Abstract
Background
Blastic plasmacytoid dendritic cell neoplasm (BPDCN) is a rare, aggressive blood cancer from plasmacytoid dendritic cell precursors. It’s marked by CD4, CD56, CD123, and CD303/CD304 expression and involves molecular disruptions like chromatin deletions, mutations, and chromosomal translocations.
Methods
The current study employed a comprehensive method with clinical samples, histology, FACS immunophenotyping, karyotype analysis, transcriptome and protein structure analysis, and single-cell sequencing to explore BPDCN’s molecular basis.
Results
The study discovered a new MYB-ZFAT gene fusion in a BPDCN patient and showed a diverse cell population, contradicting a single cell type theory. It found four major clusters (Cluster 1,2,3,8 ) and one cluster (clulster 12) with unique profiles and roles in disease progression. The research noted Key pathways include T cell receptor signaling, NK cell cytotoxicity, and hematopoiesis are involved in pathogenesis. The study emphasized MYB activation’s role in BPDCN’s cellular clustering and identity.
Conclusion
The study indicates BPDCN’s complexity with varied cellular origins and a significant role for MYB activation in its development. This research deepens our comprehension of BPDCN’s pathogenesis and cell populations.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12935-025-03899-4.
Introduction
Blastic plasmacytoid dendritic cell neoplasm (BPDCN) is a very rare and aggressive blood cancer originating from plasmacytoid dendritic cell precursors, characterized by abnormal CD4, CD56, and CD123,CD303/CD304 expression [1]. While BPDCN’s exact etiology is not fully understood, it’s likely linked to various molecular disruptions such as chromatin deletions, genetic mutations, and chromosomal translocations, all of which are considered part of its pathogenetic mechanisms [2]. Renosi et al. reviewed the genetic and epigenetic features of neoplasms involving plasmacytoid dendritic cells (pDC). BPDCN is characterized by complex karyotypes, frequent deletions involving immune and tumor suppressor genes, and MYC/MYB rearrangements, while pDC-AML is marked by RUNX1 mutations [3].
Suzuki’s study found that all five pediatric and four adult BPDCN patients had MYB gene rearrangements. This mutation is common and enhances MYB’s transcriptional activity, possibly by shortening its regulatory domain, which may contribute to the disease’s development [4]. Booth et al. investigated the role of MYB fusions in BPDCN. They showed that MYB fusions, found in about 20% of BPDCN cases, aberrantly regulated G2/M cell cycle genes, impaired dendritic cell differentiation, and induced leukemia in vivo. Using CUT&RUN chromatin profiling and in vivo models, they demonstrated that MYB fusions increased binding at cell cycle gene promoters, leading to higher expression of these genes [5]. Erica A. K. DePasquale analyzed 52,803 single-cell transcriptomes, including 18,779 T-cells, to understand BPDCN’s impact on the tumor environment. The study found increased interferon alpha (IFNA) response and decreased tumor necrosis factor-alpha (TNFA) signaling in BPDCN patients. It also revealed significant T-cell exhaustion linked to T-cell clonotype expansion, showing a complex tumor-immune interaction in BPDCN [6].
In this study, we identified a new MYB-ZFAT gene fusion in a BPDCN patient. We analyzed the chimeric protein’s structure with the SMART database, created 3D models with I-TASSER and PyMOL, and used single-cell sequencing to explore the fusion gene’s role in BPDCN’s pathophysiology. This approach offers a detailed view of the molecular mechanisms behind this rare cancer.
Case report
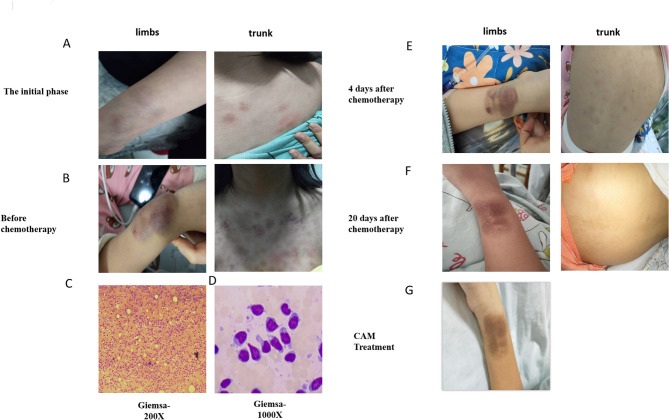
In April 2021, a 12-year-old girl was admitted to Hunan Children’s Hospital with a month-long illness. She had firm, non-attached masses all over her body and non-itchy, non-painful red, peeling rashes on her head, neck, face, chest, back, and arms. A mass near her right wrist grew to egg-sized. The rash turned into purpuric petechiae, showing tiny skin hemorrhages. She also had subcutaneous nodules and occasional knee joint swelling and discomfort (Fig. 1A -E).
Fig. 1.
(A) At the initial phase of the disease, the patient developed rashes on the wrists and back. The rash turned into purpuric petechiae, showing tiny skin hemorrhages on the wrists and Chest before chemotherapy. (B) BPDCN cells stained with Giemsa may appear as large blasts with irregular nuclear contours and scant cytoplasm. (C) The typical morphological features observed in the patient’s bone marrow include: large cell size, high nuclear-to-cytoplasmic ratio, finely granular chromatin, prominent nucleoli, abundant cytoplasm, and the presence of scant azurophilic granules. (D) After treatment with VDLD (Vincristine, Dexamethasone, Daunorubicin, Pemetrexed )for 4 days, the patient’s skin lesions on the wrists and back have shown improvement. (E) After a 20-day treatment with VDLD, there has been significant improvement in the patient’s skin lesions on the wrists and back, with the rashes on the back being almost unnoticeable. (F) After treatment with CAM(Cyclophosphamide, Cytarabine, Mercaptopurine), the patient’s skin lesions on the wrist have been alleviated
Based on the complete blood count (CBC) report, the patient exhibited a white blood cell (WBC) count of 3.24 × 10^9 per liter, a hemoglobin (HGB) concentration of 102.00 g per liter, and a platelet (PLT) count of 164.00 × 10^9 per liter. Furthermore, the absolute neutrophil count (ANC), denoted as NE#, was recorded at 1.39 × 10^9 per liter.
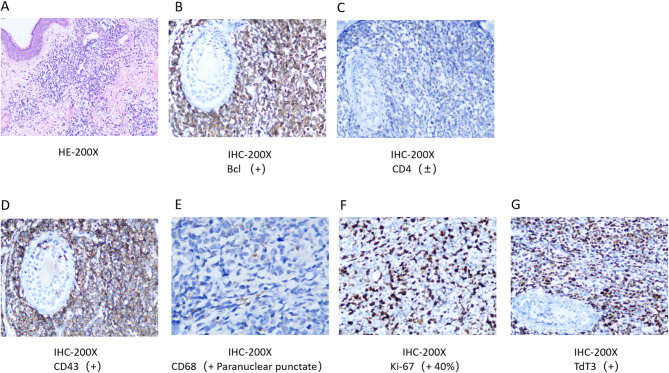
HE staining showed diffuse infiltration of tumor cells in the dermis and subcutaneous tissue(Figure 2A). Immunohistochemical analysis yielded the following results: Ki-67 with a strong positive index of 50%, indicative of high proliferative activity, and terminal deoxynucleotidyl transferase (TdT) with a score of 3+. The panel of markers demonstrated negativity for CD3, CD20, PAX-5, CD30, CD35, CD21, CD23, and CD38, while positivity was observed for CD4 and CD43. Additionally, myeloperoxidase (MPO) showed a negative reaction, whereas CD99 and CD68 exhibited positive staining. CD117 and CD34 were both negative (Fig. 2B -G).
Fig. 2.
(A) HE staining of affected skin biopsy sample. Red arrow showed diffuse infiltration of tumor cells in the dermis and subcutaneous tissue. (B) IHC analysis of affected skin biopsy sample. Brown precipitate stained with DAB indicated strongly Positive of Bcl. (C) IHC analysis of affected skin biopsy sample showed weakly Positive of CD4. (D) IHC analysis of affected skin biopsy sample showed strongly Positive of CD43. (E) IHC analysis of affected skin biopsy sample showed para-nuclear punctate of CD68. (F) IHC analysis of affected skin biopsy sample showed Moderately Positive of Ki-67. (G) IHC analysis of affected skin biopsy sample showed Moderately Positive of TdT3
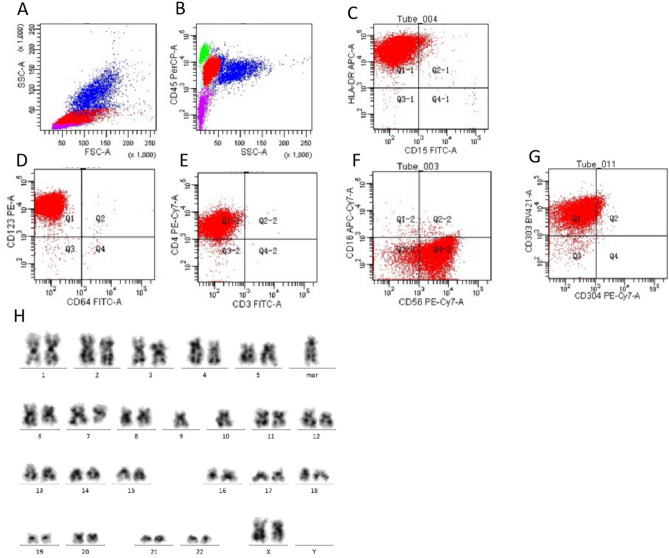
Flow cytometric analysis (FACS) of the bone marrow aspirate uncovered the presence of aberrant cell populations characterized by diminished CD45 expression relative to lymphocytes and an elevated side scatter (SSC) that was modestly greater than that of lymphocytes. These atypical cells constituted approximately 65% of the nucleated cellularity and were predominantly positive for a spectrum of antigens, including human leukocyte antigen-DR (HLA-DR), CD4, CD7, CD56, CD123, and CD303. The expression of this panel of markers is consistent with the immunophenotypic profile associated with blastic plasmacytoid dendritic cell neoplasm (BPDCN). (Figure 3A - G, Figure S1). The specimen from this patient was analyzed for 20 metaphase cells, and 9 cells showed a complex karyotype with chromosomal abnormalities. Karyotype analysis results showed 43–45,XX,-C, del(6)(q21), del(7)(q32),+mar, inc[cp9]/46,XX [11] (Figure. 3 H). These findings suggest BPDCN.
Fig. 3.
(A) The FSC and SSC profiles show a typical distribution pattern of cells. (B) The flow cytometry analysis showed that cells were divided into 4 populations using CD45 and SSC. Cells with high CD45 expression are typically indicative of lymphocytes (green).CD45- cells are typically non-hematopoietic cells (purple).Low CD45 expression may indicate monocytes or other non-lymphoid cells (Red & Blue). (C) The flow cytometry analysis demonstrated the cell population with HLA-DR positive and CD15 negative. (D) The flow cytometry analysis demonstrated the cell population with CD123 positive and CD15 negative. (E) The flow cytometry analysis demonstrated the cell population with CD4 positive and CD3 negative. (F) The flow cytometry analysis demonstrated the cell population with CD16 negetive and CD56 positive. (G) The flow cytometry analysis demonstrated the cell population with CD303 positive and CD304 negative. (H) The karyotype analysis showed abnormal chromosomal arrangement: 43–45,XX,-C, del(6)(q21),del(7)(q32),+mar, inc[cp9]/46,XX[11]
Methods
Clinical sample collection
The patient visited the Hunan Children’s Hospital and received a diagnosis of BPDCN based on the 2008 WHO Classification of Myeloid Neoplasms and Acute Leukemia [7]. To conduct further research, bone marrow, peripheral blood, and skin biopsies were collected from the patient with informed consent, which was obtained in accordance with the Declaration of Helsinki. The Human Research Ethics Committee of the Second Xiangya Hospital approved the studies.
HE staining
Samples of tissue were taken from the affected skin lesions. These samples were then placed in a 10% formalin solution and sent to the Pathological Laboratory of the Second Xiangya Hospital. Once labeled, the samples were embedded in paraffin, sliced into sections that were 5–7 mm thick, and finally stained with HE (artificial hematoxylin and eosin).
Immunohistochemistry
Samples of tissue were obtained from the affected skin lesions and sliced into sections that were 4 μm thick. The sections were dewaxed and washed using heated citrate buffer. Afterward, they were incubated with specific antibodies (anti-CD4, anti-Bcl, anti-CD43, anti-CD68, anti-Ki67, and anti-TdT3). After Washing with PBS for 3 times, they were incubated HRP-conjugated secondary antibody. DAB solution and hematoxylin were added to the slices, and the staining results were evaluated by the pathologists of the Second Xiangya Hospital.
FACS
For immunophenotyping, bone marrow cells collected from the patient were incubated with IgG1 FITC, IgG1 PE, IgG1 PerCP, IgG1 APC, IgG1 PE/Cy7, IgG1 V450, IgG1 APC Cy7, CD45 V500, cIgG1 FITC, cIgG1 PE, cIgG1 PerCP, cIgG1 APC, cIgG1 PE Cy7, cIgG1 V450, cIgG1 APC Cy7, CD45 V500, CD33 FITC, CD117 PE, CD34 PerCP, CD56 APC, CD13 PE Cy7, CD11b Pacific blue, HLA-DR APC Cy7, CD45 V500, CD14 FITC, CD304 PE, CD34 PerCP, CD123 APC, CD4 PE/Cy7, HLA-DR APC Cy7, CD45 V500, CD56 Pacific blue, CD61 FITC, CD36 PE, CD45 PerCP, CD34 APC, D16 FITC, CD13 PE, CD45 PerCP, CD24 APC, CD15 FITC, CD371 PE, CD34 PerCP, CD123 PE Cy7, CD33 APC, CD45 V500, CD56 Pacific blue, HLA-DR APC Cy7, CD4 FITC, CD13 PE, CD123 PE Cy7, CD56 APC, CD45 V500, CD7 Pacific blue, and HLA-DR APC Cy7 antibodies (all antibodies were purchased from Becton, Dickinson and Company (BD)), New York, USA). Fluorescence intensity and positive cell percentages were determined with a BD FACS Canto (Becton, Dickinson and Company (BD)), and data were analyzed using BD FACS Diva (Becton, Dickinson and Company (BD)). (Table 1)
Table 1.
FACS antibodies
| BD Biosciences catalog | Cell Surface Markers |
|---|---|
| 664,934 | CD45 PerCP |
| 662,854 | CD10 PE |
| 337,899 | CD22PE |
| 335,828 | CD20PE-CY7 |
| 663,494 | CD38 PE-Cy7 |
| CD58APC | |
| 665,750 | CD14 APC |
| 555,527 | CD64FITC |
| 566,919 | CD123PE |
| 557,758 | CD16APC-CY7 |
| 663,487 | CD56 PE-Cy7 |
| 664,995 | CD11b PE |
| 665,003 | CD15 FITC |
| 665,330 | HLA-DR APC |
| 662,910 | CD13 PE-CY7 |
| CD71APC-CY7 | |
| 665,336 | CD5 APC |
| 555,327 | CD2PE |
| 663,493 | CD4 PE-Cy7 |
| 349,201 | CD3 FITC |
| 663,521 | CD8 APC-Cy7 |
| 664,936 | CD117 PE |
| 664,935 | CD7 FITC |
| 664,937 | CD33 APC |
| 652,804 | CD19 APC |
| 348,801 | CD34PE-CY7 |
| 566,427 | CD303BV421 |
| 354,508 | CD304PE-CY7 |
| 665,342 | CD79a PE |
| 665,337 | MPO FITC |
| 663,526 | CD3 PE |
| 332,791 | TDT APC |
Chromosome karyotype analysis
Chromosome Prep: Short-term Culture involves counting bone marrow cells, culturing in 1640 medium at 37 °C for 24–48 h with colchicine, and CPG stimulant for 72 h. Post centrifugation, hypotonic treatment, and fixation, samples are stored at 4 °C. G-banding involves centrifuging for a cell suspension, air-drying on slides, baking at 75 °C, trypsin digestion, saline rinse, Giemsa stain, and final rinse and dry. Chromosome Analysis uses the Metafer workstation for automatic scanning and capturing of 40 metaphase images per slide for detecting abnormalities.
Transcriptome sequencing and MYB-ZFAT verifying
Total RNA was extracted from cell pellets with an RNeasy Mini Kit, treated with DNase I, and purified on Qiagen columns. RNA quality and quantity were measured by spectrophotometry. PolyA + RNA was isolated using an Oligotex kit. First-strand cDNA was synthesized from 1 µg PolyA + RNA with random primers and Superscript II. Second-strand synthesis was done at 16 °C, followed by purification. Illumina GAII sequencing was performed for short reads, with the RefSeq gene set and human genome build 36 as references. CLC Bio Genomic Work Bench was used to map reads with a strict cutoff for unique mapping and mismatch allowance. Expression levels were calculated in RPKM. The existence of MYB-ZFAT fusion gene was verified by PCR combined with Sanger sequencing.
Protein structure analysis
Using SMART (Simple Modular, Architecture Research Tool), the domains of the wild-type MYB and MYB-ZFAT fusion proteins were identified. The prabi database analyzed the MYB-ZFAT’s secondary structure (https://prabi.ibcp.fr/htm/site/web/aboutUs/teaching). I-TASSER Suite modeled their 3D structures (http://zhanglab.ccmb.med.umich.edu/I-TASSER/download/), and PyMOL 1.8.6 visualized the PDB Models (https://sourceforge.net/projects/pymol/files/pymol/1.8/).
Single cell sequencing
The document outlines a single-cell sequencing experiment with several key steps. Initially, human bone marrow mononuclear cells were extracted and stored in an EDTA anticoagulant tube for sample preparation. For library construction, the 10x Genomics platform was used, leveraging microfluidic technology to encapsulate cells and barcoded beads into droplets. Within these droplets, cells were lysed, allowing mRNA to bind to the barcodes, forming GEMs. Reverse transcription was then conducted to create cDNA libraries, which were indexed for sequencing. The cDNA libraries were sequenced to generate raw data. Quality control was performed using Cell Ranger software, which aligned reads to the reference genome with STAR. Metrics such as the number of high-quality cells, mean reads per cell, median genes per cell, sequencing saturation, and mapping rates were assessed. In the data analysis phase, low-quality cells and artifacts were filtered out. High-quality cells were subjected to dimensionality reduction and clustering using algorithms like MNN and t-SNE to identify distinct cell populations. Marker genes were identified, and cell types were annotated based on reference datasets. Differential gene expression analysis was conducted to find significantly upregulated or downregulated genes between different cell types or conditions. Additionally, functional enrichment analysis (GO and KEGG) and protein-protein interaction network analysis were performed to explore the biological functions and pathways associated with the identified genes.
Results
Fusion protein
The existence of MYB-ZFAT fusion gene was verified by PCR combined with Sanger sequencing (Fig. S2, Figure S2 & S3). We explore the fusion protein’s complexities, clarifying its biological function and structural analysis. This detailed molecular characterization is key to grasping the protein’s impact on the disease’s pathophysiology. The MYB-ZFAT fusion protein’s structure was closely studied and compared to the normal MYB protein. This fusion arises from the chromosomal translocation t(6;8)(q23;q24), the cause of its abnormal activation (Fig. 4A -C). A premature stop codon at the 347th amino acid in the MYB sequence results in a shortened MYB-ZFAT fusion protein with three DNA-binding domains (R1: AA 40–86, R2: AA 92–138, R3: AA 144–189) and a transcriptional activation domain (AA 267–313). The fusion omits the essential negative regulatory domain (NR: AA 518–677), which may lead to uncontrolled MYB activation and drive neoplasia.(Figure 4D and E). Rearrangements of the MYB oncogene have been identified in pediatric patients with recurrent chronic myelomonocytic leukemia (CMML), in addition to those afflicted with blastic plasmacytoid dendritic cell neoplasm (BPDCN) [8]. These genetic alterations are considered significant as they may play a pivotal role in disease pathogenesis and could have implications for therapeutic strategies [9].
Fig. 4.
A. The fusion gene is formed by the juxtaposition of two distinct genes. MYB in chromosome 6 and ZFAT in chromosome 8. The breakpoint in MYB is chr6:135515598. The breakpoint in ZFAT is chr8:135529901. B. This image showed how MYB-ZFAT fusion gene organized in chromsome. C. This image showed retained protein domains in MYB-ZFAT fusion protein: Myb-like-DNA-binding domain (green) and LMSTEN motif (purple). D. The diagram identifieds the structure of MYB WT composed with DNA-binding domain consists of three imperfect repeats labeled (R1, R2, R3); the transcriptional activation domain (TA) and the C-terminal negative regulatory domain (NR). E. MYB-ZFAT only composed withDNA-binding domain consists of three imperfect repeats labeled (R1, R2, R3) and the transcriptional activation domain (TA). F. The 3D model of the WT MYB protein generated with I-TASSER. G. The 3D model of the MYB-ZFAT fusion generated with I-TASSER
The 3D models of the WT MYB protein and the MYB-ZFAT fusion were generated with I-TASSER. The 730-AA WT MYB has three ligand-binding sites: Zinc ions at AA 119, 122, 135, 139, 147, 150, 163, 167, 175, 178, 191, 195; chloride ions at AA 203, 206, 208; and glycerol at AA 237, 240, 244, 245, 261, 262, 263. The 347-AA MYB-ZFAT fusion protein has five predicted binding sites: cholesterol at AA 37 and 64; extra Zinc ions at AA 40 and 58; n-decyl-beta-D-thiomaltoside at AA 11, 17, 18, 21, 76, 77; phosphate groups at AA 15, 18, 24; and calcium ions at AA 34, 45, 48. These structural details are key to understanding the MYB-ZFAT fusion’s molecular effects. (Fig. 4G).
Single cell sequencing
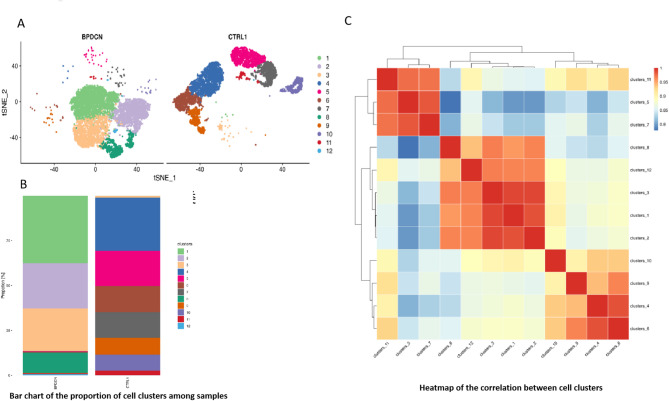
Single-cell sequencing reveals greater uniformity in BPDCN’s neoplastic cells compared to the diverse normal bone marrow cells. It shows distinct BPDCN cell populations with little overlap with the varied cells in healthy bone marrow (Fig. 5A). The cell populations in BPDCN patients are predominantly composed of four major clusters, namely Cluster 1, Cluster 2, Cluster 3, and Cluster 8, which together form the most significant proportion of the cellular landscape (Fig. 5B). The heatmap analysis shows strong links between Clusters 11, 5, and 7, and also among Clusters 8, 12, 3, 1, and 2, indicating shared cellular traits. Clusters 10, 9, 4, and 6 also correlate closely. Single-cell sequencing offers a detailed view of BPDCN’s cellular diversity and interactions (Figure 5C).
Fig. 5.
(A) The dimensionality reduction results based on MNN (Mutual Nearest Neighbors) are visualized for single-cell clustering through t-SNE, with the clustering algorithm using SNN, ultimately achieving the optimal cell grouping.The horizontal and vertical axes represent the first and second principal components of the dimensionality reduction, respectively. Each point in the graph represents a cell, with cells from different groups distinguished by different colors. (B) Bar chart of the proportion of cell clusters among samples. The horizontal axis represents different samples, and the vertical axis represents the percentage of cell counts in different groups. (C) Heatmap of the correlation between cell clusters. The horizontal and vertical axes represent different cell groups, and the numbers in the graph are Pearson correlation coefficients. The higher the value, the redder the color of the heatmap, indicating a higher degree of correlation between the cell groups
Given BPDCN’s characteristic markers CD4, CD56, CD123, and CD303/CD304, our patient’s bone marrow showed positivity for CD4, CD123, CD56, and CD303. We identified a new MYB-ZFAT fusion gene linked to MYB oncogene activation. We then analyzed expression of CD4, CD123, CD303, CD56, MYB, and lineage markers in cellular clusters. This thorough evaluation helped define the disease’s immunophenotype and molecular basis in the patient.
Cluster 1
In controls, Cluster 1 matched normal pDCs with CD4, CD123, CD303 expression but lacked CD56 and MYB. In BPDCN, Cluster 1 showed an altered profile with CD4, CD123, CD56, MYB expression and lost CD303, a late pDC marker. Several genes including LCNL1, MPPED2, SMIM5, VIPR2, PCDH9, PLXNA4, KIF17, and SLITRK5 (Figure 6 & Figure S4-S9) (Tables 2 and 3).
Fig. 6.
(A) Violin plot of CD4 gene expression levels.The horizontal axis represents cell group numbers, and the vertical axis represents the normalized gene expression values. Green represents the BPDCN group, and purple represents the CTRL group. (B) Violin plot of CD123 gene expression levels.The horizontal axis represents cell group numbers, and the vertical axis represents the normalized gene expression values. Green represents the BPDCN group, and purple represents the CTRL group. (C) Violin plot of CD303 gene expression levels.The horizontal axis represents cell group numbers, and the vertical axis represents the normalized gene expression values. Green represents the BPDCN group, and purple represents the CTRL group. (D) Violin plot of CD56 gene expression levels.The horizontal axis represents cell group numbers, and the vertical axis represents the normalized gene expression values. Green represents the BPDCN group, and purple represents the CTRL group. (E) Violin plot of MYB gene expression levels.The horizontal axis represents cell group numbers, and the vertical axis represents the normalized gene expression values. Green represents the BPDCN group, and purple represents the CTRL group
Table 2.
Lineage markers, BPDCN markers and MYB expression of each Cluster
| CLuster | Early stage markers | Myeloid markers | B cell lineage markers | T cell lineage markers | Erythroblast markers | Megakayocyte lineage markers | BPDCN markers | MYB |
|---|---|---|---|---|---|---|---|---|
| Cluster1 (Ctrl) |
CD34- HLA-DR+ |
CD36+ |
Kappa+ Lamda- |
CD4+ CD7+ CD8- |
CD71+ CD36+ |
CD36+ |
CD4+ CD123+ CD303+ CD56- |
Negative |
|
Cluster1 (BPDCN) |
CD34- HLA-DR+ |
Negative |
Kappa+ Lamda+ |
CD4+ CD7+ CD8- |
CD71+ CD36- |
Negative |
CD4+ CD123+ CD303- CD56+ |
Positive |
| Cluster2 (Ctrl) |
CD34- HLA-DR+ |
Negative | Negative | Negative | Negative | Negative | Negative | Negative |
|
Cluster2 (BPDCN) |
CD34- HLA-DR+ |
Negative |
Kappa+ Lamda+ |
CD4+ CD7+ CD8- |
CD71+ CD36- |
Negative |
CD4+ CD123+ |
Positive |
| Cluster3 (Ctrl) |
CD34- HLA-DR+ |
CD36+ |
Kappa+ Lamda- |
CD4+ CD7- CD8- |
CD71+ CD36+ |
CD36+ |
CD4+ CD123+ CD303+ CD56- |
Negative |
|
Cluster3 (BPDCN) |
CD34- HLA-DR+ |
Negative |
Kappa+ Lamda+ |
CD4+ CD7- CD8- |
CD71+ CD36- |
Negative |
CD4+ CD123+ CD303+ CD56+ |
Positive |
| Cluster 4 (Ctrl) |
CD34- HLA-DR- |
Negative |
Kappa- Lamda- |
CD8+ CD2+ CD3E+ CD3D+ CD3G+ CD7+ CD1A- TCR+ |
Negative | Negative | Negative | Negative |
| Cluster 4 (BPDCN) |
CD34- HLA-DR- |
Negative |
Kappa- Lamda- |
CD8+ CD2+ CD3E+ CD3D+ CD3G+ CD7+ CD1A- TCR- |
Negative | Negative | Negative | Negative |
| CLuster 5 (Ctrl) |
CD34- HLA-DR+ |
CD13+ CD33+ CD11b+ CD14+ CD64+ CD36+ CD11c+ |
Kappa- Lamda- |
CD4+ | CD36+ | CD36+ |
CD4+ CD123- CD303- CD56- |
Negative |
| Cluster 5 (BPDCN) |
CD34- HLA-DR+ |
CD13+ CD33+ CD11b+ CD14+ CD64+ CD36+ CD11c+ |
Kappa+ Lamda+ |
CD4+ | CD36+ | CD36+ |
CD4+ CD123- CD303- CD56- |
Negative |
| Cluster 6 (Ctrl) |
CD34- HLA-DR+ |
Negative |
Kappa- Lamda- |
CD4+ CD2+ CD3E+ CD3D+ CD3G+ CD7+ TCR+ |
Negative | Negative |
CD4+ CD123- CD303- CD56- |
Negative |
| Cluster 6(BPDCN) |
CD34- HLA-DR+ |
CD36+ |
Kappa+ Lamda+ |
CD4- CD2+ CD3E+ CD3D+ CD3G+ CD7- TCR- |
CD71+ CD36+ |
CD36+ |
CD4- CD123- CD303- CD56- |
Negative |
| Cluster 7 (Ctrl) |
CD34- HLA-DR+ |
CD13+ CD33+ CD11b+ CD14+ CD64+ CD36+ CD11c+ |
Kappa+ Lamda- |
CD4+ |
CD36+ CD71- |
CD36+ |
CD4+ CD123- CD303- CD56- |
Negative |
| Cluster 7 (BPDCN) |
CD34- HLA-DR+ |
CD13- CD33+ CD11b+ CD14+ CD64- CD36- CD11c+ |
Kappa+ Lamda+ |
CD4+ |
CD36- CD71+ |
CD36- |
CD4+ CD123- CD303- CD56- |
Negative |
| Cluster 8 (Ctrl) | Negative | Negative | Negative | Negative | Negative | Negative | Negative | Negative |
| Cluster 8 (BPDCN) |
CD34- HLA-DR+ |
Negative |
Kappa+ Lamda+ |
CD4+ CD7+ |
CD71+ | Negative |
CD4+ CD123+ CD303+ CD56+ |
Positive |
| Cluster 9 (Ctrl) |
CD34- HLA-DR+ |
CD11b+ | Negative |
CD2+ CD3E+ CD3D+ CD3G+ CD7+ TCR+ |
Negative | Negative | Negatvie | Negatvie |
| Cluster 9 (BPDCN) |
CD34- HLA-DR+ |
Negative |
Kappa+ Lamda+ |
CD2+ CD3E+ CD3D+ CD3G+ CD7+ TCR+ |
Negative | Negative | Negatvie | Negatvie |
| Cluster 10 (Ctrl) |
CD34- HLA-DR+ |
Negative |
CD79a+ CD19+ CD22+ CD20+ Kappa+ Lamda- |
Negative | Negative | Negative | Negatvie | Negatvie |
| Cluster 10 (BPDCN) |
CD34- HLA-DR+ |
Negative |
CD79a+ CD19- CD22+ CD20+ Kappa+ Lamda+ |
CD3E+ CD7+ |
Negative | Negative | Negatvie | Negatvie |
| Cluster 11 (Ctrl) |
CD34- HLA-DR+ |
CD13+ CD33+ CD11b+ CD14+ CD64+ CD36+ CD11c+ |
Kappa+ Lamda- |
CD4+ CD8A+ CD8B+ CD2+ CD3E+ CD3D+ CD3G+ CD7+ TCR+ |
CD36+ |
CD36+ CD42b- |
CD4+ CD123- CD303- CD56- |
Negative |
| Cluster 11 (BPDCN) |
CD34- HLA-DR+ |
CD13+ CD33- CD11b- CD14- CD64- CD36- CD11c- |
Kappa- Lamda+ |
CD4+ CD8A- CD8B- CD2- CD3E- CD3D- CD3G- CD7- TCR- |
Negative |
CD36- CD42b+ |
CD4+ CD123- CD303- CD56- |
Negative |
| Cluster 12 (Ctrl) |
CD34- HLA-DR+ |
CD13+ MPO+ CD33+ CD15+ CD11b+ CD14+ CD64+ CD36+ CD11c+ |
Kappa+ Lamda- |
CD4+ CD2+ CD3G+ TCR+ |
CD71+ CD36+ |
CD36+ |
CD4+ CD123+ CD303+ CD56- |
Negative |
| Cluster 12 (BPDCN) |
CD34- HLA-DR+ |
CD11b+ |
Kappa+ Lamda+ |
CD4+ CD7+ |
CD71+ CD36- |
CD36- |
CD4+ CD123+ CD303+ CD56+ |
Positive |
Table 3.
Top genes and Functions of Each Cluster
| Cluster | Definition | Top genes | Function | Reference |
|---|---|---|---|---|
| Cluster 1 | Primitive pDC population in BPDCN, at an early stage of differentiation, and related to tumor migration, microenvironment. | LCNL1 | Lung cancer | PMID: 38,180,474 |
| Bladder cancer | PMID: 34,059,019 | |||
| MPPED2 | Gliblastoma | PMID: 33,734,003 | ||
| Neuroblastoma | PMID: 22,262,177 | |||
| Breast cancer | PMID: 31,181,813 | |||
| Colorectal cancer | PMID: 30,846,004 | |||
| Squamous cell Cacinoma | PMID: 27,698,780 | |||
| VIPR2 | tumor cell migration | PMID: 36,237,322 | ||
| Glioblastoma | PMID: 35,456,975 | |||
| Lung cancer | PMID: 34,329,340 | |||
| PCDH9 | Breast caner | PMID: 36,936,167 | ||
| Prostate cancer | PMID: 36,936,167 | |||
| Glioma | PMID: 35,084,653 | |||
| PLXNA4 | Cytotoxic T cell trafficking in cancer | PMID: 34815265P | ||
| Endometrial cancer | MID: 33,012,523 | |||
| Thyroid carcinoma | PMID: 32,065,787 | |||
| Osteocarsoma | PMID: 32,614,239 | |||
| KIF17 | Breast cancer | PMID: 32,322,170 | ||
| SLITRK5 | Gastric cancer | PMID: 37,772,256 | ||
| SMIM5 | Renal cell carcinoma | PMID: 24318988P | ||
| Oral squamous cell carcinoma | MID: 30,275,705 | |||
| PTCRA | T cell development | PMID: 16,754,716 | ||
| Cluster 2 | A group of cells originally did not have clear lineage marker expression, but after MYB activation, they acquired part of the T-, B-, and erythroid lineages as well as the plasma cell DC phenotype, and are a mixed lineage tumor-associated cell population. | CLLU1 | Chronic lymphocytic leukemia | PMID: 17,284,524 |
| PMID: 16,529,606 | ||||
| PMID: 22,899,580 | ||||
| PMID: 22,738,889 | ||||
| PMID: 16,339,396 | ||||
| PMID: 19,212,335 | ||||
| PAX2 | Target of tumor suppressor gene WT1 | PMID: 14,603,255 | ||
| PMID: 21,778,682 | ||||
| CDCA7 | C-myc target gene | PMID: 37,248,764 | ||
| PMID: 11598121P | ||||
| MID: 16,580,749 | ||||
| BCAT1 | C-myc target gene | PMID: 33760210P | ||
| MID: 23,758,864 | ||||
| PMID: 8,692,959 | ||||
| SIX1 | Regulates C-myc, CCND1 | PMID: 16,488,997 | ||
| PMID: 30,379,332 | ||||
| IGLC2 | Trigger clonal expansion and differentiation of B lymphocytes into immunoglobulins-secreting plasma cells | PMID: 22,158,414 PMID: 20,176,268 | ||
| IGLC3 | Membrane-bound or secreted glycoproteins produced by B lymphocytes | PMID: 27,994,173 | ||
| FCGR2B | Fc Gamma Receptor IIb, B cell receptor signalling pathway | PMID: 17,522,256 | ||
| PMID: 15,506,939 | ||||
| Cluster 3 | Compared to the Ctrl group, the BPDCN group Cluster3 acquired Lamda, CD7, CD56, MYB, and lost CD36 This is a group of cells originally present in normal cells, with a mixed phenotype of partial myeloid lineage, B lineage, T lineage, erythroid lineage and plasma cell DC, and after MYB activation in BPDCN, it lost the myeloid phenotype, acquired Lamda, and CD56. A tumor-associated cell population. | CLEC4C (CD303) | BPDCN dendritic cell development | PMID: 11,748,283 |
| Kinase related genes | PMID: 21,880,719 | |||
| IL1R2 | ERK signaling | PMID: 31,744,444 | ||
| Esophageal cancer | PMID: 33,277,616 | |||
| Gastric cancer | PMID: 31,921,558 | |||
| Breast cancer | PMID: 36,029,680 | |||
| Renal cell carcinoma | ||||
| TIFAB | P53, Malignant Hematopoiesis | PMID: 32,101,751 | ||
| HOXA9, AM | PMID: 34,877,491 | |||
| TRAF6,MDS | PMID: 26,458,771 | |||
| HTR3A | 5 H tryptophan | PMID: 37,795,441 | ||
| prognosis of breast | PMID: 37,231,904 | |||
| Gastric cancer | PMID: 35,757,399 | |||
| Lung cancer | PMID: 33,042,464 | |||
| SLC18A2 | Dopamine | PMID: 19,228,741 | ||
| Norepinephrine | PMID: 26,905,753 | |||
| Serotonin | PMID: 30,954,278 | |||
| Histamine | PMID: 31,240,161 | |||
| prostate cancer | PMID: 32,084,491 | |||
| TDRD1 | prostate cancer | PMID: 36,497,036 | ||
| Non small cell lung cancer | PMID: 33,381,926 | |||
| Ovarian cancer | PMID: 36,865,141 | |||
| PMID: 25,788,493 | ||||
| PMID: 37,609,819 | ||||
| PMID: 33,374,923 | ||||
| Cluster 4 | This population of cells normally mainly expressed markers of the T lineage, expressing Kappa across the lines. Kappa and TCR were lost in the BPDCN. It can be defined as a population of T cells that did not develop into a tumor cell population. | CCR7 | Dendritic cell maturation, | PMID: 38,267,413 |
| Tumor related Dendritic cells | PMID: 24,691,220 | |||
| PMID: 34,780,080 | ||||
| CD8B | Surface glycoprotein on cytotoxic T lymphocytes | PMID: 1,672,332 | ||
| PMID: 37,781,365 | ||||
| NELL2 | Oncogenesis | PMID: 34,365,093 | ||
| PMID: 10,548,494 | ||||
| LRRN3 | Extracellular matrix and extracellular space, Vascular Skin Disease, melanoma, phechromocytomas | PMID: 34,552,928 | ||
| PMID: 19,661,783 | ||||
| APBA2 | Breast carcinogenesis | PMID: 34,773,240 | ||
| AML | PMID: 16,849,538 | |||
| EPHX2 | Prostate cancer | PMID: 32,687,069 | ||
| Colon cancer | PMID: 32,687,069 | |||
| Hepatocellular carcinoma | PMID: 34,278,494 | |||
| SCGB3A1 | Lung cancer | PMID: 38,505,085 | ||
| breast cancer | PMID: 30,405,052 | |||
| Testicular cancer | PMID: 17,029,216 | |||
| Cluster 5 | This population of cells mainly expressed myeloid markers and CD4 across lines. In the BPDCN, the expression of Lamda was obtained. The remaining phenotypes remained unchanged. It can be considered that it is mainly a myeloid cell population that does not develop into a tumor cell population | CYP1B1 | Colorectal cancer | PMID: 37,059,712 |
| Triple nagative Breast cancer | PMID: 36,077,068 | |||
| Lung cancer | PMID: 24,989,113 | |||
| AML | PMID: 29,793,312 | |||
| ALL | PMID: 23,757,320 | |||
| CES1 | Prostate cancer | PMID: 34,185,414 | ||
| Hepatocellular carcinoma | PMID: 36,472,914 | |||
| Liver cancer | PMID: 33,206,527 | |||
| THBS1 | Platelet aggregation | PMID: 33,538,796 | ||
| Angiogenesis | PMID: 36,196,150 | |||
| GliomaTumorigenesis | PMID: 33,664,245 | |||
| Tumor Microenvironment | PMID: 33,925,464 | |||
| MCEMP1 | Mast cell differentiation | PMID: 37,041,174 | ||
| Immune responses | ||||
| QPCT | Breast cancer | PMID: 38,417,050 | ||
| AML | PMID: 37,381,175 | |||
| Renal cell carcinoma | PMID: 34,036,385 | |||
| Thyroid cancer | PMID: 37,060,824 | |||
| STEAP4 | Prostate cancer | PMID: 36,011,027 | ||
| Hepatocellular carcinoma | PMID: 35,390,632 | |||
| Colorectal cancer | PMID: 36,187,390 | |||
| CRISPLD2 | Neutrophil extracellular traps | PMID: 37,178,049 | ||
| CLEC4D | clearance of the antigen, processing and further presentation to T-cells | PMID:14,971,047 | ||
| Cluster 6 | This population of cells, under normal conditions, mainly expressed T lineage markers, expressed Kappa across the lines, and in the case of MYB activation, lost Kappa gained Lamda, acquired CD36, and lost CD4, CD7, TCR. It can be defined as a group of T cell populations that vary in phenotype due to the tumor. | TRAT1 | Ovarian cancer | PMID: 34,868,239 |
| Non-small cell lung cancer | ||||
| MAF | Induce monocytic differentiation and apoptosis inbipotent myeloid progenitors | PMID: 10,477,683 | ||
| Interact with c-Myb to down regulate Bcl−2 expression and increase apoptosis in peripheral CD4 cells | PMID: 17,823,980 | |||
| CD40LG | T-B Cell-Activating Molecule | PMID: 8,617,933 | ||
| PMID: 19,426,221 | ||||
| GATA 3 | Regulator of T-cell development. | PMID: 23,287,858 | ||
| TNFRSF25 | Stimulates NF-kappa B activity and regulate cell apoptosis. | PMID: 9,430,227 | ||
| Lymphocyte proliferation induced by T-cell activation. | PMID:22,956,587 | |||
| TNFRSF4 | Activate NF-kappaB, CD4 + T cell response, T cell-dependent B cell proliferation and differentiation | PMID:22,956,587 | ||
| PMID: 10,754,303 | ||||
| KLRB1 | Natural killer (NK) cell cytotoxicity | PMID: 17,295,095 | ||
| CD28 | enhances CD40L-mediated activation of NF-kappa-B and kinases MAPK8 and PAK2 in T-cells | PMID: 15,067,037 | ||
| NPDC1 | Colon cancer | PMID: 37,822,345 | ||
| Gastric Cancer | PMID: 36,578,802 | |||
| AML | PMID: 31,235,852 | |||
| SLAMF1 | T cell-B cell cross-talk | PMID: 30,058,241 | ||
| Stimulation Toll-like receptor | PMID: 29,440,514 | |||
| Dendritic Cells Developmental | PMID: 16,317,102 | |||
| Cluster 7 | Under normal conditions, this population of cells mainly expressed the markers of the myeloid lineage, expressing both CD4 and Kappa across the lines. In BPDCN, this population lost a fraction of myeloid Markers: CD13, CD64, CD36; Lamda, and erythroid marker CD71. It can be considered a population of myeloid cell populations whose phenotypes change due to the tumor. | CXCL10 | Monocytes, natural killer and T-cell migration | PMID: 12,750,173 |
| PMID: 12,663,757 | ||||
| TCF7L2 | Wnt signaling pathway/ MYC | PMID: 32,248,976 | ||
| PMID: 32,068,780 | ||||
| HES4 | promotes early T-cell development | PMID: 31,919,081 | ||
| Bladder cancer | PMID: 38,370,367 | |||
| Colorectal cancer | PMID: 36,672,336 | |||
| CLEC10A | Immunological role and prognostic potential of CLEC10A in pan-cancer | PMID: 35,702,069 | ||
| CTSL | lysosomal cysteine protease tumor progression | PMID: 26,299,995 | ||
| BATF3 | Development of classical dendritic cells (cDCs) and CD103(+) dendritic cells | PMID: 37,392,735 | ||
| PMID: 19,008,445 | ||||
| PMID: 28,486,109 | ||||
| TPPP3 | Glioblastoma | PMID: 37,863,960 | ||
| Oral squamous cell carcinoma | PMID: 36,058,091 | |||
| Clear cell sarcomas | PMID: 31,488,818 | |||
| Nasopharyngeal | PMID: 34,668,461 | |||
| PTGIR | Ovarin cancer microenviroment | PMID: 36,551,640 | ||
| Oropharyngeal cancer | PMID: 33,003,642 | |||
| Cluster 8 | This group of cells, under normal conditions and did not express any lineage-related markers, obtained HLA-DR in BPDCN, some T lines B, some erythroid lines, and typical BPDCN markers, which can be regarded as the emergence of this tumor-associated cell population due to MYB activation. | KLF4 | G-S cell cycle, P53, maintaining ES, Dendritic cells development | PMID: 34,680,943 |
| PMID: 30,578,299 | ||||
| PMID: 16,904,174 | ||||
| PMID: 36,827,419 | ||||
| SPC25 | Lung cancer | PMID: 34,746,885 | ||
| Breast cancer | PMID: 35,965,791 | |||
| Hepatocellualr carcinoma | PMID: 32,742,802 | |||
| Prostate cancer | PMID: 29,552,205 | |||
| PMID: 29,552,205 | ||||
| MCM10 | Preventing DNA damage | PMID: 34,645,815 | ||
| NK cell deficiency | PMID: 32,865,517 | |||
| PBK | MAPK family, DNA damage, P27 expression, phosphotylates P38, TP53, activation of lymphoid cells | PMID: 17,160,018 | ||
| PMID: 17,160,018 | ||||
| PMID: 25,466,965 | ||||
| E2F8 | Lung cancer | PMID: 37,863,324 | ||
| Colon cancer | PMID: 26,089,541 | |||
| Prostate cancer | PMID: 27,683,099 | |||
| Ovarian | PMID: 32,823,614 | |||
| G1/s phase transition | PMID: 26,992,224 | |||
| Wnt/beta-catenin | PMID: 37,635,486 | |||
| PKMYT1 | ALKBH5, gastic cancer | PMID: 35,114,989 | ||
| Promising therapeutic strategy for CCNE1-amplified cancers | PMID: 35,444,283 | |||
| TMSB15A | Breast cancer | PMID: 23,079,573 | ||
| Neck squamous cell carcinoma | PMID: 37,161,060 | |||
| Gastic cancer | PMID: 38,577,455 | |||
| DIAPH3 | Diagnostic cancer biomarker | PMID: 36,750,200 | ||
| Lung adenocarcinoma | PMID: 31,586,548 | |||
| Cervical cancer | PMID: 34,659,408 | |||
| Beta-catenin/TCF, hepatocellular carcinoma | PMID: 28,795,316 | |||
| Cluster 9 | This is a group of cells mainly expressing T lines, expressing CD11b, acquiring B lines Kappa and Lamda, in BPDCN, losing myeloid marker CD11b, and can be considered a population of NK cells which phenotype changes due to the tumor. | GZMH | Apoptosis | PMID: 17,765,974 |
| Natural Killer cell | PMID: 17,409,270 | |||
| KLRD1 | NK cells and some cytotoxic T-cells | PMID: 12,480,700 | ||
| Oral squamous cell carcinoma | PMID: 37,491,735 | |||
| Esophageal cancer | PMID: 36,588,538 | |||
| FGFBP2 | Cytotoxic lymphocytes | PMID: 30,993,913 | ||
| KLRF1 | NK cells and stimulates their cytoxicity and cytokine release | PMID:18,922,855 | ||
| PTGDR | Allergic inflammation | PMID: 30,986,261 | ||
| XCL2 | Chemotactic activity for lymphocytes | PMID: 30,986,261 | ||
| FCRL6 | Mature cytotxcic lymphocytes, B cell chronic lymphocytic leukemia | PMID: 18,991,291 | ||
| S1PR5 | NK cells | PMID: 19,808,259 | ||
| Homestasis of patrolling monocytes | PMID: 23,519,784 | |||
| EOMES | Necessary for the differentiation of effector CD8 + T cells | PMID: 36,580,919 | ||
| Cluster 10 | This population of cells expressed only the markers of the B line in normal control, and in BPDCN, lost CD19, while obtaining the markers of two T lines, CD3E and CD7, can be considered as a population of cells that cause phenotypic changes due to tumor. | VPREB3 | Representative of all stages of B-cell differentiation | PMID: 22,210,545 |
| MYC translocated lymphomas | PMID: 20,823,132 | |||
| B-cell lymphomas | PMID: 24,493,312 | |||
| CD22 | The localization of B-cells in lymphoid tissues | PMID: 30,323,814 | ||
| PAX5 | Early stages of B-cell differentiation | PMID: 22,127,921 | ||
| Acute lymphoblastic leukemia | PMID: 9,442,394 | |||
| PMID: 30,643,249 | ||||
| FCRL1 | B-cells activation and differentiation. | PMID: 37,822,931 | ||
| B-cell lymphoma | PMID: 36,409,389 | |||
| Immunotherapy target of CLL | PMID: 17,895,404 | |||
| Hairy cell lymphoma | ||||
| B-cell non hodgkin lymphoma | ||||
| FCRL2 | Regulatory role in normal and neoplastic B cell development. | PMID: 19,682,311 | ||
| PMID: 21,068,405 | ||||
| Prognostic marker for chronic lymphocytic leukemia | PMID: 18,314,442 | |||
| PMID: 31,092,813 | ||||
| POU2AF1 | Nodular lymphocyte predominant hodgkin lymphoma | PMID: 7,859,290 | ||
| Essential for response of B-cells to antigens | PMID: 7,779,176 | |||
| CD19 | ell surface protein is restricted to B cell lymphocytes | PMID:2,463,100 | ||
| PMID:1,373,518 | ||||
| COL19A1 | Maintain the integrity of the extracellular matrix | PMID: 35,346,795 | ||
| BACE2 | Aspartic protease | PMID: 10,683,441 | ||
| Cluster 11 | It can be argued that in tumors, this group of cells has lost almost all lineage marker and retained only a few myeloid and T lineage marker, but transformed to megakaryocytes, and the significance of whether this transition is due to tumorigenesis is unclear. | CYP1B1 | NADPH-dependent electron transport pathway | PMID: 22,210,049 |
| PTAFR | Melanoma metastasis | PMID: 32,168,105 | ||
| Breast cancer | PMID: 30,008,927 | |||
| Eosinophilic leukemia | PMID: 30,866,528 | |||
| CLEC4E | Activation of plasmacytoid Dendritic cells | PMID: 27,183,629 | ||
| TREM1 | Activatin of myeloid cells promtes antitumor immunity | PMID: 37,647,386 | ||
| Augment antitumor T cell immunity by inhibiting meyloid derived suppressor cells and restraning anti-PD-1 resistance | PMID: 37,651,197 | |||
| ALDH1A1 | AML | PMID: 37,298,333 | ||
| Hematopoietic progenitors | PMID: 35,028,852 | |||
| Maintains leukemia stem cell properties | PMID: 37,761,947 | |||
| PMID: 18,082,256 | ||||
| PMID: 31,768,017 | ||||
| THBS1 | A marker for acute myeloid leukemia and plays a role in tumor invasion | PMID: 32,117,788 | ||
| THBS1 producing tumor infiltrating monocyte-like cells contribute to immunosuppression and metastasis | PMID: 37,749,092 | |||
| CRISPLD2 | Gstric cancer Tumor microenviroment | PMID: 37,600,223 | ||
| Bladder cancer | PMID: 37,178,049 | |||
| Attenuate pro-inflammatory cytokines | PMID: 34,150,000 | |||
| Cluster 12 | This is a group of mixed across lineage markers of cells, in BPDPN, the multilineage phenotype have changed, and obtained the CD7, CD56 tumor related markers, can be regarded as a group of relatively naive cell group, its originally has a variety of lineage marker expression, in tumor, the phenotype changes, but still is mixed with other tumor related cell group. | CRISP3 | Prostate cancer | PMID: 32,357,309 |
| Mammay carcinoma | PMID: 30,609,035 | |||
| MMP8 | Tumor microenviroment | PMID: 36,941,602 | ||
| Invasive of Breast cancer | PMID: 38,017,545 | |||
| Inflammatory disorders and Cancer progression | PMID: 21,388,856 | |||
| CYP4F3 | Mediator of inflammation | PMID: 8,486,631 | ||
| Promyelocytic leukemic cell | PMID: 18,577,768 | |||
| AML | PMID: 19,029,204 | |||
| PMID: 14,715,252 | ||||
| ANXA3 | Triple-negative breast cancer | PMID: 37,139,408 | ||
| Colon cancer | PMID: 35,117,344 | |||
| Cervical cancer cells | PMID: 37,843,781 | |||
| CEACAM8 | High expression in acute lymphoblastic leukemia | PMID: 17,909,799 | ||
| CD177 | Beta-catenin signaling | PMID: 32,042,113 | ||
| Modulate the Tumor regulatory T cells | PMID:34,599,187 | |||
| TFF3 | Lung cancer | PMID: 36,909,373 | ||
| Breast cancer | PMID: 28,687,783 | |||
| Colorectal cancer progression | PMID: 34,262,017 | |||
| TCN1 | Colorectal cancer | PMID: 35,686,504 | ||
| Gastic cancer | PMID: 34,549,663 | |||
| Colon cancer | PMID: 32,686,693 | |||
| ARG1 | Target for cancer treatment | PMID: 35,316,752 | ||
| Tumor-associated macrophages | PMID: 30,613,266 | |||
| Immune suppression in pancreatic cancer | PMID: 36,727,849 | |||
| CLC | Essential for Treg | PMID: 35,274,950 | ||
| Associated with inflammation | PMID: 12,031,912 | |||
| Myeloid leukemia | PMID: 12,399,964 | |||
| Myeloid-specific genes in childhood ALL |
Cluster 2
In controls, the cluster lacked BPDCN markers. In BPDCN, it gained B cell (Kappa, Lambda), T cell (CD4, CD7), and erythroid (CD71) markers, plus CD123 and MYB, but not CD303 or CD56. The gene profile was rich in oncogenesis and c-myc pathway genes (Figure 6 & Figure S5-S9) (Tables 2 and 3).
Cluster 3
In BPDCN, Cluster 3 gained B cell (Lambda), NK cell (CD56), T cell (CD7), and MYB markers, losing CD36, a myeloid marker. It showed a mixed phenotype of myeloid, B, T, erythroid lineages, and pDCs. MYB activation led to a shift from myeloid to a tumor-associated cell population with B and NK cell markers. The top 10 genes expressed are linked to dendritic cell development (Figure 6 & Figure S4-S9) (Tables 2 and 3).
Cluster 4
Compared to controls, BPDCN’s Cluster 4 lacked Kappa light chains and TCR complex, suggesting a T cell subset not transformed into tumor cells. Normally, this cluster shows T lineage markers with Kappa co-expression. The top 10 gene expressions relate to T cell oncogenesis, tumor immunology, and dendritic cell functions (Figure 6 & Figure S4-S9) (Tables 2 and 3).
Cluster 5
Cluster 5 in BPDCN, unlike the control group, expressed Lambda immunoglobulin light chains alongside myeloid and CD4 markers (Figure 6 & Figure S4-S9) (Tables 2 and 3).
Cluster 6
Cluster 6 in BPDCN showed a shift with Lambda gain, Kappa loss, CD36 upregulation, and CD4, CD7, TCR downregulation, plus MYB oncogene acquisition. Normally T lineage marker-expressing with Kappa, these cells change post-MYB activation, losing Kappa, gaining Lambda and CD36, and T cell markers. (Figure 6 & Figure S4-S9) (Tables 2 and 3).
Cluster 7
Cluster 7 in BPDCN lost myeloid markers CD13, CD64, CD36, and gained erythroid marker CD71 and Lambda light chain, unlike controls with myeloid markers and CD4, Kappa. This indicates a tumor-influenced phenotype shift. Classified as a myeloid subset. Its top 10 genes are linked to immune regulation, cancer progression, and T and dendritic cell development. (Figure 6 & Figure S4-S9) (Tables 2 and 3).
Cluster 8
Cluster 8 in BPDCN showed a shift with new expressions of HLA-DR, Kappa, Lambda, CD4, CD7, CD71, and BPDCN-specific markers CD56, CD123, CD303, not seen in controls. The top genes in this cluster relate to dendritic cell development, chromosomal stability, DNA damage response, TP53, and cell cycle regulation (Figure 6 & Figure S4-S9) (Tables 2 and 3).
Cluster 9
Cluster 9 in BPDCN showed a loss of myeloid marker CD11b and gained B cell markers Kappa and Lambda, differing from controls with T lineage markers and CD11b. The top 10 gene expressions linked to NK cell function regulation (Figure 6 & Figure S4-S9) (Tables 2 and 3).
Cluster 10
Cluster 10 in BPDCN, unlike controls with only B lineage markers, lost CD19 and gained Lambda, CD3E, and CD7, indicating a T cell-like shift due to the tumor. Gene expression showed top genes crucial for B cell function, development, and lymphocytic leukemia pathogenesis (Figure 6 & Figure S4-S9) (Tables 2 and 3).
Cluster 11
Cluster 11 in BPDCN lost myeloid (CD33, CD11b, etc.), T lineage (CD8A, CD3E, etc.), and B (Lambda) markers, and gained CD42b, unlike controls with myeloid, B, and T markers. Top gene expressions link to leukemia pathogenesis, pDC activation, and tumor microenvironment dynamics, (Figure 6 & Figure S4-S9) (Tables 2 and 3).
Cluster 12
Cluster 12 in BPDCN, unlike controls, lost myeloid markers but gained Lambda, CD56, and CD2, and lost CD3G and CD7 but gained CD7 and MYB. Normally expressing myeloid and variably T and pDC markers, in BPDCN it retained only CD11b, and upregulated CD56. The top 10 gene expressions are enriched in cancer progression, ALL, and Wnt/Beta-catenin signaling regulation (Figure 6 & Figure S4-S9) (Tables 2 and 3).
Pseudotime analysis
Cluster 1, 2, 3, 5, 6, 7, 8, 11, and 12 expressing CD4 are cell populations that may differentiate into plasma cell DC (pDC).
Cluster 1, 2, 3, 7, 8, and 12 expressing both CD4 and CD123 are likely to be the cell populations that begin to differentiate into plasma cell DC (pDC).
Cluster 3, 8, and 12 expressing CD4, CD123, and CD303 are late-stage cell populations that have differentiated into plasma cell DC (pDCs).
Cluster 3 and 8, which express CD4, CD123, CD303, and CD56 simultaneously, can be defined as a cell population typical of BPDCN.
The cell population with high MYB expression can be divided into three types:
Cluster 1 is a group of BPDCN cells that are highly expressed in MYB and express CD4, CD123, and CD56, but not CD303, and should be defined as a group of BPDCN cells with malignant potential that appear earlier.
Cluster 12, which highly expresses MYB and expresses CD4, CD123, and CD303 but does not express CD56, should be defined as a population of plasma cell DC (pDC) cells that lack the characteristics of BPDCN but possess malignant potential.
Cluster 3 and Cluster 8, both of which express high levels of MYB and all of the genes CD4, CD123, C303, and CD56, should be defined as typical BPDCN cell clusters (Figure 7).
Fig. 7.
A. Based on the results calculated by Monocle2, the trajectory is plotted in the graph, where the points represent cells, the colors represent different cell clusters, group information, cell types, sample information, and state status, with the black solid line indicating the cell differentiation trajectory. B-D. The cell trajectory graph based on the cluster, cell type, sample, and group information in Fig. 7A has been split, with each cluster, cell type, sample, and group displayed separately on the trajectory graph. In the graph, the points represent cells, the colors represent different cell clusters, group information, cell types, sample information, and state status, with the black solid line indicating the cell differentiation trajectory. E-F. The cell trajectory graph of the cells from BPDCN group and CTRL group. Green cell population representing the BPDCN group and purple cell population representing the CTRL group in the illustration. G. A pie chart showing the proportion of different states in BPDCN and CTRL samples
KEGG analysis
KEGG analysis found that The main differentially expressed genes between BPDCN and normal control are enriched in the following three pathways: T cell receptor signaling pathway, Natural killer cell mediate cytotoxity, Hematopoietic cell lineage (Fig. 8).
Fig. 8.
(A) Arrange the fold changes of differentially expressed genes from largest to smallest, and select the top 25 up-regulated and down-regulated genes to draw a heatmap: The horizontal axis represents the differential grouping information, and the vertical axis represents the top 25 up-regulated and down-regulated genes (if there are fewer than 25 differentially expressed genes in either up- or down-regulation, all genes will be plotted; mitochondrial genes and ribosomal genes are not plotted by default). Yellow indicates high expression, and purple indicates low expression. (B) KEGG Enrichment Analysis Top 20 (select pathways with corresponding differential gene counts greater than 2, sorted by each item’s -log10Pvalue from highest to lowest) bubble chart. The horizontal axis Enrichment Score represents the enrichment score, the larger the bubble, the more differential protein-encoding genes it contains. The bubble color changes from purple to blue to green to red, and the smaller the enrichment p Value, the more significant it is
Discussion
Blastic plasmacytoid dendritic cell neoplasm (BPDCN) is a rare, aggressive blood cancer originating from plasmacytoid dendritic cell precursors. It can affect tissues like skin, bone marrow, lymph nodes, and blood, and is identified by markers CD4, CD56, CD123, and either CD303 or CD304 [10]. The precise etiology of BPDCN is not fully understood but involves various molecular disruptions. This includes deletions affecting tumor regulators and suppressor genes, and mutations in multiple genes like CDKN2A/B, CDKN1B, RB1, BCL2, CCND1, ATM, TP53, ASXL1, IDH2, NPM1, TET2, KRAS, NRAS, IKZF1/2/3, and ZEB2. BPDCN also features chromosomal rearrangements involving genes such as ALK, ETV6, and MYC. These genetic changes disrupt cell processes and likely drive the disease’s development [11].
The role of the MYB gene in BPDCN
In 2017, Suzuki’s team studied 14 BPDCN patients, including 5 children and 9 adults, through RNA sequencing and transcriptome analysis. They found MYB gene rearrangements in all children and 4 adults. The study identified gene fusions like MYB-ZFAT, MYB-PLEKHO1, MYB-DCPS, and MYB-MIR3134, present at diagnosis and relapse but absent in remission. The frequent MYB rearrangements are linked to increased MYB transcriptional activity in BPDCN [9]. The study also found MYB and MYC gene rearrangements in some BPDCN patients. These rare translocations in myeloid malignancies may cause the MYB protein’s regulatory domain to be shortened, possibly leading to its overactivation and playing a role in BPDCN’s oncogenesis [12–14]. These insights into the molecular pathology of BPDCN have expanded our understanding of the disease’s genetic underpinnings and may have implications for targeted therapeutic strategies [5].
Single cell sequencing of BPDCN in previous study
Erica A. K. DePasquale analyzed 52,803 single-cell transcriptomes, including 18,779 T-cells, to study the effects of malignant BPDCN cell growth on the tumor environment. The study showed increased interferon alpha (IFNA) response and decreased tumor necrosis factor alpha (TNFA) signaling in BPDCN patients. It also found significant T-cell exhaustion, linked to T-cell clonotype expansion, offering insights into BPDCN’s immune interactions [6].
Characterization of each cell cluster in the current study
Cluster 1
Top genes expressed in cluster 1 are correlated with tumor spread, microenvironment, and prognosis. This suggested the molecular signatures’ link to BPDCN’s clinical traits.
Cluster 2
The gene profile of cluster2 was rich in oncogenesis and c-myc pathway genes, indicating a key role in tumorigenesis. It’s suggested that post-MYB activation, this initially unmarked cell group transforms into a mixed lineage phenotype with T-, B-, erythroid, and pDC traits, forming a diverse tumor cell population. This reflects the tumor microenvironment’s plasticity and the complex interaction of lineage and oncogenic factors in BPDCN.
Cluster 3
The top 10 genes expressed in cluster 3 are linked to dendritic cell development, highlighting their role in BPDCN’s pathogenesis and the complexity of cell differentiation and oncogenic signaling.
Cluster 4
The top 10 gene expressions in cluster 4 relate to T cell oncogenesis, tumor immunology, and dendritic cell functions, indicating a complex T cell role in BPDCN’s immunological and neoplastic processes.
Cluster 5
This cluster, mainly myeloid, did not seem to become tumorigenic. However, some of its most highly expressed genes were linked to oncogenesis and the tumor microenvironment, indicating that these cells, though not turning into tumor cells, are affected by BPDCN’s neoplastic processes. This reveals the myeloid compartment’s role in the disease’s tumorigenic landscape.
Cluster 6
This T cell population’s variable phenotype is influenced by BPDCN. Highly expressed genes in this group relate to cancer development, T cell activation, T-B cell communication, dendritic cell maturation, and cancer-associated pathways like C-MYB and NF-kappaB. This suggests a complex role for these T cells in BPDCN’s pathophysiology, potentially in immune response and tumor progression.
Cluster 7
The phenotype of cluster 7 indicates a tumor-influenced phenotype shift. Classified as a myeloid subset, it shows phenotypic changes due to tumorigenesis. Its top 10 genes are linked to immune regulation, cancer progression, and T and dendritic cell development, intersecting with Wnt and MYC pathways critical for cellular transformation and tumor maintenance. This reveals the myeloid compartment’s complex interaction with the BPDCN tumor microenvironment.
Cluster 8
This cell group, once without specific markers, has transformed in BPDCN, adopting a profile with T, B lymphoid, erythroid elements, and BPDCN markers, likely due to MYB oncogene activation. This indicates a new tumor-associated cell population. The top genes in this cluster relate to dendritic cell development, chromosomal stability, DNA damage response, TP53, and cell cycle regulation. These suggest a complex network in BPDCN’s pathogenesis, showing these genes’ roles in tumor-associated cell development and behavior.
Cluster 9
The phenotypic change of cluster 9 in BPDCN may due to the tumor suggests a role in modulating NK cell activity, with the top 10 gene expressions linked to NK cell function regulation, potentially impacting the immune response and BPDCN’s pathophysiology.
Cluster 10
The phenotype and top genes expression in Cluster 10 suggested a role in B cell-T cell interplay and BPDCN’s neoplastic process. This implicates Cluster 10 in disease progression and identifies potential B and T cell regulatory network targets.
Cluster 11
This suggests a shift towards a megakaryocyte-like state, with the role in tumorigenesis to be clarified. Top gene expressions of this cluster offered insights into cellular adaptations in BPDCN’s neoplastic landscape.
Cluster 12
This heterogeneous cluster shows a multilineage shift with tumor-associated markers, suggesting an immature cell population that changes in the tumor microenvironment. The top 10 gene expressions of Cluster 12 indicating a complex network influencing this cell population’s tumorigenic potential and behavior in BPDCN.
Based on the findings above, our study’s single-cell sequencing categorized cells into four main clusters: Cluster 1 as primitive plasmacytoid dendritic cells (pDCs); Cluster 2 and 3 as mixed lineage tumor-associated cells; and Cluster 8 as a tumor-associated cell population due to MYB activation. All clusters are MYB-positive but show varied expression of pDC markers, suggesting diverse cellular states and roles in BPDCN’s pathophysiology.
In the control group, Cluster 1 is marked by CD303, a mature pDC marker. In BPDCN, there’s a loss of CD303 with the gain of CD56 and MYB, indicating a reversion to an immature pDC stage due to the oncogenic MYB gene activation.
Cluster 2 lacks clear lineage markers in controls but shows significant immunophenotypic changes with MYB activation, gaining CD4, CD123, CD7, and Kappa/Lambda light chains, indicating a mixed lineage cell population emergence.
Cluster 3, a minor pDC group in controls, expresses CD4, CD123, CD303. In BPDCN, MYB activation leads to increased CD56 and other markers, indicating a large mixed phenotype tumor cell population.
Cluster 8, like Cluster 2, is unmarked in controls but gains CD4, CD123, CD303, CD56, and immunoglobulin light chains with MYB activation, showing a tumor-associated cell population with an aberrant phenotype. This highlights MYB’s significant role in BPDCN’s cellular differentiation and identity.
The study indicates that BPDCN’s tumor cellularity mainly consists of four unique cell populations, with some possibly evolving from pDCs. The origins of Cluster 2 and 8 from specific precursors are still unclear.
KEGG pathways
T cell receptor signaling pathway
The TCR signaling pathway is essential for activating T lymphocytes, key to the immune system’s ability to respond effectively. It starts with TCR binding to peptide-MHC complexes and is modulated by molecules like CD28, leading to T cell functions including proliferation and cytokine production.
In dendritic cells (DCs), maturation involves cytokine secretion like IL-12, crucial for T cell response initiation. Mature DCs also express chemokine receptors like CCR7, aiding migration to lymphoid tissues for immune response regulation. The interaction between TCR signaling and DC function is fundamental to the immune response’s coordination and effectiveness.
Natural killer cell mediate cytotoxity
NK cells are vital in the immune response to infections and diseases, with the ability to perform antibody-dependent cellular cytotoxicity (ADCC). They target IgG-coated “foreign” cells like virus-infected or cancerous cells by binding to the Fc portion of antibodies that have attached to the cells.
NK cells and dendritic cells (DCs) interact, which is crucial for initiating and coordinating adaptive immunity against cancer. This interaction has been associated with improved survival and responses to anti-PD-1 therapy in metastatic melanoma patients. Immature DCs are vulnerable to NK-cell cytolysis, while mature DCs are resistant, allowing NK cells to regulate DC function by eliminating immature DCs and activating other immune cells.
Hematopoietic cell lineage
Dendritic cells (DCs) are essential for the immune system, facilitating antigen presentation for immunity and tolerance. Originating from multipotent, self-renewing hematopoietic stem cells (HSCs), DCs contribute to the development of all blood cell types, including immune cells and components like erythrocytes and platelets.
DCs are present in various tissues, interacting with T and B cells to regulate innate and adaptive immune responses. Their communication with these cells is crucial for immune activation against pathogens and for maintaining self-tolerance.
Conclusion
The MYB-ZFAT fusion gene produces a 347-amino-acid protein, causing the native MYB gene’s inhibitory domain to be removed. This leads to uncontrolled MYB activation, a key abnormality in BPDCN’s development. The loss of this domain may trigger neoplastic change by disrupting the gene’s normal role, thus fueling the disease’s severity.
Single-cell sequencing shows BPDCN has a diverse cell population, contradicting the idea of a uniform cell type. The varied origins of tumor cells highlight the need for complex treatment strategies. In BPDCN patients with the MYB-ZFAT fusion, a significant cell subset with high MYB expression likely fuels the disease. Conversely, the smaller fraction without MYB expression mainly consists of non-BPDCN cells, indicating a more complex cellular makeup. Understanding this heterogeneity is crucial for developing targeted, personalized treatments.
The KEGG analysis points to key biological pathways in BPDCN tumorigenesis. The T cell receptor (TCR) signaling, vital for T lymphocyte activation, is a significant factor. The NK cell cytotoxicity pathway, important for immune defense against abnormal cells, is also involved in BPDCN. The hematopoietic cell lineage, involving blood cell development from stem cells, is linked to disease progression. Further research into these pathways’ interactions is needed to understand BPDCN’s pathogenesis. Targeting these processes could reveal new therapeutic approaches and improve treatment effectiveness.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
The authors would like to thank the patients for participation and coordination in this study.
Author contributions
Dr. Ruijuan Li and Dr. Wenyong Kuang performed main experiments. Miss Kexin Zhao and Miss Weiyi Fang helped with partial experiments. Dr. Wenyong Kuang provided patients’ sample. Dr. Haixia Yang and Dr. Benshan Zhang helped with diagnosis and treatment of patient. Dr. Zhao Cheng designed the study, analyzed data, and composed manuscript. Dr. Xianming Fu. provided the funding to support this study. Professor Hongling Peng supervised this study. All authors read and approved the final manuscript.
Funding
National Natural Science Foundation of China, 81400093 (Zhao Cheng); Natural Science Foundation of Hunan Province, 2018JJ3757 (Zhao Cheng); Natural Science Foundation of Hunan Province, 2021JJ30937 (Ruijuan Li); Natural Science Foundation of Hunan Province, 2023JJ60415 (Zhao Cheng); Scientifc Program of Health Commission of Hunan Province, 202203042723 (Ruijuan Li); Scientifc Program of Health Commission of Hunan Province, B202303046108 (Zhao Cheng); Scientifc Program of Health Commission of Hunan Province, 202104020628(Xianming Fu); Scientific Program of Health Commission of Hunan Province, 20200639 (Wenyong Kuang); Changsha Municipal Natural Science Foundation, kq2014234 (Ruijuan Li); Changsha Municipal Natural Science Foundation, kq2208319 (Zhao Cheng).
Data availability
No datasets were generated or analysed during the current study.
Declarations
Compliance with ethical standards
The experimental protocol was established according to the ethical guidelines of the Helsinki Declaration and was approved by the Human Ethics Committee of The Second Xiangya Hospital, Central South University. P.R. China. Written informed consent was obtained from individual or guardian participants.
Competing interests
The authors declare no competing interests.
Footnotes
Ruijuan Li and Wenyong Kuang contributed equally.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Zhao Cheng, Email: echocz@126.com.
Xianming Fu, Email: xianmingfu@csu.edu.cn.
References
- 1.Sweet K. Blastic plasmacytoid dendritic cell neoplasm: diagnosis, manifestations, and treatment. Curr Opin Hematol. 2020;27:103–7. 10.1097/MOH.0000000000000569. [DOI] [PubMed] [Google Scholar]
- 2.Cuglievan B, Connors J, He J, Khazal S, Yedururi S, Dai J, Garces S, Quesada AE, Roth M, Garcia M, et al. Blastic plasmacytoid dendritic cell neoplasm: a comprehensive review in pediatrics, adolescents, and young adults (AYA) and an update of novel therapies. Leukemia. 2023;37:1767–78. 10.1038/s41375-023-01968-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Renosi F, Callanan M, Lefebvre C. Genetics and epigenetics in neoplasms with plasmacytoid dendritic cells. Cancers (Basel). 2022;14. 10.3390/cancers14174132. [DOI] [PMC free article] [PubMed]
- 4.Suzuki Y, Kato S, Kohno K, Satou A, Eladl AE, Asano N, Kono M, Kato Y, Taniwaki M, Akiyama M, Nakamura S. Clinicopathological analysis of 46 cases with CD4(+) and/or CD56(+) immature haematolymphoid malignancy: reappraisal of blastic plasmacytoid dendritic cell and related neoplasms. Histopathology. 2017;71:972–84. 10.1111/his.13340. [DOI] [PubMed] [Google Scholar]
- 5.Booth CAG, Bouyssou JM, Togami K, Armand O, Rivas HG, Yan K, Rice S, Cheng S, Lachtara EM, Bourquin JP, et al. BPDCN MYB fusions regulate cell cycle genes, impair differentiation, and induce myeloid-dendritic cell leukemia. JCI Insight. 2024;9. 10.1172/jci.insight.183889. [DOI] [PMC free article] [PubMed]
- 6.DePasquale EAK, Ssozi D, Ainciburu M, Good J, Noel J, Villanueva MA, Couturier CP, Shalek AK, Aranki SF, Mallidi HR, et al. Single-Cell multiomics reveals clonal T-Cell expansions and exhaustion in blastic plasmacytoid dendritic cell neoplasm. Front Immunol. 2022;13:809414. 10.3389/fimmu.2022.809414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vardiman JW, Thiele J, Arber DA, Brunning RD, Borowitz MJ, Porwit A, Harris NL, Le Beau MM, Hellstrom-Lindberg E, Tefferi A, Bloomfield CD. The 2008 revision of the world health organization (WHO) classification of myeloid neoplasms and acute leukemia: rationale and important changes. Blood. 2009;114:937–51. 10.1182/blood-2009-03-209262. [DOI] [PubMed] [Google Scholar]
- 8.Patnaik MM, Lasho T, Howard M, Finke C, Ketterling RL, Al-Kali A, Pardanani A, Droin N, Gangat N, Tefferi A, Solary E. Biallelic inactivation of the retinoblastoma gene results in transformation of chronic myelomonocytic leukemia to a blastic plasmacytoid dendritic cell neoplasm: shared clonal origins of two aggressive neoplasms. Blood Cancer J. 2018;8:82. 10.1038/s41408-018-0120-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Suzuki K, Suzuki Y, Hama A, Muramatsu H, Nakatochi M, Gunji M, Ichikawa D, Hamada M, Taniguchi R, Kataoka S, et al. Recurrent MYB rearrangement in blastic plasmacytoid dendritic cell neoplasm. Leukemia. 2017;31:1629–33. 10.1038/leu.2017.101. [DOI] [PubMed] [Google Scholar]
- 10.Shumilov E, Mazzeo P, Ghandili S, Kunstner A, Weidemann S, Banz Y, Strobel P, Pollak M, Kolloch L, Beltraminelli H, et al. Diagnostic management of blastic plasmacytoid dendritic cell neoplasm (BPDCN) in close interaction with therapeutic considerations. Ann Hematol. 2024;103:1587–99. 10.1007/s00277-023-05587-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cazzato G, Capuzzolo M, Bellitti E, De Biasi G, Colagrande A, Mangialardi K, Gaudio F, Ingravallo G. Blastic plasmocytoid dendritic cell neoplasm (BPDCN): clinical features and histopathology with a therapeutic overview. Hematol Rep. 2023;15:696–706. 10.3390/hematolrep15040070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boddu PC, Wang SA, Pemmaraju N, Tang Z, Hu S, Li S, Xu J, Medeiros LJ, Tang G. 8q24/MYC rearrangement is a recurrent cytogenetic abnormality in blastic plasmacytoid dendritic cell neoplasms. Leuk Res. 2018;66:73–8. 10.1016/j.leukres.2018.01.013. [DOI] [PubMed] [Google Scholar]
- 13.Sakamoto K, Katayama R, Asaka R, Sakata S, Baba S, Nakasone H, Koike S, Tsuyama N, Dobashi A, Sasaki M, et al. Recurrent 8q24 rearrangement in blastic plasmacytoid dendritic cell neoplasm: association with immunoblastoid cytomorphology, MYC expression, and drug response. Leukemia. 2018;32:2590–603. 10.1038/s41375-018-0154-5. [DOI] [PubMed] [Google Scholar]
- 14.Sumarriva Lezama L, Chisholm KM, Carneal E, Nagy A, Cascio MJ, Yan J, Chang CC, Cherry A, George TI, Ohgami RS. An analysis of blastic plasmacytoid dendritic cell neoplasm with translocations involving the MYC locus identifies t(6;8)(p21;q24) as a recurrent cytogenetic abnormality. Histopathology. 2018;73:767–76. 10.1111/his.13668. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No datasets were generated or analysed during the current study.