Abstract
3D-printing has emerged as a leading technology for fabricating personalized scaffolds for bone regeneration. Among the 3D-printing technologies, vat photopolymerization (VP) stands out for its high precision and versatility. It enables the creation of complex, patient-specific scaffolds with advanced pore architectures that enhance mechanical stability and promote cell growth, key factors for effective bone regeneration. This review provides an overview of the advances made in vat photopolymerization printing of calcium phosphates, covering both the fabrication of full ceramic bodies and polymer-calcium phosphate composites. The review examines key aspects of the fabrication process, including slurry composition, architectural design, and printing accuracy, highlighting their impact on the mechanical and biological performance of 3D-printed scaffolds. The need to tailor porosity, pore size, and geometric design to achieve both mechanical integrity and biological functionality is emphasized by a review of data published in the recent literature. This review demonstrates that advanced geometries like Triply Periodic Minimal Surfaces and nature-inspired designs, achievable with exceptional precision by this technology, enhance mechanical and osteogenic performance. In summary, VP's versatility, driven by the diversity of material options, consolidation methods, and precision opens new horizons for scaffold-based bone regeneration.
Keywords: Additive manufacturing, 3D printing, Vat polymerization, Hydroxyapatite, Scaffold, Bone regeneration
Graphical abstract
Highlights
-
•
Vat photopolymerization excels in the fabrication of precise and personalized 3D calcium phosphate-based scaffolds.
-
•
Vat photopolymerization supports fabricating both full ceramic and composite calcium phosphate scaffolds.
-
•
The advanced pore designs enabled by Vat photopolymerization promote efficient bone regeneration.
-
•
Vat Photopolymerization enables the fabrication of complex calcium phosphate scaffolds with tailored mechanical properties.
Structured abstractPurpose: 3D printing has steadily garnered increasing popularity for fabricating bone scaffolds. In the last years, vat photopolymerization has emerged as a groundbreaking 3D printing technique for calcium phosphate scaffolds, owing to its high versatility, exceptional printing precision, and material options. This review aims to provide a comprehensive and novel analysis of the advances in vat photopolymerization of calcium phosphate-based scaffolds for bone regeneration, highlighting innovative approaches and strategies.Procedure: This review was conducted through an advanced bibliographic search using Scopus, Web of Science, and PubMed. The search focused on a combination of the following areas of interest: vat photopolymerization techniques, the inclusion of calcium phosphate bioceramics, and bone related applications. The selected studies were meticulously analyzed for key aspects such as resin formulations, manufacturing parameters, rheological properties, post-printing processes, mechanical properties, and, finally, biological performances. A particular emphasis was placed on comparing the mechanical performance of the scaffolds based on their composition and architecture, offering a unique categorization and comparative analysis.Results: This study offers a deep insight of the latest studies, by providing details with a novel approach to understand the field's progress. Two primary fabrication routes were determined, each leading to distinct structural and functional properties. Namely, over 75 % of the studies focused on creating full ceramic bodies, while the remaining 25 % explored composite structures. Additionally, this study discusses the latest strategies performed by researches to enhance both the mechanical and biological performance of bone scaffolds. These include advanced innovative scaffold compositions, post-processing techniques, and architectural designs.
1. Introduction
Bone is a highly hierarchical and metabolically active tissue that provides support and protection to vital organs, stores essential ions, and plays a critical role in maintaining bone homeostasis. Despite its regenerative capacity, bone healing can be impaired by systemic factors like age, diabetes or malnutrition, and local factors like poor blood supply, infection, or the presence of large defects caused by trauma or tumor resection. In such scenarios, bone grafting is a common procedure to restore bone function and structure. Worldwide, an estimated 2.2 million bone grafting procedures are performed annually, and are expected to grow by 13 % each year [1]. While autologous bone grafts, so-called autografts, remain the gold standard, the use of synthetic bone grafts for orthopedic interventions strongly surged from 11.8 % in 2008 to 23.9 % in 2018 [2]. In the last ten years, the demand for synthetic-based biomaterials increased by 134 % compared to autografts, which varied by 74 %, and allografts, whose demand decreased by 14 % [2].
Bone tissue engineering addresses large bone defects by integrating biomaterials, cells, and signaling molecules with scaffolds. These temporary structures support bone remodeling while minimizing complications [3]. They enable defect filling with personalized shapes, providing mechanical support, and guiding new tissue growth [4]. Designed to mimic the extracellular matrix, scaffolds facilitate cell adhesion, proliferation, differentiation, and the exchange of nutrients and waste while gradually degrading and being replaced by newly formed bone [5,6].
Synthetic bone grafts have been extensively developed and have achieved clinical relevance, particularly calcium phosphate (CaP) bioceramics. CaPs closely resemble the mineral phase of natural bone in terms of composition and have been shown to be biocompatible, bioactive and osteoconductive. Moreover, in certain configurations, they have demonstrated osteoinductive properties, promoting the differentiation of cells into the bone lineage [7]. One of the main motivations fostering CaP research is the key role of Ca mineralization in endochondral ossification during early fetal development, leading to bone tissue formation. On the other hand, the degradation of certain synthetic CaP-based biomaterials can be integrated into the physiological process of bone remodeling. Finally, the presence of calcium (Ca2+) and phosphorous (Pi), at specific concentrations, can enhance the proliferation and differentiation of osteoprogenitor cells [8]. Despite their success, their application is largely limited to non-load-bearing scenarios due to their inherent brittleness. To address these structural limitations, calcium phosphates have been combined with biocompatible polymers, providing enhanced elasticity and toughness [9].
Despite the close resemblance to the natural bone mineral phase, CaP geometrical design has been a limiting factor in their overall structural integrity and implementation. Often produced in standardized shapes or blocks, CaPs pre-formed designs fail to adapt to patient-specific bone defects, requiring manual adjustments and tailoring by surgeons, which can further compromise material properties and the clinical outcome. Traditional methods such as particulate leaching [10,11], emulsions, freeze-drying, foaming [12], or the use of templates enable the fabrication of porous scaffolds with high interconnectivity and porosity, similar to bone natural structure [13]. However, while offering simplicity and versatility, these techniques yield poor reproducibility and low patient-specificity [14]. The emergence of cutting edge technologies such as additive manufacturing (AM) has enabled the creation of complex customized scaffolds, with interconnected macroporous architectures that mimic the structure and hierarchy of natural bone [4]. Additive manufacturing (AM), and more precisely 3D printing offers a promising approach to personalized medicine. It allows fabricating patient-specific scaffolds with complex geometries, with high reproducibility and accuracy, not only of the external shape but also of the internal architecture [6].
The ASTM F2792-12a standard identifies seven distinct 3D printing technologies: binder jetting, directed energy deposition, material extrusion, material jetting, powder bed fusion, sheet lamination, and vat photopolymerization. Regarding ceramic 3D printing, these seven technologies can be categorized into four groups: i) Powder bed-based AM, where a roller or scraper system spreads a layer of powder or slurry onto the printing substrate, and a device selectively binds the desired regions; ii) Dispensing-based AM, which uses a dispensing device controlled by a 3D positioning system to progressively deposit material layer by layer, forming a 3D structure; iii) Lamination-based AM, where sheets of material are layered on the printing area, and a cutting device defines the regions of interest; and iv) Vat photopolymerization AM, based on the polymerization of liquid polymer precursors or suspensions with the incorporation of colloidal ceramic particles in the case of ceramic printing [15]. Each of these techniques has its advantages and limitations for the printing of calcium phosphate-based scaffolds, in terms of resolution and material selection.
Dispensing-based AM techniques, such as fused deposition modelling (FDM) and direct ink writing (DIW) are very versatile in terms of materials, allowing to print different polymers loaded with calcium phosphate particles, which can be subsequently sintered or not, resulting in full ceramic or composite scaffolds. Ceramic-FDM works by depositing a composite filament from a nozzle by reducing its viscosity through melting. It has been widely adopted with CaPs to create suitable and robust composites by incorporating biocompatible and bioresorbable thermoplastic polymers such as polycaprolactone (PCL) [16,17] or polylactic acid (PLA) [[18], [19], [20]] without requiring solvents. This approach has enabled the fabrication of dimensionally accurate and mechanically enhanced scaffolds [17]. However, FDM has limitations in terms of ceramic loading, typically achieving optimum loadings ranging from 5 to 15 wt%, with some exceptions incorporating up to 30 to 50 wt% [20]. These values are significantly lower than the mineral content of natural bone, which is around 60–70 wt% [21]. In contrast, DIW, which entails the extrusion of a paste, operates at low temperatures (e.g., room temperature), offering new possibilities for bone scaffold manufacturing [9]. For instance, it can be made compatible with cell printing, which is not possible when using FDM [22]. Additionally, DIW supports higher ceramic loading, enabling the extrusion of highly loaded ceramic pastes that, after post-processing, result in full-ceramic 3D printed porous bioceramic scaffolds [[23], [24], [25]]. To overcome the brittleness of full-ceramic scaffolds, biocompatible polymers such as poly-lactic-co-glycolic acid (PLGA) [26], and PCL [9,27] have also been incorporated into the inks for DIW, resulting in composite scaffolds with enhanced toughness. This approach, compared to FDM, allows increasing the ceramic content, reaching up to 70 wt% and showing improved mechanical and biological properties. However, material extrusion AM requires ceramic suspensions with appropriate viscosity and shear-thinning behavior [28]. To ensure smooth and steady extrusion, the nozzle size is generally large in order to meet a feasible extruding force, ranging from hundreds to thousands of newtons for high-load suspensions. This creates a trade-off between printing accuracy and printability [29]. As a result, printing resolutions are typically above 100 μm [30], limiting the possibility of creating complex structures with high precision that could mimic the architecture of natural bone, even when support structures are used [31].
Higher resolution AM methods such as powder bed-based techniques are also commonly used for scaffold fabrication. Powder bed fusion (PBF) uses a high-energy laser or electron beam to selectively melt (Selective Laser Melting; SLM) or sinter (Selective Laser Sintering; SLS) the spread powder, bonding the particles into a dense structure. In contrast, Binder Jetting (BJ) uses a dispensing nozzle to selectively deposit a liquid binder onto the powder, bonding the particles together in specific areas. These AM techniques allow the production of complex geometries and architectures with the great advantage of not needing support structures in the printing process. However, each technique has its trade-offs when fabricating CaP scaffolds. SLS-based strategies pose a number of challenges in both processing and post-processing. CaPs exhibit very high melting temperatures, making them hard to process, as they require high-power lasers capable of heating ceramic powders to densify particles into stacked 2D layers [32]. While PBF provides high mechanical properties, high precision, and no need for binders, the melting/sintering process may cause phase transformations, temperature gradients and internal residual stresses and cracks [33]. One possible alternative is the incorporation of lower-melting-point thermoplastic polymers such as PCL, poly-L-lactic acid (PLLA), polyether ether ketone (PEEK), or polyamide (PA) to obtain composite structures [34]. However, achieving high ceramic loadings is challenging, as it requires increased laser energy, creating a compromise between ceramic loading, printability, and energy consumption. As a result, composite scaffolds fabricated with PBF normally contain 5-50 wt% ceramic content [34]. In opposition, BJ operates at low processing temperature, resulting in lower probability of cracks and no residual stresses. This technique also enables higher ceramic loadings, reaching >70 wt% [35]. Nonetheless, BJ-printed scaffolds experience lower mechanical strength, and lower resolution due to potential binder infiltration, affecting the scaffold's final porosity. Overall, powder bed-based AM techniques are promising approaches for creating complex scaffolds with high precision, achieving layer thicknesses of about 30–200 μm for SLS/SLM and 50–200 μm for BJ [32,36]. However, they are often limited in terms of resulting composition, as a sintering process is needed to remove the binder and fuse the ceramic particles together. Additionally, the large size and high cost of these machines are limiting factors when adapting their integration into clinical or hospital settings.
In this context, Vat Photopolymerization (VP) appears as an innovative technique that has gained increasing attention in medical applications, especially due to its high precision, achieving resolutions of a few tens of micrometers. Compared to other AM techniques, VP offers significant advantages in addressing some of the previously mentioned challenges. Specifically, calcium phosphate particles are mixed into liquid resins to form ceramic suspensions, which are processed in vats where light selectively solidifies layers at low temperatures. Unlike FDM, which requires the material to pass through heated nozzles, VP enables the use of resins heavily loaded with calcium phosphate particles (10–70 wt%) [[37], [38], [39]]. While VP does present challenges related to light-material interactions, it offers greater design capability than material extrusion techniques, enabling the fabrication of complex geometries similar to the architecture of natural bone. Additionally, although challenging, the rheological demands are less stringent than in material extrusion techniques. Furthermore, compared to SLS/SLM, VP does not operate with highly powered lasers and does not require high temperatures, making this technique a more affordable variant that could potentially be integrated into clinical and hospital settings. The risk of defects such as cracks, phase transformations, and dimensional distortions during printing is reduced. However, VP requires extensive post-processing, including cleaning, which can be difficult with tight and low-porous designs, often leading to pore occlusions and residual uncured resin inside the structure. Moreover, common resins often contain toxic components, requiring a binder removal step followed by sintering, resulting in mechanically weak full-ceramic parts. Recent advances in the formulation of biocompatible resins have expanded the potential for obtaining composite structures, a promising approach to overcome post-processing limitations, and enhance mechanical and biological performances.
This review aims to summarize the progress and future perspectives of VP manufacturing of calcium phosphate-based scaffolds for bone tissue engineering, including: (1) the principles of CaP VP printing and the slurry requirements; (2) the composition and processing strategies of VP-printed CaP-scaffolds; (3) the effect of the material and processing routes on the mechanical and biological response; and (4) the strategies that are being explored to improve the mechanical and biological performance of the scaffolds. Although ceramic VP has been used with various bioceramics and bioactive glasses, this study focuses specifically on calcium phosphates as key materials in bone grafting due to their close resemblance to the mineral phase of bone. Furthermore, the growing interest toward incorporating these materials into advanced VP approaches cannot be overlooked, as it holds significant relevance for an increasingly engaged research community (Fig. S1 in Supplementary information). This review was conducted through a bibliographic search of scientific articles using three databases, Scopus, Web of Science, and PubMed. Other sources of information such as patents or clinical study reports may contain valuable but more fragmented and less accessible information, and were considered outside the scope of this study. Similarly, this review is specifically focused on calcium phosphate for bone regeneration and does not address other types of ceramics that may serve different applications with distinct structural and functional requirements. The keywords and search strategy are detailed in the Supplementary information.
2. Vat photopolymerization (VP) techniques
VP manufacturing involves selectively curing a photosensitive material, usually polymers, using a light source, typically in the ultraviolet range. During this process, a tank containing the photosensitive resin is exposed to light, forming the layers of the final geometry which is attached to a building platform. This exposure can either be from the bottom up through a transparent film into the tank containing the resin (bottom-up approach), or from the top down to the tank containing the resin (top-down approach). The light travels from the light source and penetrates the photosensitive resin, colliding with the building platform. The material in between solidifies and attaches to the moving building platform, also known as the build plate, becoming the first layer. The build plate retraces back and the following layers solidify and adhere to one another progressively, creating the final piece layer by layer.
VP techniques can be categorized depending on the employed light sources during photocuring. Basically, laser-based (Fig. 1A) and projection-based sources can be used (Fig. 1B). Laser-based techniques create a linear pattern for each layer. Common examples include Stereolithography (SLA), which uses a laser beam to cure the resin layer by layer linearly (either bottom-up or top-down), and Two-Photon Polymerization (2PP), which utilizes femtosecond lasers to cure the resin at a precise focal point. Projection-based techniques irradiate an entire pattern directly onto the resin, curing it all at once. Typical examples include Digital Light Processing (DLP), which uses a digital micromirror device (DMD) to project the image; Continuous Liquid Interface Production (CLIP), which creates a dead zone hampering the curing of the resin (enabling continuous production); and LCD-DLP 3D printing, also known as Masked Stereolithography (mSLA), which is similar to DLP but uses an LCD panel to mask the UV light source [40].
Fig. 1.
Schematic representation of Vat Photopolymerization techniques, categorized as: (A) laser-based techniques, such as Stereolithography (SLA) and Two-photon Polymerization (2PP); and (B) projection-based techniques, such as Digital Light Processing (DLP), Liquid Crystal Display-based masking projection (LCD-DLP), or masked stereolithography apparatus (mSLA), and Continuous Liquid Interface Production (CLIP).
The most popular techniques for VP printing of calcium phosphate-based materials are DLP, accounting for over 75 % of the reviewed publications, and SLA, used in around 25 % of the publications.
2.1. Stereolithography (SLA)
SLA is considered one of the earliest VP techniques, first developed in 1986 by Charles Hull [29]. SLA involves using a laser that scans a photosensitive liquid resin leaving behind a solidified line of crosslinked polymer. The photosensitive resin is placed in a vat and the laser scans linearly, selectively irradiating specific areas which become a solidified layer. This process is repeated layer by layer, solidifying and adhering consecutive layers to each other, and resulting in the final print. Typical commercial printers use tens of microwatts lasers (such as a 30 mW [41]), and can be set to hundreds of microwatts (100 mW [42]), and even higher, up to 300 W [43] when employing custom-made SLA printers. The laser beam penetrates solidifying tens of μm high in the z-axis linear patterns. In the x-y plane, the resolution depends on the laser beam-spot size, as represented in Fig. 1A, which is easily adapted to create complex and detailed geometries. However, SLA has some limitations in terms of printing time, as the laser scan results in a slow printing speed.
2.2. Digital Light Processing (DLP)
DLP is a projection-based technique which involves projecting a pattern of light at once onto a photosensitive liquid resin using LED arrays projected towards a Digital Micromirror Device (DMD) or a liquid crystal display (LCD). First, the build plate is immersed into a ceramic-loaded photosensitive resin (or slurry). The movable build plate descends beneath the liquid surface leaving a programmed gap (layer height) between the build plate and the bottom of the transparent vat. Then, the light source, typically a lamp or LED array, irradiates onto the DMD or the LCD, which directs it to the bottom of the tank in the designed pattern. In the case of DMD, multiple micrometric mirrors selectively move to either reflect light towards the vat, or hinder the exposition at that specific location. This way, each micromirror acts as a pixel that solidifies a quantified volume or voxel. Once the slurry solidifies, the platform separates from the bottom of the tank enabling the slurry to flow back underneath. Then, the build plate with the first layer attached to its surface descends again leaving the latter layer height between the hardened layer and the bottom of the tank. The process is repeated until the scaffold is formed layer by layer [44]. Since it prints the entire layer simultaneously, DLP is faster than SLA. On the other hand, LCD screens are controlled by a computer, selectively blocking the light and exposing only the transmission areas that will form the layer. One key advantage of this technology compared to DMD is its relatively more affordable cost, making it the most common commercially available option. However, LCD has some limitations in terms of printing size, which is restricted by the LCD panel, and less lifespan when compared to a projector with DMD [29].
The printing resolution in the z-axis, ranging from 20 to 100 μm, is associated with the layer height and is highly dependent on the stepper motor or linear actuators controlling Z-axis platforms. The most typical layer heights are 25–50 μm, although lower values, down to 20 μm, have been achieved [45,46]. The resolution in the x-y axes, on the other hand, can significantly vary between DMD or LCD due to their differences in their light modulation mechanics and optics. Typically, the resolution is limited to the pixel size that the projection system creates on the resin, resulting in a solidified voxel as the minimum printing volume, represented in (Fig. 1B). While DMD x-y resolutions typically range from 50 to 100 μm, LCD systems can achieve 50–75 μm; however, DMDs provide higher pixel size consistency and uniformity compared to LCD systems where light intensity can vary across the build area and reduce precision.
3. Photocurable resins for Vat photopolymerization of calcium phosphate-based materials
VP of calcium phosphate-based scaffolds has garnered much attention in the field of bone regeneration. Calcium phosphate-loaded photosensitive resins consist of a colloidal suspension of calcium phosphate particles in a liquid photosensitive polymeric resin. Under irradiation, a continuous cross-linked polymeric network containing the calcium phosphate particles is obtained [3,47,48]. The polymeric resin contains polymeric monomers/oligomers, light-sensitive initiator molecules (photoinitiators), and small concentrations of other additives. The selection of the appropriate components and their concentrations plays a crucial role in determining the polymerization resolution, printing time, and accuracy, thereby tailoring the functional properties of the 3D printed structure [30]. These components are typically mixed before printing, using either a planetary ball milling or centrifugal mixers. The elements in the slurry must be carefully selected to ensure high precision, and will be described in the following sections.
3.1. Photocurable polymeric resins
3.1.1. Calcium phosphate-loaded photocurable resins
The central components of the resin are the photocurable prepolymers (monomers or oligomers), which act as the building blocks of the final solidified part. These molecules contain crosslinking functional groups that interact with each other, forming a network. The most commonly used prepolymers are synthetic acrylated prepolymers like poly(ethylene glycol) diacrylate (PEGDA) [37,[49], [50], [51], [52], [53]], 1,6-hexanediol diacrylate (HDDA) [[54], [55], [56], [57], [58], [59]], trimethylolpropane triacrylate (TPMTA) [55,[60], [61], [62], [63], [64]], poly(trimethylene carbonate)-methacrylate (PTMC-MA) [[65], [66], [67], [68], [69]], among others. These synthetic polymers provide adequate, mechanically robust structures and are non-cytotoxic, although they are inert in terms of cell-material interactions and often face poor cell adhesion. Natural polymers such as gelatin, collagen, silk-fibroin, or alginate in their acrylated forms (methacrylated gelatin (GelMA) [[70], [71], [72], [73]], methacrylated collagen (ColMA), methacrylated silk-fibroin (SilMA) [74], methacrylated alginate (AlgMA) [75]) have been more recently explored in the field of VP printing for medical applications. Bone-derived decellularized extracellular matrix methacrylate (bdECM-MA), rich in collagen-, glycosaminoglycans- (GAGs), and other bone-specific ECM proteins, is another promising material recently used in VP bioprinting [76]. These materials closely resemble the polymeric composition of natural bone, potentially exhibiting better specificity in cell-material interactions. Another prepolymer that has been recently proposed for the preparation of CaP-containing resins is CSMA-2 ((3R, 3aR, 6S, 6aR)-hexahydrofuro [3,2-b] furan-3,6-diyl)bis(oxy)) bis(ethane-2,1-diyl))bis(oxy))bis(carbonyl))bis(azan ediyl))bis(3,3,5 trimethylcyclohexane-5,1-diyl))bis (azanediyl))bis(carbonyl))bis(oxy))bis(ethane-2,1-diyl) bis(2-methylacrylate)). This novel synthetic bio-based prepolymer has shown excellent printability, good mechanical properties and biocompatibility in vitro and in vivo [39,77], with great osteogenic and angiogenic potential [78].
3.1.2. Photoinitiators
Under light irradiation, the prepolymers start to crosslink to form the solidified network due to the action of photoinitiators. These are molecules that create reactive species in the form of free radicals or cations/anions when exposed to radiation, initiating the polymerization reaction by interacting with the monomers/oligomers. Thereby, the crosslinking mechanisms are categorized as either radical polymerization or cationic polymerization [79]. Some of the most used monomers/oligomers and photoinitiators are displayed in Table 1, detailing their chemical composition and optimal operating wavelength.
Table 1.
Common monomers/oligomers and initiator molecules used in the formulation of photocurable resins. Typical (meth)acrylated monomers/oligomers are selected for their double bonds, which react with each other to form covalent bonds, thus enabling chemical crosslinking (TMPTA molecule figure taken from Ref. [69], and CSMA-2 from Ref. [39]). The initiation and propagation of this reaction are driven by photoinitiator molecules, which convert photolytic energy into reactive species that initiate the polymerization process (photoinitiator molecule figures taken from Ref. [79]).
| Monomer/Oligomer | Molecule | Initiator | Molecule |
|---|---|---|---|
| Poly(ethylene glycol) diacrylate (PEGDA) |  |
Diphenyl(2,4,6-trimethylbenzoyl) phosphine oxide (TPO) |  |
| λpeak 380 nm | |||
| 1,6-hexanediol diacrylate (HDDA) |  |
Ethyl phenyl(2,4,6-trimethylbenzoyl) phosphinate (TPO-L) |  |
| λpeak 379 nm | |||
| Trimethylolpropane triacrylate (TMPTA) |  |
Lithium phenyl-2,4,6 trimethyl-benzoyl phosphinate (LAP) |  |
| λpeak 450 nm | |||
| Poly(trimethylene carbonate)-methacrylate (PTMC-MA) |  |
Phenyl bis (2,4,6-trimethylbenzoyl) phosphine oxide (BAPO) |  |
| λpeak 370 nm | |||
| CSMA-2 | |||
The most commonly used mechanism in VP printing is radical photopolymerization. The mechanism is based in the reaction of monomer/oligomer functional groups with free radicals, forming covalent bonds between prepolymers and resulting in crosslinked chains [80]. The process follows three steps: (i) radical generation: under light irradiation photoinitiator molecules react with photons of a specific energy (wavelength), and are responsible of converting this energy into reactive species; (ii) initiation: these species react with prepolymer molecules initiating the polymer chain reaction; and (iii) propagation: polymerization process [30,79,81]. Free radical initiators themselves can be categorized as Norrish-type I (also called α-cleavage), and Norrish-type II. Type I free radical initiators are the most used photoinitiators in VP printing with calcium phosphates. They undergo photo-cleavage resulting in two radical species, both being capable of initiating the polymerization. The wavelength and intensity of light needed to trigger cleavage vary based on the chemical structures of the photoinitiators [79]. Common type I initiators include phosphine oxide-containing molecules, such as diphenyl(2,4,6-trimethyl benzoyl)phosphine oxide (TPO; λpeak ∼ 380 nm), ethyl phenyl(2,4,6-trimethylbenzoyl)phosphinate (TPO-L; λpeak ∼ 379 nm), and phenylbis(2,4,6-trimethyl-benzoyl)-phosphineoxide (BAPO; λpeak ∼ 370 nm) (Table 2). In fact, TPO and BAPO, and their commercial branding names (Irgacure®, Omnirad®) are widely used because of their efficiency. Other type I initiators used in VP printing include lithium phenyl-2,4,6 trimethyl-benzoyl phosphinate (LAP; λpeak ∼ 405 nm) [82]. Free-radical type II initiators generate radicals in the presence of a co-initiator, typically hydrogen donating compounds, such as amines, thiols or alcohols. The most commonly used type II initiators are camphorquinones (CQ, λpeak ∼ 480 nm) and thioxanthones [79], such as 2-isopropyl-9h-thioxanthen- 9-one (ITX) [37]. However, compared to type I initiators, type II initiators such as CQ have low photoreactivity, often addressed with the addition of tertiary amines as electron/proton donors or reducing agents [83].
Table 2.
Resin formulations and rheological properties, printing parameters and post-printing processes for vat photopolymerization printing of calcium phosphate full-ceramic scaffolds (D: debinding; CD: chemical debinding; S: sintering; d: dose, I: intensity, P: power, LH: layer height, λ: curing wavelength).
| CaP | Loading | Slurry composition | VP printing | Slurry's viscosity | Post-process | Ref. |
|---|---|---|---|---|---|---|
| HA | _ | Commercial slurry: LithaBone HA 480E (Lithoz GmbH, Vienna, Austria) | DLP (Cerafab7500) | _ | D: up to 800 °C, or CD: LithaSol 30 | [123,124,126,130] |
| LH: 25 μm | S: 1275–1300 °C for 2 h | |||||
| λ: 460 nm | ||||||
| d: 150 mJ/cm2 | ||||||
| 48 wt% | Commercial slurry: HAPM100T01 (CERHUM, Belgium) | DLP (Propmaker V6000) | _ | S: 1170 °C, 1270 °C for 5–90 h | [128,129] | |
| LH: 50 μm | ||||||
| λ: 365 nm | ||||||
| 45 wt% | Commercial slurry: Shanghai Guangyi Chemical Co., Ltd., China | DLP | <12 Pa s | D: 500 °C for 4 h | [101] | |
| Dispersant: SPA | LH: 100 μm | S: 1400 °C for 1.5 h | ||||
| λ: 405 nm | ||||||
| I: 11 mW/cm2 | ||||||
| Protected | SLA (3D Ceram) | _ | D: 240-460-800 °C | [99,131] | ||
| S: 1050 °C | ||||||
| 40 vol% | Monomer: PEGDA (Mn = 250) | DLP (custom) | 0.18 Pa s | S: at 1300 °C for 2 h | [51] | |
| Initiator: 2 wt% PPO (BAPO) | LH: 100 μm | |||||
| Dispersant: 3 wt% Triton X-100 | λ: 380–420 nm | |||||
| I: 0.5 mW/cm2 | ||||||
| 50 vol% | Monomers: HDDA + HEMA + TMPTA (6:3:1) | DLP (AutoCera) | 10–15 Pa s at 50 Hz | S: 1250 °C | [60] | |
| Initiator: TPO | LH: 25 μm | |||||
| Dispersant: 2 wt% Solsperse 17000 | λ: 405 nm | |||||
| I: 8 mW/cm2 | ||||||
| 50 wt% | Commercial resin: Shanghai, China | DLP (custom) | _ | S: 1500 °C for 3 h | [132] | |
| 35 vol% | Commercial resin: Rigid resin (XYZ Printing inc. Taiwan) + HDDA (∼3:5) | SLA (Novel 1.0, XYZ) | 3.6–6 Pa s | S: 1250 °C | [102] | |
| Dispersant: 1.5 wt% BYK 180 | LH: 50 μm | |||||
| λ: 405 nm | ||||||
| 30 vol% | Monomer: HDDA | DLP (Photon, Anycubic) | _ | S: 1250 °C for 2 h | [59] | |
| Initiator: BAPO (Omnirad 819) | LH: 50 μm | |||||
| Dispersant: 0.15 wt% OA | ||||||
| 55 wt% | Monomer: HDDA | DLP | 380 mPa s at 52 Hz | S: 1250 °C for 3 h | [106] | |
| Initiator: TPO | LH: 30 μm | |||||
| Dispersant: 3 wt% BYK (not specified) | λ: 405 nm | |||||
| I: 15 mW/cm2 | ||||||
| 50 vol%/68 wt% | Monomers: OPPEA + HDDA (mass ratio 1:1) | SLA (Ceramaker 100) | 1.25 Pa s at 100 Hz | S: 1100, 1200, 1300 °C for 2 h | [41] | |
| Initiator: TPO | LH: 50 μm | |||||
| Dispersant: 0.2 wt% S18 | λ: 405 nm | |||||
| P: 30 mW | ||||||
| 30 vol% | Monomers: HDDA + TMPTA (4:1) | DLP (AutoCera) | _ | S: 1250 °C for 2 h | [104] | |
| Initiator: TPO | LH: 50 μm | |||||
| Dispersant: Solsperse KOS163 | λ: 405 nm | |||||
| I: 0.9 mW/cm2 | ||||||
| 45 vol% | Monomers: HDDA + HEMA + TMPTA (6.3:1) | DLP (AutoCera) | _ | S: 1250 °C for 2 h | [64] | |
| Initiator: 1.5 wt% TPO (regarding resin) | LH: 25 μm | |||||
| Dispersant: 2 wt% Solsperse 17000 (regarding powder) | λ: 405 nm | |||||
| I: 8 mW/cm2 | ||||||
| 60 wt% | Monomer: Not specified | DLP (Admaflex 130) | _ | S: 1150 °C for 2 h | [107] | |
| Initiator: TPO | ||||||
| Dispersant: BYK 2155 | ||||||
| 46 vol% | Commercial: LithaBone HA400 (Lithoz GmbH) | DLP (CeraFab7500) | _ | S: 1300 °C | [125] | |
| LH: 25 μm | ||||||
| I: 56 mW/cm2 | ||||||
| 50 vol% | Commercial: Dentifix-3D (FunToDo®) | mSLA (Phrozen shuffle) | 0.065 Pa s at 100 Hz, <5 Pa s at 1 Hz, TSI<2 | S: 1250 °C for 2 h | [117] | |
| Diluent: 35 vol% PEG-200 | LH: 100 μm | |||||
| λ:405 nm | ||||||
| P: 50.000 mW | ||||||
| 38 vol% | Commercial: 62 vol% LithaBone, not specified (Lithoz GmbH) | DLP (CeraFab7500) | _ | CD: LithasSol 80 | [133] | |
| LH: 25 μm | S: 900–1300 °C | |||||
| 40 vol% | Monomer: Acrylate oligomers (ACMO) | LCD-DLP (Phrozen) | 1,2 Pa s - 1.8 Pa s at 10 Hz | S: 1250 °C for 9 ha | [115] | |
| Absorber: Light Stabilizer 292 | LH: 50 μm | |||||
| Dispersant: SPA | λ: 460 nm | |||||
| 55 wt% | Commercial: Sylgard 184 silicone elastomer kit (3DCeram Sinto, France) | SLA (3DCeram Sinto) | _ | S: 1280 °C for 1 h | [134] | |
| LH: 100 μm | ||||||
| P: 48 mW | ||||||
| 0-60 wt% | Monomer: Cyracures UVR-6105 | SLA (custom) | <3 Pa s at 100 Hz | _ | [43] | |
| Initiator: Cyracures UVI-6976 | LH: 100 μm | |||||
| λ: 370 nm | ||||||
| P: 300 W | ||||||
| 27 wt% | Monomer: methacrylate-based (not specified) | DLP (custom) | _ | S: 1300 °C for 2 h | [135] | |
| LH: 50 μm | ||||||
| I: 28 mW/cm2 | ||||||
| 50 wt% | Polyfunctional acrylic resins (not specified) | SLA (Prodways V6000) | _ | S: 1125 °C, for 5 h | [136] | |
| LH: 50 μm | ||||||
| 50-56 vol% | Monomer: MBAM | SLA (SPS450B) | <3 Pa s at 30 Hz | S: 1080 °C | [96] | |
| Initiator: photocure-1173 | LH: 100 μm | |||||
| Dispersant: ammonium polyacrylate | P: 300 mW, d: 20.3 mJ/cm2 | |||||
| 10-45 wt% | Commercial: DSM's Somos (ABSlike) | DLP (LAYING II 1510P) | _ | D: 500 °C | [137] | |
| LH: 50 μm | S: 1250 °C | |||||
| 5, 10, 20 wt% | Commercial resin: FormLabs Ceramic RS-F2-CEWH-01 | SLA (FormLabs Form2) | 3.4–4.1 Pa s at 12 rpm | D: up to 300 °C | [138] | |
| λ: 405 nm | S: 1270 °C | |||||
| LH: 100 μm | ||||||
| 60 wt% | Monomers: PUA + PEGDA (Mn 400) (3:1 ratio) | DLP (Admaflec 130 plus) | _ | S: 1150 °C for 2 h | [139,140] | |
| Coating: GelMA (20 wt%) + Icariin (for drug release) | ||||||
| Initiator: 2 % TPO-L | ||||||
| Dispersant: BYK 2155 | ||||||
| 50 wt% | Monomers: HDDA | LCD-DLP (Sonic 4K, Phrozen) | <150 mPa s | D: up to 600 °C | [141] | |
| Initiator: 3 wt% TPO | LH: 50 μm | S: 1200 °C for 2 h | ||||
| Dispersant: BYK 111 | ||||||
| 50 wt% | Monomers: UA + PEGDA (Mn400) (3:1) | DLP | _ | S: 1150 °C for 2h | [142] | |
| Initiator: TPO | LH: 50 μm | |||||
| Dispersant: BYK 2155 | ||||||
| 45 wt% | Monomer: HDDA | DLP (Autocera-R) | 1 Pa s at 50 Hz | S: 1200 °C | [95] | |
| Initiator: 1.5 wt% BAPO (regarding resin) | λ: 405 nm | |||||
| Dispersant: 5 wt% Solsperse 41000 (regarding ceramic) | ||||||
| Absorbers: 0.2 wt% MEHQ (regarding resin) | I: 5 mW/cm2 | |||||
| Porogen: ethylene glycol | LH: 50 μm | |||||
| 50-70 wt% | Monomers: UA + PEGDA-400 (3:1) | DLP (Admaflec 130+) | 0.3–2 Pa s | S: 1050-1150-1250 °C for 2h | [49,50] | |
| Initiator: TPO | LH: 65 μm | |||||
| Dispersant: BYK 2155 | ||||||
| HA + Al2O3 | 20-80 wt% | Monomers: Trimethylolpropane formal acrylate + PEGDA | DLP (CeraStation 160) | _ | S: 1400 °C | [143] |
| Initiator: BAPO | λ: 405 nm | |||||
| Dispersant: SP710 | I: 127.23 mW/cm2 | |||||
| LH: 50 μm | ||||||
| HA + ZrO2 | 45-70 wt% | Commercial: (Shanghai Guangyi Chemical Co., Ltd.) and (Shanghai Prismlab Co., Ltd.) respectively | DLP (custom, SU-100A) | _ | D: 500 °C for 4 h | [45,46] |
| Dispersant: 2 wt% SPA | LH: 20 μm | S: 1400 °C for 1.5h | ||||
| λ: 405 nm | ||||||
| I: 10 mW/cm2 | ||||||
| 70 wt% | Monomer: PEGDA (Mn 600) | DLP (custom) | _ | S: 1200, 1300, 1400 °C | [37] | |
| Initiator: TPO | λ: 405 nm | |||||
| Absorber: Sudan red | I: 0.9 mW/cm2 | |||||
| Dispersant: KH-570 | ||||||
| _ | Monomers: PEGDA + Hydroxyethyl methacrylate phosphate | DLP | _ | S: 1200 °C | [144] | |
| Initiator: 0.5 % TPO | λ: 405 nm | |||||
| Dispersant: KH-570 + ACMO | LH: 40 μm | |||||
| Absorber:3·10−5 wt% Sudan | ||||||
| 10-60 wt% | Monomers: 45 % HDDA + 35 % ACMO + 15 % TMPTA + 5 % hyperbranched polyester acrylate | DLP (custom) | 10–100 mPa s at 100 Hz | D: up to 600 °C | [63] | |
| Initiator: 0.5 % BAPO (Omnirad® 819) | λ: 405 nm | S: 1100–1250 °C | ||||
| Dispersant: KH-570 + OA + Castor oil | ||||||
| HA + AK (9:1) | 40 vol% | Monomers: 60 wt% HDDA + TPGDA (7:3) | DLP (Autocera-M) | _ | D: 600 °C for 3 h | [[56], [57], [58]] |
| Initiator: 0.5 wt% TPO | LH: 50 μm | S: 1000–1250 °C for 2h | ||||
| Dispersant: 4 wt% Solsperse 41000 | d: 8 mJ/cm2 | |||||
| 40 vol% + nano-Fe3O4 | Monomers: HDDA + TPGDA (7:3) | DLP (Autocera-M) | _ | S: 1100 °C for 2 h | [105] | |
| Initiator: 0.5 wt% TPO (regarding resin) | ||||||
| Dispersant: 4 wt% Solsperse 41000 | ||||||
| Si-HA | 55 vol% | Monomer: amine modified polyester acrylate | DLP (PμSLA) (Xianlin 3D) | 2 Pa s at 150 Hz (<5 Pa s) | S: 1160–1200 °C for 2 h | [88] |
| Initiator: EDMD | LH: 250 μm | |||||
| Dispersant: phosphate ester | λ: 365 nm | |||||
| HA + SiO2 | 35 wt% | Monomer: 60 wt% SR454NS acrylic resin | DLP | <4 Pa s | S: 1200 °C for 3 h | [120] |
| Initiator: 0.5 wt% Ethyl 4-(Dimethylamino) benzoate | LH: 25 μm | |||||
| Dispersant: 2 wt% TAEA | ||||||
| HA + Sr2+ + Mg2+ + Zn2+ | 38 vol% | Commercial resin: 62 vol% Lithoz GmbH | DLP (CeraFab 7500) | _ | D: up to 500–600 °C | [133] |
| LH: 25 μm | S: 900, 1000, 1100, 1200. 1300 °C | |||||
| HA + CaSiO2 + SrPO4 + CaSO4 | 30 wt% | Monomers: acrylic, acrylate monomer | DLP (custom) | _ | D: up to 715 °C for 3 h | [116] |
| Dispersant: SPA | LH: 40 μm | S: 1300 °C for 2 h | ||||
| λ: 405 nm | ||||||
| HA + BR | 55 wt% | Monomers: HDDA + TPGDA (7:3) | DLP | 0.5–1 Pa s at 30 Hz | D: up to 600 °C | [145] |
| Initiator: 0.5 wt% TPO | S: 1300 °C for 2 h | |||||
| Dispersant: 4 wt% BYK 111 | ||||||
| HA + BG (8:2) | 50 wt% | Commercial rigid resin (Anycubic) | LCD-DLP | _ | D: 600 °C at 5 °C/min for 2h | [146] |
| S: 1300 °C at 5 °C/min | ||||||
| HA + ZnO | 60 wt% (95–15, 90–10) | Monomers: PUA + PEGDA (Mn 400) (3:1 ratio) | DLP (Admaflec 130 plus) | _ | S: 1150 °C for 2 h | [147] |
| Initiator: 2 % TPO-L | ||||||
| Dispersant: BYK 2155 | ||||||
| HA + BT (5:5, 3:7, 1:9) | 55 wt% | Monomers: HDDA + TPGDA | DLP (Admaflex 130) | _ | D: up to 600 °C at 1 °C/min | [148] |
| Initiator: Initiator 819 (BAPO) | λ: 405 nm | S: 1300 °C for 3h at 3 °C/min | ||||
| Absorber: HEMQ | LH: 40 μm | |||||
| Dispersant: Triton X-100 | ||||||
| HA + BT (2:8) | 45 vol% | Monomers: HDDA | DLP (Cerafab7500) | 0.287 Pa s | D: up to 468 °C at 0.5–1 °C/min | [149] |
| Initiator: Aladdin, Shanghai | λ: 453 nm | S: 1300 °C for 3h at 2 °C/min | ||||
| Dispersant: 2 wt% KH-570 | I: 87 mW/cm2 | |||||
| LH: 50 μm | ||||||
| HA + BT (3/7) + ZnO | _ | Monomers: 80 % PEGDA (Mn 600) | DLP | _ | D: up to 600 °C | [150,151] |
| Initiator: 2 % TPO-L | I: 2.5 mW/cm2 | S: 1250 °C for 2 h | ||||
| Dispersant: 15 % KH-570 | LH: 20 μm | |||||
| Absorber: 3 % Sudan red | ||||||
| Whitlockite | 75 wt% | Monomers: HDDA + TPGDA (7:3) | DLP (Anycubic D2) | 0.5 Pa s at 30 Hz | D: up to 600 °C | [152] |
| Initiator: 3 wt% TPO | S: 1000 °C for 2 h | |||||
| Dispersant: 18.7 wt% BYK 111 | ||||||
| β-TCP | 60 wt% | Monomers: PEGDA (Mw = 200) + β-CEA + HDDA (30: 5.2: 4.8) + HA-DA | DLP (custom) | _ | S: 1150 °C | [153] |
| Initiator: TPO | LH: 50 μm | |||||
| Dispersant: KH-570 | λ: 405 nm | |||||
| 60 wt% | Monomers: PEGDA (Mw = 200) + β-CEA + HDDA | DLP (custom) | 2–3 Pa s (<3 Pa s at 30 Hz) | D: up to 536 °C | [97] | |
| Initiator: TPO | LH: 50 μm | S: 1150 °C for 4 h | ||||
| Dispersant: 1 wt% KH-570 | λ: 405 nm | |||||
| 70 wt% | Monomers: AM + MBAM | SLA (custom) | _ | D: 80 °C/h to 660 °C, 115 °C/h to 700 °C | [38] | |
| Initiator: 0.02 wt% photocure-1173 | S: 360 °C/h to 1150 °C for 1 h | |||||
| Dispersant: SPMA | ||||||
| 65 wt% | Commercial: CryoBeryl Software, France | SLA (CryoCeram) | _ | S: 1050 °C for 3 h at 5 °C/min | [154] | |
| LH: 50 μm | ||||||
| λ: 350–400 nm | ||||||
| I: 5 mW/cm2 | ||||||
| 71 wt% | Monomers: 50 wt% HDDA + TGDA + HEMA + TTA + PEGDA | SLA (Admaflex 130) | 3.5–4.4 Pa s (5–10 Pa s < 300 Hz) | S: 1100 °C for 3h | [155] | |
| Initiator: 1 wt% TPO | λ: 405 nm | |||||
| Dispersant: 0.5 wt% Zelec P312 | ||||||
| Diluent: 10 wt% PEG200 | ||||||
| 52 vol% | Monomers: HDDA + OPPEA | DLP (CeraRay CR-1) | 5.76 Pa s at 100 Hz | D: up to 450 °C | [93] | |
| Initiator: 1 wt% TPO | LH: 100 μm | S: 1000 °C for 2 h | ||||
| Dispersant: 2 wt% S18 | λ: 405 nm | |||||
| 50 vol% | Commercial: HDDA, TMPTA, epoxy acrylic resin (Liangzhi chemical, Germany) | SLA (Ceramaker) | _ | S: 1100 °C | [108,109] | |
| Dispersant: 2.5 wt% BYK 110 | λ: 355 nm | |||||
| P: 180 mW | ||||||
| 30 wt% | Commercial: photosensitive resin (Anycubic Co, Shenzhen, China) | DLP (custom) | _ | S: 1150 °C for 3 h | [119] | |
| Dispersants: 2 wt% PEG-600 + 3 wt% 1,5-pentanediol | LH: 50 μm | |||||
| 40-60 wt% | Commercial: acrylic resin (FTD Standard Blend 3D Printing resin, Fun To Do, Alkmaar, The Netherland) | DLP (3DLPrinter-HD 2.0) | _ | S: 1200 °C for 2 h | [156,157] | |
| Dispersant: 0.1 wt% OA (regarding resin) | LH: 25 μm | |||||
| λ: 400–500 nm | ||||||
| I: 10 mW/cm2 | ||||||
| 40 vol% | Commercial: acrylic resin (FTD Standard Blend 3D Printing resin, Fun To Do, Alkmaar, The Netherland) | DLP (3DLPrinter-HD 2.0) | 1.9 Pa s at 10 Hz | S: RSA (rapid sintering) 5 min dwell, CSA (conventional sintering) 2 h dwell, SPS (vacuum) 5 min dwell at 1200, 1300, 1400, 1500 °C | [[158], [159], [160]] | |
| Diluent: 30 wt% Camphor | LH: 25–50 μm | |||||
| Dispersant: 0.1 wt% OA (regarding resin) | λ: 385 nm | |||||
| I: 31 mW/cm2 | ||||||
| 40 vol% | Commercial: resin (ELEGOO) | DLP (3DLPrinter-HD 2.0) | _ | S: Conventional sintering (CS): 1200 °C for 3 h; 2-step sintering (2SS): 1250/1270/1290/1310 °C for 2 min + 1000 °C for 3 h | [121] | |
| Dispersant: 6 wt% Disperbyk 110 | LH: 25 μm | |||||
| λ: 405 nm | ||||||
| I: 13.2 mW/cm2 | ||||||
| 40 wt% | Commercial: 60 wt% FLGPCL02, Formlabs | SLA (SEPS) (custom) | _ | S: 1250 °C for 3 h | [161] | |
| LH: 100 μm | ||||||
| λ: 405 nm | ||||||
| 47 vol% | Commercial: Lithabone TCP 300 (Lithoz GmBH, Austria) | DLP (LCM) (Cerafab7500) | 6–12 Pa s | D: up to 850 °C | [103] | |
| LH: 25 μm | S: 1200 °C | |||||
| I: 101 mW/cm2 | ||||||
| _ | Commercial: LithaBone TCP 380 D | DLP (Cerafab7500) | _ | D: 96 h | [122] | |
| LH: 25 μm | S: 1200 °C for 2 h | |||||
| 45 wt% | Commercial: resin (WANHAO Co.) | DLP (Autocera-M) | _ | S: 1150 °C for 3 h | [162] | |
| LH: 50 μm | ||||||
| 50 wt% | Commercial: resin (Suzhou Ding'an Technology Co.) | SLA (custom) | _ | _ | [163] | |
| Dispersant: SPA | I: 10 mW/cm2 | |||||
| 40 vol% | Monomers: TMPTA + HDDA (1:1) | DLP (M-Jewelry U30) | <3 Pa s at 30 Hz | D: 600 °C for 2 h | [55,61,62] | |
| Initiator: BAPO (Omnirad® 819) | LH: 15–65 μm | S: 1100 °C for 2 h | ||||
| Absorber: graphite | λ: 405 nm | |||||
| Dispersant: KH-550 | I: 2.19 mW/cm2 | |||||
| 68 wt% | Commercial resin | SLA (CryoCeram) | _ | D: 600 °C for 1 h at 1 °C/min | [164] | |
| Dispersant: Darvan C + B1001 | LH: 50 μm | S: 1000, 1050, 1120 °C for 3 h at 5 °C/min | ||||
| 60 wt% _ |
Monomers: Aliphatic UA + HDDA (6:4) | DLP (Autocera-M) | _ | S: 1100 °C | [165] | |
| Initiator: Hydroxy cyclohexyl phenyl ketone | LH: 25 μm | |||||
| Dispersant: phosphoric acid ester | I: 10 mW/cm2 | |||||
| Monomer: PEGDA (Mw = 200) | DLP | _ | D: up to 440 °C | [166] | ||
| Initiator: TPO | LH: 50 μm | S: 1150 °C for 3 h | ||||
| λ: 405 nm | ||||||
| 43.1 vol% | Monomers: HDDA + TMPTA | SLA (Ceramaker 300) | _ | S: 1200 °C | [167] | |
| Initiator: 3 % TPO | LH: 100 μm | |||||
| Dispersant: 5 % JOS-110 | λ: 365 nm | |||||
| I: 52 mW/cm2 | ||||||
| 45 wt% | Commercial resin: 50 % SP700 photosensitive acrylic resin | DLP (Shaoxing) | _ | S: 1160 °Cat 2 °C /min | [110] | |
| Dispersant: 5 % BYK 111 | LH: 50 μm | |||||
| 50 wt% | Monomer: 49 wt% HDDA | SLA (Cerafab 8500) | _ | D: 200 °C for 16 h | [168] | |
| Initiator: 1 wt% CQ | LH: 25 μm | S: 1200 °C for 4 h | ||||
| λ: 406 nm | ||||||
| I: 200 mW/cm2 | ||||||
| β-TCP + MgO | 43 wt% | Commercial: 57 wt% resin, shanghai guangyi chemical co. | DLP (custom) | _ | S: 1500 °C for 3 h | [169,170] |
| Dispersant: 4 wt% SPA | ||||||
| 50 vol% | Monomers: HDDA + TPGDA (7:3) | DLP (Autocera-M) | _ | S: 1250 °C for 2 h | [171] | |
| Initiator: 0.5 wt% TPO | LH: 25 μm | |||||
| Absorber: 0.1 wt% P-hydroxyanisole | d: 12.4 mJ/cm2 | |||||
| Dispersant: Solsperse 41000 (Lubrizol) | ||||||
| β-TCP + BG-58S (8:2) | 45-60 wt% | Monomer: PEGDA | DLP | 30.5–85.92 Pa s at 10 Hz | _ | [53] |
| Initiator: TPO | LH: 50 μm | |||||
| Dispersants: DCA-1228 + PPG | ||||||
| β-TCP + BG | 52 vol% | Monomers: HDDA + OPPEA | DLP (CeraRay 1) | _ | D: 370, 420, 460 °C for 2 h | [172] |
| Initiator: 1 % TPO | LH: 100 μm | S: 710 °C | ||||
| β-TCP + Laponite | 50-60 wt% | Monomer: PEDGA (200) | DLP | _ | _ | [52] |
| Initiator: 0.5 % TPO | LH: 50 μm | |||||
| Dispersant: DCA-1228 | λ: 405 nm | |||||
| β-TCP + α-CS | 55 vol% | Monomers: TPGDA + TMP3EOTA | SLA (3DCeram C900) | 40–50 Pa s | S: 1100 °C for 3 h | [173] |
| Initiator: 2,2-Dimethoxy-2-phenylbenzene | LH: 50 μm | |||||
| Dispersants: KH-560 (3–5 %) + KOS110 + Ammonium polyacrylate | λ: 355 nm | |||||
| P: 128 mW | ||||||
| TCP (not specified) | 60 vol% | Acrylate resin (not specified) | SLA (B9Creator) | 0.1–1 Pa s at 1 Hz (<1000 cP) | _ | [174] |
| Dispersant: Surfactant Darvan C (Vanderbilt, USA) | ||||||
| BCP (15/85) | _ | Monomer: Commercial resin type B-0#, Ten Dimensions Technology | DLP (Autocera-L) | _ | S: 1200 °C | [175] |
| Initiator: TPO | λ: 405 nm | |||||
| Dispersant: Solsperse 17000 | I: 24.5 mW/cm2 | |||||
| LH: 25 μm | ||||||
| BCP (1:1) | 35 vol% | Monomer: HDDA | DLP (3DP-21ODS) | 0.47–0.10 Pa s at 0.1–100 Hz | S: 1200 °C for 3 h | [113] |
| Initiator: 1.5 wt% BAPO (Omnirad 819) | LH: 25 μm | |||||
| Dispersant: 4 wt% BYK 2001 | λ: 405 nm | |||||
| Diluent: 40 wt% Camphor | I: 16.4 mW/cm2 | |||||
| 40-70 vol% | Monomers: HDDA + PMMA (as porogen agent) | DLP | 0.26–0.55 Pa s at 10 Hz | D: 600 °C | [112] | |
| Initiator: 2 wt% PPO (BAPO) | LH: 100 μm | S: 1200 °C for 3 h | ||||
| Absorber: 4 wt% benzopurpurin 4B | ||||||
| Dispersant: Disperbyk 2001 | ||||||
| Diluent: 40 wt% Camphor | ||||||
| 70 wt% | Monomers: IBOA, HDDA, PEGDA (1:3:1) | DLP | 0.8 Pa s at 40 Hz (<3 Pa s) | D: 700 °C | [176,177] | |
| Initiator: 1 wt% TPO (regarding resin) | d: 10 mJ/cm2 | S: 1200 °C for 2 h | ||||
| Dispersant: 4 wt% BYK 111 (regarding ceramic) | LH: 35 μm | |||||
| 65 wt% | Monomer: 28.86 wt% HDDA | DLP (Asiga Max) | 400 mPa s at 50 Hz | S: 1100, 1200, 1300 °C | [47] | |
| Initiator: 1 wt% TPO | ||||||
| Dispersant: 9 wt% BYK 111 | ||||||
| BCP (6:4) | 40-60 wt%a _ |
Monomers: UDMA + camphene-camphor (ratio 2:1) | DLP | _ | S: 1250 °C for 3 h | [178] |
| Initiator: 2 wt% TPO | LH: 220 μm | |||||
| Dispersant: 3 wt% KD4 (Croda, Everberg, Belgium) | ||||||
| Commercial: acrylic monomers, trade secret of Genoss® | DLP (Cubicon Lux) | _ | _ | [179] | ||
| LH: 50 μm | ||||||
| 50 wt% | Commercial: polyfunctional acrylic resin (Sirris, belgium) | SLA (Optoform) | _ | S: 1125 °C, for 5 h | [180] | |
| LH: 50 μm | ||||||
| 64 wt% | Monomers: Acrylic monomers (proprietary info) | DLP (Cubicon Lux) | _ | S: 1250 °C for 10 h | [181] | |
| Initiator: TPO | ||||||
| 40 wt% | Monomers: HDDA + TPGDA (7:3) | DLP (Autocera-M) | <5 Pa s over 60 Hz | S: 1100 °C for 2 h | [100] | |
| Initiator: 0.5 wt% TPO | LH: 50 μm | |||||
| Absorber: 0.1 wt% MEHQ | d: 12.5 mJ/cm2 | |||||
| Dispersant: 4 wt% Solsperse 41000 (Lubrizol) | ||||||
| BCP (7:3) | 20 vol% | Commercial: resin FA1260T; SKCytec | DLP (pMSTL) (custom) | _ | S: 1400 °C | [182] |
| 65 wt% | Photosensitive resin (not specified) | DLP (Admaflex 130+) | _ | D: 800 °C for 2.7 h | [183] | |
| LH: 50 μm | S: 1100 °C for 5 h | |||||
| _ | Monomers: UA + PEGDA (Mn400) (3:1) | DLP (Admatec 130) | _ | S: 1050 °C for 2 h | [111] | |
| Initiator: TPO | λ: 405 nm | |||||
| Dispersant: BYK 2155 | ||||||
| BCP | 50 wt% | Not specified + toners as pore forming agents | DLP (Autocera-M) | _ | _ | [184] |
| Dispersant: MAEP | LH: 50 μm | |||||
| λ: 405 nm | ||||||
| 50 wt% | Not specified + 2 wt% toners as pore-forming agents | DLP (Autocera-M) | 3 Pa s at 30 Hza | S: 1100 °C for 2 h | [185] | |
| Dispersant: MAEP | LH: 50 μm | |||||
| λ: 405 nm | ||||||
| 50-60 wt% | Monomer: HDDA | DLP (Autocera-M) | 5 Pa s at 30 Hza | S: 1100 °C for 2 h | [54] | |
| Initiator: BAPO | λ: 405 nm | |||||
| Dispersant: steric acid, sebacic acid, OA, MAEP | I: 10–34 mW/cm2 | |||||
| 50 wt% | Monomer: HDDA | DLP (Autocera-R) | _ | D: up to 466 °C | [91] | |
| Initiator: 2 wt% Irgacure® 819 (BAPO) | λ: 405 nm | S: 1250 °C | ||||
| Dispersant: 5 wt% Solsperse 41000 | I: 5 mW/cm2 | |||||
| Absorber: 2 wt% MEHQ | LH: 30 μm | |||||
| 45 wt% | Monomers: HDDA + TMP3EOTA | DLP (Autocera-M) | _ | D: up to 550 °C | [55] | |
| Initiator: BAPO (Omnirad® 819) | λ: 405 nm | S: 1100 °C | ||||
| Dispersant: Disperbyk 111 | I: 3.1–7.4 mW/cm2 | |||||
| BCP (6:4) + BG | 30 vol% | Monomers: HDDA + TPGDA + PEG (54:23:23) | DLP (Autocera-M) | _ | D: up to 650 °C for 1 h | [186] |
| Initiator: 0.5 wt% TPO (regarding resin) | λ: 405 nm | S: 1200 °C for 2 h | ||||
| Dispersant: 5 wt% Solsperse 41000 + 1 wt% RAD2500 | LH: 50 μm | |||||
| Absorber: 0.1 wt% Easepi 590 | ||||||
| BCP + BG 45S5® | 40 vol% | Monomers: HDDA + TPGDA | DLP (Autocera-M) | 0.1–2.2 Pa s at 20 Hz | S: 1200 °C for 2, 4, 6 h | [57] |
| Initiator: TPO | LH: 50 μm | |||||
| Absorber: MeHQ | λ: 405 nm | |||||
| Dispersant: Solsperse 41000 | d: 12.54 mJ/cm2 | |||||
| α-TCP | 45 wt% | Commercial: 50 % SP700 photosensitive acrylic resin | DLP (Shaoxing) | _ | S: 1240 °C | [110] |
| Dispersant: 5 wt% BYK 111 | LH: 50 μm | |||||
| Ca2,5Na(PO4)2 | _ | Monomers: Laromer 8889 + HDDA | DLP (Ember) | _ | S: 1200 °C for 12 h | [114] |
| Initiator: TPO-L | LH: 30–50 μm | |||||
| Absorbers: Sudan II orange + Carbon black | d: 170 mJ/cm2 | |||||
| Dispersant: Triton X-100 | ||||||
Abbreviations: α-β-TCP: tricalcium phosphate, α-CS: α-calcium silicate, β-CEA: β-carboxyethyl acrylate, ACMO: acryloylmorpholin (4-(1-oxo-2-propenyl)-morpholine), AK: akermanite, AM: acrylamide, BAPO: phenylbis(2,4,6-trimethyl-benzoyl)-phosphineoxide, BCP: biphasic calcium phosphate, BG: bioglass, CEA: β-carboxyethyl acrylates, CQ: camphorquinone, EDMD: ethanone, 2,2-dimethoxy-1,2-diphenyl, HA: hydroxyapatite, HA-DA: hyaluronic acid-dopamine, HDDA: 1,6-hexanediol diacrylate, HEMA: 2-hydroxyethyl methacrylate, IBOA: isobornyl acrylate, MAEP: monoalcohol ethoxylate phosphate, MBAM: N-N′ methylenebisacrylamide, MeHQ: p-hydroxyanisole, OA: oleic acid, OPPEA: 2-([1,1′-biphenyl]- 2-yloxy) ethylacrylate, PEG: poly(ethylene glycol), PEGDA: poly(ethylene glycol) diacrylate, PMMA: poly(methyl methacrylate), PPG: polypropylene glycol, PPO: phenylbis (2,4,6-trimethylbenzoyl) phosphine oxide, SPA: sodium polyacrylate, SPMA: sodium polymethacryate, TAEA: tris(2-Hydroxyethyl) amine, TGDA: tetraethylene glycol diacrylate, TMP3EOTA: ethoxylated trimethylolpropane triacrylate, TMPTA: trimethylol-propane triacrylate, TPGDA: tripropylene glycol diacrylate, TPO: diphenyl(2,4,6-trimethylbenzoyl)phosphine oxide, TPO-L: ethyl phenyl(2,4,6-trimethylbenzoyl)phosphinate, TTA: trimethylolpropane trimethacrylate, UA: urethane acrylate, UDMA: diurethane dimethacrylate.
Commercial chemicals: Irgacure® 819/Omnirad® 819 (BAPO): phenylbis(2,4,6-trimethyl-benzoyl)-phosphineoxide, KH-550: 3-aminopropyl triethoxy silane, KH-560: γ-glycidyloxy-propyltrimethoxy silane, KH-570: γ-methacryloxy-propyltrimethoxy silane, Photocure-1173: 2-hydroxy-2-methylpropiophenone, SR454NS: ethoxylated (3) trimethylolpropane triacrylate, Triton® X-100: t-octilfenoxipolietoxietanol, Zelec P312: alcohol phosphates.
Values taken indirectly from graphs and not explicitly described on article's text.
3.1.3. Photoabsorbers
One intrinsic challenge of VP is the precision of the projected light. Light must travel through different materials, from the light source to the tank's bottom film, and finally to the photosensitive resin contained in the building gap. These materials present different refraction indices causing changes in the behavior of light waves. These changes can cause reflection, refraction, and finally scattering phenomena, with loss of light directionality and spreading of the light beam spots [30], thus reducing printing precision. Light-absorbing molecules (photoabsorbers), which attenuate the light scattering are often added to improve the printing resolution and pattern fidelity [30], although they might require longer exposure times and higher photoinitiator concentrations [84]. Improved printing resolution is obtained by a balance between these additives. Typical absorbing molecules include Orasol Orange G (absorbing λopt.range 480–500 nm) [[65], [66], [67], [68], [69]], Quinoline Yellow (absorbing λopt_range 400–450 nm) [85], Tartrazine (absorbing λopt.range 425–450 nm) [86], and Sultan I (absorbing λopt.range 385–415 nm [87].
Resin composition and printing parameters are intrinsically dependent and thus, they need to be tightly balanced to obtain optimal printability. Two key indicators often used to assess optimal printability are cure depth (Cd) and resin viscosity. Cure depth is the farthest point where the light is able to cure the photosensitive resin, thus is highly dependent on the interaction between light and resin. These interactions can be modified either by tailoring the resin formulation or by adjusting the light exposure parameters such as energy, intensity or exposure time, depending on the printing device. To ensure printability, the distance between layers (layer height) must be smaller than Cd.
The reactivity of a resin is commonly evaluated by curing it in a build plate-free volume, where the light penetration is unlimited. By varying the energy dose (i.e., light intensity) and exposure times, different thicknesses of cured resins are obtained. The Cd is represented versus the logarithm of the exposure times while keeping the light energy constant, or versus the logarithm of the light intensities while keeping the layer height constant. These calculations allow identifying the critical energy dose, according to Jacobs's equation (Equation (2)), characteristic of each resin formulation [88]. This empirical equation is commonly used in the literature to analyze experimental data on cure depth to determine the depth of penetration (Dp) and the empirical constant of critical energy (Ec). These values are then used to determine the layer thickness of each layer for light-based fabrication [89].
| Equation 2 |
where Cd represents the cure depth, Ei is the energy dosage per area, Ec represents the “critical” energy dosage, and Dp refers to the “depth of penetration” of the laser beam into the solution, which is inversely proportional to the molar extinction coefficient and the concentration of photoinitiator.
Finally, a key parameter in VP approaches is the rheological behavior of the resin. Resins require a shear-thinning behavior which implies that the resin's apparent viscosity decreases when the shear stress increases, and is due to shear-induced disentanglement of the long polymeric chains. The polymeric chains, which are entangled at rest, align upon shearing, reducing the internal resistance to flow and, thus, its viscosity [90], ensuring printability [91]. Shear-thinning behavior favors an easier flow of the resin underneath the build plate, allowing layer-by-layer printing. This type of characteristic behavior is described by the Herschel-Burkley model (Equation (3)), which allows calculating some rheological parameters, such as the yield stress (), which can be used to characterise the properties of the VP resins.
| Equation 3 |
3.2. Calcium phosphate-loaded photocurable resins
The addition of CaPs into VP resins affects key parameters needed for printability such as the rheological properties of the resins and the light interactions with the light sources. Light penetration is hindered by the suspended particles causing light scattering and light absorption [92]. As a result, these changes in light interaction affect the cure depth (Equation (2)) of the resins. On the other hand, CaPs addition to the resin highly affects the resin's rheological behavior. Resin viscosity increases with the addition of ceramic fillers and can compromise printability. Furthermore, the ceramic filler hydrophilicity, when combined with common hydrophobic resin monomers, can cause agglomeration and sedimentation [93]. The main effect due to the incorporation of ceramic particles is on the shear-thinning behavior of the resins, typically increasing their yield stress (, see Equation (3)). However, high yield stress is commonly considered to be an obstacle to the spreading of new layers and the yield stress tends to rise with increasing solid content [94].
A strategy widely used to modify the rheological properties and suspension stability in ceramic-loaded resins is by adding dispersant molecules which help stabilize the viscosity during the printing process. Dispersants form a protective film on ceramic particles preventing particle collision and maintaining the resin viscosity stable [60]. As dispersant molecules start to adsorb on the surface of ceramic particles, the repulsive forces between particles increase, reducing the viscosity of the slurry. Nonetheless, there is a limit to dispersant adsorption on ceramic particles due to the limited number of dispersant molecules that can be adsorbed onto their surface [41]. When excessive dispersant is added, excess dispersant results in flocculation and subsequent viscosity increase [47,91,95]. This phenomenon also limits the ceramic loading capacity. Generally, the reported viscosity limit for slurries ranges from 3 Pa s [39,43,62,[96], [97], [98]] to 5 Pa s [54,86,99,100], although higher viscous slurries have been successfully used [[101], [102], [103]]. Commercially available dispersants, such as commercial Solsperse® variants [57,60,64,104,105], BYK® variants [47,49,50,102,[106], [107], [108], [109], [110], [111], [112], [113]], or common surfactants such as Triton® X-100 [51,114], or sodium polyacrylates [45,46,101,115,116], beyond others, are used to lower the viscosity of the slurry, tuning their printability.
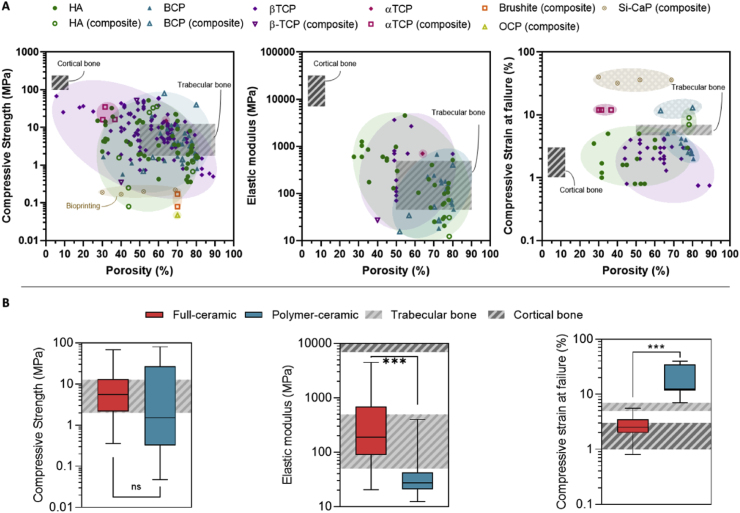
In the case of calcium phosphates, the most commonly used ceramic fillers are hydroxyapatite (HA), β-tricalcium Phosphate (β-TCP), and a combination of both known as biphasic calcium phosphate (BCP) [60,113,[117], [118], [119], [120]]. In fact, over 90 % of the reviewed literature use these three CaPs, purely or together with other ceramic fillers such as zirconia (ZrO2), magnesium oxide (MgO), and bioglass, among others (Fig. 2B).
Fig. 2.
(A) Schematic representation of post-printing processes resulting in composite or full ceramic parts. Composite scaffolds, consist of a continuous polymeric matrix containing dispersed ceramic particles. Full ceramic scaffolds are obtained through a high-temperature treatment consisting in debinding and sintering. The resulting microstructure consists of ceramic particles bound together. (B) Schematic representation of different calcium phosphate ceramics and other inorganic components used on both the full-ceramic route (in red) and composite route (in blue), following Table 2, Table 3 (Abbreviations: α-CS: α-Calcium silicate, AK: akermanite, BCP: biphasic calcium phosphate, BG: bioglass, BR: bregidite, BT: barium titanate, CPP: calcium pyrophosphate, HA: hydroxyapatite, MAEP: monoalcohol ethoxylate phosphate, MCPM: mono-calcium phosphate monohydrate, OCP: octacalcium phosphate, Si-CaP: silicon-calcium phosphate, SWCNT: single-walled carbon nanotube, TCP: tricalcium phosphate).
4. Fabrication routes
The resin composition, formulation and printing configuration are closely linked to the fabrication route used. In the literature, two main approaches are identified: producing fully ceramic scaffolds or polymeric-ceramic composite scaffolds. The resin composition and characteristics vary depending on the selected route. In the case of full ceramic scaffolds, the polymeric resin serves a critical role in the printing process but is subsequently removed through high-temperature treatments. Careful consideration of the debinding and sintering processes is essential to ensure structurally robust ceramic frameworks. In contrast, composite scaffolds maintain the polymeric phase, which remains an integral part of the final structure.
Once printed, the scaffolds can be further processed to adjust their physicochemical, mechanical or structural properties. Full ceramic bodies can be obtained by applying a thermal treatment that includes a debinding and a sintering step. The polymer matrix is removed during the debinding process, and the ceramic particles are fused together by solid-state diffusion during the sintering step. Alternatively, the polymeric matrix can be maintained, resulting in a composite scaffold based on a continuous polymeric matrix with dispersed ceramic particles (Fig. 2A). These two processing routes result in two distinct types of scaffolds, with different properties mainly in terms of mechanical and biological response. The first route is the most commonly followed, accounting for 75 % of the analyzed publications. Conversely, only 25 % of the published studies have investigated composite scaffolds (Fig. 2B).
4.1. Full ceramic scaffolds
Full ceramic scaffolds are obtained by removing the polymeric matrix and sintering the ceramic particles through thermal treatment. In this approach the polymeric resin is used as a sacrificial support, and the final goal is to obtain a full-ceramic part.
Commonly, the thermal treatment consists first of a debinding step to decompose the polymeric matrix. The temperature applied is usually slightly above the degradation temperature of the polymer used in the resin. The debinding temperature is often determined by Thermogravimetric analysis (TGA-DTG), which allows to identify the temperature range where the crosslinked polymer decomposes, and a thermal treatment is designed including a slow heating ramp and a dwell time to ensure total polymer debinding and simultaneously preserve the structural integrity of the scaffold [113,121]. Debinding steps are usually programmed with several steps including dwelling times at each step which ensures a homogeneous decomposition of the polymer without affecting the structural integrity of the scaffolds. After eliminating the polymeric phase, the scaffolds undergo a sintering process at high temperature to allow for solid-state grain boundary atomic diffusion, resulting in the consolidation of a full-ceramic part. The sintering temperature, heating ramp, and dwell times affect the grain growth and have a clear effect on the resulting microstructure. This process is commonly set depending on the material to avoid cracks or inconsistent gaps and undesired porosity. Low sintering temperatures lead to loose grains resulting in an increased number of pores, whereas higher temperatures promote grain coarsening, abnormal growth, and may even cause ceramic cracking and failure [41]. Resin formulations and processing parameters reported in the literature for the fabrication of full CaP ceramic scaffolds by VP are summarized in Table 2, including several commercially available CaP-resins, such as Lithabone® from Lithoz GmbH [103,[122], [123], [124], [125], [126]], Dental resin DETAX® [127], Cerhum® [128,129], for which the exact composition is often confidential [40]. Abbreviations can be found in footnotes bellow the table or in Table S1 (supplementary information).
4.2. Composite scaffolds
The high brittleness of full ceramic CaP scaffolds has encouraged the development of composite CaP specimens [157]. These composites must meet certain requirements of biocompatibility and resorbability. Therefore, the polymers used in the resin formulation should also be biocompatible and bioresorbable, degrading upon contact with organic fluids, and disappearing completely from the organism once the defected area is healed without any acidic degradation by-products [65]. In this approach the crosslinked polymeric structure is preserved, resulting in a composite material where a polymer matrix embeds ceramic particles that act as reinforcing agents. Polymer-ceramic printed scaffolds offer a promising solution for bone grafts, combining the strength and flexibility of both components. In this context, the photocrosslinkable resin is no longer a sacrificial phase and becomes an integral part of the final scaffold.
Commonly used resins in this particular approach entail the use of acrylated groups capable of reacting upon light irradiation. However, acrylated resins can exhibit high irritancy levels or even cytotoxicity in the uncured state. The leaching of unreacted monomers/oligomers or photoinitiators, as a result of low double-bond conversion rates, which is used as an indicator for the extent of the reaction, may cause health risks. Chen et al. illustrated the cytotoxicity problems associated to low crosslinking degrees, the concentration of the photoinitiator being a key factor influencing photopolymerization efficiency. When the photoinitiator concentration was too low, the energy to trigger the polymerization reaction was insufficient, leading to unpolymerized monomers, which can have toxic effects. Conversely, if the concentration was too high, excessive light absorption caused rapid polymerization of the surface layers, resulting in incomplete polymerization [73,99,131]. This phenomenon has been already addressed previously. Lee et al. demonstrated the existence of a critical photoinitiator concentration for which the curing depth is maximized for photopolymerization reactions. They reported experimental evidence that there must clearly be an “optimal” concentration to maximize the curing of the gel. In fact, when the photoinitiator concentration is low, just a small fraction of the photons is absorbed, resulting in few free radicals to start the reaction which are unable to form a gel. However, when the concentration of photoinitiator increases, the resulting radical initiation increases which results in higher double-bond conversion. At high photoinitiator concentrations, the photon absorption is so intense that light penetration diminishes, remaining confined near the surface of the resin. This fact induces the formation of a tightly cross-linked, thin layers [89]. To add up, this phenomenon is further aggravated by the presence of ceramic particles in the resin, which absorb part of the light radiation.
The printed parts must be thoroughly rinsed and ultrasonically cleaned, to wash out unpolymerized monomers or loose particles. In some cases, additional curing of the printed structure is carried out to completely crosslink any unpolymerized residue [86,98,187,188]. Moreover, in the case of reactive ceramics that are able to undergo a self-hardening process by a cement-like reaction, a subsequent process can be applied to transform the ceramic phase, as reported by Oliver-Urrutia et al. for an α-TCP-loaded resin [189]. A detailed summary of resin formulations and processing parameters reported in the literature for the fabrication of CaP/polymer composite scaffolds by VP are summarized in Table 3. Abbreviations can be found in footnotes bellow the table or in Table S1 (supplementary information).
Table 3.
Resin formulation, rheological properties and printing parameters for vat photopolymerization printing of calcium phosphate composite scaffolds. Acronyms (LSS: laser spot size, E: energy, d: dose, I: intensity, P: power, LH: layer height, λ: curing wavelength).
| CaP | Loading | Slurry composition | VP printing | Slurry's viscosity | Ref. |
|---|---|---|---|---|---|
| HA | 40 wt% | Monomer: TATO alkene + TATO thiol | SLA (Peopoly Moai 130) | _ | [87] |
| Initiator: 0.68 wt% TPO | LH: 60 μm | ||||
| Absorbers: Sultan I + PYG | λ: 400 nm | ||||
| Diluents: TMPMP, PETMP, ETTMP | |||||
| 10-20 wt% | Monomer: 60 wt% PEGDA (Mn = 700) | SLA (custom solid-oodle) | _ | [190] | |
| Initiator: 0.5 wt% BAPO | LH: 400 μm | ||||
| Diluent: PEG (Mw = 300) | λ: 355 nm | ||||
| I: 40 mW/cm2 | |||||
| 55 wt% | Monomer: OL-MA (Mn 1420 g/mol) + TEGDMA (1:1) | SLA | _ | [187] | |
| Initiator: Irgacure® 819 (BAPO) | LSS: 100 μm | ||||
| λ: 355 nm | |||||
| 40 wt% | Monomer: 60 wt% PEGDA (Mn 700) | SLA | 5 Pa s at 100 Hza | [131] | |
| Initiator: 0.5 wt% Irgacure® 2959 | LH: 50 μm | ||||
| λ: 355 nm | |||||
| P: 70 mW | |||||
| 8 wt% | Commercial: acrylic-based urethane methacrylated resin (Novafab Powerdent Temp) | DLP (Novafab Vega) | _ | [188] | |
| λ: 405 nm | |||||
| I: 2.3 mW/cm2 | |||||
| 5.2, 16.7 wt% | Monomer: 60–47.4-25.1 wt% PTMC-MA | SLA | 57.5–71.9 mPa s | [65] | |
| Initiator: 5 wt% TPO-L | LH: 50 μm | ||||
| Absorber: 0.15–0.12-0.08 wt% Orasol Orange G | |||||
| Diluent: 40-47.4-58.2 wt% Propylene carbonate | |||||
| 20, 40 wt% | Monomer: PTMC-MA | SLA (Envisiontec Perfactory III) | _ | [[66], [67], [68]] | |
| Initiator: 5 wt% TPO-L | LH: 50 μm | ||||
| Absorber: (0.15–0.1-0.08 wt%) Orange G | I: 1.80 mW/cm2 | ||||
| Diluent: propylene carbonate | |||||
| 7 wt% | Monomer: PPF (70 %) | MSTL (SLA) (custom) | _ | [191] | |
| Initiator: 1 wt% Irgacure® 819 (BAPO) | LH: 215 μm | ||||
| Diluent: DEF (30 %) | λ: 375 nm | ||||
| P: 310 mW | |||||
| 10 vol% | Monomer: PEGDA (Mw250) + AESO (1:1) | mSLA (Anycubic Photon) | 0.2–0.49 Pa s at 50 Hz | [94] | |
| Initiator: 1 wt% Irgacure® 819 (BAPO) | LH: 50 μm | ||||
| 40 wt% | Monomer: OCM-2P | SLA (LS-250) | 260 cSt | [192] | |
| Initiator: Irgacure® 671P | LH: 200 μm | ||||
| Absorber: 0.02 wt% bis-(5-methyl-3-tert-butyl-2-oxyphenyl)-methane | |||||
| Dispersant: PAA | |||||
| 20 wt% | Monomer: PDLLA | SLA (Envisiontec Perfactory Mini) | 4–7 Pa s | [193,194] | |
| Initiator: 4 wt% Lucirin®-TPO-L | LH: 25 μm | ||||
| Absorber: 0.2 wt% tocopherol inhibitor + 0.15 wt% Orange Orasol G | λ: 400–550 nm | ||||
| Diluent: 50 wt% NMP | I: 17 mW/cm2 | ||||
| 55, 75 wt% | Monomer: PLA-MA | SLA (custom) | _ | [195] | |
| Initiator: ethylene glycoxide | LSS: 70 μm | ||||
| Diluent: TEGDMA | λ: 355 nm | ||||
| P: 1.5 mW | |||||
| 10 wt% | Monomer: PEDGA (60 or 40 %) RGD modified | SLA | _ | [196] | |
| Initiator: Not specified | LH: 400 μm | ||||
| I: 25–300 mW/cm2 | |||||
| 10 w/v% | Monomer: 15 w/v% PEGDA + 10 w/v% GelMA + PLGA NPs with TGFb1 | SLA (not specified) | _ | [71] | |
| Initiator: 0.5 w/v% Irgacure® 2959 | |||||
| 10 wt% | Monomer: 10–15 % GelMA | SLA | _ | [70] | |
| Initiator: 0.5 % Irgacure® 2959 | LH: 200 μm | ||||
| E: 20 μJ at 15 kHz | |||||
| 2, 5, 10 w/w % | Monomer: 60 % PEGDA (Mn 700) | SLA (Printrbot®) | _ | [197,198] | |
| Initiator: 0.5 % Irgacure® 819 (BAPO) | LSS 190 μm | ||||
| Diluent: 40 % PEG | λ: 355 nm | ||||
| Energy: 20 μJ at 15 kHz | |||||
| 30 w/v% | Monomers: GelMA + SilMA (1:1) | DLP (BP600, EFL) | _ | [74] | |
| Initiator: 0.5 w/v% LAP | I: 15 mW/cm2 | ||||
| Absorber: 0.05 w/v% Tartrazine | LH: 50 μm | ||||
| 10-50 wt% | Monomers: 30 wt% GelMA | SLA (custom) | [73] | ||
| Initiator: 4 % (w/v) Irgacure® 2959 | P: 180–200 mW | ||||
| LH: 100 μm | |||||
| 10-30 wt% | Monomer: mAESO + PEGDA (1:1) | mSLA (Sonic XL 4K, Phrozen) | _ | [199] | |
| Initiator: 1 % Irgacure® 819 | LH: 50 μm | ||||
| 5.5 wt% | Monomer: GelMA + AlgMA | DLP (EFL-BP-8601) | _ | [75] | |
| Initiator: LAP | I: 14 mW/cm2 | ||||
| LH: 25 μm | |||||
| 5-10 wt% | Monomer: CSMA-2 | DLP (Nobel Superfine, XYZ) | 0.3–0.55 Pa s | [77,78] | |
| Initiator: 2 wt% BAPO | λ: 405 nm | ||||
| I: 5.3–6 mW/cm2 | |||||
| HA + CPP | 5 + 5 wt% | Commercial: Soybean oil-based commercial resin (Anycubic Co.) | SLA (Anycubic Photon S) | 0.501–0.839 Pa s | [200] |
| λ: 355–410 nm | |||||
| HA + Sr | 32.6–38 wt% | Monomers: 10 w/v% GelMA | DLP | [72] | |
| Initiator: 0.5 wt% LAP | λ: 405 nm | ||||
| I: 12 mW/cm2 | |||||
| HA + SWCNT | 12.5 mg/mLa + 0, 1, 2 wt% | Commercial: Dental resin, DITAX | DLP | _ | [127] |
| Dispersant: TEA | LH: 20 μm | ||||
| λ: 360–410 nm | |||||
| β-TCP | 32, 51, 60 wt% | Monomer: PTMC-MA | DLP (Envisiontec Perfactory III mini SXGA+) | _ | [69] |
| Initiator: 5 wt% TPO-L | LH: 50 μm | ||||
| Absorber: Orasol Orange G | λ: 400–550 nm | ||||
| Dispersant: Propylene carbonate | I: 7 mW/cm2 | ||||
| _ | Monomer: GelMA + HyAc-MA | DLP (LumenX, Celllink) | [82] | ||
| Initiator: LAP | LH: 50 μm | ||||
| Absorber: R1800, benzophenone-9 | λ: 400–550 nm | ||||
| I: 7 mW/cm2 | |||||
| 20 vol% | Monomer: 40 % PEGDA (Mw = 400) | DLP (MMSL) (Custom) | _ | [201] | |
| Initiators: 0,25 wt% DAROCUR-1173 + DAROCUR- TPO (2:3) | LH: 100 μm | ||||
| Dispersant: Quaternary ammonium | λ: 400–410 nm | ||||
| I: 14,98 mW/cm2 | |||||
| 5 mg/ml | 30 w/v% PEGDA (Mn 700) + Chitosan (4 mg/ml) | SLA | 100–200 mPa s | [202] | |
| Initiator: 0.5 w/v% Irgacure® 2959 | λ: 365 nm | ||||
| I: 1 mW/cm2 | |||||
| 10 % | Commercial PLA resin (Yisheng New Material) | LCD-DLP (Chuangxiang LD-002R | 200 mPa s | [203] | |
| LH: 50 μm | |||||
| _ | Monomer: PEGDA 508 | SLA (Custom) | _ | [42] | |
| Initiator: 0.5 % Irgacure® 2959 | P: 100 mW | ||||
| BCP | 22.5, 40 wt% | Monomer: 56 wt% PLLA + 20 wt% TMPTMA (crosslinker) | DLP (Kavosh economy) | 0.1–5a Pa s (<5 Pa s) | [86] |
| Initiator: 4 wt% of TPO | LH: 50 μm | ||||
| Absorber: 0.01 wt% tartrazine | λ: 405 nm | ||||
| Diluent: 20 wt% of NMP | I: 18 mW/cm2 | ||||
| 0.5, 1 w/v% | Commercial: resin Portux Print 3D Model (New stetic) | DLP (Wanhao Duplicator 7) | <3 Pa s | [98] | |
| LH: 45 μm | |||||
| λ: 405 nm | |||||
| OCP | 5 wt% | Monomer: PEGDA (50 %, 700Da) | DLP (Ember Autodesk) | _ | [85,204] |
| Initiator: 0.5 wt% Irgacure® 819 (BAPO) | LH: 200 μm | ||||
| λ: 405 nm | |||||
| I: 39.8 mW/cm2 | |||||
| Brushite | 10 wt% | Monomer: PEGDA | DLP (Ember Autodesk) | _ | [85] |
| Initiator: Irgacure® 819 (BAPO), | LH: 200 μm | ||||
| TPO or Api-180 (0.1–1 %) | λ: 405 nm | ||||
| Absorber: E104 | I: 39.8 mW/cm2 | ||||
| α-TCP | 55.4 wt% (32 vol%) | Commercial: plant-based UV resin Anycubic Co. | DLP/LCD (Photon, MonoX Anycubic) | _ | [189] |
| LH: 75 μm | |||||
| λ: 405 nm | |||||
| 60 wt% | Monomer: PEGDA (Mw 575) + PEGMA (Mw 350) (1:1 ratio) | DLP (Ember) | _ | [92] | |
| Initiator: Irgacure® 819 (BAPO) | λ: 405 nm | ||||
| I: 22–23 mW/cm2 | |||||
| CDHA | 10 vol% | Monomer: 45 vol% mAESO + 45 vol% TEGDMA | mSLA (Sonic XL 4K, Phrozen) | _ | [205] |
| Initiator: 1 wt% Irgacure® 819 (BAPO; regarding resin) | LH: 50 μm | ||||
| MCPM + TCP (1:9) | 40, 50, 60 wt% | Monomer: CSMA-2 | DLP (Nobel Superfine) | 3 Pa s | [39] |
| Initiator: 1 wt% CQ | I: 10 mW/cm2 | ||||
| Si-CaP | 0.5 mg/mL | Monomer: bdECM-MA | DLP (BP8601 Pro, EFL) | _ | [76] |
| Initiator: 0.25 % LAP | I: 20 mW/cm2 | ||||
| Cells: BMSCs (Bioprinting) | LH: 100 μm | ||||
| _ | Monomer: PEGDA | DLP (Custom) | _ | [118] | |
| Initiator: TPO | |||||
| Dispersant: DCA‐1228 | |||||
| Diluent: polypropylene glycol |
Abbreviations: α-β-TCP: tricalcium phosphate, AESO: acrylated epoxidized soybean oil, AlgMA: alginate methacrylate, BAPO: phenylbis(2,4,6-trimethyl-benzoyl)-phosphine oxide, BCP: biphasic calcium phosphate, CSMA-2: r((3R, 3aR, 6S, 6aR)-hexahydrofuro [3,2-b] furan-3,6-diyl) bis(oxy)) bis(ethane-2,1-diyl)) bis(oxy)) bis(carbonyl)) bis(azanediyl)) bis(3,3,5-trimethylcyclohexane-5,1-diyl)) bis(azanediyl)) bis(carbonyl))bis(oxy)) bis(ethane-2,1-diyl) bis(2-methylacrylate)), CPP: calcium pyrophosphate, CQ: camphorquinone, DEF: diethyl fumarate, E104: quinoline yellow food coloring, ETTMP: ethoxylated trimethylolpropane tri(3-mercaptopropionate), Gel-MA: gelatin-methacrylate, HA: hydroxyapatite, MCPM: mono-calcium phosphate monohydrate, NMP: N-Methyl-2-pyrrolidone, NPs: nanoparticles, OCP: octacalcium phosphate, OCM-2P: olygocarbonate dimethacrylate, OL-MA: methacrylated oligolactide, PAA: polyacrylic acid, PBS: phosphate-buffered saline, PDLLA: poly(DL-lactide), PEG: poly(ethylene glycol), PEGDA: poly(ethylene glycol) diacrylate, PEGMA: polyethylene glycol monomethacrylate, PETMP: pentaerythritol tetrakis(3-mercaptopropionate), PLA-MA: polylactic acid-methacrylate, PLGA: poly(lactic-co-glycolic) acid, PLLA: poly(L-lactide), PPF: poly(propylene fumarate), PTMC-MA: poly(trimethylene carbonate)-methacrylate, PYG: pyrogallol, RGD: arginine-glycine-aspartic acid peptide sequence, Si-CaP: silicon-calcium phosphate, SilMA: silk fibroin methacrylate, SWCNT: single-walled carbon nanotubes, TATO alkene: 1,3,5-tiallyl-1,3,5-triazine-2,4,6-trione, TATO thiol: tris[2-(3-mercaptopropionyloxy)ethyl]-isocyanurate, TEA: triethanolamine, TEGDMA: triethylene glycol dimethacrylate, TGFb1: transforming growth factor Beta 1, TMPMP: trimethylolpropane tris(3-mercaptopropionate), TMPTMA: trimethylolpropane trimethacrylate, TPO: diphenyl(2,4,6-trimethylbenzoyl) phosphine oxide, TPO-L: ethyl phenyl(2,4,6-trimethylbenzoyl)phosphinate.
Commercial chemicals: Api-180: 2-hydroxy-1-[3-(hydroxymethyl)phenyl]-2-methyl-1- propanone, Irgacure® 671P: 2,2-Dimethoxy-2-phenylacetophenone, Irgacure® 819/Omnirad® 819 (BAPO): phenylbis(2,4,6-trimethyl-benzoyl)-phosphineoxide, Irgacure® 2959: 2-Hydroxy-4′-(2-hydroxyethoxy)-2-methylpropiophenone, Lucirin®: ethyl phenyl(2,4,6-trimethylbenzoyl)phosphinate, DAROCUR-1173: 2-hydroxy-2-methyl-1-phenyl-1-propanone.
Values taken indirectly from graphs and not explicitly described on article's text.
5. Mechanical performance
Mimicking bone anisotropy and hierarchical structure remains a significant challenge. However, advances in the digitalization and processing of medical images such as those obtained from magnetic resonance imaging (MRI) or computed tomography (CT) have facilitated their integration into geometrical models suitable for implementation in advanced manufacturing techniques. This implementation has enabled the development of patient-specific designs and customization, but still, replicating the specific mechanical properties of natural bone persists as a hurdle in the clinical application of VP scaffolds. While the design and composition of the printed scaffolds are critical in determining their mechanical performance, the printing parameters also play a crucial role. The following sections examine and summarize the effects of printing parameters, designs, and compositions on the overall mechanical performance of VP printed scaffolds.
5.1. Effect of printing parameters on mechanical properties
The printing parameters set up during the printing process greatly affect the outcome of the printed parts. The energy dose, controlled by the light intensity and/or the exposure time has a strong effect on the polymerization process of the resins used in VP techniques. Higher polymerization degrees, achieved by higher energy dosage often result in higher stiffness in the polymeric phase. In addition, the printing orientation affects the mechanical properties of the scaffolds. Unlike other printing techniques where part orientations are highly restricted, VP printing offers a high versatility of printing orientation, allowing the anisotropy resulting from the printing process to be adjusted depending on the implantation site. Therefore, the printing orientation of the parts must be carefully designed to match the intended mechanical requirements of the printed scaffolds.
5.1.1. Light exposure
Vat photopolymerization techniques are based on the interaction between light and the material. Consequently, the light energy dosage (which measures the intensity per time of exposure) is the main parameter affecting the integrity of the printed polymeric matrix and thus affects the mechanical properties of the scaffolds due to its role in the polymerization process. A higher energy dose, resulting from either longer exposure times or higher light intensity, leads to a higher energy absorption by photoinitiator molecules yielding more radical formation, and consequently a higher degree of double bond conversion. This creates a more crosslinked polymer network which generally improves the mechanical strength of the scaffold. Oppositely, low energy dose results in insufficient energy and uncured material, negatively impacting the crosslinking degree and lowering the scaffold's mechanical properties. Many commercially available resins are optimized for a certain energy dose and have specific parameters of light exposure times to print. However, and more specifically for composite scaffolds, formulated resins need to be optimized in terms of the energy dosage by varying exposure time or intensity. This effect can be seen in Table 2, and Table 3, where various energy dosages have been used depending on the resin formulation.
5.1.2. Orientation
Typical VP printed structures, based on layered configurations, often suffer the so-called “stair-stepping” effect, which refers to the individual layers stacking along the printing direction [156,158]. This effect leads to a notable anisotropy in the strengths of the construct between load configurations [157]. Specifically, layered structures are generally weaker when subjected to compression perpendicular to the orientation of the printed layers because defects across the interlayers promote shear-driven delamination. Paredes et al. reported that parts manufactured using VP techniques exhibit interlayer defects which results in the formation of local interlayer shear cracks, thus, reducing the strength during compression perpendicular to the printing plane (perpendicular configuration). In contrast, when tested parallelly to the printing plane (parallel configuration), the stacked layers collectively bear the load until a longitudinal crack propagates through one of the interlayers at the struts’ intersections (Fig. 3A). Although this results in catastrophic failure, the stress required to initiate crack propagation in this orientation is higher than that required for failure in the perpendicular orientation. Navarrete-Segado et al. reported a less pronounced but similar trend in full ceramic HA scaffolds. Compressive strength was higher when loads were applied parallelly or at a 45° angle to the printing direction (4.8 ± 0.2 MPa and 4.9 ± 0.3 MPa, respectively) compared to perpendicular loading (4.2 ± 0.4 MPa) [206]. Accordingly, in tests on β-TCP full-ceramic scaffolds, compressive strength in the parallel configuration (22 ± 4 MPa) was nearly double that of the perpendicular configuration (12 ± 3 MPa) [156] (illustrated in Fig. 3B). Another study showed similar results, with an average compressive strength of 37 ± 8 MPa in the parallel configuration (where the failure occurred abruptly) and 19 ± 4 MPa in the perpendicular configuration, where fractures progressed more gradually [158]. Therefore, it is important to consider the final implantation site and adjust the optimal printing orientation to withstand the highest loads.
Fig. 3.
Effect of the selected printing parameters on mechanical properties; strategies proposed in the literature to improve the mechanical properties of calcium phosphate scaffolds obtained by VP. (A) the “stair-stepping” effect typically visualized in VP printing causes shear driven delamination/cracks when loaded perpendicularly to the printing plane. Photography of printed specimens at three different printing orientations, namely 0-, 45- and 90-degree angle. Figures taken from Ref. [117]. (B) In agreement with the later, β-TCP full ceramic scaffolds performed better when tested in the parallel configuration compared to the perpendicular one [156]. (C) Sintering method effects: conventional and advanced sintering methods such as Rapid Sintering in Air (RSA), and pressure-less Spark Plasma sintering (pl-SPS) have different effects on the microstructure and final compressive performance of β-TCP full ceramic scaffolds [160], red arrows pointing at microcracks.
5.1.3. Thermal treatment: debinding and sintering
It is important to mention that in the particular case of full ceramic printed scaffolds, high-temperature treatments undoubtedly play a major role in their final mechanical performance. To start, the elimination of the polymeric phase of the scaffold, performed in the first step known as debinding, leads to the release of gases that produce microcracks and internal porosities, negatively affecting the structural and mechanical performance of the specimens. Furthermore, grain coarsening and the final consolidation of the piece, achieved during the second step known as sintering, also have a meaningful effect on its microstructure and resulting mechanical performance.
Specifically, the heating ramp and dwell times during the debinding step, as well as the temperature and dwell time during the sintering step, affect the grain growth and have a clear effect on the resulting microstructure. Temperature ramps are commonly set to increase slowly and are maintained at certain critical points where the mass change, as previously observed in TGA analysis, is highest for that specific material. The dwell time at those critical points is crucial to guarantee the proper controlled removal of the polymer, minimizing microcracks that could compromise structural integrity. Once the polymer is completely removed, the sintering process follows, playing a critical role in the structure's integrity [133]. A low sintering temperature leads to lose grains resulting in an increased number of pores, whereas excessive temperatures promote grain coarsening, abnormal growth, and may even cause ceramic cracking and failure [41], and the presence of second phases. Furthermore, the sintering dwell time also plays a key role in structural integrity. Guo et al. confirmed that a 2-h dwell during debinding and sintering resulted in optimal densification and mechanical properties of HA ceramic scaffolds. Without dwelling time, high microporosity led to weak intergranular bonding and low strength. Extending the dwell time to 4 h caused excessive grain growth and grain boundary cracks, reducing the compressive strength [145]. In addition, the heat treatment process itself plays a key role in the microstructure and, consequently, in the final structure's mechanical and biological properties [121,160].
Paredes et al. investigated the effects of conventional and advanced sintering methods, including Rapid Sintering in Air (RSA), and pressure-less Spark Plasma sintering (pl-SPS), on β-TCP ceramic scaffold at various sintering temperatures [160]. Their study highlighted how heat treatment parameters influence abnormal grain growth, the formation of microcracks (indicated by red arrows in Fig. 3C), the presence of an α-TCP second phase (associated with bimodal grain size distribution and the often relation to microcracks due to volumetric changes, shown as coarse dashed line areas in Fig. 3C), and undesired microporosity caused by poor densification. These factors play a critical role in the structural integrity of the scaffolds. They found that, with rapid sintering, the grain growth is inhibited facilitating surface diffusion and accelerating densification, which in turn is related to a lower amount of surface defects. This resulted in higher compressive strengths for the scaffolds sintered by the RSA method compared to those sintered by conventional routes.
5.2. Effect of scaffold composition on mechanical performance
The scaffolds’ composition is obviously a key parameter in their mechanical performance, together with biologically relevant pathways that will be later described. The mechanical properties of scaffolds as a function of their composition considering their overall porosity is represented in Fig. 4A. The numerical values based on the results reported in the literature are provided in Table S2 in the supplementary information. Compressive strengths and elastic moduli in the range of trabecular bone have been achieved for highly porous structures. It is well known that brittle materials when subjected to stress, break with little elastic deformation and near-zero plastic deformation. Consequently, brittle materials absorb relatively little energy before the fracture, even those of high strength [206]. This is the case of full ceramic scaffolds (represented in the graphs as filled symbols). Therefore, a rapid solution to mitigate the lack of strain energy is to print polymer-ceramic composite structures by creating ceramic slurries based on biocompatible prepolymer acrylic resins that serve as a matrix, with dispersed ceramic particles. Unlike full ceramic scaffolds, these composites (represented in the graphs as empty symbols) offer a higher deformation-uptake capability, where the polymeric phase can absorb higher energy yielding more flexible materials without compromising their compressive strength. A more direct assessment of the mechanical properties of full ceramic and composite scaffolds is presented in Fig. 4B, where the data from various studies are summarized in box plots and analyzed statistically using the Mann-Whitney test. This analysis indicated no significant difference in the compressive strength of the scaffolds. However, the intrinsic differences between full ceramic and composite scaffolds are evident in their elastic moduli and strain at failure. Full ceramic scaffolds are characterized by their stiffness, brittleness, and limited flexibility, with an average strain at failure of approximately 2 %, making them more challenging to handle. In contrast, the inclusion of a polymeric matrix in composite scaffolds enhances their strain at failure, achieving an average of around 10 %, thus improving their flexibility.
Fig. 4.
Mechanical properties of calcium phosphate ceramic and composite VP-printed scaffolds. (A) Compressive strength, elastic modulus, and compressive strain at failure as a function of the porosity of the scaffold. Each dot represents one condition. The dashed grey areas represent the values of natural bone (cortical and trabecular) obtained from Ref. [207]. (B) Box plots of all the data from the above graphs, grouped by processing strategies. Polymer-ceramic composites show improved strain at failure while maintaining adequate compressive properties. A normality test was performed, resulting in a non-parametric distribution; therefore, a Mann-Whitney test was conducted, revealing no significant differences in compressive strength (p > 0.05) and significant differences in elastic modulus and compressive strain at failure (∗∗∗p < 0.01).
In addition to using biocompatible polymers as the structural matrix, researchers considered alternative strategies to improve the scaffold's flexibility and toughness. One strategy consists of infiltrating a polymeric phase into a hollow ceramic structure in order to create a hard yet less brittle phase-separated composite structure. Wu et al. demonstrated that these phase-separated composites exhibit strengths comparable to the fully dense-strut structure counterparts and enhanced toughness. These scaffolds resist loading even after the outer layer fractures under compression [208,209]. They printed β-TCP full ceramic scaffolds with an external macroporosity of 62.8 %. When subjected to compression testing, the hybrid scaffolds exhibited a compressive strength similar to fully dense scaffolds (approximately 15 MPa) but showed a notable improvement in strain energy density. A similar toughening effect was observed in bending tests. In a similar approach, Paredes et al. developed a similar co-continuous β-TCP/polycaprolactone shell-core composite obtained by infiltrating PLC into a hollow structure. They recorded enhanced toughening in both compression [157] and bending [158]. In the first study, while the hybrid scaffolds showed a slight reduction in compressive strength compared to dense scaffolds (from 11 ± 4 MPa to 9 ± 3 MPa), the strain energy density (a measure of toughness) improved by an order of magnitude, closely resembling the behavior of natural bone [157] (see Fig. 5A). It is important to note that in both studies the enhanced toughness in compression was recorded even after the scaffolds' ceramic shells began to fracture. The internal hybrid structures retained some integrity, allowing them to sustain additional loading.
Fig. 5.
Effect of the scaffold's composition on mechanical properties; strategies proposed in the literature to improve the mechanical properties of calcium phosphate scaffolds obtained by VP. (A) when infiltrating a polymer (PCL) through the hollow internal canals/pores of a β-TCP full ceramic scaffold, creating a hybrid structure, its strain energy density further increases [157]. (B) Multicomponent printing, by the addition of other components (MgO) to the β-TCP initial powder. After sintering, whitlockite forms and further influences the mechanical properties [169]. (C) Fracture mode modification by incorporating ZnO as doping agent to HA ceramic scaffolds by dispersion of particles, piezoelectric properties, and residual stress toughening [147].
Furthermore, the addition of ceramic/oxide phases to the calcium phosphates in the resin formulation has gained interest as a strategy to enhance scaffolds’ strength. Commonly used materials which act as second-phase ceramics are silicon oxides, zinc oxides, zirconia oxides, magnesium oxides/akermanite, bioglass, and to a smaller extent carbon nanotube (see Fig. 2B). The use of slurries consisting of a mixture of different agents has been performed mainly in full-ceramic routes containing high-temperature treatments. Thereafter, new phases can be obtained after sintering that enhance mechanical and biological properties. For instance, Ge et al. demonstrated that using MgO as co-ceramic filler with β-TCP, increased the compressive performance of composite scaffolds. After sintering, some of the MgO particles transformed into Whitlockite, a new ceramic phase which increased the compressive strength of the scaffolds when the amount of MgO achieved 30–40 %. The compression tests revealed that for a 50 % porous scaffolds, the compressive strength reached 4.49 MPa for the 40 % MgO-containing scaffolds compared to 2.26 MPa obtained with pure β-TCP ceramic scaffold (Fig. 5B) [169,170]. Moreover, the mechanical properties of HA scaffolds can be significantly enhanced by modifying their fracture mode. Gui et al. doped HA ceramic scaffolds with ZnO, demonstrating an improvement in their mechanical properties [147]. This improvement was attributed to three key factors: 1) dispersed ZnO reduced crack propagation through interfacial interactions such as transgranular cracking (also discussed in another study [145]), crack deflection, bridging, and pinning, increasing its hardness and strength; 2) ZnO generated an electrical charge under stress, transforming mechanical energy into electrical energy and enhancing fracture resistance; 3) due to differences in thermal expansion between ZnO and HA, residual stresses created during sintering and cooling restricted crack growth (Fig. 5C).
5.3. Effect of scaffold design on mechanical performance
The scaffold design, including the unit cell geometry and the overall scaffold architecture, is a crucial factor in the development of interconnected macroporous scaffolds as it directly influences and drives cell migration, vascularization, nutrient transport, and the mechanical stability required for successful bone regeneration and remodeling.
The advances in software processing have enabled the application of a wide range of different geometries that can be applied and further customized into the final printed scaffolds. In terms of geometrical designs, three main categories have been widely used in literature: 1) strut-based geometries; 2) Triply periodic minimal surface geometries; and 3) Nature-inspired geometries. Commonly used geometries are shown in Fig. 6A. The geometrical patterns can be obtained either by computer-aided designs (CAD) or by reverse-engineering, which involves the use of computer tomography (CT), or magnetic resonance imaging (MRI) to obtain a reconstructed 3D design [29].
Fig. 6.
Pore geometry and mechanical properties of VP-printed calcium phosphate scaffolds. A) Different pore geometries, classified as strut-based, TPMS, and nature-inspired. Figures were taken from Refs. [122,157,210,211]; B) Compressive strength as a function of porosity classified as different pore geometries for full ceramic and composite scaffolds. The original data are reported in the Supplementary information. The values for natural bone (dashed grey areas) were taken from Ref. [207].
The most common three-dimensional pore structures are the strut-based cells, also known as log piles, lattice-based, or orthogonal. The properties of these three-dimensional structures are directly related to the strut size, lattice shape, and cell arrangements. However, unlike the smooth, interconnected, and random arrangement of natural bone, they produce convex, self-intersecting rough shapes with no curvature. This makes strut-based cells suboptimal due to the abundance of stress concentration points [157]. In this line, researchers have also investigated the properties of inverse strut lattices, which are dense structures interpenetrated by linear pores, just like the negative of a strut-based lattice [60,97,206]. On the other hand, triply periodic minimal surface (TPMS) lattices are composed of infinite non-self-intersecting and periodic surfaces in three principal directions [210]. These structures are characterized as minimal surfaces, because the curvature along the principal curvature plane is equal and opposite at every point, making their overall curvature zero, and closely resembling bone natural surface. They can be designed as solid-TPMS, considering the volume bound by these minimal surfaces, hence filling out the volume inside them, or as sheet-TPMS, created by offsetting the minimal surface along its normal direction to generate a double surface, hence filling out the volume between the latter two. The smooth curvatures found in these arrangements eliminate the stress concentration often found in strut-based structures achieving mechanical properties close to trabecular bone [210]. Typical TPMS structures, used as solid or sheet-based, are Gyroid [86,98,100,118,212], Diamond [129,189], and Schwarz-primitive [54,106,130,190] among others. Finally, nature-inspired structures mimicking geometries found in nature have gained increasing interest. They can be obtained through tessellation during the scaffold modelling process. For instance, trabecular geometries consisting of trabeculae mimicking the cancellous bone hierarchy can be either obtained by CAD with Voronoi geometries [119] or CT scanned directly from natural bone [51], or sponges [130], through reverse engineering.
The mechanical performance of scaffolds is directly influenced by their design parameters. Among them, pore geometry is a critical factor extensively studied in the field of VP printing for CaP scaffolds. Fig. 6B illustrates the mechanical properties of scaffolds as a function of their porosity and pore geometrical designs, with data presented separately for full-ceramic scaffolds, and composite scaffolds. The numerical values derived from the literature are provided in Table S2 of the supplementary information. Most of the samples studied exhibit high porosity levels, ranging from 30 % to 80 %, close to the porosity of trabecular bone. However, less porous scaffolds, which mimic cortical bone, have also been developed, using mainly strut-based structures with porosities ranging from 5 to 25 %. In contrast, nature-inspired and TPMS geometrical designs seem to focus more on the trabecular bone zone. While both fabrication routes can achieve comparable compressive strengths (as seen in the previous section), the represented data reveal slight differences between these two routes. Full ceramic scaffolds generally show a reduction of the compressive strength with increasing porosity, which is a trend generally observed in porous materials [213]. Conversely, composite scaffolds show a wider dispersion of results with no clear correlation between porosity and compressive strength. This variability underscores that the lattice geometry of pores is not the only variable affecting the mechanical performance of the scaffolds. Instead, other factors, such as pore size and material composition, which are not considered in the graphs, play crucial roles. The latter is particularly significant for composite scaffolds, where the polymer matrix formulation can vary widely, contributing to the observed scatter in the results.
Pore geometry, however, does significantly affect the mechanical properties of scaffolds when comparable scaffolds (in terms of materials and porosity variables) are analyzed. Paredes et al. have registered significant differences between two identical structures but with different geometry. In this study, a strut-based β-TCP full ceramic scaffold was compared with its Schwarz Primitive counterpart with equivalent porosity [121]. The smooth-curved Schwarz Primitive geometries demonstrated superior mechanical performance under compression compared to the strut-based geometries as seen in Fig. 7A. The smooth curvatures of these structures can effectively alleviate shear stresses in comparison to simpler geometries with intersecting edges, which can act as crack initiators. In another study, Oliver-Urrutia et al. compared different TPMS pore architectures with similar porosities for CDHA-Epoxy composite scaffolds; 36.7 % for the solid-gyroid, 30.2 % for the diamond, and 31.5 % for the solid-schwarz primitive. They found significant differences in mechanical properties, with the solid-Schwarz primitive structure exhibiting a compressive strength twice that of the diamond structure. This result indicates that, for comparable porosities, the compressive strength is significantly influenced by pore architecture (see Fig. 7B) [189].
Fig. 7.
Effect of the scaffold's architecture on mechanical properties; strategies proposed in the literature to improve the mechanical properties of calcium phosphate scaffolds obtained by VP. Pore geometry plays an important role, (A) improving mechanical properties from convex-self intersecting orthogonal pattern to smooth Schwarz-TPMS of β-TCP full ceramic scaffolds [121], (B) Schwarz geometry outperforms gyroid and diamond counterparts for CDHA-epoxy composite scaffolds with similar porosities [189]. (C) Geometry modification, by elongating a BCP full ceramic gyroid structure yielded better compressive performance [113]. Blue arrows point the loading direction. (D) Porosity gradient can enhance its strength, as recorded in HA-AK full ceramic scaffolds [56].
In addition to selecting different pore geometries, another strategy to significantly enhance the mechanical performance of the scaffolds is by creating more complex pore architecture from already existing geometries. Lee et al. revealed that, by elongating the widely studied gyroid-TPMS pores, a remarkable increase of the compressive strength was obtained, from 4.3 ± 0.26 MPa to 11.5 ± 1.75 MPa, with an increase in elongation percentage from 0 to 60 % for BCP scaffolds [113] (see Fig. 7C). This suggests the possibility of enhancing the mechanical performance just by simple modifications of already-known structures. Another approach has been considered by creating gradient structures to TPMS geometries, mimicking the natural bone hierarchy, and thus promoting better mechanical properties. Deng et al. studied the effect of linear, quadratic, and exponential porosity gradients in gyroid-scaffolds. They reported an increase in compressive strength and elastic modulus for gradient-scaffolds compared to uniform ones (see Fig. 7D). More precisely, exponential gradient-scaffolds made with HA-AK (9:1 ratio) performed best of all four combinations, exhibiting twice compressive strength (from 2.10 ± 0.27 MPa to 4.45 ± 0.52 MPa) and elastic modulus (from 0.301 ± 0.036 GPa to 0.580 ± 0.034 GPa) than the other combinations [56].
6. Biological performance
Bone regenerative grafts act as sacrificial structures promoting tissue colonization, bone formation, and resorption while being replaced by new bone. Despite the vast research in biomaterials topic, there is a large discrepancy between the numbers of those reaching clinical application. Ascribed more to a lack of predictive power in the methods to test the biological performance than the biological performance itself, bone regenerative grafts must undergo a series of validations to ensure meeting clinical relevance. To comply with regulatory demands, scaffolds undergo extensive preclinical evaluations assessing cytotoxicity, bone formation capability, angiogenesis potential, and risks from biodegradation or manufacturing residues. Biomaterial validation entails both in vitro and in vivo studies. In vitro studies, usually performed following ISO-10993 standards, are aimed at testing the direct and indirect cytotoxicity of the scaffolds, besides other aspects like cell adhesion, proliferation, and osteogenic differentiation. This assessment can be performed using colorimetric assays (e.g., MTT, CCK8), staining methods (e.g., Alizarin Red), and osteogenic markers (e.g., ALP, RUNX2, OCN). On the other hand, vascularization, which is key in promoting bone regeneration and nutrient supply, is also assessed, especially in macroporous scaffolds. This is performed by examining endothelial progenitor cells (EPCs) for viability, migration, and differentiation via assays like tube formation and RT-PCR for angiogenesis-related genes (e.g., VEGF). In vivo studies involve implanting the scaffolds in orthotopic (bone) or ectopic (non-bone) sites in animal models such as rats, rabbits, and dogs. Bone formation is analyzed using histology, micro-CT, and biomechanical tests. Angiogenesis is assessed through immunohistochemical staining, angiography for 3D vascular reconstructions, and the measurement of percentage vascular volume. In/Ex ovo assays performed on a Chicken Egg Chorioallantoic Membrane (CAM) model, the vascularization potential can be assessed by measuring vascular density and bifurcation points on the embryonic membrane.
The most relevant reviewed outcomes in terms of biological evaluation of VP printed scaffolds are presented followingly, categorized as in vitro evaluations (Table 4), and in vivo evaluations (Table 5).
Table 4.
in vitro evaluation studies of VP-printed calcium phosphate-based scaffolds reported in the literature (h: hour/s, d: day/s, w: week/s, m: month/s).
| Ref | Assay | Cell type | Quantification assays | time |
|---|---|---|---|---|
| [87] | Indirect | hDF, Raw264.7 | Alamar Blue (cytotoxicity) | 24, 72 h |
| [86] | Indirect | L929 | MTT | 24, 48, 72 h |
| [153] | Indirect | mBMSC | qPCR of ALP, OCN, OPN, RUNX2 (osteogenesis genes) | 7 d |
| qPCR of CD31, VEGF, GAPDH | ||||
| [97] | Indirect | mBMSC | CCK8 (cytotoxicity) | 3 d |
| [99] | Indirect | NCTC clone L929 | CCK8 (cytotoxicity) | 3 d |
| [131] | Indirect | MSCs | CCK8 (cytotoxicity) | 1 d |
| [58] | Indirect | Primary rBMSC | CCK8 (cytotoxicity) | 1 d |
| [98] | Indirect | L929 | Cytotoxicity | 3 d |
| [132] | Indirect | MC3T3-E1 | Differentiation: Zn2+ released in medium detected using commercial kits | 4 d |
| [179] | Indirect | L929 | Microscope characterization | 1, 2 d |
| [181] | Indirect | L929 | Cytotoxicity | 24, 48 h |
| [64] | Indirect | hBMSC | MTT (proliferation) | 1, 3, 7 d |
| [114] | Indirect | NCTC L929 | MTT (cytotoxicity) | 1, 4 d |
| [171] | Indirect | rBMSC, EPC, RAW264.6 | Sirius red staining, ARS, ALP, RT_PCR (osteogenic differentiation) | 24 h, 3 d (PCR) |
| Wound healing assay, Transwell assay, Tube formation assay, RT_PCR (angiogenic differentiation) | ||||
| [100] | Indirect | rBMSC | CCK8 (cytotoxicity) | 1, 3 d |
| [137] | Indirect | MC3T3-E1 | Immunofluorescence (cell survival) | 24 h |
| [120] | Indirect | Mouse L929 | MTT (Cytocompatibility) | 1, 4, 7, 14 d |
| [166] | Indirect | rBMSC | (PCR: ALP, OCN, OPN, RUNX2), activation of MAPK | 1, 7, 14, 21 d |
| [141] | Indirect | L929 | MTT | 24 h |
| [139] | Indirect | HUVEC | Scratch wound healing, cell migration, tube formation with 24 h extracts | 6, 24 h |
| Indirect | Rabbit BMSC | ALP, ARS, qRT-PCR (COL1, RUNX2, BMP2, OPN, SPP1), Staining | 7, 10, 14 d | |
| [140] | Indirect | HUVEC | Angiogenesis (tube formation, capillary length, ARS, crystal violet staining) | 24 h |
| Indirect | BMSC | Osteogenesis (ALP, ARS) | 7, 14, 21 d | |
| [147] | Indirect | MC3T3 | Live/dead staining, CCK8 (viability), ALP | 1, 3, 5 d |
| [76] | Indirect | BMSC | ALP, ARS staining, western blot (RUNX2, OSX, ALP, COL1, OPN, OCN), qRT-PCR (RUNX2, ALP, COL1, OPN, OSX, OCN) | 3, 7, 14 d |
| Indirect | RAOEC | CD31/DAPI, tube formation, western blot (TLR4, T-PI3K, T-AKT, P-AKT) | 7 d | |
| Indirect | Macrophage | Western blot (iNOS, TNF-α, ARG-1, IL-10), flow cytometry | 1, 3, 7 d | |
| [175] | Indirect | BMSC | CCK8, Phalloidin staining, ALP, ARS | 1, 2, 3, 4, 5 d |
| [97] | Direct | mBMSC | CAM/PI staining | 7 d |
| [154] | Direct | MC3T3-E1 | Not described | 6, 12, 24, 48 h |
| [45] | Direct | MC3T3-E1 | CCK8 (adhesion + proliferation) | 1, 4, 7 d |
| [101] | Direct | MC3T3-E1 | CCK8, DAPI, Ca deposition (ARS) | 1, 3, 5 d (CCK8) |
| 24 h (DAPI) | ||||
| 14 d (ARS) | ||||
| [190] | Direct | hMSC | MTS (adhesion + proliferation), Quant-iT™ Picro-Green® dsDNA Kit, GAG, II collagen, Ca deposition reagent kit (differentiation) | 4 h, 1, 3, 5 d (adhesion) |
| 1, 2 w (differentiation) | ||||
| [187] | Direct | MC3T3-E1 | RT-PCR (COL1, OPN, OCN) | 21 d |
| [118] | Direct | BMSC | Adhesion (CCK8) | 1, 3, 5, 7, 14 d |
| Morphology and cytotoxicity (live/dead) | ||||
| Early osteogenesis (ALP) | ||||
| Mineral deposition (ARS) | ||||
| Osteogenic gene expression (COL1, OCN, OPN, RUNX2) | ||||
| [58] | Direct | primary rBMSC | DAPI, Phalloidin (cell viability) | 1 d |
| [98] | Direct | L929 | Adhesion | 8 d |
| [169] | Direct | MC3T3-E1 | CCK8 (adhesion + proliferation) | 1, 4, 7 d |
| [66] | Direct | hBMSC | Cytotoxicity | 1, 7, 14, 21, 28 d |
| [67] | Direct | hBMSC | Adhesion + proliferation, ALP (cell differentiation), ARS (calcium deposition) | 1, 7, 14, 21, 28 d |
| [132] | Direct | MC3T3-E1 | Live/dead (cytotoxicity), CCK8 (proliferation), ALP (differentiation) | 1, 4, 7 d (proliferation), 21 d (differentiation) |
| [46] | Direct | MC3T3-E1 | DAPI (adhesion, proliferation, differentiation) | 1, 4, 7 d |
| [178] | Direct | MC3T3-E1 | DAPI (adhesion), MTS (cell proliferation) | 1 d (adhesion) |
| 3 d (proliferation) | ||||
| [180] | Direct | MG-63 | MTS (cytotoxicity), DAPI-actin (morphology) | 3 d |
| [191] | Direct | MC3T3_E1 | CCK8 (adhesion + proliferation) | 2 w |
| [108] | Direct | MC3T3-E1 | Adhesion, CCK8 (proliferation), ALP (differentiation) | 3 d (adhesion) |
| 1, 7 d (proliferation) | ||||
| 7, 14, 21 d (differentiation) | ||||
| [53] | Direct | rBMSC | CCK8 | 1, 7 d (adhesion) |
| 1, 3, 7 d (proliferation) | ||||
| [106] | Direct | rBMSC | MTT (proliferation) | 1, 4, 7 d |
| [111] | Direct | HUVEC | CCK8 & live/dead assay (adhesion) | 1, 3, 5, 7 d |
| qPCR (angiogenesis) | ||||
| [104] | Direct | rBMSC | CCK8, live/dead (adhesion, proliferation), ALP (differentiation), OPN, RUNX2, COL2, VEGFR2, vWF, CD31 (protein expression) | 1, 4, 7 d |
| [107] | Direct | MC3T3-E1 | CCK8 (adhesion + proliferation) | 1, 3 d |
| [125] | Direct | U2OS, MSC | DAPI (adhesion), GloTM-MT (proliferation), ALP + ARS (differentiation), RUNX2, Sp7, Spp1 (RT-qPCR) | 66 h (proliferation) |
| 14–35 d (differentiation) | ||||
| [94] | Direct | MC3T3-E1 | XTT kit (adhesion, proliferation) | 1, 3, 7 d |
| [39] | Direct | ADSC, MC3T3 | Alamar blue (proliferation) | 1, 7, 14, 21 d |
| Phalloidin/DAPI staining (Viability) | ||||
| RUNX2, COL1A1, OCN, OPN (qPCR) | ||||
| [171] | Direct | rBMSC, EPC, RAW264.7 | DAPI (adhesion), CCK8 (proliferation), Calcium-AM/PI (Live/dead) | 1, 3, 5 d |
| Polarization of macrophages: immunofluorescence staining, RT-PCR | ||||
| Osteogenic activity of BMSCs in MCM: Alizarin red | ||||
| Angiogenic activity of EPCs in MCM | ||||
| [161] | Direct | rBMSC, hBMSC | DAPI (Adhesion), qPCR (qualitative osteogenic gene analysis (RUNX2, GAPDH, OCN, OPG) | 3, 7 d |
| [193] | Direct | hMSCs | ALP, DNA | 21 d |
| [122] | Direct | MC3T3-E1 | pNPP (ALP activity), DAPI (cell adhesion), Quanti-iT PicoGreen assay (cell viability) | 1, 7, 14 d |
| [214] | Direct | MC3T3-E1 | Cytotoxicity (agar) | 24 h |
| [182] | Direct | hTMSC | CCK8 (cytotoxicity) | 1, 4, 7 d |
| [100] | Direct | Phalloidin/DAPI staining | 1 d | |
| [134] | Direct | hFOB | CCK8 (cytotoxicity & proliferation) | 1, 4, 7 d |
| qPCR: RUNX2, COL1, ALP, OPN, OCN (differentiation) | ||||
| Actin/DAPI staining | ||||
| [184] | Direct | BMSC | Proliferation, migration, differentiation | 1,3, 5 d (proliferation) |
| 1 d (migration) | ||||
| 3, 7, 14, 21 d (differentiation) | ||||
| [195] | Direct | MC3T3-E1 | ALP (differentiation) | 21 d |
| [135] | Direct | MC3T3-E1 | Phase contrast light microscopy, SEM | 2, 7, 14 d |
| [162] | Direct | rBMSC | Adhesion, proliferation | 1, 3, 5, 7 d |
| [185] | Direct | BMSC | AlamarBlue, confocal, PCR | 1, 3, 5, 7, 14 d |
| [47] | Direct | BMSC | Proliferation, apatite formation | 1, 3, 5 d (proliferation) |
| 3 d (apatite formation) | ||||
| [54] | Direct | BMSC | CCK8 (cytotoxicity & proliferation) | 1, 4, 7 d |
| [55] | Direct | mBMSC | cell proliferation, ALP staining, live dead | 1, 3, 5 d |
| [168] | Direct | hMSC | OIM, ALP, AR, OCN + qRT-PCR + angiogenic + proteomics | 7, 14 d |
| [137] | Direct | MTT (cytotoxicity-proliferation) | 12, 24, 36, 48 h | |
| [110] | Direct | MC3T3-E1 | CCK8 (cytotoxicity & proliferation) | 1, 4, 7 d |
| F-actin (adhesion) | ||||
| [49] | Direct | MC3T3-E1 | CCK8 (cytotoxicity & proliferation) | 1, 3, 7 d |
| [50] | Direct | BMSC | CCK8 (cytotoxicity & proliferation) | 1, 3, 5 d |
| Rhodamine-phalloidin/DAPI (adhesion) | ||||
| qRT-PCR: RUNX2, COL1A1, BMP-2, OPN (differentiation) | ||||
| [120] | Direct | Mouse L929 | DAPI, SEM (morphology) | 1, 4, 7, 14 d |
| [116] | Direct | MC3T3-E1 | SEM (adhesion) | 1, 4, 7 d |
| CCK8 (cell viability) | ||||
| [63] | Direct | mBMSC | Alamar blue + live dead (cell viability) | 1, 3, 5 d |
| [42] | Direct | BMSC | ALP quantification (differentiation) | 7 d |
| [167] | Direct | BMSC | Live/dead (viability) | 1, 3, 5 d |
| CCK (proliferation) | ||||
| [196] | Direct | MSC | MTS (proliferation) | 1, 2, 3 w |
| ALP (differentiation) | ||||
| [71] | Direct | hMSC | F-actin, DAPI (proliferation) | 1, 2, 6 d |
| PCR: RUNX2, ALP, OPN, Osteocalcin, COL1 (differentiation) | 1, 2, 3, 4 w (PCR) | |||
| [70] | Direct | OB, MSC, BrCa (mono & co-culture) | Live/dead (cell viability) | 1,3,5 d, 2 w (coculture) |
| Alamar Blue (proliferation mono-culture) | ||||
| MTS (proliferation co-culture) | ||||
| CellTracker Green/orange (adhesion of co-culture) | ||||
| ALP (differentiation) | ||||
| [197] | Direct | hFOB, MDA-MB-231 | MTS (viability) | 1, 3, 5, 7 d |
| Co-culture | Red-X Phalloidin/DAPI (adhesion) | |||
| IL-8 ELISA Kit (Osteoblast cell function | ||||
| Direct | hADSC | SEM (cell attachment) | 1, 3, 7, 14, 21 d | |
| ARS (differentiation) | ||||
| RT-qPCR (OCN, OPN, RUNX2) | ||||
| [198] | Direct | MCF-7/MB-MDA231 | MTS (viability & chemosensitivity) | 1, 3, 5 d |
| Co-culture | Red-X Phalloidin/DAPI (adhesion and tumor growth) | |||
| [141] | Direct | MG63 | Live/dead | 1, 3, 7, 14 d |
| [72] | Direct | Osteoblasts | MTS | 1, 4, 7 d |
| [144] | Direct | RAW267.4 | RT-qPCR | 3, 5, 7 d |
| [139] | Direct | HUVEC | CD31/VEGF staining | 5 d |
| Direct | MC3T3 | Cell viability (live/dead), adhesion (phalloidin/DAPI staining) | 3, 5 d | |
| [140] | Direct | MC3T3 | CCK8 (cell proliferation) | 3, 7 d |
| [95] | Direct | Rat BMSC | DAPI/FITC staining, CCK8 | 1, 3, 5 d |
| [145,152] | Direct | MC3T3 | Rhodamine phalloidin/Hoechst 33342 staining, MTT, ALP | 1, 3, 7 d (adhesion, MTT) |
| 7, 14 d (ALP) | ||||
| [199] | Direct | dMC3T3-OB | Live/dead, XTT (proliferation), ALP (differentiation) | 1, 3, 7 d |
| Direct | dRAW-OC | XTT, TRAP staining, F-Actin/DAPI staining | 7 d | |
| [91] | Direct | rBMSC | CCK8, calcein AM/PI, SEM, ALP | 1, 4, 7 d |
| [186] | Direct | rBMSC | CCK8, Phalloidin/DAPI, RT-qPCR (BMP2, ALP, OPN, COL1) | 1, 3, 5 d (proliferation) |
| 7, 14 d (osteogenesis) | ||||
| [146] | Direct | UC-MSC | CD31, PDGF-α staining (vascularization) | 3 w |
| Direct | UC-MSC | OCN, COL1 staining | 1, 3, 4 w | |
| [75] | Direct | BMSC | Live/dead staining, CCK8 (proliferation) | 1, 4, 7 d |
| [74] | Direct | MC3T3-E1 | CCK8, Phalloidin/DAPI staining (proliferation) | 1, 4, 7 d |
| Direct | Rabbit BMSC | ALP, ARS, PT.PCR | 12, 18 d | |
| [203] | Direct | MC3T3-E1 | CCK8, Live/dead, hemolysis test | 1, 3, 5 d |
| Direct | MC3T3-E1 | ALP staining, ALP, ARS, RT-qPCR (BMP-2, OCN, COL1, GAPDH), Phalloidin/DAPI staining, Elisa assay (osteogenesis activity) | 7, 14 d | |
| [55,215] | Direct | MC3T3-E1 | Proliferation, ALP, ARS, PCR | 1, 3, 5 d (proliferation) |
| 7, 14 d (ALP) | ||||
| 14 d (PCR) | ||||
| 14, 21 d (ARS) |
Abbreviations: ADSC: adipose derived stem cell, ALP: alkaline phosphatase, ARS: alizarin red S, BMP-2: bone morphogenetic protein-2, BMSC: bone marrow stem cells, BrCa: breast cancer, CAM/PI: calcein-AM and propidium iodide solutions, CCK8: cell counting kit 8, CD31: cluster of differentiation 31, COL1: collagen type 1, DAPI: 2-(4-amidinophenyl)-1H -indole-6-carboxamidine, EPC: endothelial progenitor cells, GAG: glycosaminoglycans, GAPDH: glyceraldehyde-3-phosphate dehydrogenase, hBMSC: human bone marrow stem cells, hDF: human dermal fibroblasts, hFOB: human fetal osteoblast, hMSC: human mesenchymal stem cells, hTMSC: human turbinate mesenchymal stromal cells, HUVEC: human umbilical vein endothelial cells, IL-8: interleukin-8, MAPK: mitogen activated protein kinase, mBMSC: mouse bone marrow stem cell, MC3T3-E1: mouse calvaria cell line, MCF-7: breast cancer cell line, MDA-MB.231: breast epithelial cell line, MG-63: osteosarcoma derived cell line, MTS/MTT/XTT: cell viability assays, NCTC-L929: clone of mouse strain L, OCN: osteocalcin, OPN: osteopontin, PBS: Phosphate buffered saline, pNPP: nitrophenyl phosphate, RAW264,7: macrophage cell line, rBMSC: rabbit bone marrow derived mesenchymal stem cell, RT-qPCR: real-time quantitative polymerase chain reaction, RUNX2: runt-related transcription factor 2, SBF: simulated body fluid, SD rats: SPRAGUE DAWLEY®, SEM: scanning electron microscopy, Sp7: osterix, Spp1: osteopontin, TRIS-HCl: 2-Amino-2-hydroxymethyl-propane-1,3-diol, U2OS: human osteo-sarcoma cell line, VEGF: vascular endothelial growth factor, VEGFR2: vascular endothelial growth factor receptor 2, vWF: Von Willebrand factor.
∗Values taken indirectly from graphs and not explicitly described on article's text.
Table 5.
In vivo characterization studies of VP-printed calcium phosphate-based scaffolds reported in the literature. (d: day/s, w: week/s, m: month/s).
| ref | In vivo | Animal model | time |
|---|---|---|---|
| [153] | Orthotopic (forelimb) | New Zealand white rabbits | 4, 12 w |
| [128] | Orthotopic (sheep femur) | Female Sheep (ISO 109936:2016) | 6 m |
| [216] | Orthotopic (skull) | Humans' skull | 12 m |
| [99] | Orthotopic (parietal bone) | New Zealand white rabbits | 4, 8 w |
| [67] | Orthotopic (calvaria defect) | Rabbits, females | 0, 3, 6 w |
| [179] | Orthotopic (mandible) | Adult male beagle dogs | 4, 8 w |
| [180] | Orthotopic (subperiosteal cranial) | Rat | 3, 6 m |
| [108] | Orthotopic (femur) | New Zealand rabbits, male | 6, 12 w |
| [181] | Orthotopic (skull, calvaria) | Rabbit | 4, 8 w |
| [111] | Ectopic (subcutaneous back midline) | SD rats | 8 w |
| [41] | Ectopic (subcutaneous) | Not said | 2 w |
| [107] | Ectopic (dorsal muscles) | New Zealand white rabbits | 2 w |
| [39] | Orthotopic (rat femur) | Rat | 4, 8 w |
| Ex-ovo (CAM model) | Chicken egg | Embryonic d 10 | |
| [192] | Orthotopic (diastolic epiphysis of femoral bone) | White rat (line "Vistar") | 2, 4, 8 w |
| [144] | Ectopic (subcutaneous) | SD male rats | 1, 4 w |
| Orthotopic (skull) | SD male rats | 4, 8 w | |
| [139] | Ectopic (epidermal wounds) | Male New Zealand rabbits | 4 w |
| Orthotopic (femoral condyle) | Male New Zealand rabbits | 4 w | |
| [140] | Orthotopic (femoral condyle) | Male New Zealand rabbits | 4, 8 w |
| Orthotopic (calvaria) | Rat | 1, 2, 3, 6 w | |
| [175] | Orthotopic (distal femur) | New Zealand rabbits | 4, 8, 12 w |
| [186] | Orthotopic (femoral condyle) | Female Bama miniature pigs | 3, 6 m |
| [146] | Orthotopic (femur) | New Zealand rabbits | 2, 4 w |
| [75] | Orthotopic (calvaria) | SD rats | 4, 8 w |
| [74] | Ectopic (intradermal) | Nude mice | 30 d |
| Orthotopic (cranium) | New Zealand rabbits | 4, 12 w | |
| [78] | Ectopic (subcutaneous) | SD rats | 1, 7, 14, 21 d |
| Orthotopic (calvaria) | SD rats | 6 w | |
| [177] | Ectopic (subcutaneous) | Mouse | 2, 4 w |
| [142] | Ectopic (subcutaneous) | SD rats | 2, 4 w |
| Orthotopic (calvaria) | New Zealand white rabbits | 4, 8 w | |
| [171] | Orthotopic (femoral condyle bone) | New Zealand White Rabbit | 6, 12 w |
| [161] | Orthotopic (parietal bones) | Sprague Dawley rats | 4 w |
| [184] | Ectopic (rectus femoris muscle) | BALB/c mouse | 60, 90 d |
| Orthotopic (femoral condyle) | SD rat | 4, 8 w | |
| [162] | Orthotopic (femoral condyle) | Rabbit (New Zealand) | 8, 12 w |
| [85] | Orthotopic (femur diaphysis) | Rat | 3, 6 w |
| [136] | Orthotopic (calvaria) | Rat (male Wistar) | 4, 8 w |
| [185] | Ectopic (rectus femoris muscle) | Mouse (BALB/C) | 60, 90 d |
| [54] | Ectopic (dorsal muscle) | Dog (Beagle) | 3 m |
| [55] | Orthotopic (calvaria defect) | Rat | 12 w |
| [168] | Orthotopic (femoral defect) | Rabbit | 4, 8 w |
| [110] | Orthotopic | Rabbit mandible | 4, 8, 12 w |
| [49] | Orthotopic (femur) | Rabbit | 4, 16 w |
| Ectopic (dorsal muscles) | Dog (Beagle) | 4, 16 w | |
| [166] | Orthotopic (parietal bone) | Rat | 8 w |
| [172] | Orthotopic (femur) | Rabbit | 6, 12 w |
Furthermore, the following sections summarize the most relevant effects of scaffolds’ composition and their design in the overall biological performance.
6.1. Effect of scaffolds composition on biological performance
The composition of the scaffolds directly influences their biological performance when interacting with cells or tissues in animal models. The most commonly reviewed strategies used to modify scaffold composition and enhance biological performance involve either adjusting the inorganic components or the organic polymeric matrix in composite scaffolds before printing or incorporating coatings and/or molecules after the printing process.
The first strategy is the modification of the inorganic phase of the slurry, mainly focused on full ceramic scaffolds. The addition of multicomponent inorganic elements to the ceramic slurry has been addressed, as researchers have found that the release of different ions can positively favor bone ingrowth properties. Bivalent cations such as Sr2+, Mg2+, and Zn2+ can replace Ca2+ in the crystal structure, altering stability, microstructure, solubility, and ion exchange. These changes positively impact bone formation and material degradation through both chemical effects and lattice modification [217]. Following this line, Qi et al. used MgO as doping agent for full ceramic β-TCP scaffolds, as the appropriate release of Mg2+ demonstrated to regulate the immune environment and positively affect osteogenesis [171]. In this study, Mg-doped β-TCP scaffolds with a macroporosity of approximately 67 % and a pore size of 650 μm promoted osteogenesis and angiogenesis through macrophage immunomodulation in vitro and in vivo. The in vivo results reflected around 1.5-fold increase in bone formation (BV/TV) in 3 % Mg doped β-TCP compared to β-TCP controls both in 6 weeks and 12 weeks (Fig. 8A). Alternatively, adding ion-rich crystals like Whitlockite (Ca9Mg(PO4)6(PO3OH), [152]), Akermanite (Ca2MgSi2O7, [56,58]), or Bredigite (Ca7MgSi4O16, [145]) also promoted cell proliferation and osteogenic differentiation by releasing Mg2+ and Si4+. Guo et al. demonstrated that the addition of Bredigite in HA full ceramic scaffolds reduced HA grain size, increased microporosity, and reduced densification, leading to higher porosity, accelerated degradation, and Mg2+, and Si4+ release. In addition, it also accelerated the release of Ca2+ and PO43− from the HA matrix. While reducing the mechanical properties of the scaffolds, the release of Mg2+, Si4+ Ca2+, and PO43−, stimulated bone protein production, osteoblast adhesion and growth [145]. Lately, barium titanate (BaTiO3) has gained attention in HA/BT doped ceramic scaffolds. During sintering, Ba2+ can substitute Ca2+, forming Ba3(PO4)2, and CaTiO3, promoting cell differentiation [149]. Together with Zn2+ substitution, these studies highlight the synergistic effects of piezoelectric properties that support osteogenesis and angiogenesis when combined with low-intensity pulsed ultrasound (LIPUS) [150]. Finally, ZrO2, has also been employed to modify the inorganic phase of HA ceramic scaffolds. Gao et al. introduced nano zirconia to coat the HA grains, targeting the inhibition of soft tissue invasion [144]. Zirconia was used for its potential to bind to dectin-1 receptor, known to mediate soft-tissue invasion. As the regeneration of hard and soft tissue is competitive and interactive, this study aimed at potentiating osteogenesis while reducing soft tissue generation. Although not inducing bone regeneration subcutaneously, HA/zirconia scaffolds caused an anti-inflammatory environment with little fibrosis after 4 weeks. Besides, when implanted in calvaria it resulted in decreased inflammation and soft tissue invasion, and accelerated bone regeneration at 4 weeks, showing around two-fold increased BV/TV in HA/nano zirconia group compared to HA control group.
Fig. 8.
Effect of the scaffold's composition on biological performance; strategies proposed in the literature to improve the biological performance of calcium phosphate scaffolds obtained by VP. (A) Multicomponent printing, by the addition of other components (MgO) to the β-TCP initial powder, Mg-doped scaffolds performed better bone regeneration at orthotopic implantation for both 6 and 12 weeks compared to undoped full ceramic β-TCP counterpart [171]. (B) BMP-2-coated BCP ceramic scaffold performed better bone formation at ectopic and orthotopic implantation for both 2 and 3 month after implantation compared to uncoated BCP scaffolds [184]. (C) The addition of biological elements such as platelet lysates (PL) containing growth factors to a GelMA coating on BCP full ceramic scaffolds enhance the scaffold's vascularization properties in terms of vessel area/total area formation percentage [111].
Conversely, other researchers have focused on the modification of the organic part of polymer/CaP composite scaffolds made prior to printing, by formulating polymeric resins that contain biomolecules. Modified polymers are often envisioned to improve local cell-material interactions, providing textural or biologically engineered surfaces where cells can anchor through integrins in their membrane. Zhou et al. mixed GelMA with PEGDA to print HA-containing composite scaffolds, as GelMA exhibits notable biocompatibility due to the abundant arginine-glycine-aspartic acids (RGD) and matrix metalloproteinase (MMP) poly-peptide sequences, which can significantly promote cell attachment and proliferation while maintaining an adequate strength of the material using PEGDA [70,71]. In addition, in other studies, they substituted GelMA by incorporating RGD peptidic sequences directly into stronger synthetic PEGDA polymers. In fact, they demonstrated enhanced differentiation of mesenchymal stem cells (MSC) through alkaline phosphatase activity (ALP) assays on PEGDA-nHA composite scaffolds. Incorporating RGD sequences the ALP activity increased from 1.9 μmol/(L min) to 2.1 μmol/(L min) at week three. This effect was further amplified by treating the scaffold with low-intensity pulsed ultrasound (LIPUS), raising the ALP activity to approximately 2.3 μmol/(L min) at week three [196]. Moreover, Liu et al. highlighted the reduced list of natural polymers for printing bioactive scaffolds, and especially for bioprinting with cells, where GelMA is the most used polymer [76]. Their study focused on the synergistic effects of using methacrylated bone-derived decellularized extracellular matrix (bdECM-MA) and silicon-substituted CaP (Si-CaP) to bioprint bone-derived mesenchymal stem cells (BMSCs). In vitro, both materials enhanced osteogenesis and angiogenesis, with bdECM-MA promoting collagen secretion and endothelial growth, while Si-CaP boosting ALP expression. Combined, they showed synergistic effects and activated the TLR4–PI3K–AKT pathway. They also modulated the immune response by inhibiting the p38-MAPK pathway and promoting anti-inflammatory macrophage polarization. In vivo, the composite supported sequential immune modulation, enhanced vascularization, and achieved near-complete bone regeneration within 6 weeks. The findings highlighted their strong therapeutic potential and encourage further study of the immunomodulatory mechanisms.
Finally, coatings and functionalizing molecules can be included as part of the scaffold after printing. Tang et al. used Bone Morphogenetic protein-2 (BMP-2), a bone growth factor used to induce osteoblastic differentiation, by incorporating it into nanogels made of Heparin and Polyethyleneimine (PEI), which have good biocompatibility, water solubility, and biodegradation, and applying it as coating for BCP full ceramic scaffolds. Their findings revealed not only a promotion of BMSC proliferation, migration, and osteoblastic differentiation in vitro but also ectopic bone formation and accelerated bone regeneration in vivo. These results indicated a slightly increased bone formation fraction (BV/TV) for ectopic implantation for BMP-2 coated BCP ceramic scaffold compared to uncoated BCP scaffolds, showcasing the osteoinductive properties of morphogenic proteins. The most significant effect was revealed in orthotopic implantation, where BMP-2 coated BCP scaffolds resulted in a 10 % increase in bone formation after 8 weeks compared to the BCP control (Fig. 8B) [184]. In a similar approach but focusing on vascularization, Liu et al. coated a BCP full ceramic scaffold with platelet lysate (PL)-rich GelMA coating, which promoted angiogenesis [111]. Their findings revealed a significant increase in blood perfusion, number of capillaries, and vessel areas ratio with over 1.5 % improvement for PL-GelMA coated BCP scaffold compared to the uncoated control when implanted subcutaneously in the back of rat models (Fig. 8C). Finally, the integration of traditional pharmaceuticals to CaP scaffolds is gaining attention [139]. Gui et al. incorporated icariin, a primary chemical constituent extracted from plants (Epimedium genus in the Burseraceae family) in GelMA hydrogels to coat HA full-ceramic scaffolds after printing. Icariin has the promising ability to enhance stem cell osteogenic differentiation and vascular regeneration. The incorporation of icariin promoted angiogenesis and osteogenesis, outperforming the HA full ceramic groups both in vitro and in vivo. The HA/GelMA/Icariin group promoted the activation, proliferation, migration, and tube formation ability of HUVECs, and promoted the differentiation of rabbit BMSCs in vitro. Furthermore, in vivo tests concluded that icariin-loaded scaffolds promoted osteoinduction after 4 weeks of subcutaneous implantation showing ∼4 mm3 of new bone formation compared to ∼2 mm3 in the icariin-free scaffolds [139]. Furthermore, the HA/GelMA/icariin scaffolds showed a relative in-growth bone area of 9 %, compared to 5.5 % in icariin-free HA scaffolds, orthotopically after 8 weeks [140].
6.2. Effect of scaffolds design on biological performance
Similar to the mechanical performance outcomes, the overall scaffold architecture greatly affects their biological outcome. Scaffolds' designs, often dictating pore geometry, total porosity, and interconnectivity influence the cellular response, bone ingrowth and resorption rates, crucial for bone remodeling. For instance, studies have shown that smooth, curved geometries like TPMS promoted higher mechanical strength and cell proliferation than commonly designed grid-like structures in extrusion-based techniques [167]. Using VP printed β-TCP full ceramic scaffolds with a porosity of 60 % and 300 μm pore size for both geometries, in vitro studies of mouse bone marrow stem cells (mBMSCs) at day five showed a higher proliferation for gyroid compared to common grid-like counterpart (Fig. 9A). Among the various TPMS geometries, gyroid stands as one of the most promising. Recent research has demonstrated that gyroid structures promote greater bone formation in vivo compared to that of Diamond or Schwarz primitive [218]. Additionally, the influence of pore geometry on enhancing and steering cell activity and bone regeneration has garnered great interest [215]. Werner et al. showed that human bone marrow stem cells (hBMSCs) exhibited faster migration on concave surfaces compared to convex ones [219]. In line with this, previous studies using strut-based printed scaffolds have highlighted the need for concave surfaces within the scaffolds to enhance bone regeneration in vivo [220,221]. Biologically, the need for concavities capable of concentrating important ions, growth factors and proteins has been long accepted [7].
Fig. 9.
Effect of the scaffold's architecture on biological performance; strategies proposed in the literature to improve the biological performance of calcium phosphate scaffolds obtained by VP. (A) β-TCP full ceramic scaffolds with similar porosity for gyroid and grid-like geometries show different proliferation results of mouse bone marrow stem cells (mBMSCs) at day five. Gyroid-TPMS geometry showed a higher proliferation compared to common grid-like counterpart [167]. (B) flow-channel designs promote bone ingrowth and vascularization in ectopic implantation by facilitating a rapid infiltration of the HA full ceramic scaffold's inner struts and the smooth transportation of substances, forming a richer metabolic microenvironment [107]. (C) Hexagonal close-packed (HCP) BCP full ceramic structures promoted osteoinductivity, with enhanced new bone formation after 10 weeks in vivo ectopic implantation, and slightly promoted bone formation in orthotopic implantation after 8 weeks [215].
Generally, a greater bone formation is obtained with rapid cell colonization and proliferation, resulting from proper nutrient and cell diffusion throughout the printed scaffold. Therefore, different studies have focused on the promotion of permeability/flowability of the scaffolds following different strategies. Mao et al. designed interpenetrating flow channels in HA scaffolds, which appeared to be helpful for the rapid infiltration of the scaffold's inner struts and the smooth transportation of substances, forming a richer metabolic microenvironment for tissue regeneration by bringing richer nutrients and targeting cells. In fact, the flow channels revealed a higher relative bone ingrowth area than the conventional scaffold counterpart implanted in vivo in rabbit muscle (ectopic implantation) [107]. The results showed a prominent increase when comparing a control group without channels, with a relative in-growth area of ∼30 %, with scaffolds with channels, showing ∼50–70 % in-growth area (Fig. 9B). Following this idea, Gui et al. fostered bone regeneration by creating internal channels, mimicking the structure of loofah sponges. The enhanced flowability of the loofah-like scaffolds, combined with a drug-loaded coating, created a synergistic effect showing an optimum drug distribution inside the scaffold that promoted bone regeneration capability both in vitro and in vivo [139]. Regarding water penetration, Lee et al. found that elongated pores in β-TCP full ceramic scaffolds showed also much faster water penetration [113]. This discovery led them to suggest that porous CaP scaffolds with elongated pores would enhance the transport of blood, oxygen, and nutrients, thereby promoting faster bone regeneration. However, the search for better flowability properties comes with a decrease in mechanical properties. Therefore, a balance between these two properties must be considered when designing bone grafts.
Despite the decrease in mechanical properties, permeability can strongly influence the scaffold's properties in vitro and in vivo. Wu et al. tested BCP full ceramic scaffolds with varying pore architecture, namely gyroid, octahedral, diamond, and hexagonal close-packed (HCP), with comparable macroporosity and pore size (∼70 % and ∼515 μm respectively). They reported that compared to the other architectures, hexagonal close-packed (HCP) exhibited 1) a more uniformly cell growth along the pore walls, 2) significantly higher osteogenic genes and proteins expressions in vitro using MC3T3-E1, 3) higher osteoinductivity related to an enhanced new bone formation on an ectopic in vivo model after 10-week, with up to eight times greater bone formation (BV/TV) than octahedral (with an 8.02 ± 1.94 % of new bone), 4) higher new bone formation on orthotopic implantation, showing slightly higher bone formation capability for HCP with approximately around 10 % higher new bone (BV/TV) compared to orthogonal geometry after 8 weeks (Fig. 9C), 5) upregulated expression of angiogenic factors, and 6) high bioactivity regarding a full coverage of the surface with precipitated bone-like apatite after SBF immersion [215]. The role of pore geometry in bone regeneration was attributed to the round and close-packed nature of HCP pores, which diminishes permeability with a lower liquid flow rate as obtained with simulation and mercury porosimetry. This control in flow rate is thought to create a more stable environment, promoting the formation of bone-like apatite and increasing the biological performance. This is consistent with previous reports, which indicate that bone formation typically occurs in concavities rather than convexities, suggesting that concave surfaces are more effective at accumulating calcium and phosphate ions compared to convex pores [7].
7. Conclusions and future perspectives
In regenerative medicine, computer-aided three-dimensional printing stands as one of the most promising methods for fabricating bone implants. These additive manufacturing techniques offer high versatility and precision, enabling the creation of patient-specific grafts tailored to individual trauma needs. Notably, Vat photopolymerization (VP) printing has gained increasing attention in the field of bone regeneration. This review highlights the significant potential of calcium phosphate (CaP)-based scaffolds, which closely mimic the mineral phase of natural bone and possess osteoconductive and osteoinductive properties. The development of CaP scaffolds has rapidly advanced, with a clear trend towards using high-resolution AM techniques such as VP, which provides distinct advantages regarding other techniques in promoting bone healing.
The use of ceramic loaded resins in VP involves a suspension of CaP particles that must meet rigorous optical and rheological requirements. Numerous researchers have addressed this challenge, optimizing the printability of slurries containing up to 70 % ceramic in weight. Advances in understanding the interactions between slurry components have led to the development of complex compositions, such as introducing doping agents, biopolymers, and even therapeutic components, hardly considered in other AM techniques. Ongoing efforts focus on improving printing accuracy, maintaining design integrity, enhancing slurry printability, and evaluating the mechanical and biological performance of scaffolds.
VP offers the possibility of using a wide range of materials to create CaP-based bone scaffolds. In fact, resins can be extensively formulated with different photocurable polymers that provide varying characteristics. When printing with calcium phosphates, the resulting composite structures can follow two distinct processing routes, each affecting the microstructure. These routes lead either to a full ceramic body or a polymeric composite framework reinforced with ceramic particles.
Around 75 % of the reviewed studies are focused on full-ceramic scaffolds and the optimization of the thermal treatments after the printing process. Typically, full-ceramic scaffolds include high-temperature CaPs such as hydroxyapatite and beta-tricalcium phosphate, resulting in a sintered-grain microstructure. One key challenge when fabricating full-ceramic scaffolds is the inevitable shrinkage during thermal treatment and subsequent dimensional reduction, resulting in alteration of the initial CAD design and imposing rigorous dimensional characterization. In terms of mechanical properties, although sintered ceramic scaffolds have high compressive strength, they are brittle materials, which can be aggravated by the presence of cracks, abnormal grain growth, poor densification, phase transformations, and other microstructural defects. Furthermore, while showing excellent biocompatibility, sintered CaP scaffolds and especially HA have limited bone-forming capacity due to their reduced surface area and degradability. Some strategies to overcome this limitation include the incorporation of doping agents to enhance reactivity by distorting the crystal lattice, the combination with more soluble second phases, or the increase of microporosity. All of them increase ion release, able to interact with the surrounding cells, aiming at enhancing their biological activity, but in turn may compromise mechanical stability. To preserve the balance between mechanical properties and biological performance, another approach is the introduction of therapeutic-loaded biopolymers onto CaP scaffolds.
In contrast, composite scaffolds are based on biocompatible resins composed of cell-friendly monomers/oligomers, and photoinitiators, combined with CaP particles to form a composite structure. This usually results in a continuous polymeric matrix with dispersed ceramic particles, which resembles more closely the composition of natural bone. The combination of polymers and ceramics provides increased ductility and toughness while maintaining suitable compressive strength. Moreover, as no thermal treatment is required, dimensional changes from the original CAD designs are reduced or eliminated. Synthetic biocompatible polymers such as PEGDA, PTMC-MA, and HDDA, among others, confer adequate rigidity and toughness to the composite structure. However, their inert nature, lacking binding sites for cell adhesion, limits their bioactivity. Natural biopolymers derived from gelatin, silk fibroin, or bone-derived extracellular matrix can be functionalized with acrylate terminating groups, creating hydrogels that can be incorporated as the matrix of CaP composites instead of using them as coatings. In this way, osteogenesis and angiogenesis are promoted due to the increased cell-binding sites and biodegradation. In addition, the use of biopolymers may support bioprinting, with the incorporation of cells inside the structure further enhancing bone regeneration. However, despite the great potential of this family of materials, the list of possible candidates is still very narrow, and the development of new formulations of photosensitive biopolymers is emerging as one of the lines of progress for VP-printing of composite CaPs.
Another remaining challenge is to increase the CaP loading in biopolymer composite resins, as current studies use low amounts, with materials that lack sufficient mechanical properties, making manipulation, implantation, and stability in the host tissue challenging. Furthermore, the possibility of using new types of CaPs may open the way to materials with novel properties. In this regard, investigating the potential of using calcium phosphate cements (CPCs), such as α-TCP, could lead to the formation of a continuous ceramic network following the hardening reaction. This network can have the capacity to interpenetrate the solidified polymeric resin.
One critical feature that has been widely highlighted in this review is the role of the scaffold's pore architecture, which has revealed important outcomes in terms of mechanical stability and biological performance. Advanced geometries containing smooth curvatures, including Triply Periodic Minimal Surfaces (TPMS), have gained important attention, demonstrating increased mechanical stability by alleviating stress concentrations. Pore curvature is also responsible for creating an adequate environment for directing cell growth in vitro and bone regeneration in vivo. The chosen architecture and porosity also affect the scaffold's permeability, and in doing so, affect the biological response. In fact, an interconnected structure with adequate permeability is desired, permitting a proper interconnection between newly formed blood vessels, nutrients and growth factors, as well as the scaffold's degradation. However, the macroporous nature also affects the mechanical stability. Therefore, it is imperative to balance properties such as porosity, pore size, and geometrical design parameters together with the material and processing strategy selection.
One of the great advantages of VP is that it has high resolution and is capable of creating intricate structures, otherwise very difficult to replicate using other AM techniques. SLA and DLP (using DMD or LCD) are the only explored techniques to fabricate CaP scaffolds. While demonstrating exceptional resolution and the ability to create complex, curvature-rich-structures, these techniques operate in layered configurations, which lead to the so-called “stair-stepping” effect, referring to the individual layers stacked along the printing direction. This leads to certain degree of mechanical anisotropy. Although higher-resolution VP techniques, such as Continuous Liquid Interface (CLIP) and Two-Photon Polymerization (2PP) have not yet been explored, their implementation to fabricate CaP scaffolds could lead to smoother surfaces, higher isotropy, and enhanced personalization of medical implants. However, it is crucial to consider that higher resolution increases the printing time, which could jeopardize the stability of the resin during the process.
Infection risks remain a critical concern during scaffold implantation. To date, limited research has addressed this issue, with most efforts relying on antibiotic-based approaches. As pointed by the World Health Organization, antibiotic-resistant implant-related infections present a major challenge in biomedical devices, prompting diversification of treatments alternative to antibiotic-based ones. Promising strategies such as nature-inspired nano-topographies, with bactericidal properties, or antimicrobial peptides (AMPs), such as lactoferrin, have recently attracted scientific attention [222,223]. Nature-inspired nano-topographies can be replicated in CaP ceramics, where features such as crystal aspect ratio significantly influence antimicrobial activity. Acicular CDHA nanocrystals, characterized by a high aspect ratio, have demonstrated bactericidal potential [224], though this remains unexplored in macroporous 3D-printed scaffolds. Additionally, AMP functionalization of printed scaffolds, or their incorporation in the resin formulation could further offer antibacterial strategies [225,226]. Future research should continue to explore the integration of antibacterial properties, whether through mechanical design or compositional adjustments.
In conclusion, VP, combined with calcium phosphate materials and advanced pore designs, offers a powerful approach to improving bone scaffolds compared to existing additive manufacturing procedures, balancing mechanical stability and biological performance. Looking ahead, this technology holds great promise for addressing future challenges in bone tissue engineering. Its high resolution is ideally suited for creating intricate, scaffolds that mimic the complex microenvironment of native bone, paving the way for advanced in vitro models and ultimately, functional tissue regeneration. Furthermore, the versatility of VP allows for the incorporation of therapeutic agents directly into the scaffold material, offering a potent strategy to develop bone scaffolds that can release therapeutic agents in a controlled manner, offering unprecedented opportunities for personalized and dynamic bone regeneration therapies.
CRediT authorship contribution statement
Roberto Fagotto-Clavijo: Writing – original draft, Visualization, Investigation, Formal analysis, Data curation, Conceptualization. Irene Lodoso-Torrecilla: Writing – review & editing, Investigation, Data curation, Conceptualization. Anna Diez-Escudero: Writing – review & editing, Supervision, Investigation, Data curation, Conceptualization. Maria-Pau Ginebra: Writing – review & editing, Supervision, Funding acquisition, Conceptualization.
Ethics approval and consent to participate
Not applicable for this manuscript. This study did not involve animal/human participants, animal/human data, or animal/human tissue.
Declaration of competing interest
The authors declare no competing nor financial interests, or personal relationship that influenced this paper.
Acknowledgements
This work was funded by the European Union - ERC grant (BAMBBI; 101055053, doi: 10.3030/101055053). The research group is part of Maria de Maeztu Units of Excellence Programme CEX2023-001300-M/funded by MCIN/AEI/10.13039/ 501100011033, Spanish Ministry of Science and Innovation. M.-P.G. acknowledges the ICREA Academia award by the Generalitat de Catalunya. A.D.E. acknowledges the Spanish Ministry of Universities for the support received through the Maria Zambrano fellowship.
We would like to acknowledge BioRender for providing the tools to create some of the illustrations of this review. The graphical abstract, Fig. 2, and Fig. 8 were created in BioRender. Ginebra, M. (2025) https://BioRender.com/t26v848.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bioactmat.2025.05.001.
Contributor Information
Anna Diez-Escudero, Email: anna.diez-escudero@upc.edu.
Maria-Pau Ginebra, Email: maria.pau.ginebra@upc.edu.
Appendix A. Supplementary data
The following is the Supplementary data to this article.
References
- 1.Archunan M.W., Petronis S. Bone grafts in trauma and orthopaedics. Cureus. 2021;13 doi: 10.7759/CUREUS.17705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rupp M., Klute L., Baertl S., Walter N., Mannala G., Frank L., Pfeifer C., Alt V., Kerschbaum M. The clinical use of bone graft substitutes in orthopedic surgery in germany—A 10‐years survey from 2008 to 2018 of 1,090,167 surgical interventions. J. Biomed. Mater. Res. Part B Appl. Biomater. 2022;110:350–357. doi: 10.1002/jbm.b.34911. [DOI] [PubMed] [Google Scholar]
- 3.Wubneh A., Tsekoura E.K., Ayranci C., Uludağ H. Current state of fabrication technologies and materials for bone tissue engineering. Acta Biomater. 2018;80:1–30. doi: 10.1016/j.actbio.2018.09.031. [DOI] [PubMed] [Google Scholar]
- 4.Gao J., Li M., Cheng J., Liu X., Liu Z., Liu J., Tang P. 3D-Printed GelMA/PEGDA/F127DA scaffolds for bone regeneration. J. Funct. Biomater. 2023;14:96. doi: 10.3390/jfb14020096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Clarke B. Normal bone anatomy and physiology. Clin. J. Am. Soc. Nephrol. 2008;3:S131–S139. doi: 10.2215/CJN.04151206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ghorbani F., Li D., Ni S., Zhou Y., Yu B. 3D printing of acellular scaffolds for bone defect regeneration: a review. Mater. Today Commun. 2020;22 doi: 10.1016/j.mtcomm.2020.100979. [DOI] [Google Scholar]
- 7.Bohner M., Miron R.J. A proposed mechanism for material-induced heterotopic ossification. Mater. Today. 2019;22:132–141. doi: 10.1016/j.mattod.2018.10.036. [DOI] [Google Scholar]
- 8.Chai Y.C., Carlier A., Bolander J., Roberts S.J., Geris L., Schrooten J., Van Oosterwyck H., Luyten F.P. Current views on calcium phosphate osteogenicity and the translation into effective bone regeneration strategies. Acta Biomater. 2012;8:3876–3887. doi: 10.1016/j.actbio.2012.07.002. [DOI] [PubMed] [Google Scholar]
- 9.Del-Mazo-Barbara L., Johansson L., Tampieri F., Ginebra M.-P. Toughening 3D printed biomimetic hydroxyapatite scaffolds: polycaprolactone-Based self-hardening inks. Acta Biomater. 2024;177:506–524. doi: 10.1016/j.actbio.2024.02.012. [DOI] [PubMed] [Google Scholar]
- 10.Lodoso-Torrecilla I., van den Beucken J.J.J.P., Jansen J.A. Calcium phosphate cements: optimization toward biodegradability. Acta Biomater. 2021;119:1–12. doi: 10.1016/j.actbio.2020.10.013. [DOI] [PubMed] [Google Scholar]
- 11.Lodoso-Torrecilla I., Stumpel F., Jansen J.A., van den Beucken J.J.J.P. Early-stage macroporosity enhancement in calcium phosphate cements by inclusion of poly(N-vinylpyrrolidone) particles as a porogen. Mater. Today Commun. 2020;23 doi: 10.1016/j.mtcomm.2020.100901. [DOI] [Google Scholar]
- 12.Diez-Escudero A., Espanol M., Beats S., Ginebra M.-P. In vitro degradation of calcium phosphates: effect of multiscale porosity, textural properties and composition. Acta Biomater. 2017;60:81–92. doi: 10.1016/j.actbio.2017.07.033. [DOI] [PubMed] [Google Scholar]
- 13.Cinici B., Yaba S., Kurt M., Yalcin H.C., Duta L., Gunduz O. Fabrication strategies for bioceramic scaffolds in bone tissue engineering with generative design applications. Biomimetics. 2024;9:409. doi: 10.3390/biomimetics9070409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ginebra M.P., Espanol M., Montufar E.B., Perez R.A., Mestres G. New processing approaches in calcium phosphate cements and their applications in regenerative medicine. Acta Biomater. 2010;6:2863–2873. doi: 10.1016/j.actbio.2010.01.036. [DOI] [PubMed] [Google Scholar]
- 15.Raymond Y., Johansson L., Thorel E., Ginebra M.-P. Translation of three-dimensional printing of ceramics in bone tissue engineering and drug delivery. MRS Bull. 2022;47:59–69. doi: 10.1557/s43577-021-00259-1. [DOI] [Google Scholar]
- 16.Petousis M., Michailidis N., Korlos A., Papadakis V., David C., Sagris D., Mountakis N., Argyros A., Valsamos J., Vidakis N. Biomedical composites of polycaprolactone/hydroxyapatite for bioplotting: comprehensive interpretation of the reinforcement course. Polymers. 2024;16:2400. doi: 10.3390/polym16172400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Petousis M., Papadakis V., Moutsopoulou A., Spiridaki M., Argyros A., Sfakiotakis E., Michailidis N., Stratakis E., Vidakis N. The effect of hydroxyapatite particle shape, and concentration on the engineering performance and printability of polycaprolactone-hydroxyapatite composites in bioplotting. Bioprinting. 2024;44 doi: 10.1016/j.bprint.2024.e00370. [DOI] [Google Scholar]
- 18.Wang W., Zhang B., Li M., Li J., Zhang C., Han Y., Wang L., Wang K., Zhou C., Liu L., Fan Y., Zhang X. 3D printing of PLA/n-HA composite scaffolds with customized mechanical properties and biological functions for bone tissue engineering. Composites, Part B. 2021;224 doi: 10.1016/j.compositesb.2021.109192. [DOI] [Google Scholar]
- 19.Wang W., Liu P., Zhang B., Gui X., Pei X., Song P., Yu X., Zhang Z., Zhou C. Fused deposition modeling printed PLA/nano β-TCP composite bone tissue engineering scaffolds for promoting osteogenic induction function. Int. J. Nanomed. 2023;18:5815–5830. doi: 10.2147/IJN.S416098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Diez-Escudero A., Andersson B., Persson C., Hailer N.P. Hexagonal pore geometry and the presence of hydroxyapatite enhance deposition of mineralized bone matrix on additively manufactured polylactic acid scaffolds. Mater. Sci. Eng. C. 2021;125 doi: 10.1016/j.msec.2021.112091. [DOI] [PubMed] [Google Scholar]
- 21.Wang L., You X., Zhang L., Zhang C., Zou W. Mechanical regulation of bone remodeling. Bone Res. 2022;10 doi: 10.1038/s41413-022-00190-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Milazzo M., Contessi Negrini N., Scialla S., Marelli B., Farè S., Danti S., Buehler M.J. Additive manufacturing approaches for hydroxyapatite‐reinforced composites. Adv. Funct. Mater. 2019;29:1–26. doi: 10.1002/adfm.201903055. [DOI] [Google Scholar]
- 23.Raymond Y., Thorel E., Liversain M., Riveiro A., Pou J., Ginebra M.-P. 3D printing non-cylindrical strands: morphological and structural implications. Addit. Manuf. 2021;46 doi: 10.1016/j.addma.2021.102129. [DOI] [Google Scholar]
- 24.Raymond S., Maazouz Y., Montufar E.B., Perez R.A., González B., Konka J., Kaiser J., Ginebra M.-P. Accelerated hardening of nanotextured 3D-plotted self-setting calcium phosphate inks. Acta Biomater. 2018;75:451–462. doi: 10.1016/j.actbio.2018.05.042. [DOI] [PubMed] [Google Scholar]
- 25.Raymond Y., Lehmann C., Thorel E., Benitez R., Riveiro A., Pou J., Manzanares M.C., Franch J., Canal C., Ginebra M.P. 3D printing with star-shaped strands: a new approach to enhance in vivo bone regeneration. Biomater. Adv. 2022;137 doi: 10.1016/j.bioadv.2022.212807. [DOI] [PubMed] [Google Scholar]
- 26.Johansson L., Raymond Y., Labay C., Mateu-Sanz M., Ginebra M.-P. Enhancing the mechanical performance of 3D-printed self-hardening calcium phosphate bone scaffolds: PLGA-Based strategies. Ceram. Int. 2024;50:46300–46317. doi: 10.1016/j.ceramint.2024.08.473. [DOI] [Google Scholar]
- 27.del-Mazo-Barbara L., Ginebra M.-P. Self-hardening polycaprolactone/calcium phosphate inks for 3D printing of bone scaffolds: rheology, mechanical properties and shelf-life. Mater. Des. 2024;243 doi: 10.1016/j.matdes.2024.113035. [DOI] [Google Scholar]
- 28.del-Mazo-Barbara L., Ginebra M.-P. Rheological characterisation of ceramic inks for 3D direct ink writing: a review. J. Eur. Ceram. Soc. 2021;41:18–33. doi: 10.1016/j.jeurceramsoc.2021.08.031. [DOI] [Google Scholar]
- 29.Guo W., Li B., Li P., Zhao L., You H., Long Y. Review on vat photopolymerization additive manufacturing of bioactive ceramic bone scaffolds. J. Mater. Chem. B. 2023 doi: 10.1039/d3tb01236k. [DOI] [PubMed] [Google Scholar]
- 30.Goodarzi Hosseinabadi H., Nieto D., Yousefinejad A., Fattel H., Ionov L., Miri A.K. Ink material selection and optical design considerations in DLP 3D printing. Appl. Mater. Today. 2023;30 doi: 10.1016/j.apmt.2022.101721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.del-Mazo-Barbara L., Diez-Escudero A., Lodoso-Torrecilla I., Aramesh M., Persson C., Ginebra M.-P. Direct ink writing of biomimetic hydroxyapatite scaffolds with tailored concave porosity. Int. J. Bioprinting. 2024;0:3805. doi: 10.36922/ijb.3805. [DOI] [Google Scholar]
- 32.Kamboj N., Ressler A., Hussainova I. Bioactive ceramic scaffolds for bone tissue engineering by powder bed selective laser processing. A review, Materials (Basel). 2021;14 doi: 10.3390/ma14185338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Navarrete-Segado P., Frances C., Tourbin M., Tenailleau C., Duployer B., Grossin D. Powder bed selective laser process (sintering/melting) applied to tailored calcium phosphate-based powders. Addit. Manuf. 2022;50 doi: 10.1016/j.addma.2021.102542. [DOI] [Google Scholar]
- 34.Dinoro J.N., Paxton N.C., Skewes J., Yue Z., Lewis P.M., Thompson R.G., Beirne S., Woodruff M.A., Wallace G.G. Laser sintering approaches for bone tissue engineering. Polymers. 2022;14:1–29. doi: 10.3390/polym14122336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhou Z., Lennon A., Buchanan F., McCarthy H.O., Dunne N. Binder jetting additive manufacturing of hydroxyapatite powders: effects of adhesives on geometrical accuracy and green compressive strength. Addit. Manuf. 2020;36 doi: 10.1016/j.addma.2020.101645. [DOI] [Google Scholar]
- 36.Du X., Fu S., Zhu Y. 3D printing of ceramic-based scaffolds for bone tissue engineering: an overview. J. Mater. Chem. B. 2018;6:4397–4412. doi: 10.1039/c8tb00677f. [DOI] [PubMed] [Google Scholar]
- 37.Du Y., Hu T., You J., Ye Y., Zhang B., Bao B., Li M., Liu Y., Wang Y., Wang T. Study of falling‐down‐type DLP 3D printing technology for high‐resolution hydroxyapatite scaffolds. Int. J. Appl. Ceram. Technol. 2022;19:268–280. doi: 10.1111/ijac.13915. [DOI] [Google Scholar]
- 38.Bian W., Li D., Lian Q., Zhang W., Zhu L., Li X., Jin Z. Design and fabrication of a novel porous implant with pre-set channels based on ceramic stereolithography for vascular implantation. Biofabrication. 2011;3 doi: 10.1088/1758-5082/3/3/034103. [DOI] [PubMed] [Google Scholar]
- 39.Owji N., Mandakhbayar N., Cha J.-R., Padalhin A.R., Erdogan Z.K., Aldaadaa A., Shakouri T., Sawadkar P., Frost O., Kim H.-W., García-Gareta E., Knowles J.C. Inclusion of calcium phosphate does not further improve in vitro and in vivo osteogenesis in a novel, highly biocompatible, mechanically stable and 3D printable polymer. Sci. Rep. 2022;12 doi: 10.1038/s41598-022-21013-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xu X., Awad A., Robles-Martinez P., Gaisford S., Goyanes A., Basit A.W. Vat photopolymerization 3D printing for advanced drug delivery and medical device applications. J. Contr. Release. 2021;329:743–757. doi: 10.1016/j.jconrel.2020.10.008. [DOI] [PubMed] [Google Scholar]
- 41.Liu K., Wu X., Liu J., Yang H., Li M., Qiu T., Dai H. Design and manufacture of a customized, large-size and high-strength bioactive HA osteoid composite ceramic by stereolithography. Ceram. Int. 2023;49:11630–11640. doi: 10.1016/j.ceramint.2022.12.010. [DOI] [Google Scholar]
- 42.Zhang W., Lian Q., Li D., Wang K., Hao D., Bian W., Jin Z. The effect of interface microstructure on interfacial shear strength for osteochondral scaffolds based on biomimetic design and 3D printing. Mater. Sci. Eng. C. 2015;46:10–15. doi: 10.1016/j.msec.2014.09.042. [DOI] [PubMed] [Google Scholar]
- 43.Scalera F., Esposito Corcione C., Montagna F., Sannino A., Maffezzoli A. Development and characterization of UV curable epoxy/hydroxyapatite suspensions for stereolithography applied to bone tissue engineering. Ceram. Int. 2014;40:15455–15462. doi: 10.1016/j.ceramint.2014.06.117. [DOI] [Google Scholar]
- 44.Hisham M., Saravana Kumar G., Deshpande A.P. Process optimization and optimal tolerancing to improve dimensional accuracy of vat-photopolymerized functionally graded hydrogels. Results Eng. 2022;14 doi: 10.1016/j.rineng.2022.100442. [DOI] [Google Scholar]
- 45.Cao Y., Shi T., Jiao C., Liang H., Chen R., Tian Z., Zou A., Yang Y., Wei Z., Wang C., Shen L. Fabrication and properties of zirconia/hydroxyapatite composite scaffold based on digital light processing. Ceram. Int. 2020;46:2300–2308. doi: 10.1016/j.ceramint.2019.09.219. [DOI] [Google Scholar]
- 46.Jiao C., Xie D., He Z., Liang H., Shen L., Yang Y., Tian Z., Wu G., Wang C. Additive manufacturing of Bio-inspired ceramic bone scaffolds: structural design, mechanical properties and biocompatibility. Mater. Des. 2022;217 doi: 10.1016/j.matdes.2022.110610. [DOI] [Google Scholar]
- 47.Wang Y., Chen S., Liang H., Liu Y., Bai J., Wang M. Digital light processing (DLP) of nano biphasic calcium phosphate bioceramic for making bone tissue engineering scaffolds. Ceram. Int. 2022;48:27681–27692. doi: 10.1016/j.ceramint.2022.06.067. [DOI] [Google Scholar]
- 48.Chen Z., Li Z., Li J., Liu C., Lao C., Fu Y., Liu C., Li Y., Wang P., He Y. 3D printing of ceramics: a review. J. Eur. Ceram. Soc. 2019;39:661–687. doi: 10.1016/j.jeurceramsoc.2018.11.013. [DOI] [Google Scholar]
- 49.Zhang B., Gui X., Song P., Xu X., Guo L., Han Y., Wang L., Zhou C., Fan Y., Zhang X. Three-dimensional printing of large-scale, high-resolution bioceramics with micronano inner porosity and customized surface characterization design for bone regeneration. ACS Appl. Mater. Interfaces. 2022;14:8804–8815. doi: 10.1021/acsami.1c22868. [DOI] [PubMed] [Google Scholar]
- 50.Zhang B., Xing F., Chen L., Zhou C., Gui X., Su Z., Fan S., Zhou Z., Jiang Q., Zhao L., Liu M., Fan Y., Zhang X. DLP fabrication of customized porous bioceramics with osteoinduction ability for remote isolation bone regeneration. Biomater. Adv. 2023;145 doi: 10.1016/j.bioadv.2022.213261. [DOI] [PubMed] [Google Scholar]
- 51.Erbereli R., de Camargo I.L., Morais M.M., Fortulan C.A. 3D printing of trabecular bone-mimetic structures by vat photopolymerization of bovine hydroxyapatite as a potential candidate for scaffolds. J. Brazilian Soc. Mech. Sci. Eng. 2022;44:170. doi: 10.1007/s40430-022-03468-0. [DOI] [Google Scholar]
- 52.Zhang H., Han K., Dong L., Li X. Preparation and characterization of β-tricalcium phosphate/nano clay composite scaffolds via digital light processing printing. J. Inorg. Mater. 2022;37:1116. doi: 10.15541/jim20210745. [DOI] [Google Scholar]
- 53.Li X., Zhang H., Shen Y., Xiong Y., Dong L., Zheng J., Zhao S. Fabrication of porous β-TCP/58S bioglass scaffolds via top-down DLP printing with high solid loading ceramic-resin slurry. Mater. Chem. Phys. 2021;267 doi: 10.1016/j.matchemphys.2021.124587. [DOI] [Google Scholar]
- 54.Wei Y., Zhao D., Cao Q., Wang J., Wu Y., Yuan B., Li X., Chen X., Zhou Y., Yang X., Zhu X., Tu C., Zhang X. Stereolithography-based additive manufacturing of high-performance osteoinductive calcium phosphate ceramics by a digital light-processing system. ACS Biomater. Sci. Eng. 2020;6:1787–1797. doi: 10.1021/acsbiomaterials.9b01663. [DOI] [PubMed] [Google Scholar]
- 55.Wu Y., Cao Q., Wang Y., Liu Y., Xu X., Liu P., Li X., Zhu X., Zhang X. Optimized fabrication of DLP-Based 3D printing calcium phosphate ceramics with high-precision and low-defect to induce calvarial defect regeneration. Mater. Des. 2023;233 doi: 10.1016/j.matdes.2023.112230. [DOI] [Google Scholar]
- 56.Deng Z.-L., Pan M.-Z., Hua S.-B., Wu J.-M., Zhang X.-Y., Shi Y.-S. Mechanical and degradation properties of triply periodic minimal surface (TPMS) hydroxyapatite & akermanite scaffolds with functional gradient structure. Ceram. Int. 2023;49:20808–20816. doi: 10.1016/j.ceramint.2023.03.213. [DOI] [Google Scholar]
- 57.Hua S.-B., Su J., Deng Z.-L., Wu J.-M., Cheng L.-J., Yuan X., Chen F., Zhu H., Qi D.-H., Xiao J., Shi Y.-S. Microstructures and properties of 45S5 bioglass® & BCP bioceramic scaffolds fabricated by digital light processing. Addit. Manuf. 2021;45 doi: 10.1016/j.addma.2021.102074. [DOI] [Google Scholar]
- 58.Hua S.-B., Yuan X., Wu J.-M., Su J., Cheng L.-J., Zheng W., Pan M.-Z., Xiao J., Shi Y.-S. Digital light processing porous TPMS structural HA & akermanite bioceramics with optimized performance for cancellous bone repair. Ceram. Int. 2022;48:3020–3029. doi: 10.1016/j.ceramint.2021.10.003. [DOI] [Google Scholar]
- 59.Kennedy B.M., De Barra E., Hampshire S., Kelleher M.C. Investigation of oleic acid as a dispersant for hydroxyapatite powders for use in ceramic filled photo-curable resins for stereolithography. J. Eur. Ceram. Soc. 2023;43:7146–7166. doi: 10.1016/j.jeurceramsoc.2023.07.028. [DOI] [Google Scholar]
- 60.Feng C., Zhang K., He R., Ding G., Xia M., Jin X., Xie C. Additive manufacturing of hydroxyapatite bioceramic scaffolds: dispersion, digital light processing, sintering, mechanical properties, and biocompatibility. J. Adv. Ceram. 2020;9:360–373. doi: 10.1007/s40145-020-0375-8. [DOI] [Google Scholar]
- 61.Wu Y., Chen R., Chen X., Yang Y., Qiao J., Liu Y. Development of strong and tough β-TCP/PCL composite scaffolds with interconnected porosity by digital light processing and partial infiltration. Materials. 2023;16:947. doi: 10.3390/ma16030947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wu Y., Chen R., Zhao G., Chen X., Qu X., Liu Y. Effect of graphite particles as additive on the curing behaviour of β-tricalcium phosphate suspensions and scaffold fabrication by digital light processing. J. Eur. Ceram. Soc. 2020;40:4323–4331. doi: 10.1016/j.jeurceramsoc.2020.05.013. [DOI] [Google Scholar]
- 63.Zhang J., Huang D., Liu S., Dong X., Li Y., Zhang H., Yang Z., Su Q., Huang W., Zheng W., Zhou W. Zirconia toughened hydroxyapatite biocomposite formed by a DLP 3D printing process for potential bone tissue engineering. Mater. Sci. Eng. C. 2019;105 doi: 10.1016/j.msec.2019.110054. [DOI] [PubMed] [Google Scholar]
- 64.Liu R., Ma L., Liu H., Xu B., Feng C., He R. Effects of pore size on the mechanical and biological properties of stereolithographic 3D printed HAp bioceramic scaffold. Ceram. Int. 2021;47:28924–28931. doi: 10.1016/j.ceramint.2021.07.053. [DOI] [Google Scholar]
- 65.Geven M.A., Varjas V., Kamer L., Wang X., Peng J., Eglin D., Grijpma D.W. Fabrication of patient specific composite orbital floor implants by stereolithography. Polym. Adv. Technol. 2015;26:1433–1438. doi: 10.1002/pat.3589. [DOI] [Google Scholar]
- 66.Guillaume O., Geven M.A., Grijpma D.W., Tang T.T., Qin L., Lai Y.X., Yuan H., Richards R.G., Eglin D. Poly(trimethylene carbonate) and nano‐hydroxyapatite porous scaffolds manufactured by stereolithography. Polym. Adv. Technol. 2017;28:1219–1225. doi: 10.1002/pat.3892. [DOI] [Google Scholar]
- 67.Guillaume O., Geven M.A., Sprecher C.M., Stadelmann V.A., Grijpma D.W., Tang T.T., Qin L., Lai Y., Alini M., de Bruijn J.D., Yuan H., Richards R.G., Eglin D. Surface-enrichment with hydroxyapatite nanoparticles in stereolithography-fabricated composite polymer scaffolds promotes bone repair. Acta Biomater. 2017;54:386–398. doi: 10.1016/j.actbio.2017.03.006. [DOI] [PubMed] [Google Scholar]
- 68.Guillaume O., Geven M.A., Varjas V., Varga P., Gehweiler D., Stadelmann V.A., Smidt T., Zeiter S., Sprecher C., Bos R.R.M., Grijpma D.W., Alini M., Yuan H., Richards G.R., Tang T., Qin L., Yuxiao L., Jiang P., Eglin D. Orbital floor repair using patient specific osteoinductive implant made by stereolithography. Biomaterials. 2020;233 doi: 10.1016/j.biomaterials.2019.119721. [DOI] [PubMed] [Google Scholar]
- 69.Dienel K.E.G., van Bochove B., Seppälä J.V. Additive manufacturing of bioactive poly(trimethylene carbonate)/β-Tricalcium phosphate composites for bone regeneration. Biomacromolecules. 2020;21:366–375. doi: 10.1021/acs.biomac.9b01272. [DOI] [PubMed] [Google Scholar]
- 70.Zhou X., Zhu W., Nowicki M., Miao S., Cui H., Holmes B., Glazer R.I., Zhang L.G. 3D bioprinting a cell-laden bone matrix for breast cancer metastasis study. ACS Appl. Mater. Interfaces. 2016;8:30017–30026. doi: 10.1021/acsami.6b10673. [DOI] [PubMed] [Google Scholar]
- 71.Zhou X., Esworthy T., Lee S.-J., Miao S., Cui H., Plesiniak M., Fenniri H., Webster T., Rao R.D.R.D., Zhang L.G.L.G. 3D printed scaffolds with hierarchical biomimetic structure for osteochondral regeneration. Nanomedicine. 2019;19:58–70. doi: 10.1016/j.nano.2019.04.002. [DOI] [PubMed] [Google Scholar]
- 72.Codrea C.I., Baykara D., Mitran R.-A., Koyuncu A.C.Ç., Gunduz O., Ficai A. 3D-Bioprinted gelatin methacryloyl-strontium-doped hydroxyapatite composite hydrogels scaffolds for bone tissue regeneration. Polymers. 2024;16:1932. doi: 10.3390/polym16131932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Chen Q., Zou B., Wang X., Zhou X., Yang G., Lai Q., Zhao Y. SLA-3d printed building and characteristics of GelMA/HAP biomaterials with gradient porous structure. J. Mech. Behav. Biomed. Mater. 2024;155 doi: 10.1016/j.jmbbm.2024.106553. [DOI] [PubMed] [Google Scholar]
- 74.Song P., Gui X., Wu L., Su X., Zhou W., Luo Z., Zhang B., Feng P., Wei W., Fan C., Wu Y., Zeng W., Zhou C., Fan Y., Zhou Z. DLP fabrication of multiple hierarchical biomimetic gelMA/silMA/hAp scaffolds for enhancing bone regeneration. Biomacromolecules. 2024;25:1871–1886. doi: 10.1021/acs.biomac.3c01318. [DOI] [PubMed] [Google Scholar]
- 75.Ren X., Wang J., Wu Y., Zhang Y., Zhang J., Bai L., Liu J., Li G., Song P., Shi Z., Su J. One-pot synthesis of hydroxyapatite hybrid bioinks for digital light processing 3D printing in bone regeneration. J. Mater. Sci. Technol. 2024;188:84–97. doi: 10.1016/j.jmst.2024.01.001. [DOI] [Google Scholar]
- 76.Liu D., Liu J., Zhao P., Peng Z., Geng Z., Zhang J., Zhang Z., Shen R., Li X., Wang X., Li S., Wang J., Wang X. 3D bioprinted tissue‐engineered bone with enhanced mechanical strength and bioactivities: accelerating bone defect repair through sequential immunomodulatory properties. Adv. Healthcare Mater. 2024;13 doi: 10.1002/adhm.202401919. [DOI] [PubMed] [Google Scholar]
- 77.Verisqa F., Cha J.-R., Nguyen L., Kim H.-W., Knowles J.C. Digital light processing 3D printing of gyroid scaffold with isosorbide-based photopolymer for bone tissue engineering. Biomolecules. 2022;12 doi: 10.3390/biom12111692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Verisqa F., Park J.-H., Mandakhbayar N., Cha J.-R., Nguyen L., Kim H.-W., Knowles J.C. In vivo osteogenic and angiogenic properties of a 3D-Printed isosorbide-based gyroid scaffold manufactured via digital light processing. Biomedicines. 2024;12:609. doi: 10.3390/biomedicines12030609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bagheri A., Jin J. Photopolymerization in 3D printing. ACS Appl. Polym. Mater. 2019;1:593–611. doi: 10.1021/acsapm.8b00165. [DOI] [Google Scholar]
- 80.Kim S., Lee H., Choi H., Yoo K.Y., Yoon H. Investigation on photopolymerization of PEGDA to fabricate high-aspect-ratio microneedles. RSC Adv. 2022;12:9550–9555. doi: 10.1039/d2ra00189f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Enbergs S., Spinnen J., Dehne T., Sittinger M. 3D printing of bone substitutes based on vat photopolymerization processes: a systematic review. J. Tissue Eng. Regen. Med. 2023;2023 doi: 10.1155/2023/3901448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bedell M.L., Torres A.L., Hogan K.J., Wang Z., Wang B., Melchiorri A.J., Grande-Allen K.J., Mikos A.G. Human gelatin-based composite hydrogels for osteochondral tissue engineering and their adaptation into bioinks for extrusion, inkjet, and digital light processing bioprinting. Biofabrication. 2022;14 doi: 10.1088/1758-5090/ac8768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kamoun E.A., Winkel A., Eisenburger M., Menzel H. Carboxylated camphorquinone as visible-light photoinitiator for biomedical application: synthesis, characterization, and application. Arab. J. Chem. 2016;9:745–754. doi: 10.1016/j.arabjc.2014.03.008. [DOI] [Google Scholar]
- 84.Mau R., Nazir J., John S., Seitz H. Preliminary study on 3D printing of PEGDA hydrogels for frontal sinus implants using digital light processing (DLP) Curr. Dir. Biomed. Eng. 2019;5:249–252. doi: 10.1515/cdbme-2019-0063. [DOI] [Google Scholar]
- 85.Tikhonov A., Evdokimov P., Klimashina E., Tikhonova S., Karpushkin E., Scherbackov I., Dubrov V., Putlayev V. Stereolithographic fabrication of three-dimensional permeable scaffolds from CaP/PEGDA hydrogel biocomposites for use as bone grafts. J. Mech. Behav. Biomed. Mater. 2020;110 doi: 10.1016/j.jmbbm.2020.103922. [DOI] [PubMed] [Google Scholar]
- 86.Bagheri Saed A., Behravesh A.H., Hasannia S., Akhoundi B., Hedayati S.K., Gashtasbi F. An in vitro study on the key features of poly L-lactic acid/biphasic calcium phosphate scaffolds fabricated via DLP 3D printing for bone grafting. Eur. Polym. J. 2020;141 doi: 10.1016/j.eurpolymj.2020.110057. [DOI] [Google Scholar]
- 87.Badria A., Hutchinson D.J., Sanz del Olmo N., Malkoch M. Acrylate‐free tough 3D-printable thiol‐ene thermosets and composites for biomedical applications. J. Appl. Polym. Sci. 2022;139 doi: 10.1002/app.53046. [DOI] [Google Scholar]
- 88.Lasgorceix M., Champion E., Chartier T. Shaping by microstereolithography and sintering of macro–micro-porous silicon substituted hydroxyapatite. J. Eur. Ceram. Soc. 2016;36:1091–1101. doi: 10.1016/j.jeurceramsoc.2015.11.020. [DOI] [Google Scholar]
- 89.Lee J.H., Prud’homme R.K., Aksay I.A. Cure depth in photopolymerization: experiments and theory. J. Mater. Res. 2001;16:3536–3544. doi: 10.1557/JMR.2001.0485. [DOI] [Google Scholar]
- 90.Schwab A., Levato R., D'Este M., Piluso S., Eglin D., Malda J. Printability and shape fidelity of bioinks in 3D bioprinting. Chem. Rev. 2020;120:11028–11055. doi: 10.1021/acs.chemrev.0c00084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Liu Y., Zhang X., Guo J., Liu Y., Huang J., Gan R. Triply periodic minimal surfaces structured biphasic calcium phosphate bio-scaffolds with controllable porous struts from digital light processing of pickering emulsion. Ceram. Int. 2024;50:39608–39626. doi: 10.1016/j.ceramint.2024.07.341. [DOI] [Google Scholar]
- 92.Preobrazhenskiy I.I., Tikhonov A.A., Evdokimov P.V., Shibaev A.V., Putlyaev V.I. DLP printing of hydrogel/calcium phosphate composites for the treatment of bone defects. Open Ceram. 2021;6 doi: 10.1016/j.oceram.2021.100115. [DOI] [Google Scholar]
- 93.Huang X., Dai H., Hu Y., Zhuang P., Shi Z., Ma Y. Development of a high solid loading β-TCP suspension with a low refractive index contrast for DLP -based ceramic stereolithography. J. Eur. Ceram. Soc. 2021;41:3743–3754. doi: 10.1016/j.jeurceramsoc.2020.12.047. [DOI] [Google Scholar]
- 94.Mondal D., Haghpanah Z., Huxman C.J., Tanter S., Sun D., Gorbet M., Willett T.L. mSLA-based 3D printing of acrylated epoxidized soybean oil - nano-hydroxyapatite composites for bone repair. Mater. Sci. Eng. C. 2021;130 doi: 10.1016/j.msec.2021.112456. [DOI] [PubMed] [Google Scholar]
- 95.Guo J., Zhang X., Yan J., Wu J., Shi Y., Zhang S. Digital light processing bio-scaffolds of hydroxyapatite ceramic foams with multi-level pores using pickering emulsions as the feedstock. J. Eur. Ceram. Soc. 2024;44:4272–4284. doi: 10.1016/j.jeurceramsoc.2024.01.021. [DOI] [Google Scholar]
- 96.Wang Z., Huang C., Wang J., Zou B. Development of a novel aqueous hydroxyapatite suspension for stereolithography applied to bone tissue engineering. Ceram. Int. 2019;45:3902–3909. doi: 10.1016/j.ceramint.2018.11.063. [DOI] [Google Scholar]
- 97.Liu S., Mo L., Bi G., Chen S., Yan D., Yang J., Jia Y.-G., Ren L. DLP 3D printing porous β-tricalcium phosphate scaffold by the use of acrylate/ceramic composite slurry. Ceram. Int. 2021;47:21108–21116. doi: 10.1016/j.ceramint.2021.04.114. [DOI] [Google Scholar]
- 98.Duque C., Gómez-Tirado C.A., Ocampo S., Arroyave-Muñoz L.M., Restrepo-Munera L.M., Vásquez A.F., Ossa A., García C. Obtaining biocompatible polymeric scaffolds loaded with calcium phosphates through the digital light processing technique. J. Mater. Res. 2023 doi: 10.1557/s43578-023-01144-0. [DOI] [Google Scholar]
- 99.Chen Q., Zou B., Lai Q., Wang Y., Xue R., Xing H., Fu X., Huang C., Yao P. A study on biosafety of HAP ceramic prepared by SLA-3D printing technology directly. J. Mech. Behav. Biomed. Mater. 2019;98:327–335. doi: 10.1016/j.jmbbm.2019.06.031. [DOI] [PubMed] [Google Scholar]
- 100.Su J., Hua S., Chen A., Chen P., Yang L., Yuan X., Qi D., Zhu H., Yan C., Xiao J., Shi Y. Three-dimensional printing of gyroid-structured composite bioceramic scaffolds with tuneable degradability. Biomater. Adv. 2022;133 doi: 10.1016/j.msec.2021.112595. [DOI] [PubMed] [Google Scholar]
- 101.Liu Z., Liang H., Shi T., Xie D., Chen R., Han X., Shen L., Wang C., Tian Z. Additive manufacturing of hydroxyapatite bone scaffolds via digital light processing and in vitro compatibility. Ceram. Int. 2019;45:11079–11086. doi: 10.1016/j.ceramint.2019.02.195. [DOI] [Google Scholar]
- 102.Kang J.-H., Sakthiabirami K., Jang K.-J., Jang J.-G., Oh G.-J., Park C., Fisher J.G., Park S.-W. Mechanical and biological evaluation of lattice structured hydroxyapatite scaffolds produced via stereolithography additive manufacturing. Mater. Des. 2022;214 doi: 10.1016/j.matdes.2021.110372. [DOI] [Google Scholar]
- 103.Ryan E., Yin S. Compressive strength of β-TCP scaffolds fabricated via lithography-based manufacturing for bone tissue engineering. Ceram. Int. 2022;48:15516–15524. doi: 10.1016/j.ceramint.2022.02.085. [DOI] [Google Scholar]
- 104.Liu K., Zhou Q., Zhang X., Ma L., Xu B., He R. Morphologies, mechanical and in vitro behaviors of DLP-Based 3D printed HA scaffolds with different structural configurations. RSC Adv. 2023;13:20830–20838. doi: 10.1039/D3RA03080F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Pan M.-Z., Hua S.-B., Wu J.-M., Su J.-J., Zhang X.-Y., Shi Y.-S. Composite bioceramic scaffolds with controllable photothermal performance fabricated by digital light processing and vacuum infiltration. J. Eur. Ceram. Soc. 2023;43:2245–2252. doi: 10.1016/j.jeurceramsoc.2022.12.043. [DOI] [Google Scholar]
- 106.Lee Y.-H., Lee J.-W., Yang S.-Y., Lee H., Koh Y.-H., Kim H.-E. Dual-scale porous biphasic calcium phosphate gyroid scaffolds using ceramic suspensions containing polymer microsphere porogen for digital light processing. Ceram. Int. 2021;47:11285–11293. doi: 10.1016/j.ceramint.2020.12.254. [DOI] [Google Scholar]
- 107.Lee J.-W., Lee Y.-H., Lee H., Koh Y.-H., Kim H.-E. Improving mechanical properties of porous calcium phosphate scaffolds by constructing elongated gyroid structures using digital light processing. Ceram. Int. 2021;47:3252–3258. doi: 10.1016/j.ceramint.2020.09.164. [DOI] [Google Scholar]
- 108.Liang H., Wang Y., Chen S., Liu Y., Liu Z., Bai J. Nano-hydroxyapatite bone scaffolds with different porous structures processed by digital light processing 3D printing. Int. J. Bioprinting. 2022;8:502. doi: 10.18063/ijb.v8i1.502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Mao R., Lai Y., Li D., Huang Y., Wang L., Luo F., Chen Y., Lu J., Ge X., Liu Y., Fan Y., Zhang X., Jiang Q., Wang K. Flow channel performance in 3D printed hydroxyapatite scaffolds to improve metabolism and tissue ingrowth in flat bone repair. Composites, Part B. 2023;259 doi: 10.1016/j.compositesb.2023.110727. [DOI] [Google Scholar]
- 110.Li J., Guo D., Li J., Wei X., Sun Z., Yang B., Lu T., Ouyang P., Chang S., Liu W., He X. Irregular pore size of degradable bioceramic voronoi scaffolds prepared by stereolithography: osteogenesis and computational fluid dynamics analysis. Mater. Des. 2022;224 doi: 10.1016/j.matdes.2022.111414. [DOI] [Google Scholar]
- 111.Li J., Li J., Yang Y., He X., Wei X., Tan Q., Wang Y., Xu S., Chang S., Liu W. Biocompatibility and osteointegration capability of β-TCP manufactured by stereolithography 3D printing: in vitro study. Open Life Sci. 2023;18 doi: 10.1515/biol-2022-0530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Zhang B., Yin X., Zhang F., Hong Y., Qiu Y., Yang X., Li Y., Zhong C., Yang H., Gou Z. Customized bioceramic scaffolds and metal meshes for challenging large-size mandibular bone defect regeneration and repair. Regen. Biomater. 2023;10 doi: 10.1093/rb/rbad057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Liu G., Zhang B., Wan T., Zhou C., Fan Y., Tian W., Jing W. A 3D-printed biphasic calcium phosphate scaffold loaded with platelet lysate/gelatin methacrylate to promote vascularization. J. Mater. Chem. B. 2022;10:3138–3151. doi: 10.1039/D2TB00006G. [DOI] [PubMed] [Google Scholar]
- 114.Putlyaev V.I., Yevdokimov P.V., Mamonov S.A., Zorin V.N., Klimashina E.S., Rodin I.A., Safronova T.V., Garshev A.V. Stereolithographic 3D printing of bioceramic scaffolds of a given shape and architecture for bone tissue regeneration. Inorg. Mater. Appl. Res. 2019;10:1101–1108. doi: 10.1134/S2075113319050277. [DOI] [Google Scholar]
- 115.Roohani I., Entezari A., Zreiqat H. Liquid crystal display technique (LCD) for high resolution 3D printing of triply periodic minimal surface lattices bioceramics. Addit. Manuf. 2023;74 doi: 10.1016/j.addma.2023.103720. [DOI] [Google Scholar]
- 116.Zhang H., Jiao C., Liu Z., He Z., Ge Mengxing, Tian Zongjun, Wang C., Wei Z., Shen L., Liang H. 3D-printed composite, calcium silicate ceramic doped with CaSO4·2H2O: degradation performance and biocompatibility. J. Mech. Behav. Biomed. Mater. 2021;121 doi: 10.1016/j.jmbbm.2021.104642. [DOI] [PubMed] [Google Scholar]
- 117.Navarrete-Segado P., Tourbin M., Frances C., Grossin D. Masked stereolithography of hydroxyapatite bioceramic scaffolds: from powder tailoring to evaluation of 3D printed parts properties. Open Ceram. 2022;9 doi: 10.1016/j.oceram.2022.100235. [DOI] [Google Scholar]
- 118.Chen D., Chen G., Zhang X., Chen J., Li J., Kang K., He W., Kong Y., Wu L., Su B., Zhao K., Si D., Wang X. Fabrication and in vitro evaluation of 3D printed porous silicate substituted calcium phosphate scaffolds for bone tissue engineering. Biotechnol. Bioeng. 2022;119:3297–3310. doi: 10.1002/bit.28202. [DOI] [PubMed] [Google Scholar]
- 119.Liu S., Chen J., Chen T., Zeng Y. Fabrication of trabecular-like beta-tricalcium phosphate biomimetic scaffolds for bone tissue engineering. Ceram. Int. 2021;47:13187–13198. doi: 10.1016/j.ceramint.2021.01.184. [DOI] [Google Scholar]
- 120.Zhang C., Yuan Y., Zeng Y., Chen J. DLP 3D printed silica-doped HAp ceramic scaffolds inspired by the trabecular bone structure. Ceram. Int. 2022;48:27765–27773. doi: 10.1016/j.ceramint.2022.06.077. [DOI] [Google Scholar]
- 121.Paredes C., Roleček J., Miranda P. Improving the strength of β-TCP scaffolds produced by digital light processing using two-step sintering. J. Eur. Ceram. Soc. 2024;44:2571–2580. doi: 10.1016/j.jeurceramsoc.2023.11.028. [DOI] [Google Scholar]
- 122.Schmidleithner C., Malferrari S., Palgrave R., Bomze D., Schwentenwein M., Kalaskar D.M. Application of high resolution DLP stereolithography for fabrication of tricalcium phosphate scaffolds for bone regeneration. Biomed. Mater. 2019;14 doi: 10.1088/1748-605X/ab279d. [DOI] [PubMed] [Google Scholar]
- 123.Schiavi A., Fiume E., Orlygsson G., Schwentenwein M., Verné E., Baino F. High-reliability data processing and calculation of microstructural parameters in hydroxyapatite scaffolds produced by vat photopolymerization. J. Eur. Ceram. Soc. 2022;42:6206–6212. doi: 10.1016/j.jeurceramsoc.2022.06.022. [DOI] [Google Scholar]
- 124.Baino F., Magnaterra G., Fiume E., Schiavi A., Tofan L., Schwentenwein M., Verné E. Digital light processing stereolithography of hydroxyapatite scaffolds with bone‐like architecture, permeability, and mechanical properties. J. Am. Ceram. Soc. 2022;105:1648–1657. doi: 10.1111/jace.17843. [DOI] [Google Scholar]
- 125.Martinez J.S., Peterson S., Hoel C.A., Erno D.J., Murray T., Boyd L., Her J.-H., Mclean N., Davis R., Ginty F., Duclos S.J., Davis B.M., Parthasarathy G. High resolution DLP stereolithography to fabricate biocompatible hydroxyapatite structures that support osteogenesis. PLoS One. 2022;17 doi: 10.1371/journal.pone.0272283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.D'Andrea L., Gastaldi D., Baino F., Verné E., Saccomano G., D'Amico L., Longo E., Schwentenwein M., Vena P. Mechanical characterization of miniaturized 3D-printed hydroxyapatite parts obtained through vat photopolymerization: an experimental study. J. Mech. Behav. Biomed. Mater. 2023;141 doi: 10.1016/j.jmbbm.2023.105760. [DOI] [PubMed] [Google Scholar]
- 127.Akbari-Aghdam H., Bagherifard A., Motififard M., Parvizi J., Sheikhbahaei E., Esmaeili S., Saber-Samandari S., Khandan A. Development of porous photopolymer Resin-SWCNT produced by digital light processing technology using for bone femur application. Arch. Bone Jt. Surg. 2021;9:445–452. doi: 10.22038/abjs.2020.43409.2189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Bouakaz I., Drouet C., Grossin D., Cobraiville E., Nolens G. Hydroxyapatite 3D-printed scaffolds with gyroid-triply periodic minimal surface (TPMS) porous structure: fabrication and an in vivo pilot study in sheep. Acta Biomater. 2023;170:580–595. doi: 10.1016/j.actbio.2023.08.041. [DOI] [PubMed] [Google Scholar]
- 129.Bouakaz I., Sadeghian E., Meille S., Schrijnemakers A., Preux N., Geris L., Nolens G., Grossin D., Dupret-bori A. 3D printed triply periodic minimal surfaces calcium phosphate bone substitute : the effect of porosity design on mechanical properties. Ceram. Int. 2023 doi: 10.1016/j.ceramint.2023.10.238. [DOI] [Google Scholar]
- 130.Baino F., Schwentenwein M., Verné E. Modelling the mechanical properties of hydroxyapatite scaffolds produced by digital light processing-based vat photopolymerization. Ceramics. 2022;5:593–600. doi: 10.3390/ceramics5030044. [DOI] [Google Scholar]
- 131.Chen Q., Zou B., Lai Q., Zhu K. SLA-3d printing and compressive strength of PEGDA/nHAP biomaterials. Ceram. Int. 2022;48:30917–30926. doi: 10.1016/j.ceramint.2022.07.047. [DOI] [Google Scholar]
- 132.He Z., Jiao C., Wu J., Gu J., Liang H., Shen L., Yang Y., Tian Z., Wang C., Jiang Q. Zn-doped chitosan/alginate multilayer coatings on porous hydroxyapatite scaffold with osteogenic and antibacterial properties. Int. J. Bioprinting. 2023;9:668. doi: 10.18063/ijb.v9i2.668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Ressler A., Zakeri S., Dias J., Hannula M., Hyttinen J., Ivanković H., Ivanković M., Miettinen S., Schwentenwein M., Levänen E., Frankberg E.J. Vat photopolymerization of biomimetic bone scaffolds based on Mg, Sr, Zn-substituted hydroxyapatite: effect of sintering temperature. Ceram. Int. 2024;50:27403–27415. doi: 10.1016/j.ceramint.2024.05.038. [DOI] [Google Scholar]
- 134.Tang Q., Li X., Lai C., Li L., Wu H., Wang Y., Shi X. Fabrication of a hydroxyapatite-PDMS microfluidic chip for bone-related cell culture and drug screening. Bioact. Mater. 2021;6:169–178. doi: 10.1016/j.bioactmat.2020.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Tesavibul P., Chantaweroad S., Laohaprapanon A., Channasanon S., Uppanan P., Tanodekaew S., Chalermkarnnon P., Sitthiseripratip K. Biocompatibility of hydroxyapatite scaffolds processed by lithography-based additive manufacturing. Bio Med. Mater. Eng. 2015;26:31–38. doi: 10.3233/BME-151549. [DOI] [PubMed] [Google Scholar]
- 136.Van hede D., Liang B., Anania S., Barzegari M., Verlée B., Nolens G., Pirson J., Geris L., Lambert F. 3D‐Printed synthetic hydroxyapatite scaffold with in silico optimized macrostructure enhances bone formation in vivo. Adv. Funct. Mater. 2022;32 doi: 10.1002/adfm.202105002. [DOI] [Google Scholar]
- 137.Zeng Y., Yan Y., Yan H., Liu C., Li P., Dong P., Zhao Y., Chen J. 3D printing of hydroxyapatite scaffolds with good mechanical and biocompatible properties by digital light processing. J. Mater. Sci. 2018;53:6291–6301. doi: 10.1007/s10853-018-1992-2. [DOI] [Google Scholar]
- 138.Rstakyan V., Mkhitaryan L., Baghdasaryan L., Ghaltaghchyan T., Karabekian Z., Sevoyan G., Aghayan M., Rodríguez M.A. Stereolithography of ceramic scaffolds for bone tissue regeneration: influence of hydroxyapatite/silica ratio on mechanical properties. J. Mech. Behav. Biomed. Mater. 2024;152 doi: 10.1016/j.jmbbm.2024.106421. [DOI] [PubMed] [Google Scholar]
- 139.Gui X., Song P., Zhang B., Lei H., Wu L., Sun J., Tang R., Zhang H., Qin Y., Su Z., Sun J., Zhao Z., Han M., Wei W., Fan Y., Zhou C. Natural loofah sponge inspired 3D printed bionic scaffolds promote personalized bone defect regeneration. Composites, Part B. 2025;288 doi: 10.1016/j.compositesb.2024.111920. [DOI] [Google Scholar]
- 140.Gui X., Zhang B., Song P., Su Z., Gao C., Xing F., Liu L., Wei W., Hui D., Gu L., Liu M., Wu Y., Zhou C., Fan Y. 3D printing of biomimetic hierarchical porous architecture scaffold with dual osteoinduction and osteoconduction biofunctions for large size bone defect repair. Appl. Mater. Today. 2024;37 doi: 10.1016/j.apmt.2024.102085. [DOI] [Google Scholar]
- 141.Chen H., Lo W.-L., Lee S.-Y., Lin Y.-M. Controlling sintering temperature for biphasic calcium phosphate scaffolds using submicron hydroxyapatite slurries for LCD 3D printing. Ceram. Int. 2024;50:11060–11074. doi: 10.1016/j.ceramint.2024.01.007. [DOI] [Google Scholar]
- 142.Xiong S., Zhang Y., Zeng J., Zhou J., Liu S., Wei P., Liu H., Yi F., Wan Z., Xiong L., Zhang B., Li J. DLP fabrication of HA scaffold with customized porous structures to regulate immune microenvironment and macrophage polarization for enhancing bone regeneration. Mater. Today Bio. 2024;24 doi: 10.1016/j.mtbio.2023.100929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Liu Q., Tang Q., Huang Z., Li Z., Wang X., Wen P., Bai Y., Chen F. Functionally graded triply periodic minimal surface scaffold of HA-Al2O3 via vat photopolymerization 3D printing. Addit. Manuf. 2024;84 doi: 10.1016/j.addma.2024.104117. [DOI] [Google Scholar]
- 144.Gao X., Yang J., Gan X., Lin Y., Xu J., Shan Z., Han Z., Chen S., Huang B., Fan B., Chen Z. Optimized DLP 3D-printed high-resolution nano zirconia-hydroxyapatite scaffold with craniomaxillofacial soft tissue invasion resistance and pro-osteogenic properties via dectin-1/syk inflammatory axis. Chem. Eng. J. 2024;491 doi: 10.1016/j.cej.2024.152044. [DOI] [Google Scholar]
- 145.Guo W., Li P., Wei Y., Zhao L., Pang Y., Huang Y., Ye X., Wang S., Liu B., You H., Long Y. Ionic substitution through bredigite doping for microstructure and performance adjustment in DLP 3D-printed TPMS porous HA bone scaffolds. Virtual Phys. Prototyp. 2024;19 doi: 10.1080/17452759.2024.2423840. [DOI] [Google Scholar]
- 146.Luo S., Wang Z., He J., Tang G., Yuan D., Wu Z., Zou Z., Yang L., Lu T., Ye C. Bioceramic modular tissue-engineered bone with rapid vascularization for large bone defects. Ceram. Int. 2024;50:18275–18283. doi: 10.1016/j.ceramint.2024.02.311. [DOI] [Google Scholar]
- 147.Gui X., Zhang B., Qin Y., Lei H., Xia X., Li Y., Lei H., Zhou X., Tan Y., Dong Z., You Q., Zhou C., Fan Y. Structural and material double mechanical enhancement of HAp scaffolds promote bone defect regeneration. Compos. Part A Appl. Sci. Manuf. 2025;189 doi: 10.1016/j.compositesa.2024.108600. [DOI] [Google Scholar]
- 148.Xue R., Yuan P., Zhao B., Jing F., Kou X., Yue W., Wang Y., Wang D., Sewvandi G.A., Hu D. DLP printing of BT/HA nanocomposite ceramic scaffolds using low refractive index BT crystals. J. Mater. 2024;10:1036–1048. doi: 10.1016/j.jmat.2023.11.004. [DOI] [Google Scholar]
- 149.Zhao X., Chen J., Zeng Y. Study on performance regulation of electro-mechanical properties 3D printed BaTiO3/HA porous structure composite ceramic. Ceram. Int. 2024;50:49021–49032. doi: 10.1016/j.ceramint.2024.09.243. [DOI] [Google Scholar]
- 150.Chen K., Wang F., Sun X., Ge W., Zhang M., Wang L., Zheng H., Zheng S., Tang H., Zhou Z., Wu G. 3D-printed zinc oxide nanoparticles modified barium titanate/hydroxyapatite ultrasound-responsive piezoelectric ceramic composite scaffold for treating infected bone defects. Bioact. Mater. 2025;45:479–495. doi: 10.1016/j.bioactmat.2024.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Chen K., Wang Y., Wu C., Du Y., Tang H., Zheng S., Zhou Z., Zheng H., Wu G. Ln vitro assessment of immunomodulatory and osteogenic properties in 3D-printed hydroxyapatite/barium titanate piezoelectric ceramic scaffolds. Ceram. Int. 2024;50:8751–8759. doi: 10.1016/j.ceramint.2023.12.192. [DOI] [Google Scholar]
- 152.Guo W., Zhao L., Li P., Wang E., Pang Y., Wei Y., Li B., Huang Y., Liu B., Wang S., You H., Long Y. All-natural ceramic composite bone scaffolds of whitlockite/wollastonite fibers: DLP additive manufacturing, microstructure, and performance. J. Mater. Res. Technol. 2024;33:7391–7405. doi: 10.1016/j.jmrt.2024.11.077. [DOI] [Google Scholar]
- 153.Bi G., Mo L., Liu S., Zhong X., Yang J., Yuan Z., Chen S., Ren L. DLP printed β-tricalcium phosphate functionalized ceramic scaffolds promoted angiogenesis and osteogenesis in long bone defects. Ceram. Int. 2022;48:26274–26286. doi: 10.1016/j.ceramint.2022.05.310. [DOI] [Google Scholar]
- 154.Bouchart F., Vidal O., Lacroix J.M., Spriet C., Chamary S., Brutel A., Hornez J.C. 3D printed bioceramic for phage therapy against bone nosocomial infections. Mater. Sci. Eng. C. 2020;111 doi: 10.1016/j.msec.2020.110840. [DOI] [PubMed] [Google Scholar]
- 155.Goutagny C., Hocquet S., Hautcoeur D., Lasgorceix M., Somers N., Leriche A. Development of calcium phosphate suspensions suitable for the stereolithography process. Open Ceram. 2021;7 doi: 10.1016/j.oceram.2021.100167. [DOI] [Google Scholar]
- 156.Paredes C., Martínez-Vázquez F.J., Elsayed H., Colombo P., Pajares A., Miranda P. Evaluation of direct light processing for the fabrication of bioactive ceramic scaffolds: effect of pore/strut size on manufacturability and mechanical performance. J. Eur. Ceram. Soc. 2021;41:892–900. doi: 10.1016/j.jeurceramsoc.2020.09.002. [DOI] [Google Scholar]
- 157.Paredes C., Martínez-Vázquez F.J., Elsayed H., Colombo P., Pajares A., Miranda P. Using ductile cores for enhancing the mechanical performance of hollow strut β-TCP scaffolds fabricated by digital light processing. Ceram. Int. 2021;47:10163–10173. doi: 10.1016/j.ceramint.2020.12.165. [DOI] [Google Scholar]
- 158.Paredes C., Martínez-Vázquez F.J., Pajares A., Miranda P. Co-continuous calcium phosphate/polycaprolactone composite bone scaffolds fabricated by digital light processing and polymer melt suction. Ceram. Int. 2021;47:17726–17735. doi: 10.1016/j.ceramint.2021.03.093. [DOI] [Google Scholar]
- 159.Paredes C., Roleček J., Pejchalová L., Miranda P., Salamon D. Impact of residual carbon after DLP and SPS-sintering on compressive strength and in-VITRO bioactivity of calcium phosphate scaffolds. Open Ceram. 2022;11 doi: 10.1016/j.oceram.2022.100281. [DOI] [Google Scholar]
- 160.Paredes C., Roleček J., Pejchalová L., Spusta T., Salamon D., Miranda P. Evaluating the suitability of fast sintering techniques for the consolidation of calcium phosphate scaffolds produced by DLP. J. Eur. Ceram. Soc. 2023;43:6493–6503. doi: 10.1016/j.jeurceramsoc.2023.05.052. [DOI] [Google Scholar]
- 161.Remy M.T., Akkouch A., He L., Eliason S., Sweat M.E., Krongbaramee T., Fei F., Qian F., Amendt B.A., Song X., Hong L. Rat calvarial bone regeneration by 3D-Printed β-Tricalcium phosphate incorporating MicroRNA-200c. ACS Biomater. Sci. Eng. 2021;7:4521–4534. doi: 10.1021/acsbiomaterials.0c01756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Tian Y., Ma H., Yu X., Feng B., Yang Z., Zhang W., Wu C. Biological response of 3D-printed β-tricalcium phosphate bioceramic scaffolds with the hollow tube structure. Biomed. Mater. 2023;18 doi: 10.1088/1748-605X/acc374. [DOI] [PubMed] [Google Scholar]
- 163.Wang D., Hou J., Xia C., Wei C., Zhu Y., Qian W., Qi S., Wu Y., Shi Y., Qin K., Wu L., Yin F., Chen Z., Li W. Multi-element processed pyritum mixed to β-tricalcium phosphate to obtain a 3D-printed porous scaffold: an option for treatment of bone defects. Mater. Sci. Eng. C. 2021;128 doi: 10.1016/j.msec.2021.112326. [DOI] [PubMed] [Google Scholar]
- 164.Wojcik T., Chai F., Hornez V., Raoul G., Hornez J.-C. Engineering precise interconnected porosity in β-Tricalcium phosphate (β-TCP) matrices by means of top–down digital light processing. Biomedicines. 2024;12:736. doi: 10.3390/biomedicines12040736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Zhang F., Yang J., Zuo Y., Li K., Mao Z., Jin X., Zhang S., Gao H., Cui Y. Digital light processing of β-tricalcium phosphate bioceramic scaffolds with controllable porous structures for patient specific craniomaxillofacial bone reconstruction. Mater. Des. 2022;216 doi: 10.1016/j.matdes.2022.110558. [DOI] [Google Scholar]
- 166.Zhang H., Zhang H., Xiong Y., Dong L., Li X. Development of hierarchical porous bioceramic scaffolds with controlled micro/nano surface topography for accelerating bone regeneration. Mater. Sci. Eng. C. 2021;130 doi: 10.1016/j.msec.2021.112437. [DOI] [PubMed] [Google Scholar]
- 167.Zhang Y., Zhang Q., He F., Zuo F., Shi X. Fabrication of cancellous-bone-mimicking β-tricalcium phosphate bioceramic scaffolds with tunable architecture and mechanical strength by stereolithography 3D printing. J. Eur. Ceram. Soc. 2022;42:6713–6720. doi: 10.1016/j.jeurceramsoc.2022.07.033. [DOI] [Google Scholar]
- 168.Yang Y., Xu T., Bei H.-P., Zhang L., Tang C.-Y., Zhang M., Xu C., Bian L., Yeung K.W.-K., Fuh J.Y.H., Zhao X. Gaussian curvature–driven direction of cell fate toward osteogenesis with triply periodic minimal surface scaffolds. Proc. Natl. Acad. Sci. 2022;119 doi: 10.1073/pnas.2206684119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Ge M., Xie D., Jiao C., Yang Y., Shen L., Qiu M., Zhang H., He Z., Liang H., Tian Z. Mechanical properties and biocompatibility of MgO/Ca3(PO4)2 composite ceramic scaffold with high MgO content based on digital light processing. Ceram. Int. 2022;48:21175–21186. doi: 10.1016/j.ceramint.2022.04.010. [DOI] [Google Scholar]
- 170.Ge M., Xie D., Yang Y., Tian Z. Sintering densification mechanism and mechanical properties of the 3D-printed high-melting-point-difference magnesium oxide/calcium phosphate composite bio-ceramic scaffold. J. Mech. Behav. Biomed. Mater. 2023;144 doi: 10.1016/j.jmbbm.2023.105978. [DOI] [PubMed] [Google Scholar]
- 171.Qi D., Su J., Li S., Zhu H., Cheng L., Hua S., Yuan X., Jiang J., Shu Z., Shi Y., Xiao J. 3D printed magnesium-doped β-TCP gyroid scaffold with osteogenesis, angiogenesis, immunomodulation properties and bone regeneration capability in vivo. Biomater. Adv. 2022;136 doi: 10.1016/j.bioadv.2022.212759. [DOI] [PubMed] [Google Scholar]
- 172.Zhu H., Li M., Huang X., Qi D., Nogueira L.P., Yuan X., Liu W., Lei Z., Jiang J., Dai H., Xiao J. 3D printed tricalcium phosphate-bioglass scaffold with gyroid structure enhance bone ingrowth in challenging bone defect treatment. Appl. Mater. Today. 2021;25 doi: 10.1016/j.apmt.2021.101166. [DOI] [Google Scholar]
- 173.Song S., Gao Z., Xu H., Bao C., Lu B., Zheng B., Dong W., Ma H. Rapid preparation and performance of degradable ceramic scaffolds based on stereolithography. J. Asian Ceram. Soc. 2022;10:58–68. doi: 10.1080/21870764.2021.2008102. [DOI] [Google Scholar]
- 174.Putlyaev V.I., Evdokimov P.V., Filippov Y.Y., Safronova T.V., Tikhonov A.A. Investigation of highly concentrated calcium phosphate suspensions for forming bioceramic with complex architecture. Glass Ceram. 2018;74:378–381. doi: 10.1007/s10717-018-9998-4. [DOI] [Google Scholar]
- 175.Liu X., Gao J., Liu J., Cheng J., Han Z., Li Z., Chang Z., Zhang L., Li M., Tang P. Three-dimensional-printed spherical hollow structural scaffolds for guiding critical-sized bone regeneration. ACS Biomater. Sci. Eng. 2024;10:2581–2594. doi: 10.1021/acsbiomaterials.3c01956. [DOI] [PubMed] [Google Scholar]
- 176.Wang Y., Chen S., Liang H., Bai J., Wang M. Design and fabrication of biomimicking radially graded scaffolds via digital light processing 3D printing for bone regeneration. J. Mater. Chem. B. 2023;11:9961–9974. doi: 10.1039/d3tb01573d. [DOI] [PubMed] [Google Scholar]
- 177.Wang Y., Liu Y., Chen S., Francis Siu M.-F., Liu C., Bai J., Wang M. Enhancing bone regeneration through 3D printed biphasic calcium phosphate scaffolds featuring graded pore sizes. Bioact. Mater. 2025;46:21–36. doi: 10.1016/j.bioactmat.2024.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Kim J.-W., Lee J.-B., Koh Y.-H., Kim H.-E. Digital light processing of freeze-cast ceramic layers for macroporous calcium phosphate scaffolds with tailored microporous frameworks. Materials. 2019;12:2893. doi: 10.3390/ma12182893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Kim J.-W., Yang B.-E., Hong S.-J., Choi H.-G., Byeon S.-J., Lim H.-K., Chung S.-M., Lee J.-H., Byun S.-H. Bone regeneration capability of 3D printed ceramic scaffolds. Int. J. Mol. Sci. 2020;21:4837. doi: 10.3390/ijms21144837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Le Guéhennec L., Van hede D., Plougonven E., Nolens G., Verlée B., De Pauw M., Lambert F. In vitro and in vivo biocompatibility of calcium‐phosphate scaffolds three‐dimensional printed by stereolithography for bone regeneration. J. Biomed. Mater. Res., Part A. 2020;108:412–425. doi: 10.1002/jbm.a.36823. [DOI] [PubMed] [Google Scholar]
- 181.Lim H.-K., Hong S.-J., Byeon S.-J., Chung S.-M., On S.-W., Yang B.-E., Lee J.-H., Byun S.-H. 3D-Printed ceramic bone scaffolds with variable pore architectures. Int. J. Mol. Sci. 2020;21:6942. doi: 10.3390/ijms21186942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Seol Y.-J., Park D.Y., Park J.Y., Kim S.W., Park S.J., Cho D.-W. A new method of fabricating robust freeform 3D ceramic scaffolds for bone tissue regeneration. Biotechnol. Bioeng. 2013;110:1444–1455. doi: 10.1002/bit.24794. [DOI] [PubMed] [Google Scholar]
- 183.Yang Z., Xie L., Zhang B., Zhang G., Huo F., Zhou C., Liang X., Fan Y., Tian W., Tan Y. Preparation of BMP-2/PDA-BCP bioceramic scaffold by DLP 3D printing and its ability for inducing continuous bone formation. Front. Bioeng. Biotechnol. 2022;10 doi: 10.3389/fbioe.2022.854693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Tang Y., Wang J., Cao Q., Chen F., Wang M., Wu Y., Chen X., Zhu X., Zhang X. Dopamine/DOPAC-assisted immobilization of bone morphogenetic protein-2 loaded Heparin/PEI nanogels onto three-dimensional printed calcium phosphate ceramics for enhanced osteoinductivity and osteogenicity. Biomater. Adv. 2022;140 doi: 10.1016/j.bioadv.2022.213030. [DOI] [PubMed] [Google Scholar]
- 185.Wang J., Tang Y., Cao Q., Wu Y., Wang Y., Yuan B., Li X., Zhou Y., Chen X., Zhu X., Tu C., Zhang X. Fabrication and biological evaluation of 3D-printed calcium phosphate ceramic scaffolds with distinct macroporous geometries through digital light processing technology. Regen. Biomater. 2022;9 doi: 10.1093/rb/rbac005. rbac005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Luo D., Su J., Zou Y., Hua S., Cheng L., Qi D., Yuan X., Zhu H., Liu C., Shi Y., Xiao J. 3D-printed triply periodic minimal surface bioceramic scaffold for bone defect treatment with tunable structure and mechanical properties. Ceram. Int. 2024;50:39020–39031. doi: 10.1016/j.ceramint.2024.07.268. [DOI] [Google Scholar]
- 187.Channasanon S., Kaewkong P., Chantaweroad S., Tesavibul P., Pratumwal Y., Otarawanna S., Kirihara S., Tanodekaew S. Scaffold geometry and computational fluid dynamics simulation supporting osteogenic differentiation in dynamic culture. Comput. Methods Biomech. Biomed. Eng. 2024;27:587–598. doi: 10.1080/10255842.2023.2195961. [DOI] [PubMed] [Google Scholar]
- 188.Dokuz M.E., Aydın M., Uyaner M. Production of bioactive various lattices as an artificial bone tissue by digital light processing 3D printing. J. Mater. Eng. Perform. 2021;30:6938–6948. doi: 10.1007/s11665-021-06067-7. [DOI] [Google Scholar]
- 189.Oliver-Urrutia C., Drotárová L., Gascón-Pérez S., Slámečka K., Ravaszová S., Čelko L., Montufar E.B. Hydroxyapatite-resin composites produced by vat photopolymerization and post-processing via in situ hydrolysis of alpha tricalcium phosphate. Ceramics. 2023;6:2282–2294. doi: 10.3390/ceramics6040139. [DOI] [Google Scholar]
- 190.Castro N.J., O'Brien J., Zhang L.G. Integrating biologically inspired nanomaterials and table-top stereolithography for 3D printed biomimetic osteochondral scaffolds. Nanoscale. 2015;7:14010–14022. doi: 10.1039/C5NR03425F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Lee J.W., Ahn G., Kim D.S., Cho D.-W. Development of nano- and microscale composite 3D scaffolds using PPF/DEF-HA and micro-stereolithography. Microelectron. Eng. 2009;86:1465–1467. doi: 10.1016/j.mee.2008.12.038. [DOI] [Google Scholar]
- 192.Popov V.K., Evseev A.V., Ivanov A.L., Roginski V.V., Volozhin A.I., Howdle S.M. Laser stereolithography and supercritical fluid processing for custom-designed implant fabrication. J. Mater. Sci. Mater. Med. 2004;15:123–128. doi: 10.1023/B:JMSM.0000011812.08185.2a. [DOI] [PubMed] [Google Scholar]
- 193.Ronca A., Ambrosio L., Grijpma D.W. Design of porous three-dimensional PDLLA/nano-hap composite scaffolds using stereolithography. J. Appl. Biomater. Funct. Mater. 2012;10:249–258. doi: 10.5301/JABFM.2012.10211. [DOI] [PubMed] [Google Scholar]
- 194.Ronca A., Ambrosio L., Grijpma D.W. Preparation of designed poly(d,l-lactide)/nanosized hydroxyapatite composite structures by stereolithography. Acta Biomater. 2012;9:5989–5996. doi: 10.1016/j.actbio.2012.12.004. [DOI] [PubMed] [Google Scholar]
- 195.Tanodekaew S., Channasanon S., Kaewkong P., Uppanan P. PLA-HA scaffolds: preparation and bioactivity. Procedia Eng. 2013;59:144–149. doi: 10.1016/j.proeng.2013.05.104. [DOI] [Google Scholar]
- 196.Zhou X., Castro N.J., Zhu W., Cui H., Aliabouzar M., Sarkar K., Zhang L.G. Improved human bone marrow mesenchymal stem cell osteogenesis in 3D bioprinted tissue scaffolds with low intensity pulsed ultrasound stimulation. Sci. Rep. 2016;6 doi: 10.1038/srep32876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Zhu W., Castro N.J., Cui H., Zhou X., Boualam B., McGrane R., Glazer R.I., Zhang L.G. A 3D printed nano bone matrix for characterization of breast cancer cell and osteoblast interactions. Nanotechnology. 2016;27 doi: 10.1088/0957-4484/27/31/315103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Zhu W., Holmes B., Glazer R.I.R.I., Zhang L.G.L.G. 3D printed nanocomposite matrix for the study of breast cancer bone metastasis. Nanomedicine. 2016;12:69–79. doi: 10.1016/j.nano.2015.09.010. [DOI] [PubMed] [Google Scholar]
- 199.Haghpanah Z., Mondal D., Momenbeitollahi N., Mohsenkhani S., Zarshenas K., Jin Y., Watson M., Willett T., Gorbet M. In vitro evaluation of bone cell response to novel 3D‐printable nanocomposite biomaterials for bone reconstruction. J. Biomed. Mater. Res., Part A. 2024;112:1725–1739. doi: 10.1002/jbm.a.37719. [DOI] [PubMed] [Google Scholar]
- 200.Shams M., Mansurov Z., Daulbayev C., Bakbolat B. Effect of lattice structure and composite precursor on mechanical properties of 3D-Printed bone scaffolds. Eurasian Chem. J. 2021;23:257. doi: 10.18321/ectj1129. [DOI] [Google Scholar]
- 201.Wu X., Lian Q., Li D., Jin Z. Biphasic osteochondral scaffold fabrication using multi-material mask projection stereolithography. Rapid Prototyp. J. 2019;25:277–288. doi: 10.1108/RPJ-07-2017-0144. [DOI] [Google Scholar]
- 202.Zhou X., Zou B., Chen Q., Yang G., Lai Q., Wang X. Construction of bilayer biomimetic periosteum based on SLA-3D printing for bone regeneration. Colloids Surf. B Biointerfaces. 2025;246 doi: 10.1016/j.colsurfb.2024.114368. [DOI] [PubMed] [Google Scholar]
- 203.Wang B., Ye X., Chen G., Zhang Y., Zeng Z., Liu C., Tan Z., Jie X. Fabrication and properties of PLA/β-TCP scaffolds using liquid crystal display (LCD) photocuring 3D printing for bone tissue engineering. Front. Bioeng. Biotechnol. 2024;12 doi: 10.3389/fbioe.2024.1273541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Tikhonov A.A., Klimashina E.S., Evdokimov P.V., Kukueva E.V., Biryukov A.S., Putlayev V.I., Scherbackov I.M., Dubrov V.E. Dicarboxylate-substituted octacalcium phosphates for hydrogel filling and fabrication of resorbable ceramics. Inorg. Mater. Appl. Res. 2021;12:421–432. doi: 10.1134/S2075113321020441. [DOI] [Google Scholar]
- 205.Diederichs E.V., Mondal D., Willett T.L. The effects of physiologically relevant environmental conditions on the mechanical properties of 3D-printed biopolymer nanocomposites. J. Mech. Behav. Biomed. Mater. 2024;159 doi: 10.1016/j.jmbbm.2024.106694. [DOI] [PubMed] [Google Scholar]
- 206.Navarrete-Segado P., Tourbin M., Frances C., Grossin D. Masked stereolithography of hydroxyapatite bioceramic scaffolds: from powder tailoring to evaluation of 3D printed parts properties. Open Ceram. 2022;9 doi: 10.1016/j.oceram.2022.100235. [DOI] [Google Scholar]
- 207.Henkel J., Woodruff M.A., Epari D.R., Steck R., Glatt V., Dickinson I.C., Choong P.F.M., Schuetz M.A., Hutmacher DiW. Bone regeneration based on tissue engineering Conceptions-A 21st century perspective. Bone Res. 2013;1:216–248. doi: 10.4248/BR201303002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Wu Y., Chen X., Zhao G., Chen R., Liu Y., Ren H., Qu X., Liu Y. β-Tricalcium phosphate/ԑ-polycaprolactone composite scaffolds with a controllable gradient: fabrication and characterization. Ceram. Int. 2019;45:16188–16194. doi: 10.1016/j.ceramint.2019.05.140. [DOI] [Google Scholar]
- 209.Feng B., Zhang M., Qin C., Zhai D., Wang Y., Zhou Y., Chang J., Zhu Y., Wu C. 3D printing of conch-like scaffolds for guiding cell migration and directional bone growth. Bioact. Mater. 2023;22:127–140. doi: 10.1016/j.bioactmat.2022.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Yeranee K., Rao Y. A review of recent investigations on flow and heat transfer enhancement in cooling channels embedded with triply periodic minimal surfaces (TPMS) Energies. 2022;15:8994. doi: 10.3390/en15238994. [DOI] [Google Scholar]
- 211.Blanquer S.B.G., Werner M., Hannula M., Sharifi S., Lajoinie G.P.R., Eglin D., Hyttinen J., Poot A.A., Grijpma D.W. Surface curvature in triply-periodic minimal surface architectures as a distinct design parameter in preparing advanced tissue engineering scaffolds. Biofabrication. 2017;9 doi: 10.1088/1758-5090/aa6553. [DOI] [PubMed] [Google Scholar]
- 212.Hang Z., Songfeng X., Yinze X., Ruining G., Xiang L. Fabrication of porous β β -TCP bioceramics using digital light processing. J. Mech. Eng. 2019;55:81. doi: 10.3901/JME.2019.15.081. [DOI] [Google Scholar]
- 213.Lu F., Wu R., Shen M., Xie L., Liu M., Li Y., Xu S., Wan L., Yang X., Gao C., Gou Z. Rational design of bioceramic scaffolds with tuning pore geometry by stereolithography: microstructure evaluation and mechanical evolution. J. Eur. Ceram. Soc. 2021;41:1672–1682. doi: 10.1016/j.jeurceramsoc.2020.10.002. [DOI] [Google Scholar]
- 214.Seol Y.-J., Kim J.Y., Park E.K., Kim S.-Y., Cho D.-W. Fabrication of a hydroxyapatite scaffold for bone tissue regeneration using microstereolithography and molding technology. Microelectron. Eng. 2009;86:1443–1446. doi: 10.1016/j.mee.2009.01.053. [DOI] [Google Scholar]
- 215.Wu Y., Liu P., Feng C., Cao Q., Xu X., Liu Y., Li X., Zhu X., Zhang X. 3D printing calcium phosphate ceramics with high osteoinductivity through pore architecture optimization. Acta Biomater. 2024;185:111–125. doi: 10.1016/j.actbio.2024.07.008. [DOI] [PubMed] [Google Scholar]
- 216.Brie J., Chartier T., Chaput C., Delage C., Pradeau B., Caire F., Boncoeur M.-P., Moreau J.-J. A new custom made bioceramic implant for the repair of large and complex craniofacial bone defects. J. Cranio-Maxillofacial Surg. 2013;41:403–407. doi: 10.1016/j.jcms.2012.11.005. [DOI] [PubMed] [Google Scholar]
- 217.Lodoso-Torrecilla I., Klein Gunnewiek R., Grosfeld E.-C., de Vries R.B.M., Habibović P., Jansen J.A., van den Beucken J.J.J.P. Bioinorganic supplementation of calcium phosphate-based bone substitutes to improve in vivo performance: a systematic review and meta-analysis of animal studies. Biomater. Sci. 2020;8:4792–4809. doi: 10.1039/D0BM00599A. [DOI] [PubMed] [Google Scholar]
- 218.Maevskaia E., Ghayor C., Bhattacharya I., Guerrero J., Weber F.E. TPMS microarchitectures for vertical bone augmentation and osteoconduction: an in vivo study. Materials. 2024;17:2533. doi: 10.3390/ma17112533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Werner M., Petersen A., Kurniawan N.A., Bouten C.V.C. Cell‐perceived substrate curvature dynamically coordinates the direction, speed, and persistence of stromal cell migration. Adv. Biosyst. 2019;3 doi: 10.1002/adbi.201900080. [DOI] [PubMed] [Google Scholar]
- 220.Barba A., Maazouz Y., Diez-Escudero A., Rappe K., Espanol M., Montufar E.B., Öhman-Mägi C., Persson C., Fontecha P., Manzanares M.C., Franch J., Ginebra M.P. Osteogenesis by foamed and 3D-printed nanostructured calcium phosphate scaffolds: effect of pore architecture. Acta Biomater. 2018;79:135–147. doi: 10.1016/j.actbio.2018.09.003. [DOI] [PubMed] [Google Scholar]
- 221.Barba A., Diez-Escudero A., Maazouz Y., Rappe K., Espanol M., Montufar E.B., Bonany M., Sadowska J.M., Guillem-Marti J., Öhman-Mägi C., Persson C., Manzanares M.-C., Franch J., Ginebra M.-P. Osteoinduction by foamed and 3D-Printed calcium phosphate scaffolds: effect of nanostructure and pore architecture. ACS Appl. Mater. Interfaces. 2017;9:41722–41736. doi: 10.1021/acsami.7b14175. [DOI] [PubMed] [Google Scholar]
- 222.Ivanova E.P., Hasan J., Webb H.K., Truong V.K., Watson G.S., Watson J.A., Baulin V.A., Pogodin S., Wang J.Y., Tobin M.J., Löbbe C., Crawford R.J. Natural bactericidal surfaces: mechanical rupture of Pseudomonas aeruginosa cells by cicada wings. Small. 2012;8:2489–2494. doi: 10.1002/smll.201200528. [DOI] [PubMed] [Google Scholar]
- 223.Ganjian M., Modaresifar K., Ligeon M.R.O., Kunkels L.B., Tümer N., Angeloni L., Hagen C.W., Otten L.G., Hagedoorn P., Apachitei I., Fratila‐Apachitei L.E., Zadpoor A.A. Nature helps: toward bioinspired bactericidal nanopatterns. Adv. Mater. Interfac. 2019;6 doi: 10.1002/admi.201900640. [DOI] [Google Scholar]
- 224.Diez-Escudero A., Espanol M., Ginebra M.-P. High-aspect-ratio nanostructured hydroxyapatite: towards new functionalities for a classical material. Chem. Sci. 2024;15:55–76. doi: 10.1039/D3SC05344J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Godoy-Gallardo M., Wang Z., Shen Y., Manero J.M., Gil F.J., Rodriguez D., Haapasalo M. Antibacterial coatings on titanium surfaces: a comparison study between in vitro single-species and multispecies biofilm. ACS Appl. Mater. Interfaces. 2015;7:5992–6001. doi: 10.1021/acsami.5b00402. [DOI] [PubMed] [Google Scholar]
- 226.Godoy-Gallardo M., Mas-Moruno C., Yu K., Manero J.M., Gil F.J., Kizhakkedathu J.N., Rodriguez D. Antibacterial properties of hLf1–11 peptide onto titanium surfaces: a comparison study between silanization and surface initiated polymerization. Biomacromolecules. 2015;16:483–496. doi: 10.1021/bm501528x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.