Abstract
Rigorous studies have characterized the aa-tRNA selection mechanism in bacteria, which is essential for maintaining translational fidelity. Recent investigations have identified critical distinctions in humans, such as the requirement of subunit rolling and a tenfold slower proofreading step. Although these studies captured key intermediates involved in tRNA selection, they did not elucidate the transitions of aa-tRNA between intermediates. Here, we simulated 1,856 aa-tRNA accommodation events into the human ribosomal A site, revealing the requirement of a distinct ~30° pivoting of aa-tRNA about the anticodon stem within the accommodation corridor. This pivoting is crucial for navigating the crowded accommodation corridor, which becomes more constrained due to subunit rolling. Subunit rolling-dependent crowding increases the steric contributions of the accommodation corridor during aa-tRNA accommodation, consistent with the 10-fold reduction in the rate of proofreading. The pivoting of the aa-tRNA enables precise alignment within the accommodation corridor, allowing it to traverse the narrower passage. Furthermore, we found that domain III of eEF1A interacts with the accommodating aa-tRNA through conserved basic residues, providing a steric block to prevent dissociation from the A site. Together, these findings provide a structural framework for understanding the distinctions between bacterial and human aa-tRNA selection and demonstrate that the alignment of the aa-tRNA relative to the ribosomal catalytic sites is a critical determinant of translational fidelity.
INTRODUCTION
Protein synthesis is dependent on megadalton ribonucleoprotein complexes known as ribosomes, which decode genetic information into polypeptide sequences. Ribosomes decode genetic information - in the form of messenger RNA (mRNA) - three nucleotides at a time (codon), using aminoacyl-tRNA (aa-tRNA) as a substrate. During this process the mRNA forms three Watson-Crick base pairs with the aa-tRNA (codon-anticodon interactions) to select which amino acid to incorporate into the peptide. Maintaining the fidelity of translation during decoding is essential to the integrity of the proteome and has been evolutionarily fine-tuned to an error rate of 10−3 to 10−4 or 10−4 to 10−5 amino acids are miscoded per codon in bacteria and eukaryotes, respectively1-10. The importance of maintaining fidelity during decoding is exemplified by several antibiotics, including aminoglycosides, that disrupt this process in bacteria11-13 and by the fact that perturbation of translational fidelity can lead to protein aggregates which have been associated with neurodegenerative diseases14. Furthermore, in humans, new therapies have been designed to target tRNA selection as a strategy for treating cancer, viral infections, and certain monogenic disorders15-17.
mRNA decoding and aa-tRNA selection are well studied in bacteria where it occurs through a two-step process involving initial selection and kinetic proofreading, which are separated by irreversible GTP hydrolysis18,19. This mechanism has been extensively biochemically characterized and structurally validated through various methodologies including single molecule Fluorescence (Förster) Resonance Energy Transfer (smFRET)20-30, cryo-electron microscopy (cryo-EM)31-39, X-ray crystallography25,29,40-59, and pre-steady state kinetics60-63. Initial selection involves Elongation factor Tu (EF-Tu, or eEF1A in eukaryotes) binding to the small subunit (SSU) of the ribosome as a ternary complex composed of EF-Tu, guanosine triphosphate (GTP), and aa-tRNA (Initial Binding complex - IB). Formation of Watson-Crick base-pairs between the mRNA and aa-tRNA follow, bending the tRNA into the A/T position (Codon Recognition complex – CR). The closing of the SSU shoulder facilitates docking of EF-Tu onto the Sarcin-Ricin loop (SRL) of the large subunit (LSU) (GTPase Activated complex – A)28,58,61,62,64,65. GTP hydrolysis separates initial selection from proofreading where the release of inorganic phosphate and conformational rearrangement of EF-Tu allows for the aa-tRNA to begin transition through the accommodation corridor to enter the A site of the ribosome (Accommodated complex – AC)61,62,66. Proofreading during aa-tRNA movement into the A site, through reversable fluctuations30, promotes opportunities for EF-Tu to reengage the aa-tRNA23 or to reject the aa-tRNA if it is non-cognate. These intermediate conformations of eEF1A and aa-tRNA in complex with eukaryotic ribosomes have also been identified by smFRET and cryo-EM investigations67-69. Although these intermediates have been identified, the tRNA selection process is less understood in eukaryotes.
Human aa-tRNA selection has been determined to be tenfold slower than in bacteria and is rate-limited by proofreading68. These findings, showing that aa-tRNA selection in humans is kinetically different, suggest a distinct barrier for aa-tRNA selection during proofreading. To achieve the accommodated position, additional movements of the tRNA perpendicular to the intersubunit space towards the LSU were observed in humans, accompanied by subunit rolling68-70. Subunit rolling was observed to occur between the GA and AC complexes and led to the closing of the A site and opening of the E site. These additional vectors of movement indicate that tRNA trajectories into the A site of the ribosome in humans diverge from those observed in bacteria. These insights capture critical intermediates of tRNA selection but do not resolve transitions of aa-tRNA from one intermediate to another, nor do they identify the barrier leading to tenfold reduced rate of tRNA selection.
A remarkably powerful tool for describing protein folding71, oligomerization72, tRNA selection and tRNA translocation have been structure-based molecular simulations30,73. Simulations have illustrated the accommodation corridor that aa-tRNA must navigate through reversible fluctuations as it enters the A site of the ribosome30,74,75. Moreover, they have described the roles of EF-Tu23,76-79, the A-site finger (helix 38 of the 23S rRNA)80, L11 flexibility81, tRNA selection82, and tRNA diffusion83, as well as ribosome-stimulated EF-Tu GTP hydrolysis84-89. Here, we employ structure-based simulations to compare the structural dynamics of tRNA selection in H. sapiens and in E. coli to determine the structural distinctions between these systems during proofreading. In doing so, we identified that intersubunit rolling occurs early during proofreading and that there is a distinct pivoting of the aa-tRNA during human aa-tRNA selection. This pivoting is required due to the increased contact surface between the tRNA and the accommodation corridor caused by subunit rolling. Furthermore, we identified that domain III (DIII) of eEF1A interacts with the elbow domain of the accommodating aa-tRNA through conserved basic amino acids, elucidating how it contributes to aa-tRNA selection. The requirement of tRNA pivoting and eEF1A interactions with the aa-tRNA provides a structural framework for understanding why tRNA selection is tenfold slower in H. sapiens compared to E. coli and supports previously proposed studies suggesting that tRNA alignment is essential to selection.
RESULTS
Simulations of aa-tRNA proofreading in humans
Accommodation of aa-tRNA into the A site of the human ribosome was simulated using structure-based simulations, which were previously used to simulate bacterial tRNA selection30,75-77,80,90. Simulations were initiated in the post-GTP hydrolysis state of eEF1A in complex with aa-tRNA, GDP, and the ribosomal GTPase-activating center (GAC) (Figs. 1A, B). During simulations, the tRNA approached the A site of the ribosome where the attractive term for native contacts of the accommodated position were defined by potential 1 (attractive 6-12 potential) or potential 2 (attractive Gaussian potential) and non-native contacts were given a repulsive term (Figs. 1C, D; Methods). Native contacts are defined as atom pairs within 4.5 Å of each other in the ribosome when the aa-tRNA is in the accommodated A/A conformation, while non-native contacts refer to interactions not present in this conformation. Similarly, coordinates of eEF1A in the open conformation were defined by native contacts, enabling the protein to undergo conformational change from a compacted to extended conformation during release and accommodation of the aa-tRNA. Simulations with potential 1 allowed us to capture the reversible fluctuations of the aa-tRNA as it traversed the accommodation corridor of the ribosome. In these simulations the aa-tRNA fluctuates between elbow-accommodated conformations (EA-1 and EA-2) that were previously identified in bacteria by cryo-EM during aa-tRNA selection37. Simulations with potential 2 enabled us to identify early- and late-stage events during aa-tRNA accommodation as the 3’-CCA end achieved the fully accommodated conformation and facilitated comparisons between bacterial and eukaryotic aa-tRNA accommodation (Supplemental Material, Movies 1, 2). Although simulations with potential 2 enabled us to characterize early and late stage-events of aa-tRNA accommodation, each trajectory only captured 1 accommodation event. An accommodation event was considered when the distance between the aa-tRNA and peptidyl-tRNA elbow domains reached a distance of <32.5 Å. The convergence of both types of simulations were defined by the typical measurement of backbone RMSD of the ribosome or aa-tRNA in addition to the pointwise RMSD between successive free energy landscapes and of , (Supplemental Figs. 1, 2). These simulations were determined to be converged when RMSD or the Conv(t) value approaches a plateau as described previously90,91. Simulations using potential 1 converged at 50-500 μs and simulations using potential 2 converged at 5-20 μs, depending on if RMSD or Conv(t) was used. From these simulations, we identified 1769 elbow accommodation events with potential 1 and 87 with potential 2. In using potential 2 we observed in 13 simulations where the aa-tRNA dissociates prior to accommodation, representing rejected aa-tRNA. Within these simulations 1 reduced unit of time was approximated to be 1 ns, in line with previous investigations using structure-based simulations to quantify tRNA accommodation30,75,90.
Figure 1. Conformations of ribosomes during eukaryotic aa-tRNA accommodation.
(A) All-atom structural representation of the pre-accommodated GTPase activated state with aa-tRNA in the A/T position. (B) Simplified model of the GTPase activated state with aa-tRNA in the A/T position. (C) All-atom structural representation of the accommodated state with aa-tRNA in the A/A position. (D) Simplified model of the accommodated state with aa-tRNA in the A/A position. In these models aa-tRNA (yellow), mRNA (green), peptidyl-tRNA (orange), 28S, 5S, 5.8S rRNA (white), 18S (grey), ribosomal proteins (blue) and eEF1A (purple) are represented.
To achieve reversible fluctuations of the aa-tRNA in the simulations with potential 1 we scaled the weight of the contacts for the tRNA in the A/A position in the ribosome. By titrating the weight of the native contacts for the A/A position of the tRNA we were able to fine-tune our simulations to be evenly distributed between the two elbow accommodated positions (EA-1 and EA-2) in the simulations at a contact weight of 0.13 (Supplemental Fig. 3). Similarly, the contacts for the A/A position in potential 2 simulations were scaled by 0.4 to enable fluctuations of the aa-tRNA during accommodation, as previously performed in bacterial systems90. A rescaling of 0.4 enables the tRNA to accommodate into the A-site through reversible fluctuations, reflecting tRNA trajectories seen in smFRET studies28,30. The interactions between the mRNA and tRNA were scaled by a factor of 0.8 to compare to bacterial studies where it was rescaled by this value to reflect tRNA rejection frequencies90.
Human aa-tRNA accommodation requires movement of the elbow domain and 3’-CCA end
Bacterial aa-tRNA accommodation is known to occur through an elbow-first mechanism where the elbow domain of the aa-tRNA (D Loop and T Loop, nucleotides 14-21 and 54 to 60, respectively) enters the A site of the ribosome prior to the 3’-CCA end. In humans, we measured the trajectory of the aa-tRNA elbow domain into the ribosomal A site by tracking the distance between the O3' atoms of U60 and U8 on the aa-tRNA and peptidyl-tRNA, respectively (Fig. 2A). Additionally, we monitored the accommodation of the 3'CCA end of the tRNA, conjugated to either the nascent polypeptide chain or amino acid, by measuring the distance between the A76 residues of both the peptidyl and aa-tRNA (Fig. 2B). We observed the tRNA elbow accommodation as decreased from ~57 Å to ~32 Å, a distance change of ~25 Å (Fig. 2C; Supplemental Fig. 4). Similarly, the 3’-CCA end of the aa-tRNA moved into the A site of the ribosome as it goes from ~80 Å to ~8 Å, a 72 Å change (Fig. 2D; Supplemental Fig. 5). The measurement shows a clear intermediate position at 20-40 Å, an intermediate position previously identified in bacteria where the tRNA contacts regions of the accommodation corridor such as H71 and H89 of the 23S rRNA (Fig. 2D). We observed that this intermediate for human aa-tRNA accommodation contacts the same regions of the ribosome in the intermediate position (Supplemental Fig. 6).
Figure 2. Dynamics of human aa-tRNA accommodation.
(A) Distance measured between O3’ atoms of U60 and U8 of the aa-tRNA and peptidyl-tRNA, respectively, to characterize the elbow accommodation dynamics of the aa-tRNA (). (B) Distance measurement between the O3’ atoms of A76 of the aa-tRNA and peptidyl tRNA during accommodation into the A site of the ribosome . (C) Representative distance of during simulation using potential 2. (D) Representative distance of during a simulation using potential 2. (E) Representative distance of during a simulation using potential 1, showing reversible fluctuations. (F) Representative distance of during a simulation using potential 1, showing reversible fluctuations.
At longer timescales (up to 1.5 ms) of simulation using potential 1, we observe the reversible fluctuation of the aa-tRNA into the A-site of the ribosome, reflecting repetitive accommodation attempts. This is captured in both the value of the simulations and in the value of the simulations (Figs. 2E, F; Supplemental Figs. 7, 8). In these trajectories, we see that the movement of the elbow domain and of the 3’CCA region are highly coupled meaning that as one approaches the A/A or A/T position, the other follows. By using the number of timesteps required for each accommodation event in our simulations we could estimate the barrier height during transition from the A/T position to the transition state using previously determined rates of accommodation (Supplemental Methods). From these measurements we estimate that the barrier height of E. coli accommodation is 9.3 – 10.4 , similar to previously determined values, while in humans the barrier is 11-13.4 75.
Human aa-tRNA accommodates through a distinct 3’-CCA first approach
From simulations of the reversible accommodation of aa-tRNA into the A site of the ribosome, we generated a Boltzmann-weighted approximate free energy landscape comparing and . In this landscape, we observe two dominant positions, one at a and value of ~42 Å and ~60 Å, respectively denoted elbow accommodated-1 (EA-1) and another at ~32 Å and ~25 Å, respectively denoted elbow accommodated-2 (EA-2) (Fig. 3A). The nomenclature of EA-1 and EA-2 are used instead of A/T and A/A as the aa-tRNA in these positions differ from the and of the A/T and A/A from resolved structures. In the A/T position the and are 57 Å and 79 Å, respectively, and in the A/A state they are 32 Å and 8 Å, respectively. The EA-1 and EA-2 positions reflect the elbow-accommodation aa-tRNA position that has been observed in E. coli previously37; which are on-path towards the A/A tRNA position. Both the EA-1 and EA-2 positions were observed in our simulations of bacterial accommodation (Supplemental Fig. 9). The and values of the EA-1 position in E.coli structures are ~36 Å and ~47 Å and the EA-2 position are ~35 Å and 22 Å , respectively. In comparing bacterial and human elbow accommodated positions, EA-1 has distinct and distances compared to humans but the EA-2 position is similar between species.
Figure 3. Human aa-tRNA accommodation occurs through a 3’-CCA end dependent pathway.
(A) Approximate free energy landscape of tRNA accommodation as measured by comparison of the and distance measurements from simulations using potential 1. (B) Representative structure of EA-1. Complete 80S structure of EA-1 (i). Representation of the accommodation corridor of the ribosome with aa-tRNA engaging H89 in EA-1 (ii). Zoom in of accommodation corridor with aa-tRNA engaging H89 in EA-1 (iii). Representation of the accommodation corridor in EA-1 from the LSU perspective (iv). Zoom in of the 3’ CCA end of the aa-tRNA interacting with H71 in EA-1 (v). (C) Representative structure of the accommodation corridor during transition path from EA-1 to EA-2 (Path). Complete 80S structure of Path (i). Representation of the accommodation corridor of the ribosome with aa-tRNA engaging H71 in Path (ii). Zoom in of the Path position with the aa-tRNA interacting with H89 (iii). Representation of the accommodation corridor in Path from the LSU perspective (iv). Zoom in of the 3’ CCA end of the aa-tRNA interacting with H71 in Path (v). (D) Representative structure of EA-2. Complete 80S structure of EA-2 (1). Representation of the accommodation corridor of the ribosome wiith aa-tRNA engaging H71 after passing H89 (ii). Zoom in of the EA-2 position with the aa-tRNA interacting with H89 (iii).Representation of the accommodation corridor in EA-2 from the LSU perspective (iv). Zoom in of the 3’ CCA end of the aa-tRNA interacting with H71 in EA-2(v). In these models highlighted in blue and highlighted in red, while the rRNA (white and grey), ribosomal proteins (blue), peptidyl-tRNA (orange), aa-tRNA (yellow), H89 (grey), H90 (mauve), H71 (green), h18 (purple), H44 (red), and mRNA (green) are represented.
EA-1 reflects an aa-tRNA arrangement whereby the aa-tRNA elbow domain and the 3’-CCA end have initiated movement into the ribosome and have contacted H89, which sterically restricts the tRNA movement (Fig. 3B). In EA-2 the aa-tRNA has passed H89 and is approaching final A/A position, however, the 3’-CCA contacts H71, posing as a steric barrier for tRNA movement (Fig. 3C). An observable difference between H. sapiens and E. coli tRNA accommodation is that the tRNA 3’-CCA end moves towards the A-site prior to the elbow domain in the transition between EA-1 and EA-2 (Fig. 3A). This pathway (Path) appears to be distinct in eukaryotic tRNA selection and involves the 3’-CCA end of the tRNA approaching the H71 barrier prior to overcoming the H89 barrier (Fig. 3D). This indicates that the steric barrier imposed by H89 is larger in humans than it is in bacteria, which could account for the tenfold reduction in the rate of tRNA selection.
Human tRNA pivots during accommodation into the A site
For aa-tRNA to accommodate into the ribosomal A-site using the 3’-CCA end first pathway that we observe between EA-1 and EA-2, then the tRNA would have to be positioned differently than it is in bacteria. To quantify the different positioning of the tRNA, we measured the change in angle of the tRNA over time () (Figs. 4A, B; Supplemental Fig. 10A). Specifically, is the change in angle of a vector perpendicular to the plane of the tRNA generated from atoms O3’ of C4, A35, and G56. This angle reflects the pivoting of the tRNA with respect to the codon-anticodon interaction along the acceptor stem and D-loop of the tRNA. In a manner distinct from bacteria, we observed key pivoting of the human aa-tRNA during accommodation into the A site (Supplemental Fig. 10B). This pivoting occurs at the early stages of tRNA accommodation as measured by and appears to match the same temporal regime (2-6 μs) as it takes the tRNA to approach the intermediate position (Fig. 2D). No pivoting was observed in our bacterial simulations (Supplemental Fig. 9B). When comparing with the distance on an approximate free energy landscape we observe that as distances decrease, indicating the tRNA is moving from EA-1 to EA-2, that the angle of the tRNA displays variability of −30 to 30° (Fig. 4C). This pivoting of the aa-tRNA narrows to an energetic minimum of ~0° at a value of ~ 38Å, a distance between EA-1 and EA-2 where the aa-tRNA is in contact with H89 (Figs 3A, C). Similarly, we compared the value compared to the distance measurement. Through this comparison we see a similar trend whereas the tRNA transitions from EA-1 to EA-2, the tRNA goes through an energy minimum of ~0°, although variability in the tRNA angle is available (Fig. 4D). Here, we see that the narrowing of the tRNA angle occurs at an value of ~35 Å, occurring prior to 3’CCA end contacts H71, distance ~25 Å (Fig. 2D). This means that the tRNA pivoting in the accommodation corridor is not to surpass the H71 barrier. The pivoting aligns with the timing required for the tRNA to surpass the H89 barrier. This indicates that the tRNA is flexible in the accommodation corridor in eukaryotes, enable the 3’-CCA end to reach the H71 barrier, however, to overcome the H89 barrier, the tRNA has to achieve a of ~0° to enter the A site.
Figure 4. Human aa-tRNA pivoting during accommodation into the ribosomal A site.
(A) Structural representation of aa-tRNA pivoting () during accommodation into the A site. Perspective from the central protuberance (left), LSU (middle), and P site (right) are represented to highlight the pivoting of the aa-tRNA. Peptidyl-tRNA (orange), mRNA (green), pre-pivot aa-tRNA (yellow), and post-pivot aa-tRNA (blue) are represented and the axis of the pivoting along the anticoding stem to the elbow domain is highlighted in red. is the angle of the tRNA as it moves into the ribosomal A site as measured by changes in the vector perpendicular to the plane of the tRNA (Methods). (B) Cartoon representations of the aa-tRNA pivoting into the ribosomal A site. aa-tRNA begins accommodation into the ribosome in the bent non-pivoted position (left), the tRNA then needs to pivot and accommodate through a 3’CCA-end first mechanism (middle), then the tRNA returns to a non-pivoted position once the elbow of the tRNA enters the A site. (C) Approximate free energy landscape of human tRNA accommodation into the ribosomal A site generated by comparing the distance and angle, generated from potential 1 simulations. (D) Approximate free energy landscape of human tRNA accommodation into the ribosomal A site generated by comparing the distance and angle, generated from potential 1 simulations. (E) Representative structure of the open A site of the ribosome in the GA complex, prior to accommodation (F) Representative structure of the closed A site of the ribosome in the AC complex, after accommodation. (G) Approximate free energy landscape of human ribosome rolling during tRNA accommodation generated by comparing and , generated from potential 2 simulations. (H) Approximate free energy landscape of human ribosome rolling during tRNA accommodation generated by comparing and , generated from potential 2 simulations. (I) Histogram of distances between atoms A35 and C61 of the accommodating H. sapiens and E. coli aa-tRNA, representing the compression of tRNA during accommodation (), generated from potential 2 simulations. (J) Solvent accessible surface area (SASA) of aa-tRNA during H. sapiens and E. coli during accommodation, generated from potential 2 simulations.
The human accommodation corridor is compacted by subunit rolling
As subunit rolling is known to occur during aa-tRNA accommodation between the GA and AC complexes68-70, we investigated if it contributes to the aa-tRNA pivoting observed in structure-based simulations (Supplemental Fig. 11). To quantify subunit rolling, we measured the distance between the O3’ atoms of A465 of h14 and U4559 of the SRL (Figs. 4E, F). At the beginning of our simulations, we observed a distance of 33.4 Å, and at the end of the simulation, this distance narrowed to 27.5 Å, consistent with subunit rolling, leading to a closure of the A site of the ribosome (Figs. 4C, D). We investigated how subunit rolling correlates with the aa-tRNA accommodation to investigate if the closure of the A site could influence the tRNA pivoting. By comparing with on an approximate free energy landscape, we identified that subunit rolling occurs prior to elbow accommodation (Fig. 4G). As subunit rolling occurred early in our simulations, we had to perform these comparisons in simulations using potential 2. These landscapes show that decreases from 33.4 Å to ~27.5 Å prior to shifts from ~57 Å to 32 Å (Fig. 4G). Similar behavior is observed when comparing with (Fig. 4H). We observed that decreased prior to shifts from ~80 Å to ~8 Å (Fig. 4H). This suggests that as the aa-tRNA accommodates, subunit rolling has already occurred.
To provide an understanding as to how subunit rolling impacts the accommodating aa-tRNA, we investigated the steric constraints that the accommodation corridor imposes on the aa-tRNA. Initially, we investigated the compression of the tRNA (the distance between residues A35 and C61 of the tRNA) to identify whether the accommodation corridor is narrower (Fig. 4I). This measurement showed that in humans, to enter the A site, the tRNA is compressed by 3.7 Å, as from our Gaussian fit, we identify the tRNA compression to be 54.4 ± 0.8 Å in H. sapiens and 58.1 ± 0.9 Å in E. coli (Fig. 4I). Similarly, we measured the solvent accessible surface area (SASA) of the tRNA in the accommodation corridor (Fig. 4J). Here, we observe H. sapiens tRNA having 13079 ± 121 Å2 SASA and E. coli tRNA having 14390 ± 206 Å2 SASA, indicating that the tRNA is surrounded by more of the ribosome in H. sapiens than in E. coli (Fig. 4J). Surprisingly, the SASA values did not appear to change much throughout the course of our trajectories, indicating that subunit rolling induced crowding of the accommodating tRNA provides uniform contacts between the tRNA and ribosome or eEF1A throughout accommodation (Supplemental Fig. 12). Together, these data support that the accommodation corridor in humans is more compact than in bacteria due to subunit rolling.
H89 of the tRNA accommodation corridor influences tRNA pivoting angles
To identify which region of the aa-tRNA accommodation corridor influences the pivoting angles of aa-tRNA during accommodation, we examined all regions of the 28S, 18S, and ribosomal proteins that are within 4 Å of the aa-tRNA during accommodation (Figs. 5A, B; Supplemental Fig. 13). Using this method to identify the ribosomal regions that contact the aa-tRNA, we found that H38, H69, H71, and H89 make the majority of contacts with the tRNA in the 28S rRNA (Fig. 5A). Within the 18S rRNA, h18, h30, h31, h34, and h44 make contact with the aa-tRNA (Fig. 5B). We observed regions such as H69, h30, h31, and h44 making contacts for the majority of the frames, as the aa-tRNA remains in constant contact with these regions throughout the simulation. These ribosomal elements are near the codon-anticodon interactions, which remain relatively stationary during the accommodation of aa-tRNA. Compared to the rRNA, ribosomal proteins made fewer contacts with the aa-tRNA; however, ribosomal proteins uL16, uL14, uL6, and eS30 made the most contacts with the aa-tRNA (Supplemental Fig. 13). These ribosomal regions are consistent with what we observe in simulations as the aa-tRNA moves through the accommodation corridor (Fig. 5C).
Figure 5. aa-tRNA interactions with the accommodation corridor.
(A) Regions of the 28S rRNA that are within 4 Å of the aa-tRNA during accommodation simulations of the tRNA into the ribosomal A site. (B) Regions of the 18S rRNA that are within 4 Å of the aa-tRNA during accommodation simulations of the tRNA into the ribosomal A site. (C) Structural representation of rRNA and ribosomal protein uL14 that are in proximity to aa-tRNA during accommodation. (D) Histogram of during accommodation of tRNA, captured from N ≥ 5 potential 2 simulations composed of full ribosome or with no H69/71 or no H89.
To determine which regions of the ribosomal accommodation corridor influence the pivoting of the aa-tRNA we performed structure-based simulations using potential 2 with a virtual removal of H69 and H71 to remove interactions with the acceptor stem and for 3’-CCA accommodation. Secondly, we virtually removed H89 from simulations to identify its influence on aa-tRNA accommodation. H38 or the A-site finger was not removed from simulations as it is structural dynamic and is therefore unlikely to restrict aa-tRNA movements92. From these simulations we measured during the simulation (Fig. 5D). In these simulations, we observed a similar distribution of for the full ribosome ~-20 to 25°, with a mean of 7.1 ± 10.5° (Fig. 5D). When we deleted H69 and H71, we observed a similar distribution with a mean of 2.6 ± 8.4° (Fig. 5D). This indicates that the pivoting of the aa-tRNA is not dependent on these two helical elements. When we removed H89, we observed an increased distribution of angles during the simulation, now ranging from −20 to 65° (Fig. 5D). Furthermore, the distribution no longer centered around the mean of 7.1°, indicating that the tRNA is no longer required to restrict the angle distribution to enter the ribosomal A site. This data supports that the aa-tRNA during accommodation must approach a narrow angular distribution to surpass H89 to enter the ribosome and that H89 creates a larger barrier for aa-tRNA entry into the ribosome.
Switch I of eEF1A interacts with the 3’-CCA end of the accommodating aa-tRNA
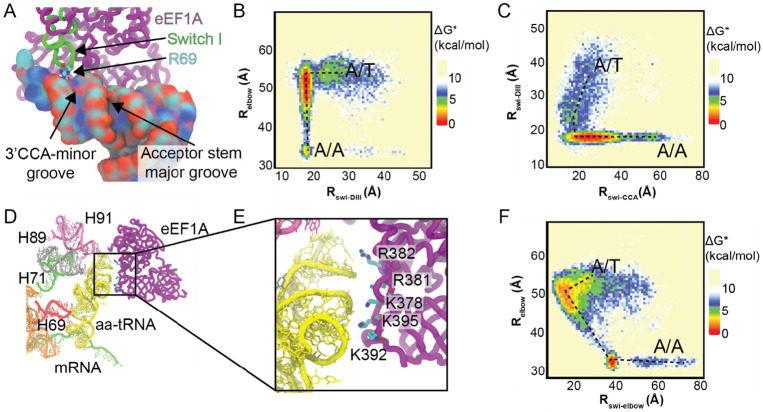
Previously, we have reported on the conserved arginine of the switch I element of EF-Tu (R58) that interact with the minor and major grooves of the acceptor stem of the accommodating aa-tRNA before docking onto domain III of eEF1A during early stage accommodation events into the A site of the ribosome76,90. To investigate if eEF1A interacts similarly with the accommodating tRNA in humans, we investigated the interactions of R67 and R69 of eEF1A switch I with the acceptor stem of aa-tRNA. Here we observe R69 of switch I interacts with the phosphodiester backbone of the aa-tRNA during early accommodation movements of the aa-tRNA (Fig. 6A). To quantify this, we measured the distance of R69 to and A425 of eEF1a to describe the position of switch I relative to domain III and compared it to (Fig. 6B). This measurement showed that the distance decreased prior to the distance, indicating that switch I docks onto domain III of eEF1A prior to accommodation of the aa-tRNA. This requirement is also found in bacteria where switch I needs to interact with and traverse the grooves of the aa-tRNA prior to aa-tRNA accommodation76. Secondly, we wanted to identify which grooves of the aa-tRNA does R69 interacts with during accommodation. By comparing with the distance of R69 to A76 of the 3’-CCA end of the aa-tRNA , we could identify which grooves of the aa-tRNA R69 passes through (Fig. 6C). Here, we identify only one dominant path that R69 passes through at an distance of ~22 Å, we see that the distance is ~15-25 Å. This distance is consistent with R69 passing through the 3’CCA-minor groove of the aa-tRNA and not with it passing through the major groove of the acceptor stem (Fig. 6A). This behavior is distinct from bacteria where R58 can pass through both the 3’CCA-end minor groove and the acceptor stem major groove. As switch I can only traverse the 3’CCA-end minor groove, it indicates that the initial distribution of possible trajectories of the aa-tRNA into the A site is narrower than it is in bacteria.
Figure 6. eEF1A interacts with accommodating aa-tRNA through R69 and conserved basic amino acids in Domain III.
(A) R69 of switch I of eEF1A interacts with the minor groove of the aa-tRNA adjacent to the 3’CCA end. R69 is highlighted interacting with the phosphodiester backbone of the tRNA. (B) Approximate free energy landscape of in comparison to the distance between eEF1A R69 and A425 of domain III , generated from potential 2 simulations. This landscape indicates that for aa-tRNA to accommodate, R69 of switch I needs to pass through the 3’CCA-minor groove of the aa-tRNA and dock on domain III. (C) Approximate free energy landscape of with respect to the distance of R69 and A76 of aa-tRNA, generated from potential 2 simulations. This landscape highlights that only the path where R69 passes by the 3’CCA-minor groove is available. (D) Interactions of eEF1A domain III with in proximity to the accommodating aa-tRNA. (E) Zoomed in image of domain III of eEF1A interacting with the accommodating aa-tRNA. Basic amino acids are highlighted that could interact with the aa-tRNA. (F) Approximate free energy landscape of distance with respect to K378 to U55 O3’ distance, generated from potential 2 simulations. This demonstrates that domain III approaches the accommodating aa-tRNA in all simulations at an of ~52 Å.
Domain III of eEF1A interacts with the aa-tRNA elbow through conserved basic amino acids
To identify any other basic amino acids that make direct contact with aa-tRNA during accommodation, we measured all basic amino acids of eEF1A that are within 4 Å of the accommodating aa-tRNA during the simulation (Supplemental Fig. 14). This analysis showed amino acids that line the aa-tRNA binding site, but also revealed that the basic amino acids K378, K392, K395, R381, and R382, which are part of domain III and distant from aa-tRNA, at the start of the simulation, interact with the accommodating aa-tRNA. From our simulations we observe that as eEF1A domain separation occurs and domain I rotates relative to domain II and III, domain III becomes positioned to interact with the elbow domain of the aa-tRNA (Figs. 6D, E; Movie 1). The amino acids K378, K392, K395, R381, and R382 are positioned to interact with the phosphodiester backbone of the accommodating aa-tRNA (Figs. 6D, E). In this position eEF1A would remain bound to the ribosome long enough to facilitate aa-tRNA accommodation by providing a steric barrier for aa-tRNA dissociation from the A site. To determine the likelihood of these interactions being critical to aa-tRNA accommodation, we looked at the conservation of these basic amino acids (Supplemental Fig. 15). By comparing 154 eEF1A sequences across eukaryotes, we identified the conservation of these basic amino acids (Table 1). From this analysis we find that K378 and K395 are highly conserved at 94.84% and 96.13%, respectively, and are not known to be post-translationally modified, indicating that their basic properties are conserved93. Furthermore, if these amino acids are not lysine, they are frequently arginine, indicating that their charge is conserved. The conservation of nucleotides of the tRNA that interact with domain III of eEF1A were also considered, either amongst humans or throughout different domains of life (G1, U51, G52, A64, G65, and U66) (Supplemental Table 2). Although the conservation of these nucleotides varied (0.43% to 99.31%), all interactions between the tRNA and domain III of eEF1A were mediated by backbone interactions, indicating that the nucleoside identity is not essential for these interactions (Figs. 6 D,E). Together, these findings indicate that the role these positive amino acids play in aa-tRNA accommodation is conserved.
Table 1.
Conservation of basic amino acids of domain III of eEF1A that interact with the elbow of the aa-tRNA
| Amino Acid | Conservation | Other possible identities |
|---|---|---|
| K378 | 94.84% | R, T |
| K392 | 58.71% | A, G, S, H, D, I, P, V, E, Q |
| K395 | 96.13% | A, Q, R |
| R381 | 58.44% | A, P, K |
| R382 | 78.57% | A, S, K |
DISCUSSION
Here, we identified a pivoting of the aa-tRNA during accommodation into the ribosomal A site of the ribosome which is not observed in bacteria. The ribosomal accommodation corridor appears to be more crowded in humans, as observed by the decreased SASA and increased compaction of the aa-tRNA (Fig. 7A). Increased crowding of the aa-tRNA accommodation corridor is consistent with subunit rolling occurring prior to aa-tRNA accommodation, compacting the A site. Increased crowding appears to increase the steric contribution of H89 during accommodation, making it a larger barrier to overcome during accommodation. Surpassing this barrier is aided by the pivoting of the aa-tRNA (up to ~30°) that aligns the elbow domain minor groove and acceptor stem major groove of the tRNA properly to accommodate into the A site (Fig. 7B). This increased barrier that H89 provides is consistent with the tenfold reduced rate in aa-tRNA accommodation into the A site as observed with smFRET studies68.
Figure 7. Model of aa-tRNA pivoting into the ribosomal A-site.
(A) Crowded environment of the human accommodation corridor enables aa-tRNA accommodation to be 10-fold slower. Additional eEF1A stays in complex with the ribosome long enough form transient interactions between domain III of eEF1A and the elbow domain of the accommodating aa-tRNA (B) aa-tRNA in human approaches the accommodation corridor until it reaches the H89 steric barrier. It then requires pivoting of the aa-tRNA to properly align with all of the elements of the accommodation corridor to enter the A site.
In the studies that identified the tenfold reduced rate in aa-tRNA accommodation, subunit rolling was also identified between the GA and AC states of the ribosome68,69. It appears that the subunit rolling that occurs during this transition is responsible for the increased barrier of aa-tRNA accommodation, namely by H89. The aa-tRNA can overcome this barrier by swiveling to align the aa-tRNA properly with all of the elements of the accommodation corridor (Fig. 7B). Simultaneously, the accommodating aa-tRNA can fit through a narrower window to accommodate into the A-site of the ribosome. These findings suggest that the ribosome has evolved the accommodation corridor to provide an optimally crowded environment for cognate aa-tRNA selection, similar to how molecular crowding influences the thermodynamics and kinetics of RNA and protein folding94,95.
Furthermore, the requirement of the aa-tRNA pivoting provides further sup mort for the hypothesis that the geometric alignment of the aa-tRNA relative to catalytic centers of the ribosome is a critical component in maintaining the fidelity of translation90,96. In bacteria, we observed that near-cognate aa-tRNA is misaligned relative to catalytic centers in comparison to cognate aa-tRNA. If the same misalignment of near-cognate aa-tRNA occurs in humans, it would be less likely to pivot correctly to overcome the increased barrier of H89. Therefore, the misalignment of the near-cognate aa-tRNA would not be able to align with the accommodation corridor correctly, contributing to the increased accuracy of decoding observed in eukaryotes8.
The second distinction we observed in aa-tRNA accommodation is that in humans, switch I of eEF1A only interacts with 3’-CCA end minor groove of the aa-tRNA and that domain III of eEF1A made interactions through conserved lysine amino acids with the elbow domain of the aa-tRNA (Fig. 6). In bacteria, it is posited that either the switch I passes through the 3’-CCA end, or the acceptor stem groove to align the aa-tRNA during accommodation into the A site76. As human aa-tRNA selectively passes through the 3’-CCA end groove, it indicates that there is a more restrictive selection on the initial trajectory of the aa-tRNA into the ribosomal A site.
The direct interactions observed between domain III of eEF1A through conserved basic amino acids provides a structural framework for how eEF1A can contribute to aa-tRNA proofreading. It was observed that EF-Tu can lower to the barrier of aa-tRNA proofreading by providing steric contributions to aa-tRNA movements towards the A site77. Similarly, domain III of eEF1A could provide these steric barriers after domain rearrangement of eEF1A. Together, the contributions of eEF1A and aa-tRNA pivoting provide distinct structural features for how aa-tRNA accommodates into the ribosome compared to bacteria, providing a rationale for the higher accuracy observed in eukaryotes.
METHODS
Generation of ribosome models with A/T and A/A aa-tRNA.
Both the GA and AC complexes with A/T and A/A aa-tRNA positions were modeled prior to the availability of the complexes68,69. These states were modeled using the coordinates of the ribosome from PDB ID: 6Y0G. Coordinates of the eEF1A(GTP) phe-tRNAphe ternary complex bound to ribosomes were derived from PDB ID: 5LZS, a Oryctolagus cuniculus ribosomes bound to a compact post-hydrolysis eEF1A in complex with GDP, stalled in a conformation consistent with the GTP bound form by didemnin B97. A homology model of the H. sapiens eEF1A was generated using the SWISS-MODEL server, using a previously described approach and the GDP was manually converted to GTP in Visual Molecular Dynamics (VMD)98-101. Similarly, the tRNA was converted to the sequence of tRNAphe using the Swapna package in Chimera102. Phenylalanine was attached to the 3’ – adenosine of the aa-tRNA according to the specific chemistry. The A/A positioned aa-tRNA from the 6Y0G PDB was removed to model the A/T conformation and replaced with the H. sapiens homology model eEF1A(GTP) phe-tRNAphe ternary complex, representing the GA complex.
To model GDP conformation of eEF1A for the A/A aa-tRNA state the structure of eEF1A in the open, or GDP bound, conformation was used PDB ID: 4C0S103. A homology model of the eEF1A(GDP) conformation was made in the SWISS-MODEL server and was aligned to the ribosome by the P-loop of the eEF1A(GTP) model. The eEF1A(GDP) was then minimized in GROMACS 2021 to ensure that there were no overlapping atoms between eEF1A and the ribosome 104-107.
Model preparation of ribosome complexes.
Models of the GA and AC complexes were subject to explicit solvent minimization and equilibration in GROMACS 2021104-107. Initially, periodic boundaries extending 15 Å beyond the boundaries of the ribosome complex was filled with SPC/E water molecules and all explicit solvent simulation used the AMBER 99 forcefield. Ions were added at a ratio of 20 mM Mg and 200 mM K per water molecule and sufficient Cl to neutralize the system, to maintain ionic strength conditions similar to previous ribosome simulations74,91. Water was removed and the ions were simulated with a frozen ribosome, in implicit water conditions with a dielectric of 80, to allow for Mg and K to condense on the ribosome using modified ion parameters to limit ion-ion interactions108. The Van der Waals radii of the ions were increased to mimic a hydrated ion so that they would properly condense on the ribosome. SPC/E water was added back to the system and the water was minimized using a steepest decent approach followed by minimization of the ions, with their van der Waals radii returned. An NVT equilibration at 300 K of the ions and solvent was performed for 10 ns. Subsequently, an NPT equilibration was performed at 300 K and 1 atmosphere was performed for 10 ns while slowly releasing restraints on the ribosome.
Structure-based simulations of ribosome complexes.
Ribosome coordinates after 20 ns of equilibration were used as starting structures for structure-based simulations of the aa-tRNA proofreading process using SMOG 2.4 source code 109. Two different potentials were implemented in this manuscript defined as potential 1 and potential 2. These two potentials differ in how they define the contacts that define the energetic minima in the structure-based simulations.
Potential 1 used in the structure-based simulations is defined as:
| 1 |
where,
| 2 |
Where , , , , , , and . Native contacts were defined as atom pairs within 4.5 Å of each other. This potential was implemented to capture the reversible fluctuations of the aa-tRNA during proofreading. In these simulations the contacts of the aa-tRNA in the A/A tRNA position were scaled by a factor of 0.13 and contacts between the codon and anticodon were scaled by a factor of 0.8. This scaling was implemented to achieve reversible fluctuations of the aa-tRNA and to model a system where the mRNA and aa-tRNA interactions are reversible. Using this model the aa-tRNA rapidly reaches the elbow accommodated-1 (EA-1) position and rapidly fluctuates between the EA-1 and elbow accommodated-2 position (EA-2). This potential capture reversible nature of accommodation but did not capture early and late-stage events during aa-tRNA accommodation. Each system was simulated for a total of 1x106 (time reduced units) or 5x108 timesteps with a timestep of 0.002 using GROMACS 2021104-107. Simulations were performed at a constant temperature of 0.5 reduced units. Within these simulations we captured 1769 aa-tRNA accommodation into the A site events. Accommodation events were considered when the elbow domain of the aa-tRNA reached a distance of < 32.5 Å, indicating an elbow accommodated position77.
Potential 2 was similarly defined, however, the contact terms were replaced with a single Gaussian term as described previously (Supplementary Material)90. Similarly, the contacts of the final accommodated state (AC) between the aa-tRNA and the ribosome were scaled by 0.4 and between the mRNA and tRNA by 0.8. The advantage of these simulations is they enable the identification of early and late-stage accommodation events. However, they do not capture the reversible fluctuations of the aa-tRNA during accommodation. Simulations using potential 2 also enabled the comparison between aa-tRNA proofreading of prokaryotic (E. coli) and eukaryotic (H. sapiens) ribosomes (Supplemental Material). Each simulation was performed for at least 1x107 timesteps using a timestep is 0.002 . Using this simulation setup we performed 100 simulations. Of these 100 simulations we observed 87 elbow accommodation events with 13 aa-tRNA dissociating prior to accommodation (Supplemental Table 3). For simulations without H89 or H69/H71 we used potential 2, deleting each helix as described previously77.
Simulations of prokaryotic ribosomes
Prokaryotic ribosomes were prepared as previously described90. Potential 2 was used to characterize early- and late-stage accommodation events. Each simulation was performed for 1.25x107 timesteps using a timestep is 0.002 .
Distance measurements
To measure the progression of aa-tRNA during accommodation into the A site, we measured the distance between the O3’ oxygen of the U60 and U8 nucleotides of the aa-tRNA and peptidyl-tRNA respectively . This distance was used to describe the movement of the tRNA elbow domain into the A site of the ribosome, similar to measured for accommodating tRNA in E. coli90. Similarly, we measured the distance between A76 of the aa-tRNA and peptidyl tRNA to describe late-stage accommodation events of aa-tRNA, similar to as previously described in E. coli90. Subunit rolling was measured using the distance between the O3’ atoms of A465 of h14 and U4559 of the SRL. To describe the movement of switch I relative to domain III of eEF1A we measured the distance of the Cα atoms of R69 and A425 . To quantify the movements of switch I relative to the 3’-CCA end of the tRNA during accommodation we measured the distance between the Cα atom R69 of eEF1A and the O3’ atom of A76 of the accommodating aa-tRNA . Lastly, to describe the position of domain III of eEF1A relative to the elbow domain of aa-tRNA we measured the distance of the Cα atom of K378 to U55 O3’ . Average distance measurements were determined using a single gaussian distribution fit as defined by:
| 3 |
where is the peak of the distribution, is the average value of the distance, and is the standard deviation for the population.
tRNA angle Measurement
Measurements of the tRNA angle () during accommodation were performed in VMD. Here a plane was generated across the accommodating tRNA from the O3’ atoms of C4, A35, and G56. A vector perpendicular to the plane of the accommodating tRNA is generated and the change in the angle of the vector throughout the simulation is measured, using the first frame as a reference.
Approximate Free Energy Landscape
All free energy landscape approximations were generated as described previously90. In brief, two reaction coordinates (i.e. and ) are compared in a Boltzmann weighted approximated free energy landscape using equation 4.
| 4 |
Here, is the relative free energy of the landscape, is the Boltzmann coefficient, is the temperature (300 K), is the probability density function of being at state obtained from a histogram of the compared measurements, and is the maximum probability of the most observed state.
Convergence of simulations
Convergence of the structure-based simulations was calculated using the pointwise RMSD between successive free energy landscapes as previously described90,91. The pointwise RMSD convergence metric is defined by equation 5:
| 5 |
where is the approximate free energy landscape of and after sampling to time (i.e., 1000 frames of simulation) and is the approximate free energy landscape of and after sampling to time , and is the number of grid points on the free energy landscape. After plateaus, simulations were considered converged.
Supplementary Material
ACKNOWLEDGEMENTS
The authors would like to thank all members of the Girodat and Sanbonmatsu labs for their helpful comments and discussions. The authors would also like to thank LANL Institutional Computing and Digital Research Alliance of Canada for their generous support. This work was supported by NIGMS R15-GM151696-01, University of Lethbridge startup funds, and NSERC Discovery Grant (RGPIN-2025-04896) (to DG), and NIGMS 5R01GM072686 (to KYS).
Footnotes
DECLARATION OF COMPETING INTERESTS
All authors declare no competing interests.
DATA AVAILABILITY
Structures, parameters, and example trajectories for these simulations have been uploaded to Zenodo and are publicly available. This includes the initial PDB file used, the input parameters for gromacs simulations (i.e. gro, top, and mdp files), as well as the tpr files. We have also included example trajectories of the simulations. They may be accessed at doi: 10.5281/zenodo.15653228.
CODE AVAILABILITY
All scripts that were developed for the analysis of this project have been uploaded to Zenodo and are publicly available at doi: 10.5281/zenodo.15653228.
REFERENCES
- 1.Bouadloun F., Donner D. & Kurland C. G. Codon-specific missense errors in vivo. The EMBO Journal 2, 1351–1356 (1983). 10.1002/j.1460-2075.1983.tb01591.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Edelmann P. & Gallant J. Mistranslation in E. coli. Cell 10, 131–137 (1977). 10.1016/0092-8674(77)90147-7 [DOI] [PubMed] [Google Scholar]
- 3.Kramer E. B. & Farabaugh P. J. The frequency of translational misreading errors in E. coli is largely determined by tRNA competition. RNA 13, 87–96 (2007). 10.1261/rna.294907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Laughrea M., Latulippe J., Filion A. M. & Boulet L. Mistranslation in twelve Escherichia coli ribosomal proteins. Cysteine misincorporation at neutral amino acid residues other than tryptophan. Eur J Biochem 169, 59–64 (1987). 10.1111/j.1432-1033.1987.tb13580.x [DOI] [PubMed] [Google Scholar]
- 5.Ogle J. M. & Ramakrishnan V. Structural insights into translational fidelity. Annu Rev Biochem 74, 129–177 (2005). 10.1146/annurev.biochem.74.061903.155440 [DOI] [PubMed] [Google Scholar]
- 6.Parker J. Errors and alternatives in reading the universal genetic code. Microbiol Rev 53, 273–298 (1989). 10.1128/mr.53.3.273-298.1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rosenberger R. F. & Foskett G. An estimate of the frequency of in vivo transcriptional errors at a nonsense codon in Escherichia coli. Mol Gen Genet 183, 561–563 (1981). 10.1007/BF00268784 [DOI] [PubMed] [Google Scholar]
- 8.Kramer E. B., Vallabhaneni H., Mayer L. M. & Farabaugh P. J. A comprehensive analysis of translational missense errors in the yeast Saccharomyces cerevisiae. RNA 16, 1797–1808 (2010). 10.1261/rna.2201210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stansfield I. et al. Missense translation errors in Saccharomyces cerevisiae. J Mol Biol 282, 13–24 (1998). 10.1006/jmbi.1998.1976 [DOI] [PubMed] [Google Scholar]
- 10.Plant E. P. et al. Differentiating between near- and non-cognate codons in Saccharomyces cerevisiae. PLoS One 2, e517 (2007). 10.1371/journal.pone.0000517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wilson D. N. The A-Z of bacterial translation inhibitors. Crit Rev Biochem Mol Biol 44, 393–433 (2009). 10.3109/10409230903307311 [DOI] [PubMed] [Google Scholar]
- 12.Wilson D. N. Ribosome-targeting antibiotics and mechanisms of bacterial resistance. Nat Rev Microbiol 12, 35–48 (2014). 10.1038/nrmicro3155 [DOI] [PubMed] [Google Scholar]
- 13.Trylska J. & Kulik M. Interactions of aminoglycoside antibiotics with rRNA. Biochem Soc Trans 44, 987–993 (2016). 10.1042/BST20160087 [DOI] [PubMed] [Google Scholar]
- 14.Lehmkuhl E. M. & Zarnescu D. C. Lost in Translation: Evidence for Protein Synthesis Deficits in ALS/FTD and Related Neurodegenerative Diseases. Adv Neurobiol 20, 283–301 (2018). 10.1007/978-3-319-89689-2_11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Welch E. M. et al. PTC124 targets genetic disorders caused by nonsense mutations. Nature 447, 87–91 (2007). 10.1038/nature05756 [DOI] [PubMed] [Google Scholar]
- 16.White K. M. et al. Plitidepsin has potent preclinical efficacy against SARS-CoV-2 by targeting the host protein eEF1A. Science 371, 926–931 (2021). 10.1126/science.abf4058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xu Y. & Ruggero D. The Role of Translation Control in Tumorigenesis and Its Therapeutic Implications. Annual Review of Cancer Biology 4, 437–457 (2020). 10.1146/annurev-cancerbio-030419-033420 [DOI] [Google Scholar]
- 18.Ninio J. Kinetic amplification of enzyme discrimination. Biochimie 57, 587–595 (1975). 10.1016/s0300-9084(75)80139-8 [DOI] [PubMed] [Google Scholar]
- 19.Thompson R. C. & Stone P. J. Proofreading of the codon-anticodon interaction on ribosomes. Proc Natl Acad Sci U S A 74, 198–202 (1977). 10.1073/pnas.74.1.198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kavaliauskas D. et al. Structural dynamics of translation elongation factor Tu during aa-tRNA delivery to the ribosome. Nucleic Acids Res 46, 8651–8661 (2018). 10.1093/nar/gky651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu W. et al. EF-Tu dynamics during pre-translocation complex formation: EF-Tu.GDP exits the ribosome via two different pathways. Nucleic Acids Res 43, 9519–9528 (2015). 10.1093/nar/gkv856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee T. H., Blanchard S. C., Kim H. D., Puglisi J. D. & Chu S. The role of fluctuations in tRNA selection by the ribosome. Proc Natl Acad Sci U S A 104, 13661–13665 (2007). 10.1073/pnas.0705988104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Morse J. C. et al. Elongation factor-Tu can repetitively engage aminoacyl-tRNA within the ribosome during the proofreading stage of tRNA selection. Proc Natl Acad Sci U S A 117, 3610–3620 (2020). 10.1073/pnas.1904469117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tsai A. et al. The impact of aminoglycosides on the dynamics of translation elongation. Cell Rep 3, 497–508 (2013). 10.1016/j.celrep.2013.01.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang L. et al. Allosteric control of the ribosome by small-molecule antibiotics. Nat Struct Mol Biol 19, 957–963 (2012). 10.1038/nsmb.2360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gonzalez R. L. Jr., Chu S. & Puglisi J. D. Thiostrepton inhibition of tRNA delivery to the ribosome. RNA 13, 2091–2097 (2007). 10.1261/rna.499407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Juette M. F. et al. Single-molecule imaging of non-equilibrium molecular ensembles on the millisecond timescale. Nat Methods 13, 341–344 (2016). 10.1038/nmeth.3769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Geggier P. et al. Conformational sampling of aminoacyl-tRNA during selection on the bacterial ribosome. J Mol Biol 399, 576–595 (2010). 10.1016/j.jmb.2010.04.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Demirci H. et al. The central role of protein S12 in organizing the structure of the decoding site of the ribosome. RNA 19, 1791–1801 (2013). 10.1261/rna.040030.113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Whitford P. C. et al. Accommodation of aminoacyl-tRNA into the ribosome involves reversible excursions along multiple pathways. RNA 16, 1196–1204 (2010). 10.1261/rna.2035410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Agirrezabala X. et al. Structural insights into cognate versus near-cognate discrimination during decoding. EMBO J 30, 1497–1507 (2011). 10.1038/emboj.2011.58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fischer N. et al. Structure of the E. coli ribosome-EF-Tu complex at <3 A resolution by Cs-corrected cryo-EM. Nature 520, 567–570 (2015). 10.1038/nature14275 [DOI] [PubMed] [Google Scholar]
- 33.Fislage M. et al. Cryo-EM shows stages of initial codon selection on the ribosome by aa-tRNA in ternary complex with GTP and the GTPase-deficient EF-TuH84A. Nucleic Acids Res 46, 5861–5874 (2018). 10.1093/nar/gky346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Frank J. et al. The role of tRNA as a molecular spring in decoding, accommodation, and peptidyl transfer. FEBS Lett 579, 959–962 (2005). 10.1016/j.febslet.2004.10.105 [DOI] [PubMed] [Google Scholar]
- 35.Li W. et al. Recognition of aminoacyl-tRNA: a common molecular mechanism revealed by cryo-EM. EMBO J 27, 3322–3331 (2008). 10.1038/emboj.2008.243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Loveland A. B., Demo G., Grigorieff N. & Korostelev A. A. Ensemble cryo-EM elucidates the mechanism of translation fidelity. Nature 546, 113–117 (2017). 10.1038/nature22397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Loveland A. B., Demo G. & Korostelev A. A. Cryo-EM of elongating ribosome with EF-Tu*GTP elucidates tRNA proofreading. Nature 584, 640–645 (2020). 10.1038/s41586-020-2447-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schuette J. C. et al. GTPase activation of elongation factor EF-Tu by the ribosome during decoding. EMBO J 28, 755–765 (2009). 10.1038/emboj.2009.26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Villa E. et al. Ribosome-induced changes in elongation factor Tu conformation control GTP hydrolysis. Proc Natl Acad Sci U S A 106, 1063–1068 (2009). 10.1073/pnas.0811370106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fagan C. E. et al. Reorganization of an intersubunit bridge induced by disparate 16S ribosomal ambiguity mutations mimics an EF-Tu-bound state. Proc Natl Acad Sci U S A 110, 9716–9721 (2013). 10.1073/pnas.1301585110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hoffer E. D., Maehigashi T., Fredrick K. & Dunham C. M. Ribosomal ambiguity (ram) mutations promote the open (off) to closed (on) transition and thereby increase miscoding. Nucleic Acids Res 47, 1557–1563 (2019). 10.1093/nar/gky1178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Choi J. et al. 2'-O-methylation in mRNA disrupts tRNA decoding during translation elongation. Nat Struct Mol Biol 25, 208–216 (2018). 10.1038/s41594-018-0030-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nguyen H. A., Sunita S. & Dunham C. M. Disruption of evolutionarily correlated tRNA elements impairs accurate decoding. Proc Natl Acad Sci U S A 117, 16333–16338 (2020). 10.1073/pnas.2004170117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Demirci H. et al. A structural basis for streptomycin-induced misreading of the genetic code. Nat Commun 4, 1355 (2013). 10.1038/ncomms2346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jenner L., Demeshkina N., Yusupova G. & Yusupov M. Structural rearrangements of the ribosome at the tRNA proofreading step. Nat Struct Mol Biol 17, 1072–1078 (2010). 10.1038/nsmb.1880 [DOI] [PubMed] [Google Scholar]
- 46.Jenner L. et al. Structural basis for potent inhibitory activity of the antibiotic tigecycline during protein synthesis. Proc Natl Acad Sci U S A 110, 3812–3816 (2013). 10.1073/pnas.1216691110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lai J., Ghaemi Z. & Luthey-Schulten Z. The Conformational Change in Elongation Factor Tu Involves Separation of Its Domains. Biochemistry 56, 5972–5979 (2017). 10.1021/acs.biochem.7b00591 [DOI] [PubMed] [Google Scholar]
- 48.Murphy F. V. t., Ramakrishnan V., Malkiewicz A. & Agris P. F. The role of modifications in codon discrimination by tRNA(Lys)UUU. Nat Struct Mol Biol 11, 1186–1191 (2004). 10.1038/nsmb861 [DOI] [PubMed] [Google Scholar]
- 49.Ogle J. M., Murphy F. V., Tarry M. J. & Ramakrishnan V. Selection of tRNA by the Ribosome Requires a Transition from an Open to a Closed Form. Cell 111, 721–732 (2002). 10.1016/s0092-8674(02)01086-3 [DOI] [PubMed] [Google Scholar]
- 50.Rozov A. et al. Novel base-pairing interactions at the tRNA wobble position crucial for accurate reading of the genetic code. Nat Commun 7, 10457 (2016). 10.1038/ncomms10457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rozov A., Demeshkina N., Westhof E., Yusupov M. & Yusupova G. Structural insights into the translational infidelity mechanism. Nat Commun 6, 7251 (2015). 10.1038/ncomms8251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rozov A., Westhof E., Yusupov M. & Yusupova G. The ribosome prohibits the G*U wobble geometry at the first position of the codon-anticodon helix. Nucleic Acids Res 44, 6434–6441 (2016). 10.1093/nar/gkw431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rozov A. et al. Tautomeric G*U pairs within the molecular ribosomal grip and fidelity of decoding in bacteria. Nucleic Acids Res 46, 7425–7435 (2018). 10.1093/nar/gky547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Schmeing T. M. et al. The crystal structure of the ribosome bound to EF-Tu and aminoacyl-tRNA. Science 326, 688–694 (2009). 10.1126/science.1179700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Schmeing T. M., Voorhees R. M., Kelley A. C. & Ramakrishnan V. How mutations in tRNA distant from the anticodon affect the fidelity of decoding. Nat Struct Mol Biol 18, 432–436 (2011). 10.1038/nsmb.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Voorhees R. M. et al. The structural basis for specific decoding of AUA by isoleucine tRNA on the ribosome. Nat Struct Mol Biol 20, 641–643 (2013). 10.1038/nsmb.2545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Voorhees R. M., Schmeing T. M., Kelley A. C. & Ramakrishnan V. The mechanism for activation of GTP hydrolysis on the ribosome. Science 330, 835–838 (2010). 10.1126/science.1194460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ogle J. M. et al. Recognition of cognate transfer RNA by the 30S ribosomal subunit. Science 292, 897–902 (2001). 10.1126/science.1060612 [DOI] [PubMed] [Google Scholar]
- 59.Murphy F. V. t. & Ramakrishnan V. Structure of a purine-purine wobble base pair in the decoding center of the ribosome. Nat Struct Mol Biol 11, 1251–1252 (2004). 10.1038/nsmb866 [DOI] [PubMed] [Google Scholar]
- 60.Ruusala T., Ehrenberg M. & Kurland C. G. Is there proofreading during polypeptide synthesis? The EMBO Journal 1, 741–745 (1982). 10.1002/j.1460-2075.1982.tb01240.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Blanchard S. C., Gonzalez R. L., Kim H. D., Chu S. & Puglisi J. D. tRNA selection and kinetic proofreading in translation. Nat Struct Mol Biol 11, 1008–1014 (2004). 10.1038/nsmb831 [DOI] [PubMed] [Google Scholar]
- 62.Pape T., Wintermeyer W. & Rodnina M. V. Complete kinetic mechanism of elongation factor Tu-dependent binding of aminoacyl-tRNA to the A site of the E. coli ribosome. EMBO J 17, 7490–7497 (1998). 10.1093/emboj/17.24.7490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rodnina M. V., Gromadski K. B., Kothe U. & Wieden H. J. Recognition and selection of tRNA in translation. FEBS Lett 579, 938–942 (2005). 10.1016/j.febslet.2004.11.048 [DOI] [PubMed] [Google Scholar]
- 64.Gromadski K. B., Daviter T. & Rodnina M. V. A uniform response to mismatches in codon-anticodon complexes ensures ribosomal fidelity. Mol Cell 21, 369–377 (2006). 10.1016/j.molcel.2005.12.018 [DOI] [PubMed] [Google Scholar]
- 65.Gromadski K. B. & Rodnina M. V. Kinetic determinants of high-fidelity tRNA discrimination on the ribosome. Mol Cell 13, 191–200 (2004). 10.1016/s1097-2765(04)00005-x [DOI] [PubMed] [Google Scholar]
- 66.Kothe U. & Rodnina M. V. Delayed release of inorganic phosphate from elongation factor Tu following GTP hydrolysis on the ribosome. Biochemistry 45, 12767–12774 (2006). 10.1021/bi061192z [DOI] [PubMed] [Google Scholar]
- 67.Ferguson A. et al. Functional Dynamics within the Human Ribosome Regulate the Rate of Active Protein Synthesis. Mol Cell 60, 475–486 (2015). 10.1016/j.molcel.2015.09.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Holm M. et al. mRNA decoding in human is kinetically and structurally distinct from bacteria. Nature 617, 200–207 (2023). 10.1038/s41586-023-05908-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gemmer M. et al. Visualization of translation and protein biogenesis at the ER membrane. Nature 614, 160–167 (2023). 10.1038/s41586-022-05638-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Budkevich T. V. et al. Regulation of the mammalian elongation cycle by subunit rolling: a eukaryotic-specific ribosome rearrangement. Cell 158, 121–131 (2014). 10.1016/j.cell.2014.04.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sulkowska J. I., Noel J. K. & Onuchic J. N. Energy landscape of knotted protein folding. Proc Natl Acad Sci U S A 109, 17783–17788 (2012). 10.1073/pnas.1201804109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yang S., Levine H. & Onuchic J. N. Protein oligomerization through domain swapping: role of inter-molecular interactions and protein concentration. J Mol Biol 352, 202–211 (2005). 10.1016/j.jmb.2005.06.062 [DOI] [PubMed] [Google Scholar]
- 73.Nishima W. et al. Hyper-swivel head domain motions are required for complete mRNA-tRNA translocation and ribosome resetting. Nucleic Acids Res 50, 8302–8320 (2022). 10.1093/nar/gkac597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sanbonmatsu K. Y., Joseph S. & Tung C. S. Simulating movement of tRNA into the ribosome during decoding. Proc Natl Acad Sci U S A 102, 15854–15859 (2005). 10.1073/pnas.0503456102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Whitford P. C., Onuchic J. N. & Sanbonmatsu K. Y. Connecting energy landscapes with experimental rates for aminoacyl-tRNA accommodation in the ribosome. J Am Chem Soc 132, 13170–13171 (2010). 10.1021/ja1061399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Girodat D., Blanchard S. C., Wieden H. J. & Sanbonmatsu K. Y. Elongation Factor Tu Switch I Element is a Gate for Aminoacyl-tRNA Selection. J Mol Biol 432, 3064–3077 (2020). 10.1016/j.jmb.2020.01.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Noel J. K. & Whitford P. C. How EF-Tu can contribute to efficient proofreading of aa-tRNA by the ribosome. Nat. Commun. 7 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Yang H., Perrier J. & Whitford P. C. Disorder guides domain rearrangement in elongation factor Tu. Proteins 86, 1037–1046 (2018). 10.1002/prot.25575 [DOI] [PubMed] [Google Scholar]
- 79.Warias M., Grubmuller H. & Bock L. V. tRNA Dissociation from EF-Tu after GTP Hydrolysis: Primary Steps and Antibiotic Inhibition. Biophys J 118, 151–161 (2020). 10.1016/j.bpj.2019.10.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Nguyen K., Yang H. & Whitford P. C. How the Ribosomal A-Site Finger Can Lead to tRNA Species-Dependent Dynamics. J Phys Chem B 121, 2767–2775 (2017). 10.1021/acs.jpcb.7b01072 [DOI] [PubMed] [Google Scholar]
- 81.Yang H., Noel J. K. & Whitford P. C. Anisotropic Fluctuations in the Ribosome Determine tRNA Kinetics. J Phys Chem B 121, 10593–10601 (2017). 10.1021/acs.jpcb.7b06828 [DOI] [PubMed] [Google Scholar]
- 82.Satpati P., Sund J. & Aqvist J. Structure-based energetics of mRNA decoding on the ribosome. Biochemistry 53, 1714–1722 (2014). 10.1021/bi5000355 [DOI] [PubMed] [Google Scholar]
- 83.Yang H. et al. Diffusion of tRNA inside the ribosome is position-dependent. J Chem Phys 151, 085102 (2019). 10.1063/1.5113814 [DOI] [PubMed] [Google Scholar]
- 84.Aleksandrov A. & Field M. Mechanism of activation of elongation factor Tu by ribosome: catalytic histidine activates GTP by protonation. RNA 19, 1218–1225 (2013). 10.1261/rna.040097.113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Adamczyk A. J. & Warshel A. Converting structural information into an allosteric-energy-based picture for elongation factor Tu activation by the ribosome. Proc Natl Acad Sci U S A 108, 9827–9832 (2011). 10.1073/pnas.1105714108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Aqvist J., Kazemi M., Isaksen G. V. & Brandsdal B. O. Entropy and Enzyme Catalysis. Acc Chem Res 50, 199–207 (2017). 10.1021/acs.accounts.6b00321 [DOI] [PubMed] [Google Scholar]
- 87.Aqvist J. & Kamerlin S. C. Exceptionally large entropy contributions enable the high rates of GTP hydrolysis on the ribosome. Sci Rep 5, 15817 (2015). 10.1038/srep15817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Aqvist J. & Kamerlin S. C. The conformation of a catalytic loop is central to GTPase activity on the ribosome. Biochemistry 54, 546–556 (2015). 10.1021/bi501373g [DOI] [PubMed] [Google Scholar]
- 89.Wallin G., Kamerlin S. C. & Aqvist J. Energetics of activation of GTP hydrolysis on the ribosome. Nat Commun 4, 1733 (2013). 10.1038/ncomms2741 [DOI] [PubMed] [Google Scholar]
- 90.Girodat D., Wieden H. J., Blanchard S. C. & Sanbonmatsu K. Y. Geometric alignment of aminoacyl-tRNA relative to catalytic centers of the ribosome underpins accurate mRNA decoding. Nat Commun 14, 5582 (2023). 10.1038/s41467-023-40404-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Vaiana A. C. & Sanbonmatsu K. Y. Stochastic gating and drug-ribosome interactions. J Mol Biol 386, 648–661 (2009). 10.1016/j.jmb.2008.12.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Leshin J. A., Heselpoth R., Belew A. T. & Dinman J. High throughput structural analysis of yeast ribosomes using hSHAPE. RNA Biol 8, 478–487 (2011). 10.4161/rna.8.3.14453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Jakobsson M. E., Malecki J. & Falnes P. O. Regulation of eukaryotic elongation factor 1 alpha (eEF1A) by dynamic lysine methylation. RNA Biol 15, 314–319 (2018). 10.1080/15476286.2018.1440875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Cheung M. S., Klimov D. & Thirumalai D. Molecular crowding enhances native state stability and refolding rates of globular proteins. Proc Natl Acad Sci U S A 102, 4753–4758 (2005). 10.1073/pnas.0409630102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Dhar A. et al. Structure, function, and folding of phosphoglycerate kinase are strongly perturbed by macromolecular crowding. Proc Natl Acad Sci U S A 107, 17586–17591 (2010). 10.1073/pnas.1006760107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Sanbonmatsu K. Y. Alignment/misalignment hypothesis for tRNA selection by the ribosome. Biochimie 88, 1075–1089 (2006). 10.1016/j.biochi.2006.07.002 [DOI] [PubMed] [Google Scholar]
- 97.Shao S. et al. Decoding Mammalian Ribosome-mRNA States by Translational GTPase Complexes. Cell 167, 1229–1240 e1215 (2016). 10.1016/j.cell.2016.10.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Waterhouse A.et al. SWISS-MODEL: homology modelling of protein structures and complexes. Nucleic Acids Res 46, W296–W303 (2018). 10.1093/nar/gky427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Girodat D., Mercier E., Gzyl K. E. & Wieden H. J. Elongation Factor Tu's Nucleotide Binding Is Governed by a Thermodynamic Landscape Unique among Bacterial Translation Factors. J Am Chem Soc 141, 10236–10246 (2019). 10.1021/jacs.9b01522 [DOI] [PubMed] [Google Scholar]
- 100.Mercier E., Girodat D. & Wieden H. J. A conserved P-loop anchor limits the structural dynamics that mediate nucleotide dissociation in EF-Tu. Sci Rep 5, 7677 (2015). 10.1038/srep07677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Humphrey W., Dalke A. & Schulten K. VMD: Visual molecular dynamics. Journal of Molecular Graphics 14, 33–38 (1996). 10.1016/0263-7855(96)00018-5 [DOI] [PubMed] [Google Scholar]
- 102.Pettersen E. F. et al. UCSF Chimera - A visualization system for exploratory research and analysis. J Comput Chem 13, 1605–1612 (2004). 10.1002/jcc.20084 [DOI] [PubMed] [Google Scholar]
- 103.Crepin T. et al. Mammalian translation elongation factor eEF1A2: X-ray structure and new features of GDP/GTP exchange mechanism in higher eukaryotes. Nucleic Acids Res 42, 12939–12948 (2014). 10.1093/nar/gku974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Abraham M. J. et al. GROMACS: High performance molecular simulations through multi-level parallelism from laptops to supercomputers. SoftwareX 1-2, 19–25 (2015). 10.1016/j.softx.2015.06.001 [DOI] [Google Scholar]
- 105.Van Der Spoel D. et al. GROMACS: fast, flexible, and free. J Comput Chem 26, 1701–1718 (2005). 10.1002/jcc.20291 [DOI] [PubMed] [Google Scholar]
- 106.Pronk S. et al. GROMACS 4.5: a high-throughput and highly parallel open source molecular simulation toolkit. Bioinformatics 29, 845–854 (2013). 10.1093/bioinformatics/btt055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Hess B., Kutzner C., van der Spoel D. & Lindahl E. GROMACS 4: Algorithms for Highly Efficient, Load-Balanced, and Scalable Molecular Simulation. J Chem Theory Comput 4, 435–447 (2008). 10.1021/ct700301q [DOI] [PubMed] [Google Scholar]
- 108.Dang L. X. Mechanism and Thermodynamics of Ion Selectivity in Aqueous Solutions of 18-Crown-6 Ether: A Molecular Dynamics Study. Journal of the American Chemical Society 117, 6954–6960 (2002). 10.1021/ja00131a018 [DOI] [Google Scholar]
- 109.Noel J. K. et al. SMOG 2: A Versatile Software Package for Generating Structure-Based Models. PLoS Comput Biol 12, e1004794 (2016). 10.1371/journal.pcbi.1004794 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Structures, parameters, and example trajectories for these simulations have been uploaded to Zenodo and are publicly available. This includes the initial PDB file used, the input parameters for gromacs simulations (i.e. gro, top, and mdp files), as well as the tpr files. We have also included example trajectories of the simulations. They may be accessed at doi: 10.5281/zenodo.15653228.
All scripts that were developed for the analysis of this project have been uploaded to Zenodo and are publicly available at doi: 10.5281/zenodo.15653228.