Abstract
Usutu virus (USUV) is an emerging mosquito-borne flavivirus known to induce neuroinvasive disease in birds, mice, and humans in European and African countries. The mechanisms of infection and dissemination remain poorly understood. Thus, elucidating how USUV spreads in a susceptible host is crucial for identifying therapeutic targets. To investigate host defenses against USUV, we generated an infectious clone of the TC508 isolate. After characterizing its replication dynamics in cultured cells from multiple species, we investigated its pathogenesis in an array of mice with genetic perturbations. Previous studies demonstrated that whole-body deletion of type I interferon (IFN) signaling led to widespread USUV infection and fatality in mice. Here, we observed the same lethal phenotype in STAT1-deficient mice and identified hematopoietic cells specifically as central to USUV pathogenesis in a mammalian host. Deletion of STAT1 in all hematopoietic subsets, but not hepatocytes, neurons, macrophages or conventional dendritic cells, was sufficient for systemic viral dissemination and ultimate fatality. Conversely, mice lacking functional B, T, and natural killer (NK) cells but with intact myeloid cells were resistant to USUV. Our findings provide new insights into the tissue-specific barriers that regulate USUV infection and underscore the importance of innate immunity in host defense for this important emerging flavivirus.
Author summary
Mosquito-borne viruses are continual threats to human and animal health globally. With climate change, the geographic distribution of mosquitoes is projected to expand, leading to increased burden of their accompanying viruses. The flavivirus genus consists of well-known threats including yellow fever virus, Zika virus, dengue viruses, West Nile virus, and Japanese encephalitis virus as well as closely related, but understudied species, such as Usutu virus. Although most Usutu virus infections in humans are asymptomatic, symptoms of neuroinvasive disease in individuals have been recorded. Further, this virus is highly lethal in avian species and has caused massive die-offs of Eurasian blackbirds. Here, we investigated Usutu virus infection in various cell lines and mouse strains to gain insight into how this virus establishes infection in a susceptible host. Cell lines derived from humans, mice, chicken, and mosquitoes were all susceptible to Usutu virus. Immunocompetent mice were resistant to infection, but mice with disrupted innate immune signaling succumbed to lethal infection. Notably, intact innate immune signaling was essential in hematopoietic cells, but dispensable in liver and neuronal cells. Additionally, B, T, and natural killer cells were not necessary to restrict Usutu virus infection.
Introduction
Usutu virus (USUV) is an emerging flavivirus, which was first isolated in 1959 in Eswatini from Culex neavei mosquitoes [1]. USUV belongs to the Japanese encephalitis virus (JEV) serocomplex and is closely related to West Nile Virus (WNV), St. Louis encephalitis virus (SLEV), and Murray Valley encephalitis virus (MVEV). Currently, USUV is endemic in several European and African countries and was likely introduced into Europe by infected migratory birds [2]. Similar to WNV, it is maintained in the environment in an enzootic cycle between ornithophilic mosquito vectors, most commonly Culex pipiens, and avian amplifying hosts [3–6]. High pathogenicity and virulence of USUV has been observed in European wild birds, particularly Eurasian blackbirds (Turdus merula) [7–13]. USUV has been detected in a wide range of other animal hosts including human, bat, wild boar, tree squirrel, dog, horse, rat, shrew, and multimammate mouse, which are considered to be incidental hosts [2,14–20].
While the majority of USUV infections in humans are asymptomatic and have been retrospectively discovered through seroprevalence studies, such as blood donor screening [21–26], this virus has demonstrated its role as a human pathogen with a neuroinvasive capacity. In Italy in 2009, the first cases of USUV with neuroinvasive symptoms, including meningoencephalitis, distal resting tremors, dysmetria, and headache were observed in immunocompromised patients [27,28]. Subsequent neuroinvasive USUV infections have been predominantly recorded in immunocompromised patients, including one fatality [29]; however, there have also been instances of immunocompetent individuals with neurological presentation [30,31]. Separately, an ex vivo study demonstrated the ability of USUV to infect all three cell types comprising the human blood brain barrier in primary brain microvascular endothelial cells, astrocytes, and pericytes [32]. Albeit at a small scale compared to established flavivirus threats, USUV has clearly demonstrated a pathogenesis in humans. Understanding the mechanisms by which this virus causes disease in mammalian hosts is crucial for future research regarding vaccines or therapeutics and for preparedness efforts in the event that strains with increased human virulence emerge.
Utilizing small animal models, such as mice, is an invaluable method for studying viral pathogenesis. Adult immunocompetent mice are often resistant to flavivirus infection, including USUV; therefore, it is common to use immunocompromised mouse strains, such as mice with disrupted interferon signaling. Established mouse models to study USUV pathogenesis include suckling mice and interferon (IFN) alpha/beta receptor 1 deficient (Ifnar1-/-) mice. One group observed neuroinvasive USUV infection in 1-week-old outbred NMRI suckling mice marked by neuronal apoptosis and demyelination [33]. Another group found that Swiss suckling mice (4–7 days old) succumbed to lethal USUV infection and detected viral RNA in brain tissues, yet adult mice were resistant [34]. Ifnar1-/- mice also serve as a lethal infection model that can be used to study USUV neuropathogenesis [35,36] or to compare the virulence of different USUV strains [37–39].
The objective of the present study was to determine the role of innate immune signaling in controlling USUV spread in vivo. To accomplish this, we generated an infectious clone of the Vienna 2001 isolate, USUV TC508. We confirmed its infectivity in cell lines from several species, then proceeded with in vivo experiments. We determined that a variety of inoculation doses and injection routes caused uniform lethality in Ifnar1-/- and Stat1-/- mice. Notably, we found that mice lacking functional B, T, and natural killer (NK) cells were similarly resistant to infection compared to wild-type (WT) mice, which indicated the importance of innate immune responses rather than adaptive for controlling and overcoming USUV infection. Further, by generating and infecting tissue-specific STAT1 knockout (KO) mice, we discovered that functional IFN-mediated defenses in the hematopoietic compartment were essential for survival, yet dispensable in hepatocytes and neuronal cells. We also demonstrated that IFN-β treatment before or after USUV infection partially protected mice from lethality. Ultimately, we established a new mouse model to study USUV pathogenesis and narrowed down the key cellular reservoirs of USUV to hematopoietic cells that are not classical dendritic cells or macrophages.
Methods
Ethics statement
All animal experiments described in this study were performed in accordance with protocols that were reviewed and approved by the Institutional Animal Care and Use and Committee (IACUC) of Princeton University (number 3063). All mice were maintained in facilities accredited by the Association for the Assessment and Accreditation of Laboratory Animal Care (AAALAC). All work with infectious USUV was reviewed and approved by the Institutional Biosafety Committee of Princeton University (protocol registration number 1145).
Cell lines and antibodies
Hepa1-6 (CRL-1830), L929 (CCL-1), H2.35 (CRL-1995), AML12 (CRL-2254), NIH 3T3 (CRL-1658), and LMH (CRL-2117) cell lines were obtained from the American Type Culture Collection (ATCC, Manassas, VA). Huh7 (RRID:CVCL_0336), Huh7.5 (RRID:CVCL_7927) and STO5 (CRL-1503) cells were kindly provided by Dr. Charles Rice at The Rockefeller University. Hep-56.1D (RRID:CVCL_5769) cells were purchased from CLS Cell Line Service GmbH (Eppelheim, Germany). Aag2 cells were a kind gift from Dr. Raul Andino at the University of California, San Francisco. LMH cells were maintained in Waymouth’s medium (Thermo Fisher Scientific, Waltham, MA) supplemented with 10% (vol/vol) fetal bovine serum (FBS, Phoenix Scientific, Laguna Niguel, CA) and 1% (vol/vol) Penicillin-Streptomycin (P/S, Thermo Fisher Scientific, Waltham, MA). Aag2 cells were maintained in Leibovitz’s L-15 medium (Thermo Fisher Scientific, Waltham, MA) supplemented with 20% (vol/vol) FBS and 1% (vol/vol) P/S. All other cell lines were maintained in Dulbecco’s Modified Eagle Medium (DMEM) with high glucose and pyruvate (Thermo Fisher Scientific, Waltham, MA), which was supplemented with 10% (vol/vol) FBS and 1% (vol/vol) P/S. LMH cells were grown on plates coated with 0.1% (vol/vol) gelatin (MilliporeSigma, Burlington, MA) in Dulbecco’s phosphate-buffered saline (DPBS, Thermo Fisher Scientific, Waltham, MA) and incubated at 37°C with 5% CO2. Aag2 cells were incubated at 28°C without additional CO2. All other cell lines were incubated at 37°C with 5% CO2. All antibodies used in this study are listed below in Table 1.
Table 1. Primary and secondary antibodies used in this study for virus titration, flow cytometry, or histology staining.
| Antibody and stock concentration | Manufacturer | Identifier | Dilution | Final concentration |
|---|---|---|---|---|
| Anti-mouse Flavivirus group antigen (D1-4G2-4–15 (4G2)) (1 mg/mL) | Bio-Techne | NBP2–52709 | 1:100 (flow) 1:1000 (FFU) | 10 µg/mL (flow) 1 µg/mL (FFU) |
| Goat anti-mouse IgG (H + L) Cross-Adsorbed Secondary Antibody, HRP (1 mg/mL) | Thermo Fisher Scientific | G-21040 | 1:500 | 2 µg/mL |
| Goat anti-mouse IgG (H + L) Highly Cross-Adsorbed Secondary Antibody, Alexa Fluor 647 (1 mg/mL) |
Thermo Fisher Scientific | A-21236 | 1:250 | 4 µg/mL |
| Anti-rabbit Japanese encephalitis virus NS3 (1 mg/mL) | GeneTex | GTX125868 | 1:100 | 10 µg/mL |
| Anti-mouse CD45 monoclonal antibody (HI30) (0.5 mg/mL) | Thermo Fisher Scientific | 14-0459-82 | 1:100 | 5 µg/mL |
| CD68 monoclonal antibody (KP1) (0.2 mg/mL) |
Thermo Fisher Scientific | MA5–13324 | 1:50 | 4 µg/mL |
| Donkey anti-rabbit IgG (H + L) Highly Cross-Adsorbed Secondary Antibody, Alexa Fluor 488 (2 mg/mL) |
Thermo Fisher Scientific | A-21206 | 1:400 | 5 µg/mL |
| Donkey anti-mouse IgG (H + L) Highly Cross-Adsorbed Secondary Antibody, Alexa Fluor 546 (2 mg/mL) |
Thermo Fisher Scientific | A10036 | 1:400 | 5 µg/mL |
USUV reconstitution and preparation
The clinical isolate USUV TC508 was originally collected from an infected blackbird in Vienna, Austria in 2001 (GenBank: AY453411.1) and was kindly provided by Dr. Kenneth Plante at the World Reference Center for Emerging Viruses and Arboviruses (WRCEVA) at the University of Texas Medical Branch (UTMB) for this study. The lyophilized virus was resuspended in 1 mL of chilled DPBS and propagated once in Huh7.5 cells. Cell culture supernatant was collected at 48, 72, and 96 hours post-infection (hpi) and stored at 4°C between timepoints. Cellular debris was removed from the pooled supernatant using a 0.45-μm syringe filter. The filtered supernatant was subsequently concentrated using the Amicon Ultra-15 Centrifugal Filter Unit (MilliporeSigma, Burlington, MA) and aliquoted in cryovials for storage at -80°C.
Viral genome sequencing
We performed viral genome sequencing using an RNase H-based rRNA depletion method as described previously [40]. Following RNA extraction from filtered cell culture supernatant, we depleted rRNA with complementary DNA probes and RNase H and purified remaining RNA. To prepare sequencing libraries, we synthesized first-strand cDNA using random primers, synthesized second-strand cDNA using the Gubler-Hoffman method, tagmented the cDNA using the Nextera XT DNA Library Preparation Kit (Illumina, San Diego CA), and amplified cDNA fragments with indexing primers for 18 cycles of PCR. We pooled and sequenced libraries on MiSeq Micro v2 flowcells (Illumina, San Diego CA) with 2 x 151 bp reads. Following sequencing, we demultiplexed reads with Picard v2.25.6 from within viral-core v2.3.1. We performed reference-guided de novo viral genome assembly using viral-pipelines v2.1.33.14 [41,42]. Briefly, we removed human and bacterial contaminant reads, deduplicated the remaining reads, and filtered to genus-level (flavivirus) reads. We used these reads to assemble contigs, orient them relative to a reference genome, create a consensus genome assembly, and then re-map reads to the assembled genome to call intrahost single-nucleotide variants (iSNVs). The sequencing data was visualized using SnapGene ver. 8.0.1 (GSL Biotech LLC, Boston, MA). The sequencing data is available on GenBank at the BioProject accession PRJNA1263528.
Generation of an USUV infectious clone by circular polymerase extension reaction (CPER)
Circular polymerase extension cloning (CPEC) was originally established for assembling complex gene libraries [43]. In 2013, this method was first adapted for the generation of infectious flavivirus cDNAs and has since been utilized by several groups including our own [44–49]. Based on the empirically determined genomic sequence for USUV TC508, seven gBlock gene fragments were obtained (Integrated DNA Technologies (IDT), Newark, NJ) and cloned into pCR-Blunt II-TOPO vectors (Thermo Fisher Scientific, Waltham, MA). An additional fragment encoding the polyA signal, cytomegalovirus (CMV) promoter, and hepatitis delta virus ribozyme (HDVr) site was cloned into an eighth pCR-Blunt II-TOPO vector. The constructed plasmids were verified by Sanger sequencing (Eton Bioscience, San Diego, CA). In preparation for CPER, the eight fragments were generated by PCR using the Q5 High-Fidelity DNA Polymerase (New England BioLabs, Ipswich, MA) and primer pairs that have complementary ends listed in Table 2. Molar concentrations of each resulting fragment were calculated using the NEBioCalculator dsDNA Mass to Moles Converter (New England BioLabs, Ipswich, MA). To generate circular cDNA by CPER, the eight fragments were combined in equimolar concentrations (0.1 pmol each) and ligated using the PrimeSTAR GXL DNA polymerase (Takara Bio USA, San Jose, CA). The following PCR cycling was used: 98°C for 2 min, followed by 20 cycles of 10 s at 98°C, 15 s at 55°C, 12 min at 68°C, and a final extension step at 68°C for 12 min. The CPER product was stored at -20°C. For transfection, Huh7.5 cells were seeded at a density of 1e6 cells/well in a 6-well plate format. The CPER product was divided and transfected into two wells of the 6-well plate using the X-tremeGENE HP DNA Transfection Reagent (MilliporeSigma, Burlington, MA). After 24 h, the contents of the two transfected wells were combined and expanded in a 100 mm plate. Additionally, two wells of non-transfected Huh7.5 cells were similarly combined as a mock control. Cells were monitored daily for cytopathic effect (CPE). Each day post-transfection, the supernatant was aspirated and replaced with warmed DMEM containing high glucose and pyruvate + 2% (vol/vol) FBS + 0.2% (vol/vol) P/S. Beginning on the first day of observed CPE, supernatant was collected and stored at 4°C. For the following two days or until 80% of cells were lifted from the plate surface, supernatant was collected and pooled. On the final day, the pooled supernatant was centrifuged at 3,000 rpm at 4°C for 10 min. The supernatant was then passed through a 0.45-μm filter to remove cellular debris. The virus was kept on ice, aliquoted into cryovials, and stored at -80°C until future use.
Table 2. PCR primers for amplification of USUV fragments for infectious clone generation via circular polymerase extension reaction (CPER).
| Fragment # | Fragment components | Length (bp) | Primer names | F primer sequence (5’ to 3’) | R primer sequence (5’ to 3’) |
|---|---|---|---|---|---|
| 1 | 5’ UTR, C, prM, partial E | 2019 | F: PU-O-8811 R: PU-O-8812 | AGATGTTGGCCTGTGTGAGCTC | GAGATCGGAAAGTGATGCCAC |
| 2 | Partial E, NS1, partial NS2A | 1944 | F: PU-O-8813 R: PU-O-9147 |
GTGGCATCACTTTCCGATCTC | CATCCAAGCTGTGGCAGTGG |
| 3 | Partial NS2A, NS2B, partial NS3 | 2049 | F: PU-O-9148 R: PU-O-8816 |
CCACTGCCACAGCTTGGATG | CAGCACTAGCACTAGTGATTG |
| 4 | Partial NS3 | 726 | F: PU-O-8817 R: PU-O-8818 |
CAATCACTAGTGCTAGTGCTG | CGAGGAGCAAGAAAACTCCTG |
| 5 | NS4A, 2K, NS4B | 1120 | F: PU-O-8819 R: PU-O-9149 |
CAGGAGTTTTCTTGCTCCTCG | GCCTCTTTCCTGTACTTCAG |
| 6 | Partial NS5 | 1825 | F: PU-O-9150 R: PU-O-9151 |
CTGAAGTACAGGAAAGAGGC | CAATCACTCCTTCAGCTTCC |
| 7 | Partial NS5, 3’ UTR | 1506 | F: PU-O-9152 R: PU-O-8824 |
GGAAGCTGAAGGAGTGATTG | AGATCCTGTGTTCTTCTCCA |
| 8 | HDVr, CMV promoter | 1076 | F: PU-O-8825 R: PU-O-8826 |
GGTGGAGAAGAACACAGGATCTGGGTCGGCATGGCATCTCCACCTC | GAGCTCACACAGGCCAACATCTCGGTTCACTAAACGAGCTCTG |
Virus titration by focus-forming unit (FFU) assay
Huh7.5 cells were seeded in a collagen-coated 48-well plate at 80% confluency. The following day, the cells were incubated with 10-fold serial dilutions of the virus stock in DPBS for 2 h at 37°C. Each dilution was tested in triplicate. Following virus adsorption, inoculum was aspirated and warmed methylcellulose overlay solution (1% (vol/vol) methylcellulose, 1% (vol/vol) DMEM, 10% (vol/vol) FBS, 1% (vol/vol) P/S + 1.85 g NaHCO3) was added to each well. Three days later, the overlay medium was aspirated and the cell monolayer was washed twice using unsupplemented DMEM and twice using DPBS, followed by fixation with 4% (vol/vol) paraformaldehyde (PFA, MilliporeSigma, Burlington, MA) for 15 min at room temperature. Cells were then permeabilized with 0.2% (vol/vol) Triton X-100 in DPBS for 15 min, washed with PBS, then blocked with 0.2% (vol/vol) BSA in DPBS for 30 min. Each well was stained with pan-flavivirus anti-E primary antibody clone 4G2 (Bio-Techne, Minneapolis, MN) and incubated on a rotator for 1 h at room temperature. Following two washes with DPBS, cells were stained with goat anti-mouse IgG (H + L) cross-adsorbed HRP (Thermo Fisher Scientific, Waltham, MA) secondary antibody and incubated on a rotator for 1 h. All antibody dilutions and final concentrations can be found in Table 1. After two washes with DPBS, foci were visualized using DAB Substrate Kit, Peroxidase (HRP), with Nickel, (3,3’-diaminobenzidine) (Vector Laboratories, Burlingame, CA). Deionized water was used for the final wash, then the plates were laid face down on paper towels to dry. Finally, the number of foci per well was quantified and expressed as FFU per milliliter.
Flow cytometry
To determine the permissiveness and replication kinetics of USUV TC508 in different cell lines, cells were seeded in a 48-well plate at a density of 5e4 cells/well. Virus inoculation was performed with the indicated MOI for 2 h at 37°C. Following incubation, the inoculum was aspirated and replaced with 0.5 mL/well of DMEM containing high glucose and pyruvate + 10% (vol/vol) FBS + 1% (vol/vol) P/S. For replication kinetic characterization, cells were collected on 1–4 days post-infection (dpi). For testing the permissiveness of various murine cell lines to USUV, cells were only collected at 4 dpi. On collection days, cells were washed with DPBS, treated with 0.05% (vol/vol) trypsin in Hank’s balanced salt solution (Thermo Fisher Scientific), then quenched with supplemented cell culture medium. Following a wash with DPBS, cells were fixed in 4% (vol/vol) PFA. All samples were collected in triplicate. Uninfected wells for each cell line were also harvested and prepared for flow cytometry as mock controls. On staining days, cells were permeabilized using 0.1% (vol/vol) saponin + 1% vol/vol FBS in DPBS for 15 min at room temperature. Cells were stained with pan-flavivirus anti-E primary antibody clone 4G2 (Bio-Techne, Minneapolis, MN) and incubated for 1 h at 4°C covered in foil. Following two washes with DPBS, cells were stained with goat anti-mouse IgG (H + L) cross-adsorbed Alexa Fluor 647 (Thermo Fisher Scientific, Waltham, MA) secondary antibody. All antibody dilutions and final concentrations can be found in Table 1. Cells were then washed twice with PBS, resuspended in PBS, and transferred to flow cytometry cluster tubes. Samples were covered in foil until flow cytometry acquisition using the FACSymphony A3 with HTS Configuration (BD Biosciences, Franklin Lakes, NJ). The R-670 detector was used to determine the percentage of E-positive cells. Results were analyzed using FlowJo v10 software (BD Biosciences, Franklin Lakes, NJ).
Mouse strains
C57BL/6 (JAX stock #000664) [50], NRG (JAX stock #007799) [51], and mice expressing Cre recombinase under the control of the CMV (CMV-Cre, JAX stock #003465) [52], albumin (Alb-Cre, JAX stock #035593) [53], Vav1 (Vav-Cre, JAX stock #008610) [54] synapsin 1 (Syn-Cre, JAX stock #003966) [55], lysozyme 2 (LysM-Cre, JAX stock #004781) [56], or integrin alpha x gene (CD11c-Cre, JAX stock #008068) [57] promoters were commercially obtained from the Jackson Laboratory (Bar Harbor, ME). Mice deficient for type I IFN receptor (Ifnar1-/-) on a C57BL/6 genetic background were kindly provided by Dr. Sergei Kotenko at Rutgers New Jersey Medical School and generated as described previously [58]. Stat1fl/fl mice were kindly provided by Dr. Lothar Hennighausen (National Institute of Health, Bethesda, MD) [59] and backcrossed for 10 generations to the C57BL/6 background as previously described [60]. Stat1-/-, Vav-Cre/Stat1fl/fl, Alb-Cre/Stat1fl/fl, Syn-Cre/Stat1fl/fl, LysM-Cre/Stat1fl/fl, and CD11c-Cre/Stat1fl/fl mice were generated by intercrossing Stat1fl/fl and CMV-Cre, Vav-Cre, Alb-Cre, Syn-Cre, LysM-Cre, or CD11c-Cre mice, respectively. Vav-Cre/Stat1fl/fl, Alb-Cre/Stat1fl/fl, and Syn-Cre/Stat1fl/fl offspring were genotyped by PCR using Bullseye Taq-DNA Polymerase (Midwest Scientific) using primer combinations to distinguish wild-type and mutant alleles (available upon request). LysM-Cre/Stat1fl/fl, and CD11c-Cre/Stat1fl/fl mice were genotyped by the third party vendor (Transnetyx, Cordova, TN). All mice were bred in the Laboratory Animal Resource (LAR) Center of Princeton University.
Mouse infections and monitoring experiments
Adult (6- to 14-week-old) male and female mice were infected through subcutaneous injection via nape of the neck, intravenous injection via tail vein, intramuscular via hindlimb, intraperitoneal, or intradermal via footpad with doses ranging from 101–105 FFU of USUV TC508 diluted in DPBS to a final volume of 200 µL. Signs of disease progression were recorded through weight measurements and clinical scoring daily. Overall appearance was assessed using a clinical scoring matrix assigned as follows: 0, posture normal, appearance with smooth, shiny fur; 1, posture hunched, appearance with ruffled fur, loss of muscle tone, loss of weight; 2, posture hunched, trembling, shaky, loss of weight; 3, posture severely hunched, disheveled appearance, significant (greater than or equal to 20%) weight loss, buildup of white residue around the eyes; 4, death. Mice with weight loss greater than or equal to 20% were euthanized by CO2 chamber and their deaths were recorded for the following day.
Serum and tissue collection
Blood (ca. 200 µL) was collected through submandibular bleeding. Serum was separated from blood cells by centrifugation (3,500 rpm, 10 min, room temperature) and stored at -80°C in aliquots of 25 µL for later quantification of viremia. At the indicated endpoints, mice were anesthetized with ketamine/xylazine injection, and subject to cardiac puncture for a terminal blood collection. Spleen, liver, kidney, lung, and brain were harvested from mock or infected mice. Tissue sections were placed in 500 µL of RNAlater stabilization solution (Thermo Fisher Scientific, Waltham, MA) and stored at -80°C for downstream molecular analysis including RNA extraction and RT-qPCR. Tissue sections were placed in 3 mL of 10% neutral buffered formalin (MilliporeSigma) for 72 h at 4°C, then washed with DPBS and stored at 4°C in 5 mL of 70% (vol/vol) ethanol for downstream histological analysis.
RNA extraction from mouse serum and tissue
Tissue sections stored in RNAlater were thawed on ice. 10 mg of tissue was resuspended in 200 µL of MagMAX lysis/binding solution concentrate (Thermo Fisher Scientific, Waltham, MA) in a 2 mL microcentrifuge tube. A single 5-mm stainless steel bead (QIAGEN, Hilden, Germany) was placed into each tube containing tissue. Homogenization was accomplished using a TissueLyser LT (QIAGEN) set to 50 pulses per min for 2 min. After two rounds of homogenization separated by 2-min incubation on ice, the tubes were centrifuged at high speed (10,000 rpm) for 10 min at 4°C. The resulting supernatant was transferred to a 1.5-mL tube and used for viral RNA extraction. Viral RNA was isolated from 25 µL of serum or 10 mg of homogenized tissue using the MagMax mirVana Total RNA Isolation Kit (Thermo Fisher Scientific, Waltham, MA) and the KingFisher Flex machine (Thermo Fisher Scientific, Waltham, MA) according to manufacturer’s instructions. For each organ, two cuts (each 10 mg) were processed as biological replicates. For each sample, 30 µL of eluate was transferred to 0.2-mL strip tubes (USA Scientific, Ocala, FL) and subjected to DNase I treatment with Turbo DNase (Thermo Fisher Scientific, Waltham, MA). Lastly, viral RNA was purified by a 0.8x SPRI reaction using RNAClean XP beads (Beckman Coulter, Brea, CA). Purified RNA was stored in 0.2 mL strip tubes at -80°C until downstream RT-qPCR analysis.
Reverse transcription-quantitative polymerase chain reaction (RT-qPCR)
Viral RNA was quantified using single-step RT-quantitative real-time PCR (Luna Universal One-Step RT-qPCR Kit, New England BioLabs Inc., Ipswich, MA) according to manufacturer’s protocol. To detect USUV RNA, primers PU-O-5812 (CAAAGCTGGACAGACATCCCTTAC) and PU-O-5813 (CGTAGATGTTTTCAGCCCACGT) were designed complementary to a region in USUV NS5. For each reaction 2 µL of total RNA was used as sample input. An RNA standard with a stock concentration of 9.38e8 RNA copies/µL was used. Nine 10-fold serial dilutions were prepared from the undiluted standard and were used to create a standard curve from which RNA copies in each sample were determined. RT-qPCR amplification was performed using a StepOnePlus Real Time PCR System (Thermo Fisher Scientific, Waltham, MA) with the following settings: 55°C for 10 min; 95°C for 1 min; 40 cycles of 95°C for 10 sec and 60°C for 1 min; 95°C for 10 sec; 65°C for 10 sec 95°C for 10 sec; 50°C for 5 sec.
Mouse IFN-β treatment
Recombinant Mouse IFN-β1 (carrier-free) (BioLegend, San Diego, CA) was aliquoted upon arrival and stored at -80°C. To prepare injections, IFN-β1 was diluted in sterile DPBS and administered at a dose of 104 IU/200 uL via subcutaneous route. This dose was chosen based on the methods of previous groups [61–63].
Histological analysis
Freshly isolated mouse tissues were fixed in 4% (w/vol) paraformaldehyde for 24 h at 4°C, dehydrated, and embedded in paraffin. Tissue sectioning and paraffin processing was done by Saffron Scientific Histology Services, LLC (Illinois, USA). Sections of 5-µm thickness were obtained using a microtome. Tissue sections underwent staining with hematoxylin and eosin (H&E) using standard protocols. Stained slides were imaged using the Zeiss Axioscan 7 Microscope Slide Scanner.
Tissue immunostaining
Unstained tissue sections were deparaffinized in xylene and rehydrated through graded alcohol to deionized water. Antigen retrieval was achieved by steaming sections in citrate buffer (pH 6.0) at 95°C for 20 min. After cooling to room temperature, non-specific binding sites were blocked using 10% donkey serum in DPBS for 1 h at room temperature. Sections were then incubated with primary antibodies, including anti-JEV-NS3 (GeneTex, Irvine CA), anti-mouse CD45 (Thermo Scientific, Waltham, MA), and anti-mouse CD68 (Thermo Scientific, Waltham, MA), overnight at 4°C. After washing in PBS, slides were incubated with donkey anti-rabbit IgG (H + L) Highly Cross-Adsorbed Secondary Antibody, Alexa Fluor 488 or Alexa Fluor 546 (Thermo Scientific, Waltham, MA) secondary antibody for 1 h at room temperature. All antibody dilutions and final concentrations used are listed in Table 1. Cell nuclei were counterstained with 4’6-diamidino-2-phenylindole (DAPI; Thermo Scientific, Waltham, MA). Immunostaining images were acquired using a confocal laser scanning microscope (Zeiss LSM 880).
Statistical analyses
GraphPad Prism software (version 10) was used for statistical analysis. One sample t tests were used to compare quantitative data to the limit of detection (*P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001).
Results
Cell lines from several species are susceptible to USUV infection
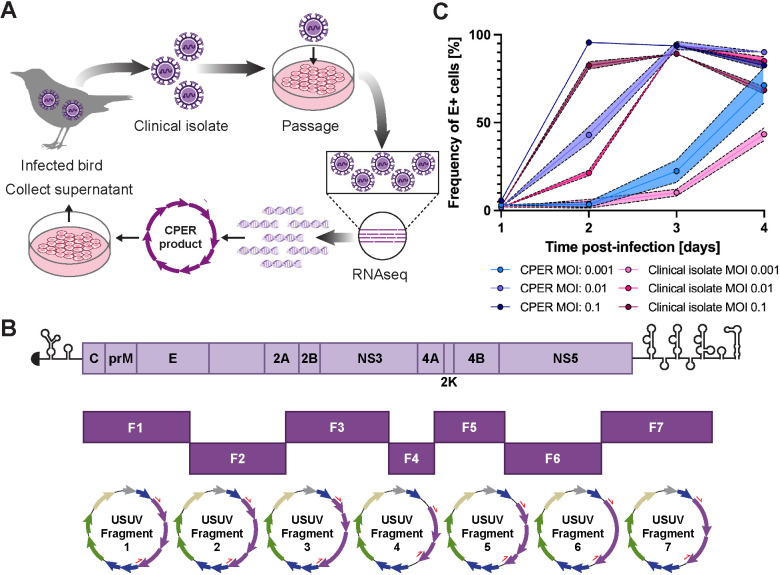
For this study, we generated an infectious clone of the Vienna 2001 isolate, USUV TC508. This isolate was originally obtained from an infected blackbird, minimally passaged in cell culture, then subjected to RNA sequencing (RNAseq). The USUV TC508 genome was subdivided into 7 fragments which were then combined using a circular polymerase extension reaction (CPER) [44,45]. Upon transfection into human Huh7.5 hepatoma cells, the resulting CPER products yielded full-length viral genomic transcripts driven by a CMV promoter with a native 3’ end produced by a hepatitis delta virus ribozyme (Fig 1A and 1B). We ensured the genome sequence of our CPER-derived virus matched the clinical isolate by RNAseq. Since hepatic damage is commonly observed during flavivirus infection and has been observed in USUV-infected birds and mice, we set out to characterize the replication kinetics of our CPER-derived USUV in cell lines originating from the liver of several species [7,12,37,67]. We first tested the permissiveness of a human (Huh7) hepatoma cell line to the CPER-derived USUV versus the original clinical isolate using three multiplicities of infection (MOI). We determined the percentage of infected cells by quantification of USUV envelope (E) antigen-bearing cells by flow cytometry. At all tested MOIs, we recorded similar replication kinetics between the CPER-derived virus and the clinical isolate; however, the CPER-derived virus was consistently higher (Fig 1C). At the lowest tested MOI (0.001), the percentage of E-positive cells was 3% at 1 and 2 dpi following exposure to the CPER-derived virus and increased to 22% on 3 dpi and 71% on 4 dpi. Similarly, using the clinical isolate, 23% of cells were E-positive on 1 and 2 dpi, which progressed to 10% on 3 dpi and rose to 43% on 4 dpi. Escalating to an MOI of 0.01, 2% of cells were E-positive at 1 dpi using the CPER-derived virus, which grew to 43% by 2 dpi, peaked to 94% at 3 dpi, and decreased to 90% at 4 dpi due to cytopathic effect (CPE) and cell death. Using the clinical isolate at the same MOI, the frequency of E-positive cells increased from 2–21% at 1–2 dpi, peaked to 94% at 3 dpi, and fell to 85% at 4 dpi. At the highest tested MOI (0.1), infection progressed quickly between 1–2 dpi as we detected an increase in E-positive cells from 6 to 96% using the CPER-derived virus or 2–83% using the clinical isolate. Infection rates remained high as 94% and 83% of CPER-derived virus-infected cells were E-positive on 3–4 dpi and 89% and 68% of clinical isolate-infected cells were E-positive on 3–4 dpi. Ultimately, we found that the intermediate MOI of 0.01 provided the most steady USUV growth kinetics from 1–4 dpi. Using the higher MOI of 0.1, the vast majority of cells were infected by 2 dpi and then decreased by 4 dpi due to cell death. Using the lower MOI of 0.001, peak infection was likely not achieved; however, we did not want to extend the experiment due to cell overgrowth. Moving forward, we proceeded with using an MOI of 0.01 to test the susceptibility of other cell lines.
Fig 1. A genetically-defined infectious clone of USUV TC508 was generated using the circular polymerase extension reaction (CPER) and remains infectious in cell culture.
(A) Schematic representation of the workflow for generating an USUV infectious clone from an avian clinical isolate. Some figure elements (bird [64], cells [65], and DNA fragments [66]) were sourced from the public domain and are listed as references. (B) Schematic representation of the USUV genome and its division into seven fragments used for CPER. Each fragment was inserted into pCR-Blunt II-TOPO vectors. The purple arrows represent the USUV genes. Other vector components include lacZa (blue), ccdB (blue), antibiotic resistance markers (green), ori (sand), and the lac promoter (gray). The red half arrows indicate the PCR primer binding sites. (C) Replication kinetics of the USUV TC508 infectious clone in human hepatoma (Huh7) cells 1–4 days post-infection (dpi) using a multiplicity of infection (MOI) of 0.001, 0.01, or 0.1. The percentage of infected cells was quantified based on envelope (E) antigen staining detected by flow cytometry. Shading indicates standard error of the mean.
Since mice serve as a common animal model for flavivirus research and birds are the amplifying host of USUV, we next determined the replication kinetics of USUV in hepatoma cell lines from these species, which had not been previously done. Additionally, to model replication in the vector, we tested the permissiveness of the embryonic mosquito cell line Aag2, which had also not been used for USUV studies. We previously saw robust infection of Huh7 cells using MOIs 0.001, 0.01, and 0.1, but captured the best steady increase in infection using the intermediate MOI of 0.01; therefore, we chose to proceed with an MOI of 0.01. Chicken hepatoma cells (LMH) had a steady progression of infection from 0% to 3% at 1–2 dpi, 22% at 3 dpi, and 67% at 4 dpi. Notably, mouse hepatoma cells (Hepa1–6) were non-permissive to USUV infection and mosquito cells (Aag2) demonstrated low permissiveness (11% infection) to USUV by 4 dpi (Fig 2A). Before proceeding with in vivo experiments, we were curious if we could find a murine cell line that was permissive to USUV. Thus, we infected the murine hepatocellular carcinoma cell lines H2.35, AML12, and Hep-56.1D and the murine fibroblast cell lines STO5, NIH 3T3, and L929, then evaluated the frequencies of E antigen-bearing cells on 4 dpi (Fig 2B). We chose this timepoint since we knew a high frequency of cells should be E-positive by 4 dpi based on our replication kinetics experiment in Huh7 cells and did not want to choose a later timepoint in which cell death or overgrowth could impede results. Hep-56.1D and STO5 cells were minimally permissive, reaching 10–15% infection at 4 dpi, while H2.35, AML12, and NIH 3T3 appeared to be resistant to USUV. L929 fibroblasts supported a comparable level of USUV infection to Huh7 and LMH cells, reaching 63% at 4 dpi. Collectively, these data demonstrate that the CPER-produced virus is infectious across multiple cell lines from various species and tissues. Further, we determined the growth kinetics of USUV in L929 cells from 1–4 dpi (Fig 2C). E-positive cells were first detected at 2 dpi and their frequency was 5%. This rose to 58% on 3 dpi and 63% on 4 dpi.
Fig 2. USUV TC508 has variable Infection kinetics in murine, avian, and mosquito cell lines.
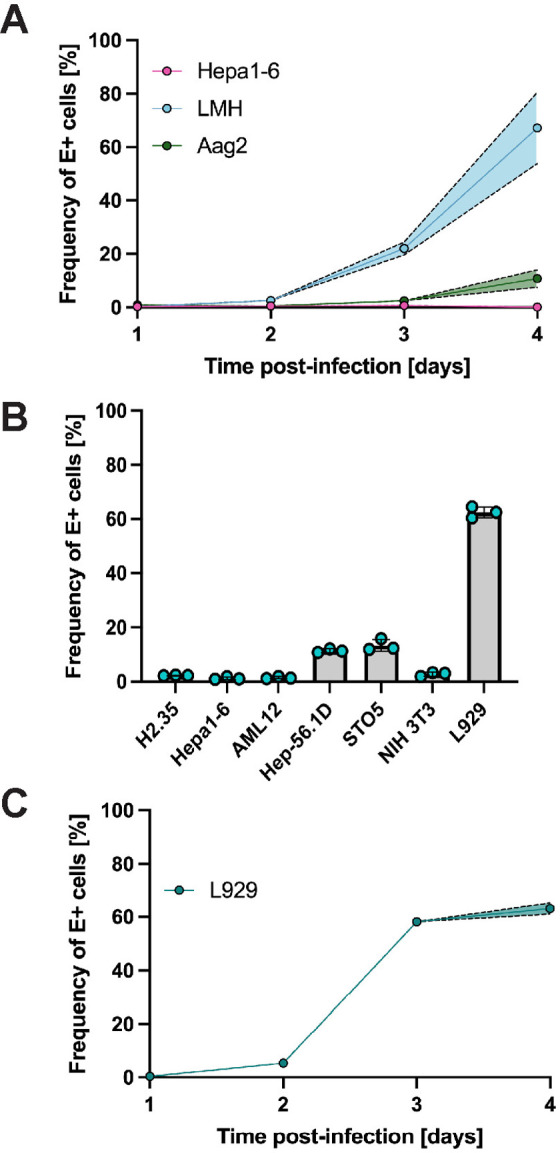
(A) Percent of E antigen-positive cells in mouse hepatoma (Hepa1-6), chicken hepatoma (LMH), and embryonic mosquito cells (Aag2) 1–4 days post-infection (dpi) using a multiplicity of infection (MOI) of 0.01. Shading indicates standard error of the mean. (B) Percent of E antigen-positive cells in various murine cell lines at 4 dpi using an MOI of 0.01. (C) Percent of E antigen-positive cells in mouse fibroblasts (L929) 1–4 dpi using an MOI of 0.01. Shading indicates standard error of the mean.
Type I interferon receptor-deficient and STAT1-deficient mice succumb to lethal USUV infection by low or high inoculation dose
To determine if our USUV infectious clone was pathogenic in vivo, we subcutaneously injected mice with disrupted IFN signaling (Ifnar1-/- and Stat1-/-) with five inoculation doses ranging from 101–105 focus-forming units (FFU) per mouse. On a daily basis, we measured change in weight, assessed clinical signs (ruffled fur, hunched posture, loss of muscle tone, decreased mobility, trembling, buildup of white residue around the eyes), and euthanized mice that reached ≤ 80% of their initial weight measured before infection.
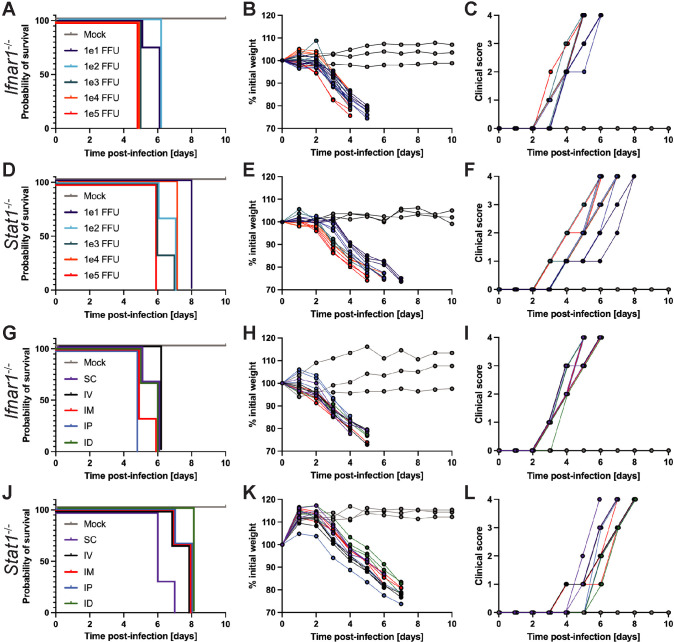
Regardless of the dose, all Ifnar1-/- and Stat1-/- mice experienced incremental weight loss, increasing clinical signs, and ultimately succumbed to lethal infection between 5–6 dpi and 6–8 dpi, respectively (Fig 3A–3F), in sharp contrast to wild-type mice of which 100% survived infection with 103 FFU of USUV. In Ifnar1-/- mice, all animals that were inoculated with 101–102 FFU of USUV died by 6 dpi while animals that received 103–105 FFU of USUV died by 5 dpi. At all doses tested, Ifnar1-/- mice succumbed to lethal infection sooner than Stat1-/- mice. In Stat1-/- mice, animals that received the highest dose (105 FFU) died by 6 dpi, animals injected with the lowest dose (101 FFU) died by 8 dpi, and those injected with 102–104 FFU of USUV died within this range. Previous studies established Ifnar1-/- mice as a lethal model for USUV infection and noted clinical signs such as lethargy, progressive weight loss, ruffled fur, and hunching [35–39]. In addition to confirming that Ifnar1-/- mice could be used as a model for our USUV infectious clone and exhibited similar disease progression to other groups, we established Stat1-/- mice as a new model for USUV investigation, and determined 103 FFU as the optimal inoculation dose to observe uniform lethality at an early time point while also conserving the amount of virus used.
Fig 3. USUV infection is lethal in mice with compromised type I interferon or STAT1-mediated antiviral defenses regardless of inoculation dose or route.
The inoculation doses tested ranged from 101 to 105 focus-forming units (FFU), all of which were administered via subcutaneous (SC) route. The inoculation routes tested were SC, intravenous (IV), intramuscular (IM), intraperitoneal (IP), and intradermal (ID) using a consistent inoculation dose of 103 FFU. (A) Survival, (B) weight changes, and (C) clinical scoring of Ifnar1-/- mice following inoculation with varying doses of USUV via SC injection (101 FFU, n = 4; 102 FFU, n = 4; 103 FFU, n = 7; 104 FFU, n = 3; 105 FFU, n = 3). (D) Survival, (E) weight changes, and (F) clinical scoring of Stat1-/- mice following inoculation with varying doses of USUV via SC injection (n = 3/group). (G) Survival, (H) weight changes, and (I) clinical scoring of Ifnar1-/- mice following inoculation with 103 FFU of USUV by various routes (n = 3/group). (J) Survival, (K) weight changes, and (L) clinical scoring of Stat1-/- mice following inoculation with 103 FFU of USUV by various routes (n = 3/group).
USUV exposure by various inoculation routes causes lethal infection in Ifnar1-/- and Stat1-/- mice
To test if lethality was dependent on the route of inoculation, we inoculated Ifnar1-/- and Stat1-/- mice with 103 FFU of USUV via subcutaneous (SC), intravenous (IV), intramuscular (IM), intraperitoneal (IP), or intradermal (ID) injection. Regardless of the inoculation route, 100% of Ifnar1-/- and Stat1-/- mice succumbed to lethal USUV infection by 6 and 8 dpi, respectively (Fig 3G and 3J). Death of Ifnar1-/- mice inoculated via SC, IM, IP, or ID routes was first observed at 5 dpi, but 100% of IV-inoculated mice died at 6 dpi. In Stat1-/- mice, lethality was observed most quickly following SC inoculation (6–7 dpi) while the longest time until death was observed by ID inoculation (8 dpi). Stat1-/- mice inoculated via IV, IM, or IP all succumbed to lethality between 7–8 dpi. Following infection, incremental weight loss (Fig 3H and 3K) and additional clinical signs listed previously were recorded in Ifnar1-/- and Stat1-/- mice (Fig 3I and 3L). Ultimately, no specific inoculation route was associated with increased or decreased USUV pathogenesis and we used the SC injection route for all subsequent experiments.
STAT1-mediated IFN signaling in the hematopoietic compartment is essential for restricting USUV infection
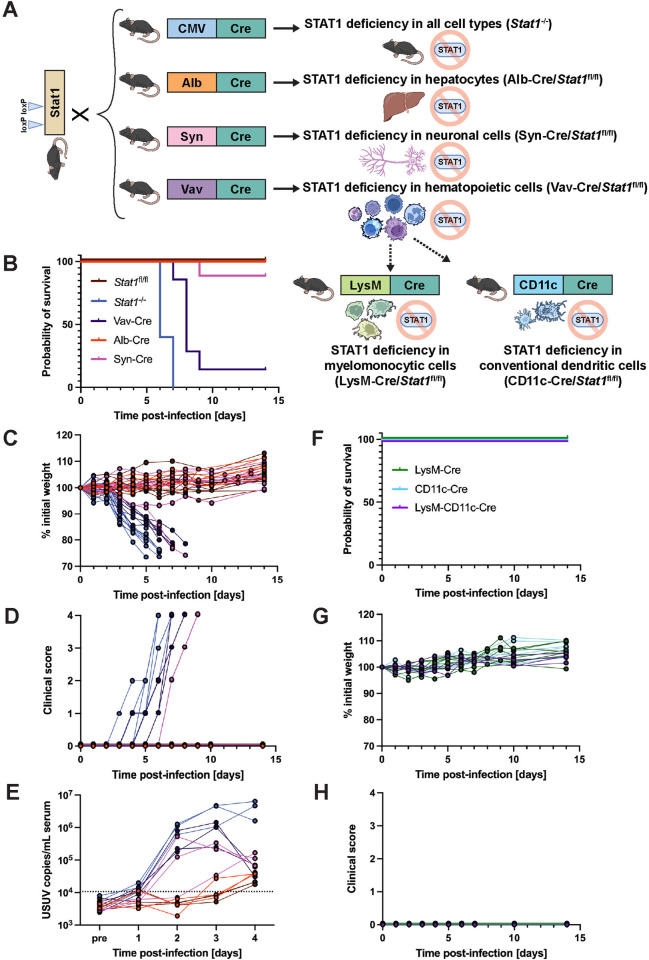
To evaluate whether STAT1 was required throughout all tissues or just in certain compartments, we generated three tissue-specific STAT1 KO mice and tested their susceptibility to USUV. We chose to generate hematopoietic cell-specific STAT1 KO mice because a previous study from our group determined that these mice were hypersusceptible to infection with other orthoflaviviruses, such as yellow fever virus strain 17D, and we hypothesized that YFV and USUV may have a similar reliance on STAT1-mediated signaling in hematopoietic cells [60]. Next, we chose to generate hepatocyte-specific STAT1 KO mice since USUV has been recorded to cause hepatomegaly and liver necrosis in birds and mice [7,12,37]. Lastly, we chose to generate neuronal-cell specific STAT1 KO mice due to the demonstrated neuroinvasive potential of USUV in humans, mice, and birds [30,33,36,67]. To generate these mice, we crossed mice that harbored the Cre recombinase gene downstream of a tissue-specific promoter (Vav-Cre for hematopoietic cell expression [68], Alb-Cre for hepatocytes [53], Syn-Cre for neuronal cells [55]) to mice with loxP sites flanking exons 1 and 3 of the Stat1 gene (Stat1fl/fl) [59] (Fig 4A). We then compared the USUV susceptibility of these mouse strains to wild-type mice (Stat1fl/fl) or full body STAT1 KO mice (Stat1-/-), which exhibited 100% survival or 100% lethality, respectively. While Alb-Cre/Stat1fl/fl and Syn-Cre/Stat1fl/fl mice had high survival rates of 100% and 89%, respectively, only 17% of Vav-Cre/Stat1fl/fl mice survived after 9 dpi (Fig 4B). In the Vav-Cre/Stat1fl/fl mice and the singular Syn-Cre/Stat1fl/fl mouse that succumbed to lethal USUV infection, we observed incremental weight loss and the development of the same clinical symptoms as recorded in Stat1-/- mice, albeit at a slightly delayed timeline (Fig 4C and 4D). While Stat1-/- mice died between 6–7 dpi, lethality in Vav-Cre/Stat1fl/fl mice occurred between 7–9 dpi and occurred at 9 dpi for the singular Syn-Cre/Stat1fl/fl mouse. Further, we longitudinally evaluated viremia in all five mouse lines and detected high levels of USUV RNA in the serum from mice that succumbed to lethal USUV infection (Fig 4E). In Syn-Cre/Stat1fl/fl, Vav-Cre/Stat1fl/fl, and Stat1-/- mice, there were 38-fold, 35-fold, and 68-fold increases in USUV RNA detected in serum between 1 and 2 dpi. In contrast, viral RNA levels in serum from Stat1fl/fl and Alb-Cre/Stat1fl/fl mice remained below the limit of detection. While viremia in Stat1-/- mice remained high 3–4 dpi, viremia in Vav-Cre/Stat1fl/fl peaked at 3 dpi and then decreased prior to death of the animals. Compared to these two strains, viremia in Syn-Cre/Stat1fl/fl mice was overall lower, but still notable compared to the low levels observed in Alb-Cre/Stat1fl/fl and Stat1fl/fl mice. Ultimately, these data suggest that IFN signaling in hematopoietic cells is critical for protection against lethal USUV infection.
Fig 4. USUV infection in tissue-specific STAT1-deficient mouse strains demonstrates a necessity for functional STAT1-mediated signaling in the hematopoietic compartment.
(A) Schematic representation of the breeding scheme used to generate whole-body, hepatocyte-specific, neuronal cell-specific, hematopoietic cell-specific, macrophage-specific, or dendritic cell-specific STAT1 KO mice. Wild-type mice harboring loxP sites within the Stat1 gene (Stat1fl/fl) were crossed with mice containing the Cre recombinase downstream of a ubiquitous (CMV) or tissue-specific (Alb, Syn, Vav, LysM, CD11c) promoter. Some figure elements (mouse [69], liver [70], neuron [71], and immune cells [72–77]) were sourced from the public domain and are listed as references. (B) Survival, (C) weight changes, and (D) clinical scoring of Stat1fl/fl (n = 11), Stat1-/- (n = 10), Vav-Cre/Stat1fl/fl (n = 7) Alb-Cre/Stat1fl/fl (n = 6), and Syn-Cre/Stat1fl/fl (n = 9) mice following inoculation with 103 focus-forming units (FFU) of USUV TC508 via SC injection. (E) Changes in viremia 1–4 days post-infection compared to a pre-bleed sample (“pre”) collected prior to infection (n = 3/mouse genotype). (F) Survival, (G) weight changes, and (H) clinical scoring of LysM-Cre/Stat1fl/fl (n = 8), CD11c-Cre/Stat1fl/fl (n = 8), and LysM-CD11c-Cre/Stat1fl/fl (n = 4) following inoculation with 103 FFU of USUV TC508 via SC injection.
It was previously shown that mice with a targeted disruption of the IFN alpha/beta receptor in macrophages and dendritic cells (DCs) were hypersusceptible to certain DENV strains [78]. To probe whether STAT1 signaling is required for USUV clearance in these particular hematopoietic cell subsets, we crossed Stat1fl/fl mice with mice expressing the Cre recombinase specifically in monocytes, mature macrophages, and granulocytes (LysM-Cre) [56], conventional DCs (CD11c-Cre) [57], or both (LysM-CD11c-Cre). All three mouse strains demonstrated 100% survival, maintained healthy weights, and did not develop any clinical signs throughout daily monitoring for 14 dpi (Fig 4F–4H). Collectively, these data demonstrate that STAT1-dependent signaling in monocytes, macrophages, granulocytes and conventional DCs is not required for controlling USUV infection in mice.
USUV has broad tissue tropism and causes pathological lesions in STAT1-deficient mice
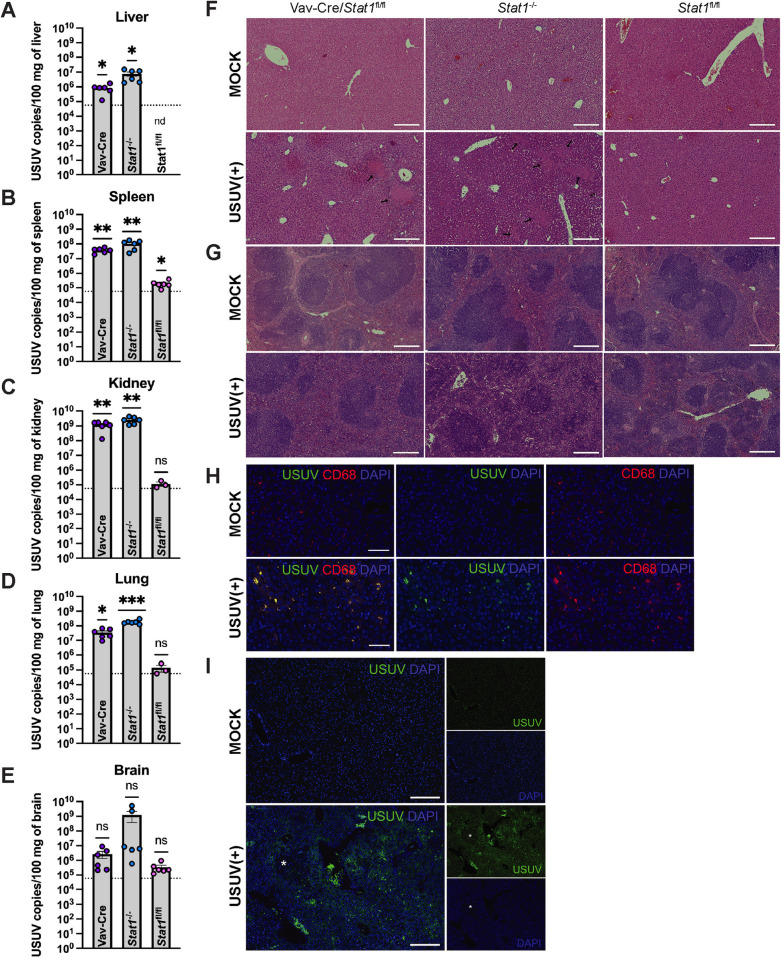
Due to the understudied nature of this virus, limited characterization of USUV replication, dissemination, and pathology in peripheral tissues has been accomplished in mouse models. Here, we detected high levels of USUV RNA in liver, spleen, kidney, lung, and brain from Stat1-/- and Vav-Cre/Stat1fl/fl mice, but not Stat1fl/fl, at 4 dpi (Fig 5A–5E). Amplification of primer dimers (based on melt curve analysis) limited our ability to quantify USUV in samples with fewer than 93.8 copies/uL of USUV RNA, including our mock tissue samples. Nevertheless, tissues with a high viral burden were still clearly identified and demonstrated a statistically significant increase in USUV RNA compared to the limit of detection. Correspondingly, histological analysis of tissues from USUV-infected mice revealed extensive necrosis and significant immune infiltration in highly vascularized organs, including the liver, spleen, and lungs, compared to mock mice. These pathological features were most pronounced in the liver and spleen of Vav-Cre/Stat1fl/fl and Stat1-/- mice (Fig 5F and 5G). Kidney, lung, and brain sections from Vav-Cre/Stat1fl/fl and Stat1-/- mice as well as all tissues from Alb-Cre/Stat1fl/fl and Syn-Cre/Stat1fl/fl mice can be found in the supporting information (S1 Fig). To confirm the presence of USUV in tissues, we detected USUV NS3 protein in liver tissue harvested from infected Stat1-/- mice using an anti-JEV NS3 antibody (Fig 5H and 5I). Further, immunostaining of tissues from Stat1-/- mice confirmed that the immune cells infiltrating the necrotic regions of the liver expressed CD68, which is a marker of histiocyte-like immune cells (Fig 5H). Counterstaining with the pan-immune cell marker CD45 can be found in the supporting information (S2 Fig). These findings demonstrate that USUV can establish infection and circulate systemically in mice with global or hematopoietic cell-specific deletion of STAT1. Further, in these mice, USUV is able to invade and cause pathology in STAT1-sufficient tissues.
Fig 5. USUV replicates in STAT1-sufficient peripheral tissues leading to spleen and liver pathology.
Vav-Cre/Stat1fl/fl, Stat1-/-, and Stat1fl/fl mice (n = 3/group) were infected with 103 focus-forming units (FFU) of USUV via subcutaneous (SC) injection and euthanized at 4 days post-infection (dpi). Tissues were collected and subjected to molecular and histological analysis. For each mouse genotype, one representative image was chosen. USUV RNA was quantified in the (A) liver, (B) spleen, (C) kidney, (D) lung, and (E) brain of Vav-Cre/Stat1fl/fl, Stat1-/-, and Stat1fl/fl mice by qPCR. Significant levels of USUV RNA were detected in the liver, spleen, kidney, and lung of Vav-Cre/Stat1fl/fl and Stat1-/- mice. In some samples collected from Stat1fl/fl mice (6 out of 6 liver samples, 5 out of 6 spleen samples, and 3 out of 6 kidney samples), the amount of USUV RNA was below the limit of detection and was not plotted (nd indicates “not detected”). For each organ harvested from infected mice, two cuts were processed and used as separate sample inputs for qPCR. The dotted line indicates the limit of detection. Statistical analysis was performed using one sample t tests comparing USUV RNA detected in samples to the limit of detection. (F) H&E staining of liver tissue collected from mock or USUV-infected mice with a 200 µm scale bar. Liver necrosis was observed in Vav-Cre/Stat1fl/fl and Stat1-/- mice and is indicated by black arrows. (G) H&E staining of spleen tissue collected from mock or USUV-infected mice with a 200 µm scale bar. Decreased red pulp due to massive immune cell infiltration was observed in Vav-Cre/Stat1fl/fl and Stat1-/- mice. H&E staining of kidney, lung, and brain tissues can be found in S1 Fig. (H) Immunostaining of liver tissue harvested from a Stat1-/- mouse using the histiocyte marker CD68, anti-JEV NS3 to mark USUV infection, and DAPI with a 50 µm scale bar. (I) Liver tissue harvested from a Stat1-/- mice and stained with anti-JEV NS3 and DAPI with a 200 µm scale bar. The white asterisk indicates an inflammatory lesion.
B, T and natural killer cells are dispensable for USUV clearance in mice
With the knowledge that STAT1-mediated IFN signaling in hematopoietic cells is essential for defense against lethal USUV infection, we questioned whether lymphocyte subsets were required. We challenged highly immunodeficient NOD-Rag1null-IL2rgnull (NRG) mice, which lack functional B, T, and natural killer (NK) cells [79]. These mice demonstrated 100% survival and the absence of weight loss and other clinical signs throughout 14 days of post-infection monitoring (Fig 6A–6C). Further, we did not detect viral RNA in serum collected from C57BL/6 nor NRG mice 1–4 dpi. NRG resistance to USUV infection indicated a nonessential role of adaptive immunity and as well as NK cell-driven innate responses in USUV clearance.
Fig 6. B, T, and natural killer cells are dispensable for restricting USUV infection.
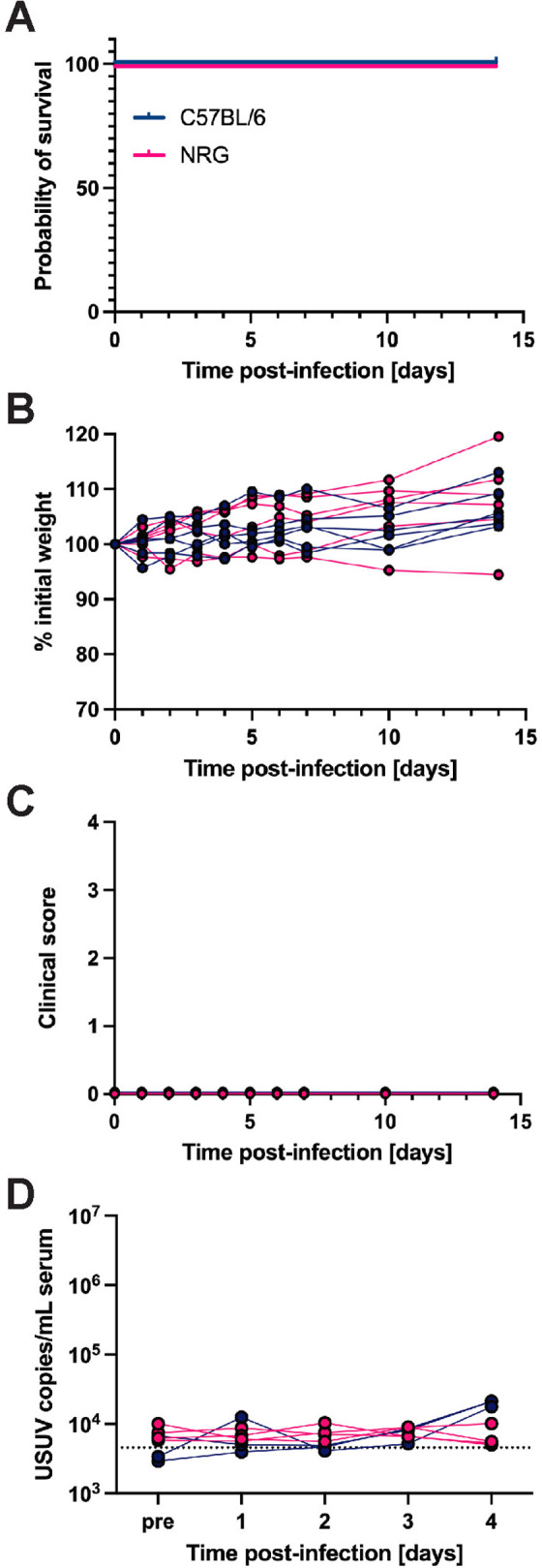
(A) Survival, (B) weight changes, and (C) clinical scoring of NRG (n = 6) and wild-type C57BL/6 (n = 6) mice following inoculation with 103 focus-forming units (FFU) of USUV TC508 via subcutaneous (SC) injection. (D) Changes in viremia 1–4 days post-infection (dpi) compared to a pre-bleed sample (“pre”) collected prior to infection in NRG (n = 4) and C57BL/6 (n = 3) mice.
IFN-β treatment following USUV exposure partially protects Vav-Cre/Stat1fl/fl mice from lethality
Vav-Cre/Stat1fl/fl mice are highly susceptible to lethal USUV infection, which may be attributable to disrupted STAT1-mediated IFN signaling in their hematopoietic cells. Thus, we investigated if administration of mouse IFN-β before or after USUV infection could provide protection against lethality. The pre-treatment group received one dose of IFN-β at 2 hours prior to USUV infection and another dose at 24 hpi. The other two treatment groups received both doses of IFN-β at two timepoints following infection: 6 and 24 hpi or 24 and 48 hpi. The untreated group did not receive any cytokine. Pre-treatment with IFN-β proved to be more effective than post-infection treatment. In the pre-treatment group (-2, 24 hpi), 5 out of 6 mice survived following USUV infection, while 4 out of 4 untreated mice succumbed to lethality by 9 dpi. In the early post-infection group (6, 24 hpi), 3 out of 8 mice survived while the others died between 8–10 dpi. In the later post-infection group (24, 48 hpi), 3 out of 7 mice survived and the rest died between 8–9 dpi. (Fig 7A). Notably, the IFN-β-treated mice that survived USUV infection had all manifested clinical signs including ruffled fur and weight loss to a similar degree as the non-surviving mice in their groups; however, they ultimately regained lost body weight and recovered (Fig 7B and 7C). Collectively, these data further affirm that early interferon signaling is critical for controlling USUV infection in vivo.
Fig 7. Administration of IFN-beta (IFN-β) partially protects Vav-Cre/Stat1fl/fl mice from lethal USUV infection.

(A) Survival, (B) weight changes, and (C) clinical scoring of Vav-Cre/Stat1fl/fl mice that received 104 IU IFN-β via SC injection at two timepoints before or after infection with 103 FFU of USUV TC508. The pre-treatment group (n = 6) received IFN-β injections at 2 hours prior to USUV infection and 24 hours post-infection (hpi). One post-treatment group received IFN-β injections 6 and 24 hpi (n = 8) and the second post-treatment group received IFN-β injections at 24 and 48 hpi (n = 7).
Discussion
Due to the continued geographical expansion of arthropod vectors, we must further monitor and investigate both well-known and understudied flaviviruses to develop prophylactic vaccines and therapeutic options beyond supportive care [80]. To this end, we generated an infectious clone of USUV isolate TC508 and demonstrated its pathogenicity both in vitro and in vivo. Similar to established flavivirus threats, such as DENV, YFV, ZIKV, and WNV, that cause more than 400 million infections annually and have been more extensively characterized [81], USUV was highly lethal in mice bearing deficiency in type I IFN signaling. This pathway was specifically essential in hematopoietic cells in defense against USUV. Our work here is one of the first characterizations of USUV pathogenesis using adult mouse models and should be an invaluable resource for further molecular characterizations of USUV and potential medical countermeasures.
USUV has the remarkable ability to replicate in wildly divergent species—mosquito, birds, and mammals—therefore, we tested its ability to replicate in cultured cells from these species. The first study of USUV in vitro tested the susceptibility of cell lines from multiple species including human, rat, monkey, bovine, equine, porcine, avian, canine, feline, rabbit, hamster, and turtle. Infection with pronounced cytopathic effect (CPE) was observed in African green monkey (Vero), porcine (PK-15), and goose embryonic fibroblasts. Noncytopathic infection with positive detection of viral antigen by IHC staining occurred in all other cell lines except for chicken embryonic fibroblasts, which were not susceptible to infection [82]. Extensive summaries of in vitro models used to study USUV have been nicely summarized by previous groups [39,83]. In the present study, we determined that USUV successfully infected and replicated in human, bird, mosquito, and mouse lines; however, the degree of permissiveness varied widely between mouse cell lines. Overall, murine fibroblasts (STO5, NIH 3T3, L929) were more permissive compared to hepatocyte or hepatoma cell lines (Hepa1–6, H2.35, AML12, Hep-56.1D). Further, fibroblasts isolated from an adult mouse (L929) were more permissive than fibroblasts isolated from embryos (STO5, NIH 3T3). Our work establishes several new cell lines as feasible models for propagating and studying USUV infection including Huh7, LMH, and L929. Further, we have determined other cell lines to be lowly permissive to USUV such as Hepa1–6, H2.35, AML12, and NIH 3T3, which may lack necessary host factors or encode restriction factors. Future work could explore the permissiveness of human and murine neuronal cell lines as well as primary cells isolated from the blood brain barrier to better characterize the neurotropic capacity of this virus in vitro and ex vivo. Some studies have successfully infected primary cell types isolated from the human brain [32,84], but analogous studies have yet to be performed in similar cell types isolated from mice.
In devising medical countermeasures against flaviviruses, new animal models and an improved understanding of USUV pathogenesis will be required. In this study, we established Stat1-/- and Vav-Cre/Stat1fl/fl mice as new models for USUV investigation and confirmed that they exhibited similar disease progression to Ifnar1-/- mice. Regardless of inoculation dose or route, USUV infection in Stat1-/- mice was uniformly lethal by 8 dpi. Using our optimized conditions of 103 FFU via SC injection, 83% of Vav-Cre/Stat1fl/fl mice succumbed to lethal USUV infection by 9 dpi. Molecularly, lethal infection was associated with USUV dissemination to a wide range of tissues—liver, spleen, lung, kidney, and brain—based on RT-qPCR assays, as well as necrosis and significant immune cell infiltration in the liver and spleen. Of note, we observed the same outcomes in male and female mice; therefore, there were no sex differences for any genotypes used in this study. As a proof-of-concept, treating USUV-challenged mice with exogenous IFN-β led to disease recovery in some cases, particularly with pre-infection treatment. Together, our study establishes inoculation parameters, as well as expected baseline pathology, for lethal USUV infection in IFN-deficient mouse models.
While multiple groups have used Ifnar1-/- mice to study USUV, the specific parameters by which IFN signaling protects against lethal USUV infection are not well-defined. Our data collectively suggest an important role of IFN signaling in non-macrophage, non-conventional dendritic cell subsets of the myeloid lineage during very early infection. First, we found that STAT1-mediated IFN signaling was essential in hematopoietic cells, using cell-specific deletion of STAT1 in Vav-expressing cells. Although STAT1 was only disrupted in the hematopoietic system, USUV was able to gain entry to, and replicate efficiently, in STAT1-sufficient peripheral organs. Second, we determined that B, T, and NK cells were not required for protection against USUV, as NRG mice were resistant to infection. Third, we redirected our focus towards myeloid cells and determined that deletion of STAT1 in macrophages (LysM-expressing) and/or conventional dendritic cells (CD11c-expressing) did not increase susceptibility to USUV.
A previous study from our group found that Vav-Cre/Stat1fl/fl mice succumbed to lethal YFV-17D infection, yet Alb-Cre/Stat1fl/fl and C57BL/6 mice exhibited 100% survival [60]. Thus, we have now demonstrated that STAT1 competency in hematopoietic cells specifically is required to protect against lethality following infection with two flaviviruses. Currently, it is not known whether STAT1 signaling competency is required in the same immune cell subsets to control YFV-17D and USUV infections; however, this could be explored through generation and infection of immune cell-specific STAT1 KO mice.
Our generation of LysM and CD11c-specific STAT1-deficient mice was inspired by a previous study, which found that mice lacking IFNAR1 in both of these compartments (LysM-CD11c-Cre/Ifnar1fl/fl) phenocopied full body Ifnar1-/- mice, succumbing to lethal DENV infection by 5 dpi. Notably, mice lacking IFNAR1 in either the myeloid subset (LysM-Cre/Ifnar1fl/fl) or dendritic cells (CD11c-Cre/Ifnar1fl/fl) were also susceptible to DENV infection, but often started to recover once the the CD8 + T cell response was initiated around 5 dpi [78]. In this study, LysM-Cre/Stat1fl/fl, CD11c-Cre/Stat1fl/fl, and LysM-CD11c-Cre/Stat1fl/fl mice were all resistant to USUV infection. This suggests that the affected immune cells may not be required for restricting USUV infection and perhaps different immune subsets are required for controlling USUV compared to DENV infection. Alternatively, the remaining STAT1-competent immune cells in LysM-Cre/Stat1fl/fl, CD11c-Cre/Stat1fl/fl, and LysM-CD11c-Cre/Stat1fl/fl mice may be able to compensate for the loss of STAT1-mediated antiviral signaling to restrict USUV, but similar compensation of STAT1-competent immune cells in LysM-CD11c-Cre/Ifnar1fl/fl may not be sufficient to restrict DENV. Future studies will have to focus on determining the specific immune subset(s) in which IFN signaling competency is essential for organism survival.
The mechanisms by which USUV establishes initial infection and spreads within a susceptible host remains poorly characterized. Understanding the immune system components that this virus interacts with or evades is essential for developing strategies to restrict infection. Here, we found that the absence of STAT1-mediated IFN signaling in hematopoietic cells allows USUV to overcome murine host defenses. We hypothesize that USUV infects certain immune cells, then exploits them as viral amplification reservoirs and/or shuttles to gain entry to peripheral tissues in a “Trojan horse” fashion. In these tissues, USUV replicates to high levels, thereby causing significant inflammation and damage. In future investigations, we aim to identify if specific immune cells become enriched during early stages of infection and how the cytokine response may accompany this. As USUV is an understudied virus with limited available reagents, this could be facilitated by the development of novel reporter viruses to mark infection in vitro and track infection in vivo.
Supporting information
H&E staining of liver, spleen, kidney, lung, and brain tissues harvested from Stat1fl/fl, Stat1-/-, Alb-Cre/Stat1fl/fl, Syn-Cre/Stat1fl/fl, Vav-Cre/Stat1fl/fl mice (n = 3/group) that were infected with 103 FFU of USUV via subcutaneous (SC) injection or corresponding mock mice. For each mouse genotype, one representative image was chosen. The scale bars for liver, spleen, kidney, and brain images are 200 µm. The scale bar for lung images is 100 µm.
(PDF)
Livers were harvested from Stat1-/- mice that were infected with 103 FFU of USUV via subcutaneous (SC) injection. Immunostaining of mouse liver tissue using the pan-immune cell marker CD45, the histiocyte marker CD68, anti-JEV NS3 to mark USUV infection, and DAPI. The scale bar indicates 50 µm.
(PDF)
Acknowledgments
We thank Raul Andino (UCSF) for providing the Aag2 cells, Charles Rice (The Rockefeller University) for the Huh7.5 and STO5 cells, Kenneth Plante (WRCEVA) for providing the USUV TC508 isolate, Lothar Hennighausen (NIDDK) for the Stat1fl/fl mice, and Sergei Kotenko (Rutgers) for the Ifnar1-/- mice. We thank Wei Wang and other members of the Genomics Core Facility of the Lewis-Sigler Institute for Integrative Genomics for their help in maintaining and loading the Illumina sequencers. We thank Christina DeCoste and Jailene Garcia at the Flow Cytometry core for independent user training and maintaining the FACSymphony A3 analyzer. We thank NIH BioArt, Bioicons, and Openclipart for providing images to the public domain that we utilized in generating several manuscript figures. We thank members of the Ploss lab for critical discussions and comments on this project.
Data Availability
Viral genome sequences have been deposited to NCBI GenBank under the BioProject accession PRJNA1263528, with the corresponding raw reads available at the Sequence Read Archive (SRA). All data supporting the conclusions of this study are reported in the paper. All primary data are available via Princeton Data Commons repository at the following link: https://doi.org/10.34770/fwqx-xw53.
Funding Statement
This study was supported by grants from the National Institutes of Health [R01AI138797, R01AI153236, R01AI146917, R01AI168048, R01AI181664, and U19A171401 (all to A.P., https://www.niaid.nih.gov/); R01AI107301 (to A.P. and R.E.S., https://www.niaid.nih.gov/); R01AA027327 (to R.E.S., https://www.niaaa.nih.gov/) and R01DK121072 (to R.E.S., https://www.niddk.nih.gov/], Open Philanthropy (to A.P., https://www.openphilanthropy.org/), the Princeton Catalysis Initiative (to A.P., https://pci.princeton.edu/), the Princeton Center for Health and Wellbeing (to A.P., https://chw.princeton.edu/), Princeton University (to A.P., https://www.princeton.edu/), the Irma T. Hirschl Trust from Weill Cornell Medicine (to R.E.S., https://weill.cornell.edu), and the Paul G. Allen Family Foundation UWSC13448 (to R.E.S., https://pgafamilyfoundation.org/). A.N.N. was supported by the National Institute of General Medical Sciences of the National Institutes of Health under grant number T32GM007388 (https://www.nigms.nih.gov). A.E.L. is a recipient of post-doctoral fellowship from the Damon Runyon Cancer Research Fund under the award number DRG-2432-21 (https://www.damonrunyon.org/). We thank Christina DeCoste, Jailene Garcia, and the Molecular Biology Flow Cytometry Resource Facility, which is partially supported by the Rutgers Cancer Institute of New Jersey (NCI-CCSG P30CA072720-5921, https://cinj.org/) and the National Institutes of Health S10 Shared Instrumentation Grant (S10OD028592, https://www.nih.gov). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.McIntosh BM. Usutu (SA Ar 1776), nouvel arbovirus du groupe B. Int Catalogue Arboviruses. 1985;3:1059–60. [Google Scholar]
- 2.Diagne MM, Ndione MHD, Di Paola N, Fall G, Bedekelabou AP, Sembène PM, et al. Usutu Virus Isolated from Rodents in Senegal. Viruses. 2019;11(2):181. doi: 10.3390/v11020181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nikolay B, Diallo M, Boye CSB, Sall AA. Usutu virus in Africa. Vector Borne Zoonotic Dis. 2011;11(11):1417–23. doi: 10.1089/vbz.2011.0631 [DOI] [PubMed] [Google Scholar]
- 4.Williams MC, Simpson DI, Haddow AJ, Knight EM. The isolation of West Nile virus from man and of Usutu virus from the bird-biting mosquito Mansonia aurites (Theobald) in the Entebbe area of Uganda. Ann Trop Med Parasitol. 1964;58:367–74. [DOI] [PubMed] [Google Scholar]
- 5.Fros JJ, Miesen P, Vogels CB, Gaibani P, Sambri V, Martina BE, et al. Comparative Usutu and West Nile virus transmission potential by local Culex pipiens mosquitoes in north-western Europe. One Health. 2015;1:31–6. doi: 10.1016/j.onehlt.2015.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Weissenböck H, Kolodziejek J, Url A, Lussy H, Rebel-Bauder B, Nowotny N. Emergence of Usutu virus, an African mosquito-borne flavivirus of the Japanese encephalitis virus group, central Europe. Emerg Infect Dis. 2002;8(7):652–6. doi: 10.3201/eid0807.020094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chvala S, Kolodziejek J, Nowotny N, Weissenböck H. Pathology and viral distribution in fatal Usutu virus infections of birds from the 2001 and 2002 outbreaks in Austria. J Comp Pathol. 2004;131(2–3):176–85. doi: 10.1016/j.jcpa.2004.03.004 [DOI] [PubMed] [Google Scholar]
- 8.Chvala S, Bakonyi T, Bukovsky C, Meister T, Brugger K, Rubel F, et al. Monitoring of Usutu virus activity and spread by using dead bird surveillance in Austria, 2003-2005. Vet Microbiol. 2007;122:237–45. [DOI] [PubMed] [Google Scholar]
- 9.Steinmetz HW, Bakonyi T, Weissenböck H, Hatt J-M, Eulenberger U, Robert N, et al. Emergence and establishment of Usutu virus infection in wild and captive avian species in and around Zurich, Switzerland--genomic and pathologic comparison to other central European outbreaks. Vet Microbiol. 2011;148(2–4):207–12. doi: 10.1016/j.vetmic.2010.09.018 [DOI] [PubMed] [Google Scholar]
- 10.Becker N, Jöst H, Ziegler U, Eiden M, Höper D, Emmerich P, et al. Epizootic emergence of Usutu virus in wild and captive birds in Germany. PLoS One. 2012;7(2):e32604. doi: 10.1371/journal.pone.0032604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Giglia G, Agliani G, Munnink BBO, Sikkema RS, Mandara MT, Lepri E, et al. Pathology and Pathogenesis of Eurasian Blackbirds (Turdus merula) Naturally Infected with Usutu Virus. Viruses. 2021;13(8):1481. doi: 10.3390/v13081481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Störk T, de le Roi M, Haverkamp AK, Jesse ST, Peters M, Fast C, et al. Analysis of avian Usutu virus infections in Germany from 2011 to 2018 with focus on dsRNA detection to demonstrate viral infections. Sci Rep. 2021;11:24191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.de Bruin E, van Irsel J, Chandler F, Kohl R, van de Voorde T, van der Linden A, et al. Usutu Virus Antibody Dynamics in Naturally Infected Blackbirds, the Netherlands, 2016-2018. Emerg Infect Dis. 2025;31:1244–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cadar D, Becker N, Campos R de M, Börstler J, Jöst H, Schmidt-Chanasit J. Usutu virus in bats, Germany, 2013. Emerg Infect Dis. 2014;20:1771–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Escribano-Romero E, Lupulović D, Merino-Ramos T, Blázquez A-B, Lazić G, Lazić S, et al. West Nile virus serosurveillance in pigs, wild boars, and roe deer in Serbia. Vet Microbiol. 2015;176(3–4):365–9. doi: 10.1016/j.vetmic.2015.02.005 [DOI] [PubMed] [Google Scholar]
- 16.Romeo C, Lecollinet S, Caballero J, Isla J, Luzzago C, Ferrari N, et al. Are tree squirrels involved in the circulation of flaviviruses in Italy?. Transbound Emerg Dis. 2018;65(5):1372–6. doi: 10.1111/tbed.12874 [DOI] [PubMed] [Google Scholar]
- 17.Durand B, Haskouri H, Lowenski S, Vachiery N, Beck C, Lecollinet S. Seroprevalence of West Nile and Usutu viruses in military working horses and dogs, Morocco, 2012: dog as an alternative WNV sentinel species? Epidemiol Infect. 2016;144(9):1857–64. doi: 10.1017/S095026881600011X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bażanów B, Jansen van Vuren P, Szymański P, Stygar D, Frącka A, Twardoń J, et al. A Survey on West Nile and Usutu Viruses in Horses and Birds in Poland. Viruses. 2018;10(2):87. doi: 10.3390/v10020087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Barbic L, Vilibic-Cavlek T, Listes E, Stevanovic V, Gjenero-Margan I, Ljubin-Sternak S, et al. Demonstration of Usutu virus antibodies in horses, Croatia. Vector Borne Zoonotic Dis. 2013;13(10):772–4. doi: 10.1089/vbz.2012.1236 [DOI] [PubMed] [Google Scholar]
- 20.De Kesel W, Vanden Broecke B, Borremans B, Fourchault L, Willems E, Ceulemans A, et al. Antibodies against medically relevant arthropod-borne viruses in the ubiquitous African rodent Mastomys natalensis. PLoS Negl Trop Dis. 2024;18(9):e0012233. doi: 10.1371/journal.pntd.0012233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cadar D, Maier P, Müller S, Kress J, Chudy M, Bialonski A, et al. Blood donor screening for West Nile virus (WNV) revealed acute Usutu virus (USUV) infection, Germany, September 2016. Euro Surveill. 2017;22(14):30501. doi: 10.2807/1560-7917.ES.2017.22.14.30501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bakonyi T, Jungbauer C, Aberle SW, Kolodziejek J, Dimmel K, Stiasny K, et al. Usutu virus infections among blood donors, Austria, July and August 2017 - Raising awareness for diagnostic challenges. Euro Surveill. 2017;22(41):17–00644. doi: 10.2807/1560-7917.ES.2017.22.41.17-00644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Aberle SW, Kolodziejek J, Jungbauer C, Stiasny K, Aberle JH, Zoufaly A, et al. Increase in human West Nile and Usutu virus infections, Austria, 2018. Euro Surveill. 2018;23(43):1800545. doi: 10.2807/1560-7917.ES.2018.23.43.1800545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gaibani P, Pierro A, Alicino R, Rossini G, Cavrini F, Landini MP, et al. Detection of Usutu-virus-specific IgG in blood donors from northern Italy. Vector Borne Zoonotic Dis. 2012;12(5):431–3. doi: 10.1089/vbz.2011.0813 [DOI] [PubMed] [Google Scholar]
- 25.Nagy A, Csonka N, Takács M, Mezei E, Barabás É. West Nile and Usutu virus seroprevalence in Hungary: A nationwide serosurvey among blood donors in 2019. PLoS One. 2022;17(4):e0266840. doi: 10.1371/journal.pone.0266840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Faggioni G, De Santis R, Pomponi A, Grottola A, Serpini GF, Meacci M, et al. Prevalence of Usutu and West Nile virus antibodies in human sera, Modena, Italy, 2012. J Med Virol. 2018;90(10):1666–8. doi: 10.1002/jmv.25230 [DOI] [PubMed] [Google Scholar]
- 27.Pecorari M, Longo G, Gennari W, Grottola A, Sabbatini A, Tagliazucchi S, et al. First human case of Usutu virus neuroinvasive infection, Italy, August-September 2009. Euro Surveill. 2009;14. [PubMed] [Google Scholar]
- 28.Cavrini F, Gaibani P, Longo G, Pierro AM, Rossini G, Bonilauri P, et al. Usutu virus infection in a patient who underwent orthotropic liver transplantation, Italy, August-September 2009. Euro Surveill. 2009;14. Available from: https://www.ncbi.nlm.nih.gov/pubmed/20070935 [PubMed] [Google Scholar]
- 29.Gaibani P, Barp N, Massari M, Negri EA, Rossini G, Vocale C, et al. Case report of Usutu virus infection in an immunocompromised patient in Italy, 2022. J Neurovirol. 2023;29:364–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Santini M, Vilibic-Cavlek T, Barsic B, Barbic L, Savic V, Stevanovic V, et al. First cases of human Usutu virus neuroinvasive infection in Croatia, August-September 2013: clinical and laboratory features. J Neurovirol. 2015;21(1):92–7. doi: 10.1007/s13365-014-0300-4 [DOI] [PubMed] [Google Scholar]
- 31.Vilibic-Cavlek T, Savic V, Sabadi D, Peric L, Barbic L, Klobucar A, et al. Prevalence and molecular epidemiology of West Nile and Usutu virus infections in Croatia in the “One health” context, 2018. Transbound Emerg Dis. 2019;66(5):1946–57. doi: 10.1111/tbed.13225 [DOI] [PubMed] [Google Scholar]
- 32.Marshall EM, Koopmans M, Rockx B. Usutu virus and West Nile virus use a transcellular route of neuroinvasion across an in vitro model of the human blood-brain barrier. Npj Viruses. 2024;2(1):32. doi: 10.1038/s44298-024-00034-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Weissenböck H, Bakonyi T, Chvala S, Nowotny N. Experimental Usutu virus infection of suckling mice causes neuronal and glial cell apoptosis and demyelination. Acta Neuropathol. 2004;108(5):453–60. doi: 10.1007/s00401-004-0916-1 [DOI] [PubMed] [Google Scholar]
- 34.Blázquez A-B, Escribano-Romero E, Martín-Acebes MA, Petrovic T, Saiz J-C. Limited susceptibility of mice to Usutu virus (USUV) infection and induction of flavivirus cross-protective immunity. Virology. 2015;482:67–71. doi: 10.1016/j.virol.2015.03.020 [DOI] [PubMed] [Google Scholar]
- 35.Martín-Acebes MA, Blázquez A-B, Cañas-Arranz R, Vázquez-Calvo Á, Merino-Ramos T, Escribano-Romero E, et al. A recombinant DNA vaccine protects mice deficient in the alpha/beta interferon receptor against lethal challenge with Usutu virus. Vaccine. 2016;34(18):2066–73. doi: 10.1016/j.vaccine.2016.03.015 [DOI] [PubMed] [Google Scholar]
- 36.Clé M, Barthelemy J, Desmetz C, Foulongne V, Lapeyre L, Bolloré K, et al. Study of Usutu virus neuropathogenicity in mice and human cellular models. PLoS Negl Trop Dis. 2020;14(4):e0008223. doi: 10.1371/journal.pntd.0008223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kuchinsky SC, Hawks SA, Mossel EC, Coutermarsh-Ott S, Duggal NK. Differential pathogenesis of Usutu virus isolates in mice. PLoS Negl Trop Dis. 2020;14(10):e0008765. doi: 10.1371/journal.pntd.0008765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bates TA, Chuong C, Hawks SA, Rai P, Duggal NK, Weger-Lucarelli J. Development and characterization of infectious clones of two strains of Usutu virus. Virology. 2021;554:28–36. doi: 10.1016/j.virol.2020.12.004 [DOI] [PubMed] [Google Scholar]
- 39.Duyvestyn JM, Marshall EM, Bredenbeek PJ, Rockx B, van Hemert MJ, Kikkert M. Dose and strain dependent lethality of Usutu virus in an Ifnar-/- mouse model. Npj Viruses. 2025;3(1):6. doi: 10.1038/s44298-025-00089-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Matranga CB, Andersen KG, Winnicki S, Busby M, Gladden AD, Tewhey R, et al. Enhanced methods for unbiased deep sequencing of Lassa and Ebola RNA viruses from clinical and biological samples. Genome Biol. 2014;15(11):519. doi: 10.1186/s13059-014-0519-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Park D, Tomkins-Tinch C, Ye S, Jungreis I, Shlyakhter I, Metsky H, et al. broadinstitute/viral-ngs: v1.25.0. 2019. doi: 10.5281/zenodo.3509008 [DOI] [Google Scholar]
- 42.Park D, Tomkins-Tinch C, Ye S, Jungreis I, Shlyakhter I, Metsky H, et al. broadinstitute/viral-pipelines: v2.1.33.14. 2022. doi: 10.5281/zenodo.7079081 [DOI] [Google Scholar]
- 43.Quan J, Tian J. Circular polymerase extension cloning of complex gene libraries and pathways. PLoS One. 2009;4(7):e6441. doi: 10.1371/journal.pone.0006441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Edmonds J, van Grinsven E, Prow N, Bosco-Lauth A, Brault AC, Bowen RA, et al. A novel bacterium-free method for generation of flavivirus infectious DNA by circular polymerase extension reaction allows accurate recapitulation of viral heterogeneity. J Virol. 2013;87(4):2367–72. doi: 10.1128/JVI.03162-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tamura T, Zhang J, Madan V, Biswas A, Schwoerer MP, Cafiero TR, et al. Generation and characterization of genetically and antigenically diverse infectious clones of dengue virus serotypes 1-4. Emerg Microbes Infect. 2022;11(1):227–39. doi: 10.1080/22221751.2021.2021808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dong H-L, He M-J, Wang Q-Y, Cui J-Z, Chen Z-L, Xiong X-H, et al. Rapid Generation of Recombinant Flaviviruses Using Circular Polymerase Extension Reaction. Vaccines (Basel). 2023;11(7):1250. doi: 10.3390/vaccines11071250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Setoh YX, Prow NA, Rawle DJ, Tan CSE, Edmonds JH, Hall RA, et al. Systematic analysis of viral genes responsible for differential virulence between American and Australian West Nile virus strains. J Gen Virol. 2015;96(Pt 6):1297–308. doi: 10.1099/vir.0.000069 [DOI] [PubMed] [Google Scholar]
- 48.Setoh YX, Prow NA, Peng N, Hugo LE, Devine G, Hazlewood JE, et al. De Novo Generation and Characterization of New Zika Virus Isolate Using Sequence Data from a Microcephaly Case. mSphere. 2017;2(3):e00190-17. doi: 10.1128/mSphereDirect.00190-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Uversky V, Longhi S. Flexible viruses: Structural disorder in viral proteins. John Wiley & Sons; 2012. [Google Scholar]
- 50.Jones MB, An A, Shi LJ, Shi W. Regional Variation in Genetic Control of Atherosclerosis in Hyperlipidemic Mice. G3 (Bethesda). 2020;10(12):4679–89. doi: 10.1534/g3.120.401856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Brehm MA, Cuthbert A, Yang C, Miller DM, DiIorio P, Laning J, et al. Parameters for establishing humanized mouse models to study human immunity: analysis of human hematopoietic stem cell engraftment in three immunodeficient strains of mice bearing the IL2rgamma(null) mutation. Clin Immunol. 2010;135:84–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schwenk F, Baron U, Rajewsky K. A cre-transgenic mouse strain for the ubiquitous deletion of loxP-flanked gene segments including deletion in germ cells. Nucleic Acids Res. 1995;23(24):5080–1. doi: 10.1093/nar/23.24.5080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Postic C, Shiota M, Niswender KD, Jetton TL, Chen Y, Moates JM, et al. Dual roles for glucokinase in glucose homeostasis as determined by liver and pancreatic beta cell-specific gene knock-outs using Cre recombinase. J Biol Chem. 1999;274(1):305–15. doi: 10.1074/jbc.274.1.305 [DOI] [PubMed] [Google Scholar]
- 54.de Boer J, Williams A, Skavdis G, Harker N, Coles M, Tolaini M, et al. Transgenic mice with hematopoietic and lymphoid specific expression of Cre. European Journal of Immunology. 2003;33(2): 314–325. doi: 10.1002/immu.200310005 [DOI] [PubMed] [Google Scholar]
- 55.Zhu Y, Romero MI, Ghosh P, Ye Z, Charnay P, Rushing EJ, et al. Ablation of NF1 function in neurons induces abnormal development of cerebral cortex and reactive gliosis in the brain. Genes Dev. 2001;15(7):859–76. doi: 10.1101/gad.862101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Clausen BE, Burkhardt C, Reith W, Renkawitz R, Förster I. Conditional gene targeting in macrophages and granulocytes using LysMcre mice. Transgenic Res. 1999;8(4):265–77. doi: 10.1023/a:1008942828960 [DOI] [PubMed] [Google Scholar]
- 57.Caton ML, Smith-Raska MR, Reizis B. Notch-RBP-J signaling controls the homeostasis of CD8- dendritic cells in the spleen. J Exp Med. 2007;204(7):1653–64. doi: 10.1084/jem.20062648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lin J-D, Feng N, Sen A, Balan M, Tseng H-C, McElrath C, et al. Correction: Distinct Roles of Type I and Type III Interferons in Intestinal Immunity to Homologous and Heterologous Rotavirus Infections. PLoS Pathog. 2016;12(6):e1005726. doi: 10.1371/journal.ppat.1005726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Klover PJ, Muller WJ, Robinson GW, Pfeiffer RM, Yamaji D, Hennighausen L. Loss of STAT1 from mouse mammary epithelium results in an increased Neu-induced tumor burden. Neoplasia. 2010;12(11):899–905. doi: 10.1593/neo.10716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Douam F, Hrebikova G, Albrecht YES, Sellau J, Sharon Y, Ding Q, et al. Single-cell tracking of flavivirus RNA uncovers species-specific interactions with the immune system dictating disease outcome. Nat Commun. 2017;8:14781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hatchwell L, Collison A, Girkin J, Parsons K, Li J, Zhang J, et al. Toll-like receptor 7 governs interferon and inflammatory responses to rhinovirus and is suppressed by IL-5-induced lung eosinophilia. Thorax. 2015;70(9):854–61. doi: 10.1136/thoraxjnl-2014-205465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jaini R, Hannaman D, Johnson JM, Bernard RM, Altuntas CZ, Delasalas MM, et al. Gene-based intramuscular interferon-beta therapy for experimental autoimmune encephalomyelitis. Mol Ther. 2006;14(3):416–22. doi: 10.1016/j.ymthe.2006.04.009 [DOI] [PubMed] [Google Scholar]
- 63.Matsuyama S, Henmi S, Ichihara N, Sone S, Kikuchi T, Ariga T, et al. Protective effects of murine recombinant interferon-beta administered by intravenous, intramuscular or subcutaneous route on mouse hepatitis virus infection. Antiviral Res. 2000;47(2):131–7. doi: 10.1016/s0166-3542(00)00097-8 [DOI] [PubMed] [Google Scholar]
- 64.OpenClipart. Blackbird Silhouette. 14 Jun 2015. Originally sourced from publicdomainpictures.net by Gordon Dylan Johnson. Available from: https://openclipart.org/detail/220754/blackbird-silhouette [Google Scholar]
- 65.NIAID Visual & Medical Arts. Generic Cells. NIAID NIH BIOART Source. 7 Oct 2024. Available from: https://bioart.niaid.nih.gov/bioart/171 [Google Scholar]
- 66.NIAID Visual & Medical Arts. DNA. NIAID NIH BIOART Source. 7 Oct 2024. Available from: https://bioart.niaid.nih.gov/bioart/123 [Google Scholar]
- 67.Bakonyi T, Erdélyi K, Ursu K, Ferenczi E, Csörgo T, Lussy H, et al. Emergence of Usutu virus in Hungary. J Clin Microbiol. 2007;45(12):3870–4. doi: 10.1128/JCM.01390-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Murat C, Guerder S. Variegate expression of Cre recombinase in hematopoietic cells in CD11c-cre transgenic mice. J Immunol Methods. 2024;525:113600. [DOI] [PubMed] [Google Scholar]
- 69.NIAID Visual & Medical Arts. Lab Mouse. NIAID NIH BIOART Source. 7 Oct 2024. Available from: https://bioart.niaid.nih.gov/bioart/279 [Google Scholar]
- 70.Bioicons - high quality science illustrations. Healthy liver. Jan Clusmann. [cited 4 Jun 2025]. Available from: https://bioicons.com/?query=liver [Google Scholar]
- 71.Bioicons - high quality science illustrations. Interneuron. DBCLS. [cited 4 Jun 2025]. Available from: https://bioicons.com/?query=interneuron [Google Scholar]
- 72.NIAID Visual & Medical Arts. Immune Cells. NIAID NIH BIOART Source. 7 Oct 2024. Available from: https://bioart.niaid.nih.gov/bioart/253 [Google Scholar]
- 73.NIAID Visual & Medical Arts. Macrophage. NIAID NIH BIOART Source. 7 Oct 2024. Available from: https://bioart.niaid.nih.gov/bioart/309 [Google Scholar]
- 74.NIAID Visual & Medical Arts. Macrophage. NIAID NIH BIOART Source. 7 Oct 2024. Available from: https://bioart.niaid.nih.gov/bioart/310 [Google Scholar]
- 75.NIAID Visual & Medical Arts. Monocyte. NIAID NIH BIOART Source. 7 Oct 2024. Available from: https://bioart.niaid.nih.gov/bioart/356 [Google Scholar]
- 76.NIAID Visual & Medical Arts. Dendritic Cell. NIAID NIH BIOART Source. 7 Oct 2024. Available from: https://bioart.niaid.nih.gov/bioart/111 [Google Scholar]
- 77.NIAID Visual & Medical Arts. Dendritic Cell. NIAID NIH BIOART Source. 7 Oct 2024. Available from: https://bioart.niaid.nih.gov/bioart/114 [Google Scholar]
- 78.Züst R, Toh YX, Valdés I, Cerny D, Heinrich J, Hermida L, et al. Type I interferon signals in macrophages and dendritic cells control dengue virus infection: implications for a new mouse model to test dengue vaccines. J Virol. 2014;88:7276–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Pearson T, Shultz LD, Miller D, King M, Laning J, Fodor W, et al. Non-obese diabetic-recombination activating gene-1 (NOD-Rag1 null) interleukin (IL)-2 receptor common gamma chain (IL2r gamma null) null mice: a radioresistant model for human lymphohaematopoietic engraftment. Clin Exp Immunol. 2008;154(2):270–84. doi: 10.1111/j.1365-2249.2008.03753.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Nelson AN, Ploss A. Emerging mosquito-borne flaviviruses. mBio. 2024;15(12):e0294624. doi: 10.1128/mbio.02946-24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Pierson TC, Diamond MS. The continued threat of emerging flaviviruses. Nat Microbiol. 2020;5:796–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bakonyi T, Lussy H, Weissenböck H, Hornyák A, Nowotny N. In vitro host-cell susceptibility to Usutu virus. Emerg Infect Dis. 2005;11(2):298–301. doi: 10.3201/eid1102.041016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Benzarti E, Garigliany M. In Vitro and In Vivo Models to Study the Zoonotic Mosquito-Borne Usutu Virus. Viruses. 2020;12(10):1116. doi: 10.3390/v12101116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Salinas S, Constant O, Desmetz C, Barthelemy J, Lemaitre J-M, Milhavet O, et al. Deleterious effect of Usutu virus on human neural cells. PLoS Negl Trop Dis. 2017;11(9):e0005913. doi: 10.1371/journal.pntd.0005913 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
H&E staining of liver, spleen, kidney, lung, and brain tissues harvested from Stat1fl/fl, Stat1-/-, Alb-Cre/Stat1fl/fl, Syn-Cre/Stat1fl/fl, Vav-Cre/Stat1fl/fl mice (n = 3/group) that were infected with 103 FFU of USUV via subcutaneous (SC) injection or corresponding mock mice. For each mouse genotype, one representative image was chosen. The scale bars for liver, spleen, kidney, and brain images are 200 µm. The scale bar for lung images is 100 µm.
(PDF)
Livers were harvested from Stat1-/- mice that were infected with 103 FFU of USUV via subcutaneous (SC) injection. Immunostaining of mouse liver tissue using the pan-immune cell marker CD45, the histiocyte marker CD68, anti-JEV NS3 to mark USUV infection, and DAPI. The scale bar indicates 50 µm.
(PDF)
Data Availability Statement
Viral genome sequences have been deposited to NCBI GenBank under the BioProject accession PRJNA1263528, with the corresponding raw reads available at the Sequence Read Archive (SRA). All data supporting the conclusions of this study are reported in the paper. All primary data are available via Princeton Data Commons repository at the following link: https://doi.org/10.34770/fwqx-xw53.