Abstract
Introduction
The IHOPE study evaluated the efficacy and safety of oral iron polysaccharide complex versus i.v. iron in Chinese patients on hemodialysis (HD).
Methods
This randomized, open-label, noninferiority trial enrolled adults (aged 18–75 years) on maintenance HD (MHD) with hemoglobin of 100 to 130 g/l, transferrin saturation (TSAT) of 20% to 50%, or serum ferritin of 100 to 500 μg/l. Patients were randomized 1:1 to receive oral iron polysaccharide complex (150 mg twice daily) or i.v. iron sucrose (100 mg biweekly) for 24 weeks. The primary outcome was TSAT at 12 weeks. Noninferiority margin was set at 7% with significance at 0.05.
Results
Among 193 participants (mean age: 55.3 years, 62.2% male), 12-week TSAT was 32.3% (95% CI: 29.39%–35.19%) in the oral group versus 33.4% (95% CI: 30.95%–35.77%) in the i.v. group, demonstrating noninferiority. At 24 weeks, TSAT remained noninferior (29.4% vs. 32.6%), and hemoglobin levels were comparable (114.6 vs. 117.4 g/l; P = 0.166). Adverse events occurred in 51.0% of oral and 47.4% of i.v. group patients, with serious adverse events in 14.6% and 13.4%, respectively.
Conclusion
Oral iron polysaccharide complex demonstrated noninferiority to i.v. iron sucrose for maintaining iron status in patients on HD, with comparable safety profiles.
Keywords: hemodialysis, oral iron supplements, renal anemia
Graphical abstract
Chronic kidney disease is a significant global public health issue that affects the quality of life of millions of people. Among the numerous complications associated with chronic kidney disease, anemia is one of the most prevalent and severe and is especially common in patients on MHD. Reports indicate that the prevalence of anemia among patients on dialysis in China is as high as 91.6% to 98.2%.1,2 Anemia not only significantly reduces quality of life but is also closely associated with an increased risk of cardiovascular disease and increased mortality rates.3
The primary causes of chronic kidney disease–related anemia include insufficient secretion of erythropoietin and iron metabolism disorders. Iron deficiency is particularly common among patients on MHD because of factors such as inadequate dietary intake, gastrointestinal absorption issues, iron loss during dialysis, and chronic inflammation. Therefore, in addition to the use of erythropoiesis-stimulating agents (ESAs), iron supplementation plays a crucial role in managing anemia in patients on MHD.4
Currently, i.v. iron supplements (e.g., iron sucrose) are the standard method for iron supplementation in patients on MHD, offering the advantages of rapid absorption and high bioavailability. However, i.v. administration has limitations, including the need for medical facility operations, potential allergic reactions, and the risk of iron overload and increased oxidative stress with long-term use.5 In contrast, oral iron supplements are convenient and have fewer adverse effects, although patient compliance is often affected by gastrointestinal irritation because of traditional oral iron supplements (e.g., ferrous sulfate).6
Polysaccharide-iron complex capsules are a new type of oral trivalent iron supplement characterized by high iron content and minimal gastrointestinal irritation. However, high-quality clinical evidence supporting its use in patients on MHD is lacking. Therefore, the aim of this study was to evaluate whether oral polysaccharide-iron complex capsules are noninferior to i.v. iron sucrose in iron supplementation for patients on MHD and to observe their safety and economic viability. These findings provide new treatment options for anemia management in patients on MHD.7
In this study, a randomized, open-label, positive-controlled, multicenter design was adopted with the aim of recruiting 186 participants from 12 HD centers across mainland China. The participants were randomly assigned to receive either polysaccharide-iron complex capsules or iron sucrose alongside standard ESA therapy. The primary end point was TSAT at 12 weeks; and the secondary end points were TSAT at 24 weeks, hemoglobin levels, serum ferritin, high-sensitivity C-reactive protein, pharmacoeconomic evaluation, quality of life, and oxidative stress markers. The study was designed to last 24 weeks.
The aim of this study was to provide high-quality evidence-based medicine for clinical practice, optimizing anemia management strategies for patients on MHD, by comparing the efficacy and safety of oral polysaccharide-iron complex capsules with those of i.v. iron sucrose in patients on MHD.
Methods
This study is a randomized, open-label, positive-controlled, multicenter study designed to evaluate whether oral polysaccharide-iron complex capsules are noninferior to i.v. iron sucrose in iron supplementation for patients on MHD and to observe their safety and economic viability. The study was conducted at 12 HD centers across mainland China, with a planned enrollment of 186 participants. The study protocol was approved by the ethics committees of all participating centers and strictly adhered to the principles of good clinical practice.
Ethics approval and consent to participate in this study protocol was approved by the Ethics Committee of Ren ji Hospital, School of Medicine, and Shanghai Jiao Tong University School of Medicine (approval number: 2019-048). Informed consent to participate will be obtained from all participants. This study was prospectively registered with the Chinese Clinical Trial Registry (ChiCTR) under registration number ChiCTR2000031166 on March 23, 2020.
Participants
Sample Size Calculation and Statistical Analysis
The study used a noninferiority trial design, with a noninferiority margin of 7% for the primary end point (TSAT at 12 weeks). Using a 1-sided alpha of 0.025 and 80% power, the required sample size was 154 participants. Accounting for a 20% dropout rate, the final planned enrollment was 186 participants, with an actual enrollment of 193 participants. Statistical analyses were performed using SAS 9.4 (SAS 9.4 Institute Inc., Cary, NC), and the primary efficacy analysis was based on full analysis set, per-protocol set, and safety set (Supplementary Material S1).
The study included patients on MHD aged 18 to 75 years. The key inclusion criteria were hemoglobin concentration ≥ 100 g/l and < 130 g/l, TSAT ≥ 20% and ≤ 50%, or serum ferritin of 100 to 500 μg/l, and treatment with ESAs or iron supplements within 12 weeks before enrollment. The key exclusion criteria were allergy or intolerance to the study drugs, acute or chronic bleeding, severe secondary hyperparathyroidism, or severe heart or liver dysfunction4 (Supplementary Material S2).
Randomization and Treatment
Eligible participants were randomly assigned at a 1:1 ratio to either the treatment group or the control group via a central randomization system. The treatment group received oral polysaccharide-iron complex capsules (150 mg, 1 capsule per dose, twice daily), whereas the control group received i.v. iron sucrose (100 mg every 2 weeks). Patients were advised to avoid concurrent administration with antacids and tetracyclines. Both groups continued with standard ESAs therapy for 24 weeks.
Outcome Measures
The primary efficacy end point was TSAT at 12 weeks of treatment. Secondary efficacy end points included TSAT at 24 weeks; hemoglobin levels, serum ferritin, and high-sensitivity C-reactive protein at baseline, 12 weeks, and 24 weeks; iron supplementation costs within 24 weeks; quality-of-life score (EQ5D-5L); and oxidative stress markers, including malondialdehyde, superoxide dismutase, and glutathione peroxidase. Safety end points included the incidence of adverse events, laboratory tests, vital signs, physical examinations, and electrocardiograms.
Follow-up and Data Collection
Follow-ups were conducted at baseline and at weeks 4, 8, 12, 16, 20, and 24 to assess efficacy and safety end points and record adverse events. Statistical analysis was performed via SAS 9.4 software. The primary efficacy end point was analyzed via a noninferiority test with a 1-sided significance level of 0.025 and a noninferiority margin of 7%. The other tests used a 2-sided significance level of 0.05. Analyses were conducted on full analysis set , per-protocol set, and safety set.
Results
In total, 262 patients were screened for this study, with 193 meeting the inclusion criteria and completing randomization. The participants were assigned to either the treatment group (n = 96) or the control group (n = 97) (Figure 1). The baseline demographic and clinical characteristics were not significantly different between the 2 groups. The mean age was 55.7 ± 10.94 years in the treatment group and 54.9 ± 12.37 years in the control group. The proportion of males was 63.5% in the treatment group and 60.8% in the control group. The median weekly ESA doses were 7500.0 IU and 8566.7 IU in the treatment and control groups, respectively, among patients receiving ESA therapy (Table 1).
Figure 1.
Patient flow chart. D, day; V, visit.
Table 1.
Baseline patient characteristics (full analysis set)
| Characteristics | Treatment group (n = 96) | Control group (n = 97) | Total (N = 193) |
|---|---|---|---|
| Demographic characteristics | |||
| Age, mean (SD), y | 55.7 (10.94) | 54.9 (12.37) | 55.3 (11.66) |
| Sex, n (%) | |||
| Male | 61 (63.5%) | 59 (60.8%) | 120 (62.2%) |
| Female | 35 (36.5%) | 38 (39.2%) | 73 (37.8%) |
| Clinical characteristics, n (%) | |||
| have been treated with iron supplements within 12 weeks before enrollment | 75 (78.1%) | 73 (75.3%) | 148 (76.7%) |
| have been treated with erythropoiesis-stimulating agents within 12 weeks before enrollment | 94 (97.9%) | 94 (96.9%) | 188 (97.4%) |
| Duration of hemodialysis, mean (SD), moa | 48.1 (38.09) | 55.4 (42.45) | 51.8 (40.40) |
| Temperature, mean (SD), °C | 36.5 (0.23) | 36.4 (0.25) | - |
| Pulse, mean (SD), beats/min | 76.2 (9.87) | 76.2 (9.26) | - |
| Systolic blood pressure, mean (SD), mm Hg | 142.6 (16.91) | 142.5 (14.15) | - |
| Diastolic blood pressure, mean (SD), mm Hg | 79.0 (10.09) | 80.2 (12.13) | - |
| Transferrin saturation, TAST, mean (SD), % | 30.8 (12.40) | 31.9 (11.49) | 31.4 (11.93) |
| high-sensitivity C-reactive protein, HsCRP, mean (SD), mg/l | 2.870 (3.6584) | 2.848 (4.0505) | - |
| Hemoglobin, Hb, mean (SD), g/l | 114.510 (7.5352) | 117.515 (23.9253) | - |
| Serum ferritin, SF, mean (SD), μg/l | 261.218 (139.2183) | 261.995 (175.3712) | - |
| Quality-of-life score, EQSD-5L, mean (SD) | 87.4 (9.50) | 87.9 (10.78) | 87.7 (10.14) |
| Glutathione peroxidase, GSH-Px, mean (SD), ng/ml | 41.904 (51.0296) | 48.523 (56.3868) | - |
| malondialdehyde, MDA, mean (SD), nmol/ml | 1.966 (2.5112) | 1.982 (1.8214) | - |
| Superoxide dismutase, SOD, mean (SD), ng/ml | 35.006 (43.2623) | 43.636 (50.5419) | - |
HsCRP, high-sensitivity C-reactive protein.
Experimental group (n = 95), control group (n = 96), Total (N = 191).
Primary Efficacy End Point
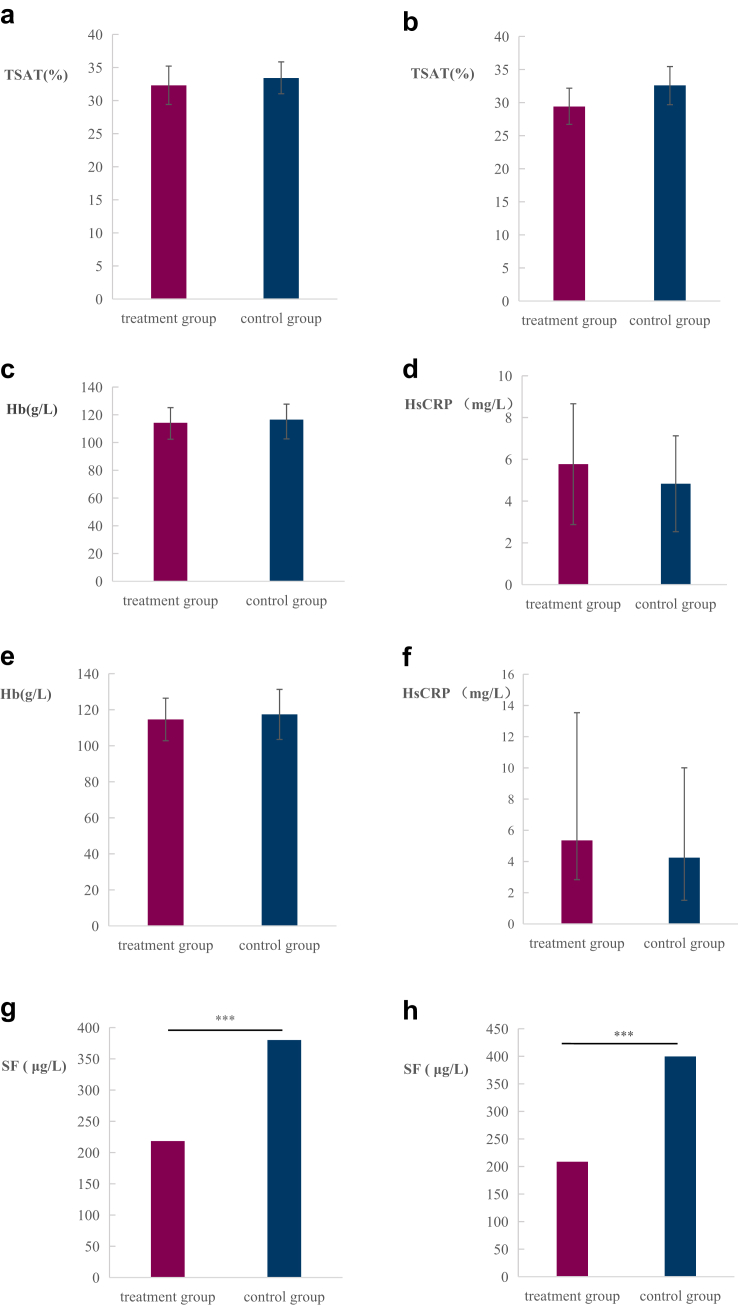
After 12 weeks of treatment, the mean TSAT in the treatment group was 32.3% (± 13.02%) [95% CI: 29.39%–35.19%], whereas it was 33.4% (± 11.44%) [95% CI: 30.95%–35.77%] in the control group. The lower limit of the 95% confidence interval for TSAT in the treatment group was 29.39%, which was greater than the mean of the control group minus the noninferiority margin (33.4%–7% = 26.4%), confirming that the treatment group was noninferior to the control group (Figure 2a).
Figure 2.
Primary and secondary efficacy end points in the treatment (n = 96) and control (n = 97) groups. (a) Mean transferrin saturation (TSAT) at week 12 (95% CI). (b) TSAT levels at week 24 (95% CI). (c) Hemoglobin levels at week 12 (P = 0.18). (d) High-sensitivity C-reactive protein (HsCRP) levels at week 12 (95% CI, P = 0.628). (e) Hemoglobin levels at week 24 (P = 0.166). (f) HsCRP levels at week 24 (95% CI, P = 0.495). (g) Serum ferritin levels at week 12 (∗∗∗P < 0.001). (h) Serum ferritin levels at week 24 (∗∗∗P < 0.001). CI, confidence interval.
Secondary Efficacy End Points
At 24 weeks, the TSAT was 29.4 ± 11.88% in the treatment group and 32.6 ± 12.97% in the control group, with no significant difference between the groups (P = 0.121) (Figure 2b). There were no significant differences in hemoglobin or high-sensitivity C-reactive protein levels at 12 weeks (Figure 2c and d) or 24 weeks (Figure 2e and f). However, the serum ferritin level was significantly lower in the treatment group than in the control group at both 12 weeks (218.4 vs. 380.0 μg/l, P < 0.0001) and 24 weeks (208.7 vs. 399.6 μg/l, P < 0.0001) (Figure 2g and h). The results of the pharmacoeconomic evaluation revealed that the mean total iron supplementation costs over 24 weeks were 2742.3 CNY for the treatment group and 2495.9 CNY for the control group (P = 0.4758). The quality-of-life score (EQ5D-5L) improved in both groups at 24 weeks, with no significant difference between the groups. Changes in the levels of oxidative stress markers (malondialdehyde, superoxide dismutase, and glutathione peroxidase) at 12 and 24 weeks were not significantly different between the groups (Supplementary Table S1).
Safety Evaluation
The incidence of adverse events was 51.0% (49/96) in the treatment group and 47.4% (46/97) in the control group. The incidence of adverse events related to the study medications was 12.5% (12/96) in the treatment group and 9.3% (9/97) in the control group. Serious adverse events occurred in 14.6% (14/96) of the treatment group and 13.4% (13/97) of the control group, with no significant difference between the groups. Most adverse events were mild to moderate, primarily involving infections, abnormalities in various tests, and gastrointestinal symptoms. No hypophosphatemia-related adverse events were reported in either group. There were no unexpected adverse reactions or serious adverse events directly related to the study medications in either group (Table 2).
Table 2.
Summary of adverse events (safety analysis set)
| Category | Treatment group SS (n = 96) |
Control group SS (n = 97) |
||||
|---|---|---|---|---|---|---|
| No. | Patients | Incidence, % | No. | Patients | Incidence, % | |
| Any AE | 96 | 49 | 51% | 106 | 46 | 47.4% |
| Any Adverse reactions | 14 | 12 | 12.5% | 11 | 9 | 9.3% |
| Skin and subcutaneous tissue diseases | 0 | 0 | 0.0% | 1 | 1 | 1.0% |
| Infectious and Infective Diseases | 0 | 0 | 0.0% | 3 | 3 | 3.1% |
| Gastrointestinal system diseases | 12 | 10 | 10.5% | 1 | 1 | 1.0% |
| Various musculoskeletal and connective tissue diseases | 0 | 0 | 0.0% | 1 | 1 | 1.0% |
| Various inspections | 1 | 1 | 1.0% | 2 | 2 | 2.1% |
| Respiratory, thoracic, and mediastinal diseases | 0 | 0 | 0.0% | 1 | 1 | 1.0% |
| Various neurological disorders | 1 | 1 | 1.0% | 2 | 1 | 1.0% |
| Serious AE | 17 | 14 | 14.6% | 18 | 13 | 13.4% |
| Diseases of the liver and gallbladder system | 2 | 1 | 1.0% | 0 | 0 | 0.0% |
| Infectious and Infective Diseases | 3 | 3 | 3.1% | 4 | 4 | 4.1% |
| Various inspections | 1 | 1 | 1.0% | 0 | 0 | 0.0% |
| Various neurological disorders | 1 | 1 | 1.0% | 1 | 1 | 1.0% |
| Various injuries, poisoning, and operational complications | 5 | 4 | 4.2% | 12 | 8 | 8.2% |
| Various surgeries and medical procedures | 1 | 1 | 1.0% | 0 | 0 | 0.0% |
| Systemic diseases and various reactions at the site of administration | 1 | 1 | 1.0% | 1 | 1 | 1.0% |
| Heart organ diseases | 2 | 2 | 2.1% | 0 | 0 | 0.0% |
| Vascular and lymphatic diseases | 1 | 1 | 1.0% | 0 | 0 | 0.0% |
| Serious Adverse reactions | 0 | 0 | - | 0 | 0 | - |
| Important AE | 68 | 36 | 37.5% | 78 | 39 | 40.2% |
| AE leading to withdrawal from the trial | 8 | 7 | 7.3% | 2 | 2 | 2.1% |
| Adverse reactions leading to withdrawal from the trial | 5 | 4 | 4.2% | 0 | 0 | - |
| Death | 2 | 2 | 2.1% | 2 | 2 | 2.1% |
| Various injuries, poisoning, and operational complications | 1 | 1 | 1.0% | 0 | 0 | 0.0% |
| Systemic diseases and various reactions at the site of administration | 1 | 1 | 1.0% | 0 | 0 | 0.0% |
| Infectious and Infective Diseases | 0 | 0 | 0.0% | 1 | 1 | 1.0% |
| Various neurological disorders | 0 | 0 | 0.0% | 1 | 1 | 1.0% |
AE, adverse event; SS, safety set.
Including cases where the investigational drug has been used at least once and has undergone safety evaluation.
Discussion
This study is the first large-scale randomized controlled trial to evaluate the efficacy of oral polysaccharide-iron complex capsules for iron supplementation in patients on MHD. These results indicate that oral polysaccharide-iron complex capsules are noninferior to i.v. iron sucrose in improving TSAT and demonstrate good safety and acceptability. This finding has significant clinical implications and application value.
Iron deficiency and metabolic disorders are key factors contributing to anemia in patients on MHD. Absolute iron deficiency manifests as decreased serum ferritin and TSAT. If serum ferritin is normal and TSAT is low, it suggests sufficient iron reserves but impaired iron utilization, known as relative iron deficiency.8
Iron supplements are categorized into oral and i.v. iron preparations. Oral iron supplements affect the body's iron metabolism more physiologically, offering safe and convenient treatment with a low risk of allergic reactions and infection. They also eliminate the need for frequent hospital visits. However, oral iron supplements are relatively slow in correcting anemia and may have gastrointestinal adverse effects. Through numerous evidence-based studies, i.v. iron supplements have been shown to be effective and safe in correcting renal anemia.9,10 Meta-analyses have demonstrated that i.v. iron can efficiently increase hemoglobin and maintain target levels while reducing the need for ESA doses and blood transfusions.11, 12, 13, 14
Despite the proven safety and effectiveness of i.v. iron supplements in clinical practice, they can cause allergic reactions, including severe, life-threatening anaphylaxis.15, 16, 17 Long-term high-dose i.v. iron treatment increases the risk of cardiovascular events and infections. Improper use can lead to iron overload, damaging vital organs such as the liver and heart.18 Any i.v. iron preparation can potentially cause life-threatening hypersensitivity reactions. During the first 60 minutes of initial i.v. iron administration, vital signs should be monitored, and emergency medications should be available. Active systemic infections are a contraindication for i.v. iron, thereby limiting its use.
In terms of efficacy, the TSAT after 12 weeks of treatment with oral polysaccharide-iron complex capsules (32.3 ± 13.02%) was comparable with that of i.v. iron sucrose (33.4 ± 11.44%), thereby confirming noninferiority. These findings suggest that oral iron supplements can serve as an effective alternative to i.v. iron, potentially improving treatment adherence because of their oral administration. The secondary efficacy end points, such as hemoglobin levels, were similar between the 2 groups, further supporting the effectiveness of the oral preparation. However, serum ferritin levels at both 12 and 24 weeks were significantly lower in the oral iron group than in the i.v. iron group, indicating potentially weaker iron reserve improvement in the oral iron group, which may be related to the inflammatory state in the i.v. iron group.
In terms of safety, the incidence of adverse events in the oral iron group was comparable with that in the control group (51.0% vs. 47.4%), with most being mild. The incidence of drug-related adverse events was also low (12.5% vs. 9.3%). Notably, there were no serious drug-related adverse events, hypophosphatemia-related events, or unexpected adverse reactions in either group, indicating good safety for oral polysaccharide-iron complex capsules.
The quality-of-life assessments revealed improvements in both groups after 24 weeks, with no significant difference between the groups, suggesting that the convenience of oral iron supplementation may enhance the quality of life of patients.
In this study, the impact of oral iron supplementation on oxidative stress was also evaluated for the first time. The results revealed no significant differences in the levels of oxidative stress markers (malondialdehyde, superoxide dismutase, and glutathione peroxidase) between the groups.
Unlike previous randomized controlled trials that primarily evaluated traditional oral iron supplements (e.g., ferrous sulfate, ferrous fumarate) in Western populations,19 our study represents several innovations. First, it is the first randomized controlled trial comparing oral polysaccharide-iron complex and i.v. iron sucrose in Chinese patients on MHD, assessing polysaccharide-iron complex, a newer-generation oral iron with higher bioavailability and fewer gastrointestinal side effects. Second, we conducted a comprehensive safety assessment, including pharmacoeconomics, oxidative stress markers, and quality-of-life (EQ5D-5L), offering a more holistic clinical evaluation. These findings provide region-specific evidence to optimize anemia management in Chinese dialysis centers; supporting oral polysaccharide-iron complex as a viable, safe, and convenient alternative to i.v. iron therapy.
However, there are several limitations to this study. First, the open-label design may introduce bias in patient selection and physician assessment. Second, the 24-week follow-up period is insufficient for assessing long-term efficacy and safety. Third, we did not measure hepcidin and inflammatory cytokine levels, which could have provided additional insights into iron metabolism and patient responsiveness to different iron formulations. Further research is needed to determine whether these results can be generalized to other racial populations.
Future research directions may include longer follow-up periods, evaluations of cardiovascular outcomes, applications in patients on MHD, and assessment of inflammatory markers including hepcidin to better identify patients who might benefit most from oral iron therapy. Considering the convenience and potential economic and social benefits of oral preparations, a more comprehensive health economic evaluation is warranted.
In conclusion, this study provides important clinical evidence for the use of oral polysaccharide-iron complex capsules in patients on MHD. It offers a new iron supplementation option that may improve treatment adherence and quality of life. More long-term studies are needed to further validate its efficacy and safety before its widespread clinical application.
Disclosure
All the authors declared no competing interests.
Acknowledgments
We would like to thank all participants and their families for participating in this clinical trial. We also thank Jiangsu Province Hospital and Nanjing Medical University First Affiliated Hospital, Taixing People's Hospital, Ningbo Hospital of Traditional Chinese Medicine, Tongren Hospital Affiliated to Shanghai Jiao Tong University School of Medicine, Sir Run Run Shaw Hospital, Zhejiang University School of Medicine, Shanghai Pudong New District Zhoupu Hospital, Wuxi People’s Hospital, and Shandong Province Qianfoshan Hospital for their contributions to the Institute as participating research centers.
Funding
The clinical trial was supported by the Clinical Research Plan of Shanghai Hospital Development Center under Grant SHDC2020CR4004.
Data Availability Statement
The investigator properly handled all the data obtained during the clinical trial and truthfully recorded all adverse events and serious adverse events during the clinical trial, to ensure the rights and privacy of the participants participating in the clinical trial. In accordance with the regulations, the right to access all test records belongs to the National Medical Products Administration, hospital ethics committee, medical inspection authorities, project managers, clinical research associate, etc., who verify the accuracy of the original data and understand the progress of the test during the trial. If the original records could not be effectively verified, the investigator assisted the inspector or auditor in further verifying the quality of the data. The group leader of this study set up a data monitoring committee (DMC) for the purpose of ensuring the safety of participants and the quality of study data. The DMC was composed of clinicians and biostatisticians from the Clinical Center for Investigation, Ren Ji Hospital Shanghai Jiao Tong University School of Medicine who were not involved in this study.
Author Contributions
ZN was responsible for the integrity of the whole research work, participation in the clinical trial protocol drafting, clinical trial concept design and evaluation, interpretation of trial data, revision, and submission of trial protocol manuscript. RL and HJ were responsible for drafting the test plan, research design, data interpretation, and participation in writing and modifying the test plan manuscript. JC, HL, XW, YQ, QL, XC, BS, NL, LS, LW, and XW were responsible for research management, data collection, coordination, and quality control. All the authors read and approved the final manuscript.
Footnotes
Supplementary Material S1. Statistical analysis plan and statistical power analysis.
Supplementary Material S2. Clinical trial protocol.
Table S1. Participants in the protocol analysis set.
Supplementary Material
Supplementary Material S1. Statistical analysis plan and statistical power analysis. Supplementary Material S2. Clinical trial protocol. Table S1. Participants in the protocol analysis set.
References
- 1.Qian G., Zhu Y., Tao S., et al. Increased hemoglobin concentration and related factors in maintenance hemodialysis patients in Anhui, China. Medicine (Baltimore) 2022;101 doi: 10.1097/MD.0000000000031397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chinese Experts Group of the Guideline for the Management of ‘CKD-PeriDialysis,’ Chinese Non-government Medical Institutions Association Chinese clinical practice guideline for the management of “CKD-PeriDialysis”-the periods prior to and in the early-stage of initial dialysis. Kidney Int Rep. 2022;7:S531–S558. doi: 10.1016/j.ekir.2022.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chinese Medical Association Nephrology Physician Branch Renal Anemia Guideline Working Group Chinese clinical practice guidelines for the diagnosis and treatment of renal anemia. Natl Med J China. 2021;101:1463–1502. doi: 10.3760/cma.j.cn112137-20210201-00309. [DOI] [Google Scholar]
- 4.Lu R., Zhang X., Cai X., et al. Efficacy and safety of polysaccharide iron complex capsules compared with iron sucrose in hemodialysis patients: study protocol for a randomized, open-label, positive control, multicenter trial (IHOPE) Trials. 2021;22:691. doi: 10.1186/s13063-021-05663-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jose A., Mahey R., Sharma J.B., et al. Comparison of ferric carboxymaltose and iron sucrose complex for treatment of iron deficiency anemia in pregnancy-randomised controlled trial. BMC Pregnancy Childbirth. 2019;19:54. doi: 10.1186/s12884-019-2200-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schröder O., Mickisch O., Seidler U., et al. Intravenous iron sucrose versus oral iron supplementation for the treatment of iron deficiency anemia in patients with inflammatory bowel disease--a randomized, controlled, open-label, multicenter study. Am J Gastroenterol. 2005;100:2503–2509. doi: 10.1111/j.1572-0241.2005.00250.x. [DOI] [PubMed] [Google Scholar]
- 7.Neogi S.B., Devasenapathy N., Singh R., et al. Safety and effectiveness of intravenous iron sucrose versus standard oral iron therapy in pregnant women with moderate-to-severe anaemia in India: a multicentre, open-label, phase 3, randomised, controlled trial. Lancet Glob Health. 2019;7:e1706–e1716. doi: 10.1016/S2214-109X(19)30427-9. [DOI] [PubMed] [Google Scholar]
- 8.Roemhild K., von Maltzahn F., Weiskirchen R., Knüchel R., von Stillfried S., Lammers T. Iron metabolism: pathophysiology and pharmacology. Trends Pharmacol Sci. 2021;42:640–656. doi: 10.1016/j.tips.2021.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kidney Disease: Improving Global Outcomes (KDIGO) Anemia Work Group KDIGO Clinical Practice Guideline for Anemia in Chronic Kidney Disease. Kidney Int. 2012;2:1–64. [Google Scholar]
- 10.Locatelli F., Aljama P., Bárány P., et al. Revised European best practice guidelines for the management of anaemia in patients with chronic renal failure. Nephrol Dial Transplant. 2004;19:ii1–ii47. doi: 10.1093/ndt/gfh1032. [DOI] [PubMed] [Google Scholar]
- 11.Shokri H., Ali I. Intravenous iron supplementation treats anemia and reduces blood transfusion requirements in patients undergoing coronary artery bypass grafting-a prospective randomized trial. Ann Card Anaesth. 2022;25:141–147. doi: 10.4103/aca.aca_209_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Elhenawy A.M., Meyer S.R., Bagshaw S.M., MacArthur R.G., Carroll L.J. Role of preoperative intravenous iron therapy to correct anemia before major surgery: a systematic review and meta-analysis. Syst Rev. 2021;10:36. doi: 10.1186/s13643-021-01579-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Buchrits S., Itzhaki O., Avni T., Raanani P., Gafter-Gvili A. Intravenous iron supplementation for the treatment of chemotherapy-induced anemia: a systematic review and meta-analysis of randomized controlled trials. J Clin Med. 2022;11:4156. doi: 10.3390/jcm11144156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shah A., Fisher S.A., Wong H., et al. Safety and efficacy of iron therapy on reducing red blood cell transfusion requirements and treating anaemia in critically ill adults: a systematic review with meta-analysis and trial sequential analysis. J Crit Care. 2019;49:162–171. doi: 10.1016/j.jcrc.2018.11.005. [DOI] [PubMed] [Google Scholar]
- 15.DeLoughery T.G. Safety of oral and intravenous iron. Acta Haematol. 2019;142:8–12. doi: 10.1159/000496966. [DOI] [PubMed] [Google Scholar]
- 16.Sivakumar C., Jubb V.M., Lamplugh A., Bhandari S. Safety of intravenous iron - CosmoFer and Monofer therapy in peritoneal dialysis and non-dialysis-dependent chronic kidney disease patients. Perit Dial Int. 2019;39:192–195. doi: 10.3747/pdi.2018.00125. [DOI] [PubMed] [Google Scholar]
- 17.Kassianides X., Hazara A.M., Bhandari S. Improving the safety of intravenous iron treatments for patients with chronic kidney disease. Expert Opin Drug Saf. 2021;20:23–35. doi: 10.1080/14740338.2021.1853098. [DOI] [PubMed] [Google Scholar]
- 18.Del Vecchio L., Longhi S., Locatelli F. Safety concerns about intravenous iron therapy in patients with chronic kidney disease. Clin Kidney J. 2016;9:260–267. doi: 10.1093/ckj/sfv142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Agarwal R., Rizkala A.R., Bastani B., Kaskas M.O., Leehey D.J., Besarab A. A randomized controlled trial of oral versus intravenous iron in chronic kidney disease. Am J Nephrol. 2006;26:445–454. doi: 10.1159/000096174. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material S1. Statistical analysis plan and statistical power analysis. Supplementary Material S2. Clinical trial protocol. Table S1. Participants in the protocol analysis set.
Data Availability Statement
The investigator properly handled all the data obtained during the clinical trial and truthfully recorded all adverse events and serious adverse events during the clinical trial, to ensure the rights and privacy of the participants participating in the clinical trial. In accordance with the regulations, the right to access all test records belongs to the National Medical Products Administration, hospital ethics committee, medical inspection authorities, project managers, clinical research associate, etc., who verify the accuracy of the original data and understand the progress of the test during the trial. If the original records could not be effectively verified, the investigator assisted the inspector or auditor in further verifying the quality of the data. The group leader of this study set up a data monitoring committee (DMC) for the purpose of ensuring the safety of participants and the quality of study data. The DMC was composed of clinicians and biostatisticians from the Clinical Center for Investigation, Ren Ji Hospital Shanghai Jiao Tong University School of Medicine who were not involved in this study.