Abstract
Staphylococcus aureus (S. aureus) is one of the main causative agents of mastitis, which results in severe economic losses. In addition, methicillin-resistant S. aureus (MRSA) has been reported in dairy farms and in water buffaloes. The present study aimed to determine the prevalence of subclinical mastitis (SCM) in water buffaloes, associated risk factors for SCM, and prevalence of MRSA in positive milk samples for SCM. Milk samples (n = 385) from buffaloes were examined using the California mastitis test (CMT), and S. aureus was detected in positive milk samples using bacteriological and biochemical tests. In addition, MRSA was identified in positive S. aureus samples using PCR targeting the mecA gene. The results revealed that the prevalence of SCM among water buffaloes in the studied areas was 43.6%, and 61.9% (104/168) were identified as MRSA based on PCR targeting the mecA gene. In vitro antibiotic susceptibility testing found cefoxitin to be resistant and linezolid to be sensitive against MRSA isolates. In addition, the statistical analysis revealed that there was no significant association between the prevalence of SCM and locality or duration of lactation. The prevalence of SCM was strongly associated with age, parity, absence of teat dipping, hand cleaning of milker hands between milking, and in animals with a history of mastitis. Regular CMT can detect early SCM and improve udder sanitation and milking hygiene. In addition, continuous testing of antimicrobial drugs against MRSA isolates is necessary due to the importance of S. aureus in public health and the development of antibiotic resistance, such as methicillin.
Keywords: antibiotic-resistant, Egypt, PCR, Staphylococcus aureus, subclinical mastitis, water buffaloes
1. Introduction
Buffaloes are integral to Egypt's agricultural economy, contributing to milk and meat production, agricultural labor, and providing valuable manure [1]. Their role is crucial in supporting the livelihoods of many rural farmers and in promoting sustainable agricultural practices. However, research studies on buffalo have received less attention than studies on cattle [2].
Infectious illnesses are a serious barrier to livestock development in Egypt, particularly among buffaloes [3, 4]. Mastitis is one of the most serious diseases among the several infectious diseases that affect bovines, including buffaloes. Staphylococcus aureus is the primary cause of mastitis in bovines, rapidly adapts to the mammary glands' environmental circumstances, and spreads across animals during milking [5, 6].
Infection with S. aureus can induce tissue damage and gangrenous mastitis. Gangrenous mastitis is characterized by blue to black quarters that slough off and is commonly associated with staphylococcal alpha toxin production [7, 8].
Staphylococcus aureus, particularly methicillin-resistant S. aureus (MRSA), is a major pathogen of both veterinary and public health concern due to its ability to acquire and disseminate antimicrobial resistance [9]. The resistance mechanism in MRSA is primarily mediated by the mecA gene, which encodes for an altered penicillin-binding protein (PBP2a) that has a low affinity for β-lactam antibiotics, rendering them ineffective. This resistance, coupled with the pathogen's ability to form biofilms and evade the host immune response, contributes to persistent infections, such as subclinical mastitis (SCM) [10, 11].
MRSA has emerged in cattle because of the widespread and uncontrolled use of antibiotics, especially penicillin groups, to treat cows. Methicillin resistance in S. aureus has an adverse effect on treating diseases in humans and animals [12–14].
Staphylococcus aureus can develop methicillin resistance by acquiring the staphylococcal cassette chromosome (SCC) mecA gene, which modifies the penicillin-binding protein (PBP2) and reduces affinity for all β-lactam drugs [15–17].
In comparison to Europe, the United States had a higher prevalence of MRSA [18]. Studies have shown that prevalence rates were less than 50% in Portugal, Italy, and Greece, and above 70% in Vietnam and South Korea. Studies carried out in Egypt revealed that S. aureus strains resistant to cefoxitin and penicillin, which were identified from SCM, were highly prevalent in bovines in the governorates of Dakahlia and Ismailia [19, 20].
This study aimed to investigate the prevalence of SCM and MRSA infections in water buffaloes, alongside identifying the potential risk factors contributing to the occurrence of SCM.
2. Materials and Methods
2.1. Ethical Statement
The study protocol followed the guidelines and was approved by the ethics committee of the Faculty of Veterinary Medicine, Benha University. In addition, all methods were performed in accordance with the relevant guidelines and regulation ethics committee of the Faculty of Veterinary Medicine, Benha University. This study was conducted according to ARRIVE guidelines.
2.2. Study Area
The study was carried out in two governorates (Kafr El Sheikh and Menofia) located in Egypt's Nile Delta between January and December 2023. These governorates are situated geographically at 31°06′42′N 30°56′45′E and 30.52°N 30.99°E (Figure 1).
Figure 1.

Map showed governorates under the study (map generated by QGis software).
The selected governorates have a subtropical desert climate (classification: BWh) and are situated at an elevation of 30–33 feet above sea level. The annual temperature of the city is 25°C (74.8°F), and receives precipitation of 4 mm. These areas are a vital agricultural area and considered as a cornerstone of food production in Egypt.
2.3. Sampling and Sample Size
The sample size was calculated based on Thrusfield's formula [21]
where n is the sample size, Z is the 95% confidence interval (CI), P is a predicted prevalence rate (50%), and absolute precision is 5%. A total of 385 buffaloes raised by individual farmers were analyzed; some of the animals displayed symptoms of mastitis, while others appeared normal (SCM).
The udder of the examined animal was washed using clean water, dried with clean tissue, and disinfected using 70% alcohol. After screening for SCM with the California mastitis test (CMT), milk samples were obtained [22]. Milk samples (8 mL) were collected from each animal in a clean, sterilized Falcon tube (15 mL) labeled with the number of the animal and the collection date.
A questionnaire was prepared in order to get animal data from farmers at the time of sampling. The questionnaire collected information on the animals, such as their age, parity, lactation stage, history of mastitis, presence of one or more lesions on the udders, and usage of antibiotics for mastitis.
2.4. Bacteriological Examination
The milk samples were thoroughly mixed and streaked on blood agar and incubated for 24 h at 37°C. In order to verify the presence of S. aureus, the bacterial colonies were streaked onto mannitol salt agar (MSA; Merck, Germany) and identified biochemically. S. aureus forms smooth, golden–yellow colonies with β-hemolysis on blood agar and ferments mannitol on MSA, turning the medium bright yellow. The suspected S. aureus colonies were confirmed microscopically by Gram staining and biochemically by coagulase and catalase tests [23].
2.5. Phenotypic Identification and Antibiotic Susceptibility Test for MRSA Strains
Fresh S. aureus colonies were adjusted to 0.5 McFarland and swabbed on Muller–Hinton agar. Oxacillin discs (1 μg) and cefoxitin (30 μg) discs were placed aseptically on Muller–Hinton agar (Oxoid, Sigma–Aldrich, UK). After that, the plate was incubated at 37°C for 24 h, and the inhibition zones were determined in millimeters using vernier calipers [24].
The susceptibility of MRSA strains to antibiotics was evaluated in vitro, taking into consideration the most common antibiotics used in treatment in the studied area. The antibiotic sensitivity test was performed against some of drugs, such as oxytetracycline (30 μg), gentamicin (10 μg), amikacin (30 μg), tylosin (15 μg), levofloxacin (5 μg), ciprofloxcin (5 μg), moxifloxacin (5 μg), linezolid (25 μg), cefoxitin (30 μg), trimethoprim + sulphamethoxazole (30 μg), and vancomycin (30 μg). The inhibition zones were compared with standard susceptibility zone according to the Clinical and Laboratory Standards Institute (CLSI) [25].
2.6. Molecular Diagnosis of mecA Gene
Samples of S. aureus that had been verified to be resistant to oxacillin and cefoxitin underwent additional processing to confirm the mecA gene using PCR. The QIAamp DNA Mini (Qiagen, Hilden, Germany) kit was used for the extraction of DNA of resistant strains. According to Galdiero et al. [26], the extracted DNA was amplified using specific pairs of primers targeting the mecA gene, forward primers P1: 5′-TGGCAT TCGTGTCACAATCG-3′ and reverse primer P2: 5′-CTGGAACTT GTTGAGCAGAG-3′, which amplify a product of 310 bp. DNA amplification was performed over 34 cycles, consisting of denaturation at 92 °C for 1 min, annealing at 56 °C for 1 min, and extension at 72 °C for 2 min. A final extension step was conducted at 72 °C for 3 min to complete the reaction. The amplified PCR product was run on a 2% agarose gel, stained with ethidium bromide, and photographed under UV illumination.
2.7. Statistical Analysis
The statistical analysis was conducted using SPSS version 24 (SPSS Inc., Chicago, U.S.A.). In a univariate analysis, the risk factors' relationships with the prevalence of SCM were evaluated using the chi-square test. All variables with p-value less than 0.25 were subjected to final multivariate logistic regression model. Risk variables were found to have a statistically significant relationship with positive SCM when their p-value was less than 0.05. Based on this finding, their odds ratios (ORs) and 95% CI were calculated [27–29].
3. Results
The overall prevalence of SCM among examined buffaloes based on CMT was 43.6% (168/385). The prevalence did not differ significantly amongst the examined governorates, with Kafr El Sheikh having the greatest prevalence (46.9%) and Gharbia having the lowest (41.6%), as shown in Table 1.
Table 1.
The prevalence of subclinical mastitis and its association with different variables.
| Variable | No. of samples | No. of positive | Percentage of positive | 95% CI | Statistic |
|---|---|---|---|---|---|
| Locality | χ 2 = 0.875 d = 2 p = 0.646 | ||||
| Kafr El Sheikh | 130 | 61 | 46.9 | 38.55–55.46 | |
| Gharbia | 125 | 52 | 41.6 | 33.34–50.36 | |
| Menofia | 130 | 55 | 42.3 | 34.16–50.9 | |
| Age | χ 2 = 32.409 d = 2 p < 0.0001∗ | ||||
| 2–4 | 160 | 48 | 30.0 | 23.44–37.5 | |
| >4–8 | 150 | 92 | 61.3 | 53.35–68.75 | |
| >8 | 75 | 28 | 37.3 | 27.25–48.64 | |
| Parity | χ 2 = 31.703 d = 2 p < 0.0001∗ | ||||
| 1 | 140 | 40 | 28.6 | 21.74–36.55 | |
| 2 | 115 | 47 | 40.9 | 32.32–50.01 | |
| ≥3 | 130 | 81 | 62.3 | 53.74–70.17 | |
| Duration of lactation (months) | χ 2 = 2.627 d = 2 p = 0.269 | ||||
| 1–3 | 50 | 27 | 54.0 | 40.4–67.03 | |
| >3–6 | 120 | 52 | 43.3 | 34.81–52.27 | |
| >6 | 215 | 89 | 41.4 | 35.02–48.08 | |
| Teat dipping | χ 2 = 83.486 d = 1 p < 0.0001∗ | ||||
| Yes | 165 | 28 | 17.0 | 12.01–23.43 | |
| No | 220 | 140 | 63.6 | 57.1–69.71 | |
| Previous history of mastitis | χ 2 = 52.677 d = 1 p < 0.0001∗ | ||||
| Yes | 155 | 33 | 21.3 | 15.58–28.39 | |
| No | 230 | 135 | 58.7 | 52.24–64.87 | |
| Hygiene of milker's hand during milking | χ 2 = 84.300 d = 1 p < 0.0001∗ | ||||
| Yes | 168 | 29 | 17.3 | 12.29–23.69 | |
| No | 217 | 139 | 64.1 | 57.48–70.15 | |
|
| |||||
| Total | 385 | 168 | 43.6 | 38.77–48.63 | |
∗ Results are significant at p-Value <0.05.
In the present study, five variables with a p-value less than 0.25 in univariate analysis were subjected to a multivariate logistic regression model. The final logistic regression model results showed that the following factors were significantly associated with SCM in water buffaloes: median age (OR = 5.5, 95% CI: 2.8–10.9), parity more than three (OR = 3.1, 95% CI: 1.6–6.1), absence of teat dipping (OR = 7.9, 95% CI: 4.2–14.8), animal with history of previous mastitis (OR = 4, 95% CI: 2.2–7.4), and absence of hand washing between milking (OR = 8, 95% CI: 4.3–14.8) (Table 2).
Table 2.
Multivariate logistic regression analysis for risk factors related to subclinical mastitis.
| Variable | B | SE | OR | 95% CI for OR | p-Value | |
|---|---|---|---|---|---|---|
| Lower | Upper | |||||
| Age | ||||||
| >4–8 | 1.711 | 0.347 | 5.5 | 2.8 | 10.9 | <0.0001 |
| >8 | 0.763 | 0.395 | 2.1 | 1.0 | 4.7 | 0.033 |
| Parity | ||||||
| 2 | 0.597 | 0.365 | 1.8 | 0.9 | 3.7 | 0.012 |
| ≥3 | 1.126 | 0.344 | 3.1 | 1.6 | 6.1 | 0.001 |
| Teat dipping | ||||||
| No | 2.066 | 0.321 | 7.9 | 4.2 | 14.8 | <0.0001 |
| Previous history of mastitis | ||||||
| No | 1.398 | 0.308 | 4.0 | 2.2 | 7.4 | <0.0001 |
| Hygiene of milker's hand during milking | ||||||
| No | 2.076 | 0.314 | 8.0 | 4.3 | 14.8 | <0.0001 |
Note: B, logistic regression coefficient
Abbreviations: CI, confidence interval; OR, odds ratio; SE, standard error.
Out of 168 S. aureus isolates, 104 (61.9%) were resistant to oxacillin and cefoxitin and classified as phenotypic MRSA. The resistant isolates were confirmed using a PCR assay targeting the mecA gene, the positive samples gave a detectable band at 300 bp (Figure 2). According to antibiotic sensitivity testing, linezolid demonstrated 100% efficacy, ciprofloxacin 90% efficacy, amikacin and trimethoprim + sulphamethoxazole 80% efficacy, while gentamicin, tylosine, and levofloxacin showed 70%, 65%, and 70% efficacy against MRSA detected in buffalo milk, respectively. In contrast, all MRSA isolates were cefoxitin-resistant (Table 3).
Figure 2.
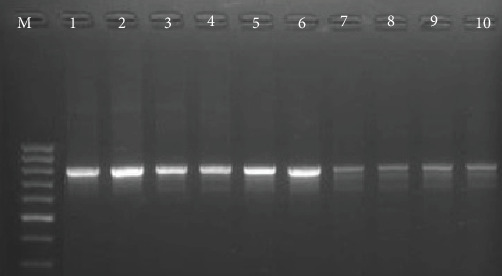
Results of PCR products of mecA gene, lane M is a 100 bp DNA ladder, and lane 1 is a positive control. Lane 2–10 are positive samples for MRSA.
Table 3.
Antimicrobial sensitivity test results against methicillin-resistant Staphylococcus aureus.
| Antibiotic disc | Potency | S | I | R |
|---|---|---|---|---|
| Oxytetracycline | 30 μg | 35 | 25 | 40 |
| Gentamicin | 10 μg | 70 | 0 | 30 |
| Amikacin | 30 μg | 80 | 20 | — |
| Tylosin | 15 μg | 65 | 20 | 15 |
| Levofloxacin | 5 μg | 70 | 15 | 15 |
| Ciprofloxcin | 5 μg | 90 | — | 10 |
| Linezolid | 25 μg | 100 | — | — |
| Trimethoprim + sulphamethoxazole | 30 μg | 80 | — | 20 |
| Cefoxitin | 30 μg | — | — | 100 |
The duration of lactation has no significant effect on the prevalence of SCM in water buffaloes. The study found a significant increase (p < 0.05) in the frequency of SCM in water buffaloes with an age group of >4–8, with a history of mastitis and a parity greater than three. Furthermore, hygienic factors, such as the absence of teat dipping and cleaning of milkers' hands between milking, significantly enhanced the prevalence of SCM (Table 3).
4. Discussion
Bovine mastitis has a significant economic impact and the most prevalent disease in dairy bovines and S. aureus is the most prevalent cause of mastitis. The development of MRSA in the last years due to misuse of antibiotics in treatment or as a growth promoter has increased the drug-resistant problems and risk to public health.
In the current study, the prevalence of SCM in water buffaloes was 43.6%, which is consistent with Salvador et al. [30] (42.76%). In addition, the prevalence rate is higher than previous reported rates in Philippines (24.22%) [31] and in Bangladesh (10.5%) [32].
According to the PCR assay targeting the mecA gene, the prevalence of MRSA is 61.9% (104/168), which is consistent with the findings of Badua et al. [31], who found that the prevalence of MRSA in buffalo milk was 61.54%. Nonetheless, the lower prevalence of MRSA was reported in Pakistan (34%) [33], Germany (16.7%) [34], India (13.1%) [35], Korea (6.3%) [36], and Wisconsin (1.8%) [37].
There are certain discrepancies in the estimation of MRSA prevalence. The prevalence of MRSA could be decreased as a result of overproduction of beta-lactamase or inadequate expression of the mecA gene, which results in inconsistent diagnosis [38, 39]. Furthermore, phenotypic expression can be influenced by the pH and osmolality of culture media, and the greater variation found in MRSA strains may contribute to diagnostic challenges [40–45].
All MRSA strains were shown to be 100% sensitive to linezolid but 100% resistant to cefoxitin, which is consistent with earlier findings [29, 33]. Furthermore, the results are consistent with other observations from Egypt, where the majority of S. aurus isolates are resistant to cefoxitin and oxytetracycline [9, 46]. Furthermore, it was discovered that none of the MRSA isolates from buffalo milk were cefoxitin sensitive. This finding is in line with the findings of Nemeghaire et al. [47], who reported that all MRSA isolates were cefoxitin resistant.
Cefoxitin and oxacillin are both used as surrogate markers for the detection of MRSA; however, discrepancies between the two can occur. Cefoxitin is considered a better inducer of the mecA gene, which encodes the penicillin-binding protein PBP2a responsible for methicillin resistance. As such, cefoxitin disk diffusion and MIC testing are generally more reliable and reproducible compared to oxacillin, especially in borderline or heteroresistant strains [48]. Studies have shown that cefoxitin testing has higher sensitivity and specificity for MRSA detection and is recommended by the CLSI over oxacillin [49]. In contrast, oxacillin may yield false-negative results due to its weaker induction of mecA, leading to underestimation of resistance in clinical isolates. Therefore, reliance on oxacillin alone could result in misclassification of MRSA strains, emphasizing the importance of cefoxitin-based testing, particularly in routine surveillance and clinical diagnostics [50].
Biofilms act as a physical barrier, impeding the penetration of antibiotics and host immune responses, thereby enhancing bacterial survival under adverse conditions. Within biofilms, bacteria exhibit altered gene expression, reduced metabolic activity, and increased horizontal gene transfer, all of which contribute to the development and maintenance of methicillin resistance [51]. Additionally, biofilm-associated MRSA infections are more difficult to treat and often result in chronic and recurrent infections [52, 53]. Therefore, understanding the interplay between biofilm formation and antibiotic resistance is essential for the development of effective control strategies.
The results of the present study revealed that the prevalence of SCM increased significantly in buffaloes of median age (4–8 years) with a high number of parities. These results are consistent with those of Badua et al. [31] and Kemal et al. [54], who found that older animals with more calvings had a higher prevalence of SCM. This could be due to pathogenic organisms being more susceptible to udders with relaxed sphincters, and primiparous cows may have more effective defensive mechanisms than multiparous animals [55].
The current findings supported previous studies that found washing the udder before milking can minimize the spread of S. aureus in dairy animals [56]. It also demonstrated that teat dipping had a substantial impact on the prevalence of S. aureus. It's interesting to note that unclean hands of milkers may be a source of infectious germs spreading during milking procedures; these results are in line with previous research [33]. The presence of S. aureus and other infectious bacteria on the udder or teat surface of infected cows is considered as a primary way for transmission of infection between infected and uninfected udder quarters. Therefore, it's clear that the causal organisms could spread quickly from infected to uninfected cows' udders or through the hands of milkers. During lactation, there is virtually little chance of recovery from S. aureus infections treated with antibiotics; many infected animals develop chronic infections and must be put to death [56, 57].
Additionally, it can be inferred that animals with a history of mastitis had a higher prevalence of S. aureus than animals without a history of mastitis, which is consistent with earlier findings by Mekibib et al. [58] and Selim et al. [44]. The resulting data imply that treating mastitis in animals might not be effective in getting rid of infections, and those infections might be transferred from one lactation to the next [59, 60]. Additionally, it has been reported that buffaloes with three calvings and a previous history of abortion seem to be the most vulnerable to mammary infections since the teat and udder are exposed to harm, and bacteria can readily attach to the teat and enter the gland tissue [56].
5. Conclusion
The results of the present study concluded that the high prevalence of SCM and MRSA among water buffaloes in the studied governorates. The prevalence of SCM was significantly associated with age, parity, absence of teat dipping and washing of milker's hand between milking and in animals with a previous history of mastitis. The in vitro antibiotic sensitivity test revealed that the sensitivity of MRSA strains to linezolid and resistance to cefoxitin. Therefore, it is possible to lower the prevalence of MRSA by implementing suitable control measures, conducting routine investigations and treatment protocols, and conducting enough surveillance.
Acknowledgments
The authors would like to acknowledge the Deanship of Scientific Research, Vice Presidency for Graduate Studies and Scientific Research, King Faisal University, Saudi Arabia (Grant KFU251996).
Contributor Information
Abdelfattah Selim, Email: abdelfattah.selim@fvtm.bu.edu.eg.
Mohamed Marzok, Email: mmarzok@kfu.edu.sa.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Consent
The authors have nothing to report.
Conflicts of Interest
The authors declare no conflicts of interest.
Author Contributions
Conceptualization, methodology, formal analysis, investigation, resources, data curation, writing – original draft preparation, writing – review and editing, funding acquisition: Abdelfattah Selim, Hattan S. Gattan, Abdelrahman M. Hereba, and Mohamed Marzok. Project administration: Mohamed Marzok. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Deanship of Scientific Research, Vice Presidency for Graduate Studies and Scientific Research, King Faisal University, Saudi Arabia (Grant KFU251996).
References
- 1.Radwan M. A. A. Cairo University; 2016. Characterization of Milk and Veal Production Chains of Buffalo Under Crop Livestock Production System in Egypt. [Google Scholar]
- 2.Shahin A. S. A. H. Adoption of Innovations in Smallholder Buffalo Dairy Farms in the Menoufia Province in Egypt . Vol. 45. Köster; 2004. [Google Scholar]
- 3.Selim A., Alshammari A., Gattan H. S., Alruhaili M. H., Rashed G. A., Shoulah S. Seroprevalence and Associated Risk Factors for Toxoplasma Gondii in Water Buffaloes (Bubalus bubalis) in Egypt. Comparative Immunology, Microbiology and Infectious Diseases . 2023;101 doi: 10.1016/j.cimid.2023.102058.102058 [DOI] [PubMed] [Google Scholar]
- 4.Selim A., Marawan M. A., Ali A.-F., Manaa E., AbouelGhaut H. A. Seroprevalence of Bovine Leukemia Virus in Cattle, Buffalo, and Camel in Egypt. Tropical Animal Health and Production . 2020;52(3):1207–1210. doi: 10.1007/s11250-019-02105-8. [DOI] [PubMed] [Google Scholar]
- 5.Benić M., Maćešić N., Cvetnić L., et al. Bovine Mastitis: A Persistent and Evolving Problem Requiring Novel Approaches for its Control-a Review. Veterinarski Arhiv . 2018;88(4):535–557. doi: 10.24099/vet.arhiv.0116. [DOI] [Google Scholar]
- 6.Campos B., Pickering A. C., Rocha L. S., et al. Diversity and Pathogenesis of Staphylococcus aureus from Bovine Mastitis: Current Understanding and Future Perspectives. BMC Veterinary Research . 2022;18(1) doi: 10.1186/s12917-022-03197-5.115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Badugela N. Limpopo, South Africa: 2020. Characterization of E. Coli and Staphylococcus aureus Isolated from Clinical and Subclinical Cases of Bovine Mastitis in the Limpopo Dairy Farm. [Google Scholar]
- 8.Javed S., McClure J., Syed M. A., et al. Epidemiology and Molecular Characterization of Staphylococcus aureus Causing Bovine Mastitis in Water Buffaloes From the Hazara Division of Khyber Pakhtunkhwa, Pakistan. PLoS ONE . 2022;17(5) doi: 10.1371/journal.pone.0268152.e0268152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Algammal A. M., Enany M. E., El-Tarabili R. M., Ghobashy M. O., Helmy Y. A. Prevalence, Antimicrobial Resistance Profiles, Virulence and Enterotoxins-Determinant Genes of MRSA Isolated From Subclinical Bovine Mastitis in Egypt. Pathogens . 2020;9(5) doi: 10.3390/pathogens9050362.362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Leonard F., Markey B. Meticillin-Resistant Staphylococcus aureus in Animals: A Review. The Veterinary Journal . 2008;175(1):27–36. doi: 10.1016/j.tvjl.2006.11.008. [DOI] [PubMed] [Google Scholar]
- 11.Pantosti A. Methicillin-Resistant Staphylococcus aureus Associated With Animals and its Relevance to Human Health. Frontiers in Microbiology . 2012;3 doi: 10.3389/fmicb.2012.00127.127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Asmelash T., Mesfin N., Addisu D., Aklilu F., Biruk T., Tesfaye S. Isolation, Identification and Drug Resistance Patterns of Methicillin Resistant Staphylococcus aureus From Mastitic Cows Milk From Selected Dairy Farms in and Around Kombolcha, Ethiopia. Journal of Veterinary Medicine and Animal Health . 2016;8(1):1–10. doi: 10.5897/JVMAH2015.0422. [DOI] [Google Scholar]
- 13.Javed M. U., Ijaz M., Fatima Z., et al. Frequency and Antimicrobial Susceptibility of Methicillin and Vancomycin-Resistant Staphylococcus aureus From Bovine Milk. Pakistan Veterinary Journal . 2021;41(4):463–468. doi: 10.29261/pakvetj/2021.060. [DOI] [Google Scholar]
- 14.Umaru G., Kabir J., Adamu N., Umar Y. A Review of Emerging Methicillin-Resistant Staphylococcus aureus (MRSA): A Growing Threat to Veterinarians. Nigerian Veterinary Journal . 2011;32(3) [Google Scholar]
- 15.Carretto E., Visiello R., Nardini P. Pet-To-Man Travelling Staphylococci . Elsevier; 2018. Methicillin Resistance in Staphylococcus aureus; pp. 225–235. [Google Scholar]
- 16.Peacock S. J., Paterson G. K. Mechanisms of Methicillin Resistance in Staphylococcus aureus. Annual Review of Biochemistry . 2015;84(1):577–601. doi: 10.1146/annurev-biochem-060614-034516. [DOI] [PubMed] [Google Scholar]
- 17.Selim A. M., Elhaig M. M., Gaede W. Development of Multiplex Real-Time PCR Assay for the Detection of Brucella spp., Leptospira spp. and Campylobacter Foetus. 2014. [DOI] [PubMed]
- 18.Maurya P. S., Rakesh R. L., Pradeep B., et al. Prevalence and Risk Factors Associated With Cryptosporidium spp. Infection in Young Domestic Livestock in India. Tropical Animal Health and Production . 2013;45(4):941–946. doi: 10.1007/s11250-012-0311-1. [DOI] [PubMed] [Google Scholar]
- 19.El-Sayed M., Algammal A., Youssef F., Hassan E. Prevalence and Genetic Characterization of S. aureus Strains Isolated From Raw Milk and its Products. Suez Canal Veterinary Medical Journal . 2019;24(2):245–256. doi: 10.21608/scvmj.2019.69848. [DOI] [Google Scholar]
- 20.Emam A., El-Diasty M., Abdelkhalek A. Prevalence of Staphyloсoссus aureus and Streptococcus agalaсtiae Isolated From Raw Milk in Dakahlia Governorate, Egypt. Zagazig Veterinary Journal . 2021;49(1):67–77. doi: 10.21608/zvjz.2021.64186.1131. [DOI] [Google Scholar]
- 21.Thrusfield M. Veterinary Epidemiology . John Wiley & Sons; 2018. [Google Scholar]
- 22.Radostits O. M., Gay C., Hinchcliff K. W., Constable P. D. A Textbook of the Diseases of Cattle, Horses, Sheep, Pigs and Goats. Veterinary Medicine . 2007;10:2045–2050. [Google Scholar]
- 23.Boerlin P., Kuhnert P., Hussy D., Schaellibaum M. Methods for Identification of Staphylococcus aureus Isolates in Cases of Bovine Mastitis. Journal of Clinical Microbiology . 2003;41(2):767–771. doi: 10.1128/JCM.41.2.767-771.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bauer A., Kirby W., Sherris J. C., Turck M. Antibiotic Susceptibility Testing by a Standardized Single Disk Method. American Journal of Clinical Pathology . 1966;45(4_ts):493–496. doi: 10.1093/ajcp/45.4_ts.493. [DOI] [PubMed] [Google Scholar]
- 25.Wayne P. Clinical and Laboratory Standards Institute (CLSI). Performance Standards for Antimicrobial Susceptibility Testing. 2015.
- 26.Galdiero E., Liguori G., D’Isanto M., Damiano N., Sommese L. Distribution of mecA Among Methicillin-Resistant Clinical Staphylococcal Strains Isolated at Hospitals in Naples, Italy. European Journal of Epidemiology . 2003;18(2):139–145. doi: 10.1023/A:1023067930211. [DOI] [PubMed] [Google Scholar]
- 27.Selim A., Abdelhady A., Alahadeb J. Prevalence and First Molecular Characterization of Ehrlichia canis in Egyptian Dogs. Pakistan Veterinary Journal . 2020;41(1):117–121. doi: 10.29261/pakvetj/2020.061. [DOI] [Google Scholar]
- 28.Selim A., Attia K. A., Alsubki R. A., Kimiko I., Sayed-Ahmed M. Z. Cross-Sectional Survey on Mycobacterium avium Subsp. Paratuberculosis in Dromedary Camels: Seroprevalence and Risk Factors. Acta Tropica . 2022;226 doi: 10.1016/j.actatropica.2021.106261.106261 [DOI] [PubMed] [Google Scholar]
- 29.Selim A., Alafari H. A., Attia K., AlKahtani M. D., Albohairy F. M., Elsohaby I. Prevalence and Animal Level Risk Factors Associated With Trypanosoma Evansi Infection in Dromedary Camels. Scientific Reports . 2022;12(1) doi: 10.1038/s41598-022-12817-x.8933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Salvador R., Beltran J., Abes N., Gutierrez C., Mingala C. Prevalence and Risk Factors of Subclinical Mastitis as Determined by the California Mastitis Test in Water Buffaloes (Bubalis Bubalis) in Nueva Ecija Philippines. Journal of Dairy Science . 2012;95(3):1363–1366. doi: 10.3168/jds.2011-4503. [DOI] [PubMed] [Google Scholar]
- 31.Badua A. T., Boonyayatra S., Awaiwanont N., Gaban P. B. V., Mingala C. N. Methicillin-Resistant Staphylococcus aureus (MRSA) Associated With Mastitis Among Water Buffaloes in the Philippines. Heliyon . 2020;6(12) doi: 10.1016/j.heliyon.2020.e05663.e05663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Aliul H., Kumar P. A., Mahmood R. M., Mizanur R., Selim A. M. Investigation of Prevalence and Risk Factors of Subclinical Mastitis of Dairy Buffaloes at Bhola District of Bangladesh. Asian Journal of Medical and Biological Research . 2020;6(4):697–704. doi: 10.3329/ajmbr.v6i4.51236. [DOI] [Google Scholar]
- 33.Aqib A. I., Ijaz M., Anjum A. A., et al. Antibiotic Susceptibilities and Prevalence of Methicillin Resistant Staphylococcus aureus (MRSA) Isolated From Bovine Milk in Pakistan. Acta Tropica . 2017;176:168–172. doi: 10.1016/j.actatropica.2017.08.008. [DOI] [PubMed] [Google Scholar]
- 34.Spohr M., Rau J., Friedrich A., et al. Methicillin-Resistant Staphylococcus aureus (MRSA) in Three Dairy Herds in Southwest Germany. Zoonoses and Public Health . 2011;58(4):252–261. doi: 10.1111/j.1863-2378.2010.01344.x. [DOI] [PubMed] [Google Scholar]
- 35.Kumar R., Yadav B., Singh R. Antibiotic Resistance and Pathogenicity Factors in Staphylococcus aureus Isolated From Mastitic Sahiwal Cattle. Journal of Biosciences . 2011;36(1):175–188. doi: 10.1007/s12038-011-9004-6. [DOI] [PubMed] [Google Scholar]
- 36.Lim S.-K., Nam H.-M., Jang G.-C., Lee H.-S., Jung S.-C., Kim T.-S. Transmission and Persistence of Methicillin-Resistant Staphylococcus aureus in Milk, Environment, and Workers in Dairy Cattle Farms. Foodborne Pathogens and Disease . 2013;10(8):731–736. doi: 10.1089/fpd.2012.1436. [DOI] [PubMed] [Google Scholar]
- 37.Makovec J. A., Ruegg P. L. Antimicrobial Resistance of Bacteria Isolated From Dairy Cow Milk Samples Submitted for Bacterial Culture: 8,905 Samples (1994–2001. Journal of the American Veterinary Medical Association . 2003;222(11):1582–1589. doi: 10.2460/javma.2003.222.1582. [DOI] [PubMed] [Google Scholar]
- 38.Selim A., Elhaig M., Taha S., Nasr E. Antibacterial Activity of Silver Nanoparticles against Field and Reference Strains of Mycobacterium tuberculosis, Mycobacterium bovis and Multiple-Drug-Resistant Tuberculosis Strains. Revue Scientifique et Technique de l’OIE . 2018;37(3):823–830. doi: 10.20506/rst.37.3.2888. [DOI] [PubMed] [Google Scholar]
- 39.Turutoglu H., Hasoksuz M., Ozturk D., Yildirim M., Sagnak S. Methicillin and Aminoglycoside Resistance in Staphylococcus aureus Isolates from Bovine Mastitis and Sequence Analysis of Their mecA Genes. Veterinary Research Communications . 2009;33(8):945–956. doi: 10.1007/s11259-009-9313-5. [DOI] [PubMed] [Google Scholar]
- 40.Abd Elmohsen M., Selim A., Abd Elmoneim A. E. Prevalence and Molecular Characterization of Lumpy Skin Disease in Cattle During Period 2016-2017. Benha Veterinary Medical Journal . 2019;37(1):172–175. doi: 10.21608/bvmj.2019.18293.1118. [DOI] [Google Scholar]
- 41.Hamdy A. S., Selim A., Shoulah S. A., Ibrahim A. M. M. Sero-Surveillance Infectious Bovine Rhinotracheitis in Ruminants and Assessment the Associated Risk Factors. Benha Veterinary Medical Journal . 2022;42(2):160–163. doi: 10.21608/bvmj.2022.128717.1507. [DOI] [Google Scholar]
- 42.Haran K., Godden S., Boxrud D., Jawahir S., Bender J., Sreevatsan S. Prevalence and Characterization of Staphylococcus aureus, Including Methicillin-Resistant Staphylococcus aureus, Isolated From Bulk Tank Milk From Minnesota Dairy Farms. Journal of Clinical Microbiology . 2012;50(3):688–695. doi: 10.1128/JCM.05214-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Selim A., Abdelhady A. Neosporosis Among Egyptian Camels and its Associated Risk Factors. Tropical Animal Health and Production . 2020;52(6):3381–3385. doi: 10.1007/s11250-020-02370-y. [DOI] [PubMed] [Google Scholar]
- 44.Selim A., Kelis K., AlKahtani M. D., Albohairy F. M., Attia K. A. Prevalence, Antimicrobial Susceptibilities and Risk Factors of Methicillin Resistant Staphylococcus aureus (MRSA) in Dairy Bovines. BMC Veterinary Research . 2022;18(1) doi: 10.1186/s12917-022-03389-z.293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Selim A., Manaa E. A., Alanazi A. D., Alyousif M. S. Seroprevalence, Risk Factors and Molecular Identification of Bovine Leukemia Virus in Egyptian Cattle. Animals . 2021;11(2) doi: 10.3390/ani11020319.319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.El-Ashker M., Gwida M., Monecke S., et al. Antimicrobial Resistance Pattern and Virulence Profile of S. aureus Isolated From Household Cattle and Buffalo With Mastitis in Egypt. Veterinary Microbiology . 2020;240 doi: 10.1016/j.vetmic.2019.108535.108535 [DOI] [PubMed] [Google Scholar]
- 47.Nemeghaire S., Argudín M. A., Haesebrouck F., Butaye P. Epidemiology and Molecular Characterization of Methicillin-Resistant Staphylococcus aureus Nasal Carriage Isolates From Bovines. BMC Veterinary Research . 2014;10:1–9. doi: 10.1186/1746-6148-10-153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Broekema N. M., Van T. T., Monson T. A., Marshall S. A., Warshauer D. M. Comparison of Cefoxitin and Oxacillin Disk Diffusion Methods for Detection of mecA-Mediated Resistance in Staphylococcus aureus in a Large-Scale Study. Journal of Clinical Microbiology . 2009;47(1):217–219. doi: 10.1128/JCM.01506-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pierce V. M., Bhowmick T., Simner P. J. Guiding Antimicrobial Stewardship Through Thoughtful Antimicrobial Susceptibility Testing and Reporting Strategies: An Updated Approach in 2023. Journal of Clinical Microbiology . 2023;61(11):e00074–00022. doi: 10.1128/jcm.00074-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ijaz M., Sabir M. J., Javed M. U., Ahmed A., Rasheed H., Jabir A. A. Molecular Insights Into Expression and Silencing of Resistance Determinants in Staphylococcus aureus. Tropical Medicine & International Health . 2024;29(6):526–535. doi: 10.1111/tmi.14000. [DOI] [PubMed] [Google Scholar]
- 51.Singh A., Amod A., Pandey P., et al. Bacterial Biofilm Infections, Their Resistance to Antibiotics Therapy and Current Treatment Strategies. Biomedical Materials . 2022;17(2):p. 022003. doi: 10.1088/1748-605X/ac50f6. [DOI] [PubMed] [Google Scholar]
- 52.Abd El-Razik K. A., Arafa A. A., Fouad E. A., Soror A. H., Abdalhamed A. M., Elgioushy M. Phenotypic and Genotypic Characterization of Erythromycin-Resistant Staphylococcus aureus Isolated From Bovine Subclinical Mastitis in Egypt. Veterinary World . 2023;16(7):1562–1571. doi: 10.14202/vetworld.2023.1562-1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ahmed A., Ijaz M., Khan J. A., Anjum A. A. Molecular Characterization and Therapeutic Insights Into Biofilm Positive Staphylococcus aureus Isolated From Bovine Subclinical Mastitis. Pakistan Veterinary Journal . 2021;42(4) [Google Scholar]
- 54.Kemal K. E., Tesfaye S., Ashanafi S., Muhammadhussien A. F. Prevalence, Risk Factors and Multidrug Resistance Profile of Staphylococcus aureus Isolated From Bovine Mastitis in Selected Dairy Farms in and Around Asella Town, Arsi Zone, South Eastern Ethiopia. African Journal of Microbiology Research . 2017;11(45):1632–1642. doi: 10.5897/AJMR2017.8529. [DOI] [Google Scholar]
- 55.Erskine R., Walker R., Bolin C., Bartlett P., White D. Trends in Antibacterial Susceptibility of Mastitis Pathogens During a 7-Year Period. Journal of Dairy Science . 2002;85(5):1111–1118. doi: 10.3168/jds.S0022-0302(02)74172-6. [DOI] [PubMed] [Google Scholar]
- 56.Abebe R., Hatiya H., Abera M., Megersa B., Asmare K. Bovine Mastitis: Prevalence, Risk Factors and Isolation of Staphylococcus aureus in Dairy Herds at Hawassa Milk Shed, South Ethiopia. BMC Veterinary Research . 2016;12:1–11. doi: 10.1186/s12917-016-0905-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kulkarni A. G., Kaliwal B. Bovine Mastitis: A Review. International Journal of Recent Scientific Research . 2013;4(5):543–548. [Google Scholar]
- 58.Mekibib B., Furgasa M., Abunna F., Megersa B., Regassa A. Bovine Mastitis: Prevalence, Risk Factors and Major Pathogens in Dairy Farms of Holeta Town, Central Ethiopia. Veterinary World. 2010;3(9):397–403. [Google Scholar]
- 59.Fesseha H., Mathewos M., Aliye S., Wolde A. Study on Prevalence of Bovine Mastitis and Associated Risk Factors in Dairy Farms of Modjo Town and Suburbs, Central Oromia, Ethiopia. Veterinary Medicine: Research and Reports . 2021;12:271–283. doi: 10.2147/VMRR.S323460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rahman M., Bhuiyan M., Kamal M., Shamsuddin M. Prevalence and Risk Factors of Mastitis in Dairy Cows. Bangladesh Veterinarian . 2009;26(2):54–60. doi: 10.3329/bvet.v26i2.4951. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.


