ABSTRACT
Aims
To compare the efficacy of enzalutamide + androgen-deprivation therapy (ADT) versus darolutamide + ADT for treatment of patients with metastatic hormone-sensitive prostate cancer (mHSPC) using a matching-adjusted indirect comparison (MAIC).
Patients and methods
Individual patient data from ARCHES (NCT02677896; enzalutamide + ADT, N = 1150) were weighted and adjusted to match published aggregated data on baseline characteristics from ARANOTE (NCT04736199; darolutamide + ADT, N = 669). The MAIC was anchored on the common comparator, placebo + ADT, and provided a (matching-adjusted) hazard ratio (HR) of enzalutamide versus darolutamide.
Results
Treatment with enzalutamide + ADT significantly prolonged the primary endpoint of radiographic progression-free survival (HR [95% confidence interval, CI]: 0.54 [0.32–0.93], p = 0.03) and time to castration resistance (HR [95% CI]: 0.57 [0.34–0.94], p = 0.03) compared with darolutamide + ADT (effective sample size: 319). Time to prostate-specific antigen progression (HR [95% CI]: 0.61 [0.29–1.30], p = 0.20) and time to initiation of new antineoplastic therapy (HR [95% CI]: 0.65 [0.34–1.24], p = 0.19) favored enzalutamide over darolutamide, albeit the difference was not statistically significant.
Conclusions
Enzalutamide + ADT showed better efficacy than darolutamide + ADT for treatment of patients with mHSPC. These findings can help inform treatment decisions in clinical practice.
KEYWORDS: Androgen receptor pathway inhibitor, enzalutamide, darolutamide, indirect treatment comparison, metastatic hormone-sensitive prostate cancer
Plain Language Summary
What is this article about?
Metastatic prostate cancer is a form of prostate cancer that has spread beyond the prostate to other parts of the body. Androgen-deprivation therapy is a form of therapy that can stop or slow down the growth of metastatic prostate cancer by reducing testosterone levels. When prostate cancer responds to androgen-deprivation therapy, it is known as hormone-sensitive or castration-sensitive prostate cancer. When androgen-deprivation therapy stops working, it is known as castration-resistant prostate cancer.
Enzalutamide and darolutamide are hormone treatments used for metastatic hormone-sensitive prostate cancer. We wanted to know if enzalutamide or darolutamide, when combined with androgen-deprivation therapy, was more efficacious in delaying how long it took patients with metastatic hormone-sensitive prostate cancer to get worse. Since there are no clinical trials that directly compare enzalutamide to darolutamide, we conducted a matching-adjusted indirect comparison of two different trials of patients with metastatic hormone-sensitive prostate cancer taking androgen-deprivation therapy combined with enzalutamide (ARCHES trial) or darolutamide (ARANOTE trial).
What were the results of the study?
It took longer for prostate cancer to get worse (progress further or lead to death) and to become castration-resistant in patients with metastatic hormone-sensitive prostate cancer who took enzalutamide with androgen-deprivation therapy compared to patients who took darolutamide with androgen-deprivation therapy. This difference in efficacy was considered clinically significant.
What do the results of the study mean?
These findings may have an impact on treatment decision-making in patients with metastatic hormone-sensitive prostate cancer.
1. Introduction
The disease burden of prostate cancer has significantly increased in recent years, with the rates of age-standardized disability-adjusted life-years rising substantially across the globe over the past three decades [1]. Thus, effective treatment options for patients with prostate cancer are crucial. Both the NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) [2] and European Association of Urology Guidelines [3] recommend androgen-deprivation therapy (ADT) with androgen receptor pathway inhibitors (ARPIs; a form of doublet therapy), as systemic treatment options for patients with metastatic hormone-sensitive prostate cancer (mHSPC; also known as metastatic castration-sensitive prostate cancer [mCSPC]) [2]. Multiple ARPIs are available for mHSPC treatment, including enzalutamide, abiraterone, apalutamide, and darolutamide [2,4,5]. Despite drug resistance remaining a challenge due to underlying mechanisms—such as androgen receptor gene alterations, increased intratumoral androgens, and androgen receptor post-translational modifications—these ARPIs have demonstrated efficacy in delaying disease progression and improving patient survival and quality of life [6].
The efficacy of enzalutamide + ADT in patients with mHSPC was assessed in the phase 3 ARCHES (NCT02677896) randomized controlled trial (RCT). Enzalutamide + ADT significantly reduced the risk of metastatic progression or death over time compared with placebo + ADT in patients with mHSPC and improved overall survival (OS) [4,7]. Similarly, the efficacy of darolutamide + ADT was assessed in the phase 3 ARANOTE (NCT04736199) RCT. Darolutamide + ADT significantly delayed radiographic progression or death compared with placebo + ADT in patients with mHSPC [5]. In terms of safety, the rate of discontinuation due to adverse events (AEs) was comparable between patients receiving enzalutamide + ADT (7.2%) [3] and patients receiving darolutamide + ADT (6.1%) [5].
With multiple ARPIs available for the treatment of mHSPC, understanding their relative efficacy can help guide treatment decisions. Although direct head-to-head RCTs serve as the gold standard to compare treatments, they are not always feasible. Indirect treatment comparisons are a valuable tool to obtain estimates of the comparative efficacy of treatments that have not been compared in an RCT [8]. The matching-adjusted indirect comparison (MAIC) method applies weights to the patients in the individual patient-level data (IPD) to match the reported baseline patient characteristics of the population in the external study, thus minimizing biases due to population differences and enabling an indirect comparison of the interventions in each of the studies [8–10]. The outcome of the MAIC is a measure of relative efficacy, such as a hazard ratio (HR), of the two interventions of interest that applies to a population such as that of the published external study [8].
The use of MAICs and other population-adjustment methods has been increasing across several therapeutic areas in recent years, and so has acceptability from regulatory and health technology assessment bodies [11–14], with indirect treatment comparisons often being used in oncology to support decision-making globally [13]. The present MAIC analysis closely follows the principles and guidelines established by organizations such as the National Institute for Health and Care Excellence (NICE) and the International Society for Pharmacoeconomics and Outcomes Research (ISPOR) [15,16].
MAICs have been previously conducted to compare efficacy between ARPIs, such as between darolutamide and apalutamide [17,18] and between enzalutamide and apalutamide [17,19], in non-metastatic castration-resistant prostate cancer (nmCRPC). However, there is a lack of direct evidence from RCTs comparing the outcomes of enzalutamide + ADT and darolutamide + ADT for patients with mHSPC. Thus, this study aimed to assess the relative efficacy of enzalutamide + ADT versus darolutamide + ADT in patients with mHSPC, using MAIC methodology and data from ARCHES and ARANOTE.
2. Patients & methods
2.1. Data sources
ARCHES was a global, double-blind, phase 3 trial wherein patients with mHSPC were randomly assigned 1:1 to enzalutamide (160 mg administered orally once daily) + ADT or placebo + ADT, stratified by disease volume and prior docetaxel chemotherapy [4]. The current study utilized IPD from the ARCHES primary analysis (median follow-up: 14.4 months) [4]. ARANOTE was a global, double-blind, phase 3 trial wherein patients with mHSPC were randomly assigned 2:1 to receive darolutamide (600 mg administered orally twice daily) + ADT or placebo + ADT [4]. The current study utilized published aggregated data from the primary analysis of ARANOTE (median follow-up: 25.3 months) [5]. In both ARCHES and ARANOTE, radiographic progression-free survival (rPFS) was the primary endpoint, while secondary endpoints included time to castration resistance, time to prostate-specific antigen (PSA) progression, and time to initiation of new antineoplastic therapy [4,5].
2.2. Identification of treatment effect modifiers
The identification of potential treatment effect modifiers to adjust for in the MAIC was informed by a targeted literature review, including the findings of subgroup analysis across RCTs in mHSPC, interviews with clinical experts, and a statistical analysis of ARCHES IPD using linear/Cox regression models featuring an interaction between treatment and the covariate of interest, for which statistical significance was analyzed (significance threshold 0.2).
Overall, the following variables were deemed to have strong effect-modifying potential: initial diagnosis (de novo/recurrent) [20], disease volume (high vs low) [21], and visceral metastatic disease [22]. The following variables were deemed to have moderate effect-modifying potential: age [23,24], Gleason score [25,26], race [27–31], region [32–35], and prior use of docetaxel [36]. The clinical expert consultations and statistical analysis supported this set of potential effect modifiers.
While Eastern Cooperative Oncology Group Performance Status (ECOG PS) is an important prognostic factor for patients with metastatic prostate cancer, many RCTs in oncology tend to focus on healthier patients, specifically those with an ECOG PS of 0 to 1 [37], which does not provide enough differentiation in terms of treatment effect-modifying potential. This is also evident from the stratification of the CHAARTED and LATITUDE studies according to ECOG PS (0/1 vs 2) [38,39], which suggests that the effect-modifying impact of ECOG PS may manifest when comparing patients with an ECOG PS of 0 or 1 to those with an ECOG PS of 2 or higher. Furthermore, ARANOTE enrolled patients with an ECOG PS of 0–2 (with only a small proportion of patients treated with darolutamide + ADT [2.7%] having an ECOG PS of 2), while ARCHES enrolled only patients with ECOG 0 or 1 [4,5].
2.3. Feasibility assessment for MAIC
To ensure that the studies of interest were sufficiently similar for performing the MAIC, a feasibility assessment was conducted. The objective of this assessment was to identify sources of potential heterogeneity that could impact the relative efficacy estimates. Specifically, comparability of ARCHES and ARANOTE was assessed across the following dimensions: study design, patient population, and outcome definitions and availability.
2.4. Statistical analyses
Using an anchored MAIC [9,10] (Figure 1), IPD from ARCHES (enzalutamide + ADT vs placebo + ADT) were adjusted to match baseline characteristics of published aggregate data from ARANOTE (darolutamide + ADT vs placebo + ADT).
Figure 1.
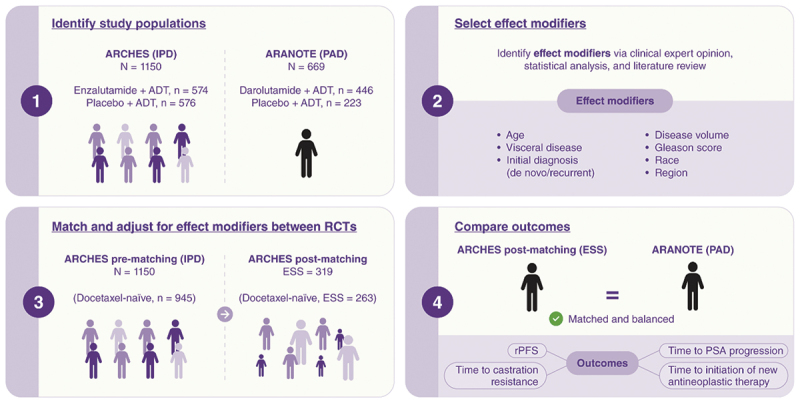
Matching-adjusted indirect comparison methodology used to compare the efficacy of enzalutamide + ADT (ARCHES) and darolutamide + ADT (ARANOTE).
ADT, androgen-deprivation therapy; ESS, effective sample size; IPD, individual patient-level data; PAD, published aggregate data; PSA, prostate-specific antigen; RCT, randomized controlled trial; rPFS radiographic progression-free survival.
Adjustment weights were estimated using the quasi-Newton optimization method [40] to account for discrepancies in any potential treatment effect modifiers across populations. The effect modifiers adjusted for in the base case of the MAIC included age, visceral metastatic disease, metastatic status at initial diagnosis (de novo/recurrent), disease volume (high vs low), Gleason score, race, and region. The distribution of MAIC weights was analyzed.
Next, the HR obtained from the weighted ARCHES IPD was compared against the published HR from ARANOTE using Cox proportional hazards models for all outcomes for which the MAIC was deemed feasible. The MAIC was performed using the intention-to-treat (ITT) population in the ARCHES study. However, because patients in ARANOTE were docetaxel-naïve [5] and prior exposure to docetaxel was considered a treatment effect modifier, the MAIC was also performed for the subgroup of docetaxel-naïve patients in ARCHES.
Analyses were also conducted using the Bucher method, which does not adjust for differences between patient populations, as it assumes that populations in the evidence base are comparable with respect to the distribution of effect modifiers [41]. This method was employed to assess the impact of adjusting for population differences between ARCHES and ARANOTE on the relative efficacy estimates.
Sensitivity analyses adjusting for ECOG PS as an additional effect modifier, or only disease volume and metastatic stage at initial diagnosis, were performed to assess the robustness of the results. Across all analyses, a 5% threshold for statistical significance was used.
Since the MAIC analyses summarize the relative efficacy of enzalutamide + ADT versus darolutamide + ADT using a Cox proportional hazards model, the plausibility of the proportional hazards assumption was assessed for each outcome and population of interest. This was done according to the methods recommended by the NICE Decision Support Unit Technical Support Document 14 [42].
3. Results
3.1. Feasibility assessment for MAIC
3.1.1. Study design
Differences in sample size, geography, and length of follow-up were detected between the ARCHES (N = 1150; enzalutamide + ADT, n = 574; placebo + ADT, n = 576; median follow-up [ARCHES primary analysis] [4]: 14.4 months) and ARANOTE [5] (N = 669; darolutamide + ADT, n = 446; placebo + ADT, n = 223; median follow-up: 25.3 months) study populations (Table 1).
Table 1.
Differences in study design between the ARCHES and ARANOTE trials.
| ARCHES | ARANOTE | |
|---|---|---|
| Efficacy population | Intention-to-treat: all randomized subjects, analyzed by treatment arm as per randomization | Full analysis set: all randomized patients, analyzed by treatment as per randomization |
| Sample size in efficacy population | Enzalutamide + ADT: 574; Placebo + ADT: 576 | Darolutamide + ADT: 446; Placebo + ADT: 223 |
| Geography | Argentina, Australia, Belgium, Canada, Chile, Denmark, Finland, France, Germany, Israel, Italy, Japan, Korea, New Zealand, Poland, Romania, Russia, Slovakia, Spain, Sweden, Taiwan, United Kingdom, United States of America | Australia, Brazil, Canada, Chile, China, India, Latvia, Lithuania, New Zealand, Peru, Russia, South Africa, Spain, Taiwan, Ukraine |
| Treatment discontinuation | Until disease progression, unacceptable toxicity, or any other discontinuation criteria were met | Until disease progression, unacceptable toxicity, initiation of new anticancer therapy, patient/physician decision, or study drug interruption for ≥ 28 consecutive days |
| Median follow-up | 14.4 months (first data cutoff date, based on rPFS) | 25.3 monthsa (first data cutoff date, based on rPFS) |
| Study unblinding and treatment crossover | Study unblinding took place after the primary analysis (first data cutoff date) and 180 (31.3%) progression-free patients assigned to placebo + ADT crossed over to open-label enzalutamide + ADT | After primary analysis, and conditional on positive results, an open-label phase may start, which would imply a crossover of patients on placebo + ADT to darolutamide + ADT |
| Schedule of visits | Treatment period: Visit 1 (Day 1); Visit 2 (Week 5); Visit 3 (Week 13) and subsequent visits every 12 weeks Safety FUP period: 30 days after last dose or before initiation of new antineoplastic therapy, whichever occurred first Long-term FUP period: approximately every 12 weeks after safety FUP periodb |
Treatment period: Visit 1 (Day 1); Visit 2 (Week 12) and subsequent visits every 12 weeks Active FUP period:
|
| Schedule of assessments | Assessed every 12 weeks
|
Assessed every 12 weeks
|
| Endpoints (primary and secondary) |
|
|
aBased on the rPFS endpoint.
bIn ARCHES: after treatment discontinuation, all patients had to undergo long-term follow-up. Long-term follow-up assessments included monitoring for survival status, new antineoplastic therapies for prostate cancer, and symptomatic skeletal events. Follow-up could have been conducted by telephone interview. Patients continued to be scanned every 12 weeks until radiographic progression was confirmed by independent review or the number of rPFS events was reached. For patients continuing with radiological assessments, if seen in clinic, QoL assessment was also completed until the start of new antineoplastic therapy for prostate cancer or the number of progression events was reached. Additional follow-up contacts could have been requested. Patients were followed for overall survival until death, lost to follow-up, final overall survival analysis, or termination of the study by the sponsor.
cIn ARANOTE, PSA was not evaluated on the date of randomization, but the first PSA assessment took place anywhere within 28 days before randomization and then 12 weeks after randomization.
ADT, androgen-deprivation therapy; BPI-SF, Brief Pain Inventory – Short Form; CT, computed tomography; EoT, end of treatment; FUP, follow-up; MRI, magnetic resonance imaging; PSA, prostate-specific antigen; QoL, quality of life; rPFS, radiographic progression-free survival.
3.1.2. Population
All patients in ARANOTE were docetaxel-naïve as patients with prior docetaxel use were excluded from this trial, whereas 17.9% of patients treated with enzalutamide + ADT and 17.7% of patients treated with placebo + ADT in ARCHES had prior docetaxel exposure (Table 2). Differences were also evident in specifications for prior use and timing of luteinizing hormone-releasing hormone (LHRH) treatment, neoadjuvant and/or adjuvant therapy, and radiotherapy (Table 3).
Table 2.
Comparison of disease and demographic characteristics at baseline between the ARCHES and ARANOTE trials.
| ARCHES |
ARANOTE |
|||
|---|---|---|---|---|
| Population | Enzalutamide + ADT, n = 574 |
Placebo + ADT, n = 576 |
Darolutamide + ADT, n = 446 |
Placebo + ADT, n = 223 |
| Median age, years (range) | 70 (46–92) | 70 (42–92) | 70 (43–93) | 70 (45–91) |
| Race, n (%) | ||||
| White | 466 (81.2) | 460 (79.9) | 251 (56.3) | 125 (56.1) |
| Asian | 75 (13.1) | 80 (13.9) | 144 (32.3) | 65 (29.1) |
| Black or African American | 8 (1.4) | 8 (1.4) | 41 (9.2) | 24 (10.8) |
| Region, n (%) | ||||
| North America | 86 (15.0) | 77 (13.4) | 0 (0.0) | 0 (0.0) |
| Europe and RoWa | 341 (59.4) | 344 (59.7) | 186 (41.7) | 88 (39.5) |
| Latin America | 32 (5.6) | 30 (5.2) | 119 (26.7) | 72 (32.3) |
| Asia | 104 (18.1) | 113 (19.6) | 141 (31.6) | 63 (28.3) |
| Other | 11 (1.9) | 12 (2.1) | 0 (0.0) | 0 (0.0) |
| ECOG PS, n (%) | ||||
| 0 | 448 (78.0) | 443 (76.9) | 235 (52.7) | 98 (43.9) |
| 1 | 125 (21.8) | 133 (23.1) | 199 (44.6) | 117 (52.5) |
| Any prior use of docetaxel, n (%) | 103 (17.9) | 102 (17.7) | 0 (0.0) | 0 (0.0) |
| Visceral disease present, n (%) | 64 (11.1) | 64 (11.1) | 53 (11.9) | 27 (12.1) |
| Any prior use of ADT, n (%) | 534 (93.2) | 514 (89.2) | 446 (100) | 223 (100) |
| Median PSA (range), ng/mL | 5.4 (0.0–4,823.5) | 5.1 (0.0–19,000.0) | 21.4 (0.02–15,915.0) | 21.2 (0.02–8,533.0) |
| Gleason score ≥ 8, n (%) | 386 (67.2) | 373 (64.8) | 311 (69.7) | 146 (65.5) |
aIncludes 2 patients from Canada in ARANOTE.
ADT, androgen-deprivation therapy; ECOG PS, Eastern Cooperative Oncology Group Performance Status; PSA, prostate-specific antigen; RoW, rest of the world.
Table 3.
Enrollment criteria for ARCHES and ARANOTE trials.
| Eligibility criteria | ARCHES | ARANOTE |
|---|---|---|
| Prior treatment with docetaxel | Included | Excluded |
| Histologically or cytologically confirmed adenocarcinoma of prostate | Included | Included |
| Metastatic prostate cancer documented by positive bone scan (for bone disease) or metastatic lesions on CT or MRI scan (for soft tissue) | Included | Included |
| Use of systemic corticosteroid with dose greater than the equivalent 10 mg of prednisone/day within 28 days before randomization | Excluded | Excluded |
| Maintained ADT with an LHRH agonist/receptor antagonist during study treatment | Included | Included |
| Prior treatment with LHRH agonist/antagonists started >3 months before randomization | Included if treated with LHRH for a period up to 6 months before randomization | Excludeda |
| Prior neoadjuvant and/or adjuvant therapy | Included if given for a duration <39 months and >9 months before randomization | Included if given for a duration ≤24 months and completed ≥12 months before randomization |
| Prior treatment with radiotherapy | Excluded if received ≤4 weeks before randomization | Excluded if received ≤2 weeks before randomization |
| ECOG PS of 0, 1, or 2 | Excluded if ECOG PS equal to 2 | Included |
aIn ARANOTE: all patients started ADT of the investigator’s choice (LHRH agonist or antagonists, or orchiectomy) within 12 weeks before initiating study treatment.
ADT, androgen-deprivation therapy; CT, computed tomography; ECOG PS, Eastern Cooperative Oncology Group Performance Status; LHRH, luteinizing hormone-releasing hormone; MRI, magnetic resonance imaging.
Imbalances between the ARCHES and ARANOTE patient populations were detected in race and region (Table 2). A higher percentage of patients were Asian in ARANOTE (31.2%) than in ARCHES (13.5%), which was noted as a potential effect modifier. When considering investigational sites in Europe, most sites in ARANOTE were in Eastern Europe, while ARCHES sites were primarily located in Northern/Western Europe. No patients from the United States were included in ARANOTE (Table 1).
When considering disease characteristics, differences were evident in the distribution of ECOG PS and baseline PSA levels (Table 2). In ARCHES, 77.4% of patients had an ECOG PS of 0, compared with 49.8% of patients in ARANOTE. Also, an ECOG PS of 2 was exclusionary in ARCHES, in contrast to ARANOTE, which included patients with an ECOG PS of 2 (Table 3). With respect to baseline PSA levels, median (range) PSA was considerably higher in ARANOTE (darolutamide + ADT: 21.4 [0.02–15,915.0] ng/mL; placebo + ADT: 21.2 [0.02–8,533.0] ng/mL) compared with ARCHES (enzalutamide + ADT: 5.4 [0.0–4,823.5] ng/mL; placebo + ADT: 5.1 [0.0–19,000.0] ng/mL), likely reflecting differences in ADT exposure before study entry. Furthermore, distribution of high-volume disease (ARCHES: 63.2%; ARANOTE: 70.5%) and metastatic disease at initial diagnosis (ARCHES: 66.7%; ARANOTE: 72.5%) were broadly comparable between trials.
3.1.3. Outcomes
Efficacy outcomes for which MAICs between ARCHES and ARANOTE were deemed feasible included rPFS, time to castration resistance, time to PSA progression, and time to initiation of new antineoplastic therapy. The definitions of these efficacy outcomes were consistent between ARCHES and ARANOTE:
rPFS was defined as the time from randomization to the first objective evidence of radiographic disease progression or death. Progressive disease was defined by Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1 for soft tissue disease, or appearance of two or more new lesions on bone scan compared with the baseline scan (ARCHES) or Prostate Cancer Working Group 3 criteria for bone metastasis (ARANOTE) [4,5].
Time to castration resistance was defined as the time from randomization to the first castration-resistant event (radiographic disease progression, PSA progression, or symptomatic skeletal event), whichever occurred first [4,5].
Time to PSA progression was defined as the time from randomization to the date of first PSA progression (i.e., ≥25% increase and an absolute increase of ≥2 ng/mL above the nadir) [4,5].
Time to initiation of new antineoplastic therapy was defined as time from randomization to the date of initiation of first subsequent systemic anticancer therapy for prostate cancer [4,5].
3.2. Matching process
While there were differences in patient baseline characteristics between ARCHES and ARANOTE before matching, the baseline characteristics were balanced after matching in both the ITT and the docetaxel-naïve populations (Table 4). The resulting effective sample size (ESS) for ARCHES was 319 patients for the ITT population and 263 for the docetaxel-naïve population. All effect modifiers adjusted for in the MAIC were binary/discrete variables.
Table 4.
Baseline characteristics in ARANOTE and ARCHES pre- and post-matching.
| ARCHES (pre-matching) N = 1150 |
ARANOTE N = 669 |
ARCHES (post-matching) N (ESS) = 319 |
|
|---|---|---|---|
| Age (years) | |||
| <65 | 26.1 | 27.4 | 27.4 |
| 65–74 | 44.4 | 43.2 | 43.2 |
| Race (%) | |||
| White | 80.4 | 56.2 | 56.2 |
| Asian | 13.5 | 31.2 | 31.2 |
| Black or African American | 1.4 | 9.7 | 9.7 |
| Region (%) | |||
| Europe and RoW | 75.7 | 41.0 | 41.0 |
| Latin America | 5.4 | 28.6 | 28.6 |
| Visceral disease present (%) | 10.8 | 12.0 | 12.0 |
| Gleason score ≥ 8 (%) | 66.0 | 68.3 | 68.3 |
| Metastasis (%) | |||
| De novo | 66.7 | 72.5 | 72.5 |
| Recurrent | 14.7 | 21.7 | 21.7 |
| High disease volume (%) | 63.2 | 70.5 | 70.5 |
ESS, effective sample size; RoW, rest of the world.
3.3. Comparative efficacy evaluation
3.3.1. MAIC analyses
Results from the MAIC analyses of the ITT population showed that patients treated with enzalutamide + ADT had a statistically significant lower risk of radiographic progression or death (HR [95% CI]: 0.54 [0.32–0.93], p = 0.03) and of progression to castration resistance (HR [95% CI]: 0.57 [0.34–0.94], p = 0.03) than those receiving darolutamide + ADT (Figure 2). Similar results were observed in the docetaxel-naïve population (rPFS HR [95% CI]: 0.47 [0.26–0.84], p = 0.01; time to castration resistance HR [95% CI]: 0.46 [0.27–0.79], p = 0.01). Results for time to PSA progression favored enzalutamide + ADT compared with darolutamide + ADT, but the difference was not statistically significant (HR [95% CI]: 0.61 [0.29–1.30], p = 0.20). Similar findings for time to PSA progression results were observed in the docetaxel-naïve population (HR [95% CI]: 0.48 [0.21–1.10], p = 0.08). Results for time to initiation of new antineoplastic therapy also favored enzalutamide + ADT over darolutamide + ADT, but the difference was not statistically significant (HR [95% CI]: 0.65 [0.34–1.24], p = 0.19). Similar results for time to initiation of new antineoplastic therapy were observed in the docetaxel-naïve population (HR [95% CI]: 0.57 [0.28–1.17], p = 0.13).
Figure 2.
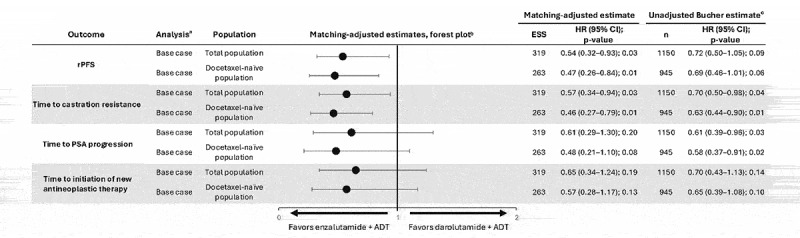
Indirect treatment comparison of enzalutamide + ADT versus darolutamide + ADT.
aEffect modifiers adjusted for in the MAIC analysis (base case): age, visceral disease, metastatic status at initial diagnosis (de novo/recurrent), disease volume, Gleason score, race, and region.
bDarolutamide + ADT served as the reference treatment for comparisons. HR < 1 favors enzalutamide + ADT.
cEstimates from the Bucher method should be interpreted with caution due to the assumption that patient populations should be balanced in effect modifiers between trials. The direction of relative effects was aligned between the two methods.ADT, androgen-deprivation therapy; CI, confidence interval; ECOG PS, Eastern Cooperative Oncology Group Performance Status; ESS, effective sample size; HR, hazard ratio; MAIC, matching-adjusted indirect comparison; n, number; PSA, prostate-specific antigen; rPFS, radiographic progression-free survival.
3.3.2. Bucher analyses
Overall, results of the Bucher analyses were broadly consistent with the MAIC results (Figure 2). Patients treated with enzalutamide + ADT had a numerically, but not significantly, lower risk of radiographic progression or death and longer time to initiation of new antineoplastic therapy. Patients treated with enzalutamide + ADT had a significantly longer time to castration resistance and time to PSA progression.
3.3.3. Sensitivity analyses
In the sensitivity analyses, adjusting for ECOG PS yielded a considerable reduction in ESS to 196 for the ITT population. These sensitivity analyses provided results consistent with the MAIC base case analyses that did not adjust for ECOG PS (Figure 3). When adjusting only for disease volume and metastatic stage at initial diagnosis—variables considered of major clinical importance in the mHSPC setting and frequently used in treatment guidelines, such as the NCCN Guidelines® [2]—the results were directionally consistent with the base case analysis (Figure 3).
Figure 3.
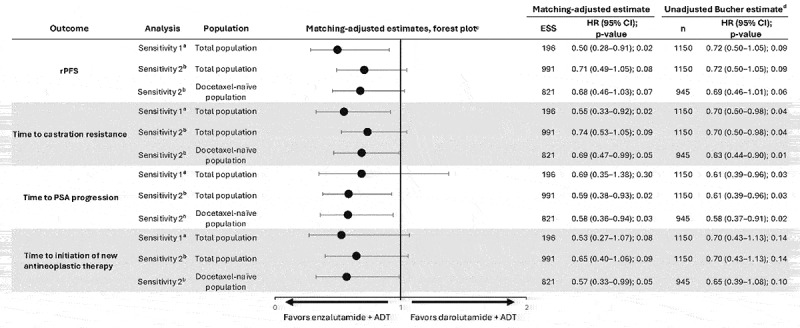
Sensitivity analyses for indirect treatment comparison of enzalutamide + ADT versus darolutamide + ADT.
aEffect modifiers adjusted for in sensitivity analysis 1: base case (age, visceral disease, metastatic status at initial diagnosis [de novo/recurrent], disease volume, Gleason score, race, and region) plus adjustment for ECOG PS.
bEffect modifiers adjusted for in sensitivity analysis 2: disease volume, metastatic status at initial diagnosis (de novo/recurrent).
cDarolutamide + ADT served as the reference treatment for comparisons. HR < 1 favors enzalutamide + ADT.
dEstimates from the Bucher method should be interpreted with caution due to the assumption that patient populations should be balanced in effect modifiers between trials. The direction of relative effects was aligned between the two methods.ADT, androgen-deprivation therapy; CI, confidence interval; ECOG PS, Eastern Cooperative Oncology Group Performance Status; ESS, effective sample size; HR, hazard ratio; MAIC, matching-adjusted indirect comparison; n, number; PSA, prostate-specific antigen; rPFS, radiographic progression-free survival.
3.4. Proportional hazards assumption
The results from the proportional hazards assessment indicated that the assumption was plausible in the base case analyses for all outcomes in both the ITT and the docetaxel-naïve populations.
4. Discussion
This study assessed the relative efficacy of enzalutamide + ADT versus darolutamide + ADT in patients with mHSPC using well-established MAIC methodology, utilizing evidence from the ARCHES and ARANOTE RCTs. Results showed that enzalutamide + ADT in patients with mHSPC was associated with a 46% lower risk of radiographic progression or death and a 43% lower risk of progression to castration resistance compared with darolutamide + ADT. Similar but slightly more favorable results were observed in the docetaxel-naïve population, in which treatment with enzalutamide + ADT showed significantly lower risk of radiographic progression or death by 53% and progression to castration resistance by 54% compared with darolutamide + ADT. There were no statistically significant differences between the trials for time to PSA progression or time to initiation of new antineoplastic treatment, albeit the point estimates suggested lower risk for patients receiving enzalutamide + ADT compared with those receiving darolutamide + ADT. These comparative efficacy results apply to a population like that of ARANOTE, which is docetaxel-naïve, and may not apply to a population with prior exposure to docetaxel. Moreover, despite being an unadjusted treatment comparison, the Bucher analyses revealed similar results to those of the MAIC analyses.
To our knowledge, this is the first MAIC analysis to assess efficacy of enzalutamide + ADT versus darolutamide + ADT in patients with mHSPC. The current findings align with previous efficacy comparisons of enzalutamide and darolutamide in patients with castration-resistant prostate cancer (CRPC) [43,44]. A network meta-analysis demonstrated favorable efficacy (as per metastasis-free survival and PSA progression-free survival) for enzalutamide over darolutamide in patients with nmCRPC [44]. Additionally, a phase 2, prospective study demonstrated numerically better efficacy (as per PSA decline and progression-free survival) with enzalutamide compared with darolutamide in patients with metastatic CRPC [43].
This MAIC utilized data from the primary analysis of ARCHES [4], rather than the final analysis [7], to avoid confounding due to crossover in the final ARCHES data cutoff. Thus, this MAIC used the latest available data for ARCHES before crossover. However, despite the differences in follow-up between the two trials, the results remained robust. Various assessments suggested that the proportional hazards assumption was plausible for the base case analyses of all outcomes assessed, alleviating potential concerns about differences in length of the follow-up between the two trials. Moreover, for rPFS, both ARCHES and ARANOTE were designed to demonstrate a benefit over placebo based on the prespecified number of rPFS events required [4,5]. This was achieved at the first data cutoff in both trials (used in the MAIC) with the required number of events for power and sample size calculations being reached (ARCHES: October 2018, 292 rPFS events; ARANOTE: June 2024, 222 rPFS events) [4,5].
While ARCHES and ARANOTE included OS as a secondary endpoint, there were a few key reasons why the present MAIC did not analyze this outcome. First, ARCHES included a final analysis of OS [7], whereas OS data from ARANOTE were available only from an interim analysis at the time of this study [5]. Second, in ARCHES, 31.3% of patients receiving placebo crossed over to receive enzalutamide [7], confounding the HR from the final data cutoff, while ARANOTE did not include a crossover [5]. Therefore, any indirect treatment comparison results using the final OS data from ARCHES might be biased to some extent due to the crossover. Third, at the first cutoff, OS data in ARANOTE were more mature than in ARCHES. ARCHES had 84 deaths (24.6% of required events) [4] while ARANOTE had 163 deaths (90.6% of required events) [5]. Therefore, comparing OS between the two trials would be subject to a high risk of bias.
4.1. Limitations
As in all MAIC analyses, the existence of any unknown or unobserved effect modifiers, which cannot be precluded, would bias the relative efficacy estimates. For example, the significant difference in median baseline PSA and the inability to adjust for it due to confounding by ADT timing is a major limitation. That is, the higher median PSA observed in the ARANOTE population may have resulted from ADT initiation occurring close to the date of randomization, as ADT initiation >3 months from randomization was exclusionary in this RCT [5]. Additionally, although the exact timing of ADT initiation was not reported in ARANOTE, the PSA assessment took place after ADT initiation within the previous 12 weeks [5]. However, a post hoc analysis of ARCHES showed that rPFS treatment effects were comparable irrespective of PSA levels measured after ADT start but before initiating enzalutamide [45], suggesting that differences in ADT initiation and timing of PSA assessments were unlikely to affect the rPFS results reported in this study. While pre-ADT treatment PSA levels would be an important effect modifier to adjust for in the MAIC, such information was not available, making baseline PSA values incomparable.
There was a poor overlap between some characteristics of the ARCHES and ARANOTE populations, namely region (i.e., location of investigational sites), race, and ECOG PS. Hence, the present MAIC adjusted for a broad set of potential effect modifiers. Including these variables in the model was crucial for reliable estimates of comparative efficacy but led to a significant (72%) reduction in ESS from the original sample size. These reductions were inevitable due to the differences between ARCHES and ARANOTE. However, similar reductions have been reported in health technology assessment submissions and other oncology studies [16,46]. Moreover, the initially large sample size of ARCHES ensured that this reduction in sample size was not prohibitive for deriving inferences. The distribution of weights reflected the observed reduction in ESS and exhibited left-skewness (median 0.44, interquartile range [0.25, 0.82]), indicating that a small subset of patients more closely aligned with the ARANOTE population received higher weights compared with the average patient enrolled in ARCHES. This pattern is common in MAICs and the distribution of weights did not suggest any major concerns.
However, biases in the relative efficacy estimates may have remained after adjustment due to inherent differences between the ARCHES and ARANOTE trials (e.g., differences in tumor genetics and/or the study timeframe), which could not be entirely adjusted for in the MAIC. Furthermore, the implied direction of bias, which may vary across outcomes, is difficult to anticipate.
Lastly, a significant gap in this analysis is that it focused on efficacy and did not consider safety outcomes. All efficacy analyses were conducted within a time-to-event framework, which inherently accounts for differences in follow-up duration. To compare safety, adjusting for treatment exposure would be necessary due to differences in observed follow-up between the ARCHES and ARANOTE trials. However, the lack of exposure-adjusted safety data from ARANOTE for the most comprehensive safety measures, such as any AE/treatment-emergent AE (TEAE) or grade 3–4 AE/TEAE, precluded a robust quantitative comparison of the safety profiles of enzalutamide + ADT versus darolutamide + ADT.
5. Conclusions
This is the first MAIC study to compare efficacy outcomes of combining ADT with enzalutamide or darolutamide for patients with mHSPC. Notably, enzalutamide + ADT resulted in a significantly lower risk of radiographic progression or death and progression to castration resistance compared with darolutamide + ADT in patients with mHSPC. As multiple ARPI options are currently available, the findings from this study, along with other patient factors, will help guide treatment decisions for management of mHSPC.
Funding Statement
This study was funded by Astellas Pharma Inc. and Pfizer Inc., the co-developers of enzalutamide.
Article highlights
Currently, no randomized controlled trials (RCTs) compare efficacy outcomes of enzalutamide and darolutamide (two androgen receptor pathway inhibitors [ARPIs] commonly used for the treatment of metastatic hormone-sensitive prostate cancer [mHSPC]) combined with androgen-deprivation therapy (ADT) for mHSPC treatment.
Matching-adjusted indirect comparisons (MAICs) enable indirect comparisons by adjusting for differences in effect modifiers between RCTs, thus minimizing biases due to population differences.
MAIC methodology was therefore used to compare the efficacy of enzalutamide + ADT (from the ARCHES trial) and darolutamide + ADT (from the ARANOTE trial) in patients with mHSPC.
Compared with darolutamide + ADT, enzalutamide + ADT significantly prolonged radiographic progression-free survival and time to castration resistance in patients with mHSPC.
Author contribution
All authors (Andrew J. Armstrong, Bhavik J. Pandya, Hemant Singh Bhadauria, Arijit Ganguli, Vagia Daki, Ana Moura, and Arun A. Azad) made a substantial contribution to study design and interpretation of study data. Vagia Daki and Ana Moura additionally made significant contributions to the acquisition and analysis of the study data.
All authors have drafted or written or substantially revised or critically reviewed the article, have agreed on the journal to which the article will be submitted, have reviewed and agreed on all versions of the article before submission, during revision, the final version accepted for publication, and any significant changes introduced at the proofing stage. All authors agree to take responsibility and be accountable for the contents of the article and to share responsibility to resolve any questions raised about the accuracy or integrity of the published work.
Disclosure statement
AJA reports having received study funding and support for medical writing/editing from Astellas; consulting fees from Astellas, AstraZeneca, Bayer, Bristol Myers Squibb, Clovis, Dendreon, Merck, and Pfizer; payment or honoraria for lectures, presentations, speakers’ bureaus, publication writing or educational events from Astellas; support for attending meetings and/or travel from Astellas; research funding from Astellas, AstraZeneca, Bayer, BeiGene, Dendreon, Bayer, Bristol Myers Squibb, Constellation, Gilead, Janssen, Merck, Novartis, Pfizer, and Genentech; has participated on a Data Safety Monitoring Board or Advisory Board for Astellas, AstraZeneca, Bayer, Bristol Myers Squibb, Clovis, Dendreon, Merck, and Pfizer; and has a patent planned, issued, or pending for circulating tumor cell novel capture technology.
BJP is an employee of Astellas Pharma Inc. and is a board member of Sky Schools.
HSB is an employee of Astellas Pharma Inc.
AG is an employee of Astellas Pharma Inc. and holds stocks in AbbVie Inc.
VD is an employee of IQVIA.
AM is an employee of IQVIA.
AAA reports having received study funding and support for medical writing/editing from Astellas; consulting fees from Amgen, Astellas, AstraZeneca, Bayer, Bristol Myers Squibb, Ipsen, Janssen, Merck, Merck Serono, Novartis, Noxopharm, Sanofi, Telix, and Tolmar; payment or honoraria for lectures, presentations, publication writing or educational events from Amgen, Arvinas, Astellas, AstraZeneca, Bayer, Bristol Myers Squibb, Daiichi Sankyo, Ipsen, Janssen, Merck Serono, Merck Sharp & Dohme, Noxopharm, Novartis, Pfizer, Sanofi, Telix, and Tolmar; payment or honoraria for speakers’ bureaus from Amgen, Astellas, Bayer, Bristol Myers Squibb, Ipsen, Janssen, Merck Serono, and Novartis; support for attending meetings and/or travel from Amgen, Astellas, Bayer, Janssen, Hinova, Merck Serono, Pfizer, Sanofi, and Tolmar; research funding from Aptevo, Astellas, AstraZeneca, Bionomics, Bristol Myers Squibb, GlaxoSmithKline, Ipsen, MedImmune, Merck Serono, Novartis, Pfizer, Sanofi, and Synthorx; and has participated on a Data Safety Monitoring Board or Advisory Board for Amgen, Astellas, AstraZeneca, Bayer, Bristol Myers Squibb, Daiichi Sankyo, Ipsen, Janssen, Merck, Merck Serono, Novartis, Noxopharm, Sanofi, Telix, and Tolmar.
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in, or financial conflict with, the subject matter or materials discussed in the manuscript, apart from those disclosed.
Reviewer disclosure
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose
Writing disclosure
Medical writing, editing, and graphic design support was provided by Kayla Stone (PhD), Arshiya Hakim (PhD), Jay Patel (PharmD), Daria Renshaw (BA), Nathaniel Grubbs (PhD), and Florencia Dobler from IQVIA, and was funded by the study sponsors.
Ethical conduct of research
This article is based on previously conducted studies and does not involve any new studies of human or animal subjects performed by any of the authors. The ARCHES study was sponsored by Astellas Pharma Inc. and Pfizer Inc., and all appropriate ethics approvals were granted. The ARANOTE study was sponsored by Bayer and Orion Pharma, and all appropriate ethics approvals were granted. Both studies were conducted in accordance with the Declaration of Helsinki and Good Clinical Practice Guidelines of the International Council for Harmonisation. All patients provided written informed consent.
Data availability statement
Details for how researchers may request access to anonymized participant-level data, trial-level data, and protocols from Astellas-sponsored clinical trials can be found at https://www.clinicaltrials.astellas.com/transparency/.
References
Papers of special note have been highlighted as either of interest (•) or of considerable interest (••) to readers.
- 1.Feng DC, Li DX, Wu RC, et al. Global burden and cross-country inequalities in urinary tumors from 1990 to 2021 and predicted incidence changes to 2046. Mil Med Res. 2025;12(1):12. doi: 10.1186/s40779-025-00599-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Referenced with permission from the NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for Prostate Cancer V.2.2025. © National Comprehensive Cancer Network, Inc; 2025. All rights reserved [cited 2025 Apr 23]. All rights reserved. [Google Scholar]
- 3.European Association of Urology . EAU - EANM - ESTRO - ESUR - ISUP - SIOG Guidelines on prostate cancer. 2024. [cited 2025 Apr 16]. Available from: https://d56bochluxqnz.cloudfront.net/documents/full-guideline/EAU-EANM-ESTRO-ESUR-ISUP-SIOG-Guidelines-on-Prostate-Cancer-2024_2024-04-09-132035_ypmy_2024-04-16-122605_lqpk.pdf
- 4.Armstrong AJ, Szmulewitz RZ, Petrylak DP, et al. ARCHES: a randomized, phase III study of androgen deprivation therapy with enzalutamide or placebo in men with metastatic hormone-sensitive prostate cancer. J Clin Oncol. 2019;37(32):2974–2986. doi: 10.1200/JCO.19.00799 [DOI] [PMC free article] [PubMed] [Google Scholar]; •• This primary analysis of the phase 3, ARCHES randomized controlled trial demonstrated that enzalutamide plus androgen-deprivation therapy (ADT) significantly reduced the risk of radiographic progression or death compared to placebo plus ADT in patients with metastatic hormone-sensitive prostate cancer. ARCHES data served as the independent patient data included in the current matching-adjusted indirect comparison.
- 5.Saad F, Vjaters E, Shore N, et al. Darolutamide in combination with androgen-deprivation therapy in patients with metastatic hormone-sensitive prostate cancer from the phase III ARANOTE trial. J Clin Oncol. 2024;42(36):4271–4281. doi: 10.1200/JCO-24-01798 [DOI] [PMC free article] [PubMed] [Google Scholar]; •• The phase 3, ARANOTE randomized controlled trial demonstrated that darolutamide plus androgen-deprivation therapy (ADT) significantly reduced the risk of radiographic progression or death compared with placebo plus ADT in men with metastatic hormone-sensitive prostate cancer. ARANOTE data served as the published aggregate data included in the current matching-adjusted indirect comparison.
- 6.Wang Z, Wang J, Li D, et al. Novel hormone therapies for advanced prostate cancer: understanding and countering drug resistance. J Pharm Anal. 2025:101232. doi: 10.1016/j.jpha.2025.101232 [DOI] [Google Scholar]
- 7.Armstrong AJ, Azad AA, Iguchi T, et al. Improved survival with enzalutamide in patients with metastatic hormone-sensitive prostate cancer. J Clin Oncol. 2022;40(15):1616–1622. doi: 10.1200/JCO.22.00193 [DOI] [PMC free article] [PubMed] [Google Scholar]; • The final analysis of the phase 3, ARCHES randomized controlled trial demonstrated that enzalutamide plus androgen-deprivation therapy (ADT) significantly prolonged overall survival compared with placebo plus ADT in patients with metastatic hormone-sensitive prostate cancer.
- 8.Aouni J, Gaudel-Dedieu N, Sebastien B.. Matching-adjusted indirect comparisons: application to time-to-event data. Stat Med. 2021;40(3):566–577. doi: 10.1002/sim.8789 [DOI] [PubMed] [Google Scholar]
- 9.Signorovitch JE, Sikirica V, Erder MH, et al. Matching-adjusted indirect comparisons: a new tool for timely comparative effectiveness research. Value Health. 2012;15(6):940–947. doi: 10.1016/j.jval.2012.05.004 [DOI] [PubMed] [Google Scholar]; • The paper introduces matching-adjusted indirect comparisons (MAICs) as a method to improve the reliability of comparative effectiveness research by using individual patient data to address differences across trials.
- 10.Phillippo DM, Ades AE, Dias S, et al. Methods for population-adjusted indirect comparisons in health technology appraisal. Med Decis Making. 2018;38(2):200–211. doi: 10.1177/0272989X17725740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thom H, Jugl S, Palaka E, et al. PRM167 matching adjusted indirect comparisons to assess comparative effectiveness of therapies: usage in scientific literature and health technology. Value Health. 2016;19(3):A100–A101. doi: 10.1016/j.jval.2016.03.1723 [DOI] [Google Scholar]
- 12.Pooley N, Kisomi M, Embleton N, et al. The increasing use of population-adjusted indirect comparisons in the NICE Health technology assessment (HTA) submission process and the response to these methods. Value Health. 2022;25(12):S49. doi: 10.1016/j.jval.2022.09.239 [DOI] [Google Scholar]
- 13.Igarashi A, Tanaka S, De Moor R, et al. Indirect treatment comparisons in healthcare decision making: a targeted review of regulatory approval, reimbursement, and pricing recommendations globally for oncology drugs in 2021–2023. Adv Ther. 2025;42(1):52–69. doi: 10.1007/s12325-024-03013-6 [DOI] [PMC free article] [PubMed] [Google Scholar]; • This review highlights the global adoption of indirect treatment comparisons for healthcare decision-making and its importance in the absence of direct evidence, particularly in oncology drug submissions.
- 14.Tanaka S, Igarashi A, De Moor R, et al. A targeted review of worldwide indirect treatment comparison guidelines and best practices. Value Health. 2024;27(9):1179–1190. doi: 10.1016/j.jval.2024.05.015 [DOI] [PubMed] [Google Scholar]
- 15.Jansen JP, Fleurence R, Devine B, et al. Interpreting indirect treatment comparisons and network meta-analysis for health-care decision making: report of the ISPOR Task Force on Indirect Treatment Comparisons Good Research Practices: part 1. Value Health. 2011;14(4):417–428. doi: 10.1016/j.jval.2011.04.002 [DOI] [PubMed] [Google Scholar]
- 16.Phillippo DM, Ades AE, Dias S, et al. NICE DSU Technical Support Document 18: Methods for population-adjusted indirect comparisons in submissions to NICE (Technical Support Documents). 2016. [cited 2025 Apr 16]. Available from: https://research-information.bris.ac.uk/ws/portalfiles/portal/94868463/Population_adjustment_TSD_FINAL.pdf; • This Technical Support Document examines methods for population-adjusted indirect comparisons in health technology assessments, focusing on matching-adjusted indirect comparisons and simulated treatment comparisons, and provides recommendations for their use in submissions to the National Institute for Health and Care Excellence (NICE).
- 17.Halabi S, Jiang S, Terasawa E, et al. Indirect comparison of darolutamide versus apalutamide and enzalutamide for nonmetastatic castration-resistant prostate cancer. J Urol. 2021;206(2):298–307. doi: 10.1097/JU.0000000000001767 [DOI] [PubMed] [Google Scholar]
- 18.Chowdhury S, Oudard S, Uemura H, et al. Apalutamide compared with darolutamide for the treatment of non-metastatic castration-resistant prostate cancer: Efficacy and tolerability in a matching-adjusted indirect comparison. Adv Ther. 2022;39(1):518–531. doi: 10.1007/s12325-021-01885-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tombal B, Sternberg CN, Hussain M, et al. Matching-adjusted indirect treatment comparison of the efficacy of enzalutamide versus apalutamide for the treatment of nonmetastatic castration-resistant prostate cancer. ESMO Open. 2022;7(3):100510. doi: 10.1016/j.esmoop.2022.100510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Francini E, Gray KP, Xie W, et al. Time of metastatic disease presentation and volume of disease are prognostic for metastatic hormone sensitive prostate cancer (mHSPC). Prostate. 2018;78(12):889–895. doi: 10.1002/pros.23645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Clarke NW, Ali A, Ingleby FC, et al. Addition of docetaxel to hormonal therapy in low- and high-burden metastatic hormone sensitive prostate cancer: long-term survival results from the STAMPEDE trial. Ann Oncol. 2019;30(12):1992–2003. doi: 10.1093/annonc/mdz396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lai GS, Chen CS, Cheng JC, et al. Impact of different visceral metastatic sites on survival in metastatic prostate cancer patients. PLOS ONE. 2024;19(9):e0309941. doi: 10.1371/journal.pone.0309941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schoen MW, Montgomery RB, Owens L, et al. Survival in patients with de novo metastatic prostate cancer. JAMA Netw Open. 2024;7(3):e241970. doi: 10.1001/jamanetworkopen.2024.1970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rajwa P, Yanagisawa T, Heidegger I, et al. Association between age and efficacy of combination systemic therapies in patients with metastatic hormone-sensitive prostate cancer: a systematic review and meta-analysis. Prostate Cancer Prostatic Dis. 2023;26(1):170–179. doi: 10.1038/s41391-022-00607-5 [DOI] [PubMed] [Google Scholar]
- 25.Fizazi K, Tran N, Fein L, et al. Abiraterone acetate plus prednisone in patients with newly diagnosed high-risk metastatic castration-sensitive prostate cancer (LATITUDE): final overall survival analysis of a randomised, double-blind, phase 3 trial. Lancet Oncol. 2019;20(5):686–700. doi: 10.1016/S1470-2045(19)30082-8 [DOI] [PubMed] [Google Scholar]
- 26.Suzuki K, Shiraishi Y, Furukawa J, et al. Clinical outcomes and risk stratification in patients with metastatic hormone-sensitive prostate cancer treated with new-generation androgen receptor signaling inhibitors. Clin Genitourin Cancer. 2024;22(5):102140. doi: 10.1016/j.clgc.2024.102140 [DOI] [PubMed] [Google Scholar]
- 27.Zhu Y, Mo M, Wei Y, et al. Epidemiology and genomics of prostate cancer in Asian men. Nat Rev Urol. 2021;18(5):282–301. doi: 10.1038/s41585-021-00442-8 [DOI] [PubMed] [Google Scholar]
- 28.Freedland SJ, Samjoo IA, Rosta E. The impact of race on survival in metastatic prostate cancer: a systematic literature review. Prostate Cancer Prostatic Dis. 2023;26(3):461–474. doi: 10.1038/s41391-023-00710-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rais-Bahrami S, Zhu Y. Disparities in prostate cancer diagnosis and management: recognizing that disparities exist at all junctures along the prostate cancer journey. Prostate Cancer Prostatic Dis. 2023;26(3):441–442. doi: 10.1038/s41391-023-00665-3 [DOI] [PubMed] [Google Scholar]
- 30.Diaz KA, Amaya SL, Garcia-Perdomo HA. Perspectives on prostate cancer: advances and pending challenges for a multidisciplinary oncological approach in South America. Int Urol Nephrol. 2024;56(1):1–7. doi: 10.1007/s11255-023-03753-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Freeman MN, Jang A, Zhu J, et al. Multi-institutional analysis of the clinical and genomic characteristics of black patients with metastatic hormone-sensitive prostate cancer. Oncologist. 2022;27(3):220–227. doi: 10.1093/oncolo/oyab057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jian T, Zhan Y, Yu Y, et al. Combination therapy for high-volume versus low-volume metastatic hormone-sensitive prostate cancer: a systematic review and network meta-analysis. Front Pharmacol. 2023;14:1148021. doi: 10.3389/fphar.2023.1148021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dasgupta P, Baade PD, Aitken JF, et al. Geographical variations in prostate cancer outcomes: a systematic review of international evidence. Front Oncol. 2019;9:238. doi: 10.3389/fonc.2019.00238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sweeney CJ, Martin AJ, Stockler MR, et al. Testosterone suppression plus enzalutamide versus testosterone suppression plus standard antiandrogen therapy for metastatic hormone-sensitive prostate cancer (ENZAMET): an international, open-label, randomised, phase 3 trial. Lancet Oncol. 2023;24(4):323–334. doi: 10.1016/S1470-2045(23)00063-3 [DOI] [PubMed] [Google Scholar]
- 35.Davis ID, Martin AJ, Stockler MR, et al. Enzalutamide with standard first-line therapy in metastatic prostate cancer. N Engl J Med. 2019;381(2):121–131. doi: 10.1056/NEJMoa1903835 [DOI] [PubMed] [Google Scholar]
- 36.Vale CL, Fisher DJ, Godolphin PJ, et al. Which patients with metastatic hormone-sensitive prostate cancer benefit from docetaxel: a systematic review and meta-analysis of individual participant data from randomised trials. Lancet Oncol. 2023;24(7):783–797. doi: 10.1016/S1470-2045(23)00230-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.West HJ, Jin JO. Performance status in patients with cancer. JAMA Oncol. 2015;1(7):998. doi: 10.1001/jamaoncol.2015.3113 [DOI] [PubMed] [Google Scholar]
- 38.Sweeney CJ, Chen YH, Carducci M, et al. Chemohormonal therapy in metastatic hormone-sensitive prostate cancer. N Engl J Med. 2015;373(8):737–746. doi: 10.1056/NEJMoa1503747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fizazi K, Tran N, Fein L, et al. Abiraterone plus prednisone in metastatic, castration-sensitive prostate cancer. N Engl J Med. 2017;377(4):352–360. doi: 10.1056/NEJMoa1704174 [DOI] [PubMed] [Google Scholar]
- 40.Nash P, McInnes IB, Mease PJ, et al. Secukinumab versus adalimumab for psoriatic arthritis: comparative effectiveness up to 48 weeks using a matching-adjusted indirect comparison. Rheumatol Ther. 2018;5(1):99–122. doi: 10.1007/s40744-018-0106-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Macabeo B, Quenechdu A, Aballea S, et al. Methods for indirect treatment comparison: results from a systematic literature review. J Mark Access Health Policy. 2024;12(2):58–80. doi: 10.3390/jmahp12020006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Latimer N. NICE DSU Technical Support Document 14: Undertaking survival analysis for economic evaluations alongside clinical trials - extrapolation with patient-level data 2011. [cited 2025 Apr 16]. Available from: https://www.ncbi.nlm.nih.gov/books/NBK395885/pdf/Bookshelf_NBK395885.pdf
- 43.Colomba E, Jonas SF, Eymard JC, et al. A randomized, open-label, cross-over phase 2 trial of darolutamide and enzalutamide in men with asymptomatic or mildly symptomatic metastatic castrate-resistant prostate cancer: patient preference and cognitive function in ODENZA. Eur Urol. 2024;85(3):274–282. doi: 10.1016/j.eururo.2023.05.009 [DOI] [PubMed] [Google Scholar]
- 44.Mori K, Mostafaei H, Pradere B, et al. Apalutamide, enzalutamide, and darolutamide for non-metastatic castration-resistant prostate cancer: a systematic review and network meta-analysis. Int J Clin Oncol. 2020;25(11):1892–1900. doi: 10.1007/s10147-020-01777-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Petrylak DP, Azad AA, Szmulewitz RZ, et al. 1398P Overall survival (OS) in patients (pts) with metastatic hormone-sensitive prostate cancer (mHSPC) who received prior androgen deprivation therapy (ADT) and reached low prostate-specific antigen (PSA) levels treated further with enzalutamide (ENZA): post hoc analyses of ARCHES. Ann Oncol. 2022;33(Suppl 7):S1183–S1184. [Google Scholar]
- 46.Atallah E, Mauro MJ, Hochhaus A, et al. Matching-adjusted indirect comparison of asciminib versus other treatments in chronic-phase chronic myeloid leukemia after failure of two prior tyrosine kinase inhibitors. J Cancer Res Clin Oncol. 2023;149(9):6247–6262. doi: 10.1007/s00432-022-04562-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Details for how researchers may request access to anonymized participant-level data, trial-level data, and protocols from Astellas-sponsored clinical trials can be found at https://www.clinicaltrials.astellas.com/transparency/.


