Abstract
Purpose
Topical eye drops combining multiple antiglaucoma agents are often required when monotherapy fails to reduce intraocular pressure (IOP). However, their effectiveness is compromised by low bioavailability and poor patient compliance. To address these issues, we developed a novel gel/microsphere eye drop (GME) containing brimonidine and timolol, aimed at enhancing ocular bioavailability, reducing dosing frequency, and improving patient compliance.
Methods
The GME system comprises separate formulations of brimonidine-loaded and timolol-loaded polymer microspheres within a thermoresponsive hydrogel, enabling simple off-the-shelf preparation. We examined the in vitro drug release, compared the biodistribution with traditional eye drops, and assessed pharmacodynamic effects in New Zealand white rabbits and Dutch Belted rabbits.
Results
Brimonidine and timolol reached high concentrations in the aqueous and vitreous humor at 1 hour following eye drop administration, declining to nearly undetectable levels by 24 hours. The GME system at a similar dose extended the drug release to 27 days with initially lower drug levels but decreasing more slowly. The GME system also resulted in significantly lower plasma drug concentrations than eye drops, suggesting a reduced risk of systemic side effects. Neither the GME nor eye drops significantly reduced IOP in normotensive rabbits.
Conclusions
The GME system presents a promising alternative to traditional eye drops for controlled drug release in ocular applications, enabling sustained drug delivery while minimizing systemic exposure and thereby potentially enhancing the safety and efficacy of ocular therapies.
Translational Relevance
The GME system bridges basic research and clinical application by providing a controlled-release platform that sustains drug delivery, reduces systemic side effects, and enhances patient adherence.
Keywords: brimonidine, timolol, drug delivery, ocular biodistribution, controlled release
Introduction
Glaucoma is a neurodegenerative ocular disease that results in damage to the optic nerve and is commonly associated with increased intraocular pressure (IOP).1 The standard first-line treatment for glaucoma involves eye drops containing a single hypotensive agent, such as beta blockers like timolol, prostaglandin analogs like bimatoprost, alpha-adrenergic agonists like brimonidine, and carbonic anhydrase inhibitors like dorzolamide.2 However, due to the multifactorial nature of glaucoma, the initial single IOP-lowering agent often fails to achieve or maintain the targeted IOP long term. In a clinical trial for ocular hypertension treatment, 39.7% of participants were prescribed 2 or more topical medications after 60 months.3 Another study on patients newly diagnosed with glaucoma treated with beta-blockers found that after 2 years, 50% of the patients in the United States and 60% in Sweden needed more intensive therapies, such as combination treatments or surgery, as the initial monotherapy was not sufficiently decreasing IOP.4
Therefore, to enhance the effectiveness of topical medications in reducing IOP, it is often necessary to combine multiple antiglaucoma agents. This approach not only helps reduce the number of dosages and the amount of preservative for patient compliance improvement but also facilitates better control of IOP. For example, Combigan is a marketed combination ophthalmic solution that contains brimonidine and timolol. Brimonidine exhibits a dual mechanism for lowering IOP, which involves reducing the production of aqueous humor and stimulating its outflow.5 In contrast, timolol operates by decreasing the formation of aqueous humor to lower IOP.6 Clinical studies have demonstrated that Combigan (twice daily) is more effective in lowering IOP than monotherapy with either brimonidine (3 times daily) or timolol (twice daily).7–9
However, the effectiveness of glaucoma eye drops is hindered by poor bioavailability and patient compliance. The bioavailability of eye drops is poor because a significant portion of the medication is either immediately ejected from the eye or carried away by tear fluid and into the nasolacrimal duct, resulting in only about 5% of the medication reaching the intended target.10 This low absorption necessitates doses much higher than what is actually needed for therapeutic purposes, which can lead to local and systemic adverse effects initially followed by a rapid decline to suboptimal levels. To maintain the therapeutic effect of eye drops, treatment regimens typically involve frequent dosing, which is associated with poor patient adherence.11 Studies have shown that only 30% of patients with glaucoma adhere adequately, with most patients’ adherence rates falling below 70%, primarily due to the required frequency of eye drop administration.12–14
New topical drug delivery systems have been developed to overcome the limitations of conventional eye drops by enhancing ocular bioavailability, maintaining therapeutic efficacy longer, reducing dosing frequency, and improving patient compliance. Clinical studies on sustained-release drug delivery systems for brimonidine or timolol have primarily focused on implant-based approaches (ClinicalTrials.gov IDs: NCT00693485 and NCT06321562). Noninvasive delivery systems, such as the ocular ring (NCT02742649) and drug-eluting contact lenses (NCT02852057), have also been investigated; however, no further updates have been reported since the completion of the phase I clinical trials. Several brimonidine and timolol combination formulations have been developed in preclinical studies. For instance, a hybrid dendrimer hydrogel/polymer nanoparticle platform exhibited an in vitro release period of 28 to 35 days,15 an in situ gel system demonstrated a rapid and complete release within 8 hours,16 and cationic liposomes provided a 12-hour in vitro drug release.17 However, the pharmacokinetic studies evaluated in these publications were limited to a maximum of 7 days.
This study aimed to investigate the long-term biodistribution of brimonidine and timolol in aqueous humor, vitreous humor, and plasma in New Zealand white rabbits, utilizing a topically applied gel/microsphere eye drop (GME) containing both drugs at similar doses to a conventional eye drop. This dual drug delivery system was developed based on our previously validated system, which includes a thermoresponsive hydrogel carrier and drug-loaded polymer microspheres.18–21 In this study, we developed separate formulations of brimonidine-loaded polymer microspheres and timolol-loaded polymer microspheres. The two types of microspheres were then combined within the thermoresponsive hydrogel at the predetermined mass ratio, facilitating a simple off-the-shelf formulation of combinatory hypotensive therapeutics. Herein, we report the in vitro drug release profiles of both brimonidine and timolol from the GME, along with a biodistribution comparison between an eye drop formulation and GME at similar drug doses. We further compare our findings in New Zealand white rabbits with those obtained in Dutch Belted rabbits to enhance understanding of the underlying pharmacodynamics.
Methods
Microsphere Fabrication and Characterization
All materials were obtained from Millipore Sigma (St. Louis, MO, USA) unless otherwise noted. Brimonidine loaded microspheres were prepared by a modified solvent evaporation method.18,22 Then, 100 mg of poly lactic-co-glycolic acid (PLGA) (Resomer RG 502 H), and 100 mg of polylactic acid (PLA) (Resomer R 203 H) were dissolved in 0.5 mL of dichloromethane and 2 mL of trifluoroethanol. To this solution, an aqueous drug solution with 20 mg of brimonidine tartrate (Santa Cruz Biotechnologies, Santa Cruz, CA, USA) in 0.3 mL MilliQ water was added. This suspension was sonicated for 10 seconds at 30% amplitude (EpiShear Probe Sonicator; Active Motif, Carlsbad, CA, USA) and transferred into a beaker with 200 mL of 5% poly(vinyl alcohol) (Polysciences, Warrington, PA, USA) and homogenized for 1 minute at 7000 rpm (Silverson L5M-A, East Longmeadow, MA, USA). This emulsion was stirred at 400 rpm for 3 hours at room temperature to allow solvent extraction and evaporation for microsphere hardening. The microspheres were washed 3 times with MilliQ water prior to lyophilization for 48 hours (Benchtop Pro; SP Scientific, Warminster, PA, USA). Dry microspheres were stored at −20°C until use. Timolol loaded microspheres were prepared by the same procedure with 20 mg of timolol maleate.
To determine the drug loading capacity, 5 mg of drug-loaded microspheres were dissolved in 0.5 mL of 1N NaOH at 37°C, and then 0.5 mL of 1N HCl was added to neutralize the solution. The 5 mg of blank microspheres were used as the control. The resulting solution was diluted 2× with water and determined at a wavelength of 248 nm for brimonidine and 300 nm for timolol (SpectraMax M5; Molecular Devices, Sunnyvale, CA, USA).
Thermoresponsive Hydrogel Fabrication
Poly(N-isopropylacrylamide) (PNIPAAm) was prepared by free radical polymerization.20 Then, 100 mg of NIPAAm were added into 2 mL of 0.5 mg/mL of ammonium persulfate (APS) in MilliQ water, followed by 5 µL of tetramethylethylenediamine (TEMED) to initiate polymerization for 12 hours at 4°C. Residual TEMED and APS were removed by repeated phase transition cycling (T > 37°C) in MilliQ water. The purified polymer was flash frozen in liquid nitrogen, lyophilized for 48 hours, and stored at 4°C. The thermoresponsive hydrogel was prepared by rehydrating 500 mg of lyophilized PNIPAAm in 5 mL of MilliQ water with 500 µL of polyethylene glycol (MW 200 kilodalton [kDa]).
In Vitro Drug Release
Each GME sample, 3 mg of brimonidine loaded microspheres and 7 mg of timolol loaded microspheres in 100 µL PNIPAAm gel, was injected into a 1.5 mL centrifuge tube at 37°C. Then, 1 mL of 37°C phosphate buffered saline (PBS) was added to initiate release. Samples were rotated at 37°C during the study. At each interval, the supernatant was replaced with fresh 37°C PBS. Blank microspheres containing no drugs were used as the controls. Brimonidine and timolol were analyzed simultaneously by high-performance liquid chromatography (HPLC; Agilent 1220 Infinity LC).23 Separations were carried out using a Thermo Hypersil BDS C18 column (4.6 × 250 mm, 5 µm). The mobile phase contained phosphate buffer (pH 5) and acetonitrile in a ratio of 78/22 (v/v). The phosphate buffer was prepared by dissolving 2.5 g potassium monohydrogen phosphate and 2.5 g potassium dihydrogen phosphate into 800 mL MilliQ water, adjusting to pH 5.0 with 0.1 M phosphoric acid solution, diluting to 1000 mL with MilliQ water, and filtering through a 0.45 µm filter. The flow rate was 1 mL/min, and the injection volume was 20 µL. UV detection was set at 248 nm for brimonidine and 300 nm for timolol.
In Vivo Studies
The University of Pittsburgh’s Institutional Animal Care and Use Committee (IACUC) reviewed and approved the protocols for all animal studies, and these studies were carried out in accordance with the Association for Research in Vision and Ophthalmology (ARVO) Statement for the Use of Animals in Ophthalmic and Vision Research.
Four-month-old male New Zealand white and Dutch Belted rabbits were purchased from Envigo (Somerset, NJ, USA). Prior to the experiments, the nictitating membrane of the right eye (OD) of each subject was removed per established methods to better mimic the human eye and to allow for long-term retention of GME in the lower fornix.18,21 This procedure was performed under systemic anesthesia with 10 mg/kg of ketamine (Ketathesia; Henry Schein Animal Health, Dublin, OH, USA) and 1 mg/kg of xylazine (AnaSed Injection; Lloyd Laboratories, Shenandoah, IA, USA). A topical 0.5% proparacaine eye drop (Bausch + Lomb, Bridgewater, NJ, USA) was applied, followed by membrane removal with a scalpel and cauterization. Post procedure, one drop of 0.3% tobramycin (Bausch + Lomb, Bridgewater, NJ, USA) and 1% prednisolone acetate (Pacifc Pharmaceuticals Inc., Rancho Cucamonga, CA, USA) were immediately administered and repeated once daily for the following 4 days to prevent infection and control inflammation. After 7 days, baseline IOP was measured using tonometry (Tonovet Plus, Icare, Finland) without anesthesia for consecutive days before eye drop or GME administration.
The brimonidine/timolol eye drop was prepared according to the Combigan product insert by mixing 8 mg of brimonidine tartrate and 27.2 mg of timolol maleate in 4 mL MilliQ water with 0.2 mg of benzalkonium chloride. The osmolality was adjusted to 274 mOsmol/kg and the pH was adjusted to 6.6 by dibasic sodium phosphate heptahydrate, monobasic sodium phosphate monohydrate, and sodium hydroxide. Each GME sample was prepared by mixing 3 mg of brimonidine-loaded microspheres and 7 mg of timolol loaded microspheres in 100 µL of PNIPAAm gel. Rabbits randomly received either a single drop (30 µL) of brimonidine/timolol eye drop or a single dose of GME in OD. At predetermined timepoints, IOP was taken for both eyes. At the endpoint, 1 mL of whole blood was collected in an EDTA-containing tube, and then centrifuged at 3000 rpm for 15 minutes to collect plasma supernatant. Then, the rabbits were euthanized with an ear vein injection of Euthasol Solution (390 mg/mL Sodium Pentobarbital, 50 mg/mL Phenytoin Sodium) following systemic anesthesia with a combination of ketamine (10 mg/kg) and xylazine (1 mg/kg). Immediately after euthanizing, both eyes were enucleated and placed on dry ice. While frozen, the cornea was excised to access aqueous humor and vitreous humor.24 Samples were stored at −80°C.
A liquid chromatography-tandem mass spectrometry (LC-MS/MS) assay for the quantification of brimonidine and timolol was developed for plasma, aqueous humor, and vitreous humor. Briefly, an LC-MS/MS system consisting of a Thermo Scientific Vanquish ultra-high-performance liquid chromatography (UPLC) and TSQ Altis mass spectrometer that was equipped with a heated ESI (HESI) source was used while the SRM transitions used for quantitation were m/z 292.0 → 212.1 for brimonidine and m/z 317.2 → 261.2 for timolol. The 50 µL of the sample was protein precipitated using 200 µL of a 75:25 (acetonitrile: methanol) mixture containing internal standard (d4-brimonidine). The sample was briefly vortexed and then centrifuged at 10,000 × g for 5 minutes before the supernatant was transferred and analyzed by LC-MS/MS. Chromatographic separation of the samples was accomplished with a Waters Acquity BEH C18 (2.1 × 100 mm, 1.7 µm) column with an isocratic elution using 40:60 (A:B, v:v) of water with 10 mM ammonium formate (A) and acetonitrile (B) at a flow rate of 0.3 mL/min. The total runtime was 4 minutes. A standard curve was created for each run to ensure the results for each day were accurate. The linear range of the standard curves for both analytes was 0.05 to 500 ng/mL.
Statistical Analysis
Data are represented as the average ± standard deviation for three samples for loading capacity and in vitro release studies. For in vivo data, only average drug concentrations are shown, as some groups lack standard deviation due to the omission of data points with concentrations below the limit of detection. The Student's t-test was used with a two-tailed distribution to compare data from New Zealand white rabbits and Dutch Belted rabbits. One-way analysis of variance (ANOVA) followed by Dunnett's multiple comparisons test was performed on baseline IOP measurements to determine statistically significant differences. Statistically significant differences were designated by a significance criterion (P value) below 0.05.
Results
In Vitro Characterization of GME
Brimonidine-loaded and timolol-loaded microspheres were fabricated individually using a solvent evaporation method. The loading capacity of brimonidine was 30.9 ± 1.51 µg per mg of microspheres, and the loading capacity of timolol was 33.7 ± 2.37 µg per mg of microspheres (Fig. 1A). Therefore, in the GME sample comprising 3 mg of brimonidine-loaded microspheres and 7 mg of timolol-loaded microspheres in 100 µL of PNIPAAm gel, the total drug content was approximately 93 µg of brimonidine and 236 µg of timolol. In the in vitro cumulative drug release study (Fig. 1B), 72.1 ± 8.77 µg brimonidine was released by day 14, after which the release rate slowed, resulting in 79.3 ± 9.93 µg released by day 28 and a total of 79.5 ± 10.03 µg by day 35. In a similar pattern, timolol released 168.4 ± 12.79 µg by day 21, and the total release reached 172.5 ± 12.15 µg by day 28 and 173.6 ± 12.22 µg by day 35 as the release rate decreased. This indicates that a single 100 µL of GME delivered a dose comparable to a 30 µL drop of brimonidine/timolol eye drop solution, which contains 60 µg of brimonidine and 204 µg of timolol.
Figure 1.
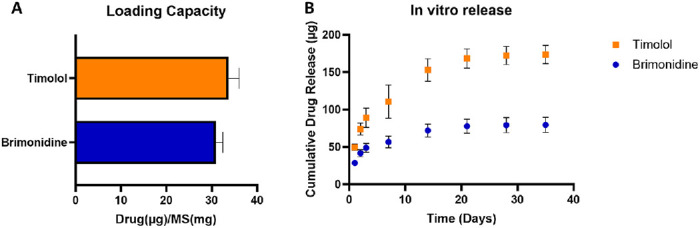
(A) Loading capacity of brimonidine and timolol loaded microspheres and (B) in vitro drug release from GME.
Ocular Biodistribution of Brimonidine and Timolol in New Zealand White Rabbits
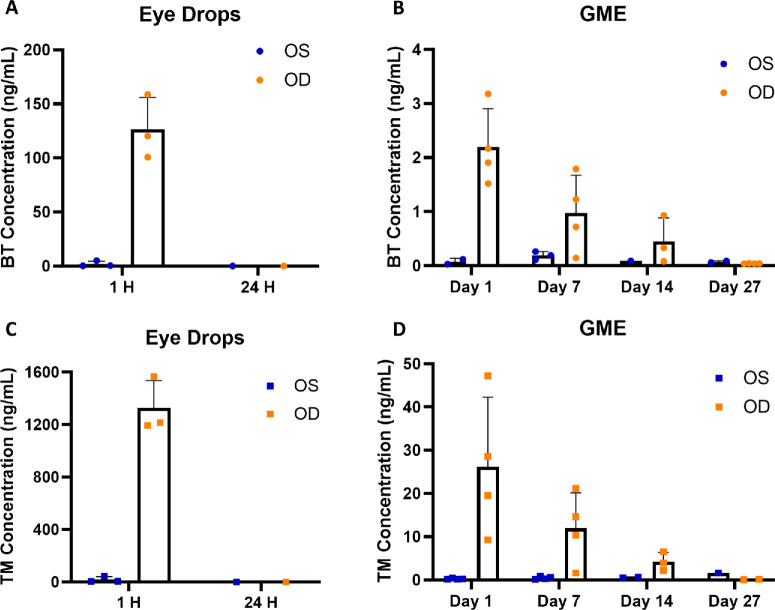
A single drop of brimonidine/timolol solution or GME was administered to the right eye (OD) of New Zealand white rabbits, whereas the left eye (OS) served as the control without treatment. Brimonidine and timolol concentrations in the aqueous humor and vitreous humor of both eyes were measured at various intervals post administration. In the eye drop group, in aqueous humor (Fig. 2A), brimonidine concentration in OD averaged 126 ng/mL at 1 hour and rapidly dropped to 0.11 ng/mL at 24 hours. In OS, brimonidine was 1.9 ng/mL at 1 hour and 0.04 ng/mL at 24 hours. A similar pattern was observed for timolol (Fig. 2C), the OD showed 1325 ng/mL at 1 hour, decreasing to 0.33 ng/mL at 24 hours. In the OS, timolol was 18.2 ng/mL at 1 hour and 0.14 ng/mL at 24 hours. However, in the GME treatment group, as shown in Figure 2B, brimonidine concentration in OD decreased gradually from 2.2 ng/mL at day 1 to 0.97 ng/mL at day 7, 0.45 ng/mL at day 14, and 0.04 ng/mL at day 27, matching levels were seen 24 hours post-eye drop administration. In the untreated OS, brimonidine levels rose from 0.075 ng/mL at day 1 to 0.19 ng/mL at day 7, and then fell to 0.09 ng/mL at day 14 and 0.072 ng/mL at day 27. Timolol followed a comparable pattern (Fig. 2D), where concentration in OD gradually decreased from 26.1 ng/mL at day 1 to 11.9 ng/mL at day 7, 4.2 ng/mL at day 14, and 0.11 ng/mL at day 27, comparable to levels 24 hours after eye drop administration. Timolol was also detected in OS, with its concentration increasing from 0.26 ng/mL at day 1 to 0.46 ng/mL at day 7, to 0.57 ng/mL at day 14, and to 1.62 ng/mL at day 27.
Figure 2.
Drug concentration (ng/mL) in aqueous humor. (A) Brimonidine concentration following eye drop administration. (B) Brimonidine concentration following GME administration. (C) Timolol concentration following eye drop administration. (D) Timolol concentration following GME administration. OS, untreated control left eye; OD, treated right eye; BT, brimonidine; TM, timolol. There were three rabbits for the eye drops group and four rabbits for the GME group, any drug concentration below the limit of detection was not shown.
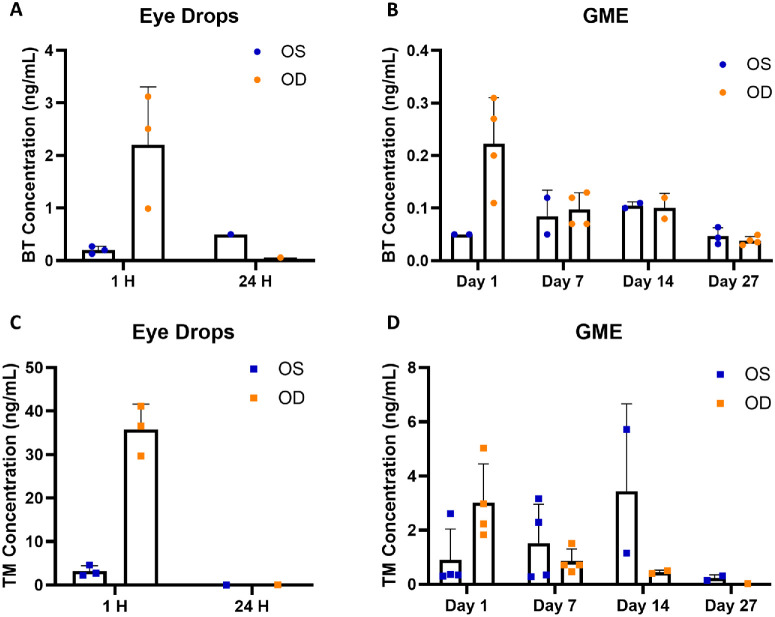
Both the eye drops and GME could deliver brimonidine and timolol to the vitreous humor through topical administration (Fig. 3). In the eye drop group, drug concentrations in vitreous humor followed a similar decline as seen in the aqueous humor. After 1 hour, brimonidine and timolol concentrations in OD in the eye drop group were significantly higher than the GME group. Specifically, brimonidine concentration decreased from 2.2 ng/mL at 1 hour to 0.06 ng/mL at 24 hours, whereas timolol concentration dropped from 35.8 ng/mL to 0.071 ng/mL. Both drugs were detected in OS at 1 hour with brimonidine at 0.2 ng/mL and timolol at 3.2 ng/mL. At 24 hours, brimonidine concentration was 0.5 ng/mL, whereas timolol was undetectable. In the GME group, brimonidine concentration in OD decreased from 0.22 ng/mL at day 1 to 0.10 ng/mL at day 7, to 0.10 ng/mL at day 14, and to 0.04 ng/mL at day 27. Timolol concentration in OD dropped from 3.0 ng/mL at day 1 to 0.85 ng/mL at day 7, to 0.45 ng/mL at day 14, and to 0.03 ng/mL at day 27. Interestingly, both brimonidine and timolol concentrations in OS increased initially before declining. Brimonidine concentration increased from 0.05 ng/mL at day 1 to 0.85 ng/mL at day 7, to 0.11 ng/mL at day 14, and decreased to 0.05 ng/mL at day 27. Timolol concentration increased from 0.91 ng/mL at day 1 to 1.52 ng/mL at day 7, to 3.44 ng/mL at day 14, and decreased to 0.23 ng/mL at day 27. A comprehensive analysis of the ocular biodistribution of brimonidine and timolol is provided in the Supplementary Material to gain a better understanding of the future dosing design of GME.
Figure 3.
Drug concentration (ng/mL) in vitreous humor. (A) Brimonidine concentration following eye drop administration. (B) Brimonidine concentration following GME administration. (C) Timolol concentration following eye drop administration. (D) Timolol concentration following GME administration. OS, untreated control left eye; OD, treated right eye; BT, brimonidine; TM, timolol. There were three rabbits for the eye drops group and four rabbits for the GME group, any drug concentration below the limit of detection was not shown.
Systemic Absorption of Brimonidine and Timolol in New Zealand White Rabbits
As shown in Figure 4, eye drop administration resulted in plasma concentrations of 0.84 ng/mL for brimonidine and 17 ng/mL for timolol at 1 hour post-administration. These finding are comparable to another study with eye drop solution of brimonidine and timolol.25 In contrast, GME maintained substantially lower plasma concentrations for both drugs, with all levels, except for a day 1 spike in timolol, remaining below 0.3 ng/mL throughout the study.
Figure 4.
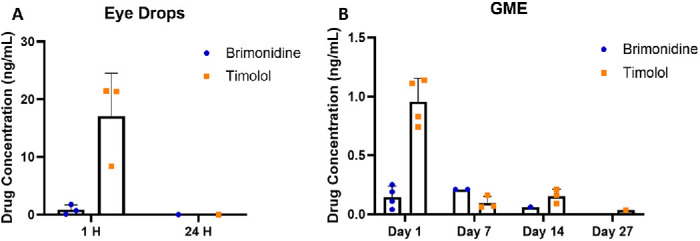
Drug concentration (ng/mL) in plasma following eye drop administration (A) and GME administration (B). There were three rabbits for the eye drops group and four rabbits for the GME group, any drug concentration below the limit of detection was not shown.
Pharmacokinetic in New Zealand White Rabbits Versus Dutch Belted Rabbits
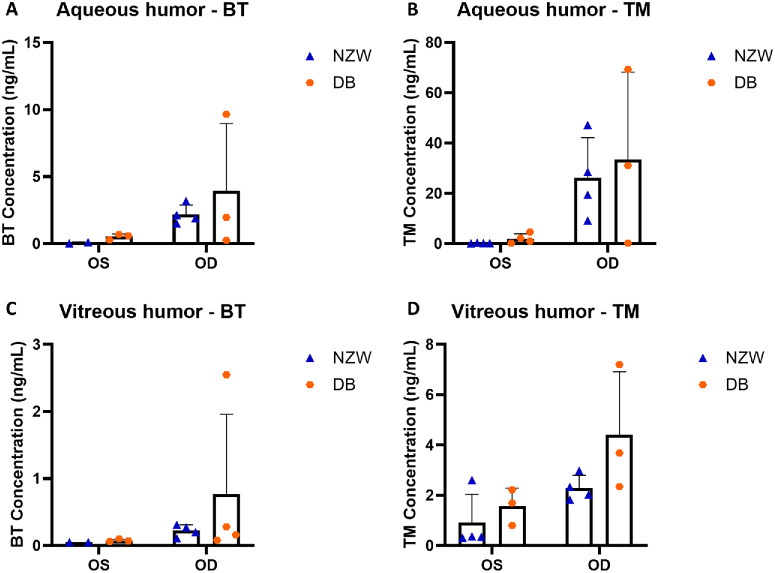
Following a 1-day administration of GME to the OD in both New Zealand white rabbits and Dutch Belted rabbits, the drug concentrations in the aqueous humor, vitreous humor, and plasma were assessed and compared. For both brimonidine and timolol, no significant differences were observed in aqueous humor or vitreous humor of either eye (Fig. 5). However, New Zealand white rabbits exhibited less variation in drug concentration in both the aqueous humor and vitreous humor compared with Dutch Belted rabbits. The only significant difference was in plasma concentration of timolol, with New Zealand white rabbits showing significantly higher levels than Dutch Belted rabbits (Fig. 6).
Figure 5.
Ocular drug concentration(ng/mL) after 1 day for different rabbit models. (A) Brimonidine concentration in aqueous humor. (B) Timolol concentration in aqueous humor. (C) Brimonidine concentration in vitreous humor. (D) Timolol concentration in vitreous humor. OS, untreated control left eye; OD, treated right eye; BT, brimonidine; TM, timolol; NZW, New Zealand white rabbits; DB, Dutch Belted rabbits. There were four rabbits in the group, any drug concentration below the limit of detection was not shown.
Figure 6.
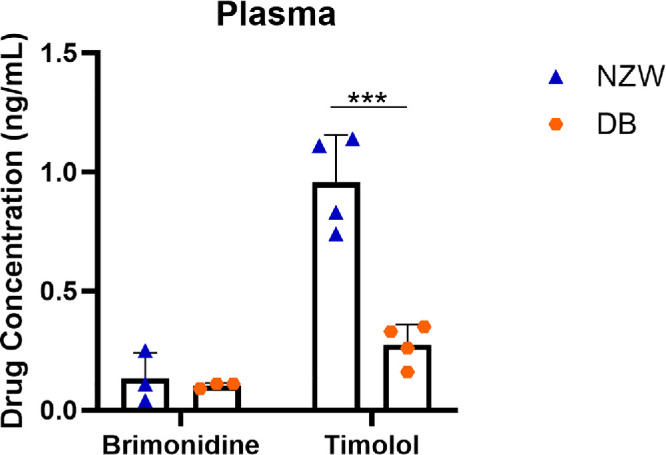
Drug concentration(ng/mL) in plasma after 1 day for different rabbit models. NZW, New Zealand white rabbits; DB, Dutch Belted rabbits. ***P < 0.001. There were four rabbits in the group, any drug concentration below the limit of detection was not shown.
Pharmacodynamic in New Zealand White Rabbits Versus Dutch Belted Rabbits
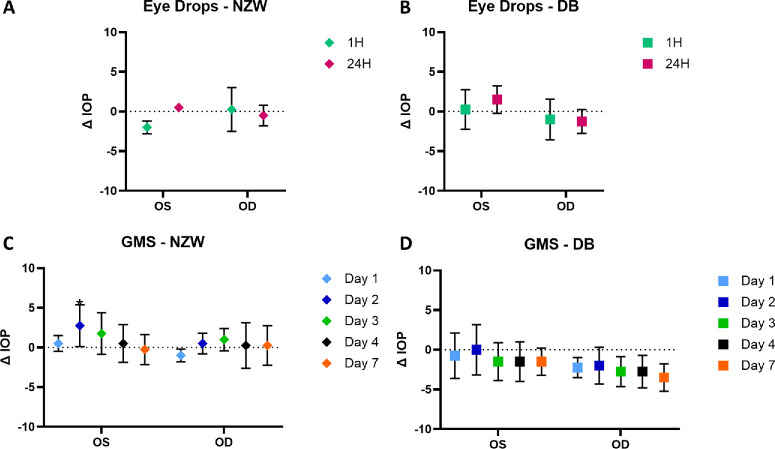
IOP was measured before and after administering drug delivery vehicles in OD. No significant IOP reduction was observed compared to baseline IOPs (Fig. 7). In New Zealand white rabbits, eye drop administration showed no IOP reduction after 1 hour. GME also exhibited no IOP reduction, and, in some cases, an increase in IOP was noted at day 2 (significant) and day 3 for both eyes. In Dutch Belted rabbits, there was an IOP reduction in both eyes 1 hour after eye drop administration, but it was not significantly different from the baseline. The GME group demonstrated a similar trend, with a slightly greater reduction in OD, although these changes were not significantly different from the baseline IOP.
Figure 7.
IOP change compared to baseline IOP. (A) IOP after eye drop administration in New Zealand white rabbits. (B) IOP after eye drop administration in Dutch Belted rabbits. (C) IOP after GME administration in New Zealand white rabbits. (D) IOP after GME administration in Dutch Belted rabbits. NZW, New Zealand white rabbits; DB, Dutch Belted rabbits; OS, untreated control left eye; OD, treated right eye. *P < 0.05. There were four rabbits in the group.
Discussion
Our dual drug delivery system, comprising drug-loaded microparticles in a thermoresponsive hydrogel, offers highly adjustable and extended drug release, easy application, and comfortable long-term topical retention. We have previously developed similar GME systems with various single drug-loaded polymer microspheres. The addition of drug-loaded microspheres had no impact on the thermoresponsive properties of PNIPAAm hydrogel with no change in lower critical solution temperature (LCST), where deswelling and gelation occur.18,19 A previous study also demonstrated the safety of the instillation technique and materials of GME, showing tolerability and transient effects resolving within 10 to 30 minutes. In addition, histological evaluation of rabbit eyelid tissue revealed no structural changes following GME application.21 In this study, the GME formulation was observed to remain in the lower fornix for up to 27 days. The presence of detectable levels of both brimonidine and timolol on day 27 suggests that the gel was not drug-depleted or a “ghost” formulation.
With similar drug doses, GME sustained brimonidine and timolol release for 27 days in both aqueous humor and vitreous humor in rabbits. In contrast, traditional eye drop formulation showed drug presence for less than 24 hours. Notably, previous studies have shown that topical brimonidine or timolol results in rapid ocular absorption, peaking within 1 hour and returning to zero after 5 to 6 hours.25–27 We believe that this study is the first to demonstrate a topical delivery system achieving over 2 weeks of drug presence in aqueous and vitreous humor, significantly surpasses traditional eye drop formulations or even prior noninvasive topical delivery systems, which lasted approximately 7 days in aqueous humor.15–17 Only a brimonidine implant has previously shown in vivo release beyond 30 days.28 This extended release ability of GME could improve patient compliance and treatment efficacy by reducing dosing frequency.
Interestingly, our study detected brimonidine and timolol in both the aqueous and vitreous humor of untreated OS, replicating the contralateral effect seen in previous rabbit and human studies using topical delivery methods for these drugs.29–32 However, this effect was absent with a polymer-based intraocular drug delivery implant, where brimonidine and timolol concentrations in the aqueous humor of untreated contralateral eyes were below quantification limits.28 The leading theory is that topically applied drugs are absorbed systemically through the nasolacrimal mucosa and transported to the contralateral eye via the bloodstream.31,33
Systemic absorption of brimonidine and timolol was assessed to evaluate their potential systemic side effects. Results suggested that the GME system significantly reduced the risk of systemic side effects compared with traditional eye drops, highlighting its potential as a favorable alternative for ocular drug delivery. Additionally, there is considerable potential to increase the drug dosage for the GME system without triggering systemic side effects, offering a promising strategy for optimizing ocular drug delivery.
Comparing drug concentrations in the aqueous humor, vitreous humor, and plasma of New Zealand white and Dutch Belted rabbits with GME showed similar ocular penetration and distribution. However, New Zealand white rabbits had significantly higher plasma timolol levels than Dutch Belted rabbits, consistent with a study reporting a 26% higher plasma timolol concentration in albino versus pigmented rabbits.34 No difference in plasma brimonidine levels was observed between the species. These results indicate that both rabbit models are suitable for ocular drug delivery studies, but the choice of model may affect systemic timolol exposure.
Our previous study demonstrated IOP reduction with brimonidine-loaded GME in normotensive New Zealand white rabbits. However, in this study, the inclusion of timolol may account for the lack of IOP reduction observed with the dual delivery of brimonidine and timolol, regardless of whether the administration was via eye drop or GME. Other studies have shown mixed results regarding timolol’s effect on IOP in normotensive rabbits. For example, timolol-loaded contact lenses reduced IOP in normotensive rabbits,35,36 whereas topical timolol (0.5% and 4%) had no significant effect.37 Additionally, timolol was found to be ineffective in lowering IOP during the light phase but effective during the dark phase of the circadian cycle. Timolol decreases IOP by reducing aqueous humor formation, which is regulated by cAMP, stimulated by endogenous catecholamines. The lack of IOP reduction with timolol in normotensive rabbits may be due to insufficient endogenous tonic stimulation or cAMP insensitivity.37–39 However, timolol has been effective in reducing IOP in rabbits with artificially elevated IOP.17,40,41 Therefore, using glaucomatous rabbits should be preferable for studies focused on evaluating the efficacy of IOP reduction. This study focused on ocular drug biodistribution; therefore, normotensive rabbits were selected instead of glaucomatous rabbits to exclude any effect of artificially elevated IOP on ocular drug biodistribution.
Overall, GME offers a promising alternative to traditional eye drops for controlled ocular drug delivery and minimizing systemic exposure, highlighting its potential for enhancing the safety and efficacy of ocular therapeutics. However, despite the extended 27-day release achieved with GME, drug concentrations in the aqueous and vitreous humor remained lower than peak levels observed with conventional eye drops. This outcome reflects the sustained release of GME and indicates that the current formulation may not provide immediate pharmacological effects. Future studies will investigate adjusted loading doses and dosing intervals (e.g. weekly) to maintain drug concentrations within a therapeutic window while preserving the advantages of reduced dosing frequency. In addition, the ability to mix and match microspheres within the hydrogel matrix offers flexibility in off-the-shelf formulating combination therapies for various medical conditions. For instance, the GME formulation can be designed for reconstitution immediately prior to use, with drug release occurring only upon administration. We are addressing key translational aspects, including formulation stability and standardization of the reconstitution-to-administration procedure, to ensure consistent dosing and prevent premature drug release during shelf life.
Supplementary Material
Acknowledgments
Supported by the Eye and Ear Foundation of Pittsburgh, an unrestricted grant from Research to Prevent Blindness, NIH CORE P30 EY008098, and UPMC Immune Transplant and Therapy Center (IPA 2019 No. 17). This project used the service of the University of Pittsburgh Small Molecule Biomarker Core facility, which was supported, in part, by the University of Pittsburgh Office of the Senior Vice Chancellor, Health Sciences, and the National Institutes of Health S10RR023461 and S10OD028540.
Author Contributions: Xin Fan: Conceptualization, Methodology, Validation, Investigation, Formal analysis, Writing – original draft; Phillip A. Harding: Writing – review & editing, Investigation; Parissa Ziaei: Methodology, Investigation; Ahmad B. Chaudhry: Investigation; Raymond E. West III: Methodology, Validation; Thomas D. Nolin: Methodology, Validation; Morgan V. DiLeo: Conceptualization, Writing – review & editing, Supervision, Funding acquisition.
Disclosure: X. Fan, None; P.A. Harding, None; P. Ziaei, None; A.B. Chaudhry, None; R.E. West III, None; T.D. Nolin, None; M.V. DiLeo, None
References
- 1. Jayaram H, Kolko M, Friedman DS, Gazzard G.. Glaucoma: now and beyond. Lancet. 2023; 402(10414): 1788–1801. [DOI] [PubMed] [Google Scholar]
- 2. Weinreb RN, Aung T, Medeiros FA.. The pathophysiology and treatment of glaucoma: a review. JAMA. 2014; 311(18): 1901–1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kass MA, Heuer DK, Higginbotham EJ, et al.. The Ocular Hypertension Treatment Study: a randomized trial determines that topical ocular hypotensive medication delays or prevents the onset of primary open-angle glaucoma. Arch Ophthalmol . 2002; 120(6): 701–713. [DOI] [PubMed] [Google Scholar]
- 4. Kobelt-Nguyen G, Gerdtham U-G, Alm A.. Costs of treating primary open-angle glaucoma and ocular hypertension: a retrospective, observational two-year chart review of newly diagnosed patients in Sweden and the United States. J Glaucoma . 1998; 7(2): 95–104. [PubMed] [Google Scholar]
- 5. Cantor LB. Brimonidine in the treatment of glaucoma and ocular hypertension. Ther Clin Risk Manag. 2006; 2(4): 337–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Coakes RL, Brubaker RF.. The mechanism of timolol in lowering intraocular pressure. In the normal eye. Arch Ophthalmol. 1978; 96(11): 2045–2048. [DOI] [PubMed] [Google Scholar]
- 7. Sherwood MB, Craven ER, Chou C, et al.. Twice-daily 0.2% brimonidine–0.5% timolol fixed-combination therapy vs monotherapy with timolol or brimonidine in patients with glaucoma or ocular hypertension: a 12-month randomized trial. Arch Ophthalmol . 2006; 124(9): 1230–1238. [DOI] [PubMed] [Google Scholar]
- 8. Frampton JE. Topical brimonidine 0.2%/timolol 0.5% ophthalmic solution: in glaucoma and ocular hypertension. Drugs Aging. 2006; 23(9): 753–761. [DOI] [PubMed] [Google Scholar]
- 9. Goñi FJ. 12-week study comparing the fixed combination of brimonidine and timolol with concomitant use of the individual components in patients with glaucoma and ocular hypertension. Eur J Ophthalmol. 2005; 15(5): 581–590. [PubMed] [Google Scholar]
- 10. Jumelle C, Gholizadeh S, Annabi N, Dana R.. Advances and limitations of drug delivery systems formulated as eye drops. J Control Release. 2020; 321: 1–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Fan X, Torres-Luna C, Azadi M, et al.. Evaluation of commercial soft contact lenses for ocular drug delivery: a review. Acta Biomater. 2020; 115: 60–74. [DOI] [PubMed] [Google Scholar]
- 12. Kholdebarin R, Campbell RJ, Jin YP, Buys YM.. Multicenter study of compliance and drop administration in glaucoma. Can J Ophthalmol. 2008; 43(4): 454–461. [DOI] [PubMed] [Google Scholar]
- 13. Rajurkar K, Dubey S, Gupta PP, John D, Chauhan L.. Compliance to topical anti-glaucoma medications among patients at a tertiary hospital in North India. J Curr Ophthalmol . 2018; 30(2): 125–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Newman-Casey PA, Robin AL, Blachley T, et al.. The most common barriers to glaucoma medication adherence: a cross-sectional survey. Ophthalmology. 2015; 122(7): 1308–1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Yang H, Tyagi P, Kadam RS, Holden CA, Kompella UB.. Hybrid dendrimer hydrogel/PLGA nanoparticle platform sustains drug delivery for one week and antiglaucoma effects for four days following one-time topical administration. ACS Nano. 2012; 6(9): 7595–7606. [DOI] [PubMed] [Google Scholar]
- 16. Taka E, Karavasili C, Bouropoulos N, et al.. Ocular co-delivery of timolol and brimonidine from a self-assembling peptide hydrogel for the treatment of glaucoma: in vitro and ex vivo evaluation. Pharmaceuticals. 2020; 13(6): 126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bigdeli A, Makhmalzadeh BS, Feghhi M, SoleimaniBiatiani E.. Cationic liposomes as promising vehicles for timolol/brimonidine combination ocular delivery in glaucoma: formulation development and in vitro/in vivo evaluation. Drug Deliv Transl Res . 2023; 13(4): 1035–1047. [DOI] [PubMed] [Google Scholar]
- 18. Fedorchak MV, Conner IP, Schuman JS, Cugini A, Little SR.. Long term glaucoma drug delivery using a topically retained gel/microsphere eye drop. Sci Rep . 2017; 7(1): 8639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bruk LA, Dunkelberger KE, Khampang P, et al.. Controlled release of ciprofloxacin and ceftriaxone from a single ototopical administration of antibiotic-loaded polymer microspheres and thermoresponsive gel. PLoS One. 2020; 15(10): e0240535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Jimenez J, Washington MA, Resnick JL, Nischal KK, Fedorchak MV.. A sustained release cysteamine microsphere/thermoresponsive gel eyedrop for corneal cystinosis improves drug stability. Drug Deliv Transl Res . 2021; 11(5): 2224–2238. [DOI] [PubMed] [Google Scholar]
- 21. Jimenez J, Resnick JL, Chaudhry AB, Gertsman I, Nischal KK, DiLeo MV.. Ocular biodistribution of cysteamine delivered by a sustained release microsphere/thermoresponsive gel eyedrop. Int J Pharm. 2022; 624: 121992. [DOI] [PubMed] [Google Scholar]
- 22. Bertram JP, Saluja SS, McKain J, Lavik EB.. Sustained delivery of timolol maleate from poly(lactic-co-glycolic acid)/poly(lactic acid) microspheres for over 3 months. J Microencapsul 2009; 26(1): 18–26. [DOI] [PubMed] [Google Scholar]
- 23. Baker MM, Belal TS.. Validated HPLC–DAD method for the simultaneous determination of six selected drugs used in the treatment of glaucoma. J AOAC Int . 2019; 101(4): 993–1000. [DOI] [PubMed] [Google Scholar]
- 24. Ahn SJ, Hong HK, Na YM, et al.. Use of rabbit eyes in pharmacokinetic studies of intraocular drugs. J Vis Exp . 2016; 113: e53878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Suzuki G, Kunikane E, Shinno K, Kozai S, Kurata M, Kawamura A.. Ocular and systemic pharmacokinetics of brimonidine and timolol after topical administration in rabbits: comparison between fixed-combination and single drugs. Ophthalmol Ther . 2020; 9(1): 115–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Acheampong AA, Small D, Baumgarten V, Welty D, Tang-Liu D.. Formulation effects on ocular absorption of brimonidine in rabbit eyes. J Ocul Pharmacol Ther . 2002; 18(4): 325–337. [DOI] [PubMed] [Google Scholar]
- 27. Huang J, Peng T, Li Y, et al.. Ocular cubosome drug delivery system for timolol maleate: preparation, characterization, cytotoxicity, ex vivo, and in vivo evaluation. AAPS PharmSciTech. 2017; 18(8): 2919–2926. [DOI] [PubMed] [Google Scholar]
- 28. Samy KE, Cao Y, Kim J, et al.. Co-delivery of timolol and brimonidine with a polymer thin-film intraocular device. J Ocul Pharmacol Ther . 2019; 35(2): 124–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Saari KM, Ali-Melkkilä T, Vuori M-L, Kaila T, Lisalo E.. Absorption of ocular timolol: drug concentrations and ß-receptor binding activity in the aqueous humour of the treated and contralateral eye. Acta Ophthalmol . 1993; 71(5): 671–676. [DOI] [PubMed] [Google Scholar]
- 30. Toris CB, Gleason ML, Camras CB, Yablonski ME.. Effects of brimonidine on aqueous humor dynamics in human eyes. Arch Ophthalmol . 1995; 113(12): 1514–1517. [DOI] [PubMed] [Google Scholar]
- 31. Piltz J, Gross R, Shin DH, et al.. Contralateral effect of topical β-adrenergic antagonists in initial one-eyed trials in the Ocular Hypertension Treatment Study. Am J Ophthalmol . 2000; 130(4): 441–453. [DOI] [PubMed] [Google Scholar]
- 32. Huupponen R, Kaila T, Salminen L, Urtti A.. The pharmacokinetics of ocularly applied timolol in rabbits. Acta Ophthalmol. 1987; 65(1): 63–66. [DOI] [PubMed] [Google Scholar]
- 33. Woodward DF, Dowling MC, Feldmann BJ, Chen J.. Topical timolol, at conventional, unilateral doses causes bilateral ocular β-blockade in rabbits. Exp Eye Res 1987; 44(2): 319–329. [DOI] [PubMed] [Google Scholar]
- 34. Urtti A, Salminen L.. A comparison between iris-ciliary body concentration and receptor affinity of timolol. Acta Ophthalmol. 1985; 63(1): 16–18. [DOI] [PubMed] [Google Scholar]
- 35. Maulvi FA, Lakdawala DH, Shaikh AA, et al.. In vitro and in vivo evaluation of novel implantation technology in hydrogel contact lenses for controlled drug delivery. J Control Release. 2016; 226: 47–56. [DOI] [PubMed] [Google Scholar]
- 36. Maulvi FA, Patil RJ, Desai AR, et al.. Effect of gold nanoparticles on timolol uptake and its release kinetics from contact lenses: in vitro and in vivo evaluation. Acta Biomater. 2019; 86: 350–362. [DOI] [PubMed] [Google Scholar]
- 37. Bartels SP, Roth HO, Jumblatt MM, Neufeld AH.. Pharmacological effects of topical timolol in the rabbit eye. Invest Ophthalmol Vis Sci. 1980; 19(10): 1189–1197. [PubMed] [Google Scholar]
- 38. Gregory DS. Timolol reduces IOP in normal NZW rabbits during the dark only. Invest Ophthalmol Vis Sci 1990; 31(4): 715–721. [PubMed] [Google Scholar]
- 39. Kamaruddin MI, Nakamura-Shibasaki M, Mizuno Y, Kiuchi Y.. Ocular hypotensive effects of a Rho-associated protein kinase inhibitor in rabbits. Clin Ophthalmol. 2017; 11: 591–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Dubey A, Prabhu P.. Formulation and evaluation of stimuli-sensitive hydrogels of timolol maleate and brimonidine tartrate for the treatment of glaucoma. Int J Pharm Investig. 2014; 4(3): 112–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Cuggino JC, Tártara LI, Gugliotta LM, Palma SD, Alvarez Igarzabal CI. Mucoadhesive and responsive nanogels as carriers for sustainable delivery of timolol for glaucoma therapy. Mater Sci Eng C Mater Biol Appl . 2021; 118: 111383. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.