Abstract
Introduction
Preoperative exercise training is recommended, when feasible, for people undergoing resection for lung cancer and has been shown to reduce the risk of postoperative pulmonary complications and improve preoperative exercise capacity. However, preoperative exercise training programmes are not commonly available in the Australian clinical practice setting due to a range of factors including resource and time restrictions. We aim to describe the protocol to evaluate the implementation of an existing preoperative exercise training programme in people undergoing lung cancer resection in an Australian setting.
Methods and analysis
This is an evaluation of a secondary objective of a study examining the effect of lung cancer resection on exercise capacity, lung function and symptoms of dyspnoea and quality of life. Participants will be prospectively recruited at the time of lung cancer diagnosis and planned surgical treatment through the lung cancer multidisciplinary team of a metropolitan hospital in Sydney, Australia. All participants will be offered the choice of participating in the preoperative exercise training programme which encompasses a hybrid gym and telerehabilitation programme of up to five sessions/week from baseline until surgical date. The programme will be evaluated using the Reach, Effectiveness, Adoption, Implementation, Maintenance Framework including both quantitative and qualitative measures which will be analysed using descriptive statistics and qualitative analysis coded inductively.
Ethics and dissemination
The study has received ethical approval through the Northern Sydney Local Health District reference 2023/ETH01643 and has been registered prospectively. Findings will be disseminated through peer-reviewed publication and scientific conference presentation.
Trial registration number
ACTRN12624000359538.
Keywords: Lung Neoplasms, REHABILITATION MEDICINE, Exercise
Strengths and limitations of this study.
The exercise training protocol development has been informed by experienced clinicians as well as people with lived experience of exercising with lung cancer and has been described in detail using the Consensus on Exercise Reporting Template.
The mixed-methods approach to evaluate the implementation of the preoperative exercise programme in clinical practice will incorporate both quantitative and qualitative methods.
This study is limited to a single site in metropolitan Australia and people older than 50 years of age, so may not be generalisable to all lung cancer services.
A further limitation of this study is that assessors will not be blinded at any time point.
Introduction
Lung cancer is a highly prevalent and difficult to cure cancer and, in Australia, is currently the fourth most diagnosed cancer and the leading cause of cancer-related mortality.1 In early-stage lung cancers, surgical resection is recommended as first-line, curative treatment resulting in increased survival rates.1 Surgical resection may involve removal of one or more segments (segmentectomy), lung lobes (lobectomy) or an entire lung (pneumonectomy). Suitability for lung cancer resection includes evaluation of cardiac risk, exercise capacity, pulmonary function and extent of resection required.2 If forced expiratory volume in 1 s (FEV1) or diffusion of carbon monoxide (DLCO) is marginal, a cardiopulmonary exercise test, incremental shuttle walk test (ISWT) or a stair climb test may be undertaken to categorise risk.3 Although poor exercise capacity increases surgical risk or may even preclude resection altogether, it is potentially modifiable through exercise training, smoking cessation and optimisation of comorbid respiratory conditions. Preoperative exercise training (PreOpET) improves exercise capacity and maximum oxygen consumption (VO2 max) prior to surgery and reduces the risk of postoperative pulmonary complications4 and is starting to be used in Australia. However, there is limited published data on how PreOpET is being implemented or its effects on exercise performance in lung cancer resection candidates in an Australian context.5
The structure of PreOpET varies widely in both research trials and clinical practice, from short twice-daily, inpatient programmes up to 1-week duration,6 7 to supervised outpatient8 9 or unsupervised home-based programmes,10 two to five times a week.11 PreOpET typically includes a component of aerobic exercise using equipment such as a stationary cycle or treadmill or ground-based walking at a moderate-high intensity for 30–180 min/day.9 12 Peripheral muscle or respiratory muscle strengthening is also included in some preoperative exercise regimens, varying from titration based on participant reported effort13 through to targeted prescription using one repetition max testing.14 While PreOpET originally involved in-person supervision,11 mobile applications15 and home-based exercise with telephone support10 have been evaluated in recent studies alongside a move towards more flexible, pragmatic approaches.16 Supervised telerehabilitation using videoconference technology improves exercise capacity and quality of life in chronic respiratory disease17 but has not been formally evaluated in people undergoing lung cancer resection.
Challenges in implementing PreOpET include short preoperative windows, other conflicting appointments and constraints to local service delivery. Consequently, a recent survey, in Australia, of pulmonary and cancer exercise programmes reported that only 11% offer PreOpET for people undergoing lung cancer resection5 and, across the spectrum of lung cancer care, utilisation of available exercise programmes is estimated to be poor.18 Increased usage of neoadjuvant chemotherapy and immunotherapy can also reduce engagement in exercise due to treatment-related toxicities19 which lead to troublesome symptoms and deconditioning. Exercise programmes have been shown to be beneficial at improving exercise capacity and quality of life during neoadjuvant therapy in other cancer populations.20 21 To maximise engagement in exercise programmes, understanding participant preferences, programme capacity and barriers to participation is vital.22 Studies exploring participant perceptions and preferences undertaking PreOpET have included only small numbers of people with lung cancer compared with other cancers and have yielded mixed perspectives.22 Some participants preferred supervised individual or group sessions, while others preferred unsupervised exercise with exercise booklets or phone calls.23 24 Commonly reported barriers were time constraints, travel difficulties and symptoms limiting participation24 25 such that between one and five sessions each week would be achievable in the preoperative period depending on an individual’s circumstances.25 As such, current Australian clinical practice includes a mix of outpatient, telerehabilitation, home-based and hybrid programmes of varied frequency and session duration,5 with a recent codesign study providing insights into the need for flexibility and individualisation of exercise programmes for people with operable lung cancer.26
Data on real-world implementation and effectiveness is required to inform service provision; therefore, the aim of this study is to evaluate the implementation of PreOpET in a real-world clinical setting using components of the Reach, Effectiveness, Adoption, Implementation and Maintenance (RE-AIM) framework.
Methods
Design
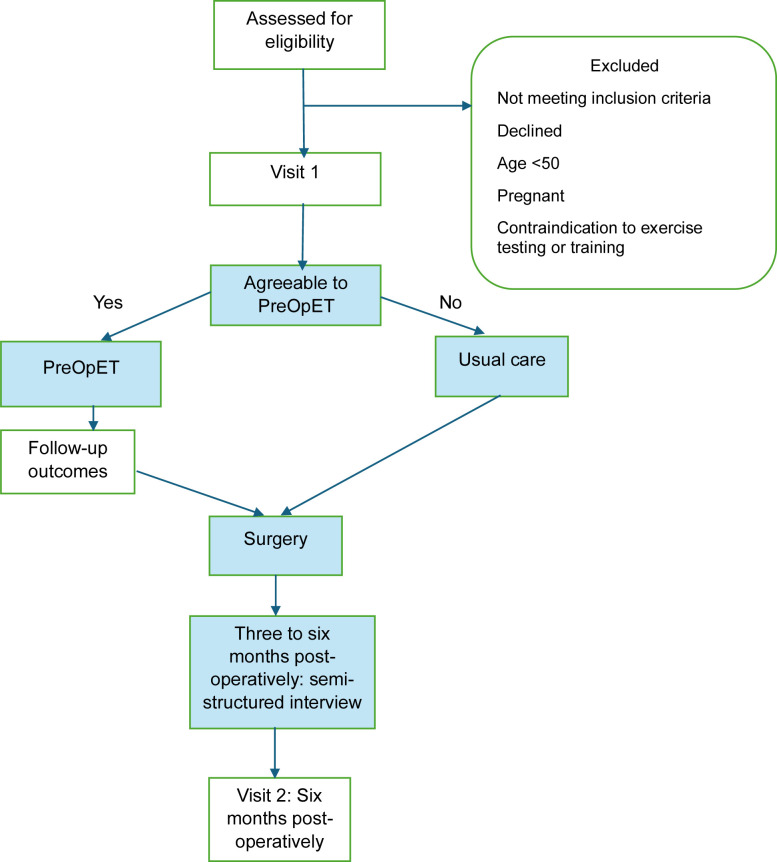
This study will be a prospective, non-randomised mixed-methods hybrid implementation-effectiveness trial evaluating a PreOpET programme in people awaiting resection surgery for lung cancer. This study is a substudy within a larger trial which is investigating the longitudinal outcomes of people with lung cancer following resection. The study design is illustrated in figure 1. All participants of the larger trial will be offered PreOpET; those who accept will form the intervention group (IG), while those who decline will form the usual care group (UCG). All participants will complete a baseline study visit (visit 1) and a follow-up visit 6 months postoperatively (visit 3) with participants in the IG and/or undergoing neoadjuvant therapy also attending an additional study visit immediately prior to their surgery (visit 2). The protocol will follow the recommendations of Standard Protocol Items: Recommendations for Interventional Trials27 and Template for Intervention Description and Replication.28
Figure 1. Study design. PreOpET, preoperative exercise training.
Participants
All people recommended for surgical resection of confirmed or suspected lung cancer by the Lung Cancer Multidisciplinary Team at Royal North Shore Hospital, New South Wales, Australia will be screened for inclusion in the study. Inclusion and exclusion criteria are described in table 1. Participants undergoing neoadjuvant therapy with the goal of progressing to surgical resection will be recruited prior to commencement of neoadjuvant treatment. Participants will be provided with written and verbal information, and written informed consent will be obtained prior to enrolment (online supplemental patient consent form) by a lead investigator (MK and NR).
Table 1. Inclusion and exclusion criteria.
| Inclusion criteria | Exclusion criteria |
|---|---|
|
|
Intervention group
Participants who consent to take part in PreOpET will be referred to their local pulmonary rehabilitation programme. The local pulmonary rehabilitation programmes have an existing rapid access pathway to allow commencement of PreOpET within 7 days of referral. The PreOpET will be a programme of supervised gym-based and/or real time supervised telerehabilitation up to five sessions/week (maximum two gym-based and other sessions via telerehabilitation) in the weeks prior to surgery. All exercise sessions will be approximately 1 hour in duration in a small group environment (up to ten participants (with mixed respiratory diseases) in gym-based sessions and up to six participants (lung cancer diagnoses only) in telerehabilitation) supervised by an experienced pulmonary rehabilitation physiotherapist and will encompass both endurance and resistance training. A detailed description of the PreOpET prescription and progression using the Consensus on Exercise Reporting Template29 is available in online supplemental material table 2.
Participants may also access the multidisciplinary team attached to the local pulmonary rehabilitation programme if desired, including dietetic review, smoking cessation support and clinical psychology, and details of what participants access will be recorded.
Usual care group
Participants who decline PreOpET will complete all other components of usual preoperative workup. Depending on individual surgeon’s protocols, this may include attendance at preadmission clinics, preoperative information booklets and review by other disciplines such as psychology or dietetics. Details of each UCG participant’s healthcare and exercise engagement will be recorded.
Schedule of assessments
The schedule of assessments is illustrated in table 2.
Table 2. Schedule of study assessments.
| Process/interventions | Visit 1 Baseline |
Visit 2* immediately prior to surgery |
Visit 3 6 months postoperatively |
|---|---|---|---|
| Informed consent | ✓ | ||
| Demographic data | ✓ | ||
| Pulmonary function (spirometry, lung volumes, diffusion) | ✓ | ✓ | |
| Airway impedance | ✓ | ✓ | ✓ |
| ISWT x2 using mobile CPET equipment | ✓ | ✓ | ✓ |
| Quality of life questionnaires | ✓ | ✓ | ✓ |
| Symptom questionnaires | ✓ | ✓ | ✓ |
| Programme adherence and completion data | ✓ | ||
| Surgical and adjuvant treatment details | ✓ | ||
| Details of other exercise and allied health involvement | ✓ | ||
| Optional semistructured interview and survey of acceptability | ✓ |
Intervention and/or neoadjuvant group only.
CPET, cardiopulmonary exercise test; ISWT, incremental shuttle walk test.
All participants will attend a baseline (visit 1) and a 6-month postoperative visit (visit 3). Participants who choose to undertake PreOpET and/or undergo neoadjuvant treatment will attend an additional assessment visit immediately prior to surgery (visit 2). If participants are unable to attend in person at visit 2 surveys, the 1 min sit to stand will be completed remotely.
Outcome measures
Primary outcome
Programme implementation
The implementation of the programme will be evaluated using elements of the RE-AIM framework30 with the primary outcome being uptake calculated by the proportion of participants that choose to undertake the PreOpET (participants in IG/total number of participants). Tables3 4 illustrate the outcomes that will be used to evaluate the different elements in the framework.
Table 3. Evaluation of success of implementation using RE-AIM framework45.
| RE-AIM element | Outcome |
|---|---|
| Reach | Uptake will be determined by the number, proportion and characteristics of eligible participants who choose to participate in PreOpET compared with those who decline. Semistructured interviews will be thematically analysed to understand the factors that impacted uptake. |
| Effectiveness | Effectiveness will be evaluated through the clinical outcomes (defined in detail in table 4) These outcomes will include exercise components (ISWT, CPET parameters and 1STS), subjective questionnaires and airway impedance. |
| Adoption | Acceptability with the implementation of PreOpET from the participant perspective determined through semistructured interviews. |
| Implementation | Implementation will be assessed through:
|
| Maintenance | Not assessed |
CPET, cardiopulmonary exercise test; HR, heart rate; ISWT, incremental shuttle walk test; PreOpET, preoperative exercise training; RE-AIM, Reach, Effectiveness, Adoption, Implementation, Maintenance; 1STS, 1 min sit to stand test.
Table 4. Clinical outcome measures.
| Outcome | Measure | Details |
|---|---|---|
| Exercise capacity | ISWT distance | Field walk test undertaken following published guidelines and distance recorded. Participants will wear portable metabolic equipment (Metamax3B, Cortex Medical, Germany) during the test. Oxygen saturation, heart rate and dyspnoea will be recorded every minute during the test. |
| Oxygen consumption and ventilator efficiency | VO2 peak and VE/VCO2 | Measured using metabolic equipment worn during ISWT |
| Dynamic hyperinflation | Inspiratory capacity | Measured during ISWT using metabolic equipment at regular intervals during the test |
| Airway impedance- resistance and reactance | Oscillometry | Measured using Resmon PRO FULL 3 (Resmon, Italy) following standardised protocol and airway resistance and reactance reported |
| Functional quadriceps endurance | 1 min sit to stand test | Completed over videoconference during telerehabilitation session in participant’s own chair. Participants will be instructed to stand up and sit down from a standard height chair as many times as possible in 1 min and the number of repetitions completed recorded. |
| Fatigue | FACIT-Fatigue | Questionnaire |
| HRQoL | EORTC QLQc30 and LC13 EQ5D |
Core questionnaire and lung cancer supplement completed) and domain scores reported Questionnaire |
| Dyspnoea | EORTC LC13 dyspnoea score MDP |
Score for dyspnoea subset questions reported Questionnaire |
EORTC QLQc30 and LC13, The European Organisation for the Research and Treatment of Cancer Quality of Life Questionnaire and Lung Cancer 13; EQ5D, EuroQol-5 Dimension; FACIT, Functional Assessment of Chronic Illness Therapy; HRQoL, health-related quality of life; ISWT, incremental shuttle walk test; MDP, multidimensional dyspnoea profile; VE/VCO2, ventilatory efficiency; VO2 peak, oxygen consumption.
Secondary outcomes
Exercise capacity
Peak exercise capacity will be measured with the ISWT conducted according to published standards.31 Participants walk around cones, 9 m apart at a speed dictated by prerecorded, timed beeps. The speed increases each minute, and participants walk until they cannot complete the 10 m shuttle before the beep, due to intolerable symptoms or if they are unable to maintain sufficient speed. Oxygen saturation and pulse rate will be continuously monitored (using Masimo Rad 5, Masimo Corporation, USA) and recorded each minute. Dyspnoea, measured by the 0–10 modified Borg scale,32 will be recorded every minute. Participants will wear portable metabolic equipment (Metamax3B, Cortex Medical, Germany) and 12-lead ECG (Custo Cardio 300, Custo Med, Germany) to record ventilation, oxygen consumption (VO2 peak), ventilatory efficiency, respiratory exchange rate and maximal heart rate. Dynamic hyperinflation will also be measured through inspiratory capacity (IC) measured at baseline, every 2 min during the ISWT and at the end of the test.
Two tests will be performed at each study time point with the best distance achieved used for analysis.
Functional quadriceps endurance will be measured by the 1 min sit to stand test.33 Participants will be instructed to stand up and sit down from a standard height chair as many times as possible in 1 min and the number of repetitions completed recorded. This test will be completed only in those participants undertaking PreOpET and will be completed during their first and final exercise training sessions using the same chair.
Respiratory measures
Airway impedance will be measured using oscillometry (Resmon PRO Full 3, Resmon, Italy). Participants will be tested sitting upright with their head in a neutral position, wearing a nose clip and supporting their own cheeks and chin. After a period of stable breathing at tidal volumes, the device superimposes a multiple frequency signal for a minimum of five breaths and airway resistance (Rrs) and reactance (Xrs) is reported for each frequency.34 Oscillometry will be measured before and after inhalation of 400 µg salbutamol through a metered dose inhaler and spacer chamber. Oscillatory reactance in Chronic Obstructive Pulmonary Disease (COPD) predicts the persistence of effects of rehabilitation,35 but this has not been studied in people with lung cancer.
Spirometry, lung volumes and DLCO will be performed according to American Thoracic Society/European Respiratory Society criteria.36
Quality of life and symptoms
Health-related quality of life (HRQoL) will be measured using The European Organisation for the Research and Treatment of Cancer Quality of Life Questionnaire (EORTC QLQ c30).37 The EORTC QLQ c30 is a 30-point questionnaire that measures HRQoL in people with cancer over the preceding week and reports a mix of functional domains as well as symptoms (including pain and fatigue) and has demonstrated reliability and sensitivity to change with rehabilitation.37 The Lung Cancer 13 (LC13) is a 13-question supplement to the EORTC QLQ c30 specific to people with lung cancer and reports single symptoms as well as the subscale of dyspnoea (using questions 3, 4 and 5).38 In both the EORTC QLQ c30 and LC13, results are described on a 0–100 scale, with lower scores on symptom domains demonstrating lower symptom burden and higher scores on functional domain and global scores indicating better HRQoL and functional capacity.
HRQoL will also be measured using the EuroQol-5 Dimensions-5 Levels of health questionnaire (EQ5D5L).39 The EQ5D5L is a generic quality of life tool that measures domains including mobility, pain and anxiety. Participant-reported global quality of life is measured as part of the EQ5D5L using a 0–100 Visual Analogue Scale.
Dyspnoea will be examined using the multidimensional dyspnoea profile (MDP). The MDP questionnaire is a reliable and valid tool for examining different dimensions of dyspnoea during an activity that is specific to the participant. Participants describe the sensations and emotions associated with their dyspnoea and rate severity on a 0–10 Numerical Rating Scale. The MDP appears to be responsive to rehabilitation40 in chronic respiratory disease, although it has not previously been used in a surgical lung cancer population.
The level of fatigue will be assessed using the thirteen-question Functional Assessment of Chronic Illness Therapy-Fatigue (FACIT-Fatigue) scale. The FACIT-Fatigue results are described between 0 and 52 with a score of less than 34 points indicating severe levels of fatigue.41 42
Participant experience
Participants in the IG will complete a short purpose-designed questionnaire examining the acceptability of PreOpET immediately prior to surgery. The survey was developed using the Theoretical Framework of Acceptability and adapted from published generic questionnaires surrounding the acceptability of healthcare interventions.43
A subset of participants from both the IG and UCG will be invited to participate in an individual semistructured interview which will take place approximately 6 months postoperatively. Participants will be invited to participate in the interview initially using a convenience sample, then a purposive sampling approach to ensure a range of participants with distribution across characteristics (eg, age ranges, gender, employment status, surgical setting and surgical location (public or private hospital) and neoadjuvant therapy). The interviews will explore participants’ beliefs about and experiences of preoperative exercise and the impact on their preoperative preparation and postoperative recovery. The interview guide was developed by investigators (MK, SW and MD) and amended through feedback from people with lived experience of lung cancer resection. The interviews will be conducted via videoconference, unless in-person is requested by the participant, by an investigator unknown to the participants (SW) to reduce bias of participant responses. All interviews will be recorded and transcribed verbatim. Thematic analysis will be undertaken using a reflexive and inductive approach44 by two researchers (MK and MD).
Data management and analysis plan
All study data will be stored securely on a Redcap database with access only available to lead investigators. All data will be deidentified, and participants will be allocated a study identification code. Only primary investigators will have access to the participant code list. Data entry will be completed by trained investigators and reviewed regularly by lead investigators. Quantitative data will be analysed using SPSS (V.22, IBM). Data analysis will occur at two time points, at completion of visit 2 (for the PreOpET implementation study) and a second time point when all participants complete visit 3 (for the larger study).
Implementation outcomes will be reported as percentages for categorical variables, for example, uptake and means (SD) for continuous variables, for example, PreOpET duration. Analysis of effectiveness as part of the RE-AIM45 framework will include the within group (IG only) change in exercise capacity (ISWT distance, V02 peak and 1 min sit to stand test) and questionnaires which will be analysed using paired t-tests with a p<0.05 considered significant. Baseline characteristics will be reported for the entire sample and a comparison between the IG and UCG will be completed, using unpaired t-tests for continuous variables and χ2tests for categorical variables, to identify any significant differences between groups at visit 1.
Data analysis for the larger, longitudinal study will focus on the primary outcome of predictors of change in ISWT from visit 1 to visit 3. Multivariate linear regression will be performed with the change in ISWT, when corrected for known variables that influence ISWT distance (age, sex and body mass index),46 examined using the variables of baseline lung function (FEV1 % predicted, forced vital capacity % predicted and DLCO % predicted), baseline ISWT, number of bronchopulmonary segments resected, neoadjuvant therapy and participation in PreOpET as a priori independent variables. Intention-to-treat analysis will be used for the PreOpET group, in that all PreOpET participants will be included in that group regardless of PreOpET volume completed.
Two multivariate logistic regression models will also be undertaken to assess the likelihood of postoperative deterioration or postoperative improvement in ISWT by more than the published minimally clinically important difference in chronic respiratory disease of 48 m47 using the same covariates described in the linear model.
All other secondary outcomes, for example, symptom questionnaires, will be analysed using the change from visit 1 to visit 3 using paired t-tests.
For the IG group only, available data from visit 2 to visit 3, as it’s anticipated that visit 2 may not be feasible for all participants or may be conducted remotely, will also be analysed using within group change for exercise capacity and questionnaires. If a participant is unable or unwilling to complete some elements of the follow-up data collection, then every effort will be made to collect as many outcomes as possible. In the event of participant withdrawals, baseline data will be compared with that of participants who do complete to ensure withdrawals do not bias the results.
Qualitative interview recordings and transcripts will be deidentified and stored on password-protected electronic drives with access only to investigators involved in the analysis. The semistructured interviews will be analysed using reflexive thematic analysis following the approach outlined by Braun and Clarke.48 The process will begin with familiarisation through repeated reading of the transcripts by two investigators (MK and MD) to become immersed in the data. Initial codes will be generated inductively, then refined using an iterative and reflexive approach before being grouped into categories based on relationships and similarities, ensuring they capture the breadth of participant experiences. The analysis will continue until the thematic schema is well-developed, coherent and provides a thorough and insightful interpretation of the data, with no new themes emerging from further data analysis.
Sample size
72 participants will be recruited for the larger study examining the outcome of lung cancer resection with all participants offered the choice to participate in the PreOpET programme. This sample size is based on the primary outcome of the larger study to identify the covariate predictors of change in peak exercise capacity (ISWT distance) 6 months postoperatively. To test a predictive model using five independent predictors (baseline lung function, baseline ISWT distance, number of bronchopulmonary segments resected, neoadjuvant therapy and participation in PreOpET), we require 62 participants to demonstrate a power of 0.6. The larger study is expected to have a low drop-out rate as participants will be attending clinic visits regularly during this time as part of standard postoperative care, so 72 participants will allow for a drop-out rate of up to 15%.
To explore the effectiveness of PreOpET (as per the RE-AIM framework),45 a further sample size calculation has been completed for the IG using the outcome of within group change in ISWT distance from visit 1 to visit 2. Assuming an SD of 120 m, 45 participants would be required to demonstrate a mean change of 50 m in ISWT distance.10
Safety and adverse events
Adverse events will be recorded in an electronic log and stored on a secure database and reported to the lead investigator. Serious adverse events are defined as any events which are life-threatening or lead to hospitalisation or disability. Serious adverse events will be reported to the ethics committee within 24 hours. Minor adverse events, including pain, falls, worsening fatigue or dyspnoea, will be logged and discussed with study committee members and the participant’s medical team where relevant. Participants will be asked at the start of each session if there were any concerns to report after the previous exercise sessions. In the instance of an adverse event, participants will be given the opportunity to withdraw from the study and offered medical care if applicable.
Committee
The lead investigators will meet at least monthly to discuss study progress and any minor adverse events. Investigators MK and SW will meet with the physiotherapists providing the PreOpET programme monthly to discuss progress and any issues arising. Reporting to the approving ethics committee will be completed through annual reports.
Participant and public involvement
Consumers have been involved in planning for the implementation trial, PreOpET development as well as the content and timing of the qualitative interviews. Consumers will also be involved in the development and dissemination of lay language trial results.
Ethics and dissemination
The study has received ethical approval through the Northern Sydney Local Health District reference 2023/ETH01643 and has been registered prospectively.
The primary results of this study, as well as any relevant subanalyses, including the qualitative interview results, will be published in peer-reviewed manuscripts. The results are also anticipated to be presented at relevant scientific conferences and local forums.
Trial status and summary
This is a mixed-methods implementation study of PreOpET in people undergoing lung cancer resection. The current protocol is Version 3 dated 26 February 2024. The study was registered and approved prospectively in the Australia and New Zealand Clinical Trials Registry (ANZCTR) and any future protocol changes will be documented and approved by both the ANZCTR and NSLHD Human Research Ethics Committee. Recruitment commenced in June 2024 with recruitment completion expected in December 2025 with final study visits occurring in June 2026.
Supplementary material
Footnotes
Funding: This study is funded by the estate of M.G. Starr.
Prepublication history and additional supplemental material for this paper are available online. To view these files, please visit the journal online (https://doi.org/10.1136/bmjopen-2025-101624).
Provenance and peer review: Not commissioned; externally peer reviewed.
Patient consent for publication: Not applicable.
Patient and public involvement: Patients and/or the public were involved in the design, or conduct, or reporting, or dissemination plans of this research. Refer to the Methods section for further details.
References
- 1.Canberra: Australian Institute of Health and Welfare; 2011. Lung cancer in Australia: an overview. [Google Scholar]
- 2.Brunelli A, Charloux A, Bolliger CT, et al. ERS/ESTS clinical guidelines on fitness for radical therapy in lung cancer patients (surgery and chemo-radiotherapy) Eur Respir J. 2009;34:17–41. doi: 10.1183/09031936.00184308. [DOI] [PubMed] [Google Scholar]
- 3.Brunelli A, Kim AW, Berger KI, et al. Physiologic evaluation of the patient with lung cancer being considered for resectional surgery: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2013;143:e166S–e190S. doi: 10.1378/chest.12-2395. [DOI] [PubMed] [Google Scholar]
- 4.Granger C, Cavalheri V. Preoperative exercise training for people with non-small cell lung cancer. Cochrane Database Syst Rev. 2022;9:CD012020. doi: 10.1002/14651858.CD012020.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Whish-Wilson GA, Edbrooke L, Cavalheri V, et al. Physiotherapy and Exercise Management of People Undergoing Surgery for Lung Cancer: A Survey of Current Practice across Australia and New Zealand. J Clin Med. 2023;12:2146. doi: 10.3390/jcm12062146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huang J, Lai Y, Zhou X, et al. Short-term high-intensity rehabilitation in radically treated lung cancer: a three-armed randomized controlled trial. J Thorac Dis. 2017;9:1919–29. doi: 10.21037/jtd.2017.06.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lai Y, Wang X, Zhou K, et al. Impact of one-week preoperative physical training on clinical outcomes of surgical lung cancer patients with limited lung function: a randomized trial. Ann Transl Med. 2019;7:544. doi: 10.21037/atm.2019.09.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Licker M, Karenovics W, Diaper J, et al. Short-Term Preoperative High-Intensity Interval Training in Patients Awaiting Lung Cancer Surgery: A Randomized Controlled Trial. J Thorac Oncol. 2017;12:323–33. doi: 10.1016/j.jtho.2016.09.125. [DOI] [PubMed] [Google Scholar]
- 9.Stefanelli F, Meoli I, Cobuccio R, et al. High-intensity training and cardiopulmonary exercise testing in patients with chronic obstructive pulmonary disease and non-small-cell lung cancer undergoing lobectomy. Eur J Cardiothorac Surg. 2013;44:e260–5. doi: 10.1093/ejcts/ezt375. [DOI] [PubMed] [Google Scholar]
- 10.Machado P, Pimenta S, Garcia AL, et al. Home-Based Preoperative Exercise Training for Lung Cancer Patients Undergoing Surgery: A Feasibility Trial. J Clin Med. 2023;12:2971. doi: 10.3390/jcm12082971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cavalheri V, Granger C. Preoperative exercise training for patients with non-small cell lung cancer. Cochrane Database Syst Rev. 2017;6:CD012020. doi: 10.1002/14651858.CD012020.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sebio García R, Yáñez-Brage MI, Giménez Moolhuyzen E, et al. Preoperative exercise training prevents functional decline after lung resection surgery: a randomized, single-blind controlled trial. Clin Rehabil. 2017;31:1057–67. doi: 10.1177/0269215516684179. [DOI] [PubMed] [Google Scholar]
- 13.Benzo R, Wigle D, Novotny P, et al. Preoperative pulmonary rehabilitation before lung cancer resection: results from two randomized studies. Lung Cancer. 2011;74:441–5. doi: 10.1016/j.lungcan.2011.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Boujibar F, Bonnevie T, Debeaumont D, et al. Impact of prehabilitation on morbidity and mortality after pulmonary lobectomy by minimally invasive surgery: a cohort study. J Thorac Dis. 2018;10:2240–8. doi: 10.21037/jtd.2018.03.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kadiri SB, Kerr AP, Oswald NK, et al. Fit 4 surgery, a bespoke app with biofeedback delivers rehabilitation at home before and after elective lung resection. J Cardiothorac Surg. 2019;14:132. doi: 10.1186/s13019-019-0951-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ulrich CM, Himbert C, Barnes CA, et al. Precision Exercise Effect on Fatigue and Function in Lung Cancer Surgery: A Randomized Clinical Trial. JAMA Surg. 2025;160:495–519. doi: 10.1001/jamasurg.2025.0130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cox NS, Dal Corso S, Hansen H, et al. Telerehabilitation for chronic respiratory disease. Cochrane Database Syst Rev. 2021;1:CD013040. doi: 10.1002/14651858.CD013040.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bowman A, Denehy L, Edbrooke L. Thoracic Cancer Exercise Services in Australia: A Point-Prevalence Survey. Asia Pac J Clin Oncol. 2025;21:328–37. doi: 10.1111/ajco.14157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Avancini A, Giannarielli D, Belluomini L, et al. Physical Exercise During Neoadjuvant Treatments for Non-Small Cell Lung Cancer: The Time is Coming. Clin Lung Cancer. 2024;25:e431–5. doi: 10.1016/j.cllc.2024.07.015. [DOI] [PubMed] [Google Scholar]
- 20.Walker RC, Pezeshki P, Barman S, et al. Exercise During Chemotherapy for Cancer: A Systematic Review. J Surg Oncol. 2024;130:1725–36. doi: 10.1002/jso.27845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Malveiro C, Correia IR, Cargaleiro C, et al. Effects of exercise training on cancer patients undergoing neoadjuvant treatment: A systematic review. J Sci Med Sport. 2023;26:586–92. doi: 10.1016/j.jsams.2023.08.178. [DOI] [PubMed] [Google Scholar]
- 22.Voorn MJJ, Bastiaansen EMW, Schröder CD, et al. A qualitative stakeholder analysis of beliefs, facilitators, and barriers for a feasible prehabilitation program before lung cancer surgery. J Cancer Res Clin Oncol. 2023;149:15713–26. doi: 10.1007/s00432-023-05298-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Eser P, Klaus C, Vetsch T, et al. Qualitative assessment of expectations on the content, form and way of delivery of a prehabilitation programme in patients with lung resection surgery - A Swiss tertiary centre experience. SAGE Open Med. 2024;12:20503121241233427. doi: 10.1177/20503121241233427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Crandall K, Maguire R, Campbell A, et al. A qualitative study exploring the views, attitudes and beliefs of patients and health professionals towards exercise intervention for people who are surgically treated for lung cancer. Eur J Cancer Care (Engl) 2018;27:e12828. doi: 10.1111/ecc.12828. [DOI] [PubMed] [Google Scholar]
- 25.Ferreira V, Agnihotram RV, Bergdahl A, et al. Maximizing patient adherence to prehabilitation: what do the patients say? Support Care Cancer . 2018;26:2717–23. doi: 10.1007/s00520-018-4109-1. [DOI] [PubMed] [Google Scholar]
- 26.Whish-Wilson GA, Edbrooke L, Cavalheri V, et al. Empowering Recovery: A Co-Designed Intervention to Transform Care for Operable Lung Cancer. Health Expect. 2025;28:e70196. doi: 10.1111/hex.70196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chan A-W, Tetzlaff JM, Altman DG, et al. SPIRIT 2013 Statement: Defining Standard Protocol Items for Clinical Trials. Ann Intern Med. 2013;158:200–7. doi: 10.7326/0003-4819-158-3-201302050-00583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hoffmann TC, Glasziou PP, Boutron I, et al. Better reporting of interventions: template for intervention description and replication (TIDieR) checklist and guide. BMJ. 2014;348:g1687. doi: 10.1136/bmj.g1687. [DOI] [PubMed] [Google Scholar]
- 29.Slade SC, Dionne CE, Underwood M, et al. Consensus on Exercise Reporting Template (CERT): Explanation and Elaboration Statement. Br J Sports Med. 2016;50:1428–37. doi: 10.1136/bjsports-2016-096651. [DOI] [PubMed] [Google Scholar]
- 30.Holtrop JS, Estabrooks PA, Gaglio B, et al. Understanding and applying the RE-AIM framework: Clarifications and resources. J Clin Transl Sci. 2021;5:e126. doi: 10.1017/cts.2021.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Holland AE, Spruit MA, Troosters T, et al. An official European Respiratory Society/American Thoracic Society technical standard: field walking tests in chronic respiratory disease. Eur Respir J. 2014;44:1428–46. doi: 10.1183/09031936.00150314. [DOI] [PubMed] [Google Scholar]
- 32.Borg GAV. Psychophysical bases of perceived exertion. Med Sci Sports Exerc. 1982;14:377. doi: 10.1249/00005768-198205000-00012. [DOI] [PubMed] [Google Scholar]
- 33.Vaidya T, de Bisschop C, Beaumont M, et al. Is the 1-minute sit-to-stand test a good tool for the evaluation of the impact of pulmonary rehabilitation? Determination of the minimal important difference in COPD. Int J Chron Obstruct Pulmon Dis. 2016;11:2609–16. doi: 10.2147/COPD.S115439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.King GG, Bates J, Berger KI, et al. Technical standards for respiratory oscillometry. Eur Respir J. 2020;55:1900753. doi: 10.1183/13993003.00753-2019. [DOI] [PubMed] [Google Scholar]
- 35.Zimmermann SC, Thamrin C, Chan AS, et al. Relationships Between Forced Oscillatory Impedance and 6-minute Walk Distance After Pulmonary Rehabilitation in COPD. Int J Chron Obstruct Pulmon Dis. 2020;15:157–66. doi: 10.2147/COPD.S225543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stanojevic S, Kaminsky DA, Miller MR, et al. ERS/ATS technical standard on interpretive strategies for routine lung function tests. Eur Respir J. 2022;60:2101499. doi: 10.1183/13993003.01499-2021. [DOI] [PubMed] [Google Scholar]
- 37.Aaronson NK, Ahmedzai S, Bergman B, et al. The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst. 1993;85:365–76. doi: 10.1093/jnci/85.5.365. [DOI] [PubMed] [Google Scholar]
- 38.Bergman B, Aaronson NK, Ahmedzai S, et al. The EORTC QLQ-LC13: a modular supplement to the EORTC Core Quality of Life Questionnaire (QLQ-C30) for use in lung cancer clinical trials. EORTC Study Group on Quality of Life. Eur J Cancer . 1994;30A:635–42. doi: 10.1016/0959-8049(94)90535-5. [DOI] [PubMed] [Google Scholar]
- 39.Herdman M, Gudex C, Lloyd A, et al. Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L) Qual Life Res. 2011;20:1727–36. doi: 10.1007/s11136-011-9903-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Williams MT, Lewthwaite H, Paquet C, et al. Dyspnoea-12 and Multidimensional Dyspnea Profile: Systematic Review of Use and Properties. J Pain Symptom Manage. 2022;63:e75–87. doi: 10.1016/j.jpainsymman.2021.06.023. [DOI] [PubMed] [Google Scholar]
- 41.Yellen SB, Cella DF, Webster K, et al. Measuring fatigue and other anemia-related symptoms with the Functional Assessment of Cancer Therapy (FACT) measurement system. J Pain Symptom Manage. 1997;13:63–74. doi: 10.1016/s0885-3924(96)00274-6. [DOI] [PubMed] [Google Scholar]
- 42.Van Belle S, Paridaens R, Evers G, et al. Comparison of proposed diagnostic criteria with FACT-F and VAS for cancer-related fatigue: proposal for use as a screening tool. Support Care Cancer. 2005;13:246–54. doi: 10.1007/s00520-004-0734-y. [DOI] [PubMed] [Google Scholar]
- 43.Sekhon M, Cartwright M, Francis JJ. Development of a theory-informed questionnaire to assess the acceptability of healthcare interventions. BMC Health Serv Res. 2022;22:279. doi: 10.1186/s12913-022-07577-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Braun V, Clarke V. Using thematic analysis in psychology. Qual Res Psychol. 2006;3:77–101. doi: 10.1191/1478088706qp063oa. [DOI] [Google Scholar]
- 45.Glasgow RE, Vogt TM, Boles SM. Evaluating the public health impact of health promotion interventions: the RE-AIM framework. Am J Public Health. 1999;89:1322–7. doi: 10.2105/ajph.89.9.1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Probst VS, Hernandes NA, Teixeira DC, et al. Reference values for the incremental shuttle walking test. Respir Med. 2012;106:243–8. doi: 10.1016/j.rmed.2011.07.023. [DOI] [PubMed] [Google Scholar]
- 47.Daynes E, Barker RE, Jones AV, et al. Determining the minimum important differences for field walking tests in adults with long-term conditions: a systematic review and meta-analysis. Eur Respir Rev. 2025;34:240198. doi: 10.1183/16000617.0198-2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Braun V, Clarke V. Conceptual and design thinking for thematic analysis. Qualitative Psychology . 2022;9:3–26. doi: 10.1037/qup0000196. [DOI] [Google Scholar]
- 49.Hill K, Dolmage TE, Woon L, et al. Comparing peak and submaximal cardiorespiratory responses during field walking tests with incremental cycle ergometry in COPD. Respirology. 2012;17:278–84. doi: 10.1111/j.1440-1843.2011.02089.x. [DOI] [PubMed] [Google Scholar]