Abstract
Rationale:
Pneumatosis cystoides intestinalis (PCI) is a rare disease characterized by gas accumulation in the intestinal wall, and is usually treated conservatively. Patients with diabetic ketoacidosis (DKA) sometimes present with severe abdominal pain as the first symptom, which may be misdiagnosed. We report a case of PCI in a patient with diabetes mellitus (DM) misdiagnosed as gastrointestinal perforation and underwent exploratory laparotomy.
Patient concerns:
A 41-year-old woman with a history of DM treated with miglitol, an α-glucosidase inhibitors (αGI), was admitted to the emergency department with severe abdominal pain. Computed tomography revealed thickening of the ascending colon wall and scattered free gas around it, and the possibility of tumor perforation was considered.
Diagnosis:
The patient was diagnosed with gastrointestinal perforation and DM but was revised to PCI and DKA after surgery.
Interventions:
The patient underwent exploratory laparotomy; however, no signs of digestive perforation were found. The patient developed DKA after surgery and received conservative treatment, including antibiotics, insulin, fluid support, oxygen therapy, and cessation of miglitol.
Outcomes:
Ketoacidosis was controlled, and the abdominal pain resolved with conservative treatment. She was discharged 16 days later and no longer required αGI therapy. She did not develop gastrointestinal symptoms or any signs of PCI on computed tomography imaging within 3 months.
Lessons:
PCI is a rare disease with great heterogeneity in etiology, treatment and prognosis and comorbidities like diabetes may increase the chances of misdiagnosis. Surgeons should pay attention to the patient’s medical history and examination and carefully identify the real disease that triggers the symptoms to avoid misdiagnosis.
Keywords: α glucosidase inhibitors, diabetes mellitus, ketoacidosis, misdiagnose, pneumatosis cystoides intestinalis
1. Introduction
Pneumatosis cystoides intestinalis (PCI) is a rare disease characterized by multiple gas cysts in the serosa or submucosa of the digestive tract. It was first identified by Du Vernoi at autopsy in the 17th century and can occur throughout the entire digestive tract, especially the ascending colon, and is easily detectable on radiological examination. The etiology and pathogenesis of PCI remain unclear, and the actual incidence of PCI cannot be accurately calculated for patients are mostly asymptomatic or have mild symptoms. Patients with PCI alone are usually treated conservatively with a good prognosis. However, many patients may be complicated with multiple concomitant diseases and are prone to complications and some of them may be misdiagnosed with intestinal ischemia or gastrointestinal perforation thus being treated with surgical procedures.
In previous publications, PCI combined with diabetes mellitus (DM) was mostly caused by taking α-glucosidase inhibitors (αGI),[1–8] and can be easily cured by ceasing αGI. Although PCI is easily misdiagnosed as gastrointestinal perforation on radiological imaging, surgeons will not easily resort to surgical treatment because the abdominal symptoms are mostly mild. We report a case of PCI combined with DM, in which the patient presented with severe abdominal pain, tachypnea, hyperpnea, and tachycardia, was misdiagnosed with gastrointestinal perforation, and then developed diabetic ketoacidosis (DKA) after surgery, which we suspected was present preoperatively. To the best of our knowledge, this may be the first reported case of PCI combined with a DKA. This case is discussed in depth, and the literature is reviewed as follows.
2. Case presentation
A 41-year-old female was admitted to the hospital with a chief complaint of right lower abdominal pain for 3 days and sudden aggravation for 2 hours, accompanied by chills, tachypnea, hyperpnea, palpitations, nausea and vomiting. The patient was diagnosed with T2DM 8 months previously, and was well controlled with miglitol, empagliflozin and insulin. She presented with a distressed expression, severe pain in the right lower abdomen, pressing tenderness and suspected peritonitis on admission accompanied by a heart rate of 117 beats per minute and rapid, deep breathing of 24 beats per minute. Laboratory tests revealed the following leukocytes 12.56 × 1012/L, neutrophils 87.1%, high-sensitivity C-reactive protein 17.04 mg/L, blood glucose 11.97 mmol/L, sodium 133.9 mmol/L, and the rest of the laboratory tests did not show any obvious abnormalities. Computed tomography (CT) revealed thickening of the ascending colon wall and scattered free gas around it (Fig. 1), and the possibility of tumor perforation was considered. The patient was diagnosed with a gastrointestinal perforation and exploratory laparotomy was performed. However, we did not find any perforation lesions nor signs of infection but only swelling of the ascending colon on carefully examination of the entire digestive tract. Thus, the abdomen was closed after ileostomy according to the principle of damage control. The patient developed diabetic ketoacidosis (DKA) (arterial pH 7.26; β-hydroxybutyrate 6.14 mmol/L, urinary ketone+++) soon postoperatively and was improved through adequate fluid support and insulin therapy. αGI was discontinued after discharge and the patient did not develop gastrointestinal symptoms again or any signs of PCI on CT imaging within 3 months, thus stoma closure was performed.
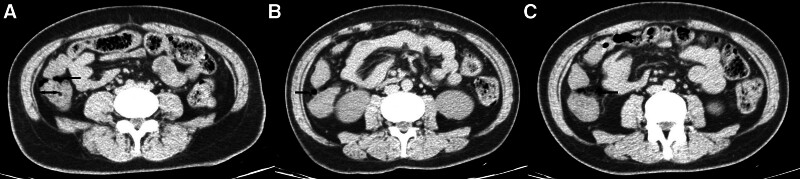
Figure 1.
The black arrow indicates PCI. (A) Thickened bowel wall (bidirectional arrow) (B) PCI on the opposite side of the mesentery (C) PCI at the mesangial border like free gas black arrow. PCI = pneumatosis cystoides intestinalis.
3. Discussion and conclusion
The diagnosis was revised to PCI after a multidisciplinary discussion, which was considered to be associated with miglitol, a kind of αGI. Severe abdominal pain at the time of admission, and swelling of the intestinal wall, and extraintestinal gas on radiological imaging were the main reasons for the misdiagnosis. The patient presented with severe abdominal pain, shortness of breath, tachypnea, and tachycardia, which could not be explained by PCI alone and was highly suspected of DKA on admission. Although the patient was normoglycemic, he was taking a sodium/glucose co-transporter-2 inhibitor, empagliflozin, which has been widely reported to cause euglycemic DKA.[9,10] The limitation of this case lies in the fact that we did not conduct arterial blood gas and ketone body tests before surgery. To our knowledge, this is the first case of hospitalization for both PCI and DKA. We conducted a search for relevant literature in PubMed, aiming to elaborate on the epidemiology, etiology, diagnosis, treatment methods, and prognosis of PCI.
3.1. Incidence
The incidence of PCI is generally considered low, and accurate statistics on its incidence are not available because many patients are asymptomatic. A cross-sectional survey showed that the detection rate of PCI using CT imaging in plateau areas of China reached 0.83%,[11] and other studies have reported that plateau areas accounted for 66.9% of all reported cases.[12] There are a few reports of PCI combined with DM, most of which are caused by αGI. We have not observed any report of misdiagnosis caused by PCI combined with DKA. We searched PUBMED with PCI and DM as key words, and found 21 English items with a total of 22 patients. The average age of these patients was 68 years, and 52.17% were female. The majority of these cases were from East Asia, with only 3 from Western countries. This is not necessarily due to racial differences, but the higher αGI use in Asians,[6] because fat accounts for a larger proportion of caloric intake in western countries,[13,14] which does not apply to the use of αGI. As we can see, 16 PCI cases were caused by αGI, and the duration of αGI use varied from 7 days to 14 years (Table 1).
Table 1.
Diagnostic information of PCI patients with DM.
| Author year | Age gender | Region | DM history | Symptoms and signs | PCI location | Other CT Feature | Blood tests | Concomitant disease and treatment |
|---|---|---|---|---|---|---|---|---|
| Dong Jin Park 2024[15] | 57F | Korea | NA | None | Ileum | Necrotizing Enteritis | Normal | Metastatic Renal Cell Carcinoma; (Sunitinib) |
| Shinya Otsuka 2021[1] | 59M | Japan | 4 yr of Voglibose (0.9 mg daily); Insulin | None | Ascending To Descending Colon | Free Gas | Normal | Lung Transplantation; immunosuppressive Therapy (Tacrolimus and PSL) |
| Andrea Police 2020[2] | 72 | France | 10 yr of Acarbose; | Abdominal pain and distention without peritonitis; | Sigmoid colon | Sigmoid Volvulus; Colonic Wall Thickened; Dolichocolon; | NA | NA |
| Divya S. Shetty 2020[16] | 85F | Indian | NA | Abdominal distention; severe abdominal pain; constipation | Small intestine | PVG, SMVG Intestinal Obstruction |
WBC 20;500/mm3 (NEU 86%) | NA |
| Minjia Wang 2020[17] | 89M | China | NA | Fever And Abdominal Distention | Small Intestine and Colon | PVG SMVG |
WBC 17,100/µL (NEU81.8%); CRP47.76 mg/L; PCT 5.99 ng/mL; PH 7.30 | COPD; CAD Hypertension; Hypercholesterolemia |
| Yong Juan Wang 2018[18] | 72F | China | 10 yr of Acarbose; Insulin |
Constipation; Diarrhea; Bloody Stool; Severe Abdominal Distension; Generalized Tenderness | Colon | NA. | Normal | |
| Eiji Suzuki 2017[19] | 71F | Japan | Voglibose | Right Malar Rash; Saddle Nose; Right Abdominal Distension; No Tenderness |
Small and Large Intestine | NA. | WBC 9;400/μL (NEU; 83.4%); HbA1c; 7.9% ESR 121 mm/h |
Granulomatosis With Polyangiitis; (PSL) |
| 70F | Japan | 10 yr of Voglibose | Severe Back Pain; Body Temperature Was Slightly High |
Colon | Free Gas | WBC 17;700/μL (NEU; 88.5%); HbA1c;5.5%; CRP; 14.63 mg/dl; ESR; 38 mm/h |
Rheumatoid Arthritis (PSL) | |
| Yen-Hsiu Liao 2017[3] | 50M | Taiwan | 1 yr of Acarbose (150 mg daily) | Dull Abdominal Pain, Fever; Diarrhea; Green Stool. |
Colon | NA. | NA. | |
| Dorota Ksiadzyna 2016[20] | 64M | Netherlands | 8 yr of Acarbose (150 mg daily) Metformin (1.5 g daily) | Diarrhea; Flatulence; Diffuse and Mild Abdominal Pain | Cecum And Splenic Flexure Colon | NA. | Normal | NA. |
| Hiroaik Makiyami 2014[21] | 80F | Japan | Voglibose (0.6 mg daily) | Abdominal Distention; Discomfort; Tenderness; Vomiting; No Peritonitis |
Ileum | PVG | WBC 15,400/μL (NEU;82.8%) | NA. |
| Shunsuke Tanabe 2013[4] | 80F | Japan | α-GI | Constipation; Abdominal Distention; Pain; Mild Tenderness; No Peritonitis |
Intestinal Tract Wall | Free Gas | Normal | Hypertension; Hemiplegia |
| Yasuhiro Shimojima 2011[22] | 48M | Japan | 6 wk of Voglibose, Glimepiride | Tenderness; Without Peritonitis | Colon; Especially Ascending Colon | Normal | Systemic Lupus Erythematosus; (PSL) |
|
| Kuniyuli Kojima 2010[5] | 58M | Japan | 2 yr of Miglitol (150 mg daily) | Abdominal Pain;Rectal Bleeding | Ileocecal and Ascending Colon | Thickening Bowel Walls, Free Gas |
Normal | NA. |
| Hideto Suzuki 2009[23] | 67F | Japan | Diet therapy | Nausea; Abdominal Pain; Mild Tenderness; Hypopnea of Bowel Sounds; | Colon | NA. | WBC (10;700/ul) | NA |
| Tatsuhiro Tsujimoto 2008[6] | 69M | Japan | 3 yr of α GI | Abdominal Distension; Constipation; Rectal Bleeding; |
Sigmoid Colon; | Free Gas | HbA1c 6.0%; | Myasthenia Gravis (PSL) |
| Yoshitaka Maeda 2007[24] | 72F | Japan | 3 yr of Voglibose (0.9 mg daily); insulin | Fever; Abdominal Pain; Transient Unconsciousness; Abdominal Tenderness; |
NA | Intestinal Lumen Narrowed | WBC 11,500/μL | Nephrotic Syndrome; Immunosuppressive Therapy (PSL And Mizoribine) |
| Akiko Hismoto 2006[7] | 56F | Japan | 7 d of Voglibose (600 mg daily) | None | Ascending Colon; | NA. | Normal | Interstitial Pneumonitis; Prednisone |
| K. Nakamura 2003[25] | 56M | Japan | NA | None | Cecum To Transverse Colon | NA | Normal | Renal Transplant; Immunosuppressive (Tacrolimus; PSL; Mizoribine; 15-Deoxyspergualin) |
| Yasushi Azami 2000[26] | 87F | Japan | 14 yr of Glibenclamide (5 mg daily), Acarbose (150 mg daily); |
Abdominal Distension; Loss of Appetite; No Peritonitis | Small Intestine | Gaseous Distension of Small Intestine | Normal | Hypothyroidism Paralytic Ileus. |
| Hayakawa T. 1999[8] | 64F | Japan | 20 yr of insulin, 2 mo of voglibose (0.6 mg/d) | Abdominal Distention | Ascending to Proximal Transverse Colon | NA. | HbA1c 7.0% | NA. |
| Bonnell H 1982[27] | 72M | American | 17 yr of Insulin | NA. | NA. | NA. | NA. | Pulmonary edema, renal failure, osteomyelitis |
CRP = C-reaction protein, CT = computed tomography, DM = diabetes mellitus, ESR = erythrocyte sedimentation rate, NA = not applicable, NEU = neutrophil, PCI = pneumatosis cystoides intestinalis, PSl = prednisolone, PVG = portal vein gas, SMVG = superior mesenteric vein gas, TPN = total parenteral nutrition, WBC = white blood cell.
3.2. Etiology
The cause of PCI is unknown and may be associated with a number of comorbidities, traumatic procedures or medication use, such as: Respiratory diseases,[28,29] connective tissue diseases,[30,31] malignancies,[15,32] Surgical complications,[33,34] gastrointestinal dysfunction,[35] Immunosuppressive agents[36,37] and so on. In the past decades, some theories about its mechanism have been gradually formed, with the most famous being the mechanics theory and microbial fermentation theory. When the physical and immune barriers of the intestine are damaged for various reasons, increased intestinal pressure or lung diseases may lead to the diffusion of gas into the submucosa and serosal layer, where bacteria colonize and ferment to convert carbohydrates into hydrogen to form cysts.[38] However, no single theory perfectly explains the pathophysiology of PCI. Several studies have shown that αGI administration is the main cause of PCI in patients with DM.[18–20,22,24,26] Patients with DM tend to use αGI more frequently in Asian countries. This may be due to the eating habits of the population, which may affect the composition of their intestinal microbiota, and the fermentation process of this microbiota may play an important role in the occurrence of PCI. Therefore, we believe that a nutritionist may also play an important role in managing these cases, as they do in the management of cancer patients.[39] However, 27% of the patients did not have a clear history of αGI, and approximately half of the patients had other underlying diseases and drug use (Table 1). Therefore, it is necessary to pay more attention to diabetes patients with no history of αGI administration when PCI occurs, as the treatment and prognosis may be quite different.
3.3. Diagnosis
Depending on the comorbidities, patients undergoing PCI may have different clinical presentations. Patients undergoing PCI alone or due to αGI are usually asymptomatic or have only mild nonspecific gastrointestinal symptoms, manifesting as abdominal distension, abdominal pain, nausea, vomiting, diarrhea, constipation, rectal bleeding, and weight loss according to the distribution in different intestines. Laboratory tests results are usually nonspecific, and the markers of infection are mostly normal or mildly elevated. However, patients may also present with severe gastrointestinal or systemic symptoms; the patient we report is a particular example, although we suspect that it was caused by DKA. Particularly, attention should be paid to the complications of PCI, such as volvulus,[2,40] intestinal ischemia,[16] intestinal obstruction,[41,42] intussusception[43–45] and uncontrolled gastrointestinal bleeding,[46,47] which may require surgical intervention and are potentially life-threatening.
CT imaging is important in the diagnosis of PCI. Multiple cystic air density shadows of different sizes were observed in the intestinal wall, some of which were connected in strings, protruding from the inside and outside of the intestinal lumen. No obvious exudation was observed around the cysts. These lesions can be distributed throughout the digestive tract, but mainly in the intestines, especially in the colon. It should be noted that PCI can also show other special imaging signs, such as extraluminal free air,[1] intestinal wall thickening,[2] and portal vein gas (PVG),[21,48] which are easily misdiagnosed as gastrointestinal perforation, intestinal necrosis, or tumor. Among the 22 patients of PCI with DM, 5 patients presented with free abdominal gas, 3 with PVG and 2 with bowel wall thickening (Table 1). A cross-sectional study in Tibet have shown that the incidence of PCI complicated with free abdominal gas is up to 60.36%,[11] which may lead to a high rate of misdiagnosis and most patients do not have severe symptoms. Therefore, CT imaging should not be over-relied upon to assess the condition of patients with PCI. Careful history-taking and physical examination are the basis for preventing misdiagnosis. In addition, endoscopy and plain abdominal radiography are diagnostic methods for PCI but appear to be less sensitive or convenient when compared with CT imaging.
3.4. Treatment
Most patients with PCI alone improved with conservative treatment. Discontinuation of αGI therapy in patients with DM is extremely important. Other treatments include fasting, rehydration, antibiotics, endoscopic therapy, hyperbaric oxygen therapy, and probiotics, the effectiveness of which is controversial (Table 2), such as oxygen therapy.[25,38] Although most patients can improve after the above treatments, some patients still require surgery even emergency surgery. A systematic analysis reported that the operation rate in China was 40.6% in 10 years,[12] among which many were nontherapeutic or misdiagnosed operations with a rate of about 15% in a report from the University of Virginia.[49] Since the probability of abdominal free gas after subserosal PCI rupture is 60.36%,[12] it must be strictly differentiated from gastrointestinal perforation and surgical exploration is recommended for patients with severe symptoms, high white blood cell count, and extensive abdominal exudation on imaging. Among the 22 patients with PCI and DM, 3 underwent surgery, 2 of whom underwent intestinal resection due to sigmoid colon torsion and small bowel ischemia caused by PCI, respectively, and the other was suspected of intestinal necrosis due to hepatic PVG on CT imaging and underwent laparotomy, but no intestinal abnormalities were found.[21] In our view, surgery is performed to address the comorbidities or complications of PCI rather than PCI itself. It can be considered for PCI patients with intestinal volvulus, intestinal obstruction, necrosis or uncontrolled gastrointestinal bleeding. Laparoscopic exploration may also be considered when vital signs are stable. However, emergency surgery is not always necessary.
Table 2.
Treatment and prognosis of PCI patients with DM.
| Author/year | Conservative treatment | Surgery | Recovery time and evaluation method | Poor prognosis |
|---|---|---|---|---|
| Park 2024[15] | TPN*†‡, ceasing oral diabetes drugs | 3 mo (CT) | ||
| Otsuka 2021[1] | Bowel rest†‡§ | 11 d (X-ray) | ||
| Police 2020[2] | – | Sigmoidectomy | 1 mo (symptoms) | – |
| Shetty 2020[16] | – | Enterectomy | – | Died |
| Wang 2020[17] | Palliative therapy | – | – | Died |
| Wang 2018[18] | Endoscopic*§ treatment; probiotics | – | 1 mo (symptoms) 6 mo (colonoscopy) | – |
| Suzuki 2017[19] | Properistaltic agents§ | – | 1 mo (CT) | – |
| * § | – | 2 wk (CT and laboratory) | – | |
| Hsiu Liao 2017[3] | § | – | NA | – |
| Ksiadzyna 2016[20] | § | – | 4 wk (symptoms, CT) | – |
| Makiyami 2014[21] | § | Laparotomy | NA (symptoms) | – |
| Tanabe 2013[4] | * † | – | 1 d (symptom), 2 d (CT) | – |
| Shimojima 2011[22] | Ceasing† glimepiride panthenol and dinoprost | – | 1 wk (X-ray free air disappeared) 19 wk (X-ray PCI disappeared) |
– |
| Kojima 2010[5] | † § | – | 12 d (colonoscopy); | – |
| Suzuki 2009[23] | Laxative; enema | – | – | Died |
| Tsujimoto 2008[6] | † § | – | 2 wk (X-ray); 3 mo (colonoscopy) | – |
| Maeda 2007[24] | Ventilator† therapy; hemofiltration | – | Rapid deterioration and eventually improved | – |
| Hismoto 2006[7] | † ‡ | – | 1 wk (symptoms) | – |
| Nakamura 2003[25] | ‡ | – | – | No efficacy |
| Azami 2000[26] | † § | – | 5 d (symptoms and CT) | |
| Hayakawa 1999[8] | † § | – | 4 d (X-ray) | |
| Bonnell 1982[27] | – | – | – | Died |
CT = computed tomography, DM = diabetes mellitus, PCI = pneumatosis cystoides intestinalis, TPN = total parenteral nutrition.
Antibiotics.
Fasting and fluid support.
Oxygen therapy.
Ceasing α-GI
3.5. Prognosis
The prognosis of PCI patients with DM is generally good. A prediction model for intestinal ischemia in PCI patients also suggested that patients with DM were less likely to undergo surgical intervention, which may be due to αGI induction in most PCI patients with DM. However, we retrieved 4 PCI patients with DM who eventually died (Table 2), and all of them were due to comorbidities,[16,17,23,27] which may mean that the prognosis of patients depends more on comorbidities or complications than on PCI itself. Patients with PCI are easily misdiagnosed and treated with nontherapeutic procedures, which may aggravate comorbidities and lead to a poor prognosis. According to an appellate study, the rate of noncurative surgery is as high as 15%.
The limitations of this review lie in its focus on the analysis of PCI combined with DM. The cited literature is mostly case reports, and some of the publications were from a long time ago, resulting in insufficient data and information. Although we made every effort to conduct a thorough review of PCI, there may still be some inadequacies that prevent readers from gaining a clear understanding of PCI.
In conclusion, PCI is a rare disease with significant heterogeneity in etiology, treatment and prognosis. It is easily misdiagnosed and its pathophysiology is still poorly understood. In patients with diabetes, the use of αGI is the most common cause of PCI, and the prognosis is good with conservative treatment. Surgery is mainly aimed at comorbidities or complications, and surgeons should pay attention to the patient’s medical history and examination and carefully identify the real disease that triggers the symptoms to avoid misdiagnosis and mistreatment.
Author contributions
Conceptualization: Wenjie Zhou.
Investigation: Wenjie Zhou.
Supervision: Yonghong Wang.
Visualization: Wenjie Zhou, Yonghong Wang.
Writing – original draft: Wenjie Zhou, Jie Dan, Mingjie Zhu, Yonghong Wang.
Writing – review & editing: Wenjie Zhou, Jie Dan, Mingjie Zhu, Ke Liu, Yonghong Wang.
Abbreviations:
- αGI
- α glucosidase inhibitors
- CT
- computed tomography
- DKA
- diabetic ketoacidosis,
- DM
- diabetes mellitus
- PCI
- pneumatosis cystoides intestinalis
This case report has obtained the informed consent of the patient. Ethical approval for this study (Ethical Committee No. LYLL 2025 KY 015) was provided by the Ethical Committee of Leshan People’s Hospital review board, Sichuan, China on February 18, 2025.
The authors have no funding and conflicts of interest to disclose.
Data sharing not applicable to this article as no datasets were generated or analyzed during the current study.
How to cite this article: Zhou W, Dan J, Zhu M, Liu K, Wang Y. Pneumatosis cystoides intestinalis and ketoacidosis in a diabetic patient: A case report and literature review. Medicine 2025;104:29(e43481).
Contributor Information
Wenjie Zhou, Email: zwj_8909@163.com.
Jie Dan, Email: 34135705@qq.com.
Mingjie Zhu, Email: 17811162@qq.com.
Ke Liu, Email: 512238588@qq.com.
References
- [1].Otsuka S, Ujiie H, Kato T, et al. Pneumatosis intestinalis after living donor lung transplantation associated with alpha-glucosidase inhibitor treatment: a case report. Transplant Proc. 2021;53:1379–81. [DOI] [PubMed] [Google Scholar]
- [2].Police A, Charre L, Volpin E, Antonopulos C, Braham H, El Arbi N. Pneumatosis cystoides intestinalis induced by the alpha-glucosidase inhibitor complicated from sigmoid volvulus in a diabetic patient. Int J Colorectal Dis. 2020;35:943–6. [DOI] [PubMed] [Google Scholar]
- [3].Liao YH, Lo YP, Liao YK, Lin C-H. Pneumatosis cystoides coli related to acarbose treatment. Am J Gastroenterol. 2017;112:984. [DOI] [PubMed] [Google Scholar]
- [4].Tanabe S, Shirakawa Y, Takehara Y, et al. Successfully treated pneumatosis cystoides intestinalis with pneumoperitoneum onset in a patient administered α-glucosidase inhibitor. Acta Med Okayama. 2013;67:123–8. [DOI] [PubMed] [Google Scholar]
- [5].Kojima K, Tsujimoto T, Fujii H, et al. Pneumatosis cystoides intestinalis induced by the α-glucosidase inhibitor miglitol. Intern Med. 2010;49:1545–8. [DOI] [PubMed] [Google Scholar]
- [6].Tsujimoto T, Shioyama E, Moriya K, et al. Pneumatosis cystoides intestinalis following alpha-glucosidase inhibitor treatment: a case report and review of the literature. World J Gastroenterol. 2008;14:6087–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Hisamoto A, Mizushima T, Sato K, et al. Pneumatosis cystoides intestinalis after alpha-glucosidase inhibitor treatment in a patient with interstitial pneumonitis. Intern Med. 2006;45:73–6. [DOI] [PubMed] [Google Scholar]
- [8].Hayakawa T, Yoneshima M, Abe T, Nomura G. Pneumatosis cystoides intestinalis after treatment with an alpha-glucosidase inhibitor. Diabetes Care. 1999;22:366–7. [DOI] [PubMed] [Google Scholar]
- [9].Sampani E, Sarafidis P, Dimitriadis C, et al. Severe euglycemic diabetic ketoacidosis of multifactorial etiology in a type 2 diabetic patient treated with empagliflozin: case report and literature review. BMC Nephrol. 2020;21:276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Morace C, Lorello G, Bellone F, et al. Ketoacidosis and SGLT2 inhibitors: a narrative review. Metabolites. 2024;14:264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Xiaoli G, Yan Z, Fengdan W, et al. Analysis of multi spiral CT findings and clinical manifestations of pneumatosis cystoides intestinalis in plateau region. J Pract Radiol. 2023;39:946–9. [Google Scholar]
- [12].Wu LL, Yang YS, Dou Y, Liu Q-S. A systematic analysis of pneumatosis cystoids intestinalis. World J Gastroenterol. 2013;19:4973–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Smyth S, Heron A. Diabetes and obesity: the twin epidemics. Nat Med. 2006;12:75–80. [DOI] [PubMed] [Google Scholar]
- [14].Wysowski DK, Armstrong G, Governale L. Rapid increase in the use of oral antidiabetic drugs in the United States, 1990-2001. Diabetes Care. 2003;26:1852–5. [DOI] [PubMed] [Google Scholar]
- [15].Park DJ, Lee D, Park IK. Pneumatosis cystoides intestinalis induced by sunitinib therapy in a patient with metastatic renal cell carcinoma: a case report. Medicine (Baltim). 2024;103:e39075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Shetty DS, Naik LP, Amarapurkar AD. , Bubbly bowel: a life-threatening condition. Indian J Pathol Microbiol. 2020;63:325–6. [DOI] [PubMed] [Google Scholar]
- [17].Wang M, Song J, Gong S, Yu Y, Hu W, Wang Y. Hepatic portal venous gas with pneumatosis intestinalis secondary to mesenteric ischemia in elderly patients: two case reports. Medicine (Baltim). 2020;99:e19810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Wang YJ, Wang YM, Zheng YM, Jiang HQ, Zhang J. Pneumatosis cystoides intestinalis: six case reports and a review of the literature. BMC Gastroenterol. 2018;18:100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Suzuki E, Kanno T, Hazama M, Kobayashi H, Watanabe H, Ohira H. Four cases of pneumatosis cystoides intestinalis complicated by connective tissue diseases. Intern Med. 2017;56:1101–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Ksiadzyna D, Peña AS. , Segmental pneumatosis cystoides coli: computed tomography-facilitated diagnosis. Rev Esp Enferm Dig. 2016;108:510–3. [DOI] [PubMed] [Google Scholar]
- [21].Makiyama H, Kataoka R, Tauchi M, Sumitomo H, Fuita R. Do alpha-glucosidase inhibitors have the potential to induce portal venous gas? – Two clinical case reports. Intern Med. 2014;53:691–4. [DOI] [PubMed] [Google Scholar]
- [22].Shimojima Y, Ishii W, Matsuda M, Tojo K, Watanabe R, Ikeda S-I. Pneumatosis cystoides intestinalis in neuropsychiatric systemic lupus erythematosus with diabetes mellitus: case report and literature review. Mod Rheumatol. 2011;21:415–9. [DOI] [PubMed] [Google Scholar]
- [23].Suzuki H, Murata K, Sakamoto A. An autopsy case of fulminant sepsis due to pneumatosis cystoides intestinalis. Leg Med (Tokyo). 2009;11(Suppl 1):S528–30. [DOI] [PubMed] [Google Scholar]
- [24].Maeda Y, Inaba N, Aoyagi M, Kanda E, Shiigai T. Fulminant pneumatosis intestinalis in a patient with diabetes mellitus and minimal change nephrotic syndrome. Intern Med. 2007;46:41–4. [DOI] [PubMed] [Google Scholar]
- [25].Nakamura K, Ohmori Y, Okamoto M, et al. Renal transplant recipient experiencing pneumatosis cystoides intestinalis: a case report. Transplant Proc. 2003;35:297–9. [DOI] [PubMed] [Google Scholar]
- [26].Azami Y. Paralytic ileus accompanied by pneumatosis cystoides intestinalis after acarbose treatment in an elderly diabetic patient with a history of heavy intake of maltitol. Intern Med. 2000;39:826–9. [DOI] [PubMed] [Google Scholar]
- [27].Bonnell H, French SW. Fatal air embolus associated with pneumatosis cystoides intestinalis. Am J Forensic Med Pathol. 1982;3:69–72. [DOI] [PubMed] [Google Scholar]
- [28].Setake M, Matsuno K, Arakaki K, Hokama A. Strongyloides stercoralis hyperinfection presenting pneumatosis intestinalis and acute respiratory distress syndrome after treatment for COVID-19. Rev Esp Enferm Dig. 2024;116:574–5. [DOI] [PubMed] [Google Scholar]
- [29].Yu G, Wang J, Kan B, Li W, Jian X. Images in acute diquat poisoning, including hepatic portal venous gas and gastrointestinal pneumatosis on computed tomography. Clin Toxicol (Phila). 2024;62:669–71. [DOI] [PubMed] [Google Scholar]
- [30].Mitsuyoshi Y, Takakura K, Kobayashi T, et al. Chronic intestinal pseudo-obstruction with pneumatosis cystoides intestinalis in a patient with systemic sclerosis: a case report. Medicine (Baltim). 2019;98:e15480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Liu J, Zhang L, Chen S, Lu X, Li S. Pneumatosis cystoides intestinalis in dermatomyositis: a case series report and literature review. Front Immunol. 2023;14:1194721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Chang CJ, Shen CI, Wu CL, Chiu C-H. Pneumatosis intestinalis induced by targeted therapy. Postgrad Med J. 2022;98:10. [DOI] [PubMed] [Google Scholar]
- [33].Castren EE, Hakeem AR, Mahmood NS, Aryal K. Case of pneumatosis intestinalis and hepatic portal venous gas following a laparoscopic right hemicolectomy. BMJ Case Rep. 2016;2016:bcr2016214431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Yeo IH, Kim YJ. Two case reports of pneumatosis intestinalis in patients with cancer: is surgical management mandatory? Clin Exp Emerg Med. 2021;8:237–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Donati F, Boraschi P, Giusti S, Spallanzani S. Pneumatosis cystoides intestinalis: imaging findings with colonoscopy correlation. Dig Liver Dis. 2007;39:87–90. [DOI] [PubMed] [Google Scholar]
- [36].Aman Bilir O, Demir AM, Akçabelen YM, et al. Pneumatosis cystoides intestinalis: a rare complication after hematopoietic stem cell transplantation. Pediatr Transplant. 2021;25:e14136. [DOI] [PubMed] [Google Scholar]
- [37].You SS, Lin ZN, Sheng LX, Lai Y-L. Acute lymphoblastic leukemia with pneumatosis cystoides intestinalis after hematopoietic stem cell transplantation: a case report. J Int Med Res. 2024;52:645631787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Alpuim CD, Modas DP, Vieira BJ. , The role of hyperbaric oxygen therapy in pneumatosis cystoides intestinalis-a scoping review. Front Med (Lausanne). 2021;8:601872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].De Felice F, Malerba S, Nardone V, et al. Progress and challenges in integrating nutritional care into oncology practice: results from a national survey on behalf of the nutrionc research group. Nutrients. 2025;17:188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Zouaghi A, Hadded D, Meryam M, et al. Case Report: an unusual case of small bowel volvulus due to appendicitis associated with pneumatosis intestinalis: review of the literature. F1000Res. 2021;10:951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Seket B, Kaczmarek D, Tiffet O. Intestinal pseudo-obstruction and pneumatosis cystoides intestinalis in a scleroderma patient. J Am Coll Surg. 2007;205:180–1. [DOI] [PubMed] [Google Scholar]
- [42].Paris MD, Fraire AE. , Intestinal obstruction from pneumatosis cystoides intestinalis. JAMA. 1981;246:622. [PubMed] [Google Scholar]
- [43].Takahashi Y, Shibagaki K, Fukuyama C, et al. Endoscopic fenestration treatment for pneumatosis cystoides intestinalis in patient with recurrent colonic intussusception. Endoscopy. 2023;55(S 01):E452–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Iio K, Hagiwara Y. Colocolic intussusception secondary to pneumatosis cystoides intestinalis: the role of abdominal radiography in the diagnosis. BMJ Case Rep. 2022;15:e252991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Otsubo Y, Ichikawa G, Yoshihara S, Kuwashima S. Pneumatosis intestinalis caused by intussusception. Postgrad Med J. 2022;98:e66. [DOI] [PubMed] [Google Scholar]
- [46].Dantas E, Coelho M, Cardoso C, Oliveira AP. A rare cause of lower gastrointestinal bleeding: pneumatosis cystoides intestinalis. Gastroenterol Hepatol. 2022;45:555–6. [DOI] [PubMed] [Google Scholar]
- [47].Laranjo A, Currais P, Veloso N, Faias S. Pneumatosis cystoides intestinalis: a rare cause of gastrointestinal hemorrhage. GE Port J Gastroenterol. 2021;28:440–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Ito M, Abe M, Maruyama T, et al. Pneumatosis cystoides intestinalis and hepatic portal venous gas on peritoneal dialysis. Clin Nephrol. 2014;82:347–50. [DOI] [PubMed] [Google Scholar]
- [49].Umapathi BA, Friel CM, Stukenborg GJ, Hedrick TL. Estimating the risk of bowel ischemia requiring surgery in patients with tomographic evidence of pneumatosis intestinalis. Am J Surg. 2016;212:762–8. [DOI] [PubMed] [Google Scholar]