Abstract
Transthoracic echocardiography (TTE) has become an essential tool for hemodynamic evaluation. However, its effect on outcomes in sepsis-associated acute kidney injury (SA-AKI) remains unclear. This study aims to explore the association of TTE with the 28-day mortality in SA-AKI patients. MIMIC-IV database was used to identify patients with Sepsis whether they underwent TTE evaluation within 24 h of sepsis diagnosis. Propensity score matching (PSM) was employed to minimize confounding, and subgroup analyses were conducted to assess the impact of TTE across different AKI severity grades. Following PSM, 121 patients were included in each group (TTE vs. no TTE). The TTE group showed improved 28-day survival compared to the no TTE group (odds ratio = 0.80, 95% CI 0.21–0.81, p = 0.011). A subgroup analysis based on different AKI stages showed no significant association between TTE and the 28-day mortality in KIDGO stage 1 or 2 patients. However, for patients with KIDGO stage 3, those who underwent TTE evaluation had a significantly lower 28-day mortality rate (OR 0.81, 95% CI 0.68–0.96, p < 0.05). Patients with SA-AKI who underwent TTE had an improvement in 28-day mortality, with this effect being more pronounced in patients with KIDGO stage 3.
Keywords: Sepsis, Acute kidney injury, Echocardiography
Subject terms: Acute kidney injury, Sepsis
Introduction
Sepsis is a life-threatening organ dysfunction caused by an infection, and it is one of the critical conditions treated in the Intensive Care Unit (ICU)1. As a common disease in the ICU, sepsis is associated with high morbidity and mortality rates2. Each year, the diagnosis and treatment of sepsis consume a significant number of medical resources, yet patients often experience poor survival outcomes that do not match the efforts expended3. Acute Kidney Injury (AKI) is a common complication of various critical illnesses, such as cardiovascular complications, chronic kidney disease, and end-stage renal disease4,5. AKI is not a single disease, but rather a syndrome encompassing various clinical conditions, from oliguria and increased serum creatinine to the need for renal replacement therapy6. The clinical outcome of AKI depends on underlying diseases, the severity and duration of kidney damage, and the patient’s baseline kidney function6. Sepsis is a common cause of AKI in the ICU, and patients with sepsis-associated AKI (SA-AKI) tend to have worse prognoses and longer hospital stays5,7. In recent years, research on SA-AKI has been ongoing, but no study has yet confirmed which interventions can significantly improve survival rates in SA-AKI patients. The impact of various interventions on patient outcomes remains under investigation.
The pathophysiological process of sepsis is often accompanied by complex hemodynamic changes, and if not properly managed, it can lead to multiple organ dysfunction8. Therefore, hemodynamic monitoring is crucial for sepsis patients, as timely monitoring is essential for clinicians to continuously assess the patient’s condition9. Conventional hemodynamic monitoring methods are primarily invasive and involve catheter insertion through vascular puncture, such as the Pulmonary Artery Catheter (PAC) and Pulse Index Continuous Cardiac Output (PiCCO) monitoring10–12. These monitoring methods typically display the patient’s status through numerical data, which requires experienced clinicians to analyze the values in combination with the patient’s overall condition at the time. As a result, there is often a delay in clinical judgment. Additionally, these invasive monitoring methods can cause certain injuries to the patient, increasing the risk of infection.
Transthoracic echocardiography (TTE) plays a crucial role in the hemodynamic monitoring of sepsis patients. As a bedside tool, TTE is non-invasive, dynamic, and intuitive visualization. These characteristics help clinicians understand the circulatory status of the patient, enabling timely feedback on changes in their condition. Society of Critical Care Medicine Guidelines on Adult Critical Care Ultrasonography suggests using critical ultrasound in adults with cardiogenic shock to improve clinical outcomes because its low complication rates13. Recently a study using a combination of echocardiographic parameters as a simple risk stratification score to predict prognosis in children with PAH14. When combined with invasive monitoring indicators, the imaging results of TTE further assist in formulating appropriate treatment plans, improving patient outcomes, and reducing mortality15. For example, some teams have developed an index called the Electrocardiographic Diastolic Index (EDI), which can predict diastolic dysfunction (DD) on TTE in adult patients16. Previous studies on TTE have primarily focused on the sepsis patient population, and the results have shown that TTE can significantly reduce mortality and improve prognosis in sepsis patients17. Although there are no data from randomized controlled trials (RCTs), it is logical to presume that identification of the forms of cardiac dysfunction with echocardiography might lead to improvement in outcomes18,19. Since SA-AKI is a serious complication of sepsis, the impact of TTE on mortality in this specific patient group remains to be further explored. Additionally, according to the 2012 Kidney Disease: Improving Global Outcomes (KDIGO) definition, the severity of AKI is classified into different stages.
Therefore, the primary aim of this study is to explore the impact of TTE on the mortality of patients with SA-AKI, as well as the effect of TTE on mortality across different AKI stages. Additionally, the study seeks to investigate the influence of TTE on certain secondary outcome indicators in these patients.
Methods
Study design
This study is reported in accordance with the STrengthening the Reporting of OBservational studies in Epidemiology (STROBE) statement20. The study is a single-center, retrospective study about whether TTE contributes to improvements in mortality and clinically important changes in the management of SA-AKI patients in the ICU. The data for this study were obtained from the Medical Information Mart for Intensive Care-IV database (MIMIC-IV).
The MIMIC-IV database is a free and publicly available relational database developed by the Massachusetts Institute of Technology (MIT) Laboratory for Computational Physiology, Beth Israel Deaconess Medical Center, and Philips Healthcare. It contains clinical information from patients admitted to the ICU at Beth Israel Deaconess Medical Center in Boston, Massachusetts, USA, between 2008 and 201921. Researchers must first complete MIT’s online ethics course and pass the examination. After obtaining the certification, they must apply for access to use the database for related research. The primary researchers of this study have completed the training and obtained the necessary permissions to access the database. Since the MIMIC-IV database is a relational database, data extraction and organization require the use of a specialized Structured Query Language (SQL).
In this study, all the involved data were extracted using SQL on the BigQuery platform. Patients who met the inclusion and exclusion criteria were selected from the MIMIC-IV database, and the relevant data were extracted, integrated, and exported using SQL. During the study period, the decision to perform a TTE was based on the clinical judgment of the medical team. There was no protocol in place or guidelines employed regarding performance of TTE in patients with SA-AKI.
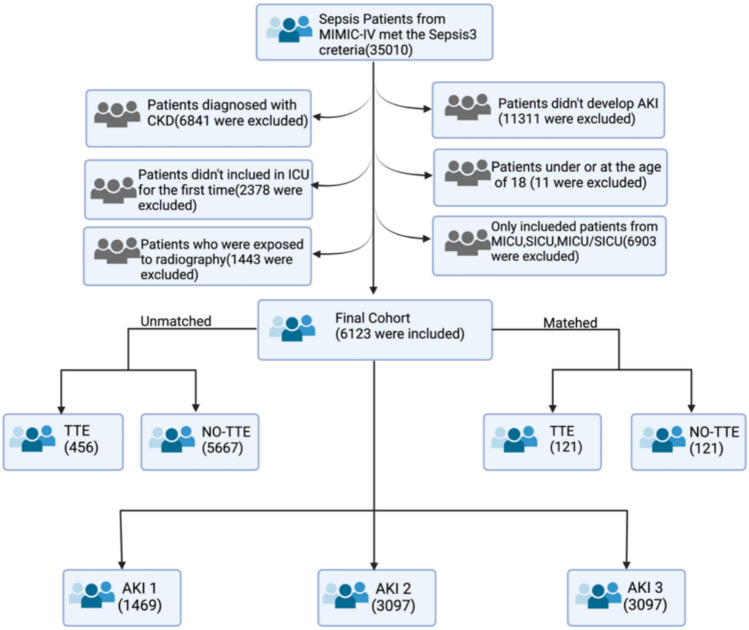
The patients underwent TTE within 24 h after being diagnosed with SA-AKI was used as the grouping criterion, thus dividing the study population into the TTE group and the non-TTE group. The specific screening process is shown in Fig. 1.
Fig.1.
Study design. Illustration of exclusion and inclusion criteria as utilized to select the final cohort of 6123 patients and subgroup analysis based on the KDIGO AKI staging criteria22.
Outcomes
The primary outcome of the study is the 28-day mortality rate of patients in the TTE group compared to the non-TTE group. The secondary outcomes include fluid intake and balance on days 1, 2, and 3 after enrollment, total intake, and total balance over the first 7 days, the maximum, minimum, and average CVP values within 7 days, and the maximum reduction in lactate levels within 7 days.
Statistical methods
Statistical analyses for this study were performed using R software (version 4.1.1; R Foundation for Statistical Computing). A QQ plot was used to verify whether continuous variables followed a normal distribution. For normally distributed variables, data are presented as mean ± standard deviation (x ± s), and t-tests were used for between-group comparisons. For non-normally distributed variables, data are presented as median (interquartile range) [median (IQR)], and non-parametric tests were used for between-group comparisons. A p-value of less than 0.05 was considered statistically significant.
The principle of Propensity Score Matching (PSM) is to balance confounding factors and group assignment by modeling. During data processing, each patient in the study population is assigned a propensity score through the constructed model. Patients with similar propensity scores are grouped together, and the final study cohort is presented in a 1:1 ratio23. This method effectively balances the influence of confounding factors on the study results and is suitable for database studies with large sample sizes and multiple confounders. PSM was used to process the patient population, resulting in a matched cohort.
Covariates
The covariates used for PSM were selected based on prior literature17, clinical relevance in the management of SA-AKI, and observed differences in baseline characteristics. These included admission information, comorbidities, vital signs, laboratory values, and early interventions. The full list is detailed below.
Admission information
Gender, age, weight. Although the SOFA score has been recorded, considering the issue of multicollinearity, the SOFA score was excluded during data processing24.
Comorbidities
Congestive heart failure (CHF), Atrial Fibrillation (AF), Chronic Liver Disease (CLF), Chronic Obstructive Pulmonary Disease (COPD), Chronic Coronary Syndromes (CCS), hypertension, diabetes, stroke, and malignant tumor.
Vital signs
Heart rate, temperature (˚C), mean arterial pressure (MAP), respiratory rate (RR), and central venous pressure (CVP) were recorded at the start of the study.
Laboratory results
White blood cell (WBC) count, hemoglobin, platelets, sodium, potassium, phosphorus, chloride, bicarbonate, total bilirubin, albumin, pH value, lactate, oxygenation index, troponin, B-type natriuretic peptide (BNP) and creatine kinase. The highest daily serum creatinine levels and urine output normalized by body weight every 6 h were extracted to determine each patient’s AKI stage based on the KDIGO classification criteria25 for subsequent analysis.
We excluded pro-BNP, creatine kinase, creatine kinase-MB, and partial pressure of carbon dioxide due to a high percentage of missing values and a lack of significant association with SA-AKI. We noticed that CVP values were not collected in each patient. Therefore, we transformed it into a binary variable indicating whether it was tested, defining it as a new covariate, which helped mitigate the impact of missing values on the study results. Similarly, we categorized variables such as total bilirubin, albumin, pH, oxygenation index, lactate, and troponin into levels of missingness and different classification levels. Finally, we performed correlation-based imputation for the remaining variables with minor missing data and used the imputed final dataset for subsequent analyses26.
Interventions
Use of Continuous Renal Replacement Therapy (CRRT), mechanical ventilation, vasopressors agents, sedatives, and analgesics drugs within 24 h of enrollment.
Results
The TTE group had a lower 28-day mortality rate
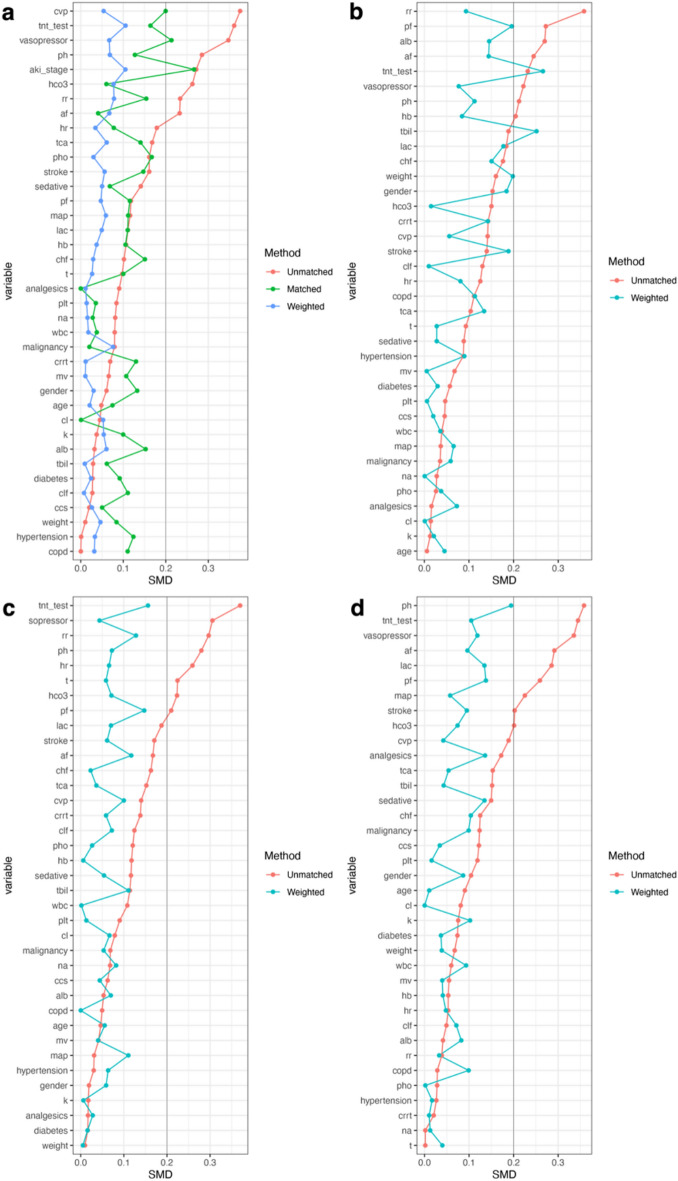
After screening based on the inclusion and exclusion criteria, the original cohort consisted of 6,123 patients, and the PSM-matched cohort consisted of 242 patients. The baseline characteristics, vital signs, laboratory test results, and mortality rates of each group are summarized in Table 1. To verify the effectiveness of propensity score matching in balancing intergroup differences, we calculated the standardized mean difference (SMD) before and after matching, as shown in Fig. 2a. We set 0.2 as the threshold, and the SMD values for various confounding factors in the matched cohort were below 0.2, indicating that propensity score matching reduced the intergroup differences between the TTE group and the non-TTE group.
Fig.2.
Standardized Mean Difference (SMD) of covariates with the 0.2 threshold. (a) The SMD of the original cohort, matched cohort, and matched-weighted cohort. (b)-(d) The SMD of three subgroups: AKI 1, AKI 2, AKI 3.
Table 1.
Comparison of the baseline characteristics between the original cohort and the PSM cohort.
| Characteristic | Original cohort | Matched cohort | ||||
|---|---|---|---|---|---|---|
| TTE | Non-TTE | SMD | TTE | Non-TTE | SMD | |
| n | 5667 | 456 | 121 | 121 | ||
| Gender (male) (%) | 3005 (53.0) | 228 (50.0) | 0.061 | 61 (50.4) | 69 (57.0) | 0.133 |
| Age (mean (SD)) | 65.26 (16.47) | 66.03 (15.29) | 0.048 | 65.55 (14.79) | 64.36 (17.06) | 0.075 |
| Weight (mean (SD)) | 83.97 (24.90) | 83.91 (25.63) | 0.002 | 85.45 (24.88) | 83.46 (22.32) | 0.084 |
| Comorbidities | ||||||
| CHF (%) | 584 (10.3) | 62 (13.6) | 0.102 | 18 (14.9) | 12 (9.9) | 0.151 |
| AF (%) | 1157 (20.4) | 139 (30.5) | 0.233 | 26 (21.5) | 24 (19.8) | 0.041 |
| CLF (%) | 682 (12.0) | 59 (12.9) | 0.027 | 10 (8.3) | 14 (11.6) | 0.111 |
| COPD (%) | 882 (15.6) | 71 (15.6) | < 0.001 | 23 (19.0) | 18 (14.9) | 0.11 |
| CCS (%) | 854 (15.1) | 72 (15.8) | 0.02 | 16 (13.2) | 14 (11.6) | 0.05 |
| Hypertension (%) | 934 (16.5) | 75 (16.4) | 0.001 | 18 (14.9) | 13 (10.7) | 0.124 |
| Diabetes (%) | 1508 (26.6) | 127 (27.9) | 0.028 | 37 (30.6) | 32 (26.4) | 0.092 |
| Stroke (%) | 485 (8.6) | 62 (13.6) | 0.161 | 8 (6.6) | 13 (10.7) | 0.147 |
| Malignancy (%) | 1316 (23.2) | 91 (20.0) | 0.079 | 26 (21.5) | 25 (20.7) | 0.02 |
| Interventions | ||||||
| mechanical ventilation use (1st 24 h) (%) | 1849 (32.6) | 163 (35.7) | 0.066 | 35 (28.9) | 41 (33.9) | 0.107 |
| CRRT use (1st 24 h) (%) | 143 (2.5) | 17 (3.7) | 0.069 | 1 (0.8) | 3 (2.5) | 0.13 |
| vasopressors agents use (1st 24 h) (%) | 1878 (33.1) | 228 (50.0) | 0.347 | 45 (37.2) | 33 (27.3) | 0.213 |
| sedatives use (1st 24 h) (%) | 3303 (58.3) | 297 (65.1) | 0.141 | 74 (61.2) | 78 (64.5) | 0.068 |
| analgesics use (1st 24 h) (%) | 3764 (66.4) | 322 (70.6) | 0.09 | 83 (68.6) | 83 (68.6) | < 0.001 |
| Vital signs | ||||||
| HR (mean (SD)) | 88.88 (19.27) | 92.48 (21.15) | 0.178 | 89.16 (19.47) | 87.72 (17.49) | 0.078 |
| MAP (mean (SD)) | 78.62 (13.87) | 76.99 (14.70) | 0.115 | 77.14 (14.55) | 78.65 (12.58) | 0.111 |
| RR (mean (SD)) | 20.24 (5.92) | 21.62 (5.98) | 0.233 | 21.12 (5.89) | 20.25 (5.36) | 0.154 |
| T (mean (SD)) (°C) | 36.95 (0.70) | 37.02 (0.78) | 0.095 | 37.08 (0.64) | 37.01 (0.74) | 0.1 |
| Laboratory tests | ||||||
| WBC (mean (SD)) | 12.89 (7.62) | 13.48 (7.82) | 0.076 | 12.76 (7.08) | 12.51 (6.27) | 0.038 |
| Hemoglobin (mean (SD)) | 10.50 (2.07) | 10.71 (2.30) | 0.099 | 10.37 (1.97) | 10.58 (1.96) | 0.105 |
| Platelet (mean (SD)) | 197.76 (117.84) | 206.16 (117.76) | 0.071 | 191.83 (102.84) | 188.19 (102.47) | 0.035 |
| Sodium (mean (SD)) | 138.35 (5.66) | 137.99 (5.73) | 0.064 | 138.57 (5.68) | 138.41 (5.45) | 0.028 |
| Potassium (mean (SD)) | 4.15 (0.67) | 4.17 (0.64) | 0.028 | 4.10 (0.67) | 4.04 (0.60) | 0.1 |
| Troponin (mean (SD)) | 8.12 (0.78) | 7.99 (0.85) | 0.167 | 8.02 (0.82) | 8.13 (0.72) | 0.141 |
| Tnt test = 1 (%)# | 1380 (24.4) | 187 (41.0) | 0.361 | 40 (33.1) | 31 (25.6) | 0.164 |
| Phosphorus (mean (SD)) | 3.67 (1.47) | 3.87 (1.48) | 0.136 | 3.66 (1.37) | 3.44 (1.16) | 0.167 |
| Chloride (mean (SD)) | 104.17 (6.81) | 103.99 (6.99) | 0.026 | 104.55 (6.36) | 104.54 (6.26) | 0.001 |
| Bicarbonate (mean (SD)) | 22.78 (5.39) | 21.40 (5.46) | 0.255 | 22.58 (5.40) | 22.89 (4.97) | 0.06 |
| Total bilirubin (%)* | 0.092 | 0.061 | ||||
| H | 1664 (29.4) | 140 (30.7) | 24 (19.8) | 27 (22.3) | ||
| L | 1957 (34.5) | 171 (37.5) | 46 (38.0) | 45 (37.2) | ||
| NA | 2046 (36.1) | 145 (31.8) | 51 (42.1) | 49 (40.5) | ||
| Albumin (%)* | 0.054 | 0.153 | ||||
| H | 115 (2.0) | 13 (2.9) | 2 (1.7) | 2 (1.7) | ||
| L | 2506 (44.2) | 198 (43.4) | 54 (44.6) | 45 (37.2) | ||
| NA | 3046 (53.7) | 245 (53.7) | 65 (53.7) | 74 (61.2) | ||
| PH (%)* | 0.305 | 0.127 | ||||
| [7.35,7.45] | 1683 (29.7) | 141 (30.9) | 38 (31.4) | 40 (33.1) | ||
| < 7.35 | 1075 (19.0) | 139 (30.5) | 26 (21.5) | 20 (16.5) | ||
| > 7.45 | 535 (9.4) | 35 (7.7) | 9 (7.4) | 10 (8.3) | ||
| NA | 2374 (41.9) | 141 (30.9) | 48 (39.7) | 51 (42.1) | ||
| Oxygenation index (%)* | 0.229 | 0.115 | ||||
| [100,200) | 984 (17.4) | 104 (22.8) | 23 (19.0) | 21 (17.4) | ||
| [200,300) | 852 (15.0) | 78 (17.1) | 19 (15.7) | 15 (12.4) | ||
| < 100 | 264 (4.7) | 31 (6.8) | 4 (3.3) | 4 (3.3) | ||
| ≥ 300 | 845 (14.9) | 72 (15.8) | 20 (16.5) | 21 (17.4) | ||
| NA | 2722 (48.0) | 171 (37.5) | 55 (45.5) | 60 (49.6) | ||
| Lactate (%)* | 0.244 | 0.11 | ||||
| < 2.0 | 1667 (29.4) | 143 (31.4) | 35 (28.9) | 37 (30.6) | ||
| ≥ 2.0 | 1095 (19.3) | 128 (28.1) | 23 (19.0) | 18 (14.9) | ||
| NA | 2905 (51.3) | 185 (40.6) | 63 (52.1) | 66 (54.5) | ||
| CVP (%)* | 0.185 | 0.2 | ||||
| H | 314 (5.5) | 48 (10.5) | 5 (4.1) | 3 (2.5) | ||
| L | 707 (12.5) | 57 (12.5) | 20 (16.5) | 13 (10.7) | ||
| NA | 4646 (82.0) | 351 (77.0) | 96 (79.3) | 105 (86.8) | ||
| The classification of AKI of KIDGO | ||||||
| The classification of AKI (%) | 0.272 | 0.267 | ||||
| 1 | 1385 (24.4) | 84 (18.4) | 26 (21.5) | 28 (23.1) | ||
| 2 | 2892 (51.0) | 205 (45.0) | 55 (45.5) | 67 (55.4) | ||
| 3 | 1390 (24.5) | 167 (36.6) | 40 (33.1) | 26 (21.5) | ||
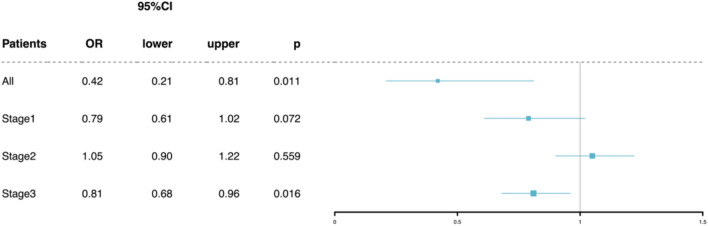
There were no statistically significant differences in baseline characteristics, vital signs, or other factors between the groups in the matched cohort. The final matched cohort consisted of 121 patients in both the TTE and non-TTE groups. In the matched cohort, the results showed that the TTE group had a lower 28-day mortality rate (13.2% vs. 26.4%) (odds ratio 0.42, 95% CI 0.21–0.81, p = 0.011), shown in Fig. 3.
Fig.3.
Primary outcome with propensity scores matched cohorts in TEE group and AKI stage1-3.
TTE evaluation resulted in a lower 28-day mortality rate for patients with KDIGO stage 3
To further explore the impact of TTE on patients with varying severity of sepsis-associated acute kidney injury (SA-AKI), this study also conducted a subgroup analysis based on different AKI stages, following the KDIGO AKI staging criteria27,28. Propensity score matching (PSM) was then applied to the three staged subgroups, and the resulting cohorts were weighted using inverse probability of treatment weighting (IPTW)29,30. The results in Fig. 3 showed no significant difference in mortality for patients with AKI stages 1 and 2 who underwent TTE, but for patients with AKI stage 3, those in the TTE group had a lower mortality rate (odds ratio 0.81, 95% CI 0.68–0.96, p = 0.016). The relationship between SMD before and after matching for the overall cohort and each AKI stage is shown in Fig. 2. Similarly, the matched and weighted new cohort had smaller intergroup differences compared to the original cohort, and PSM helped ensure that the statistical data closely aligned with randomization.
TTE Evaluation Affects Patients’ Fluid Resuscitation Strategy
The analysis of secondary outcomes for patients in the final matched cohort showed no significant statistical differences in the maximum, minimum, and average CVP values within 7 days between the two groups, although there was a high proportion of missing data. Compared to patients who did not undergo TTE, those in the TTE group had higher fluid intake on day 1, day 2, and over the total 7-day period. However, there was no statistical difference in fluid intake on day 3 or in any of the defined fluid balance metrics. Additionally, no significant statistical difference was observed in lactate changes over the 7-day period between the two groups. Detailed results are shown in Table 2.
Table 2.
Secondary outcomes comparing fluid intake, fluid balance, CVP values, and lactate reduction between the non-TTE and TTE groups over 7 days.
| Secondary outcomes | non-TTE | TTE | p value | Missing (%) |
|---|---|---|---|---|
| CVP minimum in 7 days (median [IQR]) | 7.00 [3.50, 8.00] | 6.50 [3.00, 10.75] | 0.763 | 83.1 |
| CVP average in 7 days (median [IQR]) | 10.70 [9.03, 14.50] | 12.03 [10.24, 14.62] | 0.53 | 83.1 |
| Day1 IV fluid (mean (SD)) | 3159.65 (2231.39) | 3920.37 (2896.21) | 0.023 | 0 |
| Fluid balance Day1 (mean (SD)) | 1564.81 (2555.63) | 1756.35 (2944.66) | 0.589 | 0 |
| Day2 IV fluid (mean (SD)) | 1761.88 (1898.84) | 2479.25 (1773.15) | 0.003 | 0 |
| Fluid balance Day2 (mean (SD)) | 329.51 (1902.74) | 516.07 (1820.52) | 0.437 | 0 |
| Day3 IV fluid (mean (SD)) | 1415.33 (1888.85) | 1763.99 (1771.40) | 0.14 | 0 |
| Fluid balance Day3 (mean (SD)) | 331.12 (1630.22) | 39.30 (1562.99) | 0.157 | 0 |
| Total fluid (7 days) (mean (SD)) | 9629.62 (8878.65) | 12,648.28 (11,398.50) | 0.022 | 0 |
| Total balance (7 days) (mean (SD)) | 2582.50 (7147.94) | 2630.37 (7943.86) | 0.961 | 0 |
| Serum lactate reduction (7 days) (mean (SD)) | 0.28 (0.84) | 0.29 (0.65) | 0.936 | 53.3 |
Discussion
This study explored the impact of TTE use on the prognosis of patients with SA-AKI, with the primary outcome being the 28-day mortality rate. The results showed that the use of TTE in patients with SA-AKI improved patient prognosis and reduced mortality, suggesting that TTE is a protective factor that can improve outcomes in these patients. The study population was drawn from the MIMIC-IV database and included patients who met the diagnostic criteria. With a large sample size and consideration of various influencing factors, multiple variables were included during data extraction. Propensity score matching was used to balance the differences between the two groups, leading to the final conclusions. We also explored several secondary outcomes in this study to better understand the mechanisms behind the lower mortality rate in the TTE group. Fluid input and central venous pressure (CVP) are commonly used indicators for volume status assessment, and transthoracic echocardiography (TTE) can directly influence fluid resuscitation strategies in clinical practice. Unfortunately, there were no significant changes in CVP between the two groups, which could be partly attributed to the high proportion of missing CVP data. The TTE group had higher fluid intake on days 1, 2, and over the first 7 days, which may indicate that patients in the TTE group received more adequate fluid resuscitation. Although these two indicators are nonlinearly associated with mortality, their changes may reflect the potential clinical impact of TTE on volume management. However, there were no statistically significant differences in lactate changes between the two groups. SA-AKI patients may have poor fluid responsiveness and microcirculatory dysfunction, which could limit improvements in tissue perfusion despite increased fluid resuscitation, thereby explaining the lack of significant changes in lactate levels. This finding suggests that TTE may help optimize fluid management strategies, although it may not necessarily be reflected in short-term changes in lactate levels.
Randomized controlled trials (RCTs) are widely recognized as an excellent research method and are considered the highest level of evidence for establishing causal relationships between study factors. However, the implementation of RCTs can be limited by various factors, making it difficult to achieve predetermined goals31. With the increasing digitization of medical information, the advantages of databases, such as large sample sizes and rich content, are becoming more apparent. However, the data in databases often exhibit significant heterogeneity, making effective data processing a key concern. As a result, various data integration and processing methods have emerged32. In this study, propensity score matching (PSM) and imputation of missing data were used as important components of data handling. The MIMIC database, as a crucial resource for critical care medicine, holds significant exploratory value, containing laboratory test results, vital signs, and bedside data for critically ill patients21. Many previous studies using the MIMIC-III database have yielded valuable results and provided numerous guidelines for clinical practice17,33. As a new data system, the MIMIC-IV database offers even more potential for exploration, providing support for further research21.
Throughout the course of sepsis, patients experience complex hemodynamic changes. As a non-invasive monitoring tool, TTE can promptly detect the patient’s hemodynamic status at any given moment and provide dynamic monitoring. This ability to monitor changes in real-time may be the main reason why TTE improves patient prognosis. Timely and dynamic monitoring enhances clinicians’ sensitivity to fluctuations in a patient’s condition, allowing them to intervene within an effective time window to prevent further deterioration. A retrospective study found that the left ventricular diastolic function parameter e’ and the right ventricular systolic function parameter RV-Sm were independent risk factors for 28-day outcomes in patients with sepsis34. Several studies have gradually explored the impact of TTE on mortality in patients with sepsis. A PSM analysis based on the MIMIC-III database found that septic shock patients who underwent TTE had a significantly lower 28-day mortality rate (33.2% vs. 37.7%, P = 0.019)35. Another meta-analysis indicated that bedside TTE-guided management of septic shock was associated with reduced 28-day mortality, more frequently treated with inotropic agents, and achieved faster lactate clearance, which demonstrate the potential of TTE in optimizing treatment strategies36. Analyzing echocardiographic parameters in pediatric sepsis patients suggests that left ventricular diastolic dysfunction may be important37. Although most studies support the value of TTE in the management of sepsis, some have not found a significant association between its use and reduced mortality38. However, these findings do not negate the utility of TTE in cases of diagnostic uncertainty or inadequate response to initial treatment. Our study focuses on the specific clinical subgroup of SA-AKI, with rigorous population selection and data cleaning based on the large MIMIC-IV database, aiming to reflect the real-world impact of TTE on the prognosis of SA-AKI as accurately as possible.
The study also considered the potential impact of varying degrees of disease severity in SA-AKI patients on the results. Therefore, a subsequent subgroup analysis was conducted based on KDIGO staging criteria. The results showed that the use of TTE had little effect on improving prognosis for patients with KDIGO stages 1 and 2, but for those with stage 3 kidney injury, TTE significantly reduced the 28-day mortality rate. This finding aligns with our clinical experience, as for more severely ill patients, the advantages of TTE in providing timely assessment and dynamic monitoring help clinicians gain a clearer understanding of the patient’s microcirculation, allowing for rapid responses to changes in their condition. Patients with KDIGO stage 3 often have more severe illness and significant hemodynamic fluctuations; therefore, they rely more heavily on TTE for accurate assessment of volume status and cardiac function, which can guide interventions and improve prognosis.
This study is a single-center retrospective study based on the MIMIC-IV database, extending previous research on TTE, but it also has several limitations. 1) As a retrospective study based on a database, this study cannot guarantee the completeness of clinical data for patients, and many variables have a large proportion of missing values. The study excluded variables with excessive missing data and minimal impact on the results, such as height5, and a series of adjustments were made for important variables like troponin, CVP, and total bilirubin (Tbil) to reduce the impact of missing values39,40. However, this method is merely a handling technique and cannot fully replace the missing data, so the influence of missing values and adjusted variables on the results may persist. 2) TTE, as a monitoring tool, cannot directly intervene in the pathophysiological processes of sepsis patients. While several interventions, such as vasopressors and CRRT, have been considered22,41, other unidentified factors may still affect patient outcomes. Further research is needed to verify this idea. 3) Various monitoring methods, such as PAC and PiCCO, may have influenced the results. However, in this study, very few patients underwent PiCCO monitoring, and none used PAC, so the impact of these monitoring methods was not analyzed. Nevertheless, their potential effects cannot be ruled out, and more research is required to explore this aspect. As the use of TTE in the ICU becomes more widespread, there is growing interest in the impact of ultrasound on the prognosis of critically ill patients. TTE’s non-invasive, dynamic, and timely monitoring features will provide significant support for the evaluation of critically ill patients. As an important assessment tool, TTE has further clinical applications awaiting discovery by clinicians.
Conclusion
In summary, this study based on the MIMIC-IV database shows that patients with sepsis-associated acute kidney injury who underwent transthoracic echocardiography (TTE) had a lower 28-day mortality rate, with this effect being more pronounced in patients with KDIGO stage 3.
Author contributions
Z.Q. and Z.R. was responsible for statistical analysis and manuscript drafting; L.B. conducted statistical analysis and data cleaning; Z.X., L.J., X.Y. was responsible for the acquisition and interpretation of the data; L.L. provided ultrasound technical guidance and the conception of the study; H.Y. and H.Z. designed the study and critically revised the manuscript content. All of the author reviewed the final draft.
Funding
20230158 Improvement of the diagnosis and treatment process of sepsis patients based on big data analysis, Medical Science Research Project of Hebei.
Data availability
The data used in this study were extracted from the publicly available MIMIC-IV (Medical Information Mart for Intensive Care) database. Access to the data requires credentialing and completion of the Collaborative Institutional Training Initiative (CITI) program. The dataset can be accessed at https://physionet.org/content/mimiciv/2.2/.
Declarations
Competing interests
The authors declare no competing interests.
Ethics approval
This study is based on the publicly available MIMIC-IV database, which contains de-identified patient data in compliance with HIPAA regulations. Informed consent was obtained at the time of data collection, and the need for individual consent was waived for this retrospective analysis. Access to the database requires ethical training and approval, which the researchers have completed. Additionally, patients under 18 years old were excluded from this study.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Zhang Qian and Zhu Runying contributed equally to this work.
References
- 1.Singer, M. et al. The third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA315, 801–810. 10.1001/jama.2016.0287 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sakr, Y. et al. Sepsis in intensive care unit patients: Worldwide data from the intensive care over nations audit. Open Forum. Infect. Dis.5, ofy313. 10.1093/ofid/ofy313 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Markwart, R. et al. Epidemiology and burden of sepsis acquired in hospitals and intensive care units: A systematic review and meta-analysis. Intensive Care Med.46, 1536–1551. 10.1007/s00134-020-06106-2 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hoste, E. A. J. et al. Global epidemiology and outcomes of acute kidney injury. Nat. Rev. Nephrol.14, 607–625. 10.1038/s41581-018-0052-0 (2018). [DOI] [PubMed] [Google Scholar]
- 5.Poston, J. T. & Koyner, J. L. Sepsis associated acute kidney injury. BMJ364, k4891. 10.1136/bmj.k4891 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Singbartl, K. & Kellum, J. A. AKI in the ICU: Definition, epidemiology, risk stratification, and outcomes. Kidney Int.81, 819–825. 10.1038/ki.2011.339 (2012). [DOI] [PubMed] [Google Scholar]
- 7.Bagshaw, S. M., George, C. & Bellomo, R. Early acute kidney injury and sepsis: A multicentre evaluation. Crit Care12, R47. 10.1186/cc6863 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ergin, B., Kapucu, A., Demirci-Tansel, C. & Ince, C. The renal microcirculation in sepsis. Nephrol. Dial Transpl.30, 169–177. 10.1093/ndt/gfu105 (2015). [DOI] [PubMed] [Google Scholar]
- 9.Cecconi, M. et al. Consensus on circulatory shock and hemodynamic monitoring. task force of the European society of intensive care medicine. Intensive Care Med.40, 1795–1815. 10.1007/s00134-014-3525-z (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hall, J. B. Searching for evidence to support pulmonary artery catheter use in critically ill patients. JAMA294, 1693–1694. 10.1001/jama.294.13.1693 (2005). [DOI] [PubMed] [Google Scholar]
- 11.Litton, E. & Morgan, M. The PiCCO monitor: A review. Anaesth Intensive Care40, 393–409. 10.1177/0310057x1204000304 (2012). [DOI] [PubMed] [Google Scholar]
- 12.Connors, A. F. Jr. et al. The effectiveness of right heart catheterization in the initial care of critically ill patients support Investigators. JAMA276, 889–897. 10.1001/jama.276.11.889 (1996). [DOI] [PubMed] [Google Scholar]
- 13.Díaz-Gómez, J. L. et al. Society of critical care medicine guidelines on adult critical care ultrasonography: Focused update 2024. Crit. Care Med.53, e447–e458. 10.1097/ccm.0000000000006530 (2025). [DOI] [PubMed] [Google Scholar]
- 14.Lammers, A. E., Marek, J., Diller, G. P., Haworth, S. G. & Moledina, S. Prognostic value of transthoracic echocardiography in children with pulmonary arterial hypertension. J. Am. Heart Assoc.12, e023118. 10.1161/jaha.121.023118 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Evans, L. et al. Surviving sepsis campaign: International guidelines for management of sepsis and septic shock 2021. Crit. Care Med.49, e1063–e1143. 10.1097/ccm.0000000000005337 (2021). [DOI] [PubMed] [Google Scholar]
- 16.Hayıroğlu, M. et al. A simple formula to predict echocardiographic diastolic dysfunction-electrocardiographic diastolic index. Herz46, 159–165. 10.1007/s00059-020-04972-6 (2021). [DOI] [PubMed] [Google Scholar]
- 17.Feng, M. et al. Transthoracic echocardiography and mortality in sepsis: Analysis of the MIMIC-III database. Intensive Care Med.44, 884–892. 10.1007/s00134-018-5208-7 (2018). [DOI] [PubMed] [Google Scholar]
- 18.Singh, K. & Mayo, P. Critical care echocardiography and outcomes in the critically ill. Curr. Opin. Crit. Care24, 316–321. 10.1097/mcc.0000000000000515 (2018). [DOI] [PubMed] [Google Scholar]
- 19.Singh, K. & Mayo, P. Transthoracic echocardiography and mortality in sepsis: Are we there yet?. Intensive Care Med.44, 1342–1343. 10.1007/s00134-018-5261-2 (2018). [DOI] [PubMed] [Google Scholar]
- 20.von Elm, E. et al. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: Guidelines for reporting observational studies. J. Clin. Epidemiol.61, 344–349. 10.1016/j.jclinepi.2007.11.008 (2008). [DOI] [PubMed] [Google Scholar]
- 21.Johnson, A., Bulgarelli, L., Pollard, T., Gow, B., Moody, B., Horng, S., Celi, L. A., & Mark, R. In PhysioNet. (2024).
- 22.Romagnoli, S., Ricci, Z. & Ronco, C. CRRT for sepsis-induced acute kidney injury. Curr. Opin. Crit. Care24, 483–492. 10.1097/mcc.0000000000000544 (2018). [DOI] [PubMed] [Google Scholar]
- 23.Benedetto, U., Head, S. J., Angelini, G. D. & Blackstone, E. H. Statistical primer: Propensity score matching and its alternatives. Eur. J. Cardiothorac. Surg.53, 1112–1117. 10.1093/ejcts/ezy167 (2018). [DOI] [PubMed] [Google Scholar]
- 24.Vincent, J. L. et al. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure On behalf of the working group on sepsis-related problems of the european society of intensive care medicine. Intensive Care Med.22(707), 710. 10.1007/bf01709751 (1996). [DOI] [PubMed] [Google Scholar]
- 25.Summary of Recommendation Statements. Kidney Int. Suppl.2011(2), 8–12. 10.1038/kisup.2012.7 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Austin, P. C., White, I. R., Lee, D. S. & van Buuren, S. Missing data in clinical research: A tutorial on multiple imputation. Can. J. Cardiol.37, 1322–1331. 10.1016/j.cjca.2020.11.010 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stevens, P. E. & Levin, A. Evaluation and management of chronic kidney disease: synopsis of the kidney disease: Improving global outcomes 2012 clinical practice guideline. Ann. Intern. Med.158, 825–830. 10.7326/0003-4819-158-11-201306040-00007 (2013). [DOI] [PubMed] [Google Scholar]
- 28.Section 2: AKI Definition. Kidney Int Suppl (2011)2 19–36 10.1038/kisup.2011.32 (2012). [DOI] [PMC free article] [PubMed]
- 29.Grafféo, N., Latouche, A., Le Tourneau, C. & Chevret, S. ipcwswitch: An R package for inverse probability of censoring weighting with an application to switches in clinical trials. Comput. Biol. Med.111, 103339. 10.1016/j.compbiomed.2019.103339 (2019). [DOI] [PubMed] [Google Scholar]
- 30.Seaman, S. R. & White, I. R. Review of inverse probability weighting for dealing with missing data. Stat. Methods Med. Res.22, 278–295. 10.1177/0962280210395740 (2013). [DOI] [PubMed] [Google Scholar]
- 31.Zabor, E. C., Kaizer, A. M. & Hobbs, B. P. Randomized controlled trials. Chest158, S79-s87. 10.1016/j.chest.2020.03.013 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wu, W. T. et al. Data mining in clinical big data: The frequently used databases, steps, and methodological models. Mil. Med. Res.8, 44. 10.1186/s40779-021-00338-z (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen, H. et al. Central venous pressure measurement is associated with improved outcomes in septic patients: An analysis of the MIMIC-III database. Crit. Care24, 433. 10.1186/s13054-020-03109-9 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lu, N. et al. Study on the relationship between ventricular function parameters obtained by echocardiography and prognosis of patients with sepsis. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue34, 740–745. 10.3760/cma.j.cn121430-20210826-01281 (2022). [DOI] [PubMed] [Google Scholar]
- 35.Lan, P. et al. Utilization of echocardiography during septic shock was associated with a decreased 28-day mortality: A propensity score-matched analysis of the MIMIC-III database. Ann. Transl. Med.7, 662. 10.21037/atm.2019.10.79 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Killu, K. et al. The association between integrating echocardiography use in the management of septic shock patients and outcomes in the intensive care unit: A systematic review and meta-analysis. J. Ultrasound10.1007/s40477-024-00958-w (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sanfilippo, F. et al. Echocardiographic parameters and mortality in pediatric sepsis: A systematic review and meta-analysis. Pediatr. Crit. Care Med.22, 251–261. 10.1097/pcc.0000000000002622 (2021). [DOI] [PubMed] [Google Scholar]
- 38.Blank, S. P. & Blank, R. M. Echocardiography does not reduce mortality in sepsis: A re-evaluation using the medical information mart for intensive care IV dataset. Crit. Care Med.52, 248–257. 10.1097/ccm.0000000000006069 (2024). [DOI] [PubMed] [Google Scholar]
- 39.Yamano, S. et al. Low total cholesterol and high total bilirubin are associated with prognosis in patients with prolonged sepsis. J. Crit. Care31, 36–40. 10.1016/j.jcrc.2015.09.033 (2016). [DOI] [PubMed] [Google Scholar]
- 40.Patel, J. J. et al. The association of serum bilirubin levels on the outcomes of severe sepsis. J. Intensive Care Med.30, 23–29. 10.1177/0885066613488739 (2015). [DOI] [PubMed] [Google Scholar]
- 41.Di Giantomasso, D., May, C. N. & Bellomo, R. Norepinephrine and vital organ blood flow during experimental hyperdynamic sepsis. Intensive Care Med.29, 1774–1781. 10.1007/s00134-003-1736-9 (2003). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used in this study were extracted from the publicly available MIMIC-IV (Medical Information Mart for Intensive Care) database. Access to the data requires credentialing and completion of the Collaborative Institutional Training Initiative (CITI) program. The dataset can be accessed at https://physionet.org/content/mimiciv/2.2/.