Abstract
In recent years, the role of the gut microbiome in stone formation has gained increasing attention. Specifically, certain gut microbes that metabolize oxalate may regulate oxalate levels in the body, thereby influencing the occurrence of kidney stones. This study aims to investigate the clinical characteristics of calcium oxalate stone patients, the composition of their gut microbiome, and the relationship between these factors and stone formation. This study included 159 calcium oxalate stone patients (case group) and 141 healthy controls (control group). Clinical data were collected to analyze differences in body mass index, urinary metabolic markers (urinary oxalate, calcium, and pH), and other indicators between the 2 groups. High-throughput 16S rRNA sequencing was used to compare the diversity and composition of the gut microbiome. Further, correlations between the gut microbiome and clinical metabolic indicators were analyzed, and a risk prediction model for calcium oxalate stones was developed based on clinical and gut microbiome characteristics. Significant differences in body mass index, urinary oxalate concentration, urinary calcium concentration, and urinary pH were observed between the case and control groups. Gut microbiome diversity analysis revealed that the Shannon and Chao1 indices were lower in the case group, and significant differences in microbiome composition were found. The abundance of Proteobacteria and Firmicutes changed significantly, with a notable decrease in Oxalobacter formigenes and an increase in Escherichia-Shigella species in the case group. Correlation analysis showed a negative correlation between O formigenes abundance and urinary oxalate concentration, and a negative correlation between Escherichia-Shigella and urinary pH. The random forest prediction model exhibited high predictive accuracy (area under the receiver operating characteristic curve = 0.90). The formation of calcium oxalate stones is closely related to the structure and function of the gut microbiome, particularly with the reduced abundance of the oxalate-degrading bacterium O formigenes. Gut microbiome imbalance may influence stone formation through various mechanisms. This study provides new theoretical insights for the early prediction, prevention, and treatment of calcium oxalate stones.
Keywords: calcium oxalate stones, gut microbiome, Oxalobacter formigenes, prediction model, urinary metabolism
1. Introduction
Calcium oxalate kidney stones are the most common form of urinary system stones, representing the majority of clinical cases. These stones are characterized by a high recurrence rate, which significantly impairs patients’ quality of life and imposes a substantial burden on healthcare systems.[1,2] Epidemiological studies report that approximately 50% of patients experience recurrence within 10 years, with risk increasing over time and with age.[3] These clinical challenges underscore the need for more accurate predictive tools and a deeper understanding of the underlying pathophysiology to support precision medicine approaches in stone management.
Traditionally, kidney stone formation has been attributed to factors such as urinary supersaturation of solutes, pH imbalance, low urine volume, and urinary tract infections.[4] However, emerging evidence indicates that the gut microbiome (via the so-called “gut-kidney axis”) may play a pivotal role in regulating urinary chemical composition and influencing stone development.[5–7] Specifically, certain bacterial species such as Oxalobacter formigenes possess oxalate-degrading capabilities that can reduce urinary oxalate concentrations and thereby lower the risk of calcium oxalate stone formation. Moreover, microbiota-derived metabolites, such as short-chain fatty acids, may regulate urinary pH and modulate the stone-forming environment.[8]
Recent studies have also highlighted that gut dysbiosis may contribute to stone formation indirectly, by disrupting the intestinal barrier or triggering systemic inflammation, which in turn alters the urinary metabolic milieu.[9–11] Despite these advances, most current studies are limited to animal models or small-scale clinical observations.[12] These investigations have consistently shown that stone formers tend to exhibit reduced gut microbial diversity and decreased abundance of oxalate-degrading bacteria such as O formigenes when compared to healthy individuals.[13–16]
While these findings suggest a potential role for the gut microbiota in stone pathogenesis, a critical gap remains: no studies to date have systematically integrated clinical metabolic characteristics with gut microbiome profiles to develop a predictive model for calcium oxalate stone risk in humans. Furthermore, the predictive value of microbiota-related variables has not been clearly established.
In clinical practice, current diagnostic and risk assessment strategies rely heavily on imaging techniques (e.g., ultrasound, CT) and urinary biochemical analysis.[17] While useful, these methods do not capture the complexity of host–microbiota interactions that may drive stone formation. Therefore, there is a compelling need for an integrative prediction model that combines clinical and microbial data to improve risk stratification and guide individualized prevention strategies.
In this study, we aim to address this knowledge gap by systematically analyzing the clinical metabolic features and gut microbiome composition of calcium oxalate stone patients compared to healthy controls. Using high-throughput 16S rRNA sequencing and machine learning algorithms, we construct a comprehensive risk prediction model. Our findings are expected to provide novel insights into the mechanisms of stone formation and offer a new approach for early prediction and microbiome-targeted prevention of calcium oxalate kidney stones.
2. Methods
2.1. Study participants
This study was approved by the Ethics Committee of General Hospital of Northern Theater Command. This study enrolled a total of 300 participants, including 159 calcium oxalate kidney stone patients (case group) and 141 healthy controls (control group). All participants were adults, and individuals with a history of metabolic disorders (such as diabetes, liver disease, or immune system disorders) were excluded. No significant differences were observed between the 2 groups in terms of gender, age, dietary habits, or lifestyle. All patients and controls provided written informed consent upon recruitment.
Inclusion criteria:
Aged 18 years or older, regardless of gender.
Patients with calcium oxalate kidney stones confirmed by imaging methods (e.g., CT, ultrasound) combined with infrared spectroscopy or X-ray diffraction analysis of the extracted stone composition after spontaneous passage or surgical retrieval.
Healthy controls with no history of kidney stones and no significant metabolic diseases.
Exclusion criteria:
Individuals with systemic diseases affecting metabolism, such as diabetes, liver disease, chronic kidney disease, etc.
Individuals using antibiotics, immunosuppressants, or other drugs that may impact the gut microbiome.
Pregnant or breastfeeding women.
Individuals with a history of major surgery or gastrointestinal diseases (such as inflammatory bowel disease) in the recent past.
Individuals who did not consent to participate or could not provide valid samples.
2.2. Collection of clinical data
In this study, basic clinical data were collected from each participant, including age, gender, body mass index (BMI), and urinary metabolic indicators (such as urine calcium concentration, urine oxalate concentration, and urine pH). These data were collected through standardized procedures, including routine testing methods for blood glucose, urine composition, and other relevant metrics.
2.3. Gut microbiome analysis
High-throughput 16S rRNA sequencing was used to analyze the participants’ gut microbiome. Stool samples were collected and DNA was extracted. The V3 to V4 region of the 16S rRNA gene was amplified via PCR, and sequencing was performed using the Illumina MiSeq platform. Bioinformatic analysis was conducted on the obtained sequences, calculating both alpha diversity (using Shannon and Chao1 indices) and beta diversity (using principal coordinate analysis, PCoA). Data processing and analysis were performed using QIIME2 software. LEfSe (linear discriminant analysis effect size) was used to assess the taxonomic differences in gut microbiota between the case and control groups. This analysis calculates the significant differences in microbiota classification through linear discriminant analysis and selects statistically significant microbial taxa.
2.4. Correlation analysis between clinical features and gut microbiome
Spearman correlation analysis was conducted to explore the relationship between clinical metabolic indicators and gut microbiome abundance. The correlations between urinary metabolic indicators (such as urine oxalate, urine calcium, urine pH) and gut microbiota abundance (e.g., O formigenes, Escherichia-Shigella) were statistically analyzed using SPSS software.
2.5. Development and validation of the calcium oxalate kidney stone risk prediction model
A random forest algorithm was used to develop a risk prediction model for calcium oxalate kidney stones. The model input variables included clinical metabolic parameters (such as urine calcium, urine oxalate, urine pH, and BMI) and gut microbiome features (such as the abundance of O formigenes and Escherichia-Shigella). The random forest algorithm optimized the model’s prediction performance through cross-validation, evaluating the model’s sensitivity, specificity, and area under the receiver operating characteristic (AUC) curve. Model evaluation was performed using R software.
2.6. Statistical analysis
This study adopted a case-control design, and the sample size calculation was based on differences in gut microbiome abundance and clinical metabolic indicators, especially considering the correlation between variables such as urine oxalate concentration, urine calcium concentration, and the abundance of O formigenes. Based on the expected effect size (e.g., the correlation between O formigenes abundance and urine oxalate concentration, r = ‐0.43), an alpha value (0.05), and statistical power (0.80), the sample size was calculated using G*Power software. The result indicated that at least 130 participants per group were needed to ensure sufficient statistical power (80%). To account for potential sample attrition and missing data, a final total of 159 cases and 141 controls were included to ensure the reliability and statistical effectiveness of the study.
All statistical analyses were performed using SPSS 22.0 software. Quantitative data were expressed as mean ± standard deviation, and inter-group comparisons were performed using t tests or Mann-Whitney U tests. Qualitative data were analyzed using chi-square tests. A P-value <.05 was considered statistically significant.
3. Result
3.1. Clinical characteristics of the study participants
This study included a total of 300 participants, consisting of 159 calcium oxalate kidney stone patients (case group) and 141 healthy controls (control group). The specific clinical characteristics are shown in Table 1. There were no significant differences between the 2 groups in terms of age, gender, dietary habits, and lifestyle. However, significant differences were observed between the case and control groups in terms of BMI and urinary metabolic indicators. The average BMI of the case group was 27.6 ± 3.5, while the control group had a BMI of 24.9 ± 2.7, with a statistically significant difference (P < .01). In terms of urinary metabolism, the case group had significantly higher urine calcium concentration (8.6 ± 1.3 mmol/L vs 7.2 ± 1.1 mmol/L, P < .01) and urine oxalate concentration (0.56 ± 0.14 mmol/L vs 0.38 ± 0.09 mmol/L, P < .01) compared to the control group, while the urine pH was significantly lower in the case group (5.8 ± 0.3 vs 6.2 ± 0.2, P < .01). These results indicate that calcium oxalate stone patients show significant differences in metabolic status compared to healthy controls, particularly in the abnormalities related to calcium oxalate metabolism and urine acid-base balance. On the other hand, no significant differences were observed between the 2 groups for common clinical metabolic indicators such as blood glucose, total cholesterol, triglycerides, systolic blood pressure, and diastolic blood pressure (P > .05). This finding suggests that although calcium oxalate stone patients exhibit abnormalities in BMI and urinary metabolism, they are similar to the healthy population in terms of common metabolic disease indicators.
Table 1.
Basic information of patients.
| Clinical characteristic | Case group (n = 159) | Control group (n = 141) | U/X2 value | P-value |
|---|---|---|---|---|
| Age (years) | 45.4 ± 12.2 | 44.7 ± 11.9 | U = 11,155 | .56 |
| Gender (male/female) | 93/66 | 82/59 | X² = 0.11 | .74 |
| BMI (kg/m²) | 27.6 ± 3.5 | 24.9 ± 2.7 | U = 12,125 | <.01 |
| Urinary calcium (mmol/L) | 8.6 ± 1.3 | 7.2 ± 1.1 | U = 11,890 | <.01 |
| Urinary oxalate (mmol/L) | 0.56 ± 0.14 | 0.38 ± 0.09 | U = 11,235 | <.01 |
| Urine pH | 5.8 ± 0.3 | 6.2 ± 0.2 | U = 11,285 | <.01 |
| Blood glucose (mmol/L) | 5.1 ± 0.7 | 5.0 ± 0.6 | U = 10,890 | .46 |
| Total cholesterol (mmol/L) | 4.8 ± 1.0 | 4.7 ± 0.9 | U = 11,205 | .68 |
| Triglycerides (mmol/L) | 1.5 ± 0.7 | 1.4 ± 0.6 | U = 10,930 | .47 |
| Systolic blood pressure (mm Hg) | 128.6 ± 15.2 | 127.3 ± 14.6 | U = 11,090 | .56 |
| Diastolic blood pressure (mm Hg) | 81.2 ± 9.8 | 80.6 ± 9.5 | U = 11,010 | .74 |
BMI = body mass index.
3.2. Differences in gut microbiome composition
3.2.1. Diversity analysis
High-throughput 16S rRNA sequencing was used to analyze the diversity of the gut microbiome in both the case and control groups. First, α-diversity was analyzed, with the Shannon index and Chao1 index being calculated to reflect the richness and evenness of the microbiome. The results showed that the Shannon index in the case group was 1.87 ± 0.36, which was significantly lower than that of the control group (2.13 ± 0.44, P < .01). Similarly, the Chao1 index was significantly lower in the case group (218.7 ± 47.3 vs 250.2 ± 58.5, P < .01), as shown in Table 2. These findings suggest that the gut microbiome diversity in calcium oxalate kidney stone patients is significantly reduced, which may be associated with stone formation.
Table 2.
Diversity analysis of gut microbiota.
| Index | Case group (n = 159) | Control group (n = 141) | P value |
|---|---|---|---|
| Shannon index | 1.87 ± 0.36 | 2.13 ± 0.44 | <.01 |
| Chao1 index | 218.7 ± 47.3 | 250.2 ± 58.5 | <.01 |
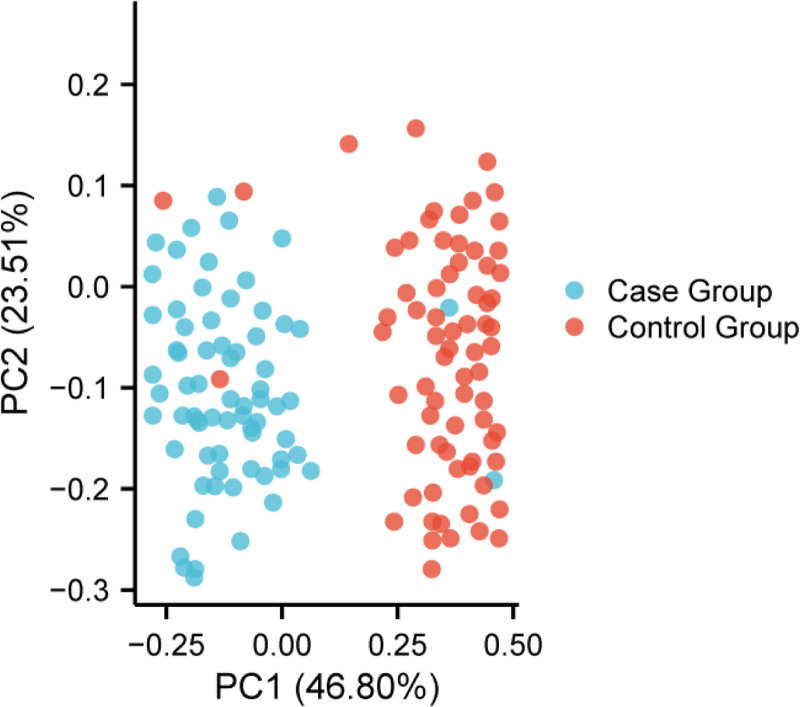
Next, β-diversity analysis was performed through PCoA to visualize the differences in microbiome composition. The analysis, shown in Fig. 1, revealed significant separation between the case and control groups (P < .01, PERMANOVA), further confirming the significant difference in microbiome composition between the 2 groups. These findings indicate that the gut microbiome structure in calcium oxalate stone patients is significantly different from that of healthy controls.
Figure 1.
PCoA analysis (β-diversity). PCoA = principal coordinate analysis.
3.2.2. Microbial taxonomic differences
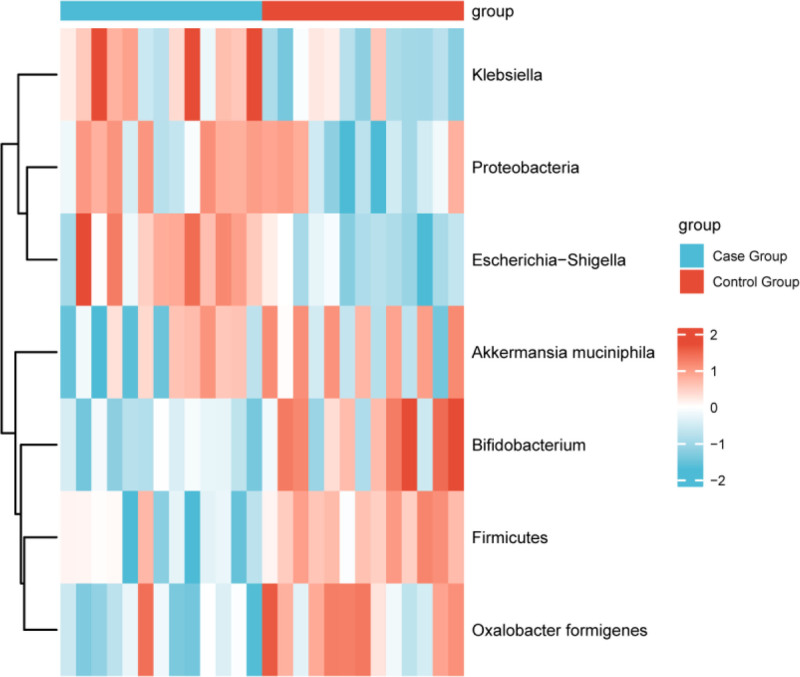
Further analysis using LEfSe was conducted to compare the taxonomic differences in the gut microbiomes between the case and control groups, as shown in Table 3 and Fig. 2. The analysis revealed significant differences in the relative abundances of several microbial phyla, including Proteobacteria (which includes the Escherichia-Shigella genus), Bacteroidetes, and Firmicutes in the gut microbiome of calcium oxalate stone patients. Specifically, the relative abundance of Proteobacteria was significantly higher in the case group (P < .05), while the abundance of Firmicutes was significantly lower (P < .01). These changes may be closely related to the formation of calcium oxalate stones.
Table 3.
Differential analysis of gut microbiota composition.
| Microbiota category | Case group (relative abundance) | Control group (relative abundance) | P value |
|---|---|---|---|
| Proteobacteria | Increased | Decreased | <.05 |
| Firmicutes | Decreased | Increased | <.01 |
| Oxalobacter formigenes | Low | High | <.01 |
| Akkermansia muciniphila | Low | High | <.05 |
| Bifidobacterium | Low | High | <.05 |
| Escherichia-Shigella | Increased | Decreased | <.05 |
| Klebsiella | Increased | Decreased | <.05 |
Figure 2.
Heatmap of gut microbiota taxonomic differences analysis.
At the genus level, the abundance of O formigenes, a bacteria known for its oxalate-degrading function, was significantly lower in the case group compared to the control group (P < .01), suggesting that this bacterium may play an important role in the prevention and treatment of calcium oxalate stones. Additionally, the abundances of Akkermansia muciniphila and Bifidobacterium were significantly lower in the case group, while the abundances of Escherichia-Shigella and Klebsiella were significantly higher (P < .05). These findings indicate that the gut microbiome imbalance may be associated with the occurrence of calcium oxalate stones.
3.3. Correlation between clinical features and gut microbiome
Through Spearman correlation analysis, we found significant correlations between clinical metabolic indicators and gut microbiome composition in calcium oxalate stone patients, particularly in metabolites associated with stone formation. The specific results are shown in Table 4.The analysis revealed a significant negative correlation between the abundance of O formigenes and urinary oxalate concentration (r = ‐0.43, P < .01). This finding suggests that higher abundance of O formigenes in the gut of calcium oxalate stone patients is associated with lower urinary oxalate levels, which may help reduce the risk of calcium oxalate stone formation. Additionally, the abundance of O formigenes was positively correlated with urinary calcium concentration (R = 0.34, P < .05). This result indicates that O formigenes may influence urinary calcium levels by metabolizing oxalate, playing a role in the balance of urinary calcium ions. The abundance of Escherichia-Shigella was found to be significantly negatively correlated with urinary pH (r = ‐0.36, P < .01). This suggests that Escherichia-Shigella may promote the formation of calcium oxalate stones by altering the urinary pH, with an increased risk of stone formation in more acidic urine environments.
Table 4.
Correlation analysis between clinical metabolic indicators and gut microbiota composition.
| Indicator | Correlation | r value | P value |
|---|---|---|---|
| O formigenes versus urinary oxalate concentration | Negative | ‐0.43 | <.01 |
| O formigenes versus urinary calcium concentration | Positive | 0.34 | <.05 |
| Escherichia-Shigella versus urinary pH | Negative | ‐0.36 | <.01 |
3.4. Construction and validation of the comprehensive prediction model
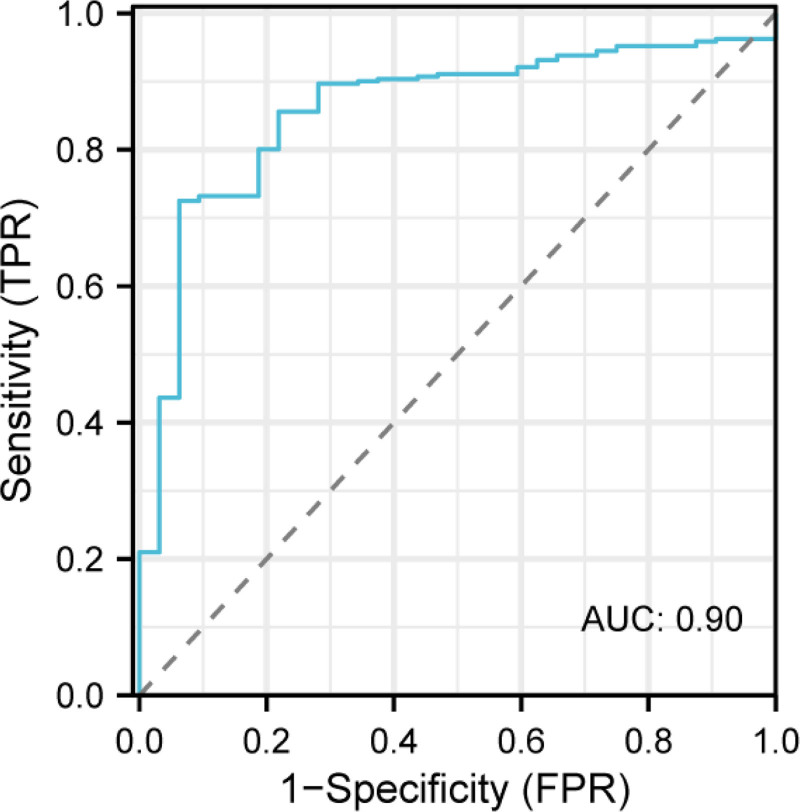
To construct a prediction model for the risk of calcium oxalate stone formation, we combined clinical features and gut microbiome characteristics using the random forest algorithm. Input variables included urinary metabolic parameters (such as urinary calcium, urinary oxalate, urinary pH, BMI, etc) and gut microbiome characteristics (such as the abundance of O formigenes and Escherichia-Shigella). The random forest algorithm effectively builds and optimizes the prediction model by handling high-dimensional data and automatically selecting important features. Through cross-validation, we evaluated the performance of the model. As shown in Fig. 3, the AUC was 0.90, with a sensitivity of 87.4% and specificity of 89.1%. These results suggest that the model has high accuracy in predicting the risk of calcium oxalate stone formation and can effectively distinguish between high-risk and low-risk individuals. Analysis of the contribution of each variable to the model revealed that urinary oxalate concentration, the abundance of O formigenes, urinary pH, and BMI were the most important factors influencing the prediction results, as shown in Table 5. Notably, the abundance of O formigenes contributed most significantly to the model’s predictive accuracy, indicating that this microbiome may play a key role in the formation of calcium oxalate stones.
Figure 3.
ROC curve of the calcium oxalate stone risk prediction model. ROC = receiver operating characteristic.
Table 5.
Variable importance analysis.
| Variable | Importance score |
|---|---|
| Urinary oxalate concentration | 0.28 |
| O formigenes abundance | 0.35 |
| Urine pH | 0.20 |
| BMI | 0.17 |
BMI = body mass index.
3.5. Oxalate metabolism and the potential mechanism of the gut–kidney axis
Further analysis, as shown in Table 6, revealed a significant negative correlation between urinary oxalate concentration and the abundance of the gut oxalate-degrading bacterium O formigenes (r = ‐0.47, P < .001). This finding suggests that O formigenes may significantly reduce urinary oxalate levels through its oxalate-degrading function, thereby lowering the risk of calcium oxalate stone formation. O formigenes is a known oxalate-degrading bacterium that metabolizes oxalate into other harmless substances in the gut, thus influencing the oxalate levels in urine.
Table 6.
Correlation analysis between oxalate concentration and gut microbiota abundance.
| Variable | r-value | P-value |
|---|---|---|
| Urinary oxalate concentration and Oxalobacter formigenes abundance | -0.47 | <.001 |
| Gut microbiota diversity and urinary pH value | 0.41 | <.01 |
In addition, we found a positive correlation between gut microbiome diversity and urinary pH (R = 0.41, P < .01). This indicates that an increase in gut microbiome diversity might regulate urinary acid–base balance through its metabolic products, such as short-chain fatty acids (SCFAs), which could affect the formation of calcium oxalate stones. SCFAs, produced in the gut, create an acidic environment that may influence urinary pH, altering the tendency for stone formation.
These results further support the potential role of the gut-kidney axis in the formation of calcium oxalate stones and provide a theoretical basis for intervening in the prevention and treatment of calcium oxalate stones through gut microbiome modulation.
4. Discussion
This study found significant differences in clinical metabolic characteristics, gut microbiota composition, and urinary metabolism between calcium oxalate stone patients and healthy controls, and explored the potential role of gut microbiota in stone formation. In recent years, increasing evidence has suggested that the gut microbiota not only influences the host’s metabolic state but may also play a key role in the development of urinary stones by regulating oxalate metabolism, urine pH, and systemic inflammatory responses.[18–20] By comprehensively analyzing microbiota diversity, the abundance of specific bacterial taxa, and their relationship with urinary metabolic parameters, this study reveals the microecological background of calcium oxalate stones and constructs a stone risk prediction model that integrates gut microbiota and urinary metabolic features, providing new insights for early clinical screening and personalized intervention.
First, in the analysis of gut microbiota diversity, we found that both the Shannon index and Chao1 index were significantly lower in stone patients than in healthy controls (P < .01), suggesting that reduced microbial diversity may be a crucial feature of stone formation. Further PCoA and LEfSe analysis revealed significant alterations in gut microbiota composition in stone patients, particularly in the abundance of O formigenes and Escherichia-Shigella. O formigenes is a bacterial species capable of directly degrading oxalate, thereby reducing intestinal oxalate absorption and lowering urinary oxalate concentrations.[21] We observed a significant reduction in the abundance of O formigenes in stone patients, with its abundance negatively correlated with urinary oxalate levels (r = ‐0.43, P < .01), further supporting its crucial role in calcium oxalate stone prevention. However, it remains unclear whether the reduction in O formigenes abundance is solely due to gut microbiota dysbiosis or is indirectly caused by host metabolic abnormalities, such as chronic inflammation or bile acid metabolism disorders. Functional studies have indicated that O formigenes not only exerts its effects through oxalate metabolism but may also influence host immune homeostasis, providing new directions for future research.
On the other hand, we observed a significant enrichment of the Escherichia-Shigella genus in stone patients, with its abundance negatively correlated with urinary pH (r = ‐0.36, P < .01). This finding suggests that this bacterial group may contribute to stone formation by secreting specific metabolic products, such as lipopolysaccharides, which induce intestinal inflammation and subsequently affect systemic metabolic status and urinary acid-base balance. Previous studies have shown that gut microbiota dysbiosis can trigger systemic inflammation, which in turn influences renal function and urinary acidification, thereby increasing the risk of stone formation.[22,23] Additionally, we found a significant increase in Proteobacteria abundance and a decrease in Firmicutes in the stone group, a trend that may be associated with the expansion of inflammation-related bacteria and the reduction of beneficial probiotics. Notably, urinary acidification may be one of the key mechanisms by which gut microbiota mediate stone formation. Future research should explore whether Escherichia-Shigella affects urinary pH through specific metabolic pathways and further assess its causal relationship with stone formation.
This study also found that stone patients had a significantly higher BMI than controls (27.6 ± 3.5 vs 24.9 ± 2.7, P < .01), along with elevated urinary calcium and oxalate levels (P < .01) and significantly lower urinary pH (P < .01). These metabolic characteristics further support the association between calcium oxalate stone formation and obesity, urinary acid-base imbalance, and oxalate metabolism disorders.[24] However, dietary oxalate intake (e.g., spinach, chocolate, nuts) was not included in our analysis, which may affect the observed correlation between gut microbiota and urinary oxalate levels. Future studies could incorporate dietary surveys or metabolomic analyses to better clarify the influence of dietary factors.
Regarding the prediction model, we employed a random forest algorithm to construct a calcium oxalate stone risk prediction model integrating urinary metabolic parameters and gut microbiota characteristics. The model achieved an AUC curve of 0.90, with a sensitivity of 87.4% and specificity of 89.1%, demonstrating excellent predictive performance. The clinical value of this model lies in its ability to assess individual stone risk based on O formigenes abundance and urinary metabolic indicators, providing a basis for early intervention. Individuals with low O formigenes abundance may benefit from microbiota-targeted strategies to reduce urinary oxalate levels and decrease the likelihood of stone formation.
Based on the findings of this study, modulating the gut microbiota may become a novel strategy for the prevention and treatment of calcium oxalate stones. Previous studies have shown that probiotic supplementation (such as Lactobacillus and Bifidobacterium) can improve the gut microbiota environment, inhibit pathogenic bacteria growth, and promote the production of SCFAs, which in turn reduces oxalate absorption and lowers stone risk.[25] Additionally, fecal microbiota transplantation, as an effective means of microbiota reshaping, has shown promising results in the intervention of metabolic diseases (such as diabetes and irritable bowel syndrome). Future studies could further explore its application in stone patients, particularly in those with significant deficiencies of O formigenes.[26] It is important to note that gut microbiota modulation should consider individualized factors, such as combining dietary control with probiotics or prebiotics supplementation, to achieve optimal outcomes. Therefore, future clinical studies should further evaluate the long-term efficacy of microbiota modulation strategies and explore their combined use with traditional stone prevention and treatment measures, such as low-oxalate diets and alkalinization therapy.
In summary, this study innovatively integrates gut microbiota and urinary metabolic data to construct a prediction model for calcium oxalate stones and reveals the potential mechanisms of microbiota imbalance in stone formation. The study found that a decrease in O formigenes may lead to elevated urinary oxalate levels, while the enrichment of Escherichia-Shigella may promote stone formation by acidifying urine or inducing inflammation.[27] These findings not only deepen the understanding of the gut-kidney axis in stone formation but also provide new intervention strategies for clinical practice. Future research should further validate the causal role of specific microbiota, assess the clinical efficacy of probiotic supplementation, fecal microbiota transplantation, and other microbiota modulation methods, and construct more precise personalized prevention and treatment plans by integrating dietary factors to reduce the risk of calcium oxalate stone formation.
5. Clinical significance
This study highlights the critical role of gut microbiome dysbiosis in calcium oxalate kidney stone formation, identifying key microbial signatures such as reduced O formigenes and increased Escherichia-Shigella as potential biomarkers. The integration of microbiome data with urinary metabolic parameters in a predictive model (AUC = 0.90) offers a novel approach for early risk assessment, paving the way for targeted interventions like probiotics or dietary adjustments. These findings underscore the gut-kidney axis’s importance in stone pathogenesis and provide a foundation for future microbiome-based preventive strategies.
6. Limitations and future directions
While our results are promising, several limitations must be acknowledged. Dietary factors, including oxalate and calcium intake, were not controlled for, which may influence both microbiome composition and urinary stone risk. Additionally, the predictive model requires validation in prospective, diverse cohorts to assess its real-world applicability, particularly in recurrent stone formers who represent the highest-risk population. Further mechanistic studies are needed to establish causality between specific microbial changes and stone formation. Addressing these gaps through dietary assessments, longitudinal designs, and interventional trials will be essential for translating these findings into clinical practice.
7. Conclusion
This study found significant differences in clinical characteristics and gut microbiome composition between calcium oxalate kidney stone patients and healthy controls. Patients exhibited urinary metabolic abnormalities, particularly in oxalate and calcium metabolism, while their gut microbiome diversity was significantly reduced. The abundance of O formigenes was negatively correlated with urinary oxalate levels, suggesting its potential key role in the formation of calcium oxalate stones. Additionally, the abundance of Escherichia-Shigella and other bacteria was negatively correlated with urinary pH, indicating that these bacteria may promote stone formation by altering urinary pH balance.
Furthermore, we developed a calcium oxalate stone risk prediction model combining clinical features and gut microbiome data, which demonstrated high predictive accuracy and provides a new tool for early screening and risk assessment. The study suggests that the gut–kidney axis may play a significant role in calcium oxalate stone formation, and future interventions to modulate the gut microbiome may offer new strategies for the prevention and treatment of calcium oxalate stones.
Author contributions
Conceptualization: Minghe Zhang, Lianhui Fan, Jian Li.
Data curation: Minghe Zhang, Lianhui Fan, Jian Li.
Formal analysis: Minghe Zhang, Lianhui Fan, Jian Li.
Validation: Jian Li.
Visualization: Jian Li.
Writing – original draft: Minghe Zhang, Jian Li.
Writing – review & editing: Minghe Zhang, Jian Li.
Abbreviations:
- AUC
- area under the receiver operating characteristic
- BMI
- body mass index
- LEfSe
- linear discriminant analysis effect size
- PCoA
- principal coordinate analysis,
- SCFAs
- short-chain fatty acids
The authors have no funding and conflicts of interest to disclose.
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
How to cite this article: Zhang M, Fan L, Li J. Prediction of calcium oxalate kidney stones: A comprehensive analysis of clinical and gut microbiome characteristics. Medicine 2025;104:29(e43103).
References
- [1].Khan SR, Pearle MS, Robertson WG, et al. Kidney stones. Nat Rev Dis Primers. 2016;2:16008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Alexander RT, Fuster DG, Dimke H. Mechanisms underlying calcium nephrolithiasis. Annu Rev Physiol. 2022;84:559–83. [DOI] [PubMed] [Google Scholar]
- [3].Song Q, Song C, Chen X, et al. FKBP5 deficiency attenuates calcium oxalate kidney stone formation by suppressing cell-crystal adhesion, apoptosis and macrophage M1 polarization via inhibition of NF-κB signaling. Cell Mol Life Sci. 2023;80:301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Mehta M, Goldfarb DS, Nazzal L. The role of the microbiome in kidney stone formation. Int J Surg. 2016;36(Pt D):607–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Kuro-O M. The Klotho proteins in health and disease. Nat Rev Nephrol. 2019;15:27–44. [DOI] [PubMed] [Google Scholar]
- [6].Li HB, Xu ML, Xu XD, et al. Faecalibacterium prausnitzii attenuates CKD via butyrate-renal GPR43 axis. Circ Res. 2022;131:e120–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Xie Y, Hu X, Li S, et al. Pharmacological targeting macrophage phenotype via gut–kidney axis ameliorates renal fibrosis in mice. Pharmacol Res. 2022;178:106161. [DOI] [PubMed] [Google Scholar]
- [8].Arvans D, Jung YC, Antonopoulos D, et al. Oxalobacter formigenes-derived bioactive factors stimulate oxalate transport by intestinal epithelial cells. J Am Soc Nephrol. 2017;28:876–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Ticinesi A, Nouvenne A, Chiussi G, Castaldo G, Guerra A, Meschi T. Calcium oxalate nephrolithiasis and gut microbiota: not just a gut–kidney axis. A nutritional perspective. Nutrients. 2020;12:548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Hunthai S, Usawachintachit M, Taweevisit M, et al. Unraveling the role of gut microbiota by fecal microbiota transplantation in rat model of kidney stone disease [published correction appears in Sci Rep. 2024 Nov 26;14(1):29360. doi: 10.1038/s41598-024-78864-8.]. Sci Rep. 2024;14:21924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Tian Y, Zhao J, Chen L, Zhang C, Chu X, Xia Y. Sanjin Paishi Decoction improves the imbalance of gut microbiota and regulates MAPK signaling pathway to inhibit calcium oxalate stones in rats. Int Urol Nephrol. 2023;55:2421–9. [DOI] [PubMed] [Google Scholar]
- [12].Siener R, Löhr P, Hesse A. Urinary risk profile, impact of diet, and risk of calcium oxalate urolithiasis in idiopathic uric acid stone disease. Nutrients. 2023;15:572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Miller AW, Penniston KL, Fitzpatrick K, Agudelo J, Tasian G, Lange D. Mechanisms of the intestinal and urinary microbiome in kidney stone disease. Nat Rev Urol. 2022;19:695–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Whiteside SA, Razvi H, Dave S, Reid G, Burton JP. The microbiome of the urinary tract—a role beyond infection. Nat Rev Urol. 2015;12:81–90. [DOI] [PubMed] [Google Scholar]
- [15].Wang Z, Zhang Y, Zhang J, Deng Q, Liang H. Recent advances on the mechanisms of kidney stone formation (Review). Int J Mol Med. 2021;48:149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Wan W, Wu W, Amier Y, et al. Engineered microorganisms: a new direction in kidney stone prevention and treatment. Synth Syst Biotechnol. 2024;9:294–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Su Y, Li S, Li X, et al. Tartronic acid as a potential inhibitor of pathological calcium oxalate crystallization. Adv Sci (Weinh). 2024;11:e2400642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Tamborino F, Cicchetti R, Mascitti M, et al. Pathophysiology and main molecular mechanisms of urinary stone formation and recurrence. Int J Mol Sci . 2024;25:3075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Ye QL, Wang DM, Wang X, et al. Sirt1 inhibits kidney stones formation by attenuating calcium oxalate-induced cell injury. Chem Biol Interact. 2021;347:109605. [DOI] [PubMed] [Google Scholar]
- [20].Wu F, Cheng Y, Zhou J, et al. Zn2+ regulates human oxalate metabolism by manipulating oxalate decarboxylase to treat calcium oxalate stones. Int J Biol Macromol. 2023;234:123320. [DOI] [PubMed] [Google Scholar]
- [21].Liang H, Song H, Zhang X, et al. Metformin attenuated sepsis-related liver injury by modulating gut microbiota. Emerg Microbes Infect. 2022;11:815–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Daniel SL, Moradi L, Paiste H, et al. Forty years of Oxalobacter formigenes, a gutsy oxalate-degrading specialist. Appl Environ Microbiol. 2021;87:e0054421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Chamberlain CA, Hatch M, Garrett TJ. Oxalobacter formigenes produces metabolites and lipids undetectable in oxalotrophic Bifidobacterium animalis. Metabolomics. 2020;16:122. [DOI] [PubMed] [Google Scholar]
- [24].Crivelli JJ, Mitchell T, Knight J, et al. Contribution of dietary oxalate and oxalate precursors to urinary oxalate excretion. Nutrients. 2020;13:62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Tian L, Liu Y, Xu X, et al. Lactiplantibacillus plantarum J-15 reduced calcium oxalate kidney stones by regulating intestinal microbiota, metabolism, and inflammation in rats. FASEB J. 2022;36:e22340. [DOI] [PubMed] [Google Scholar]
- [26].Tavasoli S, Alebouyeh M, Naji M, et al. Association of intestinal oxalate-degrading bacteria with recurrent calcium kidney stone formation and hyperoxaluria: a case–control study. BJU Int. 2020;125:133–43. [DOI] [PubMed] [Google Scholar]
- [27].Zhao J, Bai M, Ning X, et al. Expansion of escherichia-shigella in gut is associated with the onset and response to immunosuppressive therapy of IgA nephropathy. J Am Soc Nephrol. 2022;33:2276–92. [DOI] [PMC free article] [PubMed] [Google Scholar]