Abstract
In this work, magnesium (Mg)-doped silica membranes were fabricated via a facile sol–gel method and systematically evaluated for multifunctional water purification applications with a specific focus on membrane distillation (MD). In this study, controlled incorporation of magnesium into the silica network effectively improves the membrane’s hydrophobicity, structural compactness, and functional performance. This enhancement presents a novel and efficient strategy for developing high-performance membranes tailored to water desalination applications. Characterization using FTIR and SEM confirmed successful integration of Mg and trimethoxy-octyl-silane (C8TMOS) into the silica matrix, leading to the formation of a dense, uniform surface with tunable hydrophobicity. Water contact angle measurements revealed superhydrophobic behavior at higher Mg loadings, ranging from 80° to 140°, indicating reduced wettability and improved liquid entry pressure. DCMD experiments using NaCl and Na2SO4 solutions (1000 ppm) showed significant enhancement in salt rejectionup to 98.3%and a stable permeate flux of 32 L/m2·h, especially for membranes doped with 15 wt % Mg. These improvements are attributed to densification and surface modification induced by Mg cross-linking, which inhibited pore wetting and maintained membrane stability during long-term operation. EDX and XRD confirmed elemental distribution and the amorphous structure of the membranes, respectively. Furthermore, density functional theory (DFT) simulations provided insight into the role of Mg in improving electronic structure, ion repulsion, and mechanical robustness at the molecular level. The membranes also exhibited strong antibacterial efficacy against Staphylococcus aureus and Klebsiella pneumoniae, suppressing over 90% bacterial regrowth. This multi-functionality, combining desalination efficiency, antifouling resistance, and antimicrobial activity, makes Mg-doped silica membranes a promising and scalable solution for sustainable water treatment, including high-salinity brines and industrial effluents.


Introduction
An increase in global population also growing requirement for food, energy, and water, so water reserves are the basic requirement for intake, industrial, and household purposes. In order to control the upcoming crisis of freshwater, different researchers and the water industry have been developing environment-responsive membrane-based water handling technology that is sensibly low-cost and efficient. −
A composite membrane with a unique architecture can be generated using submicrometer porosity in the permeable support layer. The responsive polymer and dendrimer particles can be implanted in the pores as they correspond to a particular surface area. Stability and segregation efficiency of polymeric membranes are enhanced with increased intercellular adhesion between selective and support layers. −
RO (Reverse osmosis) is the most frequently used sheath technology of sewer water distillation, with a large dimension of RO distillate with high contaminant concentrations. Zero liquid discharge (ZLD) approaches have attracted widespread attention for the minimization of RO distillate to increase the efficacy of water reprocessing. Membrane desalination has been wished for as an auspicious technology to achieve zero liquid charge of the RO concentrate. To accomplish ZLD of the RO concentrate, the process of direct contact membrane desalination (DCMD) has been encouraged as an alternative method. The pressure of the vapor difference across the heated intake solution (60–90 °C) and the chilled drain solution drives the thermal-based DCMD mechanism. Given that DCMD is more insensitive to the intake concentration when compared to other membrane technologies that it is better at purifying exceptionally salty fluids from waste. −
Metal-doped silica made it possible to create membranes with significant antimicrobial activity. When exposed to a 1/500 nutritional risk medium for 20 h at 36 °C, colonies of E. coli and S. aureus were completely eliminated from the membrane surface as well as the liquid in connection with the sheaths. Metal release rates varied depending on the speciation of the metal and made up 0.1–0.6% of the overall metal substance of films. − MATLAB Fuzzy rule-based systems are widely utilized with several distinct methodologies and methods for parametric estimation for synthesis and characterization. − Recent studies have highlighted the effectiveness of NiO/MgO nanoparticles embedded in silica matrices for wastewater treatment due to their high surface area, structural stability, and enhanced adsorption capacity. DFT simulations further support their strong pollutant interactions and structural integrity. Advances in membrane technology, including nanomaterial integration and innovative fabrication methods, have also improved the performance by mitigating fouling and enhancing contaminant removal. These developments are crucial in addressing global water challenges through sustainable treatment solutions. −
In this study, magnesium (Mg)-doped silica membranes were synthesized by using a sol–gel method and investigated for their potential in treating reverse osmosis (RO) concentrates via direct contact membrane distillation (DCMD). The novelty of this work lies in the strategic incorporation of Mg as a cross-linking dopant to enhance the structural integrity, hydrophobicity, and multifunctionality of silica membranes, which are typically limited by brittleness and pore wetting during long-term operation. A fuzzy logic-based framework was also introduced for optimizing membrane synthesis parameters and characterizing the effects of dopant concentration on the membrane performance. The resulting membranes demonstrated improved optical transparency, thermal stability, and superhydrophobic behavior, making them highly suitable for low-surface-tension, high-salinity water treatment. Unlike conventional desalination methods such as multistage flash (MSF) and multieffect distillation (MED), which are energy-intensive and require high-pressure components, DCMD operates under mild thermal and pressure conditions, offering a more sustainable and cost-effective solution. Furthermore, DCMD enables high water recovery with minimal chemical pretreatment, positioning it as a promising alternative for zero-liquid-discharge and brine concentration applications. This research focuses on evaluating the salt rejection efficiency of Mg-doped membranes in DCMD configurations using both NaCl and Na2SO4 as model solutes. By integration of material innovation with process-level optimization, this study advances the design of silica-based membranes for next-generation water purification technologies.
Materials and Method
All chemicals used in this study were of analytical grade and used without further purification. Polyvinylpyrrolidone (PVP), with an average molecular weight of ∼40,000 g/mol and 98% purity (Sigma-Aldrich), was employed as an emulsifier and pore-forming agent. Poly(vinyl alcohol) (PVA), having a molecular weight range of approximately 85,000–124,000 g/mol and a viscosity of 4–6 cP for a 4% aqueous solution (Sigma-Aldrich, 98% purity), was used as a binder to improve membrane film integrity. Polyethylene glycol (PEG) of molecular weight 400 g/mol and 99% purity (Merck) was incorporated as a progeny to enhance membrane porosity.
Sodium citrate (Na3C6H5O7), with 99% purity (Merck), functioned as a mild reducing agent, while ammonia solution (NH4OH, 25%) was added to maintain an alkaline pH during sol–gel processing. Trimethoxy(octyl)silane (C8H18O3Si), abbreviated as C8-TMOS and having 97% purity (Sigma-Aldrich), acted as a hydrophobic surfactant to stabilize the sol and impart hydrophobic characteristics. Tetraethyl orthosilicate (TEOS), with a purity of 99.99% (Merck), served as the principal silica precursor in the sol–gel synthesis route. Nitric acid (HNO3, 68%, Fischer Scientific) was used as a catalyst to facilitate the hydrolysis of TEOS.
Magnesium nitrate hexahydrate [Mg(NO3)2·6H2O], with 99% purity (Alfa Aesar), was used as the magnesium source for doping of the silica matrix. Butanol (C4H10O, 99.8% purity, Fischer Scientific) was used as a cosolvent to promote uniform emulsification during the polymer and silica precursor mixing stage. Deionized water with a resistivity of 18.2 MΩ·cm was used throughout the experimental process for solution preparation.
Silica Membrane Fabrication
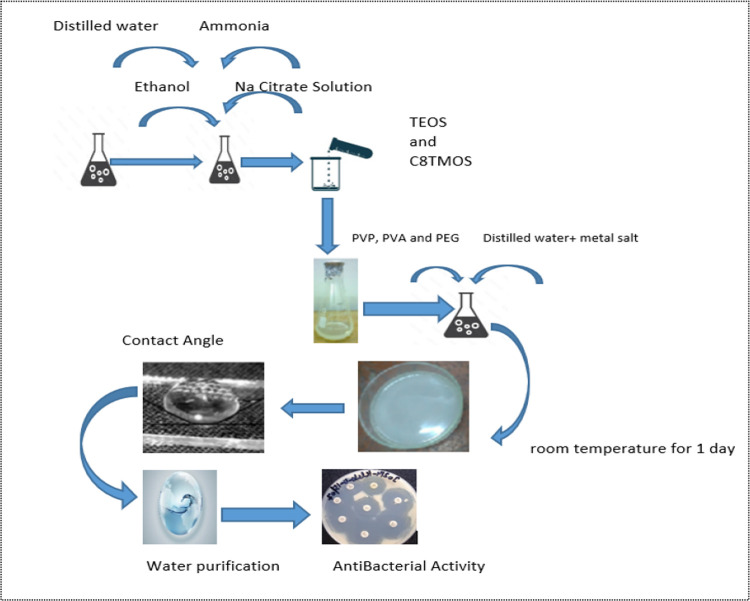
The fabrication of the Mg-doped silica membrane involved a multistep sol–gel-based emulsification process. Initially, polyvinylpyrrolidone (PVP) was dissolved in deionized water at a concentration of 3 wt %, and the solution was magnetically stirred at 560–600 rpm for 10 min to achieve complete dissolution. Subsequently, polyethylene glycol (PEG, 2 wt %) and poly(vinyl alcohol) (PVA, 2 wt %) were added in equal weight percentages to the PVP solution and stirred continuously for an additional 10 min. This polymeric blend acted as a structural and pore-forming template for membrane formation.
In a separate beaker, an aqueous solution of sodium citrate (1 wt %) and ammonia solution (25%) was prepared by stirring for approximately 5 min. This solution was then added dropwise to the polymeric mixture under constant stirring, which facilitated pH control and emulsion stabilization.
Following this, two precursor solutions were prepared separately: tetraethyl orthosilicate (TEOS, 10 mL) and trimethoxy(octyl)silane (C8TMOS, 5 mL), which were then gradually introduced into the reaction mixture. The TEOS served as the primary silica source, while C8TMOS acted as a surfactant to impart hydrophobicity and control particle agglomeration. Once the solution achieved complete homogeneity, magnesium nitrate hexahydrate [Mg(NO3)2·6H2O], previously dissolved in a minimal volume of 0.5 M nitric acid, was added to the sol in varying concentrations of 5, 10, 15, and 20 wt % relative to the total silica content to serve as the magnesium dopant. These compositions were designated as Mg5SiO2, Mg10SiO2, Mg15SiO2, and Mg20SiO2, respectively. The magnesium precursor was incorporated into the TEOS-based sol system under continuous stirring at 60 °C for 3 h to ensure uniform doping. The final molar compositions of the undiluted magnesium-doped silica solscalculated by including the structural water contentwere as follows: for Mg5SiO2: TEOS:EtOH:Mg(NO3)2·6H2O:H2O:HNO3 = 1:4.0:0.06:7.0:0.1; for Mg10SiO2: TEOS:EtOH:Mg(NO3)2·6H2O:H2O:HNO3 = 1:4.0:0.12:7.0:0.1; for Mg15SiO2: TEOS:EtOH:Mg(NO3)2·6H2O:H2O:HNO3 = 1:4.0:0.18:7.0:0.1; and for Mg20SiO2: TEOS:EtOH:Mg(NO3)2·6H2O:H2O:HNO3 = 1:4.0:0.24:7.0:0.1. In these formulations, tetraethyl orthosilicate (TEOS) acted as the silica precursor, ethanol (EtOH) served as the solvent, water (H2O) included both the added and coordinated water from the magnesium salt, and nitric acid (HNO3) was used as the acid catalyst to facilitate the hydrolysis and condensation reactions essential to the sol–gel process.
The resultant sol was left undisturbed at room temperature for 4–5 h to facilitate gelation, after which the samples were dried in a hot air oven at 50 °C for approximately 4–5 h.
The schematic representation of the complete synthesis route is illustrated in Figure .
1.
Schematic illustration for the method for the preparation of membrane.
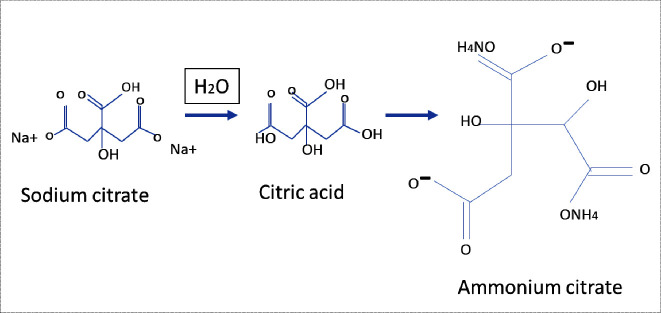
During emulsification, sodium citrate played a crucial role in stabilizing the reaction medium. Initially, the solution was slightly acidic due to free H+ ions from the water medium. Upon the addition of ammonia, these protons were neutralized, adjusting the pH to alkaline conditions suitable for sol–gel chemistry. Moreover, the interaction of sodium citrate with ammonia resulted in the formation of ammonium citrate, as depicted in the chemical pathway shown in Figure , thereby minimizing the concentration of free protons and stabilizing the colloidal system.
2.
Ammonium citrate formation by sodium citrate.
The following processes will be used by TEOS to create silica nanoparticles using the sol–gel method:
Preparation of Silica Nanoparticles
Tetraethyl orthosilicate TEOS (Si (OC2H5)4 when it reacts with water forms a silanol group. The chemical reactions are as follows.
Hydrolysis
Water condensation:
Alcohol condensation:
The purpose of sodium citrate in the water was to alleviate the hydrolyzed TEOS, and provide the silica networks.
Membrane Characterization
To evaluate the structural and surface characteristics of Mg-doped silica membranes, a series of analytical methods was employed. Fourier-transform infrared spectroscopy (FTIR) confirmed the presence of Si–O–Si linkages and indicated the successful incorporation of magnesium into the silica network. Scanning electron microscopy (SEM) revealed consistent surface texture and porosity variations corresponding to Mg content, while energy-dispersive X-ray spectroscopy (EDX) validated the elemental composition. X-ray diffraction (XRD) patterns demonstrated the amorphous structure of the membranes. Hydrophobicity was examined using contact angle measurements obtained via a 3D camera and analyzed in ImageJ software, with values reaching 140°, confirming superhydrophobic behavior. These characterizations collectively affirm the membranes’ suitability for efficient water treatment in direct contact membrane distillation (DCMD) systems.
Results and Discussion
Contact Angle and Wettability
The hydrophobicity or surface wettability of the Mg-doped silica membranes was evaluated by measuring the water contact angle using ImageJ software based on images captured with a 3D digital camera. The introduction of magnesium as a dopant significantly influenced the membrane’s surface properties. As the Mg content increased (5, 10, 15, and 20 wt %), a noticeable enhancement in surface hydrophobicity was observed, with contact angle values rising from approximately 110° to 140°. This increase in contact angle reflects a decrease in the surface’s wettability, indicating improved water repellency. The underlying mechanism can be attributed to the effect of Mg incorporation on the membrane’s microstructure and surface chemistry. Magnesium ions tend to interact with the silanol groups (Si–OH) on the silica network, leading to partial substitution and the formation of Mg–O–Si bonds. This reduces the number of hydrophilic silanol sites and simultaneously increases the membrane’s surface roughness and compactness. At higher doping concentrationsparticularly at 15 wt %the dopant uniformly covers the surface, forming a dense and less polar structure that exhibits superhydrophobic behavior. This transformation restricts water penetration and enhances the barrier properties of the membrane. which enhances the Cassie–Baxter wetting regime, resulting in increased water contact angles. These changes contribute to a more hydrophobic membrane surface suitable for desalination. Although increased hydrophobicity generally reduces water permeability, in this case, Mg doping also improves the membrane’s surface roughness and pore interconnectivity, thereby enhancing vapor flux. The net effect is an increase in both hydrophobicity and water flux, indicating successful optimization of surface chemistry and pore morphology for DCMD. Furthermore, the directional orientation of immobilized surfactant molecules during the synthesis process also contributes to the hydrophobic surface characteristics. The surfactant acts to further reduce surface energy, thereby amplifying the membrane’s water-repelling behavior. As illustrated in Figure A, dynamic images demonstrate the contact angle of water droplets on the Mg-doped membranes over time, while Figure B provides a quantitative plot showing the temporal progression of the measured contact angles across different doping levels.
3.
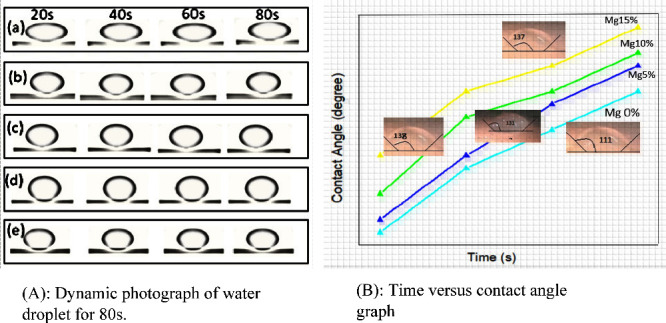
(A) Dynamic photograph of water droplet on Mg doped silica membrane at different times from 20 to 80 s. (B) Graph of time (s) vs contact angles (°) of Mg-doped silica membranes.
Membrane Morphologies
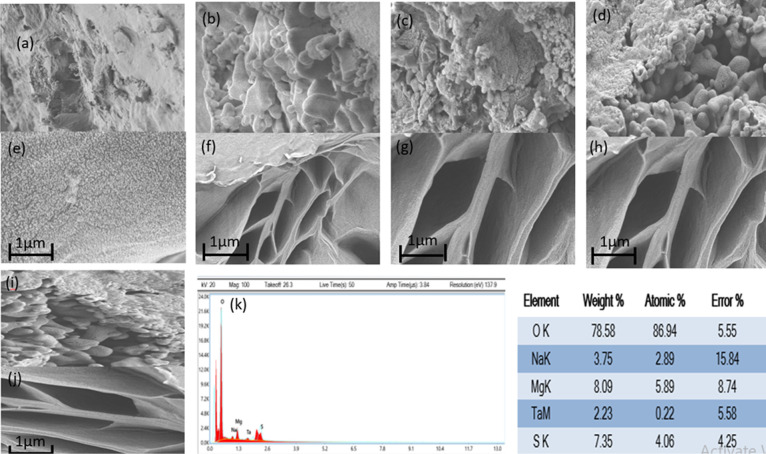
The surface and cross-sectional images of prepared membranes showed that metal doping increased the mechanical strength of membranes significantly composite membranes possessed an enhanced mechanical property, which was attributed to silica nanoparticles, intramolecular and intermolecular hydrogen bonds. The effects of Mg doping on the membrane surface geomorphologies were explored via SEM images, as shown in Figure .
4.
Scanning electron microscopy (SEM) images comparing undoped and doped silica membranes. Subfigures (a) and (e) depict the surface and cross-sectional structures, respectively, of the undoped silica membrane, revealing a porous surface morphology. Subfigures (b, c, d, i) and (f, g, h, j) display surface and cross-sectional images under high magnification (5K×) respectively, of Mg-doped silica membranes exhibiting varying concentrations of dopant, characterized by porous structures and aggregation phenomena. Subfigure (k) provides energy-dispersive X-ray (EDX) analysis confirming the presence of Mg within the doped silica membranes.
SEM images of undoped and doped silica membranes have shown nanoparticle morphology with a porous surface. 5% doping of Mg displayed a uniform porous surface with no obvious aggregations. Whereas, 10% doping of Mg prevents the enlargement of aggregations. 15% doping of Mg exhibited the same results with more confinement and no aggregation. 20% doping of Mg showed a porous and uniform surface with no aggregation. One aspect of silica nanoparticle formation is that the rounded morphology can give a fairly rough micronano architecture, which is necessary for the formation of a hydrophobic surface. Undoped membrane reveals a porous structure with a pore size of 1.6 nm and a regular surface. The membrane surface exhibited a similar architecture after Mg doping, and no apparent aggregations were seen. This was due to PVP’s capacity to dramatically diminish noncovalent coupling and stop conglomerate growth. The results showed that the Mg doping is evenly dispersed across the membranes. Mg-doped membrane revealed a rougher surface, which resulted in heavy loading of tiny indentations and large pore size (∼2 nm).
Energy Dispersive X-ray Analysis (EDX Analysis)
EDX analysis of Mg-doped silica membranes displayed the presence of Mg contents. The allocation of Mg doped in the investigated particles is similar to the findings. Every spectrum had maxima of the perforated element Mg together with maxima of S (sulfur), Ta (tantalum), Na (Sodium), and O (Oxygen). The presence of tantalum is likely attributed to the sample holder or coating material used during EDX measurements, as Ta is commonly employed as a conductive layer or support grid in electron microscopy. It was not intentionally introduced during the membrane synthesis process and did not originate from the precursor materials. Therefore, the detected Ta is considered an artifact of the analytical setup rather than a constituent of the membrane composition.
X-ray Diffraction Analysis
The XRD patterns of magnesium-doped silica membranes, shown in Figure , exhibit a broad diffuse hump without any sharp diffraction peaks, indicating the absence of a long-range crystalline order. This broad halo, typically observed around 2θ = 22°–30°, is characteristic of amorphous silica materials. The lack of well-defined peaks confirms that the silica framework retains its noncrystalline, glassy structure even after doping with magnesium. Furthermore, no secondary phases or crystalline Mg-related compounds were detected, suggesting that the Mg dopant is homogeneously incorporated into the silica network without inducing crystallization. These findings validate the formation of a stable amorphous phase across all doping concentrations, in alignment with the intended sol–gel synthesis strategy. As the XRD pattern does not display clear crystalline or amorphous peak separation, determining the Crystallinity Index (CI %) quantitatively is not applicable. Thus, the amorphous nature of the Mg-doped silica membrane is qualitatively inferred from the broad, nondistinctive XRD profile.
5.
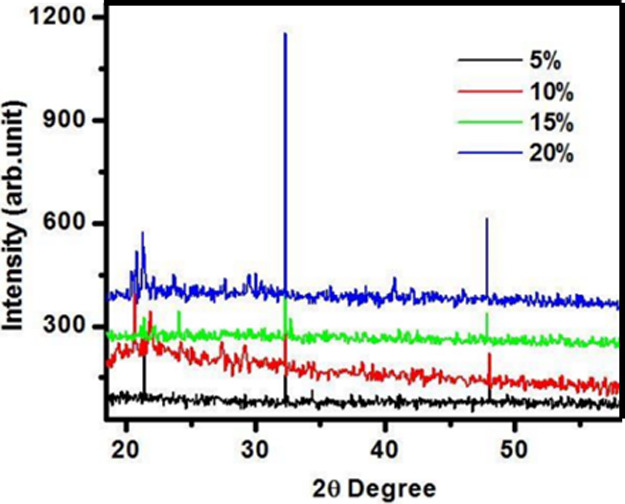
XRD diffraction pattern of Mg- doped SiO2 membranes for 5, 10, 15 and 20% conc. shown the amorphous nature of Silica membranes.
Fourier Transform Infrared Spectrum of the Samples
FTIR spectrum was used to classify the chemical bonds and functional groups, as shown in Figure . Table presents the FTIR spectrum, which exhibits a peak at 3327 cm–1 corresponding to the symmetric stretching vibration of the −CH2 group. Another broad peak observed at 2782 cm–1 is attributed to the C–C bond vibrations. The crowning at 1645 cm–1 was for the C–O bonding. The crests at 2223 and 2177 cm–1 exhibited symmetric tremors of −CH2– and −CH3– correspondingly. The peak, beginning at 1646–733 cm–1, specified the stretching tremors of the Si–Mg–Si bond.
6.
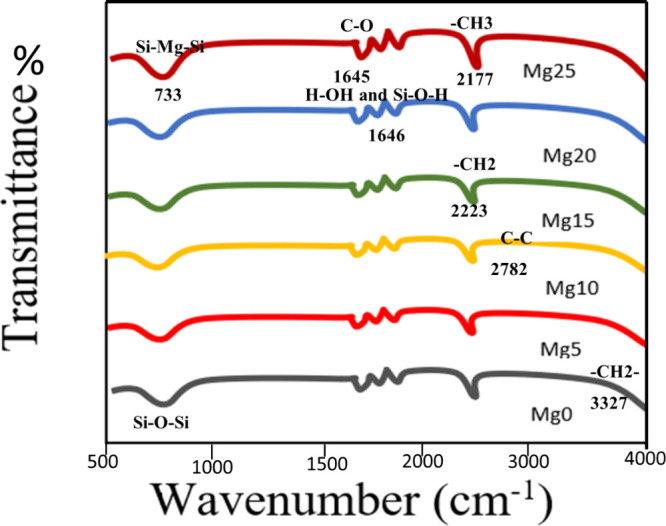
Comparison FTIR spectrum of samples with different concentrations of Mg doping.
1. Vibrational Frequencies (cm–1) in FTIR of Hybrid Silica Nanoparticles with Different Concentrations of Mg as the Dopant.
| no. | vibrational frequency (cm–1) | types of vibrations | structural units |
|---|---|---|---|
| 1 | 3327 | symmetric | –CH2 |
| 2 | 2782 | C–C stretching | C–C |
| 3 | 2223 | νs C–H | –CH2 |
| 4 | 2177 | νas C–H | –CH3 |
| 5 | 1646 | O–H stretching and SiO–H | H–O–H and SiO–H |
| 6 | 1645 | CO stretching | CO |
| 7 | 733 | stretching | Si–Mg–Si |
UV–Visible Spectroscopy
The UV–visible absorption range of prepared silica nanoparticles was disseminated in ethanol was established by UV–visible spectroscopic analysis of model U-2800 HITACHI.
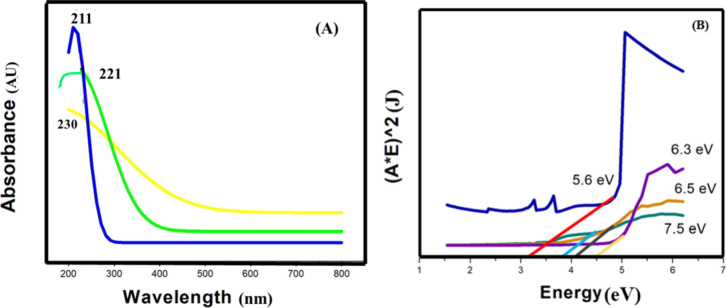
Figure A depicts that the sample created with 5% doping of Mg showed a high immersion peak at 230 nm, while the sample created with 10% doping of Mg had a peak at 221 nm. These wavelengths demonstrated the produced sample’s red shift. The increase in particle size was reflected by the red shift. The band gap in nanoparticles is influenced by the quantum confinement effect and size-dependent energy levels as particles shrink. Additionally, surface effects, associated with surface atoms, introduce extra energy levels within the band gap. This is in accordance with Beer–Lambert’s law, which states that as the concentration of nanoparticles in a solution rises, so does their absorbance value.
7.
(A) UV–visible spectrum of silica membrane absorption with 10% Mg and 15% Mg and 20% Mg doping. (B) Comparison graph for band gap shows that upon increasing of dopant concentration band gap increases.
The relation of the band gap energy and wavelength is described by the Planck–Einstein equation as follows:
In this equation, E g is the band gap energy expressed in electron volts (eV), h is Planck’s constant, υ is frequency, c is the speed of light, and λ is the wavelength.
Figure B displayed the band gaps of Mg-doped silica membranes as 5.6, 6.3, 6.5, and 7.5 eV for Mg 5, 10, 15, and 20%, respectively. The drop in particle size was accompanied by an increase in the band gap, which confirmed the size-dependent energy gap prediction. As particles decrease in size, the gap between energy levels widens. This occurs due to quantum confinement and surface effects. Quantum confinement limits electron movement, defining distinct energy levels. Simultaneously, surface effects, influenced by more surface atoms, introduce extra energy levels within the gap. Together, these factors enlarge the gap in smaller particles.
Water Desalination by DCMD (Direct Contact Membrane Distillation)
Performance Evaluation of Mg-Doped Silica Membranes
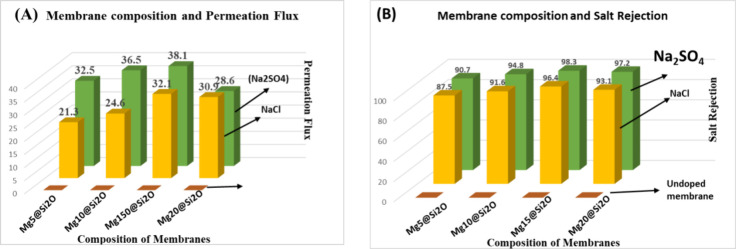
Following the successful synthesis and comprehensive characterization of the magnesium-doped silica membranes, their desalination performance was evaluated using a lab-scale direct contact membrane distillation (DCMD) setup. The performance of the Mg-doped silica membranes was evaluated by using the direct contact membrane distillation (DCMD) process. Key performance indicators included water flux, salt rejection, and operational stability. The Mg incorporation influenced the membrane’s structural and surface properties, which contributed to enhanced water vapor transport. The membranes demonstrated a stable and appreciable water flux throughout the experimental run, indicating good permeability and thermal efficiency. Additionally, the salt rejection remained consistently above 99%, confirming the membrane’s effectiveness in separating dissolved salts from water. Long-term operation further revealed that the membrane maintained its performance without significant fouling or pore wetting, demonstrating good chemical and thermal stability. These findings suggest that Mg doping not only preserved the amorphous silica network but also improved the membrane’s desalination capability under DCMD conditions. Flat-sheet membranes with an effective surface area of 9 cm2 were utilized for direct contact membrane distillation (DCMD) experiments. A temperature gradient essential for the DCMD process was maintained by heating the feed solution to 60 °C while keeping the permeate side at 25 °C. The feed and permeate solutions circulated at flow rates of 0.2 and 0.15 m/s, respectively. A 2 L saline solution containing 35 g/L NaCl was used as the feed, and 150 mL of Milli-Q water served as the permeate. To assess membrane resistance against surfactant-induced wetting, sodium dodecyl sulfate (SDS) was gradually introduced into the feed with continuous stirring at 400 rpm to ensure complete dissolution. Flux measurements were recorded in real time by using a precision balance. As shown in Figure A, increasing magnesium content in the silica membranes led to a noticeable improvement in water flux, especially in the presence of both NaCl and Na2SO4 salts. The Mg15@SiO2 membrane exhibited the highest flux performance, reaching 32.1 L/m2·h for NaCl and 38.1 L/m2·h for Na2SO4. This enhancement is linked to optimized structural properties induced by magnesium incorporation. −
8.
(A) Graph of membrane composition versus the permeation flux used for the removal of salt. (B) Salt (Na2SO4 and NaCl) rejection from water.
Complementary results in Figure B indicate a corresponding improvement in salt rejection capabilities with rising Mg content. The Mg15@SiO2 membrane achieved 98.3% NaCl and 96.4% Na2SO4 rejection, outperforming both lower and higher doping concentrations. The superior separation efficiency is attributed to increased structural densification and reduced membrane wettability due to magnesium-driven cross-linking within the silica network. This densification not only enhanced hydrophobicity but also minimized pore wetting and salt intrusion.
Collectively, these results affirm that magnesium doping strengthens membrane structure and boosts both permeability and selectivity, making Mg-doped silica membranes highly suitable for DCMD-based seawater desalination applications. −
Antibacterial Activity
A single colony of bacteria was picked and inoculated into 5 mL of Luria Broth (LB) medium. Then, LB was incubated at 37 °C for the whole night at 200 rpm. The next day, the grown preculture 106 CFU/ml of test bacteria strain was inoculated into 20 mL of LB contained in a 100 mL conical flask. Then culture media were placed in Petri dishes, and all the prepared samples of Mg-doped silica membranes were put in the culture with the final concentration of 50 μg/mL. After that, the cultures were incubated at 37 °C for 24 h. The same procedure was followed for all bacteria strains. ,
Utilizing culture obscurity as an informal indicator of cell proliferation, the antibacterial test of produced nanostructures was carried out on cutaneous microorganisms, Gram-positive S. aureus, and Gram-positive K. pneumoniae bacterial strains. Nearly 90% of the growth rate of both bacterial strains was inhibited by all of the produced membranes. The fact that produced membranes are 250 times smaller than a bacterium may be the cause of their antibacterial effectiveness. Due to their tiny sizes, they can more readily attach to the bacterial cell wall, triggering its disintegration and resulting in the death of the bacterial cell. These processes between nanoparticles and bacterial cells result in a zone of inhibition (ZOI) surrounding the nanoparticles. The bacterial effectiveness of nanoparticles effect the ZOI size. −
By using disc diffusion. method, the in vitro antibacterial. activity of the produced membranes toward diverse pathogens was evaluated.
As shown in Figure , every synthetic membrane has antibacterial action against S. aureus and K. pneumoniae bacterial strains. Undoped and doped silica membranes in the case of S. aureus create the same size zone of inhibition (ZOI), which is 40 mm. It is interesting to see that the regrowth primarily slows down with the Mg doping. The level rises before disappearing. The modification in the particle dimensions and shape may be the cause of the increased antibacterial activity of silica with metal doping. According to reports, each of these components has a significant impact on silica’s antibacterial action. In the instance of K. pneumoniae, the ZOI falls as the metal doping level rises. This discrepancy in the antibacterial. The activity of Mg-doped silica membranes, in contrast to Gram-negative and Gram-positive bacterial straining, may be caused by a variation in the bacterial species' cell wall reliability or membrane assembly. Additionally, different bacterial strains differ substantially in their susceptibilities to infection and their tolerances of various substances, including antibiotics.
9.
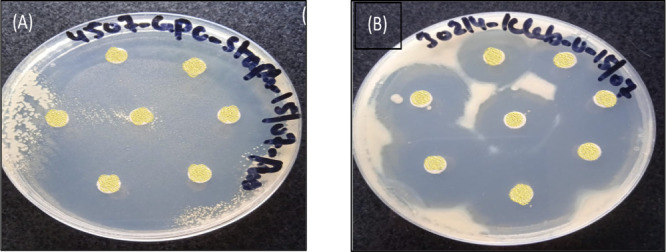
Zone of inhibition produced by undoped and Mg-doped silica membranes against (A) GPC S. aureus and (B) GNC K. pneumonia.
Fuzzy Analysis
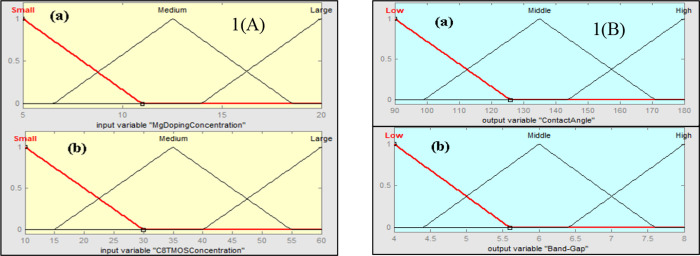
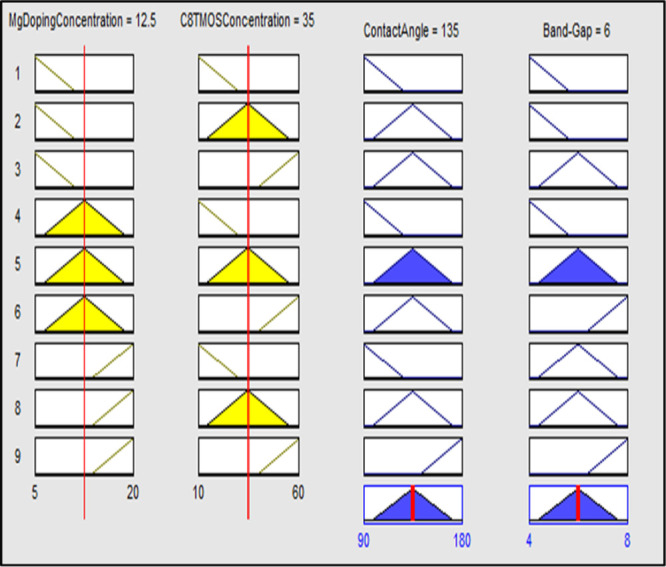
The effect of Mg metal doping concentration and tri-methoxy-octyl-silane (C8TMOS) on contact angle and band gap was also examined using fuzzy analysis in MATLAB. Input Mg dopant and C8TMOS concentration, together with their effects on output, contact angle, and band gap, were investigated. The membership functions and ranges were established for the inputs and outputs after initialization of the values. Small, middle, and large membership functions were used for the Mg doping concentration range, which is taken to be between 5 and 20%. The range for the C8TMOS input is 10–60 μL, with small, medium, and large serving as membership functions. Low, middle, and high contact angles for the output contact angle, ranging from ∼90 to 180 °C, and the band gap range ∼4 to 8 eV, with low, middle, and high being the membership functions, were used as displayed in Figure A,B. The membership function ranges for input and output were displayed in Table .
10.
(A) Membership functions for inputs (a) Mg doping concentration and (b) C8TMOS concentration. (B) Membership functions for outputs (a) contact angle and (b) band gap.
2. Membership Function on the Input Mg Doping Meditation, C8TMOS Concentration Membership, Output Contact Angle, and Band Gap.
| membership function | range | |
|---|---|---|
| Mg doping concentration | small | 1–11% |
| medium | 3–17% | |
| large | 13–20% | |
| C8TMOS concentration | small | 1–30 μL |
| medium | 15–55 μL | |
| large | 40–60 μL | |
| contact angle | low | 90–125° |
| middle | 100–170° | |
| high | 144–180° | |
| band-gap | low | 4–5.6 eV |
| middle | 4.4–7.6 eV | |
| high | 6.4–8 eV |
The membership function on the input Mg doping meditation, C8TMOS concentration membership, output contact angle, and band gap are shown in Figure .
Rules are set on the basis of logical and human-based data. The numbers of rules are set as (mn) where n is the number of inputs, and m is the number of membership functions. On the basis of the directions, the 3D charts and rule viewer were studied.
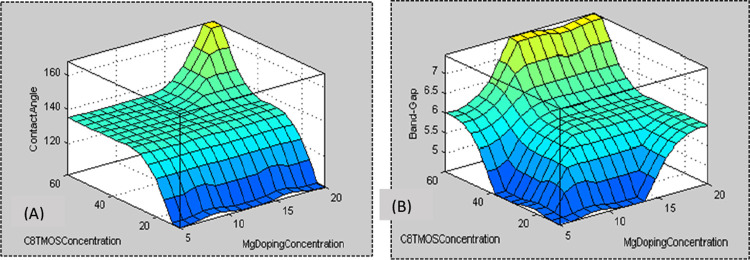
The 3D chart for contribution and consequences for Mg and C8TMOS contents along the x-axis and y-axis, with contact angle and band-gap at the z-axis, is displayed in Figure A,B, respectively.
11.
(A) 3D graph for input (C8TMOS and Mg doping concentration along x and y axis, respectively) and its effect on the contact angle along the z axis. (B) 3D graph for input (C8TMOS and Mg doping concentration along x and y axes, respectively) and its effect on the band gap along the z direction.
The calculated value for the input and its effect on output can be seen from the regulation spectator, as shown in Figure .
12.
Rule spectator for input and output of dopant concentration, silica source concentration, the contact angle, and band gap.
The error concerning the computer-generated and designed value is determined by comparing the output contact angle and band gap data from the simulated rule viewer. The simulated values are those values that were given by the software, and the designed values are those that we get after membrane fabrication and testing in the laboratory.
In terms of the contact angle, the designed value was 135°, and the simulated value closely matched 137°, resulting in a minimal error of 1.45%. Similarly, for the band gap, the designed value was 6, and the simulated value closely approached it at 5.9, yielding an error of 1.66%. These findings suggest a generally favorable agreement between the designed and simulated values for both the contact angle and band gap, affirming the accuracy of the simulation in capturing the intended characteristics.
The output value based on the inputs is calculated using the MAMDANI formula. Mamdani’s model is represented by the following method:
| 1 |
where Si is the singleton value and Mi is the value for the minimum membership function.
The values of the inputs from the rule viewer are taken to calculate the minimum membership function values, and four minimum membership functions are calculated as shown below.
B1 = 20–12.5/20 = 0.375
B2 = 1-A1 = 0.625
B3 = 180–135/180 = 0.25
B4 = 1-A3 = 0.75
The output value of contact angle and band gap was determined using the minimal membership function values and singleton values.
The precision between the simulated and calculated values based on the MAMDANI model employed in MATLAB’s fuzzy rule-based system is demonstrated by the calculated error being less than 1.7%, the values of band gap and contact angle obtained from fuzzy simulation, and the experimental results were comparable.
Density Field Analysis of Magnesium-Doped Silica Membranes via the Forcite Module
To better understand how magnesium-doped silica (Mg@SiO2) membranes enhance water desalination performance, an in-depth analysis of their density field is essential. Incorporating magnesium ions into the silica matrix notably alters the membrane’s structural and functional properties, such as permeability, selectivity, and mechanical strength. This study employs the Forcite module to evaluate the density behavior of Mg@SiO2 membranes and assess their role in improving desalination processes.
Structural Configuration and Density Distribution
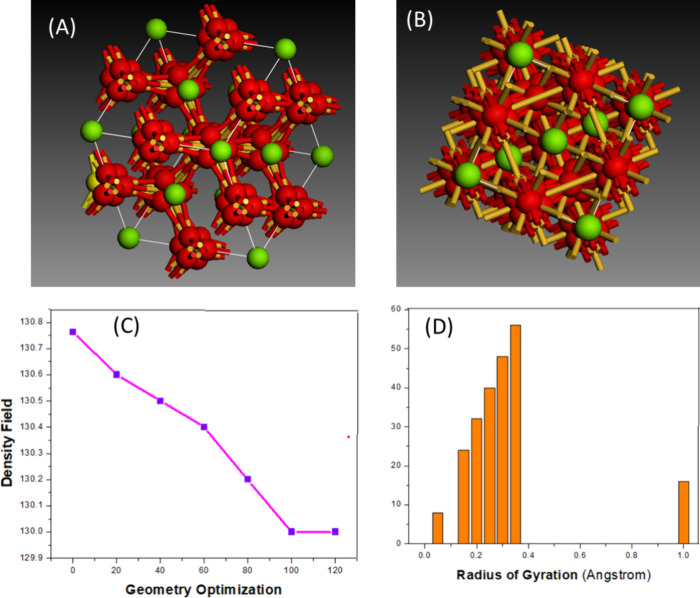
As shown in Figure A, magnesium ions (green spheres) are embedded uniformly within a silica matrix (red spheres), forming a cohesive and well-integrated framework. The even distribution of these dopants is crucial for maintaining consistent membrane characteristics. Any irregularity in ion placement could cause localized density variations, potentially disrupting the membrane’s desalination efficiency.
13.
(A) Structural model of Mg-doped silica membranes. (B) Ion size and density distribution from DFT simulations. (C) Geometry optimization trends linked to the density fields. (D) Relationship between the radius of gyration and perimeter (P).
Primary Factors Impacting Desalination Efficiency
Ion Distribution Uniformity
The consistent spread of magnesium ions across the membrane surface, as is evident in Figure A, supports stable filtration performance. A uniform ionic distribution leads to balanced water permeability and ion selectivity. In contrast, uneven doping may result in fluctuating flux rates and compromised rejection capabilities.
Effect of the Ion Size and Density
Magnesium ions, due to their size and density, directly influence the membrane structure and functionality. Higher ionic density can improve surface interactions with water molecules, boosting the level of contaminant removal. Figure B,C highlight how changes in ion size and packing density impact the membrane integrity.
Results from geometry optimization show a progressive reduction in system energy, atomic forces, and internal stress, indicating enhanced membrane stability due to magnesium incorporation.
Correlation of Radius of Gyration with the Perimeter (P)
Radius of gyration (x-axis): Reflects ion spread within the silica network.
Perimeter (y-axis): Represents density distribution trends.
Trend observed: A positive relationship between ion dispersion and perimeter, suggesting that broader dispersion leads to improved structural properties, enhancing both water permeability and ion selectivity.
Figure D provides a graphical interpretation of how the dispersion of magnesium ions (radius of gyration) correlates with structural density (perimeter, P).
Membranes with greater radii of gyration tend to exhibit higher functional efficiency due to increased interaction sites for water molecules.
Mechanical and Thermal Enhancements
Strength and Thermal Conductivity
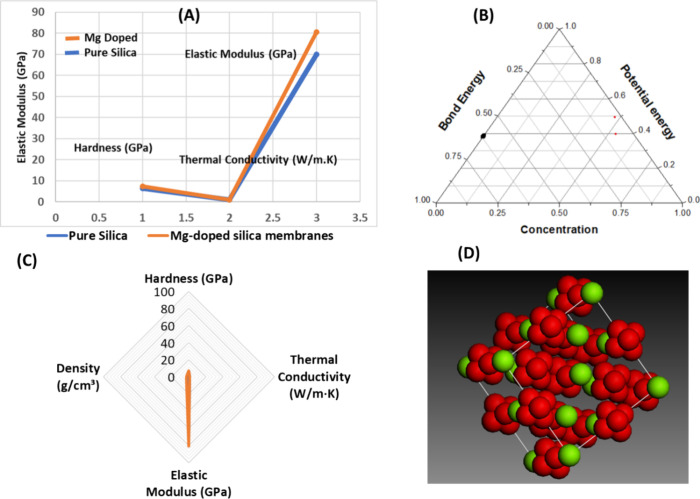
Magnesium doping significantly strengthens silica membranes. Figure A demonstrates that the elastic modulus rises from 70 GPa (pure silica) to 80.5 GPa in doped membranes, while hardness increases from 6.5 to 7.5 GPa. Thermal conductivity also improves by 17%, from 1.0 to 1.15 W/m·K, suggesting better heat resistancevital for continuous desalination use.
14.
(A) Comparative evaluation of the elastic modulus, hardness, and thermal conductivity in magnesium-doped silica (Mg@SiO2) membranes. (B) Ternary diagram illustrating the interplay among bond energy, potential energy, and magnesium content within the silica framework. (C) Correlative trends between membrane density, mechanical hardness, thermal conductivity, and elastic modulus. (D) Schematic depiction of how magnesium doping influences density distribution and pore size architecture.
Ternary Analysis: Energy Parameters and Concentration
The ternary plot in Figure B illustrates the interaction among magnesium concentration, bond energy, and potential energy. The stronger bonding introduced by Mg enhances structural integrity. Additionally, magnesium may offer catalytic or antimicrobial benefits, potentially reducing fouling and improving filtration outcomes.
Density and Structural Implications of Magnesium Integration
Magnesium-doped silica membranes exhibit a 20% increase in density (2.68 g/cm3), attributed to Mg’s higher atomic mass. Despite this increase, Figure C shows no substantial structural disruption, indicating stable integration into the silica framework.
This density adjustment could also impact the electronic behavior, possibly introducing new states within the silica band gap. Such changes may be advantageous for light-assisted desalination technologies, improving optical interactions.
Porosity, Selectivity, and Durability
As seen in Figure D, Mg doping influences the membrane porosity and pore distribution. The introduction of magnesium results in more defined pore structures, which enhances ion selectivity while maintaining water permeability. Improved mechanical characteristics from doping contribute to long-term durability and resistance to degradation.
Utilizing the Forcite module to analyze Mg-doped silica membranes reveals key structural and functional advancements. Uniform magnesium dispersion improves the density field, which supports optimal water transport and ion rejection. Additionally, the mechanical and thermal reinforcements provided by magnesium incorporation make these membranes highly suitable for sustained desalination use. Balancing the ion distribution, density, and mechanical stability is critical for maximizing membrane performance in real-world desalination systems.
Conclusions
This study demonstrates the successful fabrication of Mg-doped silica membranes via a sol–gel route with a systematic evaluation of their structure, hydrophobicity, and performance in direct contact membrane distillation (DCMD) for water desalination. Magnesium doping significantly influenced membrane morphology, leading to denser and more hydrophobic surfaces, as evidenced by increasing contact angles up to 140°, particularly at 15 wt % Mg loading. This enhanced surface property correlated to improved water vapor transport and minimized pore wetting during DCMD.
Performance testing revealed that membranes doped with 15 wt % Mg achieved optimal salt rejection98.3% for NaCl and 96.4% for Na2SO4while maintaining a stable permeate flux of 32 L/m2·h. These enhancements are attributed to the cross-linking effect of Mg, which increased the silica matrix’s packing density and mechanical integrity, thereby reducing pore intrusion and fouling. The membranes demonstrated sustained performance over a 420 min operational period and resistance against surfactant-induced wetting when challenged with 0.1 mM SDS, supporting their robustness under realistic desalination conditions.
XRD analysis confirmed the amorphous nature of the membranes, even after Mg incorporation, while EDX mapping validated the uniform distribution of Mg throughout the silica matrix. Complementary DFT simulations reinforced the experimental findings, revealing a light structure with evenly dispersed Mg ions (2.68 eV total energy), good mechanical stiffness (80.5 GPa), and strong surface resistance (7.5 eV) contributing to the membrane durability. The thermal stability was supported by a steady thermal profile (∼1.15 eV), and bandgap measurements showed negligible deviation from pure silica, preserving optical transparency and electronic insulation. The formation of well-defined microchannels improved water transport and ion exclusion, while the reactive surface reduced the microbial presence through ROS generation.
Overall, the integration of Mg into silica membranes offers a scalable, multifunctional, and thermally stable platform for efficient water purification. These membranes exhibit promising potential for sustainable treatment of high-salinity brines and complex industrial effluents, combining high ion rejection, durability, antifouling behavior, and computationally validated structural resilience.
Acknowledgments
We would like to express our sincere gratitude to all individuals and organizations that contributed to the successful completion of this research paper. Special thanks are due to our dedicated research team for their hard work and collaborative efforts. We are deeply appreciative of the resources and support provided by Lahore College for Women University in Lahore, Pakistan. We also acknowledge the valuable insights and guidance offered by the Institute of Polymer and Engineering at the University of the Punjab, Lahore, Pakistan, as well as the Georgia Institute of Technology (USA). Finally, we extend our heartfelt thanks to our families and friends for their unwavering support and encouragement throughout this endeavor.
In this study, all authors role equally to the design, synthesis, analysis, and interpretation of the results related to the samples.
No funding was received for this research.
The authors declare no competing financial interest.
References
- Nayak M. C., Isloor A. M., Lakshmi B., Marwani H. M., Khan I.. Polyphenylsulfone/multiwalled carbon nanotubes mixed ultrafiltration membranes: Fabrication, characterization and removal of heavy metals Pb2+, Hg2+, and Cd2+ from aqueous solutions. Arabian J. Chem. 2020;13(3):4661–4672. doi: 10.1016/j.arabjc.2019.10.007. [DOI] [Google Scholar]
- Priya P., Nguyen T. C., Saxena A., Aluru N. R.. Machine learning assisted screening of two-dimensional materials for water desalination. ACS Nano. 2022;16(2):1929–1939. doi: 10.1021/acsnano.1c05345. [DOI] [PubMed] [Google Scholar]
- Zou H., Huang J., Zhang M., Lin H., Teng J., Huang Z.. Mitigation of protein fouling by magnesium ions and the related mechanisms in ultrafiltration process. Chemosphere. 2023;310:136817. doi: 10.1016/j.chemosphere.2022.136817. [DOI] [PubMed] [Google Scholar]
- Zhao S., Shen L.. Advanced membrane science and technology for sustainable environmental applications. Frontiers in Chemistry. 2020;8:609774. doi: 10.3389/fchem.2020.609774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H., He D., Niu S., Qi H. T.. Tuning the microstructure of organosilica membranes with improved gas permselectivity via the co-polymerization of 1, 2-bis (triethoxysilyl) ethane and 1, 2-bis (triethoxysilyl) methane. Int. J. Hydrogen Energy. 2021;46(33):17221–17230. doi: 10.1016/j.ijhydene.2021.02.139. [DOI] [Google Scholar]
- Cho I. H., Kim D. H., Park S.. Electrochemical biosensors: Perspective on functional nanomaterials for on-site analysis. Biomater. Res. 2020;24(1):6. doi: 10.1186/s40824-019-0181-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tasleem S., Sabah A., Tahir M., Sabir A., Shabbir A., Nazir M.. Alkyl silica hybrid nanowire assembly in improved superhydrophobic membranes for RO filtration. ACS omega. 2022;7(5):3940–3948. doi: 10.1021/acsomega.1c04498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alosaimi E. H., Alsohaimi I. H., Hassan H. M., Chen Q., Melhi S., Younes A. A.. Towards superior permeability and antifouling performance of sulfonated polyethersulfone ultrafiltration membranes modified with sulfopropyl methacrylate functionalized SBA-15. Chinese Journal of Chemical Engineering. 2023;53:89–100. doi: 10.1016/j.cjche.2021.09.019. [DOI] [Google Scholar]
- Meng L., Mansouri J., Li X., Liang J., Huang M., Lv Y., Chen V.. Omniphobic membrane via bioinspired silicification for the treatment of RO concentrate by membrane distillation. J. Membr. Sci. 2022;647:120267. doi: 10.1016/j.memsci.2022.120267. [DOI] [Google Scholar]
- Rilda Y., Putra E. S., Arief S., Agustien A., Pardi H., Sofyan N.. Mucor sp. (Fungal Philospheric) of Gambir (Uncaria) Leaf surface as a biosynthetic Mg doped ZnO Nanorods media for antibacterial applications. J. Dispersion Sci. Technol. 2024;45(12):2291–2301. doi: 10.1080/01932691.2023.2263544. [DOI] [Google Scholar]
- Kahli H., Béven L., Grauby-Heywang C., Debez N., Gammoudi I., Moroté F., Cohen-Bouhacina T.. Impact of growth conditions on Pseudomonas fluorescens morphology characterized by atomic force microscopy. Int. J. Mol. Sci. 2022;23(17):9579. doi: 10.3390/ijms23179579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian B., Liu Y.. Antibacterial applications and safety issues of silica-based materials: A review. International Journal of Applied Ceramic Technology. 2021;18(2):289–301. doi: 10.1111/ijac.13641. [DOI] [Google Scholar]
- Castillo-Henríquez L., Brenes-Acuña M., Castro-Rojas A., Cordero-Salmerón R., Lopretti-Correa M., Vega-Baudrit J. R.. Biosensors for the detection of bacterial and viral clinical pathogens. Sensors. 2020;20(23):6926. doi: 10.3390/s20236926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curulli A.. Electrochemical biosensors in food safety: Challenges and perspectives. Molecules. 2021;26(10):2940. doi: 10.3390/molecules26102940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan X., Saltepe B., Yu L., Wang B.. Programming living sensors for environment, health and biomanufacturing. Microbial biotechnology. 2021;14(6):2334–2342. doi: 10.1111/1751-7915.13820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alatraktchi F. A. A.. Rapid measurement of the waterborne pathogen Pseudomonas aeruginosa in different spiked water sources using electrochemical sensing: Towards on-site applications. Measurement. 2022;195:111124. doi: 10.1016/j.measurement.2022.111124. [DOI] [Google Scholar]
- Manzoor S., Tayyaba S., Ashraf M. W.. Simulation, analysis, fabrication and characterization of tunable AAO membrane for microfluidic filtration. Journal of Intelligent & Fuzzy Systems. 2022;43(2):2099–2108. doi: 10.3233/JIFS-219309. [DOI] [Google Scholar]
- Anjum M. S., Ashraf M. W., Tayyaba S., Imran M.. Simulation, synthesis, and analysis of strontium-doped ZnO nanostructures for optoelectronics and energy-harvesting devices. Front. Mater. 2023;10:1260609. doi: 10.3389/fmats.2023.1260609. [DOI] [Google Scholar]
- Minaev Y. M., Filimonova O. Y., Minaeva Y. I.. Forecasting of fuzzy time series based on the concept of the nearest fuzzy sets and tensor models of time series. Cybernetics and Systems Analysis. 2023;59(1):165–176. doi: 10.1007/s10559-023-00551-9. [DOI] [Google Scholar]
- Liu Z.. Forecasting stock prices based on multivariable fuzzy time series. AIMS Mathematics. 2023;8(6):12778–12792. doi: 10.3934/math.2023643. [DOI] [Google Scholar]
- Jena R. M., Chakraverty S., Jena S. K., Sedighi H. M.. Analysis of time-fractional fuzzy vibration equation of large membranes using double parametric based Residual power series method. J. Appl. Math. Mech. 2021;101(4):e202000165. doi: 10.1002/zamm.202000165. [DOI] [Google Scholar]
- Mohammadi Soleymani M., Mirzadeh S.. Multi-objective optimization of operating parameters in tumbling mill with Neuro-Fuzzy network. Modares Mech. Eng. 2020;20(9):2331–2341. [Google Scholar]
- Gao Y. M., Chiu S. H., Busa P., Liu C. L., Kankala R. K., Lee C. H.. Engineered mesoporous silica-based core-shell nanoarchitectures for synergistic chemo-photodynamic therapies. International Journal of Molecular Sciences. 2022;23(19):11604. doi: 10.3390/ijms231911604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khnifira M., Boumya W., Atarki J., Sadiq M., Achak M., Bouich A., Abdennouri M.. Experimental, DFT and MD simulation combined studies for the competitive adsorption of anionic and cationic dyes on activated carbon in an aqueous medium. J. Mol. Struct. 2024;1310:138247. doi: 10.1016/j.molstruc.2024.138247. [DOI] [Google Scholar]
- Chen C., Yang Y., Graham N. J., Li Z., Yang X., Wang Z., Hou L. A.. A comprehensive evaluation of the temporal and spatial fouling characteristics of RO membranes in a full-scale seawater desalination plant. Water Res. 2024;249:120914. doi: 10.1016/j.watres.2023.120914. [DOI] [PubMed] [Google Scholar]
- Abdulazeez I., Alrajjal A. S., Ganiyu S., Baig N., Salhi B., AbdElazem S.. Facile engineering of mesoporous silica for the effective removal of anionic dyes from wastewater: Insights from DFT and experimental studies. Heliyon. 2023;9(11):e21356. doi: 10.1016/j.heliyon.2023.e21356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q., Yang X., Zhang L. Z.. Development, testing, and molecular dynamics simulation of a membrane that resists fouling by gel pollutants for humidification–dehumidification–type seawater desalination. Desalination. 2023;561:116686. doi: 10.1016/j.desal.2023.116686. [DOI] [Google Scholar]
- Galarza-Acosta G. L., Parra J. G., Hernández-Bravo R., Iza P., Schott E., Zarate X., Mujica V.. A Computational Chemistry Approach to the Molecular Design of SiO2 Nanoparticles Coated with Stearic Acid and Sodium Stearate in Ethanol Solvent. Colloids Surf., A. 2023;679:132527. doi: 10.1016/j.colsurfa.2023.132527. [DOI] [Google Scholar]
- Kuhn R., Bryant I. M., Jensch R., Böllmann J.. Applications of environmental nanotechnologies in remediation, wastewater treatment, drinking water treatment, and agriculture. Applied Nano. 2022;3(1):54–90. doi: 10.3390/applnano3010005. [DOI] [Google Scholar]
- Sahu A., Dosi R., Kwiatkowski C., Schmal S., Poler J. C.. Advanced polymeric nanocomposite membranes for water and wastewater treatment: a comprehensive review. Polymers. 2023;15(3):540. doi: 10.3390/polym15030540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Aswar E. I., Ramadan H., Elkik H., Taha A. G.. A comprehensive review on preparation, functionalization and recent applications of nanofiber membranes in wastewater treatment. Journal of Environmental Management. 2022;301:113908. doi: 10.1016/j.jenvman.2021.113908. [DOI] [PubMed] [Google Scholar]
- Hani U.. Comprehensive review of polymeric nanocomposite membranes application for water treatment. Alexandria Engineering Journal. 2023;72:307–321. doi: 10.1016/j.aej.2023.04.008. [DOI] [Google Scholar]
- Moreno Ruiz Y. P., de Almeida Campos L. A., Alves Agreles M. A., Galembeck A., Macário Ferro Cavalcanti I.. Advanced hydrogels combined with silver and gold nanoparticles against antimicrobial resistance. Antibiotics. 2023;12(1):104. doi: 10.3390/antibiotics12010104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ihsanullah I.. Boron nitride-based materials for water purification: Progress and outlook. Chemosphere. 2021;263:127970. doi: 10.1016/j.chemosphere.2020.127970. [DOI] [PubMed] [Google Scholar]
- Biswas A. K.. Water as an engine for regional development. International Journal of Water Resources Development. 2021;37(3):359–361. doi: 10.1080/07900627.2021.1890409. [DOI] [Google Scholar]
- Zhang Y., Zhao M., Cheng Q., Wang C., Li H., Han X., Li Z.. Research progress of adsorption and removal of heavy metals by chitosan and its derivatives: A review. Chemosphere. 2021;279:130927. doi: 10.1016/j.chemosphere.2021.130927. [DOI] [PubMed] [Google Scholar]
- Abujazar M. S. S., Karaağaç S. U., Amr S. S. A., Alazaiza M. Y., Bashir M. J.. Recent advancement in the application of hybrid coagulants in coagulation-flocculation of wastewater: A review. J. Cleaner Prod. 2022;345:131133. doi: 10.1016/j.jclepro.2022.131133. [DOI] [Google Scholar]
- Cui L., Li J., Gao X., Tian B., Zhang J., Wang X., Liu Z.. Human health ambient water quality criteria for 13 heavy metals and health risk assessment in Taihu Lake. Front. Environ. Sci. Eng. 2022;16:41. doi: 10.1007/s11783-021-1475-6. [DOI] [Google Scholar]
- Azarafza A., Islam M. A., Golpazirsorkheh Y., Efteghar I., Sadrzadeh M., Kamkar M., Rezakazemi M.. Aquaporin-Based Biomimetic Membranes for Low Energy Water Desalination and Separation Applications. Adv. Funct. Mater. 2023;33(21):2213326. doi: 10.1002/adfm.202213326. [DOI] [Google Scholar]
- Bayda S., Adeel M., Tuccinardi T., Cordani M., Rizzolio F.. The history of nanoscience and nanotechnology: from chemical–physical applications to nanomedicine. Molecules. 2020;25(1):112. doi: 10.3390/molecules25010112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmad M., Yang K., Li L., Fan Y., Shah T., Zhang Q., Zhang B.. Modified tubular carbon nanofibers for adsorption of uranium (VI) from water. ACS Appl. Nano Mater. 2020;3:6394–6405. doi: 10.1021/acsanm.0c00837. [DOI] [Google Scholar]
- Perveen R., Shujaat S., Qureshi Z., Nawaz S., Khan M. I., Iqbal M.. Green versus sol-gel synthesis of ZnO nanoparticles and antimicrobial activity evaluation against panel of pathogens. Journal of Materials Research and Technology. 2020;9(4):7817–7827. doi: 10.1016/j.jmrt.2020.05.004. [DOI] [Google Scholar]
- Ma S., Lin L., Wang Q., Zhang Y., Zhang H., Gao Y., Zhang Y.. A new strategy to simultaneously improve the permeability and antifouling properties of EVAL membranes via surface segregation of macrocyclic supra-amphiphiles. J. Membr. Sci. 2020;595:117562. doi: 10.1016/j.memsci.2019.117562. [DOI] [Google Scholar]
- Naseem T., Durrani T.. The role of some important metal oxide nanoparticles for wastewater and antibacterial applications: A review. Environmental Chemistry and Ecotoxicology. 2021;3:59–75. doi: 10.1016/j.enceco.2020.12.001. [DOI] [Google Scholar]
- Park S. H., Alammar A., Fulop Z., Pulido B. A., Nunes S. P., Szekely G.. Hydrophobic thin film composite nanofiltration membranes derived solely from sustainable sources. Green Chem. 2021;23(3):1175–1184. doi: 10.1039/D0GC03226C. [DOI] [Google Scholar]