Dear Editor,
The current standard of care for kidney preservation is hypothermic preservation. Nonetheless, this does not allow a safe extension of preservation time due to cold-related adenosine 5′-triphosphate (ATP) depletion and accumulation of metabolic waste products1. Perfusion at near-normothermic temperature can avoid cold ischaemic injury and restore cellular metabolism2,3. Prolonging preservation by maintaining metabolic activity may increase the donor organ pool by improving organ assessment, matching, and allocation, and allowing organ modification and repair. We investigated whether subnormothermic acellular perfusion (SNAP) at 32°C could support cellular metabolism for up to 24 h in human kidneys.
Following static cold storage (SCS), 21 human kidneys declined for transplantation were preserved for 6 h, 12 h or 24 h using SNAP (Fig. S1). In the 24 h group, kidneys were perfused with either urine replacement (UR) or urine recirculation (URC) (Fig. S1). SNAP was performed using adapted paediatric cardiopulmonary bypass technology with an acellular human serum albumin (HSA) solution. As a control, three additional kidneys were intentionally preserved for 41 h with only SCS to match the total preservation time of the 24 h SNAP group (17 h SCS + 24 h SNAP = 41 h; Fig. S1). After preservation, all kidneys underwent ex vivo reperfusion with a red-cell-based solution at 37°C to simulate post-transplant revascularization and compare the four experimental groups (Fig. S1).
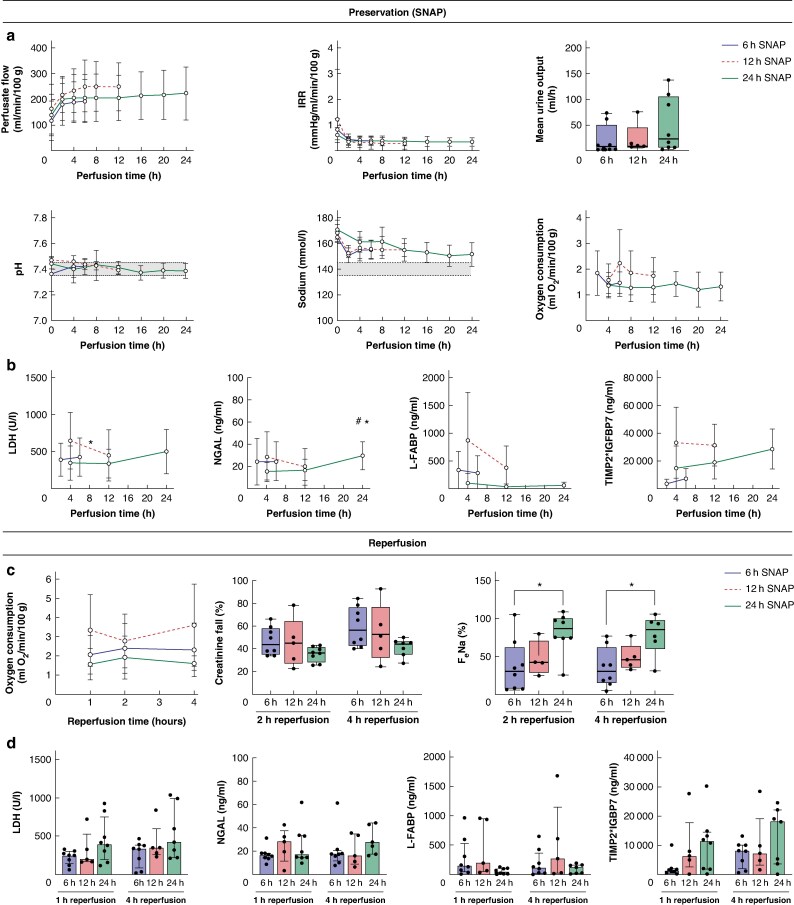
The three SNAP cohorts were well matched for donor demographics and ischaemic times (Table S1). Throughout SNAP, all kidneys maintained stable perfusion parameters and similar levels of oxygen consumption (Fig. 1a, Table S2). Levels of injury markers remained stable or decreased up to 12 h of perfusion and marginally increased from 12 to 24 h of perfusion (Fig. 1b). The 24 h SNAP subgroup analysis comparing URC and UR showed similar results (Fig. S2).
Fig. 1.
Comparison of 6 h, 12 h or 24 h of subnormothermic acellular machine perfusion (SNAP) for preservation of human kidneys
a Perfusate flow, intrarenal resistance (IRR), urine output, pH, sodium levels and oxygen consumption in kidneys perfused for 6 h (blue), 12 h (red) or 24 h (green) of SNAP. b Time course of injury markers lactate dehydrogenase (LDH) (*P < 0.05 12 h perfusion timepoint versus 4 h perfusion timepoint in 12h SNAP group, paired two-tailed Student t-test), neutrophil gelatinase-associated lipocalin (NGAL) (*P < 0.05 24 h perfusion timepoint versus 4 h perfusion timepoint in 24h SNAP group; #P < 0.05 24 h perfusion timepoint versus 12 h perfusion timepoint in 24h SNAP group; repeated measures ANOVA), liver-type fatty acid-binding protein (L-FABP), and tissue inhibitor of metalloproteinases 2 (TIMP2)*insulin-like growth factor binding protein 7 (IGFBP7) in kidneys perfused for 6 h, 12 h or 24 h of SNAP. c Comparison of oxygen consumption, creatinine fall and fractional sodium excretion (FeNa) (*P < 0.05, one-way ANOVA) during reperfusion of kidneys perfused for 6 h, 12 h or 24 h of SNAP. d Injury markers LDH, NGAL, L-FABP, and TIMP2*IGFBP7 at 1 and 4 h of reperfusion of kidneys perfused for 6 h, 12 h or 24 h of SNAP. In XY graphs and bar plots, error bars show mean ± standard deviation. For boxplots, the central mark indicates the median; bottom and top edges indicate the 25th over 75th percentiles; the whiskers extend to the most extreme data points.
At reperfusion, all kidneys demonstrated functionality with oxygen consumption, sodium reabsorption and creatinine clearance (Fig. 1c, Fig. S3). There was evidence of additional tubular dysfunction in the 24 h SNAP kidneys (Fig. 1c,d). Histological assessment demonstrated a preserved renal morphology in all kidneys (Fig. S4), with no significant difference regarding injury between the groups at the end of SNAP (P = 0.2973) and after reperfusion (P = 0.5453) (Tables S4, S5). However, the 24 h SNAP kidneys had a numerically higher injury score (Table S4).
Compared to kidneys preserved for 24 h with SNAP, SCS kidneys showed poor functionality during reperfusion with significantly lower renal blood flow, reduced creatinine clearance and numerically lower urine output (Fig. S5a). They also released higher levels of injury markers (Fig. S5b) and sustained significantly more histological damage (P = 0.0152) (Fig. S4, Tables S4, S5).
To our knowledge, this is the first report of extended kidney preservation at 32°C with an acellular perfusate. All kidneys showed functionality and metabolic activity with adequate levels of oxygen consumption during perfusion and reperfusion. In the 24 h SNAP group, there were signs of mild deterioration after 12 h with increased levels of injury markers and histological change during reperfusion. Further investigation is needed to determine if refining the nutrient requirements or perfusate would further support viability4.
Compared to previous reports of (near-)normothermic machine perfusion, the absence of red blood cells in the perfusate represents a significant advantage by reducing cost, simplifying the procedure, eliminating the risk of disease transmission and avoiding the harmful effects of haemolysis. Further, as the perfusate contains Ringer’s lactate and HSA solution as principal components with no animal-derived supplements it can be translated clinically. A comparable perfusate was recently used to perfuse porcine kidneys for 24 h at 22–25°C followed by successful autotransplantation5.
We demonstrate that SNAP enables functional and metabolic preservation of human kidneys for up to 24 h following SCS, providing a clinically feasible strategy to extend preservation time during transplantation.
Supplementary Material
Acknowledgements
We thank Cambridge Biorepository for Translational Medicine for providing access to human kidneys for part of the study. Figure 1 was created using Biorender.com with publication license EL276GM5SS. We would like to acknowledge Maja Kaczmarek and Léonie Walker-Panse for help with perfusion experiments.
Contributor Information
Sara Deffrennes, Department of Development and Regeneration, Katholieke Universiteit Leuven, Leuven, Belgium; Department of Nephrology, Dialysis and Renal Transplantation, University Hospitals Leuven, Leuven, Belgium.
Serena MacMillan, Department of Surgery, University of Cambridge, Addenbrooke's Hospital, Cambridge, UK.
Anna Paterson, Department of Histopathology, Cambridge University Hospitals NHS Foundation Trust, Cambridge, UK.
Michael L Nicholson, Department of Surgery, University of Cambridge, Addenbrooke's Hospital, Cambridge, UK.
Sarah A Hosgood, Department of Surgery, University of Cambridge, Addenbrooke's Hospital, Cambridge, UK.
Funding
This study was funded by the National Institute for Health and Care Research (NIHR) Blood and Transplant Research Unit in Organ Donation and Transplantation (NIHR203332), a partnership between NHS Blood and Transplant, University of Cambridge and Newcastle University. The views expressed are those of the author(s) and not necessarily those of the NIHR, NHS Blood and Transplant or the Department of Health and Social Care.
Author contributions
Sara Deffrennes (Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Writing—original draft), Serena MacMillan (Formal analysis, Investigation, Writing—review & editing), Anna Paterson (Formal analysis, Investigation, Writing—review & editing), Michael Nicholson (Conceptualization, Funding acquisition, Supervision, Writing—review & editing), and Sarah Hosgood (Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Supervision, Writing—review & editing)
Disclosure
The authors of this manuscript have no conflicts of interest to disclose as described by The British Journal of Surgery.
Supplementary material
Supplementary material is available at BJS online.
Data availability
The data that support the findings of this study are available in the Supplementary material of this article. Additional information is available from the corresponding author upon reasonable request.
References
- 1. Debout A, Foucher Y, Trébern-Launay K, Legendre C, Kreis H, Mourad G et al. Each additional hour of cold ischemia time significantly increases the risk of graft failure and mortality following renal transplantation. Kidney Int 2015;87:343–349 [DOI] [PubMed] [Google Scholar]
- 2. Nicholson ML, Hosgood SA. Renal transplantation after ex vivo normothermic perfusion: the first clinical study. Am J Transplant 2013;13:1246–1252 [DOI] [PubMed] [Google Scholar]
- 3. Hosgood SA, Callaghan CJ, Wilson CH, Smith L, Mullings J, Mehew J et al. Normothermic machine perfusion versus static cold storage in donation after circulatory death kidney transplantation: a randomized controlled trial. Nat Med 2023;29:1511–1519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Arykbaeva AS, de Vries DK, Doppenberg JB, Engelse MA, Hankemeier T, Harms AC et al. Metabolic needs of the kidney graft undergoing normothermic machine perfusion. Kidney Int 2021;100:301–310 [DOI] [PubMed] [Google Scholar]
- 5. Abraham N, Gao Q, Kahan R, Alderete IS, Wang B, Howell DN et al. Subnormothermic oxygenated machine perfusion (24 h) in DCD kidney transplantation. Transplant Direct 2024;10:e1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available in the Supplementary material of this article. Additional information is available from the corresponding author upon reasonable request.