnamaldehyde
- CRP
C-reactive protein
- CVD
Cardiovascular diseases
- CVH
Cardiovascular health
- DBP
Diastolic blood pressure
- eNOS
Endothelial NO synthase
- FBG
Fasting blood glucose
- FPG
Fasting plasma glucose
- GLUT-4
Glucose transporter-4
- HbA1c
Hemoglobin A1c
- HDL-C
High-density lipoprotein cholesterol
- HOMA-IR
Homeostatic model assessment of insulin resistance
- HTN
Hypertension
- IGF-1
Insulin-like growth factor-1
- IGT
Impaired glucose tolerance
- IL-6
Interleukin-6
- IR
Insulin resistance
- IRS-1
Insulin receptor substrate 1
- LDL-C
Low-density lipoprotein cholesterol
- LE8
Life’s essential 8
- MDA
Malondialdehyde
- MetS
Metabolic syndrome
- MS
Multiple sclerosis
- NAFLD
Non-alcoholic fatty liver disease
- NO
Nitric oxide
- PCOS
Polycystic ovary syndrome
- PI3-K
Phosphatidylinositol 3-kinase
- PRISMA
Preferred reporting items for systematic reviews and meta-analyses
- PROSPERO
Prospective register of systematic reviews
- QUICKI
Quantitative insulin sensitivity check index
- RA
Rheumatoid arthritis
- RCT
Randomized controlled trial
- SBP
Systolic blood pressure
- SMD
Standardized mean difference
- T2DM
Type 2 diabetes mellitus
- TAC
Total antioxidant capacity
- TC
Total cholesterol
- TG
Triglycerides
- WC
Waist circumference
- WHR
Waist-to-hip ratio
Author contributions
Designing this study: Ali Jafari. Performed this study: Ali Jafari, Helia Mardani, Amir Hossein Faghfouri Drafted the article: Alireza Alaghi, Vali Musazadeh, Minoo AhmadianMoghaddam Revised the article critically for important intellectual content: Vali Musazadeh, Ali Jafari Approved the version to be published: Ali Jafari, Helia Mardani, Amir Hossein Faghfouri, Minoo AhmadianMoghaddam, Alireza Alaghi, Vali Musazadeh.
Funding
None.
Availability of data and materials
The original data used during the current study can be obtained by contacting the corresponding author.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Vali Musazadeh, Email: Mosazadeh.vali05@gmail.com.
Alireza Alaghi, Email: alirezaalaghi@gmail.com.
References
- 1.Mensah GA, et al. Global burden of cardiovascular diseases and risks, 1990–2022. J Am Coll Cardiol. 2023;82(25):2350–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Di Cesare M, et al. World heart report 2023: Confronting the world’s number one killer. World Heart Federation: Geneva, Switzerland, 2023.
- 3.Jafari A, et al. Beneficial Effects of Okra (Abelmoschus esculentus L.) Consumption on Anthropometric Measures, Blood Pressure, Glycemic Control, Lipid Profile, and Liver Function Tests in Randomized Controlled Trials: A GRADE-Assessed Systematic Review and Dose-Response Meta-Analysis. Br J Nutr. 2025 1–87. [DOI] [PubMed]
- 4.Nikpayam O, et al. Effect of Menaquinone-7 (MK-7) Supplementation on Anthropometric Measurements, Glycemic Indices, and Lipid Profiles: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Prostaglandins & Other Lipid Mediators, 2025: p. 106970. [DOI] [PubMed]
- 5.Zhang K, et al. Effect of cinnamon supplementation on blood pressure, oxidative stress, and inflammatory biomarkers in adults: An umbrella review of the meta-analyses of randomized controlled trials. Nutrition, Metabolism and Cardiovascular Diseases, 2024. [DOI] [PubMed]
- 6.Sarmadi B, et al. The effect of cinnamon consumption on lipid profile, oxidative stress, and inflammation biomarkers in adults: An umbrella meta-analysis of randomized controlled trials. Nutr Metab Cardiovasc Dis. 2023;33(10):1821–35. [DOI] [PubMed] [Google Scholar]
- 7.Mousavi SM, et al. Anti-hypertensive effects of cinnamon supplementation in adults: A systematic review and dose-response Meta-analysis of randomized controlled trials. Crit Rev Food Sci Nutr. 2020;60(18):3144–54. [DOI] [PubMed] [Google Scholar]
- 8.De Silva ND, Wasana KGP, Attanayake AP. Cinnamon Bark (Cinnamomum Species). In: Medicinal Spice and Condiment Crops. CRC Press; 2024. p. 180–99. [Google Scholar]
- 9.Culas M, Popovich D, Rashidinejad A. Recent advances in encapsulation techniques for cinnamon bioactive compounds: A review on stability, effectiveness, and potential applications. Food Biosci. 2024;57: 103470. [Google Scholar]
- 10.Zhang R, et al. Cinnamon (Cinnamomum) as a Food-Medicine Homologue: A Review of Pharmacological Mechanisms and Potential Applications in Metabolic Diseases. Food Reviews International, 2025: p. 1–30.
- 11.Fateh HL, Amin SM. Effects of Cinnamon Supplementation on Lipid Profile: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Clin Nutr Res. 2024;13(1):74–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.de Moura SL, et al. Effects of cinnamon supplementation on metabolic biomarkers in individuals with type 2 diabetes: a systematic review and meta-analysis. Nutr Rev, 2024. [DOI] [PubMed]
- 13.Krittanawong C, et al. Association Between Cinnamon Consumption and Risk of Cardiovascular Health: A Systematic Review and Meta-Analysis. Am J Med. 2022;135(1):110–7. [DOI] [PubMed] [Google Scholar]
- 14.Keramati M, et al. Cinnamon, an effective anti-obesity agent: Evidence from an umbrella meta-analysis. J Food Biochem. 2022;46(8): e14166. [DOI] [PubMed] [Google Scholar]
- 15.Zarezadeh M, et al. The effect of cinnamon supplementation on glycemic control in patients with type 2 diabetes or with polycystic ovary syndrome: an umbrella meta-analysis on interventional meta-analyses. Diabetol Metab Syndr. 2023;15(1):127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moher D, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev. 2015;4:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Higgins JP, et al. Assessing risk of bias in a randomized trial. Cochrane handbook for systematic reviews of interventions, 2019: p. 205–228.
- 18.Group G. Grading quality of evidence and strength of recommendations. BMJ. 2004;328(7454):1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chandler J, et al. Cochrane handbook for systematic reviews of interventions. Hoboken: Wiley; 2019. [Google Scholar]
- 20.Follmann D, et al. Variance imputation for overviews of clinical trials with continuous response. J Clin Epidemiol. 1992;45(7):769–73. [DOI] [PubMed] [Google Scholar]
- 21.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21(11):1539–58. [DOI] [PubMed] [Google Scholar]
- 22.Orsini N, Bellocco R, Greenland S. Generalized least squares for trend estimation of summarized dose–response data. Stand Genomic Sci. 2006;6(1):40–57. [Google Scholar]
- 23.Xu C, Doi SA. The robust error meta-regression method for dose–response meta-analysis. JBI Evid Implement. 2018;16(3):138–44. [DOI] [PubMed] [Google Scholar]
- 24.Khan A, et al. Cinnamon improves glucose and lipids of people with type 2 diabetes. Diabetes Care. 2003;26(12):3215–8. [DOI] [PubMed] [Google Scholar]
- 25.Mang B, et al. Effects of a cinnamon extract on plasma glucose, HbA1c, and serum lipids in diabetes mellitus type 2. Eur J Clin Invest. 2006;36(5):340–4. [DOI] [PubMed] [Google Scholar]
- 26.Vanschoonbeek K, et al. Cinnamon supplementation does not improve glycemic control in postmenopausal type 2 diabetes patients. J Nutr. 2006;136(4):977–80. [DOI] [PubMed] [Google Scholar]
- 27.Ziegenfuss TN, et al. Effects of a water-soluble cinnamon extract on body composition and features of the metabolic syndrome in pre-diabetic men and women. J Int Soc Sports Nutr. 2006;3(2):45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Blevins SM, et al. Effect of cinnamon on glucose and lipid levels in non-insulin-dependent type 2 diabetes. Diabetes Care. 2007;30(9):2236–7. [DOI] [PubMed] [Google Scholar]
- 29.Crawford P. Effectiveness of cinnamon for lowering hemoglobin A1C in patients with type 2 diabetes: a randomized, controlled trial. The J Am Board Family Med. 2009;22(5):507–12. [DOI] [PubMed] [Google Scholar]
- 30.Roussel A-M, et al. Antioxidant effects of a cinnamon extract in people with impaired fasting glucose that are overweight or obese. J Am Coll Nutr. 2009;28(1):16–21. [DOI] [PubMed] [Google Scholar]
- 31.Soni R, Bhatnagar V. Effect of cinnamon (Cinnamomum cassia) intervention on blood glucose of middle aged adult male with non insulin dependent diabetes mellitus (NIDDM). Stud Ethno-Med. 2009;3(2):141–4. [Google Scholar]
- 32.Akilen R, et al. Glycated haemoglobin and blood pressure-lowering effect of cinnamon in multi-ethnic Type 2 diabetic patients in the UK: a randomized, placebo-controlled, double-blind clinical trial. Diabet Med. 2010;27(10):1159–67. [DOI] [PubMed] [Google Scholar]
- 33.Radhia Khan RK, Zakkia Khan ZK, Shah SH. Cinnamon may reduce glucose, lipid and cholesterol level in type 2 diabetic individuals. 2010 430-433.
- 34.KHADEM HH et al. Effect of cinnamon supplementation on blood glucose and lipid levels in type2 diabetic patients. 2011.
- 35.Wainstein J, et al. Dietary cinnamon supplementation and changes in systolic blood pressure in subjects with type 2 diabetes. J Med Food. 2011;14(12):1505–10. [DOI] [PubMed] [Google Scholar]
- 36.Khan R, et al. Cinnamon on the functions of liver and kidney in type 2 diabetic individuals. Ann Pak Inst Med Sci. 2012;8(2):145–9. [Google Scholar]
- 37.Lu T, et al. Cinnamon extract improves fasting blood glucose and glycosylated hemoglobin level in Chinese patients with type 2 diabetes. Nutr Res. 2012;32(6):408–12. [DOI] [PubMed] [Google Scholar]
- 38.Sharma P, et al. A randomised double blind placebo control trial of cinnamon supplementation on glycemic control and lipid profile in type 2 diabetes mellitus. Aust J Herb Med. 2012;24(1):4–9. [Google Scholar]
- 39.Vafa M, et al. Effects of cinnamon consumption on glycemic status, lipid profile and body composition in type 2 diabetic patients. Int J Prev Med. 2012;3(8):531. [PMC free article] [PubMed] [Google Scholar]
- 40.Abdil Razzaq Mohammed Noori A. A potential role of Cinnamon in improvement of glycemic control in untreated diabetic patients. Karbala J Pharm Sci. 2013;4(6):85–91. [Google Scholar]
- 41.Hasanzade F, et al. The effect of cinnamon on glucose of type II diabetes patients. J Tradit Complement Med. 2013;3(3):171–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zahmatkesh M, Khodashenas-Roudsari M. Comparing the therapeutic effects of three herbal medicine (cinnamon, fenugreek, and coriander) on hemoglobin A1C and blood lipids in type II diabetic patients. Chronic Dis J. 2013;1(2):74–82. [Google Scholar]
- 43.Askari F, Rashidkhani B, Hekmatdoost A. Cinnamon may have therapeutic benefits on lipid profile, liver enzymes, insulin resistance, and high-sensitivity C-reactive protein in nonalcoholic fatty liver disease patients. Nutr Res. 2014;34(2):143–8. [DOI] [PubMed] [Google Scholar]
- 44.Azimi P, et al. Effects of cinnamon, cardamom, saffron, and ginger consumption on markers of glycemic control, lipid profile, oxidative stress, and inflammation in type 2 diabetes patients. Rev Diabet Stud RDS. 2014;11(3):258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hosseini SA, et al. Impact of short–term intake of Cinnamon on serum glucose and lipid profile in patients with type 2 diabetes mellitus. J Appl Environ Biol Sci. 2014;4(2):295–8. [Google Scholar]
- 46.Kort DH, Lobo RA. Preliminary evidence that cinnamon improves menstrual cyclicity in women with polycystic ovary syndrome: a randomized controlled trial. Am J Obstet Gynecol. 2014;211(5):487. [DOI] [PubMed] [Google Scholar]
- 47.Wickenberg J, et al. Cassia cinnamon does not change the insulin sensitivity or the liver enzymes in subjects with impaired glucose tolerance. Nutr J. 2014;13:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tangvarasittichai S, et al. Effect of cinnamon supplementation on oxidative stress, inflammation and insulin resistance in patients with type 2 diabetes mellitus. Int J Toxicol Pharm Res. 2015;7(4):56–9. [Google Scholar]
- 49.Anderson RA, et al. Cinnamon extract lowers glucose, insulin and cholesterol in people with elevated serum glucose. J Tradit Complement Med. 2016;6(4):332–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Azimi P, et al. Effect of cinnamon, cardamom, saffron and ginger consumption on blood pressure and a marker of endothelial function in patients with type 2 diabetes mellitus: A randomized controlled clinical trial. Blood Press. 2016;25(3):133–40. [DOI] [PubMed] [Google Scholar]
- 51.Mirfeizi M, et al. Controlling type 2 diabetes mellitus with herbal medicines: A triple-blind randomized clinical trial of efficacy and safety: 使用草药来控制 2 型糖尿病: 一项验证有效性与安全性的三盲随机临床试验. J Diabetes. 2016;8(5):647–56. [DOI] [PubMed] [Google Scholar]
- 52.Sengsuk C, et al. Effect of cinnamon supplementation on glucose, lipids levels, glomerular filtration rate, and blood pressure of subjects with type 2 diabetes mellitus. Diabetol Int. 2016;7:124–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gupta Jain S, et al. Effect of oral cinnamon intervention on metabolic profile and body composition of Asian Indians with metabolic syndrome: a randomized double-blind control trial. Lipids Health Dis. 2017;16:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Talaei B, et al. Effects of cinnamon consumption on glycemic indicators, advanced glycation end products, and antioxidant status in type 2 diabetic patients. Nutrients. 2017;9(9):991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Borzoei A, Rafraf M, Asghari-Jafarabadi M. Cinnamon improves metabolic factors without detectable effects on adiponectin in women with polycystic ovary syndrome. Asia Pac J Clin Nutr. 2018;27(3):556–63. [DOI] [PubMed] [Google Scholar]
- 56.Borzoei A, et al. Effects of cinnamon supplementation on antioxidant status and serum lipids in women with polycystic ovary syndrome. J Tradit Complement Med. 2018;8(1):128–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hajimonfarednejad M, et al. Insulin resistance improvement by cinnamon powder in polycystic ovary syndrome: A randomized double-blind placebo controlled clinical trial. Phytother Res. 2018;32(2):276–83. [DOI] [PubMed] [Google Scholar]
- 58.Pishdad S, et al. Effect of cumin and cinnamon on lipid profile in middle-aged women with dyslipidemia: A double blind, randomized controlled clinical trial. Prog Nutr. 2018;20:232–7. [Google Scholar]
- 59.Shishehbor F, et al. Cinnamon consumption improves clinical symptoms and inflammatory markers in women with rheumatoid arthritis. J Am Coll Nutr. 2018;37(8):685–90. [DOI] [PubMed] [Google Scholar]
- 60.Mirmiran P, et al. A randomized controlled trial to determining the effect of cinnamon on the plasma levels of soluble forms of vascular adhesion molecules in type 2 diabetes mellitus. Eur J Clin Nutr. 2019;73(12):1605–12. [DOI] [PubMed] [Google Scholar]
- 61.Zare R, et al. Efficacy of cinnamon in patients with type II diabetes mellitus: A randomized controlled clinical trial. Clin Nutr. 2019;38(2):549–56. [DOI] [PubMed] [Google Scholar]
- 62.Davari M, et al. Effects of cinnamon supplementation on expression of systemic inflammation factors, NF-kB and Sirtuin-1 (SIRT1) in type 2 diabetes: a randomized, double blind, and controlled clinical trial. Nutr J. 2020;19:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Romeo GR, et al. Influence of cinnamon on glycemic control in individuals with prediabetes: A randomized controlled trial. J Endocr Soc. 2020;4(11):bvaa094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zare R, et al. Analysis of the efficacy of cinnamon for patients with diabetes mellitus type II based on traditional Persian medicine syndrome differentiation: a randomized controlled trial. Shiraz E-Med J. 2020;21(7):e95609. [Google Scholar]
- 65.Zareie A, et al. Effect of cinnamon on migraine attacks and inflammatory markers: A randomized double-blind placebo-controlled trial. Phytother Res. 2020;34(11):2945–52. [DOI] [PubMed] [Google Scholar]
- 66.Delaviz E, et al. Effect of cinnamon on inflammatory factors, pain and anthropometric indices in progressive-relapsing multiple sclerosis patients: a randomized controlled trial. Jundishapur J Nat Pharm Prod. 2021;16(1):e14505. [Google Scholar]
- 67.Lira Neto JCG, et al. Efficacy of cinnamon as an adjuvant in reducing the glycemic biomarkers of type 2 diabetes mellitus: a three-month, randomized, triple-blind, placebo-controlled clinical trial. J Am Nutr Assoc. 2022;41(3):266–74. [DOI] [PubMed] [Google Scholar]
- 68.Shirzad F, et al. Cinnamon effects on blood pressure and metabolic profile: A double-blind, randomized, placebo-controlled trial in patients with stage 1 hypertension. Avic J Phytomed. 2021;11(1):91. [PMC free article] [PubMed] [Google Scholar]
- 69.Dastgheib M, et al. A comparison of the effects of cinnamon, ginger, and metformin consumption on metabolic health, anthropometric indices, and sexual hormone levels in women with poly cystic ovary syndrome: A randomized double-blinded placebo-controlled clinical trial. Front Nutr. 2022;9:1071515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Al Dhaheri AS, et al. The Effect of Therapeutic Doses of Culinary Spices in Metabolic Syndrome: A Randomized Controlled Trial. Nutrients. 2024;16(11):1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Peivandi S, et al. Metabolic and endocrine changes induced by cinnamon in women with polycystic ovarian syndrome: A pilot study. Avic J Phytomed. 2024;14(2):242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zareie A, et al. Effects of cinnamon on anthropometric indices and headache-related disability of patients with migraine: A randomized double-blind placebo-controlled trial. Avic J Phytomed. 2024;14(1):1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Qin B, Panickar KS, Anderson RA. Cinnamon: potential role in the prevention of insulin resistance, metabolic syndrome, and type 2 diabetes. J Diabet Sci Technol. 2010;4(3):685–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Abraham K, et al. Toxicology and risk assessment of coumarin: focus on human data. Mol Nutr Food Res. 2010;54(2):228–39. [DOI] [PubMed] [Google Scholar]
- 75.De Silva DAM, et al. Clean vs dirty labels: Transparency and authenticity of the labels of Ceylon cinnamon. PLoS ONE. 2021;16(11): e0260474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Daemi A, et al. Topical application of Cinnamomum hydroethanolic extract improves wound healing by enhancing re-epithelialization and keratin biosynthesis in streptozotocin-induced diabetic mice. Pharm Biol. 2019;57(1):799–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Yang CH, Li RX, Chuang LY. Antioxidant activity of various parts of Cinnamomum cassia extracted with different extraction methods. Molecules. 2012;17(6):7294–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zhu R, et al. Age- and sex-specific effects of a long-term lifestyle intervention on body weight and cardiometabolic health markers in adults with prediabetes: results from the diabetes prevention study PREVIEW. Diabetologia. 2022;65(8):1262–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Medagama AB. The glycaemic outcomes of Cinnamon, a review of the experimental evidence and clinical trials. Nutr J. 2015;14:108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Silva ML, et al. Cinnamon as a Complementary Therapeutic Approach for Dysglycemia and Dyslipidemia Control in Type 2 Diabetes Mellitus and Its Molecular Mechanism of Action: A Review. Nutrients. 2022;14(13):2773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Adisakwattana S. Cinnamic Acid and Its Derivatives: Mechanisms for Prevention and Management of Diabetes and Its Complications. Nutrients. 2017;9(2):163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lee JS, et al. Cinnamate supplementation enhances hepatic lipid metabolism and antioxidant defense systems in high cholesterol-fed rats. J Med Food. 2003;6(3):183–91. [DOI] [PubMed] [Google Scholar]
- 83.Ismail BS, et al. Cinnamaldehyde Mitigates Atherosclerosis Induced by High-Fat Diet via Modulation of Hyperlipidemia, Oxidative Stress, and Inflammation. Oxid Med Cell Longev. 2022;2022(1):4464180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Mandal A, et al. Impact of cassia bark consumption on glucose and lipid control in Type 2 diabetes: An updated systematic review and meta-analysis. Cureus. 2021;13(7):e16376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Chen P, et al. Cinnamic Aldehyde, the main monomer component of Cinnamon, exhibits anti-inflammatory property in OA synovial fibroblasts via TLR4/MyD88 pathway. J Cell Mol Med. 2022;26(3):913–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Chen P, et al. Cinnamic aldehyde inhibits lipopolysaccharide-induced chondrocyte inflammation and reduces cartilage degeneration by blocking the nuclear factor-kappa B signaling pathway. Front Pharmacol. 2020;11:949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ashfaq MH, Siddique A, Shahid S. Antioxidant activity of Cinnamon zeylanicum:(A review). Asian J Pharm Res. 2021;11(2):106–16. [Google Scholar]
- 88.Yang L, et al. Proanthocyanidins against Oxidative Stress: From Molecular Mechanisms to Clinical Applications. Biomed Res Int. 2018;2018:8584136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Baradaran A, Nasri H, Rafieian-Kopaei M. Oxidative stress and hypertension: Possibility of hypertension therapy with antioxidants. J Res Med Sci. 2014;19(4):358–67. [PMC free article] [PubMed] [Google Scholar]
- 90.Rao PV, Gan SH. Cinnamon: a multifaceted medicinal plant. Evid Based Complement Alternat Med. 2014;2014: 642942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Earley S. TRPA1 channels in the vasculature. Br J Pharmacol. 2012;167(1):13–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Manneck D, et al. The TRPA1 Agonist Cinnamaldehyde Induces the Secretion of HCO(3)(-) by the Porcine Colon. Int J Mol Sci. 2021;22(10):5198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ettehad D, et al. Blood pressure lowering for prevention of cardiovascular disease and death: a systematic review and meta-analysis. Lancet. 2016;387(10022):957–67. [DOI] [PubMed] [Google Scholar]
- 94.Hardy ST, et al. Reducing the blood pressure–related burden of cardiovascular disease: impact of achievable improvements in blood pressure prevention and control. J Am Heart Assoc. 2015;4(10): e002276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Schaap LA, et al. Changes in body mass index and mid-upper arm circumference in relation to all-cause mortality in older adults. Clin Nutr. 2018;37(6):2252–9. [DOI] [PubMed] [Google Scholar]
- 96.DPPR Group. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. New England J Med. 2002;346(6):393–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Lee E-J, et al. Toxicity of cassia and cinnamon oil compounds and cinnamaldehyde-related compounds to Sitophilus oryzae (Coleoptera: Curculionidae). J Econ Entomol. 2008;101(6):1960–6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The original data used during the current study can be obtained by contacting the corresponding author.