Abstract
Background
The coronary artery disease–based polygenic risk score (PRS-CAD) estimates risk of acute myocardial infarction (AMI), but its performance across AMI subtypes in younger individuals, especially women, remains uncertain.
Objectives
The authors assessed PRS-CAD's performance in AMI subtypes.
Methods
We included 2,079 AMI patients aged 18 to 55 years with a 2:1 female-to-male ratio from the VIRGO (Variation in Recovery: Role of Gender on Outcomes of Young Acute Myocardial Infarction Patients) study and 3,761 controls from the MESA (Multi-Ethnic Study of Atherosclerosis) study. AMI subtypes were classified using the VIRGO taxonomy. We evaluated PRS-CAD’s association with AMI subtypes using multinomial logistic regression and with 1-year outcomes in AMI subtypes using Cox regression.
Results
PRS-CAD was significantly associated with MI due to coronary artery disease (N = 1,876; OR: 1.82 per 1-SD increase; 95% CI: 1.67-1.97; P < 0.001) but not with MI with nonobstructive coronary artery disease (N = 188; OR: 1.13 per 1-SD increase; 95% CI: 0.96-1.34; P = 0.14). PRS-CAD’s performance did not differ by sex. A 1-SD increase in PRS-CAD was associated with higher risk of 1-year hospitalization or death in patients with MI with nonobstructive coronary artery disease (HR: 1.50; 95% CI: 1.08-2.10; P = 0.02) but not in patients with MI due to coronary artery disease (HR: 0.98; 95% CI: 0.91-1.07; P = 0.67).
Conclusions
PRS-CAD’s association with AMI varied by subtype but not by sex in young adults, warranting caution in application.
Key words: acute myocardial infarction, polygenic risk score, subtypes
Central Illustration
Polygenic risk scores (PRS) aggregate the cumulative risk associated with various genetic variants to predict the likelihood of specific conditions, such as acute myocardial infarction (AMI).1, 2, 3 In a previous study with a cohort of 2,081 patients, predominantly women, with early-onset AMI from the VIRGO (Variation in Recovery: Role of Gender on Outcomes of Young Acute Myocardial Infarction Patients) study, we demonstrated that those with the highest PRS had a 3-fold increased odds of early-onset AMI compared to control participants.4 PRS has also been used to predict AMI prognosis in some studies, although results have been mixed.5, 6, 7, 8
PRS is calculated by summing the risk alleles present in an individual, each weighted by the effect size estimated in a base genome-wide association study (GWAS) on the same phenotype. However, discrepancies between the base cohort used for GWAS and the target cohort for PRS application can reduce the predictive utility of PRS.9 Most PRS models for AMI have been developed from GWAS summary statistics of coronary artery disease (CAD) populations (PRS-CAD).1, 2, 3 For instance, the most widely used summary statistics come from the GWAS study of the CARDIoGRAMplusC4D Consortium, which includes 60,801 CAD cases, encompassing patients with AMI, acute coronary syndrome, chronic stable angina, or coronary stenosis >50%; however, specific AMI subtypes are not distinguished.10
Increasing evidence shows that the pathomechanisms of AMI are heterogeneous, particularly among younger individuals and especially among women. AMI cases can be classified into two main groups: MI due to coronary artery disease (MI-CAD) and MI with nonobstructive coronary artery disease (MINOCA),11 which can be further subdivided into 5 distinct subtypes.12, 13, 14 Patients with MINOCA tend to present with fewer traditional cardiovascular risk factors, such as hypertension, dyslipidemia, and diabetes, than those with MI-CAD.11 Prior PRS studies have focused on MI-CAD, yet the relationships between PRS-CAD and various AMI subtypes remain unexplored. Understanding this relationship is crucial given the unique risk factors and outcomes associated with different AMI subtypes.
Therefore, we aimed to evaluate the association of PRS-CAD with AMI subtypes in young adults, with a particular focus on women, and to examine subsequent outcomes, including mortality and rehospitalization. Using the PRS calculated from 2,079 individuals with AMI aged 18 to 55 years with a 2:1 female-to-male enrollment ratio in the VIRGO study,15 along with 3,761 CAD-free controls from the MESA (Multi-Ethnic Study of Atherosclerosis) with comparable whole-genome sequencing (WGS) data,16 we sought to provide valuable insights into the performance of a CAD-based PRS in young adults with AMI.
Methods
Study population
The study population comprises patients with AMI from the VIRGO study (cases)15 and individuals without AMI (controls) from the MESA study.16 The VIRGO study15 recruited 3,501 patients with early-onset (ages 18-55) AMI with a 2:1 female-to-male enrollment ratio from 103 U.S. hospitals and 24 Spanish hospitals between August 2008 and January 2012. Participants were eligible if they had elevated cardiac biomarkers (at least one biomarker—troponin I or T, or creatine kinase-myocardial band—above the 99th percentile upper reference limit) within 24 hours of admission. Additional evidence of AMI was required, including either ischemic symptoms or electrocardiographic changes indicating new ischemia. Of these participants, 2,101 provided DNA samples and consented to genetic analysis; 2,081 of these had WGS data that met quality control criteria.4 A total of 2,079 patients with phenotype classification data were included in our analysis (Supplemental Figure 1).
The MESA study16 included 6,814 individuals aged 45 to 84 years without baseline cardiovascular disease (CVD), recruited from 2000 to 2002 from 6 U.S. communities. Participants were of White, African American, Hispanic, and Asian (Chinese descent) backgrounds. Individuals were excluded if genetic data were unavailable or if they developed CVD events (eg, myocardial infarction, coronary revascularization, angina, peripheral arterial disease, stroke, resuscitated cardiac arrest, cardiovascular death) by the last follow-up in December 2014. CVD events during follow-up were identified through death certificates, hospital records, autopsy reports, and participant interviews or questionnaires. Ultimately, 3,932 participants had WGS data, of whom 3,761 passed quality filtering as described in a prior study.4
Data collection
For VIRGO, trained personnel collected demographic characteristics and family history of AMI through standardized in-person interviews, while data on traditional risk factors and medications were obtained from medical records.15 Data on 12-month mortality and rehospitalizations were also collected and are described in the “Outcome Definitions” section. In MESA, demographic characteristics, smoking status, and medication use were obtained via questionnaires. Hypertension, diabetes, and lipid levels were based on measurements of blood pressure, blood glucose, and blood lipids. Protocols are available at www.mesa-nhlbi.org.16 Biological ages of both VIRGO and MESA subjects were recorded at the time of enrollment.
Calculation of PRS and ancestry genetic principal components
Individual genetic principal components (PCs) and PRS for VIRGO and MESA were previously computed (2019).4 Genetic PCs were calculated using principal components analysis (PCA). The PRS was generated using 6,286,512 single nucleotide polymorphisms and summary statistics from CARDIoGRAMplusC4D,10 followed by ancestry adjustment. Specifically, the first 4 PCs from the PCA were used for ancestry adjustment. The PRS percentile was determined using MESA controls as a reference.10
Definitions of AMI subtypes
AMI endotypes were defined based on a taxonomy developed in the VIRGO study for young AMI patients.13,14 This taxonomy builds on the third universal definition of MI12 and captures mechanisms prevalent in younger individuals. It classifies AMI into 5 categories: plaque-mediated culprit lesion (class I), supply-demand mismatch with obstructive CAD (class II), supply-demand mismatch with nonobstructive CAD (class III), MI with nonatherosclerotic mechanisms (eg, vasospasm, spontaneous coronary artery dissection (SCAD), embolism, Takotsubo) (class IV), and indeterminate (class V).13,14 Classes I and II are grouped as MI-CAD, while classes III and IV are grouped as MINOCA.11
Outcome Definitions
In the VIRGO dataset, mortality events were obtained through interviews with family members and verified with death certificates, hospital records, or obituaries.15 Hospitalizations were defined as any hospital or observation stay longer than 24 hours within 12 months post discharge. Hospitalizations were further categorized into cardiovascular, noncardiovascular, or unknown rehospitalizations. Cardiovascular rehospitalizations included MI, heart failure, stable/unstable angina, stroke, and other cardiac causes (eg, pericarditis, valvular disease, arrhythmias). Noncardiovascular rehospitalizations included all other hospital events. All hospitalization records were collected through a 2-stage process and adjudicated as described previously.17 Time zero was defined as the time of discharge, and censoring was applied for patients who withdrew from the study or had no adverse events within the 12-month postdischarge period.
Statistical analysis
First, we examined the unadjusted and adjusted associations of PRS with each AMI type using multinomial logistic regression with both VIRGO cases and MESA controls. The independent variable was PRS, and the dependent variable was either a specific AMI type in VIRGO or non-AMI in MESA. The unadjusted model included PRS as the sole covariate, while the adjusted model included age, sex, and the first four PCs from the genomic PCA as additional covariates. We also assessed the association of genetics-related variables with different AMI subtypes using the chi-square test for categorical variables and the Kruskal-Wallis rank-based test for continuous variables. Categorical variables were presented as counts (%) and continuous variables as median (Q1, Q3). Continuous variables included PRS and PRS percentile, while categorical variables included family history of MI, familial hypercholesterolemia mutation, and PRS percentiles (top 5, 10, 20, 30, or bottom 30, 20, 10, and 5 percentiles). Given the established variation in PRS predictive power between male and female AMI patients,1 we also compared the same set of variables between males and females with MI-CAD or MINOCA using the methods described earlier.
Next, we examined whether PRS was associated with subsequent rehospitalization events vs no rehospitalization events in VIRGO cases, stratified by AMI type, using survival analysis. Three outcomes were considered: all-cause hospitalizations or death, cardiovascular hospitalizations or death, and noncardiovascular hospitalizations. Kaplan-Meier curves were generated to compare outcomes between cases in the top 10th percentile of PRS and those in the bottom 90th percentile within MI-CAD and MINOCA cohorts, respectively, and the log-rank test was used for comparison. Cox proportional-hazards regression was used to model outcomes with PRS as a continuous variable, and the proportional hazards assumption was assessed by testing the independence between scaled Schoenfeld residuals and time. Both unadjusted and adjusted models were analyzed, with age, sex, and the first 4 PCs included as covariates in the adjusted model. We further conducted competing risk analysis to consider death and other rehospitalizations as competing events when evaluating cardiovascular and noncardiovascular rehospitalizations. We used both the Cox regression model which treats competing events as censoring to assess the cause-specific hazard and the Fine-Gray regression model18 which treats them as competing risks to assess the subdistribution hazard. For each individual, only the first hospitalization event was included in the competing risk analysis.
All statistical tests were two-sided. For tests involving the top 5th to 30th and bottom 5th to 30th PRS percentiles, a significance level of 0.00625 (0.05/8) was applied following Bonferroni multiple-testing correction. All other tests had a significance level of 0.05. P-values presented are nominal, and confidence intervals were not adjusted for multiple comparisons; therefore, they should be interpreted with caution. Analyses were conducted in R v4.0.3.
The project received institutional review board approval at all participating sites and followed the STROBE guidelines.
Results
We examined the PRS-CAD in 2,079 patients with early-onset AMI. Among them, 1,816 (87.3%) were classified as class I MI, 60 (2.9%) as class II, 163 (7.8%) as class III, 25 (1.2%) as class IV, and 15 (0.7%) as class V (Supplemental Table 1). In addition, 1,876 (90.2%) cases were classified as MI-CAD, while 188 (9.0%) were classified as MINOCA (Table 1). Patients with different AMI subtypes differed in demographic characteristics and traditional risk factors (Supplemental Table 1, Table 1). Compared with MI-CAD cases, MINOCA cases included more Black women and individuals with fewer traditional risk factors (Table 1). Compared with MESA controls, VIRGO AMI cases included more White individuals, women, younger individuals, and had more traditional risk factors (Table 1).
Table 1.
Baseline Characteristics in AMI Patients and Controls
| MI-CAD (n = 1,876) | MINOCA (n = 188) | Controls (n = 3,761) | |
|---|---|---|---|
| Race/ethnicity | |||
| White | 1,405 (74.9%) | 124 (66.0%) | 1,544 (41.1%) |
| Black | 288 (15.4%) | 45 (23.9%) | 962 (25.6%) |
| Asian | 35 (1.9%) | 2 (1.1%) | 504 (13.4%) |
| Hispanic | 148 (7.9%) | 17 (9.0%) | 751 (20.0%) |
| Female | 1,190 (63.4%) | 167 (88.8%) | 2,030 (54.0%) |
| Age, y, mean (SD) | 47.8 (5.7) | 45.9 (7.0) | 60.3 (9.7) |
| Hypertension | 1,231 (65.6%) | 109 (58.0%) | 1,467 (39.0%) |
| Diabetes | 694 (37.0%) | 40 (21.3%) | 358 (9.5%) |
| Current smoker | 991 (52.9%) | 61 (32.4%) | 437 (11.6%) |
| Statin use | 532 (28.4%) | 40 (21.3%) | 527 (14.0%) |
| Lipid level, mg/dL | |||
| LDL-C, mean (SD)a | 114.6 (42.3) | 98.9 (35.9) | 117.1 (30.5) |
| HDL-C, mean (SD) | 39.7 (13.1) | 46.4 (16.1) | 51.6 (14.8) |
| Triglycerides, median (Q1-Q3) | 138.0 (96.0, 214.2) | 109.0 (70.2, 159.8) | 110.0 (77.0, 159.0) |
AMI = acute myocardial infarction; HDL-C = high-density lipoprotein cholesterol; LDL-C = high-density lipoprotein cholesterol; MI-CAD = myocardial infarction due to coronary artery disease; MINOCA = MI with nonobstructive coronary artery disease.
LDL-C values were adjusted by dividing them by 0.7 if statin use was reported.
Association of PRS-CAD with different AMI subtypes
A 1-SD increase in PRS-CAD was significantly associated with class I and class II AMI compared to controls (class I = OR: 1.81; CI: 1.67-1.97; P < 0.001, class II = OR: 2.09; CI: 1.60-2.72; P < 0.001) (Figure 1), while no significant odds increase was observed for class III, IV, and V AMI (class III = OR: 1.11; CI: 0.93-1.32; P = 0.24, class IV = OR: 1.27; CI: 0.84-1.91; P = 0.26, class V = OR: 0.82; CI: 0.48-1.41; P = 0.48). Similarly, patients with 1-SD higher PRS-CAD had a 1.82-fold (CI: 1.67-1.97; P < 0.001) increase in odds of MI-CAD, but only a 1.13-fold (CI: 0.96-1.34; P = 0.14) increase in odds of MINOCA.
Figure 1.
Multinomial Logistic Regression of PRS With AMI Subtypes
The association of PRS-CAD with class I-IV AMI subtypes, as well as with MI-CAD and MINOCA, were assessed as compared to controls using multinomial logistic regression. Both the unadjusted model (blue box) and the model adjusted for sex, age, and the first four principal components (red box) are shown. AMI = acute myocardial infarction; MI-CAD = myocardial infarction due to coronary artery disease; MINOCA = MI with nonobstructive coronary artery disease; PRS-CAD = coronary artery disease–based polygenic risk score.
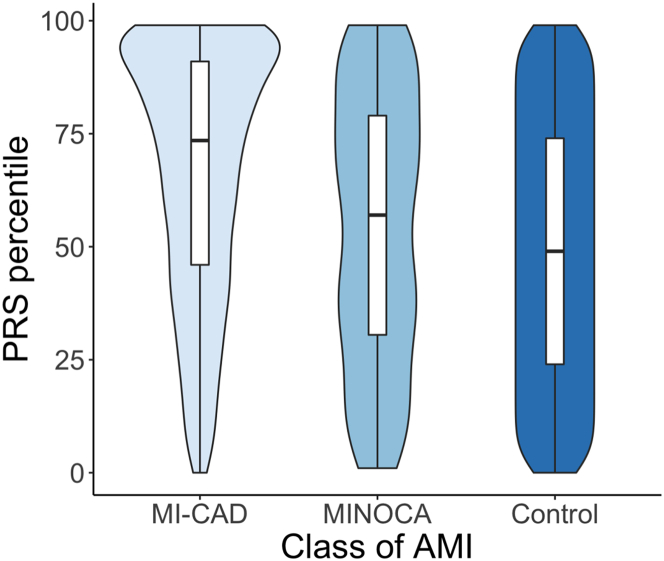
Among AMI subtypes, the median PRS percentile for MI-CAD patients, calculated using MESA as a reference, was 74th, significantly higher than the 57th percentile in MINOCA patients (P < 0.001; Figure 2, Table 2). When broken down into 5 classes, class I (73rd) and class II (76th) AMI cases had generally higher PRS-CAD scores than class III (57th), IV (52nd), and V (42nd) (Supplemental Figure 2, Supplemental Table 2). The MI-CAD cohort included significantly more participants in the top 5th, 10th, 20th, and 30th PRS percentiles and fewer participants in the bottom 30th, 20th, 10th, and 5th percentiles (Table 2). Consistently, patients with MI-CAD were more likely to have a family history of MI than those with MINOCA (75.1% vs 66.3%, P < 0.001; Table 2). All but one patient with familial hypercholesterolemia mutations were classified as MI-CAD (Table 2).
Figure 2.
PRS Percentile Distribution in MI-CAD, MINOCA, and Control
PRS-CAD percentile relative to controls is shown as violin plot. AMI = acute myocardial infarction; MI-CAD = myocardial infarction due to coronary artery disease; MINOCA = MI with nonobstructive coronary artery disease; PRS-CAD = coronary artery disease–based polygenic risk score.
Table 2.
Differences of PRS in MI-CAD vs MINOCA
| MI-CAD (n = 1,876) | MINOCA (n = 188) | Total (n = 2,064) | P Value | |
|---|---|---|---|---|
| Family history of MI | 1,408 (75.1%) | 119 (63.3%) | 1,527 (74.0%) | <0.001 |
| Familial hypercholesterolemia mutation | 35 (1.9%) | 1 (0.5%) | 36 (1.7%) | 0.183 |
| PRS, median (Q1, Q3) | 4.67e-09 (−5.92e-10, 9.64e-09) | 1.28e-09 (−3.63e-09, 5.81e-09) | 4.34e-09 (−8.70e-10, 9.37e-09) | <0.001 |
| PRS percentile, median (Q1, Q3) | 73.5 (46.0, 91.0) | 57.0 (30.5, 79.0) | 72.0 (45.0, 91.0) | <0.001 |
| Top 5th percentile PRS | 342 (18.2%) | 17 (9.0%) | 359 (17.4%) | 0.002 |
| Top 10th percentile PRS | 532 (28.4%) | 28 (14.9%) | 560 (27.1%) | <0.001 |
| Top 20th percentile PRS | 812 (43.3%) | 46 (24.5%) | 858 (41.6%) | <0.001 |
| Top 30th percentile PRS | 1,033 (55.1%) | 71 (37.8%) | 1,104 (53.5%) | <0.001 |
| Bottom 30th percentile PRS | 256 (13.6%) | 47 (25.0%) | 303 (14.7%) | <0.001 |
| Bottom 20th percentile PRS | 152 (8.1%) | 37 (19.7%) | 189 (9.2%) | <0.001 |
| Bottom 10th percentile PRS | 75 (4.0%) | 19 (10.1%) | 94 (4.6%) | <0.001 |
| Bottom 5th percentile PRS | 33 (1.8%) | 8 (4.3%) | 41 (2.0%) | 0.019 |
15 indetermined cases were not included; FH mutation: familial hypercholesterolemia mutation. Top 5th-30th and bottom 5th-30th percentile PRS are subjected to multiple-testing correction. The significant P value cutoff is 0.00625 = 0.05/8 after Bonferroni correction.
MI-CAD = myocardial infarction due to coronary artery disease; MINOCA = MI with nonobstructive coronary artery disease; PRS = polygenic risk score.
The distribution of PRS-CAD and its association with AMI subtypes are similar when stratified among White individuals (Supplemental Figures 3 and 4).
The median PRS-CAD percentiles for male and female participants did not significantly differ within either the MI-CAD or MINOCA cohort (Supplemental Figure 5, Supplemental Table 3). Similarly, the proportion of male and female participants with high or low PRS-CAD was not significantly different (Supplemental Figure 5, Supplemental Table 3).
Association of PRS-CAD with subsequent rehospitalization events in different AMI subtypes
For MI-CAD, the 12-month rates of all-cause hospitalization and death were similar between patients in the top 10th PRS and those in the bottom 90th PRS (Supplemental Table 4, Supplemental Figure 6). However, in the MINOCA cohort, patients in the top 10th PRS percentile were more likely to be hospitalized within 12 months of discharge (42.9% vs 19.4%, P = 0.006) (Supplemental Table 4, Supplemental Figure 6). This trend persisted for cardiovascular hospitalizations, including MI, heart failure, stable/unstable angina, stroke, and other cardiac causes, but not for noncardiovascular hospitalizations.
Furthermore, a 1-SD increase in PRS-CAD was associated with an increased risk of 1-year hospitalization or death in MINOCA patients, after adjusting for sex, age, and PC1–4 (HR: 1.50; 95% CI: 1.08-2.10; P = 0.017), but not in MI-CAD patients (HR: 0.98; 95% CI: 0.91-1.07; P = 0.672) (Figure 3). The association between PRS-CAD and outcomes in MINOCA persisted when restricted to cardiovascular hospitalization (HR: 1.83; 95% CI: 1.18-2.83; P = 0.007), but not for noncardiovascular hospitalization (HR: 1.13; 95% CI: 0.75-1.70; P = 0.569) (Figure 3), considering the competing risk from death or other hospitalization events did not change the trend of the results (Supplemental Figures 7 and 8). Among cardiovascular hospitalizations, a higher rehospitalization rate due to stable/unstable angina was observed in MINOCA patients with the top 10th PRS vs those with the bottom 90th PRS, but not in MI-CAD patients (Supplemental Table 4). Although not statistically significant, increased HRs were observed in AMI, stable/unstable angina, and other cardiac rehospitalizations per 1-SD increase in PRS (Supplemental Figures 9 and 10).
Figure 3.
Cox Proportional-Hazards Regression of PRS With 12-Month Outcomes in MI-CAD and MINOCA Cases
The association of PRS-CAD with 12-month rehospitalization or death was analyzed using Cox proportional-hazards regression in MI-CAD and MINOCA separately. Further analysis was conducted for subcategories including death, cardiovascular rehospitalization, and noncardiovascular rehospitalization. For MINOCA cases, the regression analysis was not performed for death as there was only one death event. MI-CAD = myocardial infarction due to coronary artery disease; MINOCA = MI with nonobstructive coronary artery disease; PRS-CAD = coronary artery disease–based polygenic risk score.
Discussion
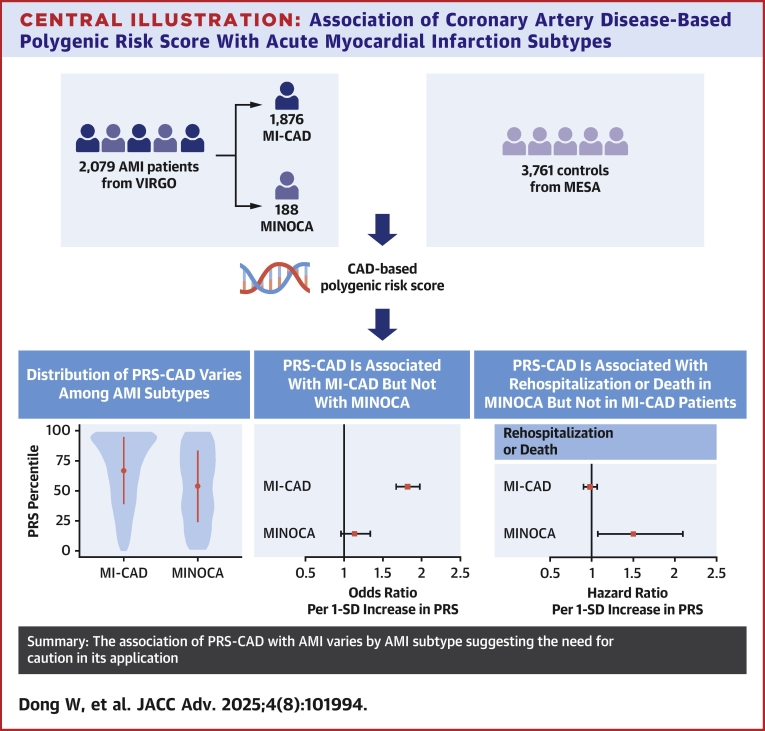
In this large study of younger adults, predominantly women, with AMI, we found that a CAD-based PRS was significantly associated with MI-CAD but not MINOCA, with this association showing similar patterns in both women and men. These results highlight the utility of PRS within a specific subset of MI in a younger population. In cases of MI-CAD, the PRS did not provide additional prognostic information. These findings offer valuable insights into the future use of PRS in assessing AMI occurrence and prognosis (Central Illustration).
Central Illustration.
Association of Coronary Artery Disease-Based Polygenic Risk Score With Acute Myocardial Infarction Subtypes
The association of a CAD-based PRS with AMI subtypes was evaluated in 2,079 VIRGO AMI cases and 3,761 MESA controls, revealing subtype-specific differences in PRS-CAD associations. AMI = acute myocardial infarction; MESA = Multi-Ethnic Study of Atherosclerosis; MI-CAD = myocardial infarction due to coronary artery disease; MINOCA = MI with nonobstructive coronary artery disease; PRS-CAD = coronary artery disease–based polygenic risk score.
This study extends the current literature in several important ways. First, it demonstrates that PRS-CAD may not be reliably used across all AMI types in younger individuals. Currently, most studies use CAD-based PRS for AMI risk and outcome predictions without accounting for AMI subtypes. Our study, however, shows a strong association of PRS-CAD with MI-CAD, but only a marginal association with MINOCA. This finding has at least two potential explanations. First, MINOCA may have less polygenic contribution than MI-CAD, as suggested by a lower rate of family history in MINOCA patients. Second, PRS-CAD may not capture the genetic landscape of MINOCA, which has distinct genetic contributors, as shown in several GWAS studies. A GWAS study of nonobstructive CAD identified top risk loci that differ from those in CAD.19 In addition, a SCAD GWAS study found that many identified risk loci in SCAD had effects in the opposite direction compared to CAD.20 Another study indicated that high PRS-CAD conferred a protective effect against SCAD.21 Thus, large-scale, high-quality GWAS studies specific to MINOCA are needed to develop PRS scores that accurately reflect polygenic risk in MINOCA cases.
The relationship between PRS-CAD and various AMI subtypes remains largely unexplored. Understanding this relationship is critical given the unique risk factors and outcomes associated with different AMI subtypes. For instance, MINOCA patients have fewer traditional risk factors than those with MI-CAD.11 Genetic risk loci differ between obstructive and nonobstructive CAD cases.19,20 In cases of obstructive CAD, high PRS scores have been linked to higher all-cause mortality following cardiac catheterization compared to lower PRS scores, a trend not observed in nonobstructive CAD cases.6
Second, our results provide insights into the association between PRS-CAD and post-MI adverse events. We found no association between PRS-CAD and subsequent adverse events in MI-CAD cases, but a moderate association was observed in MINOCA cases, especially for cardiovascular events. The point estimates suggested higher risks of stable/unstable angina, AMI, and other cardiac causes, although the analysis was underpowered. This novel finding differs from previous studies with mixed results regarding PRS’s link to post-MI complications5,6,8 Possible explanations are as follows: First, PRS-CAD may be more effective in identifying first-time CAD events than subsequent ones, consistent with prior findings showing stronger associations in patients without established CAD.8 Second, as some risk loci for MINOCA may be protective against CAD,20,21 a high PRS-CAD in MINOCA patients suggests that their polygenic risk overcomes the effect, which predisposes them to a higher CAD risk. Third, some MINOCA cases may have shared pathology with MI-CAD cases but did not receive adequate management, as supported by a lower rate of discharge medications in MINOCA,11 leading to worse outcomes. Lastly, patients in the VIRGO cohort have less severe CAD as evidenced by lower 1-year mortality,11,22 potentially weakening the association of PRS-CAD with adverse outcomes in this context. Future studies with larger cohort sizes are required for validation.
Our findings have important clinical implications. PRS holds potential for CAD prevention and management, as it provides a cumulative genetic risk score available from birth, is increasingly affordable, and often enhances CAD predictive accuracy.23,24 However, improving PRS accuracy remains challenging, with inconsistent results regarding its incremental benefit when added to standard CAD risk-prediction models.25 Our findings suggest that aligning AMI subtypes in base and target cohorts may improve PRS accuracy; for example, a MINOCA-specific PRS could potentially enhance risk assessment for MINOCA. In addition, our study suggests that PRS-CAD may be associated with cardiovascular rehospitalization in specific MINOCA subtypes, although further data are needed to confirm its validity. This work is especially important for young women, who experience more diverse MI subtypes than men.
Study limitations
This study has several limitations. First, the sample sizes for some AMI subtypes, especially MINOCA, are relatively small, limiting our power to evaluate the association of PRS-CAD with these subtypes and their subsequent adverse events. Second, the rehospitalization data lack detailed information to classify AMI subtypes, preventing us from determining whether PRS-CAD is associated with subsequent MI-CAD or MINOCA events, which limits interpretation. Third, the VIRGO and MESA datasets are not fully matched in age, sex, race, and traditional risk factors and may have batch differences in genomic sequencing, potentially confounding association tests. Fourth, the GWAS used to develop PRS primarily included individuals of European ancestry. Although ancestry correction was applied, PRS-CAD likely performs less effectively for Black, Hispanic, and Asian participants. Therefore, optimized PRS models for non-European ancestry groups are needed.
Conclusions
Our findings indicate that the association between a CAD-based PRS and AMI risk varies by AMI subtype in younger individuals, underscoring the need for subtype-specific PRS models in future research.
Perspectives.
COMPETENCY IN SYSTEM-BASED PRACTICE: PRS quantifies cumulative genetic risk and is used to assess AMI risk. Our study demonstrated that coronary artery disease–based PRS exhibits varied association with AMI risk and prognosis across different subtypes. Therefore, clinicians should be aware of these differences and exercise caution when incorporating PRS into AMI risk-prediction models or applying it in clinical practice for AMI prevention and management.
TRANSLATIONAL OUTLOOK: Our study suggested that aligning AMI subtypes in both base and target cohorts when calculating PRS may improve its accuracy when assessing AMI risk and prognosis. Therefore, it is important to have larger-scale genome-wide association study studies for less common AMI subtypes, such as myocardial infarction with nonobstructive coronary artery disease, to enable the development of subtype-specific PRS and enhance risk assessment of AMI.
Funding support and author disclosures
The VIRGO study was supported by grant R01 HL081153 from the National Heart, Lung, and Blood Institute (NHLBI). Whole genome sequencing (WGS) for the Trans-Omics in Precision Medicine (TOPMed) program was supported by the NHLBI. WGS for “NHLBI TOPMed: Multi-Ethnic Study of Atherosclerosis (MESA)” (phs001416.v3.p1) was conducted at the Broad Institute of MIT and Harvard (3U54HG003067-13S1). Centralized read mapping and genotype calling, along with variant quality metrics and filtering, were provided by the TOPMed Informatics Research Center (3R01HL-117626-02S1). Phenotype harmonization, data management, sample identity quality control, and general study coordination were provided by the TOPMed Data Coordinating Center (3R01HL-120393-02S1) and TOPMed MESA Multi-Omics (HHSN2682015000031/HSN26800004). The MESA projects are conducted and supported by the NHLBI in collaboration with MESA investigators. Funding support for MESA is provided by contracts 75N92020D00001, HHSN268201500003I, N01-HC-95159, 75N92020D00005, N01-HC-95160, 75N92020D00002, N01-HC-95161, 75N92020D00003, N01-HC-95162, 75N92020D00006, N01-HC-95163, 75N92020D00004, N01-HC-95164, 75N92020D00007, N01-HC-95165, N01-HC-95166, N01-HC-95167, N01-HC-95168, N01-HC-95169, UL1-TR-000040, UL1-TR-001079, UL1-TR-001420, UL1TR001881, DK063491, R01HL105756, and R01HL146860. In the past 3 years, Dr Sawano has been partially supported by research funding from Polybio, Pfizer, and Novartis through Yale University; he has also received lecture honoraria from Boehringer Ingelheim. Dr Krumholz has received options for Element Science and Identifeye and payments from F-Prime for advisory roles; and he is a co-founder of and holds equity in Hugo Health, Refactor Health, and ENSIGHT-AI. He is associated with research contracts through Yale University from Janssen, Kenvue, Novartis, and Pfizer. Dr Lu has received support from the Sentara Research Foundation, the National Heart, Lung, and Blood Institute of the National Institutes of Health (under awards R01HL69954 and R01HL169171), and the Patient-Centered Outcomes Research Institute (under award HM-2022C2-28354) outside of the submitted work. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Acknowledgment
The authors are grateful to the patients who participated in this research. The authors extend their thanks to the MESA investigators, staff, and participants for their valuable contributions. A full list of participating MESA investigators and institutions can be found at http://www.mesa-nhlbi.org.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
Appendix
For supplemental methods, tables, and figures, please see the online version of this paper.
Supplementary data
References
- 1.Manikpurage H.D., Eslami A., Perrot N., et al. Polygenic risk score for coronary artery disease improves the prediction of early-onset myocardial infarction and mortality in men. Circ Genom Precis Med. 2021;14(6) doi: 10.1161/CIRCGEN.121.003452. [DOI] [PubMed] [Google Scholar]
- 2.Isgut M., Sun J., Quyyumi A.A., Gibson G. Highly elevated polygenic risk scores are better predictors of myocardial infarction risk early in life than later. Genome Med. 2021;13(1):13. doi: 10.1186/s13073-021-00828-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Douville N.J., Surakka I., Leis A., et al. Use of a polygenic risk score improves prediction of myocardial injury after non-cardiac surgery. Circ Genom Precis Med. 2020;13(4) doi: 10.1161/CIRCGEN.119.002817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Khera A.V., Chaffin M., Zekavat S.M., et al. Whole-genome sequencing to characterize monogenic and polygenic contributions in patients hospitalized with early-onset myocardial infarction. Circulation. 2019;139(13):1593–1602. doi: 10.1161/CIRCULATIONAHA.118.035658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xu H., Hon C., Kaiser S., et al. Coronary artery disease polygenic risk score identifies patients at higher risk for recurrent cardiovascular events in the CANTOS trial. Circ Genom Precis Med. 2021;14(6) doi: 10.1161/CIRCGEN.121.003440. [DOI] [PubMed] [Google Scholar]
- 6.Levin M.G., Kember R.L., Judy R., et al. Genomic risk stratification predicts all-cause mortality after cardiac catheterization. Circ Genom Precis Med. 2018;11(11) doi: 10.1161/CIRCGEN.118.002352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aittokallio J., Kauko A., Vaura F., et al. Polygenic risk scores for predicting adverse outcomes after coronary revascularization. Am J Cardiol. 2022;167:9–14. doi: 10.1016/j.amjcard.2021.11.046. [DOI] [PubMed] [Google Scholar]
- 8.Howe L.J., Dudbridge F., Schmidt A.F., et al. Polygenic risk scores for coronary artery disease and subsequent event risk amongst established cases. Hum Mol Genet. 2020;29(8):1388–1395. doi: 10.1093/hmg/ddaa052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Choi S.W., Mak T.S., O'Reilly P.F. Tutorial: a guide to performing polygenic risk score analyses. Nat Protoc. 2020;15(9):2759–2772. doi: 10.1038/s41596-020-0353-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nikpay M., Goel A., Won H.H., et al. A comprehensive 1,000 genomes-based genome-wide association meta-analysis of coronary artery disease. Nat Genet. 2015;47(10):1121–1130. doi: 10.1038/ng.3396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Safdar B., Spatz E.S., Dreyer R.P., et al. Presentation, clinical profile, and prognosis of young patients with myocardial infarction with nonobstructive coronary arteries (MINOCA): results from the VIRGO study. J Am Heart Assoc. 2018;7(13) doi: 10.1161/JAHA.118.009174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thygesen K., Alpert J.S., Jaffe A.S., et al. Third universal definition of myocardial infarction. Circulation. 2012;126(16):2020–2035. doi: 10.1161/CIR.0b013e31826e1058. [DOI] [PubMed] [Google Scholar]
- 13.Spatz E.S., Curry L.A., Masoudi F.A., et al. The variation in recovery: role of gender on outcomes of young AMI patients (VIRGO) classification system: a taxonomy for young women with acute myocardial infarction. Circulation. 2015;132(18):1710–1718. doi: 10.1161/CIRCULATIONAHA.115.016502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sciria C.T., Dreyer R.P., D'Onofrio G., Safdar B., Krumholz H.M., Spatz E.S. Application of the VIRGO taxonomy to differentiate acute myocardial infarction in young women. Int J Cardiol. 2019;288:5–11. doi: 10.1016/j.ijcard.2019.03.054. [DOI] [PubMed] [Google Scholar]
- 15.Lichtman J.H., Lorenze N.P., D'Onofrio G., et al. Variation in recovery: role of gender on outcomes of young AMI patients (VIRGO) study design. Circ Cardiovasc Qual Outcomes. 2010;3(6):684–693. doi: 10.1161/CIRCOUTCOMES.109.928713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bild D.E., Bluemke D.A., Burke G.L., et al. Multi-ethnic study of atherosclerosis: objectives and design. Am J Epidemiol. 2002;156(9):871–881. doi: 10.1093/aje/kwf113. [DOI] [PubMed] [Google Scholar]
- 17.Dreyer R.P., Raparelli V., Tsang S.W., et al. Development and validation of a risk prediction model for 1-year readmission among young adults hospitalized for acute myocardial infarction. J Am Heart Assoc. 2021;10(18) doi: 10.1161/JAHA.121.021047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Austin P.C., Fine J.P. Practical recommendations for reporting Fine-Gray model analyses for competing risk data. Stat Med. 2017;36(27):4391–4400. doi: 10.1002/sim.7501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Weng L., Taylor K.D., Chen Y.D.I., et al. Genetic loci associated with nonobstructive coronary artery disease in Caucasian women. Physiol Genomics. 2016;48(1):12–20. doi: 10.1152/physiolgenomics.00067.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Adlam D., Berrandou T.E., Georges A., et al. Genome-wide association meta-analysis of spontaneous coronary artery dissection reveals common variants and genes related to artery integrity and tissue-mediated coagulation. medRxiv. 2022 doi: 10.1038/s41588-023-01410-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Saw J., Yang M.L., Trinder M., et al. Chromosome 1q21.2 and additional loci influence risk of spontaneous coronary artery dissection and myocardial infarction. Nat Commun. 2020;11(1):4432. doi: 10.1038/s41467-020-17558-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Smolderen K.G., Buchanan D.M., Gosch K., et al. Depression treatment and 1-year mortality after acute myocardial infarction: insights from the TRIUMPH registry (Translational Research Investigating Underlying Disparities in Acute Myocardial Infarction Patients' Health Status) Circulation. 2017;135(18):1681–1689. doi: 10.1161/CIRCULATIONAHA.116.025140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Phulka J.S., Ashraf M., Bajwa B.K., Pare G., Laksman Z. Current state and future of polygenic risk scores in cardiometabolic disease: a scoping review. Circ Genom Precis Med. 2023;16(3):286–313. doi: 10.1161/CIRCGEN.122.003834. [DOI] [PubMed] [Google Scholar]
- 24.Marston N.A., Pirruccello J.P., Melloni G.E.M., et al. Predictive utility of a coronary artery disease polygenic risk score in primary prevention. JAMA Cardiol. 2023;8(2):130–137. doi: 10.1001/jamacardio.2022.4466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Groenendyk J.W., Greenland P., Khan S.S. Incremental value of polygenic risk scores in primary prevention of coronary heart disease: a review. JAMA Intern Med. 2022;182(10):1082–1088. doi: 10.1001/jamainternmed.2022.3171. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.