Abstract
Objective
Frontotemporal lobar degeneration with TDP-43 pathology (FTLD) usually presents with frontotemporal dementia, semantic aphasia or progressive nonfluent aphasia. Corticobasal syndrome and atypical parkinsonism have been occasionally reported, but progressive supranuclear palsy (PSP) syndrome (also known as Richardson syndrome (RS)) has not been reported in patients with FTLD-TDP. In this study we report clinical and pathologic characteristics of FTLD-TDP, clinically diagnosed as PSP syndrome (FTLD-TDP-PSP).
Methods
We reviewed clinical information of 270 patients with FTLD-TDP from the Mayo Clinic brain bank and identified 5 patients with FTLD-TDP-PSP. As a control group, we selected ten consecutive patients of pathologically confirmed PSP with clinical presentations of PSP syndrome (PSP-RS). We compared the clinical and pathological features of FTLD-TDP-PSP and PSP-RS.
Results
The most common clinical symptoms in FTLD-TDP-PSP were memory loss (100%) followed by parkinsonism (80%), early falls (60%), and behavioral variant FTD (60%). All patients with PSP-RS met the Movement Disorder Society’s criteria for probable PSP, while only one FTLD-TDP-PSP met the probable PSP. Two of the five patients with FTLD-TDP-PSP had moderate or severe neuronal loss in the substantia nigra and one had moderate or severe neuronal loss in the putamen and globus pallidus.
Conclusion
A small subset of patients with FTLD-TDP can, in rare instances, present with symptoms associated with PSP. Therefore, FTLD-TDP may be considered in differential diagnosis, especially in patients who do not meet the diagnostic criteria. Our findings emphasize the need for further clinical and biomarker studies of FTLD-TDP.
Supplementary Information
The online version contains supplementary material available at 10.1186/s40478-025-02058-0.
Keywords: Frontotemporal Lobar degeneration, TDP-43, Progressive supranuclear palsy
Introduction
Frontotemporal lobar degeneration with TDP-43 pathology (FTLD-TDP) is pathologically characterized by neuronal loss and gliosis in the frontal and temporal lobes as well as TDP-43 positive neuronal cytoplasmic inclusions (NCI), and variable dystrophic neurites (DN) and neuronal intranuclear inclusions [24]. It typically presents with frontotemporal dementia, semantic aphasia, logopenic progressive aphasia or frontotemporal dementia with or without motor neuron disease [15, 34]. Atypical parkinsonism [22] and corticobasal syndrome (CBS) have also been reported in patients with FTLD-TDP [18, 26]; however, patients presenting with the progressive supranuclear palsy (PSP) syndrome are uncommon in FTLD-TDP.
PSP often presents with postural instability, falls, vertical gaze palsy, and levodopa-unresponsive parkinsonism, and this syndrome has been referred to as Richardson syndrome (RS), recognizing that there are other clinical presentations of PSP, such as CBS and parkinsonism. The most common pathology in patients with PSP syndrome is PSP, but occasionally corticobasal degeneration can mimic PSP syndrome. In a previous study, we characterized clinical presentations of upper-motor-neuron-predominant motor neuron disease with or without FTLD-TDP, and we found that a subset of patients presented with PSP syndrome [30]. In such patients, it is thought that clinical features of motor neuron disease can mimic PSP syndrome. It remains unclear whether FTLD-TDP without motor neuron disease can also present with PSP syndrome. The present study aimed to determine the frequency of patients who were clinically diagnosed with PSP, although their underlying pathology was confirmed FTLD-TDP (FTLD-TDP-PSP) and to characterize their clinical and pathological features, focusing on neuronal loss and TDP-43 pathology. To characterize the clinical features of FTLD-TDP-PSP, we compared them to patients with PSP syndrome and pathologically confirmed PSP (PSP-RS).
Methods
Case selection
The present study included 270 patients with a pathological diagnosis of FTLD-TDP from the Mayo Clinic brain bank for neurodegenerative disorders accessioned between 2000 and 2022. Cases were identified by searching the brain bank database for entries with a pathological diagnosis of FTLD-TDP.
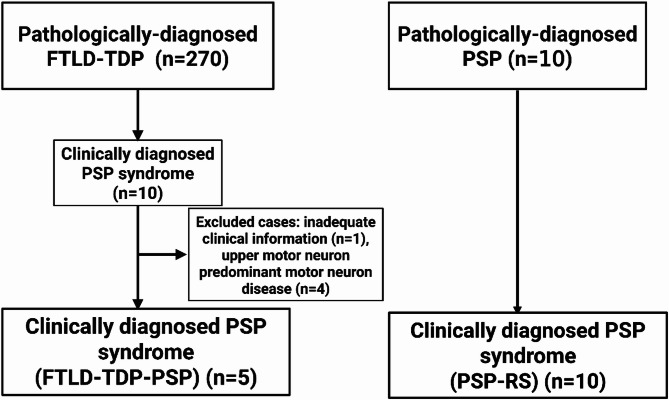
Upon reviewing medical records, ten of these patients had a final clinical diagnosis of PSP. Cases were excluded if they had a pathological diagnosis of upper-motor-neuron-predominant motor neuron disease (n = 4) or if they lacked adequate medical records (n = 1). The final cohort consisted of 5 patients with FTLD-TDP-PSP. As a control group, we selected 10 consecutive patients who had a clinical diagnosis of PSP and were pathologically confirmed as PSP; these cases were defined as PSP-RS. The study design is shown in Fig. 1.
Fig. 1.
Flow chart of study design. FTLD-TDP = frontotemporal lobar degeneration with TDP-43; FTLD-TDP-PSP = frontotemporal lobar degeneration with TDP-43, clinically diagnosed as progressive supranuclear palsy syndrome; PSP-RS = progressive supranuclear palsy with PSP syndrome; PSP = progressive supranuclear palsy; RS = Richardson syndrome
Clinical assessment
Two neurologists (A.M., S.K.) abstracted the following information from medical records collected throughout the course of the disease and entered the information into a database. The following variables were assessed: sex, age at symptomatic onset, age at death, family history of neurologic disease, clinical diagnoses, initial symptoms (i.e., motor dysfunction or cognitive dysfunction), signs and symptoms during the disease course and their timing, as well as neurologic findings as documented by a neurologist or movement disorder specialist. For each patient, a particular clinical symptom or sign was considered present if specifically noted in the medical records. If clinical symptoms or signs were not described, they were considered to be absent for the purpose of analysis. The following symptoms and neurologic signs were abstracted from medical records: parkinsonism, bradykinesia, axial/limb rigidity, orobuccal or limb apraxia, myoclonus, dystonia, aphasia, falls, early falls (defined as occurring within 3 years of symptomatic onset), ocular motor dysfunction, and memory loss, pseudobulbar affect, and features of behavioral variant frontotemporal dementia (bvFTD), including behavioral disinhibition, apathy or inertia, loss of sympathy or empathy, perseverative or compulsive/ritualistic behavior, and hyperorality or dietary changes. Response to levodopa therapy was recorded as good or no good. The Movement Disorder Society’s criteria for PSP [12] were used for the diagnosis of FTLD-TDP-PSP and PSP-RS. When available the neuropsychological profile was reviewed and data from brain MRI or CT were recorded. The clinical information was obtained from medical records, pathology records summarizing the clinical history and the CurePSP brain bank questionnaire filled out by the family or someone with close knowledge of the patient [25]. Given the retrospective nature of the study, the quality of available medical records was variable from case to case.
Neuropathologic assessment
Most of the brains were received with the left hemibrain fixed in 10% formalin; the right hemibrain frozen at -80 °C. All cases underwent systematic and standardized sampling with neuropathologic evaluation by an experienced neuropathologist (DWD). Paraffin-embedded 5-µm thick sections mounted on glass slides were stained with hematoxylin and eosin (H&E) and thioflavin S. H&E-stained sections from the substantia nigra, corticobulbar tract in the cerebral peduncle, hippocampus with parahippocampal gyrus, subthalamic nucleus, putamen, globus pallidus, amygdala, nucleus basalis of Meynert, superior frontal gyrus, middle frontal gyrus, inferior parietal gyrus, superior temporal gyrus, occipital temporal gyrus, and motor cortex were reviewed by three observers (A.M., S.K., D.W.D.) to assess neuronal loss on four-point semiquantitative scores (0 = absent; 1 = mild; 2 = moderate; 3 = severe) as previously reported [17, 30].
To confirm the neuropathological diagnosis of FTLD-TDP, immunohistochemistry for phospho-TDP43 (pTDP-43) (pS409/410, mouse monoclonal, 1:5000, Cosmo Bio, Tokyo, Japan) or TDP-43 (MC2085, 1:3000, gift from Dr. Leonard Petrucelli, Mayo Clinic, Jacksonville, Florida) was performed on sections of mid-frontal cortex, superior and middle temporal cortex, parietal cortex, anterior cingulate gyrus, amygdala, striatum and globus pallidus, hippocampus with parahippocampal gyrus, and midbrain including substantia nigra. To exclude coexisting tauopathies, such as PSP, corticobasal degeneration [7, 11, 23], immunohistochemistry for phospho-tau (CP13, Ser202, mouse monoclonal, 1:1000, from the late Dr Peter Davies, Feinstein Institute, North Shore Hospital, NY) was performed on sections of mid-frontal cortex, superior and middle temporal cortex, parietal cortex, anterior cingulate gyrus, amygdala, striatum and globus pallidus, hippocampus with parahippocampal gyrus, subthalamic nucleus, midbrain, pons, and cerebellum.
Braak neurofibrillary stage and Thal amyloid phase were assigned based upon lesion density and distribution with thioflavin S fluorescent microscopy according to published criteria [3, 21, 37]. Alzheimer’s type neuropathologic change was based on the consensus criteria for the neuropathologic diagnosis of Alzheimer’s disease [13]. Lewy-related pathology was assessed by α-synuclein immunohistochemistry (NACP, rabbit polyclonal, 1:3000; Mayo Clinic, Jacksonville, Florida) [28] in the amygdala, basal forebrain, brainstem, cingulate gyrus, and temporal lobe, and classified as the brainstem, limbic and diffuse types. Neuronal inclusions were detected using p62/sequestosome-1 immunohistochemistry (p62 lck ligand, mouse monoclonal, 1:250, BD Transduction, Franklin Lakes, New Jersey) in cerebellum [1], which have been described in subjects with C9ORF72 hexanucleotide repeats.
Assessment of TDP-43 pathology
All slides were reviewed simultaneously by two observers (A.M., S.K.) who agreed on the presence of TDP-43 immunoreactivity, defined as NCI, DN, glial cytoplasmic inclusion, or neuronal intranuclear inclusions, in the midbrain, basal ganglia, amygdala, hippocampus, superior temporal cortex, middle frontal cortex, and motor cortex. The severity of TDP-43 pathology was graded semi-quantitatively on a five-point scale (0 = absent, 1 = rarely, 2 = mild, 3 = moderate, 4 = severe) as previously reported [29].
Assigning FTLD-TDP subtype
Each case was classified into an FTLD-TDP subtype based on the criteria [24]. Type A is characterized by abundant NCI and short DN in neocortical layer II and the presence of lentiform neuronal intranuclear inclusions. Type B is characterized by abundant NCI and few DN in all cortical layers. Type C is characterized by abundant long DN and few NCI in all cortical layers. Cases were assigned Type A, Type B or Type C.
Statistical methods
All statistical analyses were performed using R version 4.0.5 (The R Foundation for Statistical Computing, Vienna, Austria) and EZR (Saitama Medical Center, Jichi Medical University Saitama, Japan), which is a graphical interface for R [16]. A Fisher’s exact test was performed for group comparisons of categorical data, as appropriate. Analysis of t-test or Mann–Whitney U test was used for analyses of continuous variables, as appropriate. p-values < 0.05 were considered statistically significant. Hierarchical cluster analysis using Euclidean distance and average linkage clustering was performed on patients and region-specific variables reflecting the severity of the neuronal loss or TDP-43 pathology as previously reported [30].
Results
Demographic and pathological features
Table 1 summarizes the demographic and pathologic features of FTLD-TDP-PSP and PSP-RS patients. The average age at onset in FTLD-TDP-PSP patients was 66 ± 4 years ranging from 61 to 70, and the average disease duration was 8.2 ± 6.5 years ranging from 1.3 years to 17.7 years. Family history of amyotrophic lateral sclerosis was noted in 40% of FTLD-TDP-PSP patients, and one patient had a family history of dementia. There were no significant differences in the proportion of male/female, age at onset, disease duration to death between FTLD-TDP-PSP and PSP-RS. Pathologic features, including brain weight, Braak neurofibrillary tangle stage, Thal amyloid phase, and Lewy-related pathology did not differ between the two groups. One patient had C9ORF72 repeat expansion based upon p62 immunohistochemistry in the cerebellum.
Table 1.
Demographic and pathological features of FTLD-TDP-PSP and PSP-RS
| Clinical features | FTLD-TDP-PSP | PSP-RS | P-value |
|---|---|---|---|
| Number | 5 | 10 | |
| Male | 40% (2) | 40% (4) | |
| Age at onset, years | 66 ± 4 | 67 ± 8 | 0.77 |
| Age at death, years | 74 ± 5 | 73 ± 8 | 0.89 |
| Disease duration, years | 8.2 ± 6.5 | 6.7 ± 2 | 0.49 |
| Pathological features | |||
| Brain weight, grams | 934 ± 222 | 1101 ± 202 | 0.17 |
| Braak stage | II (II, II) | III (II, III) | 0.70 |
| Thal phase | 1 (0, 3) | 1 (0, 1) | 0.60 |
| Hippocampal sclerosis | 40% (2) | 0% (0) | 0.08 |
| Lewy-related pathology | 0% (0) | 10% (1) | 1.00 |
FTLD-TDP-PSP = frontotemporal lobar degeneration with TDP-43, clinically diagnosed as progressive supranuclear palsy syndrome; PSP-RS = progressive supranuclear palsy presenting with progressive supranuclear palsy syndrome; Data are displayed as n (%), mean ± SD, and median (25th, 75th range)
Clinical features of FTLD-TDP-PSP and comparing FTLD-TDP-PSP versus PSP-RS
Supplemental Table lists the frequency of clinical features in FTLD-TDP-PSP and PSP-RS. The most common clinical symptoms were parkinsonism (80%) and memory loss (80%), followed by early falls (60%), and bvFTD symptoms (60%). No patients with FTLD-TDP-PSP met criteria for bvFTD [32]. Among those with parkinsonism, 40% showed asymmetrical symptoms. Two patients were prescribed antidepressants after the onset of parkinsonism and clinical diagnosis of PSP, suggesting that drug-induced parkinsonism was unlikely.
We next compared the clinical features of FTLD-TDP-PSP with PSP-RS (Table 2). Eight patients with PSP-RS met the Movement Disorder Society criteria for probable PSP with Richardson’s syndrome and two patients met criteria for PS with predominant parkinsonism, while only one FTLD-TDP-PSP patient met criteria for probable PSP with Richardson’s syndrome. Two FTLD-TDP-PSP met criteria for suggestive PSP with predominant postural instability. Of note, all PSP-RS patients had eye movement dysfunction defined by the Movement Disorder Society’s criteria for PSP (i.e., vertical supranuclear gaze palsy and slow velocity of vertical saccades), but only 2 of 5 FTLD-TDP-PSP patients had this sign, one of whom had a C9ORF72 repeat expansion. Other features, such as the frequency of parkinsonism, early falls, bvFTD, aphasia, L-dopa responsiveness, memory loss, and apraxia, did not significantly differ between the two groups.
Table 2.
Clinical features in FTLD-TDP-PSP vs. PSP-RS
| FTLD-TDP-PSP | PSP-RS | P-value | |
|---|---|---|---|
| Parkinsonism | 80% (4) | 100% (10) | 0.33 |
| Early falls | 60% (3) | 80% (8) | 0.56 |
| Oculomotor Dysfunction | 40% (2) | 100% (10) | 0.02 |
| bvFTD | 60% (3) | 30% (3) | 0.33 |
| Aphasia | 40% (2) | 30% (3) | 1 |
| L-dopa responsiveness | 25% (1/4) | 80% (8) | 0.09 |
| Memory loss | 80% (4) | 80% (8) | 1 |
| Apraxia | 0% (0) | 20% (2) | 1 |
| MDS-PSP criteria | < 0.01 | ||
| Probable PSP | 20% (1) | 100% (10) | |
| Possible PSP | 0% (0) | 0% (0) | |
| Suggestive PSP | 40% (2) | 0% (0) | |
| Neuroimaging | n = 5 | n = 8 | |
| Midbrain atrophy | 20% (1) | 50% (4) | 0.56 |
| Frontotemporal atrophy | 40% (2) | 12.5% (1) | 0.51 |
| Duration final imaging study from death, months | 68 ± 19 | 40 ± 18 | 0.52 |
bvFTD = behavioral variant frontotemporal dementia; FTLD-TDP-PSP = frontotemporal lobar degeneration with TDP-43, clinically diagnosed as progressive supranuclear palsy syndrome; PSP-RS = progressive supranuclear palsy presenting with progressive supranuclear palsy syndrome
Imaging was performed using MRI in all FTLD-TDP-PSP cases, and in the PSP-RS group, 8 cases underwent MRI and 2 underwent CT. Data are displayed as n (%), mean ± SD, and median (25th, 75th range)
Neuropathological characteristics in FTLD-TDP-PSP
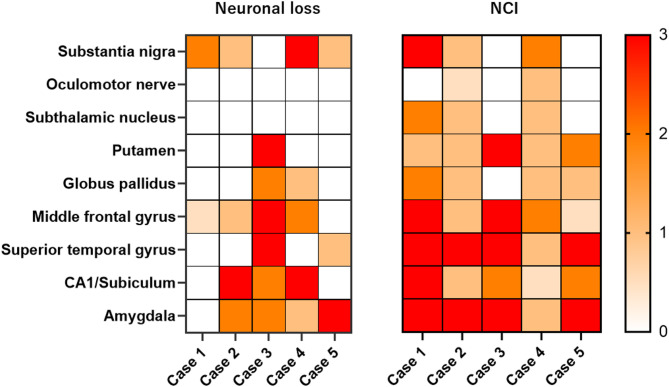
We examined the distribution of neuronal loss in eight brain regions of FTLD-TDP-PSP (Fig. 2). As shown in the heatmap, four patients had neuronal loss in the substantia nigra (Cases 1, 2, 4, and 5), and two patients (Cases 1 and 4) had moderate or severe neuronal loss. The remaining one patient (Case 3) did not have neuronal loss in the substantia nigra, but did have severe neuronal loss in the putamen and a moderate neuronal loss in the globus pallidus. No FTLD-TDP-PSP had significant neuronal loss in the subthalamic nucleus, where most PSP-RS patients had moderate to severe neuronal loss.
Fig. 2.
Heatmap based on neuronal loss and neuronal cytoplasmic inclusions in 5 cases of FTLD-TDP-PSP. The heatmap reflects the severity of neuronal loss and density of neuronal cytoplasmic inclusions in each brain region. The color scale is on the right. According to the Movement Disorder Society progressive supranuclear palsy (PSP) diagnostic criteria, Case 1 fulfilled the criteria for probable PSP, Cases 2 and 3 for suggestive PSP, and Cases 4 and 5 did not meet the diagnostic criteria. NCI = TDP-43-positive neuronal cytoplasmic inclusions
The severity of TDP-43 pathology in eight brain regions is summarized in Fig. 2. Two patients (Cases 1 and 4) had more than moderate TDP-43 pathology in the substantia nigra. Three patients had NCI in the subthalamic nucleus (Cases 1, 2 and 4). All cases had NCI in the putamen. The FTLD-TDP subtypes of the cases included 3 cases (Cases 1, 2, and 4) with Type A, who all had NCIs, and 2 cases (Cases 3 and 5) with Type B. Representative macroscopic and histopathologic images of FTLD-TDP-PSP cases are shown in Fig. 3. Detailed neuropathological findings for each FTLD-TDP-PSP case are provided in Supplementary Data.
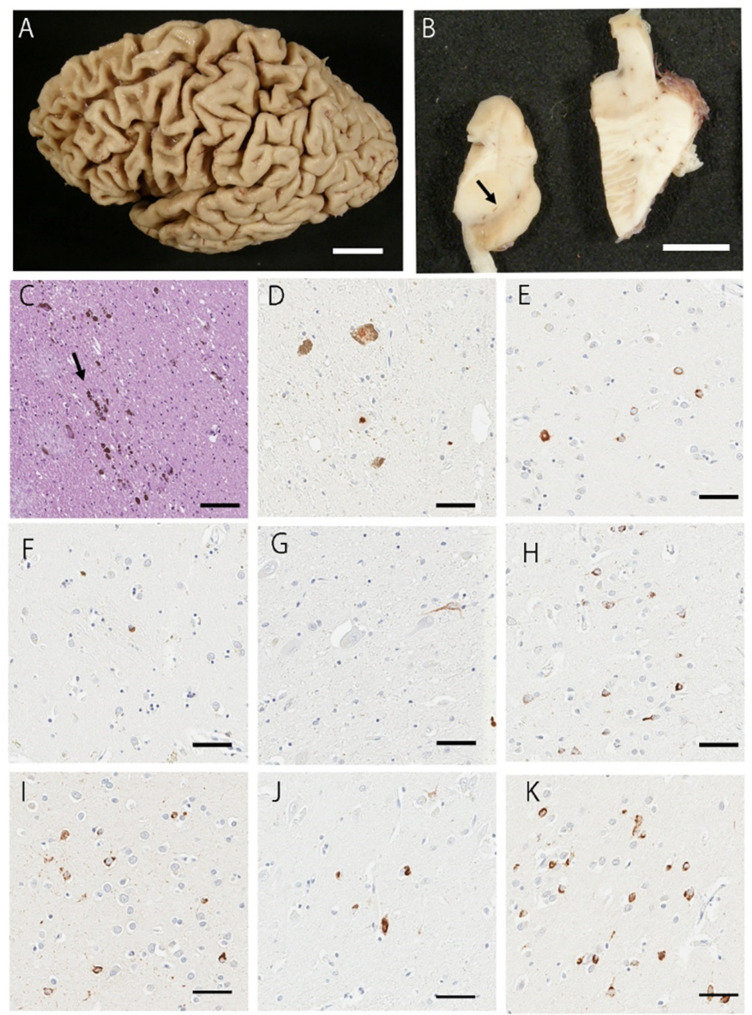
Fig. 3.
Representative macroscopic and histopathologic images. (A) The brain shows frontotemporal atrophy. (B) The substantia nigra has reduced pigment (arrow). (C) The H&E staining of the substantia nigra shows a moderate neuronal loss. Free melanin is observed (arrow). (D-K) pTDP43-positive NCIs and glial cytoplasmic inclusions are observed in the substantia nigra (D), globus pallidus (E), putamen (F), subthalamic nucleus (G), middle frontal gyrus (H), superior temporal gyrus (I), subiculum (J), and amygdala (K). B: H&E staining; D-K: pTDP43 immunostaining. Scale bars: A = 3 cm; B = 1 cm; C-K = 50 μm
Discussion
The aim of this study was to identify patients with FTLD-TDP-PSP and characterize their clinicopathological features. In our brain bank, only 5 of 270 patients (2%) with FTLD-TDP had a final clinical diagnosis of PSP, consistent with FTLD-TDP-PSP, without coexisting primary tauopathies. A review of clinical features revealed that vertical gaze palsy was uncommon in patients with FTLD-TDP-PSP, with only one of the five meeting the rigorous diagnostic criteria for probable or possible PSP. Neuropathological evaluation revealed that three patients had moderate to severe neuronal loss in the substantia nigra or putamen, which may represent the neuropathologic correlate of parkinsonism. All three also had TDP-43 pathology in the substantia nigra. These findings suggest that although rare, FTLD-TDP can present with symptoms associated with PSP and is often associated with neuronal loss and TDP-43 pathology in the striatonigral system.
The findings of this study expand the understanding of the clinical presentations of FTLD-TDP by identifying FTLD-TDP-PSP as a novel clinical phenotype. TDP-43 pathology has been implicated as an underlying pathology of PSP syndrome, particularly in studies on C9ORF72 repeat expansions. A review of six different genetic studies that evaluated C9ORF72 repeat expansions in patients with PSP syndrome [2, 10, 19, 20, 31, 35, 40] identified three FTLD-TDP-PSP cases with abnormal C9ORF72 repeat expansions among 240 patients. All three showed parkinsonism and eye movement dysfunction, including vertical supranuclear palsy or gaze palsy, features suggestive of RS. Additionally, two patients had frontal lobe syndrome. Imaging studies revealed midbrain atrophy in one patient and frontotemporal lobar atrophy in another. Similarly, in the present study, the FTLD-TDP-PSP patient with a C9ORF72 repeat expansion exhibited downward gaze palsy and square wave jerks. Although the genetic findings strongly suggest an association with TDP-43 pathology, autopsy confirmation was not available in these patients. There have also been reports of patients with non-pathogenic progranulin variants presenting with PSP [6, 27]. While TDP-43 pathology was suspected as a possible underlying pathology in these cases, autopsy was not performed, and it remains unclear whether the underlying pathology was tau or TDP-43. Given that PSP pathology coexisting with TDP-43 pathology has been reported [33], the possibility of concomitant tau pathology in these patients cannot be excluded. The present study is the first to demonstrate that patients with FTLD-TDP can present with PSP syndrome.
Comparison of clinical features between FTLD-TDP-PSP and PSP-RS provided some clues that may help distinguish between the two. One of the key differences was the frequency of vertical gaze palsy, which was lower in FTLD-TDP-PSP than PSP-RS. Only one FTLD-TDP-PSP patient met criteria for probable PSP, while all ten PSP-RS patients met the criteria for probable PSP. Regarding imaging findings, midbrain atrophy was noted in only 20% of FTLD-TDP-PSP patients compared to 50% of PSP-RS patients. In contrast, frontotemporal atrophy was more frequent in FTLD-TDP-PSP than PSP-RS. Although these findings were not statistically significant due to the small sample size, these imaging features might be helpful in differentiating FTLD-TDP-PSP from PSP-RS.
The neuropathologic substrate of PSP syndrome in FTLD-TDP remains unclear. The substantia nigra has been the most investigated brain region that is associated with parkinsonism. More than moderate neuronal loss in the substantia nigra and the putamen is associated with parkinsonism [8]. In FTLD-TDP, patients presenting with parkinsonism have also been found to have neuronal loss of the substantia nigra [36, 39]. Given that the putamen plays a key role in both the direct and indirect pathways of the basal ganglia, this lesion may contribute to parkinsonism [4]. In our study, three out of five FTLD-TDP-PSP patients had moderate or severe neuronal loss in the substantia nigra or putamen. Interestingly, a recent study demonstrated that a subset of patients with FTLD-TDP have parkinsonism but lack significant neuronal loss in the substantia nigra. Instead, the severity of TDP-43 pathology in the substantia nigra correlated with parkinsonism [9]. We found TDP-43-positive NCI in the substantia nigra in 3 of 5 cases of FTLD-TDP-PSP. Nevertheless, the pathological substrate of parkinsonism in one of the cases (Case 5) remains unknown, since there was neither neuronal loss nor TDP-43 pathology in the substantia nigra. Taken together, both neuronal loss and TDP-43 pathology in the substantia nigra may contribute to parkinsonism. Vertical gaze palsy is one of the core features of RS, and is a key differential feature for FTLD-TDP-PSP and PSP-RS; however, the pathological correlates of this sign were not identified. Although the brain regions responsible for vertical gaze palsy are not fully understood, no significant neuronal loss or TDP-43 pathology was observed in the tegmentum or subthalamic nucleus—regions typically affected in PSP-RS and considered to be involved in oculomotor dysfunction [5, 14, 38]—in our FTLD-TDP-PSP cases. This may partly explain the lower frequency of oculomotor symptoms in FTLD-TDP-PSP cases.
There are several limitations to the present study. First, we were unable to assess the laterality of pathology because only one hemi-brain was available for the pathologic assessment in all but one case. Second, the retrospective nature of this brain bank study limited the availability of clinical information in some cases. To better evaluate potential correlation between neuronal loss, TDP-43 pathology, and clinical features associated with antemortem diagnoses, it will be important to evaluate patients who undergo autopsy as part of prospective clinical studies. Third, the small sample size limited our ability to identify significant differences in clinicopathologic features between clusters. A larger cohort may help define distinct clinicopathologic subtypes of FTLD-TDP-PSP.
In conclusion, the present study demonstrates that FTLD-TDP pathology can present with symptoms resembling PSP. Therefore, FTLD-TDP should be considered in the differential diagnosis of PSP syndrome, particularly in patients who do not fulfill the criteria for clinically probable PSP syndrome. Our findings expand the clinical spectrum of FTLD-TDP and emphasize the need for further clinical, biomarker, and genetic studies of FTLD-TDP-PSP.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
We would like to thank the patients and their families who donated brains to help further the scientific understanding of neurodegeneration. The authors would also like to acknowledge Virginia Phillips, for histologic support, Monica Castanedes-Casey (Mayo Clinic, Jacksonville) for immunohistochemistry support. Rachel LaPaille-Harwood provides valuable outreach to families wishing to donate brains to the Mayo Clinic brain bank.
Author contributions
Aya Murakami: Drafting/revision of the article for content, including medical writing for content; major role in the acquisition of data; study concept or design; and analysis or interpretation of data, Shunsuke Koga: Drafting/revision of the article for content, including medical writing for content; major role in the acquisition of data; study concept or design; and analysis or interpretation of data, Hiroaki Sekiya: Major role in the acquisition of data; editing of the manuscript, Masataka Nakamura: Editing of the manuscript, Yusuke Yakushiji: Editing of the manuscript, Dennis W. Dickson: Drafting/revision of the article for content, including medical writing for content; editing of the manuscript; major role in the acquisition of data; study concept or design; and analysis or interpretation of data.
Funding
This study was supported in part by The Rainwater Charitable Foundation, the Albertson Parkinson’s Research Foundation, and the Spastic Paraplegia Foundation, Inc., as well as NIH grants P30-AG062677, U54-NS100693, P01-AG03949, R01-AG062348, 1U19AG063911, RF1-NS123052, R01-NS121125 and FAIN: U19AG063911. Fellowships from the Japanese Society of Neurology, the Yukihiko Miyata Memorial Trust for ALS Research, the Katano Foundation, and the Research Foundation for Dementia of Osaka provided support for AM. The authors declare that there are no conflicts of interest relevant to this work.
Data availability
Data that support the findings of this study are available from the corresponding author upon request.
Declarations
Ethics approval and consent to participate
Brain autopsies were obtained after consent of the legal next-of-kin and studies of this type are considered exempt from human subject research. The Mayo Clinic brain bank operates under protocols approved by the Mayo Clinic Institutional Review Board.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Financial disclosures of all authors for the preceding 12 months
AM received speaker fees (royalties) from Chugai Pharmaceutical Co., Ltd. And supported by a JSPS KAKENHI Grant-in-Aid for Early-Career Scientists #23K14764. YY received speaker fees (royalties) from the following companies: Daiichi Sankyo Company, Limited; Otsuka Pharmaceutical Co., Ltd.; Eisai Co., Ltd.; Chugai Pharmaceutical Co., Ltd.; Takeda Pharmaceutical Co., Ltd.; Kyowa Kirin Co., Ltd.; Alexion Pharmaceuticals, Inc.; EA Pharma Co., Ltd.; Ono Pharmaceutical Co., Ltd.; Kowa Co., Ltd.; Novartis Pharma K.K.; UCB Japan Co., Ltd.; and Eli Lilly Japan K.K. and supported by a JSPS KAKENHI Grant-in-Aid for Scientific Research (C) #24K13494. SK, HS, and DWD have no financial disclosures to declare.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Shunsuke Koga, Email: shunsuke.koga@pennmedicine.upenn.edu.
Dennis W. Dickson, Email: dickson.dennis@mayo.edu
References
- 1.Al-Sarraj S, King A, Troakes C, Smith B, Maekawa S, Bodi I, Rogelj B, Al-Chalabi A, Hortobágyi T, Shaw CE (2011) p62 positive, TDP-43 negative, neuronal cytoplasmic and intranuclear inclusions in the cerebellum and hippocampus define the pathology of C9orf72-linked FTLD and MND/ALS. Acta Neuropathol 122:691–702. 10.1007/s00401-011-0911-2 [DOI] [PubMed] [Google Scholar]
- 2.Bourinaris T, Houlden H (2018) C9orf72 and its relevance in parkinsonism and movement disorders: A comprehensive review of the literature. Mov Disord Clin Pract 5:575–585. 10.1002/mdc3.12677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Braak H, Braak E (1991) Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol 82:239–259. 10.1007/bf00308809 [DOI] [PubMed] [Google Scholar]
- 4.Calabresi P, Picconi B, Tozzi A, Ghiglieri V, Di Filippo M (2014) Direct and indirect pathways of basal ganglia: a critical reappraisal. Nat Neurosci 17:1022–1030. 10.1038/nn.3743 [DOI] [PubMed] [Google Scholar]
- 5.Chen AL, Riley DE, King SA, Joshi AC, Serra A, Liao K, Cohen ML, Otero-Millan J, Martinez-Conde S, Strupp M, Leigh RJ (2010) The disturbance of gaze in progressive supranuclear palsy: implications for pathogenesis. Front Neurol 1:147. 10.3389/fneur.2010.00147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Deng B, Zheng Z, Zheng J, Yang W, Huang Y, Luo Y, Jin D, Shen L, Jin K, Wang Q (2021) FTD-PSP is an unusual clinical phenotype in A frontotemporal dementia patient with A novel progranulin mutation. Aging Dis 12:1741–1752. 10.14336/ad.2021.0309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dickson DW, Bergeron C, Chin SS, Duyckaerts C, Horoupian D, Ikeda K, Jellinger K, Lantos PL, Lippa CF, Mirra SS, Tabaton M, Vonsattel JP, Wakabayashi K, Litvan I (2002) Office of rare diseases neuropathologic criteria for corticobasal degeneration. J Neuropathol Exp Neurol 61:935–946. 10.1093/jnen/61.11.935 [DOI] [PubMed] [Google Scholar]
- 8.Dickson DW, Braak H, Duda JE, Duyckaerts C, Gasser T, Halliday GM, Hardy J, Leverenz JB, Del Tredici K, Wszolek ZK, Litvan I (2009) Neuropathological assessment of parkinson’s disease: refining the diagnostic criteria. Lancet Neurol 8:1150–1157. 10.1016/s1474-4422(09)70238-8 [DOI] [PubMed] [Google Scholar]
- 9.Fiondella L, Gami-Patel P, Blok CA, Rozemuller AJM, Hoozemans JJM, Pijnenburg YAL, Scarioni M, Dijkstra AA (2023) Movement disorders are linked to TDP-43 burden in the substantia Nigra of FTLD-TDP brain donors. Acta Neuropathol Commun 11:63. 10.1186/s40478-023-01560-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Galimberti D, Fenoglio C, Serpente M, Villa C, Bonsi R, Arighi A, Fumagalli GG, Del Bo R, Bruni AC, Anfossi M, Clodomiro A, Cupidi C, Nacmias B, Sorbi S, Piaceri I, Bagnoli S, Bessi V, Marcone A, Cerami C, Cappa SF, Filippi M, Agosta F, Magnani G, Comi G, Franceschi M, Rainero I, Giordana MT, Rubino E, Ferrero P, Rogaeva E, Xi Z, Confaloni A, Piscopo P, Bruno G, Talarico G, Cagnin A, Clerici F, Dell’Osso B, Comi GP, Altamura AC, Mariani C, Scarpini E (2013) Autosomal dominant frontotemporal Lobar degeneration due to the C9ORF72 hexanucleotide repeat expansion: late-onset psychotic clinical presentation. Biol Psychiatry 74:384–391. 10.1016/j.biopsych.2013.01.031 [DOI] [PubMed] [Google Scholar]
- 11.Hauw JJ, Daniel SE, Dickson D, Horoupian DS, Jellinger K, Lantos PL, McKee A, Tabaton M, Litvan I (1994) Preliminary NINDS neuropathologic criteria for Steele-Richardson-Olszewski syndrome (progressive supranuclear palsy). Neurology 44:2015–2019. 10.1212/wnl.44.11.2015 [DOI] [PubMed] [Google Scholar]
- 12.Höglinger GU, Respondek G, Stamelou M, Kurz C, Josephs KA, Lang AE, Mollenhauer B, Müller U, Nilsson C, Whitwell JL, Arzberger T, Englund E, Gelpi E, Giese A, Irwin DJ, Meissner WG, Pantelyat A, Rajput A, van Swieten JC, Troakes C, Antonini A, Bhatia KP, Bordelon Y, Compta Y, Corvol JC, Colosimo C, Dickson DW, Dodel R, Ferguson L, Grossman M, Kassubek J, Krismer F, Levin J, Lorenzl S, Morris HR, Nestor P, Oertel WH, Poewe W, Rabinovici G, Rowe JB, Schellenberg GD, Seppi K, van Eimeren T, Wenning GK, Boxer AL, Golbe LI, Litvan I (2017) Clinical diagnosis of progressive supranuclear palsy: the movement disorder society criteria. Mov Disord 32:853–864. 10.1002/mds.26987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hyman BT, Phelps CH, Beach TG, Bigio EH, Cairns NJ, Carrillo MC, Dickson DW, Duyckaerts C, Frosch MP, Masliah E, Mirra SS, Nelson PT, Schneider JA, Thal DR, Thies B, Trojanowski JQ, Vinters HV, Montine TJ (2012) National Institute on Aging-Alzheimer’s association guidelines for the neuropathologic assessment of alzheimer’s disease. Alzheimers Dement 8:1–13. 10.1016/j.jalz.2011.10.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jantz JJ, Watanabe M, Levy R, Munoz DP (2017) Evidence for a task-dependent switch in subthalamo-nigral basal ganglia signaling. Nat Commun 8:1039. 10.1038/s41467-017-01023-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Josephs KA, Hodges JR, Snowden JS, Mackenzie IR, Neumann M, Mann DM, Dickson DW (2011) Neuropathological background of phenotypical variability in frontotemporal dementia. Acta Neuropathol 122:137–153. 10.1007/s00401-011-0839-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kanda Y (2013) Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transpl 48:452–458. 10.1038/bmt.2012.244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Koga S, Sanchez-Contreras M, Josephs KA, Uitti RJ, Graff-Radford N, van Gerpen JA, Cheshire WP, Wszolek ZK, Rademakers R, Dickson DW (2017) Distribution and characteristics of transactive response DNA binding protein 43 kda pathology in progressive supranuclear palsy. Mov Disord 32:246–255. 10.1002/mds.26809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Koga S, Josephs KA, Aiba I, Yoshida M, Dickson DW (2022) Neuropathology and emerging biomarkers in corticobasal syndrome. J Neurol Neurosurg Psychiatry 93:919–929. 10.1136/jnnp-2021-328586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Le Ber I, Camuzat A, Guillot-Noel L, Hannequin D, Lacomblez L, Golfier V, Puel M, Martinaud O, Deramecourt V, Rivaud-Pechoux S, Millecamps S, Vercelletto M, Couratier P, Sellal F, Pasquier F, Salachas F, Thomas-Antérion C, Didic M, Pariente J, Seilhean D, Ruberg M, Wargon I, Blanc F, Camu W, Michel BF, Berger E, Sauvée M, Thauvin-Robinet C, Mondon K, Tournier-Lasserve E, Goizet C, Fleury M, Viennet G, Verpillat P, Meininger V, Duyckaerts C, Dubois B, Brice A (2013) C9ORF72 repeat expansions in the frontotemporal dementias spectrum of diseases: a flow-chart for genetic testing. J Alzheimers Dis 34:485–499. 10.3233/jad-121456 [DOI] [PubMed] [Google Scholar]
- 20.Lesage S, Le Ber I, Condroyer C, Broussolle E, Gabelle A, Thobois S, Pasquier F, Mondon K, Dion PA, Rochefort D, Rouleau GA, Dürr A, Brice A (2013) C9orf72 repeat expansions are a rare genetic cause of parkinsonism. Brain 136:385–391. 10.1093/brain/aws357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liesinger AM, Graff-Radford NR, Duara R, Carter RE, Hanna Al-Shaikh FS, Koga S, Hinkle KM, DiLello SK, Johnson MF, Aziz A, Ertekin-Taner N, Ross OA, Dickson DW, Murray ME (2018) Sex and age interact to determine clinicopathologic differences in alzheimer’s disease. Acta Neuropathol 136:873–885. 10.1007/s00401-018-1908-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lindquist SG, Duno M, Batbayli M, Puschmann A, Braendgaard H, Mardosiene S, Svenstrup K, Pinborg LH, Vestergaard K, Hjermind LE, Stokholm J, Andersen BB, Johannsen P, Nielsen JE (2013) Corticobasal and ataxia syndromes widen the spectrum of C9ORF72 hexanucleotide expansion disease. Clin Genet 83:279–283. 10.1111/j.1399-0004.2012.01903.x [DOI] [PubMed] [Google Scholar]
- 23.Litvan I, Hauw JJ, Bartko JJ, Lantos PL, Daniel SE, Horoupian DS, McKee A, Dickson D, Bancher C, Tabaton M, Jellinger K, Anderson DW (1996) Validity and reliability of the preliminary NINDS neuropathologic criteria for progressive supranuclear palsy and related disorders. J Neuropathol Exp Neurol 55:97–105. 10.1097/00005072-199601000-00010 [DOI] [PubMed] [Google Scholar]
- 24.Mackenzie IR, Neumann M, Baborie A, Sampathu DM, Du Plessis D, Jaros E, Perry RH, Trojanowski JQ, Mann DM, Lee VM (2011) A harmonized classification system for FTLD-TDP pathology. Acta Neuropathol 122:111–113. 10.1007/s00401-011-0845-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Martin NB, Sekiya H, Kim M, Dickson DW, Koga S (2023) Brain bank questionnaire helps in differential diagnosis of movement disorders: an autopsy study of 150 patients. Mov Disord Clin Pract 10:1131–1135. 10.1002/mdc3.13788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Masellis M, Momeni P, Meschino W, Heffner R Jr., Elder J, Sato C, Liang Y, St George-Hyslop P, Hardy J, Bilbao J, Black S, Rogaeva E (2006) Novel splicing mutation in the progranulin gene causing Familial corticobasal syndrome. Brain 129:3115–3123. 10.1093/brain/awl276 [DOI] [PubMed] [Google Scholar]
- 27.McAnnis KE, Mbydeen TH, Jonnakuti VS, Davila PA, Laylani NA, Jurkute N, Ondo WG, Lee AG (2024) Candidate GRN variant in patient with progressive supranuclear palsy. J Neuroophthalmol. 10.1097/wno.0000000000002192 [DOI] [PubMed] [Google Scholar]
- 28.McKeith IG, Boeve BF, Dickson DW, Halliday G, Taylor JP, Weintraub D, Aarsland D, Galvin J, Attems J, Ballard CG, Bayston A, Beach TG, Blanc F, Bohnen N, Bonanni L, Bras J, Brundin P, Burn D, Chen-Plotkin A, Duda JE, El-Agnaf O, Feldman H, Ferman TJ, Ffytche D, Fujishiro H, Galasko D, Goldman JG, Gomperts SN, Graff-Radford NR, Honig LS, Iranzo A, Kantarci K, Kaufer D, Kukull W, Lee VMY, Leverenz JB, Lewis S, Lippa C, Lunde A, Masellis M, Masliah E, McLean P, Mollenhauer B, Montine TJ, Moreno E, Mori E, Murray M, O’Brien JT, Orimo S, Postuma RB, Ramaswamy S, Ross OA, Salmon DP, Singleton A, Taylor A, Thomas A, Tiraboschi P, Toledo JB, Trojanowski JQ, Tsuang D, Walker Z, Yamada M, Kosaka K (2017) Diagnosis and management of dementia with lewy bodies: fourth consensus report of the DLB consortium. Neurology 89:88–100. 10.1212/wnl.0000000000004058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Murakami A, Koga S, Sekiya H, Oskarsson B, Boylan K, Petrucelli L, Josephs KA, Dickson DW (2022) Old age amyotrophic lateral sclerosis and limbic TDP-43 pathology. Brain Pathol 32:e13100. 10.1111/bpa.13100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Murakami A, Koga S, Fujioka S, White AE, Bieniek KF, Sekiya H, DeJesus-Hernandez M, Finch NA, van Blitterswijk M, Nakamura M, Tsuboi Y, Murray ME, Wszolek ZK, Dickson DW (2024) Upper motor neuron-predominant motor neuron disease presenting as atypical parkinsonism: A clinicopathological study. Brain Pathol:e13286. 10.1111/bpa.13286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Origone P, Verdiani S, Ciotti P, Gulli R, Bellone E, Marchese R, Abbruzzese G, Mandich P (2013) Enlarging the clinical spectrum associated with C9orf 72 repeat expansions: findings in an Italian cohort of patients with parkinsonian syndromes and relevance for genetic counselling. Amyotroph Lateral Scler Frontotemporal Degener 14:479–480. 10.3109/21678421.2013.774020 [DOI] [PubMed] [Google Scholar]
- 32.Rascovsky K, Hodges JR, Knopman D, Mendez MF, Kramer JH, Neuhaus J, van Swieten JC, Seelaar H, Dopper EG, Onyike CU, Hillis AE, Josephs KA, Boeve BF, Kertesz A, Seeley WW, Rankin KP, Johnson JK, Gorno-Tempini ML, Rosen H, Prioleau-Latham CE, Lee A, Kipps CM, Lillo P, Piguet O, Rohrer JD, Rossor MN, Warren JD, Fox NC, Galasko D, Salmon DP, Black SE, Mesulam M, Weintraub S, Dickerson BC, Diehl-Schmid J, Pasquier F, Deramecourt V, Lebert F, Pijnenburg Y, Chow TW, Manes F, Grafman J, Cappa SF, Freedman M, Grossman M, Miller BL (2011) Sensitivity of revised diagnostic criteria for the behavioural variant of frontotemporal dementia. Brain 134:2456–2477. 10.1093/brain/awr179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Riku Y, Yoshida M, Iwasaki Y, Sobue G, Katsuno M, Ishigaki S (2022) TDP-43 proteinopathy and tauopathy: do they have pathomechanistic links?? Int J Mol Sci 23. 10.3390/ijms232415755 [DOI] [PMC free article] [PubMed]
- 34.Rohrer JD, Lashley T, Schott JM, Warren JE, Mead S, Isaacs AM, Beck J, Hardy J, de Silva R, Warrington E, Troakes C, Al-Sarraj S, King A, Borroni B, Clarkson MJ, Ourselin S, Holton JL, Fox NC, Revesz T, Rossor MN, Warren JD (2011) Clinical and neuroanatomical signatures of tissue pathology in frontotemporal Lobar degeneration. Brain 134:2565–2581. 10.1093/brain/awr198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schottlaender LV, Polke JM, Ling H, MacDoanld ND, Tucci A, Nanji T, Pittman A, de Silva R, Holton JL, Revesz T, Sweeney MG, Singleton AB, Lees AJ, Bhatia KP, Houlden H (2015) Analysis of C9orf72 repeat expansions in a large series of clinically and pathologically diagnosed cases with atypical parkinsonism. Neurobiol Aging 36:1221e1221–1221e1226. 10.1016/j.neurobiolaging.2014.08.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tando S, Kasai T, Mizuta I, Takahashi H, Yaoi T, Saito K, Hojo T, Mizuno T, Hasegawa M, Itoh K (2021) An autopsy case of corticobasal syndrome due to asymmetric degeneration of the motor cortex and substantia Nigra with TDP-43 proteinopathy, associated with alzheimer’s disease pathology. Neuropathology 41:214–225. 10.1111/neup.12723 [DOI] [PubMed] [Google Scholar]
- 37.Thal DR, Rüb U, Orantes M, Braak H (2002) Phases of A beta-deposition in the human brain and its relevance for the development of AD. Neurology 58:1791–1800. 10.1212/wnl.58.12.1791 [DOI] [PubMed] [Google Scholar]
- 38.Waldthaler J, Sperlich A, Stüssel C, Steidel K, Timmermann L, Pedrosa DJ (2023) Stimulation of non-motor subthalamic nucleus impairs selective response Inhibition via prefrontal connectivity. Brain Commun 5:fcad121. 10.1093/braincomms/fcad121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yamashita R, Beck G, Yonenobu Y, Inoue K, Mitsutake A, Ishiura H, Hasegawa M, Murayama S, Mochizuki H (2022) TDP-43 proteinopathy presenting with typical symptoms of parkinson’s disease. Mov Disord 37:1561–1563. 10.1002/mds.29048 [DOI] [PubMed] [Google Scholar]
- 40.Yeh TH, Lai SC, Weng YH, Kuo HC, Wu-Chou YH, Huang CL, Chen RS, Chang HC, Traynor B, Lu CS (2013) Screening for C9orf72 repeat expansions in parkinsonian syndromes. Neurobiol Aging 34:1311e1313–1311e1314. 10.1016/j.neurobiolaging.2012.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data that support the findings of this study are available from the corresponding author upon request.