Abstract
Background
Acute acquired concomitant esotropia (AACE) causes diplopia and asthenopia, severely affecting quality of life. This study aimed to explore the association between dry eye (DE) tear film biomarkers and Type III AACE.
Methods
We enrolled 52 patients (52 eyes) with Type III AACE and 50 controls (50 eyes). Assessments included tear meniscus height (TMH), tear film non-invasive break-up time (NIBUT), corneal fluorescein staining (CFS), Schirmer's test (ST), and the Dry Eye-Related Quality of Life Score (DEQS). Tear samples were analyzed for cytokine levels using a multiplex immunoassay. Logistic regression analyses identified risk factors, and a random forest classifier evaluated predictive performance.
Results
Patients with Type III AACE without DE showed significantly reduced NIBUT and elevated levels of cytokines (e.g., MMP-2, MMP-13, and IFN-γ) compared to normal controls (all P < 0.05). Near-distance work duration showed a moderate positive correlation with Galectin-3 (r = 0.64, P < 0.05). MMP-2, MMP-13, and IFN-γ were identified as independent risk factors (arear under the curve (AUC): 0.91, 0.91, 0.94). The random forest model achieved an AUC of 1.00.
Conclusion
Elevated tear levels of MMP-2, MMP-13, and IFN-γ were strongly associated with Type III AACE, highlighting their potential as predictive biomarkers.
Keywords: Acute acquired concomitant esotropia, Dry eye, Tear film biomarkers, Random forest models, Risk factors
Introduction
Acute acquired concomitant esotropia (AACE) is an ocular disorder characterized by acute onset of inward eye deviation, mainly affecting children over 5 years aged old and adults. It is typically accompanied by uncomfortable diplopia [1]. AACE is classified into three types based on clinical features and underlying etiology: Type I (Swan type), which occurs following occlusion; Type II (Franceschetti type), associated with minimal hypermetropia and lacking an accommodative component; and Type III (Bielschowsky type), which is linked to myopia [2]. In recent years, the majority of AACE patients seeking treatment have been diagnosed with Type III. This form of AACE significantly impacts the quality of life and can compromise visual function [3, 4]. The deviation angle remains constant across all gaze directions, without paralysis of the extraocular muscles or impairment of ocular motility. This misalignment leads to increased exposure of the bulbar conjunctiva on the temporal side, which may contribute to tear film instability and hyperosmolarity [5].
Dry eye (DE) is a multifactorial disorder of the tear film. The Tear Film and Ocular Surface Society Dry Eye Workshop II (TFOS DEWS II) consensus emphasizes the inflammatory vicious cycle of DE that leads to abnormalities in the tear film [6]. This cycle is typically initiated by stress-related inflammatory signaling, which prompts the release of pro-inflammatory factors and the infiltration of immune cells, exacerbating damage to the ocular surface [7, 8]. The cytokines present in tears may serve as reliable biomarkers for ocular disease severity and progression [9]. Our previous research demonstrated that DE-related inflammatory cytokines were upregulated in the tear film of patients with concomitant exotropia compared to controls [10]. Although the onset of DE symptoms following strabismus surgery has been documented, it is attributable to changes in corneal sensitivity, increased tear film instability, and a reduction in goblet cells (GC) [11, 12]. Limited information is available regarding ocular surface changes in patients with Type III AACE who have not undergone strabismus surgery.
Treatment methods for Type III AACE include refractive correction, prism therapy, pharmacological therapy, botulinum toxin injection, and strabismus surgery [13, 14]. The optimal timing of surgery remains controversial and is generally recommended after the deviation angle stabilizes after at least 6 months of conservative treatment [15]. Research has demonstrated that eliminating behavioral factors, including the use of electronic devices and excessive near work [16], can reduce the severity of Type III AACE, as well as decrease its recurrence post-surgery [17]. For patients with a mild deviation angle, early intervention to alleviate DE, and ameliorate asthenopia may be a prerequisite before implementing other treatments.
The purpose of the current study was to determine whether there are differences in DE-related biomarkers within the tear film of Type III AACE patients compared to healthy controls and to investigate the potential relationship between Type III AACE and DE. Additionally, this study aimed to explore whether these biomarkers could predict the development of Type III AACE to help determine the optimal timing for surgical intervention.
Materials and methods
Subjects
Patients diagnosed with Type III AACE at the ophthalmic center of the First Affiliated Hospital of Fujian Medical University from January 2022 to September 2024 were enrolled in this study. This study was approved by the Ethics Committee of the First Affiliated Hospital of Fujian Medical University (MRTCA, ECFAH of FMU [2022] 323). Informed consent forms were obtained from the patients or their guardians. The flowchart of the study is shown in Fig. 1.
Fig. 1.
Flowchart of our study. AACE acute acquired concomitant esotropia, DE dry eye, BCVA best-corrected visual acuity, LogMAR logarithm of the minimum angle of resolution, SE spherical equivalents, NDA near deviation angle, DDA distant deviation angle, DNW duration of near-distance work, TMH tear meniscus height, NIBUT non-invasive break-up time, CFS corneal fluorescein staining, ST Schirmer’s test, DEQS Dry Eye-Related Quality of Life Score
Patients included in this prospective study met the following criteria for Type III AACE:
Sudden onset of acquired concomitant esotropia with a deviation difference of less than 5 prism diopters (PD) in all gaze directions.
Myopic refractive error preceding the onset of deviation.
Age > 5 years at the onset of diplopia.
Corrected visual acuity better than 20/25 in both eyes.
No limitations in ocular motility.
No history of systemic disease, head trauma, neurological disorders, latent or manifest strabismus during infancy, ophthalmic disease, or surgery. No use of systemic medications in recent 1 years, including NSAIDs, diuretics, vasodilators, analgesics/antipyretics, anti-ulcer agents, sulfonylureas, cardiac glycosides, anxiolytics/benzodiazepines, anti-infectives, antidepressants/antipsychotics, antihypertensives, and antihistamines.
No combination of keratitis, meibomian gland dysfunction, eyelid diseases, or Sjögren’s syndrome was included.
No combination of dissociated vertical deviation (DVD), superior oblique muscle palsy (SOP), or other vertical strabismus < 5 PD.
No history of botulinum toxin injection.
All participants underwent ophthalmic and orthoptic examinations, as well as cranial and orbital computed tomography (CT) or magnetic resonance imaging (MRI). For each participant, data from one eye was randomly selected using a computer-generated random number method to ensure that the assumption of independence was met. DE was diagnosed according to the Asia Dry Eye Society (ADES) diagnostic criteria based on tear film breakup time < 5 s and DE symptoms (DEQS ≥ 15 points) [7, 18].
Cycloplegic refraction was performed using 0.5% tropicamide eye drops (age ≥ 12 years) or 1% atropine ointment (age < 12 years). The refractive values were converted to spherical equivalents (SE), calculated as the algebraic sum of the sphere and half of the cylinder. Best-corrected visual acuity (BCVA) was recorded as logMAR. The deviation angle was measured after refractive correction, and both eyes were assessed separately.
The objective deviation of esotropia was evaluated using the prism and alternate cover test for near (33 cm) and distance (5 m) targets. Base-out prisms gradually increased until exotropia occurred and then decreased until the eye remained stable; the near deviation angle (NDA) and distant deviation angle (DDA) showed significant findings in PD. The duration of near work (≤ 30 cm) per day, including hours spent on laptops, smartphones, and non-video display terminals (non-VDT), was documented for all participants.
Sample size estimation
Sample size estimation was conducted using G*Power 3.1 software (Heinrich Heine University, Düsseldorf, Germany) for a two-group comparison using independent samples t-test (equivalent to comparing biomarker levels between Type III AACE and normal controls). Assuming a medium effect size (Cohen’s d = 0.5), a stringent significance level (α) of 0.01, and statistical power (1–β) of 0.80, the minimum sample size required was calculated to be approximately 86 participants (43 per group). This sample size provided sufficient power to detect clinically meaningful differences in DE-related tear biomarker levels between the groups.
A total of 52 patients (52 eyes) with Type III AACE were included, along with 50 outpatients (50 eyes) without a Type III AACE diagnosis from the same ophthalmic center, who served as the control group.
Tear collection
Tear samples from all participants were collected by the same examiner within the same time window, between 2:00 PM and 3:00 PM, prior to any other ocular examinations. Tear samples were collected without anesthesia using calibrated 10-µL glass microcapillary tubes (Drummond Microcaps, Merck, USA) from the temporal inferior tear meniscus to minimize ocular surface irritation. The samples were labeled, placed in Eppendorf tubes, and stored at −80 °C until analysis.
Tear analysis
Tear samples were diluted 1:20 in phosphate-buffered saline to a final volume of 100 µL according to the protocols provided by the multiplex immuno-bead assay kit (Luminex Human® Discovery Assay, LXSAHM-30, R&D, USA). The cytokine concentrations were measured using a Luminex 200 system (DiaSorin, USA).
Sign evaluation
An ocular surface analyzer (Keratograph 5 M; OCULUS, Wetzlar, Germany) was used to evaluate the tear film, including measurements of tear meniscus height (TMH) and non-invasive tear film break-up time (NIBUT). Ocular surface damage was assessed by the corneal fluorescein staining (CFS) using the National Eye Institute/Industry (NEI) grading scale [19], which divides the cornea into five sections, scoring staining from 0 (absent) to 3 (severe) in each section, and summing the scores.
To evaluate tear secretion, the standard Schirmer’s test (ST) without topical anesthesia was performed more than 30 min after collecting tear samples, determining the NIBUT or staining scores to avoid affecting the results of these tests. The fluorescein strips and ST strips were obtained from Tianjin Jingming New Technology Development Co., Ltd. (Tianjin, China).
Symptom evaluation
All participants completed the DE-Related Quality of Life Score (DEQS) questionnaire [20] to assess DE symptoms.
Statistical analysis
Statistical analyses were conducted using PyCharm (Edition 2020.1.2 × 64) integrated with Python (Version 3.8) on the Windows 11 operating system. Continuous variables were recorded as means and standard deviations (SD), whereas categorical variables were recorded as numbers. The consistency of variables with a normal distribution was investigated using both visual (histogram and probability graphics) and analytical (Kolmogorov–Smirnov test) methods. Due to non-normally distributed, all cytokine data were therefore log-transformed (log10) prior to statistical analyses. Student's t-test or paired t-test was used to analyze continuous data. The chi-square test was used to analyze categorical data. For non-normally distributed variables, the Kruskal–Wallis test was applied. Correlations between tear cytokine levels and other ocular examination findings were analyzed using the Spearman’s correlation analysis. Cluster Heatmap was obtained to study the relationship among the variables. Risk factors were analyzed using univariate and multivariate logistic regression models. Univariate logistic regression analysis was performed to identify significant risk factors, and variables with statistically significances were then included in the multivariate logistic regression analysis to select independent risk factors. To ensure the validity of the multivariate logistic regression model, we assessed multicollinearity by calculating the Variance Inflation Factor (VIF) for all variables entered into the model. All VIF values, except for those of NDA and DDA, were below 5.0, indicating no significant multicollinearity. During the model fitting process, the NDA variable was excluded due to multicollinearity issues, as it exhibited a higher VIF value than DDA, which prevented successful model convergence. To confirm the predictive factors for Type III AACE, the dataset was divided into training (80%) and testing (20%) sets to evaluate the performance of the model on unseen data. A Random Forest Classifier was trained on the training data and evaluated using a confusion matrix, classification report (precision, recall, and F1-score), error analysis, ROC-AUC score, and fivefold cross-validation. P values of < 0.05 considered statistically significant.
Results
Baseline clinical features of the study population
Fifty two patients (52 eyes) with Type III AACE were enrolled, comprising 39 eyes with Type III AACE without DE (AACE+ DE− group) and 13 eyes with Type III AACE with DE (AACE+ DE+ group). 50 outpatients (50 eyes) without a Type III AACE diagnosis from the same ophthalmic center served as the control group; among these, 37 eyes were normal controls without DE (control group) and 13 eyes had DE without Type III AACE (AACE− DE+ group). The basic demographics and clinical characteristics of the study population are summarized in Table 1. There were no significant differences in age, sex, SE, or BCVA among the four groups (all P > 0.05, Table 1). However, the mean duration of near-distance work (DNW) was significantly higher in the AACE+ DE− group than in the normal control group (P < 0.05, Table 1).
Table 1.
Basic information and ocular parameters of the subjects included in this study
| AACE+ DE− | Control | AACE+ DE+ | AACE− DE+ | P | |
|---|---|---|---|---|---|
| Subjects | 39 patients (39 eyes) | 37 patients (37 eyes) | 13 patients (13 eyes) | 13 patients (13 eyes) | – |
| Sex (male/female) | 17/22 | 15/22 | 8/5 | 10/3 | 0.79# |
| Age (Year) | 26.69 ± 10.27 | 25.19 ± 7.27 | 30.38 ± 11.56 | 25.23 ± 16.88 | 0.10* |
| BCVA (LogMAR) | −0.00 ± 0.04 | −0.00 ± 0.02 | 0.15 ± 0.04 | 0.15 ± 0.04 | 0.10* |
| SE(D) | −4.26 ± 2.26 | −3.94 ± 1.71 | −4.75 ± 1.62 | −4.54 ± 2.05 | 0.57* |
| NDA (PD) | 34.69 ± 23.56 | – | 30.08 ± 19.77 | – | < 0.001* |
| DDA (PD) | 32.67 ± 21.82 | – | 30.62 ± 18.97 | – | < 0.001* |
| DNW (Hours/day) | 5.95 ± 1.81 | 4.43 ± 1.44 | 5.38 ± 1.76 | 5.00 ± 1.87 | < 0.001* |
| TMH (mm) | 0.19 ± 0.03 | 0.19 ± 0.02 | 0.18 ± 0.02 | 0.20 ± 0.03 | 0.10* |
| NIBUT (s) | 6.00 ± 2.95 | 6.24 ± 3.28 | 2.92 ± 3.45 | 1.46 ± 1.61 | < 0.001* |
| CFS (score) | 1.21 ± 1.81 | 1.59 ± 1.82 | 2.00 ± 2.16 | 1.23 ± 1.64 | 0.50* |
| ST (mm) | 12.04 ± 8.65 | 14.08 ± 8.78 | 3.92 ± 6.38 | 7.38 ± 5.97 | < 0.001* |
| DEQS (score) | 29.31 ± 19.74 | 21.76 ± 16.10 | 53.07 ± 17.19 | 42.36 ± 18.34 | < 0.001* |
AACE acute acquired concomitant esotropia, DE dry eye, AACE+ DE− Type III AACE without DE group, Control normal control group, AACE+ DE+ Type III AACE with DE group, AACE− DE+ DE without Type III AACE group, BCVA best-corrected visual acuity, LogMAR logarithm of the minimum angle of resolution, SE spherical equivalents, NDA near deviation angle, DDA distant deviation angle, DNW duration of near-distance work, TMH tear meniscus height, NIBUT non-invasive break-up time, CFS corneal fluorescein staining, ST Schirmer’s test, DEQS Dry Eye-Related Quality of Life Score
*Kruskal–Wallis’s test
#Chi-square test
Comparison of DE diagnostic indicators among the four groups
Mean NIBUT was < 5 s and was significantly lower in the AACE⁺ DE⁺ and AACE⁻ DE⁺ groups compared with the control and AACE⁺ DE⁻ groups (all P < 0.05, Table 1). No significant differences in ST, TMH, CFS score, or DEQS score were observed between the control and AACE⁺ DE⁻ groups or between the AACE⁺ DE⁺ and AACE⁻ DE⁺ groups (all P > 0.05, Table 1).
Multiplex cytokine analysis of Type III AACE and control subjects
To investigate the relationship between Type III AACE and DE-related biomarkers, we performed a Luminex assay to measure cytokine levels in the tears of both AACE⁺ DE⁻ and control participants. We observed significantly higher concentrations of the inflammatory mediators Interleukin-6 (IL-6), IL-8, IL-17/IL-17A, Interferon-γ (IFN-γ), and Tumor Necrosis Factor α (TNF-α) in the tears of AACE⁺ DE⁻ patients than in those of control subjects (all P < 0.05, Table 2). Additionally, the tear concentrations of Matrix Metalloproteinases-1 (MMP-1), MMP-2, MMP-9, MMP-13, and MUC16 were significantly elevated in the AACE⁺ DE⁻ group compared to the control group (all P < 0.05, Table 2). Increased levels of Galectin-1, Galectin-3, and Galectin-9 were also found in the AACE⁺ DE⁻ group (all P < 0.05, Table 2). No significant differences were detected in the levels of inflammatory mediators (IL-1β, IL-2, IL-3, IL-4, IL-5, IL-10, IL-12, and IL-13), chemokines (CCL2, CCL4, CCL5, and CX3CL1), basic Fibroblast Growth Factor (bFGF), Granulocyte–Macrophage Colony Stimulating Factor (GM-CSF), or MUC-1 between the two groups (all P > 0.05, Table 2).
Table 2.
Comparison of DE-related biomarkers levels in the tear film among the groups
| Variable | AACE+ DE− | Control | AACE+ DE+ | AACE− DE+ | P |
|---|---|---|---|---|---|
| IL-1β | 1.15 ± 0.57 | 0.97 ± 0.50 | 1.20 ± 0.58 | 1.07 ± 0.45 | 0.48 |
| IL-2 | 1.25 ± 0.20 | 1.29 ± 0.27 | 1.19 ± 0.20 | 1.17 ± 0.29 | 0.28 |
| IL-3 | 1.47 ± 0.14 | 1.45 ± 0.20 | 1.52 ± 0.14 | 1.41 ± 0.17 | 0.16 |
| IL-4 | 2.05 ± 0.22 | 1.94 ± 0.30 | 1.99 ± 0.24 | 1.86 ± 0.32 | 0.24 |
| IL-5 | 0.55 ± 0.22 | 0.50 ± 0.21 | 0.56 ± 0.19 | 0.41 ± 0.22 | 0.13 |
| IL-6 | 0.72 ± 0.33 | 0.39 ± 0.42 | 0.83 ± 0.31 | 0.34 ± 0.64 | < 0.001 |
| IL-8 | 2.33 ± 0.32 | 1.98 ± 0.38 | 2.37 ± 0.35 | 2.02 ± 0.39 | < 0.001 |
| IL-10 | 0.80 ± 0.36 | 0.68 ± 0.36 | 0.87 ± 0.40 | 0.71 ± 0.39 | 0.20 |
| IL-12 | 1.72 ± 0.26 | 1.78 ± 0.31 | 1.93 ± 0.31 | 1.64 ± 0.34 | 0.09 |
| IL-13 | 2.68 ± 0.19 | 2.59 ± 0.21 | 2.67 ± 0.19 | 2.55 ± 0.14 | 0.10 |
| IL-17/IL-17A | 1.54 ± 0.49 | 1.31 ± 0.47 | 1.49 ± 0.48 | 1.05 ± 0.39 | < 0.05 |
| TNF-α | 1.28 ± 0.58 | 0.87 ± 0.60 | 1.11 ± 0.57 | 0.96 ± 0.49 | 0.03 |
| IFN-γ | 1.42 ± 0.13 | 1.19 ± 0.09 | 1.47 ± 0.15 | 1.22 ± 0.14 | < 0.001 |
| MMP-1 | 1.16 ± 0.15 | 0.98 ± 0.17 | 1.11 ± 0.12 | 0.94 ± 0.13 | < 0.001 |
| MMP-2 | 3.52 ± 0.14 | 3.20 ± 0.19 | 3.44 ± 0.10 | 3.12 ± 0.24 | < 0.001 |
| MMP-9 | 3.79 ± 0.69 | 3.47 ± 0.64 | 4.08 ± 0.48 | 3.70 ± 0.56 | < 0.001 |
| MMP-13 | 2.67 ± 0.11 | 2.07 ± 0.44 | 2.62 ± 0.15 | 2.14 ± 0.40 | < 0.001 |
| Galectin-1 | 3.97 ± 0.34 | 3.43 ± 0.50 | 3.70 ± 0.50 | 3.31 ± 0.48 | < 0.001 |
| Galectin-3 | 3.95 ± 0.30 | 3.79 ± 0.28 | 3.75 ± 0.30 | 3.85 ± 0.30 | < 0.001 |
| Galectin-9 | 4.51 ± 0.22 | 4.22 ± 0.32 | 4.55 ± 0.22 | 4.03 ± 0.44 | < 0.001 |
| CCL2 | 1.77 ± 0.48 | 1.86 ± 0.53 | 1.80 ± 0.43 | 1.93 ± 0.58 | 0.72 |
| CCL4 | 2.54 ± 0.28 | 2.41 ± 0.27 | 2.39 ± 0.27 | 2.47 ± 0.23 | 0.14 |
| CCL5 | 1.65 ± 0.39 | 1.40 ± 0.48 | 1.53 ± 0.31 | 1.34 ± 0.42 | 0.04 |
| CX3CL1 | 3.54 ± 0.19 | 3.46 ± 0.27 | 3.56 ± 0.18 | 3.35 ± 0.28 | 0.10 |
| bFGF | 0.92 ± 0.29 | 0.92 ± 0.34 | 0.86 ± 0.29 | 0.97 ± 0.41 | 0.87 |
| GM-CSF | 0.83 ± 0.27 | 0.82 ± 0.28 | 0.66 ± 0.37 | 0.99 ± 0.31 | 0.19 |
| MUC-1 | −0.08 ± 0.67 | 0.01 ± 0.69 | 0.01 ± 0.56 | 0.03 ± 0.59 | 0.90 |
| MUC16 | 3.20 ± 0.18 | 3.02 ± 0.17 | 3.11 ± 0.18 | 2.94 ± 0.12 | < 0.001 |
AACE acute acquired concomitant esotropia, DE dry eye, AACE+ DE− Type III AACE without DE group, Control normal control group, AACE+ DE+ Type III AACE with DE group, AACE− DE+ DE without Type III AACE group
*Kruskal–Wallis test
Correlation of tear cytokines and clinical parameters of Type III AACE patients
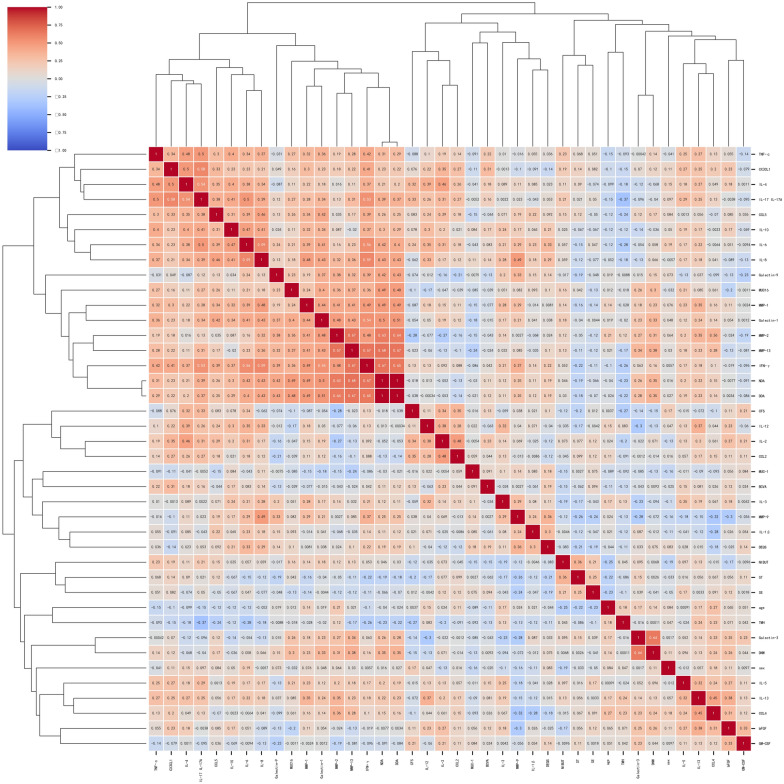
A cluster heatmap was used to visualize the correlations among a large set of variables using color gradients, where red indicates positive correlations and blue indicates negative correlations. A strong positive correlation was observed between NDA and DDA (r = 1, P < 0.05), leading to NDA exclusion to avoid collinearities. Galectin-3 showed a moderate positive correlation with DNW (r = 0.64, P < 0.05). The heatmap revealed two prominent clusters of cytokines with moderate correlations. The larger cluster included IFN-γ, MMP-13, and MMP-2, whereas the smaller cluster comprised CX3CL1, IL-4, and IL-17/IL-17A (Fig. 2).
Fig. 2.
Correlation of tear cytokines and clinical parameters in patients with Type III AACE. A cluster heatmap was used to visualize the correlations among a large set of variables using color gradients, where red indicates positive correlations and blue indicates negative correlations. The absolute value 0.8–1.0 is a high correlation, 0.5–0.8 is a medium correlation, 0.3–0.5 is a weak correlation, and 0–0.3 is no correlation. The heatmap revealed two prominent clusters of cytokines with moderate correlations. The larger cluster included IFN-γ, MMP-13, and MMP-2, whereas the smaller cluster comprised CX3CL1, IL-4, and IL-17/IL-17A. AACE acute acquired concomitant esotropia, BCVA best-corrected visual acuity, LogMAR logarithm of the minimum angle of resolution, SE spherical equivalents, NDA near deviation angle, DDA distant deviation angle, DNW duration of near-distance work, TMH tear meniscus height, NIBUT non-invasive break-up time, CFS corneal fluorescein staining, ST Schirmer’s test, DEQS Dry Eye-Related Quality of Life Score. *Spearman correlation analysis
Logistic regression analyze the risk factors for Type III AACE
Univariate logistic regression analysis indicated that DDA, ST, DEQS, TMH, DNW, inflammatory mediators (IL-1β, IL-4, IL-5, IL-6, IL-8, IL-10, IL-13, IL-17/IL-17A, TNF-α, IFN-γ), MMPs (MMP-1, MMP-2, MMP-9, MMP-13), Galectins (Galectin-1, Galectin-3, Galectin-9), chemokines (CCL4, CCL5), and MUC-16 were individually associated with an increased risk of type III AACE (all P < 0.05, Table 3). Furthermore, multiple logistic regression analysis identified DDA, TMH, IFN-γ, MMP-2, MMP-9, MMP-13, Galectin-9, and MUC-16 as independent risk factors for type III AACE, with MMP-2 ranking the highest (Table 3). TMH showed an odds ratio (OR) of 0.60 with a statistically significant P-value in the multiple logistic regression analysis (P < 0.001). Receiver Operating Characteristic (ROC) curve analysis was employed to evaluate these 8 risk factors (Table 4). MMP-2, MMP-13, and IFN-γ, with corresponding area under the curve (AUC) values of 0.91, 0.91, and 0.94, respectively, demonstrated excellent predictive ability.
Table 3.
Univariate and multivariate logistic regression analyses for Type III AACE risk factors
| Univariate logistic regression analysis | Multivariate logistic regression analysis | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Variable | P-value | OR | 95% CI Lower |
95% CI Upper |
Variable | P-value | OR | 95% CI Lower |
95% CI Upper |
| IL-1β | < 0.05 | 1.39 | 1.11 | 1.74 | IL-1β | 0.20 | 1.20 | 0.91 | 1.58 |
| IL-2 | 0.14 | 0.84 | 0.67 | 1.06 | IL-4 | 0.53 | 1.12 | 0.78 | 1.62 |
| IL-3 | 0.22 | 1.15 | 0.92 | 1.44 | IL-5 | 0.77 | 1.05 | 0.77 | 1.42 |
| IL-4 | < 0.001 | 1.46 | 1.17 | 1.83 | IL-6 | 0.63 | 0.91 | 0.63 | 1.32 |
| IL-5 | 0.03 | 1.28 | 1.02 | 1.60 | IL-8 | 0.72 | 1.08 | 0.71 | 1.66 |
| IL-6 | < 0.001 | 2.41 | 1.93 | 3.02 | IL-10 | 0.76 | 1.05 | 0.77 | 1.44 |
| IL-8 | < 0.001 | 2.82 | 2.25 | 3.53 | IL-13 | 0.09 | 1.34 | 0.96 | 1.88 |
| IL-10 | < 0.05 | 1.40 | 1.12 | 1.75 | IL-17/IL-17A | 0.76 | 0.94 | 0.64 | 1.39 |
| IL-12 | 0.07 | 0.81 | 0.65 | 1.02 | TNF-α | 0.55 | 0.89 | 0.62 | 1.29 |
| IL-13 | < 0.001 | 1.58 | 1.26 | 1.98 | IFN-γ | < 0.05 | 2.19 | 1.35 | 3.56 |
| IL-17/IL-17A | < 0.001 | 1.59 | 1.27 | 1.99 | MMP-1 | 0.19 | 1.28 | 0.88 | 1.85 |
| TNF-α | < 0.001 | 1.97 | 1.57 | 2.47 | MMP-2 | < 0.001 | 2.82 | 1.83 | 4.35 |
| IFN-γ | < 0.001 | 8.27 | 6.60 | 10.35 | MMP-9 | < 0.001 | 1.75 | 1.26 | 2.42 |
| MMP-1 | < 0.001 | 2.98 | 2.38 | 3.73 | MMP-13 | < 0.05 | 1.96 | 1.26 | 3.05 |
| MMP-2 | < 0.001 | 9.47 | 7.56 | 11.85 | Galectin-1 | 0.08 | 1.35 | 0.96 | 1.90 |
| MMP-9 | < 0.001 | 1.62 | 1.30 | 2.03 | Galectin-3 | 0.71 | 0.93 | 0.63 | 1.37 |
| MMP-13 | < 0.001 | 12.24 | 9.78 | 15.33 | Galectin-9 | < 0.001 | 2.02 | 1.41 | 2.89 |
| Galectin-1 | < 0.001 | 3.86 | 3.08 | 4.83 | CCL4 | 0.73 | 0.94 | 0.66 | 1.33 |
| Galectin-3 | < 0.001 | 1.71 | 1.36 | 2.14 | CCL5 | 0.66 | 1.08 | 0.77 | 1.53 |
| Galectin-9 | < 0.001 | 3.02 | 2.41 | 3.78 | CX3CL1 | 0.09 | 0.72 | 0.50 | 1.05 |
| CCL2 | 0.14 | 0.84 | 0.67 | 1.06 | MUC16 | < 0.001 | 1.99 | 1.51 | 2.63 |
| CCL4 | < 0.001 | 1.62 | 1.29 | 2.03 | ST | 0.64 | 1.07 | 0.81 | 1.40 |
| CCL5 | < 0.001 | 1.74 | 1.39 | 2.18 | DEQS | 0.52 | 1.10 | 0.83 | 1.46 |
| CX3CL1 | < 0.05 | 1.41 | 1.12 | 1.76 | TMH | < 0.001 | 0.60 | 0.44 | 0.81 |
| bFGF | 0.92 | 0.99 | 0.79 | 1.24 | DDA | < 0.001 | 5.63 | 4.11 | 7.71 |
| GM-CSF | 0.90 | 1.01 | 0.81 | 1.27 | DNW | 0.17 | 1.31 | 0.89 | 1.94 |
| MUC-1 | 0.24 | 0.87 | 0.70 | 1.09 | |||||
| MUC16 | < 0.001 | 2.78 | 2.22 | 3.49 | |||||
| sex | 0.61 | 1.06 | 0.85 | 1.33 | |||||
| age | 0.16 | 1.18 | 0.94 | 1.47 | |||||
| NIBUT | 0.51 | 0.93 | 0.74 | 1.16 | |||||
| ST | 0.05 | 0.80 | 0.64 | 1.00 | |||||
| DEQS | < 0.001 | 1.50 | 1.20 | 1.88 | |||||
| SE | 0.18 | 0.86 | 0.69 | 1.07 | |||||
| BCVA | 0.94 | 0.99 | 0.79 | 1.24 | |||||
| CFS | 0.07 | 0.81 | 0.65 | 1.02 | |||||
| TMH | 0.01 | 0.75 | 0.60 | 0.94 | |||||
| DDA | < 0.001 | 33.14 | 26.47 | 41.50 | |||||
| DNW | < 0.001 | 2.53 | 2.02 | 3.16 | |||||
Univariate logistic regression analysis was performed to identify significant risk factors, and variables with statistically significances were then included in the multivariate logistic regression analysis to select independent risk factors
AACE acute acquired concomitant esotropia, BCVA best-corrected visual acuity, SE spherical equivalents, DDA distant deviation angle, DNW duration of near-distance work, TMH tear meniscus height, NIBUT non-invasive break-up time, CFS corneal fluorescein staining, ST Schirmer’s test, DEQS Dry Eye-Related Quality of Life Score
Table 4.
Receiver operating characteristic (ROC) analysis for Type III AACE risk factors
| Variable | AUC | 95% CI lower | 95% CI upper | Sensitivity | Specificity | Best cut-off |
|---|---|---|---|---|---|---|
| IFN-γ | 0.94 | 0.81 | 1.07 | 1.00 | 0.88 | 0.58 |
| MMP-2 | 0.91 | 0.75 | 1.06 | 0.88 | 0.88 | 0.59 |
| MMP-9 | 0.64 | 0.36 | 0.92 | 0.50 | 0.88 | 0.59 |
| MMP-13 | 0.91 | 0.75 | 1.06 | 1.00 | 0.88 | 0.62 |
| Galectin-9 | 0.69 | 0.42 | 0.95 | 0.63 | 0.88 | 0.68 |
| MUC16 | 0.91 | 0.75 | 1.06 | 1.00 | 0.75 | 0.48 |
| TMH | 0.80 | 0.58 | 1.03 | 0.75 | 0.88 | 0.57 |
| DDA | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 0.33 |
TMH tear meniscus height, DDA distant deviation angle, AUC arear under the curve
Machine learning model evaluated the risk factors for Type III AACE
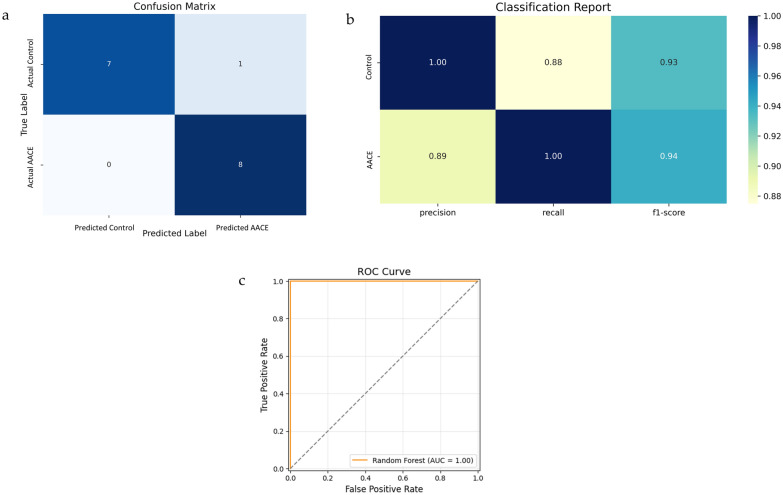
To confirm the risk factors for Type III AACE, a random forest model was trained and evaluated. The confusion matrix showed only one false negative, indicating that the model missed a very small number of positive cases, whereas it successfully identified all the negative cases (Fig. 3a). The classification Report showed that F1 scores for class 0 (control group) and class 1 (AACE group) were 0.93 and 0.94, respectively, reflecting a good balance between precision and recall (Fig. 3b). Error analysis indicated mean prediction error (MPE), standard deviation (SD), mean absolute error (MAE), and median absolute error (MedAE) values of −0.063, 0.242, 0.063, and 0.000, respectively. The AUC value was 1.00 (Fig. 3c). This random forest model exhibited an excellent predictive performance.
Fig. 3.
Evaluation of the predictive algorithm of the Random Forest model. a Confusion Matrix. b Classification Report Heatmap. c Receiver operating characteristic (ROC) Curve for dataset
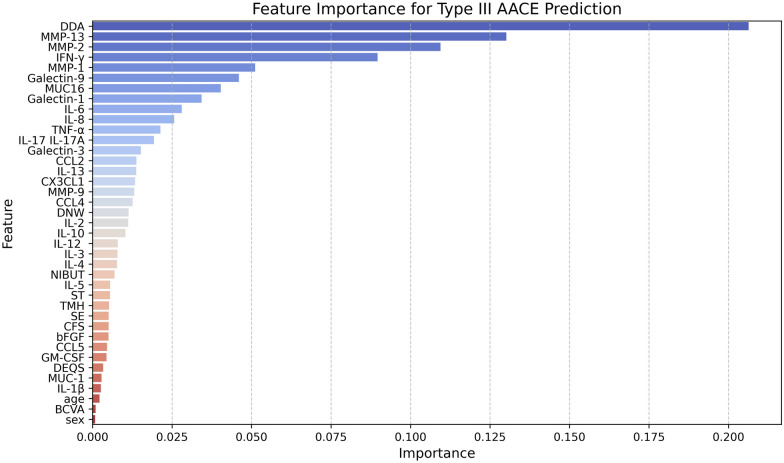
MMP-2, MMP-13, and IFN-γ, demonstrated higher feature importance scores for Type III AACE prediction than other tear cytokines (Fig. 4), indicating a high predictive accuracy. To further explore the importance of MMP-2, MMP-13, and IFN-γ in prediction, we constructed separate random forest models using the top 3, top 4, and top 5 features of tear cytokines based on their feature importance rankings. fivefold cross-validation was used to verify the accuracy of the three random forest models and tested using paired t-tests (Fig. 5). The mean cross-validation scores for the top 3 features (MMP-13, MMP-2, and IFN-γ), the top 4 features (MMP-13, MMP-2, IFN-γ, and MMP-1) and the top 5 features (MMP-13, MMP-2, IFN-γ, MMP-1, and Galectin-9) were 0.96, 0.96, and 0.95, respectively. No significant differences were found among the groups (top 3 features vs. top 4 features: t = −0.58, P = 0.59; top 3 features vs. top 5 features: t = 1.11, P = 0.33; top 4 features vs. top 5 features: t = 1.31, P = 0.26). Cutoff values for the top 3 features of tear cytokines (Fig. 6) using Youden’s J statistic were as follows: MMP-13: 2.56 (363.08 pg/mL), MMP-2: 3.36 (2290.87 pg/mL), IFN-γ: 1.32 (20.89 pg/mL).
Fig. 4.
Importance of features for Type III AACE prediction. AACE acute acquired concomitant esotropia, BCVA best-corrected visual acuity, SE spherical equivalents, DDA distant deviation angle, DNW duration of near-distance work, NIBUT non-invasive break-up time, CFS corneal fluorescein staining, ST Schirmer’s test, TMH tear meniscus height, DEQS Dry Eye-Related Quality of Life Score
Fig. 5.
Cross-validation scores (five-fold) for top features of tear cytokines. The mean cross-validation scores for top 3 features (MMP-13, MMP-2, IFN-γ), top 4 features (MMP-13, MMP-2, IFN-γ, and MMP-1) and top 5 features (MMP-13, MMP-2, IFN-γ, MMP-1, and Galectin-9) were 0.96, 0.96, and 0.95, respectively. No significant differences were found among the groups (all P > 0.05)
Fig. 6.
ROC curve for top 3 features of tear cytokines. Cutoff values based on Youden's Index: MMP-13: 2.56 (363.08 pg/mL), MMP-2: 3.36 (2290.87 pg/mL), IFN-γ: 1.32 (20.89 pg/mL)
Discussion
This study demonstrated that various inflammatory mediators, Galectin-3, and MUC16 were significantly elevated in the tear film of Type III AACE patients without DE compared to healthy controls. Our findings suggest a positive link of ocular surface inflammation with the development of Type III AACE. Type III AACE is characterized by its association with myopia and has a significant impact on quality of life. The increased DNW observed in the AACE group compared to the controls suggests that prolonged near work may be a contributing factor to the development of Type III AACE. This finding aligns with previous research demonstrating that behavioral factors, such as excessive near work and electronic device use, can exacerbate the severity of AACE and increase its recurrence rate post-surgery [17]. Therefore, addressing these factors through early intervention may be crucial for improving patient outcomes. Furthermore, DE-related tear biomarkers, particularly MMP-2, MMP-13, and IFN-γ, may serve as potential indicators associated with Type III AACE and could contribute to risk stratification and early identification in clinical practice.
Our study determined that the expression levels of MMP-2, MMP-13, and IFN-γ were upregulated in the tear film of patients with Type III AACE. The TFOS DEWS II and ADES consensus emphasize that ocular surface inflammation plays a central role in the vicious cycle of DE, which is driven by both intrinsic and extrinsic factors [6, 7]. The tear film, a fluid layer on the mucosal surface of the eye, plays a homeostatic role through its cytokine content, with imbalances linked to ocular surface diseases [21]. Tear hyperosmolarity has been shown to upregulate the expression of collagenase MMP-13 and gelatinase MMP-2 in human corneal epithelial cells [22]. MMP-2 expression is also upregulated in the extraocular muscles of patients with strabismus [23]. In patients with AACE, increased exposure of the bulbar conjunctiva due to ocular misalignment could disrupt lipid distribution and induce hyperosmotic stress [5]. Previous studies have demonstrated that DE induction leads to enhanced IFN-γ expression [24, 25]. As the only type II interferon, IFN-γ can reduce tear secretion and conjunctival GC counts, damage corneal epithelial cells, and promote the secretion of inflammatory cytokines and chemokines in the cornea and lacrimal glands [26]. IFN-γ treatment inhibited mucin secretion in GC cultures while reducing GC proliferation [27]. This contributes to tear film instability and the release of inflammatory mediators, which perpetuate ocular surface inflammation. In addition to these findings, our study identified MMP-2, MMP-13, and IFN-γ as strong independent risk factors for Type III AACE, with high predictive values. Increased levels of these cytokines in tears may indicate inflammation and hyperosmotic stress on the ocular surface. The random forest model displayed excellent predictive performance, with MMP-2, MMP-13, and IFN-γ showing high feature importance scores for predicting Type III AACE. Our findings suggest that tear film inflammation may be interconnected with Type III AACE. Elevated levels of MMP-2, MMP-13, and IFN-γ exceeding their respective cutoff values may indicate an increased risk of Type III AACE development, warranting surgical intervention.
Notably, we observed a moderate positive correlation between Galectin-3 levels and DNW, suggesting a potential link between prolonged visual tasks and ocular surface inflammation. Galectin-3 is a 35 kDa protein initially identified as the only chimera-type galectin [28]. In healthy eyes, the binding between transmembrane mucin glycans and the carbohydrate recognition domains (CRDs) of Galectin-3 forms pentamers, which are essential for maintaining the glycocalyx barrier function [29]. In DE, Galectin-3 is secreted into the tear film [30, 31], and our findings also showed upregulated level of Galectin-3 in the tears of Type III AACE patients without DE compared to controls. MUC16, a critical transmembrane-associated mucin that is part of the glycocalyx barrier on the ocular surface, is disrupted due to the binding of MUC16 glycans to the CRDs of oligomerized Galectin-3 [32–34]. We found that MUC16 levels were significantly elevated in the tears of patients with Type III AACE, suggesting glycocalyx barrier disruption. This impairment reduces ocular surface protection and lubrication, thereby increasing the risk of microtrauma from blinking [30, 35]. This inflammation likely contributes to the elevated levels of MMPs and other cytokines observed in patients with Type III AACE, further aggravating both DE and AACE symptoms. Our previous study demonstrated that DNW is an independent risk factor for Type III AACE [36]. Prolonged DNW, particularly the extended use of video display terminals (VDT), increases incomplete blinking, leading to tear film instability and exacerbating DE symptoms [37, 38]. Even as little as 1–2 h of daily VDT use can significantly impact the tear film and ocular surface, potentially contributing to both DE and AACE [39]. Our findings suggest that while prolonged DNW may serve as a common behavioral factor contributing to ocular surface instability, the distinct cytokine profiles observed in Type III AACE patients appear to reflect more than just the impact of DE or near-work-related behavioral patterns. The similarity in tear film parameters between AACE⁺ DE⁻ patients and controls supports the notion that tear cytokine alterations in Type III AACE may occur even in the absence of clinically diagnosed DE. Additionally, TMH showed an OR < 1 with a significant P-value in the multivariate analysis, suggesting its potential role as a protective factor in the context of Type III AACE pathophysiology and ocular surface homeostasis. This implies that supplementation with artificial tears may help reduce the risk of developing Type III AACE.
This study had some limitations. First, the sample size was small, which may induce potential biases. Second, this was a cross-sectional study which can only demonstrate the association of Type III AACE with DE-related biomarkers. Further longitudinal studies are warranted to confirm the effectiveness of these biomarkers in predicting Type III AACE progression. Third, the predictive model was internally validated without external validation. This means that while the model demonstrated excellent performance within our specific dataset, its generalizability to other populations or settings remains uncertain. Future research should focus on external validation using independent datasets from different cohorts or clinical centers. This would allow for a more robust assessment of model accuracy and reliability across diverse populations. Additionally, external validation could help to identify potential biases or confounding factors that may not be evident within the internal validation framework. By conducting external validation studies, we can further refine the model and enhance its applicability in clinical practice, ultimately improving the prediction and management of Type III AACE. Nevertheless, this study underscores the potential of tear analysis as a predictive tool for Type III AACE.
In conclusion, this study demonstrated a significant association between DE-related tear biomarkers and Type III AACE. Specifically, elevated levels of MMP-2, MMP-13, and IFN-γ were identified as potential predictive biomarkers of Type III AACE. These findings highlight a possible mechanistic link between ocular surface inflammation and the development of Type III AACE, providing a foundation for future diagnostic and therapeutic strategies.
Acknowledgements
The authors thank all the participants who agreed to participate in the current study.
Abbreviations
- AACE
Acute acquired concomitant esotropia
- BCVA
Best-corrected visual acuity
- LogMAR
Logarithm of the minimum angle of resolution
- SE
Spherical equivalents
- NDA
Near deviation angle
- DDA
Distant deviation angle
- DNW
Duration of near-distance work
- TMH
Tear meniscus height
- NIBUT
Non-invasive break-up time
- CFS
Corneal fluorescein staining
- ST
Schirmer’s test
- DEQS
Dry eye-related quality of life score
- MMP
Matrix metalloproteinase (MMP)
- IL
Interleukin
- TNF-α
Tumor necrosis factor-α
- IFN-γ
Interferon-γ
Author contributions
Conceptualization, L.H. and W.Z.; methodology, L.H. and S.T.; software, L.H.; validation, L.H.; formal analysis, L.H. and S.T.; investigation, W.W. and Y.L.; resources, W.W. and Y.L.; data curation, Y.L. and S.T.; writing—original draft preparation, L.H. and S.T.; writing—review and editing, L.H. and W.Z.; visualization, L.H.; supervision, W.Z.; project administration, S.T. and W.Z.; funding acquisition, L.H. All the authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Joint Funds for the Innovation of Science and Technology, Fujian Province (No.2021Y9114). The APC was funded by the Joint Funds for the Innovation of Science and Technology of Fujian Province.
Data availability
The datasets used and analyzed during the current study are available from the corresponding author upon reasonable request.
Declarations
Ethics approval and consent to participate
The study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Board of Ethics Committee of The First Affiliated Hospital of Fujian Medical University’s approved study protocol (MRTCA, ECFAH of FMU [2022] 323). Informed consent was obtained from all the participants involved in the study.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Yijun Lin and Shumin Tang have contributed equally to this work.
Contributor Information
Weidong Zheng, Email: weidongzheng@fjmu.edu.cn.
Libin Huang, Email: hlibin@sina.com.
References
- 1.Chen J, Deng D, Sun Y, Shen T, Cao G, Yan J, et al. Acute acquired concomitant esotropia: clinical features, classification, and etiology. Medicine (Baltimore). 2015;94:e2273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Burian HM, Miller JE. Comitant convergent strabismus with acute onset. Am J Ophthalmol. 1958;45:55–64. [DOI] [PubMed] [Google Scholar]
- 3.Zhao S, Hao J, Liu J, Cao K, Fu J. Fusional vergence dysfunctions in acute acquired concomitant esotropia of adulthood with myopia. Ophthalmic Res. 2023;66:320–7. [DOI] [PubMed] [Google Scholar]
- 4.Hu Y, Wang S, Wu L, Xi S, Wen W, Zhao C. Deficits of visual cortex function in acute acquired concomitant esotropia patients. Invest Ophthalmol Vis Sci. 2023;64:46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Giannaccare G, Versura P, Sebastiani S, Fariselli C, Pellegrini M, Campos E. Dry eye disease in strabismus patients: does eye deviation harm ocular surface? Med Hypotheses. 2018;111:15–8. [DOI] [PubMed] [Google Scholar]
- 6.Bron AJ, de Paiva CS, Chauhan SK, Bonini S, Gabison EE, Jain S, et al. TFOS DEWS II pathophysiology report. Ocul Surf. 2017;15:438–510. [DOI] [PubMed] [Google Scholar]
- 7.Tsubota K, Yokoi N, Shimazaki J, Watanabe H, Dogru M, Yamada M, et al. New perspectives on dry eye definition and diagnosis: a consensus report by the Asia dry eye society. Ocul Surf. 2017;15:65–76. [DOI] [PubMed] [Google Scholar]
- 8.Kumar NR, Praveen M, Narasimhan R, Khamar P, D’Souza S, Sinha-Roy A, et al. Tear biomarkers in dry eye disease: progress in the last decade. Indian J Ophthalmol. 2023;71:1190–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen Y, Mallem K, Asbell PA, Ying GS. A latent profile analysis of tear cytokines and their association with severity of dry eye disease in the Dry Eye Assessment and Management (DREAM) study. Sci Rep. 2024;14:526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gao F, Hong X, Ding F, Huang S, Lian W, Wang H, et al. High level of inflammatory cytokines in the tears: a bridge of patients with concomitant exotropia and dry eye. Oxid Med Cell Longev. 2021;2021:5662550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang Y, Tang XJ, Liu Q, Chen L. The incidence and risk factors for dry eye after pediatric strabismus surgery. Ophthalmol Ther. 2023;12:87–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang S, Guo W, Gong Y, Wang J, Chen L, Zhao J, et al. Application of vitamin A palmitate eye gel and nurse value of Watson’s theory of caring in children with dry eye after strabismus surgery: a randomized trial. Transl Pediatr. 2021;10:2335–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guo S, Zhou Y, Xi S, Zhao C, Wen W. Advances in the diagnosis and treatment of acute acquired comitant esotropia. Int Ophthalmol. 2024;44:315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wen W, Farzavandi SK, Sato M, Quah BL, Ko ST, Surendran TS, et al. Clinical practices on acute acquired comitant esotropia: a consensus statement proposed by the Council of Asia-Pacific Strabismus and Pediatric Ophthalmology Society. Asia Pac J Ophthalmol (Phila). 2025;14:100134. [DOI] [PubMed] [Google Scholar]
- 15.Nouraeinejad A. To prioritize treatment options for acute acquired comitant esotropia. Eur J Ophthalmol. 2023;33:NP145–6. [DOI] [PubMed] [Google Scholar]
- 16.Nouraeinejad A. Letter to the Editor: Environmentally driven abnormalities in accommodation and vergence can be one of the causes of acute acquired comitant Esotropia. Optom Vis Sci. 2023;100:238. [DOI] [PubMed] [Google Scholar]
- 17.Lim CW, Lee J, Kim WJ. Changes in the number and characteristics of patients with acute acquired concomitant esotropia over time: an 8-year retrospective study. Medicine (Baltimore). 2023;102: e33986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zou X, Nagino K, Okumura Y, Midorikawa-Inomata A, Eguchi A, Yee A, et al. Optimal cutoff value of the dry eye-related quality-of-life score for diagnosing dry eye disease. Sci Rep. 2024;14:4623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bron AJ, Evans VE, Smith JA. Grading of corneal and conjunctival staining in the context of other dry eye tests. Cornea. 2003;22:640–50. [DOI] [PubMed] [Google Scholar]
- 20.Sakane Y, Yamaguchi M, Yokoi N, Uchino M, Dogru M, Oishi T, et al. Development and validation of the dry eye-related quality-of-life score questionnaire. JAMA Ophthalmol. 2013;131:1331–8. [DOI] [PubMed] [Google Scholar]
- 21.Krok M, Wroblewska-Czajka E, Lach-Wojnarowicz O, Bronikowska J, Czuba ZP, Wylegala E, Dobrowolski D. Analysis of cytokine and chemokine level in tear film in keratoconus patients before and after corneal cross-linking (CXL) treatment. Int J Mol Sci. 2024;25:1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Deng R, Su Z, Hua X, Zhang Z, Li DQ, Pflugfelder SC. Osmoprotectants suppress the production and activity of matrix metalloproteinases induced by hyperosmolarity in primary human corneal epithelial cells. Mol Vis. 2014;20:1243–52. [PMC free article] [PubMed] [Google Scholar]
- 23.Agarwal AB, Feng CY, Altick AL, Quilici DR, Wen D, Johnson LA, von Bartheld CS. Altered protein composition and gene expression in strabismic human extraocular muscles and tendons. Invest Ophthalmol Vis Sci. 2016;57:5576–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yang X, Zuo X, Zeng H, Liao K, He D, Wang B, Yuan J. IFN-gamma facilitates corneal epithelial cell pyroptosis through the JAK2/STAT1 pathway in dry eye. Invest Ophthalmol Vis Sci. 2023;64:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Coursey TG, Tukler Henriksson J, Barbosa FL, de Paiva CS, Pflugfelder SC. Interferon-gamma-induced unfolded protein response in conjunctival goblet cells as a cause of mucin deficiency in Sjogren syndrome. Am J Pathol. 2016;186:1547–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ko JH, Kim S, Ryu JS, Song HJ, Oh JY. Interferon-gamma elicits the ocular surface pathology mimicking dry eye through direct modulation of resident corneal cells. Cell Death Discov. 2023;9:209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Garcia-Posadas L, Hodges RR, Li D, Shatos MA, Storr-Paulsen T, Diebold Y, Dartt DA. Interaction of IFN-gamma with cholinergic agonists to modulate rat and human goblet cell function. Mucosal Immunol. 2016;9:206–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nabi IR, Shankar J, Dennis JW. The galectin lattice at a glance. J Cell Sci. 2015;128:2213–9. [DOI] [PubMed] [Google Scholar]
- 29.Argueso P, Guzman-Aranguez A, Mantelli F, Cao Z, Ricciuto J, Panjwani N. Association of cell surface mucins with galectin-3 contributes to the ocular surface epithelial barrier. J Biol Chem. 2009;284:23037–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Uchino Y, Mauris J, Woodward AM, Dieckow J, Amparo F, Dana R, et al. Alteration of galectin-3 in tears of patients with dry eye disease. Am J Ophthalmol. 2015;159:1027–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hata-Mizuno M, Uchino Y, Uchino M, Shimmura S, Ogawa Y, Tsubota K, Negishi K. Analysis of the association between galectin-3 concentration in tears and the severity of dry eye disease: a case-control study. J Clin Med. 2021;11:66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hattrup CL, Gendler SJ. Structure and function of the cell surface (tethered) mucins. Annu Rev Physiol. 2008;70:431–57. [DOI] [PubMed] [Google Scholar]
- 33.Uchino Y. The ocular surface glycocalyx and its alteration in dry eye disease: a review. Investig Ophthalmol Vis Sci. 2018;59:DES157–62. [DOI] [PubMed] [Google Scholar]
- 34.Ablamowicz AF, Nichols JJ. Ocular surface membrane-associated mucins. Ocul Surf. 2016;14:331–41. [DOI] [PubMed] [Google Scholar]
- 35.Portal C, Gouyer V, Gottrand F, Desseyn JL. Ocular mucins in dry eye disease. Exp Eye Res. 2019;186: 107724. [DOI] [PubMed] [Google Scholar]
- 36.Zhang J, Chen J, Lin H, Huang L, Ma S, Zheng W. Independent risk factors of type III acute acquired concomitant esotropia: a matched case-control study. Indian J Ophthalmol. 2022;70:3382–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fjaervoll H, Fjaervoll K, Magno M, Moschowits E, Vehof J, Dartt DA, Utheim TP. The association between visual display terminal use and dry eye: a review. Acta Ophthalmol. 2022;100:357–75. [DOI] [PubMed] [Google Scholar]
- 38.Kamoy B, Magno M, Noland ST, Moe MC, Petrovski G, Vehof J, Utheim TP. Video display terminal use and dry eye: preventive measures and future perspectives. Acta Ophthalmol. 2022;100:723–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lee HS, Park SW, Heo H. Acute acquired comitant esotropia related to excessive smartphone use. BMC Ophthalmol. 2016;16:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and analyzed during the current study are available from the corresponding author upon reasonable request.