Abstract
Objectives
Leucine-rich alpha-2-glycoprotein 1 (LRG1) was previously reported to regulate inflammation and arthritis progression. This study aimed to investigate the correlation of serum LRG1 level with disease features and response to biologics in rheumatoid arthritis (RA) patients.
Methods
Seventy-eight RA patients who underwent biologics treatment were analyzed. Serum LRG1 level was detected by enzyme-linked immunosorbent assay at baseline (before biologics were initiated) and at weeks 6 and 12. Treatment response, low disease activity (LDA), and remission were analyzed on the basis of disease activity score in 28 joints. Moreover, serum LRG1 level in another 20 health controls was also analyzed.
Results
LRG1 was greater in RA patients than in health controls (46.3 versus 28.6 µg/mL, P < 0.001), with an area under the curve of 0.795 for differentiating them according to the receiver operator characteristic curve. By correlation analysis, LRG1 was correlated with a greater body mass index (P = 0.007) and C-reactive protein level (P = 0.013) in RA patients and tended to be associated with swollen joint count but was not statistically significant (P = 0.052). Furthermore, LRG1 decreased from baseline to week 12 after biologics treatment in RA patients (P < 0.001). However, baseline LRG1 was not correlated with treatment response (P = 0.987), LDA (P = 0.405), or remission (P = 0.763) in RA patients. A decrease in LRG1 at week 12 (P = 0.028) was related to response achievement, and a decrease in LRG1 at week 6 (P = 0.047) and week 12 (P = 0.019) was related to LDA achievement.
Conclusion
LRG1 may aid in RA disease supervision, but further validation is needed.
|
Key Points • LRG1 level can distinguish RA patients from health controls with a high AUC at 0.795. • LRG1 level is correlated with higher BMI and CRP level, and tends to be related to elevated SJC in RA patients. • LRG1 level after treatment is correlated with clinical response and LDA to biologics in RA patients, while its level before treatment fails to do so. • Collectively, LRG1 level shows a potential to be a biomarker for RA disease supervision. |
Keywords: Disease activity, Health controls, Response to biologics, Rheumatoid arthritis, Serum LRG1
Introduction
Rheumatoid arthritis (RA) is a chronic autoimmune disease that affects mainly females, with an estimated global prevalence of 0.46% [1, 2]. Profiting from the progress of the biopharmaceutical industry, several novel kinds of drugs have been developed in recent years, such as biologics or biosimilars (etanercept, adalimumab, infliximab, golimumab, tocilizumab, etc.) and targeted synthetic disease-modifying anti-rheumatic drugs (DMARDs) (tofacitinib, baritinib, etc.), which greatly improve the outcomes of RA patients [3–5]. However, a nonnegligible number of RA patients insufficiently respond to these therapies or relapse/flare early after achieving an initial response [6–8].
To help resolve this issue, the identification of markers that can predict the treatment response to identify risky patients in advance is a possible solution that can be used to personalize treatment drugs and strategies to improve RA patients’ outcomes [9, 10]. For example, the plasma resistin level is positively correlated with disease activity and can predict 5-year radiological progression in RA patients treated with methotrexate, sulfasalazine, hydroxychloroquine, or prednisolone with or without infliximab [11]; the serum JNK pathway-associated phosphatase (JKAP) level is inversely associated with multiple inflammatory and disease-activity-related indexes, and its increase over 24 weeks is related to the response to etanercept in RA patients [12]. These findings imply the importance of biomarkers for RA management.
Leucine-rich alpha-2-glycoprotein 1 (LRG1) is a glycoprotein with a carbohydrate content of 23% and 5 estimated glycosylation sites that widely modulates immune, proinflammatory, metabolic, fibrotic, oncogenic, and vasculopathic biological processes [13]. Specific to immune and inflammatory regulation, LRG1 is reported to regulate TGF-β-mediated hematopoietic progenitor functions, neutrophil activation and extravasation, M1 macrophage polarization, and Th17 cell proliferation [14–18]. Furthermore, LRG1 is elevated quickly in the blood following inflammatory stimulation [19, 20]. LRG1 is also dysregulated in several autoimmune diseases, such as psoriasis, lupus nephritis, vasculitis, and RA [21–24]. Moreover, a very recent study reported that LRG1 was correlated with C-reaction protein (CRP), erythrocyte sedimentation rate (ESR), and traditional biomarkers in RA patients receiving IL-6 inhibitor, which remained elevated despite suppression of CRP and ESR, indicating it might serve as an alternative disease activity biomarker for RA [24]. However, its longitude variation and correlation with the response to biologics is still unclear.
Therefore, the present study aimed to detect the serum LRG1 level variation during treatment, and explore its correlation with disease risk, activity, and response to biologics in RA patients.
Methods
Patients
This study consecutively included 78 RA patients underwent biologics treatment. The inclusion criteria were as follows: (i) diagnosed as RA according to ACR/EULAR 2010 criteria, (ii) at moderate-severe active disease status defined as 28-joint disease activity score using ESR (DAS28ESR) above 3.2, (iii) about to initiate biologics treatment, (iv) considered to be able to be followed up regularly for at least 3 months, and (v) signed the informed consent. The exclusion criteria were as follows: (i) complicated with malignancies or other inflammatory diseases, (ii) complicated with active infection or hepatitis B, (iii) was taking the biologics or targeted synthetic DMARDs at the time of enrollment, and (iv) pregnancies or during lactation period. In addition, another 20 health controls with age and gender matched to RA patients were included, which were defined as no abnormities (including comorbidities) by physical examination. The Ethics Review Borad of Wuhan No.1 Hospital approved this study.
Data collection, treatment, and outcomes
The baseline data of RA patients, which included demographics, treatment history, antibody positivity, and disease activity indexes, were collected. RA patients underwent biologics treatment, including adalimumab, etanercept, and infliximab. For adalimumab, 40 mg of drug was given every 2 weeks; for etanercept, 25 mg of drug was given twice every week, or 50 mg of drug was given once every week; for infliximab, 3 mg/kg drug was given at 0, 2, and 6 weeks and then every 8 weeks. DAS28ESR was assessed at week 0, week 6 (±1 week), and week 12 (±2 weeks) after the initiation of biologics treatment as outcomes. Patients who did not have follow-up DAS28ESR data were excluded from the analysis. In brief, seven patients lost to follow-up and had been already excluded from the analysis of this study.
Definitions
Response was defined as a decline in DAS28ESR ≥ 2.0 from baseline. Low disease activity (LDA) was defined as a DAS28ESR < 3.2 and ≥ 2.6. Remission was defined as a DAS28ESR < 2.6. The treatment response, LDA, and remission were assessed at week 6 and week 12 according to the DAS28ESR.
LRG1 measurement
Serum was acquired at week 0, week 6, and week 12 from RA patients and at inclusion in health controls. LRG1 was then detected by the enzyme-linked immunosorbent assay (ELISA) kits (Elabscience, China).
Statistical analysis
SPSS 26.0 (IBM, USA) was used for statistical analysis. The data are presented as numbers (%), means ± standard deviations, or medians (interquartile ranges). Comparisons were analyzed via the Wilcoxon rank sum test or the Friedman test. Correlations were analyzed via the Spearman test. The ability of LRG1 to distinguish RA patients from health controls was analyzed via receiver operator characteristic (ROC) curves. P < 0.05 was considered statistically significant.
Results
Characteristics of RA patients
The analyzed RA patients consisted of 82.1% females and 17.9% males, with an age of 53.5 ± 11.6 years (Table 1). The disease duration of RA was 5.0 (2.4–11.3) years, all patients had treatment history of conventional DMARDs, 10.3% patients had treatment history of targeted synthetic DMARDs, and 21.8% of patients had undergone biologics treatment. The baseline DAS28ESR was 5.6 ± 0.8 and the baseline clinical disease activity index (CDAI) was 25.3 ± 9.3.
Table 1.
RA patients’ characteristics
| Items | RA patients (N = 78) |
|---|---|
| Age (years) | 53.5 ± 11.6 |
| Sex | |
| Females | 64 (82.1%) |
| Males | 14 (17.9%) |
| BMI (kg/m2) | 22.9 ± 3.1 |
| Treatment history | |
| Conventional DMARDs | 78 (100.0%) |
| Targeted synthetic DMARDs | 8 (10.3%) |
| Biologics | 17 (21.8%) |
| RF status | |
| Positive | 68 (87.2%) |
| Negative | 10 (12.8%) |
| ACPA status | |
| Positive | 71 (91.0%) |
| Negative | 7 (9.0%) |
| Disease duration (years) | 5.0 (2.4–11.3) |
| Baseline disease activity assessment | |
| TJC (counts) | 8.3 ± 4.4 |
| SJC (counts) | 5.8 ± 3.2 |
| ESR (mm/h) | 53.5 (36.7–75.7) |
| CRP (mg/L) | 32.7 (20.0–59.9) |
| DAS28ESR (points) | 5.6 ± 0.8 |
| PGA (points) | 5.7 ± 1.5 |
| PhGA (points) | 5.5 ± 1.6 |
| CDAI (points) | 25.3 ± 9.3 |
RA rheumatoid arthritis, BMI body mass index, DMARDs disease-modifying anti-rheumatic drugs, RF rheumatoid factor, ACPA anti-citrullinated protein autoantibody, TJC tender joint count, SJC swollen joint count, ESR erythrocyte sedimentation rate, CRP C-reactive protein, DAS28ESR 28-joint disease activity score using erythrocyte sedimentation rate, PGA patient’s global assessment, PhGA physician’s global assessment, CDAI clinical disease activity index
LRG1 level in RA patients
LRG1 level was 46.3 (36.3–63.2) µg/mL in RA patients, which was much higher than that in health controls (28.6 (16.2–41.3) µg/mL, P < 0.001, Fig. 1A). The ROC curve revealed that LRG1 had an acceptable ability to distinguish RA patients from health controls (area under the curve (AUC) = 0.795, 95%CI: 0.678–0.911, Fig. 1B). By cutting off at 20, 30, 40, 50, and 60 µg/mL of LRG1 level, LRG1 threshold of 30 µg/mL showed the highest Youden index of 0.435 (Fig. 1C).
Fig. 1.
Dysregulated LRG1 level. Comparison of LRG1 level between RA patients and health controls (A). ROC curve analysis of LRG1 level for distinguishing RA patients from health controls (B). Diagnostic indexes of LRG1 level by different cutoffs for RA (C)
Correlation between LRG1 level and characteristics in RA patients
LRG1 level was positively correlated with body mass index (BMI, r = 0.305, P = 0.007) and CRP (r = 0.279, P = 0.013), and tended to be associated with swollen joint count (SJC) but not statistically significant (r = 0.221, P = 0.052) (Table 2). However, LRG1 was not correlated with other characteristics in RA patients.
Table 2.
Correlation between LRG1 level and RA patients’ characteristics
| Items | r correlation coefficient | P value |
|---|---|---|
| Age | 0.124 | 0.279 |
| Sex-females | 0.108 | 0.345 |
| BMI | 0.305 | 0.007 |
| Treatment history | ||
| Conventional DMARDs | - | - |
| Targeted synthetic DMARDs | 0.058 | 0.613 |
| Biologics | 0.020 | 0.862 |
| RF status-positive | 0.136 | 0.234 |
| ACPA status-positive | 0.178 | 0.118 |
| Disease duration | 0.152 | 0.183 |
| Baseline disease activity assessment | ||
| TJC | 0.153 | 0.181 |
| SJC | 0.221 | 0.052 |
| ESR | 0.093 | 0.418 |
| CRP | 0.279 | 0.013 |
| DAS28ESR | 0.178 | 0.119 |
| PGA | 0.133 | 0.245 |
| PhGA | 0.125 | 0.275 |
| CDAI | 0.200 | 0.078 |
LRG1 leucine rich alpha-2-glycoprotein 1, RA rheumatoid arthritis, BMI body mass index, DMARDs disease-modifying anti-rheumatic drug, RF rheumatoid factor, ACPA anti-citrullinated protein autoantibody, TJC tender joint count, SJC swollen joint count, ESR erythrocyte sedimentation rate, CRP C-reactive protein, DAS28ESR 28-joint disease activity score using erythrocyte sedimentation rate, PGA patient’s global assessment, PhGA physician’s global assessment, CDAI clinical disease activity index
Treatment information and outcomes in RA patients
All RA patients started biologics treatment, among them 43.6% of patients received adalimumab treatment, 42.3% of patients received etanercept treatment, and 14.1% of patients received infliximab treatment (Table 3). In addition, 96.2% of patients received the combination of conventional DMARDs.
Table 3.
Current treatment information in RA patients
| Items | RA patients (N = 78) |
|---|---|
| Initiation of biologics | 78 (100.0%) |
| Type of biologics | |
| Adalimumab | 34 (43.6%) |
| Etanercept | 33 (42.3%) |
| Infliximab | 11 (14.1%) |
| Combined conventional DMARDs | 75 (96.2%) |
RA rheumatoid arthritis, DMARDs disease-modifying anti-rheumatic drug
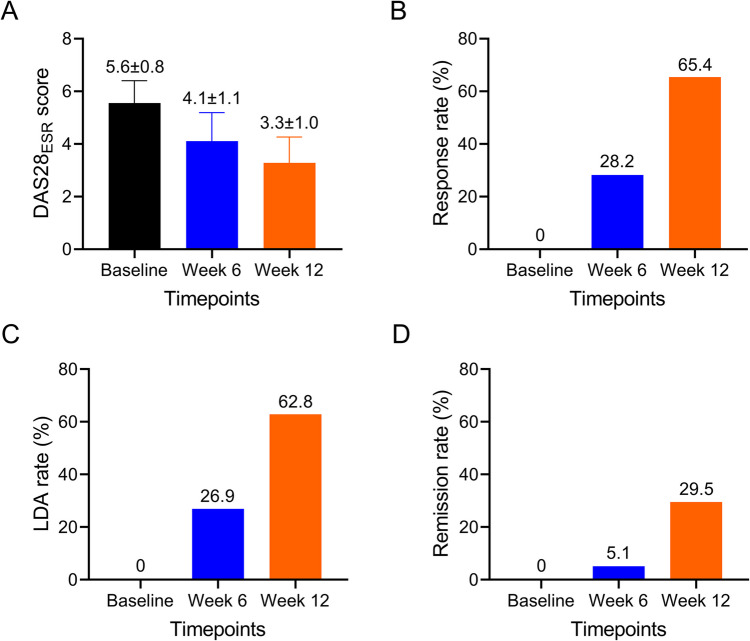
The DAS28ESR gradually decreased from 5.6 ± 0.8 at baseline, and to 4.1 ± 1.1 at week 6, to 3.3 ± 1.0 at week 12 in RA patients (Fig. 2A). A total of 28.2% and 65.4% of RA patients realized treatment response at week 6 and week 12, respectively (Fig. 2B); 26.9% and 62.8% RA patients realized LDA at week 6 and week 12, respectively (Fig. 2C); 5.1% and 29.5% RA patients realized remission at week 6 and week 12, respectively (Fig. 2D).
Fig. 2.
Treatment outcomes at different timepoints. DAS28ESR score at baseline, week 6, and week 12 (A). Response rate at baseline, week 6, and week 12 (B). LDA rate at baseline, week 6, and week 12 (C). Remission rate at baseline, week 6, and week 12 (D)
Correlation between LRG1 decrement during biologics treatment and patients’ outcomes
LRG1 level was gradually decreased from baseline to week 12 in RA patients (P < 0.001, Fig. 3A). After comparison, LRG1 level at baseline (P = 0.987) and week 6 (P = 0.128) was not different, while its level at week 12 (P = 0.028) was lower, in response patients versus no response patients (Fig. 3B). LRG1 level at baseline (P = 0.405) was not varied, but its level at week 6 (P = 0.047) and week 12 (P = 0.019) was reduced, in LDA patients versus no LDA patients (Fig. 3C). In addition, LRG1 level at any timepoints was not different between remission patients and no remission patients; but it should be stated that LRG1 level at week 6 (P = 0.068) and week 12 (P = 0.073) showed a tendency to be lower in remission patients versus no remission patients, even though not statistically (Fig. 3D).
Fig. 3.
LRG1 decrement related to treatment outcome to some extent. LRG1 level at baseline, week 6, and week 12 (A). Comparison of LRG1 level at baseline, week 6, and week 12, between response RA patients and no response RA patients (B), between LDA RA patients and no LDA RA patients (C), between remission RA patients and no remission RA patients (D)
Discussion
LRG1 has been shown to be aberrantly expressed in several autoimmune diseases [21, 25, 26]. For example, serum LRG1 level was elevated in psoriasis patients and psoriatic arthritis patients compared to health controls [21]; serum LRG1 level was also increased in ulcerative colitis patients compared with health controls [25]. Regarding RA, a study revealed that LRG1 level was much greater in RA patients than that in health controls [26]. This study revealed that serum LRG1 level was elevated in RA patients compared to health controls, which was in line with the findings of a previous study [26]. In addition, serum LRG1 was able to well distinguish RA patients from health controls (AUC = 0.795); moreover, with a cutoff level of 30 µg/mL, LRG1 showed diagnostic utility for RA, with a sensitivity of 0.885, a specificity of 0.550, and a Youden index of 0.435. These findings indicated that LRG1 had the potential to be a diagnostic biomarker for RA.
LRG1 has also been shown to be correlated with disease severity indexes in autoimmune diseases [25–27]. For example, serum LRG1 is positively correlated with both clinical activity and endoscopic activity in ulcerative colitis patients [25]; it is also positively associated with CRP level, ferritin level, and disease activity in systemic juvenile idiopathic arthritis patients [27]. In terms of RA, a previous study reported that serum LRG1 was correlated with higher DAS28, CRP, and ESR levels [26]. In this study, serum LRG1 was positively correlated with BMI and CRP, and tended to be associated with SJC but not statistically. These findings were partially in accordance with those of a previous study [26]. The correlation between LRG1 and BMI could be explained by the relationship of LRG1 with thyroid hormones, insulin, angiogenesis, and obesity-related markers [28–32]. The correlation between LRG1 and CRP could be explained by the relationship between LRG1 and inflammation [33]. Furthermore, a latest study reported that LRG1 was associated with CRP, ESR, and traditional biomarkers, but remained high despite suppression of CRP and ESR in RA patients receiving IL-6 inhibitor [24]. Compared to the abovementioned previous study, this study found that LRG1 level was much higher in RA patients than that in health controls, and it had potential as a biomarker aiding in RA diagnosis. In addition, this study also explored the longitude variation of LRG1 during treatment and its correlation with the response to biologics, which provided new evidence of LRG1 as a potential biomarker for RA.
Regarding the correlation between LRG1 level and treatment outcomes in patients with autoimmune diseases, only one previous study reported this topic, which revealed that LRG1 gene expression was dysregulated in canakinumab responders compared with canakinumab non-responders among systemic juvenile idiopathic arthritis patients [34]. This study revealed that LRG1 level gradually decreased from baseline to week 12 in RA patients who underwent biologics treatment, which could be explained by the anti-inflammatory effect of biologics on the level of LRG1 (an inflammatory marker) [35–37]. In addition, and importantly, a decrease in LRG1 was related to response and LDA to biologics treatment in RA patients and tended to be related to remission (not statistically significant), indicating the potential of LRG1 as a prognostic marker for biologics in RA patients. Moreover, LRG1 decrement reflected on-treatment response more than pre-treatment prediction. However, more data and evidences are needed for validation. It could be mentioned that DAS28CRP may underestimate disease activity and overestimate EULAR response criteria compared to DAS28ESR in RA patient [38], and DAS28ESR is commonly applied in our clinical practice and in previous studies [39, 40], DAS28ESR was applied for assessment in this study.
The limitations of this study include the following: (i) The limited sample size restricted the generalizability of the study findings. (ii) RA patients receiving biologics were analyzed; therefore, the clinical value of LRG1 in RA patients receiving other treatment regimens is not clear. (iii) A 12-week follow-up period made radiographic progression evaluation unnecessary; therefore, the relationship of LRG1 level with radiographic progression risk in RA patients could be evaluated in future studies.
In conclusion, LRG1 may aid in RA disease supervision and biologics treatment response prediction, but further validation is needed.
Author contribution
Liang Zou and Qiuyu Fan: conceptualization, project administration, supervision and writing—review and editing; Ya Liu: data curation, formal analysis, methodology, and writing—original draft; Hao He and Chao Jia: investigation and validation; all authors have read and approved the final manuscript.
Data availability
The datasets generated and analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval
All procedures have been approved by the ethics committee of Wuhan No.1 Hospital and performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments.
Informed consent
All participants signed the informed consent.
Disclosures
None.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Liang Zou and Qiuyu Fan contributed equally to this work.
References
- 1.Di Matteo A, Bathon JM, Emery P (2023) Rheumatoid arthritis. Lancet 402(10416):2019–2033. 10.1016/S0140-6736(23)01525-8 [DOI] [PubMed] [Google Scholar]
- 2.Almutairi K, Nossent J, Preen D, Keen H, Inderjeeth C (2021) The global prevalence of rheumatoid arthritis: a meta-analysis based on a systematic review. Rheumatol Int 41(5):863–877. 10.1007/s00296-020-04731-0 [DOI] [PubMed] [Google Scholar]
- 3.Smolen JS, Landewe RBM, Bergstra SA, Kerschbaumer A, Sepriano A, Aletaha D, Caporali R, Edwards CJ, Hyrich KL, Pope JE, de Souza S, Stamm TA, Takeuchi T, Verschueren P, Winthrop KL, Balsa A, Bathon JM, Buch MH, Burmester GR, Buttgereit F, Cardiel MH, Chatzidionysiou K, Codreanu C, Cutolo M, den Broeder AA, El Aoufy K, Finckh A, Fonseca JE, Gottenberg JE, Haavardsholm EA, Iagnocco A, Lauper K, Li Z, McInnes IB, Mysler EF, Nash P, Poor G, Ristic GG, Rivellese F, Rubbert-Roth A, Schulze-Koops H, Stoilov N, Strangfeld A, van der Helm-van Mil A, van Duuren E, Vliet Vlieland TPM, Westhovens R, van der Heijde D (2023) EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2022 update. Ann Rheum Dis 82(1):3–18. 10.1136/ard-2022-223356 [DOI] [PubMed] [Google Scholar]
- 4.Patel JP, Konanur Srinivasa NK, Gande A, Anusha M, Dar H, Baji DB (2023) The role of biologics in rheumatoid arthritis: a narrative review. Cureus 15(1):e33293. 10.7759/cureus.33293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhao JH, Ma S, Li CY, Zhang HC, Zhao LJ, Zhang ZY (2023) Clinically approved small-molecule drugs for the treatment of rheumatoid arthritis. Eur J Med Chem 256:115434. 10.1016/j.ejmech.2023.115434 [DOI] [PubMed] [Google Scholar]
- 6.Ochi S, Saito K, Mizoguchi F, Kato S, Tanaka Y (2020) Insensitivity versus poor response to tumour necrosis factor inhibitors in rheumatoid arthritis: a retrospective cohort study. Arthritis Res Ther 22(1):41. 10.1186/s13075-020-2122-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Terslev L, Ostergaard M (2021) Rheumatoid arthritis relapse and remission - advancing our predictive capability using modern imaging. J Inflamm Res 14:2547–2555. 10.2147/JIR.S284405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Naniwa T, Iwagaitsu S, Kajiura M (2020) Successful cessation of tumor necrosis factor inhibitor treatment in rheumatoid arthritis patients and potential predictors for early flare: an observational study in routine clinical care. Mod Rheumatol 30(6):948–958. 10.1080/14397595.2019.1702253 [DOI] [PubMed] [Google Scholar]
- 9.Savvateeva E, Smoldovskaya O, Feyzkhanova G, Rubina A (2021) Multiple biomarker approach for the diagnosis and therapy of rheumatoid arthritis. Crit Rev Clin Lab Sci 58(1):17–28. 10.1080/10408363.2020.1775545 [DOI] [PubMed] [Google Scholar]
- 10.Myasoedova E (2021) New era for outcomes and management of rheumatoid arthritis: facing the individualized treatment challenge. Joint Bone Spine 88(3):105066. 10.1016/j.jbspin.2020.08.001 [DOI] [PubMed] [Google Scholar]
- 11.Vuolteenaho K, Tuure L, Nieminen R, Laasonen L, Leirisalo-Repo M, Moilanen E, Group NE-RS (2022) Pretreatment resistin levels are associated with erosive disease in early rheumatoid arthritis treated with disease-modifying anti-rheumatic drugs and infliximab. Scand J Rheumatol 51(3):180–185. 10.1080/03009742.2021.1929456 [DOI] [PubMed]
- 12.Sun L, Tu J, Chen X, Dai M, Xia X, Liu C, Zhou Y (2021) JNK pathway-associated phosphatase associates with rheumatoid arthritis risk, disease activity, and its longitudinal elevation relates to etanercept treatment response. J Clin Lab Anal 35(4):e23709. 10.1002/jcla.23709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Camilli C, Hoeh AE, De Rossi G, Moss SE, Greenwood J (2022) LRG1: an emerging player in disease pathogenesis. J Biomed Sci 29(1):6. 10.1186/s12929-022-00790-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Druhan LJ, Lance A, Li S, Price AE, Emerson JT, Baxter SA, Gerber JM, Avalos BR (2017) Leucine rich alpha-2 glycoprotein: a novel neutrophil granule protein and modulator of myelopoiesis. PLoS One 12(1):e0170261. 10.1371/journal.pone.0170261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu C, Teo MHY, Pek SLT, Wu X, Leong ML, Tay HM, Hou HW, Ruedl C, Moss SE, Greenwood J, Tavintharan S, Hong W, Wang X (2020) A multifunctional role of leucine-rich alpha-2-glycoprotein 1 in cutaneous wound healing under normal and diabetic conditions. Diabetes 69(11):2467–2480. 10.2337/db20-0585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yu B, Yang L, Song S, Li W, Wang H, Cheng J (2021) LRG1 facilitates corneal fibrotic response by inducing neutrophil chemotaxis via Stat3 signaling in alkali-burned mouse corneas. Am J Physiol Cell Physiol 321(3):C415–C428. 10.1152/ajpcell.00517.2020 [DOI] [PubMed] [Google Scholar]
- 17.Wang J, Wang J, Zhong J, Liu H, Li W, Chen M, Xu L, Zhang W, Zhang Z, Wei Z, Guo J, Wang X, Sui J, Liu X, Zhang S, Wang X (2024) LRG1 promotes atherosclerosis by inducing macrophage M1-like polarization. Proc Natl Acad Sci U S A 121(35):e2405845121. 10.1073/pnas.2405845121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Urushima H, Fujimoto M, Mishima T, Ohkawara T, Honda H, Lee H, Kawahata H, Serada S, Naka T (2017) Leucine-rich alpha 2 glycoprotein promotes Th17 differentiation and collagen-induced arthritis in mice through enhancement of TGF-beta-Smad2 signaling in naive helper T cells. Arthritis Res Ther 19(1):137. 10.1186/s13075-017-1349-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Codina R, Vanasse A, Kelekar A, Vezys V, Jemmerson R (2010) Cytochrome c-induced lymphocyte death from the outside in: inhibition by serum leucine-rich alpha-2-glycoprotein-1. Apoptosis 15 (2):139-152. 10.1007/s10495-009-0412-0 [DOI] [PubMed]
- 20.Shirai R, Hirano F, Ohkura N, Ikeda K, Inoue S (2009) Up-regulation of the expression of leucine-rich alpha(2)-glycoprotein in hepatocytes by the mediators of acute-phase response. Biochem Biophys Res Commun 382(4):776–779. 10.1016/j.bbrc.2009.03.104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nakajima H, Serada S, Fujimoto M, Naka T, Sano S (2017) Leucine-rich alpha-2 glycoprotein is an innovative biomarker for psoriasis. J Dermatol Sci 86(2):170–174. 10.1016/j.jdermsci.2017.01.008 [DOI] [PubMed] [Google Scholar]
- 22.Yang Y, Luo R, Cheng Y, Liu T, Dai W, Li Y, Ge S, Xu G (2020) Leucine-rich alpha2-glycoprotein-1 upregulation in plasma and kidney of patients with lupus nephritis. BMC Nephrol 21(1):122. 10.1186/s12882-020-01782-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yanagimachi M, Fukuda S, Tanaka F, Iwamoto M, Takao C, Oba K, Suzuki N, Kiyohara K, Kuranobu D, Tada N, Nagashima A, Ishii T, Ino Y, Kimura Y, Nawa N, Fujiwara T, Naruto T, Morio T, Doi S, Mori M (2021) Leucine-rich alpha-2-glycoprotein 1 and angiotensinogen as diagnostic biomarkers for Kawasaki disease. PLoS One 16(9):e0257138. 10.1371/journal.pone.0257138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fujimoto M, Hosono Y, Serada S, Suzuki Y, Ohkawara T, Murata O, Quick A, Suzuki K, Kaneko Y, Takeuchi T, Naka T (2024) Leucine-rich alpha2-glycoprotein as a useful biomarker for evaluating disease activity in rheumatoid arthritis. Mod Rheumatol 34(5):1072–1075. 10.1093/mr/road112 [DOI] [PubMed] [Google Scholar]
- 25.Shinzaki S, Matsuoka K, Iijima H, Mizuno S, Serada S, Fujimoto M, Arai N, Koyama N, Morii E, Watanabe M, Hibi T, Kanai T, Takehara T, Naka T (2017) Leucine-rich alpha-2 glycoprotein is a serum biomarker of mucosal healing in ulcerative colitis. J Crohns Colitis 11(1):84–91. 10.1093/ecco-jcc/jjw132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ha YJ, Kang EJ, Lee SW, Lee SK, Park YB, Song JS, Choi ST (2014) Usefulness of serum leucine-rich alpha-2 glycoprotein as a disease activity biomarker in patients with rheumatoid arthritis. J Korean Med Sci 29(9):1199–1204. 10.3346/jkms.2014.29.9.1199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shimizu M, Inoue N, Mizuta M, Nakagishi Y, Yachie A (2019) Serum leucine-rich alpha2-glycoprotein as a biomarker for monitoring disease activity in patients with systemic juvenile idiopathic arthritis. J Immunol Res 2019:3140204. 10.1155/2019/3140204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kay RG, Barton C, Velloso CP, Brown PR, Bartlett C, Blazevich AJ, Godfrey RJ, Goldspink G, Rees R, Ball GR, Cowan DA, Harridge SD, Roberts J, Teale P, Creaser CS (2009) High-throughput ultra-high-performance liquid chromatography/tandem mass spectrometry quantitation of insulin-like growth factor-I and leucine-rich alpha-2-glycoprotein in serum as biomarkers of recombinant human growth hormone administration. Rapid Commun Mass Spectrom 23(19):3173–3182. 10.1002/rcm.4237 [DOI] [PubMed] [Google Scholar]
- 29.Masood A, Benabdelkamel H, Ekhzaimy AA, Alfadda AA (2020) Plasma-based proteomics profiling of patients with hyperthyroidism after antithyroid treatment. Molecules 25 (12). 10.3390/molecules25122831 [DOI] [PMC free article] [PubMed]
- 30.Wang X, Abraham S, McKenzie JAG, Jeffs N, Swire M, Tripathi VB, Luhmann UFO, Lange CAK, Zhai Z, Arthur HM, Bainbridge J, Moss SE, Greenwood J (2013) LRG1 promotes angiogenesis by modulating endothelial TGF-beta signalling. Nature 499(7458):306–311. 10.1038/nature12345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.He S, Ryu J, Liu J, Luo H, Lv Y, Langlais PR, Wen J, Dong F, Sun Z, Xia W, Lynch JL, Duggirala R, Nicholson BJ, Zang M, Shi Y, Zhang F, Liu F, Bai J, Dong LQ (2021) LRG1 is an adipokine that mediates obesity-induced hepatosteatosis and insulin resistance. J Clin Invest 131 (24). 10.1172/JCI148545 [DOI] [PMC free article] [PubMed]
- 32.Alhammad R, Abu-Farha M, Hammad MM, Thanaraj TA, Channanath A, Alam-Eldin N, Al-Sabah R, Shaban L, Alduraywish A, Al-Mulla F, Rahman A, Abubaker J (2022) Increased LRG1 levels in overweight and obese adolescents and its association with obesity markers, including leptin, chemerin, and high sensitivity C-reactive protein. Int J Mol Sci 23 (15). 10.3390/ijms23158564 [DOI] [PMC free article] [PubMed]
- 33.Sarkar A, Chakraborty D, Kumar V, Malhotra R, Biswas S (2022) Upregulation of leucine-rich alpha-2 glycoprotein: a key regulator of inflammation and joint fibrosis in patients with severe knee osteoarthritis. Front Immunol 13:1028994. 10.3389/fimmu.2022.1028994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Verweyen EL, Pickering A, Grom AA, Schulert GS (2021) Distinct gene expression signatures characterize strong clinical responders versus nonresponders to canakinumab in children with systemic juvenile idiopathic arthritis. Arthritis Rheumatol 73(7):1334–1340. 10.1002/art.41640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rider P, Carmi Y, Cohen I (2016) Biologics for targeting inflammatory cytokines, clinical uses, and limitations. Int J Cell Biol 2016:9259646. 10.1155/2016/9259646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yoshimura T, Mitsuyama K, Sakemi R, Takedatsu H, Yoshioka S, Kuwaki K, Mori A, Fukunaga S, Araki T, Morita M, Tsuruta K, Yamasaki H, Torimura T (2021) Evaluation of serum leucine-rich alpha-2 glycoprotein as a new inflammatory biomarker of inflammatory bowel disease. Mediators Inflamm 2021:8825374. 10.1155/2021/8825374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chong PF, Sakai Y, Torisu H, Tanaka T, Furuno K, Mizuno Y, Ohga S, Hara T, Kira R (2018) Leucine-rich alpha-2 glycoprotein in the cerebrospinal fluid is a potential inflammatory biomarker for meningitis. J Neurol Sci 392:51–55. 10.1016/j.jns.2018.07.006 [DOI] [PubMed] [Google Scholar]
- 38.Matsui T, Kuga Y, Kaneko A, Nishino J, Eto Y, Chiba N, Yasuda M, Saisho K, Shimada K, Tohma S (2007) Disease activity score 28 (DAS28) using C-reactive protein underestimates disease activity and overestimates EULAR response criteria compared with DAS28 using erythrocyte sedimentation rate in a large observational cohort of rheumatoid arthritis patients in Japan. Ann Rheum Dis 66(9):1221–1226. 10.1136/ard.2006.063834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.He K, He S, Su M (2022) Inter-alpha-trypsin inhibitor heavy chain 4: a serologic marker relating to disease risk, activity, and treatment outcomes of rheumatoid arthritis. J Clin Lab Anal 36(3):e24231. 10.1002/jcla.24231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Song D, Zhu X, Wang F, Sun J (2021) Longitudinal monitor of Jun N-terminal kinase pathway associated phosphatase reflects clinical efficacy to triple conventional disease-modifying anti-rheumatic drugs treatment in rheumatoid arthritis patients. Inflammopharmacology 29(4):1131–1138. 10.1007/s10787-021-00823-w [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated and analyzed during the current study are available from the corresponding author on reasonable request.