Abstract
Background
MDRO infections are increasingly problematic in ICUs, especially among elderly patients with lung infections, but knowledge about these infections in this group is limited. This study aimed to assess the status and risk factors of MDRO infections in elderly ICU patients and develop a risk prediction model to aid clinical decisions.
Methods
Using a retrospective cohort study, a total of 494 elderly patients with lung infections admitted to the ICU from January 2017 to December 2022 were selected, and the patients were divided into the MDRO group (259) and the non-MDRO group (235) based on whether or not the patients developed MDRO infections. Lasso and multifactorial logistic regression were applied to analyze the independent risk factors for multidrug-resistant bacterial infections in elderly patients with pulmonary infections, and to construct a nomogram model of the risk of MDRO infections. The differentiation, consistency and clinical benefit of the model were evaluated by receiver operating characteristic curve(ROC), calibration curves and decision curve analysis, respectively, and the stability of the model was verified by Bootstrap method.
Results
Duration of hospitalization before MDRO diagnosis, chronic obstructive pulmonary disease, personal history of cerebrovascular disease, tracheotomy and prior carbapenem exposure were found to be independent risk factors for multidrug-resistant bacterial infections in elderly patients with pulmonary infections in the intensive care unit (all p < 0.05). The nomogram model, constructed based on the results of logistic regression analysis, exhibited an area under the ROC curve of 0.748 with a 95% confidence interval of 0.705–0.790. The Hosmer-Lemeshow test indicated that the model predicted a good fit (p = 0.75), and the DCA curve suggested that the model had a good clinical utility.
Conclusion
Risk prediction model is effective in predicting the risk of MDRO infection in the ICU elderly pulmonary infection population and can be used to assess risk and inform preventive treatment and nursing interventions.
Keywords: Multidrug resistant bacteria, Risk factors, Elderly people
Introduction
The advent of antibiotics in modern medicine marked a revolutionary stride in the battle against infectious diseases. Yet, their widespread and often indiscriminate use has given rise to multidrug resistant organisms (MDROs), posing a formidable challenge to global public health [1]. These organisms can resist multiple antibiotics, undermining their efficacy and transforming once manageable infections into potentially fatal threats.
Intensive care units (ICUs) are particularly vulnerable to the menace of MDROs, as patients there frequently undergo invasive procedures such as mechanical ventilation and central venous catheterization, which significantly heighten the risk of infection [2]. The exigency of their condition necessitates prompt empirical antibiotic treatment, further complicated by the presence of MDROs. Given the complexity and difficulty of treating drug-resistant bacterial infections, it is imperative to focus on risk analysis and identification of MDROs to protect the health of vulnerable populations [3].
In recent years, national and international researchers have begun to focus on the risk factors for MDROs infection. Studies have primarily focused on identifying host factors, medical behaviors, and environmental conditions associated with MDRO infection [4-6]. However, the elderly ICU population is less studied, which is of particular concern as the global population ages. They are more susceptible to pulmonary infections, and their infections are complex and difficult to treat, due to declining immune function, coexisting chronic diseases and impaired organ function [7]. The aim of this study was to collect and analyze clinical data from elderly patients with lung infections in intensive care unit, identify independent risk factors associated with MDRO infections and develop a risk prediction model. The results of the study will provide a scientific basis to guide clinical practice, optimize treatment options and improve the prognosis of elderly patients.
Materials and methods
Study population
We retrospectively collected data on 520 elderly patients with positive culture results for pathogenic bacteria who were hospitalized in the Eastern ICU of the Affiliated Hospital of Xuzhou Medical University between January 2017 and December 2022.
Inclusion criteria: ① Participants must be 60 years of age or older. ② Each case must meet the standards for pneumonia diagnosis. ③ Sputum sample should be satisfactory and meet the requirements of > 25 leucocytes, < 10 epithelial cells or > 2.5 leucocytes/epithelial cells at low magnification. There are clear results for pathogenic bacteria and drug sensitivity.
Exclusion criteria: ① Clinical and laboratory examination data are incomplete. ②There are multiple ICU admissions within the same hospitalization period.
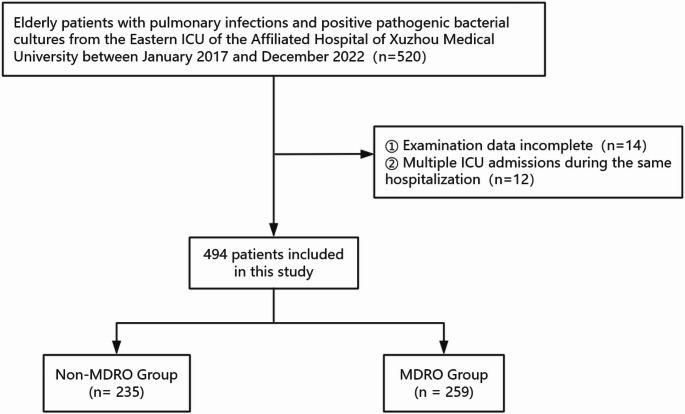
After applying the inclusion and exclusion criteria, 12 patients admitted to the ICU during the same hospitalization and 14 patients were excluded from the study due to lack of complete clinical and laboratory data. After screening, a total of 494 patients were included in this study (Fig. 1).
Fig. 1.
Study population flowchart
Multidrug-resistant organisms are bacteria that are resistant to more than three commonly used antimicrobial drugs. In this study, doctors made the first diagnosis of MDROs infections, which the hospital infection control department then reviewed and confirmed.
Clinical data collection
This study identified factors linked to infections caused by multidrug-resistant organisms through a comprehensive literature review and expert interviews. Using the hospital’s electronic medical record system, the research team collected clinical data on patients in intensive care for further analysis.
The following data were collected: age, sex, coronary heart disease, hypertension, diabetes mellitus, chronic obstructive pulmonary disease, atrial fibrillation, history of tumor, duration of hospitalization, duration of ventilator use, duration of central venous use, cerebrovascular disease, history of surgery, tracheotomy, prior carbapenem exposure and combination antimicrobials.
Duration of hospitalization, ventilator use, and central venous catheter use were calculated only for the period prior to MDRO diagnosis, representing the cumulative time from ICU admission until first microbiological confirmation of MDRO. “Prior carbapenem exposure” was any carbapenem use within 30 days pre-admission or during hospitalization before MDRO diagnosis.
Statistical analysis
In order to ensure the integrity of the data, a database was created in Excel with a dual-entry and verification system. Software such as SPSS version 26.0 and R version 4.1.3 were used for statistical analysis.
Quantitative data were presented as frequencies and percentages, and the chi-squared test or Fisher’s exact test was used for their analysis. Measurement data that conformed to a normal distribution were presented as the mean plus or minus the standard deviation (Mean ± SD), and group comparisons were made using the t-test. Nonconforming measurement data were described using the median along with the minimum and maximum values (M (min, max)), and the nonparametric Mann-Whitney U test was used for group comparisons.
A nomogram was developed using the results from a multivariate logistic regression analysis, which included variables identified through the Lasso regression model for predictive feature selection. This analysis ensured that only the most significant variables were included, with a threshold of P < 0.05, to construct a reliable and accurate predictive tool.
The ability of the model to discriminate was assessed by determining the area under the curve (AUC). Internal validation was performed using the bootstrap method with 1000 resamples. Calibration of the model was verified by the Hosmer-Lemeshow test and by reviewing the calibration plots. In addition, the clinical applicability of the model was evaluated by Decision Curve Analysis (DCA) performed using the rmda package in R.
Results
Patient characteristics
The baseline characteristics table (Table 1) presents key demographic and clinical features of the study population. The comparison between the non-MDRO and MDRO groups revealed similar mean ages (72 ± 7 vs. 72 ± 8, p = 0.714) and distribution of gender (p = 0.669). There were no significant differences in the prevalence of hypertension, diabetes, coronary artery disease, or history of tumor between the two groups. However, the MDRO group had a significantly higher prevalence of chronic obstructive pulmonary disease (9.65% vs. 3.40%, p = 0.005), cerebrovascular disease (22.01% vs. 11.49%, p = 0.002), tracheostomy (37.07% vs. 20.00%, p < 0.001), and the use of carbapenems (41.70% vs. 20.00%, p < 0.001). Additionally, the MDRO group had a longer median hospital stay (7 days vs. 3 days, p < 0.001) but similar median durations of mechanical ventilation and central venous catheter use compared to the non-MDRO group.
Table 1.
Basic characteristics of the patient population
| Variable | Non-MDRO Group (n = 235) |
MDRO Group (n = 259) |
P value |
|---|---|---|---|
| Age, years | 72 ± 7 | 72 ± 8 | 0.714 |
| Gender, n(%) | 0.669 | ||
| Female | 86 (36.60) | 90 (34.75) | |
| Male | 149 (63.40) | 169 (65.25) | |
| Hypertensive, n(%) | 0.183 | ||
| No | 123 (52.34) | 151 (58.30) | |
| Yes | 112 (47.66) | 108 (41.70) | |
| Diabetes, n(%) | 0.983 | ||
| No | 188 (80.00) | 207 (79.92) | |
| Yes | 47 (20.00) | 52 (20.08) | |
| Coronary heart disease, n(%) | 0.865 | ||
| No | 202 (85.96) | 224 (86.49) | |
| Yes | 33 (14.04) | 35 (13.51) | |
| Chronic obstructive pulmonary disease, n(%) | 0.005 | ||
| No | 227 (96.60) | 234 (90.35) | |
| Yes | 8 (3.40) | 25 (9.65) | |
| Atrial fibrillation, n(%) | 0.551 | ||
| No | 210 (89.36) | 227 (87.64) | |
| Yes | 25 (10.64) | 32 (12.36) | |
| Medical history of a tumour, n(%) | 0.614 | ||
| No | 211 (89.79) | 236 (91.12) | |
| Yes | 24 (10.21) | 23 (8.88) | |
| Cerebrovascular disease, n(%) | 0.002 | ||
| No | 208 (88.51) | 202 (77.99) | |
| Yes | 27 (11.49) | 57 (22.01) | |
| Duration of hospitalization, days | 3 (1, 7) | 7 (3, 13) | < 0.001 |
| Duration of ventilator use, days | 0.00 (0.00, 15.09) | 0.00 (0.00, 24.05) | 0.756 |
| Duration of Intravenous catheter, days | 0.00 (0.00, 28.99) | 0.00 (0.00, 46.96) | 0.105 |
| Surgery, n(%) | 0.848 | ||
| No | 110 (46.81) | 119 (45.95) | |
| Yes | 125 (53.19) | 140 (54.05) | |
| Tracheotomy, n(%) | < 0.001 | ||
| No | 188 (80.00) | 163 (62.93) | |
| Yes | 47 (20.00) | 96 (37.07) | |
| Combined use of antibiotics, n(%) | 0.835 | ||
| No | 24 (10.21) | 25 (9.65) | |
| Yes | 211 (89.79) | 234 (90.35) | |
| Carbapenems drug exposure, n(%) | < 0.001 | ||
| No | 188 (80.00) | 151 (58.30) | |
| Yes | 47 (20.00) | 108 (41.70) |
Analysis of predictive factors
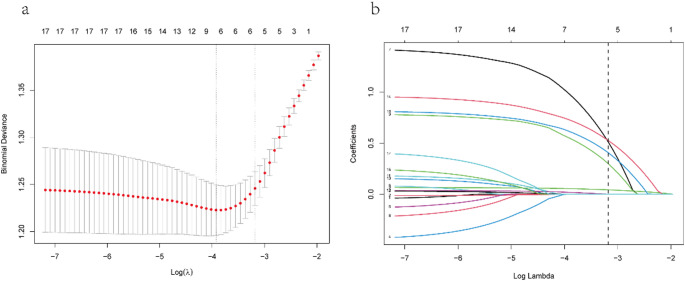
After analyzing a set of 16 variables collected from patients, five were identified as significant based on their non-zero regression coefficients obtained from the LASSO method (Fig. 2). Factors selected included length of hospital stay before MDRO diagnosis, presence of cerebrovascular disease, chronic obstructive pulmonary disease, cases requiring tracheostomy, and prior carbapenem exposure.
Fig. 2.
LASSO regression with ten-fold cross-validation was used to select predictive variables. (a) The best lambda for LASSO regression was found by analyzing deviance using the minimum (left dotted line) and 1-SE criteria (right dotted line). (b) A plot of coefficient profiles versus the log of lambda values was created. Using the 1-SE criterion, five variables with non-zero coefficients were selected
Nomogram’s Building and apparent performance
Based on the aforementioned five variables chosen by the LASSO regression, multivariable logistic regression analysis was carried out to develop a predictive model for MDRO. It was discovered that duration of hospitalization before MDRO diagnosis, cerebrovascular disease, chronic obstructive pulmonary disease, tracheotomy and prior carbapenem exposure were independently linked to MDRO infections (Table 2). Using these variables, we created a nomogram that predicts the probability of MDRO infections in older individuals suffering from lung infections (Fig. 3). Every variable has a numerical value; the higher the number, the higher the likelihood of MDRO infection in older individuals with lung infections.
Table 2.
Multivariate logistic regression analysis of MDRO infection in elderly patients with pulmonary infection
| Variables | β | SE | Wald | OR | 95%CI | P value |
|---|---|---|---|---|---|---|
| LOS before MDRO diagnosis | 0.073 | 0.015 | 22.491 | 1.075 | 1.044 ~ 1.108 | < 0.001 |
| COPD | 1.518 | 0.438 | 12.025 | 4.565 | 1.935 ~ 10.770 | < 0.001 |
| Cerebrovascular disease | 0.846 | 0.273 | 9.619 | 2.331 | 1.365 ~ 3.978 | 0.002 |
| Prior carbapenem exposure | 0.936 | 0.223 | 17.650 | 2.550 | 1.648 ~ 3.946 | < 0.001 |
| Tracheotomy | 0.856 | 0.225 | 14.497 | 2.353 | 1.515 ~ 3.656 | < 0.001 |
| Constant | -1.182 | 0.173 | 46.518 | 0.307 | - | < 0.001 |
COPD mean chronic obstructive pulmonary disease; LOS mean length of hospital stay
Fig. 3.
Nomogram for the prediction of multidrug-resistant bacterial infections in elderly patients with lung infections. COPD mean chronic obstructive pulmonary disease, MDRO multidrug resistant organisms, LOS mean length of hospital stay
Assessment of the accuracy
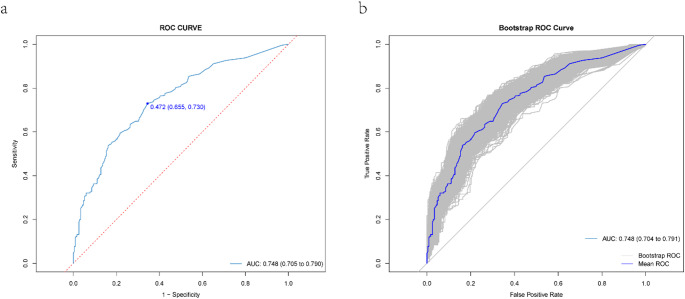
The analysis of the ROC curve related to this nomogram revealed an AUC of 0.748, with a 95% Confidence Interval ranging from 0.705 to 0.790, which indicates a good level of discriminative ability. To further evaluate the reliability of the nomogram, we used an internal bootstrapping validation method. After conducting 1000 iterations of bootstrapping, we found that the AUC for the bootstrap model was also 0.748, with a 95% Confidence Interval of 0.704 to 0.791, demonstrating comparable statistical efficacy with the original model (Fig. 4).
Fig. 4.
ROC curve for the predictive model and internal validation. (a) displays the model’s AUC, and (b) presents the internal validation AUC via bootstrap. The gray area indicates ROC curves from 1,000 bootstrap cross-validations
Assessment of the degree of calibration for the risk prediction model
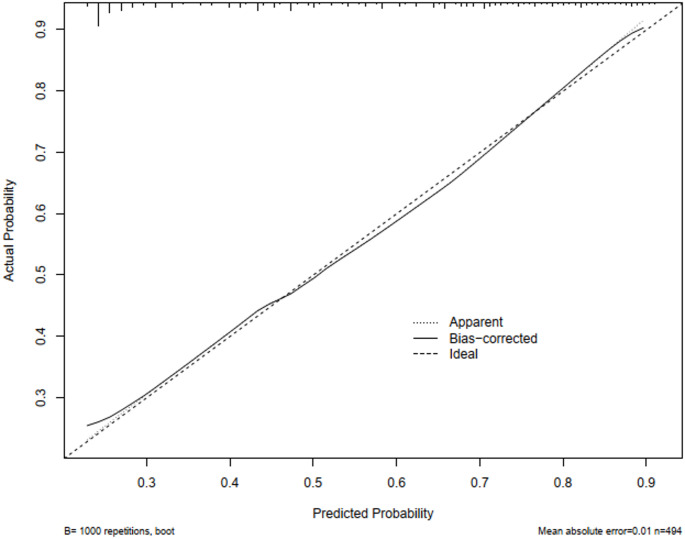
In the study, a calibration curve was introduced to evaluate the precision of the risk prediction model for multidrug resistant bacterial infections in elderly patients with pulmonary infections. As depicted in Fig. 5, the x-axis represents the model’s predicted risks, and the y-axis represents the actual occurrences of infections in this demographic. The graph includes a solid diagonal line signifying the ideal model’s predictions and a dashed line indicating the actual predictive performance. The predictive ability of the model increases as the dashed line gets closer to the diagonal. The Hosmer-Lemeshow test confirmed that the model had a good calibration, indicating that the model’s predictions closely matched the observed results (χ2 = 5.071, P = 0.750).
Fig. 5.
Calibration plot for cohort nomogram prediction. The x-axis represents the nomogram’s predicted probability, while the y-axis represents its actual probability
Clinical net benefit
The study employed Decision Curve Analysis (DCA) to assess the potential of the risk prediction model to improve clinical decision-making, as illustrated in Fig. 6. The graph’s y-axis measures net benefit, while the x-axis corresponds to the threshold probability. The green line signifies no action, with a net benefit of zero. The red line indicates the intervention, showing a negative slope for net benefit. The blue line represents the model’s actual performance in predicting multidrug resistant bacterial infections.
Fig. 6.
Decision curve analysis for the nomogram
Across a risk threshold of 23–100%, the model’s DCA curve outperformed both extreme scenarios, resulting in a positive clinical net benefit for patients. This suggests that the prediction model is highly applicable in clinical practice.
Discussion
The aim of this study was to identify the influencing factors of MDRO infections in ICU wards and to further construct a prediction model based on the influencing factors. A total of 494 elderly ICU patients were included in our study, we found that length of hospital stay before MDRO diagnosis, chronic obstructive pulmonary disease, history of cerebrovascular disease, tracheotomy, and prior carbapenem exposure were important factors for contracting MDRO infections in elderly patients with pulmonary infections in the ICU, and a prediction model was constructed using these risk factors.
The predictive model, visualized for ease of use, enables healthcare providers to estimate the risk of MDRO infection for individual patients by taking into account their specific risk factors. With an AUC of 0.748 from the ROC curve, the model demonstrated a great ability to detect MDRO infections in elderly patients with lung infections in the ICU. Furthermore, the calibration curve confirmed the accuracy of the model in predicting the likelihood of MDRO infection in elderly ICU patients. In addition, Decision curve analysis was performed, highlighting the practical utility of the model in clinical settings.
Currently, there are few predictive models for MDRO infections in elderly populations with pulmonary infections in intensive care units. Our study is similar to the clinical studies of Shu Wang [8], which developed models for predicting pulmonary multidrug-resistant bacterial infections in the elderly population, and the results showed the independent influence of cerebrovascular disease and respiratory failure on the role of MDRO in the elderly population with pulmonary infections. However, significant differences remain. Our model focuses on ICU-related risk factors, taking into account the length of stay in the ICU, time of invasive operations such as tracheal intubation and central venous, etc. among elderly patients. Multidrug-resistant bacterial infections occur mainly in the intensive care unit [9], and the findings are important for guiding clinical practice and preventing multidrug-resistant bacterial infections.
Length of hospital stay before MDRO diagnosis is an independent predictor of increased risk of MDRO infection. Our study is similar to the results of Oliveira [10] and Marina [11] et al. Duration of hospitalization was significantly associated with an increased risk of infection with drug-resistant bacteria. This phenomenon may be attributed to the interaction of several factors. Patients with prolonged hospitalization may be at higher risk of MDRO infection due to more medical interventions, potential cross-infections, and longer antibiotic exposure [12] Notably, prolonged hospitalization is associated with a poorer prognosis for patients with resistant bacterial infections, including higher mortality and longer mechanical ventilation. Therefore, clinicians and public health professionals need to take measures such as judicious use of antibiotics, enhanced infection control and prophylaxis measures, and optimization of the patient discharge process in order to reduce length of stay and lower the risk of MDRO infection.
The elderly population is distinguished by a high prevalence of multiple comorbidities. As individuals age, they undergo a gradual decline in physical function, rendering them more susceptible to the development of various chronic diseases. This study significantly demonstrates the association between the medical history of the elderly population and infections caused by multidrug-resistant organisms. The study encompassed conditions such as hypertension, coronary heart disease, atrial fibrillation, diabetes, chronic lung disease, and cerebrovascular disease. Ultimately, chronic obstructive pulmonary disease and cerebrovascular disease were identified as independent risk factors for infection with multidrug-resistant bacteria in elderly patients. In patients with chronic obstructive pulmonary disease, compromised mucociliary clearance and the frequent administration of antibiotics diminish the lung’s defense mechanisms, facilitating the colonization and proliferation of drug-resistant strains [13, 14]. Patients with cerebrovascular disease may have an elevated risk of respiratory infections as a consequence of dysphagia and aspiration [15]. These patients often require more frequent medical interventions, such as prolonged mechanical ventilation and invasive manoeuvres, which may further increase the chances of infection with drug-resistant strains.
Tracheotomy is a commonly used treatment in the ICU to relieve dyspnea and improve ventilation. However, this procedure may also increase the risk of MDRO infection. In a study of neurorehabilitation wards, the research team investigated risk factors for MDRO infections in patients with CNS disorders and found that tracheotomized patients were more likely to develop MDRO infections than those who were not tracheotomized(OR = 4.458) [16]. In this study, it was found that elderly tracheotomized patients with lung infections in the intensive care unit exhibited a higher likelihood of being infected with multidrug-resistant bacteria (OR = 2.353). Studies have shown that tracheotomy bypasses the normal protective barrier of the respiratory tract and provides a direct pathway for bacteria to enter the lower respiratory tract. The formation of a biofilm in the tracheotomy tube provides a protective environment for drug-resistant bacteria, making it more difficult for these bacteria to be removed, thus increasing the incidence of infection [17, 18]. And tracheotomized patients often require extended mechanical ventilation and intricate care. Therefore, effective preventive measures, including continuous balloon pressure monitoring and suctioning of vocal secretions, are essential in reducing infections from multidrug-resistant bacteria [19].
Our study identified previous exposure to carbapenem as an independent risk factor for the acquisition of MDROs in elderly patients with pulmonary infections within the ICU. This finding is consistent with previous studies such as Liu [20] and Bian [21], which also report an increased risk of MDRO infection with carbapenem exposure. The potential mechanisms underlying this association may involve the selective pressure exerted by carbapenems on bacterial populations, leading to the enrichment of resistant strains. Additionally, the widespread use of carbapenems could facilitate the horizontal gene transfer of resistance genes, further contributing to the spread of MDROs [22]. Unlike established risk factors (e.g., COPD), prior carbapenem exposure during the current admission is a potentially modifiable variable. Restricting unnecessary carbapenem use in low-risk patients may reduce MDRO acquisition, thus aligning with antimicrobial stewardship goals.
The model quantifies the risk of MDROs in individual patients using routinely available admission data (e.g. comorbidities and prior antibiotic exposure). Its primary value lies in identifying high-risk patients, which enables targeted enhanced microbiological surveillance (e.g. serial cultures), timely antimicrobial stewardship consultations and pre-emptive isolation protocols for those at the greatest risk. Unlike rigid prediction scores, the tool provides scenario-adaptive decision support for dynamic clinical judgements. It informs antibiotic selection, justifying broader empirical coverage when clinical suspicion aligns with higher scores, and it informs resource allocation, directing infection control resources to the patients at the highest risk.
This study has limitations: it’s a retrospective, single-center study in a tertiary hospital, which may affect the generalizability of the results and introduce bias. In addition, the predictive model was only internally validated and lacked external cohort confirmation, which is necessary to verify its validity and reliability in different populations.
Conclusions
In addition, this study identified several significant risk factors associated with the incidence of multidrug-resistant organism infections in elderly patients with pulmonary infections in the intensive care unit. The implementation of nomogram modeling could enhance the early detection of MDRO infections in this patient population and inform appropriate clinical management strategies.
Acknowledgements
We sincerely thank all those who helped us during the experiment.
Author contributions
BW designed the study and analyzed the data; SZ contributed to the study design and collected data; LM provided research ideas and experimental materials; JF supported for data analysis and revised the manuscript.
Data availability
No datasets were generated or analysed during the current study.
Declarations
Ethical approval
This study was conducted in accordance with the Declaration of Helsinki and received Ethics Committee approval from the Affiliated Hospital of Xuzhou Medical University.
Informed consent
Ethics Committee did not require patient consent since no personal information was disclosed, and no interventions were performed.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Bo Wang and Suming Zhang contributed equally to this work.
References
- 1.Ikhimiukor OO, Odih EE, Donado-Godoy P, Okeke IN (2022) A bottom-up view of antimicrobial resistance transmission in developing countries. Nat Microbiol 7:757–765. 10.1038/s41564-022-01124-w [DOI] [PubMed] [Google Scholar]
- 2.Wang N, Wang X, Yang J et al (2023) Health care-Associated infection in elderly patients with cerebrovascular disease in intensive care units: A retrospective cohort study in taizhou, China. World Neurosurg 178:e526–e532. 10.1016/j.wneu.2023.07.114 [DOI] [PubMed] [Google Scholar]
- 3.Zaragoza R, Vidal-Cortés P, Aguilar G et al (2020) Update of the treatment of nosocomial pneumonia in the ICU. Crit Care Lond Engl 24:383. 10.1186/s13054-020-03091-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Garcia ER, Vergara A, Aziz F et al (2022) Changes in the gut microbiota and risk of colonization by multidrug-resistant bacteria, infection, and death in critical care patients. Clin Microbiol Infect 28:975–982. 10.1016/j.cmi.2022.01.004 [DOI] [PubMed] [Google Scholar]
- 5.Gross AE, Van Schooneveld TC, Olsen KM et al (2014) Epidemiology and predictors of multidrug-resistant community-acquired and health care-associated pneumonia. Antimicrob Agents Chemother 58:5262–5268. 10.1128/AAC.02582-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ziegler MJ, Babcock HH, Welbel SF et al (2022) Stopping hospital infections with environmental services (SHINE): A Cluster-randomized trial of intensive monitoring methods for terminal room cleaning on rates of Multidrug-resistant organisms in the intensive care unit. Clin Infect Dis 75:1217–1223. 10.1093/cid/ciac070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lin H, Gao Y, Qiu Y et al (2022) The prognostic factors of bloodstream infection in immunosuppressed elderly patients: A retrospective, Single-center, Five-year cohort study. Clin Interv Aging Volume 17:1647–1656. 10.2147/CIA.S386922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang S, Li J, Dai J et al (2023) Establishment and validation of models for the risk of Multi-Drug resistant Bacteria infection and prognosis in elderly patients with pulmonary infection: A multicenter retrospective study. Infect Drug Resist 16:6549–6566. 10.2147/IDR.S422564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kalayci Cekin Z (2023) Bloodstream infections caused by multidrug resistant bacteria: clinical and Microbiological features and mortality. Sisli Etfal Hastan Tip Bul 57:416–425. 10.14744/SEMB.2023.31697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.De Oliveira BS 1 et al (2022) A, Hirassawa Sacillotto2 G, Francisco Balthazar Neves2 M, Prevalence, outcomes, and predictors of multidrug-resistant nosocomial lower respiratory tract infections among patients in an ICU. J Bras Pneumol 49:e20220235. 10.36416/1806-3756/e20220235 [DOI] [PMC free article] [PubMed]
- 11.Munari M, Franzoi F, Sergi M et al (2022) Extensively drug-resistant and multidrug-resistant gram-negative pathogens in the neurocritical intensive care unit. Acta Neurochir (Wien) 164:859–865. 10.1007/s00701-020-04611-3 [DOI] [PubMed] [Google Scholar]
- 12.Folic MM, Djordjevic Z, Folic N et al (2021) Epidemiology and risk factors for healthcare-associated infections caused by Pseudomonas aeruginosa. J Chemother 33:294–301. 10.1080/1120009X.2020.1823679 [DOI] [PubMed] [Google Scholar]
- 13.Ibrahim ME (2023) Risk factors in acquiring multidrug-resistant Klebsiella pneumoniae infections in a hospital setting in Saudi Arabia. Sci Rep 13:11626. 10.1038/s41598-023-38871-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dantas LF, Dalmas B, Andrade RM et al (2019) Predicting acquisition of carbapenem-resistant Gram-negative pathogens in intensive care units. J Hosp Infect 103:121–127. 10.1016/j.jhin.2019.04.013 [DOI] [PubMed] [Google Scholar]
- 15.Wu B, Peng M, Tong Y et al (2023) Distribution of bacteria and risk factors in patients with multidrug-resistant pneumonia in a single center rehabilitation ward. Med (Baltim) 102:e35023. 10.1097/MD.0000000000035023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jiang W, Li L, Wen S et al (2022) Gram-negative multidrug-resistant organisms were dominant in neurorehabilitation ward patients in a general hospital in Southwest China. Sci Rep 12:11087. 10.1038/s41598-022-15397-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Drożdż K, Ochońska D, Ścibik Ł et al (2022) The frequency of occurrence of resistance and genes involved in the process of adhesion and accumulation of biofilm in Staphylococcus aureus strains isolated from tracheostomy tubes. Microorganisms 10:1210. 10.3390/microorganisms10061210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ścibik Ł, Ochońska D, Golda-Cepa M et al (2022) Microbiological analysis of tracheostomy tube biofilms and antibiotic resistance profiles of potentially pathogenic microorganisms. Otolaryngol Pol 76:8–21. 10.5604/01.3001.0015.8827 [DOI] [PubMed] [Google Scholar]
- 19.Pawlik J, Tomaszek L, Mazurek H, Mędrzycka-Dąbrowska W (2022) Risk factors and protective factors against Ventilator-Associated Pneumonia-A Single-Center mixed prospective and retrospective cohort study. J Pers Med 12:597. 10.3390/jpm12040597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu Y, Wang Q, Zhao C et al (2020) Prospective multi-center evaluation on risk factors, clinical characteristics and outcomes due to carbapenem resistance in Acinetobacter baumannii complex bacteraemia: experience from the Chinese antimicrobial resistance surveillance of nosocomial infections (CARES) network. J Med Microbiol 69:949–959. 10.1099/jmm.0.001222 [DOI] [PubMed] [Google Scholar]
- 21.Bian W, Chen W, Gu X et al (2020) Analysis of related factors of carbapenem resistant Klebsiella pneumoniae infection in patients with artificial airway. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue 32:1324–1330. 10.3760/cma.j.cn121430-20200601-00431 [DOI] [PubMed] [Google Scholar]
- 22.Sharma S, Das A, Banerjee T et al (2021) Adaptations of carbapenem resistant Acinetobacter baumannii (CRAB) in the hospital environment causing sustained outbreak. J Med Microbiol 70. 10.1099/jmm.0.001345 [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No datasets were generated or analysed during the current study.