Abstract
Postoperative pain (POP) is one of the leading clinical challenges of patients undergoing major surgeries, including abdominal surgeries. Opioid analgesics are considered the gold standard for POP. However, their use is associated with a high incidence of adverse events, including nausea and vomiting, which has prompted clinicians to look for alternative regimens that are not opioid-based. This review aims to provide an overview of the existing evidence regarding the effectiveness of non-opioid analgesics (NOAs) in the management of POP in patients who have undergone major abdominal surgeries.
A comprehensive search was conducted on four databases: Google Scholar, PubMed, CENTRAL, and Science Direct. The studies that met the inclusion criteria were then included in the review. The reported outcomes were pooled using the Review Manager software (RevMan 5.4, The Cochrane Collaboration, London, UK).
The literature search identified 657 articles, among which 18 were included in the review according to the inclusion criteria. Our study found that the mean postoperative opioid consumption was significantly lower among individuals treated with non-opioid analgesics than with opioid analgesics SMD -1.88; 95% CI (-2.40, -1.36); p < 0.0001). Further analysis showed that the mean opioid consumption was also lower in those who received NSAIDs and other atypical analgesics (SMD -2.24; 95% CI (-2.94, -1.55); p < 0.00001) and (SMD -1.18; 95% CI (-2.18, -0.17); p = 0.02), respectively. However, in those who received paracetamol, the mean opioid consumption was comparable to that of controls (SMD -1.09; 95% CI (-2.21, 0.03); p = 0.06). Secondly, our study found that the incidence of opioid-related nausea was reduced in patients who received NOA than in controls (OR 0.38; 95% CI (0.22, 0.66); p = 0.0005). However, the incidence of vomiting was equivalent across both groups (OR 0.64; 95% CI (0.39, 1.04); p = 0.07).
This study found that NOAs are good adjuvants in pain management in patients undergoing major abdominal surgery. They aid in reducing the dosage of opioids required for adequate analgesia and thus also reduce the incidence of some of the related adverse events.
Keywords: analgesia, non-opioid analgesics, (nsaid) non-steroidal anti-inflammatory drugs, postoperative pain, surgery, systematic review and meta analysis
Introduction and background
More than 200 million major surgeries are performed worldwide, of which about 20% are major abdominal surgeries [1]. Severe postoperative pain (POP) is one of the significant clinical challenges faced by clinicians and patients. Severe POP has been estimated to occur in about 20 to 40% of all patients who have undergone major surgery [2].
One of the mainstay management approaches for POP is the use of patient-controlled analgesia (PCA) to enable patients to self-administer morphine postoperatively [3]. This analgesic approach is the gold standard for alleviating POP in patients undergoing major surgery [4]. While morphine is regarded as a reference analgesic, it has its limitations, which have prompted clinicians to look for alternative analgesic approaches. These limitations include moderate efficacy in relieving pain when the patient moves and the incapacitating side effects associated with morphine use, such as nausea and vomiting. It may also be prudent to include gastrointestinal manifestations like gastroparesis, reduced bowel motility, and constipation [5].
One of the alternative analgesia strategies is the use of balanced analgesia instead of morphine alone [6]. This strategy, which was proposed more than three decades ago, aims to improve POP management while reducing the use of morphine and its associated side effects. [6] It entails the use of combinations of different analgesics, such as non-steroidal anti-inflammatory drugs (NSAIDs) and other weak opioids, either as monotherapy or in combination with morphine [7].
Different trials have investigated the efficacy of varying monotherapy regimens in managing POP after major surgeries. These trials have investigated the use of monotherapy with or without morphine compared to placebo on POP and postoperative nausea and vomiting (PONV). Furthermore, a summary of these trials has been conducted previously [8,9]. However, none of these meta-analyses focused on POP after major abdominal surgery. This review and meta-analysis, therefore, aim to determine the comparative efficacy of non-opioid analgesics in managing POP after major abdominal surgery.
Review
Methodology
Protocol and Registration
This review's methodology followed the PRISMA 2020 guidelines (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) [10]. However, the study protocol was not registered in any publicly available database.
Literature Search
Two authors conducted the process independently through a detailed and comprehensive literature search for relevant articles published until October 2024. The search was conducted from the inception of each database until October 2024. Only studies published in English were eligible for inclusion. No restrictions were applied to the publication date or study setting. The search strategy used specified search criteria for each of the four databases, i.e., PubMed, ScienceDirect, CENTRAL, and Google Scholar. Boolean operators ("AND" and "OR") were used to combine relevant keywords and MeSH terms. The full search for PubMed was: (Non-opioid) AND (Analgesics) AND (Acetaminophen OR Paracetamol OR Panadol) AND (NSAIDs OR COX-Inhibitors) AND (Pain OR POP) AND (Major Abdominal surgery). Following the database search, the reviewers manually screened the reference lists from the included studies to identify any additional relevant articles that may have been missed in the electronic search.
Eligibility Criteria
All studies from the three databases were evaluated based on predefined eligibility criteria. Those that fulfilled the inclusion requirements outlined below were selected for the review as described in Table 1.
Table 1. Inclusion and Exclusion Criteria for Study Selection.
| Domain | Inclusion Criteria | Exclusion Criteria |
| Population | Patients who had undergone major abdominal surgery, as defined by Earl (1917) [11]. | Patients who had undergone other types of major surgeries, such as cardiac or orthopedic surgeries. |
| Intervention | Studies investigating the use of non-opioid analgesics (including nonsteroidal anti-inflammatory drugs, nefopam, acetaminophen, cyclooxygenase-2 inhibitors, and metamizole) in combination with morphine administered solely via patient-controlled analgesia for at least 24 hours. | Studies that did not include non-opioid analgesics as one of the interventions. Studies involving continuous morphine infusion in addition to patient-controlled analgesia were excluded. |
| Comparison | Trials comparing non-opioid analgesics to placebo, different dosages, or other pharmacological drug classes. | — |
| Outcomes | Changes in morphine consumption and changes in postoperative pain intensity measured using the visual analog scale. | Studies that did not report any of the outcomes of interest. |
| Study Design | Randomized controlled trials evaluating non-opioid analgesics used either alone or in combination with other therapies. | Abstracts presented at conferences without full-text articles, single-arm studies, and secondary literature such as systematic reviews or editorial commentaries. |
Study Selection and Data Extraction
Study selection was carried out by independent reviewers in multiple phases. Initially, duplicate records were removed. This was followed by a title and abstract screening, and finally, a full-text review of the remaining articles. Abstracts of non-duplicate studies were first assessed against the inclusion criteria. If eligibility could not be determined based on the abstract alone, the full text was retrieved and reviewed.
In addition, the reviewers manually examined results from Phase III clinical trials to identify potentially eligible studies that had not yet been published in peer-reviewed journals.
Once the selection process was completed, the reviewers independently extracted relevant data using standardized, pilot-tested data extraction forms. Data were collected across all reported time points for analysis. Extracted variables included the first author's last name and publication year, the study setting, and the sample size.
Risk of Bias Assessment
The risk of bias across the included randomized trials was evaluated using the revised Cochrane Risk of Bias Tool (RoB 2) [12]. This tool assesses five key domains: the randomization process, deviations from intended interventions, missing outcome data, measurement of outcomes, and selection of the reported results.
Each domain was rated as follows: ‘low risk’ if the criteria were appropriately addressed, ‘some concerns’ if there were issues or uncertainties in the methods, ‘high risk’ if the domain was inadequately addressed, and ‘no information’ when insufficient data were available to make a judgment.
An overall risk of bias rating was determined for each study: ‘low’ if all domains were rated low risk, ‘some concerns’ if one or more domains raised some concerns, and ‘High’ if one or more domains were judged to be at high risk.
Statistical Analysis
Meta-analyses were conducted using Review Manager (RevMan 5.4, The Cochrane Collaboration, London, UK). Continuous outcomes were analyzed using standardized mean differences (SMD), while dichotomous outcomes were analyzed using odds ratios (OR), both with 95% confidence intervals (CI). Heterogeneity was assessed using the I² statistic. Given the anticipated heterogeneity across studies, all analyses were performed using a random-effects model (DerSimonian and Laird method).
Statistical significance was defined as a p-value of ≤ 0.05, and 95% confidence intervals (CIs) were applied to all effect estimates. To assess variability among studies, heterogeneity was evaluated using the I² statistic. Values of I² less than 25% were interpreted as low heterogeneity, 25-50% as moderate, and greater than 50% as high.
Given the anticipated variability across studies, a random-effects model was employed for all meta-analyses.
Results
Search Results
The comprehensive literature search initially identified 663 records across the databases outlined in Table 2.
Table 2. Database Search Strategies and Number of Records Retrieved.
| Database | Search Strategy | Records Retrieved |
| PubMed | ("Non-opioid") AND ("Analgesics") AND ("Acetaminophen" OR "Paracetamol" OR "Panadol") AND ("NSAIDs" OR "COX-Inhibitors") AND ("Pain" OR "POP") AND ("Major Abdominal Surgery") | 210 |
| ScienceDirect | ("Non-opioid analgesics" AND "postoperative pain" AND "major abdominal surgery") | 155 |
| CENTRAL (Cochrane) | ("Non-opioid analgesics" AND "postoperative pain" AND "major abdominal surgery") | 180 |
| Google Scholar | ("Non-opioid analgesics" AND "postoperative pain" AND "major abdominal surgery") | 118 |
| Citation Tracking | Manual reference list screening | 0 |
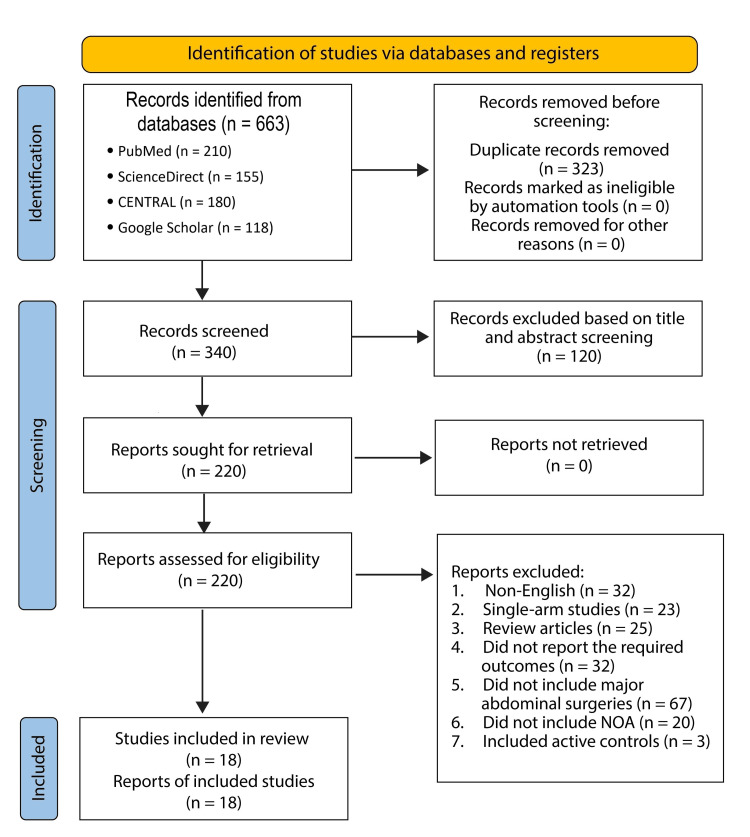
Duplicate records were identified and removed using EndNote 20 reference management software (Clarivate Analytics, Philadelphia, PA), followed by manual verification to ensure completeness. In total, 323 duplicates were excluded. 340 unique abstracts were screened for relevance to the research topic. Of these, 120 were excluded based on their abstract content. The remaining 220 articles were retrieved in full and assessed against the predefined eligibility criteria. Ultimately, only 18 studies met all inclusion criteria and were included in the final review. The reasons for exclusion of the remaining studies are detailed below:22 were not published in English, 23 were single-arm studies, 15 were review articles, 22 did not report the required outcomes, 67 included surgeries other than major abdominal surgeries, 12 did not include non-opioid analgesics as one of their interventions, and eight did not have placebo controls. A PRISMA diagram summarizing the search strategy is outlined in Figure 1.
Figure 1. A PRISMA flow diagram summarizing the search strategy.
Characteristics of the Included Studies
This review included 18 randomized controlled trials summarising data from 1894 patients. The RCT was conducted in different settings, including the United Kingdom (UK), the United States of America (USA), Croatia, Australia, South Africa, Egypt, China, Taiwan, Malaysia, Saudi Arabia, Norway, and France. The included patients underwent different procedures, including colorectal surgeries, abdominal hysterectomies, bariatric surgeries, and other major abdominal surgeries. The different NOAs included in the various studies include paracetamol, ketoprofen, ibuprofen, flurbiprofen axetil, naproxen, rofecoxib, ketorolac, lornoxicam, dexmedetomidine, allopurinol, opiranserin, and nefopam. Most of the drugs were given as post-operative continuous doses. On the other hand, a few of the drugs were given pre-operatively as continuous doses, and only one drug was given intraoperatively. The characteristics of the included studies are shown in Table 3.
Table 3. The characteristics of the included studies.
Oberhofer et al., 2005. [13], Rao et al., 2000. [14], Thompson et al., 2000. [15], Celik et al., 2002. [16], Kvalsvik et al., 2003. [17], Chen et al., 2004. [18], Karaman et al., 2006. [19], Bakhamees et al., 2007. [20], Lin et al., 2009. [21], Mowafi et al., 2012. [22], Silinsky et al., 2021. [23], Schmidt et al., 2021. [24], Rindos et al., 2019. [25], Nedeljkovic et al., 2021. [26], Ciftci et al., 2019. [27], Subramaniam et al., 2021. [28], Wang et al., 2020. [29], Raymond et al., 2004. [30]
RCT: randomized controlled trial
| Author ID | Study Design | Setting | Type of surgery | Analgesic | Sample size | Mean age | Time of administration | Dosage | Type of administration |
| Oberhofer et al., 2005. [13] | RCT | Croatia | Colon surgeries, gastric surgery, liver resection, and biliary digestive anastomosis. | Ketoprofen | 21 | 66 ± 8.3 | Postoperative | 100 mg | Continuous |
| Placebo | 22 | 63 ± 11.6 | Postoperative | 0 | |||||
| Rao et al., 2000 [14] | RCT | Malaysia | Resective bowel surgery | Ketoprofen | 20 | 38.9 ± 6.25 | Postoperative | 100 mg | Continuous |
| Placebo | 19 | 43.1 ± 7.1 | Postoperative | ||||||
| Thompson et al., 2000 [15] | RCT | UK | Abdominal hysterectomy | Meloxicam | 18 | 43.8 (31–70) | Pre-operative | 15 mg | Single dose |
| Placebo | 19 | 40.9 (30–54) | |||||||
| Celik et al., 2002 [16] | RCT | Turkey | Abdominal hysterectomy | Naproxen | 20 | 50 ± 9 | Pre-operative | 550 mg | Single dose |
| Rofecoxib | 20 | 52 ± 11 | 50 mg | ||||||
| Placebo | 20 | 52 ± 8 | |||||||
| Kvalsvik et al., 2003 [17] | RCT | Norway | Abdominal hysterectomy | Paracetamol | 30 | 45.0 (39-64) | Postoperative | 1 g | Continuous |
| Placebo | 30 | 45.0 (35-55) | |||||||
| Chen et al., 2004 [18] | RCT | Taiwan | colorectal surgery | Morphine + ketorolac | 39 | 64.5 (48.5-71.0) | Postoperative | 120 mg plus 100 mg morphine | Continuous |
| Morphine | 35 | 68 (47.8-74.0) | 100 mg morphine. | ||||||
| Karaman et al., 2006 [19] | RCT | Turkey | Abdominal hysterectomy | Ketoprofen | 20 | 52.3 ± 12.8 | Preoperative | 100 mg | Single |
| Lornoxicam | 20 | 55.6 ± 11.4 | 8 mg | ||||||
| Placebo | 20 | 53.7 ± 11.9 | |||||||
| Bakhamees et al., 2007 [20] | RCT | Egypt | Laparoscopic gastric bypass. | Dexmedetomidine | 40 | 30 ± 6 | Preoperative | 0.8 mg/kg | Continuous |
| Placebo | 40 | 29 ± 8 | |||||||
| Lin et al., 2009 [21] | RCT | Turkey | Abdominal hysterectomy | Dexmedetomidine | 50 | 43.5 (25–57) | Postoperative | 500 μg | Continuous |
| Placebo | 48 | 43.5 (25–59) | |||||||
| Mowafi et al., 2012. [22] | RCT | Saudi Arabia | Abdominal hysterectomy, abdominal myomectomy and radical prostatectomy. | Lornoxicam | 20 | 52.8 ± 16.1 | Postoperative | 16 mg | Continuous |
| Paracetamol | 19 | 49.4 ± 18.4 | 1 g | ||||||
| Placebo | 19 | 48.5 ± 14.4 | |||||||
| Silinsky et al., 2021 [23] | RCT | USA | Laparoscopic or open colorectal surgery. | Meloxicam | 26 | 58.8 ± 11.2 | Perioperative | 30 mg | Continuous |
| Placebo | 27 | 60.6 ± 11.1 | |||||||
| Schmidt et al., 2021 [24] | RCT | Brazil | Allopurinol | 27 | 50.6 ± 1.7 | Preoperative | 300 mg | Single | |
| Control | 27 | 49.9 ± 1.7 | |||||||
| Rindos et al., 2019 [25] | RCT | USA | Laparoscopic hysterectomy | Paracetamol | 91 | 41.8 ± 8.3 | Perioperative | 1 g | Continuous |
| Placebo | 92 | 42.1 ± 8.2 | |||||||
| Nedeljkovic et al., 2021 [26] | RCT | USA | Laparoscopic colorectal surgery. | VVZ-149 | 40 | 53.9 ± 11.8 | Post-operative | 1.8 mg/kg loading dose and 1.3mg/kg maintenance dose | Continuous |
| Placebo | 20 | 53.9 ± 11.8 | |||||||
| Ciftci et al., 2019 [27] | RCT | Turkey | Laparoscopic sleeve gastrectomy. | Ibuprofen | 30 | 50.16 ± 14.46 | Pre-operative | 800 mg | Single dose |
| Acetaminophen | 30 | 48.10 ± 16.01 | 1000 mg | ||||||
| Placebo | 30 | 43.93 ± 8.58 | |||||||
| Subramaniam et al., 2021 [28] | RCT | USA | Major abdominal surgeries. | Acetaminophen | 76 | 62 (55-71) | Post-operative | 1000 mg | Continuous |
| Placebo | 78 | 64 (54- 74) | |||||||
| Wang et al., 2020 [29] | RCT | China | Upper abdominal surgery | Flurbiprofen axetil | 118 | 51.5 (23-75) | Postoperative | 100 mg | Continuous |
| Tramadol | 121 | 55 (21-75) | 100 mg. | ||||||
| Raymond et al., 2004 [30] | RCT | USA | Lower abdominal surgery | Rofecoxib | 16 | 45.6 ± 11.6 | Preoperative | 25 mg | Single dose |
| 16 | 50.1 ± 13.3 | 50 mg | |||||||
| Placebo | 18 | 48.8 ± 9.8 |
Risk of bias
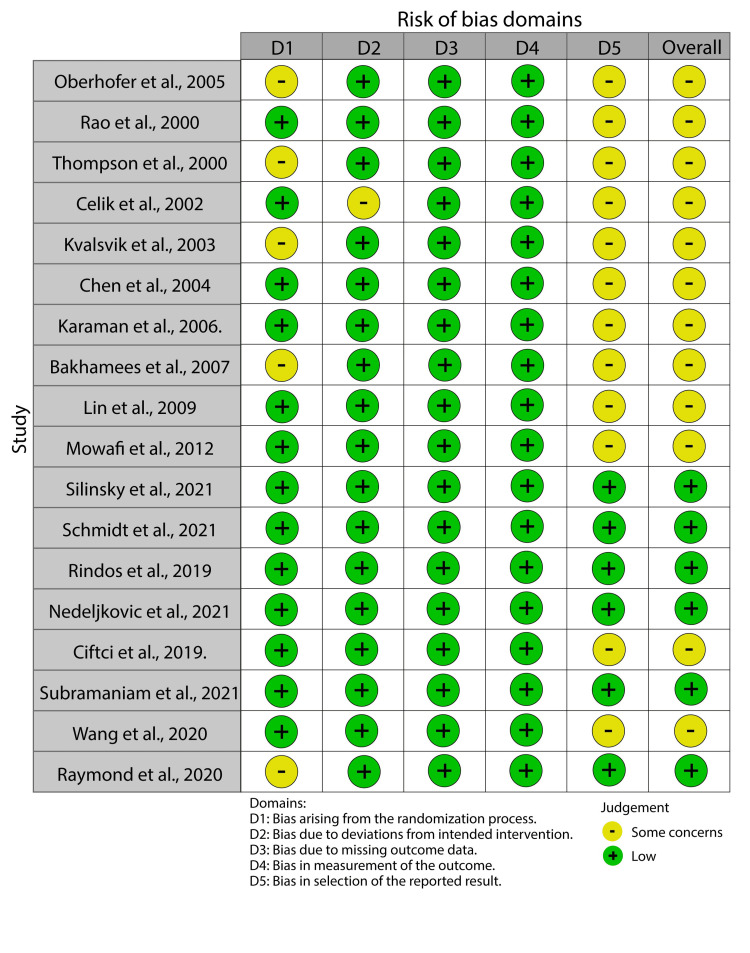
The main risk of bias in the included studies was selective reporting bias (Figure 2).
Figure 2. A risk of bias summary showing the risk of bias of the included studies.
Oberhofer et al., 2005. [13], Rao et al., 2000. [14], Thompson et al., 2000. [15], Celik et al., 2002. [16], Kvalsvik et al., 2003. [17], Chen et al., 2004. [18], Karaman et al., 2006. [19], Bakhamees et al., 2007. [20], Lin et al., 2009. [21], Mowafi et al., 2012. [22], Silinsky et al., 2021. [23], Schmidt et al., 2021. [24], Rindos et al., 2019. [25], Nedeljkovic et al., 2021. [26], Ciftci et al., 2019. [27], Subramaniam et al., 2021. [28], Wang et al., 2020. [29], Raymond et al., 2004. [30]
This bias occurred because most of the studies, especially those conducted between 2000 and 2010, did not register the study protocol before its commencement.
Post-operative Opioid Consumption
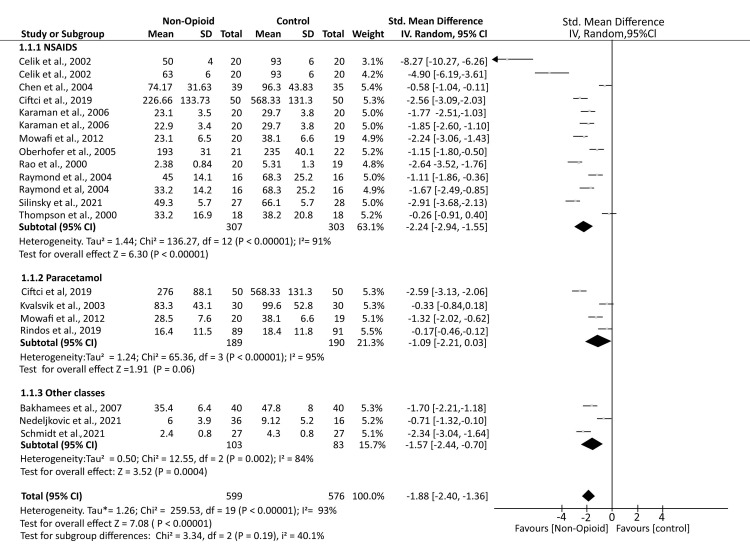
A total of 16 studies reported postoperative opioid consumption. The analysis demonstrated that patients who received non-opioid analgesics (NOA) had significantly lower opioid consumption compared to controls who received a placebo (SMD -1.88; 95% CI (-2.40, -1.36); p < 0.00001). Subgroup analyses showed that patients receiving NSAIDs (SMD -2.24; 95% CI (-2.94, -1.55); p < 0.00001) and other classes of analgesics, including dexmedetomidine, allopurinol, and opiranserin (SMD -1.57; 95% CI (-2.44, -0.70); p = 0.0004), had significantly reduced opioid consumption compared to controls. However, for patients receiving paracetamol, opioid consumption was comparable to controls (SMD -1.09; 95% CI (-2.21, 0.03); p = 0.06). The analysis demonstrated substantial heterogeneity (I² = 93%) (Figure 3).
Figure 3. A forest plot showing the mean opioid consumption in the postoperative period.
Oberhofer et al., 2005 [13], Rao et al., 2000 [14], Thompson et al., 2000 [15], Celik et al., 2002 [16], Kvalsvik et al., 2003 [17], Chen et al., 2004 [18], Karaman et al., 2006 [19], Bakhamees et al., 2007 [20], Lin et al., 2009 [21], Mowafi et al., 2012 [22], Silinsky et al., 2021 [23], Schmidt et al., 2021 [24], Rindos et al., 2019 [25], Nedeljkovic et al., 2021 [26], Ciftci et al., 2019 [27], Subramaniam et al., 2021 [28], Wang et al., 2020 [29], Raymond et al., 2004 [30].
Postoperative Pain Scores (POP)
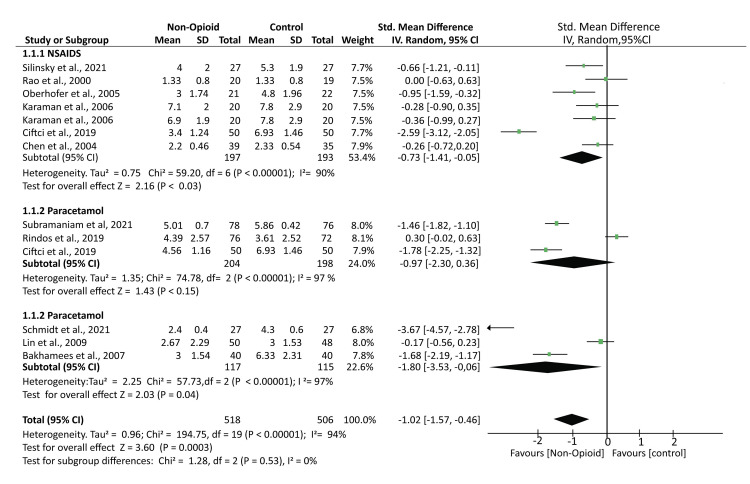
A total of 11 studies reported POP rating outcomes using different scales, including numerical and visual analog scales. A pooled analysis of the reported outcomes showed that the mean pain scores were significantly lower in patients treated with NOA than controls (SMD -1.02; 95% CI (-1.57, -0.46); p = 0.0003). Further subgroup analysis found that the mean pain scores were also significantly lower in patients who received NSAIDs and the other atypical analgesics (SMD -0.73; 95% CI (-1.41, -0.05); p < 0.03) and (SMD -1.80; 95% CI (-3.53, -0.06); p = 0.04). However, the mean pain scores in patients who received paracetamol were comparable to those of controls (SMD -0.97; 95% CI (-2.30, 0.36); p < 0.15). The analysis had high heterogeneity: I² = 94% (Figure 4).
Figure 4. A forest plot showing the mean pain outcomes based on the various pain rating scales.
Rao et al., 2000 [14], Chen et al., 2004 [18], Karaman et al., 2006 [19], Bakhamees et al., 2007 [20], Lin et al., 2009 [21], Silinsky et al., 2021 [23], Schmidt et al., 2021 [24], Rindos et al., 2019 [25], Ciftci et al., 2019 [27], Subramaniam et al., 2021 [28]
Opioid-Related Adverse Events
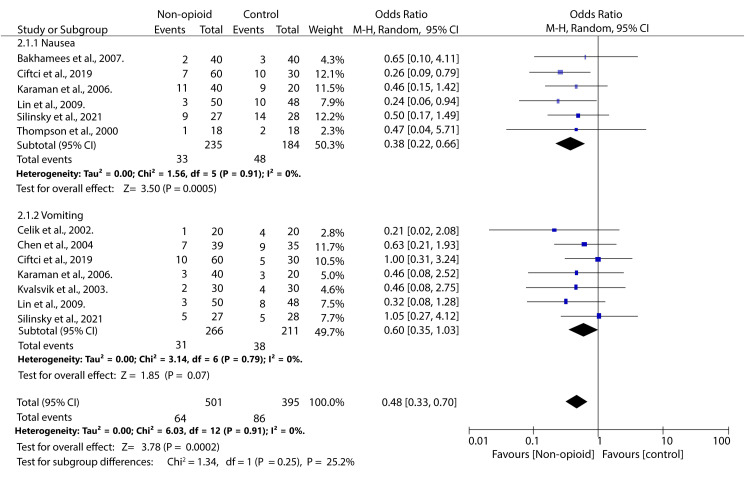
We analysed the incidence of two opioid-related adverse events, i.e., nausea and vomiting. Our analysis found that the incidence of nausea was significantly lower in patients who received NOA than in the controls (OR 0.38; 95% CI (0.22, 0.66); p = 0.0005). No significant difference was observed in the incidence of vomiting between patients treated with non-opioid analgesics and those in the control group (OR 0.60; 95% CI (0.35, 1.03); p = 0.07). There was no heterogeneity across the studies (I2 = 0%) (Figure 5).
Figure 5. Forest plot illustrating the incidence of adverse events among patients treated with non-opioid analgesics compared to controls.
Thompson et al., 2000 [15], Celik et al., 2002 [16], Kvalsvik et al., 2003 [17], Chen et al., 2004 [18], Karaman et al., 2006 [19], Bakhamees et al., 2007 [20], Lin et al., 2009 [21], Silinsky et al., 2021 [23], Ciftci et al., 2019 [27]
Discussion
Our study found that non-opioid analgesics had superior efficacy to placebo in reducing post-operative opioid consumption in patients who have undergone major abdominal surgeries. Consequently, they provided superior pain relief and also reduced the incidence of opioid-related adverse events in these patients.
Our study analysed the NOA in three groups: NSAIDs, which included traditional NSAIDs such as ibuprofen, and cox-2 inhibitors such as rofecoxib. The second subgroup included studies that investigated the use of acetaminophen. Lastly, the third subgroup included other groups of analgesics, such as dexmedetomidine, an alpha-2 agonist with analgesic effects, and opiranserin, a glycine transporter two agonist. Among the different classes, our study found that NSAIDs had superior efficacy in reducing post-operative opioid consumption. Similar results were found by Martinez et al., who found that NSAIDs had superior efficacy to other analgesics, such as acetaminophen, in managing POP after major surgeries. Martinez et al. further found that combination therapies were even more effective than monotherapies in reducing post-operative opioid consumption and pain scores [5]. However, none of our included studies used combination therapies, and thus, we did not establish the clinical importance of using combination therapies in patients undergoing major abdominal surgeries.
The efficacy of various paracetamol formulations in managing POP has been demonstrated in previous studies [31]. However, our analysis did not show a significant reduction in opioid consumption or POP scores among patients who received paracetamol. In contrast to our findings, a meta-analysis by McDaid et al. reported that paracetamol significantly reduced postoperative opioid consumption following major surgeries [32]. It is, however, essential to note that the efficacy of paracetamol in the meta-analysis was inferior to that of NSAIDs and selective COX inhibitors. These results indicate that paracetamol may not be that efficacious in the management of POP in patients who have undergone major surgeries.
Furthermore, the inferior efficacy of paracetamol in pain management to other analgesics was also demonstrated by Tan et al., who found that ibuprofen was superior in managing pain in children compared to paracetamol [33]. However, the results of our analysis should be interpreted with caution since they are based on a limited number of clinical trials. Further investigation is warranted to establish the efficacy of paracetamol compared to placebo and other analgesics in the management of POP after major abdominal surgeries.
This study also investigated other analgesics, such as dexmedetomidine, an alpha-2 agonist. Dexmedetomidine has been shown to have different beneficial effects in patients who have undergone major surgeries. For instance, a meta-analysis by Poon et al., 2022, found that dexmedetomidine was associated with a reduced mean duration of intensive care and reduced risk of short-term mortality in patients who had undergone cardiac surgery [34]. Furthermore, like our study, a meta-analysis by Zhang et al. found that dexmedetomidine reduced the pain scores of patients and the incidence of postoperative nausea and vomiting in patients who had undergone bariatric surgeries [35]. The last group of drugs investigated in the study is allopurinol, a xanthine oxidase inhibitor mainly used for hyperuricemia [24]. Although not primarily indicated for analgesia, the results of the RCT by Schmidt et al. have shown its efficacy as a preanesthetic analgesic drug [23]. Furthermore, these results indicate that the purinergic system in which allopurinol exerts its mechanism of action is a potential therapeutic target for analgesics [24]. Therefore, further research is warranted to establish its efficacy in analgesia.
The primary aim of introducing NOAs in POP management was due to the associated side effects of opioids that patients face post-operatively [7]. Furthermore, these regimens aimed to reduce the mean opioid consumption and thus reduce the associated side effects [7]. Our study found that using NOAs decreased the incidence of post-operative nausea. However, it is also important to note that caution must be observed since these NOAs also have their associated side effects, which should be closely monitored [35]. For instance, NSAIDs are associated with an increased incidence of bleeding and thus would be a concern in patients, especially after a significant surgical procedure [36]. Fortunately, the analysed studies reported no incidence of postoperative hemorrhage.
Limitations
The main limitation of our study was the high heterogeneity across the studies. The first may be attributed to the different operative procedures the patients in the included studies underwent. Since there is no consensus on the definition of major surgeries, all the surgeries included were of diverse origin from the upper abdomen and the lower abdomen. Furthermore, the operations were either open or minimally invasive laparoscopic surgeries. This diversity in the techniques may have contributed to the heterogeneity observed. Secondly, the reporting of the included outcomes was varied across the studies. For instance, in postoperative opioid consumption, some studies reported the outcomes using the mg while others used milliequivalents; some reported the mean opioid consumption while others reported the cumulative opioid consumption. While the authors used the SMD to enable adjustment of the effect size, this would not adjust for all the variations in the methodology of the different studies.
Conclusions
The results of this study indicate that non-opioid analgesics (NOAs) are a valuable adjunct to perioperative pain management and contribute significantly to reducing opioid consumption and related adverse events in post-operative patients undergoing major abdominal surgery. Among the evaluated classes, NSAIDs showed the most consistent efficacy, while the role of paracetamol remains less specific. Although dexmedetomidine and other novel agents show promise, further validation is needed.
To further strengthen clinical practice, future studies should focus on head-to-head comparisons of different NOA classes, explore the effectiveness of combination therapies, and investigate patient-specific factors that influence analgesic outcomes. Standardizing outcome measures and surgical classifications across studies will enhance comparability and evidence synthesis. Ultimately, integrating non-opioid analgesics (NOAs) into comprehensive multimodal analgesia protocols-including regional anesthetic techniques such as fascial plane blocks, epidural and intrathecal analgesia, as well as adjuncts like lidocaine and ketamine-may offer a safer and more effective strategy for postoperative pain management in major abdominal surgeries.
Disclosures
Conflicts of interest: In compliance with the ICMJE uniform disclosure form, all authors declare the following:
Payment/services info: All authors have declared that no financial support was received from any organization for the submitted work.
Financial relationships: All authors have declared that they have no financial relationships at present or within the previous three years with any organizations that might have an interest in the submitted work.
Other relationships: All authors have declared that there are no other relationships or activities that could appear to have influenced the submitted work.
Author Contributions
Concept and design: Hany A. Zaki, Benny Ponappan, Mohammed Gafar Abdelrahim, Eman Shaban, Ahmed Shaban, Amira Shaban
Acquisition, analysis, or interpretation of data: Hany A. Zaki, Ali Elkandow, Mohammed F. Abosamak, Eman Shaban, Ahmed Shaban, Mujeeb Ur Rehman
Drafting of the manuscript: Hany A. Zaki, Benny Ponappan, Ali Elkandow, Mohammed Gafar Abdelrahim, Eman Shaban, Ahmed Shaban, Mujeeb Ur Rehman
Critical review of the manuscript for important intellectual content: Hany A. Zaki, Mohammed F. Abosamak, Eman Shaban, Ahmed Shaban, Amira Shaban
Supervision: Hany A. Zaki, Eman Shaban, Ahmed Shaban
References
- 1.An estimation of the global volume of surgery: a modelling strategy based on available data. Weiser TG, Regenbogen SE, Thompson KD, Haynes AB, Lipsitz SR, Berry WR, Gawande AA. Lancet. 2008;372:139–144. doi: 10.1016/S0140-6736(08)60878-8. [DOI] [PubMed] [Google Scholar]
- 2.Pain intensity on the first day after surgery: a prospective cohort study comparing 179 surgical procedures. Gerbershagen HJ, Aduckathil S, van Wijck AJ, Peelen LM, Kalkman CJ, Meissner W. Anesthesiology. 2013;118:934–944. doi: 10.1097/ALN.0b013e31828866b3. [DOI] [PubMed] [Google Scholar]
- 3.Patient controlled opioid analgesia versus conventional opioid analgesia for postoperative pain. Hudcova J, McNicol E, Quah C, Lau J, Carr DB. Cochrane Database Syst Rev. 2006:0. doi: 10.1002/14651858.CD003348.pub2. [DOI] [PubMed] [Google Scholar]
- 4.Patient-controlled analgesia in the management of postoperative pain. Momeni M, Crucitti M, De Kock M. Drugs. 2006;66:2321–2337. doi: 10.2165/00003495-200666180-00005. [DOI] [PubMed] [Google Scholar]
- 5.Non-opioid analgesics in adults after major surgery: systematic review with network meta-analysis of randomized trials. Martinez V, Beloeil H, Marret E, Fletcher D, Ravaud P, Trinquart L. Br J Anaesth. 2017;118:22–31. doi: 10.1093/bja/aew391. [DOI] [PubMed] [Google Scholar]
- 6.Prevention of postoperative pain by balanced analgesia. Dahl JB, Rosenberg J, Dirkes WE, Mogensen T, Kehlet H. Br J Anaesth. 1990;64:518–520. doi: 10.1093/bja/64.4.518. [DOI] [PubMed] [Google Scholar]
- 7.Practice guidelines for acute pain management in the perioperative setting: an updated report by the American Society of Anesthesiologists Task Force on Acute Pain Management. Anesthesiology. 2012;116:248–273. doi: 10.1097/ALN.0b013e31823c1030. [DOI] [PubMed] [Google Scholar]
- 8.Perioperative single dose systemic dexamethasone for postoperative pain: a meta-analysis of randomized controlled trials. De Oliveira GS Jr, Almeida MD, Benzon HT, McCarthy RJ. Anesthesiology. 2011;115:575–588. doi: 10.1097/ALN.0b013e31822a24c2. [DOI] [PubMed] [Google Scholar]
- 9.Does multimodal analgesia with acetaminophen, nonsteroidal antiinflammatory drugs, or selective cyclooxygenase-2 inhibitors and patient-controlled analgesia morphine offer advantages over morphine alone? Meta-analyses of randomized trials. Elia N, Lysakowski C, Tramèr MR. Anesthesiology. 2005;103:1296–1304. doi: 10.1097/00000542-200512000-00025. [DOI] [PubMed] [Google Scholar]
- 10.The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Page MJ, McKenzie JE, Bossuyt PM, et al. BMJ. 2021;372:0. doi: 10.1186/s13643-021-01626-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Definition of major and minor surgery: a question and an answer. Earl R. Ann Surg. 1917;65:799. doi: 10.1097/00000658-191706000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.RoB 2: a revised tool for assessing risk of bias in randomised trials. Sterne JA, Savović J, Page MJ, et al. BMJ. 2019;366:0. doi: 10.1136/bmj.l4898. [DOI] [PubMed] [Google Scholar]
- 13.Intravenous ketoprofen in postoperative pain treatment after major abdominal surgery. Oberhofer D, Skok J, Nesek-Adam V. World J Surg. 2005;29:446–449. doi: 10.1007/s00268-004-7612-0. [DOI] [PubMed] [Google Scholar]
- 14.Morphine-sparing effect of ketoprofen after abdominal surgery. Rao AS, Cardosa M, Inbasegaran K. Anaesth Intensive Care. 2000;28:22–26. doi: 10.1177/0310057X0002800103. [DOI] [PubMed] [Google Scholar]
- 15.Effect of meloxicam on postoperative pain after abdominal hysterectomy. Thompson JP, Sharpe P, Kiani S, Owen-Smith O. Br J Anaesth. 2000;84:151–154. doi: 10.1093/oxfordjournals.bja.a013395. [DOI] [PubMed] [Google Scholar]
- 16.A comparative study of the effect of rofecoxib (a COX 2 inhibitor) and naproxen sodium on analgesic requirements after abdominal hysterectomy. Celik JB, Tuncer S, Reisli R, Sarkilar G, Celik C, Akyürek C. Arch Gynecol Obstet. 2003;268:297–300. doi: 10.1007/s00404-002-0377-5. [DOI] [PubMed] [Google Scholar]
- 17.Randomized, double-blind, placebo-controlled study of the effect of rectal paracetamol on morphine consumption after abdominal hysterectomy. Kvalsvik O, Borchgrevink PC, Hagen L, Dale O. Acta Anaesthesiol Scand. 2003;47:451–456. doi: 10.1034/j.1399-6576.2003.00080.x. [DOI] [PubMed] [Google Scholar]
- 18.Effect of adding ketorolac to intravenous morphine patient-controlled analgesia on bowel function in colorectal surgery patients--a prospective, randomized, double-blind study. Chen JY, Wu GJ, Mok MS, et al. Acta Anaesthesiol Scand. 2005;49:546–551. doi: 10.1111/j.1399-6576.2005.00674.x. [DOI] [PubMed] [Google Scholar]
- 19.The effect of pre-operative lornoxicam and ketoprofen application on the morphine consumption of post-operative patient-controlled analgesia. Karaman S, Gunusen I, Uyar M, Firat V. J Int Med Res. 2006;34:168–175. doi: 10.1177/147323000603400206. [DOI] [PubMed] [Google Scholar]
- 20.Effects of dexmedetomidine in morbidly obese patients undergoing laparoscopic gastric bypass. Bakhamees HS, El-Halafawy YM, El-Kerdawy HM, Gouda NM, Altemyatt S. https://pubmed.ncbi.nlm.nih.gov/18044282/ Middle East J Anaesthesiol. 2007;19:537–551. [PubMed] [Google Scholar]
- 21.Effect of combining dexmedetomidine and morphine for intravenous patient-controlled analgesia. Lin TF, Yeh YC, Lin FS, Wang YP, Lin CJ, Sun WZ, Fan SZ. Br J Anaesth. 2009;102:117–122. doi: 10.1093/bja/aen320. [DOI] [PubMed] [Google Scholar]
- 22.Intravenous lornoxicam is more effective than paracetamol as a supplemental analgesic after lower abdominal surgery: a randomized controlled trial. Mowafi HA, Elmakarim EA, Ismail S, Al-Mahdy M, El-Saflan AE, Elsaid AS. World J Surg. 2012;36:2039–2044. doi: 10.1007/s00268-012-1649-2. [DOI] [PubMed] [Google Scholar]
- 23.Preoperative intravenous meloxicam for moderate-to-severe pain in the immediate post-operative period: a Phase IIIb randomized clinical trial in 55 patients undergoing primary open or laparoscopic colorectal surgery with bowel resection and/or anastomosis. Silinsky JD, Marcet JE, Anupindi VR, et al. Pain Manag. 2021;11:9–21. doi: 10.2217/pmt-2020-0061. [DOI] [PubMed] [Google Scholar]
- 24.Allopurinol attenuates postoperative pain and modulates the purinergic system in patients undergoing abdominal hysterectomy: a randomized controlled trial. Schmidt AP, de Oliveira ED, Fagundes AC, et al. J Anesth. 2021;35:818–826. doi: 10.1007/s00540-021-02983-z. [DOI] [PubMed] [Google Scholar]
- 25.Intravenous acetaminophen vs saline in perioperative analgesia with laparoscopic hysterectomy. Rindos NB, Mansuria SM, Ecker AM, Stuparich MA, King CR. Am J Obstet Gynecol. 2019;220:373–378. doi: 10.1016/j.ajog.2019.01.212. [DOI] [PubMed] [Google Scholar]
- 26.Exploratory study of VVZ-149, a novel analgesic molecule, in the affective component of acute postoperative pain after laparoscopic colorectal surgery. Nedeljkovic SS, Song I, Bao X, et al. J Clin Anesth. 2022;76:110576. doi: 10.1016/j.jclinane.2021.110576. [DOI] [PubMed] [Google Scholar]
- 27.Comparison of intravenous ibuprofen and paracetamol for postoperative pain management after laparoscopic sleeve gastrectomy. A randomized controlled study. Ciftci B, Ekinci M, Celik EC, Kaciroglu A, Karakaya MA, Demiraran Y, Ozdenkaya Y. Obes Surg. 2019;29:765–770. doi: 10.1007/s11695-018-3613-1. [DOI] [PubMed] [Google Scholar]
- 28.The effect of scheduled intravenous acetaminophen in an enhanced recovery protocol pathway in patients undergoing major abdominal procedures: a prospective, randomized, and placebo-controlled clinical trial. Subramaniam K, Esper SA, Mallikarjun K, et al. Pain Med. 2022;23:10–18. doi: 10.1093/pm/pnab272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Flurbiprofen axetil for postoperative analgesia in upper abdominal surgery: a randomized, parallel controlled, double-blind, multicenter clinical study. Wang RD, Sheng XR, Guan WX, et al. Surg Today. 2020;50:749–756. doi: 10.1007/s00595-019-01951-1. [DOI] [PubMed] [Google Scholar]
- 30.Preoperative rofecoxib oral suspension as an analgesic adjunct after lower abdominal surgery: the effects on effort-dependent pain and pulmonary function. Sinatra RS, Shen QJ, Halaszynski T, Luther MA, Shaheen Y. Anesth Analg. 2004;98:135–140. doi: 10.1213/01.ANE.0000085637.00864.D7. [DOI] [PubMed] [Google Scholar]
- 31.Unlocking the optimal analgesic potential: a systematic review and meta-analysis comparing intravenous, oral, and rectal paracetamol in equivalent doses. Ibrahim T, Gebril A, Nasr MK, Samad A, Zaki HA. Cureus. 2023;15:0. doi: 10.7759/cureus.41876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Paracetamol and selective and non-selective non-steroidal anti-inflammatory drugs (NSAIDs) for the reduction of morphine-related side effects after major surgery: a systematic review. McDaid C, Maund E, Rice S, Wright K, Jenkins B, Woolacott N. Health Technol Assess. 2010;14:1-153, iii-iv. doi: 10.3310/hta14170. [DOI] [PubMed] [Google Scholar]
- 33.Comparison of acetaminophen (paracetamol) with ibuprofen for treatment of fever or pain in children younger than 2 years: a systematic review and meta-analysis. Tan E, Braithwaite I, McKinlay CJ, Dalziel SR. JAMA Netw Open. 2020;3:0. doi: 10.1001/jamanetworkopen.2020.22398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dexmedetomidine for adult cardiac surgery: a systematic review, meta-analysis and trial sequential analysis. Poon WH, Ling RR, Yang IX, et al. Anaesthesia. 2023;78:371–380. doi: 10.1111/anae.15947. [DOI] [PubMed] [Google Scholar]
- 35.Dexmedetomidine reduces postoperative pain and speeds recovery after bariatric surgery: a meta-analysis of randomized controlled trials. Zhang Y, Zhou Y, Hu T, et al. Surg Obes Relat Dis. 2022;18:846–853. doi: 10.1016/j.soard.2022.03.002. [DOI] [PubMed] [Google Scholar]
- 36.Adverse effects of nonsteroidal antiinflammatory drugs: an update of gastrointestinal, cardiovascular and renal complications. Harirforoosh S, Asghar W, Jamali F. J Pharm Pharm Sci. 2013;16:821–847. doi: 10.18433/j3vw2f. [DOI] [PubMed] [Google Scholar]