ABSTRACT
People with human immunodeficiency virus-hepatitis B virus (HIV-HBV) co-infection have faster rates of liver disease progression and an increase in hepatocellular carcinoma compared to people with HBV mono-infection. Given that HIV can infect multiple cells in the liver, including hepatocytes, we hypothesized that HIV will impact HBV replication through HIV viral proteins that can impact HBV replication either directly or indirectly, via effects on cellular pathways. Following infection of sodium taurocholate co-transporting polypeptide (NTCP)-expressing HepG2 cells with HBV and vesicular stomatitis virus G protein (VSV.G)-pseudotyped HIV, we found that productive HIV infection led to a twofold upregulation of HBV surface (HBs) mRNA and a marked increase in intracellular production and cellular retention of HBs antigen (HBsAg). Overexpression of HIV Tat protein, but not other HIV proteins, by DNA plasmid transfection in the HBV-producing cell line AD38 significantly stimulated HBs mRNA expression. This could be rescued by CDK9 inhibition with BAY-1251152. This study provides new insights into the mechanisms by which HIV directly impacts HBV replication and has implications for understanding adverse liver outcomes in people living with HIV and HBV.
IMPORTANCE
People with both human immunodeficiency virus (HIV) and hepatitis B virus (HBV) face faster liver disease progression and a higher risk of liver cancer than those with HBV alone. This study investigated how HIV affects HBV replication in liver cells and found that HIV infection increases the production of a key HBV surface protein (HBsAg) by enhancing the expression of its gene (HBs). This effect is driven by the HIV Tat protein. Notably, blocking the CDK9 pathway prevented this increase, suggesting a possible explanation for the adverse liver outcomes in co-infected individuals. Our findings have implications for interventions aiming to cure HIV through latency reversal, as these interventions can specifically increase the Tat protein. Future exploratory treatment strategies, such as Tat inhibitors, could play a role in the management of people with HIV and HBV at high risk of liver disease.
KEYWORDS: human immunodeficiency virus, hepatitis B virus, co-infection, Tat, liver, CDK9, HBsAg, hepatocyte
INTRODUCTION
Approximately 7.4% of people living with HIV (PLWH) are co-infected with hepatitis B virus (HBV), meaning that about 2.7 million people are living with both viruses worldwide (1). Current treatment with HBV-active antiretroviral therapy (ART) including antivirals, such as tenofovir (TDF), tenofovir alafenamide (TAF), lamivudine (LMV), or emtricitabine (FTC), can fully suppress both HIV and HBV replication leading to a significant reduction in liver-related mortality (2, 3). However, overall mortality, liver-related mortality, hospital utilization rates, and the risk of hepatocellular carcinoma (HCC) remain significantly higher in people co-infected with HIV and HBV on HBV-active ART compared to those with HIV or HBV mono-infection (4, 5). Furthermore, we and others have shown that there is liver disease progression in 10–20% of people living with HIV-HBV co-infection despite HBV-active ART (6–9). Understanding how HIV and HBV interact within hepatocytes could potentially identify novel approaches to reduce adverse liver outcomes.
HBV infects hepatocytes leading to the establishment of an episomal covalently closed circular (ccc) DNA mini-chromosome, which serves as a very stable template for HBV RNA transcription (10). Production of RNA includes pregenomic (pg) RNA, which undergoes reverse transcription resulting in HBV DNA that gets packaged into new virions; and pre-S1 and pre-S2 RNA, which are translated to form HBV surface antigen (HBsAg). Antiviral nucleos(t)ide reverse transcriptase inhibitors block the production of HBV DNA but have minimal impact on the ongoing production of HBsAg (11, 12). HBsAg can also be produced from mRNA derived from integrated HBV DNA, which is the dominant source of HBsAg in people with hepatitis B e antigen negative chronic HBV (13). HBsAg can inhibit adaptive immunity and the effective production of anti-HBs antibodies, which are required for long-term HBV control (11). If synthesized in large quantities, HBsAg can also cause direct damage to the hepatocyte through activation of Fas ligand-mediated apoptosis (14). Persistent high levels of HBsAg have also been shown to increase the risk of HCC in untreated HBV mono-infection (15, 16). Therefore, increased production of HBsAg can have a significant impact on a range of clinical outcomes in people living with HBV.
In addition to CD4+ T cells, HIV can also infect multiple cells in the liver, potentially impacting liver disease outcomes. We and others have shown that HIV can infect cells in the liver, using in vitro, ex vivo, and in vivo models, including HIV infection of hepatocytes (17, 18), Kupffer cells (19–22), hepatic stellate cells (HSC) (23), and infiltrating T-cells (24). Using a cell line model of immortalized hepatocytes producing HBV, we previously demonstrated that HIV could infect these cells via either CCR5 or CXCR4, despite very low expression of both coreceptors, leading to an increase in intracellular HBsAg (25). Whether the increase in intracellular HBsAg was due to increased production of HBsAg or reduced release remains unknown, as does the mechanism by which HIV impacts levels of intracellular HBsAg.
RESULTS
Pseudotyped HIV infection of HBV-expressing hepatocytes leads to efficient HIV integration and production
Direct interaction between HIV and HBV can result in significant adverse outcomes in co-infected cells, even though wild-type HIV only infects hepatic cell lines at very low levels (17, 25). To study these rare but potentially impactful events, we used a vesicular stomatitis virus G protein (VSV.G) pseudotyped, HIV envelope deleted virus that expressed green fluorescent protein (GFP) (VSV.G-NL4-3-ΔEnv-EGFP). This system ensures high and consistent levels of single-round HIV infection. AD38 cells, an immortalized HBV-producing hepatocyte cell line, were first infected with the pseudotyped HIV virus at a multiplicity of infection (MOI) of 0.5 for 4 days. A mean (range) of 67.3% (64.1–71.0%; N = 3) cells expressed GFP (Fig. 1A and S1A), with a mean frequency of 650,228 (384,091–1,011,736; N = 3) copies of integrated HIV DNA per million cells (Fig. 1B). Addition of the HIV integrase inhibitor raltegravir or the non-nucleoside reverse transcriptase inhibitor efavirenz effectively eliminated GFP expression and detection of HIV integration (Fig. 1A and B and Fig. S1A).
Fig 1.
High levels of HIV-HBV co-infection in AD38 and HepG2-NTCP cells using VSV.G pseudotyped HIV virus. (A) Percentage of AD38 cells expressing green fluorescent protein (GFP) 5 days post VSV.G-pseudotyped HIV infection. Cells were treated with or without 10 µM raltegravir (RAL) or 300 nM efavirenz (EFV) 24 h before and immediately after HIV infection. GFP expression was determined by flow cytometry (N = 3). (B) Frequency of HIV integration in AD38 cells 5 days post VSV.G-pseudotyped HIV infection with or without RAL/EFV treatment. HIV integration was measured by real-time PCR for Alu-LTR and normalized to CCR5 copy number. The detection limit for the Alu-LTR was 200 copies/106 cells and is shown as a dashed line (N = 3). (C) GFP and HBsAg expression in mock, HBV-mono, HIV-mono, and HIV-HBV co-infected HepG2-NTCP cells. HepG2-NTCP cells were infected with HBV inoculum derived from AD38 cells for 10 days and VSV.G-pseudotyped HIV virus for another 5 days. Cells were immunostained for GFP (green) and HBsAg (red). DNA was counterstained with DAPI (blue). Bars, 10 µm. Results are representative of at least three experiments. (D) Percentage of HepG2-NTCP cells expressing GFP following HBV and VSV.G-pseudotyped HIV infection. Cells were treated with or without 300 nM EFV 24 h before and after HIV infection (N = 3). (E) HIV integration in HepG2-NTCP cells following HBV and VSV.G-pseudotyped HIV infection with or without EFV treatment (N = 4). In all graphs, the horizontal bar represents the mean.
To mimic HBV infection in vivo, the sodium taurocholate co-transporting polypeptide (NTCP) expressing cell line, HepG2-NTCP, was next infected with HBV virions concentrated from the supernatant of AD38. We inoculated 800 viral genome equivalents (VGE) per cell for 10 days in the presence or absence of single-round HIV infection using an MOI of 1. GFP expression was detected using fluorescent microscopy with a mean (range) of 69.8% (62.3–72.1%; N = 3) of the cells infected with HIV. Staining of intracellular HBsAg confirmed HBV production in a mean (range) of 22.5% (16.7–28.9%; N = 3) of the HBV mono-infected cells (Fig. 1C). In cells infected with both HIV and HBV, the expression of HBsAg and GFP was detected in 22.6% (12.8–37.0%; N = 4) and 67.6% (62.8–76.1%; N = 4) of cells, respectively. 15.5% (9.0–26.0%; N = 3) of the cells expressed both HBsAg and GFP (arrowhead in Fig. 1C). Infection with HIV resulted in high levels of GFP expression and HIV integration in the presence or absence of HBV infection, and as expected, HIV infection was inhibited in the presence of efavirenz (Fig. 1D and E). These results demonstrated that HIV-HBV co-infection could be established in the HepG2-NTCP cell line.
Productive HIV infection induces a significant increase in intracellular HBsAg
We then assessed the impact of HIV co-infection on HBV protein production in both AD38 and HepG2-NTCP cell lines. Following HIV infection of AD38 cells (as described above), we detected a substantial increase in intracellular HBsAg (L and M forms) as detected by Western blot using an anti-preS2 antibody (Fig. 2A). This aligns with our previous study showing increases in all HBsAg, including the S form, in the context of HIV co-infection (25). Similar findings were observed following HIV-HBV co-infection of HepG2-NTCP cells (Fig. 2B). Antiretroviral drugs inhibited the increase in HBsAg in both AD38 (Fig. 2A) and HepG2-NTCP cells (Fig. 2B). There was no significant increase in HBsAg in the supernatant following HIV infection of either HBV-infected AD38 or HepG2-NTCP cells (Fig. S1B and S1C), consistent with our previous findings using wild-type HIV infection (25). We also quantified HBV DNA by Southern blot in both models of HBV infection and found marked increases in HBV DNA following HIV infection of AD38 cells but not following HIV infection of HBV-infected HepG2-NTCP cells (Fig. S1D and S1E). These results demonstrate a direct impact of HIV infection on the intracellular protein level of HBsAg in hepatocytes, independent of an effect on HBV DNA.
Fig 2.
Productive HIV infection leads to an increase in intracellular HBsAg in AD38 and HepG2-NTCP cells. (A) Western blot with anti-HBV PreS2 and anti-GAPDH antibodies using lysates from AD38 cells 5 days post-infection with VSV.G-pseudotyped HIV treated with or without 10 µM RAL or 300 nM EFV 24 h before and after HIV infection. Results are representative of at least three experiments. (B) Western blot with anti-HBV PreS2 and anti-GAPDH antibodies using lysates from HepG2-NTCP cells infected with HBV, VSV.G-pseudotyped HIV infection or both and treated with or without 300 nM EFV 24 h before and immediately after HIV infection. Results are representative of at least three experiments. Results are representative of at least three experiments. (C) HIV integration in AD38 cells with or without productive HIV infection. GFP+ and GFP− cells were collected by flow sorting 5 days post VSV.G-pseudotyped HIV infection. HIV integration was quantified using real-time PCR for Alu-LTR and normalized to CCR5 copy numbers as a housekeeping gene. The detection limit for the Alu-LTR was 200 copies/106 cells and is shown as a dashed line (N = 2). (D) Representative example (from two separate experiments) of intracellular HBsAg levels in AD38 cells with or without productive HIV infection. GFP+ and GFP− cells were collected by flow sorting 5 days post VSV.G-pseudotyped HIV infection. Cell lysates of unsorted and sorted samples were examined by Western blot with anti-HBV PreS2 and anti-GAPDH antibodies. (E) HIV integration in AD38 cells infected with an HIV virus expressing GFP or a luciferase (luc) reporter. Cells were treated with or without 300 nM EFV 24 h before and immediately after HIV infection (N = 2). (F) Representative example (from two independent experiments) of intracellular HBsAg levels in AD38 cells 5 days post VSV.G-pseudotyped HIV infection expressing GFP or a luciferase (luc) reporter. Cells were treated with or without 300 nM EFV 24 h before and immediately after HIV infection. AD38 cell lysates were examined by Western blot with anti-HBV PreS2 and anti-GAPDH antibodies. In all graphs, the horizontal bar represents the mean.
To determine if the increase in HBsAg was a direct effect of HIV infection and not a bystander effect in uninfected cells, we sorted GFP+ and GFP− cells following HIV infection and quantified HBsAg. We detected HIV integration in both GFP+ and GFP− cells but at much lower levels in GFP− cells, as expected (Fig. 2C). Despite detecting HIV integration in both subsets, we only observed an increase in HBsAg in GFP+ cells compared to mock infection (Fig. 2D). These findings suggest that productive infection rather than integration of HIV alone is associated with elevated intracellular HBsAg. Finally, to demonstrate that GFP itself in the pseudotyped HIV virus was not driving increased HBsAg, we infected AD38 cells with another VSV.G-pseudotyped HIV virus, which carries a luciferase reporter instead of GFP and also had deletions in both envelope and vpr HIV genes (VSV.G-NL4-3-ΔEnv-ΔVpr-Luc) for 4 days. Consistent with findings using a GFP-expressing virus, infection with the luciferase virus led to high levels of HIV integration and an upregulation of intracellular HBsAg (Fig. 2E and F). Taken together, our results demonstrate that productive HIV infection is associated with an increase in HBsAg expression in hepatocytes producing or infected by HBV, and this is a result of direct infection and not a bystander effect.
HIV infection upregulates HBs mRNA level in HBV-expressing hepatocytes
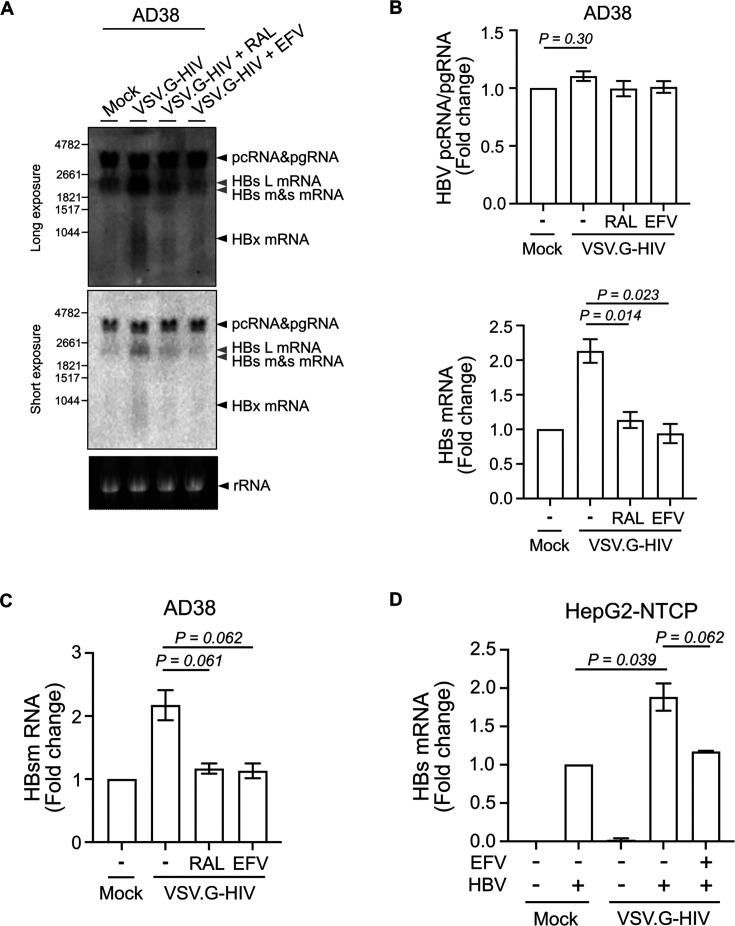
The increase in intracellular HBsAg could be a consequence of increased production or reduced release of HBsAg. We showed in an earlier study that HBsAg in the supernatant was not changed by HIV co-infection, consistent with HIV driving increased HBsAg production, coupled with an increase in intracellular retention (25). We, therefore, next quantified HBV RNA levels by Northern blot in the AD38 cell line following HIV infection. HBV pregenomic RNA (pgRNA) remained unchanged in the presence or absence of VSV.G-HIV-EGFP infection. In contrast, HBV surface (HBs) mRNA was increased by twofold upon HIV infection, and the increase was reduced in the presence of raltegravir or efavirenz (Fig. 3A and B).
Fig 3.
Productive HIV infection leads to a twofold increase in HBs mRNA in AD38 and HepG2-NTCP cells. (A) Northern blot of RNA extracted from AD38 cells 5 days post VSV.G-pseudotyped HIV infection. Cells were treated with or without 10 µM RAL or 300 nM EFV 24 h before and immediately after HIV infection. A genomic length HBV-DNA probe or a ribosomal probe was used. A long (upper panel) and short (lower panel) exposure time is shown. Arrows in black indicate pcRNA/pgRNA, HBx mRNA or ribosomal RNA. Arrows in gray indicate large (L), medium (M), and small (S) HBs mRNA; Representative example from three separate experiments. (B) Fold change of HBV pcRNA/pgRNA and HBs mRNA on Northern blot (A) using image density (N = 3). (C) Fold change of HBs mRNA expression in AD38 cells 5 days post VSV.G-pseudotyped HIV infection. Cells were treated with or without 10 µM RAL or 300 nM EFV 24 h before and after HIV infection. HBs mRNA was quantified by real-time PCR using two sets of specific primers, which quantified either pcRNA/pgRNA (3.5 kb) only or together with HBs mRNAs (3.5 kb + 2.4 kb + 2.1 kb). HBV PreC (pcRNA/pgRNA) was subtracted from PreC and S (pcRNA/pgRNA with HBs mRNAs) and normalized to the expression of the housekeeping gene RPLP0 (N = 3). (D) Fold change of HBs mRNA level in HepG2-NTCP cells following HBV and VSV.G-pseudotyped HIV infection. Cells were treated with or without 300 nM EFV 24 h before and after HIV infection. HBs mRNA was quantified by real-time PCR and normalized to the expression of the housekeeping gene RPLP0 (N = 3). In all graphs, the columns and error bars represent mean and SEM.
Next, we performed real-time PCR to precisely quantify the mRNA levels of HBs in both AD38 cells and HBV-infected HepG2-NTCP with and without infection with VSV.G-HIV-EGFP. Because the genome for HBV pcRNA/pgRNA overlaps with HBs RNA, we used two sets of specific primers, which quantified either pcRNA/pgRNA (3.5 kb) only or together with HBs mRNAs (3.5 kb + 2.4 kb + 2.1 kb) and subtracted HBV PreC (pcRNA/pgRNA) from PreC and S (pcRNA/pgRNA with HBs mRNAs). HBs mRNA levels were upregulated two-fold following HIV infection of either AD38 cells or HBV-infected HepG2-NTCP, and this increase was inhibited by both raltegravir or efavirenz (Fig. 3C and D). However, pcRNA/pgRNA levels did not change significantly in either cell line (Fig. S2A and S2B). These results indicate that the increase in HBsAg protein level was a result of increased transcription of HBs mRNA in hepatocytes following productive HIV infection.
HIV Tat stimulates HBs transcription via CDK9
To determine whether HIV proteins directly upregulated HBs mRNA level, leading to a higher expression of intracellular HBsAg following productive HIV infection, we transfected AD38 cells with either a plasmid DNA construct expressing the HIV genome with a deletion in envelope (NL4-3-ΔEnv) or a plasmid expressing different HIV proteins with a FLAG or GFP tag. Following a 72-h transfection, the cells were enriched by flow sorting based on GFP expression or intracellular staining of FLAG, and total RNA was then extracted. HBs mRNA was quantified by real-time PCR. Transfection of the NL4-3-ΔEnv plasmid led to an increase in the HBs mRNA level by 2.5-fold (P = 0.0025), similar to what we observed with near full-length pseudotyped virus infection. Transfection of the plasmid expressing Tat but not Gag, Nef, Rev, Vpr, or Vpu also led to a twofold increase in HBs mRNA level compared to the relevant negative control (P = 0.015; Fig. 4A and S2C). To confirm these findings using a different method, we synthesized the first exon of Tat as mRNA, encapsulated the mRNA into lipid nanoparticles (LNPs), and delivered Tat-LNP to AD38 and TZM-bl cells, as a positive control. TZM-bl cells contain a luciferase reporter under the control of the HIV long terminal repeat (LTR), and following treatment with the Tat-LNP, a dose-dependent increase in luciferase was observed (Fig. S3A). Tat-LNP in AD38 cells also led to a dose-dependent increase in intracellular HBsAg, but with a much lower magnitude than what was demonstrated in the TZM-bl cell line, with a maximum 1.8-fold elevation of HBs mRNA (Fig. 4B, C and S3B), consistent with Tat mediating an increase in HBs transcription.
Fig 4.
HIV Tat upregulates the transcription level of HBs via CDK9/cyclin T2. (A) Fold change of HBs mRNA in AD38 cells following transfection of plasmids expressing either HIV NL4-3-GFP or Gag-GFP (left panel) or FLAG-tagged Nef, Rev, Tat, Vpr, or Vpu (right panel). The cells were transfected with plasmid DNA and enriched following flow sorting, based on expression of either GFP expression or FLAG intracellular staining. HBs mRNA was quantified by real-time PCR and normalized to the expression of the housekeeping gene RPLP0 (N = 5). (B) Western blot with anti-HBV PreS2 and anti-α-tubulin antibodies using lysates from AD38 cells 2 days post-transfection with lipid nanoparticles (LNPs), which were empty or co-formulated with Tat mRNA (Tat-LNP) ranging from 50 to 400 ng/mL. Results are representative of three experiments. (C) Fold change of HBs mRNA level in AD38 cells 2 days post Tat-LNP transfection. HBs mRNA was quantified by real-time PCR and normalized to the expression of the housekeeping gene RPLP0 (N = 3). (D) Western blot with anti-RNA-Pol2-S2p, anti-HBV PreS2, and anti-α-tubulin antibodies. AD38 cells were transfected with or without 200 ng/mL Tat-LNP for 48 h and treated with or without 1 µM BAY1251152 for another 16 h. Results are representative of two experiments. (E) Fold change of HBs mRNA level in AD38 cells transfected with or without 200 ng/mL Tat-LNP for 48 h followed by 1 µM BAY1251152 treatment for another 16 h. HBs mRNA was quantified by real-time PCR and normalized to the expression of the housekeeping gene RPLP0 (N = 2). In all graphs, the columns and error bars represent mean and SEM.
Given that Tat has been shown to directly activate CDK9 in vitro and CDK9 increases HBV transcription and translation (26–28), we tested whether the effects of Tat on HBV replication were mediated by CDK9 by inhibiting P-TEFb/CDK9 with BAY-1251152. We added BAY-1251152 (1 µM) to AD38 cells 48 h following Tat-LNP treatment, and after a further 16 h confirmed activity, as demonstrated by a reduction in phosphorylation of RNA polymerase II (RNA-Pol2-S2p) measured by Western blot (Fig. 4D). Although both pcRNA/pgRNA and HBs mRNA levels were dramatically decreased by the inhibition of CDK9, consistent with inhibition of HBV viral replication, HBs expression remained unchanged with additional Tat-LNP treatment compared to BAY-1251152 alone (Fig. 4E and S3C). These data suggest that the upregulation of HBs transcription by HIV Tat could be rescued by the inhibition of CDK9. However, intracellular protein levels of HBsAg on Western blots were not decreased by this inhibition (Fig. 4D), possibly due to a longer half-life of HBsAg than HBs mRNA within the limited incubation time with BAY-1251152 that we used in these experiments. These results are consistent with a direct upregulation of HBs mRNA by HIV Tat via P-TEFb/CDK9.
DISCUSSION
In this study, we used a new model of HBV infection of HepG2-NTCP cells with HBV-free virions, followed by single-round infection with a VSV.G-pseudotyped HIV virus to assess how replication of HBV is impacted by co-infection with HIV. We found that productive HIV infection led to a twofold upregulation of HBs mRNA and a marked increase in intracellular production and cellular retention of HBsAg. Overexpression of HIV Tat protein, but not other HIV proteins, by DNA plasmid transfection in the HBV-producing cell line AD38 significantly stimulated HBs mRNA expression. This could be rescued by CDK9 inhibition with BAY-1251152. This study provides new insights into the mechanisms by which HIV directly impacts HBV replication and has implications for understanding adverse liver outcomes in people living with HIV and HBV.
We found a clear increase in both mRNA for HBs and all forms of intracellular HBsAg in HBV-infected hepatocytes following co-infection with HIV (Fig. 2B and 3A), consistent with our previous study using AD38 cells (25). AD38 cells stably express HBV under the control of a tetracycline-responsive cytomegalovirus promoter, while HBV infection of HepG2-NTCP cells more accurately replicates in vivo HBV infection (29). The overall HBV infection frequency in HepG2-NTCP cells was approximately 20% by immunofluorescence staining. Given the lower MOI, it is not surprising that the frequency of HBV infection was lower than prior reports (30).
Increased HBV integration could potentially contribute to the upregulation of HBs in HIV co-infected cells. However, we believe this is unlikely as HBV integration is infrequent in chronically infected cell lines (31). Since the budding of both HIV and HBV relies on the endosomal sorting complex required for the transport system (32), HIV co-infection in hepatocytes may also restrict the secretion capacity of HBsAg. Indeed, HBsAg levels in the supernatant were similar in mono and co-infected hepatocytes (Fig. S1B and S1C), consistent with no change in the release of HBsAg from the cytosol. Taken together, this would lead to the accumulation of HBsAg, which can damage infected hepatocytes in chronic hepatitis B (33), via several pathways. First, the intracellular level of L surface protein has been associated with the severity of liver disease caused by coagulative necrosis of hepatocytes in transgenic mice (34). Second, expression of L or S proteins in vitro has been shown to induce apoptosis in hepatocyte cell lines (35, 36). Therefore, the accumulation of HBsAg in the presence of HIV-HBV co-infection, even if only in a subset of HIV-infected cells, may well be a driver of adverse liver outcomes in co-infection.
We clearly demonstrated that Tat rather than other HIV viral proteins stimulated HBs mRNA expression. Tat is known to promote HIV transcription by binding to the transactivation response element on the HIV LTR and activating RNA Polymerase II (37, 38). This involves the recruitment of positive transcription elongation factor (P-TEFb) containing CDK9 to the super elongation complex (SEC) (39). The SEC, together with BRD4, can also promote HBV transcription (26), and HBV replication can be inhibited by CDK9 inhibitors (27). We showed that inhibition of CDK9 caused a significant decrease in HBV transcription, including the HBs mRNA (Fig. 4E and S3C). Given that Tat did not increase HBsAg in the presence of CDK9 inhibition, Tat may activate HBs transcription by enhancing the recruitment of P-TEFb to the preS1 and preS2 promoters.
It is possible that Tat could also have an indirect impact on an HBV-infected cell and, therefore, will affect hepatocytes beyond those that are infected with HIV. For example, soluble Tat can be secreted from HIV-infected cells and impact bystander cells (40, 41). Given that soluble Tat can be taken up by neighbouring uninfected cells, and there is only a very small proportion of hepatocytes infected with HIV in the liver (25, 42, 43), Tat could be released from either infected hepatocytes or infected CD4+ T cells that migrate through the liver, resulting in a continuous stimulus for HBsAg production. Consistent with this mechanism, soluble Tat can induce apoptosis in neurons in the absence of HIV infection (44). Our findings have implications for some investigative HIV cure strategies such as latency reversal (reviewed in Tanaka et al.) (45), which could potentially be harmful in the setting of HIV-HBV co-infection should there be an increase in Tat protein (46). In addition, the recent identification of Tat inhibitors, although still in pre-clinical development, could play a beneficial role in the management of people with HIV and HBV at high risk of liver disease (47).
This is the first study to show the direct effects of Tat on HBsAg transcription. However, we acknowledge several limitations in our approach. First, we used immortalized cell lines, which have clear differences in gene expression compared to primary hepatocytes (48). However, isolation and adaptation of primary cell culture from liver biopsy can also have a significant unintended impact on the gene expression level (49). Three-dimensional liver organoids derived from adult stem cells have been shown to robustly mimic the functionality of adult hepatocytes (50). Future studies using liver organoids co-cultured with primary T cells could offer further insights into the interactions between HIV and HBV replication. Second, infection with a pseudotyped HIV virus is an artificial model of what happens in vivo. Although hepatocytes express very low levels of the HIV receptor (CD4) and co-receptors (CXCR4 or CCR5), we previously showed that, in vitro, HIV could infect hepatocytes at very low levels (25). Multiple studies using in situ hybridization and, more recently, DNA and RNA scope by our group have clearly identified HIV RNA and DNA in hepatocytes in liver biopsies from patients with HIV off-ART, confirming that HIV can indeed infect hepatocytes in vivo (42, 43, 51). The use of a pseudotyped HIV virus allowed for consistent and highly reproducible HIV infection, which greatly facilitated our ability to capture the infrequent but significant impact of HIV infection on HBV replication within a co-infected cell.
In summary, we established an in vitro model of HIV-HBV co-infection of hepatocyte cell lines using HBV and VSV.G-pseudotyped HIV viruses and showed a direct and significant impact of HIV co-infection on the HBV life cycle. We showed that the HIV Tat protein increased HBs transcription, production, and intracellular accumulation of HBsAg, which was mediated by CDK9. Inhibiting Tat protein, as currently being explored as part of an HIV cure strategy, could also potentially benefit people with HIV-HBV co-infection. Our findings provide new insights into the direct effects of HIV on HBV replication and have implications for future exploratory treatment strategies for people living with HIV and HBV, specifically those aimed at the cure of either chronic infection.
MATERIALS AND METHODS
Cell culture
The human hepatic cell lines HepG2, AD38, and HepG2-NTCP cells were cultured on 0.01% collagen (C9791; Sigma-Aldrich, St. Louis, MO) coated culture dishes in complete medium: minimal essential media (MEM), Dulbecco’s modified Eagle’s medium (DMEM)/F-12 with 400 µg/mL G418 and DMEM with 5 µg/mL puromycin, respectively, supplemented with 10% fetal bovine serum and 2 mM l-glutamine at 37°C in a humidified atmosphere of 5% CO2. HEK 293T and TZM-bl cells were cultured in DMEM supplemented with 10% fetal bovine serum and 2 mM l-glutamine at 37°C in a humidified atmosphere of 5% CO2.
HIV pseudotyped virus production and infection
HEK 293T cells in T75 flask were co-transfected with 16 µg NL4-3-ΔEnv with an enhanced GFP (EGFP) gene (a kind gift from Damien Purcell, University of Melbourne, Australia) or NL4-3-ΔEnv-ΔVpr with a luciferase reporter gene (NIH AIDS reagent program) and 4 µg of the envelope plasmid expressing VSV.G using 60 µL FuGENE 6 (E2692; Promega, Madison, WI) according to the manufacturer’s instructions. After overnight incubation, the cells were washed two times and cultured with 12 mL of DMEM complete medium for another 48 h. The supernatant containing pseudotyped HIV was harvested, filtered through a 0.45 µm Filtropur S 0.45 filter (84.1826; Sarstedt, Nümbrecht, Germany) and applied onto 6 mL of 20% sucrose, followed by centrifugation at 26,500 rpm for 1 h in a Sorvall RC-90 ultracentrifuge equipped with an AH-629 rotor. The virus pellet was re-suspended in 1 mL of DMEM complete medium and stored at −80°C until use. Viral stocks were titrated in hepatocytes using the serial dilution method. An MOI of 0.5 was determined by 50% of the hepatocytes expressing EGFP 4 days post-infection. For HIV infection, pseudotyped HIV virus was added directly to the culture medium. The cells were washed two times with phosphate-buffered saline (PBS) 24 h post-infection and incubated for another 72 h until harvest.
Drug treatment
To inhibit HIV infection, the cells were treated with 10 µM raltegravir or 300 nM efavirenz (NIH AIDS reagent program) 24 h before and immediately after HIV infection. To inhibit CDK9, AD38 cells were treated with 1 µM BAY-1251152 (S8730, Selleckchem, Houston, TX) 48 h after Tat-LNP treatment and incubated for another 16 h until harvest.
HBV inoculum and infection
Supernatant from AD38 cells containing HBV viral particles was incubated overnight at 4°C with 6% polyethylene glycol (PEG) 8000 (P5431; Sigma-Aldrich), concentrated 100-fold in PBS supplemented with 10% FBS by centrifugation at 11,000 rpm for 1 h at 4°C and stored at −80°C until use. The HBV DNA concentration in VGE per mL was quantified using the COBAS AmpliPrep/COBAS TaqMan HBV test kit v2.0 (Roche Diagnostics, Basel, Switzerland). The concentrated HBV inoculum in DMEM complete medium (600 µL/well of a 12-well plate) supplemented with 4% PEG 8000 was applied onto HepG2-NTCP cells at 800 VGE/cell. The cells were washed two times with PBS 16 h post-infection and incubated for 10 days until harvest, with culture medium change every 2 or 3 days. Secreted HBsAg was quantified as described previously (52).
RNA extraction, cDNA synthesis, real-time PCR
Cell pellets were homogenized in TRIzol reagent (15596026; Invitrogen, Waltham, MA). Total RNA was extracted and treated with DNase I according to the manufacturer’s instructions. RNA was converted to cDNA using SuperScript III Reverse Transcriptase (18080085; Invitrogen). The quantitative RT-PCR reaction was performed in a Mx3005P QPCR System using Brilliant II SYBR Green QPCR Master Mix (Agilent Technologies, Santa Clara, CA) according to the manufacturer’s instructions. The relative HBs mRNA level was quantified by subtracting HBV PreC (pcRNA and pgRNA) from PreC and S (pcRNA, pgRNA, PreS/L-mRNA, and S-mRNA) after normalization to RPLP0. Primer sequences were, for HBV PreC: forward, 5′-GCCTTAGAGTCTCCTGAGCA-3′; reverse, 5′-GAGGGAGTTCTTCTTCTAGG-3′; for HBV S: forward, 5′-ACCCCTTCTCGTGTTACAGG-3′; reverse, 5′-GAGTGATTGGAGGTTGGGGA-3′; and for RPLP0: forward, 5′-AGATGCAGCAGATCCGCAT-3′; reverse, 5′-GGATGGCCTTGCGCA-3′.
DNA constructs and transfection
The HIV Tat expressing construct pcDNA3.1-Tat101AD8-FLAG was a kind gift from Professor Damien Purcell, University of Melbourne, Australia (53). pPA-GFP-Gag, pCMV6-Nef-FLAG, pCMV6-Vpr-FLAG, and pCMV6-Vpu-FLAG were obtained from the NIH AIDS reagent program. HIV Rev sequence was generated by flanking PCR product of Rev cDNA (forward primer: 5′-CGGAATTCCACC ATGGCAGGAAGAAGCGGAG-3′, reverse primer: 5′-CGACGCGT TTCTTTAGTTCCTGACTCCAATA-3′) and cloned into pCMV6-FLAG vector using EcoRI and MluI sites. Plasmid transfection to AD38 cells was performed using FuGENE 6 according to the manufacturer’s instructions. The cells were harvested 72 h post-transfection.
mRNA synthesis and LNPs
The coding region of NL4-3-ΔEnv-EGFP was amplified, and a T7 promoter sequence (5′-GAAATTAATACGACTCACTATAGG-3′) and a 37 nt poly-A sequence were added through overhang PCR using Platinum Taq High Fidelity polymerase (11304011; Invitrogen). Resulting reaction products were cleaned up using a QIAquick PCR purification kit (28104; Qiagen, Hilden, Germany) as per the manufacturer’s instructions. In vitro transcription was performed using the mMESSAGE mMACHINE T7 Transcription Kit (AM1344; Invitrogen) as per the manufacturer’s instructions, which includes a GG Cap0 for co-transcriptional mRNA capping. 400–600 ng of PCR product was loaded into each 20 µL reaction. Reactions were left for 4 h to maximize mRNA yield. Quality control of the synthesized mRNA was performed in two ways: mRNA size, integrity, and purity were assessed through gel electrophoresis using a TapeStation instrument (Agilent Technologies), after which the concentration was determined through fluorescence detection using the Quant-iT RiboGreen RNA Assay Kit (R11490, Invitrogen) as per the manufacturer’s low-range protocol.
LNPs encapsulating HIV Tat mRNA were synthesized through microfluidic mixing using a NanoAssemblr Spark (Precision Nanosystems, Vancouver, Canada). Briefly, ionizable lipids, D-Lin-MC3-DMA (MC3) (S6683, Selleckchem), 1,2-distearoyl-sn-glycero-3-phosphocholine (DSPC) (850365P, Avanti Polar Lipids, Alabaster, AL), cholesterol (C8667, Sigma-Aldrich) and 1,2-Dimyristoyl-rac-glycero-3-methoxypolyethylene glycol-2000 (DMG-PEG2000) (880151P, Avanti Polar Lipids) were resuspended in 100% ethanol at 10 mM and stored at −30°C until further use. Lipids were mixed at a molar ratio of 50:10:38.5:1.5 of MC3:DSPC:cholesterol:DMG-PEG2000. Immediately prior to microfluidic mixing, RNA constructs were diluted to 150 ng/µL in 30 mM RNase-free sodium acetate buffer, pH 4.0. Aqueous and organic phases were then mixed at a flow rate ratio of 1.8 (aqueous):1 (organic) (N/P ratio of 6:1), and resulting LNPs were diluted fourfold into PBS to neutralise the pH. LNP size and polydispersity were determined using dynamic light scattering (Zetasizer Ultra, Malvern Panalytical, Malvern, UK). RNA encapsulation efficiency and total RNA concentration of the LNP solution were determined using a modified RiboGreen assay. Briefly, RNA concentration measurements were performed on LNP samples as per the manufacturer’s low-range assay protocol after 30-min incubation in either standard TE buffer (for free RNA measurement) or in TE buffer with 1% Triton X-100 to release encapsulated RNA (for total RNA measurement). LNPs were either stored at 4°C for a maximum of 2 weeks or frozen at −80°C in the presence of 20% wt/vol sucrose for cryoprotection.
Quantification of HIV integration
Cells were lysed in 10 mg/mL proteinase K buffer. HIV integrated DNA was quantified as described previously and normalized to the CCR5 gene as a surrogate for the number of cells per reaction (54–56).
Antibodies
Commercial primary antibodies used for Western blotting, immunofluorescence and flow cytometry were rabbit anti-HBV preS2 (PA5-22820; Invitrogen), rabbit anti-HBsAg (NB100-62652; Novas Biologicals, Littleton, CO), mouse anti-FLAG M2 (F1804; Sigma-Aldrich), rabbit anti-GFP (ab290; Abcam, Cambridge, UK), rabbit anti-GAPDH (2118; Cell Signaling Technology, Danvers, MA), rabbit anti-RNA polymerase II CTD, phospho S2 (ab193467; Abcam), and SureLight APC mouse anti-DDDDK tag (ab72569; Abcam). Anti-mouse IgG-HRP and anti-rabbit IgG-HRP or Alexa Fluor 488 and 568 (A11001, A11008, A11011, and A11031; Invitrogen) were used as secondary antibodies for Western blotting or immunofluorescence.
Immunofluorescence microscopy
HepG2-NTCP cells grown on collagen-coated coverslips were washed with PBS, fixed with 4% paraformaldehyde (PFA) for 15 min, permeabilized with 0.5% Triton X-100 for 5 min and blocked for 1 h in 5% bovine serum albumin (BSA; A7906; Sigma-Aldrich) in PBS at room temperature. Coverslips were incubated with primary antibodies diluted in blocking buffer at 4°C overnight, followed by three washes with 0.05% Tween-20 in PBS. The cells were then incubated with secondary antibodies conjugated with Alexa Fluor 488 or 568 for 1 h at room temperature. After three washes with 0.05% Tween-20 in PBS, DNA was counterstained with DAPI (D1306; Invitrogen). Coverslips were mounted with FluorSave reagent (345789; Merck, Kenilworth, NJ). Images were obtained using a confocal fluorescence microscope LSM700 (Carl Zeiss, Jena, Germany) equipped with a 63× Plan Apochromat oil immersion objective (NA 1.4) and built-in laser scanning unit, at RT. Images were acquired and analyzed using ZEN (Carl Zeiss).
Analysis of HBV protein, RNA, and DNA
For Western blotting, the cells were harvested, washed once with PBS and lysed in radioimmunoprecipitation assay buffer (50 mM Tris-HCl pH7.4, 150 nM NaCl, 1% NP-40, 0.1% SDS, 50 mM NaF, and 2 mM EDTA) containing 1× Halt protease inhibitor Cocktail (1862209, Thermo Fisher Scientific, Waltham, MA). After centrifugation at 10,000 × g for 10 min at 4°C, the supernatant was mixed with 2× Laemmli sample buffer containing 200 mM dithiothreitol and boiled at 95°C for 3 min. For Northern blotting, 30 µg of RNA from each sample was electrophoresed through 1% agarose glyoxal gels before transfer to nylon membranes using the NorthernMax-Gly Kit (AM194; Invitrogen) according to the manufacturer’s instructions. Membrane was probed using a genomic length HBV-DNA probe as previously described (57). For Southern blotting, a DIG-labeled 2.5 kb DNA probe (forward, 5′-AAGGTGGGAAACTTTACTGGGC-3′; reverse, 5′-GGCAAAAACGAGAGTAACTC-3′) was used as previously described (Roche Diagnostics) (52).
Flow cytometry
The cells were dispersed to single-cell suspension using Trypsin-EDTA, passed through a 40 µm strainer (Sarstedt, Nümbrecht, Germany), washed once with PBS and incubated with Fixable Viability Stain 450 (562247; BD Biosciences, Franklin Lakes, NJ) for 20 min at room temperature. The cells were washed again with PBS and fixed in PBS containing 1% formaldehyde. All data were acquired on a LSRFortessa Cell Analyzer (BD Biosciences) and were analyzed using FlowJo 9.9.6. Live and single cells were gated using forward and side scatter plots. For sorting, the cells were re-suspended in PBS containing 10% BSA and 10 mM EDTA instead of 1% formaldehyde and sorted on a FACSAria III Cell Sorter (BD Biosciences) based on GFP expression. Isolation of high-quality RNA following intracellular sorting was performed using the method for analysing RNA following intracellular sorting (MARIS)(58). The cells were fixed and permeabilized in 4% PFA supplemented with 100 U/mL RNAse OUT (1077019; Invitrogen) for 30 min on ice. The cells were pelleted by centrifugation at 500 × g for 3 min at 4°C, washed in wash buffer: PBS containing 0.2% BSA and RNAse OUT and incubated with SureLight APC anti-FLAG antibody M2 (ab72569; Abcam) diluted in PBS with 1% BSA and RNAse OUT for 1 h at 4°C. The cells were washed two times, re-suspended in PBS with 0.5% BSA and RNAse OUT and sorted on a BD FACSAria III Cell Sorter based on the expression of FLAG.
Statistical analysis
All data shown represent the mean value of at least three independent experiments. Error bars represent the standard error of the mean. P values for bar plots were obtained by paired t-test and represent a comparison of all cells analyzed in the indicated cell populations. For all multiple comparisons, one-way ANOVA with Geisser-Greenhouse correction was performed. A P value of less than 0.05 was considered significant. All analysis was performed using GraphPad Prism 9 (GraphPad, La Jolla, CA).
ACKNOWLEDGMENTS
We thank Damian Purcell for the kind gift of plasmids used in this study. We also thank the Victorian Infectious Diseases Reference Laboratory (VIDRL) and the Biological Optical Microscopy Platform (BOMP), University of Melbourne, for their assistance. The Novo Nordisk Foundation Center for Stem Cell Medicine, reNEW, is supported by a Novo Nordisk Foundation (grant number NNF21CC0073729).
This work was funded by the National Health and Medical Research Council (APP606615, APP1149994; APP1131581; APP2026490).
W.Z.: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Validation, Writing—original draft preparation, Writing—review and editing. M.C.: Conceptualization, Funding acquisition, Methodology, Project administration, Writing—review and editing. J.A.: Conceptualization, Funding acquisition, Methodology, Project administration, Supervision, Writing—review and editing. S.R.L.: Conceptualization, Funding acquisition, Methodology, Project administration, Resources, Supervision, Validation, Writing—original draft preparation, Writing—review and editing. F.J.R.: Investigation, Methodology, Validation, Writing—original draft preparation, Writing—review and editing. K.S.: Investigation, Methodology, Writing—review and editing. T.S.: Investigation, Writing—review and editing. J.M.Z.: Investigation, Methodology, Writing—review and editing. C.T.: Investigation, Writing—review and editing. D.F.: Investigation. P.C.: Methodology, Writing—review and editing. L.M.: Methodology, Writing—review and editing. D.R.P.: Methodology, Writing—review and editing. J.S.: Methodology, Writing—review and editing. J.A. (Jenny Anderson): Methodology, Writing—review and editing. P.R.: Methodology, Writing—review and editing. S.G.: Methodology, Supervision, Writing—review and editing. M.R.: Methodology, Writing—review and editing. A.R.: Project administration, Writing—review and editing.
Contributor Information
Sharon R. Lewin, Email: sharon.lewin@unimelb.edu.au.
Vaithilingaraja Arumugaswami, David Geffen School of Medicine at UCLA, Los Angeles, California, USA.
SUPPLEMENTAL MATERIAL
The following material is available online at https://doi.org/10.1128/spectrum.00809-25.
Pseudotyped HIV infection of HBV-expressing hepatocytes leads to efficient HIV integration and production.
HIV infection up-regulates HBs mRNA level in HBV-expressing hepatocytes.
HIV Tat stimulates HBs transcription via CDK9.
Legends for Fig. S1 to S3.
ASM does not own the copyrights to Supplemental Material that may be linked to, or accessed through, an article. The authors have granted ASM a non-exclusive, world-wide license to publish the Supplemental Material files. Please contact the corresponding author directly for reuse.
REFERENCES
- 1. Platt L, French CE, McGowan CR, Sabin K, Gower E, Trickey A, McDonald B, Ong J, Stone J, Easterbrook P, Vickerman P. 2020. Prevalence and burden of HBV co-infection among people living with HIV: a global systematic review and meta-analysis. J Viral Hepat 27:294–315. doi: 10.1111/jvh.13217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Smith CJ, Ryom L, Weber R, Morlat P, Pradier C, Reiss P, de Wit S, Law M. 2014. Trends in underlying causes of death in people with HIV from 1999 to 2011 (D:A:D): a multicohort collaboration. Lancet 384:241–248. doi: 10.1016/S0140-6736(14)60604-8 [DOI] [PubMed] [Google Scholar]
- 3. Boyd A, Gozlan J, Maylin S, Delaugerre C, Peytavin G, Girard PM, Zoulim F, Lacombe K. 2014. Persistent viremia in human immunodeficiency virus/hepatitis B coinfected patients undergoing long-term tenofovir: virological and clinical implications. Hepatology 60:497–507. doi: 10.1002/hep.27182 [DOI] [PubMed] [Google Scholar]
- 4. Klein MB, Althoff KN, Jing Y, Lau B, Kitahata M, Lo Re V 3rd, Kirk GD, Hull M, Kim HN, Sebastiani G, et al. 2016. Risk of end-stage liver disease in HIV-viral hepatitis coinfected persons in North America from the early to modern antiretroviral therapy eras. Clin Infect Dis 63:1160–1167. doi: 10.1093/cid/ciw531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Dezanet LNC, Kassime R, Miailhes P, Lascoux-Combe C, Chas J, Maylin S, Gabassi A, Rougier H, Delaugerre C, Lacombe K, Boyd A. 2021. Effect of viral replication and liver fibrosis on all-cause mortality in HIV/HBV coinfected individuals: a retrospective analysis of a 15-year longitudinal cohort. MedRxiv. doi: 10.1101/2021.04.13.21255432 [DOI] [PubMed] [Google Scholar]
- 6. Vinikoor MJ, Sinkala E, Chilengi R, Mulenga LB, Chi BH, Zyambo Z, Hoffmann CJ, Saag MS, Davies MA, Egger M, Wandeler G. 2017. Impact of antiretroviral therapy on liver fibrosis among human immunodeficiency virus-infected adults with and without HBV coinfection in Zambia. Clin Infect Dis 64:1343–1349. doi: 10.1093/cid/cix122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Coffin CS, Osiowy C, Myers RP, Gill MJ. 2013. Virology and clinical sequelae of long-term antiviral therapy in a North American cohort of Hepatitis B virus (HBV)/human immunodeficiency virus type 1 (HIV-1) co-infected patients. J Clin Virol 57:103–108. doi: 10.1016/j.jcv.2013.02.004 [DOI] [PubMed] [Google Scholar]
- 8. Audsley J, Robson C, Aitchison S, Matthews GV, Iser D, Sasadeusz J, Lewin SR. 2016. Liver fibrosis regression measured by transient elastography in human immunodeficiency virus (HIV)-Hepatitis B virus (HBV)-coinfected individuals on long-term HBV-active combination antiretroviral therapy. Open Forum Infect Dis 3:ofw035. doi: 10.1093/ofid/ofw035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Dezanet LNC, Miailhes P, Lascoux-Combe C, Chas J, Maylin S, Gabassi A, Rougier H, Delaugerre C, Lacombe K, Boyd A. 2021. Profiles of liver fibrosis evolution during long-term tenofovir treatment in HIV-positive patients coinfected with hepatitis B. Liver Int 41:2874–2884. doi: 10.1111/liv.15019 [DOI] [PubMed] [Google Scholar]
- 10. Revill P, Testoni B, Locarnini S, Zoulim F. 2016. Global strategies are required to cure and eliminate HBV infection. Nat Rev Gastroenterol Hepatol 13:239–248. doi: 10.1038/nrgastro.2016.7 [DOI] [PubMed] [Google Scholar]
- 11. Seto W-K, Wong DK-H, Fung J, Huang F-Y, Lai C-L, Yuen M-F. 2013. Reduction of hepatitis B surface antigen levels and hepatitis B surface antigen seroclearance in chronic hepatitis B patients receiving 10 years of nucleoside analogue therapy. Hepatology 58:923–931. doi: 10.1002/hep.26376 [DOI] [PubMed] [Google Scholar]
- 12. Chevaliez S, Hézode C, Bahrami S, Grare M, Pawlotsky J-M. 2013. Long-term hepatitis B surface antigen (HBsAg) kinetics during nucleoside/nucleotide analogue therapy: finite treatment duration unlikely. J Hepatol 58:676–683. doi: 10.1016/j.jhep.2012.11.039 [DOI] [PubMed] [Google Scholar]
- 13. Wooddell CI, Yuen M-F, Chan H-Y, Gish RG, Locarnini SA, Chavez D, Ferrari C, Given BD, Hamilton J, Kanner SB, Lai C-L, Lau JYN, Schluep T, Xu Z, Lanford RE, Lewis DL. 2017. RNAi-based treatment of chronically infected patients and chimpanzees reveals that integrated hepatitis B virus DNA is a source of HBsAg. Sci Transl Med 9:eaan0241. doi: 10.1126/scitranslmed.aan0241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Xu ZC, Yen TSB. 1996. Intracellular retention of surface protein by a hepatitis B virus mutant that releases virion particles. J Virol 70:133–140. doi: 10.1128/JVI.70.1.133-140.1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Tseng TC, Liu CJ, Yang HC, Su TH, Wang CC, Chen C-L, Kuo S-T, Liu C-H, Chen P-J, Chen D-S, Kao J-H. 2012. High levels of hepatitis B surface antigen increase risk of hepatocellular carcinoma in patients with low HBV load. Gastroenterology 142:1140–1149. doi: 10.1053/j.gastro.2012.02.007 [DOI] [PubMed] [Google Scholar]
- 16. Yang Y, Gao J, Li H-L, Zheng W, Yang G, Zhang W, Ma X, Tan YT, Rothman N, Gao YT, Chow WH, Shu XO, Xiang YB. 2016. Dose-response association between hepatitis B surface antigen levels and liver cancer risk in Chinese men and women. Int J Cancer 139:355–362. doi: 10.1002/ijc.30086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kong L, Cardona Maya W, Moreno-Fernandez ME, Ma G, Shata MT, Sherman KE, Chougnet C, Blackard JT. 2012. Low-level HIV infection of hepatocytes. Virol J 9:157. doi: 10.1186/1743-422X-9-157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Iser DM, Avihingsanon A, Wisedopas N, Thompson AJ, Boyd A, Matthews GV, Locarnini SA, Slavin J, Desmond PV, Lewin SR. 2011. Increased intrahepatic apoptosis but reduced immune activation in HIV-HBV co-infected patients with advanced immunosuppression. AIDS 25:197–205. doi: 10.1097/QAD.0b013e3283410ccb [DOI] [PubMed] [Google Scholar]
- 19. Mosoian A, Zhang L, Hong F, Cunyat F, Rahman A, Bhalla R, Panchal A, Saiman Y, Fiel MI, Florman S, Roayaie S, Schwartz M, Branch A, Stevenson M, Bansal MB. 2017. Frontline science: HIV infection of Kupffer cells results in an amplified proinflammatory response to LPS. J Leukoc Biol 101:1083–1090. doi: 10.1189/jlb.3HI0516-242R [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kandathil AJ, Sugawara S, Goyal A, Durand CM, Quinn J, Sachithanandham J, Cameron AM, Bailey JR, Perelson AS, Balagopal A. 2018. No recovery of replication-competent HIV-1 from human liver macrophages. J Clin Invest 128:4501–4509. doi: 10.1172/JCI121678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Denton PW, Long JM, Wietgrefe SW, Sykes C, Spagnuolo RA, Snyder OD, Perkey K, Archin NM, Choudhary SK, Yang K, Hudgens MG, Pastan I, Haase AT, Kashuba AD, Berger EA, Margolis DM, Garcia JV. 2014. Targeted cytotoxic therapy kills persisting HIV infected cells during ART. PLoS Pathog 10:e1003872. doi: 10.1371/journal.ppat.1003872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. North TW, Higgins J, Deere JD, Hayes TL, Villalobos A, Adamson L, Shacklett BL, Schinazi RF, Luciw PA. 2010. Viral sanctuaries during highly active antiretroviral therapy in a nonhuman primate model for AIDS. J Virol 84:2913–2922. doi: 10.1128/JVI.02356-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Tuyama AC, Hong F, Saiman Y, Wang C, Ozkok D, Mosoian A, Chen P, Chen BK, Klotman ME, Bansal MB. 2010. Human immunodeficiency virus (HIV)-1 infects human hepatic stellate cells and promotes collagen I and monocyte chemoattractant protein-1 expression: implications for the pathogenesis of HIV/hepatitis C virus-induced liver fibrosis. Hepatology 52:612–622. doi: 10.1002/hep.23679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Singh KP, Crane M, Audsley J, Avihingsanon A, Sasadeusz J, Lewin SR. 2017. HIV-hepatitis B virus coinfection: epidemiology, pathogenesis, and treatment. AIDS 31:2035–2052. doi: 10.1097/QAD.0000000000001574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Iser DM, Warner N, Revill PA, Solomon A, Wightman F, Saleh S, Crane M, Cameron PU, Bowden S, Nguyen T, Pereira CF, Desmond PV, Locarnini SA, Lewin SR. 2010. Coinfection of hepatic cell lines with human immunodeficiency virus and hepatitis B virus leads to an increase in intracellular hepatitis B surface antigen. J Virol 84:5860–5867. doi: 10.1128/JVI.02594-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Francisco JC, Dai Q, Luo Z, Wang Y, Chong RH, Tan YJ, Xie W, Lee GH, Lin C. 2017. Transcriptional elongation control of hepatitis B virus covalently closed circular DNA transcription by super elongation complex and BRD4. Mol Cell Biol 37:e00040-17. doi: 10.1128/MCB.00040-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Tanaka T, Okuyama-Dobashi K, Murakami S, Chen W, Okamoto T, Ueda K, Hosoya T, Matsuura Y, Ryo A, Tanaka Y, Hagiwara M, Moriishi K. 2016. Inhibitory effect of CDK9 inhibitor FIT-039 on hepatitis B virus propagation. Antiviral Res 133:156–164. doi: 10.1016/j.antiviral.2016.08.008 [DOI] [PubMed] [Google Scholar]
- 28. Kim YK, Bourgeois CF, Isel C, Churcher MJ, Karn J. 2002. Phosphorylation of the RNA polymerase II carboxyl-terminal domain by CDK9 is directly responsible for human immunodeficiency virus type 1 Tat-activated transcriptional elongation. Mol Cell Biol 22:4622–4637. doi: 10.1128/MCB.22.13.4622-4637.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ladner SK, Otto MJ, Barker CS, Zaifert K, Wang GH, Guo JT, Seeger C, King RW. 1997. Inducible expression of human hepatitis B virus (HBV) in stably transfected hepatoblastoma cells: a novel system for screening potential inhibitors of HBV replication. Antimicrob Agents Chemother 41:1715–1720. doi: 10.1128/AAC.41.8.1715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Iwamoto M, Watashi K, Tsukuda S, Aly HH, Fukasawa M, Fujimoto A, Suzuki R, Aizaki H, Ito T, Koiwai O, Kusuhara H, Wakita T. 2014. Evaluation and identification of hepatitis B virus entry inhibitors using HepG2 cells overexpressing a membrane transporter NTCP. Biochem Biophys Res Commun 443:808–813. doi: 10.1016/j.bbrc.2013.12.052 [DOI] [PubMed] [Google Scholar]
- 31. Tu T, Zhang H, Urban S. 2021. Hepatitis B virus DNA integration: in vitro models for investigating viral pathogenesis and persistence. Viruses 13:180. doi: 10.3390/v13020180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Prange R. 2012. Host factors involved in hepatitis B virus maturation, assembly, and egress. Med Microbiol Immunol 201:449–461. doi: 10.1007/s00430-012-0267-9 [DOI] [PubMed] [Google Scholar]
- 33. Wang H-C, Wu H-C, Chen C-F, Fausto N, Lei H-Y, Su I-J. 2003. Different types of ground glass hepatocytes in chronic hepatitis B virus infection contain specific pre-S mutants that may induce endoplasmic reticulum stress. Am J Pathol 163:2441–2449. doi: 10.1016/S0002-9440(10)63599-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Chisari FV, Filippi P, McLachlan A, Milich DR, Riggs M, Lee S, Palmiter RD, Pinkert CA, Brinster RL. 1986. Expression of hepatitis B virus large envelope polypeptide inhibits hepatitis B surface antigen secretion in transgenic mice. J Virol 60:880–887. doi: 10.1128/JVI.60.3.880-887.1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Zhao C, Zhang W, Tian X, Fang C, Lu H, Yuan Z. 2010. Proteomic analysis of cell lines expressing small hepatitis B surface antigen revealed decreased glucose-regulated protein 78 kDa expression in association with higher susceptibility to apoptosis. J Med Virol 82:14–22. doi: 10.1002/jmv.21654 [DOI] [PubMed] [Google Scholar]
- 36. Foo NC, Ahn BY, Ma X, Hyun W, Yen TSB. 2002. Cellular vacuolization and apoptosis induced by hepatitis B virus large surface protein. Hepatology 36:1400–1407. doi: 10.1053/jhep.2002.36819 [DOI] [PubMed] [Google Scholar]
- 37. Dayton AI, Sodroski JG, Rosen CA, Goh WC, Haseltine WA. 1986. The trans-activator gene of the human T cell lymphotropic virus type III is required for replication. Cell 44:941–947. doi: 10.1016/0092-8674(86)90017-6 [DOI] [PubMed] [Google Scholar]
- 38. Kao SY, Calman AF, Luciw PA, Peterlin BM. 1987. Anti-termination of transcription within the long terminal repeat of HIV-1 by tat gene product. Nature 330:489–493. doi: 10.1038/330489a0 [DOI] [PubMed] [Google Scholar]
- 39. Liu R, Wu J, Shao R, Xue Y. 2014. Mechanism and factors that control HIV-1 transcription and latency activation. J Zhejiang Univ Sci B 15:455–465. doi: 10.1631/jzus.B1400059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Westendorp MO, Frank R, Ochsenbauer C, Stricker K, Dhein J, Walczak H, Debating K-M, Krammer PH. 1995. Sensitization of T cells to CD95-mediated apoptosis by HIV-1 Tat and gp120. Nature 375:497–500. doi: 10.1038/375497a0 [DOI] [PubMed] [Google Scholar]
- 41. Ensoli B, Barillari G, Salahuddin SZ, Gallo RC, Wong-Staal F. 1990. Tat protein of HIV-1 stimulates growth of cells derived from Kaposi’s sarcoma lesions of AIDS patients. Nature 345:84–86. doi: 10.1038/345084a0 [DOI] [PubMed] [Google Scholar]
- 42. Cao YZ, Dieterich D, Thomas PA, Huang YX, Mirabile M, Ho DD. 1992. Identification and quantitation of HIV-1 in the liver of patients with AIDS. AIDS 6:65–70. doi: 10.1097/00002030-199201000-00008 [DOI] [PubMed] [Google Scholar]
- 43. Housset C, Lamas E, Courgnaud V, Boucher O, Girard PM, Marche C, Brechot C. 1993. Presence of HIV-1 in human parenchymal and non-parenchymal liver cells in vivo. J Hepatol 19:252–258. doi: 10.1016/s0168-8278(05)80579-3 [DOI] [PubMed] [Google Scholar]
- 44. Shi B, Raina J, Lorenzo A, Busciglio J, Gabuzda D. 1998. Neuronal apoptosis induced by HIV-1 Tat protein and TNF-alpha: potentiation of neurotoxicity mediated by oxidative stress and implications for HIV-1 dementia. J Neurovirol 4:281–290. doi: 10.3109/13550289809114529 [DOI] [PubMed] [Google Scholar]
- 45. Tanaka K, Kim Y, Roche M, Lewin SR. 2022. The role of latency reversal in HIV cure strategies. J Med Primatol 51:278–283. doi: 10.1111/jmp.12613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Khoury G, Lee MY, Ramarathinam SH, McMahon J, Purcell AW, Sonza S, Lewin SR, Purcell DFJ. 2021. The RNA-binding proteins SRP14 and HMGB3 control HIV-1 Tat mRNA processing and translation during HIV-1 latency. Front Genet 12:680725. doi: 10.3389/fgene.2021.680725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Kessing CF, Nixon CC, Li C, Tsai P, Takata H, Mousseau G, Ho PT, Honeycutt JB, Fallahi M, Trautmann L, Garcia JV, Valente ST. 2017. In vivo suppression of HIV rebound by didehydro-cortistatin A, a “Block-and-Lock” strategy for HIV-1 treatment. Cell Rep 21:600–611. doi: 10.1016/j.celrep.2017.09.080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Boess F, Kamber M, Romer S, Gasser R, Muller D, Albertini S, Suter L. 2003. Gene expression in two hepatic cell lines, cultured primary hepatocytes, and liver slices compared to the in vivo liver gene expression in rats: possible implications for toxicogenomics use of in vitro systems. Toxicol Sci 73:386–402. doi: 10.1093/toxsci/kfg064 [DOI] [PubMed] [Google Scholar]
- 49. Lamontagne J, Mell JC, Bouchard MJ. 2016. Transcriptome-wide analysis of hepatitis B virus-mediated changes to normal hepatocyte gene expression. PLOS Pathog 12:e1005438. doi: 10.1371/journal.ppat.1005438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Huch M, Dorrell C, Boj SF, van Es JH, Li VSW, van de Wetering M, Sato T, Hamer K, Sasaki N, Finegold MJ, Haft A, Vries RG, Grompe M, Clevers H. 2013. In vitro expansion of single Lgr5+ liver stem cells induced by Wnt-driven regeneration. Nature 494:247–250. doi: 10.1038/nature11826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Zerbato JM, Avihingsanon A, Singh KP, Zhao W, Deleage C, Rosen E, Cottrell ML, Rhodes A, Dantanarayana A, Tumpach C, Tennakoon S, Crane M, Price DJ, Braat S, Mason H, Roche M, Kashuba ADM, Revill PA, Audsley J, Lewin SR. 2023. HIV DNA persists in hepatocytes in people with HIV-hepatitis B co-infection on antiretroviral therapy. EBioMedicine 87:104391. doi: 10.1016/j.ebiom.2022.104391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Sozzi V, Walsh R, Littlejohn M, Colledge D, Jackson K, Warner N, et al. 2016. In vitro studies show that sequence variability contributes to marked variation in hepatitis B virus replication, protein expression, and function observed across genotypes.. J Virol 90:10054–10064. doi: 10.1128/JVI.01293-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Khoury G, Mota TM, Li S, Tumpach C, Lee MY, Jacobson J, Harty L, Anderson JL, Lewin SR, Purcell DFJ. 2018. HIV latency reversing agents act through Tat post translational modifications. Retrovirology (Auckl) 15:36. doi: 10.1186/s12977-018-0421-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Hooker DJ, Mobarok M, Anderson JL, Rajasuriar R, Gray LR, Ellett AM, Lewin SR, Gorry PR, Cherry CL. 2012. A new way of measuring apoptosis by absolute quantitation of inter-nucleosomally fragmented genomic DNA. Nucleic Acids Res 40:e113. doi: 10.1093/nar/gks334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Vandergeeten C, Fromentin R, Merlini E, Lawani MB, DaFonseca S, Bakeman W, McNulty A, Ramgopal M, Michael N, Kim JH, Ananworanich J, Chomont N. 2014. Cross-clade ultrasensitive PCR-based assays to measure HIV persistence in large-cohort studies. J Virol 88:12385–12396. doi: 10.1128/JVI.00609-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Lewin SR, Murray JM, Solomon A, Wightman F, Cameron PU, Purcell DJ, Zaunders JJ, Grey P, Bloch M, Smith D, Cooper DA, Kelleher AD. 2008. Virologic determinants of success after structured treatment interruptions of antiretrovirals in acute HIV-1 infection. J Acquir Immune Defic Syndr 47:140–147. doi: 10.1097/QAI.0b013e31815dbf7f [DOI] [PubMed] [Google Scholar]
- 57. Chin R, Shaw T, Torresi J, Sozzi V, Trautwein C, Bock T, Manns M, Isom H, Furman P, Locarnini S. 2001. In vitro susceptibilities of wild-type or drug-resistant hepatitis B virus to (-)-beta-D-2,6-diaminopurine dioxolane and 2’-fluoro-5-methyl-beta-L-arabinofuranosyluracil. Antimicrob Agents Chemother 45:2495–2501. doi: 10.1128/AAC.45.9.2495-2501.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Hrvatin S, Deng F, O’Donnell CW, Gifford DK, Melton DA. 2014. MARIS: method for analyzing RNA following intracellular sorting. PLoS ONE 9:e89459. doi: 10.1371/journal.pone.0089459 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Pseudotyped HIV infection of HBV-expressing hepatocytes leads to efficient HIV integration and production.
HIV infection up-regulates HBs mRNA level in HBV-expressing hepatocytes.
HIV Tat stimulates HBs transcription via CDK9.
Legends for Fig. S1 to S3.