Abstract
Background
T4 is one of the most important prognostic factors in localized colon cancer (CC), especially in stage II (pT4N0). However, the optimal adjuvant treatment in this subset of patients remains unclear. We present a large, multicenter, international, real-world analysis of pT4N0 CC patients.
Patients and methods
A real-world database regarding clinicopathological characteristics of patients with stage II pT4N0 CC surgically removed between 2010 and 2021 was queried. Primary endpoints were overall survival (OS) and relapse-free survival (RFS), and analyses were adjusted on age (with a cut-off of 75 years) to reduce selection bias.
Results
Our study included 492 patients; outcomes data were available for 390 patients. Median age was 73 years. Microsatellite status was assessed in 294 (75%), including 74 (25%) mismatch repair deficient (dMMR)/microsatellite instability (MSI). Adjuvant chemotherapy was prescribed in 204 patients (52%), mostly oxaliplatin-based (70%). After a median follow-up of 46.8 months, 6 months of adjuvant chemotherapy was associated with a significant improvement in OS [hazard ratio (HR) age-adjusted 0.22, P < 0.001] when compared with no adjuvant. The benefit was seen also with 3 months of adjuvant chemotherapy, even if the benefit was lower (HR age-adjusted 0.60, P < 0.001). Similar results were observed in terms of RFS, with a statistically significant benefit both in the 6-month group (HR age-adjusted 0.47, P = 0.001) and in the 3-month group (HR age-adjusted 0.71, P = 0.001). Considering the regimen and the duration of treatment, 6 months of oxaliplatin-based chemotherapy was associated with a significant improvement in both OS and RFS (P < 0.001). In univariate analysis, MMR status was not associated with OS nor RFS.
Conclusions
T4 was confirmed to be a poor prognostic factor. Adjuvant chemotherapy provided a large benefit, with a significant reduction in risk of recurrence and death. The benefit was proportional to its duration, and oxaliplatin-based chemotherapy may be better than monotherapy.
Key words: pT4N0, stage II colon cancer, adjuvant chemotherapy, real-word dataset
Highlights
-
•
This study used a large international, multicenter real-world dataset of stage II pT4N0 colon cancer patients.
-
•
We showed that 6 months of oxaliplatin-based adjuvant chemotherapy is significantly associated with improved OS and RFS.
-
•
We offer clinically relevant guidance for optimizing treatment in this specific high-risk stage II CC patient subgroup.
Introduction
According to data from the World Health Organization’s GLOBOCAN database, colorectal cancer (CRC) is the second leading cause of cancer-related mortality globally.1 Stage at diagnosis is the most important predictor of survival, with 5-year relative survival ranging from 91% for localized disease to 73% for regional disease and 14% for metastatic disease.2
Approximately 70%-80% of patients have a radically resectable disease at the time of the diagnosis and ∼35% of them will relapse. Recurrence is more frequent in the first 5 years following surgery, with 80% of cases within the initial 3 years.3
Among stage II colon cancer (CC), pT4 is considered as the major prognostic factor, as it is associated with an increased risk of relapse and poorer oncological outcomes both in deficient (dMMR) and proficient (pMMR) mismatch repair tumors.4, 5, 6, 7 In pMMR/microsatellite stable (MSS) CC patients, 5-year disease-free survival (DFS) with surgery alone is reduced from 65% in pT3N0 to 51% in pT4N0,8 and 5-year overall survival (OS) is estimated to be 93% in stage I, 84% in stage IIA (pT3N0), 76% in pT4aN0, and 59% in pT4bN0 CC.9,10 It is noteworthy that patients with pT4N0 CC experience worse outcomes than those with stage IIIA (pT1-2N1), with a 5-year OS of 72% and 83%, respectively.11 Nevertheless, the role of adjuvant chemotherapy in this setting is still unclear, since the absolute benefit in reduction of recurrence is ∼3%-4% with fluorouracil alone.12,13 Stage II CC is in fact a very heterogeneous subgroup, and patients should be treated according to their risk group. According to the European Society of Medical Oncology (ESMO) guidelines, in the presence of a major risk factor (pT4 or inadequate lymph node sampling) or more than one minor risk factor (emergency clinical presentation, lympho-vascular invasion (LVI), perineural invasion (PNI), and poorly differentiated histology4, 5, 6,14), patients should be treated with a doublet chemotherapy regimen [5-fluorouracil (5-FU) or capecitabine plus oxaliplatin—FOLFOX/XELOX] regardless of the MMR status. On the other hand, in the presence of only one minor risk factor in MSS tumors, a monotherapy with fluoropyrimidine could be sufficient.15 Indeed, the absolute benefit of the oxaliplatin is still debated, since it is associated with a nonsignificant DFS benefit in high-risk CC,16 but studies are not consistent17 and, moreover, the definition of high risk varies among the studies.
In the absence of high-risk factors such as pT4, stage II dMMR/microsatellite instability (MSI) CC is associated with a more favorable prognosis than pMMR disease, with 5-year OS rates of 90% and 66%, respectively, resulting in a limited benefit from adjuvant chemotherapy.18, 19, 20, 21, 22, 23 Fluoropyrimidine monotherapy has not demonstrated a survival benefit and may even have a detrimental effect in this subgroup.19,24 For this reason, adjuvant chemotherapy is indicated only with a double oxaliplatin-based regimen and only in the presence of a major risk factor.
However, despite international guideline recommendations, the real benefit in survival of adjuvant chemotherapy in stage II patients and particularly in pT4N0 is not yet certainly demonstrated; in addition, the interaction between MMR status and high-risk features remains unclear. pT4N0 patients are a particular subgroup with a severe prognosis,18 and the prescription of adjuvant chemotherapy should be based on a comprehensive evaluation of the disease, and also of the molecular profile, the patient’s characteristics, and their attitude.
For this reason, our study aimed to investigate the oncological outcomes of pT4N0 CC patients and to evaluate the impact of adjuvant chemotherapy in this specific population, the optimal regimen and duration, and other prognostic factors.
Materials and methods
Patients who underwent curative resection surgery between 2010 and 2021 for a pT4N0 CC, defined by the 8th edition TNM classification, were included. Participants were collected from nine Italian and one French oncological center. Data were sourced from hospital databases and medical records for retrospective analysis. Collected patient information included age, sex, comorbidities, Eastern Cooperative Oncology Group (ECOG) performance status, primary tumor sidedness, type of surgery, histopathology, T4 stage, grade, number of removed lymph nodes, LVI, PNI, and molecular factors (dMMR/MSI, RAS, BRAF). Additionally, data regarding adjuvant chemotherapy (type and duration) and recurrence were recorded. The primary endpoints of the study were OS and recurrence-free survival (RFS). Exclusion criteria included inadequate medical records, a follow-up period of ≤2 years, and the presence of distant metastasis at diagnosis.
The study was approved by the institutional ethics committee and complied with the Declaration of Helsinki and good clinical practice guidelines.
Statistical analysis
Descriptive statistics were used to summarize clinicopathological characteristics. The primary outcome measures examined in this study were RFS and OS. RFS and OS were defined as the time from the surgery on primary tumor to the first evidence of disease progression or death, whichever occurred first, and as the time from surgery on primary tumor to death due to any cause, respectively. Survival curves were estimated by the Kaplan–Meier method and compared with the log-rank test. Hazard ratios (HRs) with 95% confidence interval (CI) were estimated with a Cox proportional hazards model. Chi-square test or Kruskal–Wallis test were used whenever appropriate to compare clinical and molecular baseline characteristics between patients treated with adjuvant treatment for 3 or 6 months or with follow-up. The impact of treatment arm, age, gender, type of resection, depth of invasion, number of lymph nodes, lymphatic vessel invasion, vascular invasion, PNI, obstruction/perforation, MSI, and primary site on RFS and OS was firstly assessed in univariate analyses. Significantly prognostic covariates (P ≤ 0.10) were included in a multivariable Cox proportional hazards model. To address the potential confounding effect of patient age across treatment groups, age-adjusted analyses were also carried out for RFS and OS, using a cut-off of 75 years old. Moreover, we assessed 3-year RFS, defined as the proportion of patients free from disease recurrence 3 years after treatment. Similarly, 3-year OS was evaluated as the proportion of patients alive 3 years after initial surgery. Follow-up duration was estimated using the reverse Kaplan–Meier method. The data cut-off for the present analysis was 31 August 2024. All analyses were carried out in SAS, version 9.4 (SAS Institute, Inc., Cary, NC).
Results
Baseline patients and tumor characteristics
Our retrospective study included 492 patients, of whom 102 were excluded due to missing outcome data, resulting in a final cohort of 390 patients for analysis. Baseline clinical characteristics, summarized in Table 1, were generally well balanced across treatment groups, with age being the only significant difference. The median age of the cohort was 73 years; however, patients in the no-adjuvant group had a median age of 80 years, notably older than the median age of 68 years in the adjuvant chemotherapy group.
Table 1.
Patient characteristics
| Characteristic | 3 months ACT (n = 58) | 6 months ACT (n = 146) | No adjuvant (n = 186) | Total (N = 390) | P value |
|---|---|---|---|---|---|
| Age, years, median (IQR) | 68 (53-74) | 68 (59-74) | 80 (74-85) | 73 (64-81) | <0.0001a |
| Age group, n (%) | <0.0001b | ||||
| ≤75 years | 47 (81.0) | 119 (81.5) | 54 (29.0) | 220 (56.4) | |
| >75 years | 11 (19.0) | 27 (18.5) | 132 (71.0) | 170 (43.6) | |
| Gender, n (%) | 0.4766b | ||||
| Female | 30 (51.7) | 64 (43.8) | 92 (49.5) | 186 (47.7) | |
| Male | 28 (48.3) | 82 (56.2) | 94 (50.5) | 204 (52.3) | |
| Primary site, n (%) | 0.9973b | ||||
| Right colon | 24 (41.4) | 65 (44.5) | 88 (47.3) | 177 (45.4) | |
| Left colon | 24 (41.4) | 60 (41.1) | 71 (38.2) | 155 (39.7) | |
| Rectum | 5 (8.6) | 9 (6.2) | 12 (6.5) | 26 (6.7) | |
| Transverse colon | 4 (6.9) | 9 (6.2) | 12 (6.5) | 25 (6.4) | |
| Multiple sites | 1 (1.7) | 3 (2.1) | 3 (1.6) | 7 (1.8) | |
| Depth of invasion (pT4), n (%) | 0.5194b | ||||
| a | 29 (60.4) | 76 (65.0) | 121 (68.8) | 226 (66.3) | |
| b | 19 (39.6) | 41 (35.0) | 55 (31.3) | 115 (33.7) | |
| Missing | 10 | 29 | 10 | 49 | |
| Type of resection, n (%) | 0.5574b | ||||
| R0 | 56 (96.6) | 143 (97.9) | 178 (96.7) | 377 (97.2) | |
| R1 | 2 (3.4) | 2 (1.4) | 6 (3.3) | 10 (2.6) | |
| R2 | 0 (0.0) | 1 (0.7) | 0 (0.0) | 1 (0.3) | |
| Missing | 0 | 0 | 2 | 2 | |
| Type of treatment, n (%) | 0.6432b | ||||
| Doublets | 39 (67.2) | 103 (70.5) | 0 (0) | 142 (69.6) | |
| Monotherapy | 19 (32.8) | 43 (29.5%) | 0 (0%) | 62 (30.4) | |
| Missing | 0 | 0 | 186 | 186 | |
| Type of treatment, n (%) | 0.6757b | ||||
| XELOX | 25 (43.1) | 61 (41.8) | 0 (0) | 86 (42.2) | |
| FOLFOX | 14 (24.1) | 42 (28.8) | 0 (0) | 56 (27.5) | |
| Capecitabine | 18 (31.0) | 37 (25.3) | 0 (0) | 55 (27.0) | |
| 5-FU | 1 (1.7) | 6 (4.1) | 0 (0) | 7 (3.4) | |
| Missing | 0 | 0 | 186 | 186 | |
| Nodes harvested (n), median (IQR) | 20 (15-28) | 20 (14-28) | 20 (14-28) | 20 (14-28) | 0.7493a |
| Grade, n (%) | 0.3780b | ||||
| Grade 1 | 9 (16.4) | 12 (9.2) | 22 (13.7) | 43 (12.4) | |
| Grade 2 | 28 (50.9) | 70 (53.4) | 86 (53.4) | 184 (53.0) | |
| Grade 3 | 18 (32.7) | 49 (37.4) | 50 (31.1) | 117 (33.7) | |
| Grade 4 | 0 (0.0) | 0 (0.0) | 3 (1.9) | 3 (0.9) | |
| Missing | 3 | 15 | 25 | 43 | |
| Lymphatic vessel invasion, n (%) | 0.3081b | ||||
| No | 42 (84.0) | 99 (78.6) | 145 (85.3) | 286 (82.7) | |
| Yes | 8 (16.0) | 27 (21.4) | 25 (14.7) | 60 (17.3) | |
| Missing | 8 | 20 | 16 | 44 | |
| Vascular invasion, n (%) | 0.7009b | ||||
| No | 31 (58.5) | 87 (64.4) | 113 (64.6) | 231 (63.6) | |
| Yes | 22 (41.5) | 48 (35.6) | 62 (35.4) | 132 (36.4) | |
| Missing | 5 | 11 | 11 | 27 | |
| Perineural invasion, n (%) | 0.3834b | ||||
| No | 40 (76.9) | 92 (68.7) | 127 (74.7) | 259 (72.8) | |
| Yes | 12 (23.1) | 42 (31.3) | 43 (25.3) | 97 (27.2) | |
| Missing | 6 | 12 | 16 | 34 | |
| Obstruction/perforation, n (%) | 0.4104b | ||||
| No | 43 (79.6) | 103 (75.7) | 124 (71.3) | 270 (74.2) | |
| Yes | 11 (20.4) | 33 (24.3) | 50 (28.7) | 94 (25.8) | |
| Missing | 4 | 10 | 12 | 26 | |
| Other risk factors, n (%) | 0.8534b | ||||
| No | 18 (36.0) | 50 (37.9) | 58 (34.7) | 126 (36.1) | |
| Yes | 32 (64.0) | 82 (62.1) | 109 (65.3) | 223 (63.9) | |
| Missing | 8 | 14 | 19 | 41 | |
| Microsatellite instability, n (%) | 0.2528b | ||||
| MSI | 12 (27.3) | 22 (19.8) | 40 (28.8) | 74 (25.2) | |
| MSS | 32 (72.7) | 89 (80.2) | 99 (71.2) | 220 (74.8) | |
| Missing | 14 | 35 | 47 | 96 | |
| Lynch syndrome, n (%) | 0.2787b | ||||
| No | 25 (92.6) | 58 (95.1) | 46 (86.8) | 129 (91.5) | |
| Yes | 2 (7.4) | 3 (4.9) | 7 (13.2) | 12 (8.5) | |
| Missing | 31 | 85 | 133 | 249 | |
5-FU, 5-fluorouracil; ACT, adjuvant chemotherapy; IQR, interquartile range; MSI, microsatellite instability; MSS, microsatellite stability.
Kruskal–Wallis P value.
Chi-square P value.
Primary tumor location varied, with the right-sided colon being the most common site (45%), followed by the left-sided colon (40%), rectum (7%), and transverse colon (6%). A small subset of patients (2%) presented with synchronous tumors in different segments of the colon.
Pathological staging data indicated that 226 patients (58%) had pT4a stage tumors, while 115 patients (29%) had pT4b stage tumors. This information was unavailable for 49 patients (13%).
Surgical performance was optimal, with 97% of patients achieving an R0 resection status.
MMR status was available in 294 patients (75%), with 74 patients (25%) identified as having dMMR/MSI CC and 220 patients (75%) pMMR/MSS. Among the dMMR/MSI group, 12 patients (16%) were diagnosed with Lynch syndrome.
Additional histopathological risk factors were also noted. G3 differentiation was present in 117 patients (34%), and 44 patients (11%) had <12 lymph nodes harvested. LVI-positive was observed in 192 patients (49%), PNI-positive in 97 patients (25%), and obstruction or perforation in 94 patients (24%). In terms of adjuvant treatment, nearly half of the patients (n = 186, 48%) did not receive adjuvant chemotherapy, while 204 patients (52%) underwent adjuvant chemotherapy. Among those treated with adjuvant chemotherapy, 58 patients (28%) received a 3-month regimen, whereas 146 patients (72%) completed a 6-month regimen. Notably, age appeared to strongly influence treatment decisions. Of the 170 patients classified as elderly (aged >75 years), 132 (78%) did not receive adjuvant chemotherapy.
Among the 204 patients treated with adjuvant chemotherapy, the majority (n = 142, 70%) received doublet chemotherapy regimens (FOLFOX or XELOX), while the remaining 62 patients (30%) received monotherapy (capecitabine or 5-FU). Within the 3 months’ adjuvant chemotherapy subgroup, 39 patients (67%) received doublet therapy and 19 patients (33%) received monotherapy. In the 6 months’ adjuvant chemotherapy group, 103 patients (72%) received doublet therapy, and 43 patients (28%) received monotherapy.
Clinical outcomes
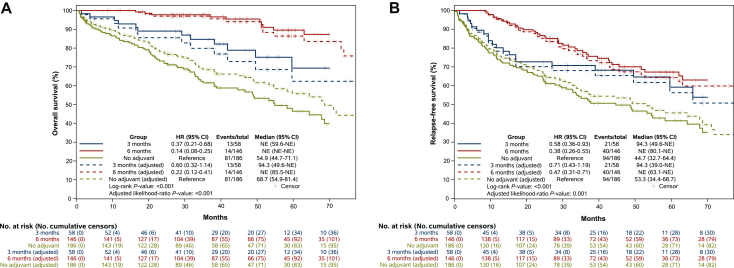
A significant difference in OS was shown among the three treatment groups. After a median follow-up of 46.8 months (range 41.4-52.3 months), patients who received 6 months of adjuvant chemotherapy had significantly longer survival than both 3-month and no-adjuvant chemotherapy subgroups (Figure 1A). When compared with no-adjuvant treatment, 6 months of adjuvant chemotherapy was associated with a significant improvement in OS, with an HR of 0.14 (95% CI 0.08-0.25, P < 0.001). The benefit was also significant in the 3-month group, even if lower, with an HR of 0.37 (95% CI 0.21-0.68, P < 0.001). The 3-year OS rates were 97.7% for the 6-month subgroup, compared with 84.6% for the 3-month subgroup and 61.5% for those who did not receive any adjuvant treatment.
Figure 1.
(A) Overall survival and age-adjusted overall survival. (B) Relapse-free survival and age-adjusted relapse-free survival. CI, confidence interval; HR, hazard ratio; NE, not evaluable.
Furthermore, a direct comparison between the two adjuvant chemotherapy durations showed a significant OS benefit for the 6-month subgroup over the 3-month subgroup (HR 0.36, 95% CI 0.17-0.77, P = 0.006).
The age-adjusted analysis confirmed the OS benefit of 6 months of adjuvant treatment (HR 0.22, 95% CI 0.12-0.41, P < 0.001) and of the 3-month regimen (HR 0.60, 95% CI 0.32-1.14).
Similar results were observed in the RFS analysis (Figure 1B). Patients who received 6 months of adjuvant chemotherapy showed a statistically significant RFS benefit compared with the no-adjuvant group, with an HR of 0.38 (95% CI 0.26-0.55, P < 0.001), while patients who received 3 months of adjuvant chemotherapy had an HR of 0.58 (95% CI 0.36-0.93, P < 0.001).
The 3-year RFS rates were 77.7% for the 6-month subgroup, 70.7% for the 3-month subgroup, and 53.5% for those who did not receive adjuvant chemotherapy.
Again, in the age-adjusted analysis, a statistically significant benefit was maintained both in the 6-month group (HR 0.47, 95% CI 0.31-0.71) and in the 3-month group (HR 0.71, 95% CI 0.43-1.19).
The use of an oxaliplatin-based doublet chemotherapy was associated with a significant benefit both in OS and RFS when compared with monotherapy, with HRs of 0.21 (95% CI 0.10-0.46, P < 0.001) and 0.48 (95% CI 0.29-0.80, P = 0.004), respectively, regardless of treatment duration (Figure 2A and B).
Figure 2.
(A) Overall survival according to chemotherapy regimen (doublet versus monotherapy). (B) Relapse-free survival according to chemotherapy regimen (doublet versus monotherapy). (C) Overall survival according to treatment and treatment duration. (D) Relapse-free survival according to treatment and treatment duration.CI, confidence interval; HR, hazard ratio; NE, not evaluable.
The age-adjusted analysis confirmed the benefit to OS of doublet therapy over monotherapy (HR 0.24, P = 0.004), and a trend of better RFS was seen (HR 0.62, 95% CI 0.34-1.11, P = 0.1).
Regarding the different treatment strategies and durations, the greatest benefit in terms of OS was observed with doublet chemotherapy for 6 months, followed by doublet for 3 months, monotherapy for 6 months, and, lastly, monotherapy for 3 months, with HRs of 0.05, 0.25, 0.38, and 0.67, respectively (Figure 2C). A similar trend was observed for RFS (Figure 2D).
Regarding dMMR/MSI status, there was no statistically significant difference, either in OS (HR 1.03, 95% CI 0.60-1.74, P = 0.93) or in RFS (HR 0.76, 95% CI 0.48-1.19, P = 0.23), between MSS and MSI patients, regardless of treatment (Supplementary Figure S1A and S1B, available at https://doi.org/10.1016/j.esmoop.2025.105496).
For OS (Table 2), univariate analysis identified a significant survival benefit with adjuvant treatment, with 3-month and 6-month regimens reducing mortality risk (HR 0.38 and 0.14, respectively, P < 0.001). Older age (>75 years, HR 4.53, P < 0.001), vascular invasion (HR 1.62, P = 0.019), and obstruction/perforation (HR 1.56, P = 0.031) were associated with worse survival. There was no significant impact from MMR status (HR 1.03, P = 0.93).
Table 2.
Univariate and multivariate analyses of overall survival
| Characteristics |
N |
Months | Univariate analysis |
Multivariate analysis |
||
|---|---|---|---|---|---|---|
| 390 | HR (95% CI) | P | HR (95% CI) | P | ||
| Treatment arm | ||||||
| No adjuvant | 186 | 54.9 | 1 | <0.001 | 1 | 0.002 |
| 3 months | 58 | NE | 0.38 (0.21-0.68) | 0.56 (0.31-1.00) | ||
| 6 months | 146 | NE | 0.14 (0.08-0.25) | 0.45 (0.29-0.72) | ||
| Age | ||||||
| ≤75 years | 220 | NE | 1 | <0.001 | 1 | 0.12 |
| >75 years | 170 | 58.3 | 4.53 (2.97-6.91) | 1.39 (0.92-2.10) | ||
| Gender | ||||||
| Female | 186 | 85.5 | 1 | 0.88 | ||
| Male | 204 | 94.3 | 1.03 (0.71-1.50) | |||
| Type of resection | ||||||
| R0 | 377 | 94.3 | 1 | 0.37 | ||
| R1/R2 | 11 | NE | 1.58 (0.58-4.29) | |||
| Missing | 2 | |||||
| Depth of invasion | ||||||
| A | 226 | 85.5 | 1 | 0.70 | ||
| B | 115 | 81.4 | 1.08 (0.72-1.63) | |||
| Missing | 49 | |||||
| N nodes | ||||||
| <12 nodes | 44 | 71.9 | 1 | 0.88 | ||
| ≥12 nodes | 346 | NE | 0.96 (0.55-1.68) | |||
| Lymphatic vessel invasion | ||||||
| No | 286 | 94.3 | 1 | 0.74 | ||
| Yes | 60 | 71.9 | 0.91 (0.52-1.59) | |||
| Missing | 44 | |||||
| Vascular invasion | ||||||
| No | 231 | 94.3 | 1 | 0.019 | 1 | <0.001 |
| Yes | 132 | 80.1 | 1.62 (1.08-2.42) | 1.99 (1.41-2.79) | ||
| Missing | 27 | |||||
| Perineural invasion | ||||||
| No | 259 | 94.3 | 1 | 0.34 | ||
| Yes | 97 | 80.1 | 1.24 (0.79-1.94) | |||
| Missing | 34 | |||||
| Obstruction/Perforation | ||||||
| No | 270 | NE | 1 | 0.031 | 1 | 0.29 |
| Yes | 94 | 71.1 | 1.56 (1.04-2.35) | 1.22 (0.85-1.77) | ||
| Missing | 26 | |||||
| Microsatellite instability | ||||||
| No | 220 | 74 | 1 | 0.93 | ||
| Yes | 74 | NE | 1.03 (0.60-1.74) | |||
| Missing | 96 | |||||
| Primary site | ||||||
| Right colon | 202 | 80.9 | 1 | 0.78 | ||
| Left colon | 181 | NE | 0.95 (0.65-1.39) | |||
Bold values represent statistically significant results.
CI, confidence interval; HR, hazard ratio; NE, not evaluable.
In multivariate analysis, adjuvant treatment remained significant, with HRs of 0.56 (P = 0.002) for 3 months and 0.45 (P = 0.002) for 6 months. Vascular invasion remained an independent negative prognostic factor (HR 1.99, P < 0.001), while age and obstruction/perforation lost significance.
For RFS (Table 3), univariate analysis showed a significant reduction in relapse risk with adjuvant treatment (HR 0.57 for 3 months, HR 0.38 for 6 months, P < 0.001). Negative prognostic factors included age >75 years (HR 2.09, P < 0.001), vascular invasion (HR 2.01, P < 0.001), LVI (HR 1.59, P = 0.020), and PNI (HR 1.44, P = 0.043). MMR status did not have a significant impact (HR 0.76, P = 0.23) on RFS.
Table 3.
Univariate and multivariate analyses of relapse-free survival
| Characteristics |
N = |
Months | Univariate analysis |
Multivariate analysis |
||
|---|---|---|---|---|---|---|
| 390 | HR (95% CI) | P | HR (95% CI) | P | ||
| Treatment arm | ||||||
| No adjuvant | 186 | 44.7 | 1 | <0.001 | 1 | 0.009 |
| 3 months | 58 | 94.3 | 0.57 (0.36-0.92) | 0.64 (0.36-1.15) | ||
| 6 months | 146 | NE | 0.38 (0.26-0.55) | 0.79 (0.43-1.42) | ||
| Age | ||||||
| ≤75 years | 220 | NE | 1 | <0.001 | 1 | 0.039 |
| >75 years | 170 | 48.7 | 2.09 (1.52-2.88) | 1.52 (1.02-2.28) | ||
| Gender | ||||||
| Female | 186 | 80.1 | 1 | 0.29 | ||
| Male | 204 | 62.1 | 1.19 (0.86-1.63) | |||
| Type of resection | ||||||
| R0 | 377 | 68.7 | 1 | 0.46 | ||
| R1/R2 | 11 | 34.4 | 1.40 (0.57-3.41) | |||
| Missing | 2 | |||||
| Depth of invasion | ||||||
| a | 226 | 66.0 | 1 | 0.93 | ||
| b | 115 | 71.1 | 1.02 (0.72-1.44) | |||
| Missing | 49 | |||||
| Number of nodes | ||||||
| <12 nodes | 44 | 42.7 | 1 | 0.18 | ||
| ≥12 nodes | 346 | 71.1 | 0.74 (0.47-1.15) | |||
| Lymphatic vessel invasion | ||||||
| No | 286 | 81.4 | 1 | 0.020 | 1 | 0.25 |
| Yes | 60 | 52.7 | 1.59 (1.07-2.35) | 1.28 (0.84-1.95) | ||
| Missing | 44 | |||||
| Vascular invasion | ||||||
| No | 231 | 79.8 | 1 | <0.001 | 1 | <0.001 |
| Yes | 132 | 37.3 | 2.01 (1.44-2.79) | 2.07 (1.39-3.08) | ||
| Missing | 27 | |||||
| Perineural invasion | ||||||
| No | 259 | 68.7 | 1 | 0.043 | 1 | 0.90 |
| Yes | 97 | 44.7 | 1.44 (1.01-2.06) | 1.03 (0.67-1.57) | ||
| Missing | 34 | |||||
| Obstruction/perforation | ||||||
| No | 270 | 68.7 | 1 | 0.15 | ||
| Yes | 94 | 53.3 | 1.30 (0.91-1.85) | |||
| Missing | 26 | |||||
| Microsatellite instability | ||||||
| No | 220 | 49.2 | 1 | 0.23 | ||
| Yes | 74 | NE | 0.76 (0.49-1.19) | |||
| Missing | 96 | |||||
| Primary site | ||||||
| Right colon | 202 | 67.9 | 1 | 0.93 | ||
| Left colon | 181 | 66.0 | 0.99 (0.72-1.36) | |||
Bold values represent statistically significant results.
In multivariate analysis, 3-months’ treatment lost significance, while 6-months’ treatment remained protective (HR 0.79, P = 0.009). Age >75 years (HR 1.52, P = 0.039) and vascular invasion (HR 2.07, P < 0.001) remained independent negative prognostic factors, whereas LVI and PNI lost significance.
Discussion
The treatment of stage II CC has represented a clinical dilemma for decades, due to its variable prognosis and the unclear benefit of adjuvant chemotherapy.7 Despite the well-known negative prognostic impact of pT4, specific data on this subpopulation are missing. Our study is the largest real-world analysis collecting clinical, pathological, and survival data of 390 pT4N0 patients that shows the benefit of the use of adjuvant chemotherapy, especially if prolonged (6 months) and with the addition of oxaliplatin.
As often happens in real-world retrospective studies, age was not balanced between the two subgroups, with older patients in the nonadjuvant chemotherapy group. However, approximately one out of four patients >75 years old received adjuvant chemotherapy, despite the absence of proven benefit,17,25 probably reflecting the poor prognosis that clinicians associate with this stage. However, all the others relevant factors (e.g. sex, sidedness, depth of invasion, R0 resections, lymph–vascular invasion) were balanced between the two groups.
We found in the overall population an R0 rate >97% and only 11% of patients with <12 lymph nodes harvested, highlighting the high level of the surgery carried out, since we collected data from high-volume referral center, thus making our results robust and reliable. These results suggest also the importance of correct preoperative staging and the evaluation of resectability in a multidisciplinary team. However, preliminary data on neoadjuvant chemotherapy26 showed a potential benefit in selected cases such as bulky cT4b tumors, but more data are still needed to consider it as clinical practice.27 It is possible that for initially unresectable pT4 patients, an intensification of preoperative treatments could be the future.28
In terms of survival, 5-year RFS and OS, regardless of treatment, was 54% and 66%, respectively. The prognostic impact of pT4 in stage II is highlighted by these RFS and OS rates, which are similar to those of high-risk stage III colorectal cancer.29 While international guidelines recommend 6 months of dual adjuvant chemotherapy for high-risk stage III patients,15,30 no clear benefit to prolonged adjuvant treatment has yet been demonstrated for this subgroup of stage II. Our cohort showed a survival benefit from the addition of adjuvant chemotherapy: 3-year OS was improved by 23% with 3 months (85% versus 62%, HR 0.37) and by 36% with 6 months (98% versus 62%, HR 0.14, P < 0.001) compared with no-adjuvant treatment. However, given the selection bias among nontreated patients (elderly, those with other compelling cause of death), OS may not represent the best clinical endpoint for adjuvant benefit. Therefore, RFS was evaluated and, similar to OS, 6 months of adjuvant chemotherapy was associated with a significant improvement in 3-year RFS (78% versus 53%, P < 0.001). To account for potential bias due to age imbalance between the groups, both OS and RFS were adjusted for age, and the benefit of adjuvant treatment remained significant (P < 0.001).
Considering the type of chemotherapy, international guidelines recommend the use of oxaliplatin for high-risk stage II CC, defined as pT4 or with <12 lymph nodes removed. This recommendation is based on the results of the MOSAIC trial,16 in which the addition of oxaliplatin showed a nonsignificant DFS benefit only in patients with high-risk stage II CRC. A recent pooled analysis of stage II patients enrolled both in the MOSAIC and in the NSABP C0731 evaluated oxaliplatin-specific survival benefit. The authors concluded that adjuvant oxaliplatin should not be the standard of care for stage II CC, even in high-risk patients (220 patients, 14% of the population, had a pT4N0 tumor), due to the absence of survival benefit. However, caution in interpreting these data is needed, since the reduction of risk of recurrence was highly clinically relevant (HR for time to recurrence 0.75) and the absence of statistical significance could be related to underpowered analyses, as this analysis is an unplanned post hoc analysis, and the number of patients was not powered for these results. Lastly, improvement in the clinical management of the patients could reduce the accuracy of analyses of trials conducted >10 years ago.32
In our analysis, data regarding the type of adjuvant chemotherapy were available for 204 patients, thus limiting the power to draw conclusions based on our data. However, when compared with 5FU/capecitabine monotherapy, the use of doublet chemotherapy was associated with a reduction of both risk of recurrence (HR 0.48, P = 0.004) and risk of death (HR 0.21, P < 0.001), suggesting that, when clinically feasible, an oxaliplatin-based adjuvant chemotherapy is recommended in all pT4 patients.
In line with the actual ESMO guidelines, which recommend adjuvant chemotherapy despite MSS in high-risk stage II patients, dMMR/MSI was not significant in univariate analysis, suggesting that MSI does not confer a better prognosis in pT4N0 CC patients. However, it is possible that this standard of treatment is going to change in the next future with the introduction of neoadjuvant immunotherapy, which showed excellent results in recent trials.33 However, caution is warranted given the risk of severe and long-lasting adverse events associated with immune checkpoint inhibitors (ICIs). Moreover, computed tomography scan staging at baseline could lead to mistakes, especially in terms of overestimation of lymph node involvement26 and, consequently, overtreatment, and predictive biomarkers are highly needed.
More importantly, circulating tumor DNA (ctDNA) has proven to be the strongest prognostic marker in localized CC34 and its measurement is probably going to become part of our clinical practice. Indeed, it could allow risk stratification based on the detection of minimal residual disease (MRD) after surgery and/or after adjuvant chemotherapy, and its monitoring over time allows the identification of patients with sustained clearance who are potentially cured.35,36
In the last update of the DYNAMIC trial,37 the use of a ctDNA-guided approach seemed to increase RFS when compared with standard management, also in pT4N0 patients (5-year RFS 81% versus 70%), but a 5-year risk of recurrence of 20% was significantly higher than that of 10% observed in the overall population. Moreover, in the pT4N0 subgroup analysis of the GALAXY trial, despite the absence of statistical significance, an improvement in 2-year DFS of 8% was associated with the use of adjuvant chemotherapy also in MRD-negative patients, and this benefit was not seen in other MRD-negative subgroups of patients. A possible explanation could be related to metastatic spread: due to local extension and infiltration, pT4 tumors often give peritoneal metastases, which are known to be, together with lung metastases, low ctDNA shedder sites.38,39 Therefore, more data are needed to better understand the limits of ctDNA in this subgroup of patients, in which clinical factors should probably be considered for a correct evaluation of individual risk.
The greater limitation of our research is its retrospective nature. Missing data, especially in terms of molecular analyses, could reduce the accuracy of the analysis; dMMR/MSI status is unknown for approximately one-third of the patients. Moreover, no ctDNA data were collected since they was not accessible during the study period.
Conclusion
In this large, multicenter retrospective study of 390 patients with pT4N0 CC, pathological T4 was confirmed to be a negative prognostic factor. Our findings demonstrate a significant benefit to OS and RFS with adjuvant chemotherapy, particularly with longer treatment durations and oxaliplatin-based doublets. However, given the retrospective design and potential for unmeasured confounders influencing treatment decisions, these results should be interpreted cautiously.
The International Duration Evaluation of Adjuvant Chemotherapy (IDEA) collaboration, which pooled prospective data from several large randomized controlled trials in stage II and III CC, continues to offer the most reliable evidence to guide treatment duration and regimen selection.
Integration of biomarkers such as ctDNA and novel strategies like neoadjuvant ICIs may change clinical management in the future, but further data are still needed.
Acknowledgments
Funding
We acknowledge the support of AIRC IG 26330 to GT, AIRC MFAG no. 27367 to LS.
Disclosure
MAC reports travel support and hospitality from Pierre Fabre, Amgen, Merck, Servier, and Bayer, and has served on an advisory board for Merck. VF reports honoraria from Amgen, Merck, Servier, Merck Sharpe & Dohme (MSD), Bristol Myers Squibb (BMS), and Pierre Fabre. SS is an advisory board member for Agenus, AstraZeneca, Bayer, BMS, CheckmAb, Daiichi Sankyo, GSK, MSD, Merck, Novartis, Pierre Fabre, Pfizer, Seagen, and T-One Therapeutics. CS reports consultant fees from Amgen and Bayer. AS acted as speaker/consultant for Johnson & Johnson, Stryker, and Oasis. GT reports consulting or advisory roles for BMS, AstraZeneca, MSD, Merck, and Servier. JT has received honoraria as a speaker and/or in an advisory role from Amgen, Astellas, AstraZeneca, Boehringer, BMS, Brenus Pharma, Bicara Therapeutics, Oxford Biotherapeutics, Proskope, Merck KGaA, MSD, Novartis, ONO Pharmaceutical, Pierre Fabre, Natera, Sanofi, Servier, and Takeda. AP reports honoraria from GlaxoSmithKline, Takeda, Bayer, Daiichi Sankyo, MSD, Amgen, BeiOne, Pierre Fabre, Servier, BMS, Merck Serono for consultancy, advisory boards or invited speaker; research funding (to the Institution) from GlaxoSmithKline, Amgen; travel Grants from AstraZeneca. All outside the submitted work. LS reports consulting or advisory roles for Pierre Fabre, AstraZeneca, Bayer, Servier, Merck, Amgen, GSK, Incyte, LEO Pharma, MSD, and Takeda.
All other authors have declared no conflicts of interest.
Footnotes
Adjuvant oxaliplatin improves OS and RFS in pT4N0 colon cancer: real-world multicenter data.
Contributor Information
A. Puccini, Email: alberto.puccini@hunimed.eu.
L. Salvatore, Email: lisa.salvatore@policlinicogemelli.it.
Supplementary data
References
- 1.Sung H., Ferlay J., Siegel R.L., et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Siegel R.L., Wagle N.S., Cercek A., Smith R.A., Jemal A. Colorectal cancer statistics, 2023. CA Cancer J Clin. 2023;73(3):233–254. doi: 10.3322/caac.21772. [DOI] [PubMed] [Google Scholar]
- 3.Sargent D., Sobrero A., Grothey A., et al. Evidence for cure by adjuvant therapy in colon cancer: observations based on individual patient data from 20,898 patients on 18 randomized trials. J Clin Oncol. 2009;27:872–877. doi: 10.1200/JCO.2008.19.5362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brierley J.D., Gospodarowicz M.K., Wittekind C. 8th ed. John Wiley; Oxford: 2016. TNM Classification of Malignant Tumours. [Google Scholar]
- 5.Roth A.D., Delorenzi M., Tejpar S., et al. Integrated analysis of molecular and clinical prognostic factors in stage II/III colon cancer. J Natl Cancer Inst. 2012;104:1635–1646. doi: 10.1093/jnci/djs427. [DOI] [PubMed] [Google Scholar]
- 6.Wells K.O., Hawkins A.T., Krishnamurthy D.M., et al. Omission of adjuvant chemotherapy is associated with increased mortality in patients with T3N0 colon cancer with inadequate lymph node harvest. Dis Colon Rectum. 2017;60:15–21. doi: 10.1097/DCR.0000000000000729. [DOI] [PubMed] [Google Scholar]
- 7.Rebuzzi S.E., Pesola G., Martelli V., Sobrero A.F. Adjuvant chemotherapy for stage II colon cancer. Cancers (Basel) 2020;12(9):2584. doi: 10.3390/cancers12092584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gill S., Loprinzi C.L., Sargent D.J., et al. Pooled analysis of fluorouracil-based adjuvant therapy for stage II and III colon cancer: who benefits and by how much? J Clin Oncol. 2004;22:1797–1806. doi: 10.1200/JCO.2004.09.059. [DOI] [PubMed] [Google Scholar]
- 9.Gao P., Song Y.X., Wang Z.N., et al. Is the prediction of prognosis not improved by the seventh edition of the TNM classification for colorectal cancer? Analysis of the surveillance, epidemiology, and end results (SEER) database. BMC Cancer. 2013;13:123. doi: 10.1186/1471-2407-13-123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Park J.S., Huh J.W., Park Y.A., et al. Clinically suspected T4 colorectal cancer may be resected using a laparoscopic approach. BMC Cancer. 2016;16:714. doi: 10.1186/s12885-016-2753-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim M.J., Jeong S.-Y., Choi S.-J., et al. Survival paradox between stage IIB/C (T4N0) and stage IIIA (T1-2N1) colon cancer. Ann Surg Oncol. 2015;22(2):505–512. doi: 10.1245/s10434-014-3982-1. [DOI] [PubMed] [Google Scholar]
- 12.Benson A.B., Schrag D., Somerfield M.R., et al. American Society of Clinical Oncology recommendations on adjuvant chemotherapy for stage II colon cancer. J Clin Oncol. 2004;22:3408–3419. doi: 10.1200/JCO.2004.05.063. [DOI] [PubMed] [Google Scholar]
- 13.Köhne C.H. Should adjuvant chemotherapy become standard treatment for patients with stage II colon cancer? Against the proposal. Lancet Oncol. 2006;7:516–517. [PubMed] [Google Scholar]
- 14.Achilli P., Crippa J., Grass F., et al. Survival impact of adjuvant chemotherapy in patients with stage IIA colon cancer: analysis of the National Cancer Database. Int J Cancer. 2021;148:161–169. doi: 10.1002/ijc.33203. [DOI] [PubMed] [Google Scholar]
- 15.Argilés G., Tabernero J., Labianca R., et al. Localised colon cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2020;31:1291–1305. doi: 10.1016/j.annonc.2020.06.022. [DOI] [PubMed] [Google Scholar]
- 16.André T., Boni C., Navarro M., et al. Improved overall survival with oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment in stage II or III colon cancer in the MOSAIC trial. J Clin Oncol. 2009;27:3109–3116. doi: 10.1200/JCO.2008.20.6771. [DOI] [PubMed] [Google Scholar]
- 17.Yothers G., O’Connell M.J., Allegra C.J., et al. Oxaliplatin as adjuvant therapy for colon cancer: updated results of NSABP C-07 trial, including survival and subset analyses. J Clin Oncol. 2011;29:3768–3774. doi: 10.1200/JCO.2011.36.4539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dienstmann R., Mason M.J., Sinicrope F.A., et al. Prediction of overall survival in stage II and III colon cancer beyond TNM system: a retrospective, pooled biomarker study. Ann Oncol. 2017;28(5):1023–1031. doi: 10.1093/annonc/mdx052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sargent D.J., Marsoni S., Monges G., et al. Defective mismatch repair as a predictive marker for lack of efficacy of fluorouracil-based adjuvant therapy in colon cancer. J Clin Oncol. 2010;28:3219–3226. doi: 10.1200/JCO.2009.27.1825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ribic C.M., Sargent D.J., Moore M.J., et al. Tumor microsatellite-instability status as a predictor of benefit from fluorouracil-based adjuvant chemotherapy for colon cancer. N Engl J Med. 2003;7:47–257. doi: 10.1056/NEJMoa022289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sinicrope F.A., Foster N.R., Thibodeau S.N., et al. DNA mismatch repair status and colon cancer recurrence and survival in clinical trials of 5-fluorouracil-based adjuvant therapy. J Natl Cancer Inst. 2011;103:863–875. doi: 10.1093/jnci/djr153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tejpar S., Saridaki Z., Delorenzi M., Bosman F., Roth A.D. Microsatellite instability, prognosis and drug sensitivity of stage II and III colorectal cancer: more complexity to the puzzle. J Natl Cancer Inst. 2011;103:841–844. doi: 10.1093/jnci/djr170. [DOI] [PubMed] [Google Scholar]
- 23.Kim J.E., Hong Y.S., Kim H.J., et al. Defective mismatch repair status was not associated with DFS and OS in stage II colon cancer treated with adjuvant chemotherapy. Ann Surg Oncol. 2015;22:630–637. doi: 10.1245/s10434-015-4807-6. [DOI] [PubMed] [Google Scholar]
- 24.Des Guetz G., Nicolas P., Perret G.Y., Morere J.F., Uzzan B. Does delaying adjuvant chemotherapy after curative surgery for colorectal cancer impair survival? A meta-analysis. Eur J Cancer. 2010;46:1049–1055. doi: 10.1016/j.ejca.2010.01.020. [DOI] [PubMed] [Google Scholar]
- 25.Tournigand C., André T., Bonnetain F., et al. Adjuvant therapy with fluorouracil and oxaliplatin in stage II and elderly patients (between ages 70 and 75 years) with colon cancer: subgroup analyses of the Multicenter International Study of Oxaliplatin, Fluorouracil, and Leucovorin in the Adjuvant Treatment of Colon Cancer trial. J Clin Oncol. 2012;30(27):3353–3360. doi: 10.1200/JCO.2012.42.5645. [DOI] [PubMed] [Google Scholar]
- 26.Morton D., Seymour M., Magill L., et al. Preoperative chemotherapy for operable colon cancer: mature results of an international randomized controlled trial. J Clin Oncol. 2023;41:541–1552. doi: 10.1200/JCO.22.00046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Taieb J., Karoui M. FOxTROT: are we ready to dance? J Clin Oncol. 2023;41:1514–1517. doi: 10.1200/JCO.22.02108. [DOI] [PubMed] [Google Scholar]
- 28.Zhang Z.T., Xiao W.W., Li L.R., et al. Neoadjuvant chemoradiotherapy versus neoadjuvant chemotherapy for initially unresectable locally advanced colon cancer: short-term outcomes of an open-label, single-centre, randomised, controlled, phase 3 trial. eClinicalMedicine. 2024;76 doi: 10.1016/j.eclinm.2024.102836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sobrero A.F., Puccini A., Shi Q., et al. A new prognostic and predictive tool for shared decision making in stage III colon cancer. Eur J Cancer. 2020;138:182–188. doi: 10.1016/j.ejca.2020.07.031. [DOI] [PubMed] [Google Scholar]
- 30.National Comprehensive Cancer Network (NCCN) NCCN Harmonized Guidelines for Sub-Saharan Africa—Colon Cancer. Version 2. NCCN. 2018:1–5. [Google Scholar]
- 31.Chibaudel B., Raeisi M., Cohen R., et al. Assessment of the addition of oxaliplatin to fluoropyrimidine-based adjuvant chemotherapy in patients with high-risk stage II colon cancer: an ACCENT pooled analysis. J Clin Oncol. 2024;42(35):4187–4195. doi: 10.1200/JCO.24.00394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Taieb J., Gandini A., Seligmann J.F., Gallois C. The clinical dilemma of high-risk stage II colon cancer: are we truly prepared to withdraw oxaliplatin? ESMO Open. 2024;9 doi: 10.1016/j.esmoop.2024.104072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chalabi M., Verschoor Y.L., Tan P.B., et al. Neoadjuvant immunotherapy in locally advanced mismatch repair–deficient colon cancer. N Engl J Med. 2024;390:1949–1958. doi: 10.1056/NEJMoa2400634. [DOI] [PubMed] [Google Scholar]
- 34.Pantel K., Alix-Panabières C. Minimal residual disease as a target for liquid biopsy in patients with solid tumours. Nat Rev Clin Oncol. 2024;22:65–77. doi: 10.1038/s41571-024-00967-y. [DOI] [PubMed] [Google Scholar]
- 35.Kotani D., Oki E., Nakamura Y., et al. Molecular residual disease and efficacy of adjuvant chemotherapy in patients with colorectal cancer. Nat Med. 2023;29:127–134. doi: 10.1038/s41591-022-02115-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kasi P.M., Aushev V.N., Ensor J., et al. Circulating tumor DNA (ctDNA) for informing adjuvant chemotherapy (ACT) in stage II/III colorectal cancer (CRC): interim analysis of BESPOKE CRC study. J Clin Oncol. 2024;42:9. [Google Scholar]
- 37.Tie J., Cohen J.D., Lahouel K., et al. Circulating tumor DNA analysis guiding adjuvant therapy in stage II colon cancer. N Engl J Med. 2022;386:2261–2272. doi: 10.1056/NEJMoa2200075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tie J., Cohen J.D., Wang Y., Ginestet P.G., et al. Circulating tumor DNA analysis informing adjuvant chemotherapy in locally advanced rectal cancer: the randomized AGITG DYNAMIC-Rectal study. J Clin Oncol. 2024;42:12. [Google Scholar]
- 39.Lonardi S., Montagut C., Pietrantonio F., et al. The PEGASUS trial: post-surgical liquid biopsy-guided treatment of stage III and high-risk stage II colon cancer patients. J Clin Oncol. 2020;38 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.