Abstract
Dental caries is a widespread chronic disease that affects a large proportion of both adults and children globally. Streptococcus mutans is widely recognized as the primary pathogen responsible for dental caries, while Candida albicans frequently coexists with it, often forming a synergistic relationship. Despite this, the specific virulence mechanisms of these microorganisms, both individually and in coaggregation, as well as their collective impact on cariogenic potential, remain incompletely understood. This comprehensive review aims to examine both original and review articles addressing the virulence characteristics of these species, both independently and in coaggregation, and to assess how these interactions contribute to tooth demineralization, polysaccharide production, and the expression of virulence genes. The research reviewed here provides valuable insights into the physiological interactions between the two species, showing that these interactions lead to increased acid production within their coexisting biofilm, which enhances the cariogenic potential. These insights could guide future studies aimed at developing targeted strategies for preventing or mitigating dental caries.
Keywords: candida albicans, caries progression, dental caries, microbiota, polysaccharides, review article, streptococcus mutans, virulence
Introduction and background
Dental caries is characterized by the demineralization of tooth enamel resulting from the metabolic activity of acid-producing bacteria within the dental biofilm. It is a dynamic and potentially reversible disease, influenced by the delicate balance between demineralization and remineralization processes [1], a balance often disrupted by frequent exposure to dietary fermentable carbohydrates [2]. Given that untreated caries in both permanent and deciduous teeth ranks among the most prevalent global health conditions [3], understanding the cariogenic microbiota and its pathogenic mechanisms is essential for the development of effective preventive and therapeutic strategies. Among these microbes, Streptococcus mutans, a Gram-positive bacterium, plays a central role due to its ability to produce extracellular polymeric substances (EPS) and organic acids from metabolized carbohydrates, particularly sucrose [4]. Its cariogenic potential is further enhanced by its acid tolerance, allowing it to thrive in low-pH environments and outcompete noncariogenic species within dental biofilm [4]. EPS production is critical for microbial adhesion, cell-to-cell aggregation, and the mechanical stability of the biofilm matrix [5]. To synthesize EPS, S. mutans secretes glucosyltransferases (GTFs), enzymes that hydrolyze sucrose, a disaccharide with an atypical glycosidic linkage involving glucose and fructose [6]. Although S. mutans is the most extensively studied cariogenic microorganism, other species, such as Lactobacillus spp., Actinomyces spp., Streptococcus sanguinis, and particularly the fungus Candida albicans, also contribute significantly to the initiation and progression of dental caries [1-7]. In this review, we emphasize the significance of C. albicans coaggregating with S. mutans and its emerging role in caries pathogenesis.
C. albicans exhibits multiple morphological forms, including yeast, pseudohyphae, and hyphae, with the latter being the most pathogenic [8]. Its hydrophobic cells exhibit strong adhesion to soft and hard tissues [9], and it secretes proteinases capable of degrading host proteins [10]. While C. albicans typically coexists harmlessly with the host, immune suppression can trigger its transition to a pathogenic state [11]. The virulence of C. albicans is attributed to its morphological plasticity, adhesin expression, biofilm formation, and the production of hydrolytic enzymes, which enable colonization and infection across multiple host sites [10]. Biofilm formation further enhances its resistance to antifungals and immune defenses [12], and microbial interactions within biofilms can be either cooperative or antagonistic, involving competition for nutrients or mutualistic exchanges of metabolites and growth stimulators [10]. In the oral cavity, co-adhesion between C. albicans and S. mutans has been linked to increased caries severity, particularly in early childhood caries, root caries, and recalcitrant infections [13-16]. Their combined pathogenicity is amplified by biofilm formation [17], where quorum sensing (QS), a density-dependent communication system, plays a critical role in regulating microbial behavior [18]. QS involves small signaling molecules called autoinducers, which accumulate with microbial growth and modulate gene expression [19]. In C. albicans, farnesol serves as the primary QS molecule, inhibiting the transition from yeast to hyphae, a key virulence factor [20]. At high concentrations, farnesol suppresses hyphal formation, tissue invasion, and even induces apoptosis, although it does not affect preexisting hyphae [12]. A second QS molecule, tyrosol (a derivative of tyrosine), has also been identified [21]. Unlike farnesol, tyrosol shortens the lag phase of fungal growth and promotes germ tube formation, accelerating hyphal development [22]. Thus, C. albicans morphogenesis is finely regulated by the opposing effects of tyrosol (stimulatory) and farnesol (inhibitory) [22].
This review examines the interactions between S. mutans and C. albicans in the pathogenesis of dental caries, with a focus on their combined effects on tooth demineralization, EPS production, and the regulation of virulence genes, both individually and in coaggregation. Relevant studies published within the last ten years were identified through systematic searches in Google Scholar, PubMed, and Scopus, using the following keywords: Streptococcus mutans, Candida albicans, tooth demineralization, polysaccharide production, expression of virulence genes, and dental caries. Articles were initially screened by title, followed by abstract review and full-text assessment. Only studies that specifically investigated the virulence mechanisms of these microorganisms and their interactions were included. To guide the selection process, we applied the PICO framework as follows: Population (P): In vitro and in vivo studies involving S. mutans, C. albicans, or both in coaggregation; Intervention (I): Evaluation of virulence traits, including biofilm formation, EPS production, gene expression, and demineralization capacity; Comparison (C): Analyses comparing the behavior of individual species versus their interactions; Outcome (O): Increased cariogenic potential, characterized by enhanced virulence factor expression and greater structural impact on dental tissues.
Review
Exploring the virulence mechanisms of S. mutans and C. albicans
The Streptococci genus comprises spherical or oval Gram-positive bacteria (0.5-0.75 μm in diameter) that typically arrange in chains [23]. As facultative anaerobes of the phylum Firmicutes, most streptococcal strains are α-hemolytic or nonhemolytic [24]. These opportunistic pathogens are globally distributed, affecting populations across diverse ethnic, geographic, and socioeconomic backgrounds, underscoring their pandemic nature [25]. Its pathogenicity is largely influenced by the production of organic acids (e.g., lactic, formic, acetic, and propionic acids), which contribute to pH reduction and enhance biofilm virulence [26]. The acid environment promoted by S. mutans generates rapid pH modulations on the biofilm, which can drop the pH from neutral to acidic in less than 20 min [27]. The microorganisms’ ability to thrive in acidic environments results from the acid tolerance response, which is the capacity to adjust to acidic stress through previous exposure to low or sub-lethal pH levels [27].
The synthesis of exopolysaccharides is a critical virulence factor of S. mutans. These polysaccharides promote bacterial adhesion to tooth surfaces, including smooth ones, contribute to the structural integrity of biofilms, and modify their porosity. Such changes facilitate sugar diffusion within the biofilm matrix, leading to more pronounced pH reductions [28]. Acidification of the polysaccharide-rich matrix favors the growth of microorganisms that are tolerant to acid stress and promotes demineralization of dental enamel [26,29]. S. mutans produces three types of GTFs (GtfB, GtfC, and GtfD) encoded by the gtfB, gtfC, and gtfD genes, respectively, whose cooperative action is essential for bacterial cell adherence with strong sucrose dependence [30]. These enzymes exhibit distinct functions: GtfB produces water-insoluble glucans, GtfC produces water-insoluble and water-soluble glucans, while GtfD exclusively generates water-soluble glucans [28]. When excess sugar is available, S. mutans produces organic acids and extracellular matrix (ECM) and synthesizes intracellular glycogen-like polysaccharides that serve as carbohydrate reserves and help reduce osmotic stress [31].
S. mutans is classified into four serotypes (c, e, f, and k) based on the chemical composition of their cell surface rhamnose-glucose polymers [32]. These serotypes differ in their surface antigens, which enable bacterial adhesion to dental structures like enamel and dentin, thereby facilitating colonization [33]. The biosynthesis of rhamnose-glucose polysaccharide (RGP), a major cell wall component, is an important surface antigen that contributes to the bacterium’s structural identity and forms the basis for serotype classification [34]. Serotype variation arises from cell surface polysaccharide composition differences, which are mediated by enzymes like RgpG [34]. Among these, serotype c is most prevalent in human populations [35], while serotypes e, f, and k show more restricted geographic distributions [36]. This genetic and structural diversity influences virulence traits, including oral surface adherence, biofilm formation, and interactions with other oral microbiota members [33].
Certain strains of S. mutans express collagen-binding proteins, such as Cnm and Cbm, which promote adhesion to collagen-rich surfaces like dentin [18]. In addition to contributing to colonization and pathogenicity in carious lesions, these proteins also modulate interspecies interactions, particularly with C. albicans [14].
C. albicans is a natural oral cavity resident that can transition from commensal to pathogenic under immunosuppression or host environment changes, leading to various infections [37]. First identified microscopically in thrush swabs by von Langenbeck in 1839 [38], C. albicans has since been recognized as a major pathogenic yeast in human infections. Its morphological versatility - existing as yeast, pseudohyphae, or hyphae - significantly contributes to pathogenesis, with hyphae enabling tissue penetration while yeast forms facilitate dissemination and less invasive infections [39].
C. albicans biofilm development occurs through four phases: adherence, initiation, maturation, and dispersal. During adherence, hydrophobic yeast-form planktonic cells attach to substrates, colonizing hard and mucosal surfaces [39]. The initiation phase sees microcolony formation, germ tube production, and early ECM synthesis. As biofilms mature, cells undergo morphological transitions that enhance ECM production [40]. This ECM comprises polysaccharides, proteins, extracellular DNA (eDNA), and other essential components that are crucial for the integrity of microorganisms and the resistance of biofilms. The accumulation of these components confers increased resistance to antimicrobial agents and host immune defenses [40].
This biofilm-forming capacity and metabolic adaptability enable C. albicans to colonize diverse body regions and evade host immune responses [39]. In the oral cavity, C. albicans co-adhesion with bacteria is essential for biofilm colonization and persistence [41]. The fungus provides adhesion sites and growth-stimulating factors for bacteria, which in turn produce lactate that serves as a carbon source for yeast proliferation [42]. Figure 1 shows an image obtained via scanning electron microscopy, showing that C. albicans provides adhesion sites for S. mutans.
Figure 1. Scanning electron micrograph showing co-adhesion of Streptococcus mutans with Candida albicans.
Image acquired by Wanessa Fernandes Matias Regis
Synergistic interactions between S. mutans and C. albicans in biofilm acidogenesis and dental tissue demineralization
The development of dental caries is fundamentally linked to the production of acid within microbial biofilms. Carbohydrate fermentation by cariogenic microorganisms generates acids that lower biofilm pH [27], disrupting the demineralization-remineralization balance and leading to dental tissue breakdown when sustained over time [43]. Frequent sugar consumption exacerbates this process by creating a dysbiotic plaque environment, where acid-tolerant species, such as S. mutans and C. albicans, dominate [43].
C. albicans, an acidogenic and fermentative microorganism with cellular hydrophobicity, has been identified as a significant caries risk factor [13]. When coexisting with S. mutans, C. albicans increases sucrose metabolism and biofilm acidogenicity, rendering an acidic environment with a pH below 5.5 [13]. Although C. albicans lacks GTF enzymes for sucrose metabolism, it can metabolize fructose, glucose, and lactose to produce short-chain carboxylic acids, which reduce environmental pH [44], thereby facilitating hard tissue demineralization [45]. In contrast, S. mutans can hydrolyze sucrose into fructose and glucose, enabling cross-feeding interactions [46].
The cariogenic potential of these microorganisms is particularly concerning given C. albicans’ ability to colonize various dental surfaces, including enamel, dentin, and cementum, with penetration into dentin tubules [47]. Dual-species biofilms of S. mutans and C. albicans produce more numerous and severe smooth-surface lesions than single-species infections [48]. This enhanced virulence stems from several synergistic mechanisms: S. mutans excretes lactate, which serves as a carbon source for yeast growth while creating the low-oxygen tension preferred by Streptococci [41]. Additionally, the catalase produced by C. albicans improves S. mutans’ oxidative stress tolerance, promoting mutual survival within the biofilm [49].
Comparative studies demonstrate that dual-species biofilms cause greater enamel demineralization than S. mutans monocultures [13], with their synergistic interactions accelerating acid production and caries progression [43]. C. albicans plays a crucial role in biofilm maturation and maintaining the acidic environment necessary for enamel demineralization [13], although its cariogenic potential varies depending on strain characteristics and experimental conditions.
The ECM of dual-species biofilms exhibits significant differences from those of single-species communities. C. albicans presence increases EPS production, resulting in greater biomass and higher S. mutans cell counts [8]. The interaction between C. albicans cell wall components and S. mutans-produced EPS enhances biofilm robustness on both enamel and dentin, thereby increasing the potential for tissue demineralization [46].
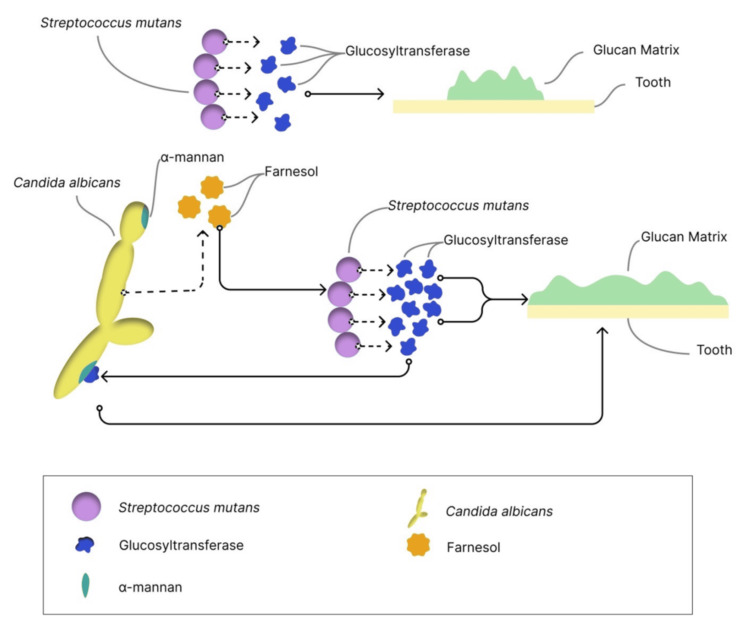
The glucan matrix, a key component of cariogenic biofilms, provides microbial adhesion sites, mechanical stability, and acidic microenvironments [50,51]. Both organisms contribute to this sticky matrix: S. mutans through GTF-mediated glucan synthesis from sucrose [31], and C. albicans through cell wall α-mannans that may facilitate Gtf binding and glucan matrix formation [52]. This protective matrix shields microorganisms from antimicrobial agents and host defenses [7]. Figure 2 provides an overview of the key pathogenic mechanisms employed by S. mutans and C. albicans in forming the glucan matrix.
Figure 2. Overview of the key pathogenic mechanisms involved in the production of the glucan matrix by Streptococcus mutans and Candida albicans.
S. mutans synthesizes the glucan matrix through the activity of its Gtfs enzymes. The presence of C. albicans enhances this process through interaction between C. albicans α-mannan and S. mutans Gtfs, thereby promoting increased matrix formation. Fungal-derived farnesol can also upregulate gtfB expression, thereby enhancing glucan production and biofilm matrix formation.
Gtf: glucosyltransferase
Image created by Wanessa Fernandes Matias Regis
The interaction between C. albicans and S. mutans appears to be highly context-dependent, with studies revealing synergistic and antagonistic dynamics. Sampaio et al. [53] demonstrated a synergistic relationship, showing that C. albicans enhances the cariogenic potential of S. mutans by increasing dentine demineralization within dual-species biofilms. Similarly, Klinke et al. [54] supported the cariogenic role of C. albicans, highlighting its capacity to produce acid from various carbohydrates, thereby contributing to the acidic microenvironment essential for tooth demineralization. In contrast, Dos Santos et al. [55] reported an antagonistic interaction in vivo, where secreted products from S. mutans inhibited C. albicans hyphal formation and reduced its virulence. Similarly, Barbosa et al. [56] suggest S. mutans has an attenuating effect on the virulence of C. albicans. These contrasting findings suggest that the outcome of S. mutans-C. albicans interactions may vary depending on environmental conditions, host context, and microbial composition, underscoring the complexity of their role in oral diseases.
The cariogenic potential of S. mutans is deeply rooted in its genomic repertoire, with complete genome sequencing of strains UA159, NN2025, LJ23, and GS-5 revealing key virulence determinants [57]. Among these, proteins harboring the LPXTG motif (leucine-proline-any amino acid-threonine-glycine) are critical for sortase-mediated anchoring to the bacterial cell wall, facilitating adhesion to tooth enamel, biofilm formation, and immune evasion [58,59]. Six conserved LPXTG-anchored proteins - Pac, FruA, DexA, GbpC, WapA, and WapE - further underscore the adaptability of S. mutans across different strains [56]. Major surface antigens, including Gtfs and glucan-binding proteins (Gbps), play pivotal roles in biofilm matrix assembly and caries pathogenesis [31,60].
The symbiotic relationship between bacteria and fungi within biofilms is mediated by extracellular signaling molecules and intercellular physical interactions, which facilitate the establishment and growth of cariogenic biofilms, accelerating caries development [61]. Notably, the co-aggregation of S. mutans and C. albicans enhances biofilm stability and acidogenicity, exacerbating caries progression. Frequent sugar consumption further amplifies acid production, while C. albicans secretes collagen-degrading enzymes under acidic conditions, intensifying biofilm virulence [46]. Acid production from carbohydrate fermentation by cariogenic microorganisms is a key driver of dental caries, as it reduces biofilm pH and disrupts the demineralization-remineralization equilibrium [27,43]. This acidogenic shift promotes a dysbiotic plaque environment, favoring the growth of acid-tolerant species. In the presence of S. mutans and C. albicans, enhanced acid production leads to a significant drop in biofilm pH, often to around 4.0-4.5 within 24 hours, which in turn promotes enamel demineralization and consequent calcium ion release from hydroxyapatite [41,62]. This synergistic interaction accelerates the cariogenic process.
Virulence gene expression and cross-kingdom interactions in dual-species cariogenic biofilms of S. mutans and C. albicans
S. mutans exhibits a symbiotic yet competitive relationship with C. albicans in mixed-species biofilms. GtfB binds strongly to α-mannan on the C. albicans cell surface, facilitating coaggregation and synergistic biofilm development [52]. This interaction is further modulated by fungal-derived farnesol, a QS molecule that, at high concentrations, disrupts S. mutans glycolytic activity and membrane permeability, reducing bacterial fitness [61]. Paradoxically, farnesol also upregulates gtfB expression, enhancing glucan production and biofilm matrix formation [44]. Moreover, S. mutans GtfB stimulates the expression of C. albicans adhesin genes (hwp1, als1, and als3), promoting fungal accumulation within biofilms [63].
Previous studies highlight the role of S. mutans membrane vesicles (MVs) in cross-kingdom interactions. MVs enriched with Gtfs integrate into C. albicans biofilms, boosting EPS production and upregulating fungal biofilm-related genes [64]. In contrast, MVs derived from ΔgtfBC mutants, which have the gtfB and gtfC genes deleted, fail to enhance biofilm formation, highlighting the crucial role of these genes in the biofilm development process [65].
The regulatory dynamics between these species are complex. The S. mutans VicRK two-component system, which plays a critical role in sensing environmental stress and regulating biofilm formation, is upregulated in the presence of C. albicans, resulting in increased EPS production and heightened cariogenic potential [66]. On the fungal side, C. albicans expresses Hwp1, a hyphal adhesin essential for epithelial attachment and biofilm stability, which interacts with S. mutans GtfB to enhance coaggregation [67,68]. Additional physical interactions, such as the binding of S. mutans to C. albicans hyphae via SspA/B proteins, further reinforce the structural and functional integrity of the mixed-species biofilm, with implications for increased tissue invasion and collagen degradation [44]. Tao et al. [69] demonstrated that S. mutans also modulates C. albicans morphology by secreting mutanocyclin, a tetramic acid compound that inhibits fungal filamentation via the Ras1-cAMP/PKA signaling pathway, specifically targeting the Tpk2 subunit. Their findings also identified key transcriptional regulators - Sfl1, Ahr1, Nrg1, and Fcr1 - as critical mediators of this suppressive effect, highlighting the genetic basis of cross-kingdom antagonism. Moreover, the VicRK two-component system, conserved in both S. mutans and C. albicans, acts as a global regulator of stress responses, adhesion, and biofilm development [4,70]. In C. albicans, VicRK controls genes involved in hyphal formation and ECM production, further contributing to biofilm architecture and virulence [70]. Although hyphal structures are known to enhance biofilm integrity, their precise role in facilitating cross-kingdom interactions remains to be fully elucidated. In parallel, S. mutans expresses a group of surface proteins containing the LPXTG motif, a conserved amino acid sequence that enables anchoring of proteins to the bacterial cell wall. These anchored proteins, particularly GtfB, play a key role in enhancing biofilm cohesion and mediating physical interactions with C. albicans [33].
Key fungal genes, including als1, als3, and hwp1, contribute to adhesion and biofilm formation, with potential modulation by S. mutans-derived factors [68]. Farnesol sensitizes S. mutans to oxidative stress, downregulating virulence genes, while C. albicans upregulate vicR and vicK in S. mutans, enhancing EPS biosynthesis and cariogenicity [71]. The hwp1 gene further amplifies coaggregation through interactions with GtfB. Lu et al. [72] demonstrated that RNase III family coding genes in S. mutans significantly modulate dual-species biofilm development. Disruption of these genes altered the expression of QS and stress-response pathways, thereby attenuating biofilm formation. Feldman et al. [73] further characterized the transcriptional impact of pharmacological intervention using thiazolidinedione-8, a QS inhibitor targeting C. albicans. They observed altered expression of key virulence genes in both C. albicans and S. mutans, including downregulation of genes involved in adhesion (e.g., ALS3) and EPS production (gtfB), weakening the structural and cooperative integrity of the biofilm. In line with this, Bachtiar and Bachtiar [74] used qPCR to evaluate gene expression profiles in dual-species biofilms from clinical samples. They reported that co-culture conditions enhanced the expression of S. mutans gtfB and gtfC, along with increased transcription of C. albicans adhesion-associated genes such as HWP1 and ALS1. This upregulation reflects a coordinated transcriptional response that reinforces biofilm cohesion and virulence. Environmental context also influences gene expression.
Guo et al. [75] revealed that eDNA, a key structural component of the ECM, modulates biofilm development by influencing the transcription of biofilm- and stress-related genes in both organisms. Their study pointed to increased expression of gtfB and gtfC in the presence of eDNA, suggesting that extracellular cues contribute to transcriptional regulation and adaptive responses within the biofilm. Kim et al. [62] found that C. albicans strains deficient in genes involved in N- and O-linked mannan biosynthesis showed significantly reduced binding of S. mutans GtfB, impairing S. mutans’ ability to anchor its EPS onto C. albicans, weakening biofilm development, and highlighting the crucial role of gene-regulated receptor-ligand interactions in cross-kingdom synergy. Wu et al. [76] proteomic analysis revealed that 170 proteins in C. albicans were significantly altered upon exposure to S. mutans MVs, with 73 upregulated and 97 downregulated. Gene ontology and KEGG (Kyoto Encyclopedia of Genes and Genomes) pathway analyses indicated that these proteins primarily involve oxidation-reduction processes and galactose metabolism. Metabolomic profiling further confirmed that S. mutans MVs increased the expression of metabolites related to carbohydrate metabolism in C. albicans.
While several studies, including those by Ikono et al. [77] and Li et al. [78], evaluated the effects of antimicrobial agents such as nanochitosan and curcumin, these investigations did not extensively explore gene expression changes. However, their reduced biofilm biomass and microbial viability findings imply indirect transcriptional repression of genes associated with adhesion, EPS synthesis, and stress tolerance. The impact of probiotics on gene expression within cross-kingdom biofilms is also noteworthy. Bao et al. [79] and Huang et al. [80] demonstrated that Lactobacillus plantarum downregulates key virulence and adhesion genes in S. mutans and C. albicans in a dose-dependent manner. These observations suggest modulation of transcriptional responses as part of the biofilm-inhibitory mechanism.
These studies highlight the pivotal role of gene regulation in the formation, stability, and pathogenicity of S. mutans-C. albicans dual-species biofilms. Their coaggregation is orchestrated by a complex network of shared virulence factors, reciprocal gene regulation, and synergistic biofilm development, collectively enhancing their cariogenic potential. Although considerable progress has been made in unraveling these interactions, particularly involving GtfB, farnesol, and adhesins, further investigation is needed to fully define the molecular mechanisms underlying their pathogenic synergy.
Table 1 summarizes the virulence mechanisms of S. mutans and C. albicans in single-species and dual-species contexts, focusing on demineralization capacity, EPS production, and biofilm architecture.
Table 1. Summary of virulence mechanisms of SM and CA in single- and dual-species biofilms, focusing on demineralization, extracellular polysaccharides production, and biofilm architecture.
+: low production; ++: medium production; +++: high production
CA: Candida albicans; Ins: insoluble polysaccharides; NA: not applicable; SM: Streptococcus mutans; Sol: soluble polysaccharides
| Reference | Strain | Substrate | Demineralization | Extracellular polysaccharides | Biofilm Architecture | |||||||||||||
| SM | CA | SM | CA | SM+CA | SM | CA | SM+CA | SM | CA | SM+CA | ||||||||
| Surface | Inner | Surface | Inner | Surface | Inner | Sol | Ins | Sol | Ins | Sol | Ins | |||||||
| Falsetta et al. (2014) [41] | UA159 | SC5314 | Sprague Dawley rats | ++ | NA | + | NA | +++ | NA | ++ | ++ | + | + | +++ | +++ | ++ | + | +++ |
| Eidt et al. (2019) [43] | UA159 | ATCC 90028 | Bovine enamel | +++ | ++ | + | + | ++ | ++ | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| Khoury et al. (2020) [51] | UA159 | SC5314 | Human enamel | NA | NA | NA | NA | NA | NA | ++ | ++ | + | + | +++ | +++ | ++ | + | +++ |
| Sampaio et al. (2019) [53] | UA159 | ATCC 90028 | Bovine root dentine | ++ | NA | + | NA | +++ | NA | ++ | ++ | + | + | +++ | +++ | + | + | ++ |
| Dos Santos et al. (2020) [55] | UA159 | ATCC18804 | 96-well flat-bottom plates | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | ++ | ++ | + |
| Barbosa et al. (2016) [56] | UA159 | ATCC 18804 | In vitro | NA | NA | NA | NA | NA | NA | ++ | ++ | + | + | +++ | +++ | ++ | + | +++ |
| Kim et al. (2021) [62] | UA159 | SC5314 | Hydroxyapatite discs | NA | NA | NA | NA | NA | NA | ++ | ++ | + | + | +++ | +++ | ++ | + | +++ |
| Kim et al. (2021) [62] | UA159 | SC5314 | Human enamel | ++ | NA | NA | NA | +++ | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| Ellepola et al. (2017) [63] | UA159 | SC5314 | 96-well flat bottom plates | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | ++ | +++ |
| Wu et al. (2020) [64] | UA159 | SC5314 | Culture plates | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | + | ++ |
| Yin et al. (2022) [65] | UA159 | SC5314 | 96-well flat-bottom plates | NA | NA | NA | NA | NA | NA | NA | ++ | NA | NA | NA | +++ | ++ | + | +++ |
| Martorano-Fernandes et al. (2023) [68] | UA159 | SC5314 | 96-well flat bottom plates | NA | NA | NA | NA | NA | NA | ++ | ++ | + | + | +++ | +++ | + | + | +++ |
| Wu et al. (2022) [76] | UA159 | SC5314 | Bovine coronal dentine | NA | NA | + | NA | ++ | NA | NA | NA | NA | NA | NA | NA | NA | + | ++ |
| Ikono et al. (2019) [77] | ATCC 25175 | ATCC 10231 | 96-well flat-bottom plates | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | ++ | + | +++ |
Table 2 summarizes the differential expression of key virulence genes in S. mutans and C. albicans during the formation of dual-species biofilms.
Table 2. Modulation of specific virulence genes that are upregulated or downregulated by SM and CA, along with their interactions.
+: low production; ++: medium production; +++: high production
CA: Candida albicans; NA: not applicable; SM: Streptococcus mutans
| Reference | Strain | Gene of virulence | |||||
| SM | CA | Molecule | Upregulation | Downregulation | |||
| SM | SM | SM | SM | ||||
| Dos Santos et al. (2020) [55] | UA159 | ATCC18804 | Supernatant of SM | NA | ywp1 | NA | cph1, efg1, hwp1 |
| Ellepola et al. (2017) [63] | UA159 | SC5314 | gtfB | NA | hwp1, als1, als3 | NA | NA |
| Yin et al. (2022) [65] | UA159 | SC5314 | Caffeic acid phenethyl ester | NA | NA | gtfB, gtfC, gtfD | NA |
| Tao et al. (2017) [69] | UA159 | SC5314 | Mutanocyclin | NA | NA | NA | hwp1, ece1, flo8 tec1; ihd1, hyrt, rbt5, rhd3; csa1; rbe1; als; cht2, cht3, chs1 |
| Lu et al. (2022) [72] | UA159 | SC5314 | NA | dcr1 | ndt80, efg1, brg1 | NA | NA |
| Feldman et al. (2016) [73] | UA159 | SC5314 | Thiazolidinedione-8 | gtfB, gtfC, gtfD, gbpB, brpA, luxS, nox, sodA | NA | NA | hwp1, csh1, als3, sod1, sod2, cat1, |
| Bachtiar and Bachtiar (2018) [74] | UA159 | SC5314 | NA | gtfB | NA | NA | NA |
| Guo et al. (2021) [75] | UA159 | SC5314 | eDNA | gtfC | spaP, eap1, hwp1, cdr2 | NA | als3 |
| Li et al. (2019) [78] | UA159 | SC5314 | Curcumin | NA | NA | gtfB, gtfC, gbpB, comC, comD, comE | als1, als3 |
| Bao et al. (2023) [79] | UA159 | SC5314 | Lactobacillus plantarum | atpD, eno | cht2 | lacC, lacG | hwp1, ece |
| Huang et al. (2023) [80] | UA159 | SC5314 | Galacto-oligosaccharide | atpD, eno, lacC, lacG | NA | NA | hwp1, ece |
Conclusions
The synergistic relationship between S. mutans and C. albicans represents a critical pathogenic axis in developing dental caries, with GTFs and farnesol-mediated signaling emerging as promising therapeutic targets. While these molecular interactions offer compelling opportunities for disrupting biofilm integrity, substantial knowledge gaps remain in three key areas: (1) the precise mechanistic role of C. albicans in caries progression; (2) strain-specific differences in fungal virulence; and (3) the clinical relevance and translatability of current in vitro findings. Addressing these gaps will require innovative strategies, including creating advanced polymicrobial biofilm models that more accurately replicate the oral microenvironment and exploring combination therapies targeting both bacterial exopolysaccharide synthesis and fungal adhesion mechanisms. By closing these gaps, we can transform mechanistic insights into precision-based interventions for caries prevention.
Acknowledgments
The authors would like to thank the support of the Central Analítica-UFC/CT-INFRA/MCTI-SISANO/Pró-Equipamentos CAPES.
Funding Statement
This study was funded by the Brazilian National Council for Scientific and Technological Development (CNPq) (grant number 409296/2021-0).
Disclosures
Conflicts of interest: In compliance with the ICMJE uniform disclosure form, all authors declare the following:
Payment/services info: This study was funded by the Brazilian National Council for Scientific and Technological Development (CNPq) (grant number 409296/2021-0).
Financial relationships: All authors have declared that they have no financial relationships at present or within the previous three years with any organizations that might have an interest in the submitted work.
Other relationships: All authors have declared that there are no other relationships or activities that could appear to have influenced the submitted work.
Author Contributions
Acquisition, analysis, or interpretation of data: Beatriz Panariello, Wanessa Fernandes Matias Regis, Francisco Ruliglésio Rocha, Ramille Araújo Lima, Anderson da Cunha Costa, Simone Duarte, Raimunda Sâmia Nogueira Brilhante, Lidiany Karla Azevedo Rodrigues
Critical review of the manuscript for important intellectual content: Beatriz Panariello, Wanessa Fernandes Matias Regis, Francisco Ruliglésio Rocha, Ramille Araújo Lima, Anderson da Cunha Costa, Simone Duarte, Raimunda Sâmia Nogueira Brilhante, Lidiany Karla Azevedo Rodrigues
Concept and design: Wanessa Fernandes Matias Regis, Raimunda Sâmia Nogueira Brilhante, Lidiany Karla Azevedo Rodrigues
Drafting of the manuscript: Wanessa Fernandes Matias Regis, Raimunda Sâmia Nogueira Brilhante, Lidiany Karla Azevedo Rodrigues
Supervision: Lidiany Karla Azevedo Rodrigues
References
- 1.A multispecies biofilm in vitro screening model of dental caries for high-throughput susceptibility testing. Heersema LA, Smyth HD. High Throughput. 2019;8:14. doi: 10.3390/ht8020014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Root surface caries - rationale behind good diagnostic practice. Fejerskov O, Nyvad B. Monogr Oral Sci. 2017;26:43–54. doi: 10.1159/000479306. [DOI] [PubMed] [Google Scholar]
- 3.Global burden of oral conditions in 1990-2010: a systematic analysis. Marcenes W, Kassebaum NJ, Bernabé E, Flaxman A, Naghavi M, Lopez A, Murray CJ. J Dent Res. 2013;92:592–597. doi: 10.1177/0022034513490168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Streptococcus mutans: a new Gram-positive paradigm? Lemos JA, Quivey RG, Koo H, Abranches J. Microbiology (Reading) 2013;159:436–445. doi: 10.1099/mic.0.066134-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Oral biofilms: pathogens, matrix, and polymicrobial interactions in microenvironments. Bowen WH, Burne RA, Wu H, Koo H. Trends Microbiol. 2018;26:229–242. doi: 10.1016/j.tim.2017.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Biology of Streptococcus mutans-derived glucosyltransferases: role in extracellular matrix formation of cariogenic biofilms. Bowen WH, Koo H. Caries Res. 2011;45:69–86. doi: 10.1159/000324598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Binding force dynamics of Streptococcus mutans-glucosyltransferase B to Candida albicans. Hwang G, Marsh G, Gao L, Waugh R, Koo H. J Dent Res. 2015;94:1310–1317. doi: 10.1177/0022034515592859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Antifungal drug resistance: molecular mechanisms in Candida albicans and beyond. Lee Y, Puumala E, Robbins N, Cowen LE. Chem Rev. 2021;121:3390–3411. doi: 10.1021/acs.chemrev.0c00199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cell wall mannan and cell surface hydrophobicity in Candida albicans serotype A and B strains. Masuoka J, Hazen KC. Infect Immun. 2004;72:6230–6236. doi: 10.1128/IAI.72.11.6230-6236.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Candida albicans pathogenicity mechanisms. Mayer FL, Wilson D, Hube B. Virulence. 2013;4:119–128. doi: 10.4161/viru.22913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Candida albicans morphogenesis and host defence: discriminating invasion from colonization. Gow NA, van de Veerdonk FL, Brown AJ, Netea MG. Nat Rev Microbiol. 2011;10:112–122. doi: 10.1038/nrmicro2711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Biofilm formation by Candida albicans and Streptococcus mutans in the presence of farnesol: a quantitative evaluation. Fernandes RA, Monteiro DR, Arias LS, Fernandes GL, Delbem AC, Barbosa DB. Biofouling. 2016;32:329–338. doi: 10.1080/08927014.2016.1144053. [DOI] [PubMed] [Google Scholar]
- 13.Candida albicans stimulates Streptococcus mutans microcolony development via cross-kingdom biofilm-derived metabolites. Kim D, Sengupta A, Niepa TH, et al. Sci Rep. 2017;7:41332. doi: 10.1038/srep41332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Association of Candida albicans and Cbp+ Streptococcus mutans with early childhood caries recurrence. Garcia BA, Acosta NC, Tomar SL, Roesch LF, Lemos JA, Mugayar LR, Abranches J. Sci Rep. 2021;11:10802. doi: 10.1038/s41598-021-90198-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dual-species biofilms of Streptococcus mutans and Candida albicans exhibit more biomass and are mutually beneficial compared with single-species biofilms. Lobo CI, Rinaldi TB, Christiano CM, De Sales Leite L, Barbugli PA, Klein MI. J Oral Microbiol. 2019;11:1581520. doi: 10.1080/20002297.2019.1581520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Candida albicans induces oral microbial dysbiosis and promotes oral diseases. Kashyap B, Padala SR, Kaur G, Kullaa A. Microorganisms. 2024;12:2138. doi: 10.3390/microorganisms12112138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Effect of curcumin-mediated photodynamic therapy on Streptococcus mutans and Candida albicans: a systematic review of in vitro studies. Picco DC, Cavalcante LL, Trevisan RL, Souza-Gabriel AE, Borsatto MC, Corona SA. Photodiagnosis Photodyn Ther. 2019;27:455–461. doi: 10.1016/j.pdpdt.2019.07.010. [DOI] [PubMed] [Google Scholar]
- 18.Quorum sensing in bacteria. Miller MB, Bassler BL. Annu Rev Microbiol. 2001;55:165–199. doi: 10.1146/annurev.micro.55.1.165. [DOI] [PubMed] [Google Scholar]
- 19.Effect of bioactive compounds on the regulation of quorum sensing network-associated genes and virulence in Streptococcus mutans—a systematic review. Rocha FR, Regis WF, Duarte S, Muniz FW, Rodrigues LK. Arch Oral Biol. 2020;119:104893. doi: 10.1016/j.archoralbio.2020.104893. [DOI] [PubMed] [Google Scholar]
- 20.Quorum sensing in the dimorphic fungus Candida albicans is mediated by farnesol. Hornby JM, Jensen EC, Lisec AD, et al. Appl Environ Microbiol. 2001;67:2982–2992. doi: 10.1128/AEM.67.7.2982-2992.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tyrosol is a quorum-sensing molecule in Candida albicans. Chen H, Fujita M, Feng Q, Clardy J, Fink GR. Proc Natl Acad Sci U S A. 2004;101:5048–5052. doi: 10.1073/pnas.0401416101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Production of tyrosol by Candida albicans biofilms and its role in quorum sensing and biofilm development. Alem MA, Oteef MD, Flowers TH, Douglas LJ. Eukaryot Cell. 2006;5:1770–1779. doi: 10.1128/EC.00219-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.On the bacterial factor in the aetiology of dental caries. Clarke JK. https://pmc.ncbi.nlm.nih.gov/articles/PMC2047899/ Br J Exp Pathol. 1924;5:141–147. [Google Scholar]
- 24.Cross-feeding and interkingdom communication in dual-species biofilms of Streptococcus mutans and Candida albicans. Sztajer H, Szafranski SP, Tomasch J, Reck M, Nimtz M, Rohde M, Wagner-Döbler I. ISME J. 2014;8:2256–2271. doi: 10.1038/ismej.2014.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Spolidorio DM, Duque C. São Paulo, Brazil: Artes Médicas Editora; 2013. Microbiology and General and Dental Immunology, Volume 2 [Portuguese] [Google Scholar]
- 26.Frequency of sucrose exposure on the cariogenicity of a biofilm-caries model. Díaz-Garrido N, Lozano C, Giacaman RA. Eur J Dent. 2016;10:345–350. doi: 10.4103/1305-7456.184163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Acid tolerance mechanisms utilized by Streptococcus mutans. Matsui R, Cvitkovitch D. Future Microbiol. 2010;5:403–417. doi: 10.2217/fmb.09.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Regulatory mechanisms of exopolysaccharide synthesis and biofilm formation in Streptococcus mutans. Zheng T, Jing M, Gong T, et al. J Oral Microbiol. 2023;15:2225257. doi: 10.1080/20002297.2023.2225257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Streptococcus mutans-derived extracellular matrix in cariogenic oral biofilms. Klein MI, Hwang G, Santos PH, Campanella OH, Koo H. Front Cell Infect Microbiol. 2015;5:10. doi: 10.3389/fcimb.2015.00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Molecular mechanisms of inhibiting glucosyltransferases for biofilm formation in Streptococcus mutans. Zhang Q, Ma Q, Wang Y, Wu H, Zou J. Int J Oral Sci. 2021;13:30. doi: 10.1038/s41368-021-00137-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.The biology of Streptococcus mutans. Lemos JA, Palmer SR, Zeng L, et al. Microbiol Spectr. 2019;7 doi: 10.1128/microbiolspec.gpp3-0051-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Demonstration of Streptococcus mutans with a cell wall polysaccharide specific to a new serotype, k, in the human oral cavity. Nakano K, Nomura R, Nakagawa I, Hamada S, Ooshima T. J Clin Microbiol. 2004;42:198–202. doi: 10.1128/JCM.42.1.198-202.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Role of Streptococcus mutans surface proteins for biofilm formation. Matsumoto-Nakano M. Jpn Dent Sci Rev. 2018;54:22–29. doi: 10.1016/j.jdsr.2017.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.A novel gene required for rhamnose-glucose polysaccharide synthesis in Streptococcus mutans. Yamashita Y, Shibata Y, Nakano Y, Tsuda H, Kido N, Ohta M, Koga T. J Bacteriol. 1999;181:6556–6559. doi: 10.1128/jb.181.20.6556-6559.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Streptococcus mutans serotypes and collagen-binding proteins Cnm/Cbm in children with caries analysed by PCR. Momeni SS, Ghazal T, Grenett H, Whiddon J, Moser SA, Childers NK. Mol Oral Microbiol. 2019;34:64–73. doi: 10.1111/omi.12254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Streptococcus mutans and Streptococcus sobrinus contributions in dental caries in Iranian and Afghan children: a report from serotype distribution and novel STs. Elyassi M, Babaeekhou L, Ghane M. Arch Oral Biol. 2022;139:105431. doi: 10.1016/j.archoralbio.2022.105431. [DOI] [PubMed] [Google Scholar]
- 37.Commensalism and pathogenesis of Candida albicans at the mucosal interface. Schille TB, Sprague JL, Naglik JR, Brunke S, Hube B. Nat Rev Microbiol. 2025 doi: 10.1038/s41579-025-01174-x. [DOI] [PubMed] [Google Scholar]
- 38.The first description of an oesophageal candidosis by Bernhard von Langenbeck in 1839. Knoke M, Bernhardt H. Mycoses. 2006;49:283–287. doi: 10.1111/j.1439-0507.2006.01237.x. [DOI] [PubMed] [Google Scholar]
- 39.Candida albicans—the virulence factors and clinical manifestations of infection. Talapko J, Juzbašić M, Matijević T, Pustijanac E, Bekić S, Kotris I, Škrlec I. J Fungi (Basel) 2021;7:79. doi: 10.3390/jof7020079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Biofilm of Candida albicans: formation, regulation and resistance. Pereira R, Dos Santos Fontenelle RO, de Brito EH, de Morais SM. J Appl Microbiol. 2021;131:11–22. doi: 10.1111/jam.14949. [DOI] [PubMed] [Google Scholar]
- 41.Symbiotic relationship between Streptococcus mutans and Candida albicans synergizes virulence of plaque biofilms in vivo. Falsetta ML, Klein MI, Colonne PM, et al. Infect Immun. 2014;82:1968–1981. doi: 10.1128/IAI.00087-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Candida albicans biofilms and polymicrobial interactions. Ponde NO, Lortal L, Ramage G, Naglik JR, Richardson JP. Crit Rev Microbiol. 2021;47:91–111. doi: 10.1080/1040841X.2020.1843400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Role of Candida albicans on enamel demineralization and on acidogenic potential of Streptococcus mutans in vitro biofilms. Eidt G, Andrade CG, Negrini TC, Arthur RA. J Appl Oral Sci. 2019;27:0. doi: 10.1590/1678-7757-2018-0593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Human tooth as a fungal niche: Candida albicans traits in dental plaque isolates. Xiang Z, Wakade RS, Ribeiro AA, et al. mBio. 2023;14:0. doi: 10.1128/mbio.02769-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.The role of sucrose in cariogenic dental biofilm formation—new insight. Paes Leme AF, Koo H, Bellato CM, Bedi G, Cury JA. J Dent Res. 2006;85:878–887. doi: 10.1177/154405910608501002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Current and prospective therapeutic strategies: tackling Candida albicans and Streptococcus mutans cross-kingdom biofilm. Li Y, Huang S, Du J, Wu M, Huang X. Front Cell Infect Microbiol. 2023;13:1106231. doi: 10.3389/fcimb.2023.1106231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Management of Streptococcus mutans-Candida spp. oral biofilms' infections: paving the way for effective clinical interventions. Salehi B, Kregiel D, Mahady G, Sharifi-Rad J, Martins N, Rodrigues CF. J Clin Med. 2020;9:517. doi: 10.3390/jcm9020517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Streptococcus mutans, Candida albicans, and the human mouth: a sticky situation. Metwalli KH, Khan SA, Krom BP, Jabra-Rizk MA. PLoS Pathog. 2013;9:0. doi: 10.1371/journal.ppat.1003616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Catalase produced by Candida albicans protects Streptococcus mutans from H2O2 stress-one more piece in the cross-kingdom synergism puzzle. Katrak C, Garcia BA, Dornelas-Figueira LM, Nguyen M, Williams RB, Lorenz MC, Abranches J. mSphere. 2023;8:0. doi: 10.1128/msphere.00295-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Glycosyltransferase-mediated biofilm matrix dynamics and virulence of Streptococcus mutans. Rainey K, Michalek SM, Wen ZT, Wu H. Appl Environ Microbiol. 2019;85 doi: 10.1128/AEM.02247-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.The role of Candida albicans secreted polysaccharides in augmenting Streptococcus mutans adherence and mixed biofilm formation: in vitro and in vivo studies. Khoury ZH, Vila T, Puthran TR, Sultan AS, Montelongo-Jauregui D, Melo MA, Jabra-Rizk MA. Front Microbiol. 2020;11:307. doi: 10.3389/fmicb.2020.00307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Candida albicans mannans mediate Streptococcus mutans exoenzyme GtfB binding to modulate cross-kingdom biofilm development in vivo. Hwang G, Liu Y, Kim D, Li Y, Krysan DJ, Koo H. PLoS Pathog. 2017;13:0. doi: 10.1371/journal.ppat.1006407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Candida albicans increases dentine demineralization provoked by Streptococcus mutans biofilm. Sampaio AA, Souza SE, Ricomini-Filho AP, Del Bel Cury AA, Cavalcanti YW, Cury JA. Caries Res. 2019;53:322–331. doi: 10.1159/000494033. [DOI] [PubMed] [Google Scholar]
- 54.Acid production by oral strains of Candida albicans and lactobacilli. Klinke T, Kneist S, de Soet JJ, Kuhlisch E, Mauersberger S, Forster A, Klimm W. Caries Res. 2009;43:83–91. doi: 10.1159/000204911. [DOI] [PubMed] [Google Scholar]
- 55.Streptococcus mutans secreted products inhibit Candida albicans induced oral candidiasis. Dos Santos JD, Fugisaki LR, Medina RP, et al. Front Microbiol. 2020;11:1605. doi: 10.3389/fmicb.2020.01605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Streptococcus mutans can modulate biofilm formation and attenuate the virulence of Candida albicans. Barbosa JO, Rossoni RD, Vilela SF, et al. PLoS ONE. 2016;11:0. doi: 10.1371/journal.pone.0150457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Contribution of the interaction of Streptococcus mutans serotype k strains with fibrinogen to the pathogenicity of infective endocarditis. Nomura R, Otsugu M, Naka S, et al. Infect Immun. 2014;82:5223–5234. doi: 10.1128/IAI.02164-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sortases and the art of anchoring proteins to the envelopes of gram-positive bacteria. Marraffini LA, Dedent AC, Schneewind O. Microbiol Mol Biol Rev. 2006;70:192–221. doi: 10.1128/MMBR.70.1.192-221.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Discovery of myricetin as an inhibitor against Streptococcus mutans and an anti-adhesion approach to biofilm formation. Hu P, Lv B, Yang K, Lu Z, Ma J. Int J Med Microbiol. 2021;311:151512. doi: 10.1016/j.ijmm.2021.151512. [DOI] [PubMed] [Google Scholar]
- 60.Exopolysaccharides produced by Streptococcus mutans glucosyltransferases modulate the establishment of microcolonies within multispecies biofilms. Koo H, Xiao J, Klein MI, Jeon JG. J Bacteriol. 2010;192:3024–3032. doi: 10.1128/JB.01649-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Impact of farnesol and Corsodyl® on Candida albicans forming dual biofilm with Streptococcus mutans. Černáková L, Jordao L, Bujdáková H. Oral Dis. 2018;24:1126–1131. doi: 10.1111/odi.12873. [DOI] [PubMed] [Google Scholar]
- 62.Intervening in symbiotic cross-kingdom biofilm interactions: a binding mechanism-based nonmicrobicidal approach. Kim HE, Dhall A, Liu Y, Bawazir M, Koo H, Hwang G. mBio. 2021;12 doi: 10.1128/mBio.00651-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bacterial GtfB augments Candida albicans accumulation in cross-kingdom biofilms. Ellepola K, Liu Y, Cao T, Koo H, Seneviratne CJ. J Dent Res. 2017;96:1129–1135. doi: 10.1177/0022034517714414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Streptococcus mutans membrane vesicles harboring glucosyltransferases augment Candida albicans biofilm development. Wu R, Tao Y, Cao Y, Zhou Y, Lin H. Front Microbiol. 2020;11:581184. doi: 10.3389/fmicb.2020.581184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Caffeic acid phenethyl ester (CAPE) inhibits cross-kingdom biofilm formation of Streptococcus mutans and Candida albicans. Yin W, Zhang Z, Shuai X, Zhou X, Yin D. Microbiol Spectr. 2022;10:0. doi: 10.1128/spectrum.01578-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Candida albicans CHK1 gene regulates its cross-kingdom interactions with Streptococcus mutans to promote caries. Liu Y, Wang Z, Zhou Z, et al. Appl Microbiol Biotechnol. 2022;106:7251–7263. doi: 10.1007/s00253-022-12211-7. [DOI] [PubMed] [Google Scholar]
- 67.Function of Candida albicans adhesin Hwp1 in biofilm formation. Nobile CJ, Nett JE, Andes DR, Mitchell AP. Eukaryot Cell. 2006;5:1604–1610. doi: 10.1128/EC.00194-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Candida albicans adhesins Als1 and Hwp1 modulate interactions with Streptococcus mutans. Martorano-Fernandes L, Goodwine JS, Ricomini-Filho AP, Nobile CJ, Del Bel Cury AA. Microorganisms. 2023;11:1391. doi: 10.3390/microorganisms11061391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Streptococcus mutans suppresses filamentous growth of Candida albicans through secreting mutanocyclin, an unacylated tetramic acid. Tao L, Wang M, Guan G, et al. Virulence. 2022;13:542–557. doi: 10.1080/21505594.2022.2046952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.The VicRK two-component system regulates Streptococcus mutans virulence. Lei L, Long L, Yang X, et al. Curr Issues Mol Biol. 2019;32:167–200. doi: 10.21775/cimb.032.167. [DOI] [PubMed] [Google Scholar]
- 71.ALS3 expression as an indicator for candida albicans biofilm formation and drug resistance. Deng K, Jiang W, Jiang Y, Deng Q, Cao J, Yang W, Zhao X. Front Microbiol. 2021;12:655242. doi: 10.3389/fmicb.2021.655242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.RNase III coding genes modulate the cross-kingdom biofilm of Streptococcus mutans and Candida albicans. Lu Y, Lei L, Deng Y, et al. Front Microbiol. 2022;13:957879. doi: 10.3389/fmicb.2022.957879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Thiazolidinedione-8 alters symbiotic relationship in C. albicans-S. mutans dual species biofilm. Feldman M, Ginsburg I, Al-Quntar A, Steinberg D. Front Microbiol. 2016;7:140. doi: 10.3389/fmicb.2016.00140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Relationship between Candida albicans and Streptococcus mutans in early childhood caries, evaluated by quantitative PCR. Bachtiar EW, Bachtiar BM. F1000Res. 2018;7:1645. doi: 10.12688/f1000research.16275.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Effects of extracellular DNA on dual-species biofilm formed by Streptococcus mutans and Candida albicans. Guo H, Chen Y, Guo W, Chen J. Microb Pathog. 2021;154:104838. doi: 10.1016/j.micpath.2021.104838. [DOI] [PubMed] [Google Scholar]
- 76.Streptococcus mutans membrane vesicles enhance Candida albicans pathogenicity and carbohydrate metabolism. Wu R, Cui G, Cao Y, Zhao W, Lin H. Front Cell Infect Microbiol. 2022;12:940602. doi: 10.3389/fcimb.2022.940602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Nanochitosan antimicrobial activity against Streptococcus mutans and Candida albicans dual-species biofilms. Ikono R, Vibriani A, Wibowo I, et al. BMC Res Notes. 2019;12:383. doi: 10.1186/s13104-019-4422-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Assessing the impact of curcumin on dual-species biofilms formed by Streptococcus mutans and Candida albicans. Li X, Yin L, Ramage G, et al. Microbiologyopen. 2019;8:0. doi: 10.1002/mbo3.937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Dose-dependent inhibitory effect of probiotic Lactobacillus plantarum on Streptococcus mutans-Candida albicans cross-kingdom microorganisms. Bao J, Huang X, Zeng Y, et al. Pathogens. 2023;12:848. doi: 10.3390/pathogens12060848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Anti-cariogenic properties of Lactobacillus plantarum in the utilization of galacto-oligosaccharide. Huang X, Bao J, Zeng Y, et al. Nutrients. 2023;15:2017. doi: 10.3390/nu15092017. [DOI] [PMC free article] [PubMed] [Google Scholar]