Abstract
ComFB is encoded in the comF operon of Bacillus subtilis, situated between the genes for ComFA and ComFC. The latter two proteins are essential for natural transformation, whereas ComFB is dispensable. We show here that ComFB binds specifically and with high affinity to the second messenger c-di-GMP and that ComFB acts as a c-di-GMP receptor to inhibit swarming and swimming motility, apparently by interfering with flagellar activity. We show further that in the absence of ComFC, swarming is completely abrogated by a mechanism that requires FB. These results reveal a new c-di-GMP regulatory system that controls motility independently of MotI.
INTRODUCTION
Self-propelled bacterial movement through liquids and across surfaces is widely conserved despite the high energetic cost of synthesizing and operating the relevant organelles, typically flagella (1). This cost requires that the formation and function of flagella be tightly regulated, often through mechanisms involving the second messenger bis-(3′-5′)-cyclic diguanylic acid (c-di-GMP). c-di-GMP is ubiquitous in bacteria, regulating diverse activities beyond motility, including biofilm formation, exopolysaccharide synthesis, virulence, DNA replication, and cell size (2, 3). To fulfill its regulatory roles, c-di-GMP interacts with protein or RNA receptors. The receptors then act on phenotype-specific targets. For example, the B. subtilis (Bsu) receptor MotI, when bound to c-di-GMP, interacts with the flagellar stator protein MotA to uncouple flagella from the motor force that enables swimming (4).
Recently, a cyanobacterial protein with partial sequence and structural similarity to the Bacillus subtilis (Bsu) protein ComFB (FB) was shown to bind c-di-GMP (5). In fact, the FB of Bsu also binds c-di-GMP with specificity and high affinity (shown here and in (6)). While MotI is a PilZ-type c-di-GMP binding receptor with canonical RR and (N/D)hSXXG motifs (7), FB represents a new class of cyclic-dimeric nucleoside monophosphate-binding proteins, widespread in bacterial phyla (5, 6, 8). Although FB is dispensable for genetic transformation (9), it is embedded in the comF operon of Bsu, which encodes two proteins, ComFA (FA) and ComFC (FC), needed for the transport of transforming DNA to the cytoplasm (Fig. 1A) (9–12). Firmicute comF operons consistently encode FA and FC, but only some firmicutes encode FB; a search using eggNOG v6 (13) revealed that although 447 known firmicute species encode close orthologs of FBBsu, these orthologs are sporadically distributed. For example, even among the bacilli, FB is encoded by only a few close relatives of Bsu. At the same time, orthologs with partial sequence similarity to FBBsu are encoded in distantly related bacteria, including cyanobacteria, Treponema denticola, and Vibrio cholerae (5, 6). Although the structure of the Bsu FB has been solved (9), its role remained obscure. The discovery that it binds c-di-GMP suggested a regulatory role, and we have now shown that FB inhibits flagellar motility.
Figure 1.
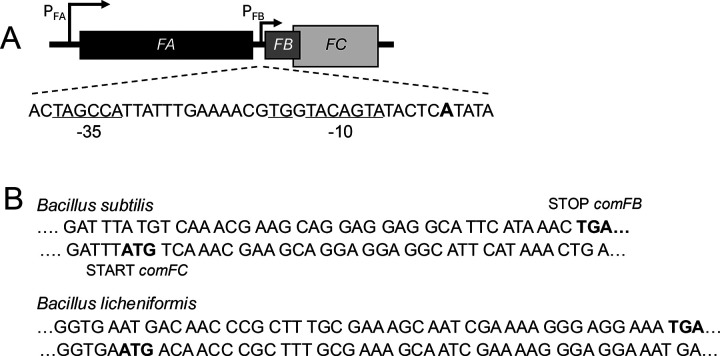
Organization of the comF operon. (A) In Bsu, the comFA, comFB, and comFC open reading frames are transcribed from PFA, a ComK-dependent promoter. PFB, a ComK-independent promoter, drives comFB and FC, which overlap. The existence of PFB is documented as discussed in the text. The +1 A-residue for PFB is shown in boldface, and the proposed promoter sequence features are underlined. (B) Two examples of bacteria that encode ComFB in their comF operons, demonstrating the overlap of their comFB and comFC coding sequences.
In Bsu, the comF operon is activated by ComK, which drives the transcription of about 20 genes needed for transformation and about 80 additional genes with largely uncharacterized roles (14–16). The semi-dormant transformation-competent “K-state” conferred by ComK is established during the transition to the stationary growth phase in a minority of cells because ComK itself is bimodally expressed (17–21). The comF operon of Bsu is preceded by a ComK-dependent promoter (PFA), which drives the transcription of comFA, comFB, and comFC (Fig. 1A) (10). A second promoter (PFB) drives transcription of the comFB and comFC genes (Fig. 1A). PB is not associated with the K-box sequences characteristic of ComK-dependent promoters (22), and is active in LB medium, where ComK is not produced. Two reports, both based on data from LB-grown cells, revealed the existence of the PFB promoter. A tiled microarray study of global transcription, with a reported resolution of 22 bases, demonstrated an uptick in transcription during growth at position 3,642,139 in the Bsu genome, 32 bases upstream of the comFB open reading frame (23). Rend-seq (24, 25), with single-nucleotide resolution, showed the presence of a transcriptional start site nearby at chromosomal position 3,642,135 (https://rendseq.org/). Plausible SigA promoter sequences are located at an appropriate distance upstream from this start site (Fig. 1A).
Here, we report that FB binds to c-di-GMP with high affinity and specificity and that it inhibits swimming and swarming motility when bound to c-di-GMP, independently of MotI. The deletion of comFC, with which comFB is translationally coupled, prevents swarming, suggesting that the joint expression of the ComFB and ComFC proteins may play a role in the regulation of motility during growth and in the K-state.
RESULTS
ComFB binds c-di-GMP specifically and with high affinity
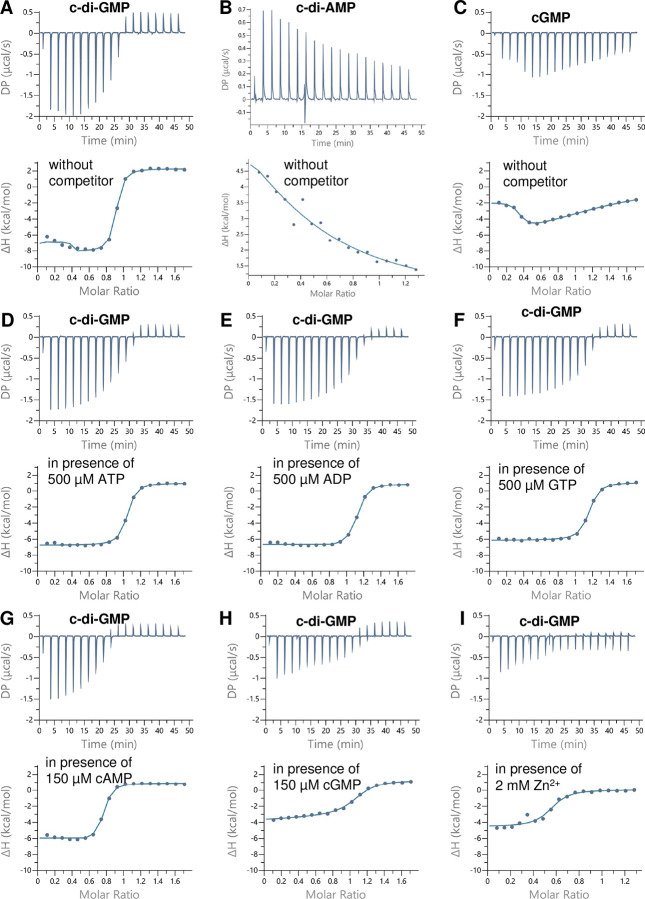
As noted above, orthologs of FB have been reported to bind cyclic dinucleotides, including c-di-GMP and c-di-AMP (5, 6, 8). To gain further insights into the affinity of Bsu FB for c-di-GMP, we heterologously purified FB in its native dimeric state (Fig. S1) and employed isothermal titration calorimetry (ITC). The raw titration data of c-di-GMP to FB were fitted using a single-site binding model to determine the average dissociation constant (KD) for all available binding sites (Table 1, Fig. 2A). ITC experiments revealed that Bsu FB has a high affinity for c-di-GMP (KD ~140 nM; Table 1), comparable to that of other high-affinity c-di-GMP binders, e.g., Pilz and FimX (26). In contrast, the FB KD value for binding to c-di-AMP (Fig. 2B) (6), which Bsu also produces (27), is nearly 500-fold higher (Table 1), above the likely physiological concentration for this dinucleotide. Also, c-di-GMP competes efficiently with c-di-AMP (at a saturating concentration of 150 μM) for FB binding (Table 1) (6).
Table 1.
c-di-GMP binding to Bus FB as determined by ITC
| Titrant | Competitor | Average KD (μM)a | ΔH (kcal mol−1)b | ΔG (kcal mol−1)c |
|---|---|---|---|---|
| c-di-GMP | ---- | 0.14 ± 0.05 | −9.6 ± 0.8 | −9.4 ± 0.15 |
| c-di-GMP | 2 mM Zn2+ | 1.2 + 0.6 | −5.7 ± 1.5 | −12.2 ± 4.2 |
| c-di-GMP (6) | 150 μM c-di-AMP | 0.24 ± 0.09 | −6 | −8.1 ± 1.8 |
| c-di-AMP | ---- | 69.1 ± 139 | 9.6 | −5.68 |
| c-di-GMP | 150 μM GTP | 0.11 + 0.09 | −8.8 ± 0.5 | −16.4 ± 0.1 |
| c-di-GMP | 500 μM GTP | 0.2 ± 0.15 | −8.1 ± 1.3 | −9.2 ± 0.5 |
| c-di-GMP | 150 μM ATP | 0.12 + 0.1 | −9.2 ± 0.6 | −13.5 ± 4.1 |
| c-di-GMP | 500 μM ATP | 0.4 ± 0.16 | −6.5 ± 1.25 | −8.8 ± 0.2 |
| c-di-GMP | 150 μM ADP | 0.25 + 0.17 | −8.2 ± 0.7 | −9.2 ± 0.7 |
| c-di-GMP | 500 μM ADP | 0.3 ± 0.03 | −7.4 ± 0.2 | −8.9 ± 0.06 |
| c-di-GMP | 150 μM cGMP | 1.9 + 0.1 | −3.7 ± 1.5 | −7.8 ± 0.3 |
| c-di-GMP | 150 μM cAMP | 0.3 + 0.06 | −6.9 | −8.94 |
| cGMP | ---- | 1.1 + 0.7 | −2.5 ± 0.9 | −8.2 ± 0.4 |
| cGMP | 150 μM c-di-GMP | No binding | ||
| GTP | ---- | No binding | ||
| ATP | ---- | |||
| ADP | ---- | No binding | ||
| cAMP | ---- | No binding | ||
The raw isothermal titration calorimetry (ITC) data were fitted using a one-site binding model for monomeric Bsu FB.
The dissociation constant (KD) values correspond to the mean of at least two independent experiments ± standard deviation. All titrations were performed in 50 mM Tris-HCl buffer (pH 7.9).
Enthalpy.
Gibbs free energy. For competition binding assays, FB protein was preincubated with 150 μM or 500 μM of the ‘competitor’ nucleotide as indicated, followed by titration with c-di-GMP.
Fig. 2.
Figure 2. Isothermal titration calorimetry (ITC) analysis of c-di-GMP, cGMP, and c-di-AMP binding to FB, as indicated. Upper panels show the raw ITC data in the form of heat produced during the titration of the respective nucleotide on the FB protein; lower panels show the binding isotherms and the best-fit curves according to the one binding site model. (A-C) ITC analysis of c-di-GMP (A), c-di-AMP (B), and cGMP (C) binding to FB in the absence of competitors. (D-I) ITC analysis of c-di-GMP binding to FB in the presence of ATP (D), ADP (E), GTP (F), cAMP (G), cGMP (H), or Zn2+ (I).
To further explore the relation between c-di-GMP binding and that of other more abundant nucleotides (ATP, ADP, and GTP), and other second messengers (cGMP and cAMP), we tested the ability of c-di-GMP to bind FB in the presence of saturating concentrations of these compounds. GTP, ATP, and ADP could not bind to FB (Fig. S2), and their presence at 150 or 500 μM did not significantly affect the c-di-GMP affinity to FB (Table 1, Figs. S1 and S2). However, at the high concentration of 500 μM, the binding enthalpy of c-di-GMP was slightly reduced (Table 1; compare isotherm of Fig. 2A with 2D), causing a slight increase in the KD values. The second messenger cAMP was not able to bind FB (Fig. S2G), but its presence at 150 μM triggered a trivial decrease in the c-di-GMP binding enthalpy to FB (Table 1), yielding weaker isotherms (compare the isotherm of Fig. 2A with 2G). In contrast, the second messenger cGMP could bind FB with a KD of ~1.1 μM. The cGMP titration curve showed a complex biphasic binding curve; in the first injections, the enthalpy increased until a maximum was reached at a cGMP concentration of ~40 μM (Fig. 2C). Subsequently, the heat isotherm gradually decreased, indicating gradual saturation of FB by cGMP (Fig. 2C). Therefore, it seems that cGMP is unable to efficiently occupy the binding sites of FB. Consistent with this conclusion, the binding of cGMP was completely abolished in the presence of a saturating concentration of c-di-GMP (150 μM) (Fig S2H). Moreover, in the presence of 150 μM cGMP, c-di-GMP was able to compete efficiently and replace cGMP for FB binding, although the apparent KD for c-di-GMP binding increased by more than 10-fold in the presence of cGMP (Table 1) as indicated by a decrease in the binding enthalpy (Table 1; compare isotherm of Fig. 2A with 2H). cGMP is produced by some bacteria (8, 28), but to our knowledge, has never been reported to exist in the Firmicutes, and its weak interaction with FB is of doubtful biological relevance. The crystal structure of Bsu FB contained a Zn2+ atom (one per monomer; PDB: 4WAI) that appeared to stabilize the protein (9) but could potentially interfere with c-di-GMP binding. Indeed, the binding assay performed in the presence of 2 mM ZnCl2 resulted in a ~8-fold higher apparent KD for c-di-GMP (Table 1, Fig. 2I). Collectively, competition studies show that GTP, ATP, ADP, and cAMP have negligible effects on c-di-GMP binding, while cGMP shows a modest competition effect, establishing the specificity of c-di-GMP binding to FB. These results indicate that FB can be regarded as a c-di-GMP binding receptor in Bsu.
The chromosomal organization of comFB in Bsu
In Bsu, the stop codon of comFB is embedded within the coding sequence of comFC with an overlap of 11 codons (Fig. 1B). A similar arrangement is found in closely related bacilli that encode FB and FC, as depicted for B. licheniformis in Fig. 1B. Such arrangements are often indicative of translational coupling (29) so that the translation of comFC may be augmented by, or even depend on, the translation of comFB.
ComFB inhibits swarming when bound to c-di-GMP
c-di-GMP binding receptors have been implicated in inhibiting swimming and swarming motility in several bacterial species (4, 30, 31). Although swimming through low-viscosity agar depends on flagellar activity, it also depends on chemotaxis (32), and a swimming assay cannot distinguish between these dependencies. Swarming, bacterial expansion across a solid surface, does not depend on chemotaxis per se (32) but rather on hyperflagellation, the association of individual cells into “rafts,” and the secretion of surfactin, which decreases surface tension. In most of the present study, we used the biologically relevant undomesticated NCIB 3610 background because domesticated strains have lost the ability to swarm (32).
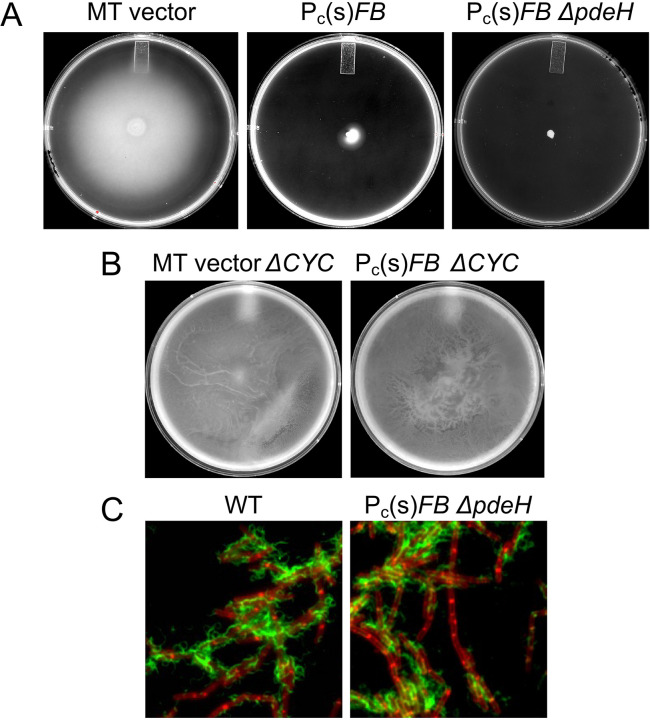
To determine whether the overexpression of FB affected swarming motility, we expressed FB from the amyE locus of NCIB 3610 under the control of a constitutive promoter (Pc) unrelated to PFA or PFB, using either a weak or a strong ribosomal binding site (RBS). The Pc promoter was derived from the inducible Phyperspac promoter but lacks the lacO operator site (33). The “weak” RBS construct (amyE::Pc(w)FB) employed the native FB ribosomal binding site, while the “strong” construct (amyE::Pc(s)FB) utilized the RBS of the highly expressed gapA gene. For these comparisons, we tested each construct both with and without the deletion of pdeH, which encodes the only c-di-GMP phosphodiesterase in Bsu (33, 34). In the absence of this enzyme, the cellular concentration of c-di-GMP increases (33). As reported previously (32), we confirmed that the inactivation of pdeH alone moderately decreased swarming (Fig. 3A). Although the amyE::Pc(w)FB construct did not affect swarming on its own, it exhibited a greater inhibitory effect when combined with ΔpdeH compared to ΔpdeH alone (Fig. 3A).
Figure 3.
FB, together with c-di-GMP, inhibits swarming. Each of the panels is a representative of at least three repeated swarming experiments, performed as described using 0.7% LB-agar plates. The expansion diameter of the cell sheet was measured at intervals and plotted against time. The symbols are consistent across all five panels, with empty symbols indicating ΔpdeH strains. MT: empty vector (pKB149 (33)) in amyE. The constructs amyE::Pc(w)FB and amyE::Pc(s)FB are described in the text. Δcyc denotes strains deleted for the three diguanlylate cyclases of Bsu (dgcP, dgcK, dgcW).
In contrast to these results, the amyE::PC(s)FB construct partially inhibited swarming on its own and eliminated it when pdeH was deleted (Fig. 3B). Western blotting showed that expression from the weak construct was slightly stronger than from the native locus, while the strong construct produced about 100-fold more than the native locus (Fig. 4). (The samples loaded on the first three lanes of the gel shown to the right of Fig. 4, were diluted 20-fold before loading, and a 10-fold reduced volume was loaded compared to the gel shown to the left of Fig. 4). The blot also shows that the expression of FB from the overproduction construct was not increased when pdeH was eliminated, suggesting that the inhibition of swarming with excess FB is not due to enhanced FB expression but may be dependent on binding to c-di-GMP.
Fig. 4.
The expression of FB and its regulation. Western blots were performed with an anti-serum raised against purified FB. As a loading control, the tops of the membranes were cut off after blotting and developed with an antiserum raised against elongation factor G (EFG). We have noticed a consistent signal, indicated by arrows, at the position expected for a dimer of FB. The Δ(FB-FC) strain was included in each panel to confirm the identity of the FB signal. The vertical dashed lines indicate that intervening lanes were omitted from a gel. For the gel to the right of panel A, the first three lanes were loaded with lysates diluted 20-fold, and only 2 μl were loaded compared to 10–20 μl of undiluted lysate for the other lanes. This is evident from the weaker EFG signals. The empty vector strain produces a low level of FB, which is increased moderately in the amyE::Pc(w)FB strain and about 100-fold over the wild-type level in the amyE::Pc(s)FB strain.
To further investigate the role of c-di-GMP, we used the amyE::Pc(s)FB construct in a background lacking dgcW, dgcP, and dgcK, the three diguanylate cyclases of Bsu (33). In this c-di-GMP null strain (ΔCYC), the overproduction of FB did not affect swarming (Fig. 3C), and Western blotting showed that the absence of the cyclases did not reduce the amount of FB (Fig. 4). Since FB is a high-affinity c-di-GMP binding protein (Table 1), these results strongly support a dose-dependent inhibition of swarming by FB when bound to c-di-GMP. MotI is a c-di-GMP receptor that prevents motility by directly interacting with the flagellar stator protein MotA (4). To determine whether MotI mediates the inhibitory effect of FB, we placed the amyE::Pc(s)FB construct in a ΔmotI ΔpdeH background. As shown in Fig. 3D, the inhibitory effect of FB on swarming is not reversed when motI is deleted. Thus, Bsu encodes two c-di-GMP receptors that independently inhibit swarming.
FB inhibits the function of flagella, not their production.
Swarming requires hyperflagellation, surfactin, raft formation, and the expression of SwrA (32, 35). Swimming, the flagellar-dependent movement of cells through a low-viscosity medium, requires neither SwrA nor surfactin (36). Thus, swimming can be measured in domesticated strains, which are deficient in the production of these factors (32). As shown in Fig. 5A, the amyE::Pc(s)FB construct inhibited swimming motility in “wild-type” domesticated Bsu even without the inactivation of pdeH. This contrasts with the partial effect of the same construct in the swarming assay (Fig. 3B) and may be due to the presence of an intact swrA gene in the NCIB3610 background, which biases the cells towards increased production of flagella (37). However, when the three diguanylate cyclases were deleted in the amyE::Pc(s)FB strain, swimming was not inhibited (Fig. 5B). We conclude that FB does not inhibit motility by interfering with surfactin or chemotaxis but likely impacts either the production or the activity of flagella. Next, we addressed whether the overexpression of FB interferes with flagella formation. Labeling with maleimide-Alexa fluor 488 to visualize the flagella of wild-type and amyE::Pc-RS-comFB ΔpdeH strains carrying the hagT209C allele (38) revealed that the overproduction of FB, which prevents swimming, does not obviously interfere with flagellar production even when pdeH is deleted (Fig. 5C), although we cannot exclude subtle effects on the number of flagella.
Fig. 5.
FB inhibits swimming but not the production of flagella. (A and B) The indicated strains were tested for swimming in 0.3% agar. The images were collected when the empty vector (MT) control strains had reached or nearly reached the outer limits of the Petri dishes. (C) The two indicated strains, both carrying the hagT209C allele, were reacted with Alexa Fluor 488 C5 maleimide (green), stained with the membrane dye FM4–64 (red), and imaged as described in Materials and Methods.
ComFC is required for swarming
The overlap of comFB and comFC suggests that the cognate proteins may be functionally related. To investigate the role of FC, we used a markerless construct in which the entire comFC coding sequence had been removed. This construct was derived from a ΔcomFC::erm deletion by removing the Erm-cassette with plasmid pDR244 (39). This was necessary because the cassette has a downward-facing promoter and readthrough from the PFA promoter during the K-state has been shown to enhance production of FlgM, leading to the loss of Hag (flagellin) synthesis (40). Surprisingly, the markerless ΔcomFC strain did not swarm (Fig. 3E). For complementation of ΔcomFC, an FC-expressing construct was placed at the thrC locus, using the Pc promoter and the gapA RBS. An identical ectopic construct with a FLAG epitope at the C-terminus of FC confirmed that the fusion protein was expressed (Fig. S3). The thrC::Pc(s)FC construct fully complemented ΔcomFC for swarming, showing that the observed phenotype is due to the lack of FC protein (Fig. 3E). When both comFB and comFC were deleted, swarming was unaffected, suggesting that the swarming-deficient phenotype of ΔcomFC is dependent on FB. These results suggest that FC may normally mitigate the ability of FB to inhibit swarming.
DISCUSSION
FB represents a new class of c-di-GMP receptors (6, 8), joining three others encoded by Bsu that regulate aspects of cell physiology; MotI inhibits motility (4), while YdaK and YkuI have been proposed to regulate the production of uncharacterized extracellular polysaccharides (41, 42). The four known c-di-GMP receptors of Bsu are classified into distinct protein families; YdaK is a degenerate GGDEF protein lacking cyclase activity, YkuI is an EAL domain protein without phosphodiesterase activity, and MotI is a PilZ domain protein (33, 34).
The overexpression of FB, when it is bound to c-di-GMP, appears to inhibit flagellar activity. The available evidence argues strongly for these conclusions; the elimination of c-di-GMP degradation by inactivation of pdeH augments FB’s activity (Fig. 3A and B), FB has no effect on motility in a cyclase null background (Fig. 3C), FB is a specific and high-affinity c-di-GMP binding protein ((6) and Table 1), and no obvious diminution in flagellar abundance was noted when FB was overexpressed even when pdeH was eliminated (Fig. 5C). This study has also revealed that FC is required for swarming, perhaps acting to mitigate the effect of FB. Clearly, the overlapping comFB and comFC genes are part of a previously undetected mechanism for the regulation of motility.
The most intriguing aspects of FB and FC are their biological roles; why are they transcribed from the PFB promoter in the absence of the K-state, and why is comFB transcribed in the K-state despite its dispensability for transformation (9)? Bsu encodes three known regulators of flagellar function: EpsE, MotI, and FB. EpsE contributes to the synthesis of a matrix polysaccharide and is transcriptionally activated as cells begin biofilm formation. In addition to its biosynthetic activity, EpsE, which does not appear to bind c-di-GMP, acts as a molecular clutch, most likely by binding to FliG, disrupting its interaction with MotA, and this activity benefits cells transitioning to a sessile state (38, 43). In contrast, MotI acts directly on MotA (4), but the rationale behind its activity and that of FB remains unclear. Since MotI and FB require c-di-GMP for their activity, they may moderate flagellar activity in response to elevated concentrations of the dinucleotide. For example, the repression of pdeH by Spo0A-P (34, 44), a regulator of various forms of development, will cause an increase in c-di-GMP as Spo0A becomes phosphorylated. This rise in c-di-GMP is expected to facilitate binding to FB by mass action, leading to reduced motility in coordination with Spo0A-P-regulated developmental processes. Given that the cellular concentration of c-di-GMP varies between cells of Bsu (44), the inhibition by FB (and MotI) may enable cells to reversibly and stochastically switch between motile and non-motile states while retaining their valuable flagella. It is noteworthy that the measured c-di-GMP binding affinities of MotI, FB, and YdaK are 0.011 μM, 0.14 μM, and 1.1 μM (Table 1 and (33)). It is possible that these differences in binding affinities allow the receptors to respond within overlapping but distinct ranges of c-di-GMP concentrations.
As noted above, the comF operon lies immediately upstream of a motility-related locus that encodes FlgM, the anti-sigma factor for SigD (45). Read-through from the ComK-dependent PFA promoter in K-state cells has been shown to inhibit flagellar synthesis via the enhanced expression of flgM (40). It has also been demonstrated that flagellar activity favors transitions to the K-state by downregulating the phosphorylation of the response regulator DegU (46, 47). Thus, while flagellar activity facilitates entry into the K-state, further flagellar synthesis is restricted by read-through to flgM after cells have transcribed the comF operon. Thus, the K-state is linked to the regulation of both flagellar synthesis and function. Interestingly, a cyanobacterial ComFB ortholog binds c-di-AMP to control natural competence (48), underscoring the relationship of this receptor with transformation, albeit in this case with a different second messenger. Gaining a biological understanding of these connections will necessitate deeper insights into the FB-FC regulatory system, identifying the FB target for motility inhibition, and exploring the potential roles of FB in the control of the K-state itself.
MATERIALS AND METHODS
Strains, strain construction, and growth conditions.
Growth was in LB Medium, except that competent cells for transformation were grown as described (49). Antibiotics were added to solid media at the following concentrations: chloramphenicol (7 μg/ml), erythromycin and kanamycin (5 μg/ml), spectinomycin (100 μg/ml), and tetracycline (20 μg/ml). Two backgrounds were used as indicated: the domesticated parental strain (IS75) is a derivative of strain 168 and is auxotrophic for histidine, leucine, and methionine.
The parental undomesticated strain (BD5776) is a comI mutant derivative of NCIB 3610, gifted by D. Kearns. Constructs were generally introduced by transformation into both backgrounds, but in some cases by transduction with bacteriophage SPP1. Strains are listed in Table S1 and plasmids in Table S2. Construction of the ΔdgcK dgcW::erm dgcP::tet knockout strain in the IS75 background was initiated by transformation with genomic DNA from DK392 (dgcP::tet), a kind gift from D. Kearns, with selection for tetracycline. The Koo et al knockout collection (39) was used as a source of dgcW::erm and dgcK::erm deletions. dgcK::erm was first introduced into the dgcP::tet strain, and the erythromycin-resistance cassette was looped out using pDR244, as described (39). The resulting transformant was then transformed with dgcW::erm to produce the triple knockout erythromycin- and tetracycline-resistant strain (Table S1). For the insertion of constructs into amyE, plasmid DNA was linearized using ScaI and transformed into IS75 or BD5776 strains, selecting for spectinomycin resistance. The resulting transformants were tested for insertion in amyE using starch plates stained with iodine. For the insertion into thrC, plasmid DNA was cut with PvuI and transformed into the appropriate Bsu strain, selecting for erythromycin resistance, followed by tests for threonine auxotrophy and the absence of spectinomycin resistance.
Measurement of c-di-GMP binding to FB
Bacillus subtilis ComFB protein was heterologously expressed as an 8xHis-tagged protein in E. coli as described previously (6). Briefly, the 8xHis-tagged constructs were expressed by overnight cultivation of E. coli cells at 20°C in the presence of 0.5 mM IPTG and purified by immobilized metal affinity chromatography using Ni2+-Sepharose resin (Cytiva™), followed by size exclusion chromatography on a Superdex 200 Increase 10/300 GL column (GE HealthCare, Munich, Germany), as described previously (50). Protein purity was assessed by Coomassie-stained SDS-PAGE, and protein concentrations were determined using Bradford assay.
Binding of c-di-GMP to FB protein was analyzed by isothermal titration calorimetry (ITC), as described previously (51). In a buffer composed of (50 mM Tris/HCl, pH 8.0, 300 mM NaCl, 0.55 mM EDTA), the ITC measurements were conducted using a MicroCal PEAQ-ITC instrument (Malvern Panalytical, Westborough, MA, USA), at 25 °C, with a reference power of 10 μcal/s. Dissociation constants KD and ΔH values were calculated using the single binding site model with the MicroCal PEAQ-ITC Analysis Software (Malvern Panalytical). In the competition binding assays, the FB protein was incubated with 150 μM or 500 μM of (ATP, ADP, GTP, cAMP or cGMP) nucleotides and titrated against 1 mM of c-di-GMP.
Before ITC experiments were conducted, analytical size exclusion chromatography coupled to a multiangle light scattering (MALS) detector was carried out at room temperature, as described previously (50, 52, 53) using the ÄKTA purifier (GE Healthcare) on Superdex™ 200 column. ASTRA software (Wyatt) was used for data analysis and molecular mass calculations using the MALS data. The elution volume was plotted against the UV signal and molecular mass.
Molecular constructs
amyE::Pc(w)FB:
The oligonucleotides FBWHinF (GCCATGAAGCTTCCGATGATGAGGAGCTG) and FBBamR (ATCGCCGGATCCGCGAATCACATAATAAACAGATC) were used in a PCR to isolate a comFB fragment containing the native RBS sequence that was then inserted between the HindIII and BamHI sites of pKB149 (33).
amyE::Pc(s)FB:
The oligonucleotide FBSHinF (GCCATGAAGCTTAAAGGAGGAAACAATCATGCTTGTCAATTCAAAAGA) was used in a PCR with FBBamR to produce a comFB fragment containing the gapA RBS that was inserted between the HindIII and BamHI sites of pKB149.
thrC::Pc(s)FB and thrC::Pc(s)FB3xFLAG:
These constructs were built using NEBuilder HiFi (New England Biolabs), and inserted into the HindIII site of pDG1664 for insertion in thrC (54). To obtain the Pc gapA RBS sequence, the oligonucleotides Pc(s)F (TGCTGCCTTCGGATCCTAGAAGCTTCTACACAGCCCAGTCCAG) and Pc(s)-R (CGTTTGACATGATTGTTTCCTCCTTTAAGCTTC) were used, with Pc(s)FB plasmid DNA as the template. To obtain the FC sequence, the oligonucleotides FC F (GGAAACAATCATGTCAAACGAAGCAGGAG) and FC-R (TAGGGTTATCGAATTCGATAAGCTTTTAGCTTCTGATCAAGGTAAAAG were used. For the equivalent FLAG-tagged construct, the FC-R-FLAG oligonucleotide (TAGGGTTATCGAATTCGATAAGCTTTCACTTGTCGTCATCGTC) was used. For the latter two PCR reactions, the template DNA was from wild-type Bsu and BD9425 (Table S1) genomic DNA, respectively.
ΔcomFC:
Strain BKE35450 (ΔcomFC::erm) (39) distributed by the Bacillus Genetic Stock Center, was the source of this deletion. After transforming into the appropriate backgrounds, the Errn-resistance cassette with its promoter was removed, using plasmid pDR244, as described (39).
Swarm and Swim tests.
Swarm expansion was measured using 0.7% LB agar Petri dishes as described by Gao et al (33). The plates were dried for about 10 minutes after spotting in the center with each strain and then incubated at 37 °C in a humidified incubator by placing a large water-filled tray on the bottom shelf. The diameter at the outermost edge of the swarm was measured at intervals. The swim assays were performed essentially as described by Hall et al (36). Petri dishes containing 0.3% LB agar were toothpick-inoculated with the desired strain and then incubated in a humidified incubator overnight at 33 °C, followed by an interval at 37 °C. The plates were imaged in a ChemiDoc MP Bio-Rad imager when the fastest-swimming strain had nearly reached the perimeter of its plate.
Western blotting.
Anti-FB Western blotting from 12.5% tricine-SDS-polyacrylamide gels (55) was carried out by semidry blotting onto nitrocellulose membranes. The blots were developed using HRP-coupled goat anti-rabbit secondary antiserum (for FB and EFG) or goat anti-mouse secondary antiserum (for FC-3xFLAG) from Abcam and ECL kits from Amersham. The images were recorded with a Bio-Rad ChemiDoc MP imager. For loading controls, the top of the membrane was cut off after blotting and developed separately with anti-elongation factor G (anti-EFG) antiserum, a kind gift from Jonathan Dworkin (Columbia University Medical School). The anti-FB polyclonal antiserum was raised in rabbits by the Thermofisher custom antibody service using FB protein, purified as described by the Burton lab (9), using the His-tagged expression construct kindly provided by B. Burton.
Flagellar labeling and microscopy
For the fluorescence microscopy of flagella filaments, cells were grown at 37 °C in LB broth until they reached an OD600 of 0.5–0.8. One milliliter of broth culture was harvested and resuspended in 50 μl of PBS containing 5 μg ml−1 Alexa Fluor 488 C5 maleimide (Thermofisher), and I μg/ml FM4–64. This suspension was incubated in the dark at room temperature for 5 min. Cells were then washed gently twice with 1 ml of PBS and resuspended to an OD600 of 10. Samples were observed by spotting 1 μl of the suspension on an agarose pad. Images were collected using a Nikon Eclipse Ti microscope equipped with an Orca Flash 4.0 Digital camera (Hamamatsu) with a Nikon TIRF 1.45 NA Plan Neofluor 100 oil immersion objective. NIS-Elements AR (v 4.40, Nikon) software was used for image acquisition.
Supplementary Material
IMPORTANCE.
Bacterial motility is subject to tight regulation, and the second messenger c-di-GMP is often involved in the production and activity of flagella. Revealing the mechanisms of these regulatory pathways is broadly important for understanding bacterial motility and of c-di-GMP-related processes. We show that ComFB is a specific, high-affinity receptor for c-di-GMP that decreases the activity of flagella to control swarming and swimming motility in Bacillus subtilis.
ACKNOWLEDGEMENTS
Work in the Dubnau lab was supported by NIH grant R01GM057720. We thank Dan Kearns (Indiana University) for his generous gift of many strains, unstinting advice, and insightful suggestions. We also thank Matthew Neiditch and Karl Forchhammer (Tübingen University) for valuable discussions throughout this work and continued support. The KAS laboratory is funded by grants from the German Research Foundation (DFG) as part of the Emmy Noether program (SE 3449/3-1), the priority research program (SPP2389; SE 3449/1-1), and by the collaborative research center SFB1381 (project number: 403222702). KAS also gratefully acknowledges the infrastructural support and funding by the collaborative research center MibiNet (SFB1535 - Project ID 458090666) and the Cluster of Excellence “Controlling Microbes to Fight Infections (CMFI)” (EXC2124-390838134). SS is funded by a scholarship from the Egyptian Ministry of Higher Education. We are also grateful to Filipp Oesterhelt (Tübingen University) for facilitating the utilization of the ITC.
REFERENCES
- 1.Schavemaker PE, Lynch M. 2022. Flagellar energy costs across the tree of life. Elife 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jenal U, Reinders A, Lori C. 2017. Cyclic di-GMP: second messenger extraordinaire. Nat Rev Microbiol 15:271–284. [DOI] [PubMed] [Google Scholar]
- 3.Sun QX, Huang M, Zhang JY, Zeng X, Zhang CC. 2023. Control of Cell Size by c-di-GMP Requires a Two-Component Signaling System in the Cyanobacterium Anabaena sp. Strain PCC 7120. Microbiol Spectr 11:e0422822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Subramanian S, Gao X, Dann CE 3rd, Kearns DB. 2017. MotI (DgrA) acts as a molecular clutch on the flagellar stator protein MotA in Bacillus subtilis. Proc Natl Acad Sci U S A 114:13537–13542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zeng X, Huang M, Sun QX, Peng YJ, Xu X, Tang YB, Zhang JY, Yang Y, Zhang CC. 2023. A c-di-GMP binding effector controls cell size in a cyanobacterium. Proc Natl Acad Sci U S A 120:e2221874120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Samir S, Elshereef AA, Alva V, Hahn J, Dubnau D, Galperin MY, Selim KA. 2024. ComFB, a new widespread family of c-di-NMP receptor proteins. bioRxiv doi: 10.1101/2024.11.10.622515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Galperin MY, Chou SH. 2020. Structural Conservation and Diversity of PilZ-Related Domains. J Bacteriol 202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Samir S, Doello S, Zimmer E, Haffner M, Enkerlin AM, Müller T, Dengler L, Lambidis SP, Sivabalasarma S, Albers S-V, Selim KA. 2023. The second messenger c-di-AMP controls natural competence via ComFB signaling protein. bioRxiv doi: 10.1101/2023.11.27.568819:2023.11.27.568819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sysoeva TA, Bane LB, Xiao DY, Bose B, Chilton SS, Gaudet R, Burton BM. 2015. Structural characterization of the late competence protein ComFB from Bacillus subtilis. Biosci Rep 35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Londono-Vallejo JA, Dubnau D. 1993. comF, a Bacillus subtilis late competence locus, encodes a protein similar to ATP-dependent RNA/DNA helicases. Mol Microbiol 9:119–31. [DOI] [PubMed] [Google Scholar]
- 11.Diallo A, Foster HR, Gromek KA, Perry TN, Dujeancourt A, Krasteva PV, Gubellini F, Falbel TG, Burton BM, Fronzes R. 2017. Bacterial transformation: ComFA is a DNA-dependent ATPase that forms complexes with ComFC and DprA. Mol Microbiol 105:741–754. [DOI] [PubMed] [Google Scholar]
- 12.Dubnau D, Blokesch M. 2019. Mechanisms of DNA Uptake by Naturally Competent Bacteria. Annu Rev Genet 53:217–237. [DOI] [PubMed] [Google Scholar]
- 13.Hernandez-Plaza A, Szklarczyk D, Botas J, Cantalapiedra CP, Giner-Lamia J, Mende DR, Kirsch R, Rattei T, Letunic I, Jensen LJ, Bork P, von Mering C, Huerta-Cepas J. 2023. eggNOG 6.0: enabling comparative genomics across 12 535 organisms. Nucleic Acids Res 51:D389–D394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Berka RM, Hahn J, Albano M, Draskovic I, Persuh M, Cui X, Sloma A, Widner W, Dubnau D. 2002. Microarray analysis of the Bacillus subtilis K-state: genome-wide expression changes dependent on ComK. Mol Microbiol 43:1331–45. [DOI] [PubMed] [Google Scholar]
- 15.Hamoen LW, Smits WK, de Jong A, Holsappel S, Kuipers OP. 2002. Improving the predictive value of the competence transcription factor (ComK) binding site in Bacillus subtilis using a genomic approach. Nucleic Acids Res 30:5517–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ogura M, Yamaguchi H, Kobayashi K, Ogasawara N, Fujita Y, Tanaka T. 2002. Whole-genome analysis of genes regulated by the Bacillus subtilis competence transcription factor ComK. J Bacteriol 184:2344–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Maier B. 2020. Competence and Transformation in Bacillus subtilis. Curr Issues Mol Biol 37:57–76. [DOI] [PubMed] [Google Scholar]
- 18.Miras M, Dubnau D. 2016. A DegU-P and DegQ-Dependent Regulatory Pathway for the K-state in Bacillus subtilis. Front Microbiol 7:1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hahn J, Tanner AW, Carabetta VJ, Cristea IM, Dubnau D. 2015. ComGA-RelA interaction and persistence in the Bacillus subtilis K-state. Mol Microbiol 97:454–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Haijema BJ, Hahn J, Haynes J, Dubnau D. 2001. A ComGA-dependent checkpoint limits growth during the escape from competence. Mol Microbiol 40:52–64. [DOI] [PubMed] [Google Scholar]
- 21.Veening JW, Smits WK, Kuipers OP. 2008. Bistability, epigenetics, and bet-hedging in bacteria. Annu Rev Microbiol 62:193–210. [DOI] [PubMed] [Google Scholar]
- 22.Hamoen LW, Van Werkhoven AF, Bijlsma JJ, Dubnau D, Venema G. 1998. The competence transcription factor of Bacillus subtilis recognizes short A/T-rich sequences arranged in a unique, flexible pattern along the DNA helix. Genes Dev 12:1539–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nicolas P, Mader U, Dervyn E, Rochat T, Leduc A, Pigeonneau N, Bidnenko E, Marchadier E, Hoebeke M, Aymerich S, Becher D, Bisicchia P, Botella E, Delumeau O, Doherty G, Denham EL, Fogg MJ, Fromion V, Goelzer A, Hansen A, Hartig E, Harwood CR, Homuth G, Jarmer H, Jules M, Klipp E, Le Chat L, Lecointe F, Lewis P, Liebermeister W, March A, Mars RA, Nannapaneni P, Noone D, Pohl S, Rinn B, Rugheimer F, Sappa PK, Samson F, Schaffer M, Schwikowski B, Steil L, Stulke J, Wiegert T, Devine KM, Wilkinson AJ, van Dijl JM, Hecker M, Volker U, Bessieres P, et al. 2012. Condition-dependent transcriptome reveals high-level regulatory architecture in Bacillus subtilis. Science 335:1103–6. [DOI] [PubMed] [Google Scholar]
- 24.Lalanne JB, Taggart JC, Guo MS, Herzel L, Schieler A, Li GW. 2018. Evolutionary Convergence of Pathway-Specific Enzyme Expression Stoichiometry. Cell 173:749–761 e38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Taggart JC, Lalanne J-B, Durand S, Braun F, Condon C, Li G-W. 2023. A high-resolution view of RNA endonuclease cleavage in Bacillus subtilis. bioRxiv doi: 10.1101/2023.03.12.532304:2023.03.12.532304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chou SH, Galperin MY. 2016. Diversity of Cyclic Di-GMP-Binding Proteins and Mechanisms. J Bacteriol 198:32–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Herzberg C, Meissner J, Warneke R, Stulke J. 2023. The many roles of cyclic di-AMP to control the physiology of Bacillus subtilis. Microlife 4:uqad043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mantovani O, Haffner M, Selim KA, Hagemann M, Forchhammer K. 2023. Roles of second messengers in the regulation of cyanobacterial physiology: the carbon-concentrating mechanism and beyond. Microlife 4:uqad008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huber M, Faure G, Laass S, Kolbe E, Seitz K, Wehrheim C, Wolf YI, Koonin EV, Soppa J. 2019. Translational coupling via termination-reinitiation in archaea and bacteria. Nat Commun 10:4006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hengge R. 2009. Principles of c-di-GMP signalling in bacteria. Nat Rev Microbiol 7:263–73. [DOI] [PubMed] [Google Scholar]
- 31.Khan F, Jeong GJ, Tabassum N, Kim YM. 2023. Functional diversity of c-di-GMP receptors in prokaryotic and eukaryotic systems. Cell Commun Signal 21:259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kearns DB, Losick R. 2003. Swarming motility in undomesticated Bacillus subtilis. Mol Microbiol 49:581–90. [DOI] [PubMed] [Google Scholar]
- 33.Gao X, Mukherjee S, Matthews PM, Hammad LA, Kearns DB, Dann CE 3rd. 2013. Functional characterization of core components of the Bacillus subtilis cyclic-di-GMP signaling pathway. J Bacteriol 195:4782–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen Y, Chai Y, Guo JH, Losick R. 2012. Evidence for cyclic Di-GMP-mediated signaling in Bacillus subtilis. J Bacteriol 194:5080–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kearns DB. 2010. A field guide to bacterial swarming motility. Nat Rev Microbiol 8:634–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hall AN, Subramanian S, Oshiro RT, Canzoneri AK, Kearns DB. 2018. SwrD (YlzI) Promotes Swarming in Bacillus subtilis by Increasing Power to Flagellar Motors. J Bacteriol 200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kearns DB, Losick R. 2005. Cell population heterogeneity during growth of Bacillus subtilis. Genes Dev 19:3083–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Blair KM, Turner L, Winkelman JT, Berg HC, Kearns DB. 2008. A molecular clutch disables flagella in the Bacillus subtilis biofilm. Science 320:1636–8. [DOI] [PubMed] [Google Scholar]
- 39.Koo BM, Kritikos G, Farelli JD, Todor H, Tong K, Kimsey H, Wapinski I, Galardini M, Cabal A, Peters JM, Hachmann AB, Rudner DZ, Allen KN, Typas A, Gross CA. 2017. Construction and Analysis of Two Genome-Scale Deletion Libraries for Bacillus subtilis. Cell Syst 4:291–305 e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu J, Zuber P. 1998. A molecular switch controlling competence and motility: competence regulatory factors ComS, MecA, and ComK control sigmaD-dependent gene expression in Bacillus subtilis. J Bacteriol 180:4243–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chandrangsu P, Helmann JD. 2016. Intracellular Zn(II) Intoxication Leads to Dysregulation of the PerR Regulon Resulting in Heme Toxicity in Bacillus subtilis. PLoS Genet 12:e1006515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bedrunka P, Graumann PL. 2017. Subcellular clustering of a putative c-di-GMP-dependent exopolysaccharide machinery affecting macro colony architecture in Bacillus subtilis. Environ Microbiol Rep 9:211–222. [DOI] [PubMed] [Google Scholar]
- 43.Guttenplan SB, Blair KM, Kearns DB. 2010. The EpsE flagellar clutch is bifunctional and synergizes with EPS biosynthesis to promote Bacillus subtilis biofilm formation. PLoS Genet 6:e1001243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Weiss CA, Hoberg JA, Liu K, Tu BP, Winkler WC. 2019. Single-Cell Microscopy Reveals That Levels of Cyclic di-GMP Vary among Bacillus subtilis Subpopulations. J Bacteriol 201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mukherjee S, Kearns DB. 2014. The structure and regulation of flagella in Bacillus subtilis. Annu Rev Genet 48:319–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Diethmaier C, Chawla R, Canzoneri A, Kearns DB, Lele PP, Dubnau D. 2017. Viscous drag on the flagellum activates Bacillus subtilis entry into the K-state. Mol Microbiol 106:367–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Holscher T, Schiklang T, Dragos A, Dietel AK, Kost C, Kovacs AT. 2018. Impaired competence in flagellar mutants of Bacillus subtilis is connected to the regulatory network governed by DegU. Environ Microbiol Rep 10:23–32. [DOI] [PubMed] [Google Scholar]
- 48.Samir S, Doello S, Enkerlin AM, Zimmer E, Haffner M, Müller T, Dengler L, Lambidis SP, Sivabalasarma S, Albers S-V, Selim KA. 2025. The second messenger c-di-AMP controls natural competence via ComFB signaling protein. bioRxiv doi: 10.1101/2023.11.27.568819:2023.11.27.568819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dubnau D, Davidoff-Abelson R. 1971. Fate of transforming DNA following uptake by competent Bacillus subtilis. I. Formation and properties of the donor-recipient complex. J Mol Biol 56:209–221. [DOI] [PubMed] [Google Scholar]
- 50.Selim KA, Lapina T, Forchhammer K, Ermilova E. 2020. Interaction of N-acetyl-l-glutamate kinase with the PII signal transducer in the non-photosynthetic alga Polytomella parva: Co-evolution towards a hetero-oligomeric enzyme. FEBS J 287:465–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Selim KA, Haase F, Hartmann MD, Hagemann M, Forchhammer K. 2018. P(II)-like signaling protein SbtB links cAMP sensing with cyanobacterial inorganic carbon response. Proc Natl Acad Sci U S A 115:E4861–E4869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Selim KA, Haffner M, Watzer B, Forchhammer K. 2019. Tuning the in vitro sensing and signaling properties of cyanobacterial PII protein by mutation of key residues. Sci Rep 9:18985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Walter J, Selim KA, Leganes F, Fernandez-Pinas F, Vothknecht UC, Forchhammer K, Aro EM, Gollan PJ. 2019. A novel Ca(2+)-binding protein influences photosynthetic electron transport in Anabaena sp. PCC 7120. Biochim Biophys Acta Bioenerg 1860:519–532. [DOI] [PubMed] [Google Scholar]
- 54.Guerout-Fleury AM, Frandsen N, Stragier P. 1996. Plasmids for ectopic integration in Bacillus subtilis. Gene 180:57–61. [DOI] [PubMed] [Google Scholar]
- 55.Schagger H, von Jagow G. 1987. Tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis for the separation of proteins in the range from 1 to 100 kDa. Anal Biochem 166:368–379. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.